Abstract
Imaging of early and subtle signs of the inflammatory arthritides exposes the radiologist to diagnostic pitfalls and, in particular, to false positives. The aim of this chapter is to present the main imaging pitfalls and mimics that may be encountered in daily practice when faced with a clinical suspicion of spondyloarthritis or rheumatoid arthritis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
- BME:
-
Bone marrow edema
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- RA:
-
Rheumatoid arthritis
- SpA:
-
Spondyloarthritis
- US:
-
Ultrasound
1 Introduction
Inflammatory arthritides represents a heterogeneous group of inflammatory disorders characterized by chronic inflammation of joints. Since their early diagnosis and treatment have been recognized as essential for the improvement of patients’ clinical outcome, imaging has been increasingly used in clinical practice. As a consequence, depiction of early and therefore subtle imaging signs of inflammatory arthritis exposes the radiologist to diagnostic pitfalls and in particular, to false positives. The aim of this chapter is to present the main imaging pitfalls that may be encountered in daily practice when faced with a clinical suspicion of inflammatory arthritides. Therefore, this chapter will be focused on the main joints affected early in these disorders (i.e., sacroiliac, spine, and peripheral joints).
2 Axial Spondyloarthritis: Sacroiliitis?
2.1 Diagnosis
The sacroiliac joints are nearly always affected in spondyloarthritis (SpA), with sacroiliitis usually being the initial manifestation. Erosions and subchondral sclerosis can be detected by radiographs, but it often takes 6–8 years before sacroiliitis is detectable on radiographs (Mau et al. 1987; Bennett et al. 2008). Computed tomography (CT) may depict subtle structural lesions more accurately, but it has the same limitations as radiographs, as these imaging modalities can detect only the structural damage of the joints resulting from inflammation and not the active inflammation itself. This is why new Assessment of Spondyloarthritis International Society (ASAS) criteria have been defined to help identify patients with early axial SpA (Rudwaleit et al. 2009a, b, c). In patients younger than 45 years with low back pain for more than 3 months, the imaging arm of these criteria requires the presence of sacroiliitis either on radiographs, which defines the ankylosing spondylitis patients, or active inflammation on magnetic resonance imaging (MRI), which defines the non-radiographic axial SpA group (Rudwaleit et al. 2009a, b). According to these criteria, bone marrow edema (BME) is regarded as essential for the definition of active sacroiliitis on MRI (Rudwaleit et al. 2009a, b).
As a consequence, MRI is nowadays frequently and widely performed for diagnostic purposes. At an early stage of the disease, BME may be uni- or bilateral, focal, multifocal, or diffuse. It is usually seen on both sides of the joint but unilateral involvement is possible. It tends to predominate on the iliac side, but a sacral predominance is also possible. It may be isolated or associated with other inflammatory or structural damage, these additional features improving diagnostic certainty. Subtle early MRI features of sacroiliitis require differentiation from other disorders that may be accompanied by BME.
2.2 Mimics and Pitfalls
2.2.1 Red Bone Marrow and Ossifying Cartilage
Particularly in young patients, BME has to be differentiated from red bone marrow and ossifying cartilage, for which signal intensity is usually less intense on fat-suppressed T2-weighted images and the distribution bilateral and more uniform (Fig. 34.1). Residual areas can also be seen along the lateral aspects of the distal sacrum. Irregularities of the sacroiliac joint surfaces can also be demonstrated until 16–18 years of age, due to the immaturity of the ossification process (Bollow et al. 1997; Sheybani et al. 2013).
2.2.2 Vessels
Vessels which are abundant in the transitional area between the cartilaginous and the ligamentous joints (Egund and Jurik 2014) can be misleading on the oblique coronal sections when the latter involve this area, mimicking synovitis or BME (Fig. 34.2). However, they are easily recognized on the oblique axial images, which are particularly useful in any doubtful cases.
