Abstract
As described in Chap. 3, hydroformylation is one of the most important applications for homogeneous catalysis. Industrial proven processes like the Ruhrchemie/Rhône-Poulenc-Process (RCh-RP) are based on a fossil feedstock mainly producing the linear n-butanal. The use of an aqueous/organic two-phase system leads on the one hand to high selectivities of C4-aldehydes and on the other hand to a highly efficient recovery of the precious rhodium catalyst complex. This process is not applicable for higher olefins (>C5) due to their low solubility in the aqueous catalyst phase [1].
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
As described in Chap. 3, hydroformylation is one of the most important applications for homogeneous catalysis. Industrial proven processes like the Ruhrchemie/Rhône-Poulenc-Process (RCh-RP) are based on a fossil feedstock mainly producing the linear n-butanal. The use of an aqueous/organic two-phase system leads on the one hand to high selectivities of C4-aldehydes and on the other hand to a highly efficient recovery of the precious rhodium catalyst complex. This process is not applicable for higher olefins (>C5) due to their low solubility in the aqueous catalyst phase [1].
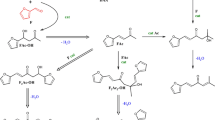
In order to develop processes for homogeneously catalysed reactions using renewable feedstocks, not only just reaction parameters and catalyst complexes have to be optimised but also innovative catalyst recycling concepts are imminent for economic operation. The hydroformylation of 1-dodecene in a continuously operated miniplant is the first step towards novel industrial processes using renewables. Terminal hydroformylation of olefins with internal C=C double bonds as well as hydroformylation of higher functionalised substrates with a terminal C=C double bond are tackled to combine both approaches in the isomerising hydroformylation of higher functionalised substrates. Figure 11.1 shows the pathway of reactions to reach, e.g. the continuous hydroformylation of methyl oleate.
Several approaches have been made to combine the hydroformylation of long chain olefins with a highly efficient catalyst recycling. Therefore investigations on the phase behaviour using surfactants, supercritical liquids or ionic liquids have been conducted [2]. Another possibility of catalyst retention is using a novel separation concept like organic solvent nanofiltration [3].
The process concept in the following is based on the thermodynamic behaviour of the mixture of one polar and one apolar solvent. The used solvents have a highly temperature-dependent miscibility gap, so that one single liquid phase is present at higher reaction temperature and two phases, one containing the substrate and products and one containing the catalyst complex, are present at lower temperature.
11.2 Batch Investigations: Catalyst Screening and Catalyst Recycling
The first step in the process development described here is the selection of an appropriate catalyst complex consisting of the catalyst precursor and the ligand. First, the model hydroformylation of 1-dodecene has been carried out in batch experiments (Table 11.1).
From several rhodium precursors (Acetylacetonato)dicarbonylrhodium(I) (Rh(acac)(CO)2) was chosen as reference and was used for further optimisation. High yields of the linear product n-tridecanal 2 and high n/iso-ratios are favourable. The use of the unmodified rhodium precatalyst Rh(acac)(CO)2 and reference conditions leads to a conversion X of 1-dodecene 1 of 55%, whereas the yield Y of n-tridecanal is at 11% and a n/iso-ratio of 40/60 is reached. Besides the hydroformylation to branched aldehydes 3, isomerisation to internal olefins 4 and hydrogenation to dodecane 5 are side reactions, as presented in Fig. 11.2.
In order to optimise the reaction towards higher n-tridecanal yields and n/iso-ratios, a wide ligand screening is carried out [4,5,6]. Figure 11.3 shows the most promising ligands for the hydroformylation of 1-dodecene, where triphenylphosphine (TPP) and triphenylphosphite represent monodentate ligands and Xantphos, Nixantphos, and Biphephos represent bidentate chelating ligands. Although the monodentate ligands lead to higher amounts of aldehydes (about 90%), the bidentate ligands seem to be more suitable for the aspired process featuring higher n/iso-ratios (about 99:1) combined with higher yields of the desired linear aldehyde n-tridecanal (about 80%).
