Abstract
Autophagy, an evolutionarily conserved process, is crucial for survival, development, and homeostasis. It serves to remove damaged cellular components, such as mitochondria and long-lived proteins. Increased understanding of autophagy within liver cancer indicates that, in addition to the role of tumor promotion, autophagy also could suppress cancer progression by various cellular machineries. This chapter summarizes recent advances about dual roles of autophagy in tumor promotion and suppression, and discusses the intrinsic mechanism and application about autophagy inducers and inhibitors in clinical treatments with experimental results developed in vivo and in vitro systems.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Liver Cancer
- Nonalcoholic Fatty Liver Disease
- Intrahepatic Cholangiocarcinoma
- Cellular Death
- Autophagic Cell Death
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
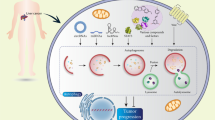
In recent years, autophagy has been a research focus. Cellular autophagy is particularly well studied in liver cancer, among the different tumor types. Confusingly, autophagy shows complex and dual roles in both suppressing and promoting tumors in liver cancer.
11.1 Autophagy in Tumor Suppression
Autophagy was proposed to be a tumor suppressor due to the direct evidence, showing that liver tumors were developed in autophagy-related gene (ATG)-deficient mouse models . Currently, some critical autophagy genes, including beclin1, ATG5 and ATG7, are proven tumor suppressor genes. In an ATG5 knockout mouse model , multiple benign tumors were developed only in the liver, but not in other tissues, indicating that continuous autophagy is important for the suppression of tumorigenesis. Moreover, swollen mitochondria , oxidative stress , and genomic damage responses were detected in the hepatic tumor cells. Similar results were also observed in liver-specific ATG7-deficient mice (Takamura et al. 2011). Beclin1 is a mammalian autophagy gene and a haploinsufficient tumor suppressor and negatively regulates tumorigenesis (Liang et al. 1999). Beclin1−/− mutant mice not only died early in embryogenesis but also suffered from a high incidence of spontaneous tumors (Qu et al. 2003; Yue et al. 2003). Heterozygous disruption of beclin1 could increase the frequency of spontaneous malignancies and accelerate the development of hepatitis B virus (HBV)-induced premalignant lesions . In addition, beclin1 heterozygous disruption also leads to an increase in cellular proliferation and reduces autophagy in vivo (Qu et al. 2003). Recently, studies revealed that the expression of beclin1 and bax in hepatocellular carcinoma (HCC) tissues might provide a synergistic effect that inhibits tumor proliferation, infiltration, metastasis, and angiogenesis (Qiu et al. 2014). All of these data from animal models provide clear evidence that autophagy is an important suppressor mechanism to prevent tumorigenesis. p62 is a selective autophagy substrate. Autophagy defects could result in sustained expression of p62 and promote tumor progression (Mathew et al. 2009). In human cancers, p62 accumulation has been detected (Inami et al. 2011), it is involved in heterozygous mutations of the beclin1 gene (Komatsu et al. 2007; Mathew et al. 2009; Qu et al. 2003; Yue et al. 2003). In a mouse model, liver-specific ATG7 knockout mice developed hepatocellular adenoma accompanied by excessive p62 accumulation and subsequent Nrf2 activation, and persistent Nrf2 activation contributes to the HCC formation (Inami et al. 2011). After a concomitant knockout of the p62 gene, tumor size in this mouse model significantly decreased (Takamura et al. 2011). Furthermore, the tumor suppressor gene p53 plays a dual role in cellular autophagy. In the cytoplasm, autophagy is suppressed by p53, and p53 degradation is also required for the induction of autophagy (Levine and Abrams 2008). Additional evidence indicates that p62 is increased in HCC tissues compared with non-tumorous liver tissues, suggesting that human HCC is autophagy defective (Bao et al. 2014). The suppression of liver cancer is also reflected in another aspect: the autophagy level is negatively correlated with the degree of HCC malignancy. Under starvation conditions, decreased basal expression of autophagic genes and their corresponding autophagic activity in HCC cell lines, and autophagy defects are correlated well with the highly malignant phenotype (Ding et al. 2008). These results suggest that autophagy defects in combination with altered apoptotic activity might facilitate tumor progression and poor prognosis because autophagy might interact with apoptosis in the regulation of HCC (Shi et al. 2009). Due to its suppression effect on liver tumor, autophagy might be of therapeutic value. Tissue injury, inflammation, and genomic instability are risk factors for carcinogenesis. In precancerous cells , these risk factors could be inhibited by autophagy, and autophagy induction may be beneficial for the prevention of liver cancer. For example, in patients with α-1 antitrypsin deficiency , inflammation and carcinogenesis could be inhibited by the upregulation of basal autophagy level in liver cells (Perlmutter 2009). It has been demonstrated that carbamazepine (CBZ) could increase the autophagy level, reduce the α-1 antitrypsin load, and lower the risks of liver fibrosis and cancer in an α-1 antitrypsin deficiency (ATD) mouse model (Hidvegi et al. 2010), indicating that increased autophagy might provide effective and preventive effects against liver cancer.
