Abstract
Muscle strength and mass decline in sedentary individuals with aging. The present study investigated the effects of both age and 21 weeks of progressive hypertrophic resistance training (RT) on skeletal muscle size and strength, and on myostatin and myogenin mRNA expression in 21 previously untrained young men (26.0 ± 4.3 years) and 18 older men (61.2 ± 4.1 years) and age-matched controls. Vastus lateralis muscle biopsies were taken before and after RT. Type I and type II muscle fiber cross-sectional areas increased more in young men than in older men after RT (P < 0.05). Concentric leg extension increased (P < 0.05) more after 10.5 weeks in young men compared to older men, but after 21 weeks no statistical differences existed. The daily energy and protein intake were greater (P < 0.001) in young subjects. Both myostatin and myogenin mRNA expression increased in older when compared with young men after RT (P < 0.05). In conclusion, after RT, muscle fiber size increased less in older compared to young men. This was associated with lower protein and energy intake and increases in myostatin gene expression in older when compared to young men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle strength declines in sedentary individuals with aging. It has been reported that maximal strength is well maintained between the ages of 30–50 years, but after the age of 50 years there is a clear drop in strength (Viitasalo et al. 1985; Häkkinen and Häkkinen 1991; Porter et al. 1995). The decrease in maximal strength from the age of 30–80 years can be as large as 30–40 %. A large proportion of this decline in strength is due to a reduction in the size and/or number of fast twitch (type II) muscle fibers (Lexell 1995). Thus, the primary reason for the reduction of muscle strength is reduced muscle mass (Lambert and Evans 2002). This age-related loss in muscle mass has been termed sarcopenia (Evans 1995). While many older people consume adequate amounts of protein, some tend to have a reduced appetite and thus consume less protein than the recommended protein (Dalbo et al. 2011; Hulmi et al. 2007, 2008, 2009a, b; Kim et al. 2005a, 2007; Mascher et al. 2008), which likely results in an accelerated rate of sarcopenia. Sarcopenia is a major health concern as well as a socioeconomic problem. Therefore, various ways to counteract these processes are extremely important. Heavy resistance training (RT) has been shown to induce hypertrophy at both the whole muscle level and in myofibers themselves in all ages and in both genders, and has also beneficial effects on body composition, physical fitness and injury prevention, as well as rehabilitation (Bamman et al. 2003; Fiatarone et al. 1994; Häkkinen et al. 1998; Hulmi et al. 2007, 2009a; Ivey et al. 2000; Kosek et al. 2006). However, some studies (Kosek et al. 2006; Moritani and deVries 1980; Welle et al. 1996), but not all (Häkkinen et al. 1998; Roth et al. 2001), suggest that older individuals may have a smaller relative muscle hypertrophy response to RT than young individuals. Different training regimens, training background or age of the older subjects may explain some of the mixed findings in the literature.
Resistance exercise produces a strong stimulus to promote skeletal muscle hypertrophy, but nutrients/amino acids will also strongly stimulate protein synthesis and ultimately the growth of muscle (Anthony et al. 2000; Dreyer et al. 2006; Fujita et al. 2007). During recent years, both positive and negative regulators of muscle growth have been studied. Of these, myostatin, a well-known negative regulator of muscle size (McPherron et al. 1997) and proteins downstream to myostatin, such as myogenic regulatory factors and cell cycle kinases, as well as their inhibitors, regulate muscle mass (Charge and Rudnicki 2004; Kuang et al. 2006; McCroskery et al. 2003; Rios et al. 2002; Wagner 2005). In humans, a single resistance exercise bout can decrease the expression of myostatin at mRNA and/or protein level in both young and older men (Hulmi et al. 2007, 2008, 2009a, b; Kim et al. 2005a, 2007; Mascher et al. 2008). Interestingly, training-induced downregulation of myostatin was observed in the quadriceps muscle of young mice, but not in older mice, although variation between different muscle types was also reported (Leiter et al. 2011). The long-term effects of RT and aging in humans on myostatin and myogenic markers are not well known. Therefore, the purpose of the present study was to investigate primarily the effects of both age and 21 weeks of RT on skeletal muscle size and strength, as well as on basal levels of myostatin and myogenin gene expression.
