Abstract
Diseases of the neurovascular unit, consisting of the endothelial vasculature and supporting cells, are incredibly prevalent in patients. Two such diseases, stroke and traumatic brain injury (TBI), share major pathological similarities, with acute and chronic pathways leading to neurodegeneration. In particular, the neuroinflammatory aspect of stroke and TBI pathology has been shown to contribute significantly to worsening outcomes. Fortunately, neuroinflammation also offers an accessible therapeutic target. Minimal treatment options currently exist for either disease, but stem cell-based therapies have demonstrated great promise in offering neuroprotection and encouraging neuroregeneration after the initial insult. Stem cells have been shown to mitigate chronic neuroinflammation as well as modulate peripheral inflammation via the spleen. Additionally, stem cells have been demonstrated to preferentially migrate to the spleen when injected after a neurovascular injury. This further validates the notion that stem cells are inflammation-honing “biologics” and confer their neuroprotection in large by ameliorating the global inflammatory response. Current research investigations are focused on understanding these cell death and neural repair processes in an effort to utilize the preclinical findings toward efficient strategies designed to employ stem cell therapies as a treatment for stroke, TBI, and other neurovascular diseases. Here, we provide scientific evidence supporting the use of stem cell therapy for neurovascular diseases through the cells’ robust ability to sequester the inflammatory response associated with the secondary cell death that plagues both stroke and TBI.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Stem cells
- Neurovascular disease
- Neurovascular unit
- Ischemic stroke
- Traumatic brain injury
- Neurodegeneration
- Regenerative medicine
Introduction
Stroke and traumatic brain injury (TBI ) are classified as neurological disorders but more precisely belong to the heterogeneous subclass of neurovascular disease. This broad field of diseases is characterized by pathological dysfunction of the cerebral vasculature which invariably results in some degree of ischemia and metabolic restriction [1]. Generally, these diseases are associated with poor clinical outcomes and an under-availability of effective therapeutic options. While stroke abides more intuitively to the definition of neurovascular disease, our improved understanding of the pathology which accompanies TBI makes it an appropriate member of this disease group as well.
In the United States, stroke affects nearly 800,000 people annually, posing a significant medical and economic burden [2]. Stroke disproportionately occurs in the aging population and is a leading cause of disability among this population [2, 3]. Stroke is defined as a temporary or permanent reduction in blood flow to a brain region which can occur in one of two ways, either ischemic or hemorrhagic, depending on the origin of the circulatory reduction [4]. Ischemic stroke s are more common, resulting from embolic vessel blockage, while hemorrhagic stroke occurs less frequently and results from the leaking or rupturing of blood vessels, reducing the appropriate circulation to downstream brain regions [4]. In both cases, the lack of blood causes a depletion of metabolic resources and triggers a cascade of events which are detrimental to neural cell health. This primary cell death can then lead to a host of progressive secondary complications.
TBI presents many pathological commonalities to stroke and is also highly prevalent in the United States. TBI is a leading cause of death and disability in both civilian and military populations [5, 6]. More recently, the implications of mild TBI—also known as concussion—have received much attention due to mild TBI’s ubiquity in sports as well as an appreciation for the long-term complications which can result from repeated impacts [7,8,9]. TBI affects 1.7 million people in the United States each year, with undiagnosed mild TBIs likely making that number a gross underestimate [10]. With the support of accumulating clinical and laboratory evidence, TBI is no longer considered only an acute injury but is now defined as a disease state which ensues a physical insult to the brain—be it blunt, penetrating, or explosive impact—and can manifest symptoms which progress for decades [11, 12]. The causative insult may result in a focal impact core (often the case with penetrating wounds) or a more diffuse area of initial damage. Regardless, this primary injury often triggers a set of secondary pathways similar to stroke, which proceed to worsen patient outcomes [13].
Developing effective clinical therapies for these neurovascular disorders has posed a steep challenge. One aspect of these diseases’ pathology in particular, neuroinflammation, has become the target of choice for most potential therapies. Particularly, attenuating the chronic neuroinflammation following stroke and TBI shows the greatest promise in providing significant functional improvements. Increasing evidence has directed researchers to approach the development of therapies in neurovascular diseases from the perspective of the entire neurovascular unit, instead of targeting a single entity. The concept of the neurovascular unit highlights the phenomenon in which a single disruption within the neurovascular unit can promote a cascade which affects all other parts of the system [14]. In a simplified view, the neurovascular unit is broken into three main parts—neurons, glial cells, and endothelial vasculature [14]. These components are connected via cell-to-cell “cross talk,” and the disturbance of one cell type can impact the whole system. New data also suggests that the neurovascular unit is further supported by non-CNS components, including important interactions observed within the spleen and the gut. In this light, a systems biology approach may be advantageous in making clinical progression for stroke and TBI research. This chapter will discuss stroke and TBI pathology, with a focus on how stem cells can be used to attack this inflammatory response on multiple fronts, with emphasis on the recent advancements in our understanding of how stem cells carry out their therapeutic mechanisms.
Acute Pathology of Stroke and TBI
Despite differing etiologies, ischemic stroke and TBI display remarkably similar pathologies, particularly in chronic stages. Because of these commonalities, much of the research on the disease progression or treatment of one disease is relevant to the other. Directly following stroke or TBI onset, a number of cells are subjected to immediate cell death, and thus the necrotic core forms. This necrotic brain tissue is quickly fixed and unable to be saved [15]. Much of the acute phase is marked by irreversible damage, primarily mitochondrial dysfunction and cell membrane disturbance [16]. Damage to both cellular systems may result from mechanical trauma or ischemic conditions. Mitochondrial dysfunction, in particular, leads to an accumulation of harmful reactive oxygen species (ROS) and a release of many cytotoxic elements [16]. Upon the necrosis caused by these factors, toxic compounds are subsequently released into the surrounding tissue. Cells within the perimeter of the necrotic core, termed the peri-infarct or penumbral region, are faced with the challenges posed by this harsh microenvironment [17]. Changes in plasma membrane permeability often occur during energy-depleted states and, without restoration, cause a solute imbalance and loss of ionic homeostasis [18]. Specifically, sodium and calcium ion infiltration follows this increased permeability and may contribute to cell death [18]. Finally, the necrotic neurons flood the extracellular space with the previously intracellular glutamate. Glutamate excitotoxicity rapidly occurs after the initial cerebral insult, and this can further accelerate the elevation of cytoplasmic calcium concentration by glutamate-mediated release of calcium stored within the endoplasmic reticulum [19]. The damage directly corresponding to glutamate toxicity is short-lived, however, as glutamate concentrations peak at only about 10–30 min after insult [20].
