Abstract
Cellulose, hemicellulose and chitin are the three most abundant polysaccharides on the earth. Cellulose and Due to the increase in the energy demand chitinases have become relatively important in the past few decades. Chitinases, produced by bacteria, fungi, insects, and plants, have been used in several applications ranging from anti-phytopathogen to antitumor cancer agents. Evolution in molecular biology and genetic engineering has given new prospects in understanding the structure functionality and catalytic mechanisms. of chitinases at molecular level. Owing to their applicability in different fields, studies related to chitinases have become very important. Synergism between the cellulases and chitinases has supported in understanding the catalytic mechanism and developing a deep understanding of the both types of the enzymes. Literature suggest that there are few reports on extremophilic chitinases production. Molecular biology and genetic engineering have only helped in the expression and understanding of extremophilic chitinases so far. This chapter focuses on the various sources of chitinases, their production and catalytic mechanisms, extremophilic chitinases molecular studies and their potential applications. This chapter also provides an insight on the various aspects of chitinases with emphasis on both research and industry.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara What Will You Learn from This Chapter?Cellulose, hemicellulose and chitin are the three most abundant polysaccharides on the earth. Due to the increase in the energy demand chitinases have become relatively important in the past few decades. Chitinases, produced by bacteria , fungi, insects, and plants, have been used in several applications ranging from anti-phytopathogen to antitumor cancer agents. Evolution in molecular biology and genetic engineering has given new prospects in understanding the structure of chitinases’ functionality and catalytic mechanisms at molecular level. Owing to their applicability in different fields, studies related to chitinases have become very important. Synergism between the cellulases and chitinases has supported in understanding the catalytic mechanism and developing a deep understanding of the both types of the enzymes. Literature suggest that there are few reports on extremophilic chitinases production. Molecular biology and genetic engineering have only helped in the expression and understanding of extremophilic chitinases so far. This chapter focuses on the various sources of chitinases, their production and catalytic mechanisms, extremophilic chitinases, molecular studies and their potential applications. This chapter also provides an insight on the various aspects of chitinases with emphasis on both research and industry.
12.1 Introduction
In the past 150 years agriculture, pharmaceutical, transport, health sectors etc. have developed at a faster rate providing the mankind with greater life expectancy, better food sources, increased standard of living and other innumerable advantages. Most of these developments have involved chemical processes which over the course of time have polluted the natural resources drastically. The extensive use of chemicals for various processes and post effluent management not only requires huge capital input but also poses threat for the environment due to improper handling. Human activities have further resulted in a profound impact on the local, regional and global environment. A vision of greener world for coming generations has necessitated development of bioprocesses to replace chemicals being used for the production of pharmaceuticals, plastics, rubber, paper, oils etc. Microorganisms and their enzymes have emerged as a viable option for the change that is required. Several key industrial processes example production and development of biofuels , acetic acid, lactic acid, milk based products (Lemes et al. 2016), biological control (Herrera-Estrella and Chet 1999), baking (Bueno et al. 2016) etc. involve use of microorganisms and their enzymes. Extremophilic enzymes have further enhanced the opportunities of their use in several chemical processes for which biological option was an outlier for last few decades. Cellulose and chitin are most abundant materials on the earth, and are being extensively investigated for their use in different fields. Chitin, has made a huge impact in several industrial sectors. The chapter discusses about the sources for chitinases and their related functions in the respective sources. It also provides different aspects of production, catalysis, developments, and applications of various chitinases including extremophilic chitinases.
12.2 Chitin and Its Derivatives
Chitin is the most abundant polysaccharide in marine environment and second most abundant polysaccharide in the terrestrial environments after cellulose. It is a crystalline, water insoluble, and recalcitrant cellulose derived homopolymer where the 2-OH group is substituted by an acetamido group on the β-1, 4-linked N-acetylglucosamine units (Eijsink et al. 2008; Bhattacharya et al. 2007).
Naturally, chitin exists in two conformations: α chitin and β chitin . In α configuration, the individual polymeric chains are arranged in antiparallel fashion whereas in β configuration these are arranged in parallel fashion (Hamid et al. 2013). Chitosan , a water-soluble chitin derivative, is derived from chitins by removing the N-acetyl groups which render in less bulky amino groups on the polymer. The solubility of chitosan in water makes it a favorable substrate in many different applications e.g., gels, fibers, and films (Rinaudo 2006).
It is commonly found as a key component in the structural make up of insects, fungi , yeast, algae , and in the internal structures of vertebrates where it functions. As per estimates, the amount of chitin observed are of the order of 1010–1011 tons on an annual basis (Tanaka et al. 1999).
Production, and processing of sea food, exoskeleton shedding, and production of other products from chitinaceous organisms generates huge quantities of chitin that pose a threat for the marine, and terrestrial environment as a potent source of pollution. Hence, the degradation of chitin materials is not only important for recycling of nutrients, but also to prevent any potential environmental hazards (Chakrabortty et al. 2012). Conventional treatment methods employed in industry involve pretreatment of chitin with HCl for demineralization, and NaOH for deproteinization (Jagadeeswari et al. 2011; Oku and Ishikawa 2006). However, due to the various hazards associated with the use of chemicals, biological control (e.g. chitinase producing organisms or direct application of enzymes) offers a potential sustainable, and green solution for the chitin waste disposal. Enzymes, such as lysozyme, some glucanases, and chitinase can hydrolyze this linear chitin polymer and among these chitinases can specifically degrade the chitin and chitin based materials.
12.3 Chitinases
Chitinases (EC 3.2.1.114) are extracellular, inducible, enzymes which hydrolyze the β-(1-4) glycosidic bond between the C1 and C4 of two consecutive N-acetylglucosamine groups producing chitooligomers (Liu et al. 2003). They are members of glycosyl hydrolase group, which is classified into 136 families (http://www.cazy.org/Glycoside-Hydrolases.html, accessed on June 16, 2016), based on amino acid sequence similarities. Most of the chitinases belong to glycosyl hydrolase family 18 and 19 (GH 18 and GH 19). GH 18 includes chitinases from bacteria , fungi , viruses, animals, and few plants. They are non-catalytic endo-acting and catalytic with exo and endo binding preferences producing chitobiose as the main product . However certain endoacting chitinases are not able to cleave trimers and tetramers yielding longer products . On the other hand, GH 19 includes almost exclusively plant chitinases (Tanaka et al. 1999).
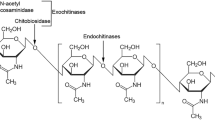
Chitin-hydrolyzing enzymes are classified into three categories (endochitinases, exochitinases, and N-acetyl-β-glucosaminidases-GlcNAc) on the basis of cleavage mechanism (Neeraja et al. 2010; Dahiya et al. 2006) (Fig 12.1).
-
1.
Endochitinases randomly cleave β-(1-4) glycosidic bonds of chitin
-
2.
Exochitinases cleave the chain from the non-reducing end to form diacetyl-chitobiose (GlcNAc2).
-
3.
N-Acetyl-β-glucosaminidases hydrolyze GlcNAc2 into GlcNAc or produce GlcNAc from the non-reducing end of N-acetyl-chitooligosaccharides.
