Abstract
In sea stars, adhesion takes place at the level of a multitude of small appendages, the tube feet. It involves the secretion of an adhesive material which, after tube foot detachment, remains on the substratum as a footprint. It was previously reported that the two main organic components of this material are proteins and carbohydrates. The carbohydrate moiety of the adhesive secretion of Asterias rubens was investigated using a set of 16 lectins which were used on sections through tube feet, on footprints, and on the proteins extracted from these footprints. After gel electrophoresis, these proteins separate into eight protein bands which were named sea star footprint proteins (Sfps). Eleven lectins label the tube foot epidermis at the level of the adhesive cells, four react with footprints, and eight with two of the extracted footprint proteins, which are therefore classified as glycoproteins. Sfp-290 appears to bear mostly N-linked oligosaccharides and Sfp-210 principally O-linked oligosaccharides. The outer chains of both glycoproteins enclose galactose, N-acetylgalactosamine, fucose, and sialic acid residues. Another part of the carbohydrate fraction of the footprints would be in the form of larger molecules, such as sialylated proteoglycans. These two types of glycoconjugates are presumably key components of the sea star temporary adhesive providing both cohesive and adhesive contributions through electrostatic interactions by the polar and hydrogen-bonding functional groups of their glycan chains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To secure themselves on immersed substrata in the sea, many marine organisms, ranging from microscopic bacteria and fungi through much larger algae and macroscopic invertebrates, use adhesive secretions (Walker 1987; Smith and Callow 2006). Most of these secretions are made up of complex blends of proteins associated with or without other components. To function effectively as a holdfast, marine adhesives must possess several characteristics such as the ability to displace water from the substratum, spread, and rapidly form strong adhesive bonds with the surface; and, in sessile organisms, the ability to cure and resist microbial degradation (Waite 1987; Kamino 2008). These characteristics are derived from the physico-chemical properties of the adhesive proteins, and in particular, from their post-translational modifications (Sagert et al. 2006). In marine adhesives, these modifications are of three main types: hydroxylation, phosphorylation, and glycosylation.
Hydroxylation is the most investigated protein modification in adhesive proteins. All the proteins identified in the attachment plaques from the byssus of mussels (the foot proteins; fp) contain 3,4-dihydroxyphenylalanine (DOPA), a residue formed by the post-translational hydroxylation of tyrosine. This modified amino acid is involved in cross-linking the different fps, thereby contributing to the cohesive strength of the adhesive plaque, and in surface-coupling, either through hydrogen bonds or by forming complexes with metal ions and metal oxides present in mineral surfaces (Waite et al. 2005; Wiegemann 2005; Lee et al. 2006; Sagert et al. 2006). Hydroxylation also concerns other amino acids in mussel fps: 4-hydroxyproline (fp1), 3,4-dihydroxyproline (fp1), 7-hydroxytryptophan (fp1), and 4-hydroxyarginine (fp3) have been detected in these organisms (Silverman and Roberto 2007; Zhao et al. 2009). All these additional hydroxyl groups could increase hydrogen bonding with surfaces (Silverman and Roberto 2007). DOPA residues were also detected in two of the cement proteins (c1 and c2) secreted by sabellariid tube-worms, in which they would presumably assume the same functions as in mussel attachment plaques (Stewart et al. 2004; Zhao et al. 2005). Recently, a halogenated derivative of DOPA, 2-chloro-DOPA, has been isolated from the tube-worm cement, demonstrating the possibility of two different modifications on a same amino acid (Sun et al. 2009). It was proposed that chloro-DOPA could play a role in defending the cement against microbial fouling and degradation, and in surface interactions (Sun et al. 2009).
Protein phosphorylation occurs in marine adhesives in the form of serine phosphorylation (Sagert et al. 2006). Phosphoserine residues were found in two mussel foot proteins (fp5 and fp6), in one tube-worm cement protein (c3), and in the adhesive released by Cuvierian tubules, the sticky defence organs of sea cucumbers (Zhao et al. 2005; Silverman and Roberto 2007; Flammang et al. 2009). In the adhesive secretions of these organisms, phosphorylation is thought to contribute both cohesive and adhesive roles to the glue (Zhao and Waite 2006; Sun et al. 2007). In the later case, it is proposed that phosphoserine residues could mediate adhesion to calcareous substrata such as the shells of mussels and barnacles (Waite and Qin 2001). They may also play a role in the condensation of the adhesive proteins in the adhesive cell secretory granules (Zhao et al. 2005).
