Abstract
Studies reviewed here implicate the extended amygdala in the negative affective states and increased drug-seeking that occur during protracted abstinence from chronic drug exposure. Norepinephrine (NE) and corticotropin-releasing factor (CRF) signaling in the extended amygdala, including the bed nucleus of the stria terminalis, shell of the nucleus accumbens, and central nucleus of the amygdala, are generally involved in behavioral responses to environmental and internal stressors. Hyperactivity of stress response systems during addiction drives many negative components of drug abstinence. In particular, NE signaling from the nucleus tractus solitarius (NTS) to the extended amygdala, along with increased CRF transmission within the extended amygdala, are critical for the aversiveness of acute opiate withdrawal as well as stress-induced relapse of drug-seeking for opiates, cocaine, ethanol, and nicotine. NE and CRF transmission in the extended amygdala are also implicated in the increased anxiety that occurs during prolonged abstinence from chronic opiates, cocaine, ethanol, and cannabinoids. Many of these stress-associated behaviors are reversed by NE or CRF antagonists given systemically or locally within the extended amygdala. Finally, increased Fos activation in the extended amygdala and NTS is associated with the enhanced preference for drugs and decreased preference for natural rewards observed during protracted abstinence from opiates and cocaine, indicating that these areas are involved in the altered reward processing associated with addiction. Together, these findings suggest that involvement of the extended amygdala and its noradrenergic afferents in anxiety, stress-induced relapse, and altered reward processing reflects a common function for these circuits in stress modulation of drug-seeking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major obstacle in drug addiction recovery is the susceptibility to relapse, even after extended periods of abstinence (O’Brien 1997). Compulsive drug-seeking and relapse may be driven by several factors, including the aversive and anxiogenic nature of acute withdrawal and protracted abstinence, increased positive and negative reinforcing properties of drugs, and external triggers such as stressors and drug-paired stimuli.
Several of these factors involve stress or anxiety, and stress-related structures in the brain, particularly the extended amygdala, have been implicated in multiple aspects of addiction. The extended amygdala is an anatomically and neurochemically interconnected system in the basal forebrain that consists of the bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and shell of the nucleus accumbens (NAc-Sh) (Heimer et al. 1993). This system has been pinpointed as having an important role in several stress-related components of drug withdrawal, and is a site where corticotropin-releasing factor (CRF) and norepinephrine (NE) transmission seem to be critical for stress effects in addiction.
Brain CRF and NE systems are implicated in stress responses both in healthy individuals (Bale and Vale 2004; Heinrichs and Koob 2004; Koob 1999a; Koob and Heinrichs 1999; Morilak et al. 2005) and in people suffering from stress-related disorders including depression and anxiety (e.g. post-traumatic stress disorder, panic disorder) (Arborelius et al. 1999; Bremner et al. 1996a, b; Clark and Kaiyala 2003; Risbrough and Stein 2006; Southwick et al. 1999; Strawn and Geracioti 2008). Due to their role in psychiatric illnesses, NE and CRF have been proposed to interact in several ways in response to chronic stress, including sensitization (via feed-forward circuits in which, e.g., CRF influences NE) and desensitization (Dunn and Swiergiel 2008; Dunn et al. 2004; Koob 1999a).
A role for the extended amygdala in stress is implicated by strong reciprocal CRF connections between CeA and BNST, CRF inputs to the extended amygdala from other amygdaloid and extra-amygdaloid structures, and NE innervation from brainstem nuclei (Berridge et al. 1997; Freedman and Cassell 1994; Hornby and Piekut 1989; Phelix et al. 1992, 1994; Sakanaka et al. 1986). Other afferents to the extended amygdala include basolateral amygdala (BLA), lateral hypothalamus (LH) and hippocampus, and efferent targets include ventral pallidum and LH (Alheid and Heimer 1988; Heimer et al. 1991). There are also reciprocal connections between the extended amygdala and the ventral tegmental area (VTA) (Carboni et al. 2000; Freedman and Cassell 1994; Georges and Aston-Jones 2001). VTA receives glutamatergic inputs from BNST, as well as CRF inputs from BNST, CeA, and paraventricular nucleus of the hypothalamus (PVN) (Georges and Aston-Jones 2002; Rodaros et al. 2007). Given the role of the VTA dopamine (DA) system in reward processing and effects of drugs of abuse (for review, see Fibiger and Phillips 1986; Wise 1978, 1996, 2004), these connections indicate that the extended amygdala may also play an important role in addiction.
The role of stress in addiction has been addressed in numerous reviews (Goeders 1997, 2003; Koob and Kreek 2007; Koob 1999b, c, 2003; Lu et al. 2003; Piazza and Le Moal 1996; Sarnyai et al. 2001; Weiss et al. 2001). Here, we have gathered a diverse set of literature and data that support a role for NE and CRF actions specifically in the extended amygdala in several stress-related components of addiction, ranging from symptoms of acute withdrawal to relapse during prolonged abstinence.
First, we discuss the well-defined role of NE inputs to the extended amygdala from A2 neurons in the caudal medulla (nucleus tractus solitarius; NTS) in the aversiveness of acute opiate withdrawal. We also review the role of NE and CRF in the enhanced anxiety that accompanies acute or protracted withdrawal from several drugs. Next, we review the extensive data supporting a role of NE and CRF signaling within the extended amygdala in stress-induced reinstatement of drug-seeking. The necessary circuitry for this reinstatement effect closely parallels that for the aversiveness of acute opiate withdrawal.
We then present data from our lab revealing an association between activation in the extended amygdala and altered reward processing during protracted drug abstinence. Fos expression in the extended amygdala and NTS is positively correlated with enhanced drug-seeking and negatively correlated with decreased food-seeking observed during protracted morphine abstinence. Our work indicates a role for the extended amygdala and its NE inputs in this changed reward-response profile, which plays an important role in the difficulty of maintaining drug abstinence in addicts. We review evidence indicating that NE affects responses to drug rewards via positive as well as negative reinforcement mechanisms. Finally, we discuss recent studies implicating connections between the extended amygdala and the orexin system in withdrawal and relapse.
These data reveal multiple ways in which the aversive and anxiogenic effects of abused drugs are associated with extended amygdala circuitry, including its NE inputs. Overall, these results support the view that changes in hedonia that accompany chronic drug exposure and withdrawal are related to anxiety and activation of stress circuitry in the extended amygdala.
Extended amygdala, norepinephrine and acute opiate withdrawal
NE has been implicated in addiction, and in particular in acute opiate withdrawal, for over a half-century (for review, see Aston-Jones et al. 1993; Maldonado 1997). Experimentally, NE neurons in locus coeruleus (LC) were found to be strongly activated by opiate withdrawal (Akaoka and Aston-Jones 1991; Aston-Jones et al. 1997; Ivanov and Aston-Jones 2001; Rasmussen et al. 1990). Clinically, the alpha-2 adrenoceptor agonist clonidine, which potently decreases activity of NE neurons as well as NE release, is used as an effective agent for reducing acute opiate withdrawal symptoms, indicating that the NE system may be involved in some of the adverse symptoms of opiate withdrawal (Gold et al. 1978).
Clinical findings were confirmed by animal studies showing that systemic administration of the beta adrenoceptor antagonists propranolol or atenolol reduced the somatic signs of opiate withdrawal induced by abstinence or by systemic administration of the opioid receptor antagonist naloxone in rats (Harris and Aston-Jones 1993a). Notably, propranolol or clonidine prevented the acquisition of a conditioned place aversion (CPA) for a withdrawal-paired environment, whereas atenolol caused only modest reductions in CPA (Harris and Aston-Jones 1993a; Kosten 1994). Atenolol has more limited access to the central nervous system following systemic administration, indicating a role for central adrenoceptors in the aversiveness of acute withdrawal.
A prime candidate for the central location of systemic propranolol and clonidine actions is ventral BNST, a structure with remarkably dense NE innervation (Fig. 1) (Phelix et al. 1992). Ventral and dorsolateral BNST showed altered neuronal activity after naltrexone-precipitated opiate withdrawal as evidenced by increased activation of the immediate early gene product Fos; this Fos response was markedly decreased by systemic propranolol (Aston-Jones et al. 1999). CeA and NAc-Sh also exhibited increased Fos after acute opiate withdrawal (Hamlin et al. 2004; Veinante et al. 2003; Walters et al. 2000). Notably, BNST, NAc-Sh, and CeA showed increased Fos following expression of opiate withdrawal CPA induced by doses of naloxone that are sub-threshold for somatic signs of withdrawal (Gracy et al. 2001). This indicates that different neural circuits may mediate the aversive and somatic symptoms of withdrawal (as do additional studies, summarized below).
