Abstract
Aging is a biological process characterized by the progressive deterioration of physiological functions known to be the main risk factor for chronic diseases and declining health. There has been an emerging connection between aging and aneuploidy, an aberrant number of chromosomes, even though the molecular mechanisms behind age-associated aneuploidy remain largely unknown. In recent years, several genetic pathways and biochemical processes controlling the rate of aging have been identified and proposed as aging hallmarks. Primary hallmarks that cause the accumulation of cellular damage include genomic instability, telomere attrition, epigenetic alterations and loss of proteostasis (López-Otín et al., Cell 153:1194–1217, 2013). Here we review the provocative link between these aging hallmarks and the loss of chromosome segregation fidelity during cell division, which could support the correlation between aging and aneuploidy seen over the past decades. Secondly, we review the systemic impacts of aneuploidy in cell physiology and emphasize how these include some of the primary hallmarks of aging. Based on the evidence, we propose a mutual causality between aging and aneuploidy, and suggest modulation of mitotic fidelity as a potential means to ameliorate healthy lifespan.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Age-Associated Aneuploidy
Aging has been linked to an increase in aneuploidy Footnote 1 for the past several decades [1]. This association has been well documented for oocytes and is considered to be the main cause of female reproductive infertility as well as of mis-carriage and birth defects in humans [2]. However, aneuploidy can also arise in somatic cells, and a number of studies have reported age-dependent increases in aneuploidy. Men are for long known to be subject to age-related loss of the Y chromosome (LOY) [3,4,5], and recent studies have suggested that LOY is associated with shorter survival and higher risk of cancer [6]. Also, loss of an X chromosome with advancing age has been reported for females [7, 8], though the physiological consequence of this phenomenon remains unclear. Similarly to sexual chromosomes , an increase in autosomal aneuploidy has been observed in elderly peripheral blood lymphocytes, bone marrow cells, myeloid cells and fibroblasts, using different techniques such as metaphase spreads and fluorescence in situ hybridization in both interphase nuclei and cytokinesis-blocked binucleated cells [9,10,11]. The results obtained have shown that aging is positively correlated with the incidence of chromosome mis-segregation ,Footnote 2 even though different chromosomes might have distinct susceptibility to mis-segregation [11, 12], or alternatively generate aneusomiesFootnote 3 more compatible with cell survival [9, 10]. Chromosome-specific aneuploidies were also found in the aging brain as well as in buccal cells from both older patients and patients with Alzheimer’s disease (AD) , suggesting they might contribute to neurodegeneration [13,14,15]. However, this became recently controversial with the advent of single-cell whole genome sequencing to measure aneuploidy [16, 17].
The relative high frequencies of multiple mis-segregation events (involving more than one chromosome) have raised the question whether there is a general dysfunction of the mitotic apparatus in aged cells [12]. Oligonucleotide microarrays in a panel of fibroblast and lymphocyte cultures from young and old individuals were used to determine changes in gene expression specific for increased aneuploidy with age [18, 19]. These analyses revealed an association between age-related aneuploidy and the expression levels of genes involved in centromereFootnote 4 and kinetochoreFootnote 5 function and in the microtubule and spindle assembly apparatus [18]. This opens the question whether dividing cells of elderly proliferative tissues exhibit any of the mitotic defects known to lead to aneuploidy. These include defective sister chromatid cohesion, weakened spindle assembly checkpoint (SAC),Footnote 6 supernumerary centrosomesFootnote 7 and abnormal dynamics of kinetochore-microtubule attachments , as reviewed [20, 21]. Proper chromosome segregation requires the maintenance of cohesion between replicated chromosomes (sister chromatids) through the G2 and M phases and then the sudden disruption at anaphase onset. The SAC acts to prevent the destruction of sister chromatid cohesion as long there are chromosomes not properly attached to spindle microtubules. Even though the back-to-back geometry of sister kinetochores favors chromosome bi-orientation (attachment to microtubules from opposite poles) [22], the stochastic nature of the attachments might result in errors such as one single kinetochore attached to microtubules from different poles (merotely). Merotely occurs naturally in early mitosis but is corrected before anaphase by specific molecular mechanisms [23]. If left uncorrected, merotely results in anaphase laggingFootnote 8 chromosomes that might generate aneuploid daughter cells. The prevalence of merotelic attachments increases with cohesion defects that impair the orderly packing of centromeric chromatin and the typical back-to-back orientation of sister kinetochores. The incidence of merotely also increases in cells with attenuated SAC activity likely due to insufficient time for correction prior to anaphase onset. Cells with extra centrosomes induce transient multipolar spindles before the coalescence of centrosomes into bipolar spindles, and this event was shown to promote merotely and lagging chromosomes [24, 25]. Finally, insults that stabilize kinetochore-microtubule attachments prevent efficient correction of wrong attachments (which acts by releasing inappropriately attached microtubules) and promote chromosome mis-segregation [26].
Interestingly, the analysis of the mitotic process in aging models and diseases, even though limited, has often revealed the presence of dysfunctional phenotypes. Thus, we next review the data supporting that the primary causes of cellular damage during aging can induce mitotic defects and aneuploidization .
7.2 Mitotic Defects Associated with Primary Hallmarks of Aging
Genomic instability , telomere loss, epigenetic drift and defective proteostasis have been classified as primary hallmarks of aging, which act as initiating triggers whose damaging consequences progressively accumulate with time leading to secondary hallmarks [27]. In this section, and for each one of these types of damage, we will (1) briefly summarize how it has been linked to aging, and (2) highlight how often it leads to mitotic defects. By compiling these data, we argue for the existence of an age-associated mitotic decline.
7.2.1 Genomic Instability
Recent evidence points to DNA damage accumulation as an important driver of the aging process [28]. DNA integrity is constantly challenged by exogenous agents such as radiation, environmental chemicals, endogenous reactive oxygen and nitrogen species. These genotoxic agents induce altogether an enormous variety of DNA lesions that include point mutations, single- or double-stranded DNA breaks, and chromosomal rearrangements, losses and gains. To counteract DNA damage, the organisms have evolved a powerful network of repair mechanisms that jointly correct most of the nuclear DNA lesions. Aging arises from a wide range of phenotypic changes at the cellular level once the repair mechanisms are not sufficient to cope with a given level of damage [29]. In addition to the direct lesions in DNA, defects in nuclear architecture, known as laminopathies,Footnote 9 also lead to genome instability and premature aging Footnote 10 [30].
7.2.1.1 Nuclear DNA Damage
7.2.1.1.1 Nuclear DNA Damage and Aging
A prominent role of genome integrity in aging has been emphasized by the perception that most multi-system premature aging syndromes are caused by mutations in DNA repair genes. Examples are Werner syndrome (WS) , Bloom syndrome (BS), Rothmund–Thomson (RTS) , Fanconi’s anemia (FA) , Cockayne syndrome (CS) , Trichothiodystrophy (TTD) , and XFE syndrome [28, 31, 32]. Mice with defects in specific DNA repair mechanisms also display many progeroid phenotypesFootnote 11 [33]. Integrative analysis of human syndromes and mouse models has tentatively assigned aging phenotypes to specific DNA lesions, although the impact of specific lesions depends additionally on the underlying DNA repair mechanism and cellular context (stage in the cell cycle, proliferation and differentiation status, overall condition) [34]. In proliferative tissues and cell compartments, such as the hematopoietic and gonadal systems, double strand breaks (DSBs) and interstrand crosslinks (ICLs) are the types of lesions translating into segmental aging features.Footnote 12 DSBs and ICLs block DNA replication, activating the DNA damage response (DDR). When not repaired by the error-prone mechanisms, these lesions lead to cell death or senescenceFootnote 13 [35]. Cell death induces loss of tissue homeostasis or depletion of somatic stem cell pools, whereas cellular senescence induces a secretory phenotype including several pro-inflammatory cytokines, leading to tissue functional decline [36]. An example of a human syndrome caused by defects in ICL repair is FA, whereas the syndromes WS, RTS and BS are all caused by defective DSB repair due to mutations in RecQ helicases. These syndromes exhibit cancer and/or aging phenotypes depending whether ICLs/DSBs are repaired or not by error-prone mechanisms, respectively. In post-mitotic tissues, such as the neuronal system, helix-distorting lesions are the type of lesions most commonly leading to tissue decline and aging. Helix-disruptive lesions block RNA polymerase elongation and repair is initiated by transcription-coupled repair (TCR), a multi-step ‘cut-and-patch’-type excision reaction that uses many core nucleotide-excision repair (NER) factors. Defective TCR repair is the main driver of premature aging phenotypes in the human disorders TTD, CS and XFE, and corresponding mouse models [37].
Finally, not only defects in DNA maintenance may lead to accelerated aging, but also there is sufficient evidence that all pathways of DNA repair become less efficient with age [38]. Even though the causes behind this decline remain elusive, several studies have identified an age-related decrease in the expression of DNA repair enzymes or their activities [39].
7.2.1.1.2 Mitotic Defects Induced by DNA Damage
The DNA damage response (DDR) is a complex signaling cascade that leads to cell cycle arrest in the presence of DNA lesions including those abovementioned that stall replication, transcription and that translate into aging phenotypes. DDR has been extensively investigated in interphase and shown to comprise two pathways, ATR/CHK1 and ATM/CHK2, that inhibit mitotic entry in order to provide sufficient time for DNA repair [40, 41]. However, recent studies have found that mitotic cells can also elicit a ‘primary DDR’ comprised of early events such as ATM activation, but then are unable to repair DNA damage due to the inhibition of 53BP1 recruitment to DNA lesions by mitotic kinase activity [42]. The rationale behind this impaired recruitment is to restrain end-to-end chromosome fusions that interfere with chromosome segregation [43] (see Sect. 7.2.2.2). Nevertheless, as lately shown, partial activation of DDR during mitosis increases the frequency of lagging chromosomes during anaphase by selectively stabilizing kinetochore-microtubule attachments and, thereby, preventing efficient correction of erroneous attachments [44] (Fig. 7.1a).
Age-associated nuclear DNA Damage leads to mitotic defects. (a–e). DNA damage accumulation or defective DNA repair mechanisms are important drivers of aging. (a) DSB and ICL lesions induce replication fork stalling and elicit a primary DDR (ATM/CHK2 activation) that leads to the phosphorylation of Aur-A and Plk-1 mitotic kinases, stabilization of kinetochore-microtubule attachments and increased frequency of lagging chromosomes during anaphase. (b) Defective ICL repair (FA) and defective WRN and BLM helicase activity result in limited resolution of anaphase ultrafine bridges (UFBs) emerging from different chromosomal loci, centromeres (C-UFBs), telomeres (T-UFBs) and fragile sites (FS-UFBs). (c) Moreover, defective Recql4 helicase (RTS) leads to premature centromere separation. (d) Bulky lesions induce transcriptional stalling and primary DDR that leads to overstable kinetochore-microtubule attachments. (e) Mutations in NER core factors, as for instance XPD and XPF, which interfere with their localization to the mitotic spindle, result in spindle defects and chromosome mis-segregation
Moreover, mutations in several DNA repair genes have been frequently reported to induce chromosome segregation errors. This is the case of FA, WS, BS and RTS human diseases in which DNA damage interferes with DNA replication. FA is caused by mutations in genes of the FANC pathway and cells from FA patients typically exhibit gross aneuploidy [45, 46]. Recent studies have evaluated the role of FA proteins in chromosome segregation. FANCD2 localizes to discrete sites on mitotic chromosomes to promote anaphase resolution of chromosome entanglements induced by replication fork stalling [47] (Fig. 7.1b). In addition, FA proteins differentially localize to structures of the mitotic apparatus generating a signal essential for SAC activity [48]. Three of the five human RecQ homologs have been shown to be associated with the autosomal recessive syndromes, WS, BS and RTS. The RecQ protein family is a highly conserved group of DNA helicases involved in recombination-related processes, the non-homologous end-joining and homologous recombination, that function in the repair of DSBs, ICLs and recovery of stalled or broken replication forks [49]. Importantly, these helicases are required to protect the genome from illegitimate recombination during chromosome segregation. Analyses of patient-derived cells have demonstrated unusually high frequencies of chromosomal abnormalities including aneuploidy [50,51,52,53,54]. Chromosomal instability is also found in the mouse models of these human diseases [55,56,57]. Cells from Recql4 mutant mice (Type II RTS model) have high frequencies of premature centromere separation and aneuploidy, suggesting a role for Recql4 in sister-chromatid cohesion [57] (Fig. 7.1c). Both WRN and BLM helicases function to resolve stalled DNA replication forks and preclude chromatin mis-segregation [58,59,60]. By unwinding various DNA structures, BLM not only prevents elevated frequency of sister chromatid homologous recombination, but also resolves ultrafine anaphase bridgesFootnote 14 during the later stages of mitosis [61]. BLM localizes to all types of ultrafine anaphase bridges (UFBs) emerging from different chromosomal loci, centromeres, telomeres and fragile sites. Centromere-UFBs are likely due to double strand DNA catenation, whereas telomere-UFBs and fragile site-UFBs most likely contain incompletely replicated DNA or hemicatenates [62]. Localization of BLM to UBFs depends on the Plk1-interacting checkpoint kinase PICH and is required to prevent the formation of supernumerary UFBs [63] (Fig. 7.1b).