2.2.3 Tiny Lesions
Since small foci of subchondral BME are sometimes seen in healthy volunteers (Weber et al. 2013), a size criterion has been proposed to increase the specificity of this sign for the diagnosis of active sacroiliitis (Rudwaleit et al. 2009a, b). If there is only one edematous lesion seen on a single slice, that lesion must be present on one of the adjacent slices (Fig. 34.3). If there is more than one lesion on a single slice, one slice may be sufficient. In summary, the lesion must be large enough and is not significant when alone/isolated and small-sized. The use of this definition should allow most of the nonsignificant small edematous areas that can be encountered in the subchondral bone marrow to be eliminated (Fig. 34.4).
2.2.4 Mechanical Overload
It is also fundamental to analyze the location of BME; as in the sacroiliac joints, mechanical overload is usually concentrated in a precise region, i.e., the anterior part of the middle third of the joint. This is why degenerative changes and osteitis condensans ilii are located or predominate in this region (Olivieri et al. 1996; Shibata et al. 2002). One should keep in mind that the most anterior oblique coronal sections involve this region. As a consequence, abnormal signal intensities of the subchondral bone marrow (edema, sclerosis, fat, or erosions) at this sole location, sometimes even on two or three adjacent slices, are not relevant for the diagnosis of sacroiliitis (Fig. 34.5). Axial sections will easily confirm the anterior location of these features, often triangular in shape, sometimes with some bony proliferation at the articular margins and often bilateral in distribution (Fig. 34.6). These changes related to mechanical stress, of which topography is the key discriminating factor, represent the main pitfall when reading MRI of the sacroiliac joints. In contrast, presence of BME in the other parts of the joint not involved by mechanical loading such as the proximal or distal third of the joint, or its posterior part, is highly suggestive of SpA, if the previously described criteria of size are respected.
2.2.5 Anatomical Variants
Several anatomical variants (e.g., accessory sacroiliac joint, iliosacral complex, semicircular defects) can be misleading on coronal images, particularly when they are associated with edema related to microtrauma or degenerative changes. Once again, axial sections are also particularly helpful for their recognition.
2.2.6 Sacral Fracture
Bone insufficiency fractures in a sagittal plane have to be kept in mind when BME is mainly located on the sacral side (Peh et al. 1996; Ahovuo et al. 2004) (Fig. 34.7). The hypointense fracture line may sometimes be difficult to identify on MR images, being embedded in the adjacent edema. However, the age of the patient is typically different, and the edema tends to predominate in the sacral wing with a relative preservation of the subchondral bone marrow, in contrast to sacroiliitis.
2.2.7 Septic Sacroiliitis
Clinical and biological symptoms are often suggestive of an infectious process, although spondylodiskitis is usually evoked. Septic sacroiliitis is typically unilateral. It is usually associated with a joint effusion and extensive BME and periarticular soft tissue changes, including abscess (Stürzenbecher et al. 2000) (Fig. 34.8).
2.2.8 Tumors
Infrequently, BME associated with tumors (such as osteoid osteoma whose nidus may be undetectable on MRI) (Llauger et al. 2000) or tumoral bone marrow infiltration such as in lymphoma (Bereau et al. 2011) can be misleading. Analysis of the topography of these abnormal signal intensity changes, located or extending far away from the joint space and/or depiction of extension into the adjacent soft tissues, may suggest the correct diagnosis.
2.2.9 Hyperparathyroidism
Bilateral subchondral bone resorption of the sacroiliac joints may be seen in hyperparathyroidism, either in its primary or secondary form, mimicking sacroiliitis (Bywaters et al. 1963). However, suggestive features include unusually intense and extensive subchondral bone resorption, only minimal cartilage surface irregularity (Tuite 2008), absent or minimal BME, and no evolution toward ankylosis (Fig. 34.9).
