Abstract
Echinoderms are quite exceptional in the sense that most species belonging to this group use adhesive secretions extensively. Two different adhesive systems may be recognised in these animals: the tube feet, organs involved in attachment to the substratum or food capture, and the Cuvierian tubules, organs involved in defence. These two systems rely on different types of adhesion and therefore differ in the way they operate, in their structure and in the composition of their adhesive. Although tube feet are present in every extant echinoderm species, only those of asteroids and regular echinoids have been studied in detail in terms of adhesion. These organs are involved in temporary adhesion, functioning as duo-gland adhesive systems in which adhesive cells release a proteinaceous secretion, while de-adhesive cells allow detachment. To date, only two adhesive proteins have been characterized in echinoderm tube feet, i.e., Sfp1 in sea stars and Nectin in sea urchins. These two proteins do not appear to be related, but they share similar protein–carbohydrate interaction domains. Cuvierian tubules occur only in some holothuroid species. These single-use organs rely on instantaneous adhesion, their contact with a surface triggering the release of the protein-based adhesive from a single cell type. Some proteins have been identified in the adhesive, but no confirmation of their adhesive function has been provided so far.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
Members of the phylum Echinodermata are among the most familiar sea creatures, and representatives, such as the sea stars, have become virtually a symbol of sea life. The phylum contains about 7250 living species of relatively large invertebrates, all being exclusively marine and largely bottom-dwellers (Ruppert et al. 2003). There are five extant classes of echinoderms: the crinoids (sea lilies and feather stars), the asteroids (sea stars), the ophiuroids (brittle stars), the echinoids (sea urchins and sand dollars) and the holothuroids (sea cucumbers). The most striking characteristics of the group are the pentamerous radial symmetry, the endodermal calcareous skeleton, the mutable collagenous tissues and the water–vascular system, a unique system of coelomic canals and surface appendages.
Echinoderms are also quite exceptional in the sense that most species belonging to this group use adhesive secretions extensively. Two different adhesive systems may be recognised in post-metamorphic individuals: the tube feet or podia, organs involved in attachment to the substratum or food capture, and the Cuvierian tubules, organs involved in defence. The former are present in every extant echinoderm species, whereas the latter occur only in some holothuroid species. These two systems rely on different types of adhesion and therefore differ in the way they operate, in their structure and in the composition of their adhesive.
9.2 Tube Feet
Being almost exclusively benthic animals, echinoderms have activities and adaptations that are correlated with a relationship with the sea bottom. Most of these activities, such as attachment to the substratum, locomotion, handling of food and burrow-building, rely on adhesive secretions allowing the animal to stick to or to manipulate a substratum. In post-metamorphic echinoderms, these adhesive secretions are always produced by specialised organs, the podia or tube feet. These are the external appendages of the water–vascular system and are also probably the most advanced hydraulic organs in the animal kingdom (Nichols 1966). Tube foot attachment is typically temporary adhesion. Indeed, although tube feet can adhere very strongly to the substratum, they are also able to detach easily and voluntarily from the substratum before reinitiating another attachment–detachment cycle (Thomas and Hermans 1985; Flammang 1996).
9.2.1 Morphology and Adhesion Strength
From their presumed origin as simple respiratory evaginations of the ambulacral system (Nichols 1966), tube feet have diversified into the wide range of specialised structures found in extant echinoderms. This morphological diversity reflects the variety of functions of tube feet (Lawrence 1987). In some groups, a single type of tube foot fulfils different functions; in others, different types of tube feet are specialised, each in one particular function. Based on their external morphology only, tube feet can be classified into six broad types: disc-ending (Fig. 9.1a), penicillate, knob-ending, lamellate, digitate (Fig. 9.1b) and ramified (Flammang 1996). Adhesive areas are organised differently according to the morphotype, and this organisation represents the first stage of specialisation of the tube feet. Tube feet that capture or manipulate small particles present an adhesive area fragmented into small, discrete zones (Flammang 1996). This is the case, for example, in the adhesive papillae scattered on the tube feet of filter-feeding ophiuroids (Fig. 9.1b). Discrete adhesive zones are presumably more efficient in the handling of small particles; conversely, a large adhesive area provides a strong attachment site for tube feet involved in locomotion or in maintaining position (Flammang 1996). Such large adhesive areas occur on the distal surface of the disc in disc-ending podia (Fig. 9.1a).
Morphological diversity in echinoderm tube feet (for comparison, tube feet have been oriented distal end up). (a) Disc-ending tube foot of the echinoid Heterocentrotus trigonarius. (b) Digitate tube foot of the ophiuroid Ophiothrix fragilis. Arrows indicate large adhesive areas and arrow heads small adhesive zones (see text for details). Modified from Santos et al. (2009a)
For practical reasons (relatively large size and high adhesion force of the tube feet), only disc-ending tube feet of asteroids and regular echinoids have been studied in detail in terms of adhesion. These tube feet consist of a basal hollow cylinder, the stem and an enlarged and flattened apical extremity, the disc (Figs 9.1a and 9.2a, b). The different constituents making up these two parts act cooperatively to make tube feet an efficient holdfast, allowing sea stars and sea urchins to resist hydrodynamically generated forces, but also to perform rather elaborate tasks such as climbing, righting, covering or shell opening (Lawrence 1987). The stem acts as a tough tether connecting the disc to the animal’s body. It is also mobile and flexible and thus gives the tube foot the capacity to perform various movements. The disc, on the other hand, makes contact with the substratum (Fig. 9.2a). It adapts to the surface profile, produces the adhesive secretion that fastens the tube foot to the substratum and encloses support structures that bear the tensions associated with adhesion. It also produces the de-adhesive secretion that allows detachment of the tube foot.
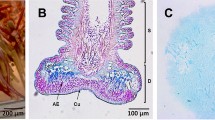
Fine structure of echinoderm tube feet (modified from Flammang 1996 and Santos et al. 2009a). (a) SEM photograph of a disc-ending tube foot of the sea star Asterias rubens attached to a textured polymer substratum. (b) Longitudinal LM section through a tube foot of A. rubens. (c, d) Longitudinal TEM sections through the adhesive epidermis of tube foot discs of the asteroid Marthasterias glacialis and of the echinoid Sphaerechinus granularis, respectively. (e–h) Ultrastructure of the secretory granules of the adhesive cells from echinoderm tube feet. Heterogeneous granules in the echinoid S. granularis (e), dense-cored granules in the ophiuroid Asteroxyx loveni (f), granules with a central filamentous bundle in the asteroid A. rubens (g), capped granules in the holothuroid Holothuria forskali (h). AE adhesive epidermis, AG adhesive secretory granule, C cap, CT connective tissue layer, CU cuticle, D disc, DG de-adhesive secretory granule, M myomesothelium, P secretory pore, S stem, SC support cell, TP terminal plate
Tube foot adhesive strength has been evaluated by measuring their tenacity, which is the adhesion force per unit area and is expressed in Pascals (Pa). The normal tenacity of single disc-ending tube feet has been quantified in several species of asteroids and echinoids under different conditions. Mean tenacity ranges from 0.17 to 0.43 MPa in asteroids and from 0.09 to 0.54 MPa in echinoids (Table 9.1). Tenacity was shown to be dependent on the chemical and physical characteristics of the surface to which the tube foot adheres (Santos et al. 2005a; Santos and Flammang 2006). In the sea urchin Paracentrotus lividus, the tenacity of single tube foot discs on four different smooth substrata was compared and showed that both the total surface energy and the ratio of polar to nonpolar forces at the surface influence tube foot attachment strength. In both asteroids and echinoids, it was demonstrated that tube feet show increased adhesion on a rough substratum in comparison to its smooth counterpart (Santos et al. 2005a). This is because the disc adhesive surface is highly compliant, replicating the substratum profile. The increase in contact area between the disc and the substratum leads to a higher adhesion force (Santos et al. 2005a). Tube foot discs and their adhesive secretions therefore appear to be well-tailored to provide an efficient attachment to natural rocky substrata, allowing echinoderms to resist hydrodynamically generated forces, but also to a large range of artificial substrata.
Suction has long been regarded as the primary functional mean for attachment in sea star and sea urchin tube feet (Nichols 1966; Lawrence 1987). However, detailed morphological and biomechanical observations clearly showed that echinoderm tube feet rely on adhesive secretions and not on suction (Thomas and Hermans 1985; Flammang et al. 1994; Hennebert et al. 2012a). Indeed, microscopy observations of tube feet rapidly fixed while they were attached to a smooth substratum showed that their distal surfaces are totally flat and lack a suction cavity (Thomas and Hermans 1985; Hennebert et al. 2012a). Moreover, detachment force and tenacity of single tube feet do not vary with pulling angle or surface perforation, as would be expected for a sucker (Santos et al. 2005a; Hennebert et al. 2012a).