11.2 Autophagy in Cancer Promotion
Growing evidences indicate that autophagy is needed by tumor cells under disadvantageous conditions, including starvation, deficiency of growth factor, hypoxia, injury, and drug medication. Generally, up-regulation of basal autophagy is required for the survival of tumor cells, implying that cancer cells use the catabolic function of autophagy to tolerate stress. The autophagosome is common in ischemic areas, and the autophagy inhibition induced by decreased levels of beclin1 causes cellular death (Degenhardt et al. 2006). In RAS-driven tumors , the basal autophagy level is increased. Moreover, RAS-expressing ATG5−/− or ATG7−/− cells displayed reduced tumor growth in vivo (Guo et al. 2011).
Recently, increasing evidences showed that HCC metastasis could be suppressed by autophagy, and the intrinsic mechanism underlying metastasis might involve facilitating anoikis resistance and lung colonization (Peng et al. 2013b, c). Moreover, studies demonstrated that hypoxia-induced autophagy contributed to HCC chemoresistance. In vitro, by enhancing cellular survival and decreasing the apoptotic potential, autophagy may contribute to the resistance of HCC cells to chemotherapeutic agents under hypoxia; thus, the cellular survivability is changed by autophagy suppression (Song et al. 2009). Because autophagy is essential for tumor survival pathways, autophagy modulation is a novel approach for enhancing the efficacy of existing cancer therapy. Current (angiogenesis inhibitors and growth factor receptors ) and conventional cancer treatments (radiotherapy and chemotherapy) could lead to metabolic stress and induce autophagy. To improve the clinical efficacy, autophagy inhibitors could be used to eliminate the protective effect of autophagy in combination therapy (Wu et al. 2012). Antimalarial drugs (chloroquine and hydroxychloroquine) have been evaluated and shown promising results in preclinical and clinical trials, and research on specific small molecule autophagy inhibitors is currently in progress (details as follows).
Autophagy could exert a strong influence on carcinogenesis and tumor progression by altering the risk factors of liver cancer , including HBV, hepatitis C virus (HCV), alcohol intake, and others. HBV infection is the most important leading cause of liver cancer, and it induces autophagy by enhancing viral DNA replication with only a slight effect on viral RNA transcription (Sir et al. 2010). During a natural HBV infection , autophagy facilitates HBV replication, which has primarily been demonstrated in cell cultures and in a mouse model (Li et al. 2011; Tian et al. 2011). HCV is also correlated with liver cancer, and studies have shown that autophagy contributes to the self-replication of HCV particles. HCV RNA replication could promote the expression of UPR and CHOP, thus leading to the accumulation of autophagosomes and activation of autophagy (Ait-Goughoulte et al. 2008; Sir et al. 2008). Some ATGs (BECN1, ATG4B, ATG5, and ATG12) are involved in the translation of viral mRNA and initiation of replication (Dreux et al. 2009). HCV RNA replication may block the maturation of the autophagosome and autolysosome (Sir et al. 2008). Furthermore, HCV seems to avoid recognition by the autophagic machinery (Alavian et al. 2011; Rautou et al. 2010). Chronic alcohol consumption has been widely recognized as one of the most common causes, and it may lead to several types of liver status: fatty liver, hepatic fibrosis, cirrhosis, and alcoholic hepatitis. The mechanism underlying the liver injury may include the induction of oxidative stress. Hepatic steatosis and oxidative stress are regulated by autophagy in liver cells, indicating the importance of autophagy in ethanol-induced liver diseases (Czaja 2011; Wang et al. 2010). Needless to say, the exact role of autophagy in alcohol-related liver cancer remains to be further studied. Nonalcoholic fatty liver disease (NAFLD) is a common chronic liver disease. The loss of lipid droplets is promoted by autophagy and is called fat endocytosis (Singh et al. 2009). In hepatic stellate cells , blocking autophagy could result in the accumulation of lipid droplets and attenuation of liver fibrosis (Hernandez-Gea et al. 2012), implying that autophagy may prevent the formation of NAFLD-related tumors.
