Abstract
Background
Critical limb ischemia (CLI) caused by peripheral arterial disease is associated with significant morbidity and mortality. This condition is associated with a 30 % amputation rate as well as mortality levels which might be as high as 25 %. There is no pharmacological therapy available, but several reports have suggested that mesenchymal stem cells (MSCs) may be a useful therapeutic option.
Methods
This study, done at a university hospital, evaluated 13 patients for a phase I trial to investigate the safety and efficacy of intra-arterial MSCs in CLI patients. Eight patients with ten affected limbs were recruited for the study. As two patients (three limbs) died of ischemic cardiac events during the 6-month follow-up period, seven limbs were finally evaluated for the study.
Results
There was significant pain relief. Visual analog scale (VAS) scores decreased from 2.29 ± 0.29 to 0.5 ± 0.34 (p < 0.05), ankle brachial pressure index (ABPI) increased significantly from 0.56 ± 0.02 to 0.67 ± 0.021 (p < 0.01), and transcutaneous oxygen pressure (TcPO2) also increased significantly in the foot from 13.57 ± 3.63 to 38 ± 3.47. Similar improvement was seen in the leg as well as the thigh. There was 86 % limb salvage and six of seven ulcers showed complete or partial healing.
Conclusion
It was concluded that intra-arterial MSCs could be safely administered to patients with CLI and was associated with significant therapeutic benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite major advances in the management of peripheral vascular disease, a substantial number of patients end up with amputations. Most of these patients have critical limb ischemia (CLI), which is diagnosed by the characteristic symptoms of rest pain, ulcers, cool limbs, and absent distal peripheral pulses. Investigations that confirm the condition include measurement of the ankle brachial index (ABI) (<60 %), low ankle pressures (<50 mmHg) and reduced transcutaneous oxygen (TcPO2) (<30–50 mmHg). Studies of a large cohort of patients with peripheral arterial disease have reported that a small but significant number, 1–3 %, suffer from CLI [1]. These patients have a miserable prognosis since conventional revascularization is possible for only <50 % of such patients and 25 % die within 1 year. Major amputation rates are about 30 % in the same period [2].
As conventional treatment protocols, including attempts at limb revascularization by surgical means, invasive radiology, or pharmacological intervention, have essentially failed these patients, clinicians have turned to attempts to revascularize these limbs by using progenitor cells which might salvage the limb. As far back as 1787, angiogenesis was identified and described by John Hunter [3] when he thus named new blood vessels in deer that had shed their antlers. Subsequent research has identified three methods by which the body can regrow blood vessels: angiogenesis, defined as the sprouting of new vessels from existing blood vessels; vasculogenesis or the de novo differentiation of mature endothelial cells from endothelial progenitor cells; and arteriogenesis, which is the remodeling of existing small vessels to form collaterals. It has long been noted that ischemic muscles secrete angiogenic factors in response to hypoxia, which leads to new vessel formation. One such factor is the vascular endothelial growth factor (VGEF) and another such factor is the cytokine fibroblast growth factor (FGF-1) [4].
It has been shown that progenitor cells released from bone marrow (BM) in response to ischemic stimuli circulate in the blood and home in on sites where new blood vessel formation is required. This has been confirmed by animal studies and there have been several clinical trials that have also suggested that varieties of progenitor cells may be useful in relieving symptoms and helping to save limbs in CLI [5–7].
This study aimed to prove the safety of allogeneic mesenchymal stem cells delivered by the intra-arterial route in patients with CLI and also to investigate the efficacy of these cells using standard efficacy parameters.
Material and methods
Patients and study design
This study was a single-center phase I open-label prospective study to investigate the effects of allogeneic BM MSC therapy in CLI. In brief, the patients included in the trial were those patients who had vascular disease of the lower extremities which was untreatable by standard surgical or interventional methods or such treatment had failed. To establish that they were essentially untreatable, the patients underwent Doppler examination, magnetic resonance angiography (MRA), and conventional angiography as indicated.