In order to design a novel continuous process for the hydroformylation of 1-dodecene, it is not sufficient to focus on the final yield and the n/iso-ratio but also on the reaction rate. In respect to a holistic process development also the catalyst recovery has to be taken into account.
One approach of recycling the homogeneous catalyst complex is the use of a thermomorphic solvent system (TMS), where two phases are used to separate the product from the catalyst. In contrast to the RCh-RP-process, the phase behaviour of the reaction mixture can be switched by temperature changes. So that both the polar phase rich in catalyst and the apolar phase rich in substrate and product form a homogenous solution at reaction temperature. Furthermore, two phases can be recreated with lowering the temperature to separate the catalyst from the product again. Using this kind of solvent system, low catalyst leaching and a high concentration of the product in the apolar solvent can be achieved [7]. In the recent process development, the TMS-system containing the apolar solvent n-decane and the polar solvent DMF results in desired phase behaviour, good catalyst separation from the product, and high reaction rates. The optimal composition was found in 50 wt% n-decane and 50 wt% DMF [8].
Using this solvent system, the five ligands described before were compared in further reactions. In these investigations, the reaction is additionally observed during the whole time span by taking samples at certain points of time. Figure 11.4 shows the yield of the main product n-tridecanal over the time of 5 h.
Comparing the n-tridecanal yield for all the used ligands after five hours, Nixantphos shows the highest yield of n-tridecanal and therefore best results as found in the experiments before (compare Fig. 11.3). In respect to an industrial process also the time to reach the final yield has to be considered, leading directly to the residence time in the continuously operated reactor. Therefore, the steep slope of the reaction using the Biphephos ligand leads to the final n-tridecanal yield after 1 h, almost reaching the yield using Nixantphos. On the whole, Biphephos shows the best performance in the reaction comparing the n-tridecanal yield, the n/iso-ratio and the reaction rate.
Based on the selection of the catalyst complex and the used solvents, further reaction parameters such as reaction temperature, pressure, and synthesis gas composition have to be optimised. The best reaction performance was reached with a synthesis gas pressure of 20 bar and a composition of 50 mol% H2 and 50 mol% CO, a reaction temperature of 90–100 °C, and a stirrer speed of 800 rpm. Using these parameters, the reaction was carried out in a Parr-autoclave in a 40 g batch. After phase separation at −10 °C in a separation funnel, the catalyst phase was fed back to the reactor and used in another reaction run. In Fig. 11.5, the procedure of reaction, catalyst separation, and recycling was repeated 30 times (Fig. 11.5). In this reaction sequence, the high n/iso-ratio of 99:1 was reached in each run, although just a very low amount of Biphephos was replenished [4, 6].
Catalyst recycling in the TMS-system. Conditions: run1: 0.026 mol 1-dodecene (4.37 g), \( 2. 6\cdot 1 0^{ - 5} \) mol Rh(acac)(CO)2 (6.7 mg), n 1-dodecene:n Rh = 1000:1, n Rh:n Biphephos = 1:20, 13.04 ml DMF (12.39 g), 16.96 ml n-decane (12.39 g), 800 rpm, 20 bar CO:H2 (1:1), T R = 100 °C, 1 h, T S = −10 °C | run2–5: 0.026 mol 1-dodecene (4.37 g), \( 7. 8\cdot 1 0^{ - 5} \) mol Biphephos (61.4 mg), 1.30 ml DMF (1.24 g), 16.96 ml n-decane (12.39 g), 800 rpm, 20 bar CO:H2 (1:1), T R = 100 °C, 1 h, T S = −10 °C | run6–30: 0.026 mol 1-dodecene (4.37 g), \( 7.8 \cdot 10^{ - 5} \) mol Biphephos (61.4 mg), DMF replenishment to 12.39 g, 16.96 ml n-decane (12.39 g), 800 rpm, 20 bar CO:H2 (1:1), T R = 100 °C, 1 h, T S = −10 °C, run14–18: 1.25 h, run19: 1.6 h, run 20–25: 2 h, run 26: 2.25 h, run 27–30: 2 h
The investigations shown in Figs. 11.4 and 11.5 lead to a determination of the residence time in a continuous reactor and prove the possibility to recycle the active catalyst complex. Although the recycling concept is proven in batch mode, these experiments are not sufficient for a scale up to build an industrial process. Therefore reaction rates, phase separation, and catalyst stability have to be investigated consecutively in a continuously operated miniplant [9].