Chronic Pathology of Stroke and TBI
Both the stroke and TBI brains are accompanied by the phenomenon of secondary cell death and its gradual progression. This notion of secondary cell death embodies the chronic phases of these diseases. Two major facets of the chronic disease progression observed in stroke and TBI are alterations in growth factor / apoptotic factor levels as well as an extensive neuroinflammatory response. Neuroinflammation has been shown to persist for years in both diseases—up to 17 years after TBI—and is a complex process involving microglia, peripheral immune cells, inflammatory cytokines, and chemokines [21,22,23]. The gradual neurodegeneration caused by the inflammatory response means the potential for worsening symptoms long after the original insult [24]. As a result of this extended time-point, a large therapeutic window exists when targeting the inflammatory states of stroke and TBI brains. Especially in TBI, the infiltration of systemic immune cells into the brain parenchyma is permitted by a loss of blood-brain barrier fidelity and can hyperactivate the brain’s immune response [25,26,27]. Therefore, the chronic inflammatory response observed in both stroke and TBI brains has been revealed as the most advantageous secondary cell death factor to attack when proposing new treatment options for the diseases. Neuroinflammation poses both a promising and challenging target though. The inflammation seen post-insult is a double-edged sword; inflammation seen in the acute phase has been shown to serve a protective role, while the chronic inflammation can become self-perpetuating and lead to significant neurodegeneration [28,29,30,31]. Precisely defining this transition from neuroprotective to neurodegenerative inflammation is nearly impossible and can pose practical challenges in developing ideal treatment plans.
Secondary cell death is caused by a host of metabolic changes, reactive species, and persistent inflammation within the regions surrounding the primary insult [32, 33]. These changes are detrimental to neural cells and have a propensity to spread to adjacent tissue, creating the outwardly expanding region of unhealthy brain tissue called the penumbra [33,34,35]. This tissue is at risk of succumbing to irreparable damage, and the expansion of the penumbra correlates to an increase in functional deficits experienced by patients. Thus, the region of dying, yet not dead, tissue in the penumbra represents a more practical therapeutic target for stroke and TBI.
Importantly, the chronic inflammatory response which accompanies neurovascular insults is now understood to be a global event. In particular, recent research has implicated the spleen as a key player in the global inflammatory response, with alterations in the brain-spleen inflammatory coupling system having been shown to affect experimental outcomes [36]. As a result, both neuroinflammation and the spleen have become highly valuable targets in developing effective stroke and TBI therapies. Current research is attempting to find traditional pharmaceuticals, as well as cellular therapy options, which can aid in abrogating the persistent and complex inflammatory response which occurs after stroke and TBI.
Stem Cell Therapy for Neurovascular Disorders
Currently, there exists an unmet clinical need for effective treatments in neurological disorders, including stroke and TBI. Only one FDA-approved drug is available for stroke patients, and it is only beneficial to a small percentage of patients [37]. This drug—tissue plasminogen activator (tPA )—is severely limited due to its required delivery within a 4.5 h window [37]. There are several other restrictions to tPA use which primarily affect patients with an increased risk for hemorrhaging, such as those taking oral anticoagulant medication [38]. In addition, patients who have undergone recent surgeries or CNS trauma may also be excluded [38]. There is a desperate need for treatment options which are available to a larger scope of patients, as well as treatments without such high risk for serious complications. Similarly for TBI victims, there is not a singular effective drug at this time; therefore, treatment for such injuries is limited to rehabilitation and symptom management. Ideal treatment options are those which possess a large therapeutic window and work to impede the progression of secondary cell death. Due to the complexities of this cell death cascade, the development of a multipronged therapeutic option will be required to observe robust clinical recovery after stroke or TBI. Accumulating research suggests that transplantation of stem cells may check off all the boxes of an optimal therapy: by rescuing the reduction of growth factors, limiting apoptotic factor levels and neuroinflammation, being chronically applicable, and potentially benefiting a larger percentage of patients.
Stem Cell Transplantation in Stroke
Researchers have established a number of mechanisms by which transplanted stem cells may be utilized to offer neuroprotection in the stroke brain (which will be discussed in the following sections). Stem cell transplantation is approaching feasibility as a stroke treatment, with numerous clinical trials completed and more in progress. With practically all stroke therapeutics having failed in the clinic, stem cell therapy offers a unique and more holistic approach by targeting multiple facets of the complex physiopathology of stroke. In both clinical cases and laboratory models of stroke, stem cell therapy has been shown to reduce infarct size, increase neuron survival, decrease chronic inflammation, and aid in blood-brain barrier repair [39,40,41,42,43]. The means by which these neuroprotective phenomena are achieved remains poorly understood; over the last 25 years of stem cell research in stroke patients, therapeutic benefits by way of cell replacement and growth factor release have been established as part of the regenerative process after transplantation yet cannot account for the entirety of the neuroregeneration displayed. Thus, less intuitive and poorly understood mechanisms must be contributing to the effects displayed. Regardless, the use of stem cells within the stroke brain has been proven to offer the potential for significant improvement of functional outcomes.
