Abstract
Drug addiction is a chronic compulsion and relapsing disorder defined as a “pathological pattern of use of a substance”, and characterized by the loss of control in drug-taking-related behaviors, the pursuance of those behaviors even in the presence of negative consequences, and a strongly motivated desire to consume substances. Several brain areas and circuits are involved, encoding cognitive functions such as reward, motivation, and memory. Addiction research has moved the focus to those psycho-neurobiological mechanisms that have a crucial role on the transition from an occasional use to the abuse of drugs. It has been hypothesized that drug addiction may start as a “goal-directed behavior”; later, with the maintenance of the “instrumental behavior”, it can turn into a “habitual behavior”, inducing a form of habit-based learning. At a brain level, it has been suggested that DA-ergic/GLU-ergic/NE-ergic meso-cortico-limbic transmission may have a crucial role in the pathological habit-based learning of a drug-seeking behavior.
The present chapter reviews the more recent studies on drug addiction, investigating the psycho-neurobiological hypotheses concerning what drives the transition from an occasional use to abuse of drugs. Then, a “habit learning” theory of drug addiction is described. Further, the possibility of an engagement of different memory systems in a “learned drug-seeking” behavior is discussed. The next section describes the role of prefrontal NE-ergic neurotransmission in drug addiction. Finally, the chapter raises some questions about a conceptual framework linking pathological learning with memory and drug addiction.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Addiction in Latin (addictus) means “slave to debt” or “subjugate”, and it is very closely associated with the concept of psychological dependence from pharmacological substances. Drug addiction is a chronically compulsive and recidivist disorder that affects individuals more psychologically than physically. The life of an addict is a progressive top-down circle of searching for, obtaining, using, and recovering from drug effects, in spite of related illness, disrupted relationships, and work/life failures. The social burden created by addiction can be quantified and measured in social and health contexts, in order to express the overall severity of this psychological disease. The extent of problematic drug use—by regular drug users—remains stable at between 16 million and 39 million people [1]; globally, these rates of drug abuse are relatively stable despite the fact that it is on the rise in developing countries. Substances of abuse reduce socio-economic development and boost organized crime, instability, and national insecurity [1].
Addiction has recently been defined by the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-V) [2] as a “pathological pattern of use of the substance”, where the loss of control over drug-seeking/drug-taking behavior, the persistence of drug-taking behavior despite negative consequences, and a high motivation to take drugs at the expense of other activities are the main features. The loss of control, persistence, and high motivation to take drugs can be analyzed and conceptualized at several levels, from psychological to molecular. In particular, a major hypothesis guiding experimental research considers the level of exposure to the substance as a key factor that leads to addiction [3,4,5,6]. Primarily, addiction is a chronic disease that involves brain systems related to reward, motivation, and memory, forming circuitry between each other. Secondarily, dysfunction in these circuits leads to bio-psycho-social manifestations of pathological behavior. Addiction researchers have recently hypothesized that there is a crucial role of the prefrontal cortex (pfC) in the limbic circuit of reward, and the pfC is considered to be one of the major components of the neurobiology of addiction [7,8,9,10,11].
The major aim of this chapter is to review recent studies highlighting the key features of drug addiction, and the nature of a transition from occasional to compulsive use of pharmacological substances. First, this chapter overviews two major hypotheses currently driving drug addiction research, all of which indicates that the level of exposure to the substance is a key factor leading to addiction [3,4,5,6]. Second, this chapter highlights a recent hypothesis related to “habit learning” that can explain the transition from occasional to compulsive drug use. The third part of this chapter discusses the possibility of the engagement of different memory systems in a “learned drug-seeking” behavior. The fourth part deals with a neurobiological conceptualization of addiction in relation to the “habit learning” and “habit memory” hypotheses. The fifth part of this chapter focuses on the role of catecholaminergic transmission in the pfC in addiction. In conclusion, this chapter highlights several questions about a conceptual framework linking pathological learning and memory with drug addiction.
An Aberrant Motivation and a Hedonic Dysregulation Driving Drug-Seeking Behavior
In this section we review two major psychological theories explaining the passage from occasional use to pharmacological substance abuse: the “incentive-sensitization” theory and the “hedonic dysregulation” theory.
The “Incentive-Sensitization” Theory
Following the “drive-reduction theories”, psychobiology of addiction has pointed out to a link between reward brain system and motivation. Motivational concepts can help us to understand how and why limbic brain systems are evolved to mediate psychological processes that guide drug seeking/taking behavior. Hedonic reward is a key concept in motivated behavior [4, 5], and cognitive expectations together with physiological internal states can modulate hedonic incentives [5]. It can be argued that learned Pavlovian incentive stimuli become both consummatory phase (“liking”, hedonic value) and appetitive phase (“wanting”, incentive salience), as a consequence of reward learning. It has been found that unconditioned affective reaction patterns elicited by sucrose and quinine solutions are essentially normal in rats after 6-hydroxydopamine (6-OHDA) lesions of the “striatal–accumbal” dopamine (DA) system [12]. Moreover, rats with extensive dopamine depletions can change their hedonic evaluation of a stimulus based on predictive relations with another event, meaning that reward learning models have posited dopamine systems to play a similar role in learned increments and learned decrements in prediction of hedonic rewards [12]. Pre-clinical research on drug addiction has found concepts to explain the compulsive use of drugs as well as the phenomenon of relapse in an interesting “motivation-based theory”. Different drugs of abuse with different pharmacological actions cause sensitization via the alteration of the mesolimbic DA system. Sensitization happens when repeated drug administration leads to an enhancement of outcomes related to that drug or to another addictive substance (cross-sensitization, [5, 13, 14]). Compulsive drug seeking/taking behavior and the relapse (through the exposition to stimuli associated with the substance or due to stress) are attributable to modifications in the motivational system related to the appetitive phase (wanting). This phenomenon was explained by Berridge and Robinson with the “incentive-sensitization” theory [15]. They consider that long-term drug use leads to mounting neuroadaptations at the “brain reward system” level, enhancing the sensitization to the substances of abuse and to associated stimuli. Repeated use of a drug induces specific associations between stimuli and, consequently, induces specific actions tagging a specific behavior such as the rewarded outcome. Increasing of drug-stimuli pairings increases the incentive value of the stimuli, leading addicts to want to take drugs, even they do not particularly like them [5]. However, even if this theory explains many aspects of human addiction, such as excessive preoccupation with the drug and with seeking it out, the intense craving, and relapse, it fails to explain a central feature of drug addiction: the inability of addicts to regulate or stop the use of a drug despite negative consequences and the self-destructive nature of its prolonged use. This theory doesn’t deny the pleasure obtained from the drug, the withdrawal, or habits such as reasons why people become addicted. However, it suggests that a sensitized wanting could better explain long-term compulsive drug seeking/taking behavior.
