Abstract
Scenedesmus spp. have been reported as potential microalgal species used for the lipid production. This study investigated the effects of light intensity (at three levels: 50, 250, and 400 μmol photons m−2 s−1) on the growth and lipid production of Scenedesmus sp. 11-1 under N-limited condition. Carotenoid to chlorophyll ratio was higher when algae 11-1 grew under 250 and 400 μmol photons m−2 s−1 than that under 50 μmol photons m−2 s−1, while protein contents was lower. Highest biomass yield (3.88 g L−1), lipid content (41.1 %), and neutral lipid content (32.9 %) were achieved when algae 11-1 grew at 400 μmol photons m−2 s−1. Lipid production was slight lower at 250 μmol photons m−2 s−1 level compared to 400 μmol photons m−2 s−1. The major fatty acids in the neutral lipid of 11-1 were oleic acid (43–52 %), palmitic acid (24–27 %), and linoleic acid (7–11 %). In addition, polyunsaturated fatty acids had a positive correlation with total lipid production, and monounsaturated fatty acids had a negative one.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Consumption of fossil fuels is one of the most concerned factors that contribute to the climate change. It is a pressing mission for humans to find an alternative fuel. The biodiesel based on soybean, Trachycarpus fortunei, rapeseed, and other oil plants has been reported to be suitable as a transport fuel. However, oil plants have low energy intensities per hectare due to the large amount of land use; it is still difficult for biodiesel from oil plants to compete with fossil fuel.
Presently, microalgae are considered to be a potential feedstock for biodiesel production [1]. Compared to other oil plants, usage of microalgae would not be a direct threat of food supplies due to its low cultivation space requirement and high lipid productivity. It was reported that microalgae started to accumulate lipids when some nutrients are exhausted. Lipid contents of various microalgal species range from 20 to 50 % [1], and the lipid content in the same species changes in response to cultivation conditions. Former studies indicated that Scenedesmus spp. was a potential microalgal species for lipid production, and the production was relative to incubation conditions, including CO2 supplement, nutrient condition, and temperature. [2–4].
The fatty acid composition of lipid is a key factor impacting the properties of biodiesel. It was reported that the fatty acid composition of Scenedesmus sp. responded to different conditions, including nitrogen concentration and temperature [4, 5]. In addition to nutrient levels and temperature, the light intensity is also an important factor affecting the growth and lipid accumulation, and it could also affect fatty acid composition in the microalgae Parietochloris incise and Dunaliella viridis [6, 7]. However, effect of the light intensity on lipid production and fatty acid composition in Scenedesmus spp. has not been reported.
Microalgae were frequently cultivated at nitrogen deficiency mediums that allowed a nitrogen-free period present during its cultivation to attain higher lipid productivity [5, 8, 9]. In this study, we investigated the effect of light intensity on the growth characteristics, total and neutral lipids accumulations, and fatty acid compositions of neutral lipids of Scenedesmus sp. 11-1 under nitrate deficiency condition.
Methods
Strain and Culturing Conditions
Microalga strain 11-1 was isolated from a wastewater pond in Qingdao, China, conserved in culture bank of Qingdao Institute of Bioenergy and Bioprocess Technology, and identified as Scenedesmus sp. by 18S rRNA gene sequencing and morphology characterization. The culture medium was modified BG-11, in which the concentration of NaNO3 was decreased to 4.41 mmol L−1, one fourth of normal BG-11 [10]. The strain was cultivated in a column with a diameter of 42 mm, effective volume of 0.6 L, and blowing air supplemented with CO2 (2 %, v/v) at bottom at 25 °C. Trichromatic lamps (Y228-T5, NVC Lighting Technology Corporation, China) were used as the light source, and their spectrum range was 380–780 nm. All of the six cultures were cultivated at the light intensity of 50 μmol photons m−2 s−1 for the first 2 days. They were then divided into three groups grown under high light intensity (HL, 250 μmol photons m−2 s−1 on each side, 400 μmol photons m−2 s−1 in total), medium light intensity (ML, 250 μmol photons m−2 s−1 on one side) and low light intensity (LL, 50 μmol photons m−2 s−1 on one side), respectively. This experiment was conducted in duplicate.
