Abstract
Rationale
Amygdala-related circuitry helps translate learned Pavlovian associations into appetitive and aversive motivation, especially upon subsequent encounters with cues.
Objectives
We asked whether μ-opioid stimulation via microinjections of the specific agonist d-Ala2, N-MePhe4, Gly-ol)-enkephalin (DAMGO) in central nucleus of amygdala (CeA), or the adjacent basolateral amygdala (BLA) would magnify sucrose or sex “wanting”, guided by available cues.
Materials and methods
CeA or BLA DAMGO enhancement of cue-triggered “wanting” was assessed using Pavlovian to instrumental transfer (PIT). Unconditioned food “wanting” was measured via intake, and male sexual “wanting” for an estrous female was measured in a sexual approach test. Sucrose hedonic taste “liking” was measured in a taste reactivity test.
Results
CeA (but not BLA) DAMGO increased the intensity of phasic peaks in instrumental sucrose seeking stimulated by Pavlovian cues over precue levels in PIT, while suppressing seeking at other moments. CeA DAMGO also enhanced food intake, as well as sexual approach and investigation of an estrous female by males. DAMGO “wanting” enhancements were localized to CeA, as indicated by “Fos plume”-based anatomical maps for DAMGO causation of behavioral effects. Despite increasing “wanting”, CeA DAMGO decreased the hedonic impact or “liking” for sucrose in a taste reactivity paradigm.
Conclusions
CeA μ-opioid stimulation specifically enhances incentive salience, which is dynamically guided to food or sex by available cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In humans and other animals, encounters with unconditioned rewards or their Pavlovian cues can trigger intense seeking to obtain and consume these rewards. Clinically, cue-triggered motivation contributes to relapse in both drug addiction and binge eating. However, the motivational potency of such cues can vary dynamically from one encounter to the next. For example, an addict may resist drug cues many times yet relapse upon another encounter, and everyone finds food more powerfully tempting when hungry than when full.
What causes such fluctuations in the motivational potency of Pavlovian cues or rewards? The incentive salience hypothesis suggests that the level of “wanting” triggered by a reward stimulus is not merely a static function of the stimulus, or its association with reward. Instead, incentive salience is dynamically generated by mesocorticolimbic circuits at the moment of cue encounter, and differences in the state of mesocorticolimbic circuits (including in central amygdala opioid and mesolimbic dopamine circuits) when cues are encountered determines the level of motivation generated (Berridge 2001; Zhang et al. 2009). In this way, the same stimulus can yield different levels of reward “wanting” on different occasions, depending on mesocorticolimbic state at the time of cue encounter. Therefore, understanding how amygdala and related mesocorticolimbic systems modulate the incentive salience of encountered cues and rewards is a major concern for understanding normal motivation, as well as aberrant motivation in addiction (Robinson and Berridge 1993; Zhang et al. 2009).
Amygdala and related circuits constitute an anatomical crossroads for translating stimulus perceptions and learned Pavlovian associations into focused appetitive and aversive motivation (Ambroggi et al. 2008; Everitt et al. 2003; Gabriel et al. 2003; Ishikawa et al. 2008; LeDoux 2000). Previously, we showed that stimulation of μ-opioid receptors within the central amygdala nucleus (CeA) enhanced appetitive and consumption-like behaviors directed at one of two available previously learned cues in an autoshaping (sign tracking) task, suggesting that CeA opioids enhance and focus “wanting” selectively for the best available CS+ (Mahler and Berridge 2009). Opioid signals therefore appear important to amygdala’s role in translating learning into motivation.
Our hypothesis here was that μ-opioid stimulation in amygdala, especially in CeA, enhances peaks of “wanting” to obtain a UCS reward in a focused, associative fashion at moments when Pavlovian reward cues are encountered. Likewise for UCS percepts, opioid stimulation enhances and focuses “wanting” toward a particular UCS reward when innate reward stimuli are encountered. Accordingly, the central amygdala contains high levels of endogenous enkephalin, and expresses μ-opioid receptors on both presynaptic and postsynaptic neurons (Chieng et al. 2006; Finnegan et al. 2005; Kang-Park et al. 2009; Wilson et al. 2002; Zhu and Pan 2005).
Regarding the generation of incentive motivation, lesion studies have shown that CeA is necessary for transforming reward cues into motivation in tasks including autoshaping and single-reward Pavlovian to instrumental transfer (PIT; Hall et al. 2001; Holland and Gallagher 2003). In contrast to CeA, the basolateral amygdala (BLA) nucleus is more necessary for a food cue to elicit eating (Petrovich et al. 2002), and for tracking associative specificity and stimulus identity when multiple cue/reward associations are involved, such as in multiple-reward PIT and specific devaluation studies (Corbit and Balleine 2005; Johnson et al. 2009; Shiflett and Balleine 2010). Additionally, BLA opioid transmission modulates new learning about the cognitive incentive value of rewards (Wassum et al. 2009, 2011). However, the roles played by opioid elevations in CeA versus BLA in translating prior learning about reward cues into motivation for associated reward are largely unexplored.
An important feature of the incentive salience hypothesis is that cues trigger transient surges in “wanting” for associated rewards, especially when mesocorticolimbic systems are highly reactive (Berridge 2001; Bindra 1978; Toates 1986). Such cue-triggered reward “wanting” can be specifically measured in animal experiments with the PIT paradigm (Balleine 1994; Estes 1943; Hall et al. 2001; Holland and Gallagher 2003; Peciña et al. 2006; Walker 1942; Wyvell and Berridge 2000, 2001). Therefore, we examined here whether μ-opioid stimulation of CeA or BLA would enhance instrumental reward seeking in PIT. Next, we asked whether CeA opioid stimulation could flexibly target enhanced incentive motivation toward different categories of rewards (food or sex), depending on which stimuli were present. Finally, we asked whether CeA opioid stimulation effects were restricted to incentive salience aspects of motivation (“wanting”), or instead also increased reward hedonic impact or “liking” (as opioid stimulation is known to do in nucleus accumbens and ventral pallidum hedonic hotspots), by measuring effects of (d-Ala2, N-MePhe4, Gly-ol)-enkephalin (DAMGO) in CeA or BLA on the palatability of sweet or bitter stimuli in a taste reactivity paradigm.
Taken together, results of these experiments indicate a specific role for CeA μ-opioid circuits in targeting incentive salience upon the most relevant available reward-associated stimuli. This modulation by CeA opioids of cue-triggered motivation likely evolved to help direct appetitive behavior toward appropriate rewards, but it may also create vulnerability to cue-triggered addictive urges.
Materials and methods
All procedures were approved by the University of Michigan’s IACUC
Subjects
Male and female Sprague–Dawley rats (n = 98), 250–450 g were single- or double-housed under a reverse light/dark cycle, in 23 × 20 × 45 cm transparent tub cages (Ancare, Bellmore, NY) with bedding and unrestricted water and food (except during PIT training and testing, when they were slightly food-restricted to 20 g chow/day).
Surgical procedures
Rats were anesthetized with ketamine (80 mg/kg), xylazine (7 mg/kg), and atropine (0.04 mg/kg) prior to surgery, and recovered for 7 days before behavioral testing. To prevent infection, 0.1 ml of penicillin was administered s.c. at the completion of surgery, and once again 48 h later.
Intracranial cannulae
Bilateral 23 gauge, 14 mm cannulae (anchored with screws and dental acrylic) were aimed 2 mm dorsal to targeted points within CeA (placements varied among rats between −1.8 to −3.0 AP; ±3.4 to ±4.6 ML; and −5.8 to −6.5 DV) or BLA (−1.8 to −3.2 AP; ±4.6 to 5.2; -8.2 to 9.2 DV; Fig. 1). Additional animals were implanted with cannulae in anatomical control sites, centered in the endopiriform cortex (−1.6 AP; ±5.6 ML; -9.1 DV), the interstitial nucleus of the posterior limb of the anterior commissure (−1.0 AP; ±3.8 ML; -8.0 DV), or dorsal striatum (−1.8 AP; ±4.6 ML; -7.0 DV). These control sites were chosen since they are immediately adjacent to CeA and BLA, where microinjected drugs could have diffused to produce behavioral effects.
Amygdala in context. Microinjections of the μ-opioid agonist DAMGO (0.05 and 0.1 μg) or vehicle were placed in either the central nucleus (CeA) or basolateral nucleus (BLA) of amygdala (shown in coronal, sagittal, and horizontal planes). Top panels show behavioral causation maps for DAMGO amplification of CS+ −triggered peaks of “wanting” in PIT (orange > ~3× elevations in PIT compared to vehicle; white = no change). Microinjection sites for individual animals are represented by hexagonal symbols proportional in radius to CeA DAMGO Fos plumes, color-coded for intensity of cue-triggered “wanting” enhancement in a PIT test in individual rats (0.1 μg DAMGO vs. vehicle; see Fig. 5 for more details). The bottom panel shows the placement of CeA and BLA in respect to other mesolimbic reward structures that were Fos activated by DAMGO microinjected into CeA or BLA (see results for details; VTA not Fos-activated by CeA or BLA DAMGO). Map background inset shows the background used for mapping Figs. 3, 4, 5 and 6, stained for Substance P to help identify boundaries of amygdala nuclei. NAc nucleus accumbens (core and shell), VP ventral pallidum (rostral and caudal), IPAC/BNST interstitial nucleus of the posterior limb of the anterior commissure/bed nucleus of the stria terminalis, LH lateral hypothalamus, VTA ventral tegmental area
Intra-oral cannulae
Taste reactivity rats were also implanted in the same surgery with bilateral oral cannulae (PE-100 tubing) to allow for intraoral infusions of sucrose or quinine solutions. Oral cannulae were inserted lateral to the first maxillary molar, threaded behind the zygomatic arch, and exited through the dorsal head where they were cemented to skull screws (Berridge et al. 1984; Grill and Norgren 1978).
Ovariectomy
Female rats used as sexual stimuli were ovariectomized in a dorsal procedure, and were allowed two weeks for recovery and clearance of residual gonadal hormones before exogenous hormonal priming and testing.
Drugs and microinjections
DAMGO (Sigma) was dissolved in an artificial cerebrospinal fluid (ACSF) vehicle to a dose of 0.05 or 0.1 μg/0.2 μl/side. These doses were chosen based on our prior report than 0.1 μg/0.5 μl DAMGO enhanced the “motivational magnet” properties of reward cues in an autoshaping task (Mahler and Berridge 2009). On test days, rats were gently hand-held while they received bilateral, syringe pump-driven microinjections of DAMGO or vehicle over 60 s via injector cannulae (29 gauge) extending 2 mm beyond guide cannulae into target sites. Microinjection cannulae were left in place for 60 additional seconds to allow for drug diffusion.