Studies of NER mutations in human patients and mouse models suggest that, like replication stalling, also transcriptional blocking might compromise chromosome segregation (Fig. 7.1d). Whereas clinical phenotypes of highly elevated cancer predisposition, such as in Xeroderma pigmentosa (XP), are caused by mutations that affect the global genome NER pathway, those of accelerated aging, such as in Cockayne Syndrome (CS), are caused by mutations that affect the transcription-coupled NER pathway [37]. However, following DNA damage recognition, both pathways converge into common NER factors that unwind the helix (XPB, XPD and XPA) and remove the damaged DNA strand (ERCC1, XPF, and XPG). Mutations in NER core factors, for example XPD, cause diseases with combined phenotypes of XP and CS, such as XP/CS and Trichothiodystrophy (TTD). Evidence for a role of transcription-coupled NER in mitotic fidelity have come from reports showing increased aneuploidy in CS cells [53] as well as in XP-D and XP-D/CS cells [64]. XPD forms a specific complex called MMXD that localizes to the mitotic spindle and is required for proper chromosome segregation [64] (Fig. 7.1e). XPF, mutated in XFE syndrome, also localizes to the mitotic spindle [65] and, in addition, co-localizes with FANCD2 on mitotic chromosomes playing a role in processing of replication stress at fragile sites until mitosis [66].
It is possible that the distinct mitotic defects found in association with mutations in different DNA repair enzymes , do actually concur in an age-associated mitotic decline, if we consider that all pathways of DNA repair become less efficient during natural aging [38].
7.2.1.2 Nuclear Lamina Defects
Defects in the nuclear lamina, a structure near the inner nuclear membrane and the peripheral chromatin, compromise nuclear architecture and can cause genomic instability [67]. LMNA is a gene differentially expressed and spliced to produce the nuclear intermediate filament proteins lamins A and C, the major components of the nuclear lamina that direct and indirectly act in the maintenance of nuclear structure, gene expression, chromatin organization, cell cycle regulation and apoptosis [68].
7.2.1.2.1 Nuclear Structure and Aging
In the past 20 years, an increasing number of mutations in lamins and lamin-binding proteins was found to be linked to at least 15 different diseases, called laminopathies , of which Hutchinson-Gilford Progeria Syndrome (HGPS), Restrictive Dermopathy (RD) and Néstor-Guillermo Progeria Syndrome (NPGS) exhibit segmental premature aging features [69]. Classic HGPS is caused by a point mutation in the LMNA gene that activates a cryptic donor splice site leading to partial deletion of exon 11 and generation of a mutant lamin A (LA) protein termed “progerin/LAΔ50”. Normally, mature lamin A is generated from a prelamin A precursor through substantial post-translational modification of its C-terminal CaaX motif , which includes cysteine farnesylation, cleavage of the aaX amino acids by the FACE-1/Zmspte24 metalloproteinase, carboxymethylation of the farnesylated cysteine and, finally, a second cleavage of the 15 terminal residues also by FACE-1 [70]. The progerin-truncated isoform remains permanently farnesylated and carboxymethylated since it cannot be secondarily cleaved by FACE-1. This leads to toxic accumulation of progerin at the nuclear envelope of HGPS patient cells causing nuclear shape abnormalities and chromatin stress [71]. Several studies have supported the dominant-negative disruption of lamin-related functions by progerin. Expression of LAΔ50 in normal cells recapitulates the nuclear abnormalities of HGPS cells [71], whereas expression of nonfarnesylated and carboxymethylated versions of progerin [72] does not show the classical HGPS phenotypes. Moreover, administration of farnesyl transferase inhibitors rescues some of the HGPS phenotypes [73, 74]. Another progeroid syndrome, RD, is caused by homozygous loss of FACE-1/ZMPSTE24 [75, 76]. Again accumulation of farnesylated prelamin A associates with the disease phenotypes, and mice deficient in Zmpste24 extensively phenocopy HGPS. The NGPS is caused by mutations in the barrier to autointegration factor BAF/BANF1 gene which codes for an essential DNA-binding protein involved in many pathways, namely the maintenance of nuclear structure through interaction with lamins and nuclear membrane proteins [77].
Remarkably, studies of progeroid laminopathies and lamin A-related mouse models have generated significant insight into the normal aging process. Fibroblast samples from elderly wild type individuals have been shown to exhibit increased DNA damage, morphological abnormalities and changes in histone modifications comparable to those of HGPS patients [71, 78]. In addition, the appearance of progerin in fibroblasts and skin samples from elderly individuals [79, 80], further suggests that progerin may play a role in normal aging. Progerin synthesis due to sporadic use of the cryptic splice site in the LMNA gene and decreased levels of lamin B1 are putative determinants of the age-related nuclear defects [80,81,82] .
7.2.1.2.2 Mitotic Defects in Progeroid Laminopathies
Early studies measuring aneuploidy in cultured cells from human progeria syndromes found significantly increased levels compared to controls [53]. More recently, expression of progerin/LAΔ50 in human cells was shown to cause chromosome segregation defects [83, 84]. Stable farnesylation and carboxymethylation of the mutant LAΔ50 cause an abnormal association with membranes during mitosis as well as formation of insoluble cytoplasmic aggregates. The abnormal dynamic behavior of progerin interferes with the mitotic membrane network morphogenesis, leading to a significant increase of lagging chromosomes at anaphase, a delay in the onset and progression of cytokinesis, and often binucleation (Fig. 7.2a). Moreover, similar mitotic defects correlating with the presence of progerin membrane-like aggregates and increasing with cell culture passage number were found in both HGPS and normal cells. In addition, cells from Lmna/Disheveled hair and ears(Dhe) heterozygous mice exhibit many phenotypes of human HGPS cells, including perturbations of the nuclear shape and lamina, increased DNA damage, and slow growth rates due to mitotic delay. Reduced levels of active hypophosphorylated RB1 and of its target NCAP-D3, a mitosis-specific centromere condensin subunit, were suggested to account for the chromosome segregation defects and consistent aneuploidy in the Lmna(Dhe/+) fibroblasts [85] (Fig. 7.2b). Lack of FACE-1/Zmspte24 metalloproteinase, also induces formation of lobulated nuclei and micronucleiFootnote 15 [86, 87]. Recently, it was shown that lamin-A/C are part of a stable complex with LAP2α and BAF that binds the actin filaments in the cell cortex and membranous spindle matrixFootnote 16 to the spindle-associated dynein, thereby ensuring proper spindle assembly and positioning [88] (Fig. 7.2c). However, the existence and functional role of a spindle matrix remain controversial as well as its molecular and structural composition [89]. Regarding BAF, the proper control of its association with other proteins and with DNA seems to be critical at multiple mitotic stages , being its dynamic phosphorylation and dephosphorylation particularly essential to drive nuclear disassembly and reassembly, respectively [77].
Mitotic defects associated with loss of nuclear architecture (a–d). (a) Accumulation of mutant lamin A or progerin in HGPS or RD patients as well as in elderly individuals, interferes with the mitotic membrane network morphogenesis causing chromosome segregation and cytokinesis defects. (b) Progerin also leads to reduced levels of active pRB1 and its transcriptional target, centromere condensin subunit NCAP-D3, causing merotelic attachments (inset). (c) In NGS, disruption of the lamin A/C-LAP2α-BAF complex, that binds the actin filaments in the cell cortex and the membranous spindle matrix to the spindle-associated dynein (inset), prevents proper spindle assembly and positioning. (d) Decreased levels of lamin B1 in HGPS or elderly cells lead to spindle defects due to disruption of the spindle matrix architecture that requires the interaction between lamin B1 bound to membrane vesicles and the NudEL/dynein complex at spindle microtubules (inset)
Finally, because B-type lamins lack a C-terminal CaaX motif, they remain bound to membrane vesicles through their farnesyl anchor during mitosis, and possibly make part of the spindle matrix controlling mitotic spindle assembly and orientation [90, 91] (Fig. 7.2d). Interestingly, a decreased amount of lamin B1 has been found in HGPS cells and in cells entering replicative senescence [78, 81], suggesting that mitotic abnormalities associated with lamin B1 repression may contribute to aging.
7.2.2 Telomere Attrition
In humans, telomeres shorten throughout the life span. The degree of shortening is roughly proportionate to risks of common diseases of aging as well as mortality risk [92]. Telomeres are dynamic complexes at chromosome ends containing tandem short DNA repeats and associated proteins [93]. The telomeres protect the genomic DNA by two means. First, telomeres are bound by the multiprotein complex shelterin to prevent the end of the linear chromosomal DNA from being recognized as DNA breaks [94]. Whereas this deflects the action of DNA repair mechanisms that would lead to chromosome rearrangements and instability, it turns DNA damage more persistent at telomeres contributing to cellular senescence and/or apoptosis [95, 96]. Second, the ribonucleoprotein enzyme telomerase adds telomeric repeat sequences to the chromosome ends to prevent the attrition arising from the inability of the DNA polymerases to completely replicate the extreme ends of linear chromosomes [97]. However, the levels of telomerase (or of its action on telomeres) are limiting in most mammalian somatic cells, causing progressive loss of telomere-protective sequences.
7.2.2.1 Age-Related Telomere Erosion
Cells with critically short or sufficiently damaged telomeres elicit a sustained DNA signaling, which leads to the loss of proliferative capacity known as replicative senescence (or Hayflick limit) [98, 99], unless telomerase is ectopically expressed [100]. However, the idea of telomere length as a mitotic clock ticking during normative agingFootnote 17 is too simplistic as telomerase enrichment in stem cells ensures their capacity to constantly renew somatic tissue cells in vivo [101] and it is unknown how much cellular senescence or death can arise from other causes than telomere erosion [102]. Also, extrapolating findings from aging studies using short-lived animal models has been limited because of the much longer time frame of human aging. Unless telomere maintenance is experimentally repressed genetically, laboratory animal models normally die of old age with relatively long telomeres [103]. But the experimental deletion of a telomerase component or a telomere protective protein does cause accelerated aging phenotypes in short-lived animals. The challenge has been to establish the extent by which telomere attrition contributes to normative aging phenotypes in the human. The study of monogenetic disorders of telomere maintenance has been valuable in this regard. Inactivating mutations are known in 11 genes that encode either a telomerase component or a telomere-binding protein [92]. These mutations are associated with premature development of diseases, such as pulmonary fibrosis, dyskeratosis congenita, and aplastic anemia, which involve the loss of the regenerative capacity of different tissues [104]. They parallel many phenotypes of experimental mouse models that are null for a telomere maintenance gene [105]. Further supporting telomere loss as a hallmark of aging, evidence indicates that aging can be reverted by telomerase activation [106].