12.4 Sources of Chitinases
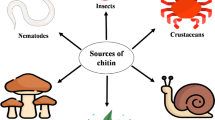
Chitinases can be obtained from a wide range of organisms including bacteria , fungi , insects, plants and animals. Depending on the source of their production, they perform different functions ranging from acting as an agent in anti-phytopathogenic to that in cell differentiation. The chitinase producing organisms can be isolated from the sites having sufficient amount of chitin that includes chitin waste sites, crab and shrimp food industries, trees infected with fungi and others. A chitinase producing gene Chi18H8 isolated from soil and was considered to be suppressive towards club root disease of cabbage (Hjort et al. 2014). A thermostable and alkaline chitinase producing strain Bacillus thruingiensis subsp. kurstaki HBK-51 was isolated from a mixture of crabs, campus soil, and compost (Kuzu et al. 2012). Chitinase BoCHI3-1 was obtained from the suspension cultured bamboo cells (Onaga et al. 2011). Chitinase producing Paenibacillus sp. D1 was obtained from a common effluent plant (Singh and Chhatpar 2011). A broad classification of the chitinases on the basis of their source is discussed in the following sections.
12.4.1 Bacterial Chitinases
Bacterial chitinases are produced both by all three domains—Bacteria, Archaea, and Eukarya. Chitinases from bacterial origin belong to GH (glycosyl hydrolase) family 18 with an exception of chitinase from Streptomyces griseus HUT 6037 which belongs to GH family 19 (Ohno et al. 1996). Bacterial chitinases have a molecular weight of about 20–60 kDa comparable to that of chitinases obtained from plant species (40–85 kDa). Bacterial chitinases have been reported from various genera including Serratia, Thermococcus, Pyrococcus, Streptomyces, Bacillus, and Aeromonas (Bhattacharya et al. 2007).
The optimum temperature and pH for bacterial chitinases can vary with the bacterial species in the same genera. An endochitinase from Streptomyces violaceusniger has optimum temperature of 28 °C whereas the optimum temperature of 80 °C has been reported for a thermostable chitinase produced by Streptomyces thermoviolaceus OPC-520 (Shekhar et al. 2006; Tsujibo et al. 1993). Similarly chitinase from Bacilus cereus 6E1 had optimum temperature of 35 °C as compared to 50 °C of Bacillus sp. BG-11 (Bhushan and Hoondal 1998; Wang et al. 2001). Extremophilic chitinases, which can withstand extreme conditions for example extreme temperature and pH, high salt concentrations etc., are obtained mostly from archaea and actinomycetes. There are few reports of extremophilic chitinases from fungi as well as from plants. Table 12.1 shows the list of different extremophilic chitinases available.
The chitinase from hyperthermophile archaeon, Pyrococcus furiosus, is active at 90 °C and pH 6.0–7.5. This chitinase gene has two catalytic domains and two substrate-binding domains, which is uncommon in bacterial chitinases . There is only one report of chitinase having more than one catalytic domain from a bacterium Thermococcus kodakaraensis (Oku and Ishikawa 2006). A thermostable chitinase found in Bacillus thuringiensis subsp. Kustaki HBK-51 is isolated from chitin wastes (crabs, campus soil and compost) on chitinase detection agar. The chitinase produced has an optimal activity at 110 °C and at pH 9.0. The enzyme could retain about 75–98% of activity over a wide range of temperature 30–120 °C and pH 3–12 after 3 h incubation (Kuzu et al. 2012).
Another thermostable chitinase Tk-ChiA obtained from Thermococcus kodakarensis has optimal temperature 85 °C and pH 5.0 for colloidal chitin. This enzyme has two catalytic domains and three substrate binding domains. Mutation studies with deletion of certain sequences showed that the two catalytic sites in the enzyme function independent of each other. Their combined hydrolytic effect is additive instead of being synergistic (Tanaka et al. 1999). Paenibacillus sp., a ubiquitously found bacterial species, not only produce chitinases but also aid in the plant growth by nitrogen fixation, mineral solubilization and production of siderophores and phytohormones. Paenibacillus sp. D1 isolated from a common effluent treatment plant produced chitinase which could withstand the pH 8.0 at a temperature of 45–60 °C in the presence of fungicides (Singh and Chhatpar 2011).
12.4.2 Fungal Chitinases
Chitinases have been found in several fungi including Trichoderma, Oenicillium, Penicillium, Lecanicillium, Neurospora, Mucor, Beauveria, Lycoperdon, Aspergillus, Myrothecium, Conidiobolus, Metharhizium, Stachybotrys, Agaricus (Karthik et al. 2014; Hamid et al. 2013). The cell wall in certain fungi is composed of chitin, which is water insoluble. Thus it is highly essential to break down the chitin into its precursor components, which then can be used by fungi for growth. Chitinases can effectively break down the chitin into precursor components, which can then be utilized by fungi for hyphal growth, hyphal extension, hyphal fusion, autolysis etc. Fungal chitinases mostly belong to GH 18 family and show high amino acid homology with class III plant chitinases (Dahiya et al. 2006; Takaya et al. 1998). Chitinases have vital physiological and biological roles including morphogenetic, autolytic, nutritional, and parasitic roles. A chitinase gene (CTS1) is required for the cell separation after division and cell clumping in Saccharomyces cerevisiae, while functional expression of chitosanase and chitinase have been reported to influence morphogenesis in the yeast (Schizosaccharomyces pombe) (Shimono et al. 2002).
Optimum temperature for most fungal chitinases is 40–50 °C. Certain thermophilic fungal species are known to produce chitinases , which can function at high temperature and pH (Li et al. 2010; Karthik et al. 2014). Chitinase from Rhizopus oryzae is active at temperature of 60 °C (Chen et al. 2013). Two thermophilic fungal species, Thermoascus aurantiacus var. levisporus, and Chaetomium thermophillum, have been reported to produce two novel thermostable chitinases TaCHIT1 and CtCHIT1 which can withstand a temperature of 60°C (Li et al. 2010). Chitinase from Talaromyces emersonii has an optimum activity at a temperature of 65°C and pH 5.5–6.5 (Mccormack et al. 1991). A chitinase of Thermomyces lanuginosus exhibits optimum catalytic activity at 55 °C and the half-life time of the enzyme at 65 °C is 25 min (Guo et al. 2005).
12.4.3 Plant Chitinases
Chitinases are found in higher plants monocotyledons and dicotyledons. They play a imperative role in the defensive mechanism of plants and are also included in the class of pathogen related (PR) protein s. On the basis of the amino acid sequence s, plant chitinases have been categorized into 5 or 6 classes. The key structure of the class I, II and IV enzymes includes a main structural unit consisting of two α-rich globular domains. While 8 α-helices and 8 β-strands form the class III and V plant chitinases. The former carries out the hydrolysis of the β-1, 4-glycosidic linkage by means of an inverting mechanism, and the latter through a retaining mechanism (Tamo et al. 2003). Chitinases from plant origin generally have molecular weight from 25 to 36 kDa and may be either acidic or basic depending on the amino acids present (Punja and Zhang 1993).
Many plant endochitinases, especially those with a high isoelectric point, exhibit an additional lysozyme or lysozyme like activity (Brunner et al. 1998). Plant chitinases have shown inhibitory activity towards the fungal spore germination and mycelial growth in disc plate diffusion against common fungal pathogens Trichoderma, Fusarium, and Alternaria (Hamid et al. 2013; Onaga et al. 2011; Dahiya et al. 2006). In addition to their roles with the pathogen management in the plants , these enzymes also play a significant role in certain physiological activities including embryogenesis and ethylene synthesis, legume nodulation (Fukamizo et al. 2003; Kasprzewska 2003; Punja and Zhang 1993). They degrade and deactivate the bacterial chitooligosaccharides when the bacterial-plant interaction is not compatible. In mycorrhizal fungi , the chitinases degrade the inducers in compatible interactions and bind to receptors during non-compatible interactions chitinases inducing an immune reaction, which in turn enhances the production of pathogen related proteins (Kasprzewska 2003; Collinge et al. 1993).