11.3 Miniplant Process
Figure 11.6 shows the simplified flow sheet of the miniplant for further investigations on the hydroformylation of 1-dodecene in continuously operated mode. Therefore the two main apparatus and the inlet-, outlet-, and recycle streams are presented. The hydroformylation takes place in a 1000 ml Büchi-autoklave which is modified to operate as continuously stirred tank reactor (CSTR). After the reaction is carried out, the homogeneous mixture is transferred into the settler subsequently, where the two phases are separated from each other, on the one hand the apolar phase rich in product and on the other hand the polar phase rich in catalyst. The substrate 1-dodecene is fed by a piston pump combined with the apolar solvent n-decane into the CSTR. The catalyst solved in the polar solvent DMF is continuously circulated from the settler back to the reactor with a gear pump. The product n-tridecanal and the n-decane are removed out of the active process through an overflow pipe [10].
As shown in Fig. 11.4, the maximum yield of n-tridecanal is reached after 40–45 min. in batch experiments using the Biphephos ligand. This knowledge was transferred to the design of the CSTR so that a residence time of 41 min. was chosen. Therefore, the liquid feed stream was set to 60 ml (27.6 wt% 1-dodecene and 72.4 wt% n-decane) and a recycle stream of 380 ml/h to realise the calculated residence time was established. Conductivity measurements showed a real residence time of 39 min. [10].
For the phase separation, a settler with two concentric segments, which are separated trough a lead pipe from each other, is used. The liquid hold-up is adjusted with two overflow pipes, the lower one in the inner segment for the polar catalyst phase and the upper pipe in the outer segment for the apolar product phase. The separation principle of the used settler is shown in detail in Fig. 11.7 [10].
The polar catalyst phase is recirculated to the reactor with a volume flow of 380 ml/h as described before and the apolar product phase is removed from the process.
Both, the catalyst phase and the product phase are filled into the reactor to start the miniplant process. Therefore the total liquid hold-up of the miniplant and the desired composition of the liquids are used. The reactor is operated for 4 h in batch mode and then switched to continuous mode, where the reaction mixture leaves the reactor through an overflow pipe and enters the settler, to be separated into two liquid phases. This step is defined as switching point from batch to continuous operation (and defined as 0 h in following diagrams). In Fig. 11.8, the yields at certain times are presented for the whole continuous operation [11].
First miniplant investigations. Conditions: p = 20 bar CO:H2 (1:1), T R = 90 °C, T S = 5 °C, 750 rpm, m DMF = 274.65 g, m Rh(acac)(CO)2 = 0.0755 g, \( \dot{m}_{\text{Biphephos}} \) = 1.1500 g, \( \dot{m}_{{ 1- {\text{dodecene}}}} \) = 12.23 g/h, \( \dot{m}_{{n - \text{decane}}} \) = 32.07 g/h, *from 40 h: \( \dot{m}_{\text{Biphephos}} \) = 31 mg/h
During the first 20 h in continuous mode, the yield of the desired product TDC increases constantly and finally reaches about 70%. During this period of time, the same n/iso-ratio, compared to the batch investigations, is detected. After that, the overall yield in aldehydes still increases for another 20 h to reach the maximum of 87%. Meanwhile, the selectivity decreases and reaches a n/iso-ratio of 65:35. In order to recreate the desired n/iso-ratio small amounts of the Biphephos ligand is added to the process after 40 h constantly.
