Abstract
In the last decades, orthotopic bladder substitution has evolved to an established form of urinary diversion in women undergoing radical cystectomy for bladder cancer. Factors that have contributed to this development are based on an improved understanding of the functional anatomy of the female pelvis and oncological risk factors that determine the risk of recurrence in women with ileal neobladder.
In order to further improve functional outcomes, variations to the standard extent of radical cystectomy with preservation of the anterior vaginal wall and/or uterus have recently shown to result in markedly improved functional outcomes. In the evolving era of organ-sparing treatment of urological malignancies, genital-sparing cystectomy has the potential to further improve functional outcomes and quality of life of women after radical cystectomy without endangering oncological outcomes. Clinical expertise will be crucial for optimized patient selection in order to avoid compromise of oncological safety. In order to implement genital-sparing cystectomy, standardization of the inclusion criteria and the technical approach within prospective trials are needed.
The author declares no conflicts of interest in relation to the contents of this book chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Anatomical Considerations for Orthotopic Bladder Substitution in Women
In order to understand the oncological and functional aspects of orthotopic urinary diversion in women, a profound knowledge about the anatomy of the female pelvic floor is important which is briefly described in this subchapter.
The female urethral sphincter consists of the internal urethral sphincter (IUS) and external urethral sphincter (EUS). The EUS consists of striated muscle fibers which are innervated by the pudendal nerve and can be divided into two portions. The superior horseshoe-like section covers the anterolateral aspects of the superior urethra and is opened at its dorsal part. The inferior part exhibits large “muscular wings” which cover both the lateral part of the inferior urethra and the lateral portion of the anterior vagina. Both parts of the EUS are not attached to the bony pelvis. Instead, a tendinous connection of the inferior EUS to medial portion of the levator ani muscle (LAM) exists. This “muscle-to-muscle” connection has implications for maintenance of sufficient urethral closing pressure.
The IUS consists of smooth muscle and covers basically circumferentially the superior urethra. It is thickest at its dorsal part. At the inferior urethral level, it covers only the anterolateral part but does not extend as far caudally as the inferior EUS. The superior EUS is covered laterally by a tendinous arch to the pelvic fascia which surrounds the puborectal muscle of the LAM. At its dorsal side, the superior EUS is connected to the anterior vaginal wall via connective tissue. As a result, proper function of the EUS for contraction and competent urethral closure depends on the integrity of the LAM. Contraction of the LAM results in a force that pulls the rectum and vagina (the so-called rectovaginal complex) anteriorly and superiorly which, in turn, results in a compression of the dorsal aspect of the superior urethra. Simultaneous contraction of the EUS results in a force that compresses the urethra inferiorly and posteriorly. These forces bend the urethra in its mid part as the contraction of the EUS and LAM exerts parallel forces on different urethral levels. When the LAM is functionally impaired (i.e., its attachments to the pelvic wall), the fixation spots of the EUS become displaced, and dysfunction of the EUS can result [1]. By contrast to the EUS which is innervated by the pudendal nerve, the LAM has a dual somatic innervation by the levator ani nerve (LAN) as its constant and main supply with communicating branches between the levator ani nerve and pudendal nerve in half of the cases [2]. These basic anatomical considerations are of particular importance for urethral dissection during radical cystectomy (RC).
Oncological Prerequisites for Orthotopic Urinary Diversion in Women
At the end of the 1980s, OBS began to emerge as a modality of urinary diversion for men undergoing RC for bladder cancer [3]. Yet, at that time, it was not considered to be a viable option for women. Skepticism that persisted during that time was that the remaining urethral length was considered to be too short to provide sufficient urethral closing pressure. In addition, tumor multifocality and the anatomical proximity to the urethra and pelvic floor raised concerns about the risk of uncontrolled local disease in case of urethral recurrence.