The constitutive production of chitinase is very low but it is triggered and enhanced along with other PR protein s in presence of various, abiotic agents (ethylene, salicylic acid, salt solutions, ozone, and UV light) and by biotic factors (fungi , bacteria , viruses, viroids, fungal cell wall components, and oligosaccharides ) in their environment (Kasprzewska 2003). Two chitinase genes FaChi2-1 and FaChi2-2, from strawberry plants were effective against Colletotrichum fragariae or Colletotrichum acutatum, which are known to cause severe strawberry disease anthracnose crown rot in southeastern US and in many parts of the world (Khan and Shih 2004). Most of the chitinases that are obtained from plants are active in moderate temperature and pH range. Only a few reports of extremophilic chitinases from plant origin are available in literature. A thermostable chitinase PLChi A obtained from Ananas comosus has half-life of more than 5.2 days at 70 °C and pH 4.5. Even at 80 °C, the half-life is 65 min at pH 4.0 (Onaga et al. 2011). Two different isoforms of chitinases obtained from cultured cells of Bambusa oldhamii were stable at 70 °C and 80 °C at a pH of 3.0 and 4.0 respectively. The two isoforms were observed to effectively control the growth of Scolecobasidium longiphorum and stable after storing for 1 year at 4 °C (Kuo et al. 2008).
12.4.4 Insect Chitinases
Chitin forms a crucial component of insect endoskeleton and its appropriate level should be maintained for the upholding of insect growth. Insect chitinases have a molecular weight range of 40–85 kDa. These play important roles as degradative enzymes during ecdysis where endochitinases randomly break the cuticle to chitooligosaccharides which are subsequently hydrolyzed by exoenzymes to N-acetyl-glucosamine. The monomers are reused for new cuticle synthesis (Koga et al. 1997; Kramer et al. 1993). As the new cuticle is produced it is protected from the chitinolytic activity of chitinases by a cuticle organizing protein Chaudhari et al. (2011). Hormones regulate the enzyme production during the transformation of the larvae. Two main sources of insect chitinases are Manduca sexta and Bombyx mori (Koga et al. 1997; Kramer et al. 1993). The expression and levels of the chitinase enzyme in Manduca sexta is very tightly regulated during the morphogenesis of insect. The chitinase is active for very short time during the larval-larval, larval-pupal and pupal-adult molting (Kramer et al. 1993). Insect chitinases have been found to possess transglycosylation activity that can produce oligosaccharides of pharmaceutical significance (Lee et al. 2002; Shen et al. 2009). No extremophilic chitinases have been reported from insects so far.
12.5 Catalysis Mechanism and Molecular Insights of Chitinases
Chitinases from different sources including microbes, fungi , plants and insects belong to either family GH 18 or GH 19. Different isoforms of chitinases are found in different organisms e.g. Serratia marcecens, Aeromona, Bacillus licheniformis X-74, and Streptomyces griseus etc. These isoforms are believed to be the product of post-translational modification when glycosylation or proteolysis occurs (Dahiya et al. 2006). Chitin and cellulose share common similarities in terms of abundance, water insolubility, β-1, 4 glycosidic bond s, and crystalline structure. A similar correlation has been found in the chitinases studied so far showing the presence of catalytic and substrate binding domains in a similar fashion to the cellulases . Reese et al. (1950) first demonstrated that an accessory protein (now called as carbohydrate binding module -CBM) is involved in the cellulose hydrolysis by cellulases , which makes the cellulose more accessible for the hydrolytic enzymes. It was only in 2005 after the discovery of an accessory protein CBP 21 in chitinase from Serratia marscens that this hypothesis was accepted (Eijsink et al. 2008). However, the number of catalytic and substrate binding domains varies in different chitinases.
The chitinases have CBMs which can adhere and disrupt the surface of polysaccharide (Merzendorfer 2013). The substrate-binding domain accumulates catalytic sites on the surface of substrates and also disrupts the hydrogen bonds in the crystalline region of substrates and thereby facilitating subsequent hydrolysis by the catalytic domains (Tanaka et al. 2001). Chitin-binding domain of ChiB, a chitinase from Serratia marcecens, interacts with the reducing end of substrates that extend beyond subsites in the active-site cleft (Zakarlaseen et al. 2009). During hydrolysis, chitinases show processive action in which they remain attached to the substrate in subsequent hydrolysis. This processive action is energetically favorable for chitinases as the individual polymer chains do not attach to the insoluble material during hydrolysis.
The substrate-binding sites in processive chitinases have more aromatic (tryptophan) residues. These residues function as a flexible and hydrophobic sheath along which the polymer chain can slide during the processive mode of action (Zakarlassen et al. 2009). Point mutation s in the tryptophan residues near the catalytic center resulted in loss of processivity of ChiB for water insoluble substance s (e.g. chitin) but a 29-fold increase in activity towards the water soluble oligomeric and polymeric substance s (e.g. chitosan ) (Horn et al. 2006). Thus processivity is a requirement for hydrolyzing insoluble substrates but can reduce the efficiency of the enzyme toward more accessible substrates.
CBP 21, a binding protein , helps in binding to β-chitin while certain other CBP21 like proteins aid in binding to the α chitin. CBP has been observed as a member of CBM family 33 (Levasseur et al. 2013). It has been observed that CBP21 like proteins can enhance the infectivity of insect virus suggesting its possible role in substrate binding. Several crystal structure and site-directed mutagenesis studies confirm the above made suggestion regarding the chitinases activity towards insoluble substrates. It has been found that CBP21 contains conserved polar residues that are crucial for its synergistic effect. Catalytic efficiency of SpChiD, chitinase D from Serratia proteamaculans, in degradation of insoluble chitin substrates was improved by fusing polycystic kidney disease (PKD) domain and chitin binding protein 21 (CBP21) (Madhuprakash et al. 2015). Point mutation in an aromatic residue near to the catalytic center in chitobiohydrolase chitinase B (ChiB) from Serratia marcescens resulted in loss of activity against the water insoluble chitin but enhanced activity for polymeric chitin and chitin hexamer oligosaccharide. Thus it was established that enzyme speed for chitin processivity is compromised at the expense of processing speed for chitosan and other related chitin isomers (Horn et al. 2006; Katouno et al. 2004).
The chitinases carry out their function by acid catalyzed glucose hydrolysis, which could be achieved by two methods. Stereochemistry retention of the anomeric oxygen at C-1 relative to the initial configuration (i) and inversion of stereochemistry (ii). Stereochemistry retention involves the double displacement mechanism in which the β-1, 4 glycosidic oxygen is protonated to produce an oxocarbenium intermediate. This oxocarbenium intermediate is stabilized by a second carboxylate from nearby sugar residue by covalent or electrostatic interactions. The nucleophilic attack by water produces the hydrolysis products that retain the stereochemistry. This type of mechanisms is commonly found in family 19 chitinases. During stereochemistry inversion for family 18 chitinases a bound water molecule acts as a nucleophile. The reaction initiates with the protonation of glycosidic oxygen by a protonated residue. N-acetyl moiety of the -1 amino acid residue near the catalytic center carries a nucleophilic attack, which results in cleavage of the sugar chain and formation of an oxazolinium ion intermediate. Subsequent hydrolysis of this ion commences the reaction completion (Merzendorfer 2013; Dahiya et al. 2006; Horn et al. 2006; Brameld and Goddard 1998a, b; Tews et al. 1997).
