Abstract
Motile microorganisms such as the green Euglena gracilis use a number of external stimuli to orient in their environment. They respond to light with photophobic responses, photokinesis and phototaxis, all of which can result in accumulations of the organisms in suitable habitats. The light responses operate synergistically with gravitaxis, aerotaxis and other responses. Originally the microscopically obvious stigma was thought to be the photoreceptor, but later the paraxonemal body (PAB, paraflagellar body) has been identified as the light responsive organelle, located in the trailing flagellum inside the reservoir. The stigma can aid in light direction perception by shading the PAB periodically when the cell rotates helically in lateral light, but stigmaless mutants can also orient with respect to the light direction, and negative phototaxis does not need the presence of the stigma. The PAB is composed of dichroically oriented chromoproteins which is reflected in a pronounced polarotaxis in polarized light. There was a long debate about the potential photoreceptor molecule in Euglena, including carotenoids, flavins and rhodopsins. This discussion was terminated by the unambiguous proof that the photoreceptor is a 400 kDa photoactivated adenylyl cyclase (PAC) which consists of two α- and two β-subunits each. Each subunit possesses two BLUF (Blue Light receptor Using FAD) domains binding FAD, which harvest the light energy, and two adenylyl cyclases, which produce cAMP from ATP. The cAMP has been found to activate one of the five protein kinase s found in Euglena (PK.4). This enzyme in turn is thought to phosphorylate proteins inside the flagellum which result in a change in the flagellar beating pattern and thus a course correction of the cell. The involvements of PAC and protein kinase have been confirmed by RNA interference (RNAi). PAC is responsible for step-up photophobic responses as well as positive and negative phototaxis, but not for the step-down photophobic response, even though the action spectrum of this resembles those for the other two responses. Analysis of several colorless Euglena mutants and the closely related Euglena longa (formerly Astasia longa) confirms the results. Photokinesis shows a completely different action spectrum. Some other Euglena species, such as E. sanguinea and the gliding E. mutabilis, have been investigated, again showing totally different action spectra for phototaxis and photokinesis as well as step-up and step-down photophobic responses.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Astasia
- Euglena longa
- Euglena gracilis
- Euglena mutabilis
- Flavin
- Photoactivated adenylyl cyclase
- Photokinesis
- Photophobic reactions
- Photoreceptor
- Phototaxis
- Protein kinase
- Pterin
- Sensory transduction
1 Introduction
Many unicellular microorganisms as well as cell colonies are motile and orient themselves with respect to external physical and chemical parameters in their environment such as temperature (Häder et al. 2014), pH, oxygen (Colombetti and Diehn 1978; Porterfield 1997), chemicals and pollutants (Govorunova and Sineshchekov 2005; Ozasa et al. 2013; Azizullah et al. 2014), mechanical stimuli (Mikolajczyk and Diehn 1979; Fenchel 2013), the magnetic field of the Earth (de Araujo et al. 1986; Kavaliers and Ossenkopp 1994) and even electrical currents (Umrath 1959; Votta and Jahn 1972; Kim 2013). Many swimming cells orient themselves in the gravity field of the Earth using a mechanism called gravitaxis (see Chap. 12) (Richter et al. 2002; Nasir 2014).
Photosynthetic organisms such as flagellates require the presence of light for energy harvesting, so that it is not astonishing that they orient with respect to light to guide their migrations (Häder 1979; Häder and Lebert 2009; Peacock and Kudela 2014). But also heterotrophic organisms use phototactic orientation for habitat selection (Hu et al. 2014). Motile flagellates often move toward the light source at low irradiances (positive phototaxis, Fig. 11.1a) (Liu and Häder 1994; Giometto et al. 2015). Since excessive radiation can be detrimental for the cells, many organisms move away from the light source at high irradiances (negative phototaxis, Fig. 11.1b) (Lenci et al. 1984; Josef et al. 2005; Ma et al. 2012). In some cases an orientation at a specific angle to the light direction (e.g. perpendicular to a light beam, Fig. 11.1c) has been found, a behavior called diaphototaxis (Häder and Lipson 1986; Nultsch and Häder 1988; Rhiel et al. 1988). This behavior enables the organisms to swim horizontally at constant optimal light intensity.
(a) Positive phototaxis in E. gracilis strain Z swimming in a horizontal cuvette with white light at 10 W m−2 impinging from 0°. Tracks of swimming cells were recorded by automatic image analysis and the angles deviating from 0° (direction toward the light source) were binned in 64 sectors. The circular histogram shows the percentage of tracks in each angular sector. (b) Negative phototaxis in E. gracilis strain Z swimming in a horizontal cuvette with white light at 100 W m−2 impinging from 0° (modified from (Lebert and Häder 2000). (c) Diaphototaxis in the colorless E. gracilis strain FB swimming in a horizontal cuvette with white light at 1000 W m−2 impinging from 0° redrawn from (Lebert and Häder 1997)
In addition, many cells show other light-induced behavioral movement responses. Upon a sudden decrease in light intensity they show a step-down photophobic response which may be a stop, a change in swimming direction or a reversal of movement (Govorunova et al. 2004; Lenci et al. 2012). Imagine cells swimming in a horizontal container which is covered by a black lid with a square opening in the center irradiated by low intensity light from above. Cells swimming in the shade may enter the irradiated zone without a response, but, if they try to leave it they undergo a step-down photophobic response at the light/dark boundary. This behavior will result in an accumulation of cells in the irradiated area over time. This reaction is exploited in the so-called light trap method used to quantify photophobic responses (Nultsch and Häder 1979). Likewise, a sudden increase in the ambient light intensity may result in a step-up photophobic response which is elicited by a sudden increase in light intensity which would occur when an organism enters a irradiated area from a shaded one (Doughty and Diehn 1984; Ntefidou et al. 2003b; Takeda et al. 2013). This usually occurs at high light intensities. In this case cells trying to cross the border from the shaded area into the brightly lit zone will undergo a step-up photophobic response while they do not react when leaving the light field. This behavior results in a depletion of cells in the lit zone and an accumulation in the dark. Photophobic responses in Euglena have also been studied by the observation of individual cells embedded in a small agar chamber (Shimmen 1981).
A dependence of the swimming speed on the ambient irradiance is called photokinesis (Zhenan and Shouyu 1983; Melkonian et al. 1986; Iwatsuki 1992). This phenomenon can also result in accumulations of cells in certain areas (Häder 1987a). Imagine cells swimming fast in light but slow in the shaded area or even stopping (positive photokinesis); these cells will accumulate in the shaded area. This has been observed e.g. in the ciliate Stentor coeruleus (Iwatsuki 1992). Also the opposite has been found: cells swimming fast in the dark and slower in light will accumulate in the irradiated field (negative photokinesis). Another mechanism for cell accumulation is phototaxis of cells toward a light field irradiated by a strong light source such as a laser beam as shown for Euglena (Itoh and Tamura 2008).
While phototaxis has been studied in many flagellates to some extent only a few were investigated in detail, such as the Chlorophyte Chlamydomonas reinhardtii (Sineshchekov et al. 2002; Schmidt et al. 2006; Inaba et al. 2014). Euglena has been established as a model system for biochemical and behavioral studies, signal transduction and molecular biology of the photoreceptor (Iseki et al. 2002; Wolken 2012; Masuda 2013; Ozasa et al. 2014; Giometto et al. 2015).
In the past, the movement and orientation of motile microorganisms was recorded and quantified by manual and video techniques (Colombetti et al. 1982), but are nowadays usually quantified using real-time image analysis and computerized cell tracking (Häder and Lebert 2000; Häder 2003).
2 The Organisms
Euglena gracilis is a photosynthetic unicellular flagellate (Buetow 1968a), but it can also live heterotrophically (Sumida et al. 2007). The size ranges between 50 μm and 80 μm length and 8 μm to 12 μm width (Buetow 1968b). Since the cell does not have a rigid cell wall its form is highly flexible ranging from almost spherical to an elongated spindle (Mikolajczyk and Diehn 1976, 1978; Mikolajczyk and Kuznicki 1981; Murray 1981). The cell body is covered with a pellicle which consists of longitudinal interlocked stripes which can slip with respect to one another (Suzaki and Williamson 1986; Leander et al. 2001), and the surface is covered with slime (Diskus 1955). The stripes are composed of the claudin-like, four-trans-membrane protein IP39 which forms linear arrays by a trimeric unit repeat which are similar to tight junctions (Capaldo et al. 2014). At the front end there is a bottle-like 5 μm × 10 μm invagination, called reservoir (Fig. 11.2), which allows pinocytotic uptake of external material (Kivic and Vesk 1974a; Bouck 2012; Omodeo 2013). A system of contractile vacuoles (one main vacuole with several accessory vacuoles) is associated with the reservoir, believed to be involved in osmoregulation (Rosati et al. 1996; Komsic-Buchmann and Becker 2012). At the base of the reservoir two flagella originate in basal bodies, but only one of them extends as a trailing flagellum from the reservoir while the other one reaches up to the paraxonemal body (PAB, also called paraflagellar body) (Tollin 1973; Piccinni and Mammi 1978), located on the long flagellum half way between the bottom and the opening of the invagination, called pharynx (Frey-Wyssling and Mühlethaler 1960; Kisielewska et al. 2015). The PAB is a small organelle (1 μm × 0.7 μm × 0.7 μm) (Wolken 1977; Omodeo 1980; Rosati et al. 1991) (Fig. 11.2). A paraxonemal rod (PAR) inside the flagellum connects the PAB to the cell matrix (Banchetti et al. 1980; Hyams 1982; Verni et al. 1992; Karnkowska et al. 2015). It consists of several proteins like in trypanosomes, the function of which is still unclear (Hyams 1982; Leedale 1982; Ngô and Bouck 1998). The membrane covering the PAB was found to be structurally specialized when observed with the freeze-fracture technique. There are large-sized (10–14 nm) intramembrane particles (Robenek and Melkonian 1983). The side next to the stigma is closely attached to the plasmalemma of the reservoir. Inside the cytoplasm, in close vicinity of the reservoir and next to the PAB it possesses a red structure, the stigma or eyespot which is so obvious that it was detected by early microscopists (Pringsheim 1937; Wolken 1956). This structure gave the flagellate its German name “Augentierchen” (small eye animal) and was considered to be the site of the photoreceptor (Cypionka 2010). The stigma consists of a number of globules (200–300 nm diameter) filled with carotenoids (Strother and Wolken 1960; Sperling et al. 1973; Benedetti et al. 1976; Heelis et al. 1979, 1980; Osafune and Schiff 1980; James et al. 1992). The main carotenoid in E. gracilis is antheraxanthin (80%) (Krinsky and Goldsmith 1960). Application of streptomycin to dark-bleached cultures of Euglena hampered the carotenoid synthesis , and electron micrographs showed a decrease in the number of stigma vesicles while no effect on the PAB was detected. Phototaxis decreased and finally disappeared after 5 weeks (Ferrara and Banchetti 1976). This does not mean that the photoreceptor is located within the stigma, but that its role as a shading device is compromised. In contrast to other algal groups, such as Chlorophytes, the stigma in Euglena is not enclosed within the chloroplast (Kivic and Vesk 1974b; Kronestedt and Walles 1975). Also in contrast to green algae, the globules are not organized in a rigid structure (Walne and Arnott 1967; Dodge 1969; Kivic and Vesk 1972a; b; Kreimer and Melkonian 1990). Therefore it does not function as an interference reflector device which is found in many stigmata of Chlorophytes such as Chlamydomonas (Kreimer 1994). Even though the stigma does not harbor the photoreceptor, as suggested by earlier authors (France 1909; Fong and Schiff 1978, 1979), it seems to be involved in photoperception, functioning as a screening device which in lateral light casts a periodic shadow on the photoreceptor (the PAB, see below) as the cell rotates around its long axis when it is propelled by its long flagellum in a forward direction (Barsanti et al. 2012). For comparison , we also shortly discuss several mutant strains of Euglena gracilis , some other species in the genus Euglena as well as the close relative E. longa (formerly Astasia longa) (Poniewozik 2014).
3 The Paraxonemal Body (PAB)
The PAB shows a paracrystalline structure (Kivic and Vesk 1972a; Forreiter and Wagner 2012). Wolken (1977) proposed a model of packed rods in a helical pattern based on optical diffraction patterns, and Piccinni and Mammi (1978) found monoclinic or slightly hexagonal cell units with the principal axes a = 8.9 nm, b = 7.7 nm, c = 8.3 nm und ß = 110°. This structure can be interpreted as an I-type 3-D crystal (Michel 1990), which is a stacks of 2-D crystal arrays stabilized by hydrophobic interactions between the planes and hydrophilic interactions with the surrounding aqueous environment. More than 100 layers of the 2-D crystals are thought to form the observed structure of the PAB (Gualtieri 1993b). 2-D type crystals have been found e.g. in the purple membrane in Halobacterium salinarium, with a very high concentration of bacteriorhodopsin to harvest light energy (Oesterhelt 1998) or in photosynthetic membranes of higher plants (Toporik et al. 2012) and purple bacteria (Sznee et al. 2014).
4 Photoresponses
As indicated above Euglena gracilis shows three types of photoresponses: both positive and negative phototaxis, step-up and step-down photophobic responses and photokinesis .