Local microinjection studies confirmed that actions of NE in withdrawal involve the extended amygdala. Intra-BNST injections of beta adrenoceptor antagonists (betaxolol + ICI-118551 cocktail, or propranolol) or alpha-2 agonists (ST-91) reduced the CPA associated with naltrexone-precipitated withdrawal, with very little effect on somatic withdrawal (Fig. 2) (Aston-Jones et al. 1999; Delfs et al. 2000). This is additional evidence for independent circuitry governing the somatic and affective responses to withdrawal. Similar effects were observed with intra-CeA injections of beta-adrenoceptor antagonists (Watanabe et al. 2003). Retrograde tracer studies revealed that the majority of NE inputs to BNST and NAc-Sh come from the ventral noradrenergic bundle (VNAB) originating in the A2 region of NTS and the A1 in the caudal brainstem, with only little contribution from the dorsal noradrenergic bundle (DNAB) originating in LC (Aston-Jones et al. 1999; Delfs et al. 1998). BNST-projecting catecholaminergic neurons in A1 and A2 showed increased Fos expression following opiate withdrawal (Fig. 3) (Aston-Jones et al. 1999; Delfs et al. 2000; Stornetta et al. 1993). Additionally, neurochemical lesions of VNAB, but not DNAB, projections reduced CPA for opiate withdrawal, with no effect on somatic withdrawal (Aston-Jones et al. 1999). These data implicate NE neurons in NTS, but not LC, in the aversiveness of acute opiate withdrawal. These results are consistent with several other studies which found that lesions of LC or its NE efferents do not alter opiate withdrawal or hinder the ability of clonidine to reduce withdrawal effects (Britton et al. 1984; Caille et al. 1999; Chieng and Christie 1995). Although structures such as cortex, hippocampus, and thalamus receive NE inputs exclusively from LC, areas such as NAc, LH, and perifornical hypothalamus receive NE inputs primarily from A1 and A2, and numerous areas receive substantial NE from both sets of NE neurons, including parts of CeA and BNST (Aston-Jones et al. 2008).
Noradrenergic drugs infused into BNST blocked CPA while having minimal effects on somatic signs associated with acute opiate withdrawal. Effects of the beta antagonists betaxolol + ICI 118,551 (a, b) or propranolol (c, d), or the alpha-2 agonist ST-91 (e, f) on aversion scores and somatic withdrawal (TC teeth chattering; ET eye twitch; WDS wet dog shakes; JUMP jumping; WR writhing; PG penile grooming; PT paw tremor). Aversion score for the withdrawal-paired environment is the mean time in seconds spent in the withdrawal side minus the non-withdrawal side on test day. n = 6 − 8 animals per dose; * P < 0.05. Taken from Delfs et al. (2000)
BNST-projecting noradrenergic cells in NTS were stimulated by acute opiate withdrawal. The retrograde tracer WGA-gold was injected into BNST, and the A2 region of NTS was triple-labeled for WGA-gold (small black particles in cell body), Fos-related antigens (dark purple-black nuclei), and tyrosine hydroxylase (light brown). Arrows indicate triple-labeled cells. Similar results were seen in A1 and LC, but fewer cells were retrogradely labeled. XII hypoglossal nucleus; scale bar: 40 um. Taken from Delfs et al. (2000)
Mechanistically, NE acting in the extended amygdala may drive dysphoria by decreasing excitatory projections from the extended amygdala to its efferent targets, including VTA. This effect may be achieved via NE actions to increase GABA and decrease glutamate inputs onto excitatory VTA-projecting neurons in BNST. NE application to neurons in ventrolateral BNST (with a physiological profile like that of VTA-projecting neurons) was found to cause increased GABA(A)-IPSC frequency during acute opiate withdrawal (Dumont and Williams 2004). This effect was mediated through alpha-1 and beta adrenoceptor mechanisms, whereas NE effects were modulated only though alpha-1 mechanisms in naive animals (Dumont and Williams 2004). These findings are consistent with those of Delfs et al. (2000) which indicate that the aversiveness of opiate withdrawal is due to NE acting at beta adrenoceptors in BNST. NE has also been shown to inhibit glutamatergic transmission in ventral BNST, via alpha-2 adrenoceptors (Egli et al. 2005; Forray et al. 1999). In addition, modulation of targets such as extended amygdala by NE inputs might amplify affective valences normally represented there via postsynaptic modulatory effects on other inputs, as has been reported for NE in other target regions (e.g. cerebral cortex (Aston-Jones et al. 2008; Berridge and Waterhouse 2003)). NE’s effects to increase GABA input and decrease glutamate input to BNST neurons may reduce excitatory projections from BNST to VTA. VTA may be strongly influenced by the extended amygdala during acute withdrawal, as indicated by the finding that acute opiate withdrawal inhibited VTA DA neuron firing and decreased extracellular DA in NAc, and that pre-treatment with clonidine prevented these effects (Diana et al. 1995; Georges and Aston-Jones 2003; Pothos et al. 1991; Spanagel et al. 1994). This inhibition of VTA DA output may contribute to withdrawal effects, as indicated by the finding that D2 agonist injection into NAc decreased opiate withdrawal behaviors (Harris and Aston-Jones 1994). Similar decreases in VTA DA firing activity accompanied acute withdrawal from chronic ethanol, cocaine, and cannabinoids (Diana et al. 1992, 1993, 1998; Mateo et al. 2005; Rossetti et al. 1992), indicating that VTA DA may be an important recipient of extended amygdala projections during withdrawal.
The increased NE levels in BNST during acute withdrawal that drive these changes may be caused by activation of NE terminals within BNST and/or by increased activity of NE cells in NTS. The strong induction of Fos in BNST-projecting A2 neurons during opiate withdrawal indicates that the latter is likely involved (Delfs et al. 2000).
Increased NE in the extended amygdala during acute opiate withdrawal is also accompanied by increased signaling of the neuropeptide CRF, a transmitter prominently involved in anxiety and other behavioral responses to stress. Some of the strong reciprocal connections between BNST and CeA, as well as some of the extra-amygdaloid inputs to the extended amygdala, utilize CRF. Studies by Nakagawa et al. (2005) suggested that projections from CeA to BNST, in particular, are important for CPA. They found that bilateral excitotoxic lesions of CeA significantly reduced opiate withdrawal-induced Fos in BNST; however, bilateral lesions of BNST had no effect on withdrawal-induced Fos in CeA. Systemic or intra-CeA administration of CRF antagonists was also found to reduce CPA associated with acute opiate withdrawal. This, plus the above results for NE, signifies that both NE and CRF signaling in BNST and CeA are involved in the aversiveness of acute opiate withdrawal. (Heinrichs et al. 1995; Stinus et al. 2005). As described later, NE and CRF connections within BNST and CeA are importantly involved also in stress-induced reinstatement of drug-seeking. This indicates that the extended amygdala may be important for the aversiveness of acute withdrawal by virtue of its role in the response to stressors. It may also play a pivotal role in other stress-related behaviors associated with drug abstinence, such as anxiety, enhanced drug-seeking, and stress-induced relapse, as discussed in the following sections.
Extended amygdala and drug abstinence-induced anxiety
NE and CRF actions in CeA and BNST have dissociable effects on autonomic and behavioral responses to stressors. Autonomic responses to stressors in rodents include activation of the HPA axis, release of adrenocorticotropic hormone (ACTH) and corticosterone, and increased blood pressure and heart rate. Behavioral responses to stressors in rodents can be measured in a variety of well-validated (anxiolytic-responsive) anxiety paradigms, including the elevated plus-maze, open field, acoustic startle reflex, and the social interaction test, and are indexed by levels of exploratory or social behavior, freezing, startle, or vocalizations (Davis 1993; File 1990; Pellow et al. 1985; Prut and Belzung 2003; Rodgers 1997). The baseline anxiety response in these paradigms can be elevated by the introduction of an acute unconditioned stressor, such as footshock, light, immobilization, swim stress, or CRF itself, to give a measure of stress-induced (or stress-enhanced) anxiety. The amount of fear displayed by the animal can be measured via the introduction of a conditioned stressor, i.e. a stimulus that has been paired with an unconditioned stressor.
The BNST has been implicated specifically in anxiety, and the CeA in fear (Davis 2006; Walker et al. 2003). Inactivation of BNST was shown to block modulation of startle related to unconditioned stressors such as light, CRF, and footshock, and inactivation of CeA blocked modulation of startle related to conditioned stressors, such as fear (Gewirtz et al. 1998; Lee and Davis 1997; Walker and Davis 1997). This role for amygdala in fear conditioning and emotional learning has been confirmed in other animals and humans (Phelps and LeDoux 2005). Cecchi et al. (2002a) showed that some anxiety effects involve NE inputs to BNST, finding that blockade of alpha-1 or beta-1,2 adrenoceptors in ventrolateral BNST reduced immobilization stress-induced anxiety in the elevated plus-maze and stress-induced rises in plasma ACTH, with no effect in a social interaction test preceded by stress. Conversely, blockade of alpha-1 receptors in CeA reduced immobilization stress-induced social interaction effects, with no effect on stress-induced anxiety in the elevated plus-maze or plasma ACTH levels (Cecchi et al. 2002b). Blockade of CRF receptors in CeA also attenuated fear-induced freezing (Swiergiel et al. 1993). These results confirm that NE and CRF actions within BNST and CeA are involved in different specific components of stress and anxiety responses.