3 Axial Spondyloarthritis: Spine Involvement?
3.1 Diagnosis
The spine is also frequently involved in SpA. On radiographs, the initial features typically start at the thoracolumbar junction, particularly at the anterior corners of the vertebral bodies, as this is a region rich in entheses (annulus fibrosus, deep fibers of the anterior longitudinal ligament). The Romanus lesion refers to marginal bone resorption or erosion of the anterior vertebral corners due to osteitis and enthesitis, which is responsible for squaring of the vertebral bodies. The healing of the inflammatory changes may result in sclerosis and bone proliferation along the peripheral fibers of the annulus fibrosus and the deep fibers of the anterior longitudinal ligament. Posterior arch involvement on radiographs cannot be identified until late in the disease.
MRI can again be particularly useful for the depiction of early features. Inflammatory anterior corners of the thoracolumbar vertebral bodies are particularly well demonstrated. The presence of at least three of them in a patient younger than 45 years of age is considered to be very suggestive of SpA (Weber et al. 2009) (Fig. 34.10). They are triangular in shape or demonstrate a more vertical distribution along the anterior cortical bone. Fatty vertebral corner can also be demonstrated (Fig. 34.11). Their diagnostic usefulness for SpA is significant when at least five are present (Hermann et al. 2012). Sometimes, BME is very extensive, with involvement of nearly the entire vertebral body. There may also be associated squaring but its assessment can be quite subjective. Less frequently, these inflammatory changes involve the posterior corners, the lateral edge, and/or the vertebral endplates. Andersson spondylodiskitis is more rarely encountered (Fig. 34.12). It is characterized by inflammatory changes of two adjacent vertebral endplates, with or without inflammatory changes of the disk, but with frequent erosions of the endplates.
Inflammatory changes may also be identified involving the posterior structures of the spine and are highly specific (Hermann et al. 2012). Changes seen in the edges of the vertebral bodies typically reflect involvement of the costovertebral and costotransverse joints and rib heads and are better demonstrated on axial images. Transverse and spinous process edema, enthesitis of the interspinal, supraspinal and flaval ligaments, and facet joints arthritis may also be encountered. However, one should keep in mind that in the DESIR cohort (patients presenting with inflammatory back pain suggestive of axial SpA), only about 4% of patients with negative sacroiliac MRI findings were reclassified as SpA patients based on spinal MRI findings (Dougados et al. 2011).
3.2 Mimics and Pitfalls
3.2.1 Vertebral Corner Lesions
Although inflammatory vertebral corners represent the classical sign suggesting the diagnosis of SpA of the spine, these lesions have also been reported in asymptomatic volunteers, with one study giving a figure as high as 26% (Weber et al. 2009). Some of these pseudo-inflammatory vertebral corners are related to microtrauma of Sharpey fibers (the most peripheral fibers of the annulus fibrosus), which may be isolated or associated with disk degeneration (spondylosis deformans). These lesions are well known and demonstrated on radiographs or CT when the cleft representing the Sharpey fibers avulsion is filled with gas opposite a vertebral corner, frequently at L3 or L4 vertebral level (Fig. 34.13). The resulting local micro-mobility is thought to explain some traction exerted on the deep fibers of the longitudinal ligament and, as a consequence, osseous metaplasia with enthesophyte formation, typically located several millimeters below the corner (Yu et al. 1989). On MRI, such microtrauma may explain mildly increased signal intensity on T2-weighted images of one, sometimes two, and exceptionally three vertebral corners (Figs. 34.14 and 34.15). A cleft of the disk is sometimes observed opposite it, filled with fluid or enhancing after contrast agent administration, which confirm the diagnosis (Fig. 34.14). T1-weighted images are also particularly helpful when they may depict the traction enthesophyte at a distance from the vertebral corner (Fig. 34.14).
Other disorders potentially associated with pseudo-inflammatory corners include degenerative disks, because radial tears extend to the insertion of Sharpey fibers or because the bulging of the disk results in stretching of the insertion of the anterior longitudinal ligament (Fig. 34.16). Modic type 1 changes may also involve the vertebral corner, but BME is frequently more extensive along the vertebral endplate. Finally, vertebral collapse, whatever its cause, may be associated with adjacent shiny corners, possibly due to traction on the anterior longitudinal ligament (Fig. 34.17). Fatty vertebral corners are even more common in asymptomatic volunteers. Demonstration of these lesions mainly or solely involving the lumbar spine should be considered cautiously, particularly when seen in isolation (Fig. 34.18).
