Abstract
Bone is a dynamic tissue with mechanical and biological functions, including adaption to load changes, storage of calcium and phosphorus ions, and bidirectional crosstalk with other tissues. In elderly, increased osteoclastic resorption and decreased osteoblastic formation activities result in osteoporosis. The pathogenetic mechanisms involved in skeletal aging are chronic inflammation, hormonal changes, genetic predisposition, low physical activity, and inadequate nutrition. Bone aging is also characterized by an inadequate mineralization of the osteoid tissue that mainly depends on impaired intestinal absorption of dietary calcium, in turn caused by hypovitaminosis D, resulting in osteomalacia. The coexistence of osteoporosis and osteomalacia (“osteoporomalacia”) increases the risk of fragility fractures, and it should be considered the typical senile bone disease. Fall is the most important risk factor for osteoporotic fractures, particularly in elderly people.
The comprehensive fracture prevention strategy is essentially based on pharmacological (bisphosphonates, denosumab, teriparatide, SERMs) and non-pharmacological approaches, such as risk of falling reduction, exercise, lifestyle modifications, adequate calcium, and vitamin D intake.
The global rehabilitative approach is a mainstay in all stages of osteoporotic fracture, from prevention to patient’s functional recovery. The main role of the physiatrist is to provide an individual rehabilitation plan (IRP) that aims to reduce mortality and disability of older patients with fragility fractures. The key element of rehabilitation is the therapeutic exercise, particularly resistance training, that is effective in both maintaining bone health and improving muscle performance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Bone Physiology and Age-Related Bone Changes
1.1 Bone Tissue Metabolism in Older Adults
Bone tissue undergoes continuous remodeling throughout life, where resorption and formation alternatively occur. From a biomechanical point of view, the aim of bone remodeling is to repair the micro-cracks and to adapt the shape and the bone density to the mechanical load which the skeleton usually undergoes. Bone tissue also serves to store various substances, in particular calcium and phosphorus ions that can be released into the blood in order to keep steady their serum concentrations.
The 5–10% of the entire adult skeleton is replaced every year through bone remodeling [1]. Bone turnover is rigorously regulated by the coupled action of osteoblasts and osteoclasts. These two types of cells build the basic multicellular units (BMUs), whose activities are controlled by several hormones and growth factors [2].
During skeletal growth, formation exceeds resorption with a consequent increase in the total body bone mass, reaching the bone peak mass (between 20 and 25 years). Bone formation and resorption are balanced till the fourth decade of life. After this time the total bone mass will start to decrease, so that at 80 years it will be reduced to the 50% of the peak value [3]. In fact, in the elderly, bone remodeling is characterized by an increase of osteoclastic resorption and a reduction of osteoblastic formation, resulting in bone mass reduction. Among the pathogenetic mechanisms involved in skeletal aging, chronic inflammation (inflammaging) has been identified as playing an important role, through the action of pro-inflammatory cytokines on bone remodeling process [4]. Several studies have shown that the levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and IL-1, elevated in elderly [5], inhibit the production of new osteoblasts and can induce their apoptosis. Moreover, in aging, there is an alteration in growth hormone (GH) and insulin-like growth factor-1 (IGF-1) serum levels with a progressive decrease of GH secretion and consequent reduction of IGF-1 levels [6], which is further decreased by the simultaneous increase in serum IGF-binding proteins that reduces the bioavailability of IGF-1 [7]. This protein is a powerful anabolic factor that increases osteoblasts number and activity [8].
Another issue with aging is the reduction of physical activity [9]. In fact, it is well known that exercise, through the increase of the mechanical load, can positively influence bone mass [10].