Several optimisations of the miniplant operation lead to steady-state miniplant runs which were carried out more than 200 h with high n/iso-ratio of 99:1 as predicted. Figure 11.9 shows the yields of the desired n-tridecanal, methyldodecanal, and the internal olefins. Since the start-up procedure is switched from batch mode at the beginning to continuous substrate supply, the start-up behaviour shown in Fig. 11.8 can be avoided. During the whole optimised miniplant run (Fig. 11.9), the Biphephos ligand has to be replenished in small amounts, to compensate ligand degradation and guarantee the high n/iso-ratio (99:1). Also, losses of the catalyst metal and the polar solvent have to be added continuously. These replenishments are served in a separate feed stream, shown in Fig. 11.6 as make-up stream [12].
Yields in the continuous miniplant process. Conditions: p = 20 bar CO:H2 (1:1), T R = 90 °C, T S = 5 °C, 750 rpm, m DMF = 274.65 g, m Rh(acac)(CO)2 = 0.0378 g, m Biphephos = 0.5750 g, \( \dot{m}_{{ 1- {\text{dodecene}}}} \) = 12.23 g/h, \( \dot{m}_{{n - {\text{decane}}}} \) = 32.07 g/h, \( \dot{m}_{\text{DMF}} \) = 4.25 g/h, \( \dot{m}_{\text{Rh(acac)(CO)2}} \)= 0.25 mg/h, \( \dot{m}_{\text{Biphephos}} \) = 31mg/h
Besides the optimisation of the process in terms of selectivity, start-up behaviour and long-term performance, the recovery of all solvents forming the TMS-system is of major interest. Therefore, the process presented in Fig. 11.6 can be extended by a thermal separation unit (e.g. distillation column) to separate the n-decane and unconverted olefins. The final process flow diagram is shown in Fig. 11.10. Here, the unpolar product phase leaving the phase separator is expanded at ambient pressure and buffered in a liquid hold-up. Subsequently the product mixture is fed to the distillation column, which is operated at an absolute pressure of 10 mbar. The recovered n-decane and olefins are buffered in a liquid hold-up again and fed back to the reactor [13].
Figure 11.11 shows the yields of the continuous miniplant process presented in Fig. 11.10. During the start-up of the process, the obtained yields in the reactor are comparable to the previously presented operation. During this period, 1-dodecene and n-decane were fed to the reactor, so that the conditions only differ in the flow rate (1-dodecene + n-decane: Fig. 11.9 60 mL/h; Fig. 11.11 120 mL/h). Subsequently (10–20 h), the distillate stream of the distillation column was recycled to the reactor and feed streams were adjusted so that the ratio of olefins (1-dodecene + internal-olefins) and n-decane remained constant, still having a flow rate of 120 mL/h. After this start-up phase, the reaction behaviour was evaluated in the presented way (Fig. 11.11).
Yields in the continuous miniplant process. Conditions: p R,S = 20 bar CO:H2 (1:1), T R = 90 °C, T S = 5 °C, 750 rpm, m DMF = 274.65 g, m Rh(acac)(CO)2 = 0.0378 g, m Biphephos = 0.5750 g, \( \dot{m}_{{ 1- {\text{dodecene}}}} \) = 24.5 g/h, \( \dot{m}_{\text{DMF}} \) = 3 g/h, \( \dot{m}_{{{\text{Rh}}\left( {{\text{acac}})({\text{CO}}} \right) 2}} \) = 0.25 mg/h, \( \dot{m}_{\text{Biphephos}} \) = 31 mg/h, \( \dot{V}_{{{\text{feed}},{\text{column}}}} \) = 120 mL/h, p column = 10 mbar, reflux ratio = 1
Due to the isomerisation and highly selective linear hydroformylation, the recycle of the internal olefins leads to a significant reduction of the isomerisation of the substrate 1-dodcene, from initially 15–3%. Over the course of investigation (20–150 h), the yield of the linear tridecanal decreases from 64 to 51%, while a l/b-ratio of 95/5 is preserved during the total operation. These results show the presence of the active catalyst species within the reactor, since the l/b-ratio does not change over time. Furthermore, the recycle of the internal olefins leads to the decrease of linear hydroformylation, since the combination of isomerisation and linear hydroformylation is slower than linear hydroformylation of the terminal olefin.