3.2.2 Infection
Infection may represent a tough differential diagnosis when SpA is revealed by Andersson spondylodiskitis or facet joints arthritis. Besides the different clinical and biological features, infectious spondylodiskitis and facet joint arthritis are rarely multifocal, and they are not associated with MRI signs of chronicity. The latter features include fat or sclerosis of the subchondral bone (T1-hyperintense or T2-hypointense band, respectively) and BME with well-limited margins predominating at a distance from the subchondral bone (Fig. 34.12).
4 Peripheral Spondyloarthritis
4.1 Diagnosis
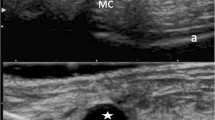
This disorder is notably characterized by the presence of peripheral arthritis, enthesitis, and/or dactylitis plus additional features, including psoriasis or inflammatory bowel disease (Rudwaleit et al. 2011). Peripheral arthritis may involve one or several joints. The hand and foot are most frequently involved, particularly at an early stage. Enthesitis is a key pathological lesion in peripheral SpA. It is defined by inflammation of the entheses, the sites where tendons, ligaments, fascias, or capsules insert into the bone. It frequently involves the heel, either at the Achilles tendon insertion or plantar aponeurosis, but any entheses can be affected (Fig. 34.19). Both ultrasound (US) imaging and MRI can demonstrate inflammatory features involving these entheses, but MRI may show the associated BME when present. Dactylitis, or the “sausage digit,” is defined as a diffuse swelling of a digit. It has long been recognized as one of the cardinal features of psoriatic arthritis. US imaging and MRI studies demonstrate that dactylitis is not only related to inflammation of the digital flexor tendon sheaths but also of the adjacent joints, periosteum, entheses, or soft tissue (Healy et al. 2008; Bakewell et al. 2013).
4.2 Mimics and Pitfalls
4.2.1 Other Joint Disorders
Septic arthritis is, of course, the main differential diagnosis when only one joint is affected. Imaging is neither sensitive nor specific for this diagnosis at an early stage, and its recognition relies on the identification of the organism (Mathews et al. 2007). The frequent involvement of the distal interphalangeal (DIP) joints and/or of the entire digit allows differentiation from rheumatoid arthritis and calcium pyrophosphate crystal deposition disease. In contrast, differentiation between osteoarthritis (OA) and psoriatic arthritis (psoA) can be difficult, as both of these diseases can be associated with DIP joint space narrowing, chronic bony proliferation, and no periarticular osteoporosis on radiographs. However, when present, erosive changes are typically marginal in psoriatic arthritis and central in OA; bony proliferations are more fluffy, spiculated, and located at a distance from the joint space in psoA in contrast to the well-defined osteophytes in OA (Fig. 34.20). When MRI is performed, inflammatory changes can sometimes be depicted in the enthesis of the DIP joints affected by osteoarthritis, but they are much less marked than in psoriatic arthritis (Tan et al. 2006). Other joints such as the metacarpophalangeal (MCP) joints or the carpal joints can also be affected in psoA, with such distribution being more unusual in OA.
4.2.2 Complex Regional Pain Syndrome
Radiographic patchy osteopenia and/or patchy BME areas on MRI (Darbois et al. 1999) can be seen in complex regional pain syndrome (CRPS) and may be confusing, particularly when the foot is involved. Erosions and joint space narrowing are however absent. Moreover, BME typically predominates in the subchondral areas, in contrast to the BME seen in SpA which frequently predominates in the marginal areas or in front/opposite of entheses (Fig. 34.21). BME in CRPS is also transient and sometimes migratory between two examinations (Malghem et al. 2003).