It should be noted that the majority of current research is focused on HCC , but autophagy also plays a similar role in two other types of liver cancer, including intrahepatic cholangiocarcinoma (ICC) and hepatoblastoma . In nutrient starvation and xenograft cholangiocarcinoma cells, autophagy is activated (Hou et al. 2011). Inhibiting autophagy (by an autophagy inhibitor or beclin1 siRNA) could induce apoptosis during starvation and increase the sensitivity of cholangiocarcinoma cells to chemotherapy (Hou et al. 2011). Similarly, blocking autophagy by regulating autophagic genes could suppress the formation of hepatoblastoma. However, the exact mechanism of autophagy in ICC and hepatoblastoma requires in-depth studies.
11.3 Autophagy as a New Therapeutic Target
Due to its critical role in liver cancer, autophagy has been considered a new therapeutic target, but there is currently no consensus about autophagy therapy in clinics. Theoretically, autophagy inhibitors could eliminate or weaken the protective roles of chemotherapy induced by autophagy, which could enhance the tumor-killing effect and promote the therapeutic effect. In addition, autophagy inducers could lead to cellular autophagic death when combined with chemotherapy . By activating the PI3K-Akt and Akt signaling pathways, sorafenib or bortezomib treatment could activate autophagy, and combining these treatments with chloroquine (CQ) or 3-methyladenine (3-MA) could improve their cytotoxicity (Hui et al. 2012; Shimizu et al. 2012; Yu et al. 2013). Cisplatin and oxaliplatin share similar effects (Ding et al. 2011; Xu et al. 2012). However, the molecule machinery of certain drugs is not similar to those above. Anticancer drugs (nilotinib, cannabinoids tetrahydrocannabinol, JWH-015, and SAHA) can upregulate autophagy by activating the AMPK signaling pathway , but their treatment efficacy was decreased when they were combined with inhibitors. Autophagy may protect cancer cells, but the induced autophagy mediated by the AMPK signaling pathway seems to induce cytotoxicity. The above results indicate the complexity of autophagy treatments in liver cancer.
11.4 Autophagy Inducers and Inhibitors
Owing to the multi-faceted role of autophagy, inhibitor applications depend on the specific circumstances (Amaravadi et al. 2011, 2007). Autophagy is considered a cellular death mechanism that promotes apoptosis or autophagic cell death, leading to better treatment (Edinger and Thompson 2004; Eisenberg-Lerner et al. 2009; Shen and Codogno 2011). In apoptosis-deficient tumor cells , induced autophagy activity and increased autophagic cell death are effective ways to facilitate cellular death (Maycotte and Thorburn 2011). The autophagy inducers and inhibitors that have been developed using in vitro or in vivo systems and have been used in clinical trials are summarized in Table 11.1.