Finally, glycosylation is a post-translational modification which occurs in a large diversity of organisms (Caldwell and Pagett 2010). It is encountered in the adhesive mucus of gastropod molluscs (the 140 kDa protein in the limpet Lottia limatula and the 36 and 41 kDa proteins in the marsh periwinkle Littoraria irrorata; Smith et al. 1999; Smith and Morin 2002), in the byssal attachment plaques of the green mussel Perna viridis (Pvfp-1; Ohkawa et al. 2004), in the cement of barnacles (cp-68k; Kamino 2006, 2008), and in the adhesive of the zoospores produced by the green alga Ulva (Stanley et al. 1999). Despite the detection of glycoproteins in the adhesives of such a wide range of organisms, little is known about the composition of their carbohydrate fraction. Information are available for two of these organisms. In the green alga, at least one of the adhesive proteins is an N-linked glycoprotein probably containing mannose and glucose residues (Stanley et al. 1999; Michael 2009). In the green mussel, Pvfp-1 encloses N-acetylglycosyl-fucose O-linked to threonine residues, C-mannosylated tryptophan, and C-mannosylated hydroxytryptophan (Ohkawa et al. 2004; Zhao et al. 2009). This latter resembles DOPA in having attributes that contribute to both cohesive and adsorptive interactions necessary for adhesion (Zhao et al. 2009). In the other groups, however, the role of protein glycosylation in adhesion is not yet understood. Moreover, in some organisms, it was demonstrated that adhesive proteins are associated with carbohydrates, but it is not known whether these carbohydrates are covalently attached to the proteins or not. This remains the case for the adhesive material released by Cuvierian tubules of sea cucumbers and for the adhesive secretion produced by sea stars (Flammang et al. 1998; De Moor et al. 2003).
The aim of this work was to characterise the carbohydrate moiety of the adhesive secretion produced by sea star tube feet. These tube feet are composed of a proximal cylinder, the stem, and an enlarged and flattened apical extremity, the disc. When the tube foot makes contact with the substratum, two types of adhesive cells present in the disc epidermis release their secretions through pores uniformly distributed on the disc surface, thus fastening the tube foot on the substratum (Hennebert et al. 2008). This attachment is temporary, and after the tube foot has become voluntarily detached, the adhesive material remains firmly bound to the substratum as a footprint (Thomas and Hermans 1985; Flammang 2006; Hennebert et al. 2008). Flammang et al. (1998) showed that the two main organic components in the adhesive material are proteins and carbohydrates, which represent 21% and 8% of the footprint dry weight, respectively. To further investigate the carbohydrate moiety of the adhesive secretion from sea star tube feet, we used lectins, proteins specifically recognizing oligosaccharidic motifs. Different lectins were used on tube foot sections, on whole footprints, and on protein blots to identify and localise adhesive glycoproteins.
Materials and Methods
Collection and Maintenance of Sea Stars
Individuals of Asterias rubens Linné, 1758 were collected intertidally in Audresselles (Pas-de-Calais, France). They were kept in a marine aquarium with closed circulation (13°C, 33 psu) and fed with mussels (Mytilus edulis, Linné, 1758).
Collection of Footprint Material and Electrophoretic Analysis
Sea star footprints were obtained by allowing individuals to walk across and/or attach to the bottom of clean glass Petri dishes filled with filtered sea water for 8 h. The Petri dishes were thoroughly rinsed with ultra pure water and placed in a freeze dryer (Christ Alpha 1–2). The lyophilised footprint material was then scraped off using a razor blade and stored at −20°C (Flammang et al. 1998).
Sea star footprint proteins (Sfps) were extracted from this footprint material according to the protocol developed by Hennebert et al. (in prep.). Briefly, 3.2 mg of lyophilised footprint material were suspended in a 10 mM sodium phosphate buffer (pH 6.0) containing 6 M guanidine hydrochloride (GuHCl). After centrifugation, the supernatant (“fraction 1”) was collected and the pellet was re-suspended in a 1.5 M Tris–HCl buffer (pH 8.5) containing 7 M GuHCl, 0.5 M dithiothreitol (DTT), and 20 mM ethylenediaminetetraacetate (EDTA). After an incubation time of 1 h at 60°C, the sample was centrifuged and the resulting supernatant was recovered as “fraction 2”. This fraction (which contains the majority of the Sfps; Hennebert et al., in prep.) was dialysed against 1% (v/v) acetic acid at 4°C and centrifuged. The pellet was then solubilised in Laemmli sample buffer (Bio-Rad) containing 5% (v/v) mercaptoethanol (βMSH), heated for 2 min at 90°C and submitted to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the gel was stained with Coomassie R-250 (Imperial protein stain, Pierce).