9.2.2 Histology and Ultrastructure
The histological structure of the tube feet is remarkably constant for all echinoderm species. Their tissue stratification consists of four layers: an inner myomesothelium surrounding the water–vascular lumen, a connective tissue layer, a nerve plexus and an outer epidermis covered externally by a cuticle (Flammang 1996). At the level of the tube foot disc, these tissue layers are specialised for strong attachment. The discs of both asteroid and echinoid tube feet consist of two superposed layers of approximately equal thickness: a proximal supporting structure bearing the tensions associated with adhesion and a distal adhesive pad making contact with the substratum and producing the adhesive secretion that fastens the tube foot to this substratum (Fig. 9.2b; Santos et al. 2005a, 2009a). There are, however, differences in the organisation of these layers between sea star and sea urchin discs.
The supporting structure consists mostly of a circular plate of connective tissue, the so-called terminal plate that is composed of densely packed collagen fibres (Fig. 9.2b). In both sea stars and sea urchins, numerous branching connective tissue septa (made up mostly of collagen fibres) emerge from the distal surface of the terminal plate, manoeuvring themselves between the epidermal cells of the adhesive pad. The thinnest, distal branches of these septa attach apically to the support cells of the epidermis. In sea stars, these septa are arranged as well-defined radial lamellae (Fig. 9.2b), whereas in sea urchins they form a more irregular meshwork (Santos et al. 2005a). On its proximal side, the terminal plate is continuous with the connective tissue sheath of the stem.
The adhesive pad is composed of a thick adhesive epidermis reinforced by the branching connective tissue septa (Fig. 9.2b). This epidermis is much thicker than the stem epidermis. Externally, it is covered by a well-developed, multilayered glycocalyx, the so-called cuticle (Ameye et al. 2000). As a general rule, epidermal adhesive areas of echinoderm tube feet always consist of four cell categories: support cells, sensory cells, adhesive cells of one (in echinoid tube feet) or two types (in asteroid tube feet) and de-adhesive cells (see Flammang 1996, and Santos et al. 2009a, for review). The study of the ultrastructure of the adhesive and de-adhesive cells during a complete cycle of attachment–detachment of the tube foot in Asterias rubens demonstrated that they function as a duo-gland adhesive system as originally proposed by Hermans (1983), and in which adhesive cells (types 1 and 2) release an adhesive secretion and de-adhesive cells a de-adhesive secretion (Flammang et al. 1994; Flammang 1996; Flammang et al. 1998; Hennebert et al. 2008). In this species, polyclonal antibodies have been raised against footprint material and were used to locate the origin of footprint constituents in the tube feet (Flammang et al. 1998). Extensive immunoreactivity was detected in the secretory granules of both types of adhesive cells, confirming that their secretions make up together the bulk of the adhesive material.
Two modes of granule secretion can be recognised according to the morphology of the apex of the adhesive cell (McKenzie 1988; Flammang and Jangoux 1992; Flammang 1996; Santos et al. 2009a). In ‘apical duct’ cells, secretory granules are extruded through a duct delimited by a ring of microvilli and opening onto the tube foot surface as a cuticular pore (Fig. 9.2c). This kind of adhesive cell occurs in asteroid, ophiuroid and crinoid tube feet, as well as in holothuroid locomotory tube feet (Flammang 1996). In ‘apical tuft’ cells, secretory granules are released at the tip of microvillar-like cell projections which are arranged in a tuft at the cell apex (Fig. 9.2d). This second kind of adhesive cell has been observed only in echinoid tube feet and holothuroid locomotory tube feet and buccal tentacles (Flammang 1996).
Although the ultrastructure of the de-adhesive cell granules is remarkably constant from one echinoderm taxon to another (Fig. 9.2c,d), the one of the adhesive cell granules varies extensively. These secretory granules are usually made up of at least two materials of different electron density, which gives them a complex ultrastructure. Five broad categories can be recognised (Flammang 1996):
-
1.
Homogeneous granules apparently made up of only one material;
-
2.
Heterogeneous granules in which two different materials are mixed in an irregular pattern (Fig. 9.2e);
-
3.
Dense-cored granules consisting of an electron-denser core surrounded by less dense material (Fig. 9.2f);
-
4.
Granules with a central filamentous bundle resembling granules of the previous group but in which the core is made up of a parallel arrangement of fibrils or rods (Fig. 9.2g);
-
5.
Capped granules in which an electron-lucent material is covered, on one side, by a cap of electron-dense material (Fig. 9.2h).
The significance of these ultrastructural differences between different echinoderm taxa is unknown at present. However, in asteroids, Engster and Brown (1972) pointed out a relationship between the internal organisation of adhesive cell secretory granules and species habitat: asteroids confined to hard rocky substratum have complex granules enclosing a highly organised core, whereas soft substratum dwelling species have granules of considerably simpler ultrastructure. They suggested that the different substructure of the adhesive cell granules would depend on the nature and composition of their contents that, in turn, could be related to the possible adhesive strength of the tube feet.
9.2.3 Fine Structure and Composition of the Adhesive Material
In all echinoderm species investigated so far, after detachment of the tube foot, the adhesive secretion usually remains firmly bound to the substratum as a footprint (Fig. 9.3a). The material constituting these footprints can be stained, allowing the observation of their morphology under the light microscope (Chaet 1965; Thomas and Hermans 1985; Flammang 1996; Santos and Flammang 2006; Hennebert et al. 2008). In both sea stars and sea urchins, the footprints have the same shape and the same diameter as the distal surface of the tube foot discs (Fig. 9.3a). Various techniques have been used to study the fine structure of the material constituting the footprints, and whatever the method used, the adhesive material always appears as a foam-like or sponge-like material made up of a fibrillar matrix with numerous holes in it (Flammang et al. 1994; Flammang et al. 1998; Flammang 2006; Hennebert et al. 2008; Santos et al. 2009a). This aspect has been observed in LM, SEM (Fig. 9.3b) and AFM (Fig. 9.3c); and it does not differ according to whether the footprint has been fixed or not (Flammang et al. 1998; Flammang 2006; Hennebert et al. 2008; Higgins and Mostaert 2013). In both asteroid and echinoid footprints, one can distinguish a very thin and homogeneous priming film covering the substratum on which the fibrillar matrix is deposited (Fig. 9.3b,c). In sea stars, the fibrils tend to form a loose meshwork with relatively large meshes, about 2–5 μm in diameter (Fig. 9.3b, c). The walls delimiting the meshes may be quite thick (up to 1 μm), and under the AFM, they appear as strings of little beads (Fig. 9.3c; Hennebert et al. 2008). In sea urchin and sea cucumber footprints, the meshwork appears denser, with smaller meshes (<1 μm) delimited by very fine fibrils (about 50 nm in diameter) (Santos et al. 2009a). These differences in ultrastructure could be linked to the way the adhesive secretions are delivered to the substratum, viz. through secretory pores in asteroids and at the apex of microvillar-like cell projections in echinoids. Indeed, the loose meshwork of sea star footprints reflects approximately the distribution of the secretory pores on the tube foot disc surface (Hennebert et al. 2008), while the denser meshwork of sea urchin footprints is more reminiscent of the dense array of cell projections covering their disc surface.
Micro- and nanostructure of the adhesive footprints left by echinoderm tube feet (modified from Flammang et al. 2005; Hennebert et al. 2008, 2012a). (a) LM photograph of a footprint of Paracentrotus lividus stained with a 0.05 % aqueous solution of the cationic dye crystal violet. (b–c) Details of footprints deposited by the tube feet of Asterias rubens on pieces of glass and observed with SEM and AFM, respectively. Both views show areas where the adhesive material forms a meshwork deposited on a thin homogeneous film. (d) 3-D topographical view of a footprint from P. lividus deposited on a glass substratum and air-dried (vertical scale: 0–80 nm). HF homogeneous film, Mw meshwork
The thickness of the fibrillar matrix may vary from one footprint to another but also between different areas of the same footprint (Flammang et al. 1994; Hennebert et al. 2008; Santos et al. 2009a). However, footprint thickness is difficult to estimate. Using an interference–optical profilometer, which generated three-dimensional images of the footprint surface, the mean maximum thickness of dry footprints was found to be of 100 nm in the echinoid P. lividus (Fig. 9.3d) and of 230 nm in the asteroid A. rubens (Flammang et al. 2005). On the other hand, based on TEM observations, the thickness of the adhesive layer ranges from 0.2 to 9 μm in A. rubens and, at least, from 0.3 to 2 μm in P. lividus (Flammang et al. 1994; Hennebert et al. 2008; Santos et al. 2009a).