Due to the pioneering efforts of urologic surgeons in the 1990s [4, 5], orthotopic ileal neobladder is nowadays considered an established option for women treated with RC [6]. Yet, risk assessment for tumor recurrence involving the neobladder outlet or urethra is still an important issue for preoperative counseling and clinical decision-making. Primary tumor location and local staging are the critical determinants for assessing the oncological safety of OBS in women. It has been demonstrated that location of the primary tumor at the bladder neck increases the risk of a positive urethral margin at RC [7]. Thus, women with bladder neck involvement at RC are nowadays counseled for a non-orthotopic bladder substitute when preoperative biopsy of the bladder neck reveals malignancy [8]. Yet, the question that derives from this, admittedly, well-accepted clinical practice is whether bladder neck involvement is an absolute contraindication for an OBS or the exact level of urethral dissection should rather dictate the intraoperative clinical decision-making for an OBS. Essentially, this question remains unanswered until today as robust evidence on this issue is absent. In order to accurately assess the distal urethral margin, frozen section analysis (FSA) may provide useful information. An important prerequisite for accurate diagnosis of malignancy on FSA is that the specimen was obtained by full-thickness biopsy in order to allow for a circumferential and in-depth assessment of the urothelial margin [6]. In women, urethral frozen sections were found to exhibit a high accuracy of 90% with a specificity of 99% when compared to the final histological result [9]. On the other hand, a previous histoanatomical study on female cystectomy specimens demonstrated that the degree of concordance between malignancy at the bladder neck and the level of urethral dissection was only 40% [7]. Urethral tumors were exclusively found in the proximal and mid-urethra, whereas the distal third was found to be free. Location of the primary tumor at the bladder neck correlated with higher grade and stage as well as with concomitant urethral malignancy. In addition, with increasing distance from the bladder neck, the risk of recurrence drops considerably. In a retrospective, multicenter series, the risk of urethral recurrence was assessed in 297 women who were treated with RC and OBS [10]. Women were excluded from OBS when primary tumors that were staged cT4b and/or cN3 or preoperatively showed involvement of the bladder neck or a positive urethral margin as intraoperatively assessed by FSA. Based on these selection criteria, the rate of solitary (0.8%) or concomitant urethral recurrence (1.6%) was very low, and the only risk factor associated with urethral recurrence was found to be a positive final urethral margin status at RC. Histological tumor and nodal stage were determinants for all-cause mortality but not for urethral recurrence. None of the patients with bladder trigone involvement developed urethral recurrence after a median of 64 months. These findings are in line with previous results from single-center studies with reported rates of urethral recurrence ranging between 0.8% and 1.2% [11, 12]. Therefore, based on the available evidence, it seems justified to consider an OBS even in women with trigonal involvement or non-bulky lymph node disease provided the urethral margin on frozen section is negative.
Functional Prerequisites for Orthotopic Urinary Diversion in Women
Ileal OBS aims to store urine at low pressure while enabling spontaneous micturition and continence. Besides oncological parameters, women scheduled for orthotopic bladder substitution need to be critically assessed for various functional criteria. Renal function should be investigated by estimation of glomerular filtration rate (eGRF) or measurement of 24 h creatinine clearance in equivocal cases. A GFR above 60 ml/min is generally accepted for replacing the bladder with an OBS [13–15]. Uncontrolled diabetes mellitus does not only increase the odds for postoperative complications after major surgery but may contribute to the long-term development of metabolic acidosis after ileal neobladder [16]. Baseline hypertension and episodes of acute pyelonephritis were found to independently increase the risk of renal deterioration after urinary diversion [17]. Therefore, measures should be taken for optimized management of perioperative blood pressure and antibiotic treatment in patients with preoperative upper urinary tract infections. Besides renal function, an unrestricted hepatic function is crucial to prevent uncontrollable hematuria postoperatively [18]. Increased post-void residual urine may result in metabolic acidosis and impaired renal function, especially in the early period after OBS. Therefore, it is mandatory to preoperatively exclude the presence of physical or mental disorders which would preclude the ability of patients to catheterize their reservoir if retention occurs. Likewise, women who suffer from severe stress urinary incontinence due to external sphincter deficiency and wish to preserve postoperative continence should rather be offered a heterotopic form of continent diversion [19]. Patient factors (i.e., biological age, prior radiotherapy) and the selection of the appropriate type of diversion are also important for optimized functional outcomes and quality of life after orthotopic ileal neobladder [6].