4.1 Phototaxis
In contrast to photokinesis and photophobic responses phototaxis depends on the direction of the impinging light. Phototaxis in motile microorganisms has been known for more than a century (France 1908, 1909). At low irradiances the flagellates move toward the light source (positive phototaxis) which can be shown both in horizontal or vertical observation chambers. At higher irradiances the cells switch to negative phototaxis and swim away from the light source (Häder et al. 1981). In Euglena, the threshold for the change from positive to negative phototaxis is found between 10 and 100 W m−2, but it depends on other external factors (Häder 1987b, 1998) as well as whether the cells swim in a horizontal or vertical cuvette. In the latter case the cells orient simultaneously with respect to light and gravity (see below). At intermediate light intensities some of the cells show positive others negative phototaxis. As the ecological consequence of this behavior, Euglena cells accumulate at a certain depth in the water column at which the impinging sun light has been attenuated to about 30 W m−2 (Häder and Griebenow 1988). A similar behavior was found in the freshwater E. proxima (Hasle 1950). In comparison, the green flagellate Chlamydomonas is much more sensitive than Euglena and has a threshold for positive phototaxis at 0.001 W m−2 (Feinleib and Curry 1971). This reflects the fact that Chlamydomonas is often found much deeper in the water column with less light availability than Euglena.
The mechanism of light direction detection was debated for a long time. Some researchers held the notion that oriented movement depends on a series of photophobic responses (Jennings 1906; Mast 1911): When an organism experiences a decrease in irradiance it might make a turn or reverse the direction of movement. When this strategy is repeated over time the organism will move up the light gradient and thus show a positive phototaxis. The same reasoning holds for negative phototaxis being based on consecutive step-up photophobic responses. This hypothesis is based on a two-instant mechanism (Feinleib 1975): The photoreceptor takes readings of the ambient irradiance and compares it to a previous measurement. This orientation mechanism has also been described as “biased random walk ” (Hill and Vincent 1993; Hill and Häder 1997), which is also found in chemotaxis of bacteria (Wadhams and Armitage 2004). This theory implies that the photoreceptors for phototaxis and photophobic responses are identical. For Euglena gracilis the hypothesis that light direction detection is based on a series of repetitive photophobic responses can be rejected at least for negative phototaxis, since in this flagellate the threshold for negative phototaxis is much lower than that for the step-up photophobic response. We will also see below that the molecular analysis of the photoreceptor showed that the step-down photophobic response in Euglena is not mediated by the same photoreceptor which drives phototaxis.
Alternatively, the organism detects the light direction with a more sophisticated sensor than just a light-intensity measuring photoreceptor. This is based on a one-instant mechanism. E.g., some ciliates posses a complicated optical organelle which like a primitive eye, is capable of discerning the direction of the impinging light (Omodeo 1975; Selbach et al. 1999).
Buder devised an ingenious experiment to tackle the question whether phototaxis is controlled by an orientation along a spatial gradient of light or the result of a directional movement with respect to the light direction. He passed light through a biconvex lens so that he produced a converging light beam. The cells swam toward the light source, passed through the focal point and continued to swim toward the light source even though the light intensity decreased with the distance from the focal point (Buder 1919).
In negative phototaxis the rear end of the cell with its chloroplasts casts a shadow on the photoreceptor. Circular histograms of the swimming directions indicate that a course correction is initiated when the cell deviates by more than 25° from the light direction (Häder et al. 1986) indicating that in this position the rear end does no longer shade the photoreceptor.
When exposed to two perpendicular light beams, at low fluence rates which induce positive phototaxis, the cells do not orient on the mathematical resultant of the two vectors but orient themselves with respect to either light beam (Häder 1993). At equal light intensities approximately half of the population swims toward one light source and the remainder toward the other light source. When the intensity of one of the light beams exceeds that of the other by more than 10%, almost all cells move in the direction of the stronger light beam (Lebert and Häder 2000). In contrast, at high fluence rates which induce negative phototaxis the cells orient on the resultant away from the two perpendicularly impinging light beams. When the cells orient with respect to light they are simultaneously exposed to the gravity field of the Earth unless they are under zero-g conditions such as on a satellite or on the International Space Station (ISS). In a vertical cuvette they show a gravitactic orientation. Shortly after inoculation into new medium the cells swim downward. In contrast, older cells (>10 days) swim upward. When they simultaneously perceive a (horizontal) light beam perpendicular to the gravitational field of the Earth the cells swim on an intermediate track on the resultant; the angular deviation from the vertical depends on the irradiance of the light beam (Kessler et al. 1992). In order to estimate the effect of the simultaneously operating gravitaxis on the precision of phototaxis a space experiment was carried out on a sounding rocket flight inside a TEXUS rocket (Kühnel-Kratz et al. 1993). As expected, under microgravity (μg) conditions the precision of phototaxis was higher than in the 1-g control but the inversion from positive to negative phototaxis was the same for a sample taking shortly after landing of the rocket as in the control cells which had been exposed to 1 g all the time. In addition, phototactic orientation was reached faster at μg than at 1-g conditions (Häder 1997).
The mechanism of changing movement direction is closely linked to the rotation of the cell and the position of the flagellum. While swimming, a cell rotates at about 1 Hz around its long axis describing a cone with a ca. 15° opening angle at the front end. The flagellum is more or less parallel to the long axis. When a low intensity light beam (which induces a positive phototaxis) impinges perpendicular to the long axis, the flagellum swings out at an angle from the long axis, when during rotation the flagellum is oriented away from the light beam. This impulse triggers a small angular turn of the front end toward the light source. This is also seen in the output of a computer simulation (Häder 1993). This course correction is repeated as long as the long axis is not yet aligned with the light direction. When the flagellum is oriented away from the light direction, the stigma intercepts the light path onto the PAB photoreceptor (Häder 1998). Thus it modulates the light intensity seen by the PAB with the frequency of the cell’s rotation with a minimum when the flagellum is opposed to the light direction. This was regarded as a proof for the shading hypothesis (Clayton 1964) and seemed to confirm the notion that phototaxis is brought about by a repetitive step-down photophobic response. For negative phototaxis this process has to be just the opposite with the additional shading from the rear end with its chloroplasts.
In order to determine the angle of the course correction a circular cuvette was rotated by an electric motor in a strong lateral light beam (30 klx) which induced negative phototaxis (Häder et al. 1986). The moving cells were recorded from below using a dark red monitoring light (>690 nm) which does not induce a visible movement response. When the cuvette was rotated at a low angular velocity (<20°/s) the cells still showed negative phototaxis but the mean swimming direction was shifted in the direction of the rotation. At higher rotational speeds the precision of orientation decreased and the swimming was more random. This indicates that the cells were capable of course corrections of up to 20°/s. Since the time for one rotation is about 1 s, the cells reorient by about 20° per rotation.
However, some mutants of E. gracilis lack a stigma but still show negative phototaxis, but no positive phototaxis (Vavra 1962; Checcucci et al. 1976; Häder 1993). In contrast, mutants which lack both PAB and stigma neither show positive nor negative phototaxis (Pringsheim 1948; Vavra 1962; Lebert and Häder 1997). Though many mutants are known lacking stigma, PAB and/or chloroplasts (Schiff et al. 1971, 1980; Shneyour and Avron 1975; Falke et al. 1997), only a few have been analyzed regarding phototaxis (Lebert and Häder 1997). These mutants have not been induced but occurred spontaneously and were isolated by accident. In one investigation, three of the studied mutants were stable, while one included some revertants which may be due to the fact that Euglena is polyploid.
Euglena longa , a close relative of E. gracilis, possesses a stigma but no PAB and does not show phototaxis (Suzaki and Williamson 1983; Mikolajczyk 1984a, 1984b) but step-up photophobic responses, while step-down responses were not observed, indicating that separate receptors exist for step-up and step-down photophobic responses. This was later confirmed by the molecular biological identification for the photoreceptor in Euglena (Iseki et al. 2002). All these findings indicate that the PAB is the true photoreceptor in E. gracilis and that the stigma only has an accessory role (at least in positive phototaxis). Thus, the classical shading hypothesis had to be discarded.
As indicated above, earlier electron microscopic analyses have revealed a quasicrystalline structure within the PAB (Kivic and Vesk 1972b). Could that mean that the photoreceptor molecules are dichroically oriented in a specific pattern with reference to the long axis and the location of the stigma? In order to answer this question, experiments have been carried out with polarized light impinging from above onto a horizontal swimming chamber with E. gracilis (Bound and Tollin 1967; Creutz and Diehn 1976; Häder 1987b). The assumption is that light is only absorbed when the absorption vector is perpendicular to the light direction. When the electric dipole of the polarized light was oriented in a specific direction, the cells swam at an angle of about 30° clockwise from the electric dipole (Fig. 11.3a). This behavior was confirmed and also seen in a computer simulation which assumes the same orientation of the photoreceptor molecules as indicated in the electron micrographs (Häder 1993). Further experiments with polarized light in all three dimensions revealed the orientation of the absorption vectors in 3D with respect to the cell axes (Häder 1987b). Tracing the cells in a vertical cuvette with polarized light impinging from above indicated that the absorption vectors of the photoreceptor pigments are dichroically oriented predominantly 60° counterclockwise from the flagellar plane (when one looks onto the front end of the cell, Fig. 11.3b) (Häder 1987b). These results were confirmed in a mathematical model for the signal received by the dichroic photoreceptor in E. gracilis when irradiated by polarized light (Hill and Plumpton 2000). Diehn assumed that Euglena has two perpendicularly oriented photoreceptor systems responsible for positive and negative phototaxis, respectively (Diehn 1969c). During the revolution of the cell around its long axis a dichroically oriented photoreceptor has two positions with maximal absorption for light hitting perpendicularly to the swimming direction. This could explain the diaphototactic orientation found in the 1F mutant which does not have a screening stigma. The 1F mutant lacks chloroplasts, stigma and PAB. It shows only diaphototaxis and neither positive or negative phototaxis. In the wild type cells one of the absorption maxima is excluded by the presence of the stigma, so that the cells orient only in one direction. In negative phototaxis the rear end of the cell assumes the role of a shading device and the presence of the stigma is not required for negative phototaxis.
(a) Euglena cells swimming in a horizontal cuvette orient themselves 30° clockwise from the electric vector of a polarized light beam swinging in a plane 0–180° impinging from above (inset), (b) absorption vectors of the photoreceptor pigments are dichroically oriented predominantly 60° counterclockwise from the flagellar plane when one looks onto the front end of the cell. Redrawn after (Häder 1987b)
Euglena shows a strong circadian rhythm which affects orientational responses (Verworn 1889; Bruce and Pittendrigh 1956; Tollin and Robinson 1969; Edmunds 1984; Petersen-Mahrt et al. 1994) as well as other reactions such as cell division and cellular cAMP concentration (Bruce and Pittendrigh 1958; Feldman and Bruce 1972; Bruce 1973; Bünning 1973; Carre et al. 1989; Lebert et al. 1999). This may also be a reason that several authors found different results in their investigations of Euglena phototaxis. Also culture conditions and age strongly affect phototactic orientation (Häder et al. 1987).
4.2 Photophobic Responses
Photophobic responses (also called phobic reactions or shock responses) were first reported by Engelmann (1883). The older literature has been reviewed (Haupt 1959; Feinleib and Curry 1967; Diehn 1973; Nultsch 1975; Nultsch and Häder 1979). Photophobic responses are elicited by rather sudden changes in irradiance. If the change occurs over a longer time period, the organisms adapt to the new condition without showing a phobic response. The phobic response occurs only when the change in irradiance exceeds a species-specific discrimination threshold (Clayton 1959), which can be as low as a few percent (Nultsch and Häder 1970). In Euglena the response is characterized by a stop and tumble which can last a few seconds; after this the cell resumes swimming in a new direction (Doughty 1991). High speed cinemicrography showed that the cells respond with the turning toward the dorsal side when exposed to a sudden increase in light intensity (Diehn et al. 1975). In this organism step-down photophobic responses occur at low irradiances and step-up responses at higher irradiances (Diehn 1969c, 1973). Ecologically this behavior can be interpreted as helping the organism to prevent swimming from a low-irradiance region into an even darker shadow or from a high-irradiance region into excessive light. Very low irradiances may not be sufficient for photosynthesis and very high irradiances may be detrimental for the cells. The action spectra for the step-up and step-down photophobic responses in Euglena differ slightly but show a resemblance to a flavin chromophore (Diehn 1969a; Barghigiani et al. 1979b; Walne et al. 1984).
4.3 Photokinesis
As described above, photokinesis describes the dependency of the swimming speed on the light intensity. This behavior was first described almost 140 years ago (Strasburger 1878). It can result in an accumulation of organisms in lighted or shaded areas (see above). In Euglena a not very pronounced positive photokinesis was described (Wolken and Shin 1958). The increase in velocity saturates at 300 lx white light. However, a 10–15 min adaptation period is needed before the velocity reaches a new steady state (Mast 1911) which might indicate that metabolic processes are involved producing higher energy. The increase in swimming speed seems to be due to an increased flagellar beating frequency (Ascoli 1975). However, also the non-photosynthetic close relative E. longa has been found to show positive photokinesis (Mast 1911).
In Euglena the action spectrum of this response is still under debate. Some researchers found an effect in red light and proposed the involvement of the photosynthetic pigments such as chlorophyll b and β-carotene (Mast 1911; Ascoli 1975). Other researchers claim a strong effect of blue light (Nultsch and Throm 1975). Therefore it is not decided whether photokinesis depends on photosynthesis or is controlled by a blue light receptor (Haupt 1959).
5 The Photoreceptor (s)
Before molecular biology tools became available the question for the photoreceptor involved in a light-dependent responses such as phototaxis was tackled using action spectroscopy (Foster 2001). Using narrow band color filters (such as interference line filters) the organism is exposed to a selected wavelength band and the response is quantified at increasing irradiances (Foster 2001). This is repeated for all relevant wavelengths. Next the inverse of the required irradiance for a certain response (e.g. 50%) is plotted versus the wavelength giving an action spectrum or in other words the efficacy of the actinic light in dependence of the wavelength. The form of the action spectrum is compared with the absorption spectrum of a presumed photoreceptor. In reality things can be more complex e.g. by the presence of shading pigments. In order to avoid this complication, the method of threshold action spectra is used which are constructed again by plotting the wavelength-dependent response versus log light intensity (Foster and Smyth 1980). The reciprocals of the threshold intensity at which no reaction occurs, obtained by the linear interpolation of the response-intensity plots, should indicate the properties of the photoreceptor molecule .