Bone is a tissue that dynamically adapts its mass and architecture to the mechanical stress of daily living. Osteocytes are considered mechano-sensors that detect mechanical afferents and translate them into biochemical messages. In physiological conditions, the osteocyte network controls osteoclastogenesis and suppresses osteoblast function, thus increasing bone resorption and inhibiting bone formation. Unloading conditions promote the osteocytes production of molecules stimulating osteoclastogenesis, whereas physical activity counteracts this function by reducing both osteoclastogenesis and at the same time stimulating osteoblast function [11]. Recently it has been shown that osteocytes produce several molecules in response to mechanical stimulation: in addition to the collagen and alkaline phosphatase, these cells produce DMP1 (dentin matrix protein 1), PHEX (phosphate-regulating neutral endopeptidase on chromosome X), MEPE (matrix extracellular phosphoglycoprotein), FGF-23 (fibroblast growth factor 23), osteocalcin, osteoprotegerin, and sclerostin [12]. In particular, sclerostin is an acid glycoprotein of 190 amino acids secreted mainly by osteocytes [13]. Its function is to antagonize the Wnt signaling pathway, thus suppressing the activity of osteoblasts and downregulating bone turnover [13]. This pathway is involved in bone response to mechanical loads.
Both adequate nutrition and physical activity are necessary to achieve the genetically determined peak bone mass, which in turn is one of the most important factors that influence bone mass in old age [14].
1.2 Calcium, Phosphorus, and Vitamin D Metabolism in the Young Adult and in the Elderly
Bone aging is characterized by an inadequate mineralization of the osteoid tissue. It mainly depends on an impaired intestinal absorption of dietary calcium. The trans-cellular active absorption is regulated by 1,25(OH)2D that induces the synthesis of the calcium-binding protein (CBP) or calbindin, which facilitates the diffusion of calcium inside the cell toward the basolateral membrane and the extracellular fluid [15]. A variable amount of vitamin D is conducted to storage sites, mainly adipose and muscle tissues, from where it is continuously mobilized. Several studies have shown that plasma levels of both 25-OH-D3 (the vitamin D metabolite usually detected in the blood) and its biologically active form (calcitriol) decrease with aging by about 50% in both men and women. Hypovitaminosis D is an extremely common condition in the elderly because of the reduction of sun exposure, of the ability to convert vitamin D by the skin, of the vitamin D intake, of the intestinal vitamin D absorption, and of the 1-α-hydroxylase activity [16].
In the elderly, the reduced calcium availability, resulting from hypovitaminosis D, leads to an abnormal calcification of osteoid tissue, named osteomalacia. The simultaneous increase in serum PTH levels, aiming to increase the calcium levels, causes an activation of osteoclasts that leads to an excessive cortical and trabecular bone resorption resulting in osteoporosis. The coexistence of these two pathological conditions gives rise to the so-called osteoporomalacia that should be considered the typical senile bone disease.
1.3 Crosstalk Among Bone and Other Tissues
Bone might be considered as an endocrine organ, since it produces molecules acting not only on the bone itself but also at distance on other tissues and organs. On the other hand, bone is a target of several substances produced by other tissues. These mutual interactions play a key role in regulating relevant metabolic activities.
1.4 Bone and Gonads
Sex steroids affect bone and muscle growth and contribute to the maintenance of the homeostasis of these tissues during adulthood, so their deficiency can contribute to the development of osteoporosis and sarcopenia in both genders.
After menopause the major estrogen source is the adipose tissue that converts androstenedione, derived from dehydroepiandrosterone, into estrone. This latter does not exert the same trophic function on bone as estradiol; therefore, it is not able to counteract the age-dependent changes of the female skeleton. That is why the loss of bone mass, architectural integrity, and strength can be observed in female senile skeleton. In elderly men bone mass decreases with the decline of gonadal function due to the loss of Leydig cells in the testes and consequent decrease of testosterone levels [17]. However, androgens appear to be less important than estrogen in the maintenance of bone health in older men [18].
1.5 Bone, Muscle, and Adipose Tissue
Bone, muscle, and adipose tissues are involved in the complex energy metabolism scenario. These tissues have the same mesodermal origin and influence each other through a biochemical crosstalk producing several molecules with paracrine and endocrine activities [19]. Both osteoblasts and muscle fibers express receptors for two hormones produced by fat tissue, leptin, and adiponectin that have direct osteogenic effects [20, 21], promoting osteoblastogenesis and inhibiting osteoclastogenesis in bone marrow stromal cells as demonstrated in animal studies [22, 23]. In muscle, adiponectin increases mitochondrial number improving the oxidative tissue metabolism [24].