The composition of echinoderm adhesive material was first investigated by histochemical tests performed on tube foot longitudinal sections and on footprints. In tube feet, acidic glycosaminoglycans (GAGs) were detected in the secretory granules of adhesive cells (Defretin 1952; Chaet and Philpott 1964; Chaet 1965; Souza Santos and Silva Sasso 1968; Engster and Brown 1972). Later, Perpeet and Jangoux (1973) also demonstrated the presence of proteins associated to these GAGs in the sea star A. rubens. The footprints of the sea urchin Sphaerechinus granularis contain GAGs but no proteins (Flammang and Jangoux 1993), whereas the footprints of the sea stars Asterias forbesi, A. rubens, Leptasterias hexactis and Marthasterias glacialis stain for both proteins and GAGs (Chaet 1965; Thomas and Hermans 1985; Flammang et al. 1994).
At present, data on the biochemical composition of the adhesive footprints is only available for the sea star A. rubens and the sea urchin P. lividus. The water content of the adhesive material has never been measured, but in terms of dry weight, footprints are made up mainly of proteins (20.6 % in sea stars and 6.4 % in sea urchins), carbohydrates (8 % in sea stars and 1.2 % in sea urchins) and a large inorganic fraction (40 % in sea stars and 45.5 % in sea urchins) (Fig. 9.7a; Flammang et al. 1998; Santos et al. 2009b). This composition is in accordance with the previously mentioned histochemical tests. In addition, lipids were also detected in footprints (5.6 % in sea stars and 2.5 % in sea urchins), although they have not been detected in the secretory granules of adhesive cells by histochemistry (Perpeet and Jangoux 1973; Flammang et al. 1998; Santos et al. 2009b).
9.2.3.1 Protein Fraction
Sea star and sea urchin adhesive footprints have been analysed in terms of amino acid composition, and due to their insolubility, all the analyses had to be performed under hydrolytic conditions (Table 9.2). The footprint material of A. rubens contains slightly more polar (55 %) than nonpolar (45 %) residues and, among the former, more charged (34 %) than uncharged residues (21 %). Sea star adhesive presents a strong bias towards asparagine/aspartic acid (11.8 %), glutamine/glutamate (10.2 %) and glycine (9.7 %), followed by threonine (7.8 %) and serine (7.6 %) (Fig. 9.7b; Flammang et al. 1998). As for sea urchins, the footprints of P. lividus present more nonpolar (57.4 %) than polar amino acids (42.6 %), and equivalent amounts of both charged (20.2 %) and uncharged residues (22.4 %). The adhesive material presents a significant bias towards glycine (14.7 %), followed by alanine (9.8 %), valine (8.9 %), serine (8.6 %) and threonine (7.4 %) (Fig. 9.7b; Santos et al. 2009b). In addition, both sea star and sea urchin footprints present higher levels of half-cystine (3.2 and 2.6 %, respectively) than the average eukaryotic proteins.
9.2.3.1.1 Adhesive Proteins
The use of strong denaturing and reducing extraction conditions allowed the solubilisation of sea star and sea urchin footprint proteins (Santos et al. 2009b; Hennebert et al. 2012b). The need for these harsh solubilisation conditions plus the above-mentioned biased amino acid composition provides evidence for the importance of non-covalent interactions and disulphide bonds between the adhesive proteins in footprint cohesion. Indeed, hydrophobic and electrostatic interactions involve nonpolar and charged polar amino acids, respectively, both present in significant amounts in sea star and sea urchin adhesives (Santos et al. 2009b; Hennebert et al. 2012b). As for disulphide bonds, they probably reinforce the cohesive strength and insolubility of the adhesive footprints, either by intermolecular bonds that covalently cross-link the proteins or by intramolecular bonds that hold proteins in the specific shape required for interaction with their neighbours (Flammang et al. 1998; Hennebert et al. 2012b). In sea urchins, the presence of disulphide bonds was further corroborated by the observation of a mobility shift for three proteins in 2D nonreducing/reducing diagonal gels, attributed to the presence of intra- or intermolecular disulphide bonds in these proteins (Santos et al. 2009b).
Upon gel electrophoresis, sea star footprint protein extracts separated into 11 major and several minor protein bands. Using mass spectrometry (MS) analyses and homology-database search, it was shown that most of the minor protein bands correspond to known intracellular proteins, presumably contaminants from cellular epidermal material, while no homolog proteins were identified for the major protein bands. The later were further analysed by tandem MS (MS/MS), yielding 43 de novo-generated peptide sequences (Hennebert et al. 2012b). The same approach was applied to sea urchin footprint protein extracts, highlighting 13 major protein bands, 6 of which were known intracellular proteins such as actins, tubulins and histones. The remaining unidentified protein bands were further processed by automated de novo peptide sequencing, but as for sea stars, no homologies were found for the deduced peptide sequences, suggesting that these adhesive proteins might be either novel or highly modified (Santos et al. 2009b).
More recently, the proteome of sea star adhesive footprints was established using high-throughput sequencing of expressed tube foot mRNAs (transcriptome analysis) combined to MS-based identification of footprint proteins. The tube foot transcripts coding for proteins identified in the adhesive footprints were then functionally annotated by similarity searches against the NCBI nr database (Hennebert et al. 2015a). The results showed that the adhesive secretion is made up of 34 proteins. Most of these proteins were not annotated in public databases and probably correspond to novel adhesive proteins (Hennebert et al. 2015a). Regarding the annotated proteins, some present a strong potential to play a role in sea star adhesion. One is similar to tachylectin-like proteins, lectins able to bind to various carbohydrates. Such a protein would be a good candidate as a component of the footprint homogeneous priming film, where it would promote adhesion to the biofilm present on the surface of the substratum. Two proteins are similar to the IgGFc binding protein, a mucin-like protein forming structural networks through oligomerisation. In sea star adhesive footprints, the two mucin-like proteins could be involved in the formation of structural networks through their potential ability to oligomerise and/or cross-link to other adhesive molecules. Finally, some footprint proteins were annotated on the basis of the presence of functional domains such as hyalin, EGF and discoidin domains, known from other studies to mediate protein–protein, protein–carbohydrate or protein–metal interactions.
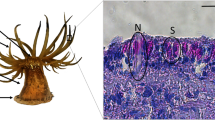
To date, only one of the unannotated proteins, the sea star footprint protein 1 (Sfp1, UniProt X2KZ73), has been fully characterised (Hennebert et al. 2014). This large protein of 3,853 amino acids is the second most abundant constituent of the secreted adhesive. It contains 23 of the 43 de novo-generated peptides obtained from the MS/MS analysis of the major protein bands (Hennebert et al. 2012b). MS and Western blot analyses showed that Sfp1 is translated from a single mRNA and then cleaved into four subunits linked together by disulphide bridges in sea star tube foot adhesive cells (Fig. 9.4a). The four subunits display specific protein-, carbohydrate- and metal-binding domains that mediate interactions with other proteins present in the adhesive material and on the tube foot surface (Hennebert et al. 2014). In situ hybridisation located the mRNA coding for Sfp1 in the tube foot adhesive epidermis (Fig. 9.4b) (Hennebert and Flammang unpubl. obs.). Using immunohisto- and immunocytochemistry, the precise location of Sfp1 was revealed at the level of the rods of the secretory granules enclosed in type 1 adhesive cells (Fig. 9.4c). Within the adhesive footprints, Sfp1 was located in the fibrillar meshwork (Fig. 9.4d) and therefore seems to provide cohesion to the adhesive layer, rather than adhesive properties (Hennebert et al. 2014).
Echinoderm adhesive proteins (adapted in part from Hennebert et al. 2014). (a) Subunits and predicted structural domains of Sfp1 (top) and Nectin (bottom). The two proteins are drawn at the same scale. Green, calcium-binding EGF-like domain; yellow, galactose-binding lectin domain; red, discoidin domain (also known as coagulation factor 5/8 C-terminal domain); blue, von Willebrand Factor; purple, C8 domain. (b) In situ hybridisation on a tube foot of Asterias rubens using probes designed on the basis of the cDNA coding for Sfp1 (whole mount). (c–e) Immunolabelling of tube feet and footprints in A. rubens with antibodies directed against Sfp1β. Longitudinal section through the adhesive epidermis in which secretory granules of adhesive cells are clearly labelled (c, immunofluorescence). Immunocytochemical localisation of Sfp1 in the secretory granules from type 1 adhesive cells (d, immunogold labelling in TEM). In the adhesive footprints left on the substratum after tube foot detachment, immunoreactivity demonstrates that Sfp1 is localised at the level of the fibrillar meshwork (e, immunofluorescence). AE adhesive epidermis, D disc, S stem
As for sea urchins, a first attempt to obtain the tube foot disc proteome successfully identified 328 nonredundant proteins, but since the disc presents a complex histological structure, only 2 % were categorised as putative adhesive proteins (Santos et al. 2013). More recently, high-resolution quantitative mass spectrometry was used to perform the first study combining the analysis of sea urchin tube foot differential proteome with the proteome of its secreted adhesive (Lebesgue et al. 2016). The differential tube foot proteome of P. lividus allowed comparing protein expression in the adhesive part (the disc) versus the nonadhesive part (the stem), resulting in the identification of 163 highly over-expressed disc proteins. In addition, the analysis of the footprint proteome yielded 611 proteins among which more than 70 % fall within five protein groups: actins (27.9 %), histones (24.4 %), tubulins (11.9 %), ribosomal proteins (7.9 %) and myosins (1.4 %). In all these proteomic studies, one protein was repeatedly pinpointed as a putative adhesive protein, Nectin (Santos et al. 2013; Lebesgue et al. 2016).