Considerations and Technique of Nerve-Sparing Radical Cystectomy in Women
Optimized functional results in women with OBS depend on the extent of preservation of neurovascular structures which are critical for an intact innervation of the urethra, vagina, and pelvic floor [20]. In women, the standard extent of RC encompasses the removal of the tumor-bearing bladder, uterus, adnexa, and anterior vaginal wall [21]. The urethra and most distal part of the anterior vaginal wall are in closest anatomical proximity (Fig. 3.1). Innervation and vascularization occurs via nerves and vessels which traverse along the anterior and lateral aspects of the vagina [20]. When oncologically safe, preservation of these structures will result in superior functional outcomes [22]. As outcomes of orthotopic urinary diversion depend strongly on the technique of cystectomy, a detailed description of a technique encompassing nerve-sparing radical cystectomy with posterior neobladder support is outlined:
-
1st: Longitudinal midline abdominal incision between the pubic symphysis and the umbilicus with incision of the fascia and dissection of rectus abdominis muscle.
-
2nd: Opening of the transversal fascia and blunt dissection of Retzius space to enter the paravesical space bilaterally.
-
3rd: V-shaped incision of the bladder peritoneum followed by dissection and ligation of the round ligament.
-
Landmark: Round Ligament
-
(Info: Preservation of maximal length of the round ligaments for suspension of the vagina later on.)
-
4th: Continuation of the peritoneal incision along the external and common iliac vessels up to the promontorium.
-
5th: Performance of pelvic lymphadenectomy up to the crossing of the ureters with common iliac vessels.
-
Landmark: Ureter
-
(Info: In case of normal findings on cross-sectional imaging lymph node dissection in the presacral region is discouraged to avoid injury of autonomous nerve fibers which traverse along the interval iliac artery and its branches to the cervix and vaginal walls [ 23 ]).
-
6th: Lateral division with identification of the ureters at their crossing with the common iliac vessels.
-
(Info: In order to preserve blood supply to the ureters, their fascial sheet should stay intact. This can be achieved by mobilization of all the tissue adjacent to the peritoneum toward the ureter.)
-
7th: Preparation of the ureters toward the bladder and ligation ca. 2–3 cm proximally to the ureteral orifice. A circumferential biopsy of both ureteral stumps should be sent to frozen section analysis [21].
-
(Info: Clipping of distal end of ureters results in temporary hydrodistention which facilitates ureteroileal anastomosis later on.)
-
8th: Ligation of the anterior pedicles as distally as possible (if oncologically possible) to minimize dissection of neurovascular structures with traverse along the branches of the internal iliac artery.
-
Landmark: Sacrouterine Ligament
-
9th: Identification of the lateral vaginal walls and posterior vaginal fornix.
-
(Info: Compression of the posterior vaginal fornix by an intravaginally placed curved sponge facilitates its anatomical identification.)
-
Landmark: Cul-de-Sac
-
10th: Identification and incision of the vaginal fornix at the level of cul-de-sac.
-
Info: Compression of the posterior vaginal fornix by an intravaginally placed curved sponge facilitates its anatomical identification (Fig. 3.2).
-
11th: Opening of the posterior vaginal fornix and identification of the cervix (Fig. 3.3).
-
Landmark: Cul-de-Sac and Cervix
-
12th: Continued dissection of the anterior from the lateral vaginal walls.
-
(Info: Identification and separation of the lateral vaginal walls from the bladder wall can be facilitated by continuous digital replacement of the catheter balloon during dissection. Ligation and dissection of the lateral from the anterior vaginal wall using hook clamps are preferred; electrocautery should be avoided whenever possible as it may result in damage of autonomic nerve fibers.)
-
13th: When dissection of the anterior vaginal wall has reached the bladder neck on both sides, a hook clamp is introduced posteriorly to the bladder neck and gently crossed to the opposite side.
-
(Info: Retraction of balloon catheter toward distal aids in the identification of the bladder neck.)
-
14th: Transverse transection of the distal end of the anterior vaginal wall.
-
(Info: A distance of at least 1.5–2 cm proximal to the level of urethral dissection should be met in order to prevent overlapping of the suture lines and decrease the risk of a neobladder-vaginal fistula formation. In vaginal-sparing cystectomy, the bladder is carefully separated from the anterior vaginal wall after opening the posterior vaginal fornix ( Fig. 3.4 ).)