5.1 Earlier Results and Hypotheses
Most flagellates have sensitivity in the UV/blue-green range (300–550 nm) of the spectrum. But there are exceptions: The action spectrum for phototaxis in Chlamydomonas reinhardii extends up to 600 nm (Foster and Smyth 1980; Johnson et al. 1991). In Ochromonas danica (Mast 1914) and Peridinium gatunense (Häder and Liu 1991) the spectrum even extends into the red region of the spectrum. These diverse spectral sensitivities probably indicate that phototaxis has evolved in multiple parallel events (Kivic and Walne 1983).
Early action spectra for photoaccumulation of green, dark-bleached and streptomycin-treated colorless Euglena, all of which possess a PAB, have been published by Checcucci et al. (1976). These spectra indicate the presence of a flavin-type photoreceptor . These measurements have been performed with the “phototaxigraph” (Lindes et al. 1965; Diehn and Tollin 1966; Checcucci et al. 1975). This instrument records the density of cells in a light trap and this is interpreted as a quantification of phototaxis when cells are attracted from the outside of the trap by light scattered from cells already inside the trap. However, as we have seen above, photoaccumulations can be brought about by several photoresponses including phototaxis. Another action spectrum for phototaxis was published by Gössel (1957).
Light microscopic images of the front end of Euglena gracilis show the reservoir with the two flagellar bases. Where they join a distinct body which represents the PAB can be seen (Fig. 11.4a). When excited with monochromatic light at 440 nm this spot shows a distinct blue fluorescence with a peak at about 520 nm (Fig. 11.4b). The red background fluorescence is derived from the chlorophyll in the cells. The flagella can be isolated with the PAB still attached (Gualtieri et al. 1986; Brodhun and Häder 1990) after the cells have been osmotically swelled in order to open the reservoir (Fig. 11.4c). Scanning electron micrographs of isolated flagella also show the PAB still attached to the flagellum (Brodhun et al. 1994). In large scale isolation experiments the chromoproteins of the PABs can be separated. SDS gel electrophoresis and isoelectric focusing shows the presence of four major proteins which are lacking in Euglena longa (which does not have a PAB) (Brodhun and Häder 1995a; Häder 1998). When excited, all four chromoproteins showed fluorescence emission spectra which were interpreted as representing flavins and pterin s (Ghetti et al. 1985; Brodhun and Häder 1990; Häder 1991; Lebert 2001). Three proteins (Mr 27, 27.5. 31.6) contained pterins and the other one a flavin (Mr 33.5) (Lebert and Häder 2000). The excitation spectrum strongly resembled the action spectrum for phototaxis confirming the notion that a flavoprotein might be the photoreceptor for this response in E. gracilis (Brodhun and Häder 1990, 1995a). In addition, microspectrofluorometric studies of the PAB confirmed the presence of a flavin chromophore which showed an emission at 520 nm when excited between 400 and 500 nm (Benedetti and Checcucci 1975; Benedetti and Lenci 1977; Schmidt et al. 1990; Sineshchekov et al. 1994b). This interpretation is further strengthened by the fact that the fluorescence emission is strongly polarized, which has to be expected from the paracrystalline structure of the PAB (Sineshchekov et al. 1994b). The PAB fluorescence quantum yield is rather low (0.005 as compared to solubilized riboflavin ~0.25) (Ghetti et al. 1985). This indicates a strong coupling of the photoreceptor molecules to the signal transduction chain. Only when the transduction chain is saturated by excessive light or the coupling of the photoperception to the subsequent steps in the transduction chain is disturbed the fluorescence yield increases. The action spectrum for phototaxis in the green strain Z of E. gracilis extends to about 500 nm (Fig. 11.5a). Also when the chloroplasts and chlorophyll are removed by cultivating the cells in an organic medium in the dark, the action spectrum of these colorless strains still has the same shape (Häder and Reinecke 1991) consistent with a flavin chromophore .
(a) Action spectrum for phototaxis in E. gracilis (blue line), after (Häder and Reinecke 1991), absorption spectrum of roseoflavin (black line) and transmission spectrum of the OG 570 cut-off filter (red line). Histograms of phototactic orientation in wild type E. gracilis grown in medium (b) and cells grown in the presence of roseoflavin (c) when exposed to actinic light filtered by OG 570 cut-off filter. Redrawn after (Häder and Lebert 1998)
Another confirmation for a flavin to be the chromophoric group in the photoreceptor for phototaxis was obtained by feeding E. gracilis over several generations with roseoflavin. This molecule is incorporated as a chromophore into the photoreceptor instead of the original flavin. Roseoflavin has an absorption peak at 500 nm and the spectrum extends up to 600 nm (Fig. 11.5a) (Häder 1998; Häder and Lebert 1998). When untreated cells were exposed to actinic light >550 nm produced by a cut-off filter they did not show phototactic orientation since the action spectrum extends only to ~500 nm (Fig. 11.5b). However, the roseoflavin-treated cells showed a clear phototaxis at wavelengths >550 nm (Fig. 11.5c). But it is of interest that the population displayed both positive and negative phototaxis. This can be easily explained by the fact that the carotenoids in the shading stigma do not absorb in this wavelength range, so that the cell cannot distinguish between light coming from the stigma side and the opposite direction.
When exposed to ultraviolet radiation the four proteins isolated by FPLC from the PAB were damaged and their amounts were significantly reduced (Brodhun and Häder 1993, 1995b). Both fluorescence excitation and emission spectra of the isolated PAB proteins decreased upon exposure to ultraviolet radiation (Häder and Brodhun 1991). As a result, exposure to solar or artificial UV-B radiation impaired phototaxis (Häder 1985, 1986; Häder and Häder 1988). However, this inhibition was not specific; chlorophyll content, photosynthetic oxygen production and motility were likewise affected (Gerber and Häder 1995; Richter et al. 2007). A polychromatic action spectrum of the inhibition confirmed that the high-energy UV-B radiation was most effective (Gerber et al. 1996). Filtering out the UV-B wavelength band from solar radiation in a field study provided some protection from excessive radiation: bleaching and immobility occurred later than in unfiltered sunlight (Gerber and Häder 1993).
Earlier work using flavin quenchers , such as KI, MnCl2 and NaN3 which are well known as effective quenchers of the electronically excited state of flavins, were effective in inhibiting the negative phototaxis in the organism (Colombetti et al. 1982; Lenci et al. 1983) but not the step-up photophobic reaction (Mikolajczyk and Diehn 1975). Neither KCN, a general metabolic inhibitor, nor KCl affected phototaxis. Both KI and MnCl2 clearly quenched the fluorescence of 1 mM aqueous solutions of riboflavin.
Galland and coworkers suggested pterin s as a possible UV-absorbing chromophore involved in phototactic photoperception of Euglena gracilis (Galland and Senger 1988a, b). Using the Okazaki large spectrograph and a computerized video motion analysis, Matsunaga and coworkers found that in addition to UV-A and blue light the action spectrum for photophobic responses of E. gracilis extended well into the UV-B with peaks at 270 nm (step-down response) and 280 nm (step-up response), respectively (Matsunaga et al. 1998) which they attributed to the combined action of 6-biopterin and FAD. In addition, fluorescence and emission spectra of the isolated PAB proteins indicated that pterins might also be involved (Galland et al. 1990). The colorless mutant 1F of Euglena gracilis does not possess flavins, as indicated by the fluorescence emission spectrum, but it shows the pterin emission band centered around 525 nm (Häder and Lebert 1998). This result indicates that the diaphototaxis found in the 1F and other mutants is not mediated by a flavin but by a pterin.
The separated flavoproteins had an apparent molecular mass of about 33,500 and the pterin-binding protein 27,000 (Brodhun and Häder 1990). Sineshchekov and coworkers suggested that there is an energy transfer from the pterins to the flavins, which can be disrupted by solubilization of the PAB (Sineshchekov et al. 1994a; Lebert and Häder 2000). It is assumed that the pterins are located on the outside of the PAB while the flavins are inside (Häder and Lebert 1998).
In order to confirm that flavins could be involved as chromophoric groups of photoreceptor pigments for phototaxis binding studies with [3H]-labeled riboflavin were carried out (Brodhun et al. 1994). Nebenführ et al. also showed riboflavin-binding sites associated with flagella of E.gracilis (Nebenführ et al. 1991). Also Neumann isolated a riboflavin-binding protein from the flagella of E. gracilis (Neumann and Hertel 1994).
High and saturable binding was found in Euglena flagella with, but also without, attached PABs. In contrast, Astasia did not show any binding activity.
To be exhaustive, it should be mentioned that carotenoids have also been discussed as photoreceptors for phototaxis in Euglena (Bendix 1960; Wolken 1960, 1977; Batra and Tollin 1964; Bensasson 1975; Gualtieri 1993a; b). In fact carotenoids, especially in the form of rhodopsins (Walne et al. 1998), have been found to be involved in photoorientation in many organisms such as Chlamydomonas, Halobacterium or Paramecium (Nakaoka et al. 1991; Govorunova et al. 2004; Kim et al. 2009). Gualtieri and others, based on theoretical considerations, absorption spectroscopy and gas chromatography-mass spectrometry, proposed that the photoreceptor pigment for Euglena phototaxis is a rhodopsin (James et al. 1992; Barsanti et al. 1993a; Gualtieri 2001). This hypothetical rhodopsin was thought to undergo a photocycle which has been analyzed by fluorescence emission spectroscopy (Evangelista et al. 2003). Hydoxylamine reacts with free and opsin-bound retinal. It was found to block the formation of the PAB and impaired photoaccumulation of the cells in the phototaxigraph (Barsanti et al. 1993b). Application of nicotine, an effective inhibitor of carotenoid biosynthesis blocked the biosynthesis of retinal by inhibiting the formation of the cyclohexylidin ring. In Euglena it prevented the formation of the PAB and impaired the accumulation of cells in a light field, interpreted as the result of phototaxis (Barsanti et al. 1992). However, when this experiment was repeated by growing E. gracilis cells up to 4 months at the highest possible concentration of nicotine (4 mM) the cells survived and neither positive nor negative phototaxis was impaired (Häder and Lebert 1998), indicating that retinal is not likely the chromophoric group of the photoreceptor. It should be mentioned that the accumulation in a light field can be brought about by a number of photoresponses such as phobic reactions or photokinesis (see Sects. 11.4.2 and 11.4.3). In fact, a photoaccumulation of Euglena was found in red light fields (Checcucci et al. 1974). This behavior was interpreted as an aerotactic attraction to the photosynthetically produced oxygen by organisms inside the light field. So the underlying mechanism in the experiment by Barsanti et al. might have been inhibited by nicotine, but it was not phototaxis.
5.2 Molecular Biology of the Photoreceptor for Phototaxis and the Step-Up Photophobic Response
All the discussions and speculations about the photoreceptor for photomovement in E. gracilis have been resolved by the molecular biological identification of the chromoproteins involved (Iseki et al. 2002). Iseki and coworkers succeeded in isolating sufficient quantities of PABs for biochemical analysis by separating the photoreceptors from the flagella followed by subcellular fractionation using a sucrose density gradient centrifugation. They separated a 400-kDa protein that binds flavins from the isolated PAB preparations by liquid chromatography. The protein is composed of four subunits: two subunits (named PACα) which consist of 1019 amino acids each with a molecular weight of 105 kDa and two more subunits (named PACβ) which consist of 859 amino acids each with a molecular weight of 90 kDa. The α and β subunits are similar to each other. Each of the four subunits has two flavin-binding BLUF (Blue Light receptor Using FAD ) domains and two adenylyl cyclase catalytic domains (Fig. 11.6). The 400-kDa flavoprotein purified from the PAB preparations showed adenylyl cyclase activity that was induced by blue-light irradiation and was accordingly named photoactivated adenylyl cyclase (PAC). The photoactivation of PAC was extensively studied by Yoshikawa et al. (2005). PAC activity was dependent on both the photon fluence rate and the duration of irradiation, between which reciprocity held well within the range of 2–50 μmol m−2 s−1 (a total fluence of 1200 μmol m−2). Intermittent irradiation also activated PAC in a photon fluence-dependent manner irrespective of the cycle periods, which implies that the increase of PAC activity occurred only during the light period and that elevated PAC activity decreased within 100 ms after the irradiation had stopped (Yoshikawa et al. 2005). Such a sharp switching property of PAC is suitable to be used as a tool to control various cellular processes by light, i.e., optogenetics. In fact, attempts to optogeneticically control cAMP levels in Aplysia neurons (Nagahama et al. 2007), Xenopus oocytes, cultured mammalian cells, Drosophila brains (Schröder-Lang et al. 2007), and Caenorhabditis neurons (Stierl et al. 2011; Weissenberger et al. 2011) have been reported.
The biological functions of PAC in Euglena cells were examined using RNAi knockdown of the genes. When the double stranded RNAs encoding PACα (the 105-kDa subunit) and/or PACβ (the 90-kDa subunit) were introduced into Euglena cells, they significantly suppressed the gene expression of PAC, which resulted in the loss of the step-up photophobic response but not the step-down photophobic response (Iseki et al. 2002). Ntefidou et al. (2003b) found that the RNAi knockdown of PAC also effectively suppressed both the positive and negative phototaxis of Euglena. Inhibition of either PACα or of PACβ completely blocked negative phototaxis. Knockout of both genes had the same effect. Obviously both genes are required for a functioning phototaxis. From these observations PAC was concluded to act as a sensor for the step-up photophobic response and phototaxis in Euglena (Häder et al. 2005; Lüdtke and Häder 2007). However, it is important to emphasize that PAC does not control the step-down photophobic response.