Molecular insights have demonstrated that the stearic movement of residues Asp140, Asp142, and Glu144 is very critical in bringing the water molecule close to the reaction center and further nucleophilic attack. Ser 93 and Tyr 10 aid in the catalysis of the chitinases by stabilizing Asp 140 and Asp 142. Conformational changes are observed in Trp-97 and Trp-220 as soon as the chitin binds the protein creating a hydrophobic sandwich between sugar residues at +1 and +2. In double displacement reaction, the anomeric carbon is positively polarized due to the electron withdrawing effect of the oxazolinium ring. Glu-144 polarizes the water molecule and two other water-mediated hydrogen bonds to the protein and place the water molecule in vicinity of the catalytic site to favor the nucleophilic attack on the anomeric carbon C1, retaining the β-anomeric stereochemistry (Zakarlassen et al. 2009). The activity of the chitinases is also dependent on the amount of chitin present and the ease of accessibility of it to the enzymes. Studies on a rice chitinase cloned in Pichia pastoris showed that the chitinase activity on fungi R. stolonifer, B. squamosa, A. niger, and P. aphanidermatum was dependent on exposure of the chitin to the chitinase and amount of chitin present in the fungal cell wall (Yan et al. 2008).
12.6 Chitinase Production
Fermentation , e.g. solid state, submerged, is the main process involved in the chitinase production . Chitinases are extracellular enzymes and their production is governed by a cumulative effect of several physical and biochemical factors e.g. carbon and nitrogen sources, agitation rate, pH, dissolved oxygen (DO), temperature, media composition, and inoculum levels. These affect the success and efficiency of a fermentation process and hence enzyme production. Table 12.2 provides information regarding the various conditions maintained for chitinase production in different organisms. Maximum chitinase production has been observed when chitin is used as a sole carbon and nitrogen source. Easily metabolizable sugars e.g. glucose supported growth but reduced the chitinase production (Jholapara et al. 2013; Chakrabortty et al. 2012; Faramarzi et al. 2009; Patidar et al. 2005). No significant change in chitinase production by Masilla tominae was observed with different organic nitrogen sources (Faramarzi et al. 2009).
The chitinases production is also influenced by agitation rate. Instead of a low (75 RPM) and high (225 RPM) a moderate (150 RPM) agitation rate enhanced the chitinase production and activity in Verticillium lecanii F091 (Liu et al. 2003). High agitation rates can cause negative effects such as rupture of cells, change in morphological state, decrease in productivity, vacuolation, and autolysis whereas at lower agitation rates there is not enough mixing of the components. pH not only decides the growth of the organism and chitinase production but also the secretion of chitinase from the organism into the medium. Optimum pH and temperature range varies with the different organisms. Usually chitinases from organisms functional at high temperatures have also shown wide range of favorable pH range (Table 12.2). Effect of inoculum size on chitinase production depends on the species type. Two different isolates of Penicillium PPCS1 and PPCS2 showed different effects of inoculum size on the chitinase production. In PPCS1 the chitinase production increased up to a point and then started decreasing while in PPCS2 the production continuously was increasing with increase in inoculum size (Patidar et al. 2005).
Optimum levels of MgSO4 enhance the chitinase production in submerged and submerged substrate fermentation both. As observed the chitinase production increases to a level with increasing concentrations of MgSO4 after which it becomes constant (Jholapara et al. 2013; Bhushan and Hoondal 1998). Addition of non-ionic detergents e.g. Tween 80, Tween 20, Triton X-100 also increase the chitinase production (Patidar et al. 2005). These non-ionic detergents disrupt the cell walls and aid in extracellular secretion of the chitinases that in turns results in higher chitinase production . Lab scale to pilot scale production of chitinases from Verticillium lecanii F091 has resulted in higher chitinase activity (Liu et al. 2003). Following production chitinases can be purified using different methods ammonium chloride precipitation , ammonium sulfate precipitation , ion exchange chromatography and ethanol-glycol etc. (Faramarzi et al. 2009).
12.7 Chitinase Assay
Enzyme assays help in finding the amount, type, and various factors affecting the activity. To determine the chitinase activity, different methods have been proposed using the colloidal chitin, carboxymethylchitin-Remazol Brilliant Violet 5R (CM-chitin- RBV; Loewe Biochemica GmbH, Otterfing, Germany), chitin-azure or p-nitrophenol based substrate (Chakrabortty et al. 2012; Dahiya et al. 2005; Tanaka et al. 1999). Insoluble colloidal chitin has been most commonly used as a substrate for determining the chitinase activity. Colloidal chitin is prepared by processing the chitin obtained from of shells crabs, oysters, shrimps etc. thorough different steps that involve the use of acids and precipitation. As per method developed by Rodriguez-Kabana et al. (1983), 50 gm chitin is finely crushed with pestle and mortar and followed by grinding in mixer. This chitin is partially hydrolyzed with 400 ml 10 N HCl for 2–3 h with continuous shaking at room temperature. After hydrolysis the chitin appears in colloidal form. The colloidal chitin is washed several times with large volumes of distilled water to adjust pH 7.0 (Khan and Khan 2014). It is very important during preparation of colloidal chitin to ground it to a fine powder in order to separate the chitin chunks from precipitated chitin.
A modified approach to prepare colloidal chitin given by Murthy and Bleakly (2012) is economical and less time consuming. Crab shell flakes, are grinded in a mortar and pestle for 5 min and sieved through 130 mm two piece polypropylene Buchner filter. Twenty grams of sieved flakes are treated with 150 ml of ~12 M HCl, which is added and stirred continuously with a glass pipette for 5 min and later is done at an interval of 5 min for 60 min at room temperature. The mixture is then passed through 8 layers of cheesecloth to remove large chitin chunks. The filtrate obtained is treated with 2 l of ice-cold water to allow colloidal chitin precipitation . The filtrate with ice water can be kept overnight at 4 °C to get better precipitation. The mixture is then filtered through two layers of coffee filter paper under vacuum and is washed with 3 l of tap water to raise the pH to 7.0. The colloidal chitin obtained is then pressed between the coffee filter papers to remove the moisture and placed in glass beaker and autoclaved at 121 °C, 15 psi (STP) for 20 min. The autoclaved colloidal chitin has cake like texture and can be stored at 4 °C until further use (Murthy and Bleakley 2012).
For chitinase assay the cultures (microbial or fungal) are grown to obtain an extract by filtration, separation, or centrifugation. The cultural filtrate serves as the source for the extracellular chitinase enzymes. This cultural filtrate is incubated with colloidal chitin at ambient temperature and pH depending upon the organism from which they are obtained. Chitinase hydrolyze the colloidal chitin into chitooligosaccharides, which can be measured by different methods used to estimate the reducing sugars. Common method for reducing sugar measurement is by using dinitrosalicyclic acid (DNS). For this method 1 ml of the supernatant obtained after centrifuging the cultures at 5000 rpm for 20 min is added to 1 ml of 1% (w/v) colloidal chitin in citrate phosphate buffer pH 5.5 and incubated at 50 °C for 30 min. Incubating the reaction mixtures in boiling water bath for 3–5 min stops the reaction. The solutions are then centrifuged at 5000 rpm for 10 min. To 1 ml of the supernatant obtained 1.5 ml of dinitrosalycilic acid (DNS) is added and kept in boiling water bath for 5 min. The change in color is observed depending on the amount of reducing present in the solution. Absorbance at 540 nm using a UV-VIS spectrophotometer is taken to compare the amount of reducing sugars in the solution against the standard. Using different concentrations of the reducing sugars with dinitrosalycilic acid (DNS) a standard curve can be plotted following the same protocol as for the enzyme assays. One chitinase enzyme unit is defined as the amount of enzyme which catalyzes the release of one μg of reducing sugar per ml per minute under the reaction conditions (Chakrabortty et al. 2012).