The “Hedonic Dysregulation” Theory
This theory describes a vicious “top-down” circle from occasional and controlled drug use into addiction passing through at least three stages: “preoccupation/anticipation”, “binge/intoxication”, and “withdrawal/negative effect” [16].
The first theories on drug addiction considered that drugs prevented or relieved psycho-physical negative states resulting from abstinence (i.e., withdrawal) or from adverse environmental circumstances (i.e., stress). While initial drug use is motivated by the hedonic rewarding properties of the drug itself, it has been hypothesized that drug use becomes motivated more by a “negative reinforcement” (abstinence symptoms avoidance) than by a positive reinforcement (euphoric high state, [17]). Negative reinforcement can be defined as the process by which removal of an aversive stimulus (i.e., negative emotional state of drug withdrawal) increases the probability of a response (i.e., dependence-induced drug intake, [18]). Drug users progress from occasional use to addiction, and the factors motivating drug use are hypothesized to shift in importance, in which impulsivity often dominates early stages, and compulsivity dominates terminal stages. A shift occurs from impulsivity to compulsivity, and a similar shift occurs from positive reinforcement to negative reinforcement, driving the motivated behavior.
On the other hand, the role of sensitization in addiction has been explained as a shift in an incentive-salience state, described as “wanting”, attributed to a pathological “over-activity” of mesolimbic dopamine function. Other factors such as an increased secretion of glucocorticoids may function in the long-term maintenance of this sensitized state [19]. Drug-taking follows the pattern of intoxication, tolerance, escalation in intake, profound dysphoria, physical discomfort, and somatic withdrawal signs during abstinence. The “craving” is an intense preoccupation/desire to obtain substances that often precedes the somatic signs of withdrawal, having a crucial role in compulsive seeking-behavior and in relapse. Moreover, craving has a role in the associated stimuli related to drug-taking behavior and to withdrawal. Finally, craving is a key part in the vicious circle of addiction, and it has been considered important in the three stages driving to drug addiction: “preoccupation/anticipation”, “binge/intoxication”, and “withdrawal/negative affect” [16]. These three stages are conceptualized as interacting with each other, becoming more intense, dysregulating the hedonic homeostasis of the reward system, and ultimately leading to the pathological state known as addiction [4]. The transition from occasional drug use to addiction involves neuroplasticity in all of these elements, and may begin with initial drug use in vulnerable individuals or individuals at particularly vulnerable developmental periods (i.e., in adolescence).
The preoccupation/anticipation (craving) stage of the addiction cycle has long been hypothesized to be a key element of relapse in humans, and it defines addiction as a chronic relapsing disorder. The binge/intoxication stage has a pattern of intake characterized by high intake of the drug except during periods of sleep and negative emotional states during abstinence, including dysphoria, irritability, and intense craving. Such “binges” can last hours or days, and are often followed by a withdrawal characterized by extreme dysphoria and inactivity. When craving is driven by environmental cues, intense substance-craving can anticipate withdrawal, signifying the availability of the substance and internal states linked to negative emotional states and stress (withdrawal/negative effect stage).
The hedonic dysregulation theory elucidates the passage from use to abuse of drugs as a “top-down” vicious circle, considering the key role of a sort of imbalance in the hedonic status of drug users. However, the theory fails to explain the role of other main features of drug addiction such as an abnormal sensitization to the substance and the instrumental behaviors to obtain the substance.
The Neural Basis of a Drug-Motivated Behavior
In addition to the behavioral criteria described above, different studies in the neurobiology of addiction also support the idea that DA plays a crucial role in drug-motivated behavior. The clearest mechanism in drug-seeking/taking behavior is the activation of the DA-ergic transmission in the brain reward circuitry [20,21,22]; and DA-ergic mesolimbic/nigrostriatal pathways are thought to be mainly involved in drug-induced neuroplastic changes. Furthermore, it has been widely shown that increased DA-ergic transmission in the nucleus accumbens (NA) plays a mediating role in the rewarding/reinforcing effects of addictive drugs [6, 23,24,25,26].
One of the two NA subnuclei (the “shell”) receives DA-ergic innervations from the ventral tegmental area (VTA), and is crucial in the modulation of “motivational salience”, also contributing to the establishment of “Pavlovian” learned associations between motivational events and concurrent environmental perceptions [27, 28]. The NA shell also projects to subcortical structures, such as the lateral hypothalamus (LHyp, which mediates autonomic responses), permitting regulatory activity in hunger/satiety modulation of food motivation and reward [28]. Neurochemical lesions of the NA DA-ergic pathways or receptor-blocking drugs reduce the “wanting” to eat, but do not reduce facial expressions of “liking” for the same reward [12, 29, 30]. Furthermore, opiates increase extracellular DA in the NA [31], and drug priming reinstates drug-seeking behavior by activating the mesolimbic DA-ergic incentive motivation system [12, 28]. Adaptive behavioral responses to the motivational situation occur under DA release, inducing cellular changes that establish learned associations with the event [32]. By contrast, in a repeated drug administration, DA release is no longer induced by a particular event, as a motivational event becomes familiar by repeated exposure [33]. In this case, the behavioral response remains goal-directed and well learned, and further DA-induced neuroplastic changes are not necessary.