Methods
Subjects
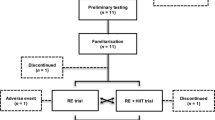
Subjects in the experimental groups were 21 previously untrained young (26.0 ± 4.3 years) and 18 older (61.2 ± 4.1 years) men (see Table 1). Serum testosterone was 17.6 ± 4.2 nmol/l in young men and 15.6 ± 4.8 nmol/l in older men (P = 0.17). Body fat percentage was smaller (P < 0.001) in young men (16.9 ± 4.0 %) compared to older men (24.4 ± 4.1 %). Nineteen men (42.5 ± 20.0 years) consisting of a combined group of young (n = 9; age 24.9 ± 2.7 years) and older (n = 10; age 62.1 ± 8.3 years) men served as controls (no RT). For the clarity of the results and equality of the subject number compared to the experimental groups in the statistics, the young and older controls were pooled. The subjects were recruited through local advertisements and screened by a medical doctor. None of the included subjects had contraindications to performing RT (e.g., a resting and exercise EKG was performed by all subjects). The exclusion criteria included the following: (1) those who engaged in moderate to heavy resistance RT within the past 5 years, (2) vegetarians, (3) those who regularly ingested nutritional supplements (e.g., protein drink or creatine) or pharmacological or hormonal substances that would have been expected to affect the responses, (4) elite athletes, (5) obese persons (body mass index >30) and (6) those with neuromuscular and cardiovascular diseases. The subjects were all moderately physically active. In the last few years, they had taken part in various low-intensity physical activities, such as walking, bicycling and swimming. The subjects were carefully informed about the design of the study with special information on possible risks and discomfort that might result. Thereafter, the subjects signed written informed consent to participate in the study, which was approved by the ethics committee of the university. The study was conducted according to the Declaration of Helsinki.
Experimental design
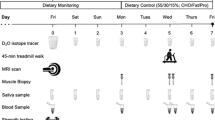
The total duration of the present study was 24 weeks where the first 3 weeks acted as a control period in which no experimental RT was carried out and the subjects maintained their normal recreational activities. After the pre-strength training (pre-ST) tests, the subjects were matched in pairs based on age, body composition and one repetition maximum (1RM) strength and randomly assigned to either the experimental groups (YOUNG or OLDER) or the control group (CONT) involving no RT, but continuing the habitual activity levels. The maximal strength of the training group subjects was tested four times: at week −3 and at week 0 (control period), as well as after 10.5 and 21 weeks. In the controls, all similar measurements were taken except at the 10.5-week time point. Furthermore, anthropometry and blood samples were collected in the morning after an overnight fast, and muscle biopsies without fasting were obtained at weeks 0 and 21 from all subjects, at about the same time of the morning. Diet diaries of the training group subjects were recorded at weeks 0, 10.5 and 21.
Experimental strength training
During the 21-week study period, RT sessions were carried out twice a week. A minimum of 2 days of rest was required between the two sessions each week. All training sessions were surveyed and supervised by the authors or assistant researchers. The following exercises were used in each training session: two exercises for the leg extensor muscles, the bilateral leg press exercise and bilateral knee extension exercise, and one exercise for the leg flexors, the bilateral knee flexion exercise. The RT program also included other exercises for the other main muscle groups of the body: chest and shoulders, upper back, trunk extensors and flexors, upper arms, ankle extensors, and hip abductors and adductors.
RT took place with progressive training loads of 40–80 % of the 1RM strength measured at 0 week. The number of the sets in each resistance exercise increased and the number of repetitions in each set decreased during the 21-week training period. During the first 7 weeks of RT, the subjects trained with loads of 40–60 % (10–15 repetitions) and 60–70 % (8–10 repetitions) of 1RM. The subjects performed two to four sets of each exercise. During the next 7 weeks, the loads were 60–70 % (8–10 repetitions) and 70–80 % (5–8 repetitions) of 1RM. During the last 7 weeks of RT, the subjects performed five to eight repetitions per set and two to five sets. The loads were mostly 70–80 % of 1RM. Rest periods were always 2–3 min between the sets. To produce improvements specifically in explosive strength during the present RT period, the subjects also performed a small part (~20 %) of all knee/leg extension exercises and bench press with light loads of 40–50 % of 1RM to fulfill the requirements of a typical explosive RT protocol. In general, the loads in all exercises were individually determined during the training sessions throughout the RT period. This type of RT program has been used in previous studies in our laboratory (e.g., Häkkinen et al. 2000) and is in line with the ACSM position stand (2009) recommendations of progression models in RT. Subjects were advised to continue their normal recreational physical activities such as walking, skiing, cycling and swimming during the RT program.