Estimation of chitinase activity can also be done using a chromogenic substance . A chromogenic substrate on being incubated with the enzyme solutions releases chromogenic products , which are measured spectrophotometrically at different wavelengths. Measuring the chitinolytic activity by using p-nitrophenyl-N-acetyl-β-D-glucosaminide (pNP-GlcNAc) is based on estimating the amount of released p-nitrophenol (pNP). The reaction mixture consists of 0.5 ml enzyme solution, 0.5 ml 10 mm pNP-GlcNAc solution, and 0.5 ml 0.1 M citrate-phosphate buffer pH 5.5. The mixture is incubated at 60 °C for 30 min and the reaction is stopped by adding 0.5 ml 1 M Na2CO3 to the mixture. The release of pNP is spectrophotometrically measured at 400 nm, and enzyme activity is calculated using a standard curve for known concentrations of pNP. One chitinase enzyme unit is defined as the amount of enzyme that can release 1 μmole pNP per hour under assay conditions (Tasharrofi et al. 2011).
The chitinase activity can also be measured using the SDS-PAGE , which gives an advantage of molecular weight determination along with enzyme activity. In gel chitinase activity measurement is carried in polyacrylamide gel electrophoresis (PAGE) having 5% stacking gel, and 12% resolving gel, which has 0.66 mg/ml carboxymethylchitin-Remazol Brilliant Violet 5R (CM-chitin-RBV). After electrophoresis the gel is incubated with 100 mM sodium phosphate buffer and 0.1% of Triton X-100 for 2 h, at room temperature, which helps in the renaturation of the protein s. Clear zones are developed after the incubation due to the in-gel degradation of CM-chitin- RBV by chitinases . The gel is stained by Coomassie Brilliant Blue R-250 or silver nitrate, which terminates the enzyme reaction and aids in the appearance of protein bands (Wang et al. 2001).
12.8 Genetic Engineering and Molecular Biology
Techniques of protein engineering and directed protein evolution have been continuously used to maximize the effectiveness and efficiency of hydrolytic enzymes. Multiple approaches allow the identification of mutant enzymes possessing desirable qualities such as increased activity, modified specificity , selectivity, or cofactor binding. Site directed mutagenesis or deletion s give an insight about the functioning of the individual amino acids and their role in the catalysis or substrate binding in the chitinases .
Recombinant technology has been critical in modification of chitinases in-terms of stability and overexpression. Pyrococcus furiosus and Thermococcus kodakarensis share gene homology among the two chitinases genes. Although no chitinase activity was observed in culture supernatant of Pyrococcus furiosus grown in media with chitin as the sole carbon source. Genetic engineering of the sequence encoding chitinases protein into E. coli after single nucleotide deletion at position 1006 resulted in recombinant strains able to show chitinase activity in cell culture extract. The cell culture extract from the recombinant E. coli strains showed significant chitinase activity with optimum temperature and pH 90 °C and 6.0–7.5 respectively (Oku and Ishikawa 2006). cDNA for two chitinase genes Tachit1 and Ctchit1 from thermophilic fungi Thermoascus aurantiacus var. levisporus and Chaetomium thermophilum, respectively was prepared and genetically engineered in a yeast Pichia pastoris. The genes were added to a shuttle vector pPIC9K which has AOX1 promoter and the Saccharomyces cerevisiae a-factor secretion signal located immediately upstream of the multiple cloning site. Restriction endonuclease digestion and later ligation of the amplified products to above mentioned plasmid s resulted in new plasmids having both the chitinase genes. The new recombinant plasmids were cut with restriction endonuclease SacI and incorporated into competent Pichia pastoris cells by electroporation (Li et al. 2010).
Mostly chitinase reported are extracellular enzymes but both extracellular and intracellular enzymes were obtained in two plant origin chitinase genes LbCHI31 and LbCHI32 from Limonium bicolor on transformation in E. coli. However, the extracellular counterparts exrCHI31 and exrCHI32 of these two chitinase genes showed more activity than the intracellular counterparts inrCHI31 and inrCHI32 (Liu et al. 2013). Sometimes the recombination of the chitinase gene sequences into other organism does not result in chitinase activity. Molecular biology studies can be very helpful in studying the machinery for the synthesis and secretion of chitinases . Tanaka et al. (2001) observed that incorporation of Chi A gene isolated did not result in the periplasmic secretion of enzyme in recombinant E. coli. It was only after the signal sequence of ChiA was replaced by a bacterial signal sequence, periplasmic secretion of enzymes could be achieved. Thus a signal peptide , which directs the secretion of enzymes, can affect the success of the recombination. The wild type organism might have the suitable signal peptide for chitinase secretion but it might not be suited for the recombinant species. The recombinant species might be able to produce the gene but not able to secrete it in absence of proper signal peptide.
Genetic engineering and molecular biology studies can also give insights for the catalysis and substrate binding efficiency of the enzymes. Site directed and point mutation s in the amino acids provide information about their role during catalysis and substrate binding. Mutants obtained by point mutations in wild-type chitinase A DNA from Vibrio carchariae helped in studying the catalysis mechanism of chitinases . Mutation s in Trp 275 and Trp 397 emphasized their role in the binding of soluble substrates. Mutations of Trp168, Tyr171, Trp570, and D392N resulted in loss of the hydrolyzing activity against colloidal chitin, and reduced the hydrolyzing activity against the pNP substrate. Mutations in Trp 168 and Trp 171 to glycine indicated their importance in bringing the chitin chain to the binding cleft (Suginta et al. 2007).
Site directed mutation in the exposed aromatic amino acids of chitinase B gene from Serratia marcescens 2170 also provided information about the significant role of these residues in the chitin hydrolyzing and binding activity. Replacement of tyrosine and tryptophan with alanine residues resulted in reduction in substrate binding and chitin hydrolysis establishing their significance in these activities. Although no change was observed when an exposed phenylalanine residue was replaced with alanine. Thus the difference in substrate binding and catalysis of two different chitinases Chi A and Chi B from Serratia marcescens were studied with the help of these mutational studies (Katouno et al. 2004).
Mutation studies also help us to understand the difference in activity of several isoforms and hence differentiate them form one another. Deletion mutants in isoforms Thermococcus kodakarensis showed that the two isoforms work independently of each other. Mutant studies confirmed that the activity of Tk-ChiA is due to the additive effect of activities in region A (Tk-ChiAA3) and region B (Tk-ChiAA2). Only mutants Tk-ChiAA2 and ChiAA4 with region B., exhibited high thermostability and retain more than 70% activity even after heat treatment at 100 °C for 3 h (Tanaka et al. 1999, 2001).
Interspecies genetic engineering of chitinase genes helps in the enhancement of desirable characteristics of species. Transformation of a chitinase gene pGL2 from rice enhanced the antifungal property of grape vine. Somatic embryos obtained from grape vine were transformed with Agarobacterium tumifaciens strain LBA4404 having a vector pGL2 with chitinase coding region from rice. Up to two folds higher chitinase activity was observed among the transformed plants . Reduced rate of lesion formation was observed in the transformed plants as compared to the non-transformed plants that correlated with the increase of chitinase activity in the transformed plants (Nirala et al. 2010). Recombinant and genetic engineering studies assist in modification of chitinase producers at intra and interspecies level. Biochemical and molecular biology studies have helped us to understand the catalytic, secretory, and binding processes of chitinases .