PIT procedures
A timeline of PIT procedures is shown in Fig. 2, top panel.
Instrumental, Pavlovian, and extinction training in Pavlovian to instrumental transfer. a Pavlovian to instrumental transfer timeline: The timeline of instrumental, Pavlovian, and extinction training, as well as PIT testing and other procedures are shown progressing from left to right. b Instrumental training: Animals were trained to press one of two available levers to receive sucrose on an increasing variable interval schedule (3 days fixed ratio 1, 3 days each variable interval (VI) 5, 15, 30, 45 s schedule). No discrete cues were presented during instrumental training. c Pavlovian CS+ training: In the next phase of training, animals were trained to associate 30 s auditory cues (tone, white noise, or click; randomized across animals) with delivery of three sucrose pellets at the end of the cue. No levers were present during Pavlovian training. Over the course of 8 training days, animals showed many more anticipatory entries into the food cup in the 30 s of the cue (total entries during all four cue presentations/session shown), compared to the 30 s prior to each cue. d Single instrumental extinction session: Finally, one instrumental extinction session was performed prior to PIT testing, to reduce the number of lever presses driven by cognitive expectations or other cue-independent factors during subsequent PIT tests
Apparatus
Chambers were 30.5 × 24.1 × 21.0 cm, with steel front and back plates, and clear plastic sides, ceiling, and floor, enclosed in a sound attenuating box with ventilation fans to mask external noise. A red house light was mounted on the top of the back wall, which was lit during all training sessions. Two retractable levers were present on either side of the front of the chamber, which were extended at the beginning of instrumental training, instrumental extinction, and PIT testing sessions. A sucrose delivery cup (food cup) was located between the levers near the floor of the front of the box, in which an infrared beam was incorporated to measure the number of head entries in the 30 s prior to, and during 30 s CS presentations. Sucrose pellets, rather than liquid sucrose were used for comparison of these findings with previous PIT studies in our lab (Wyvell and Berridge 2000, 2001). Tone, white noise, and clicker CS stimuli were presented diffusely throughout the chamber from speakers near the back of the box (opposite the levers and food cup) during Pavlovian training and PIT testing. A computer equipped with MED-PC software (Med Associates, Inc.) controlled all events and recorded lever presses and food cup entries, and a video camera under the box allowed video analysis of lever interactions and other behaviors.
Habituation
PIT rats (n = 47) were handled for 3 days, then given 20, 45 mg sucrose pellets/rat in their home cages prior to training. On training day 1, sucrose pellets were delivered into the food cup on a variable time 60 s (VT-60) schedule for 20 min, to habituate rats to taking sucrose from the cup.
Instrumental training
Rats were then trained to press one of two available levers for sucrose pellets over 15 daily 30 min sessions, working up to a variable interval (VI)-45 s schedule (days 1–3: fixed ratio-1 (FR1), days 4–6: VI-5, days 7–9: VI-15, days 10–12: VI-30, days 13–15:VI-45; Fig. 2B). Pressing on the other lever delivered no rewards.
Pavlovian training
Following instrumental training, levers were removed from the chambers, and rats received twelve 30 min Pavlovian training sessions. Cues (CS+ and CS- randomly assigned) consisted of 30 s presentations of a 2.9 kHz tone or white noise pulsing at 0.5 Hz, or a 2 Hz click, followed by delivery of three sucrose pellets (CS+), or nothing (CS-). Rats received 4 CS+/sucrose pairings/day for 8 days (Fig. 2C), then an additional 4 days of CS+/CS- discrimination training, in which they received alternate presentations of their CS+ and CS- four times each.
Surgery and retraining
After Pavlovian training, rats were implanted with bilateral cannulae into CeA (n = 13), BLA (n = 11), or control sites (n = 23). They then were given three additional VI-45 instrumental “refresher” training sessions to ensure maintenance of instrumental behavior, followed by one 30 min instrumental extinction session to reduce cue-independent sucrose lever pressing on subsequent PIT tests (lever presses yielded no rewards; Fig. 2C).
PIT testing
Rats received 3 PIT test sessions in which excitatory effects of the Pavlovian CS+ on lever pressing was measured. The CS+ and CS- stimuli were presented intermittently four times each, separated on average by 3 min. Lever pressing and food cup entries were measured during the 30 s CS+ and CS- presentations, and during the 30 s baseline periods before each cue. Rats received microinjections of DAMGO (0.05 and 0.1 μg) and vehicle in counterbalanced order, 15 min before PIT tests.
Sexual incentive test
Naive male rats were implanted with bilateral cannulae aimed at the CeA (n = 8) or control sites (n = 3), then habituated to a testing chamber for 10 min (in the absence of females) for 2 days. The CeA was chosen based on incentive salience enhancements from CeA stimulation in the experiments above, in order to assess whether increases in food “wanting” extend also to sexual “wanting”. Eight ovariectomized females were used as sexual and control stimuli, and were separately habituated to the sex testing chamber. Since familiarity with the particular females used as estrous and control stimuli can affect males’ investigation of them, both estrous and nonestrous females were novel to each male on both of his test sessions. Before test days, females were either hormonally induced to estrus [β-estrodial 3-benzoate (50 μg/kg) 48 h before testing, and progesterone (2.5 mg/kg) 4 hours before testing (both in peanut oil vehicle)], or not induced (oil injections only). Estrus induction was later confirmed in females via examination of vaginal cell morphology following testing (Becker et al. 2005). Estrous and nonestrous females were enclosed in perforated plastic containers (19 × 11.5 × 11.5 cm; Rubbermaid) on either side of a larger, Plexiglas testing chamber (61 × 29 × 46 cm). All chambers contained bedding, but no food or water. Males received counterbalanced DAMGO (0.1 μg) and vehicle microinjections 15 min prior to each of two 30 min test sessions. Females become sexually receptive for around 2 h starting 4 h after the progesterone injection in this procedure, so testing sessions began at this point (Becker et al. 2005; Nocjar and Panksepp 2002). During each test session, males were able to freely explore both females, but not to contact or mate with either. Approaches, sniffs, and proximity to each female were recorded for subsequent analysis.
Taste reactivity procedures
A separate group of naive rats (n = 24) was implanted with bilateral CeA (n = 13), BLA (n = 9), or control site (n = 2) guide cannulae, and intraoral cannulae. Rats had four testing sessions, before which they received microinjections of DAMGO (0.1 μg/0.2 μl) or vehicle, followed by three 1 ml/min infusions of tastants (3 mM sucrose and 0.3 mM quinine) at 15 min intervals (15, 30, and 45 min after microinjection). Animals received sucrose or quinine on separate, counterbalanced days to avoid cross-contamination of tastants. Orofacial reactions to sucrose and quinine tastes were recorded for later analysis.
Food intake and general behavioral testing procedures
Rats in PIT, sexual incentive, and taste reactivity experiments were also examined for DAMGO effects on food intake and other behaviors. All intake test sessions were held in clear tub cages with bedding and unrestricted food and water, on separate days from other behavioral tests (except in the case of taste reactivity rats). PIT rats underwent three, 1 h food intake tests conducted 15 min after counterbalanced vehicle and DAMGO (0.05 and 0.1 μg) microinjections (these rats received six total microinjections). Sexual incentive rats underwent two food intake tests (0 and 0.1 μg DAMGO; four total microinjections). Taste reactivity rats were transferred from taste reactivity chambers into food intake testing chambers 1 h after microinjections. Chow intake and other behaviors were then measured for 1 h in all cases.
Behavioral video analyses
All video analyses were scored in slow motion (1/10th to ½ actual speed) by observers blind to experimental conditions. For PIT testing sessions, lever and food cup looks (orientation of the head toward the lever or cup, and the nose coming within 1 cm of it but not touching), rearing, bouts of corner sniffing, and sudden orientation shifts (sudden movement of the head and body at least 90° within 1 s) were coded for the 30 s before, and 30 s of each CS+ and CS- presentation.
In food intake tests, scored behaviors included time spent eating, number of initiations of eating behavior, food sniffing initiations, time drinking, number of drinking bouts, front–back cage crosses, and rears.
For sexual incentive tests, scored behaviors included the time spent on the same half of the large testing chamber as either the estrous or nonestrous female, the number of sniffs directed at each female (nose within 1 cm of female cage with concurrent vibrissae movement), time in contact with either female’s cage, and the number of grooming bouts.
Hedonic, aversive, and neutral taste reactivity response patterns were scored using time bin scoring procedures developed to assess hedonic vs. aversive taste valuations (Berridge 2000; Berridge et al. 1984), with Observer software (Noldus, Netherlands). A time bin scoring procedure was used to ensure that taste reactivity components of different relative frequencies were balanced in their contributions to the final affective hedonic/aversive totals (Berridge 2000). Hedonic responses included rhythmic midline tongue protrusions, lateral tongue protrusions, and paw licks. Aversive responses included gapes, head shakes, face washes, forelimb flails, and chin rubs. Neutral responses, which are less consistently linked to hedonic/aversive taste valuation, included passive dripping of solution out of the mouth, ordinary grooming, and rhythmic mouth movements without tongue protrusions. Individual totals were calculated for hedonic vs. aversive categories for each rat by adding all response scores within an affective category for that rat. Hedonic “liking” was defined as the sum of scores for lateral tongue protrusions, rhythmic tongue protrusions, and paw licks. Similarly, aversive “disliking” was the sum of gapes, head shakes, face washes, forelimb flails, and chin rubs.
Fos measurements of DAMGO-induced local Fos plumes and distant limbic activation
Behavioral causation maps were constructed to identify localization of opioid stimulation effects within amygdala and vicinity, showing anatomical patterns in DAMGO stimulation of PIT behavior. Similar behavioral causation maps were constructed to show anatomical patterns for DAMGO enhancement of food consumption and sexual incentive behaviors. However, repeatedly administering microinjections can reduce the impact of intracranial drugs and shrink observed Fos plumes, making it important to assess functional drug spread on the first microinjection in order to estimate maximum drug spread in behavioral tests (Richard and Berridge 2011). We therefore used an independent group of rats (microinjected under conditions similar to behaviorally tested rats on their first day of testing) to assess Fos plumes, used to provide information on diffusion of drug impact in behavioral causation maps. Rats in the Fos plume group (n = 16) were handled for 3 days, then microinjected bilaterally in the CeA or BLA with DAMGO (0.1 μg; CeA n = 6, BLA n = 4) or vehicle (n = 5; CeA n = 3, BLA n = 2) as described above, or handled equivalently for uninjected control rats (n = 4).