Stem Cell Transplantation in TBI
Originally, TBI was categorized as an acute injury, but is now recognized to possess chronic pathological symptoms—particular secondary cell death driven by aberrant neuroinflammation—and is closely associated with lifetime behavioral deficits [44, 45]. Currently, the treatment options for TBI are limited [46] and typically consist solely of rehabilitation therapy [47,48,49,50,51]. Bearing in mind the extensive secondary cell death facilitating the progression of symptoms with TBI, new potential treatments have gravitated toward targeting the wide therapeutic window of TBI pathology, aiming to promote “neuroregeneration” instead of the relatively narrow window for “neuroprotection” associated with the acute TBI phase [52, 53]. Stem cell-based therapeutics have become a central theme in regenerative medicine , displaying promising results in animal models of TBI [54,55,56,57] but have reached scarce success in reaching the clinic [58]. Additional translational research is needed to gain a better understanding regarding the mechanisms of stem cell action and their capacity to confer neuroregeneration in the brain, as well as establish optimal treatment regimens—all in an effort to drive successful trials into the clinic. In addition, identifying a well-defined stem cell source is necessary for assuring quality of graft origins, as well as being a measure for insuring validity and reproducibility of experimental results. The establishment of optimal cell populations that are both safe and effective is also an area that requires further investigative efforts. There is, however, accumulating evidence to suggest that stem cells produce neuroprotective effects via multipronged neuroregenerative pathways including anti-inflammation and enhanced neurogenesis [59,60,61], in addition to improving angiogenesis and vasculogenesis [62,63,64]. Also, poor graft survival has been reported in the TBI brain, likely attributed to the harsh conditions manifested from the secondary neuroinflammatory response [15, 65, 66]. These data suggest significant survival may not be a prerequisite for behavioral recovery; however, abrogating the hostile microenvironment in which stem cells are transplanted may achieve higher graft survival and boost the degree to which bystander effects occur. In view of that, it is thought that by taming the incompatible microenvironment (i.e., reducing neuroinflammation), stem cell therapy can be optimized, therefore appealing to the advancement of regenerative medicine for treating the injured brain.
Mechanisms of Stem Cell Therapy
Stem cell therapy has received an increasing amount of attention within the realm of regenerative medicine . Multiple neurological diseases, including stroke and TBI, have been a special area of focus for stem cell therapy research. It was initially proposed that stem cell transplantation into the CNS would result in the replacement of dead or dying neuronal cells, as this is the most intuitive mechanism. However, it was observed in many studies that stem cell transplantation into damaged tissue resulted in poor engraftment rates. Interestingly, a robust functional recovery and reduction of infarct core were observed in animals that received stem cell transplantation despite this poor retention [37]. Thus, the therapeutic effects of stem cells appear to not be dependent on their long-term survival and differentiation as initially anticipated.
A far more prominent mechanism of stem cell action has since been brought to light. This mechanism involves the secretion of neurotrophic factors from the transplanted cells. Growth factors play a role in pro-survival pathways; therefore, increasing their concentration has the capacity to thwart impending apoptosis in vulnerable tissues such as the regions of the penumbra in stroke or TBI [67, 68]. Stand-alone administration of BDNF [69], VEGF [70], GDNF [71], SDF-1α [72], and SCF [73] has been shown to have a positive effect on neurological disease outcomes. However, it has been suggested that individual treatment with any one of these growth factors would not result in significantly improved clinical outcomes. Stem cells provide an impressive cocktail of growth factors which contribute to an overall anti-inflammatory and anti-apoptotic effect. In addition, stem cell therapy avoids the complication of establishing the correct dosage of growth factors by having self-regulating secretion. Importantly, it has been observed that drug-induced overproduction of growth factors may lead to detrimental neurological effects, such as the development of epilepsy as a consequence of BDNF overexpression [74, 75].
A third mechanism of action for stem cell transplantation was first observed in a rat model of TBI. This newly discovered mechanism functions to enhance the host’s natural neuroprotective processes which are initiated upon injury through the activation of endogenous stem cells. Until recently, a long-standing belief was held that mammalian adults lacked the ability to generate new neurons. This paradigm was quickly reversed after the discovery of endogenous stem cells, found predominantly within the SVZ of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus [76, 77]; these regions also may be referred to as the neurogenic niches. The capacity for neurogenesis therefore extends into adulthood and is amplified following neurological insults such as stroke [78]. The host’s attempt to reverse or stop the gradual progression of damaged tissue by stem cell mobilization is highly inefficient. Endogenous stem cells are limited in their ability to differentiate, commit to neuronal lineage, and migrate from these neurogenic niches [79]. Proper migration of endogenous stem cells is imperative if they are to elicit therapeutic benefit to regions distant from their origin in the SVC or SGZ. Recent research revealed assisted transportation of host stem cells via transplantation of exogenous stem cells. The first study to observe this phenomenon used a TBI model in which a controlled cortical impact (CCI) was delivered to the frontal cortex—a region too distal for significant endogenous stem cell migration—followed by intracerebral injection of mesenchymal stem cells (MSCs) [80]. Interestingly, observations utilizing immunohistochemistry and laser capture microdissection exposed the presence of transplanted MSC creating a cellular pathway connecting the neurogenic niche to the impacted cortex [80]. This discovery led to the biobridge theory as a third and novel mechanism in which transplanted stem cells provide a neuroprotective and/or neuroregenerative effect. The biobridge is thought to facilitate the migration of endogenous stem cells from their residential origin to the injured region, therefore rendering the host’s natural regenerative mechanism more effective [80]. This novel mechanism is entirely unique to stem cells and is yet to be achieved by any other therapy.
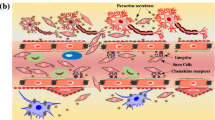
Finally, recent data have revealed a fourth mechanism of action executed by stem cells. The vast secretome produced by stem cells has displayed the ability to confer therapeutic effects on neurovascular diseases. Microvesicles and exosomes secreted from stem cells contain growth factors, cytokines, chemokines, microRNAs, and long noncoding (lncRNA), all of which may contribute to the therapeutic effect observed following transplantation [81]. Treatment with isolated exosomes and microvesicles derived from multipotent MSCs has been explored in liver, kidney, cardiovascular, and lung disease with promising results [81]. In the transition to neurovascular diseases, studies focusing on exosome transplantation following CNS insult include an in-depth analysis of neuroinflammation, as the magnitude and persistence of inflammation in neurological disorders are rather unique. As emphasized previously, neuroinflammation is an important target for the establishment of an effective therapy in neurovascular diseases.
The Promise of Combination Therapies with Stem Cells
While stem cells alone have been shown to be therapeutic in many applications, an increasing body of research is exposing the additive effects that certain combination therapies can afford. Combination therapies may take multiple forms, such as pairing multiple stem cell types, delivering stem cells in conjunction with a more traditional therapeutic agent, or delivering stem cells with biostructural material. In some cases, these adjunct agents serve as independent therapeutics which complement the stem cells, while other adjuncts play a supporting role by helping to bolster the effectiveness of stem cells. Both approaches possess merit and may serve to improve patient outcomes in the future.