12.9 Applications
Research over the years have identified several commercial applications for the chitinase enzymes. Use of biological measures (e.g. microorganism or microbial products ) to control the plant pathogens offers sustainable solution without posing any threats to the natural soil, water and air resources. As an antiphytopathogenic agents role of chitinases have been well researched and established. Chitinase Chi18H8 isolated from soil showed antiphytopathogenic activity against Alternaria alternata, Colletotrichum gloeosporiodies, Fusarium graminarium and Fusarium oxysporum (Hjort et al. 2014). Due to the absence of chitin in plant tissues the chitinases are better suited for phytopathogenic control as compared to other glucanases (Neeraja et al. 2010).
The chitinase produced by Enterobacter sp. NRG4 shows antifungal activity towards Fusarium moniliforme, Aspergillus niger, Mucor rouxi, and Rhizopus nigricans (Dahiya et al. 2005). Recombinant rice chitinase from Pichia pastoris exhibited antifungal property against Rhizopus stolonifer (Ehrenb. et Fr.) Vuill, Botrytis squamosa Walker, Pythium aphanidermatum (eds.) Fitzp, and Aspergillus niger van Tiegh. The antifungal activity of the chitinase was affected by the ease of chitin availability to enzyme and chitin amount in the fungal cell wall (Yan et al. 2008). Culture filtrate of Streptomyces hygroscopicus strain SRA14 possess antifungal properties because of extracellular chitinase enzyme (Prapagdee et al. 2008).
Chitinase from Paenibacillus sp. D1 has high tolerance towards commonly used fungicides (example Captan, Carbendazim, and Mancozeb) in the fields. In presence of Captan half-life of chitinase was 119.17 min at 80 °C and was able to withstand wide range of temperature (40–60 °C) and pH (pH 4.0–8.0). Thus it is a suitable candidate for application in field where huge variations can be found. The chitinase from Paenibacillus sp. D1 thus can be used in integrated pest management to control of soil-borne fungal phytopathogens (Singh and Chhatpar 2011).
Chitinases can be very influential in studying the growth patterns in fungi . They along with other hydrolyzing enzymes can hydrolyze the chitin in the fungal cell wall giving access to the protoplast. Several fungal studies related to cell wall synthesis, enzyme synthesis and secretion have been done with the help of chitinases . Chitinase from Enterobacter sp. NRG4 was used to obtain protoplasts from Trichoderma reesei, Pleurotus florida, Agaricus bisporus, and Aspergillus niger (Dahiya et al. 2005; Hamid et al. 2013).
Chitinases have also found application in controlling the morphogenesis in mosquito and hence controlling the diseases transmitted by them. Chitinases obtained from a saprophytic fungus Myrothecium verrucaria can control the spread of Aedes aegypti, a vector of yellow fever and dengue (Mendonsa et al. 1996). A numerous medicinal applications of chitinases have been found. Chitinases can augment the activity in antifungal ointments and drugs for against several fungal diseases. Solid waste (CaCO3, chitin, and protein ) from shellfish processing has been used to produce single cell protein s with the help of chitinases. Chitinase from Serratia marcescens is used in combination with yeast , Pichia kudriavzevii, to produce SCP where the chitinase hydrolyzes the chitinous material and yeast produces the single cell protein . In a similar fashion the chitinase from Myrothecium verrucaria and Saccharomyces cerevisiae has been used to produce SCP from chitinous waste. Myrothecium verrucaria chitinase preparation is used for chitin hydrolysis, and Saccharomyces cerevisiae for SCP (Dahiya et al. 2006; Wang and Hawang 2001; Hamid et al. 2013).
Studies on Acid mammalian chitinase (AMCase) revealed that the chitinases are important in mediating several inflammatory responses in human beings example asthma, allergic diseases, atopic dermatitis etc. AMCase has been found to be involved in T helper cells 2 mediated inflammatory response responsible for mediating the onset of asthma. This was confirmed by the fact that administration of anti-AMCase antibody leads to a decrease of T helper type 2 (Th2)-inflammation, tissue eosinophilia and lymphocyte accumulation (Zhu et al. 2004). Certain medical applications for chitin have also been developed. Chitin film and fiber can be used as materials for wound dressing and controlled drug release (Kanke et al. 1989; Kato et al. 2003). Chitin is also used as an excipient and drug carrier in film, gel or powder form for applications involving mucoadhesivity. Chitin derivative hydroxyapatite-chitin-chitosan (composite bone-filling material) forms a self-hardening paste for guided tissue regeneration in treatment of periodontal bony defects (Ito et al. 1999).
Products of chitin hydrolysis chitooligosaccharides, glucosamines, and GlcNAc are used in different pharmaceuticals. Chitopentose and Chitoheptose have shown antitumor activities. Hydrolysate produced by crude enzyme solution from Bacillus amyloliquefaciens V656 had (GlcNAc)6 showed higher antitumor activity. Hydrolysates of water soluble chitosan inhibited the growth rate of CT26 cells and survival rate to 34% in 1 day (Liang et al. 2007).
12.10 Conclusions
Chitinases have been studied for more than 40 years now and research is still being carried on these enzymes because of their several applications in various areas. They have found their roles in plant pathogenesis, morphogenesis, growth related studies, single cell protein formations, pharmaceutical industries and biofuels . Very few of the chitinases being used are from extremophilic species which can tolerate high temperature or wide range of pH’s. Finding new robust enzymes capable of withstanding extreme conditions will definitely enhance their applications in already proved commercial aspects. Studies related to the structure, catalysis and substrate binding can aid us in better understanding of certain unearthed concepts which might create new milestones in research. Use of protein engineering and molecular biology can confer certain desirable characteristics to the existing chitinases . Thus in the future, there is a great possibility and opportunity for generating chitinases with novel functions.
Take Home Message
-
Chitin is a crystalline, water insoluble, and recalcitrant cellulose derived homopolymer. Chitin exists in two conformations namely α chitin and β chitin . The individual polymeric chains are arranged in antiparallel fashion in α configuration. The individual polymeric chains are arranged in parallel fashion in β configuration.
-
Chitosan , a water-soluble chitin derivative, is derived from chitins by removing the N-acetyl groups which render in less bulky amino groups on the polymer. The solubility of chitosan in water makes it a favorable substrate in many different applications e.g., gels, fibers, and films. It is commonly found as a key component in the structural make up of insects, fungi , yeast , algae , and in the internal structures of vertebrates.
-
Enzymes, such as lysozyme, some glucanases, and chitinase can hydrolyze this linear chitin polymer and among these chitinases can specifically degrade the chitin and chitin based materials. Chitinases are classified into three categories namely endochitinases, exochitinases, and N-acetyl-β-glucosaminidases based on the cleavage mechanism. The enzyme endochitinase randomly mediates the cleave β-(1-4) glycosidic bond s of chitin, the enzyme exochitinases catalyzes cleave the chain from the non-reducing end to form diacetyl-chitobiose and the enzyme N-Acetyl-β-glucosaminidases hydrolyzes diacetyl-chitobiose into N-Acetyl-D-glucosamine or produce N-Acetyl-D-glucosamine from the non-reducing end of N-acetyl-chitooligosaccharides. The chitinolytic activity of the enzyme can be assessed by using p-nitrophenyl-N-acetyl-β-D-glucosaminide (pNP-GlcNAc) based on estimating the amount of released p-nitrophenol (pNP) using spectrophotometric technique.