The study was approved by the Ethics Committee of the University of Malaya (reference no. 732.17, dated 31 July 2009). The study was included in the University of Malaya database of research studies. All patients were carefully counseled by the investigating team and informed consent was obtained. The consent form had been previously approved by the Ethics Committee. The study conformed to the Declaration of Helsinki and to the Malaysian Guidelines for Good Clinical Practice (MOH, 2004). The study was supported by a grant from the University of Malaya. Stempeutics Research Malaysia provided the allogeneic MSCs for the study free of cost.
BM MSCs
MSCs are present as a rare population in BM, representing 0.001–0.01 % of the nucleated cells, but they can rapidly grow in culture without losing their stemness. MSCs can be expanded in vitro ≥2 million-fold and retain their ability to differentiate into several mesenchymal lineages [8]. Phenotypically, MSCs express the markers CD105, CD73, CD44, CD90 (Thy-1), CD71, and Stro-1, as well as the adhesion molecules CD106 (VCAM-1), CD166 (ALCAM), ICAM-1, and CD29. Adult human MSCs do not express the hematopoietic markers CD45, CD34, CD14, or CD11. They also do not express CD80, CD86, and CD40 (costimulatory molecules), or CD31 (PECAM-1), CD18 (LCAM), and CD56 (NCAM-1) (adhesion molecules) [9, 10].
Objectives of the study
The primary objective of the study was to assess the safety following a single dose of allogeneic BM-derived MSCs administered by intra-arterial injection in CLI patients. Safety was measured by the following parameters: (1) type of adverse events AE(s), number of AE(s), and proportion of patients with AE(s); (2) assessment of clinical laboratory parameters; (3) assessment of vital signs; and (4) assessment of electrocardiogram (ECG) parameters. The secondary objective was to explore the efficacy of BM MSCs by assessing the alleviation the symptoms of CLI. Efficacy was measured by (1) relief of the rest pain; (2) healing of all necroses and ulcerations in the target limb, assessed by independent physician and documented by photography at the end of 6 months; (3) increase in transcutaneous partial oxygen pressure (TcPO2); (4) ankle brachial pressure index (ABPI) measured by Doppler; (5) vasculogenesis by magnetic resonance angiogram; and (6) prevention of amputation of the target limb within study period.
Inclusion criteria
The study included patients from both sexes between 18 and 80 years of age with established CLI due to thromboangiitis obliterans (TAO) or atherosclerosis, clinically and hemodynamically confirmed to be Rutherford classification II-4, III-5, or III-6. They were confirmed as noncandidates for interventional or surgical revascularization. All were confirmed as no-option patients by a vascular surgeon who examined the patient clinically and also reviewed the MRA. All patients had infrapopliteal disease that could not be treated by surgical or interventional methods. Patients were required to have normal hepatic and renal function and controlled diabetes (if a diabetic).
Exclusion criteria
Patients who were suitable for surgical or interventional revascularization were excluded. In addition, a history of infectious disease (HIV, hepatitis B, and hepatitis C), a history of stroke or myocardial infarction within the last 3 months or concurrent serious illness, and documented terminal illness with an expected life span of less than 3 months also excluded the patient from the study.
BM MSC isolation
The cells were isolated from BM aspirates from unmatched, unrelated healthy donors. The donors were volunteers who agreed to participate without any payment. They are being regularly followed up. Screening of the donor was carried out as per the 21 CFR 640 and FDA norms [11]. This includes a complete blood count, blood glucose levels, kidney and liver function tests, routine urine examination, electrocardiogram, echocardiography, and chest X-ray. The donors were also screened for HIV1 and 2, HBV, HCV, and syphilis. Mesenchymal stem cells were expanded and a donor cell bank established. The expanded cells were tested for endotoxins, mycoplasma, and sterility. After up-scaling, a production lot was established, the cells were tested again for mycoplasma, endotoxin, and sterility and infective markers. The production was done in a Current Good Manufacturing Practice (cGMP) certified laboratory in our facility at Kuala Lumpur. The facility was audited by the National Pharmaceutical Control Bureau (NPCB) of the Ministry of Health, Government of Malaysia.