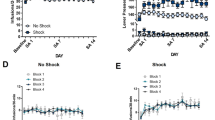
Anxiety and stress are major contributors to drug-craving and relapse in humans, as discussed in more detail in the following section (Childress et al. 1994; Kosten et al. 1986; Sinha 2001; 2007). In rats, elevated anxiety was seen during the first 48 h after withdrawal from chronic cocaine, opiates, ethanol, and cannabinoids (Basso et al. 1999; Harris and Aston-Jones 1993b; Rassnick et al. 1993a; Rodriguez de Fonseca et al. 1997; Sarnyai et al. 1995). Elevated anxiety persisted for at least two weeks of cocaine or morphine abstinence, and for at least six weeks of cocaine or ethanol abstinence (Harris et al. 2001; Harris and Aston-Jones 2001; Valdez et al. 2003). Following chronic cocaine or opiate exposure, increased withdrawal anxiety was observed in the defensive burying paradigm, which was alleviated by systemic administration of the beta antagonists propranolol and atenolol (Fig. 4) (Harris and Aston-Jones 1993b). The defensive burying paradigm is a widely used measure of stress-induced anxiety, in which the rat buries a probe after it receives a shock from the probe (Treit et al. 1981). This beta antagonist effect is consistent with the view that attenuation of the aversive aspects of acute opiate withdrawal by NE agents is due, at least in part, to actions in the ventral BNST and alleviation of elevated anxiety.
Beta adrenoceptor antagonists reduced the increased anxiety observed in the defensive burying paradigm 48 h after forced abstinence from chronic cocaine or morphine. Withdrawn animals showed a shorter latency to begin burying a shock-prod (a) and longer duration of burying (b) after chronic treatment with cocaine or morphine, as compared to chronic saline. Pre-treatment with propranolol (5 mg/kg) or atenolol (5 mg/kg) reduced this anxiety, as compared to saline pre-treatment. Asterisks denote statistically significant differences from saline/saline group; * P < 0.05; ** P < 0.01. Morphine/atenolol is significantly different from morphine/saline, P < 0.05; no other between group comparisons are different. Modified from Harris and Aston-Jones (1993b)
There is some evidence to suggest that the elevated anxiety observed after withdrawal is specific to stress-induced anxiety and does not occur in tests of baseline anxiety. This corresponds with a role for the extended amygdala in withdrawal-induced anxiety, as CeA and BNST are involved in the modulation of anxiety by conditioned or unconditioned stressors, as discussed above. Although some reports indicate that cocaine and ethanol withdrawal increased anxiety observed in the elevated plus-maze, other experiments found increased anxiety exclusively in the defensive buying paradigm (which includes a shock as a stressor to induce burying), or only if elevated plus-maze testing was preceded by restraint stress (Basso et al. 1999; Rasmussen et al. 2001; Rassnick et al. 1993a; Sarnyai et al. 1995; Valdez et al. 2002, 2003). In all of these cases, intracerebroventricular (i.c.v.) or intra-CeA administration of CRF antagonists reversed withdrawal-induced increases in anxiety (Basso et al. 1999; Rassnick et al. 1993a; Sarnyai et al. 1995; Valdez et al. 2003).
Alleviation of enhanced anxiety during withdrawal with either NE or CRF antagonists indicates that signaling in these systems may be amplified during withdrawal. In fact, increased levels of extracellular CRF in CeA have been measured following acute withdrawal from cocaine, ethanol, and cannabinoids (Merlo Pich et al. 1995; Richter and Weiss 1999; Rodriguez de Fonseca et al. 1997). Additionally, elevated levels of extracellular NE in ventral BNST were observed at least 48 h after naloxone-precipitated morphine withdrawal in rats, after somatic signs of withdrawal had dissipated, indicating that increased NE release in the extended amygdala continues to influence stress and anxiety systems in the brain for some time following acute withdrawal (Fuentealba et al. 2000). These data also indicate that acute and protracted abstinence may cause heightened anxiety due to exaggerated stress responses in the extended amygdala. Changes in the VTA DA system may be an important corollary of the increased NE and CRF levels in the extended amygdala during protracted abstinence. Reduced VTA DA firing was observed for at least 72 h following withdrawal from chronic ethanol or morphine, after physical symptoms had subsided (Diana et al. 1996, 1999). As noted above, decreased DA function may contribute to the aversiveness of the withdrawal experience.
Extended amygdala and stress-induced reinstatement of drug-seeking
Stress is a significant risk factor for opiate and cocaine relapse in humans (Kosten et al. 1986; Sinha et al. 2006). In the laboratory, personalized stress imagery, as well as drug-associated cues, caused significant increases in subjective ratings of craving and anxiety in cocaine, opiate, and alcohol abuse patients; cocaine and alcohol abusers also showed increased activation of the HPA axis and physiologic measures of stress (Fox et al. 2007; Hyman et al. 2007; Sinha et al. 1999, 2003). In addition, high-frequency abusers of cocaine or ethanol had significantly greater craving and anxiety, as well as HPA and cardiovascular responses, in response to stress and cue imagery as compared to low-frequency users, indicating that increased exposure might increase susceptibility to stress-induced relapse (Fox et al. 2005).
In animal models of stress-induced reinstatement of drug-seeking, animals are trained to self-administer a drug across several sessions and then given non-reinforced trials to extinguish the drug-seeking response. Intermittent footshock delivered in the operant self-administration chamber potently reinstates lever-pressing in the absence of drug reward; this is taken as a measure of drug-seeking (for review, see Lu et al. 2003; Shaham et al. 2000a; 2003). The work of several laboratories has shown that such reinstatement of drug-seeking is heavily dependent upon the extended amygdala in a manner very similar to the aversiveness of acute opiate withdrawal. Thus, inactivation of BNST, CeA, NAc-Sh, or VTA blocks footshock-induced reinstatement of cocaine-seeking (McFarland et al. 2004).
Systemic administration of alpha-2 agonists attenuated footshock-induced reinstatement of drug-seeking for cocaine, heroin, ethanol, and nicotine (Erb et al. 2000; Le et al. 2005; Shaham et al. 2000b; Zislis et al. 2007). Local administration of beta adrenoceptor antagonists into BNST reduced footshock-induced reinstatement of cocaine-seeking, whereas administration into CeA completely blocked reinstatement (Fig. 5a); neither had any effect on cocaine prime-induced reinstatement (Leri et al. 2002). Intra-BNST alpha-2 agonists also blocked footshock-induced reinstatement of a conditioned place preference (CPP) for morphine (Wang et al. 2001). Alpha-2 agonists had no effect on stress-induced reinstatement for opiates when injected into LC (which presumably inhibited LC impulse activity), but lesions of VNAB significantly inhibited stress-induced reinstatement (Shaham et al. 2000b; Wang et al. 2001). This points to the importance of NE innervation of the extended amygdala originating from A2 NE neurons in NTS (rather than LC) in stress-induced reinstatement of drug-seeking. Again, these results also point to the similarity in circuitries involved in the aversiveness of acute opiate withdrawal (reviewed above) and stress-induced reinstatement of drug seeking.
Stress-induced reinstatement was reduced by local administration of beta antagonists in BNST or CeA, or local unilateral administration of CRF antagonists in BNST combined with contralateral CeA inactivation. a Effects of the beta antagonists betaxolol + ICI 118,551 on footshock-induced reinstatement when infused locally into BNST (P < 0.05) or CeA (P < 0.001). Mean responses on the active and inactive lever during 3-h test sessions. Modified from Leri et al. (2002). b Effects of unilateral injection of CRF antagonists (D-Phe CRF12–41) into BNST and/or contralateral injection of TTX into CeA during 3-h test sessions preceded by footshock or no shock. Mean responses on the active and inactive lever. Only combined D + T injections effectively reduced reinstatement; * different from no shock, # different from D + T shock, P < 0.05. Taken from Erb et al. (2001)
Systemic or i.c.v. administration of CRF antagonists also reduced footshock-induced reinstatement of drug-seeking for cocaine, heroin, ethanol, and nicotine (Erb et al. 1998; Le et al. 2000; Shaham et al. 1997, 1998; Zislis et al. 2007). Systemic or i.c.v. administration of CRF antagonists blocked footshock-induced reinstatement of morphine CPP as well (Lu et al. 2000). Local administration studies showed that injections of CRF antagonists into BNST or VTA, and not amygdala, attenuated footshock-induced reinstatement of cocaine-seeking (Erb and Stewart 1999; Wang et al. 2005, 2007). Similarly, injections of CRF antagonists into BNST, and not amygdala or NAc, attenuated footshock-induced reinstatement of morphine CPP, although injections of CRF antagonists into amygdala and NAc, and not BNST, attenuated drug prime-induced reinstatement of morphine CPP (Wang et al. 2006). Erb et al. (2001) found that unilateral inactivation of amygdala via TTX combined with injection of a CRF antagonist into the contralateral BNST blocked stress-induced reinstatement of cocaine-seeking (Fig. 5b), demonstrating that CRF projections from CeA to BNST are critical for this stress-induced reinstatement.
Although there is substantial overlap in the circuitry governing acute withdrawal and stress-induced relapse, acute withdrawal does not drive drug-seeking (Shaham et al. 1996; Shaham and Stewart 1995). Lu et al. (2005) found that acute morphine withdrawal did not reinstate an extinguished morphine CPP; however, after repeated pairings of naloxone-precipitated withdrawal with the CPP chamber, re-exposure to the CPP chamber in a drug-free state elicited reinstatement, as well as increased blood corticosterone levels and some somatic signs of withdrawal. All of these cue-elicited responses were blocked by CRF antagonists (Lu et al. 2005). This indicates that withdrawal-associated cues, but not withdrawal itself, are able to drive relapse.