Lipid Extraction
Microalgal cells were harvested by centrifugation at 3,000 rpm for 10 min. Cell pellets were lyophilized using a freeze drier (ALPHA1-2LD plus, Martin Christ, Germany). The microalgal cells were harvested by centrifugation at 8,000 rpm for 10 min and lyophilized using a freeze drier. The total lipids contained in the algal cells were extracted with a modified chloroform–methanol–water solvent system [11]. Dried algal powder (30–50 mg), 4 ml chloroform, and 2 mL methanol were added into 10-mL glass tube I and shaken for 10 s to disperse the powder. Tube I was incubated at 200 rpm for 12 h at 30 °C, centrifuged for 10 min at 3,500 rpm, and 6 mL of the supernatant was collected and transferred to tube II. Two milliliters methanol and 3.6 ml deionized water were added to reach a final chloroform/methanol/water ratio of 10:10:9. The chloroform layer in tube II was transferred into a preweighed tube III (w1) and dried for 30 min under a flow of N2 at 60 °C after centrifuging at 3,500 rpm for 5 min. Tube III was dried using a vacuum-drying oven (Lantian DZF-6050, Hangzhou, China) at 0.09 MPa and 60 °C for 1.5 h and then weighed (w2). The lipid weight was calculated by subtracting w1 from w2. The extraction was performed in duplicates for each sample.
Separation of Neutral Lipid and Transesterification
The neutral lipid was separated from the total lipid by column chromatography [12] and weighted.
Methyl esters were extracted from the microalgae lipids by heating in 2 % H2SO4–methanol solution at 85 °C for 2.5 h. After cooling to room temperature, 1 mL of high-performance liquid chromatography grade hexane and 1 mL of saturated NaCl solution were added. The mixture was centrifuged for 10 min at 3,000 rpm, and the upper layer containing fatty acid methyl esters (FAMEs) was obtained for further analysis.
FAME samples were analyzed on a gas chromatography (7890A, Agilent, USA)–mass spectrometry (5975C, Agilent, USA) in electron impact mode with a HP-Innowax polyethylene glycol column (30 m × 250 μm × 0.25 μm). The carrier gas was helium. The injection temperature was 250 °C. The initial column temperature was 25 °C. It was elevated to 200 °C at a temperature gradient of 25 °C min−1 and was then elevated to 230 °C at a temperature gradient of 3 °C min−1. The temperature was kept constant at 230 °C for 11 min. The fatty acid compositions were recorded as percentages of total fatty acids.
Determination of Nitrate Concentration
The nitrate concentrations were detected by a UV spectrophotometer (Cary 50, Varian, USA) at 220 and 275 nm as described before [13].
Determination of Pigment Concentration
Two milliliters microalgal cell suspension was centrifuged at 3,000 rpm for 5 min, and the cell sediments were resuspended in 5 ml 95 % (v/v) ethanol in dark for 12 h. Afterwards, the cell suspension was centrifuged for 10 min at 3,000 rpm. The absorbance (A) of the supernatant was measured at 665, 649, and 470 nm using a spectrophotometer (Cary 50, Varian, USA). The concentrations of pigments, including chlorophyll a, chlorophyll b, chlorophyll, and carotenoid, were calculated by the following equations [14]:
C a, C b, C chl, and C car represented the concentration (mg L−1) of chlorophyll a, chlorophyll b, chlorophyll, and carotenoid, respectively.
Determination of Protein Yield and Elements Analysis
One milliliter microalgal cells were obtained by centrifugation at 3,000 rpm for 5 min. The cells were resuspended and incubated in a 0.5 mol L−1 NaOH solution for 10 min at 100 °C, and total protein yield was then measured by BCA Protein Assay Kit (Bestbio, China).
The elements of algae powder were analyzed using an elemental analyzer (Vario EL cube, Elementer, Germany). The heating value Q (kJ g−1) was calculated according to Dulong’s formula [15]:
where C, H, and O are weight percentages of carbon, hydrogen, and oxygen in algal dried cells, respectively.