Another actor in bone/muscle/fat crosstalk is osteocalcin, a protein produced by osteoblasts and routinely used as a marker of bone formation. Osteocalcin stimulates the synthesis of adiponectin by adipocytes, increasing insulin sensitivity in muscles and enhancing the testosterone release in males [25].
1.6 Bone and Nervous System
Bone is an extremely dynamic tissue, as it continuously remodels itself, requiring a large amount of energy as well as muscle activity. Fat tissue provides the energy used to drive these complex metabolic processes. Through the mechanism of appetite regulation, the hypothalamus controls the amount of fat tissue. This anatomical region also modulates reproductive mechanisms, thus representing the link between energy metabolism and reproduction and bone metabolism, with osteocalcin as a modulator of these interactions. Hypothalamic cells present leptin receptors that stimulate the sympathetic activity, which, via osteoblastic adrenergic receptors, induces the overexpression of the gene coding for the RANKL, thus promoting bone resorption. Therefore, leptin inhibits the appetite and promotes the reproduction, energy consumption, and bone resorption.
It is likely that, within the central nervous system (CNS), there is the “control room” of the integrated management of bone-muscle-fat energy metabolism [26]. Recently, there have been identified four stages for these complex mechanisms. In the first stage, bone detects the energy requirements, and fat deposits send afferent signals to the hypothalamus. In the second stage, there is the activation of the hypothalamus and other complex neuronal network that promotes adrenergic activity in order to regulate bone metabolism (third stage), since osteoblasts express beta-adrenergic receptors. These cells modulate the synthesis of adiponectin by the adipocytes, completing the crosstalk circle between bone tissue and CNS (fourth stage). It is likely that these complex mechanisms might change with aging because of the alterations of the single components (bone, muscle, and fat) and also of the “control room” within the CNS (Fig. 27.1).
2 Osteo-metabolic Disorders in Elderly: Osteoporosis and Osteomalacia
2.1 Osteoporosis
2.1.1 Definition
Osteoporosis is a systemic skeletal disease characterized by a generalized decrease in bone density and deterioration of the microarchitecture of bone tissue that predispose to skeletal fragility resulting in increased risk of fractures [27].
From an operational point of view, the WHO defined osteoporosis when the bone mineral density (BMD) is less than 2.5 standard deviations than the mean peak bone mass of healthy young adults [27], based on a BMD measured by dual-energy X-ray absorptiometry (DXA). The normal BMD corresponds to a T-score ≥ −1; osteopenia is defined as a T-score between −1 and −2.5; severe osteoporosis as a T-score ≤ −2.5 with a fracture.
2.1.2 Epidemiology
The incidence of osteoporosis increases with aging affecting most of the population over 80 years old. It is estimated that about one out of two women over the age of 50 will sustain a fragility fracture [28]. In the Italian people aged over 50, the incidence of hip fractures is more than 90,000, and vertebral fracture was detected in over 20% of people over 65 in both men and women [29].
2.1.3 Physiopathology
Primary or idiopathic osteoporosis includes juvenile, postmenopausal, and senile osteoporosis.
Secondary osteoporosis instead includes all clinical conditions in which the bone involvement is not the main pathologic finding, but the bone is one of the targets of primary disease or related treatments, especially those that include the use of glucocorticoids.
The main pathogenetic factor of postmenopausal osteoporosis is the estrogen depletion that worsens the age-related bone loss occurring from the age of 40 [30]. Senile osteoporosis comes from the combination of various factors: tissue aging, hormone depletion, nutritional disorders, and decrease in physical activity.
Secondary osteoporosis is caused by several diseases such as endocrine disorders and hematologic, gastrointestinal, rheumatic, and kidney diseases or by the use of medications such as glucocorticoids, anticoagulants, and other drugs [31].
Male osteoporosis can be considered primary only in 40% of cases [32], whereas most frequently it is secondary to other conditions, such as hypogonadism, alcoholism, multiple myeloma, hyperparathyroidism, malabsorption, and corticosteroid use [33], but also to androgen deprivation therapy for prostate cancer [34].