Although acute withdrawal has no effect on reinstatement, stress-induced reinstatement is heightened following protracted abstinence. Shalev et al. (2001) tested the ability of footshock to drive reinstatement after 1, 6, 12, 25, and 66 days of heroin withdrawal. Maximal responding was observed on days 6 and 12, but footshock was unable to drive reinstatement on day 1 of heroin withdrawal. Additionally, stress-induced reinstatement of drug-seeking for ethanol, cocaine, and heroin was heightened in animals made previously dependent or given extended access to daily self-administration (Ahmed et al. 2000; Liu and Weiss 2002; Mantsch et al. 2008).
Extended amygdala and enhanced drug-seeking associated with protracted abstinence
Prolonged exposure to drugs causes neuronal and behavioral changes that exist long after symptoms of acute withdrawal have dissipated. These alterations cause a variety of maladaptive effects, including increased anxiety, vulnerability to stress-induced relapse, and enhanced drug-seeking. A series of experiments from our laboratory have shown that post-dependent animals process reward-related stimuli in an aberrant manner, such that preference is increased for stimuli associated with drug, whereas stimuli associated with natural rewards elicit less preference than normal. For these experiments, rats were made dependent (via morphine pellets or daily cocaine injections) for two weeks and then subjected to abstinence withdrawal for 2–5 weeks. Post-dependent and drug-naive animals were then conditioned with drug- and vehicle-associated environments in a CPP paradigm. Non-conditioned control animals received unpaired drug injections in the home cage and received similar exposure to the CPP environment drug-free.
Enhanced drug-seeking during protracted abstinence Post-dependent animals showed similarly enhanced morphine CPP after 2–5 weeks of abstinence (Harris and Aston-Jones 2001, 2003b). Enhanced cocaine CPP was also found following two weeks of abstinence; longer abstinence periods have not been tested (Harris et al. 2001). This enhanced drug-seeking during protracted abstinence was accompanied by decreased CPP for food and novel objects (Fig. 6) (Harris and Aston-Jones 2003a, 2007; Harris et al. 2007). Previous studies have shown enhanced drug preference following prolonged drug exposure, but did not include a period of prolonged abstinence (Contarino et al. 1997; Lett 1989; Shippenberg and Heidbreder 1995).
Previously morphine-dependent animals (M) showed decreased CPP for food after 2–5 weeks of abstinence, as compared to non-dependent animals (P) (upper graph), and increased CPP for morphine after 5 weeks of abstinence (lower graph). Preference for food- or morphine- paired environments is expressed as the mean time in seconds spent in the rewarded side minus the non-rewarded side on test day. *P < 0.01. Taken from Harris and Aston-Jones (2003a)
NE and CRF signaling within the extended amygdala may become more heavily involved in drug-taking responses following a period of chronic drug exposure. CRF antagonists selectively decreased the increased self-administration of ethanol or cocaine observed in animals made dependent or given extended drug access, with no effects on non-dependent groups (Chu et al. 2007; Funk et al. 2006, 2007; Specio et al. 2007). Even after 3–5 weeks of abstinence, CRF antagonists reduced increased responding for ethanol in post-dependent animals only (Valdez et al. 2002). Wang et al. (2005) found that in cocaine-experienced rats, but not cocaine-naïve rats, CRF acquired control over local glutamate release in VTA and activated DA neurons, which was responsible for footshock-induced reinstatement. Similarly, the alpha-2 adrenoceptor agonist clonidine reduced morphine CPP in previously-dependent animals, with no effect in non-dependent animals (Nader and van der Kooy 1996).
Fos in extended amygdala associated with altered reward-seeking To determine whether the extended amygdala plays a role in the enhanced drug-seeking observed in CPP tests during protracted morphine abstinence, neuronal activity was investigated using immunohistochemistry for the immediate early gene protein Fos. For these experiments, animals were sacrificed 2 h following the CPP test session, so that Fos expression reflects re-exposure to, and response to, the drug-paired environment. Elevated Fos was observed in anterior cingulate, NAc core and shell, ventral BNST, BLA, CeA, LH, and NTS in post-dependent rats after 5 weeks of protracted abstinence, as compared to non-dependent and non-conditioned rats (Aston-Jones and Harris 2004; Harris and Aston-Jones 2003b; 2007). Fos in anterior cingulate and BLA correlated with CPP in all conditioned animals; however, Fos in BNST correlated with CPP in post-dependent rats only (Harris and Aston-Jones 2003b). This indicates that activation in BNST is uniquely associated with the elevated preference found during protracted abstinence. Importantly, there were no elevations in Fos for non-conditioned post-dependent animals in response to the CPP environment alone, indicating that the increased Fos following CPP in post-dependent rats was specific for exposure to drug-associated stimuli. These data, in view of NE’s strong innervation and effects in the extended amygdala, led our lab to hypothesize that drug-conditioned stimuli can activate A1 and A2 NE neurons that release NE into the extended amygdala. This was proposed to cause increased anxiety and produce negative reinforcement for drug rewards given during conditioning sessions. These negatively reinforcing effects were proposed to summate with positively reinforcing effects to increase motivation to seek out drugs (Aston-Jones and Harris 2004).
Animals also exhibited decreased food CPP during protracted morphine abstinence. As with morphine CPP, Fos in anterior cingulate and BLA correlated with food CPP for all conditioned animals. Decreased food CPP in post-dependent rats was accompanied by decreased Fos in NAc-Sh, LH, and BLA, as compared to non-dependent animals (Aston-Jones and Harris 2004; Harris and Aston-Jones 2007). These limbic areas showed increased Fos during enhanced morphine CPP, indicating that they are particularly important for the altered reward processing that occurs during protracted abstinence. These brain areas are all afferents to VTA, and all excitatory except for NAc-Sh, suggesting that VTA may receive decreased excitatory drive in relation to natural rewards during protracted abstinence. Fos was negatively correlated with food CPP in CeA, ventrolateral BNST and NTS in previously morphine-dependent rats (Fig. 7) (Harris and Aston-Jones 2007). This, in view of the increased drug preference (described above), indicates that Fos activation in BNST and CeA in the extended amygdala, and their major NE afferent in NTS, is associated with enhanced drug preference and decreased food preference. This indicates that increased activity in these stress systems may decrease the rewarding properties of natural rewards, while increasing the reward associated with drug rewards. These findings add to others reviewed above indicating that activation in the extended amygdala is strongly associated with altered reward processing during protracted abstinence.
Previously morphine-dependent animals showed an increase in Fos-activated neurons in ventrolateral BNST, CeA, and NTS following a food CPP test after 5 weeks of abstinence, as compared to non-dependent animals (placebo). Graphs show mean Fos counts for conditioned and non-conditioned animals. † significantly different from non-conditioned rats, P < 0.01; * significantly different between conditioned groups, P < 0.01. Photomicrographs of frontal sections of brain areas show representative animals in morphine-withdrawn (left) and placebo (right) groups following food CPP test. Medial is to the right; arrows on NTS section indicate cells double-labeled for Fos and TH. Modified from Harris and Aston-Jones (2007)
Possible mechanisms of enhanced drug-seeking Additional studies in our laboratory showed that morphine preference is enhanced during protracted abstinence only if CPP conditioning occurs during abstinence (Smith et al. 2004). Thus, we found that animals trained for morphine CPP prior to dependence and withdrawal did not show elevated morphine CPP when tested shortly after subsequent chronic drug exposure or after one to six weeks of abstinence. However, these same animals exhibited enhanced CPP of the expected amplitude when they were re-conditioned during protracted abstinence. This indicates that animals need to experience the stimulus-drug association during abstinence in order to acquire increased drug-seeking behavior, and that the positive or negative reinforcing properties of the drug may be altered selectively during drug abstinence.
To test whether NTS is involved in the development of altered drug reinforcement during protracted abstinence, we measured Fos in NTS following the first morphine-environment CPP pairing. We found that noradrenergic A2 neurons in NTS in post-dependent animals (after 7 weeks of protracted morphine abstinence) showed increased Fos in response to a morphine CPP conditioning trial compared to rats that were not previously drug-exposed (Fig. 8; unpublished data, 2005). It is possible that this Fos effect was partially due to the stress of the injection, however, these Fos levels are comparable to those seen following a morphine CPP test session in post-dependent and non-dependent rats in other experiments, and therefore seem unlikely to be a result of the injection itself (Harris and Aston-Jones 2007). Although opiates are traditionally thought to exert an inhibitory influence on NE neurons in LC, Olson et al. (2006) also reported an increased Fos response in tyrosine hydroxylase-containing (TH+) neurons of NTS in mice following an acute morphine injection given in the CPP chamber. Rodriguez de Fonseca et al. (1997) also found increased Fos expression in NTS, along with BNST, CeA, and NAc-Sh, following acute cannabinoid exposure or acute withdrawal from chronic exposure. Electrophysiological recordings have revealed that morphine can both inhibit spontaneous activity and induce bursting in NE–LC neurons (Aston-Jones et al. 1992). The inhibition likely reflects direct effects of morphine on opioid receptors in LC (Bird and Kuhar 1977; Christie 1991; Korf et al. 1974), whereas increased bursting is presumably due to modulation of excitatory afferents to LC in the waking intact animal (Aston-Jones et al. 1992, 1993). These studies suggest that NE systems in the behaving animal can be activated by both acute drug exposure and drug withdrawal, and that NE may play a diverse role in several stages of addiction.
