Abstract
In suspected and diagnosed rheumatoid arthritis (RA), magnetic resonance imaging (MRI) allows detection of all relevant pathologies, such as synovitis, tenosynovitis, bone marrow edema (osteitis), bone erosion and cartilage damage. MRI is more sensitive than clinical examination for monitoring disease activity (i.e., inflammation) and more sensitive than conventional radiography and ultrasonography for monitoring joint destruction. In suspected RA, MRI bone marrow edema predicts development of RA, and in early RA patients, it predicts subsequent structural damage progression. CT is the standard reference imaging modality for visualizing bone damage, including bone erosions in RA, but lacks sensitivity for soft-tissue changes, including synovitis and tenosynovitis. CT has a minimal role in RA clinical trials and practice, except in selected patients where MRI is contraindicated or not available or if crystal arthritis such as gout or pseudo-gout is suspected. MRI has documented utility in diagnosis, monitoring and prognostication of patients with RA and is increasingly used for these purposes in clinical practice and particularly clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early diagnosis combined with early initiation of appropriate therapy and tight control of inflammation has been recognized as essential for optimal clinical outcomes in rheumatoid arthritis (RA) [1]. This has increased the need for powerful, sensitive techniques that at an early stage can diagnose and indicate the prognosis of patients with RA and accurately monitor the efficacy of treatment.
Conventional radiography is able to detect structural joint damage in patients with established disease, but is not sensitive for detecting early disease manifestations such as soft-tissue changes and bone damage at its earliest stages [2].
Computed tomography (CT) is a three-dimensional radiographic technique favored by the lack of projectional superimposition and by an exquisite inherent contrast between bone and soft tissue, which makes it a gold standard reference for the detection of bone damage, including bone erosion, new bone formation, calcifications and sclerosis. CT is therefore ideally suited to the investigation of erosion in RA and other inflammatory arthritides. However, CT is hampered by very limited ability to visualize soft-tissue changes using conventional monochromatic CT units, but the introduction of newer CT technologies such as dual-energy or multispectral CT that allows better chemical composition quantification and tissue separation may change this in the near future.
Magnetic resonance imaging (MRI) is a non-ionizing imaging technique that has excellent soft-tissue visualization and allows multiplanar tomographic imaging of the body in any plane without projectional superimposition and geometric distortions associated with projectional techniques, such as radiography. Thus, early bone involvement and inflammatory soft-tissue changes of synovitis (Fig. 1) and tenosynovitis (Fig. 2), which are not detectable by conventional clinical, biochemical and radiographic methods, can be directly visualized and evaluated in detail by MRI [3]. Disadvantages of MRI include relatively higher costs, longer examination times and lower availability than both radiography and CT, and except for whole-body MRI (WBMRI), MRI has restricted anatomical coverage per imaging session. MRI, as compared to ultrasonography (US), offers greater anatomical coverage than ultrasonography (US) does, because US cannot penetrate bone, and thus cannot visualize structures hidden in acoustic shadows. Further, US cannot visualize inflammation within the bone marrow, i.e., osteitis, which has been shown to be highly predictive of subsequent bone erosion. MRI is able to visualize all joints in the hand and wrist and all regions of these joints, including osteitis. In clinical trials, the advantage of MRI compared to US highly predominates, due to the possibility of standardized image acquisition, centralized reading with full blinding of both image acquisition and reading. In clinical practice, US is favored by providing results immediately and thus allows more rapid therapeutic decision making as well as potential for imaging-guided punctures, aspirations, biopsies and injections, but this requires a skilled ultrasonographer and in most instances high-end US equipment in the outpatient clinic [3].
MRI of the wrist of 30-year-old woman with early rheumatoid arthritis. Axial pre- (a, c) and post- (b, d) contrast T1-weighted images obtained before treatment (a, b) and at 1-year follow-up during treatment with intraarticular glucocortocoids and methotrexate. At baseline, marked post-contrast enhancement, indicating synovitis (arrows), is seen, while only minimal synovitis (arrows) is seen at follow-up
MRI of the wrist of a patient with established rheumatoid arthritis. Coronal short tau inversion recovery (STIR) images before and 1 year after initiation of tumor necrosis factor inhibitor therapy show bone marrow edema (osteitis, arrows) in several carpal joints at baseline, which has disappeared at follow-up
This review article will focus on the potential uses of MRI and CT in the clinical management of patients with suspected or definite RA. After sections on technical aspects, it will describe the current knowledge on MRI and CT, respectively, for early detection of RA manifestations, diagnosis of RA, monitoring of disease activity and joint damage progression and its role in determining prognosis. The usefulness in both clinical trials and routine practice will be discussed.
Magnetic resonance imaging (MRI)
Technical considerations
High-resolution, T1-weighted (T1w) often three-dimensional (3D) imaging sequences, obtained before and after intravenous contrast injection with or without fat saturation (Fig. 1), and a “water sensitive” technique, such as T2-weighted (T2w) spin echo with FS or short tau inversion recovery (STIR) (Fig. 3), constitute the standard sequences obtained in RA [4].
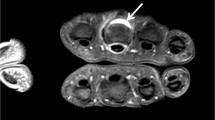
Coronal T1w 3D gradient echo images post-contrast with axial reconstructions in the level of the wrist (lower right) and MCP1 (upper right) in a 57-year-old male with RA for 8 years show moderate flexor tendon tenosynovitis of the common flexor retinaculum of the wrist (arrows) that can be followed on the axial images involving the flexor pollicis longus and fifth flexor tendons. Dashed lines mark the position of the axial images
For evaluating structural changes, such as bone erosion, T1-weighted images are preferred (Fig. 4) [2, 4,5,6]. They provide good anatomical detail and have the ability to visualize tissues with high perfusion and permeability, including the inflamed synovium, after intravenous contrast injection with paramagnetic gadolinium compounds (Gd) (Fig. 1). Intravenous contrast injection increases the sensitivity for synovitis in peripheral joints [7]. Specific sequences designed for optimal cartilage evaluation can also be applied, e.g., dual echo steady state (DESS) 3D gradient echo sequences. New MRI techniques continue to be developed, and a variety of sequences are now available for detecting free water in otherwise fatty bone marrow, indicative of inflammation—so called “bone marrow edema” (BME) or “osteitis.” These include T2w spin echo with fat saturation (T2 FS), proton density-weighted with fat saturation (PdW FS) or water-excitation (T2WE) and short tau inversion recovery (STIR) sequences, but also hybrid sequences, and techniques applying chemical shift-based fat–water separation, such as the Dixon technique [8]. The Dixon technique is increasingly being employed, though T2 FS and STIR sequences are still most frequent, because of their high general reliability, consistency across different manufacturers and, for STIR, applicability at lower magnetic field strengths (< 1 T).