Cell characterization
Mesenchymal stem cells were analyzed by flow cytometry using CD34, CD45, CD73, CD90, CD166 markers and the product was more than 80 % positive for CD73, CD90, and CD166 but less than 10 % positive for CD34 and CD45. This was done on a Guava Technologies flow cytometer, and the results were analyzed using Cytosoft ver. 5.2 (Guava Technologies, Hayward, CA, USA).
Cell delivery
Expanded mesenchymal stem cells were then frozen and stored in Plasmalyte-A (Baxter) containing 5 % human serum albumin and 10 % DMSO. Cells were aseptically filled and transferred into cryobags containing 200 million cells per bag in 15 ml of freezing medium. The product was cryopreserved and stored in liquid nitrogen vapor phase. On requisition from the site, the cells were thawed, washed with Dulbecco phosphate-buffered saline (DPBS), resuspended in 10 ml of Plasmalyte A, and aseptically filled a syringe. The syringe was delivered in an insulated box at 2–8 °C.
BM MSC therapy
The contralateral common femoral arteries were punctured under ultrasound guidance (a 8–12 MHz linear probe) using a 21G micropuncture needle (Micropuncture Introducer set, Cook Medical, Bloomington, IN). Once good arterial flow was achieved, a 40-cm-long 0.018-in. microwire was used to cannulate the artery and over which a 4F sheath was then placed in the artery. The cells were then slowly infused over 5 min through the sheath. In patients in whom the cells needed to be injected into both limbs, the 4F sheath was changed to a 60-cm 4F Cobra catheter. Using a 0.038-in. glide wire, the contralateral external iliac artery was cannulated and the cells were delivered in similar fashion. Once the Cobra catheter was withdrawn, the cells were injected through the 4F arterial sheath.
After the cells were delivered, the sheath or catheter was removed and hemostasis was secured by compression for 10 min. No formal angiography of the lower limbs was performed during the stem cell injection. Iodinated contrast (iopromide, Ultravist 300, Bayer, Wayne, NJ, USA) was used sparingly <30 ml) when the contralateral external iliac needed to be cannulated. The patients were advised not to ambulate for 3 h and avoid any strenuous activity for 24 h.
Delivery of the cells was successful in all patients. There were no complications related to vascular access or delivery of cells.
Cell dose
The dose used in this study was 2 × 106 cells/kg body weight. Previous studies using our MSCs showed that a single dose of up to 20 × 106 cells/kg body weight was well tolerated in the rat (unpublished observations). It was shown that the minimum lethal dose, the maximum lethal dose, and the median lethal dose was more than 20 × 106 cells/kg. One tenth of this dose was selected for dosing in humans. In a phase I/II study conducted by our group (ClinicalTrials.gov Identifier: NCT00883870), the 2 × 106 cells/kg body weight dose was well tolerated. A manuscript based on this study is under review.
Data and statistical analysis
Ulcer healing, amputations, and MRA findings are descriptively narrated. Rest pain scores, ABPI, and TcPO2 data are expressed as mean ± SEM. A paired t test was used to analyze the change from baseline in pain scores, ABPI, and TcPO2 values at different follow-up times using GraphPad InStat ver. 3 for Windows 95 (GraphPad Software, San Diego, CA, USA). A p value of ≤0.05 was considered statistically significant for all the tests. No statistical test was applied for ulcer healing, analysis of amputations, and MRA.
Results
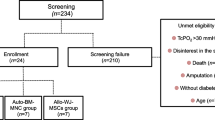
Thirteen patients (15 limbs) were screened for inclusion in the study in the period between November 2009 and July 2011. Of them, five patients (five limbs) were excluded because they did not meet the inclusion criteria (Fig. 1). They were followed up for at least 6 months after injection. A total of eight patients were enrolled for the study, of which two patients had both their lower limbs included in the study so ten limbs were considered for evaluation. Seven limbs were Rutherford classification III-5/6 and three limbs were Rutherford III-4. One patient had undergone a femoral-popliteal bypass that failed. The rest received medical care alone for their CLI and standard care for their ulcers if present.