Statistical Analysis
Two-tailed paired t tests were applied to ascertain significant differences using SPSS 10, and the level of statistical significance was P < 0.05.
Results and Discussion
The Effect of the Light Intensity on the Growth of Scenedesmus sp. 11-1
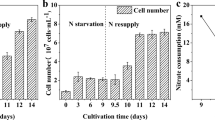
As shown in Fig. 1, it took 6, 8, and 10 days for strain 11-1 to reach stationary phase under the light intensities of 400, 250, and 50 μmol photons m−2 s−1, respectively. The total biomass yields were 3.88, 3.62, and 2.55 g L−1 under HL, ML, and LL, respectively (Fig. 1).
The difference of growth rates among the HL, ML, and LL cultures indicated that 400 μmol photons m−2 s−1 was more suitable for the growth of strain 11-1 than other light intensities if nutrients were abundant in the medium. However, nitrogen deficiency became the more important factor to limit the growths of HL and ML cultures after day 4 than light intensity, which could explain why the two cultures had a similar level of final biomass yield. An increased growth rate corresponding to the light intensity was also noted in Dunaliella tertiolecta when it was cultivated at 100, 200, and 350 μmol photons m−2 s−1 [16]. In the study about microalgae D. viridis, the growth rate rose with the increased light intensity below the level of 700 μmol photons m−2 s−1 but decreased when the light intensity changed from 700 to 1,500 μmol photons m−2 s−1 [17]. The typical midday outdoor light intensity is about 2,000 μmol photons m−2 s−1 in equatorial regions. The light saturation constants of some algae are much lower than the value of sunlight. For example, the light saturations were 185 [18] and 200 μmol photons m−2 s−1 [19] for Phaeodactylum tricornutum and Porphyridium cruentum, respectively. Thus, the high lipid productivity should be achieved in a photobioreactor reducing the light intensity below the light saturation constant when a microalga was cultivated outdoor.
Effect of Light Intensity on Pigment Content
The volumetric contents of carotenoid and chlorophyll both reached their highest level on day 4 in HL and ML cultures (Fig. 2a, b), while they reached the highest level on day 6 in LL culture. The contents of pigments (percentage of dry cell weight) especially chlorophyll decreased significantly from day 2 (Fig. 2a, b). The ratios of the chlorophyll content on day 12 were approximately 1:77, 1:26, and 1:4 of those on day 2 in HL, ML, and LL cultures, respectively. These reductions may be caused by the nitrate depletion since nitrogen is an essential element for the synthesis of chlorophyll. Meanwhile, the light available per cell was reduced due to the increased cells density.
The ratios of carotenoid to chlorophyll increased from 0.16 on day 2 to 1.01, 0.49, and 0.20 on day 12 under HL, ML, and LL, respectively (Fig. 2c), which indicated that the ratios had a positive relationship with light intensity. Given that the spectral absorption peak of carotenoid is in blue light band and that of chlorophyll in both of red and blue light band, the blue light would be more preferable for microalgal growth than the red light if that ratio increased. Previous reports reported that carotenoid contents would increase in the condition of nitrogen starvation [20] or high light intensity [21, 22]. Astaxanthin, as a main part of carotenoids, may play a role in protection against photo damages by reducing amount of light available to the light harvesting pigment–protein complex [23] and acts as an antioxidant-inhibiting lipid preoxidation [24]. The increase in the ratio of carotenoid to chlorophyll was even found to correlate closely with the volumetric content of total fatty acids [25].
Effect of Light Intensity on Protein Content
The percentages of nitrogen element content of dried algal cells were 1.37, 1.47, and 2.14 % in HL, ML, and LL cultures on day 12, respectively (Fig. 3a). There was a negative correlation between nitrogen and lipid content in algal cells, which confirmed the accelerate effect of nitrogen limitation on lipid accumulation.
The contents of protein were 6.1, 6.4, and 11.1 % in dried algal cells from HL, ML, and LL cultures on day 12, respectively (Fig. 3b). The microalgae in LL culture had higher protein content than those in ML and HL cultures after day 4. Previous studies presented similar results: When cells of P. tricornutum were grown at 18, 36, and 72 μmol photons m−2 s−1, the lower light was responsible for a lower biomass yield and lower lipid content, but higher protein content [6].