Autophagy inducers : important cellular autophagy inducers for liver cancer (based on the concept that autophagy could improve the treatment effect by enhancing cellular death) include the following: (1) rapamycin and its analogs : in HCC, the PI3K/Akt/mTOR pathway is the key signaling cascade because it modulates cellular growth, proliferation, angiogenesis, and apoptosis (Sabatini 2006), and this pathway is activated in 15–41% of HCC tumors. mTOR inhibitors have anti-HCC activity (Sieghart et al. 2007). Rapamycin (sirolimus), an mTOR inhibitor, is widely used as an autophagy inducer, and it has been applied for its anti-proliferation and anti-angiogenesis effects. In clinical research, rapamycin and its analogs (everolimus ) have proven anti-tumor activity (Huynh et al. 2009). (2) Tyrosine kinase inhibitors : tyrosine kinase plays an important role in tumor progression, and it has been utilized in cancer treatment. In combination with the HDAC inhibitor SAHA, sorafenib can enhance liver cancer death by inducing autophagy (Martin et al. 2009; Park et al. 2008). However, adriamycin (DOX)-induced autophagic cell death can be inhibited by sorafenib, thus promoting the cell cycle progress, improving the survival rate, and decreasing cancer autophagy (Manov et al. 2011). Therefore, the potent antagonistic effect of sorafenib and adriamycin treatment needs to be further considered . (3) Others: a new naphthalimide polyamine conjugate can induce autophagy and apoptosis, and the involvement of the mTOR pathway in the autophagy mediated by NPC-16 has been confirmed (Xie et al. 2011). Berberine , which inhibits the mTOR pathway by up-regulating the AMPK signaling pathway, can also induce autophagic cellular death in liver cancer (Wang et al. 2010a; Yu et al. 2014). The cannabinoid Δ9-THC and its receptor agonist (JWH-015) could inhibit autophagy induction and decrease the growth of subcutaneous xenografts through the Akt-mTORC1 axis and stimulation of the AMPK pathway. Evidence demonstrated that Δ9-THC and the agonist JWH-015 led to the death of liver cancer. As a new type of antitumor agent, fangchinoline can induce autophagic death in liver cancer through the p53/sestrin2/AMPK pathway (Wang et al. 2011). Furthermore, MLN4924 (a small molecule inhibitor of NEDD8-activating enzyme) can suppress the growth of liver cancer by inducing autophagy in vivo and in vitro. The intrinsic mechanism might be the suppression of mTOR activity by Deptor (mTOR-binding protein) (Luo et al. 2012).
Autophagy inhibitors : autophagy is an essential survival mechanism, through which cancer cells can survive under various stress conditions, including drug treatment. Therefore, inhibitors can increase the treatment effect by abolishing the protective effect of autophagy, increasing cytotoxicity, and limiting treatment resistance. (1) CQ and hydroxychloroquine (HCQ) : CQ and HCQ are widely used as autophagy inhibitors. Many reports have demonstrated that drug treatments, including CQ and HCQ treatments, have a sensitizing effect on cell apoptosis both in vivo and in vitro. For example, CQ-induced autophagy increases the cellular death caused by oxaliplatin, improving the chemosensitivity in liver cancer, and a similar result was shown by ding et al. (Ding et al. 2011). In HCC cell lines and a nude mouse model, the sorafenib group combined with CQ significantly suppressed the tumor growth compared with that for sorafenib alone (Shi et al. 2011; Shimizu et al. 2012). Currently, 25 autophagy-related clinical trials are in progress that use CQ/HCQ alone or in combination with other drugs to treat tumors (http://www.clinicaltrials.gov). However, no related clinical trials are being performed for liver cancer. (2) siRNA and shRNA : due to the high specificity and selectiveness of siRNA or shRNA, side effects could be avoided to a large extent by silencing certain autophagy genes. Many reports have reported cancer treatments that knock out specific ATG genes, but there are no clinical trials targeting liver cancer. Chen demonstrated that suppressed autophagy, which was mediated by knocking down beclin1, promoted increased cellular death in liver cancer (Chen et al. 2011). Beclin1 silencing also facilitates the H22 cellular death induced by melatonin and improves the antitumor effects of melatonin (Liu et al. 2012). Overall, silencing-specific ATG genes to inhibit autophagy could improve the clinical effect of chemotherapy. However, it is still unclear whether the siRNA-induced treatment effect would occur in liver cancer. Via the construction of viral vectors carrying shRNA or miRNA targeting critical autophagy genes, autophagy inhibition in vivo might be a new field in the future. An autophagy-related and specific shRNA designed by Peng et al. was demonstrated to be of great value in suppressing autophagy and lung metastasis both in vivo and in vitro (Peng et al. 2013a, c). (3) microRNAs (miRNAs) can regulate autophagy through multiple genes and pathways (Kim and Kim 2014). miRNAs could regulate autophagy in liver cancer by acting on ATG genes. Many miRNAs (such as miR-101, miR-30a, miR-34a, miR-204, and miR-375) could serve as inhibitors. In liver cancer, hypoxia-induced autophagy could be suppressed effectively by the direct actions of miR-375 on ATG7 (Kim and Kim 2014). Transfection with a miR-375 mimic attenuated the protective effect of autophagy and impaired the vitality of tumor cells (Pastore et al. 2013). By down regulating STMN, RAB5A, and ATG4D, miR-101 could suppress HCC autophagy. When combined with adriamycin and fluorouracil, miR-101 could increase their chemosensitivity. In liver cancer patients treated with cisplatin, the miR-199a-5p level is decreased significantly; this miRNA acts on ATG7 and abolishes the drug resistance induced by autophagy. Autophagy inhibition by miRNA is becoming a new focus. However, some miRNAs will induce autophagy. miR-100 facilitates HCC autophagy by preventing mTOR and IGF-1R expression. Overexpression of miR-100 can lead to cellular death, and this effect can be inhibited by ATG7 silencing and CQ, providing a potent target for HCC treatment . (4) Others: 20(S)-Ginsenoside Rg3 is a new type of autophagy inhibitor that can improve the adriamycin treatment effect (Kim et al. 2014). Adriamycin-induced autophagy has a protective effect in liver cancer. Ginsenoside Rg3 inhibits late-period autophagy. Combined with doxorubicin, Rg3 is able to kill HCC cells, which exhibit good tolerance to Rg3, and able to inhibit liver cancer xenografts in mice. The combination of inhibitors and traditional chemotherapy drugs is becoming an effective strategy in liver cancer treatment.
