Abstract
Wound healing is a well-orchestrated, if operative, process that involves the specific coordination of various cellular and molecular events. There are many therapeutic approaches to facilitate wound healing; however the treatment of cutaneous wounds remains a challenge, particularly in complex wounds. This chapter will discuss the therapeutic potential of stem cells, undifferentiated and multipotent cells, in the treatment of acute and complex cutaneous wounds. We will address current treatments in deficient healing (e.g., diabetes and aging) and excessive healing (e.g., hypertrophic scars and keloids) and emerging treatments that utilize adult stem cells. Finally, we will discuss the methods of delivering these stem cells as well as the challenges stem cell therapies face in regenerative medicine.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Millions of people in the United States and around the world will suffer from acute and chronic wounds. It is estimated that 300,000 people are hospitalized each year in the United States due to acute wounds along with 11 million people affected (Hostetler et al. 2006). Chronic wounds affect approximately 6.5 million people, and it costs an excess of 25 billion annually to treat these wounds, a number that represents 2% of annual health care spending (Sen et al. 2009). These alarming numbers are mainly the result of an aging population and a rise in the incidence of diabetes. For example, roughly five million people suffering from diabetes will develop chronic wounds that will fail to heal (National Diabetes Statistical Report 2014). In recent decades, clinicians and scientists have expanded upon their knowledge of the mechanisms of wound repair and wound care (Dreifke et al. 2015). As a result, the goal of wound therapy has shifted from managing symptoms to a more practical approach that will ultimately promote optimal wound healing and improve patients’ quality of life. Despite these advancements wound healing remains a challenge. Stem cells are essential for tissue homeostasis and repair, and their versatility holds tremendous potential for tissue regeneration in a number of different clinical applications, including acute and chronic wounds. The unique self-renewal and differentiation capacity of stem cells make them attractive alternative to traditional wound treatments (Cha and Falanga 2007). Stem cell therapy aims to enhance cutaneous regeneration by completely restoring the structure and function of tissue so it is indistinguishable from its native state. In this chapter, we will focus on current and emerging stem cell-based treatments in the management of acute and chronic skin wounds . We will address what type of stem cells are used, how these stem cells are administered to damaged tissue, and finally the challenges and future directions that stem cell therapy faces.
2 Wound Healing
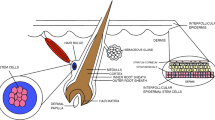
The skin is composed of two main layers: the epidermis which forms a barrier to the external environment and the dermis which is made of connective tissue that provides the skin with its mechanical properties. The mammalian epidermis is made up of stratified keratinized epithelium with hair follicles and glands scattered throughout (Heng 2011). In contrast, the dermis is subdivided into the papillary region and reticular dermis. The papillary region is superficial to the dermis, while the reticular dermis is a deeper and thicker layer; both regions consist of collagen, elastic fibers, and extrafibrillar matrix (Watt and Fujiwara 2011). Following injury to the skin and if operative, there is a coordinated and complex response that aims to achieve tissue integrity and homeostasis. There are a number of intracellular and extracellular events that become activated, and the intensity of the response depends on the severity of the injury, the size of wound, and the type of wound (Gurtner et al. 2008; Bielefeld et al. 2013).
2.1 Stages of Wound Healing
The healing process can be summarized in three phases that coincide: hemostasis/inflammation, proliferation, and tissue maturation and remodeling. The hemostasis phase is responsible for preventing blood loss and infection at the wound site. There is vascular constriction and platelet aggregation that form a fibrin clot to temporarily seal the wound (Gurtner et al. 2008; Schultz et al. 2011; Guo and Dipietro 2010). The formation of the fibrin clot is crucial to the infiltration of inflammatory cells such as neutrophils, macrophages, and lymphocytes (Gurtner et al. 2008; Schultz et al. 2011; Guo and Dipietro 2010). Neutrophils remove foreign objects and bacteria, while macrophages phagocytose foreign particles and dead neutrophils (Mahdavian Delavary et al. 2011). The infiltration of these cells also results in the release of cytokines and growth factors such as transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), tumor necrosis factor (TNF)-α, interleukin (IL), and fibroblast growth factor (FGF) (Gurtner et al. 2008). The release of these cytokines and growth factors stimulates the migration and proliferation of fibroblasts and keratinocytes into the wound, a process that is crucial to initiating the subsequent healing process (Singer and Clark 1999).
The proliferative phase occurs approximately 3 days after injury in humans and is marked by angiogenesis, formation of granulation tissue, and reepithelization of the epidermis (Singer and Clark 1999). The dermis is repaired by fibroblasts that produce fibronectin, promote collagen deposition, and secrete growth factors (Bielefeld et al. 2013). Matrix metalloproteinases (MMPs) cleave the ECM allowing for the passage of fibroblasts and keratinocytes into the wound bed (Singer and Clark 1999). As a consequence, a temporary matrix is formed that epithelial cells can migrate on and proliferate leading to the restoration of a functional epidermal layer (Clark 1990). A number of different growth factors such as keratinocyte growth factor (KGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), TGF-β, heparin-binding EGF, FGF-2, and vascular endothelial growth factor (VEGF) help facilitate the proliferative phase (Gurtner et al. 2008; Bielefeld et al. 2013; Werner and Grose 2003). This includes regulating the proliferation and mobility of keratinocytes (Gurtner et al. 2008) and supporting the formation of new capillaries and blood vessels (Werner and Grose 2003). Lastly, the proliferative stage is augmented by stem cell populations within the hair follicles and epidermis that contribute to the reepithelization of the epidermis in a wound environment (Vagnozzi et al. 2015). Stem cells from the hair follicle and interfollicular epidermis migrate to the wound site in mouse full-thickness wounds (Mascre et al. 2012; Tumbar et al. 2004). Interestingly, lineage tracing studies have demonstrated that hair follicle stem cells (marked by K15) transiently contribute to wound reepithelization and are subsequently lost from the epidermis weeks later (Ito et al. 2005). These stem cells produce transit amplifying cells which can later differentiate into the stratified epithelial layer. This suggests that stem cells from the hair follicle are not necessary for the long-term maintenance of the epidermis after injury.
Once there is adequate deposition of collagen and closure of the epithelial gap, the maturation and remodeling phase begins. The properties of the tissue begin to change as type III collagen and fibronectin are slowly replaced by more organized type I collagen (Gurtner et al. 2008). Fibroblasts also transition to a more contractile myofibroblast phenotype (Darby et al. 1990). This reorganization dramatically improves the tensile strength in the new scar tissue, although the original strength of the uninjured skin is never recuperated (Singer and Clark 1999). Recently, skeletal muscle progenitors (Pax7+) have been shown to contribute to the repair of the skin by adopting a myofibroblastic phenotype through a process mediated by β-catenin (Amini-Nik et al. 2011). These skeletal muscle stem cells may potentially utilized to increase tensile strength of scar tissue. Overall, this phase can last from weeks to years and results in the contraction of the wound and formation of scar tissue. The coordinated and timely response of these three phases is required for efficient skin healing, and any complications may result in the development of acute or chronic wounds that fail to heal.
2.2 Signaling Pathways
There are several key signaling pathways that are activated during cutaneous wound repair that are also active during embryonic skin development. These include, but are not limited to, Wnt/β-catenin, TGF-β, Notch, and Sonic hedgehog signaling pathways. Despite this similarity, there are a number of critical differences between the molecular mechanisms that control embryonic skin development and postnatal skin repair. Consequently, these differences may be at fault for the inability of injured skin to recapitulate the structure and function of uninjured skin. A number of studies have shown that these signaling pathways regulate the contribution and the fate of stem cells during the process of healing (Huelsken et al. 2001; Athar et al. 2006; St-Jacques et al. 1998; Karlsson et al. 1999; Niemann et al. 2003; Chiang et al. 1999); therefore these pathways may have significant role in understanding the mechanisms of stem cells in wound repair. We will briefly discuss these developmental pathways as they may have implications in the evolution of stem cell therapies for skin repair/regeneration.
The Wnt/β-catenin pathway plays a prominent role in cutaneous wound repair and development. Numerous Wnt = proteins are highly expressed in cutaneous wounds during the first 7 days of healing (Okuse et al. 2005), and Wnt is primarily responsible for the regeneration of hair follicles (Ito et al. 2007). On the other hand, β-catenin regulates fibroblasts during the proliferative phase of wound repair by altering fibroblast numbers, cellularity, and matrix production (Cheon et al. 2002, 2005, 2006; Sato 2006). The proliferative phase is also marked by an increase in the expression of β-catenin and its target genes fibronectin and MMP7 (Cheon et al. 2005). Upregulating β-catenin results in a fibrotic phenotype characterized by scarring, increased collagen deposition, and the formation of myofibroblasts (Cheon et al. 2006). At cellular level, an essential role for β-catenin in macrophages has been shown during wound repair. Moreover, as mentioned previously, β-catenin regulates dermal repair through regulation of Pax7+ cells; knocking out β-catenin in Pax7-expressing cells in mice leads to fewer dermal fibroblast cells and a smaller scar size (Bielefeld et al. 2013; Amini-Nik et al. 2011).
TGF-β is one of numerous growth factors involved in cutaneous wound healing, and it is comprised of three isoforms: TGF-β1, TGF-β2, and TGF-β3. TGF-βs primarily exert their effects by activating (through phosphorylation) Smads 2 and 3 which subsequently allows them to translocate to the nucleus and alter gene expression (Schultz and Wysocki 2009; Owens et al. 2008; Biernacka et al. 2011). Similarly to the Wnt/β-catenin, the TGF-β pathways exert its healing effects by regulating fibroblast proliferation and behavior and matrix production (Bielefeld et al. 2013). TGF-β1 in particular has fibrosis and scar healing properties. In aged rats, treatment with topical TGF-β1 improves ECM deposition and fibroblast proliferation and migration (Puolakkainen et al. 1995). Moreover, research has demonstrated TGF-β1’s role in the synthesis of collagen I and fibronectin (Varga et al. 1987; Hocevar et al. 1999), proliferation of fibroblasts (Schreier et al. 1993), alteration of fibroblasts to a myofibroblast phenotype (Desmouliere et al. 1993), and finally bolster wound contraction (Martinez-Ferrer et al. 2010). Unlike Wnt/β-catenin signaling, however, TGF-β has been shown to inhibit reepithelization. For example, treatment of porcine burn wounds with TGF-β1 antagonist increased the rate of complete reepithelization, and deletion of Smad3 in mice accelerates proliferation (Ashcroft et al. 1999). However, in double mutant TGF-β −/− Scid−/− mice, there is a significant delay in reepithelization (Crowe et al. 2000). These data suggest that TGF-β exerts some inhibitory effects on keratinocyte proliferation. Despite its positive effects in stimulating cutaneous wound healing, excessive TGF-β1 activity may lead to the formation of hypertrophic scars through a β-catenin-mediated mechanisms (Cheon et al. 2004). In contrast, higher TGF-β3 activity is associated with reduced scar formation as evidenced by scarless embryonic wounds in rats (Soo et al. 2003). A clinical trial which administered TGF-β3 prophylactically to prevent dermal scarring showed accelerated healing in phase I/II studies testing (Ferguson et al. 2009); however phase III trials were unsuccessful later.