In contrast, the other sub-nucleus of NA (the “core”) appears to be a primary site mediating the expression of learned behaviors in response to stimuli predicting motivationally relevant events [34, 35]; and DA is released into the core in response to stimuli predicting a rewarding event, which probably modulates the expression of adaptive behaviors [34]. Therefore, in learned associations induced by repeated motivational situations, DA will likely be released as part of the overall experience. In sum, DA might have two functions. The first is to alert the organism to the appearance of novel salient stimuli, and thereby promote neuroplasticity (learning). The second is to alert the organism to the pending appearance of a familiar, motivationally relevant event, on the basis of learned associations which were previously made with environmental stimuli predicting the event [36]. Finally, a series of parallel cortico-striato-pallido-cortical loops have been defined whereby the ventral striatum, including NA, relates to emotional learning and the dorsal striatum relates to cognitive and motor functions [37, 38].
In parallel with neurobiological studies, electrophysiological studies have revealed highly heterogeneous changes in striatal neuron firing during a motivated behavior [39,40,41].
Interestingly, two major neuronal types have been identified in the NA [42, 43]: fast spiking interneurons (FSIs) and medium spiny projection neurons (MSNs). FSIs strongly inhibit MSNs and control their spike timing [43, 44], and have been shown to respond differently than MSNs to rewards [45], suggesting that FSIs and MSNs play different roles in those behaviors related to motivation and habit learning. Finally, the NA plays an important role in both appetitive and consummatory behavior. A common finding in electrophysiological studies of the NA or ventral striatum (VS) in animal models of behavior is that subpopulations of neurons respond phasically to each identifiable component of both appetitive and consummatory phases of the task [41, 46,47,48]. However, because many more NA neurons are inhibited during consumption than are excited, manipulations that inhibit the NA may enhance food consumption, not because the NA is generally inactivated, but because the specific population of neurons whose firing inhibits consumption is silenced by such manipulations. Many of the same neurons whose inhibition may drive consumption are also excited by cues, and during operant responding are excited by behaviors that are incompatible with consumption.
A “Habit-Based Learning” Hypothesis for Drug Addiction
The “Habit Learning” Theory
Recently, addiction research has placed a special focus on what happens in the real world where drug abusers have to stoke up drugs because of their not free availability [49]. In line with this idea, an animal model of drug-seeking/taking behavior has been created. In this model, rats no longer respond to stimuli in order to obtain drug infusions. Thus, it has been defined that the drug sensitivity is due to an instrumental behavior–drug administration relationship. In fact, drug-associated stimuli have a considerable effect on behaviors, and play an important role in addiction development [50, 51]. However, it has been shown that drug-seeking behavior is not affected by pharmacological effects of the drug, because the maladaptive behavior occurs prior to drug infusion [52]. If the drug-seeking behavior is still present even if the drug is not delivered, it is arguable that the drug-seeking behavior depends on the contingency in the presentation of drug-associated cues. This animal model provides an opportunity to study the neural basis of cue-associated drug-seeking behavior. Moreover, it is useful in order to address new potential treatments that would decrease cue-associated drug-seeking behavior. The main characteristics of drug addiction are the compulsive drug-seeking/taking drug behavior in spite of adverse consequences and the relapse to the substances of abuse. When desire becomes a need, the subject acts out a different kind of behavior. It leads him or her to take substances. Goal-directed behavior and habit learning perform two forms of instrumental learning: the first one is quickly acquired and tuned by its outcome, the second one is more willful, and elicited by previous stimuli rather than their consequences [53]. The psychobiology of drug addiction identifies the first of these behaviors as simply aberrant, and the second as pathological.
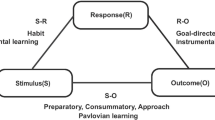
Different behavioral procedures have been developed, each of which focuses more directly on component processes. The critical procedure for demonstrating this motivational influence is the Pavlovian–instrumental transfer (PIT) design, in which the role of a separately trained conditioned stimulus (CS) on instrumental responses is assessed [6, 49, 52]. Pavlovian CSs can modulate instrumental performance. For example, a stimulus that predicts the arrival of a tasty solution will enhance lever pressing for that solution (specific PIT) or another reward (general PIT). The approach takes into account two conditions: (1) the Pavlovian processes that define sensitivity to the contingency between stimuli (S) and reinforcers (R), and (2) the instrumental processes sensitive to the contingencies between actions, or responses (R), and outcomes (O, [54, 55]). This R–O process can be contrasted with the first, S–R instrumental process in which seeking behavior is a simple habitual response triggered by the environment and drug-associated stimuli. It has been argued that drug seeking is initiated under the control of the goal-directed R–O process, but the onset of addiction becomes a compulsive habit under the control of the S–R process [52]. For example, an action such as lever-pressing works as a contingency between action and its outcomes. Combination of this contingency, along with an unconditioned stimuli (US)-induced instrumental incentive value, regulates goal-directed responses, defining a motivational incentive salience. On the other hand, the S–R process can induce an incentive learning process. Additionally, PIT provides a “motivational boost” and enhances R-O process.
Everitt considers drug addiction the final stage of several steps from the initial and controlled use of a substance [6, 49, 52, 56]. When the substance is taken voluntarily for its incentive effect, seeking behavior progressively becomes a “habit”, through a gradual loss of control. Thus, the stimulus–response mechanism plays an important role in the maintenance of an instrumental behavior. Finally, the capacity of the stimulus (substance) to act as reinforcement (conditioned reinforcer) exerts a kind of control over the seeking/taking behavior. Thus, drug addiction may start as a “goal-directed behavior”; later, with the maintenance of the “instrumental behavior”, it could turn into a “habitual behavior”, inducing a form of learning based on the habit (habit learning, [6, 49, 52, 56]).
Three major theories guide the experimental research in the field of drug addiction. The incentive-sensitization theory states that “aberrant motivation” to seek and take drugs could characterize addiction, and considers that “wanting” plays a major role in addiction development. The hedonic dysregulation theory defines a top-down spiraling, from use to abuse of drugs, and focuses on the role of dysregulation in hedonic homeostasis, taking into account a crucial role of a “liking” dysregulation. Finally, the habit learning theory highlights the role of an instrumental learning behavior that becomes habit, in order to explain the complex use/abuse transition in the drug seeking/taking behavior, and places equal weight on both the “liking” and “wanting” roles.