Effect of Light Intensity on Total Lipids and Neutral Lipids Production
The highest lipid content (41.1 %) and yield (1.60 g L−1) were both obtained from the HL culture on day 12 (Fig. 4a, b). A slightly decreased lipid content (39.2 %) and yield (1.42 g L−1) were found in the cells of ML culture. However, the lipid content (26.2 %) and yield (0.67 g L−1) were significantly lower in LL culture than those in HL and ML cultures. Thus, the biomass yield and lipid content could be promoted by increasing the light intensity from 50 μmol photons m−2 s−1 to a level at the range of 250–400 μmol photons m−2 s−1. After cultivation of 12 days, the lipid yields obtained on day 12 were 79, 127, and 245 % higher than those obtained on 6 days cultivation in HL, ML, and LL cultures, respectively (Fig. 4b). Thus, it was suggested to enhance cultivation time to achieve a higher lipid yield of microalgae.
The percentages of neutral lipids of total lipid were 80.0, 82.3, and 62.9 % in microalgal cells in HL, ML, and LL cultures on day 12, respectively (Fig. 5a), and they were corresponding to 32.9, 32.3, and 16.5 % as the percentages of dry cell weight for HL, ML, and LL cultures, respectively. The neutral lipids were only 37.0 and 36.7 % of total lipid in HL and ML cultures, respectively, on day 6. These results indicated a significant accumulation of neutral lipid from days 6 to 12. The harvesting time should be determined not only by lipid productivity but also by lipid content in algal cells in order to cut down the cost in following processes of biodiesel production. Moreover, although there was no significant enhancement in biomass in stationary phase (Fig. 1), the heating value of biomass could be increased due to the accumulation of lipid, an organic with high heating value. The heating value increased from 23.9 kJ g−1 on days 6 to 27.6 kJ g−1 on day 12 in ML culture, while it increased from 24.6 to 28.2 kJ g−1 in HL culture. It is distinctly advantageous to use the biomass with high heating value in a pyrolysis process. Therefore, it could be acceptable to delay harvesting microalgae to a moment after the greatest lipid productivity appeared. The final neutral lipid yields on day 12 were 1.28, 1.16, and 0.38 g L−1 in HL, ML, and LL cultures, respectively (Fig. 5b). HL and ML cultures significantly enhanced neutral lipid yields than LL culture, which indicated that 250–400 μmol photons m−2 s−1 would be a suitable range of light intensity for the accumulation of neutral lipid in column bioreactors used for the batch culturing system. Considering energy income, light intensity of 250 μmol photons m−2 s−1 would be better.
Triacylglycerol, the main part of neutral lipids, serves as a sink of excessive energy absorbed by photosynthetic apparatus in microalgae [26]. Triacylglycerol synthesis requires large amounts of ATP and NAD(P)H produced by the photosynthesis. Therefore, it may be helpful in the dissipation of excess light energy and prevention of the photochemical damage of algal cells [27]. Thus, more carbon flux generated from the photosynthesis is channeled to the lipid accumulation on a unit biomass basis when individual cell is exposed to a large quantity of light energy [7, 28]. Therefore, the high and medium lights could promote the final biomass yield, lipid content, and yield, especially neutral lipid content.
Fatty Acid Composition of Neutral Lipids
The major fatty acids of neutral lipid were oleic acid (43–52 %), palmitic acid (24–27 %), and linoleic acid (7–11 %) in strain 11-1 (Table 1). Eighty percent of the total fatty acids were composed of the three fatty acids. The fatty acids with carbon-16 and carbon-18 atoms were the main components in the neutral lipid, which was similar to traditional vegetable oils and adequate for biodiesel production [29–31].