4.2.3 Dactylitis
Although dactylitis can be seen in other disorders (e.g., gout, sarcoidosis, hemolytic anemia), SpA is the main diagnosis in frequency, either at the hand or at the forefoot (Rothschild et al. 1998; Healy and Helliwell 2006). The presence of extensive osteitis and periostitis is highly suggestive of SpA, when present. This requires a good quality fat suppression of these anatomical areas when a large field of view is used (Fig. 34.22).
4.2.4 Mechanical Enthesopathy
Imaging performance for the differentiation between enthesitis and mechanical enthesopathy is still debated in the literature. Increased vascularization at the junction between the bone and the enthesis on power Doppler US imaging has been reported to be highly specific for enthesitis (D’Agostino et al. 2011). However, this feature and other ones were not found to be able to discriminate between these two disorders in the study of the foot (Feydy et al. 2012). Bone marrow edema on MRI was the only abnormality specific for SpA (94%), but it is associated with a poor sensitivity (22%) (Feydy et al. 2012) (Fig. 34.23).
5 Rheumatoid Arthritis
5.1 Diagnosis
Rheumatoid arthritis (RA) is characterized by proliferative hypervascularized synovitis, which is secondarily responsible for bone erosions and cartilage damage. This disorder typically starts at the forefoot, wrist, and hand. Although radiography can provide only indirect information on synovitis and is insensitive to early bone damage, it is still widely used for bone erosion detection and remains the gold standard for evaluating structural damage. The lateral aspect of the fifth metatarsal bone; the radial aspect of the second and third metacarpal bones; the capitate, triquetrum, and lunate bones; and the ulnar styloid process are more frequently involved with bone erosions (Boutry et al. 2007). MRI and US imaging can detect pre-erosive synovitis. They can also identify early erosions before they become apparent on radiography. MRI also demonstrates BME, which may precede the development of bone erosions.
5.2 Mimics and Pitfalls
5.2.1 Synovitis
Frequent enhancement of the synovial tissue has been reported in the region of the peri-styloid process in a cohort of healthy subjects (Partik et al. 2002). However, rheumatoid synovitis is typically thick and extensive, with intense enhancement after gadolinium administration. Synovitis can also be found in many other disorders and is not specific to the disease (Boutry et al. 2005; Stomp et al. 2014). The profile of synovitis enhancement after gadolinium administration has also been studied in the literature, and although debated (Cimmino et al. 2012), rheumatoid and psoriatic arthritis do not seem to be differentiable based on this sole feature (Cimmino et al. 2005).
5.2.2 Pseudo-erosions
Because of the frequent involvement of the forefoot, wrist, and hand, analysis of these anatomical regions on radiography is commonly used for diagnosis, staging, and follow-up in RA. Analysis of these images relies heavily on the detection of bone erosions. However, pseudo-erosions are frequently observed in these anatomical regions, particularly at the wrist. They can most often be explained by the presence of ligament insertions, which are associated with bone remodeling and a thinner bone lamina (McQueen et al. 2005; Wawer et al. 2014). Other causes include absence of radiographic tangency, presence of osteophytes, arterial foramen, and mucoid cyst (McQueen et al. 2005). Wawer et al. (2014) found that pseudo-erosions were mostly seen in the distal ulnar portion of the capitate, the distal radial portion of the hamate, the proximal ulnar portion of the base of the third metacarpal, the proximal radial portion of the base of the fourth metacarpal, the distal ulnar portion of the hamate, and the proximal portion of the base of the fifth metacarpal (Wawer et al. 2014) (Fig. 34.24).
On MRI, the definition of erosions requires their visualization in two planes according to the OMERACT (outcome measures in rheumatology) RA MRI scoring system (Østergaard et al. 2003). Slice thickness and field of view should be optimized to limit misdiagnosis of true erosions due to partial volume averaging. Mucoid cysts and arterial foramen may be misleading, but analysis of their bases (larger in erosions) and absence of significant associated synovitis may be used for this differentiation (Fig. 34.25). However, rare examples of pseudo-erosion and BME have been reported at the MCP joints and wrists of subjects free from RA (Ejbjerg et al. 2004). They might be related to synovial and bony hyperemia due to increased mechanical activity.