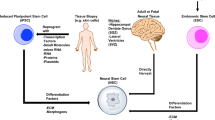
Depending on their tissue of origin, stem cells can exhibit significant variation in morphology, protein markers, differentiation capabilities, and treatment efficacy. This has been established by the use and comparison of a number of different stem cell types in the stroke brain, including mesenchymal stem cells (MSCs), bone marrow-derived stem cells (BMSC), adipose-derived stem cells (ADSC), and neural stem cells (NSC). A trail of investigation which is now being explored aims to characterize the effectiveness of heterogeneous stem cell populations. In one study, the effects of MSC-NSC combination therapy was explored in a middle cerebral artery occlusion (MCAO) rat stroke model [82]. Twenty-four hours after occlusion, MSCs were injected into the right lateral ventricle, and 6 days later, NSCs were injected in the same manner [82]. Histological and behavioral analysis revealed that the MSC + NSC group showed reduced functional deficits and smaller lesion volume when compared to sham animals, MSC-only animals, and NSC-only animals [82].
In a study utilizing a percussion model of TBI, the use of olfactory ensheathing cells (OEC)—supporting glial cells derived from the olfactory system—was delivered in conjunction with neural stem cells (NSC) [83]. The olfactory system is unique in its ability to perform significant neurogenesis throughout the mammalian life cycle [84, 85]. This experiment explored the idea that these supporting cells may be instrumental in encouraging the neural proliferation unique to this region. Using a vehicle control group, OEC alone, NSC alone, and OEC + NSC group, a notable trend was observed whereby neuron survival in the OEC + NSC was significantly greater than in either individual cell treatment, nearing sham levels [83]. Similarly, apoptosis was reduced in the OEC + NSC group to a significant degree when compared to individual cell treatments [83]. While the mechanism of this additive improvement was not studied, the ability of different cell types to synergistically ameliorate the effects of TBI supports further investigating this type of therapy.
Stem cells have also been delivered with a number of therapeutic compounds such as mannitol, granulocyte colony-stimulating factor, minocycline, and progesterone [68, 86,87,88]—often with compounding benefits. It is plausible that a major leap in stem cell effectiveness could be found in their simultaneous delivery with neurotrophic factors, anti-inflammatories, or cytoprotective agents. Stem cell treatment in neurovascular diseases has also been paired with alternative therapies such as hypothermia and hyperbaric oxygen treatment [89,90,91]. The benefit of a co-treatment with stem cells and one of these alternative therapies would be eliminating some of the complications that come along with pairing complex pharmaceuticals. Similarly, delivering stem cells in a formulated biomaterial may aid in improving the therapeutic effects of stem cells [92, 93]. Certain materials have been shown to reformat the extracellular matrix and make the brain microenvironment more conducive to stem cell survival, migration, and proliferation. In general, the search for compatible biomaterials and effective co-treatments represents a worthy endeavor and may help to expedite the transition of stem cells from the laboratory to the clinic.
The Spleen as a Novel Target for Stem Cells
Once thought to be isolated, CNS disorders, stroke, and TBI are now recognized to be affected by distal regions of the body. One organ that has been implicated in these diseases to a surprising degree is the spleen . A reciprocal relationship exists between the spleen and the brain following neurovascular insult, with the injury altering the physical size and function of the spleen and the spleen in turn, affecting brain health [94]. In fact, the spleen has been shown to release splenocytes in response to ischemic events, exacerbating neurodegeneration [94]. Additionally, studies have shown the spleen to be especially critical in the physiological processing and therapeutic mechanisms of stem cells [95]. The cornerstone of this relationship lies in the post-insult inflammatory response. We now understand the brain inflammatory response to include both central and peripheral components following stroke and TBI—local inflammation and edema persist in the brain parenchyma, while systemic inflammation helps to propagate the cerebral inflammation [96,97,98]. The peripheral aspect of neuroinflammation allows for invading immune cells—such as T cell, lymphocytes, monocytes, and macrophages— to extravasate through the compromised BBB which results from neurovascular damage [95, 98]. As mentioned previously, the chronic inflammation seen following stroke represents a prime target for therapeutics; thus, understanding both the local and the global nature of the inflammatory response is critical in attacking neuroinflammation from all possible angles.
Interestingly, studies in which the spleen was removed prior to MCAO demonstrated smaller infarct volumes, reduced infiltration of peripheral inflammatory cells, and reduced pro-inflammatory cytokines [99, 100]. This demonstrates that the spleen’s innate processes contribute to worsening outcomes following an ischemic event [94, 100]. As routine splenectomies are not a viable clinical option, the use of other approaches to attenuate the spleen’s pro-inflammatory role may serve as a practical approach.
Stem cells have been shown to preferentially migrate to the spleen when delivered intravenously, both in the acute and chronic stroke brain [95, 101]. These stem cells demonstrated an ability to downregulate pro-inflammatory molecules and immune cells which are released by the spleen, effectively altering the blood composition in a way that promotes stroke recovery [95]. This exhibits that stem cells may potentially function therapeutically, in part, by modulating this brain-spleen axis inflammatory response. Our understanding of the spleen’s implications in the stroke and TBI brain remains incomplete, but its role in the chronically inflamed state which exists after stroke gives insight into how stem cells may be an effective option in later stages of the disease. Additionally, this spleen-mediated mechanism furthers the notion that entering the brain tissue may not be a prerequisite for an effective stem cell therapy [95]. If the presence of stem cells in the blood stream can indirectly alter the brain parenchyma environment, the inability for stem cells to efficiently cross the BBB may not be a restriction to effective therapy. Importantly, this helps to circumvent the quandary whereby blood-brain barrier repair makes peripherally delivered stem cells less potent. To date, only one preclinical study has investigated the use of stem cell administration in the chronic phase of stroke [102]; understanding how stem cells confer neuroregeneration by interacting with the spleen to sequester the inflammatory response at these later time-points may pave the way for a continuous, long-term stem cell treatment plan.
