Abstract
Glioblastoma (GBM) is a highly malignant form of brain tumour for which the prognosis is generally very poor and treatment options are limited. GBM is associated with rapid and aggressive tumour growth with associated cerebral oedema. Central to the difficulty associated with treating GBM is the challenge of getting chemotherapeutic drugs to cross the blood-brain barrier (BBB). Although vasculature within and around a GBM becomes more permeable due to pathological changes in the BBB, large areas of the tumour remain resistant to systemically administered agents. Here, we will introduce the concept of the BBB and its normal role in the healthy brain before describing how it becomes compromised in cases of GBM. This will cover physiological, genetic and functional aspects of BBB function and dysfunction. Finally, the therapeutic implications of modulating BBB permeability and receptor-mediated transport will be discussed with a focus on chemotherapeutic drug delivery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
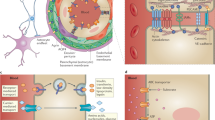
In order for the central nervous system to work effectively and efficiently, it requires a tightly regulated means of supplying neurons with nutrients and removing unwanted substrates from the cerebrospinal fluid (CSF). This is achieved by dense vascularisation of the cerebral parenchyma [1] where controlled trafficking of molecular species between the central nervous system and the periphery can occur. Access to the central nervous system is restricted by the blood-brain barrier (BBB), a complex interface between the blood stream and the cerebral parenchyma [2]. The presence of tight junctions and transporter proteins allows the BBB to selectively regulate the passage of molecules to and from the brain [3–5].
Glioblastoma (GBM) is a p articularly malignant form of brain tumour for which the prognosis is generally poor and treatment options are limited [6]. GBM is associated with rapid and aggressive tumour growth with an associated cerebral oedema, and prognosis is poor for patients diagnosed with the condition [7]. Part of the difficulty in treating in GBM is the challenge of getting chemotherapeutic drugs to cross the BBB, even though the BBB in GBM becomes more permeable due to pathological changes in the BBB [8–11]. However, dynamic changes in BBB permeability can be achieved by targeting these tight junctions and transporters with suitable treatments [12]. This can be used to increase the efficacy of drug delivery [13] or removal of pathological material from the cerebral parenchyma, for example, the removal of amyloid beta in Alzheimer’s disease [14].
The aim of this chapter will be to introduce readers to the BBB and its normal role in the healthy brain before describing how it becomes compromised in cases of GBM. This will cover physiological, genetic and functional aspects of BBB function and dysfunction. Finally, the therapeutic implications of modulating BBB permeability and receptor-mediated transport will be discussed with a focus on chemotherapeutic drug delivery.
4.2 The BBB in Normal and Pathological Conditions
Normal brain function requires rapid and controlled access to metabolic resources and molecular products from the periphery. At the same time, removal of unwanted material from the brain to the periphery is also vital. As such, the brain has evolved a pervasive and efficient vascular system that permeates through it; it is estimated that a neuron is never further than 20 μm from a capillary. Recent 3D imaging of the mouse brain shows that penetrating vessels with a diameter of approximately 23 μm access the tissue before branching into microcapillaries as small as 3 μm in diameter, ensuring a dense vascularisation of the brain [1]. The issue of supply of necessary metabolites and removal of waste is well catered for by this extensive microcapillary system, and access to the cerebral parenchyma is restricted by the BBB so that dangerous biochemical species cannot cause damage to delicate neuronal tissue.
The BBB itself is composed of a layer of endothelial cells lining the lumen of the capillary in order to create an interface between peripheral circulation and the cerebral parenchyma where substances can then enter the cerebrospinal fluid (CSF ). Endothelial cells control transcellular transport from the blood into the CSF , expressing transporters for molecules that are essential for normal metabolism and homoeostasis such as glucose, insulin and amino acids [3, 5]. The endothelial cells form adherens junctions and tight junctions between each other in order to limit paracellular transport between the vascular and cerebral compartments [12]. Interendothelial adherens junctions are mainly composed of members of the cadherin family such as vascular endothelial (VE )-cadherin and N-cadherin [15]. Tight junctions are composed of about 30 proteins including occludin, tricellulin and members of the claudins and junction adhesion molecule families [16, 17]. Tight junction proteins are anchored to the intracellular cytoskeleton of the endothelial cells by transmembrane proteins such as zonula occludens-1 (ZO-1) [18]. Tight junctions restrict molecules with a size greater than 400–450 Da from passing between endothelial cells [19, 20]. Furthermore, movement of small ions across the BBB is also restricted given its electrical resistance, on average around 1870 omega (Ω) cm2 [21]. Taken together, the endothelial cell layer can regulate access to and from the central nervous system to a high degree, with the result that approximately 98 % of drugs developed to target neurological disorders being unable to cross the BBB [22].
The microcapillaries that supply the brain are surrounded by a group of different cell types that form the neurovascular unit (NVU ), including neurons, astrocytes, microglia and pericytes. The non-neuronal cells of the NVU act both as scaffolding and as mediators of molecular transport into and out of the brain. Within the NVU , contractile pericytes form layers on the abluminal surface of the microcapillaries, regulating permeability in the mature BBB [23]. Pericytes are covered by a macromolecular layer known as the basal lamina which is in turn enclosed by perivascular endfeet from astrocytes to create a supportive sheath (the glia limitans) around the microcapillaries that supply the brain [24–26]. Microglia then form a line of protection on the brain side of the BBB, able to mount an immune response should an unwanted substance make it through the BBB [27].
Although the cerebral endothelial cell layer is the main workhorse of the BBB, the pericytic and glial support structure surrounding the microvasculature is necessary to the development and maintenance of BBB integrity and functionality. Development of the BBB is achieved by radial glia first via the production of retinoic acid to induce BBB formation and secondly by stabilisation of developing vasculature through Wnt signalling [28]. At the same time, interactions between pericytes and endothelial cells functionally regulate BBB integrity [29] via signalling involving transforming growth factor β (TGFβ) [30], platelet-derived growth factor B [31] and the forkhead transcription factor Foxf2 [32]. Once the BBB is mature, astrocytes form endfoot projections that can modulate permeability—possibly through TGFβ [33] and angiotensin signalling [34]. In the NVU, astrocytes act as a go-between for neurons and vasculature, synchronising cerebral blood flow, maintaining homoeostasis and regulating water content in the cerebral parenchyma [25]. Astrocytic endfeet are known to regulate transport of Na+ and Cl− across the endothelial cell layer [35] through intercellular Ca2+ signalling between astrocytes and endothelial cells [36]. Astrocytic endfeet also regulate neurovascular coupling via a nitric oxide-dependent intracellular Ca2+ signalling cascade [37], ensuring that metabolic supply is maintained during neuronal activation. Pericytes also play a role in controlling the environment within the NVU through the regulation of cerebral blood flow [38] and by coordinating astrocytic endfoot position on the walls of the cerebral vasculature [39].