Morphine exposure after 7 weeks of abstinence stimulated more noradrenergic cells in NTS of post-dependent rats (morphine withdrawn) than non-dependent rats (placebo). Animals were exposed to a CPP environment following an acute morphine injection (8 mg/kg), and then sacrificed 2 h later for Fos and TH double-labeling. Post-dependent rats showed a significantly greater percentage of NTS TH+ cells with Fos double-labeling (P < 0.05, n = 3−4), with no differences in the total number of TH cells or number of TH-negative cells expressing Fos. Graph shows mean Fos counts ± S.E.M.
Another study in our laboratory explored delta-FosB (a highly stable product of the immediate early gene FosB) during protracted withdrawal. Unlike the transient expression of Fos and FosB, delta-FosB is a stable transcription factor that accumulates in response to chronic treatments. Delta-FosB persists for long periods of time once induced, and therefore gives a unique perspective into long-term neuronal responses to stimuli such as drug exposure (McClung et al. 2004). Following chronic cocaine treatment, our lab found that FosB/delta-FosB was elevated in BLA as well as NAc core and shell after 2 weeks of abstinence. At this time point, FosB/delta-FosB levels in all of these brain regions were correlated positively with cocaine CPP but correlated negatively with novel object CPP. However, after 5 weeks of abstinence FosB/delta-FosB only in NAc-Sh remained positively correlated with cocaine CPP and negatively correlated with novel object CPP (Harris et al. 2007). NAc-Sh is a part of the extended amygdala, is reciprocally connected with VTA, and is a critical target of VTA DA release for behavioral responses to abused drugs and other appetitive stimuli (Di Chiara 2002). Thus, enduring changes in NAc-Sh function (as indicated by these delta-FosB results) during chronic drug exposure and withdrawal may be involved in the increased drug preference observed during protracted abstinence.
Role of noradrenergic transmission in positive drug reinforcement
Long-lasting neuroadaptations following dependence and protracted drug abstinence cause enhanced anxiety and dysphoria. This may drive increased motivation for drugs via negative reinforcement by drug exposure and consequent decreased anxiety, and also cause decreased motivation for natural rewards (Koob 1993, 1999b, 2003). This is evident in the effects of protracted drug abstinence on preference for drug and food rewards; non-dependent rats exhibit a two-fold stronger preference for food than for morphine, however, abstinent rats have a two-fold stronger preference for morphine than food, indicating that reward processing has substantially changed (Harris and Aston-Jones 2003a). As discussed above, NE transmission in the extended amygdala appears to play an important role in this reward dysregulation that accompanies chronic drug exposure, and heightened NE may result in increased negative reinforcement for drugs of abuse.
Additionally, NE may be involved in the positively reinforcing effects of drugs. Although DA has been extensively implicated in the initial reinforcing effects of drugs during self-administration and CPP, NE and other systems may play a larger role than previously suspected. For example, recent studies suggest that opiate reward may include dopamine-independent pathways (Caine et al. 2007; Hnasko et al. 2005; Laviolette et al. 2002). As reviewed in Weinshenker and Schroeder (2007), the DA hypothesis of drug reward was originally based, in part, on the role of DA in the maintenance of psychostimulant self-administration. However, other aspects of addiction (e.g., acquisition, extinction, reinstatement) were not fully explored. In addition, other studies suggested an involvement of NE in the primary reward mechanisms for opiates (discussed below) and ethanol (Amit and Brown 1982; Amit et al. 1977; Mason et al. 1979; Rassnick et al. 1993b; Weinshenker et al. 2000). Recently, the role of NE in addiction has also received interest stemming from its importance in stress-induced reinstatement, drug-induced locomotion, and opiate CPP (Weinshenker and Schroeder 2007).
In studies of opiate reward, investigators found that dopamine beta-hydroxylase (DBH) knock-out mice failed to exhibit CPP for morphine, whereas CPP for food was normal (Jasmin et al. 2006; Olson et al. 2006; Schank et al. 2006). Morphine CPP in these animals was restored if NE signaling was rescued in NTS, but not if it was rescued in LC, confirming the importance of A2 NE neurons (the primary source of NE in the extended amygdala, as reviewed above) in morphine reinforcement (Olson et al. 2006). DBH knock-out mice also displayed altered cocaine CPP, although differently for different investigators: Schank et al. (2006) found hypersensitivity to the rewarding and aversive properties of cocaine, whereas Jasmin et al. (2006) showed a lack of cocaine reward in these mutants.
These results do not rule out an important role for DA in drug reward and reinforcement as well; NE mechanisms of drug reward may be dependent on interactions with the DA system. Indeed, it has proven difficult to fully differentiate the roles of DA and NE in drug reward, and studies in this vein have often revealed discordant results. This difficulty may reflect, at least in part, redundancies in the monoamine systems, so that interruption in the functioning of one system results in compensation by the other (Hnasko et al. 2007). In addition, NE and DA neurons interact at several levels (e.g. NE binds to D4 receptors (Newman-Tancredi et al. 1997), and DA is a high-affinity substrate for the NE transporter (Carboni and Silvagni 2004)), so that alterations in the functioning of one system may often change activity in the other. Further studies are needed to more fully understand the roles of each of these catecholamines, and their interactions, in reward processes.
Orexin in the extended amygdala: recent results indicating a role in drug-seeking and withdrawal
Orexins, also called hypocretins, are recently discovered neuropeptides made from a prepro-orexin peptide exclusively in hypothalamic neurons (de Lecea et al. 1998; Sakurai et al. 1998). These neurons provide an extensive efferent plexus of projections throughout the neuraxis (Baldo et al. 2003; Date et al. 1999; Nambu et al. 1999; Peyron et al. 1998). Of interest here, the orexins innervate all areas of the extended amygdala and provide an especially dense innervation of BNST (Baldo et al. 2003; Nambu et al. 1999; Peyron et al. 1998). Extensive evidence indicates a role for these peptides in narcolepsy with cataplexy (Chemelli et al. 1999; Lin et al. 1999; Nishino et al. 2000; Siegel 2004) (for review, see Nishino 2007; Sutcliffe and de Lecea 2002; Willie et al. 2001). However, recent findings from several sources indicate that this system also plays an important role in neuroplasticity, drug reward-seeking, and reinstatement of extinguished drug-seeking (Borgland et al. 2006; Boutrel et al. 2005; Harris et al. 2005; Lawrence et al. 2006; Narita et al. 2006). Moreover, at least some of these functions may be mediated through, or influenced by, effects of orexins in the extended amygdala.
There is considerable communication between stress response systems and orexin cells in LH. The area of orexin neurons receives extensive projections from BNST, NAc-Sh, and amygdala (Sakurai et al. 2005; Yoshida et al. 2006). Orexin neurons in LH receive direct contact from CRF terminals and express CRF receptors (Winsky-Sommerer et al. 2004). Conversely, BNST has a high density of orexin-1 (OX1) receptors, complimenting the dense orexin fiber distribution in that area (discussed above) (Hervieu et al. 2001; Marcus et al. 2001; Trivedi et al. 1998). Orexin cells, particularly those in dorsomedial and perifornical hypothalamus, were found to be activated by stressors such as footshock, restraint, and cold exposure, as revealed by induction of Fos (Harris and Aston-Jones 2006; Sakamoto et al. 2004; Winsky-Sommerer et al. 2004). Stress-induced Fos activation in orexin cells was reduced in CRF receptor-1 knock-out mice (Winsky-Sommerer et al. 2004). Orexin cells are not only activated by stressors, but are also implicated in the induction of behavioral response to stressors. I.c.v. administration of orexin-A was found to activate CRF neurons in CeA and PVN, and prepro-orexin knock-out mice exhibited attenuated stress responses in a resident-intruder test (Kayaba et al. 2003; Sakamoto et al. 2004). These studies indicate that there are functional reciprocal connections between stress-related systems of CRF- and orexin-expressing neurons. NE, CRF, and orexin may act in concert in the extended amygdala to modulate stress-related components of addiction.
Orexin-mediated responses are involved in stress-associated properties of abused drugs. Acute morphine withdrawal was accompanied by increased Fos and orexin gene expression in orexin cells, and orexin knock-out mice showed attenuated somatic opiate withdrawal (Georgescu et al. 2003; Zhou et al. 2006). In addition, the OX1 receptor antagonist SB-334867 blocked stress-induced reinstatement of cocaine-seeking (Boutrel et al. 2005). Moreover, NE and CRF antagonists reduced reinstatement driven by i.c.v. injections of orexin-A (Boutrel et al. 2005). As stress-induced reinstatement of drug-seeking involves NE and CRF transmission in the extended amygdala (reviewed above), these results suggest that orexins may interact with NE and CRF projections to the extended amygdala during stress responses and drug relapse. Our lab has proposed that orexin- or stress-induced reinstatement of drug-seeking may involve orexin-induced activation of NE and CRF inputs to BNST or amygdala (Harris and Aston-Jones 2006). Given the dense orexin innervation in BNST, it is also possible that such interactions occur at a terminal level within the extended amygdala. Experiments are now underway in our lab to test the role of orexin inputs to BNST in various aspects of addiction to abused drugs.