-
15th: Visualization of the endopelvic fascia.
-
(Info: Dissection should be avoided below the level of the endopelvic fascia.)
-
16th: Ligation of the pubovesical complex by passing a clamp underneath the plexus and anteriorly to the urethra.
-
17th: Sharp dissection of the pubovesical complex from the bladder neck. Now the anterior aspect of the bladder neck and urethra can be visualized.
-
18th: Removal of the catheter slowly in order to accurately identify the transition area between the bladder neck and urethra.
-
19th: A smooth clamp is placed at the level of urethral dissection and the procedure completed by sharp dissection of the anterior and posterior urethra (Fig. 3.5).
-
(Info: A full-thickness biopsy of the urethra should be sent to frozen section analysis.)
-
20th: Closure of the anterior vaginal wall is performed initially by anchoring the long remnants of the round ligaments to the most lateral aspect of the vaginal opening.
-
(Info: This approach suspends the vaginal neofornix cranially and serves as anchoring point for the omental flap which will be placed later on onto the anterior vaginal wall to prevent backfall of the reservoir.)
-
21st: The vagina is closed symmetrically in a traverse manner using locked polyglactin sutures.
-
(Info: This technique will avoid narrowing the vaginal lumen and decrease the risk of postoperative dyspareunia.)
-
22nd: Isolation of an adequate ileal length with ileoileal anastomosis and formation of an I-Pouch neobladder reservoir (or creation of other ileal neobladder reservoirs according to other techniques [24–28]).
-
23rd: Mobilization of an omental flap (if available) along the left paracolic trough into the pelvis with fixation to the round ligament and anterior vaginal wall.
-
(Info: Preservation of vascular supply of the omental flap is mandatory.)
-
24th: Creation of an opening in the most dependent part of the omental flap at the urethral opening.
-
(Info: This approach helps to further separate the suture lines of the vaginal reconstruction and urethro-ileal anastomosis, thereby preventing fistula formation.)
-
25th: After performance of urethro-ileal anastomosis, closure of the lateral leafs of the omental flap anteriorly to the neobladder is performed (if abundant omentum available).
-
(Info: This approach will create an interlayer which may also prevent adhesion of the neobladder to the pelvic wall.)
Genital-Sparing Cystectomy in Women with Bladder Cancer
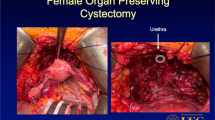
In the era of increased awareness of organ preservation and quality of life issues in lower urinary tract cancer treatment [29], variations to the standard extent of nerve-sparing RC in women that include the preservation of the anterior vaginal wall with or without hysterectomy and/or ovariectomy have been proposed [30, 31]. As a result, markedly improved functional outcomes have been reported with day- and nighttime continence rates ranging between 77–100% and 77–92%, respectively [30, 31]. These studies were conducted in highly selected young women with a median age between 37 and 42 years. The improved functional outcomes need to be weighted against the possible oncological risk of a positive soft tissue surgical margin at RC. In this regard, a recent retrospective study on 160 women found that only women with tumor location at the bladder floor and neck, with an intraoperative palpable posterior mass or clinical lymphadenopathy, exhibited female pelvic organ involvement at RC [32]. In addition, among 180 women treated with standard RC for bladder cancer, only two (1%) had uterine infiltration on final examination [33]. These results suggest that preservation of the internal genital organs at RC offers a high chance for preservation of postoperative continence and sexual function in the majority of carefully selected women. Therefore, genital-sparing cystectomy has the potential to improve functional outcomes and quality of life in women with muscle-invasive bladder cancer without endangering oncological outcomes. Clinical expertise and optimal patient selection will be the critical factors in order to implement genital-sparing cystectomy for improved functional outcomes without compromising oncological safety. Prospective trials are encouraged for standardization of the inclusion criteria and the technical approach.