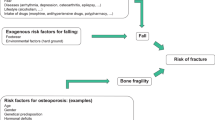
2.1.4 Risk of Falls and Fragility Fractures in Older Patients
Fall is the most important risk factor for fragility fractures, and the risk of falling increases exponentially with aging. The risk factors of falling can be categorized as intrinsic or extrinsic. The first ones include the physiological decline in age-related postural control mechanisms [35] and appendicular muscle strength, medications (diuretics, antiarrhythmics, antidepressants), orthostatic hypotension, and comorbidities. Neurological diseases, such as Parkinson’s disease, cerebellar disorders, peripheral neuropathy, myelopathy secondary to cervical spondylosis, epilepsy, and stroke, increase the risk of falling.
Extrinsic risk factors include environmental issues, such as low or soft chairs, carpets, slippery surfaces, raised thresholds, stairs (especially the first and last step), inadequate lighting, unsuitable shoes, clutter, and wires. The reduction of the risk of falling is the main target of a non-pharmacological approach for the prevention of osteoporotic fractures [36].
Fragility fractures occur when a mechanical stress applied to the bone exceeds its strength. They result from a “low-energy” trauma due to mechanical forces equivalent to a fall from a standing height or less, which would not ordinarily cause a fracture.
2.1.5 Treatment of Primary and Secondary Osteoporosis
In order to reduce the risk of fragility fractures, a preventive strategy can be taken which is essentially based on exercise and lifestyle [37]. The National Osteoporosis Foundation (NOF) suggested the use of different prevention measures that include an adequate calcium and vitamin D intake, constant and regular physical activity, smoking cessation, alcoholism identification and treatment, and fall prevention. Physical activity is strongly recommended as an effective strategy to reduce the risk of osteoporosis and fracture through its beneficial effects on bone, muscle, and risk of falling [38].
The estimated risk necessary to establish the threshold of the pharmacological intervention is based on both the BMD and clinical fracture risk factors. Integrated assessment of multiple risk factors can be done through algorithms validated as the FRAX®.
The drugs commonly used for the treatment of osteoporosis and their related evidence are shown in Tables 27.1 and 27.2.
2.2 Osteomalacia
2.2.1 Definition
Osteomalacia is a metabolic bone disorder characterized by the presence of a normal bone mass, with reduced mineral content for an inadequate mineralization of the organic matrix. Patients with osteomalacia may complain of bone pain and muscle weakness and present vertebral deformity and/or pseudofractures (Looser-Milkman striae) [39].
2.2.2 Epidemiology
Nowadays, osteomalacia is a fairly rare disease. According to the National Institutes of Health (NIH), its incidence is less than 1/1000 [39].
2.2.3 Pathophysiology
Osteomalacia is linked to a reduced availability or to an altered metabolism of vitamin D or to alterations of the renal tubular reabsorption of phosphorus. The most common causes of osteomalacia are shown in Table 27.3 [40].
In the elderly osteomalacia due to vitamin D deficiency is very common [41]. Hypovitaminosis D is also very common in people with darker skin pigmentation [42], obese, and people who undergo gastrectomy or suffer from liver cirrhosis [43].
2.2.4 Treatment
For the treatment of osteomalacia, it is essential to identify and promptly treat the underlying cause to prevent the occurrence of fractures; furthermore it is necessary to adopt simple corrective measures regarding nutrition and sun exposure. We should encourage patients to have a higher nutritional intake (oily fish, cod liver oil, egg yolk, mushrooms, cereals, and margarine enriched of vitamin D). Early pharmacological treatment is mandatory, consisting of 50–125 μg per day of calcifediol, or 5000–10,000 IU per day of cholecalciferol for 1–2 months, with a maintenance therapy of 20 μg per day of calcifediol, or 800 IU per day of cholecalciferol.