Daily nutrient intake
Macronutrient intake was recorded with 4-day diaries of the training group subjects at weeks 0, 10.5 and 21. Before the beginning of the study, each subject was provided with specific verbal and written instructions and procedures for reporting detailed dietary intake, including how to record portions by using household measures, exact brand names and preparation techniques. Dietary intake was obtained from food diaries and analyzed using the Micro Nutrica nutrient-analysis software version 3.11 (The Social Insurance Institution of Finland).
Maximal strength testing
Maximal bilateral isometric strength of the leg extensor muscles was measured on an electromechanical dynamometer at a knee angle of 107° and hip angle of 110° (Häkkinen et al. 2001b; Hulmi et al. 2007). A minimum of three trials were completed for each subject, and the best performance trial (i.e., the highest force) was used in the subsequent statistical analysis. The force signal of the isometric measurements was recorded and analyzed with Signal software version 2.15 (Cambridge Electronic Design Ltd., Cambridge, UK). A David 210 dynamometer (David Health Solutions Ltd, Finland) was used to measure maximal bilateral dynamic concentric strength of the leg extensors (hip and knee extensors). Separate trials were performed for concentric 1RM testing. After each repetition, the load was increased until the subject was unable to extend his legs to the full-extended ~180° knee angle position from ~60° knee angle. The highest load successfully lifted was determined as the 1RM. The subjects were carefully familiarized with the test procedures and had several warm-up contractions in all devices.
Anthropometry
After an overnight fast, body mass (kg) and fat percentage were measured. Body fat was measured using a skinfold caliper (biceps and triceps brachii, subscapularis and iliac crest) (Durnin and Womersley 1974). All the measurements were preceded by at least 2 days of rest from physical activity.
Muscle biopsy
Muscle biopsies were obtained before and after the 21-week training period. Biopsies were taken from the left vastus lateralis muscle, midway between the patella and the greater trochanter, in an area where the muscle was thickest and where no major nerves and blood vessels were located. The post-training biopsy was taken 1 week after the last training session after full recovery from the last exercise bout. Muscle samples were immediately frozen in liquid nitrogen and then stored at −80° C until analysis.
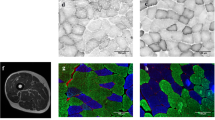
Muscle fiber cross-sectional area (CSA)
Serial 8-μm-thick transverse sections were cut on a cryomicrotome (Leica CM 3000) at –24 °C. Fiber type was classified by staining using the myofibrillar ATPase method. Fiber sarcolemma was visualized with an antibody against dystrophin (DYS2, Novocastra Laboratories, UK) using an avidin–biotin peroxidase kit (Vectastain PK-4002, Vector Laboratories, USA) with diaminobenzidine (Abbott Laboratories, USA) as a chromogen. The measurements of fiber CSA comprised an average of 106 ± 63 type I and 118 ± 62 type II muscle fibers. Stained cross sections were analyzed by Tema Image-Analysis System (Scan Beam) using a microscope (Olympus BX 50) and color video camera (Sanyo High-Resolution CCD).
Analysis of muscle messenger RNA
Total RNA isolation, reverse transcription and cDNA synthesis
Homogenization of the muscle samples was done with FastPrep (Bio101 Systems, USA) tubes and total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. An OD260/OD280 ratio of 1.8–2.0 and gel electrophoresis showed that our extraction yielded DNA free and undegraded RNA, respectively. Three μg of total RNA was reverse transcribed to synthesize cDNA according to the manufacturer’s instructions using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, Ca, USA).
Real-time RT-PCR
The mRNA expression levels were quantified with a real-time reverse transcriptase-PCR (RT-PCR) assay using an Abi 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The probes and primers used were pre-designed transcripts validated by Applied Biosystems bioinformatics design pipelines. The gene bank accession numbers and Applied Biosystems assay IDs, respectively, were: NM 005259 and Hs00193363_m1 (myostatin), NM 001106 and Hs00609603_m1 (activin receptor IIb), NM 005860 and Hs00610505_m1 (follistatin related gene protein, FLRG), NM 002478 and Hs00159528_m1 (MyoD), NM 002479 and Hs00231167_m1 (myogenin), NM_078467.1 and Hs00355782_m1 (p21), NM_052827.1 and Hs00608082_m1 (cdk2), NM002046, Hs99999905_m1 (GAPDH), NM_148177.1, and NM_058229.2 and Hs00369714_m1 (MAFbx) (Hulmi et al. 2007, 2008; Mascher et al. 2008).