The Neural Basis of a Drug-Habit-Learned Behavior
Accumulating evidence suggests a critical role for dissociable neurochemical mechanisms in the basolateral amygdala (BLA) and the NA core that underlie drug-seeking behavior maintained by conditioned reinforcers [55, 57,58,59,60]. The BLA complex performs fundamental roles in memory formation and storage linked with emotional events [61, 62]. Moreover, it is involved in appetitive (positive) conditioning [63]. Distinct neurons respond to both positive and negative stimuli, but they do not group into clear anatomical nuclei [64]. Studies report that infusions of DA receptor antagonists into the BLA prevented CS-induced reinstatement of responding after extinction [65]. This could mean a special involvement of DA-ergic transmission in the BLA in drug-seeking/taking behavior. Consistent with these observations, NA core DA efflux was not increased during the response-dependent presentation of conditioned stimuli in a reinstatement procedure [66, 67], whereas glutamate (GLU) efflux was increased in the NA core of animals engaged in active cocaine seeking [68]. Finally, combined “cues + drug-primed” reinstatement conditions showed that increased DA and GLU efflux in the medial pfF (mpfC) and NA plays a role in promoting reinstatement, and may be an important mediator of drug-seeking behavior primed by multiple relapse triggers [69]. Together, these findings suggest that drug seeking maintained by drug-associated conditioned reinforcers may depend on DA-ergic mechanisms in the BLA and GLU-ergic mechanisms in the NAc core, and together in the mpFC.
This raises the question of whether these selective neurochemical mechanisms in the BLA and NA core are components of a neuroanatomical subsystem within limbic cortical–ventral striato-pallidal circuitry [70]. In part, the technique of the so-called “disconnection” indicated that the dorsal striatum (DS) and VS interact with each other serially, in a wide range of functional settings, such as PIT on goal-directed behavior [55]. Specific PIT involves the BLA and NA shell. General PIT involves the central amygdala (CeA) and NA core [59]. The VS has long been suggested to be the interface between emotion, motivation, and action on the basis of its major inputs from the limbic cortices such as the BLA, the orbitofrontal cortex (oFC), and the LHyp [55, 70, 71]. The NA core has important functions in Pavlovian conditioning, and in the interactions between Pavlovian and instrumental learning mechanisms involved in involuntary behavior [55, 57, 66]. Conversely, the role of DS in both cognitive and motor functions is well established, providing the neurobiological substrate of both goal-directed and habitual control of “instrumental learning” [72,73,74,75]. Sequential phases of Pavlovian and instrumental learning could be especially relevant for the transition from casual drug use to substance abuse, involving compulsive drug-seeking/taking behavior [49].
Recently, several experimental and functional observations support the idea of common neural circuitry forming a distinct entity into the basal forebrain, termed the “extended amygdala”. This circuit may be delegated to act on the motivational, emotional, and habitual effects of drug addiction [76,77,78,79]. The extended amygdala represents a macro-structure composed of several basal forebrain structures: the bed nucleus of the stria terminalis (BNST), the central medial amygdala (CeA), and the NA shell [76, 77]. These structures have similarities in morphology, immunohistochemistry, and connectivity [78, 79], and they receive afferent connections from limbic structures such as the hippocampus (HP), BLA, and LHyp. Key elements of the extended amygdala include not only neurotransmitters associated with the positive reinforcing effects of drugs of abuse, but also major components of the brain stress systems associated with the negative reinforcement of dependence [76].
A New Perspective on Rewarding Memories in Drug Addiction
The “Habit-Memory” Hypothesis
The implications of the psychological/neural mechanisms of drug-seeking behavior have an important role in addressing drug addiction therapies. Interestingly, recent evidence indicates that different memory systems are also used in the new learning occurring during behavioral extinction [80]. The passage from initial casual drug use to eventual addiction could involve, at least in part, a compulsive drug-seeking/taking behavior guided by dorsal striatal-dependent habit-learning mechanisms [49, 52, 72]. This suggests that when “habit-like” drug-seeking behavior is firmly acquired, the extinction of such behavior may be differentially influenced by engaging both habit and memory systems. Furthermore, a dissociation has been defined between cognitive (hippocampus-dependent) and habit (DS-dependent) memory systems, during an initial acquisition of learned behavior [81,82,83]. Recently, it has been tested whether habit and cognitive memory systems are involved in the extinction of such behaviors [84]. In the response extinction condition, rats performed a runway approach response to an empty fluid well. In the latent extinction condition, rats were placed at the empty fluid well without performing a runway approach response. Subsequently, it has been shown that the relative effectiveness of multiple memory systems was altered by oral cocaine self-administration, during extinction training [84]. Finally, it has been found that an abnormal stimulus–response habit guiding acquired approach response can affect the cocaine-induced impairment of latent extinction, thus rendering cognitive learning mechanisms inefficient during latent extinction training. Consistent with these results, drug-seeking behaviors underlying addiction may involve, at least in part, a transition from goal-directed behaviors to habitual behaviors that characterize the function of the DS memory system [49, 52, 72, 85,86,87].