Challenges in Stem Cell Therapy
A number of major hurdles stand in the way of progressing stem cell therapy to patient availability. These include difficulties concurring on the most effective dosages, establishing consistent time-points, and determining practical routes of delivery. Three collaborative meetings have produced a set of guidelines for stem cell research called STEPS—stem cells as an emerging paradigm for stroke—guided by field experts in both the laboratory and clinical setting [103]. Many of their suggestions have addressed the issues mentioned above, stressing the importance of basic science-inspired clinical trial design [103]. A common disconnect in clinical trial design exists in deciding the appropriate dosage; the number of cells administered in human patients is rarely proportional to the most effective cells-per-kilogram dosage established in animal models. This cripples clinical trials from the start and contributes to most clinical trials agreeing on the safety of stem cell therapy in stroke, but not definitively concluding on the efficacy [102, 104,105,106,107,108].
In addition to dosage concerns, defining an ideal time-point or set of time-points which produce the greatest possible patient recovery remains an elusive target. A limitless number of possible delivery time-point combinations exist, with different studies finding varied effectiveness depending on the chronology of treatment(s) [109]. Importantly, the route of administration also affects stroke and TBI outcomes. It is not surprising that injection of stem cells directly adjacent to the infarct or impact core consistently delivers the greatest functional improvements, but the practicality of intracerebral delivery for patients en masse is questionable.
The difficulty in developing treatment plans exists not only as a result of the chasm between laboratory and clinical research, but also arises from the heterogeneity of stroke and TBI. Stroke patients experience extensive variation in infarct region, stroke severity, and capacity for natural recovery [110]. TBI is equally varied, with location, severity, and affected area differing greatly depending on the circumstances of the injury. Thus, a key to pushing stem cell treatment forward is to conduct more clinical trials, produce more substantial data, and use this to tailor future experimental designs in order to define the best treatment plans for general insults or develop patient-specific plans. Of course, this must be in addition to tighter collaboration between basic scientists and clinicians.
Potential Adverse Effects of Stem Cell Therapy
While stem cells undoubtedly offer hope as a stroke treatment, the administration of stem cells is not without its risks. The two predominate concerns with cell transplantation are the danger of teratoma formation and graft rejection —a host immune response to the exogenous cells. The danger of teratoma formation is especially prevalent in embryonic-derived stem cells (ESCs) and induced pluripotent stem cells (iPSCs) [42]. When analyzing the safety of potential stem cell therapies, regulatory institutions prefer to see a loss of stemness over time, as this indicates a loss of unwanted replicative potential. All stem cells hold a certain degree of overproliferation risk, but this is largely dependent on the cell type’s differentiation capabilities. On the other hand, the risk of immune response is primarily dependent on the host; thus, immunosuppression may be necessary in certain clinical applications [111].
A novel therapeutic concept may be able to deliver the benefits associated with stem cell transplantation while curtailing these associated risks; this approach uses cellular materials which offer functional effects comparable to those seen with traditional stem cell transplantation [42]. These cell-derived therapies take advantage of an emerging notion; significant evidence now points to the secretome, the sum total of a cell’s secreted factors, as a leading contributor to stem cells’ therapeutic actions and anti-inflammatory effects observed in stroke and other neurological disorders [42, 112–115].
Cell-Free Materials and Exosomes in Neurovascular Therapy
Different means of preparing cell-derived materials exist, but the harvesting of conditioned media is perhaps the simplest. Conditioned media refers to the chemically altered, secretome-infused media which results from the culturing of a specific stem cell type [116]. This is typically achieved by growing the cells within a 3D scaffolding to maximize the desired secretions [117]. A number of studies have exposed the potential which lies in the use of conditioned media [116–119]—highlighting a reduction in apoptosis, decrease in inflammation, improved neuron survival, and cell proliferation—yet, the practicality of employing conditioned media as an effective stroke treatment in humans remains to be verified.
The cultivation of stem cell-produced exosomes is another cell-free therapeutic option for stroke which has received significant attention in recent years. As mentioned previously, exosomes are small secreted vesicles which contain a variety of cellular products—including mRNA, lncRNA, lipids, and proteins—and maintain the ability to act as both paracrine signals as well as extracellular environmental modulators. Experiments have demonstrated that these isolated exosomes retain the ability to confer neuroprotection and neuroregeneration comparable to that of stem cell transplantation [120, 121].
TBI and Stroke: Implications of Cell-Free Treatment
Currently, the use of isolated stem cell-derived exosomes to treat stroke and TBI is a relatively new area of research. This has both aided in furthering our understanding of exosomes’ role in stem cell treatments and offered a new therapeutic option all together. This subsection will detail three studies and their unique findings on this subject.
A recent study explored the utilization of human adipose-derived stem cells (hADSCs) in TBI rats using the CCI injury model [122]. In addition to treatment with transplanted stem cells, another experimental group received cultured media (CM) derived from these cells [122]. This CM contained the entire secretome extracted from the cultured cells and included the exosomes carrying growth factors, protein, microRNA, and lncRNA [122]. Previous studies of experimental TBI have evaluated hADSCs for their ability to secrete large amounts of the anti-inflammatory cytokines IL-10 and IL-4 [119, 123, 124] and reduce the production of pro-inflammatory cytokines, such as TNF-α and IFN-γ [125]. For this reason, the anti-inflammatory effect observed with CM treatment was expected. In order to characterize the importance of exosomes and their lncRNA, knockdown groups were utilized which shed light onto the vital role these specific lncRNA exosomes play in the neuroprotective properties contributed by the CM. Many types of lncRNAs are secreted by proliferating stem cells that are not in a differentiating state.
Two specific lncRNAs were selected for knockdown, nuclear-enriched abundant transcript 1 (NEAT1) and metastasis-associated lung adenocarcinoma transcript 1 (MALAT1). They were chosen because of their essential role in cell survival, inflammation, and gene expression. Specifically, these lncRNAs are modulators of cellular differentiation due to their ability to take part in the alternative splicing of numerous pre-mRNA [126–128]. Upon knockdown of the two lncRNAs, the functional recovery seen in the CM experimental group was significantly reduced. The following proteins—VEGF, stem cell factor (SCF), and tissue inhibitor of metalloproteinases-3 (TIMP3)—were analyzed for their concentrations in CM versus CM-knockdown groups. The CM-mediated improvement in VEGF and SCF levels was significantly reduced in the knockdown group, as well as an increase in TIMP3, a VEGF inhibitor [129, 130]. Based on these results, it was concluded that the stem cell-derived secretome yields a neuroprotective and anti-inflammatory effect that is largely dependent on the action of lncRNAs.