Nectin (Uniprot Q70JA0), a cell adhesion protein secreted by the eggs and embryos of P. lividus, was shown to significantly increase the binding of dissociated embryonic cells to the substratum (Matranga et al. 1992). Its identification in tube feet led to the hypothesis that it could also be involved in substratum attachment in adult sea urchin (Santos et al. 2013). Indeed, nectin contains six galactose-binding discoidin-like domains (Fig. 9.4a; Costa et al. 2010) and can therefore bind molecules bearing galactose and N-acetylglucosamine carbohydrate moieties on the substratum, on the cuticle or within the adhesive material. Several variants differing only by a few amino acids were identified in the adhesive material, totalising 1.2 % of the footprint proteins in terms of relative abundance (Lebesgue et al. 2016). All these nectin variants are also highly over-expressed (5.4- to 13-fold) in the tube foot disc relatively to the stem (Lebesgue et al. 2016). Nectin cDNA was amplified from tube foot mRNAs showing that, in addition to the known embryonic Nectin mRNA called Nectin-1, a new mRNA sequence called Nectin-2 (GenBank KT351732), differing by 15 nucleotide substitutions, is also expressed in the tube feet of adult sea urchins. These Nectin variants most likely derive from nucleotide substitutions (SNPs) during DNA replication due, for example, to high gene expression (Toubarro et al. 2016). The two Nectin mRNAs were found to be highly over-expressed in tube foot discs comparatively to stems (Toubarro et al. 2016). Finally, Nectin was successfully immunolocalised in the adhesive cells of the tube foot disc as well as in the footprints, confirming the adhesive function of the protein (Lebesgue et al. 2016). This adhesive function is further corroborated by the fact that Nectin expression might be regulated according to the hydrodynamic conditions. Indeed, its expression is significantly higher in tube feet from freshly collected sea urchins than in tube feet from sea urchins maintained in an aquarium (Toubarro et al. 2016).
9.2.3.1.2 De-adhesive Proteins
When tube foot detachment occurs, it always takes place at the level of the outermost layer of the disc cuticle, the fuzzy coat, leaving the adhesive material strongly attached to the substratum as a footprint (Fig. 9.3a) (Flammang 1996). In A. rubens, the polyclonal antibodies raised against footprint material strongly label the fuzzy coat, but no immunoreactivity is detected in the secretory granules of de-adhesive cells (Flammang et al. 1998). This pattern of immunoreactivity suggests that secretions of de-adhesive cells are not incorporated into the footprints, but instead might function enzymatically to jettison the fuzzy coat thereby allowing the tube foot to detach (Flammang 1996; Flammang et al. 1998). In this connection, enzymes (both proteases and glycosylases) were searched in the recently obtained proteomes as they could represent putative de-adhesive proteins. Two proteases were identified in the sea star footprint proteome, which are similar to enzymes presenting a metalloendopeptidase activity (Metalloproteinase SpAN and Tolloid-like protein 2) (Hennebert et al. 2015a). Similarly, several proteases and glycosylases are over-expressed in the sea urchin tube foot disc compared to the stem, indicating that they might be potential components of the de-adhesive secretions. These comprise proteases, such as aminopeptidases, dipeptidases, bleomycin hydrolase-like and cathepsin z, and glycosylases such as N-(beta-n-acetylglucosaminyl)-l-asparaginase, carbohydrate binding module 9-containing protein and sialidases (Lebesgue et al. 2016). Although the carbohydrate fraction of the adhesive of P. lividus is still poorly characterised, the identified glycosylases might be an indication that it could contain sialylated oligosaccharides presumably conjugated to proteins through asparagine residues, similarly to sea star adhesive (Hennebert et al. 2011).
9.2.3.2 Carbohydrate Fraction
Based on colorimetric assays, Flammang et al. (1998) showed that the carbohydrate fraction (in dry weight) of sea star footprints is made up of neutral sugars (3 %), amino sugars (1.5 %) and uronic acids (3.5 %) (Fig. 9.7a). In sea urchin footprints, the presence of neutral carbohydrates (1.2 % of the footprint dry weight) was also demonstrated (Santos et al. 2009b), but no quantification was yet performed for amino sugars and uronic acids (Fig. 9.7a).
In sea stars, the composition of the carbohydrate moiety was further investigated using lectins, molecules that specifically recognise carbohydrate residues. These lectins were used on tube foot histological sections, on footprints and on adhesive protein extracts upon separation on polyacrylamide gels. The results indicate that at least two glycoproteins, as well as larger molecules such as proteoglycans, compose the carbohydrate fraction of sea star footprints. The sugar chains of both glycoproteins and proteoglycans appear to enclose mannose, galactose and sialic acid residues, and to a lesser extent N-acetylgalactosamine and fucose residues (Hennebert et al. 2011).
9.3 Cuvierian Tubules
Cuvierian tubules are peculiar defence organs found in about 60 species of sea cucumbers all belonging to the family Holothuriidae. Two main types of tubules can be differentiated on the basis of their gross external morphology, lobulated and smooth (Lawrence 2001). Lobulated tubules occur exclusively in the genus Actinopyga; they are never expelled and are not sticky (VandenSpiegel and Jangoux 1993). These tubules are used as a toxic decoy to deter predators (Van Dyck et al. 2010). On the other side, smooth tubules are present in the genera Bohadschia, Holothuria and Pearsonothuria, in which they generally appear as sticky white threads that function as a defence mechanism (Hamel and Mercier 2000; Flammang et al. 2002). Indeed, once ejected, they can elongate and release a glue allowing instantaneous adhesion on any object and can therefore entangle a predator in a matter of seconds (Zahn et al. 1973; VandenSpiegel and Jangoux 1987).
Smooth Cuvierian tubules occur in great numbers (from 50 to 600 according to the species considered) in the posterior part of the body cavity of the holothuroid (Becker and Flammang 2010). Proximally they are attached to the basal part of the left respiratory tree, and their distal, blind ends float freely in the coelomic fluid. The mechanism leading to their discharge is as follows: when the animal is disturbed, it directs its posterior end toward the stimulating source and undergoes a general body contraction. Consequently, the wall of the cloaca breaks and the free ends of a few tens of tubules are expelled through the tear and the cloacal orifice. The water of the respiratory tree is then forcefully injected into the lumen of the tubules causing their elongation, up to 20 times their initial length. Upon contact with any surface (e.g., a predator integument), the lengthened tubules become instantly sticky. Finally, the elongated tubules autotomise at the attachment point on the respiratory tree and are left behind as the sea cucumber crawls away (VandenSpiegel and Jangoux 1987; Becker and Flammang 2010). Lost tubules are then regenerated in a few weeks (VandenSpiegel et al. 2000; Hamel and Mercier 2000). As only a portion of the tubules are emitted at one time, the total number may suffice for several responses (Hamel and Mercier 2000).
Four characteristics concur to make Cuvierian tubules very efficient as a defence system: (1) their large number, (2) their adhesiveness, (3) their mechanical design and (4) their regeneration capacities. Indeed, the adhesiveness of Cuvierian tubules combines with their tensile properties to entangle and immobilise potential predators (Zahn et al. 1973; Hamel and Mercier 2000; Flammang et al. 2002). On the other side, their large number, sparing use and regeneration dynamics make them almost inexhaustible line of defence (VandenSpiegel and Jangoux 1987; VandenSpiegel et al. 2000; Hamel and Mercier 2000).
9.3.1 Fine Structure and Adhesion Strength
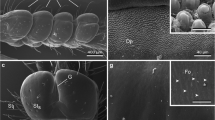
Cuvierian tubules consist of, from the inside to the outside, an epithelium surrounding the narrow lumen, a thick connective tissue layer and a mesothelium lining the surface of the tubule that is exposed to the coelomic cavity (Fig. 9.5a, b). The mesothelium is the layer responsible for adhesion. In quiescent tubules (i.e., non-expelled and non-elongated tubules), it is a pseudostratified epithelium made up of two superposed cell layers, an outer layer of peritoneocytes and an inner layer of granular cells which is highly folded along the long axis of the tubule (Fig. 9.5b). Granular cells are flattened cells filled with electron-dense membrane-bound granules enclosing a proteinaceous material (Endean 1957; VandenSpiegel and Jangoux 1987; Delmeudre et al. 2014). They are organised in V-shaped structures (Fig. 9.5c). Peritoneocytes are T-shaped, displaying a flattened apical part lining the coelomic cavity and a thin elongated basal part running between the granular cell folds (Fig. 9.5c). They bear a single cilium and a few short microvilli, and their apical cytoplasm contains mucous vesicles (VandenSpiegel and Jangoux 1987; Delmeudre et al. 2014). During elongation, the structure of the mesothelium is modified: the protective outer layer of peritoneocytes disintegrates, and the granular cell layer, now unfolded, thus becomes outermost on the tubule (Fig 9.5d; VandenSpiegel and Jangoux 1987; Delmeudre et al. 2014). Granular cells empty the contents of their granules when the elongated tubule comes into contact with a surface, resulting in adhesion (VandenSpiegel and Jangoux 1987; Delmeudre et al. 2014). Once released, this material changes in aspect, swells and spreads readily on any type of substrate where it forms a thin homogeneous adhesive layer (Fig. 9.5e; Becker and Flammang 2010; Delmeudre et al. 2014).