The Neural Basis of Habit Memory
The BLA plays a crucial role in emotion and memory [88, 89]. Numerous studies have implicated the BLA in the effects of emotional arousal on memory, mediated by HP and DS [90,91,92]. Moreover, recent evidence indicates that the relative use of cognitive and habit memory can be influenced by an organism’s emotional state [93]. On the basis that anxiety and/or stress can promote relapse into previously acquired habitual and maladaptive human behaviors such as addiction [4, 76], recent data has highlighted the mechanisms by which emotional arousal can produce a clinically significant propensity to the use of habit memory [94]. In the rat, HP and DS each receive anatomical projections from the BLA [95, 96]. Moreover, in the dual-solution plus maze task [97], BLA infusion of anxiogenic drugs may produce an inclination towards the use of habit memory by directly facilitating DS-dependent response learning. Alternatively, the infusions may indirectly bias rats towards response learning by impairing HP-dependent place learning [93, 94, 97]. Extensive evidence indicates that competitive interference between cognitive and habit memory systems can exist in some learning situations [98,99,100]. The emotional processes mediated by the BLA may also impact learning and memory by influencing the degree and nature of competitive interference among multiple memory systems. Finally, taking the considerable impact of emotion and memory on adaptive behavior, it is not surprising that the role of the amygdala in human psychopathology has received considerable empirical and theoretical attention [92, 101,102,103,104]. Recently, it has been suggested that a modulatory action may potentially provide a mechanism whereby stress or anxiety could release habit-learning systems from the competing and/or inhibitory influences of cognitive memory systems, promoting relapse into previously acquired habitual and maladaptive behaviors, as occur in drug addiction or obsessive-compulsive disorder [94].
Pre-frontal Cortical Norepinephrine Transmission in Drug Addiction
Drug addiction research is focused on the regulation of mesoaccumbens DA-ergic transmission in response to pleasant or aversive stimuli. However, recently it has been shown that mesoaccumbens DA-ergic transmission seems to be modulated by the mesocortical DA-ergic system in an inhibitory way, suggesting an inverse response relationship between them [8]. Moreover, a growing body of data considered the idea of a “hypofrontality” in drug addiction, considering that a prolonged drug use can induce an inability to inhibit responding toward the stimuli previously paired with a reward, resembling the focused and persistent drug-seeking behavior observed in drug addicts [105, 106].
It has been demonstrated that norepinephrine (NE) in the mpFC has a crucial role in NA DA release, induced by systemic amphetamine, morphine, or lithium administration [8,9,10]. Hence, studies on the involvement of brain NE-ergic systems in behavior control mostly focus on emotional memory regulation by the amygdala (AMY, [92, 104, 107]). Finally, a possible mpFC NE/DA counteractive action on subcortical DA transmission has been suggested [8,9,10]. Psycho-biological studies, which investigated different genetic backgrounds and used a useful strategy for investigating the neural basis of drug effects, have identified relationships between catecholaminergic neurotransmission and maladaptive behavior [108]. Using two well-known inbred strains of mice (DBA/2J, DBA and C57BL6/J, C57), it has been shown that DBA mice are poorly responsive to the enhancing extracellular DA induced by both natural and pharmacological substances in the NA [109,110,111,112]. Oppositely, C57 mice are highly responsive to stimulating/reinforcing effects of both natural and pharmacological substances, as shown by increased locomotor activity in amphetamine-induced conditioned place preference (CPP, [109, 110]) or by increased seeking/taking chocolate behavior in a conditioned suppression paradigm [111,112,113]. Since DA mpFC has an inhibitory role on DA NA, while NE is excitatory [7], it has been hypothesized that the NE/DA balance in the mpFC controls DA in the NA and related behavioral outcomes, making the C57 strain more responsive than DBA [8, 30]. This hypothesis was confirmed by experiments showing that selective mpFC NE depletion abolished the effects of amphetamine on DA in the NA of C57 mice [8], while selective mpFC DA depletion (preventing NE) led to DA outflow in the NAc and behavioral outcomes in DBA mice which are entirely similar to those of C57 [9, 108]. These data suggested that DA in the NA is controlled by mpFC NE, which enhances it, and by mpFC DA, which inhibits it.
Prefrontal NE transmission is known to play a critical role in regulating many cortical functions, including arousal, attention, motivation, learning, memory, and behavioral flexibility [113,114,115,116,117,118]. Moreover, both rewarding/reinforcing and aversive stimuli have been shown to increase NE release in mpFC [10, 112, 119, 120]. Furthermore, it has been shown that novel stressful experiences enhance DA release in the NA through activation of mpFC alpha-1 adrenergic receptors by high levels of released NE [121, 122].
There is some evidence to indicate that mpFC NE/DA transmission controls DA release in the NA [8, 9, 30, 117, 123]. Thus, mpFC-NA regulation partially regulates the response to rewarding (amphetamine) or aversive (stress) stimuli [10]. Further studies have provided substantial support for this view, through experimental evidence that the mpFC NE is crucial in the effects of other addictive drugs [8, 9], palatable food [11, 111,112,113], and aversive pharmacological or physical stimuli [10].
It is already known that the BLA is involved in forming associations between neutral and aversive stimuli [61, 62, 124,125,126]. The BLA receives stress-related DA projection from the VTA, suggesting that the BLA is involved in the modulation of affective stress responses, along with the NA and mpFC [127,128,129]. It has been shown that intra-BLA infusions of DA-ergic receptor antagonists enhanced DA release in NA in stressed rats, while it reduced the DA stress response in the mpFC [127]. Thus, these findings suggest that increased BLA DA-ergic transmission has opposite effects on the NAc and mpFC DA responses to stress. Moreover, the anxiety induced by withdrawal is a significant factor contributing to continued drug abuse in addicted people, and the BLA is a major brain emotional center regulating the expression of fear and anxiety [76, 130,131,132,133]. Furthermore, recent studies have suggested that central NE-ergic systems are activated during acute withdrawal from ethanol, and may have a motivational significance [134]. Moreover, electrophysiological studies have shown that interneuronal GABA-ergic activity in the “extended amygdala” may reflect the negative emotional state of motivational drug-seeking [76]. Furthermore, evidence suggests that NE enhances GABA-ergic neurotransmission via the α1 receptors [135]. Acute withdrawal from all major substances of abuse increases reward thresholds, anxiety-like responses, and AMY neurotransmission [76, 136]. Compulsive drug use associated with dependence is mediated not only by loss of function of reward systems, but also by recruitment of brain stress systems such as NE in the “extended amygdala” [76]. Finally, brain arousal/stress systems in the extended amygdala may be key components of the negative emotional states that drive dependence on drugs of abuse, and may overlap with the negative emotional components of other psychopathologies [77].