The Notch signaling pathway regulates epidermal differentiation during skin homeostasis and development, and it is vital for vascular maintenance (Moriyama et al. 2008). In terms of wound healing, blocking the expression of Notch causes delayed healing, whereas the Notch ligand, Jagged, accelerates wound closure (Chigurupati et al. 2007). Furthermore, Notch interacts with Wnt/β-catenin and Sonic hedgehog, two other signaling pathways involved in cutaneous wound healing (Okuyama et al. 2008). The exact mechanisms by which Notch promotes healing is not as well defined as Wnt/β-catenin, but there is data indicating that Notch regulates macrophage behavior and matrix formation. The Notch signaling pathway is necessary during the inflammatory phase of wound healing. Outtz et al. (2010) observed reduced TNF-α expression and macrophage recruitment in Notch1+/− mice (Outtz et al. 2010). Furthermore, they found that Notch1 regulates vascular endothelial growth factor 1 (VEGF1) expression and inflammatory cytokine expression in macrophages in vitro (Outtz et al. 2010). With regard to matrix formation, an in vitro study has shown that Notch signaling mediates the adhesion of bone marrow-derived vascular precursor cells to the ECM (Caiado et al. 2008), an essential step during wound healing.
The Sonic hedgehog (Shh) signaling pathway plays an important role in skin development (Athar et al. 2006), but like Notch its role in wound healing is not fully understood. Shh is primarily involved in dermal reconstruction. Treating diabetic mice with Shh increases wound vascularity and cellularity and even increases VEGF expression and recruitment of endothelial progenitors (Asai et al. 2006). Interestingly, the Shh inhibitor cyclopamine hinders wound vascularity and cell proliferation leading to delayed wound closure in mice (Le et al. 2008). Further research is needed to elucidate the mechanisms that allow Shh to influence dermal wound repair.
3 Acute and Complex Wounds
Acute wounds will generally heal within a few weeks in healthy individuals, and the healing process strictly follows the aforementioned wound healing phases. In contrast, complex wounds do not conform to the cellular and molecular events that lead to healthy wound healing. Instead, there may be difficulties in the phases of repair that result in the wound failing to heal. As such, complex wounds are defined as wounds that have failed to proceed through an orderly and timely reparative process to produce anatomic and functional integrity over a period of 3 months (Mustoe et al. 2006). A common theory is that complex wounds remain in the inflammatory stage for too long resulting in a wound that is unable to progress to the proliferative and remodeling phases of healing. Additionally, there may be increased free radical production, ischemia, impaired growth factor and cytokine production, infection, and diminished cellular infiltration that disrupt the healing process (Agren et al. 2000; Mast and Schultz 1996). A robust understanding of the mechanisms involved in chronic wound healing is necessary to develop effective stem cell-based therapies that go beyond the treatments currently available. The following section will discuss wound healing in diabetic patients and the elderly, two groups of people that may suffer from complex wounds that result in deficient healing.
3.1 Deficient Healing
The prolonged inflammatory response in chronic wounds causes reepithelization to stall as granulation tissue is defective and does not foster healing (Martin and Nunan 2015). In diabetic ulcer wounds, fibroblasts become defective as they are unresponsive to growth factor signals, and their ability to migrate to the wound bed is reduced (Brem et al. 2007; Lerman et al. 2003; Seibold et al. 1985). Ulcers also display a significant decrease in the number of TGF-β receptors and the TGF-β signaling cascade (Pastar et al. 2010). These aberrations combined with elevated levels of degrading MMPs reduce collagen deposition and thus affecting ECM integrity and tissue remodeling in diabetic patients (Trengove et al. 1999). Moreover, the effectiveness of immune cells such as neutrophils and macrophages to phagocytose debris is hindered resulting in a buildup of necrotic debris at the wound edge. There is an enrichment of pro-inflammatory cytokines such as TNF-α and IL-1β in the wound bed which can inhibit the growth and phenotype of fibroblasts (Zykova et al. 2000). There is also a decrease in IL-6, a cytokine that is responsible for mobilizing fibroblasts and keratinocytes to the wound bed (Werner and Grose 2003). Accordingly, this exaggerated and unbalanced production of pro-inflammatory cytokines adversely affects wound closure. In elderly patients, wound healing follows a similar trajectory to diabetic wound healing. There are several factors that lead to deficient healing in the elderly; however there are two that are well described in the literature. First, there is impaired formation of new blood vessels (neovascularization) (Chang et al. 2007). Second, aged stem cells may be dysfunctional compared to their younger counterparts, and this dysfunction may possibly be due to the microenvironment aged stem cells that are exposed and/or cell intrinsic alterations (Blau et al. 2015). Research has shown that aged bone marrow-derived stem cells (BM-MSCs) have reduced wound healing, angiogenesis, and proliferation capabilities compared to younger BM-MSCs (Choudhery et al. 2012). Moreover, it has been shown that mesenchymal stem cells (MSC) isolated from the skin of elderly burn patients have diminished cell proliferation and deficient migration (Jeschke et al. 2015).
3.2 Excessive Healing
In contrast to deficient healing, excessive healing leads to the formation of a keloid or hypertrophic scar. As mentioned previously, there is a delicate balance between ECM deposition and degradation in normal wound healing. In both keloids and hypertrophic scars, there is increased collagen deposition that is oriented in thick bundles rather than basket weave-like fibrils in normal dermis (Martin and Nunan 2015). In human hypertrophic scars, the number of macrophage cells is positively correlated with scar size and the level of β-catenin, suggesting that macrophages play a role in the development of excessive healing (Amini-Nik et al. 2014). Furthermore, deletion of β-catenin in macrophage-specific cells resulted in impaired migration, cell adhesion, and inability to produce TGF-β1 leading to inadequate wound healing (Amini-Nik et al. 2014). Moreover, there is a positive correlation between the number of inflammatory cells and expression of β-catenin (Cheon et al. 2002). Considering that β-catenin plays a role during the proliferative phase of wound healing and that increased β-catenin activity associates hypertrophic scars, it is plausible that β-catenin is partly responsible for the excessive healing. TGF-β signaling may also play a role in excessive healing as blocking the activation of TGF-β1/2 receptors prevents scarring (Ferguson and O’Kane 2004). The difference between keloids and hypertrophic scars arises primarily from the arrangement of the collagen fibers and the composition of the wound (Martin and Nunan 2015). Keloids feature collagen fibers that spill beyond the wound margin, while hypertrophic scars do not (Martin and Nunan 2015). Keloids are also characterized by occluded blood vessels and fewer fibroblasts when compared with hypertrophic scars (Martin and Nunan 2015).
4 Current Treatments of Acute and Complex Wounds
As mentioned previously, diabetes mellitus is a condition in which insufficient wound healing can result in foot ulcers, hence the term “diabetic foot” (Singh et al. 2005). Foot ulcers are a common problem in diabetic patients with some experiencing significant epidermis damage that can expose the dermis and even deeper layers like the muscle and bone (Singh et al. 2005). The International Wound Bed Preparation Advisory Board provides a systemic approach to the management of chronic wounds such as diabetic ulcers: debridement, management of exudate, and management of infection (Schultz et al. 2003; Falanga 2000). Following a severe cutaneous wound, there may be an accumulation of necrotic tissue which can lead to infection. Debridement and drainage of wound fluid is done to remove infected tissue, clean the wound, ensure adequate blood flow, and provide a moist wound environment for optimal wound healing (Tsourdi et al. 2013). After the wound is contained, frequent dressing changes and use of topical antibiotics help maintain a clean environment and curtail infection. In some cases the necrosis and infection may be uncontrollable, and as such an amputation of the leg is necessary to prevent system infection (Margolis et al. 2005). Debridement strategies can be a strenuous task and can be painful for the patient. Some diabetic patients and sufferers of chronic wounds require negative-pressure wound therapy (NPWT). This is a treatment option that uses a vacuum to reduce swelling in the wound area which allows for blood and nutrients to reach the wound site resulting in improved blood flow and removed bacterial fluid (Guffanti 2014).
Chronic wounds are often lacking in growth factors necessary for wound healing. Growth factors control many key cellular processes in normal tissue repair such as cell migration, proliferation, angiogenesis, and production of ECM components (Branski et al. 2007). Therefore, increasing growth factor concentrations in chronic wounds may enhance wound healing. Applying growth factors directly to the wound bed is a common method of delivery. For instance, human recombinant platelet-derived growth factor BB (PDGF-BB) was the first cytokine growth factor approved by the FDA (Snyder 2005). PDGF-BB is topically applied to the wound where it promotes proliferation of cells involved in cutaneous wound repair. When PDGF-BB is used in conjunction with the wound healing practices described above, there is a reduction in healing time in patients with diabetic ulcers (Signorini and Clementoni 2007). An alternative to growth factors is applying or transplanting cultured keratinocytes and/or fibroblasts to the wound in order to stimulate repair. In fact, implanting cultured keratinocytes into the wound bed has been shown to promote reepithelization in diabetic pigs (Karagoz et al. 2009).
In some cases, grafting skin from cadavers onto the wound bed may be necessary to prevent infection and promote healing; the transplanted skin provides a scaffold for epithelial cells that boost cell proliferation (Snyder 2005). The skin transplanted from a member of the same species is called an allograft, whereas an autograft is a technique in which a patient’s own skin is used for transplantation significantly reducing the risk of rejection and may be required if allografts are unsuccessful (Snyder 2005). Over the decades, bioengineering has produced dermal substitutes that can facilitate wound healing when traditional methods cannot be used or have failed. In severe burns, for example, a dermal substitute named Integra (Integra Limit Uncertainty, Plainsboro, New Jersey, USA) serves as temporary epidermis and is often used to assist wound closure (Branski et al. 2007; Jeschke et al. 2004). Integra is an acellular bilaminar structure made of silicone that provides a provisional dermal replacement which prevents fluid loss and acts as barricade against pathogens. Integra is composed of two layers: the bottom layer is made of a cross-linked matrix of bovine collagen and chondroitin-6-sulfate that is vital to preventing hypertrophic scarring, while the top layer is the silicon layer described above (Branski et al. 2007; Jeschke et al. 2004). The matrix in the bottom layer is vascularized and populated by host cells as the wound heals which forms a new dermis layer. Clinical trials have demonstrated the efficacy of Integra in promoting wound closure in addition to its ability to lessen hypertrophic scarring (Branski et al. 2007; Jeschke et al. 2004). Despite its advantages and success in the clinic, Integra is unable to recapitulate the structural properties of intact skin due to its lack of cellularity. Furthermore, it is expensive to produce, especially in the case of large total surface area burns (TBSA).
In excessive healing like hypertrophic scars and keloids, nonsurgical techniques are the most common methods of reducing scar size. The most accepted treatment of hypertrophic scars currently is the topical application of silicone gels or sheets onto the scar. The use of silicone has become standard practice among plastic surgeons, and it is effective in treating hypertrophic scars (Signorini and Clementoni 2007). Silicone decreases scar size mainly through wound hydration; silicone decreases water vapor transmission at the wound site resulting in a build of moisture in the wound (Signorini and Clementoni 2007). While it is a simple and effective treatment, silicone is difficult to apply, requires multiple applications per day, and may cause adverse skin reactions (Karagoz et al. 2009). An alternative method of reducing scar size is the use of mechanical force exerted by pressure garments. The theory behind this treatment is that the pressure limits collagen synthesis by creating a hypoxic environment that prevents the delivery of blood, oxygen, and nutrients (Puzey 2002). Pressure garments remain a prevalent treatment despite there being no conclusive data illustrating a reduction in scar size (Macintyre and Baird 2006). Lastly, an interesting and unusual treatment has emerged in the use of topical onion extract gel. The healing properties of onion extract are derived from its ability to inhibit fibroblast proliferation and hinder ECM production by increasing expression of MMP-1 (Cho et al. 2010). The efficacy of onion extract in decreasing scar size, however, is lacking in literature. Two studies observed no significant difference in scar appearance and size in patients treated with a 10% onion extract gel (Willital and Heine 1994; Chanprapaph et al. 2012). Stem cells hold great potential in producing treatments that are simple, safe, prevent complications, and go beyond typical treatments of acute and chronic wounds by fully regenerating skin. We will now describe the characteristics of different types of stem cells and how they have used in generating exciting new therapies for skin regeneration.