Treatment of Women with Functional Impairments After Orthotopic Bladder Substitution
Various types of ileal neobladder are currently used for OBS, and all of these have shown satisfactory functional and oncological outcomes [24–28, 34]. A relevant consideration for neobladder reconstruction is its propensity for postoperative complications. Although long-term preservation of renal function is crucial for any technique of OBS, uncertainty persists about the functional superiority of an antirefluxive over a refluxive ureteral implantation technique. In this regard, current evidence does not support an antirefluxive implantation technique for the protection of the upper tract. A randomized study compared both techniques with regard to long-term changes in renal function [35]. An antirefluxive ureteral implantation technique using serous-lined extramural troughs did not prevent postoperative decrease in renal function, as measured by 99 m-Tc-MAG3 scintigraphy. Likewise, pouch-related complication rates were shown to be higher in neobladders with complex antirefluxive ureteral implantation technique [25, 35]. Yet, in absolute figures, the rates of ureteral stricture between neobladder using an antirefluxive and non-refluxive ureteral implantation technique were shown to be similar [24, 26, 34]. Another important aspect on the management of patients with ileal neobladder is the easiness for accessing the upper tract during oncological surveillance after cystectomy [24]. In summary, current evidence does not strictly support the construction of a neobladder with antirefluxive ureteral implantation technique. Importantly, facilitating access to the upper tract should be also taken into account for straightforward surveillance of the upper tracts after cystectomy.
Another main problem after OBS in women is the risk of urinary retention and the need for postoperative clean intermittent catheterization (CIC). In women, the rates of CIC have shown to widely range between 20% and 58% [12, 36, 37]. The causes can be either functional, anatomical, or multifactorial [38]. Apart from oncological causes (i.e., urethral recurrence [10]), anatomical causes include an “ileal valve” of the neobladder outlet and a lack of posterior support resulting in neocystocele or urethral kinking. Hypercapacity of the neobladder can also result due to excessive length of ileum used for creation of the reservoir (“floppy bag”) or preservation of the bladder neck during RC [39]. Functional causes comprise untreated or inadequately treated urinary tract infections or the failure to relax the pelvic floor for initiation of voiding and inability to develop and maintain sufficient intra-abdominal pressure during micturition. Basic work-up of patients with urinary retention or increased post-void residual urine volume includes a detailed history with voiding diary, clinical examination and urine analysis, sonography of the kidney and neobladder, and measurement of post-void residual urine volume. Further diagnostic management consists of urethropouchoscopy, dynamic investigations with voiding pouchogram, dynamic magnetic resonance imaging of the pelvic floor, and urodynamic assessment. Formation of a neobladder-vagina fistula using a transvaginal approach with Martius or omental flap was shown to provide high success rates but may still necessitate secondary procedures for satisfactory restoration of urinary continence [40]. In one of the largest series reporting on 12 women with post-cystectomy urinary incontinence, six had undiscovered urethrovaginal fistula [38]. The use of bulking agents showed a low long-term efficacy and carried also the risk of fistula formation. Likewise, placement of a tension-free sling had only a low efficacy, and continence was only achieved with the use of obstructing slings. In summary, it has to be stated that data on surgical treatment of women with urinary incontinence after ileal neobladder reconstruction are scarce.
Conclusions
As a result of an increased awareness of clinicians and patients alike, replacing the urinary bladder with an orthotopic bladder substitute has become the diversion of choice in many academic centers of expertise. Orthotopic bladder substitution can safely be performed in women with invasive bladder cancer provided a carefully obtained bladder neck biopsy or intraoperative frozen sectional analysis of the distal urethral margin excludes malignancy. The extent of preservation of female genital structures impacts on functional outcomes of women with orthotopic bladder substitutes.
References
Wallner C, Dabhoiwala NF, DeRuiter MC, Lamers WH. The anatomical components of urinary continence. Eur Urol. 2009;55(4):932–43.
Wallner C, van Wissen J, Maas CP, Dabhoiwala NF, DeRuiter MC, Lamers WH. The contribution of the levator ani nerve and the pudendal nerve to the innervation of the levator ani muscles; a study in human fetuses. Eur Urol. 2008;54(5):1136–42.
Hautmann RE, Egghart G, Frohneberg D, Miller K. The ileal neobladder. J Urol. 1988;139(1):39–42.