Analysis of DAMGO-induced Fos plumes of neuronal activation
Our procedure for measuring drug-induced Fos plumes immediately surrounding microinjection sites followed those described previously (Mahler and Berridge 2009; Mahler et al. 2007; Peciña and Berridge 2005; Smith and Berridge 2005). Briefly, brains were perfused in 4% paraformaldehyde, cryoprotected in 30% sucrose, and sliced at 40 μm. After blocking with a 3% normal goat serum/0.3% Triton-X solution for 2 h, they were incubated for 24 h in a 1:5000 polyclonal rabbit anti-Fos primary antibody (Sigma), then for 1 h each in a biotinylated goat anti rabbit IgG secondary antibody (Santa Cruz), and avidin–biotin–peroxidase for signal amplification (both at 1:200). Fos-like immunoreactivity was visualized with a nickel DAB reaction. DAMGO-induced Fos plumes were identified by comparison with equivalent sites after vehicle or no microinjections, and maps were created based on average moderate (2× uninjected levels) and intense (3×) Fos elevation zones. In all cases, Fos-like immunoreactive cells were quantified in Adobe Illustrator as those resembling cell nuclei in size and shape, and were at least 200% background levels of grey. Importantly, we do not suggest that Fos activation around injection sites (Fos plumes) are causal of behavioral effects of DAMGO, but only that intra-amygdala DAMGO induced both behavioral effects and local Fos expression.
Behavioral causation maps (Figs. 3, 4, 5 and 6) combined the plume diameter information obtained above (represented by symbol sizes) with PIT, food intake, taste reactivity, and sexual approach data from individual rats that were behaviorally tested. In all cases, symbol colors reflect the behavioral effect of DAMGO microinjection in a given rat at a given site (compared to vehicle microinjection in the same animal).
CeA opioid stimulation enhances and temporally focuses phasic bursts of cue-triggered “wanting”. a CeA, not BLA DAMGO increases PIT: Pavlovian to instrumental transfer (PIT) reveals the ability of a non-contingent Pavlovian cue for sucrose (an auditory CS+) to trigger phasic peaks of sucrose seeking, expressed as bursts of effort in pressing a lever that previously earned sucrose pellets. DAMGO (0.1 μg) microinjection in CeA increased these baseline-relative peaks of sucrose seeking (left) but not in BLA (right). *p < 0.05, CeA vehicle vs. 0.1 μg DAMGO. b CeA DAMGO reduces non cue-related instrumental sucrose seeking: DAMGO (0.1 μg) in CeA (left) but not BLA (right) decreased total active lever presses during both CS- presentations (white lines) and baseline periods when no cues were present (grey lines), compared to vehicle days. Total active lever pressing during the CS+ (black lines) was not affected by CeA or BLA DAMGO. #p < 0.05, vehicle vs. 0.1 μg DAMGO for baseline periods; *p < 0.05, vehicle vs. 0.05 and 0.1 μg DAMGO for CS- periods. c CeA DAMGO reduces CS+ triggered food cup approaches: CeA DAMGO (0.1 μg) reduced CS+ −triggered food cup approaches (a behavior which competes with instrumental sucrose seeking during the CS+). Percent change in time spent in the food cup during CS+s, compared to the baseline periods immediately prior are shown. d CeA DAMGO-induced “wanting” comes and goes with the CS+: After vehicle or DAMGO (0.1 μg) in CeA or BLA, CS+ presentations phasically stimulated bouts of sucrose seeking. The CS-, which never was previously associated with sucrose, did not elicit comparable bursts of pressing. Intra-CeA DAMGO did not increase absolute levels of sucrose seeking during CS+ periods, but instead focused sucrose seeking more exclusively to CS+ periods. This demonstrates that CeA DAMGO primarily focused incentive salience “wanting” into periods when the sucrose-associated cue was present, at the expense of baseline and CS- sucrose seeking. In BLA, DAMGO had no effect on pressing during CS+, CS-, or baseline periods. Figures show m(SEM). DAM DAMGO (0.1 μg/0.2 μl), Veh ACSF vehicle
Functional spread of DAMGO impact around microinjection sites: a Fos plume sampling method: “Plumes” of increased Fos expression surrounding DAMGO microinjection sites were measured by counting Fos + neurons in 0.125 × 0.125 mm2 sampled at 0.125 mm intervals on eight arms extending away from the center of each microinjection site (compared to Fos expression at equivalent sites in uninjected tissue, and after control vehicle microinjections). Insets show examples of Fos expression at equivalent sites following no microinjection (normal), ACSF vehicle microinjection (Veh, 0.2 μl), or 0.1 μg/0.2 μl DAMGO microinjection. b Average plume sizes and examples of CeA and BLA Fos plumes: Mean (SEM) radii of 0.1 μg DAMGO Fos plumes were derived by comparison to vehicle microinjections, and uninjected normal tissue. Zones of >2× increase in Fos expression over normal tissue levels are shown in yellow, and zones of more intense >3× increase in Fos expression are shown in red. c, d Example CeA and BLA DAMGO plumes: DAMGO-induced Fos elevations over control vehicle microinjection levels are shown by dotted lines for >2× over vehicle, and dashed lines for >3× increases over vehicle microinjection levels of Fos. Robust Fos plumes were observed for DAMGO in CeA but only very small, vehicle-equivalent plumes in BLA. CeA Central amygdala, BLA basolateral amygdala, 1,2,3 layers of piriform cortex, BMA basomedial amygdala, MeA medial amygdala, STm amygdaloid portion of the bed nucleus of the stria terminalis, ACo cortical amygdala nucleus
Opioid stimulation of central amygdala, not basolateral amygdala (BLA), enhances cue-triggered “wanting”. PIT enhancement: DAMGO (0.1 μg) in CeA (left), but not BLA (right) focuses sucrose “wanting” tightly into periods when a Pavlovian cue is present (data from all animals with CeA or BLA cannulae shown, rat-by-rat placements shown below). In the lower panel, DAMGO-induced increases in PIT are mapped for each individual rat’s microinjection site in CeA, BLA, or control sites to show the intensity of DAMGO effects at different sites in CeA and BLA (PIT percent increase over baseline levels in sucrose lever pressing during 30 s CS+ presentations). Symbol color: Hexagonal symbol colors denote the intensity of PIT enhancement, calculated as the change from vehicle day in PIT for that rat (darker oranges indicate larger > ~3× PIT enhancement, while white indicates no change from vehicle). Symbol size: Inner hexagons represent the average diameter of 3× Fos enhancement over control levels, surrounded by semitransparent outer halos showing zones of 2× Fos enhancement (see “Materials and methods” or “Results” section for details). Bars along rostrocaudal and mediolateral axes show the average intensity of DAMGO PIT enhancement at each coordinate level [mean(SEM) increase from vehicle day; AP vs. ML dimensions: each level 0.4 mm wide and centered on the labeled coordinate. Individual plume symbols could contribute to more than one bar when they straddle two levels). Medial–lateral coordinates for CeA and BLA are offset for display purposes. *p < 0.05, vehicle vs. 0.1 μg DAMGO
Opioid stimulation of central (but not basolateral) amygdala enhances food intake: Food UCS “wanting” enhancement: DAMGO (0.1 μg) in CeA (left), but not BLA (right) robustly enhances spontaneous chow intake and eating behavior in non food-deprived rats (individual placements shown below). Darker greens indicate > ~3× increase in food intake after DAMGO (compared to after vehicle microinjection), while white indicates no change. Intake enhancement map: The lower panel shows rat-by-rat increases in food intake after DAMGO microinjections in CeA or BLA (relative to vehicle control intake in the same rat), following identical symbol logic as above. *p < 0.05 vehicle vs. 0.1 μg DAMGO
Intra-amygdala DAMGO recruitment of distant reward structures
To explore the wider brain circuits recruited by DAMGO microinjections, we also examined Fos activation by CeA or BLA DAMGO (CeA n = 5, BLA n = 3) or vehicle (CeA n = 2, BLA n = 2) of 7 reward-related structures with substantial anatomical connectivity to CeA or BLA (which were not examined in a previous analysis of CeA DAMGO-induced circuit activation (Levine et al. 2004); medial accumbens shell, lateral accumbens shell, accumbens core, rostral and caudal ventral pallidum, bed nucleus of stria terminalis (BNST) and the adjacent interstitial nucleus of the posterior limb of the anterior commissure (IPAC), lateral hypothalamus (LH), and ventral tegmental area; Fig. 1). Since these measured regions are much further from injection sites (>1 mm) than the maximal spread of local Fos plumes ever extended, it is likely that Fos elevations were due to circuit interactions of CeA with these structures, rather than effects of DAMGO diffusing and directly inducing Fos.
Fos was counted by placing a microscope eyepiece grid (composed of 5 × 5, 0.05 × 0.05 mm boxes at 20× magnification) within the center of a structure of interest (e.g. dorsal medial accumbens shell), so that all four corners of the grid were entirely within the structure. Fos was then counted in the dorsomedial, dorsolateral, ventrolateral, ventromedial, and central boxes of the grid for each measured AP and DV level of each structure, so each sampling box was 0.15 mm apart mediolaterally and dorsoventrally. These sampling boxes were averaged for each region of each structure on each slice, and further averaged to yield a per hemisphere mean for that portion of each structure. At least two slices were sampled from each brain for each subregion of each unihemispheric structure of interest. Anatomical placement of sampling grids was determined by comparison of Fos-stained slices with adjacent Substance P-stained slices and the Paxinos and Watson brain atlas (2007).
Fos activation in nucleus accumbens core, medial shell, and lateral shell was measured for each structure in three rostrocaudal bins (coordinates relative to Bregma): rostral: +2.28–2.76 mm, medial: 1.68–2.16 mm, caudal: 0.84–1.32 mm; and orthogonally in dorsal (shell: -5.9 to −7.0 mm; core: -5.8 to −6.6 mm) and ventral levels (shell: -7.2 to −8.3 mm, core: -6.6 to −7.8 mm) for core and medial shell in each rostrocaudal bin. Ventral pallidum was sampled at 2 rostral and caudal levels (~Bregma +0.5 and −0.5 mm, respectively), while LH, interstitial nucleus of the posterior limb of the anterior commissure/bed nucleus of the stria terminalis (IPAC/BNST), and ventral tegmental area (VTA) were sampled at 3 rostrocaudal levels between 1.44 and 3.1 mm (LH), 0.5 and 0.0 (IPAC/BNST), and 4.9 and 5.5 mm (VTA) caudal of Bregma.