The photoactivation mechanism of PAC still remains to be elucidated mainly due to difficulties in obtaining sufficient amounts of the protein for X-ray crystallography or vibrational spectroscopy. All attempts to express the full-length PAC subunits in Escherichia coli have so far failed. Ntefidou et al. (2006) succeeded in expressing PAC in a soluble form in insect cells, but neither crystallization nor spectroscopic analysis using the expressed protein has yet been reported. In contrast, when only the second BLUF domain of PAC (F2) was expressed in E. coli, a part of the expressed protein could be collected in a soluble form that binds flavins (Ito et al. 2005). Although most of the expressed protein collected was insoluble, sufficient amounts of soluble, flavin-binding protein were recovered by refolding the insoluble protein with added flavins after denaturation by guanidine hydrochloride. The recombinant F2 protein showed a photocycle between a dark state and a slightly red-shifted signaling state similar to other bacterial BLUF domains (Ito et al. 2005). The quantum efficiency for the phototransformation of PACαF2 (0.28–0.32) is higher than that of PACβF2 (0.06–0.08), whereas the half-life for the dark relaxation of PACαF2 (34–44 s) is longer than that of PACβF2 (3–6 s) (Ito et al. 2010). Such photocycle features of PACαF2 and PACβF2 indicates different sensitivities for the photoactivation of PACα and PACβ , which may contribute to the wide range of light sensitivity in Euglena photobehavioral responses. Iwata et al. (2011) examined the photoreaction of PACαF2 using FTIR spectroscopy and found broad positive peaks in the difference spectrum at the 2900–2400 cm−1 region, which were attributed to the O–H stretching vibration of tyrosine, providing direct evidence for the light-induced switching of the hydrogen bond network in the BLUF domain. Single molecule fluorescence spectroscopy was also applied to PACαF2 and native PAC purified from Euglena (Fujiyoshi et al. 2011). The fluorescence from a single PACαF2 molecule measured at 1.5 K decreased in one step to background levels, whereas a single PAC molecule bleached in several steps, indicating the involvement of an energy transfer between FADs in the single PAC molecule. Fujiyoshi et al. (2011) also observed reversible spectral jumps of fluorescence from single molecules, which were attributed to a structural change around the hydrogen bonds at the FAD-binding site because the Q514A mutation of PACαF2 suppressed these spectral jumps .
6 Mutants and Related Organisms
There are a number of mutants of E. gracilis (Lebert and Häder 1997) as well as the close relative Euglena longa , formerly known as Astasia longa (Krause 2008). All these mutants lack chlorophyll and photosynthesis and consequently live heterotrophically. Table 11.1 summarizes some of the characteristics of these strains. With the exception of E. longa all mutants have some form of phototaxis (diaphototaxis and/or negative phototaxis). In addition to the wild type, the FB and 9F mutants have a PAB. The FB mutant is of interest since some cells have a stigma. This mutant may not consist of a uniform population and may contain some revertant cells, which might explain the presence of a stigma in some cells. All mutants are capable of step-up photophobic responses, but only the wild type strain Z shows step-down responses. All mutants and E. longa lack positive phototaxis, but all mutants show diaphototaxis at high light intensities and the FB mutant also shows a negative phototaxis component (Lebert and Häder 1997). All strains in this group display both positive and negative gravitaxis.
Even though some of these mutants do not have a PAB, PCR showed the presence of the PACα gene in all mutants but not in E. longa (Ntefidou and Häder 2005). However, in the latter organism a largely modified PAC was found, which was dubbed AlPACα (at that time the organisms was still known under the name Astasia ) (Ntefidou et al. 2003a). The amino acid sequences of the first BLUF domain of PACα and PACβ from the wild type and the mutant strains 9F, FB, 1F and st− of E. gracilis as well as AlPACα and AlPACβ from E. longa show a homology of between 43 and 91% (Ntefidou and Häder 2005). The BLUF1 and BLUF2 subunits of the PAC proteins resemble the N-terminal end of the AppA flavoprotein found in the purple bacterium Rhodobacter sphaeroides, which also binds FAD (Gomelsky and Kaplan 1995). In this bacterium the photoreceptor regulates the expression of photosynthesis genes (Gomelsky and Kaplan 1998; Masuda and Bauer 2002). The catalytic domain of class III adenylyl cyclases is found in many organisms from bacteria to protists, fungi, trypanosomes, insects and mammals (Koumura et al. 2004). It is interesting to note that the adenylyl cyclase in the Euglena PAC genes are more closely related to those found in bacteria than those in eukaryotes including the trypanosomes. This fact supports the notion that Euglena has obtained the gene by secondary endosymbiosis, which is also reflected in the fact that the chloroplasts are covered by a triple membrane. In order to understand the evolution of PAC, protein sequences were compared from several euglenoids by reverse transcriptase-polymerase chain reaction (RT-PCR) including the photosynthetic Euglena stellata, Colacium sideropus, Eutreptia viridis, Eutreptiella gymnastica and the heterotrophic Khawkinea quartana, but not the phagotrophic Petalomonas cantuscygni (Fig. 11.7). Based on these findings the evolutionary tree of PAC starts from a common ancestor. From this the trypanosomes received the trypanosome-type adenylyl cyclases. The other line leads from the common ancestor to phagotrophic euglenoids such as Petalomonas cantuscygni (Koumura et al. 2004). The phototrophic euglenoids developed after acquisition of chloroplasts by secondary endosymbiosis which also transferred the PAC genes. The osmotrophic euglenoids such as Astasia and Khawkinea lost the chloroplasts again but kept the PAC genes.
Phylogenetic tree of the PAC proteins in the phototrophic euglenoids Euglena gracilis (Eg), Euglena stellata (Es), Colacium sideropus (Cs), Eutreptia viridis (Etv), Eutreptiella gymnastica (Etg) as well as the heterotrophic Khawkinea quartana (Kq) and Astasia longa (Al, new name Euglena longa ) constructed by the neighbor-joining method using the Clustal X program (Thompson et al. 1997). Redrawn after (Koumura et al. 2004)
Confocal immunofluorescence was used to find the localization of PAC proteins in the wild type and mutant strains. It is interesting to note that the flagella of all strains contain PAC gene products even though the fluorescence is much lower than that of the PABs. Furthermore, the RNAi knockdown of PAC also suppressed the step-up photophobic responses in the wild type and all studied mutant strains as well as in E. longa (Ntefidou et al. 2003a). In contrast, only the wild-type strain Z showed step-down photophobic responses which could not be eliminated by RNAi against PAC .
Recently, PAC-like genes were found in genome sequences of a sulfur bacterium Beggiatoa sp. (Ryu et al. 2010; Stierl et al. 2011) and a free-living amoeba Naegleria gruberi (Fritz-Laylin et al. 2010). Both of them have a BLUF domain and a cyclase domain showing high similarity to the C-terminal half of PACα and PACβ, which implies that they represent an ancestral form of PAC.
7 Signal Transduction Chain
The signal transduction chain in photomovement of Euglena is still hypothetical. Diehn and Tollin have applied a number of inhibitors and uncouplers of photosynthetic phosphorylation and concluded that the main energy source for phototaxis is photophosphorylation (Diehn and Tollin 1967). However, these experiments were carried out with the so-called phototaxigraph and the observed results probably reflect the reduced motility of the cells due to impaired ATP production since they are in the dark until they enter the light field. In addition, this assumption is ruled out since bleached cells which lack photosynthesis show phototaxis.
Some researchers have speculated about a coupling of the flavin photoreceptor to the signal transduction chain via a cytochrome, but no experimental evidence is available (Fong and Schiff 1978; Gualtieri 1993b). Based on studies with ionophores, inhibitors, on channel blockers, various pH and ion concentrations, Doughty and Diehn proposed a mechanism for the step-down photophobic response (Diehn 1969b; Barghigiani et al. 1979a; Doughty and Diehn 1979, 1982, 1983, 1984) where upon irradiation the PAB is supposed to modulate the activity of a hypothetical NA+/K+ exchange pump in the flagellar membrane. This increases the intraflagellar sodium concentration which in turn opens sodium-controlled calcium channels allowing the influx of calcium. The increased calcium concentration finally results in a change in the flagellar beating pattern. However, experiments manipulating the external Ca2+ concentration indicated that a Ca2+ influx from the medium into the flagellar space is not essential for phototaxis (Meyer and Hildebrand 1988). In a theoretical study, Bovee and Jahn (1972) assumed that the PAB has piezoelectric properties. Upon irradiation it discharges and alters the position or shape of the flagellum and thus the swimming direction. Also Froehlich and Diehn (1974) suggested an electrical type of stimulus transduction by a flavin receptor pigment embedded in a lipid matrix. However, all drugs which impair the photophobic responses did not affect phototaxis: neither the application of ouabain, a specific inhibitor of the Na+/K+ exchange pump, nor gallopamin hydrochloride, an organic calcium channel blocker, affected the phototactic orientation in Euglena (Häder et al. 1987). In contrast, heavy metal ions (lead, copper, cadmium and mercury) strongly impaired phototactic orientation (Stallwitz 1992; Stallwitz and Häder 1993). The application of triphenylmethyl phosphonium ion (TPMP+ ) which is a lipophilic membrane-penetrating cation specifically inhibited positive phototaxis and reversed it to negative phototaxis, shifting the transition from positive to negative phototaxis to lower light intensities. These findings indicate that phototaxis might be controlled by a proton or cation gradient across the membrane (Colombetti et al. 1982). Therefore a number of researchers proposed that the membrane potential might be involved (Simons 1981; Harz et al. 1992). In fact, injecting negative electric pulses as well as changing the ionic environment of the cells (Ca2+ and Mg2+) changed the flagellar beating pattern (Nichols and Rikmenspoel 1977, 1978, 1980; Nichols et al. 1980; Tamponnet et al. 1988). But exposing swimming cells to an electric field had no effect on phototactic orientation in Euglena (Häder et al. 1987). Since Euglena shows very sensitive behavioral reactions to heavy metals and other pollutants at very low concentrations it is employed in bioassays based on computerized, on-line analysis of motility and orientation (Tahedl and Häder 2001; Häder 2004; Ahmed and Häder 2011; Azizullah et al. 2013).
The application of caffeine , an inhibitor of the phosphodiesterase, reversed the negative phototaxis at high irradiances (1000 W m−2) into a positive one (Richter et al. 2006). Ammonium ions specifically enhance step-down photophobic responses, as well as l-methionine-dl-sulfoximine, an inhibitor of ammonium assimilation (Matsunaga et al. 1999). In contrast, cycloheximide, an inhibitor of eukaryotic protein synthesis, impaired the step-down photophobic response and enhanced the step-up reaction, which was interpreted as suggesting that newly synthesized proteins are specific for the photoperception and signal transduction of the step-down photophobic response .
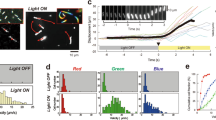
The photoreceptor for the step-up photophobic response and phototaxis has been revealed to be PAC, a light-dependent enzyme that produces cAMP upon blue light irradiation (Iseki et al. 2002; Ntefidou et al. 2003b). Intracellular cAMP levels in Euglena remarkably increased within 1 s after the onset of irradiation and then returned to the original level, corresponding well with the kinetics of the step-up photophobic response (Yoshikawa et al. 2005). Two possibilities had been postulated regarding the downstream signaling pathway from cAMP to the photobehavioral responses (Watanabe and Iseki 2005). One is that cAMP opens cyclic nucleotide-gated channels to facilitate an influx of Ca2+ that may modulate flagellar motility. The other is that cAMP activates a protein kinase A that may phosphorylate flagellar proteins to change the mode of flagellar beating. The latter seemed more plausible than the former because a catalytic subunit of a protein kinase had been cloned from Euglena (Kiriyama et al. 1999). Daiker et al. (2011) found that staurosporine, a protein kinase inhibitor, considerably blocked phototaxis as well as gravitaxis at low concentrations. Using PCR, five different kinases from Euglena were cloned. The blockage of only one of the kinases (PK.4) by RNAi suppressed both gravitaxis and phototaxis (Fig. 11.8), which suggested that PK.4 is the downstream component of cAMP signaling in both gravitaxis and phototaxis (Daiker et al. 2011). A hypothetical signaling cascade from PAC to a flagellar apparatus during step-up photophobic response and phototaxis in Euglena is summarized in Fig. 11.9. The photoreceptor molecule consisting of two PACα and PACβ subunits is activated by light absorbed by FAD bound to the BLUF domains. This activates the adenylyl cyclase domain which produces cAMP from ATP. cAMP in turn is believed to activate a protein kinase A inside the flagellum. The resulting phosphorylation of one or several proteins within the flagellum causes a change in the flagellar activity .
Inhibition of negative phototaxis by RNAi against protein kinase A PK.4 25 days after RNA knockdown (b). Control (a). Redrawn after (Daiker et al. 2011)
Assumed signal transduction chain for phototaxis and step-down photophobic responses in E. gracilis. After light activation of the photoreceptor molecule consisting of two PACα and PACβ subunits, the adenylyl cyclase domain produces cAMP from ATP which is believed to activate a protein kinase A inside the flagellum. The resulting phosphorylation of one or several proteins within the flagellum causes a change in the flagellar activity
8 Other Euglena Species
In contrast to E. gracilis, the green Euglena mutabilis does not possess flagella, but moves in a gliding fashion. The cells contain both a stigma and a PAB, which, however, differ in shape and size from the organelles found in E. gracilis. When exposed to lateral light the cells show positive phototaxis (Häder and Melkonian 1983). They swing left and right, as if to scan the light direction, and move in the direction of the light source. The precision of orientation increases with the light intensity up to 100 lx and then decreases again. Negative phototaxis was not observed. The action spectrum is completely different from that in E. gracilis as it has a number of peaks in the blue and green range of the spectrum but extends well into the red (Fig. 11.10). It can only be speculated about the nature of the photoreceptor. The peaks in the blue region might be due to the action of a flavin and the long wavelength sensitivity cold be due to the action of photosynthetic pigments as in the case of desmids (Wenderoth and Häder 1979). Since the degree of phototaxis is higher in white light than at any individual wavelength, regardless of the fluence rate, it could be speculated that phototaxis in this organism depends on the interaction of more than one photoreceptor. Since the cells do not rotate during locomotion a periodic shading mechanism can be excluded for the light direction perception.