Each sample was analyzed in triplicate. PCR cycle parameters used were for all genes: 50 °C for 2 min + 95 °C for 10 min and 37–45 cycles (depending on the mRNA analyzed) of 95 °C for 15 s and 60 °C for 1 min. Biological interpretations of expression results can depend on normalization (Flück et al. 2005). GAPDH mRNA was used as an endogenous control because it was shown to be better as a housekeeping gene than 18sRNA in our previous studies (Hulmi et al. 2007, 2008) and because GAPDH remained unchanged in both young (P = 0.2) and older (P = 0.3) subjects due to RT and thus did not affect on the results. Gene transcript results were calculated according to the Liu and Saint mathematical model (Liu and Saint 2002). SigmaPlot (version 9.0, Systat Software inc., Richmond, CA, USA) was used as a curve fitting software needed in the method.
Statistical analysis
Standard statistical methods were used for the calculation of means; standard deviations (SD) and the data from RE protocols were analyzed by a two-factor general linear model (GLM) using SPSS 12.0. Any violations of the assumptions of sphericity were explored and controlled. For each GLM with main or interaction effect, Holm–Bonferroni post hoc tests were performed to localize the effects.
Results
Training and nutrition
Experienced assistants supervised all the training bouts making sure that all the subjects trained their sets close to failure during the training session. The relative training intensity was compared in our major exercise, leg press, between the young and older individuals around weeks 7, 14 and 20 when 10 RM was tested in the gym. There was a slight but significant difference between the young and older subjects around weeks 7 and 14, but not anymore around week 20 (Fig. 1a).
a Relative average training intensity in loads compared to the measured 10 RM loads of the subjects at different time points. b Averaged protein, carbohydrate (CHO) and fat ingestion (g/kg body weight) during 4 days in week 0, 10.5 and 21, giving a total of 12 days. #Significantly (P < 0.05) greater in the young (white bars) than in the older (black bars) subjects in the training groups
In the training groups, the average (week 0, 10.5, and 21 combined) total energy intake per day in the young men (10209 ± 1915 kJ) was greater (P < 0.001) than in the older men (8002 ± 1543 kJ) and also relative to body mass (134.6 ± 22.6 kJ and 100.2 ± 23.2 kJ per kg; P < 0.001, respectively). The young men habitually ingested significantly more protein, carbohydrates and fat per body mass during RT than the older men (P < 0.05) (Fig. 1b). The amount of energy and protein tended to slightly increase from week 0 to 21 in both groups (not shown), but they were not significant due to large variation.
Body mass and fat
Body mass and body fat percentage are presented in Table 1. The older men had a greater (P < 0.001) fat percentage than young men.
Muscle fiber size
Young men had a consistent increase in muscle fiber size, also when compared with controls, in both type I and type II fibers (P < 0.01) (Fig. 2). In contrast, older men had significantly increased muscle fiber size only when I + II was averaged (P < 0.05). Only a trend existed when assessing individual fiber types I and II (P < 0.09). When this response was compared between young and older men, type I and type II, as well as the average muscle fiber CSA (type I + II), increased relatively and absolutely more in the young men than in older men after 21 weeks of training (P < 0.05). The difference between the pre- and post-distribution of fiber sizes was evident in all fiber sizes in both age groups, but the shift toward larger fiber sizes was strongest in the young subjects (Fig. 2d). The Kolmogorov–Smirnov test revealed that the fiber sizes turned out to be normally distributed in both young (P = 0.2) and old (P = 0.07) at the post-training state, unlike in the pre-training state in the young and older (P < 0.001) subjects.
Mean a Type I, b type II and c average (type I + II), and d distribution of VL muscle fiber CSA after 21-week RT or control period. *Significantly (P < 0.05) different from pre-value, #Significantly (P < 0.05) greater change in the young than in the older men, §significantly (P < 0.05) greater change in young or older training group subjects than in the controls. Both relative (not shown) and absolute changes in the means were larger in the young than in the older men and a shift toward larger fiber sizes was noticed
Maximal strength
Dynamic concentric and isometric strength values are presented in Fig. 3. At baseline, the young men were stronger in both concentric and isometric leg extension strength. Concentric leg extension 1RM increased more after 10.5 weeks in young men (21.4 ± 10.2 %) compared to the older men (9.8 ± 6.3 %) (P < 0.001), but no differences in the change existed after 21 weeks. Isometric leg extension strength increased after RT in both age groups compared to the controls (P < 0.05), but no differences existed in training-induced change between the young and older subjects.