Statistics
Repeated measures ANOVAs were used to determine DAMGO effects on PIT, food intake, and taste reactivity. For the PIT experiment, multilevel repeated measures ANOVAs were used to examine effects of DAMGO (0, 0.05, and 0.1 μg) on increases from baseline levels of sucrose seeking during CS+ and CS- presentations. Additional repeated measures ANOVAs were employed to examine the specificity of PIT enhancements to cue periods (cue vs. baseline), to the CS+ cue in particular (CS+ vs. CS-), and to the sucrose lever (sucrose vs. control lever). Effects of DAMGO in CeA, BLA, and control structures were analyzed with separate ANOVAs. No significant differences were found between behavioral effects of DAMGO at different control sites outside amygdala (F values < 1.6, n.s.), so control sites were pooled for analyses. DAMGO effects on sucrose and quinine hedonic and aversive taste reactivity were examined with separate repeated measures ANOVAs. Food intake and other behavioral effects of DAMGO were examined with repeated measures ANOVAs and t-tests. In all cases, significance levels for t-test posthocs were determined via Bonferroni correction.
Results
PIT allows measurement of phasic, cue-locked Pavlovian sucrose seeking
In this version of PIT the task (Peciña et al. 2006; Wyvell and Berridge 2000, 2001), a noncontingent 30 s Pavlovian CS+ stimulus (which rats previously learned to associate with sucrose) is presented intermittently in the presence of a lever that previously earned sucrose pellets. The PIT procedure is designed to isolate enhancements due to incentive salience from alternative mechanisms such as enhanced stimulus–response (S–R) habits, global arousal, sustained expectations, or sucrose hedonic impact. Though no sucrose was delivered during PIT testing, CS+ presentations elicited phasic increases of instrumental sucrose seeking over baseline levels. Phasic increases were triggered by Pavlovian cues, but directed into motivated instrumental sucrose UCS seeking, and were potentiated by μ-opioid stimulation of CeA (but not BLA).
CeA opioid stimulation relatively enhances and focuses sucrose seeking into CS+ presentation periods
After vehicle microinjections into CeA, BLA, and control sites, presentation of 30 s Pavlovian sucrose cues (CS+s) stimulated PIT as phasic peaks of lever pressing, increased to 440% above pre-CS+ baseline pressing levels [main effect of period (baseline vs. during CS+) on previously sucrose-delivering lever presses: F(1,46) = 14.5, p < 0.001; lever (previously active vs. never active) × period interaction: F(1,46) = 13.9, p = 0.001; Figs. 3 and 5). After vehicle microinjection, CeA and BLA groups did not differ in degree of PIT behavior emitted [no interaction of cannulae site × period: F(2,46) = 0.9, n.s.]. These cue-triggered peaks in pressing lasted the duration of the CS+ presentations, and decayed back to baseline levels shortly thereafter. In contrast, CS- presentations did not elicit increased lever pressing [main effect of cue type: F(1,46) = 8.4, p < 0.01], indicating that sucrose seeking was specifically induced by the Pavlovian sucrose cue.
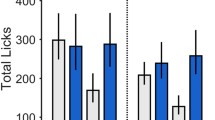
Amygdala opioid stimulation decreases hedonic “liking” reactions to sucrose. a Suppression of sucrose “liking”: DAMGO microinjection in CeA or BLA (0.1 μg) decreased hedonic orofacial responses to sucrose in a taste reactivity paradigm, compared to vehicle microinjections. b Taste reactivity components: Brain manipulations that affect hedonic affective reactions in a taste reactivity paradigm should similarly affect more than one type of reaction within the positive hedonic category. Positive hedonic reactions include midline rhythmic tongue protrusions (TPs), lateral tongue protrusions (LTPs), and paw licking (PL) behaviors, and more hedonically neutral reactions included mouth movements (MMs). The larger panel below shows rat-by-rat decreases in sucrose hedonic reactivity after DAMGO microinjections in amygdala (relative to vehicle day reactivity in the same animals), using identical symbol logic as above. Darker blues indicate suppression to < ~50% of vehicle baseline, while white indicates no change. *p < 0.05 vehicle vs. 0.1 μg DAMGO
DAMGO microinjections relatively enhanced PIT peaks in CeA but not in BLA [interaction of cannulae site × drug for percent increase from baseline pressing during the CS+: F(2,42) = 3.2, p = 0.05; CeA baseline lever pressing: mean(SEM) = 11.1(1.8), during CS+ pressing: m = 16.8(3.3); BLA: precue m = 9.6(1.9), during cue: m = 11.5(2.3)]. CeA microinjections of high dose DAMGO (0.1 μg) selectively potentiated cue-triggered peaks in instrumental seeking by >25% over vehicle levels, raising the relative increase of cue-triggered peaks of sucrose seeking to over 500% of precue levels; [main effect of CeA DAMGO: F(2,22) = 6.1, p < 0.01; 0.1 μg: t = 2.7, p < 0.05; Fig. 3, top panel). CeA DAMGO enhancements of phasic increases in pressing were directed nearly exclusively to the lever that had previously earned sucrose on training days (but not on PIT test days), and almost never to the control lever that had never yielded sucrose [active vs. control lever pressing during CS+: F(1,22) = 14.7, p < 0.001]. Increases induced by CeA DAMGO were also specific to the peaks seen in brief periods when the CS+ was present, and did not occur during the CS- [drug × CS type interaction: F(2,22) = 4.0, p < 0.05]. In contrast, CeA DAMGO reduced CS+ −triggered approach to the food cup below vehicle control levels [interaction of drug × interaction type (AL press vs. food cup entry): F(2, 20) = 3.9, p < 0.05; food cup entries during CS+ presentations; CeA: 0.05 μg: t = 2.4, p < 0.05; 0.1 μg: t = 1.0, n.s.:Fig. 3C]. This pattern suggests that DAMGO in CeA did not simply amplify all CS+ −triggered habitual behaviors, since the dominant Pavlovian S–R habit response that had previously been elicited by the CS+ during training trials was approach to the food cup.
This relative enhancement of phasic pressing on the active lever during the CS+ produced by CeA DAMGO microinjections was superimposed upon a more constant suppression of pressing at all other times in the PIT test (i.e., whenever CS+ was not physically present): during the control CS- tone that predicted nothing [main effect of DAMGO on CS- pressing: F(2,22) = 4.2, p < 0.05; 0.1 μg: t = 2.25, p < 0.05; Fig. 3B], and during precue baseline periods in the absence of any Pavlovian cue [measured 30 s immediately before each cue; F(2,22) = 5.2, p < 0.05; 0.05 μg: t = 2.1, p = 0.06; 0.1 μg: t = 2.7, p < 0.05; Fig. 3B]. This pattern of CeA DAMGO global suppression combined with relative enhancements during CS+ periods suggests a sharper temporal focusing of incentive salience, in which nearly all “wanting” was concentrated specifically into the brief 30 s periods when a physical CS+ was present.
During baseline periods in the absence of any CS, CeA DAMGO (0.05 and 0.1 μg) enhanced orientations toward the active lever, measured as turning of the head and body toward the lever with fixation for >1 s [F(2,22) = 4.4, p < 0.05; veh: m(SEM) orientations/cue presentation = 0.3(0.1); 0.05 μg: m = 0.9(0.2), t = 2.7, p < 0.05; 0.1 μg: m = 0.7(0.1), t = 2.3, p < 0.05], suggesting that animals still attended to and noticed the active lever in the absence of the CS+, though they failed to press it as often as when they received vehicle microinjections. CeA DAMGO did not alter most other behaviors emitted during noncue periods, including inactive lever pressing (which was always near zero; F(2,22) = 0.9, n.s.), spontaneous rearing (F(2,22) = 2.0, n.s.), orientation shifts (F(2,22) = 1.6, n.s.), or exploratory sniffing (F(2,22) = 0.03, n.s.), suggesting that DAMGO did not nonspecifically affect locomotor activity.
In short, CeA DAMGO dramatically changed the pattern of sucrose seeking across the PIT test session, without affecting general locomotor activity or general levels of attention to levers (in absence of CS+). In this way, CeA DAMGO relatively enhanced, or temporally focused incentive salience into moments when the Pavlovian reward cue (the sucrose-associated CS+) was present.
Localization of opioid incentive salience focusing to CeA, not BLA
Only microinjections of DAMGO centered in the CeA produced this relative enhancement and temporal focusing of sucrose “wanting”, while microinjections of DAMGO in the BLA did not (Fig. 5, top panel, and Fig. 3). BLA DAMGO failed to affect peaks in pressing during either the CS+ or CS- [no interaction of drug x cue type: F(2, 20) = 0.6, n.s.; no main effect on percent increase in lever pressing from baseline to CS+ [F(2, 20) = 1.2, n.s., Fig. 3A; no main effect on absolute lever pressing during CS+: F(2,20) = 0.4, n.s.; or CS-: F(2,20) = 1.0, n.s.; Fig. 3B]. BLA DAMGO also did not affect pressing during baseline periods in the absence of CSs [F(2,20) = 0.2, n.s.; Fig. 3B].
Finally, DAMGO microinjections also failed to potentiate PIT at control sites in structures outside the amygdala, including piriform cortex, interstitial nucleus of the posterior limb of the anterior commissure (IPAC), and dorsal striatum [No effect of drug on percent precue pressing: F(2,26) = 2.1, n.s.; Fig. 5]. No consistent differences between DAMGO effects at these different control structures were observed, and no enhancements of PIT observed for any control site (F values < 1.5, n.s.)
To more precisely confirm the localization of DAMGO effects to CeA, we produced behavioral causation maps of microinjection effects on PIT. These combined information on radius of functional drug spread within amygdala (obtained by measuring the diameters of “plumes” of increased Fos immunoreactivity around DAMGO microinjection sites) with information about the behavioral consequences in PIT tests caused by microinjections at each particular site (Fig. 5).