It is important to note that the BBB is not homogenous; different regions show variations in permeability. For example, the circumventricular organs entirely lack a layer of endothelial cells and have almost free access to peripheral blood flow [40, 41]. Even where an endothelial cell layer is well established, the permeability of the BBB is not a static value as BBB permeability is a dynamic process with up- and down-regulation of tight junction proteins occurring constantly [12]. Diurnal and seasonal effects on BBB permeability have been described [42, 43], and recent work from our group shows that concentrations of molecular regulators of BBB permeability follow a circadian rhythm (unpublished data). This reinforces the idea that the BBB is a constantly changing entity under normal conditions and this dynamism requires careful consideration in addressing questions about pathological processes and therapeutic interventions.
4.3 The Compromised BBB in GBM
GBM is a malignant class of Grade IV tumours that tends to form in the brain or spinal cord, mainly in adults aged 50–60 [44]. The prognosis for GBM is poor in many cases given the aggressive growth of the tumour and difficulties in treating GBM with standard oncological treatments [45, 46]. This means that the five-year survival of patients diagnosed with GBM is only 1.9 % in patients undergoing radiotherapy alone [7]. Typically, abnormal differentiation of brain tissue results in a mass of cancerous tissue though the exact source of the initial insult is under debate. It has been suggested that GBMs develop from aberrant glial cells but more recently the idea that cancer stem cells (CSCs ) are responsible for the condition. These CSCs develop from a suitable progenitor cell line where the normal developmental cascade has been altered leading to unregulated growth [44]. Given the fact that GBMs tend to form numerous cell types during their growth, it is likely that multipotent progenitor cells are at fault in this condition [47–50] and the location of GBM development within the brain may further influence the fate of these CSCs [51, 52]. In particular, co-activation of the Ras and Akt pathways has been identified as being necessary for GBM induction [53, 54]. The role of Ras is confirmed by the presence of altered Notch signalling in GBM cell lines [55]. Ras has also been implicated in the maintenance of GBM with suppression of Kras expression resulting in GBM apoptosis and regression in a mouse model of GBM [56].
Tumour growth and maintenance are facilitated by newly formed blood vessels that give the cancerous cells access to the peripheral blood supply; a well-established hallmark of higher-grade brain tumours is an extensive network of microcapillaries within the cancerous region [57–59]. Out of all brain cancers, GBM shows a relatively high level of biomarkers relating to proliferation and angiogenesis [60], which is unsurprising given the aggressive nature of GBM. There seems to be a reciprocal relationship between the developing tumour and the vasculature of the brain; there are high levels of perivascular nestin-positive CSCs in the early stages of GBM growth [61], nestin being a marker of angiogenesis that is upregulated in cancerous cells [62]. Calabrese and colleagues (2007) also describe direct interaction between cultured CSCs and endothelial cells and, most importantly, that endothelial cells promote GBM development in vivo. This is a subversion of the regulatory role of endothelial cells on normal neural stem cell development [63].
A number of molecular pathways regulating angiogenesis in GBMs have now been identified. Vascular endothelial growth factor (VEGF ) in particular stands out as an important regulator of angiogenesis [64]. CSCs actively promote VEGF signalling, directly acting on local endothelial cells to promote angiogenesis [65]. Normally, endothelial cells do not express the tyrosine kinase receptor for VEGF , but it is expressed on endothelial cells associated with tumour formation [64]. Inhibition of VEGF signalling following transfection of human GBM cells into the brains of nude mice was subsequently shown to inhibit GBM growth and decrease the rate of angiogenesis in vivo [66]. Specific targeting of the VEGF tyrosine kinase receptor using a diphtheria toxin conjugated to tumour-specific isoforms of the VEGF receptor has been also shown to prevent tumour-associated angiogenesis and inhibit GBM growth in vivo [67–69]. Translation of anti-VEGF treatments to human clinical cases of glioma has shown therapeutic promise; however GBM still remains resistant to treatment even with these new therapies [70–72]. Part of this resistance may be due to the issue with invasive cells that migrate away from the GBM core where vascularisation is at its most dense [73], a process that is itself dependent on VEGF signalling [74].
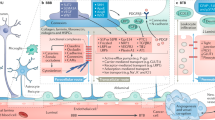
These new blood vessels develop a BBB but one that shows marked differences compared to that in normal tissue [4, 75] with alterations of both adherens [10, 11] and tight junction proteins [8, 9]. Additionally, epigenetic modulation of GBM development [76] and GBM’s susceptibility to radiation therapy [77] are associated with changes in the expression of markers for tight and adherens junctions, indicating an intimate relationship between endothelial integrity and GBM growth. It is well established that switching of cadherin expression in the adherens junction is involved in GBM development [78, 79] and these alterations have knock-on effects on tight junction stability [80, 81].
At the level of the tight junction, there is almost complete loss of claudin-1 [9] and reduced levels of claudin-3 [82], claudin-5 and occludin [9] associated with GBM. At the same time, decreased levels of claudin-1 and claudin-5 in human GBM samples are accompanied by significant increases in the expression of the adherens junction protein β-catenin [83]. Liebner and colleagues (2000) also describe alterations in plakoglobin and beta-catenin, further suggesting abnormally formed tight junctions in this pathological state. In clinical cases of GBM, these regions of abnormal tight junctions can be identified through contrast magnetic resonance imaging (MRI); a contrast agent (gadolinium) is injected into the patient and areas of high BBB permeability show a hyperintensive signal in T1-weighted scans [84, 85]. Using this method, regions of increased BBB permeability have been identified in patients [86], fitting with the molecular data from preclinical studies outlined above. This loss of tight junction integrity can be reversed in vitro using an anti-TGFβ antibody [4]. Interestingly, there appears to be a connection between TGFβ effect on endothelial cells and angiogenesis; in vitro analysis shows that TGFβ upregulates VEGF and inhibition of TGFβ signalling leads to an increase in claudin-5 levels [87], and human neuroimaging suggests that the extent of BBB “leakiness” is an indicator of patient survival [85].