Muscle mRNA
There were no differences in the pre- and post-RT GAPDH mRNA levels, validating the choice for the reference gene. Thus, all the present mRNA data were normalized to the GAPDH mRNA.
No changes were seen in the control group, whereas myostatin and myogenin mRNA increased in older men compared to young men during the 21-week RT period (P < 0.05; Fig. 4). The basal mRNA responses to RT of activin receptor IIb, FLRG, MyoD, p21, cdk2 and MAFbx were not different between the young and the older men (not shown).
Discussion
Main findings
In the present study, muscle fiber cross-sectional areas, both for type I and type II fibers of vastus lateralis, increased more in the young than in the older men after 21 weeks of resistance training. Moreover, concentric leg extension 1RM increased more after 10.5 weeks in the young compared to older men. The total energy intake per day relative to body mass was greater in the young than in the older trained subjects. More specifically, the young men ingested more protein, carbohydrates and fat per body mass during RT than older men, which may, in part, explain the more robust increase in fiber size in the young. The new finding was that both myostatin and myogenin mRNA increased in older men compared to young men during the 21-week RT period.
Muscle size and strength
RT used in this study took place with progressive hypertrophic training loads of 40–80 % of the 1RM strength and achieved large increases (10–20 %) in all muscle strength tests compared to the controls in both age groups during the 21-week periodized resistance training. These increases are consistent with a recent study in similarly previously untrained young and older men (Bickel et al. 2011). After 10.5 weeks, young men increased concentric leg extension force by 21 %, which was more than the increase of 9 % in older men. The increase continued during the second half of the study, but there were no longer age differences. A similar trend of age-specific difference was observed in isometric leg extension after 10.5 weeks and 21 weeks. The majority of strength gains was achieved during the first 10.5 weeks of training, which is consistent with the study by Bickel et al. (2011) where RT lasted 16 weeks, and by week 8 1RM improved 27 % in both young and older subjects, with additional increases of 11 and 9 %, respectively, from weeks 8 to 16.
Young subjects increased more of their myofiber CSA in both type I and type II muscle fibers than older subjects. Interestingly, our fiber size distribution results suggest that all sized fibers respond to resistance training in both younger and older subjects and the difference between the age groups was mainly in the magnitude of the change that was larger in the young. In accordance with the present study, some studies (Kosek et al. 2006; Moritani and deVries 1980; Welle et al. 1996), but not all (Häkkinen et al. 1998; Roth et al. 2001), suggest that older individuals may have a smaller relative muscle hypertrophy response to RT than young individuals. It can be speculated that months of heavy resistance training, e.g., two to three times per week of the same muscles may be too strenuous for some older individuals when compared to young ones. This is supported by our data showing that older individuals could train with slightly lower relative loads to ten repetition maximum compared to young subjects. This difference was, however, only statistically and possibly not physiologically significant. Older men may also respond better to a different training program than young men. Previously in our laboratory, Häkkinen et al. (1998) showed increases from 31 (type I) to 42 % (type II) in the vastus lateralis muscle fiber CSA in men over 60 years old following 6 months of resistance training.