MRI of the second–third metacarpophalangeal joints of a patient with established rheumatoid arthritis. Axial (a) and coronal (b) pre-contrast T1-weighted images and a coronal short tau inversion recovery (STIR) image (c) show a bone erosion (long arrows) in the metacarpal head surrounded by bone marrow edema (osteitis, short arrows)
Whole-body MRI is promising technique that allows imaging of the entire body in one examination, i.e., simultaneous assessment of peripheral and axial joints and entheses in both RA [9], spondyloarthritis and psoriatic arthritis [10,11,12], where it may provide an MRI-based total joint inflammation count. Improved image resolution and more validation are still needed for WBMRI imaging of the small joints of the hands and feet, before the method is ready for routine use.
Dynamic contrast-enhanced MRI (DCE-MRI) is based on the perfusion dynamics of synovial enhancement curves after contrast injection and has been found to correlate with synovial histopathological inflammation (Fig. 5) [13, 14]. Thus, DCE-MRI may allow accurate assessment of the disease activity based on a selected region of interest [15, 16]. Techniques using manual segmentation to quantify pathologies such as synovial volumes have been investigated for many years [17, 18], but recent automated methods using supervised machine learning techniques may increase the feasibility and also responsiveness when assessing RA pathologies [19] although both methods need further validation and internationally agreed standards.
40-year-old female with 2 years of RA starting MTX DMARD treatment with severe pain in the left wrist (upper row) and at 3-month follow-up (lower row). From left to right: coronal T1w non-fat-saturated spin echo, coronal STIR, coronal 3D gradient echo fat-saturated post-contrast VIBE sequence and coronal DCE-MRI analyzed with DYNAMIKA, Image Analysis Group, London, UK. Note the severe BME on the initial STIR and the moderate-to-severe synovitis (bright enhancing tissue) on the 3D VIBE sequence as well as the high perfusion/inflammation in the DCE-image (seen as bright and white colors). At 3-month follow-up, the pain has reduced substantially. This is associated with a significant reduction in the BME on the STIR, a slight reduction in contrast enhancing synovitis on the VIBE sequence, and a significant reduction in the perfusion in the enhancing synovium, seen as more dark red colors on the DCE-MRI map
MRI is very safe. It involves no ionizing radiation or risk of malignancy, with the major contraindications being claustrophobia or the presence of any metal devices including pacemakers or clips (though many of these are now made MRI safe). Adverse effects from Gd-based contrast agents are very rare, except in patients with severely impaired renal function [20]. The European Society of Urogenital Radiology (ESUR) guidelines recommend no patients should be denied a well-indicated Gd-enhanced MRI, and those agents with highest stability (lowest risk of nephrogenic systemic fibrosis) (e.g., macrocyclic Gd chelates, such as gadoterate meglumine and gadoteridol) should be used, at the lowest diagnostic dose [20].
MRI detection of pathology in RA
The clinical value of MRI in RA relates primarily to its ability to evaluate the wrists, hands and feet in high detail, which is also the main focus of this section. MRI is also useful for evaluating other anatomical sites involved by RA, such as the knees, hips, elbows and shoulders, and the axial skeleton, particularly the cervical spine. Further, as noted above, MRI allows assessment of all the joint structures affected by RA. Definitions of key pathologies in RA are provided in Table 1 [4, 21].
Synovitis
Synovitis (Figs. 1, 5) is the key feature of RA. Comparisons with miniarthroscopy and histopathological findings have documented that MRI synovitis, as determined by Gd-enhanced T1-weighted MRI, reflects true synovial inflammation [13, 14, 18, 22]. Low-grade MRI synovitis has been reported in healthy controls without clinical signs of synovitis [23,24,25,26], increasing with age. Since synovitis is very common in osteoarthritis (OA), including asymptomatic OA, it is not clear how many of such “normal” joints had underlying OA. Diagnostic tests are never 100% sensitive or specific, and thus, the inherent trade-off between false-negative and false-positive rates as one increases the positivity criterion for that test must be considered carefully in light of the particular role that imaging is being asked to play. If trying to identify individuals at risk of RA who would benefit from closer monitoring, a high sensitivity test may be in order, whereas to identify patients for whom costly treatment with biologics would be justified, perhaps a greater degree of specificity would be needed.
Tenosynovitis
Tenosynovitis (Fig. 2) is very common in RA, but may also occur in other arthritides, even very early in undifferentiated arthritis [27], or as a consequence of overuse [28, 29]. The tendon itself may appear normal, or it may be thickened, irregular and/or have increased signal intensity within the tendon on T2w/Pdw and STIR images (tendonitis), or with complete or incomplete tears [28].
Bone marrow edema
Bone marrow edema (Figs. 3, 4), i.e., the presence of MRI-signs of increased water in the bone marrow compartment, is frequently detected in RA. When compared with histological samples obtained at surgery in RA patients, MRI BME in RA has been shown to represent inflammatory infiltrates in the bone marrow, that is, osteitis [30, 31]. Whereas erosions reflect bone damage that has already occurred, BME appears to represent the link (“forerunner”) from joint inflammation to bone destruction where persistent osteitis leads to trabecular bone loss and an erosion [32,33,34].
Enthesitis
Enthesitis, i.e., inflammation at capsular and ligamentous insertions, is a characteristic feature in seronegative spondyloarthritides, such as PsA, but can also be seen in RA and patients without inflammatory arthritis (with presumed mechanical cause) [9, 35].
Joint cartilage
Joint cartilage can be directly visualized by MRI [36]. A number of morphological and compositional MRI markers of cartilage integrity have been studied. Cartilage assessment in small joints requires high image quality and resolution, but reliable semi-quantitative assessment systems have been developed [37,38,39].