Conclusion
We reviewed studies indicating that NE and CRF signaling within the extended amygdala are involved in the aversiveness of acute opiate withdrawal and stress-induced reinstatement of drug-seeking in a virtually identical manner. NE and CRF are also necessary for the enhanced anxiety that accompanies protracted abstinence from chronic drug exposure. We also reviewed data showing that the extended amygdala and NTS are associated with the increased drug-seeking and decreased natural reward-seeking that occurs during protracted abstinence. The orexin system may also play a prominent role in withdrawal and relapse behaviors, perhaps in part because of its strong connections with the extended amygdala and NE- and CRF-containing structures. More recent evidence indicates that NE acting within the extended amygdala is critical for the positive reinforcing properties of drugs, particularly opiates. The extended amygdala is involved in behavioral responses related to stress and anxiety, and this may explain its role in the aversiveness of withdrawal and stress-induced reinstatement. However, the role of NE in positive reinforcement remains enigmatic, and the exact mechanisms of involvement in opiate reward as well as the enhanced drug-seeking and altered reward processing aspects of addiction remain to be elucidated. We presented evidence throughout that indicates that the VTA DA system may be one important site of action for increased extended amygdala output during stress-related aspects of protracted abstinence. Taken together, the studies reviewed here indicate that acute withdrawal and protracted abstinence following chronic drug exposure are associated with increased NTS activation, increased NE release and responsivity in the extended amygdala, and increased CRF release in the extended amygdala. This amplification of stress response systems in the extended amygdala during abstinence seems to similarly account for the aversiveness of acute withdrawal, increased anxiety, stress-induced relapse, and altered reward-processing for drugs and natural rewards. Overall, these studies implicate NE and neuropeptide transmission in the extended amygdala in several stress-related components of addiction which contribute to the increased drug-seeking and relapse vulnerability that makes recovery from addiction so difficult.
References
Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421. doi:10.1016/S0893-133X(99)00133-5
Akaoka H, Aston-Jones G (1991) Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci 11:3830–3839
Alheid GF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27:1–39. doi:10.1016/0306-4522(88)90217-5
Amit Z, Brown ZW (1982) Actions of drugs of abuse on brain reward systems: a reconsideration with specific attention to alcohol. Pharmacol Biochem Behav 17:233–238. doi:10.1016/0091-3057(82)90075-2
Amit Z, Brown ZW, Levitan DE, Ogren SO (1977) Noradrenergic mediation of the positive reinforcing properties of ethanol: I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Arch Int Pharmacodyn Ther 230:65–75
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999) The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12. doi:10.1677/joe.0.1600001
Aston-Jones G, Harris GC (2004) Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology 47(Suppl 1):167–179. doi:10.1016/j.neuropharm.2004.06.020
Aston-Jones G, Rajkowski J, Kubiak P, Akaoka H (1992) Acute morphine induces oscillatory discharge of noradrenergic locus coeruleus neurons in the waking monkey. Neurosci Lett 140:219–224
Aston-Jones G, Shiekhattar R, Akaoka H, Rajkowski J, Kubiak P (1993) Opiates influence locus coeruleus neurons by potent indirect and direct actions. In: Hammer RP Jr (ed) The neurobiology of opiates. CRC Press, Boca Raton, FL, pp 175–202
Aston-Jones G, Hirata H, Akaoka H (1997) Local opiate withdrawal in locus coeruleus in vivo. Brain Res 765:331–336. doi:10.1016/S0006-8993(97)00682-3
Aston-Jones G, Delfs JM, Druhan J, Zhu Y (1999) The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci 877:486–498. doi:10.1111/j.1749-6632.1999.tb09284.x
Aston-Jones G, Mejias-Aponte CA, Waterhouse B (2008) Norepinephrine: CNS Pathways, Neurophysiology. In: Squire L, Albright T, Bloom F, Gage F, Spitzer N (eds) The New Encyclopedia of Neuroscience. Elsevier, San Diego (in press)
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237. doi:10.1002/cne.10783
Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557. doi:10.1146/annurev.pharmtox.44.101802.121410
Basso AM, Spina M, Rivier J, Vale W, Koob GF (1999) Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 145:21–30. doi:10.1007/s002130051028
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84. doi:10.1016/S0165-0173(03)00143-7
Berridge CW, Stratford TL, Foote SL, Kelley AE (1997) Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27:230–241. doi:10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E
Bird SJ, Kuhar MJ (1977) Iontophoretic application of opiates to the locus coeruleus. Brain Res 122:523–533. doi:10.1016/0006-8993(77)90462-0
Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006) Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601. doi:10.1016/j.neuron.2006.01.016
Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF et al (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA 102:19168–19173. doi:10.1073/pnas.0507480102
Bremner JD, Krystal JH, Southwick SM, Charney DS (1996a) Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23:28–38. doi:10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J
Bremner JD, Krystal JH, Southwick SM, Charney DS (1996b) Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 23:39–51. doi:10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I
Britton KT, Svensson T, Schwartz J, Bloom FE, Koob GF (1984) Dorsal noradrenergic bundle lesions fail to alter opiate withdrawal or suppression of opiate withdrawal by clonidine. Life Sci 34:133–139. doi:10.1016/0024-3205(84)90583-6
Caille S, Espejo EF, Reneric JP, Cador M, Koob GF, Stinus L (1999) Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exp Ther 290:881–892
Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF et al (2007) Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci 27:13140–13150. doi:10.1523/JNEUROSCI.2284-07.2007
Carboni E, Silvagni A (2004) Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol 16:121–128. doi:10.1615/CritRevNeurobiol.v16.i12.130
Carboni E, Silvagni A, Rolando MT, Di Chiara G (2000) Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci 20:RC102
Cecchi M, Khoshbouei H, Javors M, Morilak DA (2002a) Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience 112:13–21. doi:10.1016/S0306-4522(02)00062-3
Cecchi M, Khoshbouei H, Morilak DA (2002b) Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology 43:1139–1147. doi:10.1016/S0028-3908(02)00292-7
Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C et al (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98:437–451. doi:10.1016/S0092-8674(00)81973-X
Chieng B, Christie MJ (1995) Lesions to terminals of noradrenergic locus coeruleus neurones do not inhibit opiate withdrawal behaviour in rats. Neurosci Lett 186:37–40. doi:10.1016/0304-3940(95)11276-3
Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O’Brien CP (1994) Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat 11:17–23. doi:10.1016/0740-5472(94)90060-4
Christie MJ (1991) Mechanisms of opioid actions on neurons of the locus coeruleus. Prog Brain Res 88:197–205. doi:10.1016/S0079-6123(08)63809-1
Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ (2007) Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav 86:813–821. doi:10.1016/j.pbb.2007.03.009
Clark MS, Kaiyala KJ (2003) Role of corticotropin-releasing factor family peptides and receptors in stress-related psychiatric disorders. Semin Clin Neuropsychiatry 8:119–136. doi:10.1053/scnp.2003.50011
Contarino A, Zanotti A, Drago F, Natolino F, Lipartiti M, Giusti P (1997) Conditioned place preference: no tolerance to the rewarding properties of morphine. Naunyn Schmiedebergs Arch Pharmacol 355:589–594. doi:10.1007/PL00004988
Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K et al (1999) Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96:748–753. doi:10.1073/pnas.96.2.748
Davis M (1993) Pharmacological analysis of fear-potentiated startle. Braz J Med Biol Res 26:235–260
Davis M (2006) Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol 61:741–756. doi:10.1037/0003-066X.61.8.741
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE et al (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327. doi:10.1073/pnas.95.1.322
Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS (1998) Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806:127–140. doi:10.1016/S0006-8993(98)00672-6
Delfs JM, Zhu Y, Druhan JP, Aston-Jones G (2000) Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403:430–434. doi:10.1038/35000212
Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114. doi:10.1016/S0166-4328(02)00286-3
Diana M, Pistis M, Muntoni A, Rossetti ZL, Gessa G (1992) Marked decrease of A10 dopamine neuronal firing during ethanol withdrawal syndrome in rats. Eur J Pharmacol 221:403–404. doi:10.1016/0014-2999(92)90734-L
Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL (1993) Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA 90:7966–7969. doi:10.1073/pnas.90.17.7966
Diana M, Pistis M, Muntoni A, Gessa G (1995) Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther 272:781–785
Diana M, Pistis M, Muntoni A, Gessa G (1996) Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience 71:411–415. doi:10.1016/0306-4522(95)00482-3
Diana M, Melis M, Muntoni AL, Gessa GL (1998) Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA 95:10269–10273. doi:10.1073/pnas.95.17.10269
Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL (1999) Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J NeuroSci 11:1037–1041. doi:10.1046/j.1460-9568.1999.00488.x
Dumont EC, Williams JT (2004) Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24:8198–8204. doi:10.1523/JNEUROSCI.0425-04.2004
Dunn AJ, Swiergiel AH (2008) The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol 583:186–193. doi:10.1016/j.ejphar.2007.11.069
Dunn AJ, Swiergiel AH, Palamarchouk V (2004) Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci 1018:25–34. doi:10.1196/annals.1296.003
Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD et al (2005) Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30:657–668
Erb S, Stewart J (1999) A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19:RC35
Erb S, Shaham Y, Stewart J (1998) The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18:5529–5536
Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J (2000) Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 23:138–150. doi:10.1016/S0893-133X(99)00158-X
Erb S, Salmaso N, Rodaros D, Stewart J (2001) A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 158:360–365. doi:10.1007/s002130000642
Fibiger HC, Phillips AG (1986) Reward, motivation, cognition: psychobiology of mesotelencephalic dopamine systems. In: Mountcastle VB, Bloom FE, Geiger SR (eds) Handbook of physiology, Sect. 1: The nervous system, vol 4. American Physiological Society, Bethesda, MD, pp 647–675