In spite of these muscle fiber increases in the present study, there were no clear changes in body mass (and not in body fat percentage) during 21 weeks. This is somewhat surprising because a meta-analysis by Peterson et al. (2011), data derived from 49 studies and 81 cohorts, verified a strong association between full-body resistance exercise and increased lean body mass. The analysis revealed that after an average of 20.5 weeks of resistance training, aging men and women (over 50 years) experienced a significant main effect equal to a 1.1-kg increase in lean body mass. In older men and women, it is important to try to increase muscle mass, because of the 0.18-kg annual decline that may occur (Melton et al. 2000) through sedentary lifestyle, beyond 50 years of age. Peterson et al. (2011) concluded that the volume of training and age of participation are important determinants of effectiveness, suggesting that higher dosages result in greater adaptive response and aging individuals should consider starting a regimen of resistance training as early as possible to optimize results. Consequently, in our study there was a too low training volume for many muscle groups in order to increase whole body mass. This study especially concentrated on leg extensors and slightly less for some other muscle groups such as chest and back muscles. It should be noted also that we measured muscle fiber CSA only in one muscle, and exercise-induced muscle growth in young versus older individuals may be muscle specific. In addition, training-induced increases in muscle strength are achieved through muscle size and also neural adaptations (Harridge et al. 1999). These may explain the reason why, in muscle strength adaptation, the difference between young and older subjects was much smaller compared to the fiber size adaptations. It can be even speculated that neural adaptation can partially compensate the lower muscle fiber CSA increase in older subjects. However, this is only a speculation because direct measures for the changes in muscle activation or neural drive are lacking in the present study. Maybe even more likely, there may have been differences between the young and older subjects in muscle strength endurance adaptation such as 10–20 RM instead of the maximal dynamic and isometric strength that the present study investigated. Unfortunately, unlike concentric and isometric strengths, strength endurance was not investigated in standardized laboratory settings in the present study both before and after 21 weeks.
Nutrition
Our nutrition results from the training group subjects agree with those by Dalbo et al. (2011) who showed from 3-day food diaries that relative caloric, protein (1.6 g/kg body mass in the young and 0.8 g/kg body mass in the older subjects), carbohydrate and fat consumption were greater in young (21.0 ± 0.5 years) compared with older (66.4 ± 1.6 years) men. In the present study, the daily protein intake of ~1.4 g/kg body mass in young and ~1.1 g/kg body mass in older subjects is in the range of suggested daily intake of 1.0–1.3 g/kg body mass for older adults engaged in resistance training (Lucas and Heiss 2005). The amount should adequately and safely meet the needs of the older adults engaged in RT, provided that their energy needs are met, but this may not be optimal. Our data are associative and only allow us to speculate whether the difference between young and older men in the protein or energy intake was due to declined basal metabolism in older individuals (e.g., van Pelt et al. 2001). It is, however, possible that at least protein ingestion may have been inadequate at least in some individuals for optimal adaptations to occur (Campbell and Leidy 2003). Volpi et al. (1998) identified that a 3-h amino acid infusion stimulated muscle protein synthesis to a similar extent in young and older subjects. However, in response to ingestion of essential amino acids, the older subjects are less sensitive to smaller doses (7 vs. 15 g) compared with young subjects (Katsanos et al. 2005). It has been shown that reduced anabolic response to nutrients in older adults may be associated with dysregulated mTORC1 signaling (Cuthbertson et al. 2005; Guillet et al. 2004). Additional leucine in older subjects seems to be necessary to optimally stimulate muscle protein synthesis to a similar rate as in young adults (Katsanos et al. 2006), and this has also been observed in animal studies (Rieu et al. 2007). Therefore, it may be important for older individuals to eat a sufficient amount of protein with each meal to ensure sufficient leucine availability to maximize muscle protein synthesis during feeding (Paddon-Jones and Rasmussen 2009).
Myostatin and myogenin mRNA
Myostatin is a member of the TGF-β superfamily and is mainly expressed in skeletal muscles (McPherron et al. 1997). We found that myostatin basal mRNA increased 1.7-fold in older men, whereas in the young men there was no change during 21 weeks of training. In humans, a single resistance exercise bout can decrease myostatin gene experession in both young and older men (Dalbo et al. 2011; Hulmi et al. 2007, 2008, 2009a, b; Kim et al. 2005a, 2007; Mascher et al. 2008), which is, in theory, positive for muscle growth (McPherron et al. 1997). Interestingly, training-induced downregulation of myostatin was observed in the quadriceps muscle of young mice but not in older mice (Leiter et al. 2011), tending to partially support our finding. This was not the case, however, in other muscles.
Kim et al. (2005a) found that older females with less hypertrophy after RT showed attenuated decreases in myostatin mRNA after a resistance exercise bout. In contrast, after three sequential hypertrophic resistance exercise sessions (80 % of 1RM; 48 h between), Dalbo et al. (2011) found a significant decrease in myostatin mRNA in older men (68 years) after the third session compared with young men (28 years). It is clear that more exercise-related research comparing young and older individuals is needed to determine the role of myostatin in differentiating hypertrophy magnitude in these groups. The possible differences in myostatin results may depend on age and also on the training programs used in the studies (intensity and volume of training). Interestingly, as shown, since the time course of myostatin mRNA first declines and then increases after long-term training (Jespersen et al. 2011), the observed results may be explained by sampling 1 week after the last exercise bout. It is possible that the time course of myostatin expression after an exercise bout is different in older and young individuals, but more research is needed.