The extent of glycosylation of the Sfps was estimated using the method of Segrest and Jackson (1972). This involved running the samples on gels with different total acrylamide concentrations. In gels with low acrylamide concentrations, proteins with more than 10% carbohydrate run with a higher apparent molecular weight because the glycosylation inhibits SDS binding. At higher acrylamide concentrations, protein mobility is more dependent on the effect of sieving than on the charge. Thus, the relative mobility of heavily glycosylated proteins increases as the gel concentration increases, whereas there will be little effect on proteins with less than 10% carbohydrate (Segrest and Jackson 1972; Carlsson 1993; Smith and Morin 2002). Samples of fraction 2 were analysed on 5%, 8% and 10% (w/v) gels.
Lectin Assays
The presence of specific carbohydrate residues in the adhesive material of the tube feet was tested by lectin histochemistry and by lectin blotting. Lectins are proteins or glycoproteins of non-immune origin that are able to bind carbohydrates without chemical modification (Leathem and Atkins 1983). Sixteen biotinylated lectins, purchased from Vector Laboratories (Burlingame, CA), were used. Their full names, natural sources, and saccharide specificities are listed in Table 1.
Lectin Histochemistry
Tube feet were fixed in Bouin’s fluid for 24 h. They were subsequently dehydrated in graded ethanol, embedded in paraffin wax, and cut longitudinally into 5 μm-thick sections with a Microm HM 340 E microtome. One section was stained with Alcian blue (pH 2.6) coupled with Mayer’s hemalum and phloxine (Gabe 1968); the others were subjected to an indirect lectin-labelling method according to the following protocol. Non-specific background staining was blocked by section pre-incubation in Tris-buffered saline, pH 8.0, containing 0.05% (v/v) Tween 20 and 3% (w/v) bovine serum albumin (TBS-T-BSA). From this point, all buffers were supplemented with 5 mM CaCl2 and 5 mM MnCl2 for Con A, LCA, PSA, and PHA-L, and 5 mM CaCl2 and 5 mM MgCl2 for GSL and PNA. Biotinylated lectins diluted in TBS-T-BSA (see Table 2 for dilutions) were applied on the sections for 2 h at room temperature. After three washes of 5 min in TBS-T, the sections were incubated for 1 h in Texas-Red-conjugated streptavidin (Vector Laboratories) diluted 1:100 in TBS-T-BSA. Following three final washes of 10 min in TBS-T, the sections were mounted with Vectashield (Vector Laboratories). Control reactions were performed by substituting the lectins with TBS-T-BSA and by using the lectins saturated with their inhibitory monosaccharide (see Table 2). Sections were observed using a Leica TCS 4D confocal laser scanning microscope and/or a Zeiss Axioscope A1 microscope equipped with an AxioCam ICc 3 camera.
This method was also used on freshly collected footprints. Individual tube feet were allowed to adhere firmly to glass slides for a few minutes, and then to detach voluntarily, leaving footprints on the glass slides. Some of these footprints were stained with a 0.05% (w/v) crystal violet solution in deionised water; the others were labelled with the biotinylated lectins using the same protocol as for tube foot sections.
Lectin Blotting
Sfps separated by SDS-PAGE were blotted onto polyvinylidene fluoride (PVDF) membranes using 90 mM Tris–borate, 2.5 mM EDTA, 0.1% (w/v) SDS, and 25% (v/v) methanol as the transfer buffer. Running conditions were 200 mA constant current for 90 min. The membranes were washed with TBS-T and then blocked overnight at 4°C in TBS-T-BSA. From this point, all buffers were supplemented with 5 mM CaCl2 and 5 mM MnCl2 for Con A, LCA, PSA, and PHA-L, and 5 mM CaCl2 and 5 mM MgCl2 for GSL and PNA. Membrane strips were incubated for 1 h 30 min with one of the 16 biotinylated lectins diluted at a concentration of 1 μg/ml in TBS-T-BSA. After five washes of 5 min in TBS-T, peroxidase-conjugated streptavidin (Dakocytomation) diluted 1:5,000 in TBS-T-BSA was applied for 1 h. Finally, the membranes were washed again five times in TBS-T, and lectin-reactive bands were visualised by an ECL immunoblot detection system (Roche).
Results
Carbohydrate Localisation on Tube Foot Sections
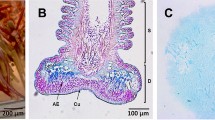
In the sea star A. rubens, the two components constituting the tube feet, the proximal stem and the distal disc, are made up of the same four tissue layers: an outer epidermis covered by a cuticle, a basiepidermal nerve plexus, a connective tissue layer, and an inner myomesothelium surrounding the water-vascular lumen (Fig. 1; see Santos et al. 2009 for review). In the stem, these different layers form concentric sheathes whilst in the disc, the connective tissue layer sends out radial septa which compartmentalise the epidermis. At this level, the epidermis is thickened and specialised for adhesion. It encloses two types of adhesive cells filled with granules in which the constituents of the adhesive material are packaged (Flammang et al. 1994; Hennebert et al. 2008). With light microscopy, the two types of adhesive cells present a same morphology and distribution in the disc epidermis and are therefore not easily distinguishable.