11.5 Conclusion
In liver cancer, autophagy has a Janus-face in that being a tumor survival mechanism, it can also lead to autophagic cell death. Accordingly, taking into account the essential role of autophagy in liver cancer and the existence of therapies targeting this process, may improve clinical efficacy of liver cancer. Exploring the potential clinical value of autophagy in liver cancer may lead to a new avenue of cancer therapeutics.
Abbreviations
- 3-MA:
-
3-methyladenine
- ATD:
-
Alpha1-antitrypsin deficiency
- ATG:
-
Autophagy-related gene
- CBZ:
-
Carbamazepine
- CQ:
-
Chloroquine
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCQ:
-
Hydroxychloroquine
- HCV:
-
Hepatitis C virus
- ICC:
-
Intrahepatic cholangiocarcinoma
- MDBs:
-
Mallory-denk bodies
- NAFLD:
-
Nonalcoholic fatty liver disease
References
Ait-Goughoulte M, Kanda T, Meyer K, Ryerse JS, Ray RB, Ray R (2008) Hepatitis C virus genotype 1a growth and induction of autophagy. J Virol 82(5):2241–2249. doi:10.1128/JVI.02093-07
Alavian SM, Ande SR, Coombs KM, Yeganeh B, Davoodpour P, Hashemi M, Ghavami S et al (2011) Virus-triggered autophagy in viral hepatitis - possible novel strategies for drug development. J Viral Hepat 18(12):821–830. doi:10.1111/j.1365-2893.2011.01530.x
Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI et al (2007) Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest 117(2):326–336. doi:10.1172/JCI28833
Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W et al (2011) Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 17(4):654–666. doi:10.1158/1078-0432.CCR-10-2634
Bao L, Chandra PK, Moroz K, Zhang X, Thung SN, Wu T, Dash S (2014) Impaired autophagy response in human hepatocellular carcinoma. Exp Mol Pathol 96(2):149–154. doi:10.1016/j.yexmp.2013.12.002
Chen LH, Loong CC, Su TL, Lee YJ, Chu PM, Tsai ML et al (2011) Autophagy inhibition enhances apoptosis triggered by BO-1051, an N-mustard derivative, and involves the ATM signaling pathway. Biochem Pharmacol 81(5):594–605. doi:10.1016/j.bcp.2010.12.011
Czaja MJ (2011) Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology 140(7):1895–1908. doi:10.1053/j.gastro.2011.04.038
Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G et al (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10(1):51–64. doi:10.1016/j.ccr.2006.06.001
Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z et al (2008) Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res 68(22):9167–9175. doi:10.1158/0008-5472.CAN-08-1573
Ding ZB, Hui B, Shi YH, Zhou J, Peng YF, Gu CY et al (2011) Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res 17(19):6229–6238. doi:10.1158/1078-0432.CCR-11-0816
Dreux M, Gastaminza P, Wieland SF, Chisari FV (2009) The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci U S A 106(33):14046–14051. doi:10.1073/pnas.0907344106
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16(6):663–669. doi:10.1016/j.ceb.2004.09.011
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16(7):966–975. doi:10.1038/cdd.2009.33
Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G et al (2011) Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 25(5):460–470. doi:10.1101/gad.2016311
Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z et al (2012) Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142(4):938–946. doi:10.1053/j.gastro.2011.12.044
Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C et al (2010) An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science 329(5988):229–232. doi:10.1126/science.1190354
Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z et al (2011) Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Investig 91(8):1146–1157. doi:10.1038/labinvest.2011.97
Hui B, Shi YH, Ding ZB, Zhou J, Gu CY, Peng YF et al (2012) Proteasome inhibitor interacts synergistically with autophagy inhibitor to suppress proliferation and induce apoptosis in hepatocellular carcinoma. Cancer 118(22):5560–5571. doi:10.1002/cncr.27586