In the present study, myogenic regulatory factor myogenin mRNA increased in older when compared with young men suggesting that there may be a training-induced elevated basal regenerative activity in older muscles. Increased regenerative activity in older muscles has been speculated also earlier (Kim et al. 2005b). Although increased myogenic regulatory factor basal levels has been seen previously in the elderly (Kim et al. 2005b), our result of increased myogenin mRNA after RT is in contrast to Kosek et al. (2006) who showed no major differences between young and older men in myogenin gene expression after RT. Future studies should investigate whether these changes are evident also at the protein level.
It should be noted that training and nutrition belong to a complex area and this study explored just part of it. The observed existence of age-related differences in this study may depend on many variables, as shown in previous studies: training programs (e.g., frequency and volume of training), the compared ages or even gender (Bamman et al. 2003; Häkkinen et al. 2001a; Ivey et al. 2000). There may be differences in the effect of protein or amino acid ingestion on protein synthesis (Cuthbertson et al. 2005; Dangin et al. 2003; Rasmussen et al. 2006; Volpi et al. 2000). There may also be an age-related decline in responsiveness of muscle satellite cells after resistance exercise (Dreyer et al. 2006) and in muscle protein synthesis response to resistance exercise (Kumar et al. 2009).
Conclusion
In conclusion, resistance training two times per week during 21 weeks with progressive hypertrophic training increased muscle size more in the young than in the older untrained men. This was accompanied by greater protein and energy intake in young men. Furthermore, older men had an increase in myostatin gene expression compared to young men.
References
American College of Sports Medicine (2009) Position stand: progression models in resistance training for health adults. Med Sci Sports Exerc 41:687–708
Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS (2000) Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130:139–145
Bamman MM, Hill VJ, Adams GR et al (2003) Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci 58:108–116
Bickel CS, Cross JM, Bamman MM (2011) Ecercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc 43:1177–1187
Campbell WW, Leidy HJ (2003) Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr 26:696S–703S
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Cuthbertson D, Smith K, Babraj J et al (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424
Dalbo VJ, Roberts MD, Sunderland KL et al (2011) Acute loading and aging effects on myostatin pathway biomarkers in human skeletal muscle after three sequential bouts of resistance exercise. J Gerontol A Biol Sci Med Sci 66:855–865
Dangin M, Guillet C, Garcia-Rodenas C et al (2003) The rate of protein digestion affects protein gain differently during aging in humans. J Physiol 549:635–644
Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA (2006) Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33:242–253
Durnin JV, Womersley J (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97
Evans W (1995) What is sarcopenia? J Gerontol 1995: 50A(special issue):5–8
Fiatarone MA, O’Neill EF, Ryan ND et al (1994) Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330:1769–1775
Flück M, Däpp C, Schmutz S, Wit E, Hoppeler H (2005) Transcriptional profiling of tissue plasticity: role of shifts in gene expression and technical limitations. J Appl Physiol 99:397–413
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshisawa F, Volpi E, Rasmussen BB (2007) Nutrient signaling in the regulation of human muscle protein synthesis. J Physiol 582:813–823
Guillet C, Prod’homme M, Balage M et al (2004) Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18:1586–1587
Häkkinen K, Häkkinen A (1991) Muscle cross-sectional area, force production and relaxation characteristics in women at different ages. Eur J Appl Physiol 62:410–414
Häkkinen K, Newton RU, Gordon SE et al (1998) Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53:B415–B423
Häkkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ (2000) Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol 83:51–62
Häkkinen K, Kraemer WJ, Newton RU, Alen M (2001a) Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand 171:51–62
Häkkinen K, Pakarinen A, Kraemer WJ, Häkkinen A, Valkeinen H, Alen M (2001b) Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol 91:569–580
Harridge S, Kryger A, Steengaard A (1999) Knee extensor strength, activation, and size in very elderly people following strength training. Muscle and Nerve 22:831–839
Hulmi JJ, Ahtiainen JP, Kaasalainen T et al (2007) Postexercise myostatin and activin IIb mRNA levels: effects of strength training. Med Sci Sports Exerc 39:289–297
Hulmi JJ, Kovanen V, Lisko I, Selanne H, Mero AA (2008) The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Eur J Appl Physiol 102:205–213
Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Häkkinen K, Mero AA (2009a) Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 37:297–308
Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA (2009b) Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol 106:1720–1729
Ivey FM, Roth SM, Ferrell RE et al (2000) Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci 55:M641–M648
Jespersen JG, Nedergaard A, Andersen LL, Schjerling P, Andersen JL (2011) Myostatin expression during human muscle hypertrophy and subsequent atrophy: increased myostatin with detraining. Scand J Med Sci Sports 21:215–223
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR (2005) Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82:1065–1073
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR (2006) A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291:E381–E387
Kim JS, Cross JM, Bamman MM (2005a) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288:E1110–E1119
Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM (2005b) Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol 99:2149–2158
Kim JS, Petrella JK, Cross JM, Bamman MM (2007) Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol 103:1488–1495
Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM (2006) Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101:531–544
Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA (2006) Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 172:103–113
Kumar V, Selby A, Rankin D et al (2009) Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587:211–217
Lambert CP, Evans WJ (2002) Effects of aging and resistance exercise on determinants of muscle strength. J Am Aging Assoc 25:73–78
Leiter JR, Peeler J, Anderson JE (2011) Exercise-induced muscle growth is muscle-specific and age-dependent. Muscle Nerve 43:828–838
Lexell J (1995) Human aging, muscle mass and fiber type composition. J Gerontol 50A (special issue):11–16
Liu W, Saint DA (2002) Validation of a quantitative method for real time PCR kinetics. Biochem Biophys Res Commun 294:347–353
Lucas M, Heiss CJ (2005) Protein needs of older adults engaged in resistance training: a review. J Aging Phys Activ 13:223–236
Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E (2008) Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab 294:E43–E51
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Melton LJ, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL (2000) Epidemiology of Sarcopenia. J Am Geriatr Soc 48:625–630
Moritani T, deVries HA (1980) Potential for gross muscle hypertrophy in older men. J Gerontol 35:672–682
Paddon-Jones D, Rasmussen BB (2009) Protein and amino acid metabolism and therapy. Dietary protein recommendations and the prevention of Sarcopenia. Curr Opin Clin Nutr Metab Care 12:1:86–90
Peterson MD, Sen A, Gordon PM (2011) Influence of resistance exercise on lean body mass in aging adults: meta-analysis. Med Sci Sports Exerc 43:249–258
Porter MM, Vandervoort AA, Lexell J (1995) Aging of human muscle structure, function and adaptability. Scand J Med Sci Sports 5:129-142
Rasmussen BB, Fujita S, Wolfe RR et al (2006) Insulin resistance of muscle protein metabolism in aging. FASEB J 20:768–769
Rieu I, Balage M, Sornet C et al (2007) Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition 23:323–331
Rios R, Carneiro I, Arce VM, Devesa J (2002) Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282:C993–C999
Roth SM, Ivey FM, Martel GF et al (2001) Muscle size responses to strength training in young and older men and women. J Am Geriatr Soc 49:1428–1433
van Pelt RE, Dinneno FA, Seals DR, Jones PP (2001) Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab 281:E633–E639
Viitasalo J, Era P, Leskinen A, Heikkinen E (1985) Muscular strength profiles and anthropometry in random samples of men aged 31–35, 51–55, and 71–75 years. Ergonomics 28:1503–1510
Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR (1998) Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 1:101(9):2000–2007
Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR (2000) The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85:4481–4490
Wagner KR (2005) Muscle regeneration through myostatin inhibition. Curr Opin Rheumatol 17:720–724
Welle S, Totterman S, Thornton C (1996) Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 51:M270–M275
Acknowledgments
The authors thank Tuomas Kaasalainen, Tuovi Nykänen, Risto Puurtinen, Liisa Kiviluoto and Aila Ollikainen for their help in data collection and analysis. We also thank the very dedicated group of subjects who made this project possible. Simon Walker is thanked for the critical reading of the manuscript and language revision, and Elina Kokkonen for the help in the statistical analysis. The Finnish Ministry of Culture and Education and the Finnish Cultural Foundation supported this research
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Rights and permissions
About this article
Cite this article
Mero, A.A., Hulmi, J.J., Salmijärvi, H. et al. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol 113, 641–650 (2013). https://doi.org/10.1007/s00421-012-2466-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2466-x