Action spectrum of positive phototaxis in E. mutabilis based on fluence-rate response curves. Abscissa, wavelength in nm; ordinate, fraction of cells moving toward the light source within a sector ±30° as percentage of the fraction expected in this sector in a randomly oriented population. Redrawn after (Häder and Melkonian 1983)
E. mutabilis also shows step-up and step-down photophobic responses (Melkonian et al. 1986). When a cell moves in the light and enters a shaded area it bends away from the shade. By repeated responses it can maneuver along the dark/light boundary. The same behavior is found when a cell glides in a dark area and suddenly hits a bright area; the sudden increase in light intensity induces a step-up photophobic response and the cell turns away from the bright area.
Photokinesis has also been observed in E. mutabilis (Melkonian et al. 1986). In darkness less than 10% of the cell population are motile. The percentage increases when exposed to light at fluence rates >20 W m−2 (ca. 4000 lx) white light and reaches about 100% between 50 and 100 W m−2 when recorded 10 min after the onset of light. The action spectra for photokinesis as well as step-up and step-down resemble each other and also that of phototaxis.
The red colored freshwater Euglena sanguinea can be occasionally found in the neuston (top layer) of ponds (Gojdics 1939). The color is due to a high concentration of carotenoids such as β-carotene, astaxanthin-diester and diadinoxanthin. The cells possess flagella and orient precisely using positive phototaxis; negative phototaxis has not been observed even at irradiances of 600 klx, which is far in excess of solar radiation (Gerber and Häder 1994). The sensitivity to light is rather low as compared with E. gracilis and reaches a plateau at about 10 klx. Further work on this interesting organism was hampered by the fact that nobody has succeeded in cultivating this flagellate.
9 Conclusions and Future Directions
The mechanism for photoperception of phototaxis has been revealed by the finding that the photoreceptor is located in the paraxonemal body inside the trailing flagellum inside the reservoir. The PAB has a dichroic structure which is reflected in the polarotaxis in polarized light. The stigma aids in light direction perception by casting a shadow on the PAB when the cell rotates in lateral light during forward locomotion, however it is not indispensable as shown by the fact that stigmaless mutants are capable of a (modified) phototaxis. The long search for the molecular identity of the photoreceptor molecules has been terminated by the molecular biological identification of a photoactivated adenylyl cyclase (PAC) consisting of two α- and β-subunits each. Upon light activation these enzymes produce cAMP from ATP which has been found to activate a specific protein kinase (PK.4). The latter enzyme is thought to phosphorylate proteins inside the flagellum which result in a reorientation and course correction of the swimming path.
While the step-up photophobic response and both positive and negative phototaxis are mediated by PAC, the receptor for the step-down photophobic reaction has not yet been identified but proven not to be PAC. Also the photoreceptor for photokinesis and those for phototaxis in the gliding E. mutabilis as well as the red colored E. sanguinea still need to be revealed having completely different action spectra extending into the red region of the spectrum. Spectrofluorometric analysis has indicated an additional role for pterin s in the photoperception of E. gracilis. Their role and location are not yet completely resolved. While the location of PAC inside the PAB (and also in the flagellum outside the reservoir) was confirmed by confocal immunofluorescence, the location of the protein kinase needs to be determined. It is also not clear if further elements are involved in the sensory transduction chain. In addition, the cooperation with the other responses to environmental stimuli has to be elucidated including gravitaxis which uses the same protein kinase (PK.4) but operates with a different adenylyl cyclase. The proteins which control the bending of the trailing flagellum as well as their molecular action have not been characterized.
References
Ahmed H, Häder D-P (2011) Monitoring of waste water samples using the ECOTOX biosystem and the flagellate alga Euglena gracilis. Water Air Soil Pollut 216(1–4):547–560
de Araujo FFT, Pires MA, Frankel RB, Bicudo CEM (1986) Magnetite and magnetotaxis in algae. Biophys J 50:375–378
Ascoli C (1975) New techniques in photomotion methodology. In: Colombetti G (ed) Biophysics of photoreceptors and photobehaviour of microorganisms. Lito Felici, Pisa, pp 109–120
Azizullah A, Murad W, Adnan M, Ullah W, Häder D-P (2013) Gravitactic orientation of Euglena gracilis—a sensitive endpoint for ecotoxicological assessment of water pollutants. Front Environ Sci 1:4
Azizullah A, Jamil M, Richter P, Häder D-P (2014) Fast bioassessment of wastewater and surface water quality using freshwater flagellate Euglena gracilis—a case study from Pakistan. J Appl Phycol 26(1):421–431
Banchetti R, Rosati G, Verni F (1980) Cytochemical analysis of the photoreceptor in Euglena gracilis Klebs (Flagellata Euglenoidina). Monit Zool Ital (NS) 14:165–171
Barghigiani C, Colombetti G, Lenci F, Banchetti R, Bizzaro MP (1979a) Photosensory transduction in Euglena gracilis: effect of some metabolic drugs on the photophobic response. Arch Microbiol 120:239–245
Barghigiani C, Colombetti G, Tranchini B, Lenci F (1979b) Photobehavior of Euglena gracilis: action spectrum for the stepdown photophobic response of individual cells. Photochem Photobiol 29:1015–1019
Barsanti L, Passarelli V, Lenzi P, Gualtieri P (1992) Elimination of photoreceptor (paraflagellar swelling) and photoreception in Euglena gracilis by means of the carotenoid biosynthesis inhibitor nicotine. J Photochem Photobiol B Biol 13:135–144
Barsanti L, Passarelli V, Lenci P, Walne PL, Dunlap JR, Gualtieri P (1993a) Effects of hydroxylamine, digitonin and triton X-100 on photoreceptor (paraflagellar swelling) and photoreception of Euglena gracilis. Vis Res 33:2043–2050
Barsanti L, Evangelista V, Passarelli V, Frassanito AM, Gualtieri P (2012) Fundamental questions and concepts about photoreception and the case of Euglena gracilis. Integr Biol 4(1):22–36
Batra PP, Tollin G (1964) Phototaxis in Euglena. I. Isolation of the eye-spot granules and identification of the eye-spot pigments. Biochim Biophys Acta 79:371–378
Bendix SW (1960) Pigments in phototaxis. In: Allen MB (ed) Comparative Biochemistry of Photoreactive. Systems Academic Press, New York, pp 107–127
Benedetti PA, Checcucci A (1975) Paraflagellar body (PFB) pigments studied by fluorescence microscopy in Euglena gracilis. Plant Sci Lett 4:47–51
Benedetti PA, Lenci F (1977) In vivo microspectrofluorometry of photoreceptor pigments in Euglena gracilis. Photochem Photobiol 26:315–318
Benedetti PA, Bianchini G, Checcucci A, Ferrara R, Grassi S (1976) Spectroscopic properties and related functions of the stigma measured in living cells of Euglena gracilis. Arch Microbiol 111:73–76
Bensasson RW (1975) Spectroscopic and biological properties of carotenoids. In: Colombetti G (ed) Biophysics of Photoreceptors and Photobehaviour of Microorganisms. Lito Felici, Pisa, pp 146–163
Bouck GB (2012) Flagella and the cell surface. Physiology 3:29
Bound KE, Tollin G (1967) Phototactic response of Euglena gracilis to polarized light. Nature 216:1042–1044
Bovee EC, Jahn TL (1972) A theory of piezoelectric activity and ion movements in the relation of flagellar structures and their movements to the phototaxis of Euglena. J Theor Biol 35:259–276
Brodhun B, Häder D-P (1990) Photoreceptor proteins and pigments in the paraflagellar body of the flagellate Euglena gracilis. Photochem Photobiol 52:865–871
Brodhun B, Häder D-P (1993) UV-induced damage of photoreceptor proteins in the paraflagellar body of Euglena gracilis. Photochem Photobiol 58:270–274
Brodhun B, Häder D-P (1995a) A novel procedure to isolate the chromoproteins in the paraflagellar body of the flagellate Euglena gracilis. J Photochem Photobiol B Biol 28:39–45
Brodhun B, Häder D-P (1995b) UV-induced damage of photoreceptor pigments and proteins in the paraflagellar body of the flagellate Euglena gracilis. Proceedings of the first European symposium on the effects of environmental UV-B radiation on health and ecosystems, EUR, vol 15607, pp 33–332
Brodhun B, Neumann R, Hertel R, Häder D-P (1994) Riboflavin-binding sites in the flagella of Euglena gracilis and Astasia longa. J Photochem Photobiol B Biol 23:135–139
Bruce VG (1973) The role of the clock in controlling phototactic rhythms. In: Pérez-Miravete A (ed) Behaviour of Microorganisms. Plenum Press, New York, pp 257–266
Bruce VG, Pittendrigh C (1956) Temperature independence in a unicellular clock. Proc Natl Acad Sci U S A 42:676–682
Bruce VG, Pittendrigh CS (1958) Resetting the Euglena clock with a single light stimulus. Am Nat 92:295–306
Buder J (1919) Zur Kenntnis der phototaktischen Richtungsbewegungen. Jahrb Wiss Bot 58:105–220
Buetow DE (1968a) The Biology of Euglena. Academic Press, New York
Buetow DE (1968b) Morphology and ultrastructure of Euglena. In: Buetow DE (ed) The Biology of Euglena. Academic Press, New York, pp 109–184
Bünning E (1973) The Physiological Clock, 3rd edn. English Univ. Press, London
Capaldo CT, Farkas AE, Nusrat A (2014) Epithelial adhesive junctions. F1000prime reports 6
Carre IA, Laval-Martin DL, Edmunds LN Jr (1989) Circadian changes in cyclic AMP levels in synchronously dividing and stationary-phase cultures of the achlorophyllous ZC mutant of Euglena gracilis. J Cell Sci 94:267–272
Checcucci A, Colombetti G, del Carratore G, Ferrara R, Lenci F (1974) Red light induced accumulation of Euglena gracilis. Photochem Photobiol 19:223–226
Checcucci A, Favati L, Grassi S, Piaggesi T (1975) The measurement of phototactic activity in Euglena gracilis Klebs. Monit Zool Ital 9:83–98
Checcucci A, Colombetti G, Ferrara R, Lenci F (1976) Action spectra for photoaccumulation of green and colorless Euglena: evidence for identification of receptor pigments. Photochem Photobiol 23:51–54
Clayton R (1959) Phototaxis of purple bacteria. Handbuch der Pflanzenphysiologie 17/1:371–387
Clayton RK (1964) Phototaxis in microorganisms. In: Giese AC (ed) Photophysiology, vol 2. Academic Press, New York, pp 51–77
Colombetti G, Diehn B (1978) Chemosensory responses toward oxygen in Euglena gracilis. J Protozool 25:211–217
Colombetti G, Häder D-P, Lenci F, Quaglia M (1982) Phototaxis in Euglena gracilis: effect of sodium azide and triphenylmethyl phosphonium ion on the photosensory transduction chain. Curr Microbiol 7:281–284
Creutz C, Diehn B (1976) Motor responses to polarized light and gravity sensing in Euglena gracilis. J Protozool 23:552–556
Cypionka H (2010) Eukaryotische Mikroorganismen. Grundlagen der Mikrobiologie 47–60
Daiker V, Häder D-P, R. RP, Lebert M (2011) The involvement of a protein kinase in phototaxis and gravitaxis of Euglena gracilis. Planta 233:1055–1062.