Conclusion
In conclusion, a good knowledge of the imaging features of the inflammatory arthritides, particularly at an early stage, is fundamental for accurate diagnosis of patients. Moreover, a good knowledge of the main imaging diagnostic pitfalls is mandatory to avoid inappropriate therapeutic management.
References
Ahovuo JA, Kiuru MJ, Visuri T (2004) Fatigue stress fractures of the sacrum: diagnosis with MR imaging. Eur Radiol 14:500–505
Bakewell CJ, Olivieri I, Aydin SZ et al (2013) Ultrasound and magnetic resonance imaging in the evaluation of psoriatic dactylitis: status and perspectives. J Rheumatol 40:1951–1957
Bennett AN, McGonagle D, O’Connor P et al (2008) Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 58:3413–3418
Bereau M, Prati C, Wendling D (2011) Sacroiliac edema by MRI does not always indicate spondylarthritis. Joint Bone Spine 78:646
Bollow M, Braun J, Kannenberg J et al (1997) Normal morphology of sacroiliac joints in children: magnetic resonance studies related to age and sex. Skeletal Radiol 26:697–704
Boutry N, Hachulla E, Flipo RM et al (2005) MR imaging findings in hands in early rheumatoid arthritis: comparison with those in systemic lupus erythematosus and primary Sjögren syndrome. Radiology 236:593–600
Boutry N, Morel M, Flipo RM et al (2007) Early rheumatoid arthritis: a review of MRI and sonographic findings. AJR Am J Roentgenol 189:1502–1509
Bywaters EGL, Dixon ASJ, Scott JT (1963) Joint lesions of hyperparathyroidism. Ann Rheum Dis 22:171–187
Cimmino MA, Parodi M, Innocenti S et al (2005) Dynamic magnetic resonance of the wrist in psoriatic arthritis reveals imaging patterns similar to those of rheumatoid arthritis. Arthritis Res Ther 7:R725–731
Cimmino MA, Barbieri F, Boesen M et al (2012) Dynamic contrast-enhanced magnetic resonance imaging of articular and extraarticular synovial structures of the hands in patients with psoriatic arthritis. J Rheumatol Suppl 89:44–48
D’Agostino MA, Aegerter P, Bechara K et al (2011) How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis 70:1433–1440
Darbois H, Boyer B, Dubayle P et al (1999) MRI symptomology in reflex sympathetic dystrophy of the foot. J Radiol 80:849–854
Dougados M, D’Agostino MA, Benessiano J et al (2011) The DESIR cohort: a 10-year follow up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine 78:598–603
Egund N, Jurik AG (2014) Anatomy and histology of the sacroiliac joints. Semin Musculoskelet Radiol 18:332–339
Ejbjerg B, Narvestad E, Rostrup E et al (2004) Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum 50:1097–1106
Feydy A, Lavie-Brion MC, Gossec L et al (2012) Comparative study of MRI and power Doppler ultrasonography of the heel in patients with spondyloarthritis with and without heel pain and in controls. Ann Rheum Dis 71:498–503
Healy PJ, Helliwell PS (2006) Dactyitis: pathogenesis and clinical considerations. Curr Rheumatol Rep 8:338–341
Healy PJ, Groves C, Chandramohan M et al (2008) MRI changes in psoriatic dactylitis – extent of pathology, relationship to tenderness and correlation with clinical indices. Rheumatology 47:92–95
Hermann KG, Baraliakos X, Van der Heijde DM et al (2012) Assessment in SpondyloArthritis international Society (ASAS). Description of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis 71:1278–1288
Llauger J, Palmer J, Amores S et al (2000) Primary tumors of the sacrum: diagnostic imaging. AJR Am J Roentgenol 174:417–424
Malghem J, Vande Berg B, Lecouvet F et al (2003) Algodystrophie du pied. In: Chevrit A (ed) Imagerie du pied et de la cheville. Sauramps, Montpellier