7.2.2.2 Mitotic Defects Associated with Telomere Dysfunction
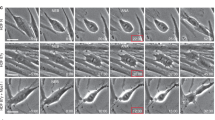
Similarly to the naturally occurring chromosome ends where DSB repair must be prevented by the telomere capping, mitosis is another condition under which DSB repair must be silenced [43, 107]. The mechanisms behind DSB repair silencing in telomeres and during mitosis both act to block downstream ubiquitin signaling in the DDR after initial upstream phosphorylation signaling occurs [108]. In the human, cellular aging leads to spontaneous accumulation of shortened intermediate-state telomeres, which activate an ATM-dependent DDR but still have sufficient levels of shelterin proteins (i.e., TRF2) to suppress non-homologous end joining DNA repair mechanisms [109]. Intermediate-state telomeres routinely pass through mitosis in increasing abundance during cellular aging, until sufficient numbers accumulate to induce senescence [110] (Fig. 7.3a). If DSB repair were active during mitosis, the transit of intermediate-state telomeres through cell division would drive genome instability in pre-senescent cells. However, under certain conditions such as excessive telomere shortening and defects in telomere-associated proteins, fully uncapped-state telomeres might occur which lack sufficient TRF2 to inhibit DSB repair [111,112,113]. Impairment of telomere function together with a compromised senescence/apoptosis response leads to chromosomal instability (CIN)Footnote 18 through end-to-end chromosome fusions entering BFB (breakage-fusion-bridge) cycles [114]. Interchromosome dicentrics or isodicentric chromatids are prone to bridge in anaphase [115] (Fig. 7.3b). If the chromatin bridges are unequally broken, the new broken ends generated will perpetuate the BFB cycle leading to structural CIN [116]. Alternatively, if chromatin bridges are not resolved by breaking, whole chromosome mis-segregation will happen [117]. Detachment of dicentric chromatids from microtubules of one or both poles during anaphase was originally proposed as the mechanism underlying chromosome bridge-induced aneuploidy [117]. However, one recent study has elucidated that dicentric chromatid bridges rarely break during mitosis and exhibit persistently bound microtubule k-fibersFootnote 19 that hardly shorten during anaphase [118]. This causes the bridged chromosomes to lag behind in anaphase leading to chromosome non-disjunction or alternatively, micronuclei formation (Fig. 7.3b). Physical isolation of chromosomes in micronuclei can lead to chromothripsis, the presence of massive chromosome rearrangements in confined genomic regions of one or a few chromosomes [119]. Fragmentation and subsequent reassembly events occur restrictively in the micronucleus chromatin in one cell division, and after mitosis the mutated chromosome can be incorporated into daughter nuclei [120]. In addition , one recent study has shown that a pattern of localized hypermutation known as kataegis may also arise during telomere crisis from the fragmentation of dicentric chromosome bridges during late mitosis [121]. The base mutations in kataegistic clusters are mainly cytosine to thymidine transitions in the context of a TpC nucleotide induced by AID/APOBEC editing deaminases in single strand DNA [122]. The mechanism by which dicentric chromosome bridges lead to kataegis involves a transient nuclear envelope breakdown at the bridge late in telophase, which makes DNA accessible to the cytoplasmic 3′ nuclease TREX1. This nuclease generates single stranded DNA that becomes a target for AID/APOBEC deamination and kataegis [121] (Fig. 7.3c).
Telomere attrition during natural aging compromises mitotic fidelity. (a) Cellular aging leads to spontaneous accumulation of shortened intermediate-state telomeres, which activate DDR but still have sufficient levels of shelterin proteins (i.e., TRF2) to suppress DSB repair. Intermediate-state telomeres routinely pass through mitosis, until sufficient numbers accumulate to induce cell cycle arrest and senescence. (b) However, excessive telomere shortening and defects in telomere-associated proteins generate uncapped-state telomeres that lack sufficient TRF2 to inhibit DSB repair and lead to telomere fusions. Interchromosome or sister telomere fusions cause bridged chromosomes to lag in anaphase. These lagging chromosomes either can lead to micronucleus formation and consequently chromothripsis, or can lead to chromosome non-disjunction and aneuploidy. (c) Excessive telomere cohesion leads to formation of anaphase ultrafine bridges (T-UBFs), which can cause micronuclei formation and chromothripsis or alternatively be resolved by the TREX1/APOBEC mechanism that generates clusters of point mutations in the DNA bridge known as kataegis
Anaphase chromatin bridges might arise not only from end-to-end chromosome fusions, but also from defects in sister chromatid resolution. Sister chromatid resolution is spatial-temporally coordinated at the centromeres, arms and telomeres. Resolution of telomere cohesion occurs in early mitosis and it is distinctly regulated from centromere and arm cohesion as it requires the poly(ADP-ribose) polymerase (PARP) tankyrase 1 [123, 124] and the isoform SA1 of the cohesin subunit Scc3 [125]. SA1 associates with the shelterin subunit TRF1 and its partner TIN2 [126], and TRF1 PARsylation by tankyrase 1 [127] releases SA1 from telomeres in prophase [128, 129]. Inhibition of tankyrase 1 and overexpression of SA1 or TIN2 induce excess cohesion at telomeres in mitosis preventing a robust and efficient anaphase [130]. Interestingly, excessive telomere cohesion was found during replicative aging of primary fibroblasts [131] (Fig. 7.3c).
7.2.3 Epigenetic Alterations
Recent evidence indicates that several of the conserved longevity pathways mediated by signaling pathways (e.g. insulin/IGF1, TOR, AMPK) and downstream transcription factors (e.g., FOXA, FOXO, NRF2) can modulate chromatin states [132]. Chromatin state is governed by a series of epigenetic modifications that include DNA methylation, histone modification and chromatin remodeling.
7.2.3.1 Epigenetic Modifications and Aging
DNA methylation represents the addition of methyl groups to cytosine residues in the context of CG dinucleotides, referred to as ‘CpG site’. The relationship between DNA methylation (DNAm) and aging remains elusive as there is global hypomethylation concurrently with loci-specific hypermethylation [133, 134], and no evidence exists thus far for lifespan extension through modulation of DNAm. Nevertheless, DNAm is perhaps the best-characterized epigenetic modification, and ‘epigenetic-signatures’ at specific CpG sites have been reported as quantitative models of both in vitro and natural aging [135,136,137]. Epigenetic drift at specific CpG sites in the genome is mediated, among other factors, by changes in the histone code. Age-associated histone modifications include increased H4K16 acetylation, H4K20 trimethylation, or H3K4 trimethylation, as well as decreased H3K9 methylation or H3K27 trimethylation [132, 138]. Multiple enzymes including acetyltransferases, deacetylases, methyltransferases and demethylases reversibly catalyze these modifications. Studies on chromatin regulators and lifespan have focused mostly on histone acetylases and deacetylases, in particular the sirtuin family . However, in the past few years, histone methyltransferases and demethylases have been also described to affect lifespan [132], even though unclear if through epigenetic mechanisms or transcriptional changes impacting on longevity signaling pathways [139]. Members of the sirtuin family of NAD-dependent protein deacetylases and ADP ribosyltransferases have been widely reported to ameliorate several aspects of aging in different organisms [140]. In mammals, not only SIRT1, which is the nearest homolog to invertebrate Sir2, but also SIRT3 and SIRT6 contribute to healthy aging through beneficial effects on genomic stability, metabolic efficiency and nutrient sensing [141,142,143,144,145,146,147,148]. Another epigenetic modification during aging is global heterochromatin loss and redistribution, caused by decreased levels of chromatin remodeling factors such as heterochromatin protein 1α (HP1α), Polycomb proteins and the NuRD complex [149,150,151]. Finally, microarray comparative analysis of young versus old tissues from different species have highlighted aging-associated transcriptional signatures affecting not only mRNAs of inflammatory, mitochondrial and lysosomal degradation pathways, but also noncoding RNAs such as a class of miRNAs that target components of longevity networks [152, 153]. Because of their reversibility, epigenetic alterations have been extensively explored for the design of novel anti-aging therapies [154].
7.2.3.2 Mitotic Defects Associated with Aging Epigenetic Marks
Supporting the functional relevance of age-related epigenetically mediated chromatin alterations in mitosis, there is a notable connection between heterochromatin formation at repeated DNA domains and chromosomal stability. In particular, heterochromatin assembly at pericentromeric regions requires trimethylation of histones H3K9 and H4K20, as well as HP1α binding, and is important for chromosomal stability [155]. Mammalian telomeric repeats are also enriched for these chromatin modifications, indicating that chromosome ends are assembled into heterochromatin domains [156, 157].
Moreover, the changes in chromatin conformation and compaction during mitosis, namely transcriptional repression and chromosome condensation, depend largely on the deacetylation of lysine residues of H3 and H4 core histones [158]. Inhibition of histone deacetylation with trichostatin A shortly before mitosis impairs proper chromosome condensation resulting in poor sister chromatid resolution (chromatin bridges) [23]. Histone hyperacetylation also causes depletion of HP1 from the pericentromeric chromatin, which in turn leads to loosened centromeres that promote the formation of merotelic attachments and lagging chromosomes [23, 159] (Fig. 7.4a). In addition, displacement of heterochromatin proteins HP1α and HP1γ from chromatin induces premature chromatid separation concomitant with delocalization of cohesion proteins from the centromeres [160] (Fig. 7.4b).
Age-associated epigenetic alterations induce mitotic defects . Changes in histone modifications (for example, decreased H3K9 methylation and increased H4K16 acetylation) and decreased levels of sirtuins (Sirt1, Sirt2) and chromatin remodeling factors (such as HP1α), are epigenetic signatures of aged cells. (a) Increased H4K16 acetylation associated with decreased H3K9me3 or Sirt2 deacetylase activity leads to depletion of HP1α from pericentromeric heterochromatin, which causes centromere relaxation and formation of merotelic attachments (inset). (b) Reduced Sirt1 activity interferes with condensin I complex loading into chromatin leading to chromosome condensation defects (inset). Moreover, reduced Sirt1 activity also causes HP1α depletion from chromatin resulting in heterochromatin loss and premature cohesion loss due to defective recruitment of Scc1 and Sgo1 proteins (inset)
Mitotic roles have been reported for several sirtuins thus suggesting that decreased sirtuin activity during aging might lead to age-associated aneuploidy. SIRT1 deficiency results in accumulation of cells in early mitotic stages due to incomplete chromosome condensation [147]. The defective chromosome condensation in sirt1−/− cells is due increased acetylation of H3K9 that impairs its trimethylation and, consequently, the recruitment of HP1α, which is required to establish a closed chromatin configuration. In addition, SIRT1 was found to associate with mitotic chromatin at prometaphase in order to mediate the chromosomal loading of histone H1 and the condensin I complex needed for chromosome condensation [161] (Fig. 7.4b). SIRT2 has strong preference for acetylated H4K16 which enzymatic conversion into its deacetylated form at G2/M may be crucial for chromatin condensation at early mitosis [162] (Fig. 7.4a). Moreover, SIRT2 was shown to regulate the anaphase-promoting complex/cyclosome (APC/C)Footnote 20 activity through deacetylation of its coactivators, APC(CDH1) and CDC20 [163]. This leads to increased levels of mitotic regulators such as Aurora-A and -B in SIRT2 deficient cells that induce centrosome amplification, aneuploidy, and mitotic cell death. Microtubule polymerization increases in SIRT3 depleted cells suggesting that SIRT3 regulates spindle dynamics [164]. SIRT6 levels, similarly to SIRT1 and SIRT2, increase in mitosis, but SIRT6 partially co-localizes with the mitotic spindle instead of being associated with condensed chromosomes [165].
Finally, even though still elusive, some noncoding RNAs implicated in the senescence and aging processes in recent years, such as geromiRs [153] might impact in mitotic function. Some examples are: miR-1 that downregulates genes involved in DNA replication and mitosis [166]; miR-34 that suppresses SIRT1 activity [167]; and let-7b that targets Aurora B, causing increased rate of aneuploidy, polyploidy and multipolarity [168].
7.2.4 Loss of Proteostasis
A progressive deterioration in the ability of cells to preserve the stability of their proteome occurs with age [169]. Protein homeostasis or proteostasis involves mechanisms for the stabilization of correctly folded proteins, most prominently the heat-shock family of proteins, and mechanisms for the degradation of proteins by the proteasome or the lysosome [170]. Dysfunction of the quality-control mechanisms and intracellular accumulation of abnormal proteins in the form of protein inclusions and aggregates occur in almost all tissues of an aged organism. Interestingly, many genes coding for components of these mechanisms have been associated with mitotic fidelity as we review next, suggesting that loss of proteostasis might contribute to aneuploidization.