The next two studies explored the potential in direct exosome transplantation following isolation from cultured MSCs. One study used a TBI model [81], the other stroke [131], and both resulted in improved cognitive and motor function in exosome-treated rats. The stroke study included a particular focus on exosomal transfer of microRNA, specifically microRNA-133b (miR-133b) [131]. The selection of miR-133b was based on prior in vitro studies that revealed an elevation in miR-133b within MSC-derived exosomes after exposure to ischemic cerebral extracts [112]. This increment in miR-133b attributed to heightened neurite growth due to transportation to astrocytes and neurons via exosomes [112]. For the following in vivo study, knock-in and knockdown experimental groups were utilized. The data showed that increased miR-133b concentration in exosomes provides a more robust neurological recovery, and a significant decrease of therapeutic capacity results when miR-133b is in reduced concentrations [131]. Exosomes with miR-133b + MSC lead to improved axonal plasticity and neurite remodeling that contributed to functional recovery [131].
The TBI study of MSC-derived exosome transplantation illustrated similar results in that the treatment was neuroprotective [114]. This study revealed the role of angiogenesis in the functional recovery seen with administration of cell-free exosomes generated by MSCs [114]. In addition, exosome treatment reduced neuroinflammation and raised the number of newly formed neuroblasts and mature neurons in the dentate gyrus [114]. Interestingly, treatment groups did not display any downsizing in cortical lesion volume but still showed improved cognitive and sensorimotor functional recovery [114]. This further exemplifies the importance of angiogenesis and sequestration of neuroinflammation and their contribution toward functional improvements following stroke or TBI.
Feasibility of Exosome Transplantation Therapies
It remains unclear whether exosomes present a practical clinical approach. For one, the difficulty of accumulating sufficient quantities of this cell-free product is both challenging and expensive. Individual stem cells secrete an unsubstantial amount of exosomal product; therapeutic dosages for humans could only be obtained with vast quantities of stem cells using presently available methods. It is also yet to be determined if the reduced risk offered by cell-free options—both conditioned media and exosomes—is marginalized by a reduction in therapeutic effectiveness. While the potential for therapeutic has already been unequivocally demonstrated, if its effects are significantly less than that offered by traditional stem cell therapies, it may be worth the associated risks to proceed with stem cell therapy as usual. Further studies comparing the neuroprotection conferred by stem cells to that offered by exosomes alone would allow for an appropriate risk-versus-reward analysis. Regardless, the study of exosomes and their constituent compounds may be instrumental in furthering our knowledge of how stem cells deliver their therapeutic benefits.
Conclusion: Connecting the Dots of Stem Cell Therapy
With such a large population of patients being affected by either stroke or TBI, the need for neurovascular therapeutics cannot be overstated. Current best medical practice for stroke involves the use of tPa if possible, yet utilizing this drug can be difficult as a result of its narrow therapeutic window and adverse effects. TBI patients are yet to have a viable option. The use of cell transplantation offers a promising solution to these clinically difficult disorders.
The therapeutic effects of stem cells have been described as paradoxically robust. As mentioned, the engraftment rate of transplanted stem cells is surprisingly low considering the significant benefits they exhibit. In reality, this paradox is just a reflection of our rudimentary understanding of how stem cells function within the body. Fortunately, great strides are being made in unveiling the complex mechanisms by which stem cells confer neuroprotection. These strides help to push stem cell therapy closer to efficient clinical applications. Specifically, the topics covered in this chapter—inflammation, the spleen’s role in neurovascular disease, and stem cells’ tendency to hone in on this organ—reveal much about how stem cell therapy for stroke and TBI operates. By targeting the pathology of neurovascular diseases in a holistic manner and by respecting the cellular interplay which is presented in the neurovascular unit concept, stem cell therapies may be able to be maximally utilized.
The role of the inflammatory response in stroke and TBI has been discussed extensively, but it is important to understand how this novel idea of splenic mediation of peripheral inflammation impacts stem cell therapy. Again, inflammation offers the most accessible target in stroke and TBI pathology. With intravenous stem cells honing in on the spleen—the newfound mediator of systemic inflammation—this provides a convenient link whereby stem cells attack the most accessible point of neurovascular pathology, the inflammatory response, at a convenient location.
Similarly, the rising notion that stem cells confer their benefits in part through the release of therapeutic exosomes is an important revelation in how stem cells function. In addition to the secretion of anti-inflammatory cytokines, growth factors, and anti-apoptotic molecules, we have recently revealed the role of exosomal secretions. Within these exosomes, important mediators of cell growth, division, and survival help to confer neuroprotection. This revelation that stem cell products—not necessarily the cells themselves—contribute to their therapeutic profile opens up new possibilities of cell-free treatments. These treatment options may take advantage of similar mechanisms as traditional stem cell therapy while circumventing the dangers that accompany it.
Stem cells are a non-conical therapy option. Because of this, transitioning stem cells from the laboratory to the clinic has proven challenging, but the difficulties associated with their use are matched by equal promise. Further research is needed to understand how stem cells operate in the human body and by which means they extend their neuroprotection. Improving our understanding of stem cell therapeutic function is a critical step in making their use widely available to patients suffering with stroke and TBI.
References
Starke RM, et al. Developments in neurovascular diseases and treatments. ScientificWorldJournal. 2015;2015:608607.
Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13(4):171–7.
Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8(3):319–29.
Sacco RL, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89.
Reid MW, Velez CS. Discriminating military and civilian traumatic brain injuries. Mol Cell Neurosci. 2015;66(Pt B):123–8.
Helmick KM, et al. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358–66.
Ellis MJ, et al. Psychiatric outcomes after pediatric sports-related concussion. J Neurosurg Pediatr. 2015;16(6):709–18.
Morgan CD, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589–98.