Abnormal tight junctions in GBMs are associated with changes in basal lamina composition, namely, decreases in agrin, a basal lamina protein associated with BBB function, and increases in tenascin, which is normally absent in the basal lamina [88]. Data from an in vitro model of BBB using cultures of rat endothelial cells suggests that there may be a transient decrease in BBB permeability given that there is a fibroblast growth factor-2-dependent increase in occludin and ZO-1 protein levels following initial exposure to human GBM cells accompanied by an increase in transendothelial electrical resistance (TEER ) [89]. However, this may be a transient increase in tight junction efficiency that is lost as the GBM becomes established in the tissue.
The role of the BBB in GBM is of further clinical significance when considering how disruption in fluid clearance can lead to serious cerebral oedema [90]. Under normal conditions, the BBB is responsible for regulating osmotic processes via the aquaporin family of proteins [91, 92]. In particular, aquaporin-4 has a clear role in water transport; it is the most abundant water channel in the central nervous system and is found throughout the glia limitans in the astrocytic endfeet lining the BBB [93]. Aquaporin-4 is implicated in multiple regulatory processes including, but not limited to, regulation of extracellular space volume, circulation of CSF, waste clearance and cell migration [94]. Importantly, directly disrupting aquaporin-4 function using aquaporin-4-immunoglobulin G causes a significant increase in BBB breakdown [95].
As noted above, the BBB becomes disturbed within the GBM and cerebral oedema has been identified in regions neighbouring the GBM [86]. Changes in aquaporin expression have been described during brain tumour development, namely, increases in aquaporin-1 [96] and aquaporin-4 [97]. Aquaporin-4 has been shown to be responsible both for the induction of cerebral oedema and for its resolution in a number of patholog ical states [98, 99]. Nevertheless, even though aquaporin-4 expression increases along with levels of cerebral oedema [100], aquaporin-4 levels are not predictive of patient survival and may follow other processes involved in tumour growth rather than oedema itself [101]. This is supported by the association between increased aquaporin-4 expression in tumours and simultaneous increases in VEGF and hypoxia-inducible factor-1α [102], suggesting a link with angiogenesis during tumour development.
Increases in BBB permeability during GBM may also be linked to aquaporin expression; in GBM the expression of aquaporin-4 moves from its polarised configuration in the astrocytic endfeet [91] and instead covers the cell bodies of the cancerous cells leading to dysregulation of the BBB [103]. Complicating the matter, aquaporin-4 seems to play a direct role in oedema during therapy against GBM; treatment with radiotherapy and chemotherapy modulates perivascular levels of aquaporin-4 leading to a reduction in cerebral oedema associated with GBM [104]. However, until recently there has been little work performed on targeting the aquaporin system directly in combating cerebral oedema in neuropathological disorders [99]. Other symptoms of GBM have been addressed by targeting aquaporins; successful attempts at modulating the aquaporin system have been made in order to attenuate angiogenesis, cell migration and growth in aquaporin-1-null mice [105] and following knockdown of aquaporin-4 expression in human cell cultures and in nude mice in vivo [106].
4.4 Therapeutic Implications of the BBB in Treating GBM
It must be considered that even though the BBB within the GBM is compromised, it is still largely functional and will continue to perform its job in excluding large molecules from the cerebral parenchyma [20]. This creates the challenge of targeting the GBM effectively using chemotherapies as many commonly used chemotherapeutic drugs are in the size range of 450–850 Da so the BBB will still greatly limit access of many therapeutic compounds to the brain [20, 22]. The current standard for GBM treatment is surgical resection [107] followed by radiotherapy with an adjuvant chemotherapy [48]. Nonetheless, response to chemotherapy in GBM is poor, meaning that therapeutic success is still very low [7], so any attempts at increasing the efficiency of drug delivery to the brain are desirable. Therefore, techniques to increase BBB permeability using osmotic, genetic and physical interventions or via receptor-mediated transport have been developed in order to aid in delivering drugs that would be too large to cross the BBB under normal circumstances.
Osmotic modulation of BBB permeability can be accomplished using a variety of techniques. The most commonly used approach is the use of a concentrated solution of the sugar mannitol to increase BBB permeability across the entire brain for several minutes [108, 109] with Na+/Ca2+ exchange governing the length of time that BBB permeability is increased [110, 111]. 20 % mannitol was shown early on as a way to improve chemotherapy survival times [112]. However, its use in treating brain cancers has been controversial as osmotic modulation tends to cause widespread opening of the BBB with debatable effects on the therapeutic index of co-administered chemotherapeutic agents [113] and mannitol can induce seizures in patients with epileptiform activity persisting for days [114]. Therefore, mannitol has fallen out of favour as methods for bypassing the BBB with fewer side effects have been introduced. These newer approaches favour selective opening of the BBB; for example, localised BBB opening has been achieved using a convection-enhanced delivery of ethylamine-human serum albumin (EA-HSA ) in order to allow greater access of systemically administered methotrexate to the cerebral parenchyma, resulting in reduced tumour growth and increased survival in a rat model of glioma [115].
RNA interference is a method of knocking down gene expression by delivery of a tailored piece of RNA via a viral or non-viral vector that shows great promise across a range of neurological disorders [116]. It has been used to directly target GBM as many of these vectors are designed to pass through the BBB [117–120]. However, RNA interference can also be used to alter BBB permeability in order to allow other therapeutic compounds to access the cerebral parenchyma. Global or selective genetic modulation of the endothelial tight junction can be achieved systemically or locally using short hairpin (sh) or small interfering (si) RNAs. Modulation of BBB permeability using siRNAs has proven to be of therapeutic use in a mouse model of Alzheimer’s disease, knocking down occludin and claudin-5 levels increases the permeability of the BBB enough to allow significant clearance of amyloid beta from the brain to the periphery [14]. For GBM, this type of approach could aid in delivering drugs as large as 1 kDa from the periphery across the BBB without inducing oedema [121, 122], allowing many standard chemotherapy drugs to enter the cerebral parenchyma. Furthermore, RNA interference can be utilised to enhance delivery of drugs that are already small enough to pass through the BBB [123], meaning that a smaller dose is needed to be systemically administered which may reduce side effects associated with a particular drug. In terms of GBM and other brain cancers, RNA interference has so far been successfully used preclinically to knock down aquaporin expression in vitro and in vivo. Knockdown of aquaporin-4 significantly reduces water mobility under normal conditions in vivo [124] and significantly reduces GBM migration and growth by disrupting pathways involved in cell invasion and adherence [106].