File SE (1990) New strategies in the search for anxiolytics. Drug Deliv 5:195–201
Forray MI, Bustos G, Gysling K (1999) Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res 55:311–320. doi:10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E
Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R (2005) Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 30:880–891. doi:10.1016/j.psyneuen.2005.05.002
Fox HC, Bergquist KL, Hong KI, Sinha R (2007) Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res 31:395–403. doi:10.1111/j.1530-0277.2006.00320.x
Freedman LJ, Cassell MD (1994) Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res 633:243–252. doi:10.1016/0006-8993(94)91545-8
Fuentealba JA, Forray MI, Gysling K (2000) Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem 75:741–748. doi:10.1046/j.1471-4159.2000.0750741.x
Funk CK, O’Dell LE, Crawford EF, Koob GF (2006) Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26:11324–11332. doi:10.1523/JNEUROSCI.3096-06.2006
Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF (2007) Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry 61:78–86. doi:10.1016/j.biopsych.2006.03.063
Georges F, Aston-Jones G (2001) Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci 21:RC160
Georges F, Aston-Jones G (2002) Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22:5173–5187
Georges F, Aston-Jones G (2003) Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology 28:1140–1149
Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ et al (2003) Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23:3106–3111
Gewirtz JC, McNish KA, Davis M (1998) Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry 22:625–648. doi:10.1016/S0278-5846(98)00028-1
Goeders NE (1997) A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology 22:237–259. doi:10.1016/S0306-4530(97)00027-9
Goeders NE (2003) The impact of stress on addiction. Eur Neuropsychopharmacol 13:435–441. doi:10.1016/j.euroneuro.2003.08.004
Gold MS, Redmond DE Jr, Kleber HD (1978) Clonidine blocks acute opiate-withdrawal symptoms. Lancet 2:599–602. doi:10.1016/S0140-6736(78)92823-4
Gracy KN, Dankiewicz LA, Koob GF (2001) Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology 24:152–160. doi:10.1016/S0893-133X(00)00186-X
Hamlin AS, Buller KM, Day TA, Osborne PB (2004) Effect of naloxone-precipitated morphine withdrawal on c-fos expression in rat corticotropin-releasing hormone neurons in the paraventricular hypothalamus and extended amygdala. Neurosci Lett 362:39–43. doi:10.1016/j.neulet.2004.02.033
Harris GC, Aston-Jones G (1993a) Beta-adrenergic antagonists attenuate somatic and aversive signs of opiate withdrawal. Neuropsychopharmacology 9:303–311
Harris GC, Aston-Jones G (1993b) Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 113:131–136. doi:10.1007/BF02244345
Harris GC, Aston-Jones G (1994) Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature 371:155–157. doi:10.1038/371155a0
Harris GC, Aston-Jones G (2001) Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology 24:75–85. doi:10.1016/S0893-133X(00)00184-6
Harris GC, Aston-Jones G (2003a) Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology 28:865–871. doi:10.1038/sj.npp.1300122
Harris GC, Aston-Jones G (2003b) Enhanced morphine preference following prolonged abstinence: association with increased Fos expression in the extended amygdala. Neuropsychopharmacology 28:292–299. doi:10.1038/sj.npp.1300037
Harris GC, Aston-Jones G (2006) Arousal and reward: a dichotomy in orexin function. Trends Neurosci 29:571–577. doi:10.1016/j.tins.2006.08.002
Harris GC, Aston-Jones G (2007) Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res 176:251–258. doi:10.1016/j.bbr.2006.10.012
Harris GC, Altomare K, Aston-Jones G (2001) Preference for a cocaine-associated environment is attenuated by augmented accumbal serotonin in cocaine withdrawn rats. Psychopharmacology (Berl) 156:14–22. doi:10.1007/s002130100693
Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. doi:10.1038/nature04071
Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G (2007) Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience 147:583–591. doi:10.1016/j.neuroscience.2007.04.050
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991) Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41:89–125. doi:10.1016/0306-4522(91)90202-Y
Heimer L, Alheid GF, Zahm DS (1993) Basal forebrain organization: An anatomical framework for motor aspects of drive and motivation. In: Kalivas PW, Barnes CD (eds) Limbic Motor Circuits and Neuropsychiatry. CRC Press, Boca Raton, pp 1–43
Heinrichs SC, Koob GF (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311:427–440. doi:10.1124/jpet.103.052092
Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L (1995) Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol 6:74–80. doi:10.1097/00008877-199501000-00011
Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA (2001) Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103:777–797. doi:10.1016/S0306-4522(01)00033-1
Hnasko TS, Sotak BN, Palmiter RD (2005) Morphine reward in dopamine-deficient mice. Nature 438:854–857. doi:10.1038/nature04172
Hnasko TS, Sotak BN, Palmiter RD (2007) Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci 27:12484–12488. doi:10.1523/JNEUROSCI.3133-07.2007
Hornby PJ, Piekut DT (1989) Opiocortin and catecholamine input to CRF-immunoreactive neurons in rat forebrain. Peptides 10:1139–1146. doi:10.1016/0196-9781(89)90005-3
Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R (2007) Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol 15:134–143. doi:10.1037/1064-1297.15.2.134
Ivanov A, Aston-Jones G (2001) Local opiate withdrawal in locus coeruleus neurons in vitro. J Neurophysiol 85:2388–2397
Jasmin L, Narasaiah M, Tien D (2006) Noradrenaline is necessary for the hedonic properties of addictive drugs. Vascul Pharmacol 45:243–250. doi:10.1016/j.vph.2005.08.030
Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I et al (2003) Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285:R581–R593
Koob GF (1999a) Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46:1167–1180. doi:10.1016/S0006-3223(99)00164-X
Koob GF (1999b) The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci 877:445–460. doi:10.1111/j.1749-6632.1999.tb09282.x
Koob GF (1999c) Stress, corticotropin-releasing factor, and drug addiction. Ann N Y Acad Sci 897:27–45. doi:10.1111/j.1749-6632.1999.tb07876.x
Koob GF (2003) Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol 13:442–452. doi:10.1016/j.euroneuro.2003.08.005
Koob GF, Heinrichs SC (1999) A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 848:141–152. doi:10.1016/S0006-8993(99)01991-5
Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159. doi:10.1176/appi.ajp.2007.05030503
Koob GF, Robledo P, Markou A, Caine SB (1993) The mesocorticolimbic circuit in drug dependence and reward–a role for the extended amygdala? In: Kalivas PW, Barnes CD (eds) Limbic motor circuits and neuropsychiatry. CRC Press, Boca Raton, pp 289–309
Korf J, Bunney BS, Aghajanian GK (1974) Noradrenergic neurons: morphine inhibition of spontaneous activity. Eur J Pharmacol 25:165–169. doi:10.1016/0014-2999(74)90045-4
Kosten TA (1994) Clonidine attenuates conditioned aversion produced by naloxone-precipitated opiate withdrawal. Eur J Pharmacol 254:59–63. doi:10.1016/0014-2999(94)90370-0
Kosten TR, Rounsaville BJ, Kleber HD (1986) A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry 43:733–738
Laviolette SR, Nader K, van der Kooy D (2002) Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res 129:17–29. doi:10.1016/S0166-4328(01)00327-8
Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759. doi:10.1038/sj.bjp.0706789
Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y (2000) The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150:317–324. doi:10.1007/s002130000411
Le AD, Harding S, Juzytsch W, Funk D, Shaham Y (2005) Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 179:366–373. doi:10.1007/s00213-004-2036-y
Lee Y, Davis M (1997) Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 17:6434–6446
Leri F, Flores J, Rodaros D, Stewart J (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–5718
Lett BT (1989) Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 98:357–362. doi:10.1007/BF00451687
Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X et al (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98:365–376. doi:10.1016/S0092-8674(00)81965-0
Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 22:7856–7861
Lu L, Ceng X, Huang M (2000) Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. NeuroReport 11:2373–2378
Lu L, Shepard JD, Scott Hall F, Shaham Y (2003) Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev 27:457–491. doi:10.1016/S0149-7634(03)00073-3
Lu L, Chen H, Su W, Ge X, Yue W, Su F et al (2005) Role of withdrawal in reinstatement of morphine-conditioned place preference. Psychopharmacology (Berl) 181:90–100. doi:10.1007/s00213-005-2207-5
Maldonado R (1997) Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev 21:91–104. doi:10.1016/0149-7634(95)00061-5
Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP (2008) Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 195:591–603. doi:10.1007/s00213-007-0950-5
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M et al (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25. doi:10.1002/cne.1190
Mason ST, Corcoran ME, Fibiger HC (1979) Noradrenaline and ethanol intake in the rat. Neurosci Lett 12:137–142. doi:10.1016/0304-3940(79)91494-0
Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR (2005) Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology 30:1455–1463. doi:10.1038/sj.npp.1300687
McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ (2004) DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132:146–154. doi:10.1016/j.molbrainres.2004.05.014
McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560. doi:10.1523/JNEUROSCI.4177-03.2004
Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF et al (1995) Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 15:5439–5447
Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S et al (2005) Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29:1214–1224. doi:10.1016/j.pnpbp.2005.08.007
Nader K, van der Kooy D (1996) Clonidine antagonizes the aversive effects of opiate withdrawal and the rewarding effects of morphine only in opiate withdrawn rats. Behav Neurosci 110:389–400. doi:10.1037/0735-7044.110.2.389
Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M et al (2005) Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience 134:9–19. doi:10.1016/j.neuroscience.2005.03.029
Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K (1999) Distribution of orexin neurons in the adult rat brain. Brain Res 827:243–260. doi:10.1016/S0006-8993(99)01336-0
Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M et al (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26:398–405. doi:10.1523/JNEUROSCI.2761-05.2006