One patient died at the 1-month follow-up (this patient had both limbs involved in the study) and another patient died at the 3-month follow-up (this patient had one limb involved in the study) so data were available for seven limbs. One patient underwent amputation of the study limb at 1-month follow-up so measurements of six limbs were available until the 6-month follow-up. Physical examination and vital signs did not vary from baseline during the follow-up period. Hematological and biochemical tests did not reveal any abnormality during the follow-up period. The two deaths reported were due to myocardial ischemia. No other acute or delayed adverse effects were reported in these patients.
ABPI
ABPI improved in all the study limbs from baseline to 6 months. The mean ABPI rose from a mean (SEM) of 0.56 (±0.02) mmHg at baseline to 0.60 (±0.022) mmHg at 1 month, 0.62 (±0.031) mmHg at 3 months, and 0.67 (±0.021) mmHg (p < 0.01) at 6 months (Fig. 2a).
Different parameters analyzed after the treatment. a Change in ABPI from baseline to 6-month follow-up (mean ± standard error). b Change in VAS score from baseline to 6-month follow-up (mean ± standard error). c Changes in TcPO2 in the foot, calf, and thigh from baseline to 6-month follow-up (mean ± standard error). ABPI ankle brachial pressure index, VAS visual analog score. The ABPI increased and VAS scores decreased significantly (p < 0.01) at 6 months. TcPO2 also increased significantly in the foot (p < 0.01) as well as in the calf and thigh (p < 0.05) (Color figure online)
Pain analysis
The rest pain was measured by the visual analog score (VAS) at the commencement of the study and at the 1-, 3-, and 6-month follow-up visits. Of the six limbs that completed the 6-month follow-up, one did not experience any relief of pain. However, all the other patients had substantial pain relief. In fact, even the patients who died during follow-up had substantial pain relief. The mean baseline pain score (VAS) was 2.29 (±0.29), which improved to 1.14 (±0.26) (p < 0.01) at 1 month, 0.6 (±0.4) (p < 0.01) at 3 months, and 0.5 (±0.34) (p < 0.01) at 6 months (Fig. 2b).
TcPO2
The transcutaneous oxygen saturation was measured using a TCM400 monitor (Radiometer Medical ApS, Bronshoj, Denmark). The measurements were done at the foot, leg, and thigh of the affected limb at the commencement of the study and at 1 month, 3 months, and the completion of 6 months of follow-up. The mean TcPO2 of the foot rose from a mean of 13.57 (±3.63) mmHg at baseline to 24.86 (3.18) mmHg (p < 0.05) at 1 month, 32.6 (±4.41) mmHg at 3 months, and 38.33 (±3.47) mmHg (p < 0.05) at 6 months. The mean TcPO2 of the calf rose from a mean of 30.29 (±3.99) mmHg at baseline to 38.71 (±5.87) mmHg (p < 0.05) at 1 month, 47.00 (±9.82) mmHg at 3 months, and 49.17 (±6.68) mmHg (p < 0·01) at 6 months. The mean TcPO2 of the thigh rose from a mean of 50.43 (±1.91) mmHg at baseline to 53.00 (±2.44) mmHg (p < 0.05) at 1 month, 60.00 (±5.68) mmHg at 3 months, and 61.67 (±4.48) mmHg (p < 0·05) at 6 months (Fig. 2c).
Ulcer healing
Six limbs completed the 6-month follow-up period. Of them, four limbs suffered from ulcers at the time of presentation and all of them showed significant improvement of the ulcer. The observed improvement ranged from complete healing to about a 70 % reduction in the ulcer area (Fig. 3).
The primary goal of this clinical study was to demonstrate the safety of transplanting allogeneic hMSCs and also to demonstrate clinical improvement by, among others, ulcer healing. a, c, e, g Preimplantation ulcer status of different limbs. b, d, f, h Gross examination of various limb ulcers showing improvement after 6 months of cell injection. All ulcers either completely or partially healed
Limb salvage
In this study the amputation rate was 14 % at 6 months (1 of 7 patients). This compares to an amputation rate of 50–60 % per year in the general PAD population.