7.2.4.1 The Protein Control Machinery and Aging
Chaperones or heat shock proteins (HSPs) are highly conserved molecules that act to assure that proteins acquire a stable folded conformation. Primary or secondary deficits in chaperone function have been reported for age-related diseases, the extent of which depends on the chaperone, the tissue and even the organism [171]. Upregulation of hsp70 in response to different stressors is decreased in fibroblasts aged in vitro and tissues from old organisms [172,173,174], due to the inability of the heat shock factor (HSF) transcriptional activator to bind the chaperone gene promoter [175, 176]. Conversely, extra copies of an hsp70 family member, as well as HSF over-expression, have been shown to increase lifespan [177,178,179].
If chaperone-driven folding attempts are unsuccessful, proteins are then delivered to the proteolytic machinery. The two main components of the ubiquitin-proteasome system (UPS) , the ubiquitinization machinery and the proteasome core, also undergo age-dependent changes that lead to loss of proteostasis such as decreased levels of free ubiquitin [180], transcriptional repression of ubiquitin-conjugating enzymes or E3 ligases [181], defective expression of proteasomal subunits or its regulatory subunits [182, 183]. On the other hand, maintained UPS activity has been shown to promote lifespan extension in different model systems [184, 185]. Also the proteolytic activities of both macroautophagy and chaperone-mediated autophagy (CMA) have been described to decrease with age. In macroautophagy, a whole region of cytosol is sequestered inside double membrane vesicles (autophagosomes), which then fuse with lysosomes to degrade their cargo [186]. Age-associated malfunctioning of macroautophagy arises from impairment of autophagosome fusion with the lysosome [187], or inhibitory effect on lysosomal proteolysis [188]. In CMA, substrate proteins are selectively recognized by the chaperone hsc70, which then binds to the lysosome-associated receptor LAMP-2A, so that translocation of the substrate across the lysosomal membrane occurs [189]. Progressively lower levels of the CMA receptor at the lysosomal membrane were found with age likely due to changes in the lipid membrane composition [190]. Increasing evidence has shown that preventing the decline in autophagic activity slows down cellular aging and preserves organ function [191].
Being aberrant protein conformers key determinants of aging, the ability to control their inheritance is crucial for avoiding aging in specific cells [192]. Several reports have shown that yeast and mammalian cells use an intricate machinery to spatially sequester misfolded proteins in inclusions that interact with organelles and cytoskeleton to ensure the polarity of their inheritance after mitosis, as reviewed in [193]. However, the rejuvenation of daughter cells by asymmetric mitotic partitioning might gradually decline with advanced age as recently found in yeast [194]. Asymmetric cell division as a key determinant of aging and age-associated diseases is reviewed by Polymenis and Kennedy in the same volume.
7.2.4.2 Mitotic Defects Associated with Loss of Proteostasis
Molecular chaperones and protein control pathways are essential for the assembly and disassembly of macromolecular complexes. They are therefore expected to assist the proper and timely assembly of cytoskeletal structures during mitosis. Indeed, HSPs have been involved in microtubule dynamics and spindle assembly during mitosis. Hsp70 is phosphorylated by the mitotic kinase Plk1 and its centrosomal localization may interfere with spindle dynamics [195] (Fig. 7.5a). Hsp72, an inducible cytoplasmic isoform of the Hsp70 family, is required for assembly of a robust bipolar spindle capable of efficient chromosome congression [196]. Targeting of Hsp72 to the mitotic spindle is dependent on phosphorylation by the Nek6 kinase. Phosphorylated Hsp72 localizes to the spindle poles and sites of kinetochore-microtubule attachment and acts not only to stabilize K-fibers through the recruitment of the ch-TOG/TACC3 complex, but also to regulate spindle positioning through the attachment of astral microtubules to the cell cortex (Fig. 7.5a). Also, the molecular chaperone Hsp90 was found to interact with the Drosophila orthologue of ch-TOG (Msps) at centrosomes and spindle, as well as to regulate the efficient localization of cyclin B at those structures [197]. Disruption of Hsp90 function by mutations in the Drosophila gene or treatment of mammalian cells with the Hsp90 inhibitor geldanamycin, results in abnormal centrosome separation and maturation, aberrant spindles and impaired chromosome segregation [198]. Moreover, the Hsp90 cochaperone Sgt1, localizes to kinetochores and its phosphorylation by Plk1 enhances the association of the Hsp90-Sgt1 chaperone with the MIS12 complex at the kinetochores to promote stable microtubule attachment and chromosome alignment [199, 200]. The Hsp90-Sgt1 complex is also required for kinetochore assembly, as Hsp90 inhibition causes delocalization of several kinetochore proteins including CENP-I and CENP-H, leading to chromosome misalignment and aneuploidy [201] (Fig. 7.5a). Interestingly, limited binding and transactivating capacity of the chaperone transcriptional regulator HSF1 was found in mitotic cells, where the chromatin is tightly compacted, turning these cells vulnerable to proteotoxicity [202]. In dividing cells, when levels of aggregation-prone proteins exceed the capacity of the proteasome to degrade them, perinuclear aggresomes accumulate and have a detrimental effect on mitosis by steric interference with chromosome alignment, centrosome positioning, and spindle formation [203] (Fig. 7.5b).
Age-associated loss of proteostasis induces mitotic defects. During natural aging there is loss of proteostasis expressed by defective chaperone activity, protein aggregation and deregulated autophagy. (a) Defective chaperone activity compromises the assembly of many protein complexes required for spindle positioning and kinetochore-microtubule (k-MT) attachment stability. For instance, defective Hsp72 activity at spindle poles and kinetochores prevents the recruitment of the ch-TOG/TACC3 complex involved in the attachment of astral microtubules to the cell cortex and stabilization of k-MT fibers. Defective Hsp70 activity at centrosomes interferes with spindle dynamics. Defective Hsp90 at kinetochores causes delocalization of several kinetochore proteins (MIS12 complex, CENP-H, CENP-I) required for stable k-MT attachments. (b) Accumulation of protein aggregates interferes sterically with spindle geometry. (c) Deregulated autophagy compromises the mitotic function of p62 and Beclin-1 autophagic proteins leading to spindle mispositioning and chromosome misalignment. The p62/HSPB8/BAG3 complex acts during mitosis to facilitate the remodeling of actin-based structures that guide spindle orientation (inset). Beclin-1 depletion causes reduction of several outer kinetochore proteins (ZW10, CENP-E, CENP-F) required for proper chromosome alignment (inset)
Even though it remains controversial whether autophagy operates during mitosis [204, 205], there is cumulative evidence suggesting that defective autophagy induces mitotic anomalies. For instance, the multichaperone complex HSPB8-BAG3 that senses damaged cytoskeletal proteins and orchestrates their seclusion by selective autophagy, acts during mitosis together with the p62/SQSTM1 autophagic receptor to facilitate the proper and timely remodeling of actin-based mitotic structures that guide spindle orientation and proper chromosome segregation [206] (Fig. 7.5c). p62/SQSTM1 has been also implicated in the concluding step of cytokinesis, suggesting that overload of the autophagic pathway might lead to impaired clearing of midbody rings and cytokinesis failure [207]. Monoallelic depletion of Beclin-1 , a subunit of the PI3K-III core complex involved in autophagy, reportedly causes chromosomal disorders such as aneuploidy and double-minute chromosomes [208]. Chromosome segregation errors associated to Beclin-1 depletion were found to arise from severe congression defects associated with a reduction in several outer kinetochore components, including ZW10, CENP-E and CENP-F [209] (Fig. 7.5c).
7.3 Aneuploidy Induces Aging
7.3.1 Aneuploidy-Driven Aging Hallmarks
In recent years, systematic analyses of disomic yeast, trisomic mouse and human cells, all cells with an extra chromosome, have elucidated the impact of aneuploidy in cellular fitness [210]. Two types of phenotypes can arise from changes in chromosome number: (1) karyotype-specific phenotypes caused by changes in copy number of specific genes and (2) phenotypes shared by different aneuploidies which reflect a decrease in cellular fitness due to a cumulative effect of copy number changes of many genes [211]. These pan-aneuploidy phenotypes include a number of aneuploidy-associated stresses found in both yeast and mammalian cells [212] that include proliferation defects [213,214,215], a gene expression profile similar to the environmental stress response (ESR) [216,217,218], multiple forms of genomic instability [219, 220], and proteotoxicity [213, 216, 221,222,223,224,225,226]. Below we summarize major findings on the impact of aneuploidy in genomic stability and protein quality-control machinery in order to emphasize how these pan-aneuploidy phenotypes recapitulate the genomic instability and proteotoxicity hallmarks of aged cells, reinforcing the causal role of aneuploidy in aging (Fig. 7.6). Whether other primary causes of cellular aging such as telomere attrition and epigenetic alterations are present in the aneuploid cell models remains unknown. Interestingly, for the human trisomy 21 or Down syndrome (DS), telomere shortening and aging epigenetic alterations have been reported. DS patients age prematurely and present early onset of Alzheimer’s disease (AD). Telomere shortening in T-lymphocytes has been proposed as a biomarker of clinical progression of AD for adults with DS [227], even though limited and conflicting data exist as to whether DS individuals have shorter telomere lengths before birth or an accelerated rate loss after birth [228]. Moreover, analysis of the quantitative DNA methylation-based biological marker of aging known as ‘epigenetic clock’ has shown that trisomy 21 significantly increases the age of blood and brain tissue on average by 6.6 years [229].
7.3.1.1 Genomic Instability
Delineating the molecular mechanisms underlying aneuploidy-driven genomic instability has remained challenging due to the difficulty of separating the effects of aneuploidy from those of other associated genetic alterations when using heterogeneous aneuploid cell populations. Recently established cell models with defined aneuploid karyotypes have facilitated the analysis of the immediate consequences of aneuploidy per se [213, 215, 218, 230]. Observations in budding and fission yeasts suggested that aneuploidy impairs the fidelity of chromosome segregation, and increases mutation and recombination rates [220, 231]. Isogenic aneuploid yeast strains with ploidies between 1N and 2N obtained through a random meiotic process were found to exhibit various levels of whole-chromosome instability, with cells with a chromosomal content closer to 1N being more stable than cells with ploidy between 1.5N and 2N [220]. Even though these results suggested inability of the mitotic system to scale continuously with an increasing number of chromosomes, the presence of specific aneuploid chromosomes also seemed to determine the rate of CIN [220]. Aneuploidy-induced chromosomal instability was also shown for human trisomies 7 and 13, which exhibit increased rate of anaphase laggings [230]. Two independent studies demonstrated that lagging chromosomes arising from merotelic attachments can in turn induce genomic instability, especially DNA damage, either due to breakage during cytokinesis [232] or due to formation of micronuclei and chromothripsis [120]. Nevertheless, untransformed damaged cells will end up activating the stress kinase p38 and the stress-induced transcription factor p53 causing cell cycle arrest [21, 232, 233].
Replication defects also seem to be a widespread consequence of the aneuploidy condition. Analysis of disomic yeast strains revealed the presence of increased levels of Rad52 foci, which formed during S phase due DSBs generated by defects in DNA replication initiation and elongation [219]. Also, a series of trisomic and tetrasomic human cells derived from near-diploid and chromosomally stable parental cell lines were recently found to exhibit anaphase UFBs and DNA damage associated with replication stress, which in turn induced genomic rearrangements at common fragile sites [234]. Importantly, reduced expression of the replicative helicase MCM2-7 was shown to account for the genomic instability phenotype [213, 217, 234] (Fig. 7.6a). Aneuploid human pluripotent stem cells (hPSCs) were also found to undergo replication stress, resulting in defective chromosome condensation and segregation [235]. However, in this study, downregulation of the transcription factor SRF and its actin cytoskeletal gene targets were identified as the molecular mechanism behind chromosomal defects. Even though aneuploidy-associated replication stress might act to promote tumorigenesis in immortalized cells, in primary cells with efficient checkpoint activities, it will most likely lead to cell death or senescence.