Morphology and ultrastructure of the Cuvierian tubules of Holothuria impatiens (a, b) and Holothuria forskali (c–e) (modified from Flammang et al. 2005, and Delmeudre et al. 2014). SEM photograph of a transversally sectioned tubule (a), and longitudinal histological section showing the arrangement of the tissue layers (b). (c) TEM of the apical part of the mesothelium of tubules before the elongation process. (d, e) TEM of the mesothelium of an elongated tubule before and after contact with a surface, respectively. AM adhesive material, CTL connective tissue layer, IE inner epithelium, G granule, GC granular cell, L lumen, ML muscle layer, M mesothelium, MV mucus vesicle, P peritoneocyte
The interspecific diversity of Cuvierian tubule histology among the three genera possessing smooth Cuvierian tubules is relatively low. However, some differences were observed in their fine structure, especially at the level of the mesothelium, with the highest variability within the genus Holothuria (Becker and Flammang 2010). In H. hilla and H. leucospilota, the granular cell layer is less folded than in H. forskali. In H. maculosa, the mesothelium presents an unusual morphology, being thicker with seemingly over-sized granular cells. In H. nobilis and in all the species from the genus Bohadschia, peritoneocytes lack mucous vesicles (Becker and Flammang 2010).
Cuvierian tubule adhesive strength on glass has been measured in seven species of sea cucumbers belonging to the genera Bohadschia, Holothuria and Pearsonothuria (Flammang et al. 2002). The mean normal tenacity observed varied from about 0.03 to 0.14 MPa. In the species H. forskali, tubule tenacity is influenced by the nature of the substratum: tubules adhere more strongly to polar than to nonpolar substrata, indicating the importance of polar interactions in adhesion (Flammang et al. 2002). A similar trend was observed for the tubules of H. dofleinii using peel tests (Peng et al. 2011). Moreover, in several species of the genus Holothuria, adhesive forces have been shown to vary with the temperature, salinity and pH of seawater (Zahn et al. 1973; Flammang et al. 2002; Peng et al. 2011).
9.3.2 Ultrastructure and Composition of the Adhesive Material
After detachment of Cuvierian tubules from a substrate, the material left on the surface is called the tubule print material (TPM). The quantity of TPM deposited on a surface varies from one tubule print to another or even within a single print. The adhesive material appears as a thin homogeneous film which clearly derives from the secretory granules of granular cells (Fig. 9.6a). Different structures such as intact granules from granular cells or contaminating collagen fibres from the connective tissue layer can be distinguished on this adhesive film (Fig. 9.6a). AFM observations demonstrated that the adhesive film is composed of nano-globular structures of about 70 nm in diameter (Fig. 9.6b) (Delmeudre et al. 2014).
Tubule print material from Holothuria forskali (modified from Delmeudre et al. 2014). (a) SEM picture showing that the adhesive material derives from the secretory granules of granular cells. (b) AFM image of the adhesive material. AM adhesive material, G granule
In H. forskali, the TPM is composed of an organic fraction consisting of 54 % protein and 36 % carbohydrate, and of an inorganic fraction accounting for 10 % of the dry weight (Fig. 9.7a; De Moor et al. 2003). The proteinaceous nature of the adhesive material is confirmed by the observation that proteolytic enzymes and protein denaturation agents reduce the adhesive strength of Cuvierian tubules (Zahn et al. 1973; Peng et al. 2011). The amino acid compositions of the protein fraction in H. forskali, H. leucospilota, B. subrubra and P. graeffei indicate that their adhesives are closely related (Table 9.3). All are rich in small side-chain amino acids, especially glycine, and in charged and polar amino acids. The amino acid composition of the TPM from H. maculosa stands apart from all other Cuvierian tubule adhesives with only half their content in glycine but a much higher proportion of glutamate/glutamine and serine (Table 9.3).
Protein extractions using strong denaturing buffers containing both chaotropic and reducing agents were performed on TPM of H. forskali and H. dofleinii. In both species, the extracts contain about ten major protein bands with apparent molecular masses ranging from 17 to 220 kDa (De Moor et al. 2003; Flammang et al. 2009; Peng et al. 2011, 2014). In H. forskali, these proteins possessed closely related amino acid compositions, rich in glycine and in glutamine/glutamic acid residues (De Moor et al. 2003). In the same species, Baranowska et al. (2011) identified an 18 kDa protein cross reacting with antibodies raised against precollagen D, a byssal protein from the mussel Mytilus galloprovincialis. These authors claimed that this 18 kDa protein would be a major adhesive protein, but no sequence was reported. More recently, Peng et al. (2014) identified some proteins extracted from the adhesive material of H. dofleinii. Among the nine protein bands detected by gel electrophoresis, tandem mass spectrometry-based sequencing of tryptic peptides allowed the authors to identify two novel proteins, one C-type lectin and three enzymes associated to the pentose phosphate cycle and glycolysis. Partial cDNA sequences of three of these proteins (one novel protein, one C-type lectin and one transketolase) were retrieved by RT–PCR experiments. No confirmation of their adhesive function was provided, however.
The second most abundant fraction composing the Cuvierian tubule adhesive, the carbohydrate fraction, was investigated by histochemical experiments. Lectin labelling was used to detect the presence of oligosaccharidic structures on tubule sections from H. forskali. No labelling was found in the granular cells using seven lectins specific for neutral sugar containing oligosaccharides (Becker and Flammang 2010). However, lectin blots performed on TPM extracts with another set of six lectins suggest that at least three glycoproteins, containing galactose, N-acetylgalactosamine, N-acetylglucosamine and sialic acid residues, would be present in the adhesive material (Demeuldre and Flammang, unpublished obs.). In addition to glycosylation, another protein post-translational modification, phosphorylation, has been highlighted in the adhesive of Cuvierian tubules (Flammang et al. 2009). Using specific antibodies, phosphoserine residues were detected in the granular cells from the tubules of three different species (B. subrubra, H. forskali and P. graeffei) (Flammang et al. 2009). Immunoblots and amino acid analyses confirmed the presence of polyphosphoproteins in the adhesive secretion of Cuvierian tubules from these species (Flammang et al. 2009).
9.4 Comparisons Between Echinoderm Adhesives and with Other Marine Bioadhesives
Adhesion (attachment with adhesive secretions) is a way of life in the sea (Waite 1983). Indeed, representatives of bacteria, protoctists (including macroalgae) and all animal phyla, living in the sea, attach to natural or artificial surfaces. Adhesion ability is particularly developed and diversified in invertebrates, which adhere during their larval and adult life (see other chapters in this book). It is involved in various functions such as the handling of food, the building of tubes or burrows and, especially, the attachment to the substratum (Walker 1987; Tyler 1988; Whittington and Cribb 2001; Flammang et al. 2005). Indeed, seawater, being a dense medium, denies gravity to hold organisms to the bottom. Thus, to withstand the hydrodynamic forces, marine organisms rely on specialised adhesive mechanisms. Adhesion to the substratum may be permanent, transitory, temporary or instantaneous (Tyler 1988; Whittington and Cribb 2001; Flammang et al. 2005). Permanent adhesion involves the secretion of a cement and is characteristic of sessile organisms staying at the same place throughout their adult life (e.g., the attachment of barnacles on rocks). Transitory adhesion allows simultaneous adhesion and locomotion: the animals attach by a viscous film they lay down between their body and the substratum and creep on this film, which they leave behind as they move (e.g., the ventral secretions of turbellarian platyhelminths). Temporary adhesion allows organisms to attach firmly but momentarily to a substratum (e.g., the adhesion of echinoderm tube feet). The boundary between transitory and temporary adhesion is not always clear, however. Indeed, gastropod molluscs may use either transitory adhesion (in conjunction with suction) when they are moving, or temporary adhesion when stationary for a long period of time; the latter giving by far the greatest adhesive strength to the animal (see, e.g., Smith et al. 1999a). Instantaneous adhesion relies on single-use organs or cells and is used in functions other than attachment to the substratum requiring a very fast formation of adhesive bonds. Prey capture by collocyte-bearing tentacles of ctenophorans and defence reaction involving Cuvierian tubules in holothuroids are typical examples of this type of adhesion (Flammang et al. 2005).