-
Thermostable chitinases can be obtained from bacterial sources such as Thermococcus kodakarensis, Pyrococcus furiosus and Bacillus thuringiensis subsp., Paenibacillus sp and fungal species such Thermoascus aurantiacus var. levisporus, Chaetomium thermophillum, Talaromyces emersonii, and Thermomyces lanuginosus.
References
Abdel-Aziz SM, Moharam ME, Hamed HA, Mouafi FE (2012) Extracellular metabolites produced by a novel strain, Bacillus alvei NRC-14:1. Some properties of the chitinolytic system. New York Sci J 5:53–62
Ali S, Wu J, Huang Z, Ren SX (2010) Production and regulation of extracellular chitinase from the entomopathogenic fungus Isaria fumosorosea. Biocontrol Sci Tech 20(7):723–738
Bhattacharya D, Nagpure A, Gupta RK (2007) Bacterial chitinases: properties and potential. Crit Rev Biotechnol 27:21–28. doi:10.1080/07388550601168223
Bhushan B, Hoondal GS (1998) Isolation, purification and properties of a thermostable chitinase from an alkalophilic Bacillus sp. BG-11. Biotechnol Lett 20:157–159. doi:10.1023/A:1005328508227
Brameld KA, Goddard WA III (1998a) Substrate distrortion to a boat conformation at subsite-1 is critical in the mechanism of family 18 chitinases. J Am Chem Soc 120:3571–3580
Brameld KA, Goddard WA III (1998b) The role of enzyme distortion in the single displacement mechanism of family 19 chitinases. Proc Natl Acad Sci U S A 120:4276–4281
Brunner F, Stintzi A, Fritig B, Legrand M (1998) Substrate specificities of tobacco chitinases. Plant J 14:225–234
Chakrabortty S, Bhattacharya S, Das A (2012) Optimization of process parameters for chitinase production by a marine isolate of Serratia marcescens. Int J Pharm Biol Sci 2:8–20
Chaudhari SS, Arakane Y, Specht CA et al (2011) Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc Natl Acad Sci U S A 108:17028–17033
Chen W, Chen C, Jiang S (2013) Purification and characterization of an extracellular chitinase from the entomopathogen Metarhizium anisopliae. J Mar Sci Technol 21:361–366. doi:10.6119/JMST-012-0518-2
Collinge DB, Kragh KM, Mikkelsen JD et al (1993) Plant chitinases. Plant J 3:31–40
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 71:773–782. doi:10.1007/s00253-005-0183-7
Dahiya N, Tewari R, Tiwari RP et al (2005) Production of an antifungal chitinase from Enterobacter sp. NRG4 and its application in protoplast production. World J Microbiol Biotechnol 21(8–9):1611–1616
Eijsink VGH, Vaaje-Kolstad G, Vårum KM, Horn SJ (2008) Towards new enzymes for biofuels: lessons from chitinase research. Trends Biotechnol 26:228–235. doi:10.1016/j.tibtech.2008.02.004
Faramarzi MA, Fazeli M, Yazdi MT et al (2009) Optimization of culture conditions for production of chitinase by a soil isolate of Massilia Timonae. Biotechnology 8(1):93–99
Fukamizo T, Sakai C, Tamoi M (2003) Plant chitinases: structure-function relationships and their physiology. Foods Food Ingredients J Jpn 208:631–632
Hamid R, Khan MA, Ahmad M et al (2013) Chitinases: an update. J Pharm Bioallied Sci 5(1):21–29. doi:10.4103/0975-7406.106559
Herrera-Estrella A, Chet I (1999) Chitinases in biological control. EXS 87:171–184
Horn SJ, Sikorski P, Cederkvist JB et al (2006) Costs and benefits of processivity in enzymatic degradation of recalcitrant polysaccharides. Proc Natl Acad Sci U S A 103(48):18089–18094
Guo RF, Li DC, Wang R (2005) Purification and properties of a thermostable chitinase from thermophilic fungus Thermomyces lanuginosus. Acta Microbiol Sin 45:270–274
Hjort K, Prest I, Elväng A et al (2014) Bacterial chitinase with phytopathogen control capacity from suppressive soil revealed by functional metagenomics. Appl Microbiol Biotechnol 98:2819–2828. doi:10.1007/s00253-013-5287-x
Ito M, Hidaka Y, Nakajima M, Yagasaki H, Kafrawy AH (1999) Effect of hydroxyapatite content on physical properties and connective tissue reactions to a chitosan–hydroxyapatite composite membrane. J Biomed Mater Res 45:204–208
Jholapara RJ, Mehta RS, Bhagwat AM et al (2013) Exploring and optimizing the potential of chitinase production by isolated Bacillus spp. Int J Pharm Pharm Sci 5(4):412
Kanke M, Katayama H, Tsuzuki S, Kuramoto H (1989) Appilcation of chitin and chitosan to pharmaceutical preparations. I.: film preparation and in vitro evaluation. Chem Pharm Bull 37(2):523–525
Karthik N, Akanksha K, Binod P, Pandey A (2014) Production, purification and properties of fungal chitinases – a review. Indian J Exp Biol 52:1025–1035
Kasprzewska A (2003) Plant chitinases-regulation and function. Cell Mol Biol Lett 8:809–824
Kato A, Kano E, Adachi I, Molyneux RJ, Watson AA, Nash RJ et al (2003) Australine and related alkaloids: easy structural confirmation by 13 C NMR spectral data and biological activities. Tetrahedron Asymmetry 14(3):325–331
Katouno F, Taguchi M, Sakurai K et al (2004) Importance of exposed aromatic residues in Chitinase B from Serratia marcescens 2170 for crystalline chitin hydrolysis. J Biochem 136:163–168. doi:10.1093/jb/mvh105
Khan AA, Shih DS (2004) Molecular cloning, characterization, and expression analysis of two class II chitinase genes from the strawberry plant. Plant Sci 166:753–762. doi:10.1016/j.plantsci.2003.11.015
Khan RS, Khan ZH (2014) Studies on chitinase isolation and thermostability between Bacillus circulance strain L2 and Bacillus Licheniformis strain 2J-1. Sci Res Report 4:1–7
Koga D, Sasaki Y, Uchiumi Y et al (1997) Purification and characterization of Bombyx mori chitinases. Insect Biochem Mol Biol 27:757–767
Kramer KJ, Corpuz LM, Choi H, Muthukrishnan S (1993) Sequence of a c-DNA and expression of the gene encoding epidermal and gut chitinases of Manduca sexa. Insect Biochem Mol Biol 23:691–701
Kuo CJ, Liao YC, Yang JH et al (2008) Cloning and characterization of an antifungal class III chitinase from suspension-cultured bamboo (Bambusa oldhamii) cells. J Agric Food Chem 56:11507–11514. doi:10.1021/jf8017589
Kuzu SB, Güvenmez HK, Denizci AA (2012) Production of a thermostable and alkaline chitinase by Bacillus thuringiensis subsp. kurstaki strain HBK-51. Biotechnol Res Int 2012:135498. doi:10.1155/2012/135498
Lee HW, Park YS, Jung JS, Shin WS (2002) Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe 8:319–324
Levasseur A, Drula E, Lombard V et al (2013) Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels 6:41. doi:10.1186/1754-6834-6-41. PMID: 23514094
Li AN, Yu K, Liu HQ et al (2010) Two novel thermostable chitinase genes from thermophilic fungi: cloning, expression and characterization. Bioresour Technol 101:5546–5551. doi:10.1016/j.biortech.2010.02.058
Liang TW, Chen YJ, Yen YH, Wang SL (2007) The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem 42(4):527–534
Liu BL, Kao PM, Tzeng YM, Feng KC (2003) Production of chitinase from Verticillium lecanii F091 using submerged fermentation. Enzyme Microb Technol 33:410–415. doi:10.1016/S0141-0229(03)00138-8