Bone erosion
Bone erosion (Fig. 4) is detected with higher sensitivity with MRI than radiography [40,41,42], and the very high level of agreement for detection of bone erosions in RA wrists and MCP-joints between MRI and CT, the gold standard reference for detection of bone damage, provides evidence that MRI erosions represent true bone damage [43, 44].
Erosions are also frequently seen in other inflammatory arthritidies and erosive OA and are thus not specific for RA. Small erosion-like lesions can also be visualized in healthy controls and OA [25, 26], so to avoid overestimation, using strict definitions of bone erosion and other pathologies is essential. As an example, bone erosions should be visible in two planes, with a cortical break visible in at least one plane to be registered (Fig. 4), and the normal anatomy and pitfalls should be kept in mind [21, 45, 46]. Control groups, sequences, slice thickness and reading methodology should be appropriately selected [47] as they can all impact the number of erosions detected.
Cervical spine
All joints, tendons and ligaments of the cervical spine can be involved in RA, leading to spine instability or subluxations. Cervical spine involvement is a serious complication of RA. Proliferating pannus from the synovial joints and anatomical malalignments (Fig. 6) can in turn lead to secondary medullary compression. The primary imaging modality is CR, but MRI can provide detailed information on bone and soft-tissue abnormalities, e.g., the pannus tissue around the odontoid process, and this can be a valuable supplement to radiographic evaluation (Fig. 7) [2, 48, 49]. MRI erosions of the atlas and reduced subarachnoid space are associated with subsequent clinical neurological dysfunction [50], and cord compression on MRI better predicts subsequent clinical deterioration than initial clinical and radiographic features [51]. The evidence-based EULAR recommendations for the use of imaging in RA clinical management state that monitoring of functional instability of the cervical spine by lateral radiograph obtained in flexion and neutral should be performed in patients with clinical suspicion of cervical involvement. Furthermore, EULAR recommends that MRI should be performed when the radiography is positive or specific neurological symptoms and signs are present [2].
CT and MRI scans of the neck of a 90-year-old female with longstanding RA and severe neck pain (same patient as Fig. 8). a Mid-sagittal 3D CT reformat of the cervical spine shows degenerative spine changes on multiple cervical levels as well as block vertebrae between C4 and C5 with approximately 5 mm anterolistesis between C3 and the block vertebrae. Arrowheads show erosions on the top of the dens axis (*, C2). b–d Corresponding MRI of the same mid-sagittal slice, b T1 weighted image without fat saturation, c T2-weighted image without fat saturation and d STIR image showing capsular hypertrophy, small erosions in the C1/C2 articulation and slight bone marrow edema of dens axis on the STIR sequence (arrow heads). Note how much better the erosions on the top of dens axis are seen on the CT scan compared to the MRI images. e A lateral right-sided 3D CT sagittal reformat at the level of the articulation between massa lateraris and axis C2 showing severe erosive changes in the articulation between massa lateralis of C1 and C2 (arrowheads) along with degenerative changes of the upper cervical facet joints (arrows). f–h Corresponding MRI of the same right-sided sagittal slice, f T1-weighted image without fat saturation, g T2-weighted image without fat saturation and h STIR showing destruction of the right-sided articulation between massa lateralis C1 and axis C2 (arrowheads) as well as severe bone marrow edema of the adjacent bone of the articulation on the STIR sequence h (arrows). Facet joint degeneration is also noted (open arrows)
X-rays and MRI of the cervical spine in patients with RA. Normal X-ray of the cervical spine with normal joint between dens axis C2 and arcus anterior of Atlas C1 (arrow). b Extension X-ray of the cervical spine shows signs of subluxation between dens axis C2 (*) of arcus anterior of atlas C1. This distance should be between 3–5 mm, but it is more than 8 mm wide. T1-weighted mid-sagittal MRI of cervical spine (c) and corresponding T2-weighted mid-sagittal image (d) in the same patient as (b) show capsular hypertrophy and erosions in the top of dens axis (C2, arrows)
CT scan of the cervical spine in a 90-year-old female with longstanding RA and severe neck pain (same patient as Fig. 6). a, b Two sagittal 3D CT reformats show degenerative spine changes on multiple cervical levels as well as a block vertebra between C4 and C5 with approximately 5 mm anterolistesis between C3 and the block vertebrae. Arrowheads show erosions on the top of the dens axis (*, C2). c Coronal reformat shows erosions in the left part of the dens axis (black arrowhead), and there are severe erosive changes in the articulation between massa lateralis of C1 and C2 on the right side. Also, note the asymmetry between massa lateralis and dens axis as sign of instability in this articulation. d Axial slice at the level of the articulation of dens axis (*) and arcus anterior C1 in the plane corresponding to the black line in c. A white arrowhead points at a left-sided erosion in dens axis, also seen in c. e Axial slice at the level of the articulation of the neck of dens axis (*), in the plane corresponding to the black dotted line in c. White arrowheads point at severe erosive changes in the articulation between massa lateralis of C1 and C2 on the right side also seen in c
Diagnosis
Longitudinal studies of undifferentiated arthritis have documented an independent predictive value of MRI in the subsequent diagnosis of RA [52, 53]. In patients with undifferentiated arthritis, a prediction model, including clinical hand arthritis, morning stiffness, positive rheumatoid factor and MRI bone edema score in metatarsophalangeal and wrist joints, correctly identified the development of RA or non-RA in 82% of patients [53]. In another study of undifferentiated arthritis, assessment of MRI tenosynovitis was helpful in predicting future RA diagnosis or disease-modifying antirheumatic drugs therapy initiation [54].
In the ACR/EULAR 2010 criteria for RA [55], classification as definite RA is based on the presence of definite clinical synovitis (swelling at clinical examination) in ≥ 1 joint, absence of an alternative diagnosis that better explains the synovitis and achievement of a total score ≥ 6 (of a possible 10) from the individual scores in four domains. In the joint involvement domain, which can provide up to 5 points of the 6 needed for an RA diagnosis, MRI and US synovitis counts. In other words, MRI can be used to determine the extent of joint involvement [55,56,57]. Synovitis/joint inflammation as found by clinical, MRI or US examination is not specific for RA, and the ACR/EULAR criteria are classification not diagnostic criteria. In the diagnostic process of the individual patient in routine clinical practice, it is important always to consider the clinical context (as with any other diagnostic test) to avoid over-diagnosis, since MRI findings are not pathognomonic. The EULAR recommendations also state that when there is diagnostic doubt, CR, ultrasound or MRI can be used to improve the certainty of a diagnosis of RA above clinical criteria alone. The recommendations also state “the presence of inflammation seen with ultrasound or MRI can be used to predict the progression to clinical RA from undifferentiated inflammatory arthritis” [2].