A third way of altering BBB permeability is the use of physical means such as focussed ultrasound which has recently been shown to be a promising non-invasive method to treat a number of cancers including prostate cancer [125] and liver carcinomas [126] as well as ablation of brain tumours in humans [127–130]. Ablation using this method is problematic at present due to side effects with overheating of nontarget brain tissue. However, at lower intensities it can also be used as a way to increase BBB permeability in vivo [131] though this has yet to be attempted in humans [132]. A 1 MHz sonication pulse can significantly increase BBB permeability in tumours in rats [133–136], and using MRI to guide focussed ultrasound application, it is possible to selectively increase BBB permeability in precise target regions of the rat brain in vivo [137]. This can then be used to allow greater access to the brain for a number of therapeutic compounds including drugs and genetic therapies: uptake of doxorubicin [138] and temozolomide [139] is significantly increased following focussed ultrasound exposure and oligonucleotides, and DNA plasmid delivery can be made even more effective when focussed ultrasound was combined with nanoparticle delivery [140, 141]. Enhanced localisation of focussed ultrasound combined with targeted drug delivery can be achieved using microbubbles preloaded with the desired drug [142, 143].
Finally, receptor-mediated transport is a method of crossing the BBB without altering its baseline level of permeability; instead drugs are bound to a ligand that can normally cross the BBB unimpeded [144]. A number of suitable endocytotic receptors have been identified including low-density lipoprotein receptor-related protein-1 and protein-2 (LRP-1 and LRP-2), transferrin receptor, insulin receptor and insulin-like growth factor receptor [144]. LRP-1 is found on endothelial cells and is responsible for transcellular transportation for multiple ligands across the BBB [145], and as such, it has become a target for the development of drug carriers that co-opt LRP-1 to carry therapeutic compounds across the BBB into the cerebral parenchyma [146]. Delivery of the chemotherapeutic drug doxorubicin and paclitaxel to the brain via LRP-1 has been achieved by binding doxorubicin to p97 [147] and by binding paclitaxel to angiopep-2 [148]. This significantly increases the effectiveness of GBM uptake of Adriamycin and paclitaxel in mouse models of glioma. Researchers have also taken advantage of transferrin’s ability to guide material across the BBB (discussed in greater detail below) with doxorubicin having been successfully delivered in this manner [149].
Nanoparticles have also been used for many years to aid the delivery of drugs across the BBB [150, 151] via receptor-mediated transport involving apolipoproteins B and E [152–154]. Doxorubicin has been successfully delivered to the rodent brain by binding it to polysorbate-coated nanoparticles [155, 156]. Not only does nanoparticle-bound doxorubicin show effectiveness in treating GBM in preclinical experiments [157], the data also suggests that binding doxorubicin to polysorbate-coated nanoparticles also reduces the drug’s systemic toxicity [158]. Similarly, methotrexate can be delivered to the brain using the same type of polysorbate-coated nanoparticles [159]; there was a significant decrease in tumour size and a significant increase in the rates of apoptosis in a rat model of glioma using an alternative nanoparticle system (methotrexate was loaded into lipid core nanocapsules) [160].
Combining RNA interference and receptor-mediated transport may result in better therapies, and research involving the transferrin receptor has seen convergence of these techniques leading to increased efficacy in the treatment of GBM [161, 162]. It has been long known that transferrin receptor levels are greatly increased in GBM [163] and these levels are significantly increased following radiotherapy [164], making them an attractive option for delivering drugs to cancerous brain tissue. Early in vitro research using transferrin conjugated to toxins showed that targeting the transferrin receptor could be a way to selectively target GBMs in vivo [165, 166]. Furthermore, RNA interference and traditional receptor-mediated transport can be made more efficient by conjugating transferrin onto nanoparticles; for example, spherical nucleic acids can be conjugated onto gold [167], cationic solid lipid [168] and hyaluronan-grafted lipid-based nanoparticles [169], whereas transferrin can be conjugated onto poly(lactic-co-glycolic acid) [170], poly(ethylene glycol)-poly(l-lactic-co-glycolic acid) [171] and gold nanoparticles [172].
Using oligonucleotides against laminin-8 (a vascular basement membrane protein that is upregulated during GBM) conjugated to an antibody against the transferrin receptor, significant decreases in GBM microvasculature density and significant increases in survival were obtained in nude rats [173]. Polypropylenimine dendrimers can be used as a non-viral alternative to deliver DNA to target cells [174], and conjugating these with transferrin has been shown to be effective in delivering treatments directly to cancerous cells with little toxicity [175]. This has allowed direct delivery of siRNA to GBM cells without using a viral vector [176]. It has also been demonstrated that conjugating microRNA to transferrin and to a nanoparticle delivery system can result in higher levels of transport across the BBB than transferrin alone [177].
4.5 Conclusion
The BBB’s tight control over access to and from the brain becomes disrupted in the presence of GBM as expression of tight junction proteins decreases, leading to an increase in BBB permeability. Increases in angiogenesis help to nurture the GBM, and alterations in aquaporin-4 levels contribute to cerebral oedema around the tumour. Despite the compromised nature of the BBB within the GBM, delivery of chemotherapeutic drugs remains problematic. BBB permeability can be further increased by osmotic modulation, RNA interference and focussed ultrasound treatment. Alternatively, receptor-mediated transport can be used to “piggyback” into the cerebral parenchyma using the transporters naturally expressed on endothelial cells. This in turn can be facilitated by the use of nanoparticle conjugates.
Abbreviations
- BBB:
-
Blood-brain barrier
- GBM:
-
Glioblastoma
- NVU:
-
Neurovascular unit
- TJ:
-
Tight junction
References
Wu J, He Y, Yang Z, Guo C, Luo Q, Zhou W, Chen S, Li A, Xiong B, Jiang T, Gong H. 3D BrainCV: simultaneous visualization and analysis of cells and capillaries in a whole mouse brain with one-micron voxel resolution. Neuroimage. 2014;87:199–208.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83(1):183–252.
Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, Virgintino D, Fontana A, Yonekawa Y, Frei K. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol. 2008;67(5):435–48.
Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136(1):82–93.
Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2014;9:CD008218.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Tani E, Nakano M, Itagaki T, Fukumori T. Cell membrane structure of human giant-celled glioblastoma. Acta Neuropathol. 1978;41(1):61–5.
Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–31.
Nikuseva-Martić T, Beros V, Pećina-Slaus N, Pećina HI, Bulić-Jakus F. Genetic changes of CDH1, APC, and CTNNB1 found in human brain tumors. Pathol Res Pract. 2007;203(11):779–87.
Zeng L, Kang C, Di C, Fee BE, Rivas M, Lin J, Adamson DC. The adherens junction-associated protein 1 is a negative transcriptional regulator of MAGEA2, which potentiates temozolomide-induced apoptosis in GBM. Int J Oncol. 2014;44(4):1243–51.
Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282(21):4067–79.
Kuo YC, Wang IH. Enhanced delivery of etoposide across the blood-brain barrier to restrain brain tumor growth using melanotransferrin antibody- and tamoxifen-conjugated solid lipid nanoparticles. J Drug Target. 2016;1–10.
Keaney J, Walsh DM, O’Malley T, Hudson N, Crosbie DE, Loftus T, Sheehan F, McDaid J, Humphries MM, Callanan JJ, Brett FM, Farrell MA, Humphries P, Campbell M. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv. 2015;1(8):e1500472.
Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901.
Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5(10):e13741.
Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2015;38:16–25.
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273(45):29745–53.
van de Waterbeemd H, Camenisch G, Folkers G, Chretien JR, Raevsky OA. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J Drug Target. 1998;6(2):151–65.
Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72.
Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241(1):49–55.
Pardridge WM. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov Today. 2002;7(1):5–7.
Parkinson FE, Hacking C. Pericyte abundance affects sucrose permeability in cultures of rat brain microvascular endothelial cells. Brain Res. 2005;1049(1):8–14.
Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53.
Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201.
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–103.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5.
Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, de Vries HE, Reijerkerk A. Retinoic acid induces blood-brain barrier development. J Neurosci. 2013;33(4):1660–71.
Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6.
Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038(2):208–15.
Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153(3):543–53.
Reyahi A, Nik AM, Ghiami M, Gritli-Linde A, Pontén F, Johansson BR, Carlsson P. Foxf2 is required for brain pericyte differentiation and development and maintenance of the blood-brain barrier. Dev Cell. 2015;34(1):19–32.
Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y. Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol. 2004;24(3):491–7.
Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, Moumdjian R, Bouthillier A, Reudelhuber TL, Prat A. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27(34):9032–42.
Sun D, Lytle C, O’Donnell ME. Astroglial cell-induced expression of Na-K-Cl cotransporter in brain microvascular endothelial cells. Am J Physiol. 1995;269(6 Pt 1):C1506–12.
Leybaert L, Paemeleire K, Strahonja A, Sanderson MJ. Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia. 1998;24(4):398–407.
Muñoz MF, Puebla M, Figueroa XF. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front Cell Neurosci. 2015;9:59.
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60.
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–61.
Gross PM, Weindl A. Peering through the windows of the brain. J Cereb Blood Flow Metab. 1987;7(6):663–72.
Miyata S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci. 2015;9:390.
Banks WA, Kastin AJ, Selznick JK. Modulation of immunoactive levels of DSIP and blood-brain permeability by lighting and diurnal rhythm. J Neurosci Res. 1985;14(3):347–55.
Lagaraine C, Skipor J, Szczepkowska A, Dufourny L, Thiery JC. Tight junction proteins vary in the choroid plexus of ewes according to photoperiod. Brain Res. 2011;1393:44–51.
Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58(6):832–46.
Nicholas MK. Glioblastoma multiforme: evidence-based approach to therapy. Expert Rev Anticancer Ther. 2007;7(12 Suppl):S23–7.
Khasraw M, Lassman A. Advances in the treatment of malignant gliomas. Curr Oncol Rep. 2010;12(1):26–33.
Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–21.
Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–400.
Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–5.
Günther HS, Schmidt NO, Phillips HS, Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal M, Lamszus K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–909.
Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9(4):424–9.
Tchoghandjian A, Baeza-Kallee N, Beclin C, Metellus P, Colin C, Ducray F, Adélaïde J, Rougon G, Figarella-Branger D. Cortical and subventricular zone glioblastoma-derived stem-like cells display different molecular profiles and differential in vitro and in vivo properties. Ann Surg Oncol. 2012;19 Suppl 3:S608–19.
Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–7.
Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61(18):6674–8.
Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106(3):417–27.
Holmen SL, Williams BO. Essential role for Ras signaling in glioblastoma maintenance. Cancer Res. 2005;65(18):8250–5.
Germano IM, Ito M, Cho KG, Hoshino T, Davis RL, Wilson CB. Correlation of histopathological features and proliferative potential of gliomas. J Neurosurg. 1989;70(5):701–6.
Plate KH, Mennel HD. Vascular morphology and angiogenesis in glial tumors. Exp Toxicol Pathol. 1995;47(2-3):89–94.
Folkerth RD. Histologic measures of angiogenesis in human primary brain tumors. Cancer Treat Res. 2004;117:79–95.
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–73.
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82.
Matsuda Y, Hagio M, Ishiwata T. Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol. 2013;19(1):42–8.
Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–40.
Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–8.
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–8.
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–4.
Ramakrishnan S, Olson TA, Bautch VL, Mohanraj D. Vascular endothelial growth factor-toxin conjugate specifically inhibits KDR/flk-1-positive endothelial cell proliferation in vitro and angiogenesis in vivo. Cancer Res. 1996;56(6):1324–30.
Arora N, Masood R, Zheng T, Cai J, Smith DL, Gill PS. Vascular endothelial growth factor chimeric toxin is highly active against endothelial cells. Cancer Res. 1999;59(1):183–8.
Wild R, Dhanabal M, Olson TA, Ramakrishnan S. Inhibition of angiogenesis and tumour growth by VEGF121-toxin conjugate: differential effect on proliferating endothelial cells. Br J Cancer. 2000;83(8):1077–83.
Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152–60.
Reardon DA, Wen PY, Desjardins A, Batchelor TT, Vredenburgh JJ. Glioblastoma multiforme: an emerging paradigm of anti-VEGF therapy. Expert Opin Biol Ther. 2008;8(4):541–53.