The evaluation of the adhesive strength in marine invertebrates is usually done by measuring their tenacity, which is the adhesion force per unit area. According to the taxonomic group considered, tenacities of marine organisms range from about 0.001 to 2 MPa (see, e.g., Walker 1987, for review). Many studies have shown that several factors may profoundly influence the tenacity of invertebrates (see, e.g., Grenon and Walker 1981). For example, the physical (e.g., roughness) as well as chemical characteristics (e.g., hydrophobicity, surface charges) of the substratum are known to change the tenacity of organisms by up to one order of magnitude (Young and Crisp 1982; Yule and Walker 1987). As a consequence, great care should be exercised when comparing values of tenacity extracted from different studies. The mean tenacity of echinoderm tube feet on polymer and glass substrata ranges from 0.09 to 0.54 MPa. These values are in the same range as those observed in other marine invertebrates known to adhere strongly to such substrata (e.g., 0.17–0.23 MPa in limpets, Grenon and Walker 1981; 0.08–0.52 MPa in barnacles, Yule and Walker 1987; 0.12–0.75 MPa in mussels, Waite 2002). On the other hand, the mean normal tenacity measured for sea cucumber Cuvierian tubules, i.e., 0.03–0.14 MPa, falls within the lower range of adhesive strengths described for marine organisms (Flammang et al. 2002).
In marine invertebrates, adhesive secretions are always predominantly made up of proteins. Yet, their biochemical composition varies from one taxonomic group to another (Flammang 2006). As a general rule, permanent adhesives consist almost exclusively of proteins. On the other hand, nonpermanent adhesives are made up of an association of proteins and carbohydrates, the latter being mostly in the form of acid and sulphated sugars (see Whittington and Cribb 2001, for review). The ratio of proteins to carbohydrates is usually about 2:1, but there may be substantial variation on this figure though there is typically more protein than carbohydrate (Grenon and Walker 1980; Davies et al. 1990; Smith et al. 1999a; Smith and Morin 2002). These adhesives usually also comprise a large inorganic fraction. The echinoderm tube foot adhesive composition corresponds to a typical nonpermanent adhesive (Fig. 9.7a). Lipids were also detected in tube foot adhesive secretions (Fig. 9.7a). This lipid fraction might come from the membranes of the adhesive granules or could be a contaminant in the footprint material (Flammang et al. 1998). However, an actual role of lipids in marine adhesion cannot be discarded since, recently, the permanent adhesive of barnacle cyprid larvae was shown to be a biphasic system containing both lipids and phosphoproteins, working synergistically to maximise adhesion to diverse surfaces under hostile conditions. Lipids were shown to be secreted first, possibly to displace water from the surface interface creating a conducive environment for introduction of phosphoproteins while simultaneously modulating the spreading of the protein phase and protecting the nascent adhesive from bacterial biodegradation (Gohad et al. 2014). The composition of the instantaneous adhesive of the Cuvierian tubule adhesive is reminiscent of nonpermanent adhesives by its association of proteins and carbohydrate in a 3:2 ratio (De Moor et al. 2003). However, it differs from them by the fact that the carbohydrate fraction is in the form of neutral sugars and not acidic sugars, and by its lower inorganic content (Fig. 9.7a).
As far as the amino acid composition of the protein fraction is concerned, all marine bioadhesives characterised so far have in common their richness in small side-chain amino acids as well as in charged and polar amino acids (Flammang 1996). These traits are common to many marine adhesives and are pointed out as key factors for their high cohesion and adhesive strength. Small side-chain amino acids are often found in large quantities in elastomeric proteins (Tatham and Shewry 2000). These proteins are able to withstand significant deformations without rupture before returning to their original state when the stress is removed (Smith et al. 1999b). Charged and polar amino acids, on the other hand, are probably involved in adhesive interactions with the substratum through hydrogen and ionic bonding (Waite 1987). Some adhesives, like those of barnacles are also rich in nonpolar, hydrophobic residues which could be involved in hydrophobic interactions with the substratum or within the adhesive material (Naldrett 1993). Adhesives from the three model echinoderm species present all these characteristics of marine adhesives. Their amino acid compositions resemble each other by the fact that they share some of their most abundant amino acids (Fig. 9.7b). However, the sea star adhesive possesses more charged residues, the sea urchin adhesive more hydrophobic residues and the sea cucumber adhesive more glycine residues (Fig. 9.7b). When these differences are quantified by the method of Marchalonis and Weltman (1971), no relatedness is found between the adhesive secretions of A. rubens, P. lividus and H. forskali (Table 9.4). The values of SΔQ for comparisons between the Cuvierian tubule adhesives of five sea cucumber species show that all these adhesives are closely related except the one of H. maculosa which stands apart and shows relatedness with the adhesive from sea star footprint (Table 9.4).
Although the detailed composition of echinoderm adhesives is only known for four species (i.e., for the tube feet of the sea star A. rubens and of the sea urchin P. lividus as well as for the Cuvierian tubules of the sea cucumbers H. forskali and H. dofleinii), the variability of the adhesive secretions within the phylum has been investigated by immunohistochemistry (Santos et al. 2005b, 2009a; Becker and Flammang 2010; Santos and Flammang 2012). This was done using polyclonal antibodies raised against the adhesive material from model species to evaluate the differences in the composition of the contents of the tube foot or Cuvierian tubule adhesive cells by looking for antibody cross reactivity on histological sections made from different species.
Polyclonal antibodies raised against the adhesive material of H. forskali were used to locate the origin of TPM constituents in the tubule. Granular cells showed extensive immunoreactivity, suggesting that their secretions make up the bulk of the adhesive material (De Moor et al. 2003). These antibodies were tested on ten other species from the genera Holothuria, Bohadschia and Pearsonothuria (Becker and Flammang 2010). Granular cells are strongly labelled in all species of the genera Bohadschia and Holothuria possessing sticky tubules, except in H. maculosa in which only the very basal part of granular cells is labelled, but not the apex. In P. graeffei, the contents of granular cells are immunoreactive, but the labelling is weaker than in Bohadschia and Holothuria. These results indicate that Cuvierian tubules adhesives are closely related, probably sharing many identical molecules or, at least, many identical epitopes on their constituents, but also that a certain variability occurs in the composition of these adhesive materials (Becker and Flammang 2010). In particular, H. maculosa is confirmed as possessing peculiar Cuvierian tubules (Demeuldre and Flammang, unpublished obs.).
A similar comparative immunohistochemical study was conducted with antibodies raised against the footprint material of the echinoid Sphaerechinus granularis on seven other sea urchin species belonging to three orders and five families. It showed that the adhesive secretions of sea urchins do not share any or little common epitopes on their constituents and thus seem to be more or less ‘species-specific’ (Santos and Flammang 2012). In sea urchins, variations in the composition of adhesive secretions could therefore explain the observed interspecific differences in disc tenacity and in adhesive granule ultrastructure reported by Santos and Flammang (2006, 2008). On the contrary, when the variability of the adhesive secretions from 14 sea star species representing five orders and families was investigated using polyclonal antibodies raised against the footprint material of A. rubens, a very strong immunolabelling was always observed at the level of the tube foot adhesive cells in every species investigated, irrespective of the taxon considered, of the tube foot morphotype or function or of the species habitat (Santos et al. 2005b). This immunoreactivity indicates that, contrary to sea urchin adhesives, sea star adhesives share many identical epitopes. However, differences in the adhesive secretion composition may exist, that are not detected by the antibodies used and that could account for the differences observed in the structure and function of asteroid tube feet (Santos et al. 2005b). For instance, Sfp1 was detected in the transcriptome of the species Pisaster ochraceus (EchinoDB; Janies et al. 2016), which belongs to the same order as A. rubens, but not in the genome of Patiria miniata (EchinoBase; Cameron et al. 2009) which belongs to another order (Hennebert and Flammang, unpublished obs.).
When the antibodies directed against sea star footprints were assayed in the other echinoderm classes, a phylogeny-related immunoreactivity pattern emerged. Crinoid and ophiuroid digitate tube feet were strongly immunoreactive, their labelling being restricted to the adhesive epidermal areas (Santos et al. 2009a). As for echinoids, of the five orders investigated, only members of the order Echinoida (the order comprising P. lividus) presented clusters of immunolabelled adhesive cells, whereas members of the other four orders did not present any labelling in the tube foot adhesive areas (Santos et al. 2009a). Finally, in holothuroids, the antibodies did not recognise the tube foot adhesive epidermis (Santos et al. 2009a). These results suggest that both crinoids and ophiuroids possess adhesive secretions sharing many similarities with the adhesive material of asteroids. On the other hand, in echinoids and holothuroids, the immunoreactivity was clearly weak or even absent indicating that there are no common epitopes between their adhesive secretions and those of A. rubens (Santos et al. 2009a). These observations are congruent with the phylogenetic hypothesis on the evolution of echinoderm adhesive systems (McKenzie 1988) according to which asteroids, crinoids and ophiuroids would share a common ancestral adhesive system in which the adhesive is extruded through apical duct cells, while a common echinoid/holothuroid adhesive system would have arisen later in the evolution in which the adhesive is released through apical tuft cells. This model fits well with the most commonly accepted echinoderm phylogeny in which echinoids and holothuroids form a derived clade, the Echinozoa (Littlewood et al. 1998, David and Mooi 1998). A drawback to this model is the moderate immunoreactivity observed in the tube feet of P. lividus, meaning that its adhesive shares some common epitopes with that of A. rubens. There is a possibility that these two species convergently acquired their similarity because of common selective pressures. Indeed, although they are clearly not homologous, Sfp1 and Nectin share the presence in their sequence of several discoidin-like domains. More studies are needed to address this question and to understand the evolution and functioning of echinoderm adhesive systems.