Aneuploidy leads to aging hallmarks. Established cell models with defined aneuploid karyotypes exhibit a number of pan-aneuploidy phenotypes that include genomic instability and proteotoxicity. (a) DNA damage, replication stress and chromosomal instability (CIN) are types of genomic instability found in the aneuploidy cell models. Decreased levels of the replicative helicase MCM2-7 were recently shown to induce replication stress particularly at fragile sites (FS) leading to increased frequency of anaphase ultrafine bridges (FS-UBFs) that promote CIN. (b) Loss of protein stoichiometry due to karyotype imbalance, leads to accumulation of protein aggregates that overwhelm the capacity of the protein-control machinery comprised by the proteasomal and autophagic degradation pathways. An impaired clearance of misfolded/aggregated proteins in autolysosomes was recently shown to activate a lysosomal stress response that upregulates the expression of transcription factor TFEB and its targets with functional role in protein-control mechanisms
7.3.1.2 Proteotoxicity
Changes in gene copy number generally translate into a corresponding change in gene expression [213, 221, 226]. Thus, gains or losses of entire chromosomes lead to massive alterations in relative abundance of many proteins. This impact in proteome composition results in proteoxicity, a state in which the protein quality-control machinery of the cell (protein chaperones, ubiquitin proteasome system, autophagy) is overwhelmed causing protein misfolding [236]. As mentioned above, yeast, mouse and human aneuploid cells exhibit upregulated expression of stress response genes, which include the chaperones [217] and autophagic proteins [213, 224]. Furthermore, analysis of disomic budding yeast revealed increased sensitivity to drugs that interfere with protein folding, synthesis and degradation [218], increased propensity to form protein aggregates, and decreased ability to fold HSP90 protein clients [223]. In line with these findings, trisomic mouse embryonic fibroblasts (MEFs) and chromosomally unstable aneuploid cancer cell lines were more sensitive to inhibition of the chaperone HSP90 than their euploid counterparts [225]. These observations clearly indicated impairment in protein quality control, either during the folding and/or disaggregation processes and/or at the level of protein degradation. Indeed, studies also uncovered mutations in the Ubp6 protein, a deubiquitinating enzyme that negatively regulates proteasome function, as conferring improved proliferation rate to a subset of disomic yeast strains, thereby implicating proteasome function in protein homeostasis in aneuploid cells [226]. In addition, effects on the autophagic protein degradation pathway were detected in mammalian aneuploid cells. Aneuploid human cell lines created by chromosome transfer and trisomic MEFs were shown to harbor significantly increased levels of LC3-II, an autophagosome-specific lipidated form of LC3, and SQSTM1/p62, the cytosolic receptor that targets ubiquitinated proteins to autophagy [213]. More recently, the molecular mechanisms behind these aneuploidy-associated phenotypes have been further elucidated. The impairment of protein folding capacity is due to defective HSF1-dependent activation of the heat shock response, as HSF1 overexpression can counteract the effects of aneuploidy in HSP90-dependent protein folding [222]. Regarding autophagy triggering, an impaired clearance of autophagosome content and accumulation of misfolded proteins in the lysosomal compartment were shown to activate a lysosomal stress response in which the transcription factor TFEB induces the expression of genes linked to autophagic protein degradation function [237] (Fig. 7.6b). It would be interesting to investigate whether these aneuploidy-induced molecular mechanisms occur in aging. In fact, inhibition of both activity and transcription of HSF1 has been described in senescent cells and shown to generate a positive feedback regulation of the p38-NF-κB-SASP senescence pathway [238].
7.3.2 Aneuploidy and Premature Aging
Studies of aneuploidy-prone mouse models exhibiting increased rate of chromosome mis-segregation uncovered a surprising link with the rate of aging and the development of age-related pathologies [239,240,241,242]. Mutant mice with low levels of the spindle assembly checkpoint protein BubR1 were found to develop progressive aneuploidy along with a variety of progeroid features, including short lifespan, growth retardation, sarcopenia, cataracts, loss of subcutaneous fat and impaired wound healing [240]. Reinforcing this link between BubR1 and aging, the majority of human patients suffering from Mosaic Variegated Aneuploidy (MVA) syndrome were found to have mutations in BUBR1 that generate unstable gene products and cause progeroid features recapitulating those in BubR1 hypomorphic mice [243, 244]. Mice doubly haploinsufficient for the mitotic checkpoint genes Bub3 and Rae1 were another aneuploidy-prone mouse strain described to exhibit an accelerated aging phenotype [245]. However, progeroid features have not been found in many other aneuploidy mouse models. Possible explanations are the premature sacrifice of mice before they start developing aging phenotypes later in life and the superficial analysis for overt age-related degeneration that might miss restricted tissue-specific phenotypes [242]. Important to mention that , even though detrimental in most cells and organisms, in some tissues such as brain and liver, aneuploidy seems to be part of normal development [246]. One possibility is that neurons and hepatocytes somehow adapt to chromosome imbalances either by accumulating chromosome-specific gene products that provide them with a selective advantage or by regulating the expression of detrimental aneuploidy-induced targets [13]. However, very recently, genome-wide high-resolution analysis of chromosome copy number variations in mouse and human tissues by single cell sequencing, has shown that aneuploidy occurs much less frequently in normal brain and liver [16] and brain with Alzheimer disease [17] than previously reported. One potential explanation is that different cell types will follow different fates (senescence vs. apoptosis) in response to aneuploidy. Senescent endothelial cells have increased sensitivity to apoptosis in comparison to senescent fibroblasts [247]. Thus, aneuploid neurons might not be detected by single cell sequencing if cleared due to apoptosis.
7.4 Concluding Remarks and Future Perspectives
Aging and aneuploidy have profound impact on most cellular functions and their association has been reported by several independent studies. However, the molecular mechanisms by which aging induces aneuploidy and by which aneuploidy triggers aging remain largely unknown. Here, we presented a detailed revision on how primary hallmarks of aging have often been associated with mitotic defects, in the attempt of gathering mechanistic insights into age-associated aneuploidy. In addition, we reviewed the systemic impacts of aneuploidy in cellular physiology, such as genomic instability and proteoxicity, and brought our perspective on how these recapitulate aging hallmarks and could be the source of aneuploidy-induced aging (Fig. 7.7). Considering all the emergent data, we propose that there is a complex positive feedback regulation between aging and aneuploidy. Age-related aneuploidy arises from mitotic function decline induced by cellular damage, and in turn contributes to secondary aging hallmarks, such as cellular senescence. It is therefore reason to ask if aneuploidy is an aging hallmark. To be an aging hallmark, aneuploidy should meet the following criteria: (1) it should manifest during normal aging; (2) its experimental aggravation should accelerate aging; and (3) its experimental amelioration should delay aging [27]. We reason these criteria are indeed met. First, we compiled a number of early and recent studies indicating that aneuploidy increases with advancing age. However, accurate karyotyping would still be needed to unequivocally establish this correlation. Each karyotyping method presently used is limited as to which cytogenetic abnormalities can be detected and in which cell type (dividing or non-dividing) [248]. Therefore, upcoming studies should consider the use of different methods to measure aneuploidy, and definitely explore the emerging karyotyping platforms that combine single cell resolution with complete karyotyping. Moreover, it would be crucial to measure aneuploidy levels in different tissues during organismal natural aging. Considering the human lifespan and limited access to human tissues, study models such as mouse and zebrafish become essential for future analyses. Another imperative aspect to investigate is whether mitotic processes exhibit an age-related decline as to expect from the aneuploidy increase during aging. Reinforcing this possibility is the observation that many of the primary aging hallmarks result in mitotic phenotypes. Secondly, we gathered evidence on how aneuploidy leads to aging. In addition to the pioneering studies in aneuploidy-prone mouse models that have shown how experimentally induced aneuploidy leads to premature aging, studies in yeast, mice and human models of constitutional aneuploidy have also supported the idea that aneuploidy contributes to aging. In particular, we highlighted the fact of many aneuploidy-associated phenotypes being primary aging hallmarks. In the future, it would be interesting to systematically investigate for the presence of other aging hallmarks in the aneuploidy models, determine if the cellular stresses associated with aneuploidy engage senescence response pathways (for instance, p16Ink4a upregulation), and compare transcriptional signatures and epigenetic marks between aneuploid and elderly cells. Interestingly, previous analyses on gene expression data from aneuploid [217] and old [18, 19] cells revealed similar impact in genes functionally linked to mitosis. The underlying mechanisms are poorly understood and may occur at both transcriptional and post-translational levels, or mitotic gene expression may merely decline as a consequence of reduced cell proliferation with aging. Third and finally, amelioration of aneuploidy acting to delay aging is the criterion for hallmark most poorly supported and difficult to achieve. Intriguingly, sustained high-level expression of BubR1 was found to reduce aneuploidy by counteracting mitotic defects possibly associated with age-related decline [239]. Attenuated aneuploidy tightly correlated with reduced senescence and tissue deterioration. Therefore, this study provided a molecular entry point for modulation of aneuploidy as an opportunity to extend healthy lifespan. One question now is whether there are mitotic genes other than BubR1 whose levels can be modulated without any overt adverse effects to prevent age-related mitotic decline and aneuploidy. In addition, it will be interesting to determine whether mutations that suppress the adverse effects of aneuploidy [226] also delay aging and extend lifespan.
Feedback positive regulation between aging and aneuploidy. Primary causes of cellular damage during natural aging, namely nuclear DNA damage, telomere attrition and protein aggregates, lead to loss of mitotic fidelity and generation of aneuploid daughter cells. Aneuploidy, in turn, induces cellular phenotypes that include primary aging hallmarks such as genomic instability and proteotoxicity. Telomere attrition in aneuploid cells is still elusive. Thus, age-related aneuploidy further enhances aging phenotypes that, in the presence of active cell cycle checkpoints, will likely lead to secondary aging hallmarks such as cellular senescence
Why should modulation of aneuploidy be a preferential opportunity to delay aging? Experimental ameliorations of genomic instability, telomere erosion, epigenetic changes and proteotoxic stress have all been shown to successfully extend lifespan, suggesting these hallmarks add-up into cellular aging. Therefore, a favored hallmark to anti-aging therapies has been difficult to assign. If in one hand genomic instability has been emphasized as a major mechanism based on the observation that most premature aging phenotypes are caused by mutations in DNA repair genes, on the other hand proteotoxic stress has been highlighted in neuronal aging. Here we have shown that all primary hallmarks end up generating aneuploidy, providing a common feature to explore in anti-aging therapy. Even though the routes to aneuploidy seem to comprise many different mechanisms depending on the type of cellular damage, one should be aware that most of these mechanisms are likely occurring jointly during chronological aging and not separately as inferred from studies in models of aging disease caused by mutations in single genes. One way to address this would be to characterize the mitotic behavior of elderly cells under advanced light microscopy. This has been largely limited by the low proliferation indexes of old tissues and/or primary cell cultures. Also, it would be important to investigate if stress-signaling pathways activated by distinct types of damage might all converge downstream to inhibit mitotic proficiency. If this assumption is correct then (1) inhibition of stress response pathways should prevent mitotic decline and (2) overexpression of a mitotic gene downstream target of stress response should increase mitotic efficiency. Inactivation of a stress pathway in humans is an unfeasible approach as it would also eliminate critical tumor-suppressive pathways and induce cancer. However, one recent strategy based in drug-induced apoptosis was designed to selectively kill stressed cells expressing the p16Ink4a gene in the context of cellular senescence [249]. If p16Ink4a-positive senescent cells come to be shown as being mainly aneuploid, then aneuploidy-selective antiproliferation compounds would be an attractive alternative to senescent cell clearance. Thus, it would be valuable to measure the extent of aneuploidy in the senescent cell population. Moreover, the aneuploidy levels induced by specific primary hallmarks could provide a means to understand the most relevant types of damage contributing to tissue-specific aging. Regarding one common downstream target of stress-signaling pathways, the Forkhead box (Fox) transcription factor family has emerged as an interesting candidate. The balance between rapid growth over maintenance of youth is largely regulated by the Fox class of transcription factors and the anaphase-promoting complex (APC) [250]. Specifically, FoxM1 and APCCdc20 function together to maintain genomic stability by regulating separation of sister chromosomes and chromatin structure, while the FoxOs and APCCdh1 regulate cellular repair and maintenance. The FoxO family has been reproducibly found to extend lifespan through reduced insulin-signaling in many model systems [250]. FoxM1 primarily drives the expression of G2/M specific genes [251] and seems to counter senescence [252]. Interestingly, artificial enrichment of FoxM1 improves liver regenerating capacity in older mice [253] and lung regeneration following injury [254], without being tumorigenic in those organs. Therefore, modulation of mitotic competence through a balanced/stoichiometric upregulation of several mitotic genes could act to prevent aneuploidy more efficiently than upregulation of specific genes such as BubR1. In the future, the crosstalk between stress response pathways and mitotic gene expression should be further investigated. Moreover, it would be interesting to investigate the impact of mitotic proficiency in cell fate decision in response to cellular stress. We support that aneuploid cells arising from age-associated mitotic decline in highly proliferating tissues and cell types are inherently more resistant to apoptosis and stay senescent than those that occasionally proliferate such as stem cells. Indeed, when chromosomal instability and aneuploidy were provoked in a tissue-specific manner, in mouse epidermis, epidermal hair follicle stem cells were rapidly depleted (likely through apoptosis), while the more committed transit amplifying cells tolerated the resulting aneuploidy quite well (even though we predict they become senescent) [255]. This could explain why aneuploid cells seem to accumulate in various somatic cell types in the ageing mouse, whereas aneuploidy in stem cell linages in the same mice remains rare [239]. In conclusion, we propose aneuploidy as a key aging hallmark and we argue for the beneficial effects of mitotic efficiency modulation at a molecular level as a strategic anti-aging therapy.