Liu Z, Huang Y, Zhang R et al (2013) Chitinase genes lbchi31 and lbchi32 from Limonium bicolor were successfully expressed in Escherichia coli and exhibit recombinant chitinase activities. Sci World J 2013:648382. doi:10.1155/2013/648382
Madhuprakash J, El Gueddari NE, Moerschbacher BM, Podile AR (2015) Catalytic efficiency of chitinase-D on insoluble chitinous substrates was improved by fusing auxiliary domains. PLoS One 10:e0116823. doi:10.1371/journal.pone.0116823
Mccormack J, Hackett TJ, Tuohy MG, Coughlan MP (1991) Chitinase production by Talaromyces emersonii. Biotechnol Lett 13:677–682
Mendonsa ES, Vartak PH, Rao JU, Deshpande MV (1996) An enzyme from Myrothecium verrucaria that degrades insect cuticles for biocontrol of Aedes aegypti mosquito. Biotechnol Lett 18(4):373–376
Merzendorfer H (2013) Insect-derived chitinases. Adv Biochem Eng Biotechnol 136:19–50. doi:10.1007/10_2013_207
Murthy N, Bleakley B (2012) Simplified method of preparing colloidal chitin used for screening of chitinase-producing microorganisms. Int J Microbiol 10:7
Neeraja C, Anil K, Purushotham P et al (2010) Biotechnological approaches to develop bacterial chitinases as a bioshield against fungal diseases of plants. Crit Rev Biotechnol 30:231–241. doi:10.3109/07388551.2010.487258
Nirala NK, Das DK, Srivastava PS et al (2010) Expression of a rice chitinase gene enhances antifungal potential in transgenic grapevine (Vitis vinifera L.) Vitis 49:181–187
Oku T, Ishikawa K (2006) Analysis of the hyperthermophilic chitinase from Pyrococcus furiosus: activity toward crystalline chitin. Biosci Biotechnol Biochem 70:1696–1701. doi:10.1271/bbb.60031
Ohno T, Armand S, Hata T, Nikaidou N et al (1996) A modular family 19 chitinase found in the prokaryotic organism Streptomyces griseus HUT 6037. J Bacteriol 178:5065–5070
Onaga S, Chinen K, Ito S, Taira T (2011) Highly thermostable chitinase from pineapple: cloning, expression, and enzymatic properties. Process Biochem 46:695–700. doi:10.1016/j.procbio.2010.11.015
Patidar P, Agrawal D, Banerjee T, Patil S (2005) Optimisation of process parameters for chitinase production by soil isolates of Penicillium chrysogenum under solid substrate fermentation. Process Biochem 40:2962–2967. doi:10.1016/j.procbio.2005.01.013
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci 4:330–337
Punja ZK, Zhang YY (1993) Plant chitinases and their roles in resistance to fungal diseases. J Nematol 25:526–540
Reese ET et al (1950) The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol 59:485–497
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. doi:10.1016/j.progpolymsci.2006.06.001
Rodriguez-Kabana R, Godoy G, Morgan-Jones G, Shelby RA (1983) The determination of soil chitinase activity: conditions for assay and ecological studies. Plant and Soil 75(1):95–106
Sandhya C, Adapa LK, Nampoothiri KM, Binod P, Szakacs G, Pandey A (2004) Extracellular chitinase production by Trichoderma harzianum in submerged fermentation. J Basic Microbiol 44(1):49–58
Shekhar N, Bhattacharya D, Kumar D, Gupta RK (2006) Biocontrol of wood- rotting fungi using Streptomyces violaceusniger XL-2. Can J Microbiol 52:805–808
Shen KT, Chen MH, Chan HY et al (2009) Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem Toxicol 47:1864–1871
Shimono K, Matsuda H, Kawamukai M (2002) Functional expression of chitinase and chitosanase, and their effects on morphologies in the yeast Schizosaccharomyces pombe. Biosci Biotechnol Biochem 2002(66):1143–1147
Singh AK, Chhatpar HS (2011) Purification and characterization of chitinase from Paenibacillus sp. D1. Appl Biochem Biotechnol 164:77–88. doi:10.1007/s12010-010-9116-8
Suginta W, Songsiriritthigul C, Kobdaj A et al (2007) Mutations of Trp275 and Trp397 altered the binding selectivity of Vibrio carchariae chitinase A. Biochim Biophys Acta Gen Subj 1770:1151–1160. doi:10.1016/j.bbagen.2007.03.012
Takaya N, Yamazaki D, Horiuchi H et al (1998) Cloning and characterization of a chitinase-encoding gene (ChiA) from Aspergillus nidulans, disruption of that decreases germination frequency and hyphal growth. Biosci Biotechnol Biochem 62:60–65
Tamo F, Chiye S, Masahiro T (2003) Structure-plant chitinases: structure – function relationships and their physiology. Foods Food Ingredients J Jpn 208:631–632
Tanaka T, Fujiwara S, Nishikori S (1999) A unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic archaeon Pyrococcus kodakar. Appl Environ Microbiol 65:5338–5344
Tanaka T, Fukui T, Imanaka T (2001) Different cleavage specificities of the dual catalytic domains in chitinase from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Biol Chem 276:35629–35635. doi:10.1074/jbc.M105919200
Tasharrofi N, Adrangi S, Fazeli M et al (2011) Optimization of chitinase production by Bacillus pumilus using Plackett-Burman design and response surface methodology. Iran J Pharm Res 10:759–768
Tews I, Scheltinga AC, Terwisscha V et al (1997) Substrate assisted catalysis unifies two families of chitinolytic enzymes. J Am Chem Soc 119:7954–7959
Tsujibo H, Minoura K, Miyamoto K et al (1993) Purification and properties of a thermostable chitinase from Streptomyces thermoviolaceus OPC-520. Appl Environ Microbiol 59:620–622. doi:10.1271/bbb.64.96
Vahed M, Motalebi E, Rigi G et al (2013) Improving the chitinolytic activity of Bacillus pumilus SG2 by random mutagenesis. J Microbiol Biotechnol 23:1519–1528. doi:10.4014/jmb.1301.01048
Wang SL, Hwang JR (2001) Microbial reclamation of shellfish wastes for the production of chitinases. Enzyme Microb Technol 28(4):376–382
Wang S-Y, Moyne A-L, Thottappilly G et al (2001) Purification and characterization of a Bacillus cereus exochitinase. Enzyme Microb Technol 28:492–498. doi:10.1016/S0141-0229(00)00362-8
Yan R, Hou J, Ding D et al (2008) In vitro antifungal activity and mechanism of action of chitinase against four plant pathogenic fungi. J Basic Microbiol 48:293–301. doi:10.1002/jobm.200700392
Zakarlassen H, Aam BB, Hom SJ et al (2009) Aromatic residues in the catalytic center of chitinase A from Serratia marcescens affect processivity, enzyme activity, and biomass converting efficiency. J Biol Chem 284:10610–10617. doi:10.1074/jbc.M900092200
Zhu Z, Zheng T, Homer RJ, Kim YK et al (2004) Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304(5677):1678–1682
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bibra, M., Navanietha Krishnaraj, R., Sani, R.K. (2017). An Overview on Extremophilic Chitinases. In: Sani, R., Krishnaraj, R. (eds) Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy. Springer, Cham. https://doi.org/10.1007/978-3-319-54684-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-54684-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54683-4
Online ISBN: 978-3-319-54684-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)