Discussion
To our knowledge this is the first study done in Southeast Asia that used allogeneic BM MSCs to treat CLI. The study was designed as an open-label prospective study because the main purpose of the study was to prove that this approach was feasible and that there were no short-term safety issues associated with the therapy. As a secondary objective, we also tried to assess the efficacy of the therapy using standard methods of measuring the improvement of blood supply to the critically ischemic limb. These were clinical parameters, including relief from pain and ulcer healing as well as limb salvage rates, that correlated with improvement of the blood supply. Objective measurements of the improvement of the blood supply, including ABPI, TcPO2, and angiography, were also carried out.
Two patients died during the course of the study. Both died of myocardial events which were documented. This is common in this group of patients as many of them have associated cardiovascular disease which often causes morbidity or death [12]. However, even those two patients as well as most (6/7) of the other limbs evaluated had relief of pain. This relief was statistically significant and clinically this is important as such patients are often difficult to treat by conventional means and severe pain can also drive such patients to suicide as one of the authors (AKD) has seen personally.
Cell therapy trials have, in general, reported fairly high levels of pain relief. This was true of the PROVASA trial [12] as well as several others [13–17]. While the VAS score is a proven outcome measure, the open-label design may have influenced the results [12]. Moreover, it has been pointed out that these patients, who have expectations for clinical benefit from an unproven therapeutic event, could have a placebo effect of 30–40 % with a subjective end point like rest pain [7].
All patients showed ulcer healing. This too is an important parameter as ischemic ulcers are notorious for their chronicity and are impervious to standard treatment methods. All ulcers healed during the period of study except two that showed an approximately 70 % decrease in ulcer diameter and depth. One limb was amputated during the course of the study. Thus, the limb salvage rate was approximately 86 %, which compares favorably with other similar studies. For instance, the TACT study [1] had a limb salvage rate of 71 % while the BM outcome study reported a salvage rate of 59 %. The PROVASA study had a similar limb salvage rate of 84 % [12].
The objective measurement indicators showed mixed results. The ABPI improved significantly; this improvement was evident at 3 months and the improvement was maintained at the 6-month follow-up. The question of ABPI has led to some debate in view of conflicting results obtained by various studies. It has been argued that the ABPI levels could be spuriously high in TAO patients [12] and that they correlate poorly with clinical outcome as measured by parameters like ulcer healing or limb salvage. In this study, however, we were able to demonstrate a significant improvement in ABPI.
Similarly, the TcPO2 levels showed a consistent improvement which was statistically significant. TcPO2 levels have been correlated to limb salvage levels and ulcer healing in previous studies and this corresponded well to our findings as well. Angiography, however, failed to demonstrate any significant improvement in collateral development. This was not unexpected as it has been shown that collateral formation is notoriously difficult to demonstrate and, in fact, one study abandoned angiography during its course due to such difficulties [7]. It is likely that the vasculogenesis that occurs is mainly in smaller vessels which do not show up in MRA. Some studies have been able to demonstrate collateral vessel formation using MRA and angiography [13, 15].
This is one of the few studies that have used allogeneic cells. We believe that this is extremely important in the treatment of CLI since using autologous cells has many limitations which are particularly important in this group of patients. CLI is basically an urgent condition that demands early relief which is not compatible with the delay associated with culturing autologous cells. Using an allogeneic cell bank as a source of cells for immediate treatment is important in such situations. It is also difficult to subject this sick patient population to the morbidity associated with BM aspiration. There is also reason to believe that BM from aged, sick patients is not as likely to achieve good results after injection as those from healthy donors [18].
Most studies in this field have used intramuscular injections of MSCs or other progenitor cells. This is one of the few studies that has used the intra-arterial route for cell delivery. The intra-arterial route has been shown to deliver the cells to the border zone of maximal ischemia. However, there is no preclinical data that shows this improves results. There are a few clinical studies that have used the intra-arterial route to deliver the cells. One such study that recruited 27 patients compared two groups: one that had a combined intra-arterial and intramuscular injection and one that had only intra-arterial injection [19]. The authors were unable to show any difference between the two groups. Another study that looked at the efficacy and safety of intra-arterial cells in diabetic patients with below-knee ischemia showed notable improvement after 6 months of follow-up [20]. A recently published Phase II study that used autologous BM showed improvement in TcPO2 as well as a much improved amputation-free survival rate [21].