Notes
- 1.
Chromosome content that is not an exact multiple of the haploid complement; imbalanced karyotype.
- 2.
Segregation of a whole chromosome to the incorrect daughter cell during mitosis.
- 3.
Gains or losses of entire chromosomes arising from mis-segregation events during cell division; generally denotes a diploid organism with subpopulations of aneuploid somatic cells.
- 4.
Part of the chromosome comprised of repetitive DNA where the sister-chromatids are connected and the kinetochores assembled.
- 5.
Large protein complex that allows the attachment of chromosomes to spindle microtubules.
- 6.
Surveillance mechanism that halts cell cycle progression until all chromosomes are correctly attached to the spindle.
- 7.
The main microtubule-organizing center of the cell, which contains the centrioles (in animal cells) and duplicates before mitosis to form a bipolar spindle.
- 8.
Chromosome that is left behind at the spindle equator when all the other chromosomes have segregated to opposite spindle poles.
- 9.
Group of rare diseases caused by mutations in genes functionally linked to formation/maintenance of nuclear lamina.
- 10.
A condition in which aging features arise early in life.
- 11.
Features of late-life aging in young individuals, as for instance grey hair, cataracts and body mass loss.
- 12.
Partially mimic an aging phenotype; do not include all signs of aging.
- 13.
Refers to an essentially irreversible growth arrest that occurs when cells that can divide encounter oncogenic stress (for instance, strong mitogenic signals); it is a secondary aging hallmark or compensatory response to primary causes of cellular damage.
- 14.
Stretched chromatin structure in between two daughter cells.
- 15.
Small nucleus in a daughter cell generated by chromosome mis-segregation.
- 16.
Vesicular membraneous matrix that embeds the microtubule spindle apparatus during mitosis.
- 17.
Natural or chronological aging.
- 18.
Increased frequency of chromosome mis-segregation events.
- 19.
Microtubule bundles that attach sister kinetochores to spindle poles and power chromosome movement during mitosis.
- 20.
Multiprotein complex with ubiquitin-ligase activity that is responsible for the ubiquitination of numerous key cell-cycle regulators.
References
Jacobs PA, Court Brown WM (1966) Age and chromosomes. Nature 212:823–824
Nagaoka SI, Hassold TJ, Hunt PA (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 13:493–504
Jacobs PA, Brunton M, Court Brown WM et al (1963) Change of human chromosome count distribution with age: evidence for a sex differences. Nature 197:1080–1081
Pierre RV, Hoagland HC (1972) Age-associated aneuploidy: loss of Y chromosome from human bone marrow cells with aging. Cancer 30:889–894
Stone JF, Sandberg AA (1995) Sex chromosome aneuploidy and aging. Mutat Res 338:107–113
Dumanski JP, Rasi C, Lönn M et al (2015) Mutagenesis. Smoking is associated with mosaic loss of chromosome Y. Science 347:81–83
Fitzgerald PH, McEwan CM (1977) Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet 39:329–337
Guttenbach M, Schakowski R, Schmid M (1994) Aneuploidy and ageing: sex chromosome exclusion into micronuclei. Hum Genet 94:295–298
Alejandro J, Payne S, Mukherjee AB, Thomas S (1996) Age-related aneuploidy analysis of human blood cells in vivo by fluorescence in situ hybridization (FISH). Mech Ageing Dev 90:145–156
Mukherjee AB, Thomas S (1997) A longitudinal study of human age-related chromosomal analysis in skin fibroblasts. Exp Cell Res 235:161–169
Nakagome Y, Abe T, Misawa S et al (1984) The “loss” of centromeres from chromosomes of aged women. Am J Hum Genet 36:398–404
Carere A, Antoccia A, Cimini D et al (1999) Analysis of chromosome loss and non-disjunction in cytokinesis-blocked lymphocytes of 24 male subjects. Mutagenesis 14:491–496
Faggioli F, Wang T, Vijg J, Montagna C (2012) Chromosome-specific accumulation of aneuploidy in the aging mouse brain. Hum Mol Genet 21:5246–5253
Thomas P, Fenech M (2008) Chromosome 17 and 21 aneuploidy in buccal cells is increased with ageing and in Alzheimer’s disease. Mutagenesis 23:57–65
Yurov YB, Vorsanova SG, Liehr T et al (2014) X chromosome aneuploidy in the Alzheimer’s disease brain. Mol Cytogenet 7(1):20
Knouse KA, Wu J, Whittaker CA, Amon A (2014) Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc Natl Acad Sci U S A 111:13409–13414
van den Bos H, Spierings DCJ, Taudt AS et al (2016) Single-cell whole genome sequencing reveals no evidence for common aneuploidy in normal and Alzheimer’s disease neurons. Genome Biol 17:116
Geigl JB, Langer S, Barwisch S et al (2004) Analysis of gene expression patterns and chromosomal changes associated with aging. Cancer Res 64:8550–8557
Ly DH, Lockhart DJ, Lerner RA, Schultz PG (2000) Mitotic misregulation and human aging. Science 287:2486–2492
Nicholson JM, Cimini D (2011) How mitotic errors contribute to karyotypic diversity in cancer. Adv Cancer Res 112:43–75
Thompson SL, Bakhoum SF, Compton DA (2010) Mechanisms of chromosomal instability. Curr Biol 20:R285–R295
Loncarek J, Kisurina-Evgenieva O, Vinogradova T et al (2007) The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature 450:745–749
Cimini D, Mattiuzzo M, Torosantucci L, Degrassi F (2003) Histone hyperacetylation in mitosis prevents sister chromatid separation and produces chromosome segregation defects. Mol Biol Cell 14:3821–3833
Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460:278–282
Silkworth WT, Nardi IK, Scholl LM, Cimini D (2009) Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One 4:e6564
Bakhoum SF, Compton DA (2012) Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest 122:1138–1143
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell 153:1194–1217
Hoeijmakers JHJ (2009) DNA damage, aging, and cancer. N Engl J Med 361:1475–1485
Erol A (2011) Genotoxic stress-mediated cell cycle activities for the decision of cellular fate. Cell Cycle 10:3239–3248
Worman HJ (2012) Nuclear lamins and laminopathies. J Pathol 226:316–325
Coppedè F, Migliore L (2010) DNA repair in premature aging disorders and neurodegeneration. Curr Aging Sci 3:3–19
Puzianowska-Kuznicka M, Kuznicki J (2005) Genetic alterations in accelerated ageing syndromes. Do they play a role in natural ageing? Int J Biochem Cell Biol 37:947–960
Schumacher B, Garinis GA, Hoeijmakers JHJ (2008) Age to survive: DNA damage and aging. Trends Genet 24:77–85
Vermeij WP, Hoeijmakers JHJ, Pothof J (2014) Aging: not all DNA damage is equal. Curr Opin Genet Dev 26:124–130
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522. doi:10.1016/j.cell.2005.02.003
Coppé J-P, Desprez P-Y, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Edifizi D, Schumacher B (2015) Genome instability in development and aging: insights from nucleotide excision repair in humans, mice, and worms. Biomol Ther 5:1855–1869
Gorbunova V, Seluanov A, Mao Z, Hine C (2007) Changes in DNA repair during aging. Nucleic Acids Res 35:7466–7474
Goukassian D, Gad F, Yaar M et al (2000) Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J 14:1325–1334
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461:1071–1078
van Vugt MATM, Gardino AK, Linding R et al (2010) A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol 8:e1000287
Benada J, Burdová K, Lidak T et al (2015) Polo-like kinase 1 inhibits DNA damage response during mitosis. Cell Cycle 14:219–231
Orthwein A, Fradet-Turcotte A, Noordermeer SM et al (2014) Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science 344:189–193
Bakhoum SF, Kabeche L, Murnane JP et al (2014) DNA-damage response during mitosis induces whole-chromosome missegregation. Cancer Discov 4:1281–1289
Mehta PA, Harris RE, Davies SM et al (2010) Numerical chromosomal changes and risk of development of myelodysplastic syndrome – acute myeloid leukemia in patients with Fanconi anemia. Cancer Genet Cytogenet 203:180–186
Pulliam-Leath AC, Ciccone SL, Nalepa G et al (2010) Genetic disruption of both Fancc and Fancg in mice recapitulates the hematopoietic manifestations of Fanconi anemia. Blood 116:2915–2920
Naim V, Rosselli F (2009) The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat Cell Biol 11:761–768
Nalepa G, Enzor R, Sun Z et al (2013) Fanconi anemia signaling network regulates the spindle assembly checkpoint. J Clin Invest 123:3839–3847
Singh DK, Ahn B, Bohr VA (2009) Roles of RECQ helicases in recombination based DNA repair, genomic stability and aging. Biogerontology 10:235–252
Honma M, Tadokoro S, Sakamoto H et al (2002) Chromosomal instability in B-lymphoblasotoid cell lines from Werner and Bloom syndrome patients. Mutat Res 520:15–24
Kaloustian Der VM, McGill JJ, Vekemans M, Kopelman HR (1990) Clonal lines of aneuploid cells in Rothmund-Thomson syndrome. Am J Med Genet 37:336–339
Melaragno MI, Pagni D, Smith MA (1995) Cytogenetic aspects of Werner’s syndrome lymphocyte cultures. Mech Ageing Dev 78:117–122
Mukherjee AB, Costello C (1998) Aneuploidy analysis in fibroblasts of human premature aging syndromes by FISH during in vitro cellular aging. Mech Ageing Dev 103:209–222
Salk D, Au K, Hoehn H, Martin GM (1981) Cytogenetics of Werner’s syndrome cultured skin fibroblasts: variegated translocation mosaicism. Cytogenet Cell Genet 30:92–107
Chang S, Multani AS, Cabrera NG et al (2004) Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet 36:877–882
Chester N, Babbe H, Pinkas J et al (2006) Mutation of the murine Bloom’s syndrome gene produces global genome destabilization. Mol Cell Biol 26:6713–6726
Mann MB, Hodges CA, Barnes E et al (2005) Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum Mol Genet 14:813–825
Chan KL, Palmai-Pallag T, Ying S, Hickson ID (2009) Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat Cell Biol 11:753–760
Chung L, Onyango D, Guo Z et al (2015) The FEN1 E359K germline mutation disrupts the FEN1-WRN interaction and FEN1 GEN activity, causing aneuploidy-associated cancers. Oncogene 34:902–911
Larsen NB, Hickson ID (2013) RecQ helicases: conserved guardians of genomic integrity. Adv Exp Med Biol 767:161–184
Chan KL, North PS, Hickson ID (2007) BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J 26:3397–3409
Liu Y, Nielsen CF, Yao Q, Hickson ID (2014) The origins and processing of ultra fine anaphase DNA bridges. Curr Opin Genet Dev 26:1–5
Ke Y, Huh J-W, Warrington R et al (2011) PICH and BLM limit histone association with anaphase centromeric DNA threads and promote their resolution. EMBO J 30:3309–3321
Ito S, Tan LJ, Andoh D et al (2010) MMXD, a TFIIH-independent XPD-MMS19 protein complex involved in chromosome segregation. Mol Cell 39:632–640
Tan LJ, Saijo M, Kuraoka I et al (2012) Xeroderma pigmentosum group F protein binds to Eg5 and is required for proper mitosis: implications for XP-F and XFE. Genes Cells 17:173–185
Naim V, Wilhelm T, Debatisse M, Rosselli F (2013) ERCC1 and MUS81-EME1 promote sister chromatid separation by processing late replication intermediates at common fragile sites during mitosis. Nat Cell Biol 15:1008–1015
Dechat T, Pfleghaar K, Sengupta K et al (2008) Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22:832–853
Gruenbaum Y, Foisner R (2015) Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem 84:131–164
Cau P, Navarro C, Harhouri K et al (2014) Nuclear matrix, nuclear envelope and premature aging syndromes in a translational research perspective. Semin Cell Dev Biol 29:125–147
Cadiñanos J, Varela I, López-Otín C, Freije JMP (2005) From immature lamin to premature aging: molecular pathways and therapeutic opportunities. Cell Cycle 4:1732–1735
Goldman RD, Shumaker DK, Erdos MR et al (2004) Accumulation of mutant lamin a causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 101:8963–8968
Yang SH, Chang SY, Ren S et al (2011) Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum Mol Genet 20:436–444