5 Stem Cells in Wound Healing
The goal of stem cell therapy is to restore skin back to its functional state after cutaneous wound injury. Unfortunately, dermal substitutes lack the biological activity necessary to regenerate the skin despite the outstanding physical support they provide to the wound bed. Incorporating stem cells and growth factors into these dermal substitutes would add the cellularity needed to accelerate skin regeneration. It is believed that the addition of these stem cells will enhance wound healing through trophic and paracrine activity that will regenerate the skin by neutralizing the damaging effects of the healing process and repairing skin by replacing lost or damaged cells (Falanga 2012).
Stem cells are groups of undifferentiated cells capable of transforming all types of cells in the human body. Using these cells as a renewable source for new tissue is the basis of regenerative medicine . The ability of these cells to self-renew and differentiate into different cell types is termed “stemness,” and it is these unique features that make stem cell-based therapies an attractive alternative to traditional treatments (Cha and Falanga 2007). Not all stem cells demonstrate true “stemness” however as only embryonic stem cells are capable of differentiating into all cell lineages. This is a serious problem for regenerative medicine as the most commonly used stem cells, adult stem cells, only partially fulfill the requirements of stemness since their differentiation potential is limited to a few cell lineages. In the following section, we will discuss recent progress in the application of different types of stem cells for cutaneous wound healing.
5.1 Embryonic Stem Cells
Embryonic stem cells (ESCs) are derived from the inner cell mass of the blastocyst of a human embryo (Cha and Falanga 2007). ECSs are the standard to which all stem cells are held due to their ability to differentiate into all three germ layers (ectoderm, mesoderm, and endoderm) given the correct stimulus, an ability termed pluripotency (Cha and Falanga 2007). ECSs can be maintained in culture in an undifferentiated or be differentiated into specialized cell types. With regard to wound healing, ESCs have been differentiated into basal keratinocytes which were used to construct a stratified epidermis. Nevertheless, there are no stem cell therapies in wound healing that utilize ESCs despite their unlimited potential for self-renewal and plasticity. This is due to ethical and legal restrictions that limit the application of ESCs in regenerative medicine . Moreover, ESCs are restricted by their allogenic nature and safety concerns regarding the risk of developing tumors (Cha and Falanga 2007). As a result, regenerative medicine has primarily focused on adult stem cells since they are able to provide some of advantages of ESCs without the rigorous ethical and legal constraints.
5.2 Bone Marrow-Derived Stem Cells
The bone marrow (BM) is comprised of a heterogeneous cell population which includes hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), two groups of cells that are highly plastic progenitor cells (Salem and Thiemermann 2010). HSCs are responsible for the bone marrow and blood, while MSCs are non-hematopoietic stem cells that are self-renewing and differentiate into various cell types such as osteoblasts, adipocytes, chondrocytes, and even dermis (Wu et al. 2007a). MSCs are marked by their ability to adhere to a plastic surface, and cultured MSCs are strongly positive for CD105, CD90, and CD73 but negative for CD34 and CD45 (Boxall and Jones 2012). During wound healing, HSCs and MSCs mobilize to the wound site where they modulate the inflammatory response so that the wound does not progress to a chronic state. BM-MSCs attenuate the inflammatory response by decreasing the secretion of pro-inflammatory cytokines while amplifying the production of anti-inflammatory cytokines in the wound bed (Aggarwal and Pittenger 2005). In addition to diminishing the inflammatory response, BM-MSCs demonstrate antimicrobial activity (Maxson et al. 2012) and secrete growth factors such as FGF and VEGF that promote fibroblast proliferation, collagen deposition, and angiogenesis (Gnecchi et al. 2008). An alternative explanation for the wound healing properties of BM-MSCs is that these stem cells migrate to the wound site and differentiate into cells necessary for the renewal and repair of damaged tissue (Wu et al. 2007a). BM-MSCs are well characterized and most widely studied stem cell population; however the isolation procedure is painful for patients, and there are few stem cells present in bone marrow aspirate.
Stem cell therapies in cutaneous wound healing have relied heavily on BM-MSCs, and numerous studies have demonstrated the usefulness of BM-MSCs in treating deficient and excessive healing in animals and humans (Table 9.1). In humans, autologous BM-MSCs delivered via a fibrin spray reduced wound size in patients with acute surgical excisional wounds (Falanga et al. 2007). In another study, direct injection of BM followed by topical application of cultured MSCs directly onto the wound bed led to complete wound closure, dermal rebuilding, and less scarring in three patients with chronic ulcer wounds (Badiavas and Falanga 2003). Moreover, examining biopsies from the healed tissue revealed significant vascularization and dermal thickness (Badiavas and Falanga 2003). The clinical benefits of BM-MSCs have also been illustrated in several trials involving diabetic ulcers where traditional therapies have failed. In one study, intramuscular injection of autologous BM-MSCs in patients with diabetic ulcers resulted in improved blood flow to the wound area (Lu et al. 2011). The injected was well tolerated, and patients experienced a significant reduction in pain and a marked improvement in healing rate of the ulcer at 24 weeks postinjection (Lu et al. 2011). Another study observed improved circulation and complete wound closure after transplantation of BM-MSCs in 18 out of 22 patients with diabetic ulcers (Kirana et al. 2012). Two other studies show that injection of BM-MSCs in patients with diabetic ulcers improves recovery time (Liu et al. 2014; Dash et al. 2009), decreases wound size (Dash et al. 2009), and enhances development of fibroblasts and inflammatory cells (Dash et al. 2009).
Animal studies involving BM-MSCs provide a more comprehensive and detailed view of the effect of these cells on cutaneous wound healing. In mouse acute excisional wounds, local injection of BM-MSCs accelerated wound closure and increased epithelization and angiogenesis (Wu et al. 2007b). Similar beneficial effects of BM-MSCs have been observed in other animals as transplantation of BM-MCSs improved tensile strength and healing in albino rat excisional wounds (Basiouny et al. 2013). As mentioned earlier, the dermis is remodeled in part through contribution of fibroblasts and deposition of collagen, and BM-MSCs may impact this process. In acute mouse wounds, BM-MSCs transcribe both collagen I and III and contributed to the production of 15–20% of the dermal fibroblast population (Fathke et al. 2004). Similar beneficial effects of BM-MSCs have been shown in diabetic wound healing in animals. Treatment with BM-MSCs has led to enhanced wound closure (Falanga et al. 2007), accelerated wound healing (Ha et al. 2010), and increased reepithelization (Fiorina et al. 2010) in various acute wound healing animal models.
The method of delivery of BM-MSCs may influence the healing effects observed. For instance, BM-MSCs seeded on a pullulan-collagen hydrogel scaffold accelerated healing and increased angiogenesis in a murine excisional wound model (Rustad et al. 2012). Interestingly, BM-MSCs seeded on this hydrogel expressed increased levels of VEGF and were able to differentiate into fibroblasts, endothelial cells, and pericytes, but not epithelial cells (Rustad et al. 2012). In contrast, injection of BM-MSCs after acute skin damage caused by irradiation speeds up wound healing and increases expression of growth factors such as TGF-β1 and SDF-1 in rats (Zheng et al. 2015). Lastly, one study observed increased healing rate, increased collagen fibers, increased tensile strength, and infiltration of CD68+ macrophages in mice after BM-MSCS were delivered topically via fibrin glue (Mehanna et al. 2015). Treatment of the BM-MSCs also has an effect on wound healing as BM-MSCs that undergo hypoxia pretreatment are more effective at healing in rats with diabetic hindlimb ischemia (Tong et al. 2015). Optimizing the method of delivery and the way BM-MSCs are prepared is crucial to developing effective stem cell therapies in cutaneous wound healing.
There are no clinical studies that examine the effect of BM-MSCs in treating hypertrophic scarring and keloids; however there are a couple animal studies that demonstrate the potential of BM-MSCs in reducing fibrosis. Infusion of human MSCs significantly reduced fibrosis in a dermal fibrosis mouse model through suppression of fibroblast proliferation (Wu et al. 2015). Another study showed that local injection of human MSCs prevented hypertrophic scar formation in rabbits through regulation of the anti-inflammatory protein, TSG-6, rather than modulating fibroblast activity like in the previous study. The efficacy of BM-MSCs in treating acute and complex wounds and the associated mechanisms need to be further explored.
5.3 Adipose-Derived Stem Cells
As mentioned earlier, harvesting BM-MSCs from patients is invasive and painful and yields relatively few cells. Adipose-derived stem cells (ASCs) have been identified as a new source of adult stem cells that appear to be promising in the field of regenerative medicine . Harvesting ASCs from adipose tissue is less invasive, less painful, and avoids the ethical and immunological concerns plaguing other stem cells (Zuk et al. 2002). Adipose tissue is also remarkable source of ASCs and can easily be isolated from a section of whole fat (biopsies) or lipoaspirate following surgery in patients (Zuk et al. 2002). For example, adipose tissue yields a 500-fold greater number of stem cells when compared to an equivalent amount of the bone marrow (Strioga et al. 2012). ASCs can differentiate into adipocytes, chondrocytes, and myogenic lineages; however one drawback is that ASCs do not offer as much plasticity as BM-MSCs (Gimble et al. 2012). Similarly to BM-MSCs, ASCs secrete a number of different cytokines and growth factors that promote wound healing (Kilroy et al. 2007). There are a couple drawbacks to ASCs however. First, the metabolic activity of ASCs (i.e., proliferation and differentiation capacity) depends on the anatomical location of the fat, age, and gender of the patient (Bailey et al. 2010). Second, it has been reported that ASCs undergo spontaneous malignant transformation after 4 months of culturing (Rubio et al. 2005); however researchers argue that this results from contamination rather than some inherent characteristic of ASCs (Garcia et al. 2010; Torsvik et al. 2010). Nevertheless, the above characteristics make ASCs an exciting alternative in the field of stem cell therapy, and a number of studies in various conditions have shown the therapeutic potential of ASCs in wound healing.
In 2012, Lee et al. (2012) showed that multiple intramuscular injections of ASCs resulted in clinical improvement in 66.7% of patients with critical limb ischemia. These patients exhibited decreased pain and improved claudication walking distance suggesting that multiple intramuscular injections of ASCs may be a viable therapeutic method of increasing blood flow in patients with critical limb ischemia (Lee et al. 2012). An interesting technique that may be used in the future to prevent the development of chronic wounds may be attaching ASCs (or other stem cells) to surgical sutures. Biodegradable surgical sutures filled with ASCs create a pro-regenerative environment in vitro with a steady release of cytokines and endothelial growth factors (Reckhenrich et al. 2014). Moreover, conditioned media from the supernatant of these cell-filled sutures significantly decreased wound area compared to controls in a wound healing assay (Reckhenrich et al. 2014).