Diehn B (1969a) Action spectra of the phototactic responses in Euglena. Biochim Biophys Acta 177:136–143
Diehn B (1969b) Phototactic responses of Euglena to single and repetitive pulses of actinic light: orientation time and mechanism. Exp Cell Res 56:375–381
Diehn B (1969c) Two perpendicularly oriented pigment systems involved in phototaxis of Euglena. Nature 122:366–367
Diehn B (1973) Phototaxis in Euglena. 1. Physiological basis of photoreception and tactic orientation. In: Pérez-Miravete A (ed) Behaviour of Microorganisms. Plenum Press, New York, pp 83–90
Diehn B, Tollin G (1966) Phototaxis in Euglena. II. Physical factors determining the rate of phototactic response. Photochem Photobiol 5:523–557
Diehn B, Tollin G (1967) Phototaxis in Euglena. IV. Effect of inhibitiors of oxidative and photophosphorylation on the rate of phototaxis. Arch Biochem Biophys 121:169–177
Diehn B, Fonseca JR, Jahn TR (1975) High speed cinemicrography of the direct photophobic response of Euglena and the mechanism of negative phototaxis. J Protozool 22:492–494
Diskus A (1955) Färbestudien an den Schleimkörperchen und Schleimausscheidungen einiger Euglenen. Protoplasma 45:460–477
Dodge JD (1969) A review of the fine structure of algal eyespots. Brit Phycol J 4:199–210
Doughty MJ (1991) A kinetic analysis of the step-up photophobic response of the flagellated alga Euglena gracilis in culture medium. J Photochem Photobiol B Biol 9:75–85
Doughty MJ, Diehn B (1979) Photosensory transduction in the flagellated alga, Euglena gracilis. I. Action of divalent cations Ca2+ antagonists and Ca2+ ionophore on motility and photobehavior. Biochim Biophys Acta 588:148–168
Doughty MJ, Diehn B (1982) Photosensory transduction in the flagellated alga, Euglena gracilis. III. Induction of Ca2+-dependent responses by monovalent cation ionophores. Biochim Biophys Acta 682:32–43
Doughty MJ, Diehn B (1983) Photosensory transduction in the flagellated alga, Euglena gracilis. IV. Long term effects of ions and pH on the expression of step-down photobehaviour. Arch Microbiol 134:204–207
Doughty MJ, Diehn B (1984) Anion sensitivity of motility and step-down photophobic responses of Euglena gracilis. Arch Microbiol 138:329–332
Edmunds LN Jr (1984) Physiology of circadian rhythms in microorganisms. In: Rose AH, Tempest DW (eds) Advances in Microbial Physiology, vol 25. Academic Press, London, pp 61–148
Engelmann TW (1883) Bakterium photometricum. Ein Beitrag zur vergleichenden Physiologie des Licht- und Farbensinnes. Pflugers Arch 30:95–124
Evangelista V, Passarelli V, Barsanti L, Gualtieri P (2003) Fluorescence behavior of Euglena photoreceptor. Photochem Photobiol 78(1):93–97
Falke JJ, Bass RB, Butler SL, Chervitz SA, Danielson MA (1997) The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol 13:457–512
Feinleib ME (1975) Phototactic response of Chlamydomonas to flashes of light. I. Response of cell population. Photochem Photobiol 21:351–354
Feinleib ME, Curry GM (1967) Methods for measuring phototaxis of cell populations and individual cells. Physiol Plant 20:1083–1095
Feinleib MEH, Curry GM (1971) The relationship between stimulus intensity and oriented phototactic response (topotaxis) in Chlamydomonas. Physiol Plant 25:346–352
Feldman JF, Bruce VG (1972) Circadian rhythm changes in autotrophic Euglena induced by organic carbon sources. J Protozool 19:370–373
Fenchel T (2013) Ecology of Protozoa: The Biology of Free-living Phagotropic Protists. Springer-Verlag, Berlin
Ferrara R, Banchetti R (1976) Effect of streptomycin on the structure and function of the photoreceptor apparatus of Euglena gracilis. J Exp Zool 198:393–402
Fong F, Schiff JA (1978) Blue-light absorbance changes and phototaxis in Euglena. Plant Physiol 61(Suppl):74
Fong F, Schiff JA (1979) Blue-light-inducted absorbance changes associated with carotenoids in Euglena. Planta 146:119–127
Forreiter C, Wagner G (2012) Photomovement versus photoadaptation. Progr Bot Genet Physiol System Ecol 64:258
Foster KW (2001) Action spectroscopy of photomovement. In: Häder D-P, Lebert M (eds) Photomovement, vol 1. Elsevier, Amsterdam, pp 51–115
Foster KW, Smyth RD (1980) Light antennas in phototactic algae. Microbiol Rev 44:572–630
France RH (1908) Experimentelle Untersuchungen über Reizbewegungen und Lichtsinnesorgane der Algen. Ztschrift Ausbau Entwicklungslehre 2:29–43
France RH (1909) Untersuchungen über die Sinnesorganfunktion der Augenflecke bei Algen. Arch Hydrobiol 4:37–48
Frey-Wyssling A, Mühlethaler K (1960) Über den Feinbau der Euglena-Zelle. Schweiz Z Hydrol 22:122–130
Fritz-Laylin LK, Prochnik SE, Ginger ML, Dacks JB, Carpenter ML, Field MC, Kuo A, Paredez A, Chapman J, Pham J (2010) The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 140(5):631–642
Froehlich O, Diehn B (1974) Photoeffects in a flavin-containing lipid bilayer membrane and implications for algal phototaxis. Nature 248:802–804
Fujiyoshi S, Hirano M, Matsushita M, Iseki M, Watanabe M (2011) Structural change of a cofactor binding site of flavoprotein detected by single-protein fluorescence spectroscopy at 1.5 K. Phys Rev Lett 106(7):078101
Galland P, Senger H (1988a) The role of flavins as photoreceptors. J Photochem Photobiol B Biol 1:277–294
Galland P, Senger H (1988b) The role of pterins in the photoreception and metabolism of plants. Photochem Photobiol 48:811–820
Galland P, Keiner P, Dörnemann D, Senger H, Brodhun B, Häder D-P (1990) Pterin- and flavin-like fluorescence associated with isolated flagella of Euglena gracilis. Photochem Photobiol 51:675–680
Gerber S, Häder D-P (1993) Effects of solar irradiation on motility and pigmentation of three species of phytoplankton. Environ Exp Bot 33:515–521
Gerber S, Häder D-P (1994) Effects of enhanced UV-B irradiation on the red coloured freshwater flagellate Euglena sanguinea. FEMS Microbiol Ecol 13:177–184
Gerber S, Häder D-P (1995) Effects of artificial UV-B and simulated solar radiation on the flagellate Euglena gracilis: physiological, spectroscopical and biochemical investigations. Acta Protozool 34:13–20
Gerber S, Biggs A, Häder D-P (1996) A polychromatic action spectrum for the inhibition of motility in the flagellate Euglena gracilis. Acta Protozool 35:161–165
Ghetti F, Colombetti G, Lenci F, Campani E, Polacco E, Quaglia M (1985) Fluorescence of Euglena gracilis photoreceptor pigment: an in vitro microspectrofluorometric study. Photochem Photobiol 42:29–33
Giometto A, Altermatt F, Maritan A, Stocker R, Rinaldo A (2015) Generalized receptor law governs phototaxis in the phytoplankton Euglena gracilis. Proc Natl Acad Sci U S A 112(22):7045–7050
Gojdics M (1939) Some observations on Euglena sanguinea Ehrbg. Trans Am Microsc Soc 58:241–248
Gomelsky M, Kaplan S (1995) appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol 177:4609–4618
Gomelsky M, Kaplan S (1998) AppA, a redox regulator of photosystem formation in Rhodobacter sphaeroides 2.4.1, is a flavoprotein. Identification of a novel FAD binding domain. J Biol Chem 273:35319–35325
Gössel I (1957) Über das Aktionsspektrum der Phototaxis chlorophyllfreier Euglenen und über die Absorption des Augenflecks. Arch Microbiol 27:288–305
Govorunova EG, Sineshchekov OA (2005) Chemotaxis in the green flagellate alga Chlamydomonas. Biochemistry (Mosc) 70(7):717–725
Govorunova EG, Jung KH, Sineshchekov OA, Spudich JL (2004) Chlamydomonas sensory rhodopsins A and B: cellular content and role in photophobic responses. Biophys J 86(4):2342–2349
Gualtieri P (1993a) A biological point of view on photoreception (no-imaging vision) in algae. J Photochem Photobiol B Biol 18:95–100
Gualtieri P (1993b) Euglena gracilis: is the photoreception enigma solved? J Photochem Photobiol B Biol 19:3–14
Gualtieri P (2001) Rhodopsin-like-proteins: light detection pigments in Leptolyngbya, Euglena, Ochromonas, Pelvetia. In: Häder D-P, Lebert M (eds) Photomovement, vol 1. Elsevier, Amsterdam, pp 281–295
Gualtieri P, Barsanti L, Rosati G (1986) Isolation of the photoreceptor (paraflgellar body) of the phototactic flagellate Euglena gracilis. Arch Microbiol 145:303–305
Häder D-P (1979) Photomovement. In: Haupt W, Feinleib ME (eds) Encyclopedia of Plant Physiology, New Series, vol 7. Springer, Berlin, Heidelberg, pp 268–309
Häder D-P (1985) Effect of UV-B on motility and photobehavior in the green flagellate, Euglena gracilis. Arch Microbiol 141:159–163
Häder D-P (1986) Effects of solar and artificial UV irradiation on motility and phototaxis in the flagellate Euglena gracilis. Photochem Photobiol 44:651–656
Häder D-P (1987a) Photomovement in eukaryotic microorganisms. Photobiochem Photobiophys, Suppl: 203–214
Häder D-P (1987b) Polarotaxis, gravitaxis and vertical phototaxis in the green flagellate, Euglena gracilis. Arch Microbiol 147:179–183
Häder D-P (1991) Phototaxis and gravitaxis in Euglena gracilis. In: Lenci F, Ghetti F, Colombetti G, Häder D-P, Song P-S (eds) Biophysics of Photoreceptors and Photomovements in Microorganisms. Plenum Press, New York, pp 203–221
Häder D-P (1993) Simulation of phototaxis in the flagellate Euglena gracilis. J Biol Phys 19:95–108
Häder D-P (1997) Gravitaxis and phototaxis in the flagellate Euglena studied on TEXUS missions. In: Cogoli A, Friedrich U, Mesland D, Demets R (eds) Life Science Experiments Performed on Sounding Rockets (1985–1994). ESTEC, ESA Publications Division, Noordwijk, pp 77–79
Häder D-P (1998) Orientierung im Licht: Phototaxis bei Euglena gracilis. Mikrokosmos 87:3–11
Häder D-P (2003) UV-B impact on the life of aquatic plants. In: Ambasht RS, Ambasht NK (eds) Modern Trends in Applied Aquatic Ecology. Kluwer Acad./Plenum Publ, New York, pp 149–172
Häder D-P (2004) Photoecology and environmental photobiology. In: Horspool W, Lenci F (eds) CRC Handbook of Organic Photochemistry and Photobiology, vol 2. CRC Press, Boca Raton, pp 1161–1167
Häder D-P, Brodhun B (1991) Effects of ultraviolet radiation on the photoreceptor proteins and pigments in the paraflagellar body of the flagellate, Euglena gracilis. J Plant Physiol 137:641–646
Häder D-P, Griebenow K (1988) Orientation of the green flagellate, Euglena gracilis, in a vertical column of water. FEMS Microbiol Ecol 53:159–167
Häder D-P, Häder MA (1988) Inhibition of motility and phototaxis in the green flagellate, Euglena gracilis, by UV-B radiation. Arch Microbiol 150:20–25
Häder D-P, Lebert M (1998) The photoreceptor for phototaxis in the photosynthetic flagellate Euglena gracilis. Photochem Photobiol 68:260–265
Häder D-P, Lebert M (2000) Real-time tracking of microorganisms. In: Häder D-P (ed) Image Analysis: Methods and Applications. CRC Press, Boca Raton, pp 393–422
Häder D-P, Lebert M 2009 Photoorientation in photosynthetic flagellates. In: Jin T, Hereld D, editors. Methods in Molecular Biology. Totowa: Humana Press. 571. p. 51–65.