3 Physiatric Approach to Older Patients with Osteo-metabolic Disorders
3.1 Background
The global rehabilitative approach has a key role in all stages of these diseases, from prevention to functional recovery after a fragility fracture. The pivotal element of rehabilitation is the therapeutic exercise. Several studies and international guidelines suggested that this intervention is effective in increasing bone mass during skeletal growth, maintaining what has been achieved in adult life, reducing bone loss in the elderly and the risk of fractures.
In the elderly, exercise can enhance cortical thickness and strength in overloaded bone tissue. These effects might result from a diminished loss of endocortical bone and/or an increase in tissue density rather than an increase in bone size (due to periosteal apposition), even if also in elderly a progressive widening of the outer diameter occurs. These geometrical adaptations are able to increase the mechanical resistance to the compression load [44].
Two types of physical exercises are suitable for elderly: aerobic activities (walking, stair climbing, jogging, tennis, tai chi, gymnastics, dancing) and resistance exercises (where joints move against an external force given by weights, machines, or the own body weight).
The most common aerobic training in older people is walking that could be effective in maintaining BMD when combined with high-impact activities, such as jogging or stepping [45].
Therapeutic exercise could exert, through the improvement of the muscle performance, an indirect effect on bone health. However, aging is characterized by a progressive decline in aerobic exercise capacity due to the reduction in cardiovascular efficiency and skeletal muscle function caused by a decrease of mitochondrial number and activity [46]. Aerobic training stimulates the synthesis of mitochondria in the skeletal muscle with a consequent reduction of the oxidative stress and the enhancement of muscular performance [47].
Several studies suggest that the muscle strengthening produces a significant increase in femoral neck BMD [48]. Furthermore, improvement in muscle strength and performance, due to resistance exercise, might reduce the risk of falls.
At a cellular level, strengthening exercises increase the transverse diameter of type I and type II muscle fibers and of the entire lean mass, with relative increase in muscle strength [49]. Fast-velocity resistance exercises (i.e., performing a concentric phase as quickly as possible followed by an eccentric 2-second contraction phase) [50] have shown to cause a greater recruitment of motor units in type II muscle fibers counteracting atrophy in aging [51].
There is a general consensus that older people should perform moderate-intensity aerobic exercises for a minimum of 30 min 5 days a week or high-intensity aerobic activity for a minimum of 20 min 3 days per week [45]. Moderate-intensity aerobic exercise is intended, from an absolute scale, as the activity that takes place at an intensity of 3.0–5.9 times the intensity at rest or, from a relative scale based on individual’s capacity (ranging from 0 to 10), an intensity of 5 or 6 times the intensity at rest. High-intensity aerobic exercise is the activity that takes place at an intensity of at least 6 times the intensity at rest on an absolute scale, an exercise intensity of 7 or 8 times the intensity at rest based on individual’s capacity. Aerobic exercise should be performed for at least 10 consecutive minutes [52].
Bodyweight exercises or training with equipment is also useful, but should be performed slowly, with 1–3 series for each type of exercise, spacing 1–3 min of rest, for 2–3 days/week [52]. Strengthening exercises are safe, feasible, and effective to induce muscle hypertrophy and to increase strength and should be performed as soon as possible in order to prevent the progression of skeletal fragility and sarcopenia. General principles of the prescription of therapeutic exercise in osteoporotic patients have been proposed by a specific task force of the NOF [53] (Table 27.4).
3.1.1 Rehabilitation of Elderly Osteoporotic Patients Without Fragility Fractures and Prevention of the Risk of Falling
The rehabilitative approach to an old osteoporotic patient at risk of a fragility fracture aims to counteract the progressive osteoporomalacia and to reduce the risk of falling. The adoption of safety measures at home, the correction of modifiable intrinsic factors such as visual disturbances, pacemaker implantation in patients with sick sinus syndrome, and the gradual reduction in the consumption of psychotropic drugs are recommended according to a Cochrane review of 2012 [54].
NICE guidelines (2013) [55] support a multidisciplinary assessment and a comprehensive intervention in order to improve both physical and psychological health. It has been shown that combined programs of balance training and muscle strengthening are effective in the prevention of falls in elderly residents in the community. On the other hand, there is no scientific evidence supporting brisk walking as a preventive measure that reduces the risk of falls.