Animal studies have demonstrated the wide variety of positive effects ASCs can have on cutaneous wound healing. Following acute wounds, injection of ASCs increases epithelization, decreases healing time, and reduces inflammation in fisher rats and rabbits (Pelizzo et al. 2015; Uysal et al. 2014). A recent study by Mendez et al. (2015) has shown that subcutaneous injection of ASCs decreases wound size and increases granulation and collagen synthesis in mice with full-thickness wounds. Interestingly, these researchers were able to develop a fibrin formulation that transforms ASCs into vascular tubes that express various endothelial markers (Mendez et al. 2015). It is thought that the growth of new blood vessels via the release of endothelial growth factors such as VEGF and HGF may be one mechanism by which ASCs exert their healing effects. In fact, preclinical studies have reported improved tissue hydration and angiogenesis after treatment with ASCs in patients with radiation-induced skin damage (Rigotti et al. 2007; Akita et al. 2010). Two animal studies also show increased vascularization and improved healing after acute wounds in mice and rats, respectively (Garg et al. 2014; Meruane et al. 2012). One study has shown that ASCs may be more effective than BM-MSCs in treating acute wounds (find reference) and found that local injection of ASCs in rabbit excisional wounds was more effective than BM-MSCs in improving tensile strength and attenuating scar formation. With regard to deficient healing, allogenic transplantation of ASCs accelerated wound healing and vascularization in a diabetic rat wound model (Kato et al. 2015). Recently, injection of ASCs had positive effects in young and old mice with pressure ulcers (Strong et al. 2015): observed accelerated wound closure, increased adipogenesis, and improved epidermal structure in these mice.
There are no human studies examining the effect of ASCs in excessive healing; however there are couple of animal studies which are promising. In fibrosis rabbit ear model, it was shown that intralesional injection of ASCs decreased expression of α-SMA and collagen type I leading to diminished collagen deposition and hence reduced hypertrophic scarring (Zhang et al. 2015). Another study revealed that ASCs delivered through an ECM patch derived from porcine small intestine submucosa (SIS) significantly reduce fibrotic scar size in mice (Lam et al. 2013). In the future, more animal studies are needed to justify the use of ASCs in treating hypertrophic scars and keloids in humans. The unique trophic functions of ASCs combined with the studies described above make them an exciting alternative in stem cell-based therapies and support their applicability in cutaneous wound healing.
5.4 Umbilical and Placental Derived Stem Cells
It is believed that umbilical cord blood is the largest potential source of hematopoietic and non-hematopoietic stem cells with naïve immune status (Knudtzon 1974). These cord blood stem cells have been shown to differentiate into hepatic, pancreatic, and neural precursor cells (McGuckin et al. 2006; Denner et al. 2007). In fact, hematopoietic stem cells from cord blood have been used to treat many pediatric and adult diseases (Branski et al. 2009). Moreover, MSCs have been isolated from umbilical cord blood and successfully differentiated into epithelial cells in vitro (Sanmano et al. 2005; Kamolz et al. 2006). In addition to the blood, different layers of the umbilical cord such as Wharton’s jelly (the gelatinous supporting matrix) and the outer lining are a source of stem cells. Stem cells isolated from the cord lining can be divided into two groups: epithelial cells and mesenchymal cells. These cells express typical stem cell markers such as Oct-4 and Nanog (In ‘t Anker et al. 2003) and are capable of differentiating into epithelial cells, hepatocytes, neural cells, and endothelium. These characteristics suggest that cord lining stem cells can potentially be used in the clinic to regenerate epithelial cells in cutaneous wound healing. While the umbilical cord is large reservoir of stem cells, isolating these cells is difficult as the umbilical cord provides 30% less cells when compared with MSCs isolated from the bone marrow (Wilson et al. 2011). Umbilical cord stem cells are a promising new source of new tissue in skin regeneration, and coadministration with other stem cells like BM-MSCs or ASCs may offer synergistic benefits in wound healing. Many clinical trials are ongoing to test the efficacy of umbilical cord-derived stem cells in treating diabetic wounds and burns. These studies have not reported any immunological rejection or tumor formation indicating that these cells pose no risk to patients.
Transplantation of Wharton’s jelly MSCs increases expression of genes in reepithelization and promoted wound healing in mouse excisional wounds (Shi et al. 2015). Other studies have reported improved wound healing after administration of Wharton’s jelly MSCs in addition to increased keratinocyte differentiation (Luo et al. 2010), reduced scar formation, and improved hair growth (Sabapathy et al. 2014). Delivering umbilical cord MSCs via a collagen-fibrin double-layered reduced healing time in a mouse full-thickness wound model further illustrating that cell delivery is an important aspect of developing stem cell therapies (Nan et al. 2015). The mechanism of action of umbilical cord-derived stem cells may be modulation of growth factors involved in the TGF-β signaling pathway. Interestingly, intraperitoneal injection of placenta-derived MSCs accelerates wound healing by increasing the expression of pro-angiogenic factors such as VEGF (Arno et al. 2014). In deficient healing, cord-derived MSCs delivered via topical application accelerated wound healing in db/db mice (Ha et al. 2010). Interestingly, TGF-β expression was significantly increased after the first week of treatment. Lastly, Wharton’s jelly MSCs increase proliferation rate of keloid fibroblasts in vitro through regulation of TGF-β2 further supporting the notion that umbilical cord-derived MSCs modulate TGF-β activity (Arno et al. 2014).
5.5 Epidermal Stem Cells
The skin has become a promising source of adult stem cells that could potentially be seeded onto dermal substitutes to create a scaffold with biological activity. In contrast to ESCs and iPSCs, epidermal stem cells are non-oncogenic, have no ethical concerns, and are easily accessible (Fuchs and Nowak 2008). As a result, these cells may solve many problems in the field of skin engineering such as immune rejection and lack of skin appendages. Epidermal stem cells are located in the basal layer of the epidermis and are unipotent and able to regenerate the epidermis in adults (Morasso and Tomic-Canic 2005). In contrast, stem cells located within the bulge region of the hair follicle are multipotent cells capable of repopulating the epidermis, sebaceous glands, and hair follicles (Blanpain et al. 2004). Additionally, these cells are K15-positive and can differentiate into smooth muscle cells, neurons, and glial cells (Amoh et al. 2010). During injury, hair follicle stem cells rapidly mobilize and migrate to repair the epidermis in vivo by contributing to reepithelization (Ito et al. 2005). This response is tightly regulated and stops once the wound has healed. Lineage tracing studies have confirmed that hair follicle stem cells are normally unipotent as they maintain their niche compartment, the hair follicle, during homeostasis (Tumbar et al. 2004).
In the treatment of deep burn wounds, transplantation of cultured epithelial cells containing epidermal stem cells and keratinocytes via a fibrin matrix is well established (Fang et al. 2014). There are also reports of cultured epidermal cells being effective in treating chronic skin ulcers and deep dermal wounds (Oshima et al. 2002). Likewise, animal studies have illustrated the potential of epidermal stem cells in treating acute and complex wounds. Transplantation of epidermal stem cells via a collagen-chitin biomimetic membrane has been shown to regenerate whole skin in mice with full-thickness skin defects (Shen et al. 2014). Comparable healing effects were also found in diabetic mice treated with stem cells isolated from the hair follicle dermal sheath (Ma et al. 2015). Furthermore, in vitro examination relieved that these stem cells secreted paracrine factors such as IL-6 and enhanced wound healing. Recently, a novel epidermal stem cell population marked by Lgr6 was found in the isthmus region of the hair follicle (Snippert et al. 2010). Similar to other hair follicle stem cells, transplantation of Lgr6-positive cells gives rise to all epidermal lineages and also migrate to and repair the interfollicular epidermis during injury (Snippert et al. 2010). Unlike other hair follicle stem cells, however, Lgr6-positive cells contribute to the maintenance of the isthmus region and the sebaceous glands. Lastly, in mice with full-thickness wounds, local injection of Lgr6-expressing epithelial cells onto the wound bed promotes hair growth, reepithelization, and angiogenesis demonstrating the potential of these cells to facilitate cutaneous wound healing (Lough et al. 2014).
5.6 Induced Pluripotent Stem Cells
The ethical and legal concerns surrounding ESCs were quickly forgotten in 2007 when a new class of stem cells was discovered which combines the advantages of embryonic and adult stem cells. Induced pluripotent stem cells (iPSCs) were generated by exposing fibroblasts cultures to a cocktail of genes such as Oct3/Oct4, Sox2, and Klf4 by retroviral delivery (Takahashi et al. 2007) and Oct4, Sox2, Nanog, and Lin278 by lentiviral vectors (Yu et al. 2007). These iPSCs were immature, pluripotent, and nonimmunogenic cells generated from adult-differentiated tissue that exhibited similar plasticity to human ESCs. Their pluripotency was confirmed by their capability of differentiating into various cell types in vitro, expression of pluripotent marker genes, and their ability to produce all three germ layers (Takahashi and Yamanaka 2006). There are, however, safety concerns regarding iPSCs as they are cancerous in an undifferentiated state, and 20% of chimeric mice develop tumors due to expression of the well-known oncogene, c-Myc (Cartwright et al. 2005). Despite these concerns, iPSCs hold great promise in regenerative medicine . Human-derived iPSCs have been differentiated into epithelial cells resulting in the development of all hair follicle lineages (Yang et al. 2014). To take this concept further, Itoh et al. (2011) generated a 3-D skin equivalent in vitro that was comprised mostly of iPSC-derived keratinocytes and fibroblasts. Lastly, iPSC-derived keratinocytes have been used to create a stratified epithelium that was subsequently used to treat recessive dystopic epidermolysis bullosa, a type of deficient healing (Sebastiano et al. 2014). Overall, iPSCs will play an important role in stem cell therapies, but further research is needed to improve safety and refine the methods of reprogramming these cells.
6 Methods of Delivering Stem Cells
The therapeutic benefit of stem cells is limited by the method of delivery, and this is a unique challenge that regenerative medicine faces. There are three main techniques that are utilized in stem cell therapy: injection-based delivery, topical application via spray, and scaffold-based delivery.
6.1 Injection-Based Delivery
Injection-based delivery is a commonly used technique to deliver stem cells, and it is well described in preclinical and early clinical studies (Falanga et al. 2007). This method involves directly injecting stem cells within a suspension or hydrogel matrix into the site of injury. While local administration of stem cells via injection is the simplest method available, it may not be the optimal method to deliver cells to a cutaneous wound. Cell viability is a major issue as the immense pressure from the injection process results in massive cell death (Garg et al. 2014). Moreover, the wound environment increases cell death and impairs cell attachment resulting in stem cells becoming disorganized and delocalized (Zhang et al. 2008). This is problematic because these stem cells may migrate and integrate with tissue away from the wound site, thus creating unwanted harmful effects in other parts of the body such as cancer.
6.2 Spray-Based Delivery
Another local administration technique is spray-based delivery of stem cells. This technique involves isolating stem cells and mixing them with a fibrinogen that forms fibrin upon administration of thrombin (Zimmerlin et al. 2013). The fibrin spray prevents the degradation of the stem cells and also facilitates the adherence of the stem cells to the wound (Falanga et al. 2007). Fibrin spray can also distribute cells across a large area unlike injection-based therapies; however this can also be a potential weakness as there might be poor control of cell density and spacing (Duscher et al. 2015). Furthermore, because the stem cells are topically applied to a non-protective and harsh wound environment, cell viability may be problematic. Regardless, many studies have proven spray-based delivery as a safe and effective technique at promoting wound healing (Falanga et al. 2007; Wu et al. 2011).