Central amygdala opioid stimulation in male rats enhances sexual incentive salience of an estrus female. a Sexual incentive testing procedure: CeA DAMGO (0.1 μg) microinjections in male rats enhanced investigational sniffs of an estrous female (confined by in a small compartment at one end of a large test chamber), but not of a nonestrous female (confined at the opposite end of the chamber), compared to vehicle levels in the same males. b CeA DAMGO enhances estrous female investigation: In CeA, but not nearby anatomical control sites, DAMGO enhanced investigation of an estrous, but not a control female. c Sexual salience enhancement map: Increases in male approaches and investigational sniffing of the estrous female after 0.1 μg CeA DAMGO microinjections are shown for each individual’s microinjection site (relative to vehicle day intake in the same animals), using identical graphical logic as above. Darker magentas indicate > ~3× increases in sexually directed motivated behavior from vehicle levels, while white indicates no change. *p < 0.05, vehicle vs. 0.1 μg DAMGO
Regarding radius of functional drug spread, DAMGO microinjection in CeA produced plumes of Fos elevation that were contained mostly within the borders of CeA, and did not spread substantially into BLA or other nearby structures. Specifically, 75% of the volume each CeA plume on average was contained within CeA boundaries (each plume filling about one sixth of CeA volume). Even for the largest plumes, only approximately 25% of Fos plumes extended beyond the borders of CeA, and even then the spread from CeA was usually not into BLA (presumably because of fiber tracts that separate the nuclei), but rather into other amygdala nuclei (such as medial amygdala), or into other adjacent structures such as dorsal striatum. The highest 0.1 μg/0.2 μl DAMGO dose produced a small intense Fos center of m = 0.12 mm radius (SEM = 0.05; volume = 0.007 mm3) that was more than triple the Fos intensity of vehicle control levels at similar sites near microinjections, surrounded by an outer halo of moderate (doubled) Fos elevation over vehicle control levels [radius = 0.31(+0.04) mm, volume = 0.12 mm3]. Center and halo together constituted a total plume radius of 0.3 mm—smaller than the plumes created by the same 0.1 μg DAMGO dose in more than a doubled microinjection volume, which produced a plume radius that averaged 0.47 mm (Mahler and Berridge 2009). Using our current lower volumes, the lower 0.05 μg/0.2 μl DAMGO dose here produced even smaller plumes than 0.1 μg DAMGO [radius = 0.16(0.04) mm, volume = 0.02 mm3; >2× control levels].
These small Fos plumes suggest that behaviorally effective CeA DAMGO microinjections acted almost exclusively within CeA. In contrast, DAMGO microinjections in BLA did not produce significant Fos elevations (Fig. 4). A lack of BLA DAMGO-induced Fos plumes might reflect any number of anatomical differences between CeA and BLA, including different localization of μ opioid receptors on presynaptic vs. postsynaptic sites (Finnegan et al. 2006; Zhu and Pan 2004, 2005), or other features of the glutamatergic cortex-type neurons of BLA, compared to the mostly GABAergic striatum-type neurons in CeA (Alheid 2003; Swanson 2003). However, given that CeA microinjections produced measurable Fos plumes, we extrapolated the size of BLA microinjection spread by assuming that DAMGO spread roughly equivalently through brain tissue in BLA and CeA. Therefore, based on a maximal spread radius of 0.3 mm, mapped onto the sites of BLA microinjections in behaviorally tested rats, we calculated that 75% of each BLA microinjection was likely to have been contained within BLA borders (each filling about 1/20th of BLA total volume). Only 25% of any BLA injection was estimated to have escaped from BLA to adjacent structures, and any spread outside BLA was most likely lateral into cortex rather than medially into CeA (again due to fiber tracts). This containment of impact may explain why BLA microinjections produced no appetitive enhancements, in contrast to CeA microinjections.
Radius information from Fos plumes was used only to assign the size of symbols in PIT enhancement maps. All other data shown in these maps (colors and columns) were obtained purely from data on behavioral consequences of DAMGO in PIT animals. Maps show PIT consequences produced by DAMGO microinjection at each site shown, compared to vehicle microinjection in the same rats.
UCS attractiveness: CeA (but not BLA) DAMGO enhances spontaneous feeding
To ask whether the incentive salience modulation in PIT observed after CeA DAMGO would translate into greater consumption of a food UCS when it was available, we tested the same rats used for PIT in subsequent free-intake tests for food pellet eating and water drinking after DAMGO (0, 0.05, and 0.1 μg). Spontaneous consumption of chow pellets was quintupled (>520%) by CeA 0.1 μg DAMGO microinjections, compared to vehicle levels [grams intake: F(2,22) = 3.7, p < 0.05; 0.05 μg: t = 1.1, n.s.; 0.1 μg: t = 4.4, p < 0.001], but no enhancement of intake was caused by DAMGO microinjections in BLA [grams intake: F(2,20) = 0.1, n.s.; Fig. 6]. DAMGO (0.1 μg) in CeA also doubled the number of feeding initiations, the total cumulative duration of feeding, and the number of sniffs of food pellets (0.1 μg; feeding initiations: t = 2.3, p < 0.05; time feeding: t = 2.8, p < 0.05; food sniffs: t = 2.3, p < 0.05; BLA: t values < 0.9, n.s.; Gosnell 1988; Levine et al. 2004; Mahler and Berridge 2009; Stanley et al. 1988). The lower 0.05 μg dose of DAMGO in CeA significantly enhanced only the number of food pellet sniffs to 140% of vehicle levels (t = 2.8, p < 0.05), though chow intake trended upward as well (t = 1.9, p = 0.07). In contrast, DAMGO in BLA failed to alter any food-related behaviors above vehicle levels at either dose (t values < 1.2, n.s.).
DAMGO never altered drinking behavior at any site or dose in either CeA or BLA (t values < 2.0, n.s.). General locomotor rears were increased by both doses of DAMGO in CeA, but not in BLA (0.05 μg: t = 2.2, p < 0.05; 0.1 μg: t = 3.6, p < 0.01; BLA: t values < 2.0, n.s.), and the 0.1 μg dose in CeA also increased cage-crossing locomotion (t = 3.1, p < 0.01). Time spent sleeping was reduced by DAMGO at the higher dose in both CeA and BLA (CeA: t = 3.3, p < 0.01; BLA: t = 3.0, p < 0.05), and also at the lower dose in CeA (t = 2.5, p < 0.05). At control sites in structures outside amygdala, DAMGO microinjection in piriform cortex, interstitial nucleus of the posterior limb of the anterior commissure, and dorsal striatum all failed to enhance food intake or eating behaviors, or to affect other behaviors (t values < 2.0, n.s.).
CeA DAMGO does not enhance hedonic reactions to sucrose, despite enhancing intake and PIT
CeA opioid enhancement of incentive salience, which increased cue-triggered sucrose seeking in PIT and consumption of food UCS in intake tests, might conceivably be accompanied by amplified taste hedonics or “liking” for sucrose reward. For example, hedonic enhancements (as well as motivational enhancements) are produced by opioid drug microinjections in hedonic hotspots within nucleus accumbens shell or ventral pallidum (Peciña and Berridge 2005; Shin et al. 2010; Smith and Berridge 2005; 2007; Smith et al. 2011). Alternatively, opioid microinjections could fail to enhance “liking” despite enhancing “wanting”, as at other sites in medial shell and in core of nucleus accumbens outside the hedonic hotspot (Peciña and Berridge 2005). To address whether DAMGO enhances the hedonic impact of sweetness, we assessed effects of DAMGO microinjections in CeA or BLA on orofacial affective reactions elicited by intraoral infusions of sucrose or quinine solutions. This taste reactivity paradigm measures drug-induced enhancements of hedonic impact across various species from mice to humans (Berridge 2000; Parker et al. 1992).
Intraoral sucrose normally elicits predominately positive hedonic reactions (e.g. tongue protrusions), with relatively few neutral mouth movements and hardly any aversive reactions (e.g., gapes; Berridge 2000). DAMGO microinjections in CeA or BLA failed to enhance any positive hedonic reactions to the taste of sucrose, but rather suppressed hedonic reactions by about 25% below vehicle levels [F(1,11) = 6.3, p < 0.05; Fig. 7]. Amygdala microinjections of DAMGO reduced levels of most reactions in the positive hedonic category elicited by sucrose [rhythmic tongue protrusions: F(1,18) = 6.2, p < 0.05; paw licks: F(1,18) = 9.9, p < 0.01; lateral tongue protrusions: F(1,18) = 2.25, p = 0.15], and simultaneously increased more affectively neutral mouth movements [F(1,18) = 40.7, p < 0.001; Fig. 7, top right]. Amygdala DAMGO failed to affect the already low number of aversive gapes, headshakes, chin rubs, forelimb flails, etc. elicited by sucrose [F(1,10) = 0.9, n.s.; individual aversive reactions: F values < 1.5, n.s.].
BLA DAMGO likewise suppressed positive hedonic reactions to sucrose, and produced no change in the (already low) level of negative aversive reactions to sucrose [hedonics: F(1,7) = 7.3, p < 0.05; aversion: F(1,7) = 0.3, n.s.; no difference in DAMGO effect in CeA vs. BLA for hedonics: F(1,18) = 0.21, n.s.; or aversion: F(1,18) = 0.9, n.s.], though BLA DAMGO did not alter PIT or food intake behavior, as described above. Infusions of a bitter quinine solution elicited high numbers of aversive reactions and few positive hedonic reactions in all cases, and neither CeA nor BLA DAMGO microinjections changed that aversive pattern [reactions to quinine; CeA aversive F(1,9) = 0.6, n.s.; hedonic F(1,7) = 2.5, n.s.; BLA aversive: F(1,7) = 1.0, n.s.; hedonic: F(1,7) = 0.3, n.s.]. Therefore, amygdala opioid stimulation in either CeA or BLA seems to specifically reduce the hedonic impact of sweet sucrose, without altering the aversive impact of bitter quinine.
Microinjections of DAMGO in anatomical control structures outside the amygdala, such as in the adjacent endopiriform cortex, did not appear to change hedonic or aversive reactions to either sucrose or quinine [F(1,1) = 1.0, n.s.; Fig. 7], though the low sample size (n = 2) requires future confirmation of site specificity for hedonic reduction effects of DAMGO in amygdala.
Finally, to verify that DAMGO enhanced “wanting” for food at the exact same sites in CeA where it suppressed “liking”, we again assessed food consumption in the same rats, in a free intake situation beginning 15 min after each rat’s taste reactivity test (i.e. 60–120 min after microinjections). CeA DAMGO microinjections again robustly enhanced food consumption, the number of feeding initiations, and the duration of feeding (grams intake: t = 2.4, p < 0.05; initiations: t = 3.8, p < 0.01; duration: t = 3.1, p < 0.01). Therefore, the very same CeA DAMGO microinjections that decreased “liking” reactions to sweetness still stimulated spontaneous food intake “wanting” in the same rats only moments later, providing a strong dissociation of CeA roles in motivation versus hedonic impact.
