Abstract
The standard thermodynamic properties of the compound Ca2CuO3 were determined electrochemically by the Electromotive Force (EMF) method. The ternary phase Ca2CuO3 was synthesized from the pure oxides CaO and CuO at CaO saturation using a ceramic route. The EMF measurements were performed over a temperature range 973 ≤ T/K ≤ 1124. The standard Gibbs energy of formation of the ternary compound Ca2CuO3 was determined using the EMF technique as:
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Phase relations and thermodynamic properties of the system Ca–Cu–O are central to understanding high temperature superconductor design [1,2,3] as well as processing of metals [4]. Sub solidus phase relations and thermochemical properties are important in the former while liquidus phase relations improve the understanding of the later [1,2,3,4].
Phase equilibria and thermochemical properties of the system Ca–Cu–O in literature are explained in six independent studies [5,6,7,8,9,10], mostly conducted in the late 1900s, between the years 1990 and 1997 [6,7,8,9]. The experimental study by Gadalla and White dating back to 1963 [5] is an exception and has since been the main reference point for all recent studies. It should be mentioned that apart from Risold et al., [7] all the studies are experimental with each one covering only some of the phase relation aspects of the system Ca–Cu–O. Risold et al. reviewed the system Ca–Cu–O using binaries at 1 bar total pressure, and on this basis performed a thermodynamic assessment to achieve an optimized description of the system. Phase relations in the system Ca–Cu–O are depicted using the CaO–CuO pseudo binary in Fig. 1. The figure is calculated with MTDATA, a thermodynamic software and using the MTOX database version 8.1 [11].
In the experimental studies, a combined Four (4) methods have been applied namely, Thermogravimetric Analysis (TGA), X-Ray Powder Diffraction (XRPD), Scanning Electron Microscopy (SEM-EDS), and Electromotive Force (EMF), to investigate the phase relations and thermochemical properties.
By the thermobalance (TGA) method, Gadalla and White [5] in their phase equilibria study established that Ca2CuO3 was the only stable ternary compound. Stability of this compound was validated by Schulze et al. [3] and that this ternary compound was only stable below 1223 K.
Roth et al. [6] investigated the phase relations in the system CaO–CuO by XRD methods. Apart from confirming the ternary compound by an earlier study of Gadalla and white, two other ternary compounds CaCu2O3 and Ca1−xCuO2 were identified. Roth et al. further proposed that; Ca2CuO3 is only stable up to 1307 K where it melts incongruently to form CaO and oxide liquid, CaCu2O3 is stable between 1258 and 1291 K and that it decomposes to Ca2CuO3 and CuO below 1258 K. on the higher side, the compound melts incongruently to form Ca2CuO3 and liquid phase, Ca1−xCuO2 is only stable in air up to 1028 K and decomposes to Ca2CuO3 and CuO.
By combining XRD and SEM-EDS methods, Tsang et al. [9] and most recently Grivel [10], studied the phase relations in systems which include the CaO–CuO pseudo binary. After observing that earlier studies of the phase relations in the Ca–Cu–O system had been done in air, Tsang et al. investigated the phase equilibria of this system in oxygen at 1 atm and in a much wider temperature range between 1073 and 1573 K. They confirmed stability of Ca2CuO3 and CaCu2O3 although the stoichiometry of the later is given as Ca3Cu7O10. Most recently, Grivel’s study [10] of sub solidus phase relations of systems that include the CaO–CuO pseudo binary, these ternary compounds are investigated.
Using isothermal ternary sections of the system Cu–Cu–O at 1073 and 1223 K, Mathews et al. [8] determined the phase relations and thermochemical properties of the system Ca–Cu–O by the EMF, XRD and EPMA methods. At 1073 K both ternary compounds Ca2CuO3 and Ca0.828CuO2 were found to be stable but only the former was found at 1223 K. They then measured thermodynamic properties of these two ternary compounds. Thermodynamic properties of the ternary compound CaCu2O3 reported in by Roth et al. [6] were not measured. It is worth mentioning that this is the single most comprehensive experimental study of this system that combines both phase equilibria and thermochemical data to establish these phase relations.
From these studies, it is evident that experimentally measured thermochemical properties data is still scarce and that even in areas where studies have been conducted, validation is still necessary. Moreover, a complete thermodynamic description of any system is a function of both phase relations and thermochemical property data [12].