Chi AS, Norden AD, Wen PY. Antiangiogenic strategies for treatment of malignant gliomas. Neurotherapeutics. 2009;6(3):513–26.
Miletic H, Niclou SP, Johansson M, Bjerkvig R. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets. 2009;13(4):455–68.
Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–99.
Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57(6):919–28.
Lu Y, Xiao L, Liu Y, Wang H, Li H, Zhou Q, Pan J, Lei B, Huang A, Qi S. MIR517C inhibits autophagy and the epithelial-to-mesenchymal (-like) transition phenotype in human glioblastoma through KPNA2-dependent disruption of TP53 nuclear translocation. Autophagy. 2015;11(12):2213–32.
Chang JH, Hwang YH, Lee DJ, Kim DH, Park JM, Wu HG, Kim IA. MicroRNA-203 modulates the radiation sensitivity of human malignant glioma cells. Int J Radiat Oncol Biol Phys. 2016;94(2):412–20.
Perego C, Vanoni C, Massari S, Raimondi A, Pola S, Cattaneo MG, Francolini M, Vicentini LM, Pietrini G. Invasive behaviour of glioblastoma cell lines is associated with altered organisation of the cadherin-catenin adhesion system. J Cell Sci. 2002;115(Pt 16):3331–40.
Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, Anastasiadis PZ. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5(10):e13665.
Adamsky K, Arnold K, Sabanay H, Peles E. Junctional protein MAGI-3 interacts with receptor tyrosine phosphatase beta (RPTP beta) and tyrosine-phosphorylated proteins. J Cell Sci. 2003;116(Pt 7):1279–89.
Lin JJ, Zhao TZ, Cai WK, Yang YX, Sun C, Zhang Z, Xu YQ, Chang T, Li ZY. Inhibition of histamine receptor 3 suppresses glioblastoma tumor growth, invasion, and epithelial-to-mesenchymal transition. Oncotarget. 2015;6(19):17107–20.
Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105(6):586–92.
Karnati HK, Panigrahi M, Shaik NA, Greig NH, Bagadi SA, Kamal MA, Kapalavayi N. Down regulated expression of Claudin-1 and Claudin-5 and up regulation of β-catenin: association with human glioma progression. CNS Neurol Disord Drug Targets. 2014;13(8):1413–26.
Brasch R, Pham C, Shames D, Roberts T, van Dijke K, van Bruggen N, Mann J, Ostrowitzki S, Melnyk O. Assessing tumor angiogenesis using macromolecular MR imaging contrast media. J Magn Reson Imaging. 1997;7(1):68–74.
Cao Y, Nagesh V, Hamstra D, Tsien CI, Ross BD, Chenevert TL, Junck L, Lawrence TS. The extent and severity of vascular leakage as evidence of tumor aggressiveness in high-grade gliomas. Cancer Res. 2006;66(17):8912–7.
Swanson KR, Chakraborty G, Wang CH, Rockne R, Harpold HL, Muzi M, Adamsen TC, Krohn KA, Spence AM. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med. 2009;50(1):36–44.
Krishnan S, Szabo E, Burghardt I, Frei K, Tabatabai G, Weller M. Modulation of cerebral endothelial cell function by TGF-β in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget. 2015;6(26):22480–95.
Rascher G, Fischmann A, Kröger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104(1):85–91.
Toyoda K, Tanaka K, Nakagawa S, Thuy DH, Ujifuku K, Kamada K, Hayashi K, Matsuo T, Nagata I, Niwa M. Initial contact of glioblastoma cells with existing normal brain endothelial cells strengthen the barrier function via fibroblast growth factor 2 secretion: a new in vitro blood-brain barrier model. Cell Mol Neurobiol. 2013;33(4):489–501.
Papadopoulos MC, Saadoun S, Davies DC, Bell BA. Emerging molecular mechanisms of brain tumour oedema. Br J Neurosurg. 2001;15(2):101–8.
Wolburg H, Noell S, Wolburg-Buchholz K, Mack A, Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15(2):180–93.
Francesca B, Rezzani R. Aquaporin and blood brain barrier. Curr Neuropharmacol. 2010;8(2):92–6.
Nico B, Ribatti D. Role of aquaporins in cell migration and edema formation in human brain tumors. Exp Cell Res. 2011;317(17):2391–6.
Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–62.
Asgari N, Berg CT, Mørch MT, Khorooshi R, Owens T. Cerebrospinal fluid aquaporin-4-immunoglobulin G disrupts blood brain barrier. Ann Clin Transl Neurol. 2015;2(8):857–63.
Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87(6):621–3.
Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72(2):262–5.
Bloch O, Manley GT. The role of aquaporin-4 in cerebral water transport and edema. Neurosurg Focus. 2007;22(5):E3.
Badaut J, Fukuda AM, Jullienne A, Petry KG. Aquaporin and brain diseases. Biochim Biophys Acta. 2014;1840(5):1554–65.
Badaut J, Brunet JF, Grollimund L, Hamou MF, Magistretti PJ, Villemure JG, Regli L. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir Suppl. 2003;86:495–8.
Warth A, Simon P, Capper D, Goeppert B, Tabatabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, Weller M, Meyermann R, Wolburg H, Mittelbronn M. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;85(6):1336–46.
Mou K, Chen M, Mao Q, Wang P, Ni R, Xia X, Liu Y. AQP-4 in peritumoral edematous tissue is correlated with the degree of glioma and with expression of VEGF and HIF-alpha. J Neurooncol. 2010;100(3):375–83.
Warth A, Kröger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol. 2004;107(4):311–8.
Nico B, Mangieri D, Tamma R, Longo V, Annese T, Crivellato E, Pollo B, Maderna E, Ribatti D, Salmaggi A. Aquaporin-4 contributes to the resolution of peritumoural brain oedema in human glioblastoma multiforme after combined chemotherapy and radiotherapy. Eur J Cancer. 2009;45(18):3315–25.
Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434(7034):786–92.
Ding T, Ma Y, Li W, Liu X, Ying G, Fu L, Gu F. Role of aquaporin-4 in the regulation of migration and invasion of human glioma cells. Int J Oncol. 2011;38(6):1521–31.
Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2015;15(2):517.
Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20(2):217–30.
Rapoport SI. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin Investig Drugs. 2001;10(10):1809–18.
Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. The effects of the Na(+)/Ca(++) exchange blocker on osmotic blood-brain barrier disruption. Brain Res. 2001;900(2):157–62.