Sexual motivation: CeA DAMGO stimulates “wanting” of a sexual incentive stimulus
To assess whether the target of CeA opioid-induced incentive motivation could be flexibly shifted from ingestive to sexual by differences in the relative availability of UCS incentive stimuli encountered in an environment, we next tested whether CeA DAMGO-induced enhancements of incentive motivation could be flexibly moved between sex and food targets in the same rats. We focused on CeA in this experiment because only CeA had produced incentive salience enhancements for food in the PIT and intake experiments. First, a group of male rats received counterbalanced CeA microinjections of DAMGO (0.1 μg) and vehicle, and then were placed in a large chamber where they were free to investigate either an estrous female in a side compartment, or a nonestrous female in the opposite side of the three compartment chamber (or neither).
CeA DAMGO increased the number of sexual investigatory sniff behaviors directed toward the estrous female [F(1,7) = 8.6, p < 0.05; Fig. 8], as well as the duration of time spent in close proximity (<20 cm) to the estrous female [F(1,7) = 21.7, p < 0.01]. DAMGO did not increase the number of approaches to the nonestrous, social control female [F(1,7) = 1.1, n.s.], but instead reduced the time spent in proximity to her [F(1,7) = 5.8, p < 0.05]. DAMGO microinjections at anatomical control sites in adjacent brain regions (IPAC and dorsal striatum) failed to alter male behavior toward either female [F values ≤ 2.0, n.s.].
Then, to assess that the target of enhanced “wanting” could be flexibly shifted to a food incentive, the same males later underwent food intake testing after receiving DAMGO or vehicle microinjections (0.1 μg DAMGO and vehicle, counterbalanced). Intake tests were run as described above in the presence of chow pellets and water, but no females). Again, CeA DAMGO enhanced food intake (grams intake: t = 2.7, p < 0.05). Food consumption was increased at the same sites where DAMGO had previously enhanced sexually appetitive behavior, confirming the targeting flexibility of CeA DAMGO-induced motivational enhancements.
Wider brain circuits recruited by CeA or BLA DAMGO microinjections
Wider mesocorticolimbic circuits extending outside amygdala must be recruited by CeA microinjections in order to cause changes in motivated behavior. To identify this circuit, we quantified Fos-like protein immunoreactivity in several distant brain structures after CeA or BLA microinjections (outside local plumes of Fos caused within amygdala; Fig. 1, bottom panel). Both unique activations, and overlaps in recruited circuitry were found after DAMGO in CeA versus BLA.
CeA unique pattern of distant Fos activation
DAMGO in CeA (but not BLA) increased Fos in the ventral half of the medial shell of nucleus accumbens to over 150% of control levels (CeA DAMGO: t 3.6, p < 0.05; BLA DAMGO: t = 2.1, n.s.).
BLA unique activations
BLA DAMGO (but not CeA DAMGO) increased Fos in the lateral shell of nucleus accumbens (t = 3.6, p < 0.01; CeA vs. BLA DAMGO effect: t = 3.1, p = 0.01), in the ventral core of nucleus accumbens (150% of vehicle, t = 2.5, p < 0.05), the caudal ventral pallidum (VP; t = 2.5, p < 0.05) and modestly in the LH (t = 2.1, p = 0.07; CeA vs. BLA: t = 2.6, p < 0.05).
Shared CeA and BLA activations
In other regions of nucleus accumbens, both CeA and BLA DAMGO increased Fos in the dorsal halves of the accumbens core and medial shell to over 170% of vehicle levels (CeA DAMGO vs. vehicle: dorsal shell: t = 3.4, p < 0.01, dorsal core: t = 3.0, p < 0.05; BLA DAMGO vs. vehicle: dorsal core: t = 5.1, p = 0.001; dorsal shell: t = 3.9, p < 0.01). In ventral pallidum, CeA and BLA DAMGO both enhanced Fos in the rostral half of the structure (rostral of Bregma: CeA DAMGO: t = 2.2, p < 0.05; BLA DAMGO: t = 3.4, p = 0.01). CeA and BLA DAMGO also increased Fos in pallidum-like components of the extended amygdala macrosystem that receive major inputs from CeA, namely, the interstitial nucleus of the posterior limb of the anterior commissure (IPAC) and the contiguous anterior portion of the bed nucleus of stria terminalis (BNST; IPAC/BNST; CeA: t = 2.8, p < 0.05; BLA: t = 3.1, p < 0.05).
In summary, CeA and BLA DAMGO recruited partly distinct, and partly overlapping, patterns of distant forebrain circuitry. CeA microinjections uniquely activated a ventral zone of the medial nucleus accumbens shell, and also activated a set of limbic circuitry including nucleus accumbens dorsal subregions, rostral ventral pallidum, and extended amygdala that was similarly recruited by BLA DAMGO microinjections. BLA DAMGO additionally activated a larger penumbra of sites including lateral accumbens shell, ventral accumbens core, caudal ventral pallidum, and LH.
Discussion
Here we demonstrated that stimulation of μ-opioid receptors in central nucleus of amygdala (CeA) helped enhance the incentive salience of learned and unlearned incentive stimuli encountered in a particular environment. In other words, CeA opioid stimulation selectively transformed conditioned and unconditioned incentive stimuli into more potent triggers of appetitive motivation, directed appropriately toward either food or sex rewards. In a PIT task, each presentation of a Pavlovian CS+ previously paired with sucrose (a reward cue) elicited a transient surge in “wanting”, reflected in larger increases from precue levels in instrumental responding on a lever that formerly earned sucrose reward. Mu opioid stimulation of CeA [but not of BLA] temporally focused instrumental sucrose seeking into brief periods when the CS+ was present, magnifying the relative increase from baseline to peak, while actually reducing seeking at other times when the CS+ was absent. In food intake and sex tests, we showed that CeA μ-opioid stimulation also robustly increased the incentive salience of innate unconditioned stimuli (UCSs) themselves. CeA DAMGO microinjections stimulated food investigation and intake when chow was present, and sexually appetitive approaches and investigation when a pheromone-emitting potential sexual partner was instead present. Enhancements of appropriately directed levels of “wanting” triggered by a reward CS+ or UCS is consistent with our previous report that CeA DAMGO microinjections enhanced and focused CS+ “wanting” on a single prepotent cue in a “winner take all” fashion when two cues were available in an autoshaping/sign tracking paradigm (Mahler and Berridge 2009).
However, despite these motivational enhancements, neither CeA nor BLA amygdala opioid stimulation enhanced hedonic “liking” of rewards in a taste reactivity test. Instead, DAMGO microinjections in CeA or BLA suppressed the number of positive hedonic orofacial reactions normally elicited by an intraoral sucrose taste (while leaving disgust reactions to a bitter quinine taste unchanged). This pattern indicates that CeA opioid stimulation specifically helps enhance or target “wanting” of the best available reward-related stimulus that is perceived in a situation, but does not accordingly increase the hedonic value or “liking” of a reward when it is actually received.
Amygdala and cues: directing incentive salience onto particular targets
The incentive salience hypothesis provides a useful framework for understanding the above pattern of effects. Reward UCSs and their Pavlovian reward cues are attractive and salient (Flagel et al. 2011; Hearst and Jenkins 1974; Holland 1977; Timberlake and Grant 1975), but the motivational power of such stimuli can vary across encounters. In PIT, cue-triggered surges in UCS-directed incentive motivation are measured as increases from baseline levels of pressing on a lever that previously delivered sucrose, triggered by presentations of an auditory CS+ that was temporally associated with the same sucrose reward.
The design of our PIT test helped rule out several alternative explanations for CeA DAMGO effects besides incentive salience modulation, such as increases in habit performance, arousal, cognitice expectations, or stress (Berridge 2001; Delamater and Holland 2008; Wyvell and Berridge 2001). First, for example, enhancement of stimulus–response habits cannot explain these results, because no stimulus–response habit ever existed between CS+ and lever pressing prior to the test (that is, the Pavlovian CS+ had never been paired with the instrumental act of pressing during any training session prior to the PIT test). In fact, the Pavlovian conditioned response most habitually associated with the CS+ in Pavlovian training sessions was approach to the food cup, but conditioned approaches to the food cup were never increased by DAMGO in the PIT test, and instead sometimes reduced. Second, our results cannot be explained by CeA opioid stimulation inducing any stable psychological state that drove lever pressing such as general arousal or stress, because such stable states would be expected to increase sucrose seeking regardless of cue presence. Instead, DAMGO in CeA channeled pressing into periods of cue presence, and actually suppressed pressing at other periods. Likewise, if CeA DAMGO bolstered stable cognitive predictions or expectations of reward, a similar sustained elevation in pressing should have resulted. Finally, CeA DAMGO could not have elevated “wanting” indirectly via enhancing “liking” for the hedonic impact of reward UCS, because no sucrose was actually delivered in the PIT test (and because CeA DAMGO actually suppressed sucrose “liking” in the taste reactivity test). Likewise, the absence of UCS delivery in PIT (under extinction conditions) prevented elevation of pressing via DAMGO-induced strengthening of associative stamping-in of response reinforcement, or of incentive learning about the outcome via retasting sucrose under DAMGO [a cognitive form of incentive learning about hedonic impact which may dissociate from core “liking” reactions (Wassum et al. 2009, 2011)].
When such alternatives are ruled out, incentive salience modulation remains as nearly the only explanation left for the PIT effects observed here. Specifically, CeA opioid stimulation seemed to focus incentive salience exclusively upon the CS+ that had a prior Pavlovian association with the reward UCS, fueling its ability to trigger peaks of Pavlovian incentive motivation for sucrose in a cue-locked manner.
It would be of interest for future experiments to examine CeA DAMGO effects in a reward-specific PIT procedure with multiple CS+s and UCSs. Our single reward PIT design is most related to “reward-general” PIT, but animals can also be trained in a “reward-specific” PIT task, where instrumental presses on different levers are associated with different rewards, and where distinct Pavlovian cues predict these different rewards (Blundell et al. 2001; Corbit and Balleine 2005; 2011; Corbit and Janak 2010; Pielock et al. 2011). These procedural variations may invoke somewhat different psychological processes and neural substrates. For example, reward-specific PIT is blocked by lesions of the BLA and nucleus accumbens shell, while reward-independent PIT is blocked by lesions of CeA or nucleus accumbens core (VTA is required for both types of PIT; Corbit and Balleine 2005, 2011; Corbit et al. 2007). Future studies using a reward-specific PIT paradigm would help elucidate additional features of incentive salience when directed towards a particular sensory-specific target.