In this study thermochemical properties of the ternary compound Ca2CuO3 are determined experimentally by the EMF method using a solid electrolyte galvanic cell.
Experimental Section
Synthesis of Ca2CuO3 Saturated with CaO
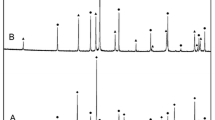
Fine powders of pure CaO (99.9%, Sigma-Aldrich, USA) and CuO (99.995%, Alfa Aesar, Germany) were thoroughly mixed with a molar ratio 3:1 in an agate mortar and pressed into a pellet. Prior to use, CaO was dried at 1123 K for 4 h in air to improve its reactivity by removal of possible volatile impurities, i.e. H2O and CO2. The pelletized sample was heat-treated at 1223 K for 36 h in pure O2 (99.999%, AGA, Finland) with an intermediate grinding and repelletizing (solid state reaction which was not complete, i.e. a certain fraction of CuO was remaining unreacted). It was further heat-treated at 1333 K for 14 h (temperature was slightly above the eutectic point in the CaO–CuO system generating a little fraction of a liquid phase which facilitated a rapid completion of the reaction) followed by annealing at 1223 K for 1 h. The pelletized sample was placed in an alumina crucible during all described cycles of heat-treatment, but a direct contact between the sample and Al2O3 was avoided with the help of a Pt substrate placed at the bottom of the crucible. This was supported by the protective sacrificial pellet made of pure CaO placed in between the Pt substrate and the sample. Purity of the sample produced as well as its biphasic Ca2CuO3–CaO phase composition was confirmed by SEM-EDS and XRPD. Figure 2 depicts the Ca2CuO3–CaO phases. The XRPD pattern is shown in Fig. 3.
Apparatus and Galvanic Cells
EMF values of a galvanic cell of the following type were measured in isothermal conditions:
where MSZ stands for magnesia-stabilized zirconia solid electrolyte.
Test electrode was formed by pressing into a pellet a mixture of synthesized Ca2CuO3 saturated with CaO and Cu2O. Cuprous oxide (Cu2O) was synthesized by oxidizing a cathode copper (99.996%, Boliden Harjavalta Oy, Finland). The pure copper was annealed in air above 1299 K for 120 h and quenched in air [13]. A mixture of CuO and Cu2O was adopted as a reference electrode.
The electrochemical cell was housed in a horizontal furnace equipped with a fused silica gas-tight work tube. The arrangement of the cell is illustrated in Fig. 4. MSZ crucible was used as a solid electrolyte and two electrodes were pressed towards inner (reference) and other (test) surfaces of its bottom (see Fig. 4). Pure Pt wires (99.99%, 0.25 mm diameter, Johnson-Matthey Noble Metals, UK) were utilized as leads. In order to separate electrode compartments from each other and due to a rather high equilibrium oxygen partial pressure of the electrodes, an alumina cement was used as a sealing. A nearly static (5–6 ml min−1 flowrate) protective atmosphere of Ar (99.999%, AGA, Finland) was provided during the entire measurement.
The temperature measurement was performed from both side of the galvanic cells with two calibrated S-type Pt–Pt/Rh thermocouples (Johnson-Matthey Noble Metals, UK) connected to a Keithley 2010 DMM multimeters. A Pt100 resistance thermometer (SKS-Group, Finland, tolerance class B 1/10) connected to a Keithley 2000 DMM multimeter was utilized as a cold junction compensation. Measurements of the EMF were performed with Keithley 6517B electrometer with high input impedance of 2 × 1014 Ω.
Results and Discussion
The reaction on the test (left hand side) electrode is:
The reaction on the reference (right hand side) electrode is:
Therefore, the overall (or virtual) cell reaction is:
The Gibbs energy change of the virtual cell reaction can be calculated from the following expression:
where E and F stand for measured EMF (V) and Faraday constant (96,485.3399 C mol−1), respectively.
Linear regression calculated for the measured EMF as a function of temperature is expressed by:
The determination coefficient is R2 is 0.9894. The experimental temperature range is 973 ≤ T/K ≤ 1124.
Therefore, the Gibbs energy change of the virtual cell reaction is given by the equation:
Meanwhile, this expression represents the standard Gibbs energy of formation of Ca2CuO3 from its component oxides, Δf(ox)G°Ca2CuO3. A comparison of the result obtained in this work with Mathews et al.’s [8] is given in Fig. 5.