Further comparisons between echinoderm adhesives and those of other marine invertebrates will require a detailed knowledge of their protein composition, of the sequences of these proteins and of their post-translational modifications. So far, none of these information are available for the Cuvierian tubule adhesive, whereas only two tube foot adhesive proteins have been characterised. In recent years, the combined use of transcriptomics and proteomics has emerged as the best way leading to the identification of novel adhesive proteins and retrieval of their complete sequences (see Hennebert et al. 2015b for review). In addition to novel proteins, attention should also be paid to proteins such as actins or histones, which have been detected in the footprints of both sea stars and sea urchin. Although these proteins have been considered as contaminants, their abundance raises the possibility that they might be specific components of the adhesive material involved in exocytosis or in protection against microbes (Lebesgue et al. 2016).
References
Ameye L, Hermann R, Dubois P, Flammang P (2000) Ultrastructure of the echinoderm cuticle after fast freezing/freeze substitution and conventional chemical fixation. Microsc Res Tech 48:385–393
Baranowska M, Schloßmacher U, McKenzie JD, Muller WEG, Schroder HC (2011) Isolation and characterization of adhesive secretion from cuvierian tubules of sea cucumber Holothuria forskali (Echinodermata: Holothuroidea). Evid Based Complement Alternat Med 2011:486845
Becker P, Flammang P (2010) Unravelling the sticky threads of sea cucumbers. A comparative study on Cuvierian tubule morphology and histochemistry. In: von Byern J, Grunwald I (eds) Biological adhesive systems—from nature to technical and medical application. Springer, Wien, pp 87–98
Cameron RA, Samanta M, Yuan A, He D, Davidson E (2009) SpBase: the sea urchin genome database and web site. Nucl Acids Res 37(Suppl 1):D750–D754
Chaet AB (1965) Invertebrate adhering surfaces: secretions of the starfish, Asterias forbesi, and the coelenterate, Hydra pirardi. Ann N Y Acad Sci 118:921–992
Chaet AB, Philpott DE (1964) A new subcellular particle secreted by the starfish. J Ultrastruct Res 11:354–362
Costa C, Cavalcante C, Zito F, Yokota Y, Matranga V (2010) Phylogenetic analysis and homology modelling of Paracentrotus lividus nectin. Mol Divers 14:653–665
David B, Mooi R (1998) Major events in the evolution of echinoderms viewed by the light of embryology. In: Mooi R, Telford M (eds) Echinoderms: San Francisco. Balkema, Rotterdam, pp 21–28
Davies MS, Jones HD, Hawkins SJ (1990) Seasonal variation in the composition of pedal mucus from Patella vulgata L. J Exp Mar Biol Ecol 144:101–112
De Moor S, Waite JH, Jangoux M, Flammang P (2003) Characterization of the adhesive from the Cuvierian tubules of the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea). Mar Biotechnol 5:37–44
Defretin R (1952) Etude histochimique des mucocytes des pieds ambulacraires de quelques échinodermes. Recueil des travaux de la Station Marine d’Endoume 6:31–33
Delmeudre M, Chinh Ngo T, Hennebert E, Wattiez R, Leclère P, Flammang P (2014) Instantaneous adhesion of Cuvierian tubules in the sea cucumber Holothuria forskali. Biointerphases 9(2):029016
Endean R (1957) The Cuvierian tubules of Holothuria leucospilota. Q J Micros Sci 98:455–472
Engster MS, Brown SC (1972) Histology and ultrastructure of the tube foot epithelium in the phanerozonian starfish, Astropecten. Tissue Cell 4:503–518
Flammang P (1996) Adhesion in echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 5. Balkema, Rotterdam, pp 1–60
Flammang P (2006) Adhesive secretions in echinoderms: an overview. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, Heidelberg, pp 183–206
Flammang P, Jangoux M (1992) Functional morphology of the locomotory podia of Holothuria forskali (Echinodermata, Holothuroidea). Zoomorphology 11:167–178
Flammang P, Jangoux M (1993) Functional morphology of coronal and peristomeal podia in Sphaerechinus granularis (Echinodermata, Echinoida). Zoomorphology 113:47–60
Flammang P, Walker G (1997) Measurement of the adhesion of the podia in the asteroid Asterias rubens (Echinodermata). J Mar Biol Ass UK 77:1251–1254
Flammang P, Demeuleneare S, Jangoux M (1994) The role of podial secretions in adhesion in two species of sea stars (Echinodermata). Biol Bull 187:35–47
Flammang P, Michel A, Van Cauwenberge A, Alexandre H, Jangoux M (1998) A study of the temporary adhesion of the podia in the sea star Asterias rubens (Echinodermata, Asteroidea) through their footprints. J Exp Biol 201:2383–2395
Flammang P, Ribesse J, Jangoux M (2002) Biomechanics of adhesion in sea cucumber Cuvierian tubules (Echinodermata, Holothuroidea). Integr Comp Biol 42:1107–1115
Flammang P, Santos R, Haesaerts D (2005) Echinoderm adhesive secretions: from experimental characterization to biotechnological applications. In: Matranga V (ed) Marine molecular biotechnology: echinodermata. Springer, Berlin, pp 201–220
Flammang P, Lambert A, Bailly P, Hennebert E (2009) Polyphosphoprotein-containing marine adhesives. J Adhes 85:447–464
Gohad NV, Aldred N, Hartshorn CM, Jong Lee Y, Cicerone MT, Orihuela B, Clare AS, Rittschof D, Mount AS (2014) Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat Commun 5:4414
Grenon JF, Walker G (1980) Biochemical and rheological properties of the pedal mucus of the limpet, Patella vulgata L. Comp Biochem Physiol B 66:451–458
Grenon JF, Walker G (1981) The tenacity of the limpet, Patella vulgata L.: An experimental approach. J Exp Mar Biol Ecol 54:277–308
Hamel J-F, Mercier A (2000) Cuvierian tubules in tropical holothurians: usefulness and efficiency as a defence mechanism. Mar Fresh Behav Physiol 33:115–139
Hennebert E, Viville P, Lazzaroni R, Flammang P (2008) Micro- and nanostructure of the adhesive material secreted by the tube feet of the sea star Asterias rubens. J Struct Biol 164:108–118
Hennebert E, Haesaerts D, Dubois P, Flammang P (2010) Evaluation of the different forces brought into play during tube foot activities in sea stars. J Exp Biol 213:1162–1174
Hennebert E, Wattiez R, Flammang P (2011) Characterisation of the carbohydrate fraction of the temporary adhesive secreted by the tube feet of the sea star Asterias rubens. Mar Biotechnol 13:484–495
Hennebert E, Santos R, Flammang P (2012a) Echinoderms don’t suck: evidence against the involvement of suction in tube foot attachment. In: Kroh A, Reich M (eds) Echinoderms 2010: proceedings of the 7th European Conference on echinoderms, Zoosymposia, vol 7., pp 25–32
Hennebert E, Wattiez R, Waite JH, Flammang P (2012b) Characterization of the protein fraction of the temporary adhesive secreted by the tube feet of the sea star Asterias rubens. Biofouling 28:289–303
Hennebert E, Wattiez R, Demeuldre M, Ladurner P, Hwang DS, Waite JH, Flammang P (2014) Sea star tenacity mediated by a protein that fragments, then aggregates. Proc Natl Acad Sci USA 111:6317–6322
Hennebert E, Leroy B, Wattiez R, Ladurner P (2015a) An integrated transcriptomic and proteomic analysis of sea star epidermal secretions identifies proteins involved in defense and adhesion. J Proteomics 128:83–91
Hennebert E, Maldonado B, Ladurner P, Flammang P, Santos R (2015b) Experimental strategies for the identification and characterization of adhesive proteins in animals: a review. Interface Focus 5:20140064
Hermans CO (1983) The duo-gland adhesive system. Oceanogr Mar Biol Ann Rev 21:281–339
Higgins LJ, Mostaert AS (2013) Qualitative and quantitative study of spiny starfish (Marthasterias glacialis) footprints using atomic force microscopy. In: Santos R, Aldred N, Gorb S, Flammang P (eds) Biological and biomimetic adhesives: challenges and opportunities. RSC Publishing, Cambridge, pp 26–37