Mehta IS, Eskiw CH, Arican HD et al (2011) Farnesyltransferase inhibitor treatment restores chromosome territory positions and active chromosome dynamics in Hutchinson-Gilford progeria syndrome cells. Genome Biol 12:R74
Yang SH, Bergo MO, Toth JI et al (2005) Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci U S A 102:10291–10296
Moulson CL, Go G, Gardner JM et al (2005) Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol 125:913–919
Navarro CL, Cadiñanos J, De Sandre-Giovannoli A et al (2005) Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin a precursors. Hum Mol Genet 14:1503–1513
Jamin A, Wiebe MS (2015) Barrier to Autointegration factor (BANF1): interwoven roles in nuclear structure, genome integrity, innate immunity, stress responses and progeria. Curr Opin Cell Biol 34:61–68
Scaffidi P, Misteli T (2005) Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med 11:440–445
McClintock D, Ratner D, Lokuge M et al (2007) The mutant form of lamin a that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One 2:e1269
Scaffidi P, Misteli T (2006) Lamin A-dependent nuclear defects in human aging. Science 312:1059–1063
Freund A, Laberge R-M, Demaria M, Campisi J (2012) Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23:2066–2075
Shimi T, Butin-Israeli V, Adam SA et al (2011) The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 25:2579–2593
Cao K, Capell BC, Erdos MR et al (2007) A lamin a protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci U S A 104:4949–4954
Dechat T, Shimi T, Adam SA et al (2007) Alterations in mitosis and cell cycle progression caused by a mutant lamin a known to accelerate human aging. Proc Natl Acad Sci U S A 104:4955–4960
Pratt CH, Curtain M, Donahue LR, Shopland LS (2011) Mitotic defects lead to pervasive aneuploidy and accompany loss of RB1 activity in mouse Lmna Dhe dermal fibroblasts. PLoS One 6:e18065
Gruber J, Lampe T, Osborn M, Weber K (2005) RNAi of FACE1 protease results in growth inhibition of human cells expressing lamin a: implications for Hutchinson-Gilford progeria syndrome. J Cell Sci 118:689–696
Liu B, Wang J, Chan KM et al (2005) Genomic instability in laminopathy-based premature aging. Nat Med 11:780–785
Qi R, Xu N, Wang G et al (2015) The lamin-A/C-LAP2α-BAF1 protein complex regulates mitotic spindle assembly and positioning. J Cell Sci 128:2830–2841
Schweizer N, Weiss M, Maiato H (2014) The dynamic spindle matrix. Curr Opin Cell Biol 28:1–7
Ma L, Tsai M-Y, Wang S et al (2009) Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat Cell Biol 11:247–256
Zheng Y (2010) A membranous spindle matrix orchestrates cell division. Nat Rev Mol Cell Biol 11:529–535
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350:1193–1198
de Lange T (2005) Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol 70:197–204
Palm W, de Lange T (2008) How shelterin protects mammalian telomeres. Annu Rev Genet 42:301–334
Fumagalli M, Rossiello F, Clerici M et al (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14:355–365
Hewitt G, Jurk D, Marques FDM et al (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3:708
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Olovnikov AM (1996) Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol 31:443–448
Bodnar AG, Ouellette M, Frolkis M et al (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Batista LFZ (2014) Telomere biology in stem cells and reprogramming. Prog Mol Biol Transl Sci 125:67–88
Simm A, Campisi J (2014) Stress and aging. Exp Gerontol 59:1–2
Blasco MA, Lee HW, Hande MP et al (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25–34
Armanios M, Blackburn EH (2012) The telomere syndromes. Nat Rev Genet 13:693–704
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622
Jaskelioff M, Muller FL, Paik J-H et al (2011) Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469:102–106
Lee D-H, Acharya SS, Kwon M et al (2014) Dephosphorylation enables the recruitment of 53BP1 to double-strand DNA breaks. Mol Cell 54:512–525
Cesare AJ (2014) Mitosis, double strand break repair, and telomeres: a view from the end: how telomeres and the DNA damage response cooperate during mitosis to maintain genome stability. BioEssays 36:1054–1061
Cesare AJ, Hayashi MT, Crabbe L, Karlseder J (2013) The telomere deprotection response is functionally distinct from the genomic DNA damage response. Mol Cell 51:141–155
Kaul Z, Cesare AJ, Huschtscha LI et al (2012) Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep 13:52–59
d'Adda di Fagagna F, Reaper PM, Clay-Farrace L et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
Takai H, Smogorzewska A, de Lange T (2003) DNA damage foci at dysfunctional telomeres. Curr Biol 13:1549–1556
van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92:401–413
Soler D, Genescà A, Arnedo G et al (2005) Telomere dysfunction drives chromosomal instability in human mammary epithelial cells. Genes Chromosomes Cancer 44:339–350
Tusell L, Pampalona J, Soler D et al (2010) Different outcomes of telomere-dependent anaphase bridges. Biochem Soc Trans 38:1698–1703
Tusell L, Soler D, Agostini M et al (2008) The number of dysfunctional telomeres in a cell: one amplifies; more than one translocate. Cytogenet Genome Res 122:315–325
Pampalona J, Soler D, Genescà A, Tusell L (2010) Whole chromosome loss is promoted by telomere dysfunction in primary cells. Genes Chromosomes Cancer 49:368–378
Pampalona J, Roscioli E, Silkworth WT et al (2016) Chromosome bridges maintain kinetochore-microtubule attachment throughout mitosis and rarely break during anaphase. PLoS One 11:e0147420
Zhang C-Z, Spektor A, Cornils H et al (2015) Chromothripsis from DNA damage in micronuclei. Nature 522:179–184
Crasta K, Ganem NJ, Dagher R et al (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 482:53–58
Maciejowski J, Li Y, Bosco N et al (2015) Chromothripsis and Kataegis induced by telomere crisis. Cell 163:1641–1654
Roberts SA, Sterling J, Thompson C et al (2012) Clustered mutations in yeast and in human cancers can arise from damaged long single-strand DNA regions. Mol Cell 46:424–435
Dynek JN, Smith S (2004) Resolution of sister telomere association is required for progression through mitosis. Science 304:97–100
Hsiao SJ, Smith S (2008) Tankyrase function at telomeres, spindle poles, and beyond. Biochimie 90:83–92
Canudas S, Smith S (2009) Differential regulation of telomere and centromere cohesion by the Scc3 homologues SA1 and SA2, respectively, in human cells. J Cell Biol 187:165–173
Canudas S, Houghtaling BR, Kim JY et al (2007) Protein requirements for sister telomere association in human cells. EMBO J 26:4867–4878
Smith S, Giriat I, Schmitt A, de Lange T (1998) Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282:1484–1487
Bisht KK, Daniloski Z, Smith S (2013) SA1 binds directly to DNA through its unique AT-hook to promote sister chromatid cohesion at telomeres. J Cell Sci 126:3493–3503
Bisht KK, Dudognon C, Chang WG et al (2012) GDP-mannose-4,6-dehydratase is a cytosolic partner of tankyrase 1 that inhibits its poly(ADP-ribose) polymerase activity. Mol Cell Biol 32:3044–3053
Kim MK, Smith S (2014) Persistent telomere cohesion triggers a prolonged anaphase. Mol Biol Cell 25:30–40
Yalon M, Gal S, Segev Y et al (2004) Sister chromatid separation at human telomeric regions. J Cell Sci 117:1961–1970
Han S, Brunet A (2012) Histone methylation makes its mark on longevity. Trends Cell Biol 22:42–49
Johnson AA, Akman K, Calimport SRG et al (2012) The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res 15:483–494
Maegawa S, Hinkal G, Kim HS et al (2010) Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res 20:332–340
Hannum G, Guinney J, Zhao L et al (2013) Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49:359–367
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115
Weidner CI, Wagner W (2014) The epigenetic tracks of aging. Biol Chem 395:1307–1314
Fraga MF, Esteller M (2007) Epigenetics and aging: the targets and the marks. Trends Genet 23:413–418
Jin C, Li J, Green CD et al (2011) Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab 14:161–172
Guarente L (2011) Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol 76:81–90
Brown K, Xie S, Qiu X et al (2013) SIRT3 reverses aging-associated degeneration. Cell Rep 3:319–327
Kanfi Y, Peshti V, Gil R et al (2010) SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 9:162–173
Kawahara TLA, Michishita E, Adler AS et al (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136:62–74
Nogueiras R, Habegger KM, Chaudhary N et al (2012) Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92:1479–1514
Oberdoerffer P, Michan S, McVay M et al (2008) SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135:907–918
Someya S, Yu W, Hallows WC et al (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–812
Wang R-H, Sengupta K, Li C et al (2008) Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14:312–323
Zhong L, D’Urso A, Toiber D et al (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140:280–293
Pegoraro G, Misteli T (2009) The central role of chromatin maintenance in aging. Aging (Albany NY) 1:1017–1022
Shumaker DK, Dechat T, Kohlmaier A et al (2006) Mutant nuclear lamin a leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A 103:8703–8708
Tsurumi A, Li WX (2012) Global heterochromatin loss: a unifying theory of aging? Epigenetics 7:680–688
de Magalhães JP, Budovsky A, Lehmann G et al (2009) The human ageing genomic resources: online databases and tools for biogerontologists. Aging Cell 8:65–72
Ugalde AP, Español Y, López-Otín C (2011) Micromanaging aging with miRNAs: new messages from the nuclear envelope. Nucleus 2:549–555
Rando TA, Chang HY (2012) Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 148:46–57
Guppy BJ, McManus KJ (2015) Mitotic accumulation of dimethylated lysine 79 of histone H3 is important for maintaining genome integrity during mitosis in human cells. Genetics 199:423–433
Blasco MA (2007) The epigenetic regulation of mammalian telomeres. Nat Rev Genet 8:299–309
Gonzalo S, Jaco I, Fraga MF et al (2006) DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol 8:416–424
Cheung P, Tanner KG, Cheung WL et al (2000) Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell 5:905–915
Inoue A, Hyle J, Lechner MS, Lahti JM (2008) Perturbation of HP1 localization and chromatin binding ability causes defects in sister-chromatid cohesion. Mutat Res 657:48–55
Shimura M, Toyoda Y, Iijima K et al (2011) Epigenetic displacement of HP1 from heterochromatin by HIV-1 Vpr causes premature sister chromatid separation. J Cell Biol 194:721–735
Fatoba ST, Okorokov AL (2011) Human SIRT1 associates with mitotic chromatin and contributes to chromosomal condensation. Cell Cycle 10:2317–2322
Vaquero A, Scher MB, Lee DH et al (2006) SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 20:1256–1261
Kim H-S, Vassilopoulos A, Wang R-H et al (2011) SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20:487–499
Choi B-S, Park JE, Jang C-Y (2014) Sirt3 controls chromosome alignment by regulating spindle dynamics during mitosis. Biochem Biophys Res Commun 444:662–669
Ardestani PM, Liang F (2014) Sub-cellular localization, expression and functions of Sirt6 during the cell cycle in HeLa cells. Nucleus 3:442–451
Hudson RS, Yi M, Esposito D et al (2012) MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic Acids Res 40:3689–3703
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105:13421–13426
Mäki-Jouppila JHE, Pruikkonen S, Tambe MB et al (2015) MicroRNA let-7b regulates genomic balance by targeting Aurora B kinase. Mol Oncol 9:1056–1070
Morimoto RI, Cuervo AM (2009) Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci 64:167–170
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10:205–215
Swindell WR, Masternak MM, Kopchick JJ et al (2009) Endocrine regulation of heat shock protein mRNA levels in long-lived dwarf mice. Mech Ageing Dev 130:393–400