Limitations of the study
This study has obvious limitations. The sample size was small and the deaths of two patients during the course of the study limited numbers even more. However, this is mitigated by the fact that the principal purpose of the study was achieved in that the procedure was shown to be safe since there were no procedure-related side effects, and no significant adverse events related to the cells were reported during the course of follow-up. It should be noted that the follow-up period was only 6 months, which is another limitation of the study. Longer follow-up periods are essential in evaluating the long-term effects of the cells.
Despite these limitations, the study has achieved its major objectives. These were to prove the feasibility of intra-arterial delivery of BM MSCs in patients with CLI. We believe that our technique of using ultrasound guidance as well as a micropuncture needle enabled us to deliver the cells without any morbidity. The allogeneic BM MSCs also did not cause any adverse reactions and were presumably able to influence the development of neoangiogenesis in the targeted vascular bed. It goes without saying that it is now necessary to do a randomized placebo-controlled trial in a larger sample size with a longer follow-up period to demonstrate the benefit that has been hinted at by this study. Only such a study can resolve all the issues related to the use of allogeneic BM MSCs in CLI.
Conclusions
This small open-label study suggests that intra-arterial administration of allogeneic BM MSCs may be a viable and useful option to treat no-option patients with CLI. The treatment is feasible and is likely to reduce symptoms, heal ulcers, and save limbs in this patient group. A large-scale randomized study is needed to prove this proposition. However, this study suggests that the intra-arterial use of allogeneic cells is likely to develop as a good option for a group of patients who are today essentially untreatable. A larger phase 2 trial is being planned by the same group to take the study forward.
References
Hirsch AT, Haskal ZJ, Hertzer NR et al (2006) Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113:e463–e654
Anon (1996) Critical limb ischaemia: management and outcome. Report of a National Survey: The Vascular Society of Great Britain and Ireland. Eur J Vasc Endovasc Surg 12:131–135
Watt SM, Athanassopoulos A, Harris AL et al (2010) Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface 7(Suppl 6):S731–S751
El Oakley RM, Seow KK, Tang TPL et al (2002) Whole bone marrow transplantation induces angiogenesis following acute ischemia. Redox Rep 7(4):215–218
Iba O, Matsubara H, Nozawa Y et al (2002) Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation 106(15):2019–2025
Kalka C, Masuda H, Takahashi T et al (2000) Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97(7):3422–3427
Murphy MP, Lawson JH, Rapp BM et al (2011) Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg 53:1565–1574
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Tse WT, Pendleton JD, Beyer WM et al (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75(3):389–397
Di Nicola M, Carlo-Stella C, Magni M et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
U.S. Food and Drug Administration, CFR Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=640. Accessed 5 Feb 2011
Walter DH, Krankenberg H, Balzer JO et al (2011) Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv 4:26–37
Tateishi-Yuyama E, Matsubara H, Murohara T et al (2002) Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360:427–435
Esato K, Hamano K, Li TS et al (2002) Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant 11:747–752
Miyamoto M, Yasutake M, Takano H et al (2004) Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transpl 13:429–437
Saigawa T, Kato K, Ozawa T et al (2004) Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J 68:1189–1193
Iafrati MD, Hallett JW, Geils G et al (2011) Early results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg 54(6):1650–1658
Levine JE, Harris RE, Loberiza FR et al (2003) A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 101(7):2476–2482
Van Tongeren RB, Hamming JF, Fibbe WE et al (2008) Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemia. J Cardiovas Surg 49:51–58
Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M et al (2011) Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transpl 20:1629–1639
Schiavetta A, Maione C, Botti C et al (2012) A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and Pietra Ligure evaluation of stem cells study. Stem Cells Trans Med 1:572–578
Acknowledgments
We acknowledge Stempeutics Research Malaysia (SRM) and University of Malaya (UM) for funding this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, A.K., Abdullah, B.J.J.B., Dhillon, S.S. et al. Intra-arterial Allogeneic Mesenchymal Stem Cells for Critical Limb Ischemia are Safe and Efficacious: Report of a Phase I Study. World J Surg 37, 915–922 (2013). https://doi.org/10.1007/s00268-012-1892-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1892-6