Monitoring disease activity and structural damage
Clinical trials and observational cohorts
To be valuable for monitoring joint inflammation and destruction, a method must be truthful, reproducible and sensitive to change. MRI allows evaluation of all RA pathologies. In observational and randomized clinical trials, semi-quantitative scoring by the OMERACT (Outcome Measures in Rheumatology) RA MRI scoring system (RAMRIS) has been the most frequently used system. It involves semi-quantitative assessment of synovitis, bone erosions, BME and, more recently, tenosynovitis and joint space narrowing in RA hands and wrists, based on consensus MRI definitions of important joint pathologies and a “core set” of basic MRI sequences [4, 21].
Good intra- and inter-reader reliability and a high sensitivity to change have been reported, demonstrating that the OMERACT RAMRIS system, after proper training and calibration of readers, is suitable for monitoring joint inflammation and destruction in RA [58], and a reference image atlas has been developed [59].
MRI allows more sensitive monitoring of inflammation (Figs. 1, 3, 5) [60] and bone erosion than clinical and radiographic assessments. Several randomized controlled trials have documented the superior ability of MRI to discriminate the effects of different therapies in inhibiting progressive structural bone and cartilage damage [38, 61,62,63]. The OMERACT erosion score is closely correlated with erosion volumes estimated by MRI and CT [64].
Due to the high responsiveness and discriminatory ability, and because MRI has demonstrated criterion validity for osteitis and synovitis with histology and construct validity for erosions when compared with CT, there has been a rapid increase in the use of MRI in RA randomized clinical trials over the past decade. A report by the imaging subcommittee of the American College of Rheumatology (ACR) Clinical Trials Task Force [65] concluded that MRI met the OMERACT validation filter for “truth, discrimination and feasibility” [66] and that MRI best serves the purpose of achieving sensitive ascertainment of structural damage in RCTs while also providing objective measures of inflammatory predictors of damage [65]. Quantitative methods, like dynamic MRI (Fig. 5) and quantitative volume determination of synovitis, BME and erosion based on supervised machine learning techniques may also prove useful for early treatment-induced changes in joint inflammation [15, 19]. Whole-body MRI also shows promise as a method of assessing total joint inflammation [9]. All these methods are used in clinical trials but require further validation before clinical use can be recommended.
Routine clinical practice
The EULAR recommendations state that “US and MRI are superior to clinical examination in the detection of joint inflammation; these techniques should be considered for more accurate assessment of inflammation” [2]. MRI may be used in clinical practice to document improvement/worsening of disease activity. However, important questions remain about when such imaging is needed and when it is cost-effective to do? There is a lack of studies to document exactly how MRI should be used for this purpose, e.g., imaging is not needed to assess disease activity if the patient has obvious clinical signs of active RA. The choice of imaging modality may depend on which expertise is present at that specific center. For instance, MRI can be replaced with US for assessment of synovitis if an objective assessment of inflammation is needed. For assessment of inflammation in the bone (osteitis), MRI is currently, however, the only available modality, and it is also the best method, except for computed tomography (CT), for monitoring of progression of erosions [30, 31, 42,43,44, 67, 68].
Predicting disease outcome
The predictive value of MRI-detected pathology in wrist and/or MCP joints for subsequent radiographic progression in bilateral hands, wrists and feet is well established. Especially, BME is known to be a strong independent predictor of subsequent radiographic progression in early RA [34, 69, 70]. Regression analyses in 3- and 5-year follow-up in two cohorts and in clinical trials have documented that MRI-bone edema is a strong predictor of short-term and long-term (3, 5 and 11 year) radiographic progression [71,72,73,74].
Thus, MRI in early RA is a useful method to predict patients with potentially worse outcomes, which may assist the clinician in the choice of treatment strategy. In agreement with this, the EULAR recommendations for the use of imaging in the clinical management of RA state “MRI bone edema is a strong independent predictor of subsequent radiographic progression in early RA and should be considered for use as a prognostic indicator” [2]. This indicates that the presence of BME could be used as an inclusion criterion in clinical trials to enrich the study population for patients with high risk of structural progression.
Utility in clinical remission
Another issue of high clinical importance is whether MRI is useful to predict the disease course in patients in clinical remission. MRI synovitis and BME are found frequently in patients in clinical remission [75, 76], and these findings are significantly related to subsequent progressive structural damage [77,78,79]. These encouraging results are acknowledged in the EULAR recommendation, which states that “MRI and US can detect inflammation that predicts subsequent joint damage, even when clinical remission is present” [2].
MRI may assist in predicting the success of tapering of biologics. In a cohort of routine care RA patients in sustained remission on biological disease-modifying antirheumatic drugs (bDMARDs), the bDMARD therapy was tapered according to a predefined treatment guideline. Successful tapering was independently predicted by ≤ 1 previous bDMARD, male gender, low baseline MRI combined inflammation score (synovitis, tenosynovitis and bone marrow edema) and/or combined damage score (erosion and joint space narrowing) [80]. The available data encourage further exploration of MRI for predicting the disease course and for evaluating disease status, including defining remission.
Computed tomography
Although still limited in soft-tissue contrast, conventional CT offers fast and reliable acquisition, high resolution and multiplanar capabilities that have promoted its use in recent years.