Fargnoli J, Kunisada T, Fornace AJ et al (1990) Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A 87:846–850
Hall DM, Xu L, Drake VJ et al (2000) Aging reduces adaptive capacity and stress protein expression in the liver after heat stress. J Appl Physiol 89:749–759
Pahlavani MA, Harris MD, Moore SA et al (1995) The expression of heat shock protein 70 decreases with age in lymphocytes from rats and rhesus monkeys. Exp Cell Res 218:310–318
Heydari AR, You S, Takahashi R et al (2000) Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res 256:83–93
Locke M, Tanguay RM (1996) Diminished heat shock response in the aged myocardium. Cell Stress Chaperones 1:251–260
Hsu A-L, Murphy CT, Kenyon C (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300:1142–1145
Morley JF, Morimoto RI (2004) Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell 15:657–664
Walker GA, Lithgow GJ (2003) Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2:131–139
Jahngen JH, Lipman RD, Eisenhauer DA et al (1990) Aging and cellular maturation cause changes in ubiquitin-eye lens protein conjugates. Arch Biochem Biophys 276:32–37
Min J-N, Whaley RA, Sharpless NE et al (2008) CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by Altered protein quality control. Mol Cell Biol 28:4018–4025
Ferrington DA, Husom AD, Thompson LV (2005) Altered proteasome structure, function, and oxidation in aged muscle. FASEB J 19:644–646
Keller JN, Huang FF, Markesbery WR (2000) Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience 98:149–156
Li W, Gao B, Lee S-M et al (2007) RLE-1, an E3 ubiquitin ligase, regulates C. elegans aging by catalyzing DAF-16 polyubiquitination. Dev Cell 12:235–246
Yun C, Stanhill A, Yang Y et al (2008) Proteasomal adaptation to environmental stress links resistance to proteotoxicity with longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105:7094–7099
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Terman A (1995) The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology 41(Suppl 2):319–326
Terman A, Brunk UT (1998) Ceroid/lipofuscin formation in cultured human fibroblasts: the role of oxidative stress and lysosomal proteolysis. Mech Ageing Dev 104:277–291
Dice JF (2007) Chaperone-mediated autophagy. Autophagy 3:295–299
Kiffin R, Kaushik S, Zeng M et al (2007) Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci 120:782–791
Zhang C, Cuervo AM (2008) Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 14:959–965
Lindner AB, Madden R, Demarez A et al (2008) Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc Natl Acad Sci U S A 105:3076–3081
Amen T, Kaganovich D (2015) Dynamic droplets: the role of cytoplasmic inclusions in stress, function, and disease. Cell Mol Life Sci 72:401–415
Zhou C, Slaughter BD, Unruh JR et al (2014) Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells. Cell 159:530–542
Chen Y-J, Lai K-C, Kuo H-H et al (2014) HSP70 colocalizes with PLK1 at the centrosome and disturbs spindle dynamics in cells arrested in mitosis by arsenic trioxide. Arch Toxicol 88:1711–1723
O’Regan L, Sampson J, Richards MW et al (2015) Hsp72 is targeted to the mitotic spindle by Nek6 to promote K-fiber assembly and mitotic progression. J Cell Biol 209:349–358
Basto R, Gergely F, Draviam VM et al (2007) Hsp90 is required to localise cyclin B and Msps/ch-TOG to the mitotic spindle in Drosophila and humans. J Cell Sci 120:1278–1287
Lange BM, Bachi A, Wilm M, González C (2000) Hsp90 is a core centrosomal component and is required at different stages of the centrosome cycle in Drosophila and vertebrates. EMBO J 19:1252–1262
Davies AE, Kaplan KB (2010) Hsp90-Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore-microtubule binding sites. J Cell Biol 189:261–274
Liu XS, Song B, Tang J et al (2012) Plk1 phosphorylates Sgt1 at the kinetochores to promote timely kinetochore-microtubule attachment. Mol Cell Biol 32:4053–4067
Niikura Y, Ohta S, Vandenbeldt KJ et al (2006) 17-AAG, an Hsp90 inhibitor, causes kinetochore defects: a novel mechanism by which 17-AAG inhibits cell proliferation. Oncogene 25:4133–4146
Vihervaara A, Sergelius C, Vasara J et al (2013) Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci U S A 110:E3388–E3397
Lu M, Boschetti C, Tunnacliffe A (2015) Long term Aggresome accumulation leads to DNA damage, p53-dependent cell cycle arrest, and steric interference in mitosis. J Biol Chem 290:27986–28000
Eskelinen E-L, Prescott AR, Cooper J et al (2002) Inhibition of autophagy in mitotic animal cells. Traffic 3:878–893
Liu L, Xie R, Nguyen S et al (2009) Robust autophagy/mitophagy persists during mitosis. Cell Cycle 8:1616–1620
Fuchs M, Luthold C, Guilbert SM et al (2015) A role for the chaperone complex BAG3-HSPB8 in actin dynamics, spindle orientation and proper chromosome segregation during mitosis. PLoS Genet 11:e1005582
Pohl C (2009) Dual control of cytokinesis by the ubiquitin and autophagy pathways. Autophagy 5:561–562
Mathew R, Kongara S, Beaudoin B et al (2007) Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 21:1367–1381
Frémont S, Gérard A, Galloux M et al (2013) Beclin-1 is required for chromosome congression and proper outer kinetochore assembly. EMBO Rep 14:364–372
Gordon DJ, Resio B, Pellman D (2012) Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13:189–203
Tang Y-C, Amon A (2013) Gene copy-number alterations: a cost-benefit analysis. Cell 152:394–405
Santaguida S, Amon A (2015) Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat Rev Mol Cell Biol 16:473–485
Stingele S, Stoehr G, Peplowska K et al (2012) Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol Syst Biol 8:608
Thorburn RR, Gonzalez C, Brar GA et al (2013) Aneuploid yeast strains exhibit defects in cell growth and passage through START. Mol Biol Cell 24:1274–1289
Williams BR, Prabhu VR, Hunter KE et al (2008) Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 322:703–709
Sheltzer JM, Amon A (2011) The aneuploidy paradox: costs and benefits of an incorrect karyotype. Trends Genet 27:446–453
Sheltzer JM, Torres EM, Dunham MJ, Amon A (2012) Transcriptional consequences of aneuploidy. Proc Natl Acad Sci U S A 109:12644–12649
Torres EM, Sokolsky T, Tucker CM et al (2007) Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317:916–924
Blank HM, Sheltzer JM, Meehl CM, Amon A (2015) Mitotic entry in the presence of DNA damage is a widespread property of aneuploidy in yeast. Mol Biol Cell 26:1440–1451
Zhu J, Pavelka N, Bradford WD et al (2012) Karyotypic determinants of chromosome instability in Aneuploid budding yeast. PLoS Genet 8:e1002719
Dephoure N, Hwang S, O’Sullivan C et al (2014) Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife 3:e03023
Donnelly N, Passerini V, Dürrbaum M et al (2014) HSF1 deficiency and impaired HSP90-dependent protein folding are hallmarks of aneuploid human cells. EMBO J 33:2374–2387
Oromendia AB, Dodgson SE, Amon A (2012) Aneuploidy causes proteotoxic stress in yeast. Genes Dev 26:2696–2708
Stingele S, Stoehr G, Storchová Z (2013) Activation of autophagy in cells with abnormal karyotype. Autophagy 9:246–248
Tang Y-C, Williams BR, Siegel JJ, Amon A (2011) Identification of aneuploidy-selective antiproliferation compounds. Cell 144:499–512
Torres EM, Dephoure N, Panneerselvam A et al (2010) Identification of aneuploidy-tolerating mutations. Cell 143:71–83
Jenkins EC, Ye L, Krinsky-McHale SJ et al (2016) Telomere longitudinal shortening as a biomarker for dementia status of adults with down syndrome. Am J Med Genet B Neuropsychiatr Genet 171:169–174
Wenger SL, Hansroth J, Shackelford AL (2014) Decreased telomere length in metaphase and interphase cells from newborns with trisomy 21. Gene 542:87
Horvath S, Garagnani P, Bacalini MG et al (2015) Accelerated epigenetic aging in down syndrome. Aging Cell 14:491–495
Nicholson JM, Macedo JC, Mattingly AJ et al (2015) Chromosome mis-segregation and cytokinesis failure in trisomic human cells. eLife 4:e05068
Sheltzer JM, Blank HM, Pfau SJ et al (2011) Aneuploidy drives genomic instability in yeast. Science 333:1026–1030
Janssen A, van der Burg M, Szuhai K et al (2011) Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333:1895–1898
Lee K, Kenny AE, Rieder CL (2010) P38 mitogen-activated protein kinase activity is required during mitosis for timely satisfaction of the mitotic checkpoint but not for the fidelity of chromosome segregation. Mol Biol Cell 21:2150–2160
Passerini V, Ozeri-Galai E, de Pagter MS et al (2016) The presence of extra chromosomes leads to genomic instability. Nat Commun 7:10754
Lamm N, Ben-David U, Golan-Lev T et al (2016) Genomic instability in human pluripotent stem cells arises from replicative stress and chromosome condensation defects. Cell Stem Cell 18:253–261
Oromendia AB, Amon A (2014) Aneuploidy: implications for protein homeostasis and disease. Dis Model Mech 7:15–20
Santaguida S, Vasile E, White E, Amon A (2015) Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev 29:2010–2021
Kim G, Meriin AB, Gabai VL et al (2012) The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell 11:617–627
Baker DJ, Dawlaty MM, Wijshake T et al (2013) Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat Cell Biol 15:96–102
Baker DJ, Jeganathan KB, Cameron JD et al (2004) BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet 36:744–749
Malureanu LA, Wijshake T, Baker DJ et al (2012) Reduced life- and Healthspan in mice carrying a mono-allelic BubR1 MVA mutation. PLoS Genet 8:e1003138
Ricke RM, van Deursen JM (2013) Aneuploidy in health, disease, and aging. J Cell Biol 201:11–21
Hanks S, Coleman K, Reid S et al (2004) Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet 36:1159–1161
Suijkerbuijk SJE, van Osch MHJ, Bos FL et al (2010) Molecular causes for BUBR1 dysfunction in the human cancer predisposition syndrome mosaic variegated aneuploidy. Cancer Res 70:4891–4900
Baker DJ, Jeganathan KB, Malureanu L et al (2006) Early aging–associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol 172:529–540
Faggioli F, Vijg J, Montagna C (2011) Chromosomal aneuploidy in the aging brain. Mech Ageing Dev 132:429–436
Childs BG, Baker DJ, Kirkland JL et al (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15:1139–1153
Bakker B, van den Bos H, Lansdorp PM, Foijer F (2015) How to count chromosomes in a cell: an overview of current and novel technologies. BioEssays 37:570–577
Baker DJ, Childs BG, Durik M et al (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184–189
Postnikoff SDL, Harkness TAA (2012) Mechanistic insights into aging, cell-cycle progression, and stress response. Front Physiol 3:183
Laoukili J, Kooistra MRH, Brás A et al (2005) FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7:126–136
Laoukili J, Stahl M, Medema RH (2007) FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta 1775:92–102
Wang X, Quail E, Hung NJ et al (2001) Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci U S A 98:11468–11473
Kalinichenko VV, Gusarova GA, Tan Y et al (2003) Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 278:37888–37894
Foijer F, DiTommaso T, Donati G et al (2013) Spindle checkpoint deficiency is tolerated by murine epidermal cells but not hair follicle stem cells. Proc Natl Acad Sci U S A 110:2928–2933
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Macedo, J.C., Vaz, S., Logarinho, E. (2017). Mitotic Dysfunction Associated with Aging Hallmarks. In: Gotta, M., Meraldi, P. (eds) Cell Division Machinery and Disease. Advances in Experimental Medicine and Biology, vol 1002. Springer, Cham. https://doi.org/10.1007/978-3-319-57127-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-57127-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57125-6
Online ISBN: 978-3-319-57127-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)