Huynh H, Chow KH, Soo KC, Toh HC, Choo SP, Foo KF et al (2009) RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med 13(7):1371–1380. doi:10.1111/j.1582-4934.2008.00364.x
Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O et al (2011) Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 193(2):275–284. doi:10.1083/jcb.201102031
Kim KM, Kim SG (2014) Autophagy and microRNA dysregulation in liver diseases. Arch Pharm Res 37(9):1097–1116. doi:10.1007/s12272-014-0439-9
Kim DG, Jung KH, Lee DG, Yoon JH, Choi KS, Kwon SW et al 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 5(12):4438–4451. doi:10.18632/oncotarget.2034
Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T et al (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131(6):1149–1163. doi:10.1016/j.cell.2007.10.035
Levine B, Abrams J (2008) p53: the Janus of autophagy? Nat Cell Biol 10(6):637–639. doi:10.1038/ncb0608-637
Li J, Liu Y, Wang Z, Liu K, Wang Y, Liu J et al (2011) Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J Virol 85(13):6319–6333. doi:10.1128/JVI.02627-10
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402(6762):672–676. doi:10.1038/45257
Liu C, Jia Z, Zhang X, Hou J, Wang L, Hao S et al (2012) Involvement of melatonin in autophagy-mediated mouse hepatoma H22 cell survival. Int Immunopharmacol 12(2):394–401. doi:10.1016/j.intimp.2011.12.012
Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D et al (2012) The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res 72(13):3360–3371. doi:10.1158/0008-5472.CAN-12-0388
Manov I, Pollak Y, Broneshter R, Iancu TC (2011) Inhibition of doxorubicin-induced autophagy in hepatocellular carcinoma Hep3B cells by sorafenib—the role of extracellular signal-regulated kinase counteraction. FEBS J 278(18):3494–3507. doi:10.1111/j.1742-4658.2011.08271.x
Martin AP, Park MA, Mitchell C, Walker T, Rahmani M, Thorburn A et al (2009) BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol 76(2):327–341. doi:10.1124/mol.109.056309
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY et al (2009) Autophagy suppresses tumorigenesis through elimination of p62. Cell 137(6):1062–1075. doi:10.1016/j.cell.2009.03.048
Maycotte P, Thorburn A (2011) Autophagy and cancer therapy. Cancer Biol Ther 11(2):127–137
Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB et al (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 7(10):1648–1662
Pastore N, Blomenkamp K, Annunziata F, Piccolo P, Mithbaokar P, Maria Sepe R et al (2013) Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med 5(3):397–412. doi:10.1002/emmm.201202046
Peng YF, Shi YH, Ding ZB, Zhou J, Qiu SJ, Hui B et al (2013a) alpha-Fetoprotein promoter-driven Cre/LoxP-switched RNA interference for hepatocellular carcinoma tissue-specific target therapy. PLoS One 8(2):e53072. doi:10.1371/journal.pone.0053072
Peng YF, Shi YH, Shen YH, Ding ZB, Ke AW, Zhou J et al (2013b) Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS One 8(9):e74407. doi:10.1371/journal.pone.0074407
Peng YF, Shi YH, Ding ZB, Ke AW, Gu CY, Hui B et al (2013c) Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy 9(12):2056–2068. doi:10.4161/auto.26398
Perlmutter DH (2009) Autophagic disposal of the aggregation-prone protein that causes liver inflammation and carcinogenesis in alpha-1-antitrypsin deficiency. Cell Death Differ 16(1):39–45. doi:10.1038/cdd.2008.103
Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao XL et al (2014) The expression of beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer 14:327. doi:10.1186/1471-2407-14-327
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A et al (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112(12):1809–1820. doi:10.1172/JCI20039
Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R (2010) Autophagy in liver diseases. J Hepatol 53(6):1123–1134. doi:10.1016/j.jhep.2010.07.006
Sabatini DM (2006) mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6(9):729–734. doi:10.1038/nrc1974