Janies DA, Witter Z, Linchangco GV, Foltz DW, Miller AK, Kerr AM, Jay J, Reid RW, Wray GA (2016) EchinoDB, an application for comparative transcriptomics of deeply-sampled clades of echinoderms. BMC Bioinf. doi:10.1186/s12859-016-0883-2
Lawrence JM (1987) A functional biology of echinoderms. Croom Helm, London
Lawrence JM (2001) Function of eponymous structures in echinoderms: a review. Can J Zool 79:1251–1264
Lebesgue N, da Costa G, Ribeiro RM, Ribeiro-Silva C, Martins GG, Matranga V, Scholten A, Cordeiro C, Heck AJR, Santos R (2016) Deciphering the molecular mechanisms underlying sea urchin reversible adhesion: a quantitative proteomics approach. J Proteomics 138:61–71
Marchalonis JJ, Weltman JK (1971) Relatedness among proteins: a new method of estimation and its application to immunoglobins. Comp Biochem Physiol B 38:609–625
Matranga V, Di Ferro D, Zito F, Cervello M, Nakano E (1992) A new extracellular matrix protein of the sea urchin embryo with properties of a substrate adhesion molecule. Roux’s Arch Dev Biol 201:173–178
McKenzie JD (1988) The ultrastructure of tube foot epidermal cells and secretions: Their relationship to the duo-glandular hypothesis and the phylogeny of the echinoderm classes. In: Paul CRC, Smith AB (eds) Echinoderm phylogeny and evolutionary biology. Clarendon, Oxford, pp 287–298
Naldrett MJ (1993) The importance of sulphur cross-links and hydrophobic interactions in the polymerization of barnacle cement. J Mar Biol Assoc UK 73:689–702
Nichols D (1966) Functional morphology of the water vascular system. In: Boolootian RA (ed) Physiology of echinodermata. Interscience Publishers, New York, pp 219–244
Paine VL (1926) Adhesion of the tube feet in starfishes. J Exp Zool 45:361–366
Peng YY, Glattauer V, Skewes TD, White JF, Nairn KM, McDevitt AN, Elvin CM, Werkmeister JA, Graham LD, Ramshaw JAM (2011) Biomimetic materials as potential medical adhesives—Composition and adhesive properties of the material coating the Cuvierian tubules expelled by Holothuria dofleinii. In: Pignatello R (ed) Biomaterials- physics and chemistry. InTech Press, Rijeka, pp 245–258
Peng YY, Glattauer V, Skewes TD, McDevitt A, Elvin CM, Werkmeister JA, Graham LD, Ramshaw JAM (2014) Identification of proteins associated with adhesive prints from Holothuria dofleinii Cuvierian tubules. Mar Biotechnol 16:695–706
Perpeet C, Jangoux M (1973) Contribution á l’etude des pieds et des ampoules ambulacraires d’Asterias rubens (Echinodermata, Asteroides). Forma et functio 6:191–209
Ruppert EE, Fox RS, Barnes RD (2003) Invertebrate zoology: a functional evolutionary approach. Saunders College Publishers, Fort Worth
Santos R, Flammang P (2006) Morphology and tenacity of tube foot disc of three common European sea urchin species: a comparative study. Biofouling 22:187–200
Santos R, Flammang P (2008) Estimation of the attachment strength of the shingle sea urchin, Colobocentrotus atratus, and comparison with three sympatric echinoids. Mar Biol 154:37–49
Santos R, Flammang P (2012) Is the adhesive material secreted by sea urchin tube feet species-specific? J Morphol 273:40–48
Santos R, Gorb S, Jamar V, Flammang P (2005a) Adhesion of echinoderm tube feet to rough surfaces. J Exp Biol 208:2555–2567
Santos R, Haesaerts D, Jangoux M, Flammang P (2005b) Comparative histological and immunohistochemical study of sea star tube feet (Echinodermata, Asteroidea). J Morphol 263:259–269
Santos R, Hennebert E, Varela Coelho A, Flammang P (2009a) The echinoderm tube foot and its involvement in temporary underwater adhesion. In: Gorb S (ed) Functional surfaces in biology, vol 2. Springer, Netherlands, pp 9–41
Santos R, da Costa G, Franco C, Gomes-Alves P, Flammang P, Coelho AV (2009b) First insights into the biochemistry of tube foot adhesive from the sea urchin Paracentrotus lividus (Echinoidea, Echinodermata). Mar Biotechnol 11:686–698
Santos R, Barreto A, Franco C, Coelho AV (2013) Mapping sea urchins tube feet proteome—a unique hydraulic mechano-sensory adhesive organ. J Proteomics 79:100–113
Smith AM, Morin MC (2002) Biochemical differences between trail mucus and adhesive mucus from marsh periwinkle snail. Biol Bull 203:338–346
Smith AM, Quick TJ, St Peter RL (1999a) Differences in the composition of adhesive and non-adhesive mucus from the limpet Lottia limatula. Biol Bull 196:34–44
Smith BL, Schäffer TE, Viani M, Thompson JB, Frederick NA, Kindt J, Belcher A, Stucky GD, Morse DE, Hansma PK (1999b) Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature 399:761–763
Souza Santos H, Silva Sasso W (1968) Morphological and histochemical studies on the secretory glands of starfish tube feet. Acta Anat 69:41–51
Tatham AS, Shewry PR (2000) Elastomeric proteins: biological roles, structures and mechanisms. Trends Biochem Sci 25:567–571
Thomas LA, Hermans CO (1985) Adhesive interactions between the tube feet of a starfish, Leptasterias hexactis, and substrata. Biol Bull 169:675–688
Toubarro D, Gouveia A, Ribeiro RM, Simões N, da Costa G, Cordeiro C, Santos R (2016) Cloning, characterization and expression levels of the Nectin gene from the tube feet of the sea urchin Paracentrotus lividus. Mar Biotechnol 18:372–383
Tyler S (1988) The role of function in determination of homology and convergence—examples from invertebrates adhesive organs. Fortsch Zool 36:331–347
Van Dyck S, Gerbaux P, Flammang P (2010) Qualitative and quantitative saponin contents in five sea cucumbers from the Indian Ocean. Mar Drugs 8:173–189
VandenSpiegel D, Jangoux M (1987) Cuvierian tubules of the holothuroid Holothuria forskali (Echinodermata): a morphofunctional study. Mar Biol 96:263–275
Vandenspiegel D, Jangoux M (1993) Fine structure and behaviour of the so-called Cuvierian organs in the holothuroid genus Actinopyga (Echinodermata). Acta Zool 74:43–50
VandenSpiegel D, Jangoux M, Flammang P (2000) Maintaining the line of defense: Regeneration of Cuvierian tubules in the holothuroid Holothuria forskali (Echinodermata). Biol Bull 198:34–49
Waite JH (1983) Adhesion in byssally attached bivalves. Biol Rev 58:209–231
Waite JH (1987) Nature’s underwater adhesive specialist. Int J Adhes Adhes 7:9–14
Waite JH (2002) Adhesion à la moule. Integr Comp Biol 42:1172–1180
Walker G (1987) Marine organisms and their adhesion. In: Wake WC (ed) Synthetic adhesives and sealants. John Wiley & Sons, Chichester, pp 112–135
Whittington ID, Cribb BW (2001) Adhesive secretions in the Platyhelminthes. Adv Parasitol 48:101–224
Young GA, Crisp DJ (1982) Marine animals and adhesion. In: Allen KW (ed) Adhesion, vol 6. Applied Sciences, London, pp 19–39
Yule AB, Walker G (1987) Adhesion in barnacles. In: Southward AJ (ed) Crustacean issues, vol 5, Biology of Barnacles. Balkema, Rotterdam, pp 389–402
Zahn RK, Müller WEG, Michaelis M (1973) Sticking mechanisms in adhesive organs from a Holothuria. Res Mol Biol 2:47–88
Acknowledgements
This work was supported in part by the Fund for Scientific Research of Belgium (F.R.S.–FNRS), by the ‘Service Public de Wallonie—Programme Winnomat 2’, by the ‘Communauté française de Belgique—Actions de Recherche Concertées’ and by COST Action TD0906. P.F. is Research Director of the F.R.S.-FNRS. RS is supported by Fundação para a Ciência e Tecnologia through a post-doctoral grant (SFRH/BPD/109081/2015). This study is a contribution from the ‘Centre Interuniversitaire de Biologie Marine’.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Flammang, P., Demeuldre, M., Hennebert, E., Santos, R. (2016). Adhesive Secretions in Echinoderms: A Review. In: Smith, A. (eds) Biological Adhesives. Springer, Cham. https://doi.org/10.1007/978-3-319-46082-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-46082-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46081-9
Online ISBN: 978-3-319-46082-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)