Häder D-P, Lipson ED (1986) Fourier analysis of angular distributions for motile microorganisms. Photochem Photobiol 44:657–663
Häder D-P, Liu SM (1991) Biochemical isolation and spectroscopic characterization of possible photoreceptor pigments for phototaxis in a freshwater Peridinium. Photochem Photobiol 54:143–146
Häder D-P, Melkonian M (1983) Phototaxis in the gliding flagellate, Euglena mutabilis. Arch Microbiol 135:25–29
Häder D-P, Reinecke E (1991) Phototactic and polarotactic responses of the photosynthetic flagellate, Euglena gracilis. Acta Protozool 30:13–18
Häder D-P, Colombetti G, Lenci F, Quaglia M (1981) Phototaxis in the flagellates, Euglena gracilis and Ochromonas danica. Arch Microbiol 130:78–82
Häder D-P, Lebert M, Di Lena MR (1986) New evidence for the mechanism of phototactic orientation of Euglena gracilis. Curr Microbiol 14:157–163
Häder D-P, Lebert M, DiLena MR (1987) Effects of culture age and drugs on phototaxis in the green flagellate, Euglena gracilis. Plant Physiol 6:169–174
Häder D-P, Ntefidou M, Iseki M, Watanabe M (2005) Phototaxis photoreceptor in Euglena gracilis. In: Wada M, Shimazaki K, Iino M (eds) Light Sensing in Plants. Springer, Tokyo, pp 223–229
Häder D-P, Richter P, Villafañe VE, Helbling EW (2014) Influence of light history on the photosynthetic and motility responses of Gymnodinium chlorophorum exposed to UVR and different temperatures. J Photochem Photobiol B Biol 138:273–281
Harz H, Nonnengässer C, Hegemann P (1992) The photoreceptor current of the green alga Chlamydomonas. Philos Trans R Soc London B 338:39–52
Hasle RG (1950) Phototactic vertical migration in marine dinoflagellates. Oikos 2:162–175
Haupt W (1959) Die Phototaxis der Algen. Handbuch der Pflanzenphysiologie 17(1):318–370
Heelis DV, Kernick W, Philips GO, Davies K (1979) Separation and identification of the carotenoid pigments of stigmata isolated from light-grown cells of Euglena gracilis strain Z. Arch Microbiol 121:207–211
Heelis DV, Heelis PF, Kernick WA, Phillips GO (1980) The stigma of Euglena gracilis strain Z: an investigation into the possible occurance of carotenoproteins and nuleic acids. Cytobios 29:135–143
Hill NA, Häder D-P (1997) A biased random walk for the trajectories of swimming micro-organisms. J Theor Biol 186:503–526
Hill N, Plumpton L (2000) Control strategies for the polarotactic orientation of the microorganism Euglena gracilis. J Theor Biol 203(4):357–365
Hill NA, Vincent RV (1993) A simple model and strategies for orientation in phototactic microorganisms. J Theor Biol 163:223–235
Hu C, Wang S, Guo L, Xie P (2014) Effects of the proximal factors on the diel vertical migration of zooplankton in a plateau meso-eutrophic Lake Erhai, China. J Limnol 73(2):375–386
Hyams JS (1982) The Euglena paraflagellar rod: structure, relationship to other flagellar components and preliminary biochemical characterization. J Cell Sci 55:199–210
Inaba K, Mizuno K, Shiba K (2014) Structure, function, and phylogenetic consideration of calaxin. In: Sexual Reproduction in Animals and Plants. Springer, Tokyo, pp 49–57
Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida C, Sugai M, Takahashi T, Hori T, Watanabe M (2002) A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 415:1047–1051
Ito S, Murakami A, Sato K, Nishina Y, Shiga K, Takahashi T, Higashi S, Iseki M, Watanabe M (2005) Photocycle features of heterologously expressed and assembled eukaryotic flavin-binding BLUF domains of photoactivated adenylyl cyclase (PAC), a blue light receptor in Euglena gracilis. Photochem Photobiol Sci 4:762–769
Ito S, Murakami A, Iseki M, Takahashi T, Higashi S, Watanabe M (2010) Differentiation of photocycle characteristics of flavin-binding BLUF domains of α-and β-subunits of photoactivated adenylyl cyclase of Euglena gracilis. Photochem Photobiol Sci 9(10):1327–1335
Itoh A, Tamura W (2008) Object manipulation by a formation-controlled Euglena group. In: Bio-mechanisms of Swimming and Flying. Springer, Tokyo, pp 41–52
Iwata T, Watanabe A, Iseki M, Watanabe M, Kandori H (2011) Strong donation of the hydrogen bond of tyrosine during photoactivation of the BLUF domain. J Phys Chem Lett 2(9):1015–1019
Iwatsuki K (1992) Stentor coeruleus shows positive photokinesis. Photochem Photobiol 55:469–471
James TW, Crescitelli F, Loew ER, McFarland WN (1992) The eyespot of Euglena gracilis: a microspectrophotometric study. Vis Res 32:1583–1591
Jennings HS (1906) Behavior of the Lower Organisms. Columbia University Press, New York
Johnson CH, Kondo T, Hastings JW (1991) Action spectrum for resetting the circadian phototaxis rhythm in the CW15 strain of Chlamydomonas. Plant Physiol 97:1122–1129
Josef K, Saranak J, Foster KW (2005) Ciliary behavior of a negatively phototactic Chlamydomonas reinhardtii. Cell Motil Cytoskeleton 61:97–111
Karnkowska A, Bennett MS, Watza D, Kim JI, Zakryś B, Triemer RE (2015) Phylogenetic relationships and morphological character evolution of photosynthetic Euglenids (Excavata) inferred from taxon-rich analyses of five genes. J Eukaryot Microbiol 62(3):362–373
Kavaliers M, Ossenkopp K-P (1994) Effects of magnetic and electric fields in invertebrates and lower vertebrates. In: Carpenter DO, Ayrapetyan S (eds) Biological Effects of Electric and Magnetic Fields. Sources and Mechanisms, vol 1. Academic Press Inc., San Diego, pp 205–240
Kessler JO, Hill NA, Häder D-P (1992) Orientation of swimming flagellates by simultaneously acting external factors. J Phycol 28:816–822
Kim D (2013) Control of Tetrahymena pyriformis as a microrobot. PhD thesis, Drexel University
Kim YJ, Chizhov I, Engelhard M (2009) Functional expression of the signaling complex sensory rhodopsin II/transducer II from Halobacterium salinarum in Escherichia coli. Photochem Photobiol 85(2):521–528
Kiriyama H, Nanmori T, Hari K, Matsuoka D, Fukami Y, Kikkawa U, Yasuda T (1999) Identification of the catalytic subunit of cAMP-dependent protein kinase from the photosynthetic flagellate, Euglena gracilis Z. FEBS Lett 450(1):95–100
Kisielewska G, Kolicka M, Zawierucha K (2015) Prey or parasite? The first observations of live Euglenida in the intestine of Gastrotricha. Eur J Protistol 51(2):138–141
Kivic PA, Vesk M (1972a) Structure and function in the euglenoid eyespot apparatus: The fine structure, and response to environmental changes. Planta 105:1–14
Kivic PA, Vesk M (1972b) Structure and function of the euglenoid eyespot. The probable location of the phototaxis photoreceptor. J Exp Bot 23:1070–1075
Kivic PA, Vesk M (1974a) Pinocytotic uptake of protein from the reservoir in Euglena. Arch Microbiol 96:155–159
Kivic PA, Vesk M (1974b) The structure of the eyespot apparatus in bleached strains of Euglena gracilis. Cytobiologie 10:88–101
Kivic PA, Walne PL (1983) Algal photosensory apparatus probably represent multiple parallel evolutions. Biosystems 16:31–38
Komsic-Buchmann K, Becker B (2012) Contractile Vacuoles in Green Algae–Structure and Function. Advances in Algal Cell Biology. Walter de Gruyter, Berlin, pp 123–141
Koumura Y, Suzuki T, Yoshikawa S, Watanabe M, Iseki M (2004) The origin of photoactivated adenylyl cyclase (PAC), the Euglena blue-light receptor: phylogenetic analysis of orthologues of PAC subunits from several euglenoids and trypanosome-type adenylyl cyclases from Euglena gracilis. Photochem Photobiol Sci 3(6):580–586
Krause K (2008) From chloroplasts to “cryptic” plastids: evolution of plastid genomes in parasitic plants. Curr Genet 54(3):111–121
Kreimer G (1994) Cell biology of phototaxis in flagellate algae. Int Rev Cytol 148:229–309
Kreimer G, Melkonian M (1990) Reflection confocal laser scanning microscopy of eyespots in flagellated green algae. Eur J Cell Biol 53:101–111
Krinsky NI, Goldsmith TH (1960) The carotenoids of the flagellated alga, Euglena gracilis. Arch Biochem Biophys 91(2):271–279
Kronestedt E, Walles B (1975) On the presence of plastids and the eyespot apparatus in a porfiromycin-bleached strain of Euglena gracilis. Protoplasma 84:75–82
Kühnel-Kratz C, Schäfer J, Häder D-P (1993) Phototaxis in the flagellate, Euglena gracilis, under the effect of microgravity. Microgravity Sci Technol 6:188–193
Leander BS, Witek RP, Farmer MA (2001) Trends in the evolution of the euglenid pellicle. Evolution 55:2215–2235
Lebert M (2001) Phototaxis of Euglena gracilis - flavins and pterins. In: Häder D-P, Lebert M (eds) Photomovement, vol 1. Elsevier, Amsterdam, pp 297–341
Lebert M, Häder D-P (1997) Behavioral mutants of Euglena gracilis: functional and spectroscopic characterization. J Plant Physiol 151:188–195
Lebert M, Häder D-P (2000) Photoperception and phototaxis in flagellated algae. Res Adv Photochem Photobiol 1:201–226
Lebert M, Porst M, Häder D-P (1999) Circadian rhythm of gravitaxis in Euglena gracilis. J Plant Physiol 155:344–349
Leedale GF (1982) Ultrastructure. In: Buetow DE (ed) The Biology of Euglena. Physiology, vol 3. Academic Press, New York, pp 1–27
Lenci F, Colombetti G, Häder D-P (1983) Role of flavin quenchers and inhibitors in the sensory transduction of the negative phototaxis in the flagellate, Euglena gracilis. Curr Microbiol 9:285–290
Lenci F, Häder D-P, Colombetti G (1984) Photosensory responses in freely motile microorganisms. In: Colombetti G, Lenci F (eds) Membranes and Sensory Transduction. Plenum Press, New York, pp 199–229
Lenci F, Ghetti F, Colombetti G, Häder D, Song P-S (2012) Biophysics of photoreceptors and photomovements in microorganisms. Springer Science & Business Media
Lindes DA, Diehn B, Tollin G (1965) Phototaxigraph: recording instrument for determination of rate of response of phototactic microorganisms to light of controlled intensity and wavelength. Rev Sci Instrum 36:1721–1725
Liu SM, Häder D-P (1994) Isolation and characterization of proteins from the putative photoreceptor for positive phototaxis in the dinoflagellate, Peridinium gatunense Nygaard. Photochem Photobiol 59:86–90
Lüdtke T, Häder D-P (2007) Molecular genetics of the novel photoreceptor PAC in euglenophytes and bacteria. In: Thangadurai D, Tang W, Pullaiah T (eds) Genes, Genomes & Genomics, vol 2. Vedams eBooks Ltd., New Delhi, pp 189–200
Ma Z, Helbling EW, Li W, Villafañe VE, Gao K (2012) Motility and photosynthetic responses of the green microalga Tetraselmis subcordiformis to visible and UV light levels. J Appl Phycol 24(6):1613–1621
Mast SO (1911) Light and Behavior of Organisms. Chapman & Hall ltd., London
Mast SO (1914) Orientation in Euglena with some remarks on tropisms. Biol Zent Bl 34:641–664
Masuda S (2013) Light detection and signal transduction in the BLUF photoreceptors. Plant Cell Physiol 54(2):171–179
Masuda S, Bauer CE (2002) AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 110:613–623
Matsunaga S, Hori T, Takahashi T, Kubota M, Watanabe M, Okamoto K, Masuda K, Sugai M (1998) Discovery of signaling effect of UV-B/C light in the extended UV-A/blue-type action spectra for step-down and step-up photophobic responses in the unicellular flagellate alga Euglena gracilis. Protoplasma 201:45–52
Matsunaga S, Takahashi T, Watanabe M, Sugai M, Hori T (1999) Control by ammonium ion of the change from step-up to step-down photophobically responding cells in the flagellate alga Euglena gracilis. Plant Cell Physiol 40:213–221
Melkonian M, Meinicke-Liebelt M, Häder D-P (1986) Photokinesis and photophobic responses in the gliding flagellate, Euglena mutabilis. Plant Cell Physiol 27:505–513
Meyer R, Hildebrand E (1988) Phototaxis of Euglena gracilis at low external calcium concentration. J Photochem Photobiol B Biol 2(4):443–453
Michel H (1990) General and practical aspects of membrane protein crystallization. In: Michel H (ed) Crystallization of Membrane Proteins. CRC Press, Boca Raton, FL, pp 73–89
Mikolajczyk E (1984a) Photophobic responses in Euglenina. 1. Effects of excitation wavelength and external medium on the step-up response of light- and dark-grown Euglena gracilis. Acta Protozool 23:1–10
Mikolajczyk E (1984b) Photophobic responses in Euglenina: 2. Sensitivity to light of the colorless flagellate Astasia longa in low and high viscosity medium. Acta Protozool 23:85–92
Mikolajczyk E, Diehn B (1975) The effect of potassium iodide on photophobic responses in Euglena: evidence for two photoreceptor pigments. Photochem Photobiol 22:269–271
Mikolajczyk E, Diehn B (1976) Light-induced body movement of Euglena gracilis coupled to flagellar photophobic responses by mechanical stimulation. J Protozool 23:144–147
Mikolajczyk E, Diehn B (1978) Morphological alteration in Euglena gracilis induced by treatment with CTAB (Cetyltrimethylammonium bromide) and Triton X-100: correlations with effects on photophobic behavioral responses. J Protozool 25:461–470
Mikolajczyk E, Diehn B (1979) Mechanosensory responses and mechanoreception in Euglena gracilis. Acta Protozool 18:591–602
Mikolajczyk E, Kuznicki L (1981) Body contraction and ultrastructure of Euglena. Acta Protozool 20:1–24
Murray JM (1981) Control of cell shape by calcium in the Euglenophyceae. J Cell Sci 49:99–117
Nagahama T, Suzuki T, Yoshikawa S, Iseki M (2007) Functional transplant of photoactivated adenylyl cyclase (PAC) into Aplysia sensory neurons. Neurosci Res 59(1):81–88
Nakaoka Y, Tokioka R, Shinozawa T, Fujita J, Usukura J (1991) Photoreception of Paramecium cilia: localization of photosensitivity and binding with anti-frog-rhodopsin IgG. J Cell Sci 99:67–72
Nasir A (2014) Analysis of the gravitaxis signal transduction chain in Euglena gracilis. 40th COSPAR Scientific Assembly. Held 2–10 August 2014, in Moscow, Russia, Abstract F1. 1–18-14. p 2234