The fatty acid profiles of neutral lipids were similar when strain 11-1 grew under HL and ML. Higher polyunsaturated fatty acid (PUFA) contents and lower monounsaturated fatty acid (MUFA) contents were noted in the cells when it grew under HL and ML compared to LL (Table 1). The increase in PUFA associated to light intensity was also noted in the microalgae Pavlova lutheri [28, 32]. By contrast, Liang et al. [33] reported that six strains of marine diatoms had higher contents of PUFA in total lipid when growing under a lower light intensity. They also found that the increase in PUFA and the decrease in MUFA were correlated with the increased neutral lipid content [33]. These results were similar to the study of Solovchenko et al. [7] but opposite to the study of Lin et al. [5]. Although the lipid containing a high content of PUFA may make the biodiesel susceptible to oxidation, it is easy to improve the saturation degree in certain chemical processes [1].
Although a higher lipid yield of 2.16 g L−1 (0.12 g L−1 day−1 as the volumetric productivity) was recorded in Scenedesmus obliquus, it should be noted that a supplementation of 1.5 % glucose in the first 10 days’ cultivation and an optimization of nutrient conditions in the next 8 days’ cultivation were present [3]. Previous studies showed a volumetric lipid productivity of 0.065 g L−1 day−1 in Neochloris oleoabundans and 0.079 g L−1 day−1 in S. obliquus. They were both considered to be excellent lipid producers [2, 9]. The highest volumetric lipid productivity of 11-1 was 0.133 g L−1 day−1 in 12-day cultivation. Meanwhile, strain 11-1 showed higher lipid productivity than the other 20 strains isolated in the same period of our lab, whose lipid productivities ranged from 0.043 to 0.080 g L−1 day−1. According to the previous reports [2, 8], Scenedesmus sp. 11-1 could be an excellent candidate used for biodiesel production due to its high lipid (especially neutral lipid) productivity and high oleic acid content. Moreover, Scenedesmus sp. could be cultivated using the nutrients in wastewater [34, 35], which provided a way to realize the economic viability in industry [36, 37]. Meanwhile, the light intensities used in the studies of Mandal and Mallick [3] and Ho et al. [2] were 75 and 60 μmol photons m−2 s−1, respectively, which may be low to achieve the highest lipid productivity. A higher light intensity, like 250 μmol photons m−2 s−1, was suggested for evaluating lipid productivity of Scenedesmus sp.
Conclusion
The high light intensities at a range of 250–400 μmol photons m−2 s−1 were found to be beneficial to the growth and lipid accumulation of Scenedesmus sp. 11-1 under nitrogen limited condition. The amounts of total and neutral lipids were significantly higher in the late growth stage (days 6–12) due to the nitrogen limitation. The increase in light intensity promoted the content of PUFA in neutral lipid and the ratio of carotenoid to chlorophyll. The highest total and neutral lipid productivities of strain 11-1 were 0.133 and 0.106 g L−1 day−1, respectively. Microalgae strain 11-1 has potential to be a promising candidate for biodiesel production.
References
Chisti, Y. (2007). Biotechnology Advances, 25, 294–306.
Ho, S., Chen, W., & Chang, J. (2010). Bioresource Technology, 101, 8725–8730.
Mandal, S., & Mallick, N. (2009). Applied Microbiology and Biotechnology, 84, 281–291.
Li, X., Hu, H., & Zhang, Y. (2010). Bioresource Technology, 102, 3098–3102.
Lin, Q., & Lin, J. (2010). Bioresource Technology, 102, 1615–1621.
Qian, K., & Michael, B. (1993). Applied Biochemistry and Biotechnology, 38, 93–103.
Solovchenko, A. E., Khozin-Goldberg, I., Didi-Cohen, S., Cohen, Z., & Merzlyak, M. N. (2008). Journal of Applied Phycology, 20, 245–251.
Griffiths, M., & Harrison, S. (2009). Journal of Applied Phycology, 21, 493–507.
Pruvost, J., Van Vooren, G., Cogne, G., & Legrand, J. (2009). Bioresource Technology, 100, 5988–5995.
Andersen, R. (2005). Algal culturing techniques. Burlington: Elsevier.
Bligh, E. G., & Dyer, W. J. (1959). Canadian Journal of Biochemistry and Physiology, 377, 911–917.
BorgstrÖM, B. (1952). Acta Physiologica Scandinavica, 25, 101–110.
Beschkov, V., Velizarov, S., Agathos, S. N., & Lukova, V. (2004). Biochemical Engineering Journal, 17, 141–145.