Shen HM, Codogno P (2011) Autophagic cell death: Loch Ness monster or endangered species? Autophagy 7(5):457–465
Shi YH, Ding ZB, Zhou J, Qiu SJ, Fan J (2009) Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy 5(3):380–382
Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW et al (2011) Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 7(10):1159–1172. doi:10.4161/auto.7.10.16818
Shimizu S, Takehara T, Hikita H, Kodama T, Tsunematsu H, Miyagi T et al (2012) Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer 131(3):548–557. doi:10.1002/ijc.26374
Sieghart W, Fuereder T, Schmid K, Cejka D, Werzowa J, Wrba F et al (2007) Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation 83(4):425–432. doi:10.1097/01.tp.0000252780.42104.95
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M et al (2009) Autophagy regulates lipid metabolism. Nature 458(7242):1131–1135. doi:10.1038/nature07976
Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH (2008) Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 48(4):1054–1061. doi:10.1002/hep.22464
Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH (2010) The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci U S A 107(9):4383–4388. doi:10.1073/pnas.0911373107
Song J, Qu Z, Guo X, Zhao Q, Zhao X, Gao L et al (2009) Hypoxia-induced autophagy contributes to the chemoresistance of hepatocellular carcinoma cells. Autophagy 5(8):1131–1144
Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S et al (2011) Autophagy-deficient mice develop multiple liver tumors. Genes Dev 25(8):795–800. doi:10.1101/gad.2016211
Tian Y, Sir D, Kuo CF, Ann DK, Ou JH (2011) Autophagy required for hepatitis B virus replication in transgenic mice. J Virol 85(24):13453–13456. doi:10.1128/JVI.06064-11
Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, Tsao SW (2010a) Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem 111(6):1426–1436. doi:10.1002/jcb.22869
Wang Y, Singh R, Xiang Y, Czaja MJ (2010b) Macroautophagy and chaperone-mediated autophagy are required for hepatocyte resistance to oxidant stress. Hepatology 52(1):266–277. doi:10.1002/hep.23645
Wang N, Pan W, Zhu M, Zhang M, Hao X, Liang G, Feng Y (2011) Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br J Pharmacol 164(2b):731–742. doi:10.1111/j.1476-5381.2011.01349.x
Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee CW, Chan FK et al (2012) The autophagic paradox in cancer therapy. Oncogene 31(8):939–953. doi:10.1038/onc.2011.295
Xie SQ, Li Q, Zhang YH, Wang JH, Mei ZH, Zhao J, Wang CJ (2011) NPC-16, a novel naphthalimide-polyamine conjugate, induced apoptosis and autophagy in human hepatoma HepG2 cells and Bel-7402 cells. Apoptosis 16(1):27–34. doi:10.1007/s10495-010-0537-1
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F, Xia Q (2012) Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun 423(4):826–831. doi:10.1016/j.bbrc.2012.06.048
Yu HC, Hou DR, Liu CY, Lin CS, Shiau CW, Cheng AL, Chen KF (2013) Cancerous inhibitor of protein phosphatase 2A mediates bortezomib-induced autophagy in hepatocellular carcinoma independent of proteasome. PLoS One 8(2):e55705. doi:10.1371/journal.pone.0055705
Yu R, Zhang ZQ, Wang B, Jiang HX, Cheng L, Shen LM (2014) Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int 14:49. doi:10.1186/1475-2867-14-49
Yue Z, Jin S, Yang C, Levine AJ, Heintz N (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 100(25):15077–15082. doi:10.1073/pnas.2436255100
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Tian, MX., Peng, YF., Wang, H., Fan, J., Shi, YH. (2017). Cell Death and Autophagy in Liver Tumorigenesis and Liver Cancer. In: Ding, WX., Yin, XM. (eds) Cellular Injury in Liver Diseases. Cell Death in Biology and Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-53774-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-53774-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53773-3
Online ISBN: 978-3-319-53774-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)