Mau W, Zeidler H, Mau R et al (1987) Outcome of possible ankylosing spondylitis in a 10 years’ follow-up study. Clin Rheumatol 6(Suppl 2):60–66
Mathews CJ, Kingsley G, Field M et al (2007) Management of septic arthritis: a systematic review. Ann Rheum Dis 66:440–445
McQueen F, Østergaard M, Peterfy C et al (2005) Pitfalls in scoring MR images of rheumatoid arthritis wrist and metacarpophalangeal joints. Ann Rheum Dis 64(Supp I):i48–i55
Olivieri I, Ferri S, Barozzi L (1996) Osteitis condensans ilii. Br J Rheumatol 35:295–297
Østergaard M, Peterfy C, Conaghan P et al (2003) OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions and the OMERACT RA-MRI scoring system. J Rheumatol 30:1385–1386
Partik B, Rand T, Pretterklieber ML et al (2002) Patterns of Gadopentetate-enhanced MR imaging of radiocarpal joints of healthy subjects. AJR Am J Roentgenol 179:193–197
Peh WCG, Khong PL, Yin Y et al (1996) Imaging of pelvic insufficiency fractures. Radiographics 16:335–348
Rothschild BM, Pingitore C, Eaton M (1998) Dactylitis: implications for clinical practice. Semin Arthritis Rheum 28:41–47
Rudwaleit M, Landewé R, van der Heijde D et al (2009a) The development of assessment of Spondyloarthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 68:770–776
Rudwaleit M, van der Heijde D, Landewé R et al (2009b) The development of Assessment of Spondyloarthritis International Society (ASAS) classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68:777–783
Rudwaleit M, Jurik AG, Hermann KGA et al (2009c) Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 68:1520–1527
Rudwaleit M, Van der Heijde D, Landewé R et al (2011) The Assessment of Spondyloarthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70:25–31
Sheybani EF, Khanna G, White AJ et al (2013) Imaging of juvenile idiopathic arthritis: a multimodality approach. Radiographics 33:1253–1273
Shibata Y, Shirai Y, Miyamoto M (2002) The aging process in the sacroiliac joint: helical computed tomography analysis. J Orthop Sci 7:12–18
Stomp W, Krabben A, Van der Heijde D et al (2014) Are rheumatoid arthritis patients discernible from other early arthritis patients using 1.5T extremity magnetic resonance imaging? A large cross-sectional study. J Rheumatol 41:1630–1637
Stürzenbecher A, Braun J, Paris S et al (2000) MR imaging of septic sacroiliitis. Skeletal Radiol 29:439–446
Tan AL, Grainger AJ, Tanner SF et al (2006) A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum 54:1328–1333
Tuite MJ (2008) Sacroiliac joint imaging. Semin Musculoskelet Radiol 12:72–82
Wawer R, Budzik JF, Demondion X et al (2014) Carpal pseudoerosions: a plain X-ray interpretation pitfall. Skeletal Radiol 43:1377–1385
Weber U, Hodler J, Kubik RA et al (2009) Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum 61:900–908
Weber U, Zubler V, Pedersen SJ et al (2013) Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis Care Res 65:977–985
Yu SW, Haughton VM, Lynch KL et al (1989) Fibrous structure in the intervertebral disk: correlation of MR appearance with anatomic sections. Am J Neuroradiol 10:1105–1110
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Felloni, P., Larkman, N., Dunca, R., Cotten, A. (2017). Inflammatory Arthritides: Imaging Pitfalls. In: Peh, W. (eds) Pitfalls in Musculoskeletal Radiology. Springer, Cham. https://doi.org/10.1007/978-3-319-53496-1_34
Download citation
DOI: https://doi.org/10.1007/978-3-319-53496-1_34
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53494-7
Online ISBN: 978-3-319-53496-1
eBook Packages: MedicineMedicine (R0)