6.3 Scaffold-Based Stem Cell Therapy
In the past decade, alternative techniques have been developed to overcome the weaknesses of spray- and injection-based delivery methods. Scaffold-based therapy has been the foundation of new stem cell delivery techniques that hope to improve therapeutic benefit. The goal of scaffold-based therapy is to seed stem cells on bioscaffolds in the hope that stem cell viability, differentiation, and engraftment into the wound are enhanced. Bioscaffolds are typically composed of collagen, hyaluronic acid, pullulan, or chitosan that mimic the native tissue environment (Jayakumar et al. 2010; Landsman et al. 2009). An important characteristic of these scaffolds is sufficient porosity to support adequate transport of nutrients and waste (Celiz et al. 2014). Moreover, these scaffolds provide protection for the stem cells and important spatial cues that mimic a natural environment, thus increasing the likelihood that these cells become functional parts of the wound. Indeed, seeding stem cells onto a pullulan-collagen matrix preserves the “stemness” of the cells and accelerate wound healing (Rustad et al. 2012).
While bioscaffolds have tremendous potential, they are still limited by poor nutrient transport and vascularization after engraftment (Meinel et al. 2004). Novel approaches need to be developed that will improve cell seeding efficiency, cell viability, cell uniformity, and cell integration. For instance, ASCs have been seeded more effectively on a hydrogel scaffold via capillary force than other methods such as injection and centrifuge and were effective at improving wound healing (Garg et al. 2014). As mentioned earlier, growth factors and cytokines play an important role in the wound healing process. Integrating these molecules, or even plasmids and vectors that modulate stem cell activity, within a scaffold could potentially help facilitate healing. Another novel approach to improving scaffold-based cell delivery is modifying the structure and composition of these scaffolds. An example would be altering the intermolecular bonds within the matrix to improve porosity, and interestingly one study has demonstrated that this can modulate cell proliferation and differentiation (Jeon and Alsberg 2013). These examples illustrate the importance of optimizing the delivery method so that stem cell-based therapies in wound healing can be readied for clinical trials. Unfortunately, bioscaffolds are limited by manufacturing costs and safety concerns (Duscher et al. 2015), but future advancements in biology and engineering may eventually produce a viable stem cell-based treatment option for acute and complex wounds.
7 Challenges and Future Directions in Stem Cell Therapy
Most stem cell-based therapies discussed in this chapter have not been proven clinically. While stem cell therapy in cutaneous wound healing has shown tremendous promise, there are still significant challenges that need to be addressed before these therapies are clinically relevant. Firstly, there is no evidence that skin and appendages regenerated by stem cell therapies are functional – flawless regeneration without scarring has yet to be achieved. There needs to be studies demonstrating confirming whether regenerated skin recapitulates the function of intact skin. Second, harvesting enough adult stem cells with high purity is obstructing the progress of developing new therapies. Lastly, the differentiation potential of stem cells may give rise to malignant cells as the niche microenvironment is crucial in directing tumor growth. Optimizing cell growth to replace damaged tissue while at the same time preventing disproportionate cell proliferation and differentiation remains a challenge for researchers. To accomplish this a robust understanding of the cellular and molecular mechanisms underlying stem cell action is necessary. Therefore, innovative approaches are needed to refine the manipulation of stem cells, improve the methods of delivering these cells, and finally validate these therapies through clinical trials so that patients can reap the benefits of regenerative medicine .
8 Conclusion
In this chapter we addressed the potential of stem cells in the treatment of acute and complex cutaneous wounds. There are various aspects to consider when developing stem cell therapies such as the signaling pathways involved in wound healing, the source of stem cells, and the method of delivery. While there may be significant barriers limiting the clinical translation of stem cell-based therapies, the clinical and animal studies discussed illustrate the therapeutic potential of stem cell therapy in regenerative medicine . More clinical studies are needed so that the plethora of research in basic science can be translated into clinical wound care.
References
Abd-Allah SH, El-Shal AS, Shalaby SM, Abd-Elbary E, Mazen NF, Abdel Kader RR (2015) The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life 67(9):701–709. doi:10.1002/iub.1427. Epub 2015/09/01; PubMed PMID: 26315141,https://www.ncbi.nlm.nih.gov/pubmed/26315141
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822. doi:10.1182/blood-2004-04-1559. Epub 2004/10/21; PubMed PMID: 15494428
Agren MS, Eaglstein WH, Ferguson MW, Harding KG, Moore K, Saarialho-Kere UK et al (2000) Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol Suppl 210:3–17. Epub 2000/07/08. PubMed PMID: 10884942
Akita S, Akino K, Hirano A, Ohtsuru A, Yamashita S (2010) Mesenchymal stem cell therapy for cutaneous radiation syndrome. Health Phys 98(6):858–862. doi:10.1097/HP.0b013e3181d3d52c. Epub 2010/05/07; PubMed PMID: 20445394
Amini-Nik S, Glancy D, Boimer C, Whetstone H, Keller C, Alman BA (2011) Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta-catenin mediated process. Stem Cells (Dayton, Ohio) 29(9):1371–1379. doi:10.1002/stem.688. Epub 2011/07/09; PubMed PMID: 21739529
Amini-Nik S, Cambridge E, Yu W, Guo A, Whetstone H, Nadesan P et al (2014) Beta-catenin-regulated myeloid cell adhesion and migration determine wound healing. J Clin Invest 124(6):2599–2610. doi:10.1172/jci62059. Epub 2014/05/20; PubMed PMID: 24837430; PubMed Central PMCID: PMCPmc4089463
Amoh Y, Katsuoka K, Hoffman RM (2010) The advantages of hair follicle pluripotent stem cells over embryonic stem cells and induced pluripotent stem cells for regenerative medicine. J Dermatol Sci 60(3):131–137. doi:10.1016/j.jdermsci.2010.09.007. Epub 2010/11/03; PubMed PMID: 21036545
Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E et al (2014) Effect of human Wharton’s jelly mesenchymal stem cell paracrine signaling on keloid fibroblasts. Stem Cells Transl Med 3(3):299–307. doi:10.5966/sctm.2013-0120. Epub 2014/01/18; PubMed PMID: 24436441; PubMed Central PMCID: PMCPmc3952928
Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C et al (2006) Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 113(20):2413–2424. doi:10.1161/circulationaha.105.603167. Epub 2006/05/17; PubMed PMID: 16702471
Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE et al (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1(5):260–266. doi:10.1038/12971. Epub 1999/11/13; PubMed PMID: 10559937
Athar M, Tang X, Lee JL, Kopelovich L, Kim AL (2006) Hedgehog signalling in skin development and cancer. Exp Dermatol 15(9):667–677. doi:10.1111/j.1600-0625.2006.00473.x. Epub 2006/08/03; PubMed PMID: 16881963
Badiavas EV, Falanga V (2003) Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol 139(4):510–516. doi:10.1001/archderm.139.4.510. Epub 2003/04/23; PubMed PMID: 12707099
Bailey AM, Kapur S, Katz AJ (2010) Characterization of adipose-derived stem cells: an update. Curr Stem Cell Res Ther 5(2):95–102. Epub 2009/11/28. PubMed PMID: 19941461
Basiouny HS, Salama NM, Maadawi ZM, Farag EA (2013) Effect of bone marrow derived mesenchymal stem cells on healing of induced full-thickness skin wounds in albino rat. Int J Stem Cells 6(1):12–25. Epub 2013/12/04. PubMed PMID: 24298370; PubMed Central PMCID: PMCPmc3840998
Bielefeld KA, Amini-Nik S, Alman BA (2013) Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci: CMLS 70(12):2059–2081. doi:10.1007/s00018-012-1152-9. Epub 2012/10/12; PubMed PMID: 23052205; PubMed Central PMCID: PMCPmc3663196
Biernacka A, Dobaczewski M, Frangogiannis NG (2011) TGF-beta signaling in fibrosis. Growth Factors (Chur, Switzerland) 29(5):196–202. doi:10.3109/08977194.2011.595714. Epub 2011/07/12; PubMed PMID: 21740331; PubMed Central PMCID: PMCPmc4408550
Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118(5):635–648. doi:10.1016/j.cell.2004.08.012. Epub 2004/09/02; PubMed PMID: 15339667
Blau HM, Cosgrove BD, Ho AT (2015) The central role of muscle stem cells in regenerative failure with aging. Nat Med 21(8):854–862. doi:10.1038/nm.3918. Epub 2015/08/08; PubMed PMID: 26248268
Boxall SA, Jones E (2012) Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int 2012:975871. doi:10.1155/2012/975871. Epub 2012/06/06; PubMed PMID: 22666272; PubMed Central PMCID: PMCPmc3361338
Branski LK, Herndon DN, Pereira C, Mlcak RP, Celis MM, Lee JO et al (2007) Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med 35(11):2615–2623. doi:10.1097/01.ccm.0000285991.36698.e2. Epub 2007/09/11; PubMed PMID: 17828040
Branski LK, Gauglitz GG, Herndon DN, Jeschke MG (2009) A review of gene and stem cell therapy in cutaneous wound healing. Burn J Int Soc Burn Inj 35(2):171–180. doi:10.1016/j.burns.2008.03.009. Epub 2008/07/08; PubMed PMID: 18603379; PubMed Central PMCID: PMCPmc3899575
Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I et al (2007) Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med (Cambridge, Mass) 13(1–2):30–39. doi:10.2119/2006-00054.Brem. Epub 2007/05/23; PubMed PMID: 17515955; PubMed Central PMCID: PMCPmc1869625
Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL et al (2014) Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 16(2):245–257. doi:10.1016/j.jcyt.2013.11.011. Epub 2014/01/21; PubMed PMID: 24438903
Caiado F, Real C, Carvalho T, Dias S (2008) Notch pathway modulation on bone marrow-derived vascular precursor cells regulates their angiogenic and wound healing potential. PLoS ONE 3(11):e3752. doi:10.1371/journal.pone.0003752. Epub 2008/11/19; PubMed PMID: 19015735; PubMed Central PMCID: PMCPmc2582964
Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S (2005) LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132(5):885–896. doi:10.1242/dev.01670. Epub 2005/01/28; PubMed PMID: 15673569
Celiz AD, Smith JG, Langer R, Anderson DG, Winkler DA, Barrett DA et al (2014) Materials for stem cell factories of the future. Nat Mater 13(6):570–579. doi:10.1038/nmat3972. Epub 2014/05/23; PubMed PMID: 24845996
Cha J, Falanga V (2007) Stem cells in cutaneous wound healing. Clin Dermatol 25(1):73–78. doi:10.1016/j.clindermatol.2006.10.002. Epub 2007/02/06; PubMed PMID: 17276204
Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N et al (2007) Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 116(24):2818–2829. doi:10.1161/circulationaha.107.715847. Epub 2007/11/28; PubMed PMID: 18040029
Chanprapaph K, Tanrattanakorn S, Wattanakrai P, Wongkitisophon P, Vachiramon V (2012) Effectiveness of onion extract gel on surgical scars in Asians. Dermatol Res Pract 2012:212945. doi:10.1155/2012/212945. Epub 2012/08/28; PubMed PMID: 22924037; PubMed Central PMCID: PMCPmc3423794
Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H et al (2002) Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A 99(10):6973–6978. doi:10.1073/pnas.102657399. Epub 2002/05/02; PubMed PMID: 11983872; PubMed Central PMCID: PMCPmc124513
Cheon SS, Nadesan P, Poon R, Alman BA (2004) Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res 293(2):267–274. Epub 2004/01/20. PubMed PMID: 14729464
Cheon S, Poon R, Yu C, Khoury M, Shenker R, Fish J et al (2005) Prolonged beta-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab Inv J Tech Methods Pathol 85(3):416–425. doi:10.1038/labinvest.3700237. Epub 2005/01/18; PubMed PMID: 15654359
Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R et al (2006) Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J: Off Publ Fed Am Soc Exp Biol 20(6):692–701. doi:10.1096/fj.05-4759com. Epub 2006/04/04; PubMed PMID: 16581977
Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK et al (1999) Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol 205(1):1–9. doi:10.1006/dbio.1998.9103. Epub 1999/01/12; PubMed PMID: 9882493
Chigurupati S, Arumugam TV, Son TG, Lathia JD, Jameel S, Mughal MR et al (2007) Involvement of notch signaling in wound healing. PLoS ONE 2(11):e1167. doi:10.1371/journal.pone.0001167. Epub 2007/11/15; PubMed PMID: 18000539; PubMed Central PMCID: PMCPmc2048753
Cho JW, Cho SY, Lee SR, Lee KS (2010) Onion extract and quercetin induce matrix metalloproteinase-1 in vitro and in vivo. Int J Mol Med 25(3):347–352. Epub 2010/02/04. PubMed PMID: 20127038
Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S (2012) Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int 36(8):747–753. doi:10.1042/cbi20110183. Epub 2012/02/23; PubMed PMID: 22352320
Clark RA (1990) Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol 94(6 Suppl):128s–134s. Epub 1990/06/01. PubMed PMID: 2161886
Crowe MJ, Doetschman T, Greenhalgh DG (2000) Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol 115(1):3–11. doi:10.1046/j.1523-1747.2000.00010.x. Epub 2000/07/08; PubMed PMID: 10886500
Darby I, Skalli O, Gabbiani G (1990) Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Inv J Tech Methods Pathol 63(1):21–29. Epub 1990/07/01; PubMed PMID: 2197503
Das AK, Bin Abdullah BJ, Dhillon SS, Vijanari A, Anoop CH, Gupta PK (2013) Intra-arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: report of a phase I study. World J Surg 37(4):915–922. doi:10.1007/s00268-012-1892-6. Epub 2013/01/12; PubMed PMID: 23307180
Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC (2009) Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 12(5):359–366. doi:10.1089/rej.2009.0872. Epub 2009/11/26; PubMed PMID: 19929258
Denner L, Bodenburg Y, Zhao JG, Howe M, Cappo J, Tilton RG et al (2007) Directed engineering of umbilical cord blood stem cells to produce C-peptide and insulin. Cell Prolif 40(3):367–380. doi:10.1111/j.1365-2184.2007.00439.x. Epub 2007/05/29; PubMed PMID: 17531081
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122(1):103–111. Epub 1993/07/01. PubMed PMID: 8314838; PubMed Central PMCID: PMCPmc2119614
Dreifke MB, Jayasuriya AA, Jayasuriya AC (2015) Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl 48:651–662. doi:10.1016/j.msec.2014.12.068. Epub 2015/01/13; PubMed PMID: 25579968; PubMed Central PMCID: PMCPmc4443476
Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M et al (2015) Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. doi:10.1159/000381877. Epub 2015/06/06; PubMed PMID: 26045256
Falanga V (2000) Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 8(5):347–352. Epub 2000/12/15. PubMed PMID: 11115147
Falanga V (2012) Stem cells in tissue repair and regeneration. J Invest Dermatol 132(6):1538–1541. doi:10.1038/jid.2012.77. Epub 2012/05/16; PubMed PMID: 22584501; PubMed Central PMCID: PMCPmc4084617
Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N et al (2007) Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13(6):1299–1312. doi:10.1089/ten.2006.0278. Epub 2007/05/24; PubMed PMID: 17518741
Fang T, Lineaweaver WC, Sailes FC, Kisner C, Zhang F (2014) Clinical application of cultured epithelial autografts on acellular dermal matrices in the treatment of extended burn injuries. Ann Plast Surg 73(5):509–515. doi:10.1097/SAP.0b013e3182840883. Epub 2013/12/11; PubMed PMID: 24322642
Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A et al (2004) Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells (Dayton, Ohio) 22(5):812–822. doi:10.1634/stemcells.22-5-812. Epub 2004/09/03; PubMed PMID: 15342945; PubMed Central PMCID: PMCPmc1388268
Ferguson MW, O’Kane S (2004) Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond Ser B Biol Sci 359(1445):839–850. doi:10.1098/rstb.2004.1475. Epub 2004/08/06; PubMed PMID: 15293811; PubMed Central PMCID: PMCPmc1693363
Ferguson MW, Duncan J, Bond J, Bush J, Durani P, So K et al (2009) Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet (London, England) 373(9671):1264–1274. doi:10.1016/s0140-6736(09)60322-6. Epub 2009/04/14; PubMed PMID: 19362676
Fiorina P, Pietramaggiori G, Scherer SS, Jurewicz M, Mathews JC, Vergani A et al (2010) The mobilization and effect of endogenous bone marrow progenitor cells in diabetic wound healing. Cell Transplant 19(11):1369–1381. doi:10.3727/096368910x514288. Epub 2010/10/28; PubMed PMID: 20977829
Fuchs E, Nowak JA (2008) Building epithelial tissues from skin stem cells. Cold Spring Harb Symp Quant Biol 73:333–350. doi:10.1101/sqb.2008.73.032. Epub 2008/11/22; PubMed PMID: 19022769; PubMed Central PMCID: PMCPmc2693088
Garcia S, Bernad A, Martin MC, Cigudosa JC, Garcia-Castro J, de la Fuente R (2010) Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res 316(9):1648–1650. doi:10.1016/j.yexcr.2010.02.016. Epub 2010/02/23; PubMed PMID: 20171963
Garg RK, Rennert RC, Duscher D, Sorkin M, Kosaraju R, Auerbach LJ et al (2014) Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. Stem Cells Transl Med 3(9):1079–1089. doi:10.5966/sctm.2014-0007. Epub 2014/07/20; PubMed PMID: 25038246; PubMed Central PMCID: PMCPmc4149299
Gimble JM, Bunnell BA, Guilak F (2012) Human adipose-derived cells: an update on the transition to clinical translation. Regen Med 7(2):225–235. doi:10.2217/rme.11.119. Epub 2012/03/09; PubMed PMID: 22397611; PubMed Central PMCID: PMCPmc3321837
Gnecchi M, Zhang Z, Ni A, Dzau VJ (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103(11):1204–1219. doi:10.1161/circresaha.108.176826. Epub 2008/11/26; PubMed PMID: 19028920; PubMed Central PMCID: PMCPmc2667788
Guffanti A (2014) Negative pressure wound therapy in the treatment of diabetic foot ulcers: a systematic review of the literature. J Wound Ostomy Continence Nurs: Off Publ Wound Ostomy Continence Nurses Soc WOCN 41(3):233–237. doi:10.1097/won.0000000000000021. Epub 2014/05/09; PubMed PMID: 24805174
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229. doi:10.1177/0022034509359125. Epub 2010/02/09; PubMed PMID: 20139336; PubMed Central PMCID: PMCPmc2903966
Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S et al (2013) A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med 11:143. doi:10.1186/1479-5876-11-143. Epub 2013/06/14; PubMed PMID: 23758736; PubMed Central PMCID: PMCPmc3688296
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321. doi:10.1038/nature07039. Epub 2008/05/16; PubMed PMID: 18480812
Ha X, Yin Q, Dong F, Jia Q, Lv T (2010) Study on bone marrow mesenchymal stem cells transfected with adenovirus hepatocyte growth factor gene promoting wounds repair in diabetic rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi = Zhongguo Xiufu Chongjian Waike Zazhi = Chin J Reparative Reconstr Surg 24(12):1520–1524. Epub 2011/01/26. PubMed PMID: 21261106
Heng MC (2011) Wound healing in adult skin: aiming for perfect regeneration. Int J Dermatol 50(9):1058–1066. doi:10.1111/j.1365-4632.2011.04940.x. Epub 2011/12/01; PubMed PMID: 22126865
Hocevar BA, Brown TL, Howe PH (1999) TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 18(5):1345–1356. doi:10.1093/emboj/18.5.1345. Epub 1999/03/04; PubMed PMID: 10064600; PubMed Central PMCID: PMCPmc1171224
Hostetler SG, Xiang H, Gupta S, Sen C, Gordillo JM (2006) Discharge patterns of injury-related hospitalizations with an acute wound in the United States. Wounds 18(12):340–351
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W (2001) Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105(4):533–545. Epub 2001/05/24. PubMed PMID: 11371349
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R et al (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102(4):1548–1549. doi:10.1182/blood-2003-04-1291. Epub 2003/08/06; PubMed PMID: 12900350
Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ et al (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11(12):1351–1354. doi:10.1038/nm1328. Epub 2005/11/17; PubMed PMID: 16288281
Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE et al (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447(7142):316–320. doi:10.1038/nature05766. Epub 2007/05/18; PubMed PMID: 17507982
Itoh M, Kiuru M, Cairo MS, Christiano AM (2011) Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci U S A 108(21):8797–8802. doi:10.1073/pnas.1100332108. Epub 2011/05/11; PubMed PMID: 21555586; PubMed Central PMCID: PMCPmc3102348
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28(1):142–150. doi:10.1016/j.biotechadv.2009.11.001. Epub 2009/11/17; PubMed PMID: 19913083
Jeon O, Alsberg E (2013) Regulation of stem cell fate in a three-dimensional micropatterned dual-crosslinked hydrogel system. Adv Funct Mater 23(38):4765–4775. doi:10.1002/adfm.201300529. Epub 2014/03/01; PubMed PMID: 24578678; PubMed Central PMCID: PMCPmc3933204
Jeschke MG, Rose C, Angele P, Fuchtmeier B, Nerlich MN, Bolder U (2004) Development of new reconstructive techniques: use of Integra in combination with fibrin glue and negative-pressure therapy for reconstruction of acute and chronic wounds. Plast Reconstr Surg 113(2):525–530. doi:10.1097/01.prs.0000100813.39746.5a. Epub 2004/02/06; PubMed PMID: 14758212
Jeschke MG, Patsouris D, Stanojcic M, Abdullahi A, Rehou S, Pinto R et al (2015) Pathophysiologic response to burns in the elderly. Biomedicine 2(10):1536–1548. doi:10.1016/j.ebiom.2015.07.040. PubMed PMID: 26629550; PubMed Central PMCID: PMC4634201
Kamolz LP, Kolbus A, Wick N, Mazal PR, Eisenbock B, Burjak S et al (2006) Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under in vitro conditions. Burns J Int Soc Burn Inj 32(1):16–19. doi:10.1016/j.burns.2005.08.020. Epub 2005/12/22; PubMed PMID: 16368194
Karagoz H, Yuksel F, Ulkur E, Evinc R (2009) Comparison of efficacy of silicone gel, silicone gel sheeting, and topical onion extract including heparin and allantoin for the treatment of postburn hypertrophic scars. Burns J Int Soc Burn Inj 35(8):1097–1103. doi:10.1016/j.burns.2009.06.206. Epub 2009/09/22; PubMed PMID: 19766399
Karlsson L, Bondjers C, Betsholtz C (1999) Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 126(12):2611–2621. Epub 1999/05/20. PubMed PMID: 10331973
Kato Y, Iwata T, Morikawa S, Yamato M, Okano T, Uchigata Y (2015) Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes 64(8):2723–2734. doi:10.2337/db14-1133. Epub 2015/03/22; PubMed PMID: 25795216
Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A et al (2007) Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 212(3):702–709. doi:10.1002/jcp.21068. Epub 2007/05/05; PubMed PMID: 17477371
Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D et al (2012) Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract 66(4):384–393. doi:10.1111/j.1742-1241.2011.02886.x. Epub 2012/01/31; PubMed PMID: 22284892
Knudtzon S (1974) In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood 43(3):357–361. Epub 1974/03/01. PubMed PMID: 4811820
Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT (2013) Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng A 19(5–6):738–747. doi:10.1089/ten.TEA.2012.0480. Epub 2012/10/18; PubMed PMID: 23072446; PubMed Central PMCID: PMCPmc3566655
Landsman A, Taft D, Riemer K (2009) The role of collagen bioscaffolds, foamed collagen, and living skin equivalents in wound healing. Clin Podiatr Med Surg 26(4):525–533. doi:10.1016/j.cpm.2009.08.012. Epub 2009/09/26; PubMed PMID: 19778686
Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I et al (2007) New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med 2(5):785–794. doi:10.2217/17460751.2.5.785. Epub 2007/10/03; PubMed PMID: 17907931
Le H, Kleinerman R, Lerman OZ, Brown D, Galiano R, Gurtner GC et al (2008) Hedgehog signaling is essential for normal wound healing. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 16(6):768–773. doi:10.1111/j.1524-475X.2008.00430.x. Epub 2009/01/09; PubMed PMID: 19128247
Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ et al (2012) Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J: Off J Jpn Circ Soc 76(7):1750–1760. Epub 2012/04/14. PubMed PMID: 22498564
Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC (2003) Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162(1):303–312. doi:10.1016/s0002-9440(10)63821-7. Epub 2003/01/01; PubMed PMID: 12507913; PubMed Central PMCID: PMCPmc1851127
Liu Y, Liu Y, Wang P, Tian H, Ai J, Liu Y et al (2014) Autologous bone marrow stem cell transplantation for the treatment of postoperative hand infection with a skin defect in diabetes mellitus: a case report. Oncol Lett 7(6):1857–1862. doi:10.3892/ol.2014.1998. Epub 2014/06/17; PubMed PMID: 24932248; PubMed Central PMCID: PMCPmc4049741
Lough DM, Yang M, Blum A, Reichensperger JD, Cosenza NM, Wetter N et al (2014) Transplantation of the LGR6+ epithelial stem cell into full-thickness cutaneous wounds results in enhanced healing, nascent hair follicle development, and augmentation of angiogenic analytes. Plast Reconstr Surg 133(3):579–590. doi:10.1097/prs.0000000000000075. Epub 2014/02/28; PubMed PMID: 24572851
Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S et al (2011) Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 92(1):26–36. doi:10.1016/j.diabres.2010.12.010. Epub 2011/01/11; PubMed PMID: 21216483
Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M et al (2010) Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 18(5):506–513. doi:10.1111/j.1524-475X.2010.00616.x. Epub 2010/09/16; PubMed PMID: 20840520
Ma D, Kua JE, Lim WK, Lee ST, Chua AW (2015) In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy 17(8):1036–1051. doi:10.1016/j.jcyt.2015.04.001. Epub 2015/05/20; PubMed PMID: 25981558
Macintyre L, Baird M (2006) Pressure garments for use in the treatment of hypertrophic scars – a review of the problems associated with their use. Burns J Int Soc Burn Inj 32(1):10–15. doi:10.1016/j.burns.2004.06.018. Epub 2006/01/18; PubMed PMID: 16413399
Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH (2011) Macrophages in skin injury and repair. Immunobiology 216(7):753–762. doi:10.1016/j.imbio.2011.01.001. Epub 2011/02/02; PubMed PMID: 21281986
Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA (2005) Diabetic neuropathic foot ulcers and amputation. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 13(3):230–236. doi:10.1111/j.1067-1927.2005.130303.x. Epub 2005/06/15; PubMed PMID: 15953040
Marino G, Moraci M, Armenia E, Orabona C, Sergio R, De Sena G et al (2013) Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 185(1):36–44. doi:10.1016/j.jss.2013.05.024. Epub 2013/06/19; PubMed PMID: 23773718
Martin P, Nunan R (2015) Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 173(2):370–378. doi:10.1111/bjd.13954. Epub 2015/07/16; PubMed PMID: 26175283; PubMed Central PMCID: PMCPmc4671308
Martinez-Ferrer M, Afshar-Sherif AR, Uwamariya C, de Crombrugghe B, Davidson JM, Bhowmick NA (2010) Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am J Pathol 176(1):98–107. doi:10.2353/ajpath.2010.090283. Epub 2009/12/05; PubMed PMID: 19959810; PubMed Central PMCID: PMCPmc2797873
Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA et al (2012) Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489(7415):257–262. doi:10.1038/nature11393. Epub 2012/09/04; PubMed PMID: 22940863
Mast BA, Schultz GS (1996) Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 4(4):411–420. doi:10.1046/j.1524-475X.1996.40404.x. Epub 1996/10/01; PubMed PMID: 17309691
Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA (2012) Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 1(2):142–149. doi:10.5966/sctm.2011-0018. Epub 2012/12/01; PubMed PMID: 23197761; PubMed Central PMCID: PMCPmc3659685
McGuckin C, Forraz N, Baradez MO, Basford C, Dickinson AM, Navran S et al (2006) Embryonic-like stem cells from umbilical cord blood and potential for neural modeling. Acta Neurobiol Exp 66(4):321–329. Epub 2007/02/03. PubMed PMID: 17269167
Mehanna RA, Nabil I, Attia N, Bary AA, Razek KA, Ahmed TA et al (2015) The effect of bone marrow-derived mesenchymal stem cells and their conditioned media topically delivered in fibrin glue on chronic wound healing in rats. Biomed Res Int 2015:846062. doi:10.1155/2015/846062. Epub 2015/08/04; PubMed PMID: 26236740; PubMed Central PMCID: PMCPmc4508387
Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L et al (2004) Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32(1):112–122. Epub 2004/02/18. PubMed PMID: 14964727
Mendez JJ, Ghaedi M, Sivarapatna A, Dimitrievska S, Shao Z, Osuji CO et al (2015) Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials 40:61–71. doi:10.1016/j.biomaterials.2014.11.011. Epub 2014/12/01; PubMed PMID: 25433608; PubMed Central PMCID: PMCPmc4268422
Meruane MA, Rojas M, Marcelain K (2012) The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg 130(1):53–63. doi:10.1097/PRS.0b013e3182547e04. Epub 2012/03/16; PubMed PMID: 22418720
Morasso MI, Tomic-Canic M (2005) Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell Auspices Eur Cell Biol Organ 97(3):173–183. doi:10.1042/bc20040098. Epub 2005/02/18; PubMed PMID: 15715523; PubMed Central PMCID: PMCPmc1283090
Moriyama M, Durham AD, Moriyama H, Hasegawa K, Nishikawa S, Radtke F et al (2008) Multiple roles of Notch signaling in the regulation of epidermal development. Dev Cell 14(4):594–604. doi:10.1016/j.devcel.2008.01.017. Epub 2008/04/16; PubMed PMID: 18410734
Mustoe TA, O’Shaughnessy K, Kloeters O (2006) Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 117(7 Suppl):35s–41s. doi:10.1097/01.prs.0000225431.63010.1b. Epub 2006/06/27; PubMed PMID: 16799373
Nan W, Liu R, Chen H, Xu Z, Chen J, Wang M et al (2015) Umbilical cord mesenchymal stem cells combined with a collagen fibrin double-layered membrane accelerates wound healing. Wounds 27(5):134–140. Epub 2015/05/13. PubMed PMID: 25965183
National Diabetes Statistical Report [Internet] (2014) Available from: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM (2003) Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A 100(Suppl 1):11873–11880. doi:10.1073/pnas.1834202100. Epub 2003/08/15; PubMed PMID: 12917489; PubMed Central PMCID: PMCPmc304101
Okuse T, Chiba T, Katsuumi I, Imai K (2005) Differential expression and localization of WNTs in an animal model of skin wound healing. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 13(5):491–497. doi:10.1111/j.1067-1927.2005.00069.x. Epub 2005/09/24; PubMed PMID: 16176457
Okuyama R, Tagami H, Aiba S (2008) Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci 49(3):187–194. doi:10.1016/j.jdermsci.2007.05.017. Epub 2007/07/13; PubMed PMID: 17624739
Oshima H, Inoue H, Matsuzaki K, Tanabe M, Kumagai N (2002) Permanent restoration of human skin treated with cultured epithelium grafting – wound healing by stem cell based tissue engineering. Hum Cell 15(3):118–128. Epub 2003/04/22. PubMed PMID: 12703542
Outtz HH, Wu JK, Wang X, Kitajewski J (2010) Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol (Baltimore, MD: 1950) 185(7):4363–4373. doi:10.4049/jimmunol.1000720. Epub 2010/08/27; PubMed PMID: 20739676; PubMed Central PMCID: PMCPmc3887523
Owens P, Han G, Li AG, Wang XJ (2008) The role of Smads in skin development. J Invest Dermatol 128(4):783–790. doi:10.1038/sj.jid.5700969. Epub 2008/03/14; PubMed PMID: 18337711
Pastar I, Stojadinovic O, Krzyzanowska A, Barrientos S, Stuelten C, Zimmerman K et al (2010) Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med (Cambridge, Mass) 16(3–4):92–101. doi:10.2119/molmed.2009.00149. Epub 2010/01/14; PubMed PMID: 20069132; PubMed Central PMCID: PMCPmc2804290
Pelizzo G, Avanzini MA, Icaro Cornaglia A, Osti M, Romano P, Avolio L et al (2015) Mesenchymal stromal cells for cutaneous wound healing in a rabbit model: pre-clinical study applicable in the pediatric surgical setting. J Transl Med 13:219. doi:10.1186/s12967-015-0580-3. Epub 2015/07/15; PubMed PMID: 26152232; PubMed Central PMCID: PMCPmc4495634
Puolakkainen PA, Reed MJ, Gombotz WR, Twardzik DR, Abrass IB, Sage HE (1995) Acceleration of wound healing in aged rats by topical application of transforming growth factor-beta(1). Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 3(3):330–339. doi:10.1046/j.1524-475X.1995.t01-1-30314.x. Epub 1995/07/01; PubMed PMID: 17173560
Puzey G (2002) The use of pressure garments on hypertrophic scars. J Tissue Viability 12(1):11–15. Epub 2002/03/13. PubMed PMID: 11887386
Reckhenrich AK, Kirsch BM, Wahl EA, Schenck TL, Rezaeian F, Harder Y et al (2014) Surgical sutures filled with adipose-derived stem cells promote wound healing. PLoS ONE 9(3):e91169. doi:10.1371/journal.pone.0091169. Epub 2014/03/15; PubMed PMID: 24625821; PubMed Central PMCID: PMCPmc3953386
Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M et al (2007) Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 119(5):1409–1422. doi:10.1097/01.prs.0000256047.47909.71. discussion 23-–4. Epub 2007/04/07; PubMed PMID: 17415234
Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC et al (2005) Spontaneous human adult stem cell transformation. Cancer Res 65(8):3035–3039. doi:10.1158/0008-5472.can-04-4194. Epub 2005/04/19; PubMed PMID: 15833829
Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J et al (2012) Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 33(1):80–90. doi:10.1016/j.biomaterials.2011.09.041. Epub 2011/10/04; PubMed PMID: 21963148; PubMed Central PMCID: PMCPmc3997302
Sabapathy V, Sundaram B, Sreelakshmi VM, Mankuzhy P, Kumar S (2014) Human Wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS ONE 9(4):e93726. doi:10.1371/journal.pone.0093726. Epub 2014/04/17; PubMed PMID: 24736473; PubMed Central PMCID: PMCPmc3988008