Newman-Tancredi A, Audinot-Bouchez V, Gobert A, Millan MJ (1997) Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. Eur J Pharmacol 319:379–383. doi:10.1016/S0014-2999(96)00985-5
Nishino S (2007) Narcolepsy: pathophysiology and pharmacology. J Clin Psychiatry 68(Suppl 13):9–15
Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E (2000) Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355:39–40. doi:10.1016/S0140-6736(99)05582-8
O’Brien CP (1997) A range of research-based pharmacotherapies for addiction. Science 278:66–70. doi:10.1126/science.278.5335.66
Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD (2006) Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science 311:1017–1020. doi:10.1126/science.1119311
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. doi:10.1016/0165-0270(85)90031-7
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG et al (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015
Phelix CF, Liposits Z, Paull WK (1992) Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull 28:949–965. doi:10.1016/0361-9230(92)90218-M
Phelix CF, Liposits Z, Paull WK (1994) Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull 33:109–119. doi:10.1016/0361-9230(94)90056-6
Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187. doi:10.1016/j.neuron.2005.09.025
Piazza PV, Le Moal ML (1996) Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36:359–378. doi:10.1146/annurev.pa.36.040196.002043
Pothos E, Rada P, Mark GP, Hoebel BG (1991) Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res 566:348–350. doi:10.1016/0006-8993(91)91724-F
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33. doi:10.1016/S0014-2999(03)01272-X
Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ (1990) Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci 10:2308–2317
Rasmussen DD, Mitton DR, Green J, Puchalski S (2001) Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res 25:999–1005. doi:10.1111/j.1530-0277.2001.tb02308.x
Rassnick S, Heinrichs SC, Britton KT, Koob GF (1993a) Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res 605:25–32. doi:10.1016/0006-8993(93)91352-S
Rassnick S, Stinus L, Koob GF (1993b) The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res 623:16–24. doi:10.1016/0006-8993(93)90004-7
Richter RM, Weiss F (1999) In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse 32:254–261
Risbrough VB, Stein MB (2006) Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav 50:550–561. doi:10.1016/j.yhbeh.2006.06.019
Rodaros D, Caruana DA, Amir S, Stewart J (2007) Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150:8–13. doi:10.1016/j.neuroscience.2007.09.043
Rodgers RJ (1997) Animal models of ‘anxiety’: where next? Behav Pharmacol 8:477–496; discussion 497–504. doi:10.1097/00008877-199711000-00003
Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F (1997) Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276:2050–2054. doi:10.1126/science.276.5321.2050
Rossetti ZL, Melis F, Carboni S, Diana M, Gessa GL (1992) Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcohol Clin Exp Res 16:529–532. doi:10.1111/j.1530-0277.1992.tb01411.x
Sakamoto F, Yamada S, Ueta Y (2004) Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept 118:183–191. doi:10.1016/j.regpep.2003.12.014
Sakanaka M, Shibasaki T, Lederis K (1986) Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res 382:213–238. doi:10.1016/0006-8993(86)91332-6
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H et al (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. doi:10.1016/S0092-8674(00)80949-6
Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y et al (2005) Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46:297–308. doi:10.1016/j.neuron.2005.03.010
Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G (1995) Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res 675:89–97. doi:10.1016/0006-8993(95)00043-P
Sarnyai Z, Shaham Y, Heinrichs SC (2001) The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev 53:209–243
Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC et al (2006) Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology 31:2221–2230
Shaham Y, Stewart J (1995) Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 119:334–341. doi:10.1007/BF02246300
Shaham Y, Rajabi H, Stewart J (1996) Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci 16:1957–1963
Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J (1997) Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–2614
Shaham Y, Erb S, Leung S, Buczek Y, Stewart J (1998) CP-154, 526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 137:184–190. doi:10.1007/s002130050608
Shaham Y, Erb S, Stewart J (2000a) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33:13–33. doi:10.1016/S0165-0173(00)00024-2
Shaham Y, Highfield D, Delfs J, Leung S, Stewart J (2000b) Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J NeuroSci 12:292–302. doi:10.1046/j.1460-9568.2000.00899.x
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20. doi:10.1007/s00213-002-1224-x
Shalev U, Morales M, Hope B, Yap J, Shaham Y (2001) Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 156:98–107. doi:10.1007/s002130100748
Shippenberg TS, Heidbreder C (1995) Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther 273:808–815
Siegel JM (2004) Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol 55:125–148. doi:10.1146/annurev.psych.55.090902.141545
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359. doi:10.1007/s002130100917
Sinha R (2007) The role of stress in addiction relapse. Curr Psychiatry Rep 9:388–395. doi:10.1007/s11920-007-0050-6
Sinha R, Catapano D, O’Malley S (1999) Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 142:343–351. doi:10.1007/s002130050898
Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ (2003) Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 170:62–72. doi:10.1007/s00213-003-1525-8
Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63:324–331. doi:10.1001/archpsyc.63.3.324
Smith RJ, Harris GC, Aston-Jones G (2004) Dependence prior to, but not subsequent to, stimulus-drug conditioning increases drug seeking during protracted morphine withdrawal. Program No. 576.13. 2004 Abstract Viewer and Itinerary Planner. Online. Society for Neuroscience, San Diego, CA
Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS (1999) Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46:1192–1204. doi:10.1016/S0006-3223(99)00219-X
Spanagel R, Almeida OF, Bartl C, Shippenberg TS (1994) Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 115:121–127. doi:10.1007/BF02244761
Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF (2007) CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl). doi:10.1007/s00213-007-0983-9
Stinus L, Cador M, Zorrilla EP, Koob GF (2005) Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology 30:90–98. doi:10.1038/sj.npp.1300487
Stornetta RL, Norton FE, Guyenet PG (1993) Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res 624:19–28. doi:10.1016/0006-8993(93)90055-R
Strawn JR, Geracioti TD Jr (2008) Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety 25:260–271. doi:10.1002/da.20292
Sutcliffe JG, de Lecea L (2002) The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3:339–349. doi:10.1038/nrn808
Swiergiel AH, Takahashi LK, Kalin NH (1993) Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res 623:229–234. doi:10.1016/0006-8993(93)91432-R
Treit D, Pinel JP, Fibiger HC (1981) Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav 15:619–626. doi:10.1016/0091-3057(81)90219-7
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75. doi:10.1016/S0014-5793(98)01266-6
Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP et al (2002) Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res 26:1494–1501
Valdez GR, Zorrilla EP, Roberts AJ, Koob GF (2003) Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol 29:55–60. doi:10.1016/S0741-8329(03)00020-X
Veinante P, Stoeckel ME, Lasbennes F, Freund-Mercier MJ (2003) c-Fos and peptide immunoreactivities in the central extended amygdala of morphine-dependent rats after naloxone-precipitated withdrawal. Eur J NeuroSci 18:1295–1305. doi:10.1046/j.1460-9568.2003.02837.x
Walker DL, Davis M (1997) Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 17:9375–9383
Walker DL, Toufexis DJ, Davis M (2003) Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463:199–216. doi:10.1016/S0014-2999(03)01282-2
Walters CL, Aston-Jones G, Druhan JP (2000) Expression of fos-related antigens in the nucleus accumbens during opiate withdrawal and their attenuation by a D2 dopamine receptor agonist. Neuropsychopharmacology 23:307–315. doi:10.1016/S0893-133X(00)00113-5
Wang X, Cen X, Lu L (2001) Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol 432:153–161. doi:10.1016/S0014-2999(01)01487-X
Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB (2005) Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25:5389–5396. doi:10.1523/JNEUROSCI.0955-05.2005
Wang J, Fang Q, Liu Z, Lu L (2006) Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 185:19–28. doi:10.1007/s00213-005-0262-6
Wang B, You ZB, Rice KC, Wise RA (2007) Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 193:283–294. doi:10.1007/s00213-007-0782-3
Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M (2003) Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl) 170:80–88. doi:10.1007/s00213-003-1504-0
Weinshenker D, Schroeder JP (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451. doi:10.1038/sj.npp.1301263
Weinshenker D, Rust NC, Miller NS, Palmiter RD (2000) Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci 20:3157–3164
Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP et al (2001) Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci 937:1–26
Willie JT, Chemelli RM, Sinton CM, Yanagisawa M (2001) To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24:429–458. doi:10.1146/annurev.neuro.24.1.429
Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T et al (2004) Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci 24:11439–11448. doi:10.1523/JNEUROSCI.3459-04.2004
Wise RA (1978) Catecholamine theories of reward: a critical review. Brain Res 152:215–247. doi:10.1016/0006-8993(78)90253-6
Wise RA (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251. doi:10.1016/S0959-4388(96)80079-1
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. doi:10.1038/nrn1406
Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE (2006) Afferents to the orexin neurons of the rat brain. J Comp Neurol 494:845–861. doi:10.1002/cne.20859
Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ (2006) Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol 191:137–145. doi:10.1677/joe.1.06960
Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW (2007) Effects of the CRF receptor antagonist d-Phe CRF((12–41)) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology 53:958–966
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, R.J., Aston-Jones, G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct 213, 43–61 (2008). https://doi.org/10.1007/s00429-008-0191-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-008-0191-3