Incentive salience of unconditioned rewards
CeA opioid stimulation also increased incentive salience that was flexibly directed toward perceived food or sex UCSs, just as it targeted incentive salience to a learned Pavlovian CS+ (Mahler and Berridge 2009). Enhancement of UCS “wanting” could be directed toward either food, or a sexually receptive potential partner—depending upon which UCS was encountered after CeA DAMGO microinjection. In the same rats, CeA DAMGO enhanced food investigation and consumption when chow was present, but instead increased sexually directed approaches and investigatory sniffs when an estrous female was present. Conversely, DAMGO decreased investigation of a nonestrous female that was present with the estrous female, indicating a focused enhancement of the sexual motivation rather than a general facilitation of all social affiliation. Therefore, we conclude that CeA DAMGO enhances incentive salience in a manner that does not indiscriminately “float all motivational boats” by increasing appetitive behavior toward all stimuli equally, but rather helps target incentive motivation selectively toward the most salient reward-related stimulus available at the moment.
Dynamic interaction of brain state and cue presence in generating incentive salience
The incentive salience hypothesis posits that enhancements in cue-triggered “wanting” such as those described here reflect a synergistic interaction between CS+/UCS presence and the mesocorticolimbic brain state at the moment of encounter (Zhang et al. 2009). This interaction determines the intensity of motivation triggered by a stimulus, while the stimulus itself controls the directional focus of motivation. Here, both CeA opioid stimulation and CS+ or UCS presence were required for maximum levels of motivation to occur. In PIT, this synergy between cue presence and CeA opioid state manifests itself as temporally phasic, cue-triggered surges of sucrose seeking. CeA DAMGO enhancement of peaks of incentive motivation over baseline levels came and went with CS+ presence, owing to an interaction between CeA μ-stimulation (which lasted most or all of a session) and the phasic reward cue (which came and went across the session, phasically triggering incentive salience). In food intake and sex tests, directional targeting of incentive salience was seen as an interaction between CeA opioid state and the nature of available UCS rewards (sight and smell of food, versus a potential sex partner).
Related incentive salience enhancements involving interactions between CS presence and mesocorticolimbic reactivity have been reported for “wanting” produced by stimulation of mesolimbic dopamine-related circuits (Smith et al. 2011; Tindell et al. 2005; Wyvell and Berridge 2000, 2001; Zhang et al. 2009), which our CeA DAMGO microinjections may well have recruited (Ahn and Phillips 2003; Phillips et al. 2008). Such motivational enhancements have been modeled computationally by Zhang and colleagues (2009) for incentive salience generated by Pavlovian cue encounters: \( \widetilde{V}({s_t}) = \widetilde{r}({r_t}\kappa ) + \gamma V({s_{{t + 1}}}) \). Our PIT results can be interpreted in terms of this model by positing that CeA DAMGO (and the wider limbic circuitry recruited by CeA DAMGO microinjections, including nucleus accumbens, ventral pallidum, and extended amygdala) increases a mesolimbic “gain” factor K that modulates incentive salience [V(S t)] triggered by CS+s that have stable, learned values. We propose that K was specifically elevated in PIT to levels >1 by CeA μ-opioid stimulation, multiplying the r t value of the learned CS+ (reflecting the CS+ values prior association with sucrose) above its normal level. This K > 1 gain factor amplifies the ability of the cue to trigger motivation for the associated UCS reward, and therefore increases the UCS-directed incentive salience generated during cue presentations (Zhang et al. 2009). For CeA DAMGO-induced UCS “wanting”, an unlearned motivational value inherent in the smell and sight of food or of the sexual partner could take the place of r t and be similarly enhanced. In this way, CeA DAMGO-induced K elevation would similarly amplify that innate UCS value to generate enhanced V(S t) incentive salience, also produced at the moment of UCS encounter.
Hedonic “liking” for sweet UCS is not enhanced, despite enhanced “wanting”
Opioid-generated enhancement of food “wanting” is sometimes accompanied by enhanced hedonic impact of palatable tastes (including “liking” reactions to sucrose), at least when μ-agonist microinjections are located within “hedonic hotspots” in nucleus accumbens or ventral pallidum (Baldo and Kelley 2007; Kelley et al. 2002; Peciña and Berridge 2000, 2005; Shin et al. 2010; Smith et al. 2010). CeA receives gustatory inputs from the pontine parabrachial nucleus and insular cortex (Kita and Arita 1996; Norgren 1976; Pitkanen 2000), raising the theoretical possibility that CeA DAMGO microinjections might also have amplified the hedonic impact of sweetness. However, we found that opioid stimulation of CeA or BLA never increased hedonic palatability, but instead reliably decreased positive “liking” reactions to sucrose taste at both sites. This CeA suppression of the hedonic impact of an otherwise pleasant taste is especially striking because only moments later, the same CeA DAMGO microinjections enhanced food intake in the same rats. This “wanting” without “liking” pattern is similar to that produced by DAMGO microinjections in nucleus accumbens (outside a cubic-millimeter dorsomedial hedonic hotspot) and related structures including caudal or ventral regions of medial accumbens shell, accumbens core, dorsal striatum, and BNST (Difeliceantonio and Berridge 2010; Jackson 2009; Peciña and Berridge 2005, 2008; Smith and Berridge 2007). It may also be relevant that CeA lesions do not disrupt “liking” reactions in rat taste reactivity studies (even when “wanting” of the same target is disrupted) further supporting a role for CeA in “wanting” rather than “liking” (Galaverna et al. 1993; Rana and Parker 2008). Finally, neuroimaging results also support the idea that human amygdala activation correlates better with subjective ratings of desire to eat than with ratings of food liking (Small et al. 2008).
BLA versus CeA roles
Like the CeA, the basolateral nucleus of amygdala (BLA) is known to be important for many aspects of Pavlovian fear and reward learning. For example, BLA lesions block feeding induced by Pavlovian cues (Holland and Gallagher 2003; Holland and Petrovich 2005) or by opioid stimulation of nucleus accumbens (Parker et al. 2010; Will et al. 2004, 2009). In addition, BLA is required for tracking stimulus identity when multiple CS-US associations are present, and for updating the cognitive incentive value of previously learned rewards (Corbit and Balleine 2005; Di Ciano and Everitt 2005; Hatfield et al. 1996; Kantak et al. 2002; Pickens et al. 2003; Whitelaw et al. 1996). BLA opioid transmission is also necessary for contexts to increase alcohol seeking (Marinelli et al. 2010). Wassum and colleagues (2009, 2011) have further shown that naloxone microinjections in BLA block new learning about increases in the cognitive incentive value of rewards, whereas BLA DAMGO conversely prevented learning about decreases in incentive value.
Unlike in CeA, opioid stimulation of BLA here did not enhance “wanting” triggered by a food reward CS+ or UCS. There are several possible explanations for this finding. First, there are important neurobiological differences between BLA and CeA. BLA contains mostly glutamatergic neurons, projects directly to frontal cortex, nucleus accumbens, and hippocampus, and occupies a cortex-level role in wider cortico-striato-pallidal and extended amygdala macrosystems (Alheid 2003; Heimer 2008; Heimer and Van Hoesen 2006; Pitkanen 2000; Swanson 2003, 2005; Zahm 2006). By contrast, CeA contains primarily GABAergic neurons, and occupies a striatal-level role in cortico-striato-pallidal and extended amygdala macrosystems (Alheid 2003; Heimer and Van Hoesen 2006; Pitkanen 2000; Swanson 2005; Zahm 2006). Such neurobiological differences might interact with opioid elevation in CeA vs. BLA to produce quite different effects on incentive salience. CeA also seems most crucial for recruiting reward-related increases in nucleus accumbens dopamine release (Phillips et al. 2008), which would be important for incentive salience generation (Berridge and Robinson 1998).
Second, some differences between experiments examining BLA roles in stimulus-triggered motivation may also partly reflect the difference between “sufficient causation” for intense increases in motivated responses versus “necessary causation” for normal baseline levels of motivated behavior. That is, neurochemical stimulation experiments such as ours aim to identify neural substrates where activation is sufficient for amplifying incentive salience to levels high above normal. By contrast, lesion or antagonist experiments aim to expose substrates necessary for normal levels of a behavior, where absence induces deficits below normal levels.
Perhaps most importantly, BLA and CeA are likely to contribute different associative/ motivational functions to reward-related behavior. For example, Pavlovian cues trigger representations of both the affective/ motivational value of the UCS, as well as of the UCS sensory identity (Dickinson and Dearing 1979; Konorski 1967). CeA appears best able to translate previously learned affective associations into intense incentive motivation for relevant cues and rewards in the moment of cue re-encounter, and may also be particularly important for generating incentive values for innate UCS rewards. By contrast, BLA may mediate more specific associative representations of stimulus identity, new learning, tracking of multiple associations, and integrating cognitive representations of a reward with experiences that update the reward’s cognitive incentive value.
Relevance to addiction and other appetitive disorders: what and when to “want”?
Excessive peaks of cue-triggered motivation to pursue or consume rewards pose a problem for drug addicts, compulsive binge eaters, gamblers, or individuals with other addictions. Cravings often occur upon exposure to conditioned stimuli, but the intensity of triggered urges can depend upon the particular state one is in when the cue is encountered (e.g. hungry, stressed, or drug-abstinent). “Wanting” can also be quite directional and target-dependent, rather than projected towards every available reward at once. Drug addicts may want only drugs, or even one drug above all other drugs (Flanagan 2011), just as a binge eater may want to eat a very particular type of food at the moment of temptation. We suggest that opioid activation in CeA circuits is an important brain component of brain circuits that amplify and target stimulus-triggered incentive salience. CeA stimulation by DAMGO microinjection may thus mimic neurobiological states of dysfunction that occur spontaneously in addiction and related compulsive pursuit disorders, and which drive excessive peaks of focused desire. By increasing mesocorticolimbic gain via amygdala-related circuits, the motivational impact of certain encountered CSs or UCSs may be dynamically amplified, producing focused surges of incentive salience or “wanting” to obtain and consume the relevant reward.