11.3.1 Hydroformylation of Renewables in a Continuous Process
The knowledge about the hydroformylation process of 1-dodecene in a miniplant using a thermomorphic solvent system served as background to develop a continuous process for the hydroformylation of renewables as shown in Fig. 11.1. Again highly selective linear hydroformylation is desired, while isomerisation of the double bond, hydroformylation to branched aldehydes and hydrogenation are side reactions (Fig. 11.12) [14].
Research by Ternel et al. on the hydroformylation of 10-undecennitril has shown that the known TMS-systems are not applicable for functionalising middle-polar substrates that already contain a polar functional group due to the high solubility of the resulting products in the catalyst phase (>90%) [15]. With this in mind, again laboratory–scale research was conducted with the aim of developing a new concept that allows for both efficient process control with high yields and selectivities as well as easy catalyst recycling. Therefore, a so-called narrow-TMS-system consisting of the solvents water and 1-butanol was investigated. This solvent system shows the main criteria of a TMS-system, which are as follows:
-
Low solubility of the product in the polar solvent
-
High solubility of the catalyst complex in the polar solvent
-
High temperature dependency of the miscibility gap
Although the reaction mixture consisting of water, 1-butanol and methyl 10-undecenoate does not form a single liquid phase at reaction temperature, high reaction rates were observed. Switching the polar solvent from DMF to water also needs for a replacement of the catalyst complex, due to low solubility of the Biphephos ligand in the aqueous phase. Therefore, the ligand SulfoXantphos was used. Figure 11.13 shows the yields of the obtained products at different temperatures. While the yields of the desired linear hydroformylation product increases from 18% at 120 °C to 79% at 150 °C, an improvement of mass transport between catalyst phase and substrate-product phase was detected. The subsequent decrease in selectivity and also hydroformylation activity can be explained by ligand decomposition at higher temperatures.
As described before, catalyst recovery and recycling were investigated in batch operation. Therefore, the liquid phases were separated in a separation funnel, the catalyst phase recycled back to the autoclave and fresh substrate was added. Catalyst recovery was shown in three consecutive hydroformylation runs at 140 °C without any decrease in selectivity and activity and without catalyst or ligand replenishment. As the miniplant process shown in Fig. 11.6 was operated in a steady state for the hydroformylation of 1-dodecene, again this process was continuously operated for the hydroformylation of methyl 10-undecenoate. After the start-up procedure, continuous miniplant operation was achieved (Fig. 11.14). A constantly high yield of the linear product at 73% was achieved over the course of miniplant operation over 21 h. In terms of the reaction behaviour of the CSTR, virtually the same selectivity was observed compared to prior experiments in the batch autoclave. Only the rhodium concentration of 15 weight-ppm in the product stream under continuous operation is higher compared to the batch separation. As the water phase decreased under continuous operation owing to its solubility in 1-butanol, catalyst leaching was likely favoured. Nevertheless, stable process conditions were maintained for 21 h without interference, which demonstrates the stability of the catalyst species under process conditions.
Continuous hydroformylation of methyl 10-undecenoate in a miniplant. Conditions: p = 20 bar, CO:H2 = 1:1, T R = 140 °C, T S = 5 °C, w n-butanol:w H2O = 1:1, m H2O = 300 g, m Rh(acac)(CO)2 = 0.071 g, m SulfoXantphos = 1.071 g, \( \dot{V}_{\text{UME}} { = 17} . 7\,{\text{ml/h}} \), \( \dot{V}_{{n{\text{ - butanol}}}} { = 42} . 3\,{\text{ml/h}} \)
11.4 Conclusions
Herein, the process development for the hydroformylation of the long chained olefin 1-dodecene was shown. Based on the selection of a suitable transition metal catalyst complex, the reaction behaviour was investigated extensively. Afterwards the catalyst recycling concept of thermomophic solvent systems was introduced. A TMS-system consisting of DMF and n-decane was used for catalyst recycling in batch mode. Following the experience, a miniplant process was designed and investigations on long-term behaviour were conducted in continuous operation, which finally resulted in steady-state operation and the recycling of the catalyst as well as all of the used solvents.