Virji-Babul N, et al. Changes in functional brain networks following sports-related concussion in adolescents. J Neurotrauma. 2014;31(23):1914–9.
Coronado VG, et al. Surveillance for traumatic brain injury-related deaths—United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1–32.
Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–40.
McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287–300.
Tajiri N, et al. A nuclear attack on traumatic brain injury: sequestration of cell death in the nucleus. CNS Neurosci Ther. 2016;22(4):306–15.
Lok J, et al. Targeting the neurovascular unit in brain trauma. CNS Neurosci Ther. 2015;21(4):304–8.
Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26(8):1191–201.
Nguyen H, et al. Growth factor therapy sequesters inflammation in affording neuroprotection in cerebrovascular diseases. Expert Rev Neurother. 2016;16(8):915–26.
Acosta SA, et al. Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson’s disease. J Cell Physiol. 2015;230(5):1024–32.
Kang X, et al. delta-Opioid receptors protect from anoxic disruption of Na+ homeostasis via Na+ channel regulation. Cell Mol Life Sci. 2009;66(21):3505–16.
Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15(11):1382–402.
Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–6.
Lozano D, et al. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 2015;11:97–106.
Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44.
Tajiri N, et al. Suppressed cytokine expression immediatey following traumatic brain injury in neonatal rats indicates an expeditious endogenous anti-inflammatory response. Brain Res. 2014;1559:65–71.
Kumar RG, Boles JA, Wagner AK. Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome at 6 and 12 months postinjury. J Head Trauma Rehabil. 2015;30(6):369–81.
Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85.
Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70.
Shlosberg D, et al. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403.
Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. 2002;35(3):419–32.
Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. J Neuropathol Exp Neurol. 2009;68(3):217–26.
Srinivasan K, et al. Untangling the brain's neuroinflammatory and neurodegenerative transcriptional responses. Nat Commun. 2016;7:11295.
Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–89.
Andrade AF, et al. The pathophysiological mechanisms following traumatic brain injury. Rev Assoc Med Bras (1992). 2009;55(1):75–81.
Abate MG, et al. Early derangements in oxygen and glucose metabolism following head injury: the ischemic penumbra and pathophysiological heterogeneity. Neurocrit Care. 2008;9(3):319–25.
Wu HM, et al. Redefining the pericontusional penumbra following traumatic brain injury: evidence of deteriorating metabolic derangements based on positron emission tomography. J Neurotrauma. 2013;30(5):352–60.
Heiss WD. The ischemic penumbra: how does tissue injury evolve? Ann N Y Acad Sci. 2012;1268:26–34.
Ajmo CT Jr, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86(10):2227–34.
Richart CH, Hayashi CY, Hedin M. Phylogenomic analyses resolve an ancient trichotomy at the base of Ischyropsalidoidea (Arachnida, Opiliones) despite high levels of gene tree conflict and unequal minority resolution frequencies. Mol Phylogenet Evol. 2016;95:171–82.
Writing Group, M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360.
Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005;18(1):59–64.
Tang G, et al. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32(12):3150–62.
Wei ZZ, et al. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. 2015;24(3):391–402.
Stone LL, Grande A, Low WC. Neural repair and neuroprotection with stem cells in ischemic stroke. Brain Sci. 2013;3(2):599–614.
Gopurappilly R, et al. Stem cells in stroke repair: current success and future prospects. CNS Neurol Disord Drug Targets. 2011;10(6):741–56.
Wang W, et al. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52(3):1572–9.
Acosta SA, et al. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;9(3):e90953.
Lin D, et al. Brain-derived neurotrophic factor signaling pathway: modulation by acupuncture in telomerase knockout mice. Altern Ther Health Med. 2015;21(6):36–46.
Siddiq I, et al. Treatment of traumatic brain injury using zinc-finger protein gene therapy targeting VEGF-A. J Neurotrauma. 2012;29(17):2647–59.
Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27(1):131–8.
Ruscher K, et al. Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J Cereb Blood Flow Metab. 2013;33(8):1225–34.
Lanfranconi S, et al. Growth factors in ischemic stroke. J Cell Mol Med. 2011;15(8):1645–87.
Kim J, et al. Developmental and degenerative modulation of brain-derived neurotrophic factor transcript variants in the mouse hippocampus. Int J Dev Neurosci. 2014;38:68–73.
Isgor C, et al. Expansion of the dentate mossy fiber-CA3 projection in the brain-derived neurotrophic factor-enriched mouse hippocampus. Neuroscience. 2015;288:10–23.
Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8.
Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702.
Merson TD, Bourne JA. Endogenous neurogenesis following ischaemic brain injury: insights for therapeutic strategies. Int J Biochem Cell Biol. 2014;56:4–19.
Liska MG, et al. Implication of biobridge concept in stem cell therapy for ischemic stroke. J Neurosurg Sci. 2016;61:173–9.
Tajiri N, et al. Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One. 2013;8(9):e74857.
Zhang Y, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2016; doi:10.1016/j.neuint.2016.08.003.
Hosseini SM, et al. Combination cell therapy with mesenchymal stem cells and neural stem cells for brain stroke in rats. Int J Stem Cells. 2015;8(1):99–105.
Liu SJ, et al. Co-grafting of neural stem cells with olfactory en sheathing cells promotes neuronal restoration in traumatic brain injury with an anti-inflammatory mechanism. J Neuroinflammation. 2014;11:66.
Barnett SC, Riddell JS. Olfactory ensheathing cells (OECs) and the treatment of CNS injury: advantages and possible caveats. J Anat. 2004;204(1):57–67.
Fairless R, Barnett SC. Olfactory ensheathing cells: their role in central nervous system repair. Int J Biochem Cell Biol. 2005;37(4):693–9.
Seyfried DM, et al. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–9.
Bilen S, et al. Treatment efficacy with bone marrow derived mesenchymal stem cells and minocycline in rats after cerebral ischemic injury. Stem Cell Rev. 2013;9(2):219–25.
Nudi ET, et al. Combining enriched environment, progesterone, and embryonic neural stem cell therapy improves recovery after brain injury. J Neurotrauma. 2015;32(14):1117–29.
Ma Z, et al. Effects of umbilical cord mononuclear cells transplantation combined with hyperbaric oxygen therapy on hypoxic-ischemic brain damage in neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(7):736–40.