References
Ahn S, Phillips AG (2003) Independent modulation of basal and feeding-evoked dopamine efflux in the nucleus accumbens and medial prefrontal cortex by the central and basolateral amygdalar nuclei in the rat. Neuroscience 116:295–305
Alheid GF (2003) Extended amygdala and basal forebrain. Ann N Y Acad Sci 985:185–205
Ambroggi F, Ishikawa A, Fields HL, Nicola SM (2008) Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron 59:648–661
Baldo BA, Kelley AE (2007) Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacol (Berl) 191:439–459
Balleine B (1994) Asymmetrical interactions between thirst and hunger in Pavlovian-instrumental transfer. Q J Exp Psychol B 47:211–231
Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673
Berridge K (2001) Reward learning: reinforcement, incentives, and expectations. In: Medin DL (ed) Psychology of Learning and Motivation. Academic Press, pp 223–278
Berridge KC (2000) Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24:173–198
Berridge KC, Flynn FW, Schulkin J, Grill HJ (1984) Sodium depletion enhances salt palatability in rats. Behav Neurosci 98:652–660
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Bindra D (1978) How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav Brain Sci 1:41–91
Blundell P, Hall G, Killcross S (2001) Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci 21:9018–9026
Chieng BC, Christie MJ, Osborne PB (2006) Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 497:910–927
Corbit LH, Balleine BW (2005) Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci 25:962–970
Corbit LH, Balleine BW (2011) The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci Off J Soc Neurosci 31:11786–11794
Corbit LH, Janak PH (2010) Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur J Neurosci 31:1312–1321
Corbit LH, Janak PH, Balleine BW (2007) General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci 26:3141–3149
Delamater AR, Holland PC (2008) The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol Anim Behav Process 34:202–222
Di Ciano P, Everitt BJ (2005) Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. Eur J Pharmacol 526:186–198
Dickinson A, Dearing MF (1979) Appetitive-aversive interactions and inhibitory processes. In: Dickinson A, Boakes R (eds) Mechanisms of learning and motivation. Lawrence Erlbaum Associates, Hillsdale, NJ, pp 203–231
Difeliceantonio AG, Berridge KC (2010) Neostriatal sites of mu opioid stimulation enhance CS motivational magnets. Society for Neuroscience, San Diego, CA
Estes WK (1943) Discriminative conditioning I: a discriminative property of conditioned anticipation. J Exp Psychol 32:150–155
Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW (2003) Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci 985:233–250
Finnegan TF, Chen SR, Pan HL (2005) Effect of the mu opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther 312:441–448
Finnegan TF, Chen SR, Pan HL (2006) Mu opioid receptor activation inhibits GABAergic inputs to basolateral amygdala neurons through Kv1.1/1.2 channels. J Neurophysiol 95:2032–2041
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H (2011) A selective role for dopamine in stimulus-reward learning. Nature 469:53–57
Flanagan O (2011) What is it like to be an addict? In: Poland J, Graham G (eds) Addiction and responsibility. MIT Press, Cambridge, MA
Gabriel M, Burhans L, Kashef A (2003) Consideration of a unified model of amygdalar associative functions. Ann N Y Acad Sci 985:206–217
Galaverna OG, Seeley RJ, Berridge KC, Grill HJ, Epstein AN, Schulkin J (1993) Lesions of the central nucleus of the amygdala. I: Effects on taste reactivity, taste aversion learning and sodium appetite. Behav Brain Res 59:11–17
Gosnell BA (1988) Involvement of mu opioid receptors in the amygdala in the control of feeding. Neuropharmacology 27:319–326
Grill HJ, Norgren R (1978) The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279
Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ (2001) Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci 13:1984–1992
Hatfield T, Han JS, Conley M, Gallagher M, Holland P (1996) Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci 16:5256–5265
Hearst E, Jenkins HM (1974) Sign tracking: the stimulus-reinforcer relation and directed action. In: Society P (ed), Austin, Tx
Heimer L (2008) Anatomy of neuropsychiatry: the new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Boston Academic Press/Elsevier, Amsterdam
Heimer L, Van Hoesen GW (2006) The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev 30:126–147
Holland PC (1977) Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Process 3:77–104
Holland PC, Gallagher M (2003) Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci 17:1680–1694
Holland PC, Petrovich GD (2005) A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav 86:747–761
Ishikawa A, Ambroggi F, Nicola SM, Fields HL (2008) Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience 155:573–584
Jackson ED (2009) The Extended Amygdala in Appetitive Motivation for Reward: Role of the Bed Nucleus of the Stria Terminalis Psychology. University of Michigan, Ann Arbor, MI
Johnson AW, Gallagher M, Holland PC (2009) The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. J Neurosci 29:696–704
Kang-Park MH, Kieffer BL, Roberts AJ, Roberto M, Madamba SG, Siggins GR, Moore SD (2009) Mu-opioid receptors selectively regulate basal inhibitory transmission in the central amygdala: lack of ethanol interactions. J Pharmacol Exp Ther 328:284–293
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22:1126–1136
Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M (2002) Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76:365–377
Kita I, Arita H (1996) Afferent projections to the central nucleus of the amygdala, evoking depressor response, tachycardia and vocalization in rat. Neurosci Res 25:229–239
Konorski J (1967) Integrative activity of the brain: an interdisciplinary approach. University of Chicago Press, Chicago, Il
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Levine AS, Olszewski PK, Mullett MA, Pomonis JD, Grace MK, Kotz CM, Billington CJ (2004) Intra-amygdalar injection of DAMGO: effects on c-Fos levels in brain sites associated with feeding behavior. Brain Res 1015:9–14
Mahler SV, Berridge KC (2009) Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29:6500–6513
Mahler SV, Smith KS, Berridge KC (2007) Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32:2267–2278
Marinelli PW, Funk D, Juzytsch W, Le AD (2010) Opioid receptors in the basolateral amygdala but not dorsal hippocampus mediate context-induced alcohol seeking. Behav Brain Res 211:58–63
Nocjar C, Panksepp J (2002) Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res 128:189–203
Norgren R (1976) Taste pathways to hypothalamus and amygdala. J Comp Neurol 166:17–30
Parker KE, McCall JG, Will MJ (2010) Basolateral amygdala opioids contribute to increased high-fat intake following intra-accumbens opioid administration, but not following 24-h food deprivation. Pharmacol Biochem Behav 97(2):262–266
Parker LA, Maier S, Rennie M, Crebolder J (1992) Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci 106:999–1010
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier, Amsterdam
Peciña S, Berridge KC (2000) Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863:71–86
Peciña S, Berridge KC (2005) Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25:11777–11786
Peciña S, Berridge KC (2008) Incentive salience mediation by opioid versus dopamine in nucleus accumbens shell and core: amplified cue-triggered ‘wanting’ for reward Society for Neuroscience. Society for Neuroscience, Washington, DC
Peciña S, Schulkin J, Berridge KC (2006) Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol 4:8
Petrovich GD, Setlow B, Holland PC, Gallagher M (2002) Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci 22:8748–8753
Phillips AG, Vacca G, Ahn S (2008) A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav 90:236–249
Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G (2003) Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci 23:11078–11084
Pielock SM, Lex B, Hauber W (2011) The role of dopamine in the dorsomedial striatum in general and outcome-selective Pavlovian-instrumental transfer. Eur J Neurosci 33:717–725
Pitkanen A (2000) Connectivity of the rat amygdaloid complex. In: Aggleton J (ed) The amygdala: a functional analysis. Oxford University Press, Oxford, U.K., pp 31–116
Rana SA, Parker LA (2008) Differential effects of neurotoxin-induced lesions of the basolateral amygdala and central nucleus of the amygdala on lithium-induced conditioned disgust reactions and conditioned taste avoidance. Behav Brain Res 189:284–297
Richard JM, Berridge KC (2011) Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci Off J Soc Neurosci 31:12866–12879
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Shiflett MW, Balleine BW (2010) At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci 32:1735–1743
Shin AC, Pistell PJ, Phifer CB, Berthoud HR (2010) Reversible suppression of food reward behavior by chronic mu-opioid receptor antagonism in the nucleus accumbens. Neuroscience 170:580–588
Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F (2008) Separable substrates for anticipatory and consummatory food chemosensation. Neuron 57:786–797
Smith KS, Berridge KC (2005) The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 25:8637–8649
Smith KS, Berridge KC (2007) Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27:1594–1605
Smith KS, Berridge KC, Aldridge JW (2011) PNAS Plus: Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA
Smith KS, Mahler SV, Pecina S, Berridge KC (2010) Hedonic hotspots: Generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC (eds) Pleasures of the brain. Oxford Univ, Press, Oxford, UK, pp 62–73
Stanley BG, Lanthier D, Leibowitz SF (1988) Multiple brain sites sensitive to feeding stimulation by opioid agonists: a cannula-mapping study. Pharmacol Biochem Behav 31:825–832
Swanson LW (2003) The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci 985:174–184
Swanson LW (2005) Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol 493:122–131
Timberlake W, Grant DL (1975) Auto-shaping in rats to the presentation of another rat predicting food. Science 190:690–692
Tindell AJ, Berridge KC, Zhang J, Pecina S, Aldridge JW (2005) Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci 22:2617–2634
Toates F (1986) Motivational Systems. Cambridge University Press, Cambridge, UK
Walker KC (1942) Effect of a discriminative stimulus transferred to a previously unassociated response. J Exp Psychol 31:312–321
Wassum KM, Cely IC, Balleine BW, Maidment NT (2011) Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosc Off J Soc Neurosci 31:1591–1599
Wassum KM, Ostlund SB, Maidment NT, Balleine BW (2009) Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A 106:12512–12517
Whitelaw RB, Markou A, Robbins TW, Everitt BJ (1996) Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacol (Berl) 127:213–224
Will MJ, Franzblau EB, Kelley AE (2004) The amygdala is critical for opioid-mediated binge eating of fat. NeuroReport 15:1857–1860
Will MJ, Pritchett CE, Parker KE, Sawani AM, Ma H, Lai AY (2009) Behavioral characterization of amygdala involvement in mediating intra-accumbens opioid-driven feeding behavior. Behav Neurosci 123:781–793
Wilson MA, Mascagni F, McDonald AJ (2002) Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett 328:160–164
Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130
Wyvell CL, Berridge KC (2001) Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci 21:7831–7840
Zahm DS (2006) The evolving theory of basal forebrain functional–anatomical ‘macrosystems’. Neurosci Biobehav Rev 30:148–172
Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW (2009) A neural computational model of incentive salience. PLoS Comput Biol 5:e1000437
Zhu W, Pan ZZ (2004) Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience 127:871–879
Zhu W, Pan ZZ (2005) Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 133:97–103
Acknowledgements
These experiments were funded by DA015188 and MH053649 to KCB, and DA021481 to SVM. We thank Stephen Chang and Brianne Dzwonek for assistance with behavioral testing and scoring, Michelle DiMondo and Phillip Hoberg for assistance with immunohistochemistry, Jill Becker for advice on estrus induction, and anonymous reviewers for helpful suggestions on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahler, S.V., Berridge, K.C. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology 221, 407–426 (2012). https://doi.org/10.1007/s00213-011-2588-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2588-6