This knowledge was transferred to the hydroformylation of the renewable resource methyl 10-undecenoate. Having main criteria of process development based on TMS-systems in mind, again the hydroformylation reaction was characterised and the catalyst recycled in batch operation. For this, a narrow-TMS-system consisting of water and 1-butanol was introduced. In the end, stable miniplant operation was shown for 20 h.
TMS-systems are promising for continuous hydroformylation processes of long chained olefins and renewable resources. With this catalyst recycling technique also high catalyst activity can be preserved, so that reaction and separation can be operated continuously. But still several points for optimisation of this concept remain, e.g. increase of operation time, improvement of catalyst separation, or variation of substrates like methyl oleate.
References
(a) Börner A Franke R (2016) Hydroformylation. Fundamentals, processes, and applications in organic systhesis, Wiley-VCH, Weinheim; (b) van Leeuwen PWNM, Claver C (2000) Rhodium Catalyzed Hydroformylation, Kluwer Academic, Dordrecht
(a) Jakuttis M, Schönweiz A, Werner S, Franke R, Wiese K-D, Haumann M, Wasserscheid P (2011) Angew Chem Int Ed 50:4492–4495; (b) Kunene TE, Webb PB, Cole-Hamilton DJ (2011) Green Chem 13:1476–1481; (c) Hamerla, N. Paul, M. Kraume, Schomäcker R (2013) Chem Ing Tech 85:1530–1539; (d) Desset TSL, Reader SW, Cole-Hamilton DJ (2009) Green Chem 11:630–637
Wiese K-D, Baumgarten G, Kuppinger FF, Moeller O, Ortmann D, Borgmann C, Büschken W (2012) US8226829 B2
Brunsch Y, Behr A (2013) Angew Chem 125:1627–1631
Brunsch Y (2012) Dissertation. TU Dortmund, Dortmund
Brunsch Y, Behr A (2013) Angew Chem Int Ed 52:1586–1589
(a) Behr A, Henze G, Obst D, Turkowski B (2005) Green Chem 7:645–649; (b) Behr A, Wintzer A (2011) Chem Ing Tech 83:1356–1370; (c) Behr A, Henze G, Schomäcker R (2006) Adv Synth Catal 348:1485–1495
Schäfer E, Brunsch Y, Sadowski G, Behr A (2012) Ind Eng Chem Res 51:10296–10306
Witte H, Zagajewski M, Behr A (2012) Chem Ing Tech 84:694–703
Zagajewski M, Behr A, Sasse P, Wittmann J (2014) Chem Eng Sci 115:88–94
Zagajewski M, Dreimann JM, Behr A (2014) Chem Ing Tech 86:449–457
Dreimann JM, Lutze P, Zagajewski M, Behr A, Górak A, Vorholt AJ (2016) Chem Eng Process Process Intensif 99:124–131
Dreimann JM, Warmeling H, Weimann JN, Künnemann K, Behr A, Vorholt AJ (2016) AIChE J 62:4377–4383
Gaide T, Dreimann JM, Behr A, Vorholt AJ (2016) Angew Chem Int Ed 55:2924–2928
Ternel J, Couturier J-L, Dubois J-L, Carpentier J-F (2013) Adv Synth Catal 355:3191–3204
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Dreimann, J., Behr, A., Vorholt, A.J. (2017). Continuously Operated Hydroformylation. In: Homogeneous Catalysis with Renewables. Catalysis by Metal Complexes, vol 39. Springer, Cham. https://doi.org/10.1007/978-3-319-54161-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-54161-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54159-4
Online ISBN: 978-3-319-54161-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)