Lichtenthaler, H. K., & Wellburn, A. R. (1983). Biochemical Society Transactions, 603, 591–592.
Minowa, T., Yokoyama, S-y, Kishimoto, M., & Okakurat, T. (1995). Fuel, 74, 1735–1738.
Tang, H., Abunasser, N., Garcia, M. E. D., Chen, M., Simon Ng, K. Y., & Salley, S. O. (2010). Applied Energy, 88, 3324–3330.
Gordillo, F., Goutx, M., Figueroa, F., & Niell, F. (1998). Journal of Applied Phycology, 10, 135–144.
Mann, J. E., & Myers, J. (1968). Journal of Phycology, 4, 349–355.
Molina, E., Acién Fernández, F. G., García Camacho, F., Camacho Rubio, F., & Chisti, Y. (2000). Journal of Applied Phycology, 12, 355–368.
Boussiba, S., & Vonshak, A. (1991). Plant & Cell Physiology, 32, 1077–1082.
Solovchenko, A. E., Khozin-Goldberg, I., Didi-Cohen, S., Cohen, Z., & Merzlyak, M. N. (2008). Russian Journal of Plant Physiology, 55, 455–462.
Zhekisheva, M., Boussiba, S., Khozin-Goldberg, I., Zarka, A., & Cohen, Z. (2002). Journal of Phycology, 38, 325–331.
Bidigare, R. R., Ondrusek, M. E., Kennicutt, M. C., Iturriaga, R., Harvey, H. R., Hoham, R. W., et al. (1993). Journal of Phycology, 29, 427–434.
Hagen, C., Braune, W., & Greulich, F. (1993). Journal of Photochemistry and Photobiology B: Biology, 20, 153–160.
Solovchenko, A., Khozin-Goldberg, I., Cohen, Z., & Merzlyak, M. (2009). Journal of Applied Phycology, 21, 361–366.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., et al. (2008). The Plant Journal, 54, 621–639.
Roessler, P. G. (1990). Journal of Phycology, 26, 393–399.
Courchesne, N. M. D., Parisien, A., Wang, B., & Lan, C. Q. (2009). Journal of Biotechnology, 141, 31–41.
Sydney, E. B., da Silva, T. E., Tokarski, A., Novak, A. C., de Carvalho, J. C., Woiciecohwski, A. L., et al. (2010). Applied Energy, 88, 3291–3294.
Abou-Shanab, R. A. I., Hwang, J., Cho, Y., Min, B., & Jeon, B. (2011). Applied Energy, 88, 3300–3306. doi:10.1016/j.apenergy.2011.01.060.
Harrington, K. J. (1986). Biomass, 9, 1–17.
Guihéneuf, F., Mimouni, V., Ulmann, L., & Tremblin, G. (2009). Journal of Experimental Marine Biology and Ecology, 369, 136–143.
Liang, Y., Mai, K., Sun, S., & Yu, D. (2001). Chinese Journal of Oceanology and Limnology, 19, 249–254.
Kim, M. K., Park, J. W., Park, C. S., Kim, S. J., Jeune, K. H., Chang, M. U., et al. (2007). Bioresource Technology, 98, 2220–2228.
Bhatnagar, A., Chinnasamy, S., Singh, M., & Das, K. C. (2010). Applied Energy, 88, 1–7.
Pittman, J. K., Dean, A. P., & Osundeko, O. (2011). Bioresource Technology, 102, 17–25.
Rawat, I., Ranjith Kumar, R., Mutanda, T., & Bux, F. (2010). Applied Energy, 88, 3411–3424. doi:10.1016/j.apenergy.2010.11.025.
Acknowledgment
This study was supported by grant KGCX2-YW-374-3 from Chinese Academy of Sciences and grant 2008ZX07422-003-5-2 from the Ministry of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Yuan, C., Hu, G. et al. Effects of Light Intensity on the Growth and Lipid Accumulation of Microalga Scenedesmus sp. 11-1 Under Nitrogen Limitation. Appl Biochem Biotechnol 166, 2127–2137 (2012). https://doi.org/10.1007/s12010-012-9639-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9639-2