Nebenführ A, Schäfer A, Galland P, Senger H, Hertel R (1991) Riboflavin-binding sites associated with flagella of Euglena: a candidate for blue-light photoreceptor? Planta 185:65–71
Neumann R, Hertel R (1994) Purification and characterization of a riboflavin-binding protein from flagella of Euglena gracilis. Photochem Photobiol 60:76–83
Ngô HM, Bouck GB (1998) Heterogeneity and a coiled coil prediction of trypanosomatid-like flagellar rod proteins in Euglena. J Eukaryot Microbiol 45:323–333
Nichols KM, Rikmenspoel R (1977) Mg2+-dependent electrical control of flagellar activity in Euglena. J Cell Sci 23:211–225
Nichols KM, Rikmenspoel R (1978) Control of flagellar motion in Chlamydomonas and Euglena by mechanical microinjection of Mg2+ and Ca2+ and by electric current injection. J Cell Sci 29:233–247
Nichols KM, Rikmenspoel R (1980) Flagellar waveform reversal in Euglena. Exp Cell Res 129:377–381
Nichols KM, Jacklet A, Rikmenspoel R (1980) Effects of Mg2+ and Ca2+ on photoinduced Euglena flagellar responses. J Cell Biol 84:355–363
Ntefidou M, Häder D-P (2005) Photoactivated adenylyl cyclase (PAC) genes in the flagellate Euglena gracilis mutant strains. Photochem Photobiol Sci 4:732–739
Ntefidou M, Iseki M, Richter P, Streb C, Lebert M, Watanabe M, Häder D-P (2003a) RNA interference of genes involved in photomovement in Astasia longa and Euglena gracilis mutants. Rec Res Dev Biochem 4:925–930
Ntefidou M, Iseki M, Watanabe M, Lebert M, Häder D-P (2003b) Photoactivated adenylyl cyclase controls phototaxis in the flagellate Euglena gracilis. Plant Physiol 133(4):1517–1521
Ntefidou M, Lüdtke T, Ahmad M, Häder D-P (2006) Heterologous expression of photoactivated adenylyl cyclase (PAC) genes from the flagellate Euglena gracilis in insect cells. Photochem Photobiol 82:1601–1605
Nultsch W (1975) Phototaxis and photokinesis. In: Carlile MJ (ed) Primitive Sensory and Communication Systems. Academic Press, New York, pp 29–90
Nultsch W, Häder D-P (1970) Bestimmungen der photo-phobotaktischen Unterschiedsschwelle bei Phormidium uncinatum. Ber Dtsch Bot Ges 83:185–192
Nultsch W, Häder D-P (1979) Photomovement of motile microorganisms. Photochem Photobiol 29:423–437
Nultsch W, Häder D-P (1988) Photomovement in motile microorganisms—II. Photochem Photobiol 47:837–869
Nultsch W, Throm G (1975) Effect of external factors on phototaxis of Chlamydomonas reinhardtii. I. Light. Arch Microbiol 103:175–179
Oesterhelt D (1998) The structure and mechanism of the family of retinal proteins from halophilic archaea. Curr Opin Struct Biol 8:489–500
Omodeo P (1975) Phototactic system morphology: Florenz
Omodeo P (1980) The photoreceptive apparatus of flagellated algal cells: Comparative morphology and some hypothesis on functioning. In: Lenci F, Colombetti G (eds) Photoreception and Sensory Transduction in Aneural Organisms. Plenum Press, New York, pp 127–154
Omodeo P (2013) Istituto di Biologia Animale de11'Università di Padova 35100 Padova, Italy. Photoreception and Sensory Transduction in Aneural Organisms 33: 127
Osafune T, Schiff JA (1980) Stigma and flagellar swelling in relation to light and carotenoids in Euglena gracilis var. bacillaris. J Ultrastruct Res 73:336–349
Ozasa K, Lee J, Song S, Hara M, Maeda M (2013) Gas/liquid sensing via chemotaxis of Euglena cells confined in an isolated micro-aquarium. Lab Chip 13(20):4033–4039
Ozasa K, Lee J, Song S, Maeda M (2014) Transient freezing behavior in photophobic responses of Euglena gracilis investigated in a microfluidic device. Plant Cell Physiol 55(10):1704–1712
Peacock MB, Kudela RM (2014) Evidence for active vertical migration by two dinoflagellates experiencing iron, nitrogen, and phosphorus limitation. Limnol Oceanogr 59(3):660–673
Petersen-Mahrt SK, Ekelund NGA, Widell S (1994) Influence of UV-B radiation and nitrogen starvation on daily rhythms in phototaxis and cell shape of Euglena gracilis. Physiol Plant 92:501–505
Piccinni E, Mammi M (1978) Motor apparatus of Euglena gracilis: ultrastructure of the basal portion of the flagellum and the paraflagellar body. Bollettino di Zoologia 45:405–414
Poniewozik M (2014) The euglenoid genera Astasia and Menoidium (Euglenozoa) from eastern Poland. Nova Hedwigia 99(1–2):193–212
Porterfield DM (1997) Orientation of motile unicellular algae to oxygen: Oxytaxis in Euglena. Biol Bull 193:229–230
Pringsheim EG (1937) Über das Stigma bei farblosen Flagellaten. Cytologia 1:234–255
Pringsheim EG (1948) The loss of chromatophores in Euglena gracilis. New Phytol 47:52–87
Rhiel E, Häder D-P, Wehrmeyer W (1988) Diaphototaxis and gravitaxis in a freshwater Cryptomonas. Plant Cell Physiol 29:755–760
Richter P, Ntefidou M, Streb C, Lebert M, Häder D-P (2002) Cellular perception and transduction mechanisms of gravity in unicellular organisms. Curr Top Plant Biol 3:143–154
Richter PR, Streb C, Häder D-P (2006) Sign change of phototaxis in Euglena gracilis. Trends Photochem Photobiol 11:57–61
Richter P, Helbling W, Streb C, Häder D-P (2007) PAR and UV effects on vertical migration and photosynthesis in Euglena gracilis. Photochem Photobiol 83:818–823
Robenek H, Melkonian M (1983) Structural specialization of the paraflagellar membrane of Euglena. Protoplasma 117:154–157
Rosati GF, Verni L, Barsanti V, Passarelli V, Gualtieri P (1991) Ultrastructure of the apical zone of Euglena gracilis: photoreceptors and motor apparatus. Electron Microsc Rev 4:319–342
Rosati G, Barsanti L, Passarelli V, Giambelluca A, Gualtieri P (1996) Ultrastructure of a novel non-photosynthetic Euglena mutant. Micron 27:367–373
Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M (2010) Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem 285:41501–41508
Schiff JA, Lyman H, Russel GK (1971) Isolation of mutants from Euglena gracilis. In: San Pietro A (ed) Methods in Enzymology: Photosynthesis. Part A, vol 23. Academic Press, New York, pp 143–162
Schiff JA, Lyman H, Russel GK (1980) Isolation in Euglena gracilis: An addendum. In: San Pietro A (ed) Methods in Enzymology: Photosynthesis and Nitrogen Fixation. Part C, vol 69. Academic Press, New York, pp 23–29
Schmidt W, Galland P, Senger H, Furuya M (1990) Microspectrophotometry of Euglena gracilis. Planta 182:375–381
Schmidt M, Geßner G, Luff M, Heiland I, Wagner V, Kaminski M, Geimer S, Eitzinger N, Reissenweber T, Voytsekh O, Fiedler M, Mittag M, Kreimer G (2006) Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell 18(8):1908–1930
Schröder-Lang S, Schwärzel M, Seifert R, Strünker T, Kateriya S, Looser J, Watanabe M, Hegemann P, Nagel G (2007) Fast manipulation of cellular cAMP level by light in vivo. Nat Methods 4(1):39–42
Selbach M, Häder D-P, Kuhlmann HW (1999) Phototaxis in Chlamydodon mnemosyne: determination of illuminance-response curve and the action spectrum. J Photochem Photobiol B Biol 49:35–40
Shimmen T (1981) Quantitative studies on step-down photophobic response of Euglena in an individual cell. Protoplasma 106:37–48
Shneyour A, Avron M (1975) Properties of photosynthetic mutants isolated from Euglena gracilis. Plant Physiol 55:137–141
Simons PJ (1981) The role of electricity in plant movements. New Phytol 87:11–37
Sineshchekov V, Geiß D, Sineshchekov O, Galland P, Senger H (1994a) Fluorometric characterization of pigments associated with isolated flagella of Euglena gracilis: evidence for energy migration. J Photochem Photobiol B Biol 23(2):225–237
Sineshchekov OA, Jung KH, Spudich JL (2002) Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 99:8689–8694
Sperling PG, Walne PL, Schwarz OJ, Triplett LL (1973) Studies on characterization of pigments from isolated eyespots of Euglenoid flagellates. J Phycol Suppl 9:20
Stallwitz E (1992) Einfluß von Schwermetallionen auf Motilität, Orientierung, Wachstum und Pigmentierung des Flagellaten Euglena gracilis. Diplom, Friedrich-Alexander University Erlangen-Nürnberg, Germany
Stallwitz E, Häder D-P (1993) Motility and phototactic orientation of the flagellate Euglena gracilis impaired by heavy metal ions. J Photochem Photobiol B Biol 18:67–74
Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gärtner W, Petereit L, Efetova M, Schwarzel M, Oertner TG, Nagel G, Hegemann P (2011) Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem 286:1181–1188
Strasburger E (1878) Wirkung des Lichtes und der Wärme auf Schwärmsporen. G. Fischer Verlag, Jena
Strother GK, Wolken JJ (1960) Microspectrophotometry of Euglena. Chloroplast and eyespot. Nature 188:601–602
Sumida S, Lyman H, Nobuhiko K, Osafune T (2007) Mechanism of conversion from heterotrophy to autotrophy in Euglena gracilis. Cytologia 72:447–457
Suzaki T, Williamson RE (1983) Photoresponse of a colorless euglenoid flagellate, Astasia longa. Plant Sci Lett 32:101–107
Suzaki T, Williamson RE (1986) Ultrastructure and sliding of pellicular structures during euglenoid movement in Astasia longa Pringsheim (Sarcomastigophora, Euglenoida). J Protozool 33:179–184
Sznee K, Crouch LI, Jones MR, Dekker JP, Frese RN (2014) Variation in supramolecular organisation of the photosynthetic membrane of Rhodobacter sphaeroides induced by alteration of PufX. Photosynth Res 119(1–2):243–256
Tahedl H, Häder D-P (2001) The use of image analysis in ecotoxicology. In: Häder D-P (ed) Image Analysis: Methods and Applications. CRC Press, Boca Raton, pp 447–458
Takeda J, Nakashima M, Ueno H, Mori T, Iseki M, Watanabe M (2013) Search for pterin-binding protein from Euglena. J Biol Macromol 13(1):13–20
Tamponnet C, Rona JP, Barbotin JN, Calvayrac R (1988) Effects of high external calcium concentrations on etiolated Euglena gracilis Z cells and evidence of an internal membrane potential. Biochim Biophys Acta 943:87–94
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Tollin G (1973) Phototaxis in Euglena. II. Biochemical aspects. In: Pérez-Miravete A (ed) Behaviour of Microorganisms. Plenum Press, New York, pp 91–105
Tollin G, Robinson MJ (1969) Phototaxis in Euglena. V. Photosupression of phototactic activity by blue light. Photochem Photobiol 9:411–416
Toporik H, Carmeli I, Volotsenko I, Molotskii M, Rosenwaks Y, Carmeli C, Nelson N (2012) Large photovoltages generated by plant photosystem I crystals. Adv Mater 24(22):2988–2991
Umrath K (1959) Galvanotaxis. Handbuch der Pflanzenphysiologie 17(1):164–167
Vavra J (1962) Instability of the stigma in apochlorotic Euglena gracilis var. bacillaris. J Protozool 9 Suppl:28–29
Verni F, Rosati G, Lenzi P, Barsanti L, Passarelli V, Gualtieri P (1992) Morphological relationship between paraflagellar swelling and paraxial rod in Euglena gracilis. Micron Microsc Acta 23:37–44
Verworn M (1889) Psychophysiologische Protistenstudien. Gustav Fischer Verlag, Jena, pp 25–130
Votta JJ, Jahn TL (1972) Galvanotaxis of Euglena gracilis. J Protozool 19(Suppl):43
Wadhams GH, Armitage JP (2004) Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol 5(12):1024–1037
Walne PL, Arnott HJ (1967) The comparative ultrastructure and possible function of eyespots: Euglena granulata and Chlamydomonas eugemetos. Planta 77:325–353
Walne PL, Lenci F, Mikolajczyk E, Colombetti G (1984) Effect of pronase treatment on step-down and step-up photophobic responses in Euglena gracilis. Cell Biol Int Rep 8:1017–1027
Walne PL, Pasarelli V, Barsanti L, Gualtieri P (1998) Rhodopsin: A photopigment for phototaxis in Euglena gracilis. Crit Rev Plant Sci 17:559–574
Watanabe M, Iseki M (2005) Discovery and characterization of photoactivated adenylyl cyclase (PAC), a novel blue-light receptor flavoprotein, from Euglena gracilis. In: Briggs WR, Spudich JL (eds) Handbook of Photosensory Receptors. Wiley-VCH, Weinheim, pp 447–460
Weissenberger S, Schultheis C, Liewald JF, Erbguth K, Nagel G, Gottschalk A (2011) PACα–an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J Neurochem 116(4):616–625
Wenderoth K, Häder D-P (1979) Wavelength dependence of photomovement in desmids. Planta 145:1–5
Wolken JJ (1956) A molecular morphology of Euglena gracilis var. bacillaris. J Protozool 3(4):211–221
Wolken J (1960) Photoreceptors: Comparative studies. In: Allen MB (ed) Comparative Biochemistry of Photoreactive Systems. Academic Press, New York, pp 145–167
Wolken JJ (1977) Euglena: the photoreceptor system for phototaxis. J Protozool 24:518–522
Wolken JJ (2012) Euglena: an Experimental Organism for Biochemical and Biophysical Studies. Springer
Wolken JJ, Shin E (1958) Photomotion in Euglena gracilis. I. Photokinesis. II. Phototaxis. J Protozool 5:39–46
Yoshikawa S, Suzuki T, Watanabe M, Iseki M (2005) Kinetic analysis of the activation of photoactivated adenylyl clclase (PAC), a blue-light receptor for photomovements of Euglena. Photochem Photobiol Sci 4:727–731
Zhenan M, Shouyu R (1983) The effect of red light on photokinesis of Euglena gracilis. In: Tseng CK (ed) Proceedings of the joint China-U.S. phycology symposium. Science in China Press, Beijing, pp 311–321
Acknowledgements
The authors thank their long-time coworkers Peter Richter, Maria Ntefidou and Sebastian Strauch, who have critically read this manuscript. The financial support for the underlying work for this review by DFG, DLR, BMBF and JSPS is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Häder, DP., Iseki, M. (2017). Photomovement in Euglena . In: Schwartzbach, S., Shigeoka, S. (eds) Euglena: Biochemistry, Cell and Molecular Biology. Advances in Experimental Medicine and Biology, vol 979. Springer, Cham. https://doi.org/10.1007/978-3-319-54910-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-54910-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54908-8
Online ISBN: 978-3-319-54910-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)