Geng CK, et al. Effect of mesenchymal stem cells transplantation combining with hyperbaric oxygen therapy on rehabilitation of rat spinal cord injury. Asian Pac J Trop Med. 2015;8(6):468–73.
Tu Y, et al. Combination of temperature-sensitive stem cells and mild hypothermia: a new potential therapy for severe traumatic brain injury. J Neurotrauma. 2012;29(14):2393–403.
Gao S, et al. Differentiation of human adipose-derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds. Tissue Eng Part A. 2014;20(7–8):1271–84.
Moshayedi P, et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–55.
Seifert HA, et al. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7(4):1017–24.
Acosta SA, et al. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46(9):2616–27.
Mohamed IN, et al. Role of inflammasome activation in the pathophysiology of vascular diseases of the neurovascular unit. Antioxid Redox Signal. 2015;22(13):1188–206.
Famakin BM. The immune response to acute focal cerebral ischemia and associated post-stroke immunodepression: a focused review. Aging Dis. 2014;5(5):307–26.
Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15(5):523–31.
Zhang BJ, et al. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J. 2013;126(12):2354–60.
Seifert HA, et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012;27(2):131–41.
Vendrame M, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199(1):191–200.
Bang OY, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–82.
Diamandis T, Borlongan CV. One, two, three steps toward cell therapy for stroke. Stroke. 2015;46(2):588–91.
Bhasin A, et al. Stem cell therapy: a clinical trial of stroke. Clin Neurol Neurosurg. 2013;115(7):1003–8.
Prasad K, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–24.
Suarez-Monteagudo C, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27(3):151–61.
Honmou O, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(Pt 6):1790–807.
Banerjee S, et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 2014;3(11):1322–30.
Jeong H, et al. Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells. 2014;7(2):63–9.
Horner RD, et al. Racial variations in ischemic stroke-related physical and functional impairments. Stroke. 1991;22(12):1497–501.
Imberti B, Monti M, Casiraghi F. Pluripotent stem cells and tolerance induction in organ transplantation. Curr Opin Organ Transplant. 2015;20(1):86–93.
Xin H, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30(7):1556–64.
Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377.
Zhang Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–67.
Chopp M, Zhang ZG. Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs. 2015;20(4):523–6.
Ban JJ, et al. Neurogenic effects of cell-free extracts of adipose stem cells. PLoS One. 2016;11(2):e0148691.
Cho YJ, et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res. 2012;90(9):1794–802.
Tsai MJ, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. 2014;21:5.
Egashira Y, et al. The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res. 2012;1461:87–95.
Doeppner TR, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–43.
Kalani A, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–9.
Dela Pena I, et al. Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J Neurosurg Sci. 2014;58(3):145–9.
Ribeiro CA, et al. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther. 2012;3(3):18.
Galindo LT, et al. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. 2011;2011:564089.
Blaber SP, et al. Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med. 2012;10:172.
Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–61.
Zhang B, et al. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2(1):111–23.
Derrien T, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89.
Qi JH, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9(4):407–15.
Yao J, et al. Tissue inhibitor of matrix metalloproteinase-3 or vascular endothelial growth factor transfection of aged human mesenchymal stem cells enhances cell therapy after myocardial infarction. Rejuvenation Res. 2012;15(5):495–506.
Xin H, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–46.
Jeong, H., et al., Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells, 2014. 7(2): p. 63–9.
Horner, R.D., et al., Racial variations in ischemic stroke-related physical and functional impairments. Stroke, 1991. 22(12): p. 1497–501.
Imberti, B., M. Monti, and F. Casiraghi, Pluripotent stem cells and tolerance induction in organ transplantation. Curr Opin Organ Transplant, 2015. 20(1): p. 86–93.
Xin, H., et al., Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 2012. 30(7): p. 1556–64.
Xin, H., Y. Li, and M. Chopp, Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci, 2014. 8: p. 377.
Zhang, Y., et al., Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg, 2015. 122(4): p. 856–67.
Chopp, M. and Z.G. Zhang, Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs, 2015. 20(4): p. 523–6.
Ban, J.J., et al., Neurogenic Effects of Cell-Free Extracts of Adipose Stem Cells. PLoS One, 2016. 11(2): p. e0148691.
Cho, Y.J., et al., Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J Neurosci Res, 2012. 90(9): p. 1794–802.
Tsai, M.J., et al., Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci, 2014. 21: p. 5.
Egashira, Y., et al., The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res, 2012. 1461: p. 87–95.
Doeppner, T.R., et al., Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med, 2015. 4(10): p. 1131–43.
Kalani, A., et al., Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol, 2016. 79: p. 360–369.
Dela Pena, I., et al., Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J Neurosurg Sci, 2014. 58(3): p. 145–9.
Ribeiro, C.A., et al., The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther, 2012. 3(3): p. 18.
Galindo, L.T., et al., Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int, 2011. 2011: p. 564089.
Blaber, S.P., et al., Analysis of in vitro secretion profiles from adipose-derived cell populations. J Transl Med, 2012. 10: p. 172.
Wapinski, O. and H.Y. Chang, Long noncoding RNAs and human disease. Trends Cell Biol, 2011. 21(6): p. 354–61.
Zhang, B., et al., The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cisregulatory role in the adult. Cell Rep, 2012. 2(1): p. 111–23.
Derrien, T., et al., The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res, 2012. 22(9): p. 1775–89.
Qi, J.H., et al., A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med, 2003. 9(4): p. 407–15.
Yao, J., et al., Tissue inhibitor of matrix metalloproteinase-3 or vascular endothelial growth factor transfection of aged human mesenchymal stem cells enhances cell therapy after myocardial infarction. Rejuvenation Res, 2012. 15(5): p. 495–506.
Xin, H., et al., MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells, 2013. 31(12): p. 2737–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Crowley, M.G., Grant Liska, M., Borlongan, C.V. (2017). Stem Cell Therapy for Neurovascular and Traumatic Brain Diseases. In: Emerich, D., Orive, G. (eds) Cell Therapy. Molecular and Translational Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-57153-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-57153-9_3
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-57152-2
Online ISBN: 978-3-319-57153-9
eBook Packages: MedicineMedicine (R0)