Recently, a Cochrane review underlined that the hip protectors, when correctly worn, might decrease hip fracture risk and both morbidity and mortality in elderly, especially in institutionalized individuals [56].
3.1.2 Rehabilitation of Elderly Patients with Fragility Fractures
The most frequent sites of osteoporotic fractures are the spine, hip, proximal humerus, and distal radius, but actually also other fractures might recognize an osteoporotic pathogenesis [29]. In many cases a surgical approach followed by a rehabilitative treatment is necessary in order to improve the functional activity and social participation and thus the quality of life of the individual. The majority of distal radius fractures is treated conservatively by closed reduction and cast/splint application for 4–6 weeks, although currently many authors prefer an internal fixation. Rehabilitation programs generally begin after cast removal and consist of range-of-motion (ROM) exercises and resistance exercise associated with occupational therapy.
Clinical vertebral fractures are commonly treated with rest and/or spinal bracing. Indications to surgery vary according to age, general clinical condition, type of fracture and spinal stability, involvement of the spinal cord, bone quality, and time from the fracture. Surgical options are frequently vertebroplasty and kyphoplasty, rarely spinal stabilization with or without fusion [57].
Rehabilitative approach begins immediately after surgery in order to recover muscle strength and spinal mobility and to enhance balance, especially during postural changes and walking. However, the strengthening of back extensor muscles is a priority [53].
Fractures of the proximal humerus are usually treated conservatively in the elderly, unless there is an unstable or displaced four-part fracture that requires surgical approach, consisting of open reduction with internal fixation or, in some cases, arthroplasty [57]. Rehabilitation starts after cast removal or surgical treatment, aiming to reduce pain and recover the ability in ADL.
Hip fracture is undoubtedly the most relevant complication of bone fragility in elderly. The main role of the physiatrist is to figure out an individual rehabilitation plan (IRP) that aims to reduce mortality and disability [58]. Hip fractures are currently treated surgically within 24–48 h from injury. Surgical intervention consists of hemiarthroplasty or total hip arthroplasty for intracapsular hip fractures, whereas osteosynthesis (screw plate or intramedullary nail) is preferred for trochanteric fractures. Considering the patient clinical condition and presence of comorbidities, rehabilitation begins immediately after surgery. Several factors should be taken into account in order to decide the timing of weight-bearing on the operated limb (Table 27.5).
The rationale for partial weight-bearing is based on the assumption that an early excessive load on the operated limb may cause micro-movements at the bone-implant interface, compromising the initial implant stability that interferes with bone healing and predispose to aseptic loosening of the implant. On the other hand, early weight-bearing after total hip arthroplasty (THA) could have potential benefits, in particular counteracting bone loss and allowing early recovery of functional activities. Patient education should be emphasized to prevent the dislocation of the prosthetic implant, through specific preventive rules. The IRP proposed by the physiatrist should include several targets that have to be addressed by rehabilitation programs as showed in Table 27.6.
Home-based programs should begin 2–3 weeks after surgery and consist of specific exercises aiming to improve muscle performance and endurance, balance, and functional ability to perform ADLs independently. Precautions to take during ADL should continue for at least 12 weeks. The rehabilitation plan proposed in outpatient setting is shown in Table 27.7.
3.1.3 Rehabilitation of Elderly Patients with Osteomalacia
Osteomalacia is characterized by skeletal fragility and muscle weakness that can interfere with the ADLs performance. The rehabilitative approach should be targeted to improve muscle strength and physical performance, through low-impact activities, such as gait training, avoiding high-intensity exercises that might cause microfractures or interfere with bone healing processes. In the advanced stages of osteomalacia, a significant reduction of the strength in proximal muscles of the lower limbs has been shown, thus resulting in balance impairment and consequent increased risk of falling. In this condition, resistance exercises could significantly improve muscle strength and reduce the risk of falling. Furthermore, occupational therapy can provide coping strategies in order to better perform ADLs [59].