Stenzl A, Colleselli K, Poisel S, Feichtinger H, Pontasch H, Bartsch G. Rationale and technique of nerve sparing radical cystectomy before an orthotopic neobladder procedure in women. J Urol. 1995;154(6):2044–9.
Stenzl A, Draxl H, Posch B, Colleselli K, Falk M, Bartsch G. The risk of urethral tumors in female bladder cancer: can the urethra be used for orthotopic reconstruction of the lower urinary tract? J Urol. 1995;153(3 Pt 2):950–5.
Gakis G, Stenzl A. Considerations for orthotopic diversions in women. Curr Opin Urol. 2015;25(6):550–4.
Stein JP, Cote RJ, Freeman JA, Esrig D, Elmajian DA, Groshen S, et al. Indications for lower urinary tract reconstruction in women after cystectomy for bladder cancer: a pathological review of female cystectomy specimens. J Urol. 1995;154(4):1329–33.
Mills RD, Studer UE. Female orthotopic bladder substitution: a good operation in the right circumstances. J Urol. 2000;163(5):1501–4.
Osman Y, Mansour A, El-Tabey N, Abdel-Latif M, Mosbah A, Hekal I, et al. Value of routine frozen section analysis of urethral margin in male patients undergoing radical cystectomy in predicting prostatic involvement. Int Urol Nephrol. 2012;44(6):1721–5.
Gakis G, Ali-El-Dein B, Babjuk M, Hrbacek J, Macek P, Burkhard FC, et al. Urethral recurrence in women with orthotopic bladder substitutes: A multi-institutional study. Urol Oncol. 2015;33(5):204. e17–23.
Stenzl A, Jarolim L, Coloby P, Golia S, Bartsch G, Babjuk M, et al. Urethra-sparing cystectomy and orthotopic urinary diversion in women with malignant pelvic tumors. Cancer. 2001;92(7):1864–71.
Jentzmik F, Schrader AJ, de Petriconi R, Hefty R, Mueller J, Doetterl J, et al. The ileal neobladder in female patients with bladder cancer: long-term clinical, functional, and oncological outcome. World J Urol. 2012;30(6):733–9.
Hautmann RE, Abol-Enein H, Davidsson T, Gudjonsson S, Hautmann SH, Holm HV, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: urinary diversion. Eur Urol. 2013;63(1):67–80.
Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, Ribal MJ, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59(6):1009–18.
Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–92.
Kim KH, Yoon HS, Yoon H, Chung WS, Sim BS, Ryu DR, et al. Risk factors for developing metabolic acidosis after radical cystectomy and ileal neobladder. PLoS One. 2016;11(7):e0158220.
Nishikawa M, Miyake H, Yamashita M, Inoue TA, Fujisawa M. Long-term changes in renal function outcomes following radical cystectomy and urinary diversion. Int J Clin Oncol. 2014;19(6):1105–11.
Oderda M, Mondino P, Lucca I, Fiorito C, Gillo A, Zitella A, et al. Fatal haematuria in a patient with an orthotopic neobladder and chronic liver failure. Urol Int. 2009;83(3):368–9.
Al Hussein Al Awamlh B, Wang LC, Nguyen DP, Rieken M, Lee RK, Lee DJ, et al. Is continent cutaneous urinary diversion a suitable alternative to orthotopic bladder substitute and ileal conduit after cystectomy? BJU Int. 2015;116(5):805–14.
Hinata N, Murakami G, Abe S, Honda M, Isoyama T, Sejima T, et al. Detailed histological investigation of the female urethra: application to radical cystectomy. J Urol. 2012;187(2):451–6.
Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45–57.
Colleselli K, Stenzl A, Eder R, Strasser H, Poisel S, Bartsch G. The female urethral sphincter: a morphological and topographical study. J Urol. 1998;160(1):49–54.
Miocinovic R, Gong MC, Ghoneim IA, Fergany AF, Hansel DE, Stephenson AJ. Presacral and retroperitoneal lymph node involvement in urothelial bladder cancer: results of a prospective mapping study. J Urol. 2011;186(4):1269–73.
Gakis G, Abdelhafez MF, Stenzl A. The “I-Pouch”: results of a new ileal neobladder technique. Scand J Urol. 2015;49(5):400–6.