Salem HK, Thiemermann C (2010) Mesenchymal stromal cells: current understanding and clinical status. Stem Cells (Dayton, Ohio) 28(3):585–596. doi:10.1002/stem.269. Epub 2009/12/08; PubMed PMID: 19967788; PubMed Central PMCID: PMCPmc2962904
Sanmano B, Mizoguchi M, Suga Y, Ikeda S, Ogawa H (2005) Engraftment of umbilical cord epithelial cells in athymic mice: in an attempt to improve reconstructed skin equivalents used as epithelial composite. J Dermatol Sci 37(1):29–39. doi:10.1016/j.jdermsci.2004.10.008. Epub 2004/12/28; PubMed PMID: 15619432
Sato M (2006) Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol 86(4):300–307. doi:10.2340/00015555-0101. Epub 2006/07/29; PubMed PMID: 16874413
Schreier T, Degen E, Baschong W (1993) Fibroblast migration and proliferation during in vitro wound healing. A quantitative comparison between various growth factors and a low molecular weight blood dialysate used in the clinic to normalize impaired wound healing. Res Exp Med Z Gesamten Exp Med Einschliesslich Exp Chir 193(4):195–205. Epub 1993/01/01. PubMed PMID: 8235072
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 17(2):153–162. doi:10.1111/j.1524-475X.2009.00466.x. Epub 2009/03/27; PubMed PMID: 19320882
Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K et al (2003) Wound bed preparation: a systematic approach to wound management. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 11(Suppl 1):S1–28. Epub 2003/03/26; PubMed PMID: 12654015
Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM (2011) Dynamic reciprocity in the wound microenvironment. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 19(2):134–148. doi:10.1111/j.1524-475X.2011.00673.x. Epub 2011/03/03; PubMed PMID: 21362080; PubMed Central PMCID: PMCPmc3051353
Sebastiano V, Zhen HH, Haddad B, Bashkirova E, Melo SP, Wang P et al (2014) Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med 6(264):264ra163. doi:10.1126/scitranslmed.3009540. Epub 2014/11/28; PubMed PMID: 25429056; PubMed Central PMCID: PMCPmc4428910
Seibold JR, Uitto J, Dorwart BB, Prockop DJ (1985) Collagen synthesis and collagenase activity in dermal fibroblasts from patients with diabetes and digital sclerosis. J Lab Clin Med 105(6):664–667. Epub 1985/06/01; PubMed PMID: 2987379
Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK et al (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17(6):763–771. doi:10.1111/j.1524-475X.2009.00543.x. PubMed PMID: 19903300; PubMed Central PMCID: PMC2810192
Shen Y, Dai L, Li X, Liang R, Guan G, Zhang Z et al (2014) Epidermal stem cells cultured on collagen-modified chitin membrane induce in situ tissue regeneration of full-thickness skin defects in mice. PLoS ONE 9(2):e87557. doi:10.1371/journal.pone.0087557. Epub 2014/02/12; PubMed PMID: 24516553; PubMed Central PMCID: PMCPmc3917838
Shi S, Jia S, Liu J, Chen G (2015) Accelerated regeneration of skin injury by co-transplantation of mesenchymal stem cells from Wharton’s jelly of the human umbilical cord mixed with microparticles. Cell Biochem Biophys 71(2):951–956. doi:10.1007/s12013-014-0292-y. Epub 2014/11/06; PubMed PMID: 25370297
Signorini M, Clementoni MT (2007) Clinical evaluation of a new self-drying silicone gel in the treatment of scars: a preliminary report. Aesthet Plast Surg 31(2):183–187. doi:10.1007/s00266-005-0122-0. Epub 2006/12/16; PubMed PMID: 17171514
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746. doi:10.1056/nejm199909023411006. Epub 1999/09/02; PubMed PMID: 10471461
Singh N, Armstrong DG, Lipsky BA (2005) Preventing foot ulcers in patients with diabetes. JAMA 293(2):217–228. doi:10.1001/jama.293.2.217. Epub 2005/01/13; PubMed PMID: 15644549
Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N et al (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science (New York, NY) 327(5971):1385–1389. doi:10.1126/science.1184733. Epub 2010/03/13; PubMed PMID: 20223988
Snyder RJ (2005) Treatment of nonhealing ulcers with allografts. Clin Dermatol 23(4):388–395. doi:10.1016/j.clindermatol.2004.07.020. Epub 2005/07/19; PubMed PMID: 16023934
Soo C, Beanes SR, Hu FY, Zhang X, Dang C, Chang G et al (2003) Ontogenetic transition in fetal wound transforming growth factor-beta regulation correlates with collagen organization. Am J Pathol 163(6):2459–2476. Epub 2003/11/25. PubMed PMID: 14633618; PubMed Central PMCID: PMCPmc1892380
St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS et al (1998) Sonic hedgehog signaling is essential for hair development. Curr Biol: CB 8(19):1058–1068. Epub 1998/10/13. PubMed PMID: 9768360
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21(14):2724–2752. doi:10.1089/scd.2011.0722. Epub 2012/04/04; PubMed PMID: 22468918
Strong AL, Bowles AC, MacCrimmon CP, Frazier TP, Lee SJ, Wu X et al (2015) Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem Cells Transl Med 4(6):632–642. doi:10.5966/sctm.2014-0235. Epub 2015/04/23; PubMed PMID: 25900728; PubMed Central PMCID: PMCPmc4449094
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024. Epub 2006/08/15; PubMed PMID: 16904174
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. doi:10.1016/j.cell.2007.11.019. Epub 2007/11/24; PubMed PMID: 18035408
Tong C, Hao H, Xia L, Liu J, Ti D, Dong L et al (2015) Hypoxia pretreatment of bone marrow-derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc. doi:10.1111/wrr.12369. Epub 2015/10/16; PubMed PMID: 26463737
Torsvik A, Rosland GV, Svendsen A, Molven A, Immervoll H, McCormack E et al (2010) Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track – letter. Cancer Res 70(15):6393–6396. doi:10.1158/0008-5472.can-10-1305. Epub 2010/07/16; PubMed PMID: 20631079
Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F et al (1999) Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 7(6):442–452. Epub 2000/01/13; PubMed PMID: 10633003
Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR (2013) Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int 2013:385641. doi:10.1155/2013/385641. Epub 2013/05/09; PubMed PMID: 23653894; PubMed Central PMCID: PMCPmc3638655
Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M et al (2004) Defining the epithelial stem cell niche in skin. Science (New York, NY) 303(5656):359–363. doi:10.1126/science.1092436. Epub 2003/12/13; PubMed PMID: 14671312; PubMed Central PMCID: PMCPmc2405920
Uysal CA, Tobita M, Hyakusoku H, Mizuno H (2014) The effect of bone-marrow-derived stem cells and adipose-derived stem cells on wound contraction and epithelization. Adv Wound Care 3(6):405–413. doi:10.1089/wound.2014.0539. Epub 2014/06/19; PubMed PMID: 24940554; PubMed Central PMCID: PMCPmc4048969
Vagnozzi AN, Reiter JF, Wong SY (2015) Hair follicle and interfollicular epidermal stem cells make varying contributions to wound regeneration. Cell Cycle (Georgetown, Tex) 14(21):3408–3417. doi:10.1080/15384101.2015.1090062. Epub 2015/09/24; PubMed PMID: 26398918
Varga J, Rosenbloom J, Jimenez SA (1987) Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J 247(3):597–604. Epub 1987/11/01. PubMed PMID: 3501287; PubMed Central PMCID: PMCPmc1148454
Vojtassak J, Danisovic L, Kubes M, Bakos D, Jarabek L, Ulicna M et al (2006) Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neurol Endocrinol Lett 27(Suppl 2):134–137. Epub 2006/12/13. PubMed PMID: 17159798
Watt FM, Fujiwara H (2011) Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol 3(4):a005124. doi:10.1101/cshperspect.a005124. Epub 2011/03/29; PubMed PMID: 21441589; PubMed Central PMCID: PMCPmc3062212
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870. doi:10.1152/physrev.00031.2002. Epub 2003/07/05; PubMed PMID: 12843410
Willital GH, Heine H (1994) Efficacy of Contractubex gel in the treatment of fresh scars after thoracic surgery in children and adolescents. Int J Clin Pharmacol Res 14(5–6):193–202. Epub 1994/01/01. PubMed PMID: 7672876
Wilson A, Butler PE, Seifalian AM (2011) Adipose-derived stem cells for clinical applications: a review. Cell Prolif 44(1):86–98. doi:10.1111/j.1365-2184.2010.00736.x. Epub 2011/01/05; PubMed PMID: 21199013
Wu Y, Wang J, Scott PG, Tredget EE (2007a) Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc 15(Suppl 1):S18–S26. doi:10.1111/j.1524-475X.2007.00221.x. Epub 2008/09/10; PubMed PMID: 17727462
Wu Y, Chen L, Scott PG, Tredget EE (2007b) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25(10):2648–2659. Epub 2007/07/07. doi: 2007-0226 [pii] 1634/stemcells.2007-0226. PubMed PMID: 17615264
Wu X, Wang G, Tang C, Zhang D, Li Z, Du D et al (2011) Mesenchymal stem cell seeding promotes reendothelialization of the endovascular stent. J Biomed Mater Res A 98(3):442–449. doi:10.1002/jbm.a.33133. Epub 2011/06/11; PubMed PMID: 21661093
Wu Y, Peng Y, Gao D, Feng C, Yuan X, Li H et al (2015) Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-beta3-dependent activation. Int J Low Extrem Wounds 14(1):50–62. doi:10.1177/1534734614568373. Epub 2015/04/11; PubMed PMID: 25858630
Yang R, Zheng Y, Burrows M, Liu S, Wei Z, Nace A et al (2014) Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nat Commun 5:3071. doi:10.1038/ncomms4071. Epub 2014/01/29; PubMed PMID: 24468981; PubMed Central PMCID: PMCPmc4049184
Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y et al (2008) Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 121(3):860–877. doi:10.1097/01.prs.0000299922.96006.24. Epub 2008/03/05; PubMed PMID: 18317135
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY) 318(5858):1917–1920. doi:10.1126/science.1151526. Epub 2007/11/22; PubMed PMID: 18029452
Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J (2008) Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng A 14(6):1025–1036. doi:10.1089/ten.tea.2007.0289. Epub 2008/05/15; PubMed PMID: 18476809
Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG (2015) Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther 6:145. doi:10.1186/s13287-015-0133-y. Epub 2015/08/19; PubMed PMID: 26282394; PubMed Central PMCID: PMCPmc4539671
Zheng K, Wu W, Yang S, Huang L, Chen J, Gong C et al (2015) Bone marrow mesenchymal stem cell implantation for the treatment of radioactivity induced acute skin damage in rats. Mol Med Rep 12(5):7065–7071. doi:10.3892/mmr.2015.4270. Epub 2015/09/02; PubMed PMID: 26323987
Zimmerlin L, Rubin JP, Pfeifer ME, Moore LR, Donnenberg VS, Donnenberg AD (2013) Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy 15(1):102–108. doi:10.1016/j.jcyt.2012.10.009. Epub 2012/12/25; PubMed PMID: 23260090; PubMed Central PMCID: PMCPmc4159959
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295. doi:10.1091/mbc.E02-02-0105. Epub 2002/12/12; PubMed PMID: 12475952; PubMed Central PMCID: PMCPmc138633
Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R (2000) Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 49(9):1451–1458. Epub 2000/09/02. PubMed PMID: 10969828
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Yousuf, Y., Amini-Nik, S., Jeschke, M.G. (2017). Use of Stem Cells in Acute and Complex Wounds. In: Pham, P. (eds) Pancreas, Kidney and Skin Regeneration. Stem Cells in Clinical Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-55687-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-55687-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55686-4
Online ISBN: 978-3-319-55687-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)