References
Parfitt AM (1982) The coupling of bone formation to bone resorption: a critical analysis of the concept and its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 4:1–6
Lee CA, Einhorn TA (2001) The bone organ system: form and function. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis, 2nd edn. Academic Press, Salt Lake City, pp 2–20
Duque G, Troen BR (2008) Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc 56(5):935–941
De Martinis M, Franceschi C, Monti D et al (2005) Inflammation-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 579:2035–2039
Nanes MSL (2003) Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene 4:1–15
Freemont AJ, Hoyland AJ (2007) Morphology, mechanisms and pathology of musculoskeletal ageing. J Pathol 21:252–259
Frystyk J (2005) Aging somatotropic axis: mechanisms and implications of insulin-like growth factor-related binding protein adaptation. Endocrinol Metab Clin North Am 34:865–876
Zofkova I (2003) Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol Res 52:657–679
Sinaki M (1998) Musculoskeletal challenges of osteoporosis. Aging Clin Exp Res 10:249–262
Felsing NE, Brasel JA, Cooper DM (1992) Effect of low and high intensity exercise on circulating growth hormone in men. J Clin Endocrinol Metab 75:157–162
Iolascon G, Resmini G, Tarantino U (2013) Mechanobiology of bone. Aging Clin Exp Res 25(Suppl 1):S3–S7
Seeman E (2006) Osteocytes—martyrs for integrity of bone strength. Osteoporos Int 17:1443–1448
Winkler D, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386
Rayal-Pandya M, Porta A et al (1998) Mechanism of action of 1,25 (OH)2 D3 on intestinal calcium absorption and renal calcium transportation. In: Holick MF (ed) Vitamin D physiology, molecular biology and clinical applications. Humana Press, Totowa, pp 163–173
Ireland P, Fordtran JS (1973) Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J Clin Invest 52:2672–2681
Orwoll ES, Bilezikian JP, Vaneerschueren D (eds) (2010) Osteoporosis in men: the effects of gender on skeletal health. Elsevier, Boston
Manolagas SC, O’Brien CA, Almeida M (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9:699–712
Reid IR (2008) Relationships between fat and bone. Osteoporos Int 19:595–606
Sáinz N, Rodríguez A, Catalán V, Becerril S, Ramírez B, Gómez-Ambrosi J, Frühbeck G (2009) Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1alpha in ob/ob mice. PLoS One 4(9):e6808
Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I (2005) Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331:520–526
Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150:3603–3610
Lee B, Shao J (2012) Adiponectin and lipid metabolism in skeletal muscle. Acta Pharm Sin B:335–434
Lee NK, Karsenty G (2008) Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 19:161–166
Buday B, Pach FP, Literati-Nagy B, Vitai M, Vecsei Z, Koranyi L (2013) Serum osteocalcin is associated with improved metabolic state via adiponectin in females versus testosterone in males. Gender specific nature of the bone-energy homeostasis axis. Bone 57(1):98–104
Gonzalez-Rozas M, Dueñas-Laita A, Perez-Castrillon JL (2015) The β-adrenergic system and bone mineral remodeling. Clin Rev Bone Miner Metab 13:114–124
World Health Organization Study Group (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129
National Osteoporosis Foundation (2013) Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington. Available at: http://nof.org/
Tarantino U, Capone A, Planta M, D’Arienzo M, Letizia Mauro G, Impagliazzo A, Formica A, Pallotta F, Patella V, Spinarelli A, Pazzaglia U, Zarattini G, Roselli M, Montanari G, Sessa G, Privitera M, Verdoia C, Corradini C, Feola M, Padolino A, Saturnino L, Scialdoni A, Rao C, Iolascon G, Brandi ML, Piscitelli P (2010) The incidence of hip, forearm, humeral, ankle, and vertebral fragility fractures in Italy: results from a 3-year multicenter study. Arthritis Res Ther 12(6):R226
Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK (2003) Bone loss and bone size after menopause. N Engl J Med 349(4):327–334
Riggs BL, Melton LJ 3rd (1983) Evidence for two distinct syndromes of involutional osteoporosis. Am J Med 75(6):899–901
Khosla S (2010) Update in male osteoporosis. J Clin Endocrinol Metab 95(1):3–10