Comparison of the standard Gibbs energy of formation of Ca2CuO3 from its component oxides acquired in this study (bold line with round symbols representing actual measurement points) with the previous work (dashed line) [8]
The absolute values measured are in a reasonable agreement between this work and Mathews et al.’s results [8], however the slope is drastically different. By extrapolating the result of the present study to the lower temperature, a decomposition of Ca2CuO3 can be expected at the temperature around 956 K, which is in a good agreement with the assessed CaO–CuO phase diagram reported by [11]. This is not in agreement with Mathews et al.’s results [8]. The difference could be attributed to the difference in the cell compartment designs. In this study, closed cell compartment compared to open isolated compartments employed in previous studies, have been used. This, however, still needs further experimental work to validate this claim.
Conclusions
The standard thermodynamic properties of solid phases were determined electrochemically by the EMF method. The ternary phase Ca2CuO3 was synthesized from the pure oxides CaO and CuO under CaO saturation using a ceramic route. The electromotive force measurements were performed over a wide temperature range which translates into more accurate extrapolations. The standard Gibbs energy of formation of the ternary compound Ca2CuO3 was found to be:
The results obtained in the present study are different from those in literature.
References
C. Sekar et al., Crystal growth of spin-ladder compound La2Cu2O5 and ladder-like compound CaCu2O3. Physica C 378, 678–682 (2002)
J.G. Bednorz, K.A. Müller, Possible High-Tc Superconductivity in the Ba-La-Cu-O System. Z. Phys. B 64(2), 189–193 (1986)
K. Schulze et al., Phase equilibria in the system Bi2O3–SrO–CaO–CuO with emphasis on the high-Tc superconducting compounds. Z. Metallkund. 81, 836–842 (1990)
J. Hamuyuni et al., Experimental determination of the liquidus of the binary system Cu2O–CaO in air from 1050 to 1500 °C. J. Chem. Thermodyn. 77(2014), 112–115 (2014)
A.M.M. Gadalla, J. White, Equilibrium relationships in the system CuO–Cu2O–CaO. Trans. Br. Ceram. Soc. 65, 181–190 (1966)
R.S. Roth et al., Phase equilibria in the systems CaO–CuO and CaO–Bi2O3. J. Am. Ceram. Soc. 74, 2148–2151 (1991)
D. Risold, B. Hallstedt, L.J. Gauckler, Thermodynamic assessment of the Ca–Cu–O System. J. Am. Ceram. Soc. 78, 2655–2661 (1995)
T. Mathews, J.P. Hajra, K.T. Jacob, Phase relations and thermodynamic properties of condensed phases in the system calcium-copper-oxygen. Chem. Mater. 5, 1669–1675 (1993)
C.F. Tsang, J.K. Meen, D. Elthon, Phase equilibria of the calcium oxide-copper oxide system in oxygen at 1 atm. J. Am. Ceram. Soc. 78, 1863–1868 (1995)
J.C. Grivel, Subsolidus Phase Relations of the CaO–REOx–CuO Systems (RE = Eu, Tb, Dy, Ho, Er, Lu and Sc) at 900 °C in Air. J. Phase Equilib. Diffus. 37, 601–610 (2016)
MTOX, Release Notes for Version 8.1 of MTOX database. (NPL, Teddington, U.K., 2015)
J. Hamuyuni, P. Taskinen, Experimental phase equilibria of the system Cu-O-CaO-Al2O3 in air. J. Eur. Ceram. Soc. 36, 847–855 (2016)
J. Hamuyuni, P. Taskinen, Liquidus experimental data for the system Cu–O–Cr2O3 in air. Thermochim. Acta 638, 96–102 (2016)
Acknowledgements
The authors are grateful for financial support of this research by Association of Finnish Steel and Metal Producers and Systems Integrated Metal Processes (SIMP) project of FIMECC.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Hamuyuni, J., Sukhomlinov, D., Lundström, M., Taskinen, P. (2017). Thermodynamic Stability of Condensed Phases in the Ternary System CaO–Cu–O by the EMF Method. In: Zhang, L., et al. Energy Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-52192-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-52192-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52191-6
Online ISBN: 978-3-319-52192-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)