Technical aspects
CT image acquisition is no longer restricted to the axial plane, and its multiplanar capability is now so pronounced that many CT scans of the body are now interpreted primarily from thin-slice coronal or sagittal reconstructions in the same way as MRI. Unlike MRI, there are no absolute contraindications to CT and scans are so fast that patient motion is rarely a problem and patient tolerance is excellent. Spatial resolution is high, usually higher than MRI, and contrast resolution between soft tissue and bone is unsurpassed by any other modality. However, despite these advantages, the application of CT in arthritis imaging still has flaws that prevent its universal application. Firstly, CT is constrained in the same way as radiography by its limited soft-tissue contrast capability. Secondly, ionizing radiation is used with increasing dose proportional to size of body part and requirements for spatial detail. While this is not a problem with the more distal extremities as the exposure doses are smaller and the tissue more radiation resistant, it remains an issue for spine, hip and shoulder CT. Consequently, in most routine clinical situations, radiography provides sufficient information of a similar nature to CT for clinical decision making and X-ray is cheaper than CT and readily available; ultrasonography is a better and cheaper way to visualize and quantify superficial soft-tissue pathology; and MRI offers superior soft-tissue contrast and bone marrow imaging. However, the technology continues to evolve and low-dose CT (LDCT) is becoming more common with iterative reconstruction techniques which reduce the radiation exposure by about 80% [81]. Exposure dose from LDCT of the sacroiliac joints is similar to radiography and should probably replace radiography as a first-line test in many cases [82].
LDCT can quantify bone formation in the spine which is undetectable with radiography [83], and cone-beam CT is a new technology that can detect bone erosion in extremities at extremely low dose [84].
Dual-energy CT (DECT) allows the separation of calcium containing bone from the soft-tissue components, thereby allowing detection of soft-tissue changes that were previously invisible with CT. For example, it has recently been reported that DECT can detect bone marrow edema (BME) with good reliability even in the small bones of the wrist in patients with RA [85, 86].
DECT can also be used for analysis of composition of some specific tissues, most particularly urate crystals which can be detected in bone and soft tissues (see below).
CT in RA
The excellent contrast between bone and soft tissue makes CT a gold standard reference for the detection of bone damage, including erosion in RA. Modern CT with isotropic voxel acquisition and three-dimensional visualization allows accurate detection and quantification of bone erosion with good intra-observer agreement, whereas radiography is limited by its two-dimensional projection and superimposition of structures. However, CT is very limited in ability to visualize soft-tissue changes and even with contrast enhancement and complex subtraction techniques; CT is still inferior to MRI and ultrasound for assessment of synovial changes such as thickening and hyperemia. Detection of erosion on CT and MRI shows very good agreement although CT is slightly more sensitive [43, 67].
Diagnosis, monitoring and prognostication
CT is not currently used in routine clinical practice for the diagnosis of RA. However, it could be potentially useful for diagnosis as CT appears to be the most sensitive technique for detection of erosion [43, 67]. CT is used for problem solving in specific cases such as examination of the cervical spine if MRI is unavailable or contraindicated.
CT may potentially be useful for longitudinal assessment of damage progression [67, 87], and CT can detect and quantify repair of erosion [88]. No CT data are available for prognostication in RA, and current use of CT in RA is very limited but since it has been shown to detect erosion reliably [89], scoring systems are in development [90].
Conclusion
MRI is a sensitive imaging modality which has documented utility in diagnosis, monitoring and prognostication of patients with RA, and important new knowledge and technical improvements are continuously being acquired. CT is the standard reference imaging modality for assessment of bone damage, including RA bone erosions. Several questions regarding the optimal use of these imaging modalities in routine practice and in clinical trials still need to be scientifically explored.
References
Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Alvaro-Gracia JM et al (2017) 2016 Update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 76(6):948–959
Colebatch AN, Edwards CJ, Østergaard M, van der Heijde D, Balint PV, d’Agostino MA et al (2013) EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis 72(6):804–814
Østergaard M, Pedersen SJ, Døhn UM (2008) Imaging in rheumatoid arthritis–status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol 22(6):1019–1044
Østergaard M, Peterfy CG, Bird P, Gandjbakhch F, Glinatsi D, Eshed I et al (2017) The OMERACT rheumatoid arthritis magnetic resonance imaging (MRI) scoring system: updated recommendations by the OMERACT MRI in arthritis working group. J Rheumatol 44(11):1706–1712
Sudol-Szopinska I, Jurik AG, Eshed I, Lennart J, Grainger A, Østergaard M et al (2015) Recommendations of the ESSR arthritis subcommittee for the use of magnetic resonance imaging in musculoskeletal rheumatic diseases. Semin Musculoskelet Radiol 19(4):396–411
Glinatsi D, Bird P, Gandjbakhch F, Mease PJ, Boyesen P, Peterfy CG et al (2015) Validation of the OMERACT psoriatic arthritis magnetic resonance imaging score (PsAMRIS) for the hand and foot in a randomized placebo-controlled trial. J Rheumatol 42(12):2473–2479
Østergaard M, Conaghan PG, O’Connor P, Szkudlarek M, Klarlund M, Emery P et al (2009) Reducing invasiveness, duration, and cost of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous contrast injection—does it change the assessment of inflammatory and destructive joint changes by the OMERACT RAMRIS? J Rheumatol 36(8):1806–1810
Del Grande F, Santini F, Herzka DA, Aro MR, Dean CW, Gold GE et al (2014) Fat-suppression techniques for 3-T MR imaging of the musculoskeletal system. Radiographics 34(1):217–233