Fitzpatrick LA (2002) Secondary causes of osteoporosis. Mayo Clin Proc 77:453–468
Adler RA, Hastings FW, Petkov VI (2010) Treatment thresholds for osteoporosis in men on androgen deprivation therapy: T-score versus FRAX. Osteoporos Int 21(4):647–653
Tinetti ME, Speechley M, Ginter SF (1988) Risk factors for falls among elderly persons living in the community. N Engl J Med 319:1701–1707
Tinetti ME, Doucette J, Claus E, Marottoli R (1995) Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc 43:1214–1221
Yates CJ, Chauchard MA, Liew D, Bucknill A, Wark JD (2015) Bridging the osteoporosis treatment gap: performance and cost-effectiveness of a fracture liaison service. J Clin Densitom 18(2):150–156
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis Foundation (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25(10):2359–2381
Whyte MP, Thakker RV (2009) Rickets and osteomalacia. Medicine 37:483–488
Field M, Burnett L, Sullivan D et al (2014) Clinical biochemistry and metabolism. In: Walker B, Colledge N, Raiston S et al (eds) Davidson’s principles and practice of medicine, 22nd edn. Churchill Livingstone Elsevier, Edinburgh
Pearce S, Cheetham T (2010) Diagnosis and management of vitamin D deficiency. BMJ 340:B3664
Holick M (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80(6 Suppl):1678S–1688S
Collier J (2007) Bone disorders in chronic liver disease. Hepatology 46:1271–1278
Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P (2010) Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med 8:47
Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C, American College of Sports Medicine, American Heart Association (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116(9):1094–1105
Staunton L, O’Connell K, Ohlendieck K (2011) Proteomic profiling of mitochondrial enzymes during skeletal muscle aging. J Aging Res. doi:10.4061/2011/908035
Forbes SC, Little JP, Candow DG (2012) Exercise and nutritional interventions for improving aging muscle health. Endocrine 42:29–38
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev (7):CD000333
Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpää T, Häkkinen K, Kovanen V, Ahtiainen JP, Selänne H (2013) Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol 113(3):641–650
Sayers SP (2007) High-speed power training: a novel approach to resistance training in older men and women. A brief review and pilot study. J Strength Cond Res 21:518–526
Bottaro M, Machado SN, Nogueira W, Scales R, Veloso J (2007) Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol 99(3):257–264
Global recommendations on physical activity for health. World Health Organization, 2011
Bonner FJ Jr, Sinaki M, Grabois M, Shipp KM, Lane JM, Lindsay R, Gold DT, Cosman F, Bouxsein ML, Weinstein JN, Gallagher RM, Melton LJ III, Salcido RS, Gordon SL (2003) Health professional’s guide to rehabilitation of the patient with osteoporosis. Osteoporos Int 14(Suppl 2):S1–22
Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, Kerse N (2012) Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev 12:12
NICE clinical guideline – June 2013 Falls: assessment and prevention of falls in older people
Santesso N, Carrasco-Labra A, Brignardello-Petersen R (2014) Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev 3:CD001255
Pietri M, Lucarini S (2007) The orthopaedic treatment of fragility fractures. Clin Cases Miner Bone Metab 4(2):108–116
Iolascon G, Gimigliano F, Piscitelli P et al (2007) Hip fracture in Italy: analysis of DRG data. Aging Clin Exp Res 19(3 Suppl):2–4
Walker J (2014) Pathogenesis, diagnosis and management of osteomalacia. Nurs Older People 26(6):32–37
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Iolascon, G., Gimigliano, F., Moretti, A., Covella, E., Gimigliano, R. (2018). Rehabilitation of Older Patients with Osteo-metabolic Disorders. In: Masiero, S., Carraro, U. (eds) Rehabilitation Medicine for Elderly Patients. Practical Issues in Geriatrics. Springer, Cham. https://doi.org/10.1007/978-3-319-57406-6_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-57406-6_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57405-9
Online ISBN: 978-3-319-57406-6
eBook Packages: MedicineMedicine (R0)