Conclusions
A few interesting questions are raised in the light of all the converging evidences presented here, starting from the theoretical/psycho-bio-physiological conceptualizations of drug addiction to the last findings about a possible conceptual framework linking pathological learning and memory with drug addiction.
The first question is whether the three theoretical conceptualizations, the “incentive-salience theory”, the “hedonic dysregulation theory”, and the “habit-based learning theory” are able to individually explain the psychopathological features of drug addiction. It is more likely, though, that these three theories can be considered as parts of a single general conceptualization that can better explain the psychopathological features of drug addiction. The hypothesis that an “aberrant motivation”, a “hedonic dysregulation” and “aberrant learning” can be individual features which can be included in the complex of psychopathological behavior should be considered.
The passage from occasional drug use to abuse is related to a change from a positive reinforcement to a negative one, with changes on motivational baseline. Drug reward is comprised of two components: one appetitive (orienting towards food) and the other consummatory (hedonic evaluation), which are also referred to as “wanting” and “liking” respectively. It has been explained that “wanting” and “liking” could act independently, defining a psychological and neuroanatomical separation between them [5, 12]. Moreover, it has been defined that craving (intense needing) and continuous neuro-plastic changes are involved in the passage from casual drug use to addiction [26]. Finally, it has been argued that only maladaptive habit-based learning could trigger drug-seeking behavior [6]. However, these three hypotheses are able to explain singular parts of the entire complex of drug addiction. Finally, motivation, hedonic dysregulation, and habit-based learning can be considered parts of the complex of the drug-addicted behavior; and neuroanatomical and neurobiological evidence discussed here are in line with this idea. However, although several studies have investigated how and when these three characteristics are involved in drug addiction, little is known about their possible temporal interpolation. Several human and animal studies have shown that the time of reward has an important role in reward processing [137, 138]. Furthermore, time intervals and rates of reward are of crucial importance for conditioning, and DA neurons are crucially involved in the processing of temporal information about the rewards. DA-ergic neurons in the meso-cortico-limbic system show reward-related responses that are sensitive to the predicted time of reward and the instantaneous reward probability [137]. This suggests a possible temporal interpolation from occasional use to abuse of substances, mediated by a meso-cortico-limbic DA-ergic circuit (Fig. 16.1). At a clinical level, this would also help to understand how and when to intervene along the continuum from occasional use to abuse of pharmacological substances.
Hypothetical timeline of the temporal interpolation. Figure describes a hypothetical timeline where the major features are defined in a single temporal interpolation from the first drug taking to the addiction. During this time, neurobehavioral changes such as the passage form a goal-directed behavior to an instrumental behavior and a functional dissociation between cognitive/HP-dependent memory and a habit/DS-dependent memory act on the hedonic dysregulation, and on the representation of the value of the drug, drastically inducing the addiction
A growing body of data hypothesizes the possibility of a conceptual framework linking the pathological learning, memory, and drug addiction. Recently, it has been hypothesized that when “habit-like” drug-seeking behavior is firmly acquired, the extinction of such behavior may be differentially influenced by engaging both habit and memory systems. Furthermore, a dissociation has been defined between cognitive (hippocampus-dependent) and habit (DS-dependent) memory systems, during an initial acquisition of learned behavior [81,82,83].
The second question is whether the three features presented above (aberrant motivation, hedonic dysregulation, and aberrant learning) underlying drug-addicted behavior could also be evaluated from a multi-emotional memory system point of view, highlighting a possible major role of aberrant learned associations between drug-associated stimuli and environmental factors, such as stress, driving the maladaptive compulsive seeking/taking behavior that is a main feature of drug addiction. Although there are emergent studies about the possible role of multi-emotional memory systems in drug addiction, little is known about the possible role of “habit memory” in psychopathological behavior characterizing drug addiction.
Finally, taken together, these four theories could contribute to better understanding the psychopathological features of drug addiction, such as the compulsive use of substances of abuse as well as the relapse. Thus, future works could aim to better understand the key elements characterizing the psycho-physio-pathological aspects of drug addiction.
References
United Nations Office on Drug and Crime. World drug report 2014. Vienna: United Nations Publications; 2014.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Nestler EJ. Molecular mechanisms of opiate and cocaine addiction. Curr Opin Neurobiol. 1997;7(5):713–9.
Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–8.
Berridge KC. Motivational concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209.
Robbins TW, Everitt BJ, Nutt DJ. Introduction: the neurobiology of drug addiction: new vistas. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3109–11. doi:10.1098/rstb.2008.0108.
Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18(7):2729–39.
Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23(5):1879–85.
Ventura R, Alcaro A, Puglisi-Allegra S. Prefrontal cortical norepinephrine release is critical for morphine-induced reward, reinstatement and dopamine release in the nucleus accumbens. Cereb Cortex. 2005;15(12):1877–86.
Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104(12):5181–6.
Latagliata EC, Patrono E, Puglisi-Allegra S, Ventura R. Food seeking in spite of harmful consequences is under prefrontal cortical noradrenergic control. BMC Neurosci. 2010;11:15. doi:10.1186/1471-2202-11-15.
Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69.
Adams E, Klug J, Quast M, Stairs DJ. Effects of environmental enrichment on nicotine-induced sensitization and cross-sensitization to d-amphetamine in rats. Drug Alcohol Depend. 2013;129(3):247–53. doi:10.1016/j.drugalcdep.2013.02.019.
Harb MR, Almeida OFX. Pavlovian conditioning and cross-sensitization studies raise challenges to the hypothesis that overeating is an addictive behavior. Transl Psychiatry. 2014;4:e387. doi:10.1038/tp.2014.28.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91.
Koob GF. Animal models of craving for ethanol. Addiction. 2000;95(Suppl 2):S73–81.
Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav. 104(1):149–56. doi:10.1016/j.physbeh.2011.04.063.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi:10.1038/npp.2009.110.
Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl). 2013;229(3):387–413. doi:10.1007/s00213-013-3224-4.
Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–60.
Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–200. doi:10.1098/rstb.2008.0107.
Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi:10.1016/j.tics.2010.11.001.
Di Chiara G, Imperato A. Drugs abused by humans preferntially increase synaptic dopamine concentrations in the mesolimbico system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8.
Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225.
Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine and amphetaemine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92(26):12304–8.
Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi:10.1016/j.neuron.2008.06.012.
Singh T, McDannald MA, Haney RZ, Cerri DH, Schoenbaum G. Nucleus accumbens core and shell are necessary for reinforcer devaluation effects on pavlovian conditioned responding. Front Integr Neurosci. 2010;4:126. doi:10.3389/fnint.2010.00126.
Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90. doi:10.3389/fnsys.2015.00090.
Peciňa S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–11.
Puglisi-Allegra S, Ventura R. Prefrontal/accumbal cathecolamine system processes high motivational salience. Front Behav Neurosci. 2012;6:31. doi:10.3389/fnbeh.2012.00031.
Di Marzo V, Ligresti A, Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond). 2009;33(Suppl 2):S18–24. doi:10.1038/ijo.2009.67.
Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69(6):375–90.
Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27.
Torregrosa MM, Gordon J, Taylor JR. Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of pavlovian cocaine cue extinction. J Neurosci. 2013;33(19):8370–7. doi:10.1523/JNEUROSCI.0489-13.2013.
Saddoris MP, Carelli RM. Cocaine self-administration abolishes associative neural encoding in the nucleus accumbens necessary for higher-order learning. Biol Psychiatry. 2014;75(2):156–64. doi:10.1016/j.biopsych.2013.07.037.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13.
Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–82.
Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–30.
Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur J Neurosci. 2009;30(5):848–59. doi:10.1111/j.1460-9568.2009.06843.x.
Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Jeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30(23):8012–23. doi:10.1523/JNEUROSCI.5749-09.2010.
Wiltschko AB, Pettibone JR, Berke JD. Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology. 2010;35(6):1261–70. doi:10.1038/npp.2009.226.
Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–56.
Matsumoto J, Urakawa S, Hori E, de Araujo MF, Sakuma Y, Ono T, et al. Neuronal responses in the nucleus accumbens shell during sexual behavior in male rats. J Neurosci. 2012;32(5):1672–86. doi:10.1523/JNEUROSCI.5140-11.2012.
Tepper JM, Plenz D. Microcircuits in the striatum: striatal cell types and their interaction. In: Grillner S, Graybiel AM, editors. Microcircuits: the interface between neurons and global brain function. Cambridge: MIT; 2006. p. 127–48.
Lansink CS, Goltstein PM, Lankelma JV, Pennartz CM. Fast-spiking interneurons of the rat ventral striatum: temporal coordination of activity with principal cells and responsiveness to reward. Eur J Neurosci. 2010;32(3):494–508. doi:10.1111/j.1460-9568.2010.07293.x.
Cacciapaglia F, Wightman RM, Carelli RM. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J Neurosci. 2011;31(39):13860–9. doi:10.1523/JNEUROSCI.1340-11.2011.
Nishijo H, Uwano T, Ono T. Representation of taste stimuli in the brain. Chem Senses. 2005;30(Suppl 1):i174–5.
Shimura T, Imaoka H, Okazaki Y, Kanamori Y, Fushiki T, Yamamoto T. Involvement of the mesolimbic system in palatability-induced ingestion. Chem Senses. 2005;30(Suppl 1):i188–9.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9.
Alderson HL, Robbins TW, Everitt BJ. Heroin self-administration under a second-order schedule of reinforcement: acquisition and maintenance of heroin-seeking behaviour in rats. Psychopharmacology (Berl). 2000;153(1):120–33.
Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl). 1998;140(3):331–44.
Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36(2-3):129–38.
Gasbarri A, Pompili A, Packard MG, Tomaz C. Habit learning and memory in mammals: behavioral and neural characteristics. Neurobiol Learn Mem. 2014;114:198–208. doi:10.1016/j.nlm.2014.06.010.
Dickinson A, Smith S, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114(3):468–83.
Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–52.
Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity and top-down cognitive control. Neuron. 2011;69(4):680–94. doi:10.1016/j.neuron.2011.01.020.
Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical–ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000;126:263–85.
Di Ciano P, Everitt BJ. Direct interactions between basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24(32):7167–73.
Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25(4):962–70.
Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703.
Tomaz C, Dickinson-Anson H, McGaugh JL. Basolateral amygdala lesions block diazepam-induced anterograde amnesia in an inhibitory avoidance task. Proc Natl Acad Sci U S A. 1992;89(8):3615–9.
Tomaz C, Dickinson-Anson H, McGaugh JL, Souza-Silva MA, Viana MB, Graeff EG. Localization in the amygdala of the amnestic action of diazepam on emotional memory. Behav Brain Res. 1993;58(1-2):99–105.
Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on β-adrenergic receptors. Learn Mem. 2008;15(2):88–92. doi:10.1101/lm.825008.
Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–70.
See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl). 2001;154(3):301–10.
Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25(3):341–60.
Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaňeda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15(5):506–14.
McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–60.
Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39(4):811–22. doi:10.1038/npp.2013.231.
Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013;23(4):564–72. doi:10.1016/j.conb.2013.01.025.
Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307.
Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–41. doi:10.1016/j.neuron.2007.12.019.
Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus–response habit formation. J Neurosci. 2005;25(11):2771–80.
Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–9.
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22(2):513–23.
Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi:10.1016/j.brainres.2009.03.038.
Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psych. 2013;4:72. doi:10.3389/fpsyt.2013.00072.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–8. doi:10.1038/nature12041.
Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology. 2014;76Pt B:320–8. doi:10.1016/j.neuropharm.2013.05.046.
Gabriele A, Packard MG. Evidence of a role for multiple memory systems in behavioral extinction. Neurobiol Learn Mem. 2006;85(3):289–99.
Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–93.
White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77(2):125–84.
Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–7.
Gabriele A, Setlow B, Packard MG. Cocaine self-administration alters the relative effectiveness of multiple memory systems during extinction. Learn Mem. 2009;16(5):296–9. doi:10.1101/lm.1253409.
Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–68.
Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci U S A. 1999;96(22):12881–6.
Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24(14):3554–62.
Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34.
Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003;417:38–50.
Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci U S A. 1994;91(18):8477–81.
Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate–putamen. Neurobiol Learn Mem. 1998;69(2):163–203.
McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28.
Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem. 2004;82(3):243–52.
Wingard JC, Packard MG. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res. 2008;193(1):126–31. doi:10.1016/j.bbr.2008.05.002.
Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–91.
Jolkonnen E, Pikkarainen M, Kemppainen S, Pitkänen A. Interconnectivity between the amygdaloid complex and the amygdalostriatal transition area: a PHA-L study in rat. J Comp Neurol. 2001;431(1):39–58.
Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72.
Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41(3):245–51.
Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82(3):230–42.
McDonald RJ, Hong NS, Devan BD. The challenges of understanding mammalian cognition and memory-based behaviours: an interactive learning and memory systems approach. Neurosci Biobehav Rev. 2004;28(7):719–45.
Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61(3):137–59.
LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84.
McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the baso-lateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985:273–93.
Roozendaal B, McEwen BS, Chattarij S. Stress, memory and the amydgala. Nat Rev Neurosci. 2009;10(6):423–33. doi:10.1038/nrn2651.
Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26(30):8004–8.
Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, et al. Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J Neurosci. 2013;33(19):8101–13. doi:10.1523/JNEUROSCI.1727-12.2013.
Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl). 2012;221(3):407–26. doi:10.1007/s00213-011-2588-6.
Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29(1):72–80.
Zocchi A, Orsini C, Cabib S, Puglisi-Allegra S. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82(2):521–8.
Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289(5478):463–5.
Di Segni M, Patrono E, Patella L, Puglisi-Allegra S, Ventura R. Animal models of compulsive eating behavior. Nutrients. 2014;6(10):4591–609. doi:10.3390/nu6104591.
Patrono E, Di Segni M, Patella L, Andolina D, Valzania A, Latagliata EC, et al. When chocolate seeking becomes compulsion: gene–environment interplay. PLoS One. 2015;10(3):e0120191. doi:10.1371/journal.pone.0120191.
Tassin JP. Norepinephrine–dopamine interactions in the prefrontal cortex and the ventral tegmental area: relevance to mental diseases. Adv Pharmacol. 1998;42:712–6.
Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience. 2000;100(4):741–8.
Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–84.
Mingote S, de Bruin JP, Feenstra MG. Noradrenaline and dopamine efflux in the prefrontal cortex in relation to appetitive classical conditioning. J Neurosci. 2004;24(10):2475–80.
Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50.
Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi:10.1146/annurev.neuro.051508.135535.
Page ME, Lucki I. Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology. 2002;27(2):237–47.
van der Meulen JA, Joosten RN, de Bruin JP, Feenstra MG. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cereb Cortex. 2007;17(6):1444–53.
Nicniocaill B, Gratton A. Medial prefrontal cortical alpha1 adrenoreceptor modulation of the nucleus accumbens dopamine response to stress in Long–Evans rats. Psychopharmacology (Berl). 2007;191(3):835–42.
Mitrano DA, Schroeder JP, Smith Y, Cortright JJ, Bubula N, Vezina P, et al. α-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission. Neuropsychopharmacology. 2012;37(9):2161–72. doi:10.1038/npp.2012.68.
Devoto P, Flore G, Pira L, Diana M, Gessa GL. Co-release of noradrenaline and dopamine in the prefrontal cortex after acute morphine and during morphine withdrawal. Psychopharmacology (Berl). 2002;160(2):220–4.
Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899(1-2):218–26.
Guarraci FA, Frohardt RJ, Young SL, Kapp BS. A functional role for dopamine transmission in the amygdala during conditioned fear. Ann N Y Acad Sci. 1999;877:732–6.
Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22(1):324–37.
Stevenson CW, Gratton A. Basolateral amygdala modulation of the nucleus accumbens dopamine response to stress: role of the medial prefrontal cortex. Eur J Neurosci. 2003;17(6):1287–95.
Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala–prefrontal cortical pathway. J Neurosci. 2007;27(8):2045–57.
Ito R, Canseliet M. Amphetamine exposure selectively enhances hippocampus-dependent spatial learning and attenuates amygdala-dependent cue learning. Neuropsychopharmacology. 2010;35(7):1440–52. doi:10.1038/npp.2010.14.
Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differetially modulate pre- and post-synaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98(6):3185–96.
Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213(1-2):43–61. doi:10.1007/s00429-008-0191-3.
Barros M, Giorgetti M, Souto AA, Vilela G, Santos K, Boas NV, et al. Persistent anxiety-like behavior in marmosets following a recent predatory stress condition: reversal by diazepam. Pharmacol Biochem Behav. 2007;86(4):705–11.
Barros M, Maior RS, Houston JP, Tomaz C. Predatory stress as an experimental strategy to measure fear and anxiety-related behaviors in non-human primates. Rev Neurosci. 2008;19(2-3):157–69.
Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162(8):1483–93.
Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24(38):8198–204.
Gilpin NW, Koob GF. Effects of β-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl). 2010;212(3):431–9. doi:10.1007/s00213-010-1967-8.
Bermudez MA, Schultz W. Timing in reward and decision processes. Philos Trans R Soc Lond B Biol Sci. 2014;369(1637):20120468. doi:10.1098/rstb.2012.0468.
Bermudez MA, Göbel C, Schultz W. Sensitivity to temporal structure in amygdala neurons. Curr Biol. 2012;22(19):1839–44. doi:10.1016/j.cub.2012.07.062.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Patrono, E., Nishijo, H., Gasbarri, A., Pompili, A., Tomaz, C. (2017). Habit Learning and Addiction. In: Gargiulo, P., Mesones-Arroyo, H. (eds) Psychiatry and Neuroscience Update - Vol. II. Springer, Cham. https://doi.org/10.1007/978-3-319-53126-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-53126-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53125-0
Online ISBN: 978-3-319-53126-7
eBook Packages: MedicineMedicine (R0)