Axelsen MB, Eshed I, Duer-Jensen A, Moller JM, Pedersen SJ, Østergaard M (2014) Whole-body MRI assessment of disease activity and structural damage in rheumatoid arthritis: first step towards an MRI joint count. Rheumatology (Oxford) 53(5):845–853
Weckbach S, Schewe S, Michaely HJ, Steffinger D, Reiser MF, Glaser C (2011) Whole-body MR imaging in psoriatic arthritis: additional value for therapeutic decision making. Eur J Radiol 77(1):149–155
Krabbe S, Østergaard M, Eshed I, Sorensen IJ, Jensen B, Moller JM et al (2018) Whole-body magnetic resonance imaging in axial spondyloarthritis: reduction of sacroiliac, spinal, and entheseal inflammation in a placebo-controlled trial of adalimumab. J Rheumatol 45:621–629
Østergaard M, Eshed I, Althoff CE, Poggenborg RP, Diekhoff T, Krabbe S et al (2017) Whole-body magnetic resonance imaging in inflammatory arthritis: systematic literature review and first steps toward standardization and an OMERACT scoring system. J Rheumatol 44(11):1699–1705
Axelsen MB, Stoltenberg M, Poggenborg RP, Kubassova O, Boesen M, Bliddal H et al (2012) Dynamic gadolinium-enhanced magnetic resonance imaging allows accurate assessment of the synovial inflammatory activity in rheumatoid arthritis knee joints: a comparison with synovial histology. Scand J Rheumatol 41(2):89–94
Humby F, Mahto A, Ahmed M, Barr A, Kelly S, Buch M et al (2017) The relationship between synovial pathobiology and magnetic resonance imaging abnormalities in rheumatoid arthritis: a systematic review. J Rheumatol 44(9):1311–1324
Boesen M, Kubassova O, Bouert R, Axelsen MB, Østergaard M, Cimmino MA et al (2012) Correlation between computer-aided dynamic gadolinium-enhanced MRI assessment of inflammation and semi-quantitative synovitis and bone marrow oedema scores of the wrist in patients with rheumatoid arthritis—a cohort study. Rheumatology (Oxford) 51(1):134–143
Waterton JC, Ho M, Nordenmark LH, Jenkins M, Dicarlo J, Guillard G et al (2017) Repeatability and response to therapy of dynamic contrast-enhanced magnetic resonance imaging biomarkers in rheumatoid arthritis in a large multicentre trial setting. Eur Radiol 27(9):3662–3668
Østergaard M, Hansen M, Stoltenberg M, Lorenzen I (1996) Quantitative assessment of the synovial membrane in the rheumatoid wrist: an easily obtained MRI score reflects the synovial volume. Br J Rheumatol 35(10):965–971
Østergaard M, Stoltenberg M, Løvgreen-Nielsen P, Volck B, Jensen CH, Lorenzen I (1997) Magnetic resonance imaging-determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum 40(10):1856–1867
Conaghan PG, Østergaard M, Bowes MA, Wu C, Fuerst T, van der Heijde D et al (2016) Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis 75(6):1024–1033
ESUR Contrast Media Safety Committee (2014) ESUR guidelines on nephrogenic sustemic fibrosis. ESUR guidelines on contrast media. 9.0 ed. Vienna, ESUR Office, 16-8
Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B et al (2003) OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 30(6):1385–1386
Ostendorf B, Peters R, Dann P, Becker A, Scherer A, Wedekind F et al (2001) Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis Rheum 44(11):2492–2502
Partik B, Rand T, Pretterklieber ML, Voracek M, Hoermann M, Helbich TH (2002) Patterns of gadopentetate-enhanced MR imaging of radiocarpal joints of healthy subjects. AJR Am J Roentgenol 179(1):193–197
Tan AL, Tanner SF, Conaghan PG, Radjenovic A, O’Connor P, Brown AK et al (2003) Role of metacarpophalangeal joint anatomic factors in the distribution of synovitis and bone erosion in early rheumatoid arthritis. Arthritis Rheum 48(5):1214–1222
Ejbjerg B, Narvestad E, Rostrup E, Szkudlarek M, Jacobsen S, Thomsen HS et al (2004) Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum 50(4):1097–1106
Mangnus L, Schoones JW, van der Helm-van Mil AH (2015) What is the prevalence of MRI-detected inflammation and erosions in small joints in the general population? A collation and analysis of published data. RMD Open 1(1):e000005
Burgers LE, Nieuwenhuis WP, van Steenbergen HW, Newsum EC, Huizinga TW, Reijnierse M et al (2016) Magnetic resonance imaging-detected inflammation is associated with functional disability in early arthritis—results of a cross-sectional study. Rheumatology (Oxford) 55:2167–2175
Rubens DJ, Blebea JS, Totterman SMS, Hooper MM (1993) Rheumatoid arthritis: evaluation of wrist extensor tendons with clinical examination versus MR imaging—a preliminary report. Radiology 187:831–838
Klarlund M, Østergaard M, Jensen KE, Madsen JL, Skjødt H, The TIRA Group (2000) Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. Ann Rheum Dis 59(7):521–528
McQueen FM, Gao A, Østergaard M, King A, Shalley G, Robinson E et al (2007) High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis 66(12):1581–1587
Jimenez-Boj E, Nobauer-Huhmann I, Hanslik-Schnabel B, Dorotka R, Wanivenhaus AH, Kainberger F et al (2007) Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum 56(4):1118–1124
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PLJ et al (1999) Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis 58:156–163
Conaghan PG, O’Connor P, McGonagle D, Astin P, Wakefield RJ, Gibbon WW et al (2003) Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum 48(1):64–71
Hetland ML, Ejbjerg B, Horslev-Petersen K, Jacobsen S, Vestergaard A, Jurik AG et al (2009) MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis 68(3):384–390
McGonagle D, Gibbon W, Emery P (1998) Classification of inflammatory arthritis by enthesitis. Lancet 352:1137–1140
Peterfy CG, van Dijke CF, Lu Y, Nguyen A, Connick TJ, Kneeland JB et al (1995) Quantification of the volume of articular cartilage in the metacarpophalangeal joints of the hand: accuracy and precision of three-dimensional MR imaging. Am J Roentgenol 165:371–375
Peterfy CG, Olech E, DiCarlo JC, Merrill JT, Countryman PJ, Gaylis NB (2013) Monitoring cartilage loss in the hands and wrists in rheumatoid arthritis with magnetic resonance imaging in a multi-center clinical trial: IMPRESS (NCT00425932). Arthritis Res Ther 15(2):R44
Peterfy C, Emery P, Tak PP, Østergaard M, Dicarlo J, Otsa K et al (2016) MRI assessment of suppression of structural damage in patients with rheumatoid arthritis receiving rituximab: results from the randomised, placebo-controlled, double-blind RA-SCORE study. Ann Rheum Dis 75(1):170–177