Skinner EC, Fairey AS, Groshen S, Daneshmand S, Cai J, Miranda G, et al. Randomized trial of studer pouch versus T-pouch orthotopic ileal neobladder in patients with bladder cancer. J Urol. 2015;194(2):433–9.
Abol-Enein H, Ghoneim MA. Functional results of orthotopic ileal neobladder with serous-lined extramural ureteral reimplantation: experience with 450 patients. J Urol. 2001;165(5):1427–32.
De Sutter T, Akand M, Albersen M, Everaerts W, Van Cleynenbreugel B, De Ridder D, et al. The N-shaped orthotopic ileal neobladder: functional outcomes and complication rates in 119 patients. Springerplus. 2016;5:646.
Mischinger J, Abdelhafez MF, Todenhofer T, Schwentner C, Aufderklamm S, Stenzl A, et al. Quality of life outcomes after radical cystectomy: long-term standardized assessment of Studer Pouch versus I-Pouch. World J Urol. 2015;33(10):1381–7.
Gakis G, Witjes JA, Comperat E, Cowan NC, De Santis M, Lebret T, et al. EAU guidelines on primary urethral carcinoma. Eur Urol. 2013;64(5):823–30.
Ali-El-Dein B, Mosbah A, Osman Y, El-Tabey N, Abdel-Latif M, Eraky I, et al. Preservation of the internal genital organs during radical cystectomy in selected women with bladder cancer: a report on 15 cases with long term follow-up. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2013;39(4):358–64.
Moursy EE, Eldahshoursy MZ, Gamal WM, Badawy AA. Orthotopic genital sparing radical cystectomy in pre-menopausal women with muscle-invasive bladder carcinoma: A prospective study. Indian J Urol J Urol Soc India. 2016;32(1):65–70.
Gregg JR, Emeruwa C, Wong J, Barocas DA, Chang SS, Clark PE, et al. Oncologic outcomes after anterior exenteration for muscle invasive bladder cancer in women. J Urol. 2016;196(4):1030–5.
Ali-El-Dein B. Oncological outcome after radical cystectomy and orthotopic bladder substitution in women. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2009;35(3):320–5.
Hautmann RE, Volkmer BG, Schumacher MC, Gschwend JE, Studer UE. Long-term results of standard procedures in urology: the ileal neobladder. World J Urol. 2006;24(3):305–14.
Harraz AM, Mosbah A, El-Assmy A, Gad H, Shaaban AA. Renal function evaluation in patients undergoing orthotopic bladder substitution: a systematic review of literature. BJU Int. 2014;114(4):484–95.
Pichler R, Zangerl F, Leonhartsberger N, Stohr B, Horninger W, Steiner H. Orthotopic bladder replacement in women: focus on functional results of a retrospective, single-centre study. Scand J Urol. 2013;47(4):295–301.
Stein JP, Dunn MD, Quek ML, Miranda G, Skinner DG. The orthotopic T pouch ileal neobladder: experience with 209 patients. J Urol. 2004;172(2):584–7.
Bailey GC, Blackburne A, Ziegelmann MJ, Lightner DJ. Outcomes of surgical management in patients with stress urinary incontinence and/or neovesicovaginal fistula after orthotopic neobladder diversion. J Urol. 2016;196(5):1478–83.
Hautmann RE, de Petriconi R, Kleinschmidt K, Gottfried HW, Gschwend JE. Orthotopic ileal neobladder in females: impact of the urethral resection line on functional results. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(4):224–9. discussion 30.
Carmel ME, Goldman HB, Moore CK, Rackley RR, Vasavada SP. Transvaginal neobladder vaginal fistula repair after radical cystectomy with orthotopic urinary diversion in women. Neurourol Urodyn. 2016;35(1):90–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Gakis, G. (2017). Orthotopic Urinary Diversion in Women. In: Daneshmand, S. (eds) Urinary Diversion . Springer, Cham. https://doi.org/10.1007/978-3-319-52186-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-52186-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52185-5
Online ISBN: 978-3-319-52186-2
eBook Packages: MedicineMedicine (R0)