Ikeda M, Bhattacharjee AK, Kondoh T, Nagashima T, Tamaki N. Synergistic effect of cold mannitol and Na(+)/Ca(2+) exchange blocker on blood-brain barrier opening. Biochem Biophys Res Commun. 2002;291(3):669–74.
Miyagami M, Tsubokawa T, Tazoe M, Kagawa Y. Intra-arterial ACNU chemotherapy employing 20% mannitol osmotic blood-brain barrier disruption for malignant brain tumors. Neurol Med Chir. 1990;30(8):582–90.
Zünkeler B, Carson RE, Olson J, Blasberg RG, DeVroom H, Lutz RJ, Saris SC, Wright DC, Kammerer W, Patronas NJ, Dedrick RL, Herscovitch P, Oldfield EH. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J Neurosurg. 1996;85(6):1056–65.
Neuwelt EA, Howieson J, Frenkel EP, Specht HD, Weigel R, Buchan CG, Hill SA. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood–brain barrier modification in glioblastoma. Neurosurgery. 1986;19:573–82.
Cooper I, Last D, Guez D, Sharabi S, Elhaik Goldman S, Lubitz I, Daniels D, Salomon S, Tamar G, Tamir T, Mardor R, Fridkin M, Shechter Y, Mardor Y. Combined local blood-brain barrier opening and systemic methotrexate for the treatment of brain tumors. J Cereb Blood Flow Metab. 2015;35(6):967–76.
Sah DW. Therapeutic potential of RNA interference for neurological disorders. Life Sci. 2006;79(19):1773–80.
Guo D, Wang B, Han F, Lei T. RNA interference therapy for glioblastoma. Expert Opin Biol Ther. 2010;10(6):927–36.
Shir A, Levitzki A, Wagner E, Klein S, Ogris M. Nucleic acid-based therapeutics for glioblastoma. Anticancer Agents Med Chem. 2011;11(8):693–9.
Nikaki A, Piperi C, Papavassiliou AG. Role of microRNAs in gliomagenesis: targeting miRNAs in glioblastoma multiforme therapy. Expert Opin Investig Drugs. 2012;21(10):1475–88.
Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part II: RNA interference as a promising way to sensitize glioblastomas to temozolomide. Drug Discov Today. 2015;20(6):772–9.
Hanrahan F, Humphries P, Campbell M. RNAi-mediated barrier modulation: synergies of the brain and eye. Ther Deliv. 2010;1(4):587–94.
Campbell M, Humphries MM, Kiang AS, Nguyen AT, Gobbo OL, Tam LC, Suzuki M, Hanrahan F, Ozaki E, Farrar GJ, Kenna PF, Humphries P. Systemic low-molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol Med. 2011;3(4):235–45.
Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O’Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar GJ, Humphries P. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10(8):930–47.
Badaut J, Ashwal S, Adami A, Tone B, Recker R, Spagnoli D, Ternon B, Obenaus A. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab. 2011;31(3):819–31.
Uchida T, Shoji S, Nakano M, Hongo S, Nitta M, Murota A, Nagata Y. Transrectal high-intensity focused ultrasound for the treatment of localized prostate cancer: eight-year experience. Int J Urol. 2009;16(11):881–6.
Aubry JF, Pauly KB, Moonen C, Haar GT, Ries M, Salomir R, Sokka S, Sekins KM, Shapira Y, Ye F, Huff-Simonin H, Eames M, Hananel A, Kassell N, Napoli A, Hwang JH, Wu F, Zhang L, Melzer A, Kim YS, Gedroyc WM. The road to clinical use of high-intensity focused ultrasound for liver cancer: technical and clinical consensus. J Ther Ultrasound. 2013;1:13.
Guthkelch AN, Carter LP, Cassady JR, Hynynen KH, Iacono RP, Johnson PC, Obbens EA, Roemer RB, Seeger JF, Shimm DS, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–84.
Ram Z, Cohen ZR, Harnof S, Tal S, Faibel M, Nass D, Maier SE, Hadani M, Mardor Y. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery. 2006;59(5):949–55.
McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323–32.
Jenne JW. Non-invasive transcranial brain ablation with high-intensity focused ultrasound. Front Neurol Neurosci. 2015;36:94–105.
Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev. 2014;72:94–109.
Burgess A, Hynynen K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem Neurosci. 2013;4(4):519–26.
Yang FY, Lin GL, Horng SC, Chang TK, Wu SY, Wong TT, Wang HE. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood-tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(5):964–70.
Yang FY, Lee PY. Efficiency of drug delivery enhanced by acoustic pressure during blood-brain barrier disruption induced by focused ultrasound. Int J Nanomedicine. 2012;7:2573–82.
Yang FY, Wang HE, Lin GL, Lin HH, Wong TT. Evaluation of the increase in permeability of the blood-brain barrier during tumor progression after pulsed focused ultrasound. Int J Nanomedicine. 2012;7:723–30.
Liu HL, Yang HW, Hua MY, Wei KC. Enhanced therapeutic agent delivery through magnetic resonance imaging-monitored focused ultrasound blood-brain barrier disruption for brain tumor treatment: an overview of the current preclinical status. Neurosurg Focus. 2012;32(1):E4.
Magnin R, Rabusseau F, Salabartan F, Mériaux S, Aubry JF, Le Bihan D, Dumont E, Larrat B. Magnetic resonance-guided motorized transcranial ultrasound system for blood-brain barrier permeabilization along arbitrary trajectories in rodents. J Ther Ultrasound. 2015;3:22.
Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901–7.
Liu HL, Huang CY, Chen JY, Wang HY, Chen PY, Wei KC. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One. 2014;9(12):e114311.
Hamano N, Negishi Y, Takatori K, Endo-Takahashi Y, Suzuki R, Maruyama K, Niidome T, Aramaki Y. Combination of bubble liposomes and high-intensity focused ultrasound (HIFU) enhanced antitumor effect by tumor ablation. Biol Pharm Bull. 2014;37(1):174–7.
Negishi Y, Yamane M, Kurihara N, Endo-Takahashi Y, Sashida S, Takagi N, Suzuki R, Maruyama K. Enhancement of blood-brain barrier permeability and delivery of antisense oligonucleotides or plasmid DNA to the brain by the combination of bubble liposomes and high-intensity focused ultrasound. Pharmaceutics. 2015;7(3):344–62.