Døhn UM, Conaghan PG, Eshed I, Boonen A, Bøyesen P, Peterfy CG et al (2014) The OMERACT-RAMRIS rheumatoid arthritis magnetic resonance imaging joint space narrowing score: intrareader and interreader reliability and agreement with computed tomography and conventional radiography. J Rheumatol 41(2):392–397
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PLJ et al (1998) Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosion at four months after symptom onset. Ann Rheum Dis 57:350–356
Lindegaard H, Vallø J, Hørslev-Petersen K, Junker P, Østergaard M (2001) Low field dedicated magnetic resonance imaging in untreated rheumatoid arthritis of recent onset. Ann Rheum Dis 60(8):770–776
Østergaard M, Hansen M, Stoltenberg M, Jensen KE, Szkudlarek M, Pedersen-Zbinden B et al (2003) New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum 48(8):2128–2131
Døhn UM, Ejbjerg BJ, Court-Payen Hasselquist M, Narvestad E, Szkudlarek M et al (2006) Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther 8(4):R110
Døhn UM, Ejbjerg BJ, Hasselquist M, Narvestad E, Møller J, Thomsen HS et al (2008) Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res Ther 10(1):R25
Østergaard M, Gideon P, Sørensen K, Hansen M, Stoltenberg M, Henriksen O et al (1995) Scoring of synovial membrane hypertrophy and bone erosions by MR imaging in clinically active and inactive rheumatoid arthritis of the wrist. Scand J Rheumatol 24(4):212–218
Conaghan P, Edmonds J, Emery P, Genant H, Gibbon W, Klarlund M et al (2001) Magnetic resonance imaging in rheumatoid arthritis: summary of OMERACT activities, current status, and plans. J Rheumatol 28(5):1158–1162
Østergaard M, Haavardsholm EA (2016) Imaging: MRI in healthy volunteers—important to do, and do correctly. Nat Rev Rheumatol 12:563
Stiskal MA, Neuhold A, Szolar DH, Saeed M, Czerny C, Leeb B et al (1995) Rheumatoid arthritis of the craniocervical region by MR imaging: detection and characterization. Am J Roentgenol 165:585–592
Oostveen JC, Roozeboom AR, van de Laar MA, Heeres J, den Boer JA, Lindeboom SF (1998) Functional turbo spin echo magnetic resonance imaging versus tomography for evaluating cervical spine involvement in rheumatoid arthritis. Spine 23(11):1237–1244
Reijnierse M, Dijkmans BA, Hansen B, Pope TL, Kroon HM, Holscher HC et al (2001) Neurologic dysfunction in patients with rheumatoid arthritis of the cervical spine. Predictive value of clinical, radiographic and MR imaging parameters. Eur Radiol 11(3):467–473
Hamilton JD, Johnston RA, Madhok R, Capell HA (2001) Factors predictive of subsequent deterioration in rheumatoid cervical myelopathy. Rheumatology (Oxford) 40(7):811–815
Tamai M, Kawakami A, Uetani M, Takao S, Arima K, Iwamoto N et al (2009) A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis Care Res 61(6):772–778
Duer-Jensen A, Horslev-Petersen K, Hetland ML, Bak L, Ejbjerg BJ, Hansen MS et al (2011) Bone edema on magnetic resonance imaging is an independent predictor of rheumatoid arthritis development in patients with early undifferentiated arthritis. Arthritis Rheum 63(8):2192–2202
Nieuwenhuis WP, van Steenbergen HW, Mangnus L, Newsum EC, Bloem JL, Huizinga TWJ et al (2017) Evaluation of the diagnostic accuracy of hand and foot MRI for early rheumatoid arthritis. Rheumatology (Oxford) 56(8):1367–1377
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III et al (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588
Østergaard M (2010) Clarification of the role of ultrasonography, magnetic resonance imaging and conventional radiography in the ACR/EULAR 2010 rheumatoid arthritis classification criteria—comment to the article by Aletaha et al. Ann Rheum Dis e-letter. Published online December 2, 2010
Aletaha D, Hawker G, Neogi T, Silman A (2011) Re: clarification of the role of ultrasonography, magnetic resonance imaging and conventional radiography in the ACR/EULAR 2010 rheumatoid arthritis classification criteria—reply to comment to the article by Aletaha et al. Ann Rheum Dis e-letter. Published online January 11, 2011
Haavardsholm EA, Østergaard M, Ejbjerg BJ, Kvan NP, Uhlig TA, Lilleas FG et al (2005) Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum 52(12):3860–3867
Østergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B et al (2005) The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 64(Suppl 1):i2–i55
Haavardsholm EA, Østergaard M, Hammer HB, Boyesen P, Boonen A, van der Heijde D et al (2009) Monitoring anti-TNFalpha treatment in rheumatoid arthritis: responsiveness of magnetic resonance imaging and ultrasonography of the dominant wrist joint compared with conventional measures of disease activity and structural damage. Ann Rheum Dis 68(10):1572–1579
Østergaard M, Emery P, Conaghan PG, Fleischmann R, Hsia EC, Xu W et al (2011) Significant improvement in synovitis, osteitis, and bone erosion following golimumab and methotrexate combination therapy as compared with methotrexate alone: a magnetic resonance imaging study of 318 methotrexate-naive rheumatoid arthritis patients. Arthritis Rheum 63(12):3712–3722
Baker JF, Conaghan PG, Emery P, Baker DG, Østergaard M (2016) Validity of early MRI structural damage end points and potential impact on clinical trial design in rheumatoid arthritis. Ann Rheum Dis 75(6):1114–1119
Peterfy C, Strand V, Tian L, Østergaard M, Lu Y, Dicarlo J et al (2017) Short-term changes on MRI predict long-term changes on radiography in rheumatoid arthritis: an analysis by an OMERACT Task Force of pooled data from four randomised controlled trials. Ann Rheum Dis 76(6):992–997
Døhn UM, Ejbjerg BJ, Hasselquist M, Narvestad E, Court-Payen Szkudlarek M et al (2007) Rheumatoid arthritis bone erosion volumes on CT and MRI: reliability and correlations with erosion scores on CT, MRI and radiography. Ann Rheum Dis 66(10):1388–1392
American College of Rheumatology Rheumatoid Arthritis Clinical Trials Task Force Imaging Group and Outcome Measures in Rheumatology Magnetic Resonance Imaging Inflammatory Arthritis Working Group (2013) Review: the utility of magnetic resonance imaging for assessing structural damage in randomized controlled trials in rheumatoid arthritis. Arthritis Rheum 65(10):2513–2523
Boers M, Brooks P, Strand CV, Tugwell P (1998) The OMERACT filter for outcome measures in rheumatology. J Rheumatol 25(2):198–199
Døhn UM, Ejbjerg B, Boonen A, Hetland ML, Hansen MS, Knudsen LS et al (2011) No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis 70(2):252–258
Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen HS, Østergaard M (2005) The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: a comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum 52(8):2300–2306
Haavardsholm EA, Bøyesen P, Østergaard M, Schildvold A, Kvien TK (2008) Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis 67(6):794–800
Nieuwenhuis WP, van Steenbergen HW, Stomp W, Stijnen T, Huizinga TW, Bloem JL et al (2016) The course of bone marrow edema in early undifferentiated arthritis and rheumatoid arthritis: a longitudinal magnetic resonance imaging study at bone level. Arthritis Rheumatol 68(5):1080–1088
Bøyesen P, Haavardsholm EA, Østergaard M, van der Heijde D, Sesseng S, Kvien TK (2011) MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis 70(3):428–433
Hetland ML, Stengaard-Pedersen K, Junker P, Østergaard M, Ejbjerg BJ, Jacobsen S et al (2010) Radiographic progression and remission rates in early rheumatoid arthritis—MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis 69(10):1789–1795
Baker JF, Østergaard M, Emery P, Hsia EC, Lu J, Baker DG et al (2014) Early MRI measures independently predict 1-year and 2-year radiographic progression in rheumatoid arthritis: secondary analysis from a large clinical trial. Ann Rheum Dis 73(11):1968–1974
Hetland ML, Østergaard M, Stengaard-Pedersen K, Junker P, Ejbjerg B, Jacobsen S et al (2018) Anti-cyclic citrullinated peptide antibodies, 28-joint disease activity score, and magnetic resonance imaging bone oedema at baseline predict 11 years’ functional and radiographic outcome in early rheumatoid arthritis. Scand J Rheumatol 48:1–8
Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E et al (2006) Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 54(12):3761–3773
Gandjbakhch F, Conaghan PG, Ejbjerg B, Haavardsholm EA, Foltz V, Brown AK et al (2011) Synovitis and osteitis are very frequent in rheumatoid arthritis clinical remission: results from an MRI study of 294 patients in clinical remission or low disease activity state. J Rheumatol 38(9):2039–2044
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG et al (2008) An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 58:2958–2967
Gandjbakhch F, Foltz V, Mallet A, Bourgeois P, Fautrel B (2011) Bone marrow oedema predicts structural progression in a 1-year follow-up of 85 patients with RA in remission or with low disease activity with low-field MRI. Ann Rheum Dis 70(12):2159–2162
Gandjbakhch F, Haavardsholm EA, Conaghan PG, Ejbjerg B, Foltz V, Brown AK et al (2014) Determining a magnetic resonance imaging inflammatory activity acceptable state without subsequent radiographic progression in rheumatoid arthritis: results from a followup MRI study of 254 patients in clinical remission or low disease activity. J Rheumatol 41(2):398–406
Brahe CH, Krabbe S, Østergaard M, Ørnbjerg LM, Glinatsi D, Røgind H et al (2019) Dose tapering and discontinuation of biological therapy in rheumatoid arthritis patients in routine care—2-year outcomes and predictors. Rheumatology (Oxford) 58(1):110–119
Vardhanabhuti V, Riordan RD, Mitchell GR, Hyde C, Roobottom CA (2014) Image comparative assessment using iterative reconstructions: clinical comparison of low-dose abdominal/pelvic computed tomography between adaptive statistical, model-based iterative reconstructions and traditional filtered back projection in 65 patients. Invest Radiol 49(4):209–216
Chahal BS, Kwan ALC, Dhillon SS, Olubaniyi BO, Jhiangri GS, Neilson MM et al (2018) Radiation exposure to the sacroiliac joint from low-dose CT compared with radiography. AJR Am J Roentgenol 211(5):1058–1062
de Koning A, de Bruin F, van den Berg R, Ramiro S, Baraliakos X, Braun J et al (2018) Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis 77(2):293–299
Aurell Y, Andersson M, Forslind K (2018) Cone-beam computed tomography, a new low-dose three-dimensional imaging technique for assessment of bone erosions in rheumatoid arthritis: reliability assessment and comparison with conventional radiography—a BARFOT study. Scand J Rheumatol 47(3):173–177
Jans L, De Kock I, Herregods N, Verstraete K, van den Bosch F, Carron P et al (2018) Dual-energy CT: a new imaging modality for bone marrow oedema in rheumatoid arthritis. Ann Rheum Dis 77(6):958–960
Wu H, Zhang G, Shi L, Li X, Chen M, Huang X et al (2019) Axial spondyloarthritis: dual-energy virtual noncalcium CT in the detection of bone marrow edema in the sacroiliac joints. Radiology 290(1):157–164
Døhn UM, Boonen A, Hetland ML, Hansen MS, Knudsen LS, Hansen A et al (2009) Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1 year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann Rheum Dis 68(10):1585–1590
Yue J, Griffith JF, Xiao F, Shi L, Wang D, Shen J et al (2017) Repair of bone erosion in rheumatoid arthritis by denosumab: a high-resolution peripheral quantitative computed tomography study. Arthritis Care Res (Hoboken) 69(8):1156–1163
Barnabe C, Toepfer D, Marotte H, Hauge EM, Scharmga A, Kocijan R et al (2016) Definition for rheumatoid arthritis erosions imaged with high resolution peripheral quantitative computed tomography and interreader reliability for detection and measurement. J Rheumatol 43(10):1935–1940
Scharmga A, Peters M, van den Bergh JP, Geusens P, Loeffen D, van Rietbergen B et al (2018) Development of a scoring method to visually score cortical interruptions on high-resolution peripheral quantitative computed tomography in rheumatoid arthritis and healthy controls. PLoS ONE 13(7):e0200331
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MØ has received speaker/consultant fees from Abbvie, BMS, Boehringer-Ingelheim, Celgene, Eli Lilly, Hospira, Janssen, Merck, Novartis, Novo, Orion, Pfizer, Regeneron, Roche and UCB, and research grants from Abbvie, Celgene, Centocor, Merck, and Novartis. MB is a shareholder of Image Analysis Group and serves as a consultant to Image Analysis Group, Eli Lilly, Esaote, Celgene, Pfizer, Abbvie, Carestream/Canon, Siemens and AstraZeneca.
Ethical standards
The manuscript does not contain clinical studies or patient data. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Østergaard, M., Boesen, M. Imaging in rheumatoid arthritis: the role of magnetic resonance imaging and computed tomography. Radiol med 124, 1128–1141 (2019). https://doi.org/10.1007/s11547-019-01014-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-019-01014-y