Ting CY, Fan CH, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33(2):704–12.
Fan CH, Ting CY, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34(8):2142–55.
Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24(9):1759–71.
Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood-brain barrier. Expert Opin Drug Deliv. 2005;2(2):299–309.
Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis. 2010;37(1):48–57.
Gabathuler R, Arthur G, Kennard M, Chen Q, Tsai S, Yang J, Schoorl W, Vitalis TZ, Jeffereies WA. Development of a potential protein vector (NeuroTrans) to deliver drugs across to the blood-brain barrier. Int Congres Series. 2005;1277:171–84.
Bertrand Y, Currie JC, Poirier J, Demeule M, Abulrob A, Fatehi D, Stanimirovic D, Sartelet H, Castaigne JP, Béliveau R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer. 2011;105(11):1697–707.
Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL, Han M. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubicin liposomes. Biomaterials. 2013;34(22):5628–39.
Schröder U, Sabel BA. Nanoparticles, a drug carrier system to pass the blood-brain barrier, permit central analgesic effects of i.v. dalargin injections. Brain Res. 1996;710(1-2):121–4.
Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2(1):108–19.
Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10(4):317–25.
Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, Kreuter J, Langer K. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317(3):1246–53.
Neves AR, Queiroz JF, Weksler B, Romero IA, Couraud PO, Reis S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: two new strategies of functionalization with apolipoprotein E. Nanotechnology. 2015;26(49):495103.
Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16(10):1564–9.
Ambruosi A, Yamamoto H, Kreuter J. Body distribution of polysorbate-80 and doxorubicin-loaded [14C]poly(butyl cyanoacrylate) nanoparticles after i.v. administration in rats. J Drug Target. 2005;13(10):535–42.
Ambruosi A, Khalansky AS, Yamamoto H, Gelperina SE, Begley DJ, Kreuter J. Biodistribution of polysorbate 80-coated doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J Drug Target. 2006;14(2):97–105.
Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhl R, Kock M, Geiger KD, Gelperina SE. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109(5):759–67.
Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int J Pharm. 2006;310(1-2):213–9.
Figueiró F, de Oliveira CP, Rockenbach L, Mendes FB, Bergamin LS, Jandrey EH, Edelweiss MI, Guterres SS, Pohlmann AR, Battastini AM. Pharmacological improvement and preclinical evaluation of methotrexate-loaded lipid-core nanocapsules in a glioblastoma model. J Biomed Nanotechnol. 2015;11(10):1808–18.
Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247(4):291–307.
Voth B, Nagasawa DT, Pelargos PE, Chung LK, Ung N, Gopen Q, Tenn S, Kamei DT, Yang I. Transferrin receptors and glioblastoma multiforme: current findings and potential for treatment. J Clin Neurosci. 2015;22(7):1071–6.
Recht L, Torres CO, Smith TW, Raso V, Griffin TW. Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg. 1990;72(6):941–5.
Kim KU, Xiao J, Ni HT, Cho KH, Spellman SR, Low WC, Hall WA. Changes in expression of transferrin, insulin-like growth factor 1, and interleukin 4 receptors after irradiation of cells of primary malignant brain tumor cell lines. Radiat Res. 2003;160(2):224–31.
Hall WA, Godal A, Juell S, Fodstad O. In vitro efficacy of transferrin-toxin conjugates against glioblastoma multiforme. J Neurosurg. 1992;76(5):838–44.
Martell LA, Agrawal A, Ross DA, Muraszko KM. Efficacy of transferrin receptor-targeted immunotoxins in brain tumor cell lines and pediatric brain tumors. Cancer Res. 1993;53(6):1348–53.
Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5(209):209ra152.
Jin J, Bae KH, Yang H, Lee SJ, Kim H, Kim Y, Joo KM, Seo SW, Park TG, Nam DH. In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 2011;22(12):2568–72.
Cohen ZR, Ramishetti S, Peshes-Yaloz N, Goldsmith M, Wohl A, Zibly Z, Peer D. Localized RNAi therapeutics of chemoresistant grade IV glioma using hyaluronan-grafted lipid-based nanoparticles. ACS Nano. 2015;9(2):1581–91.
Chang J, Paillard A, Passirani C, Morille M, Benoit JP, Betbeder D, Garcion E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29(6):1495–505.
Kang T, Jiang M, Jiang D, Feng X, Yao J, Song Q, Chen H, Gao X, Chen J. Enhancing glioblastoma-specific penetration by functionalization of nanoparticles with an iron-mimic peptide targeting transferrin/transferrin receptor complex. Mol Pharm. 2015;12(8):2947–61.
Dixit S, Miller K, Zhu Y, McKinnon E, Novak T, Kenney ME, Broome AM. Dual receptor-targeted theranostic nanoparticles for localized delivery and activation of photodynamic therapy drug in glioblastomas. Mol Pharm. 2015;12(9):3250–60.
Fujita M, Khazenzon NM, Ljubimov AV, Lee BS, Virtanen I, Holler E, Black KL, Ljubimova JY. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9(4):183–91.
Omidi Y, Hollins AJ, Drayton RM, Akhtar S. Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target. 2005;13(7):431–43.
Koppu S, Oh YJ, Edrada-Ebel R, Blatchford DR, Tetley L, Tate RJ, Dufès C. Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. J Control Release. 2010;143(2):215–21.
Perez AP, Cosaka ML, Romero EL, Morilla MJ. Uptake and intracellular traffic of siRNA dendriplexes in glioblastoma cells and macrophages. Int J Nanomedicine. 2011;6:2715–28.
Wang X, Huang X, Yang Z, Gallego-Perez D, Ma J, Zhao X, Xie J, Nakano I, Lee LJ. Targeted delivery of tumor suppressor microRNA-1 by transferrin-conjugated lipopolyplex nanoparticles to patient-derived glioblastoma stem cells. Curr Pharm Biotechnol. 2014;15(9):839–46.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Kealy, J., Campbell, M. (2016). The Blood-Brain Barrier in Glioblastoma: Pathology and Therapeutic Implications. In: Tivnan, A. (eds) Resistance to Targeted Therapies Against Adult Brain Cancers. Resistance to Targeted Anti-Cancer Therapeutics. Springer, Cham. https://doi.org/10.1007/978-3-319-46505-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-46505-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46504-3
Online ISBN: 978-3-319-46505-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)