Tissue organisation in the tube feet of the sea star Asterias rubens. a Longitudinal section stained with Alcian blue coupled with Mayer’s hemalum and phloxine. b The corresponding schematic drawing (from Santos et al. 2009). c Detail of a tube foot disc (area highlighted by the red box in b) immunolabelled with antibodies directed against the adhesive material (from Santos et al. 2009). The adhesive cells are labelled green and the nuclei appear red. AC adluminal cells, CT connective tissue, D disc, DE disc epidermis, M myomesothelium, NP nerve plexus, S stem, SE stem epidermis
The presence and location of specific oligosaccharidic motifs in the tube feet of A. rubens was investigated using 16 biotinylated lectins and the results of this histochemical labelling are summarised in Table 3. As the goal was to characterise the carbohydrate moiety of the adhesive material, the focus here is only the 11 lectins which labelled the disc epidermis at the level of the adhesive cells (Figs. 2 and 3). This labelling was moderate to strong for concavalin A (Con A), wheat germ agglutinin (WGA), Ricinus communis agglutinin (RCA), Griffonia simplicifolia lectin I (GSL I), and soybean agglutinin (SBA) (Fig. 2). Con A reacted with all the tube foot tissue layers except the connective tissue (Fig. 2a). At high magnification, spherical structures can be distinguished in the disc epidermis, showing a cortical labelling (Fig. 2b). RCA, GSL I, and SBA labelled spherical to ellipsoid structures in the disc epidermis, between the septa of connective tissue. This labelling was generally stronger in the middle part of the disc and weaker in the upper and lower parts (Fig. 2c–g). RCA reactivity was more diffuse than that of GSL I and SBA, which were in the form of distinct spots (compare Fig. 2c, e and h). WGA gave a reactivity pattern similar to those of the three aforesaid lectins, but also strongly labelled the cuticle (Fig. 2i and j). The labelling of all these lectins disappeared when they were pre-incubated with their hapten monosaccharide (Table 2). The other six lectins also labelled the disc epidermis but more weakly, and always in the form of small spots (Fig. 3). Ulex europaeus agglutinin I (UEA I), Dolichos biflorus agglutinin (DBA), and Phaseolus vulgaris leuco agglutin (PHA-L) gave a slight labelling in the lower and middle parts of the disc epidermis, whilst the reactivity of Maackia amurensis lectin II (MAL II) and Sambucus nigra agglutinin (SNA) was restricted to its upper part (Fig. 3a–e). For P. vulgaris erythro agglutinin (PHA-E), the labelled spots were clustered at scattered locations in the disc epidermis (Fig. 3f). None of this weak labelling was inhibited in the control reactions.
Lectin-fluorescent labelling of tube foot sections with Con A (a, b), GSL (c, f), RCA (d, e), SBA (g, h), and WGA (i, j). Reactive structures are labelled red. a, d, f, g, i: longitudinal sections through a tube foot disc; b, c, e, h, j: details of the adhesive epidermis and connective tissue lamellae. The disc surface is oriented towards the left in all the pictures. Cu cuticule, CT connective tissue, D disc, DE disc epidermis, S stem, SE stem epidermis
Carbohydrate Detection in Footprints
Sea star footprints consist of a heterogeneous layer of adhesive secretions made up of a fibrillar material forming a meshwork deposited on a thin homogeneous film covering the surface (Fig. 4a, d, Hennebert et al. 2008).
Among the 16 lectins tested, only four, Con A, WGA, RCA, and DBA, labelled the adhesive footprints. These four lectins were among the ones which labelled the disc epidermis at the level of the adhesive cells. The binding was strong for Con A, WGA, and RCA and weaker for DBA. WGA and RCA labelled the footprints in the form of spots which seem to correspond to the inner part of the meshes delimited by the fibrillar material (Fig. 4b, e). On the other hand, Con A and DBA labelled the whole footprints including the meshwork which was slightly distinguishable (Fig. 4c, f).
Electrophoresis and Lectin Blotting
After gel electrophoresis and Coomassie blue staining, the proteins extracted from sea star adhesive footprints separated into eight protein bands (five major and three minor) which were named “sea star footprint proteins” (Sfps). The apparent molecular weights of these Sfps were about 450 kDa (Sfp-450), 290 kDa (Sfp-290), 210 kDa (Sfp-210), 135 kDa (Sfp-135), 115 kDa (Sfp-115), 75 kDa (Sfp-75), 50 kDa (Sfp-50), and 35 kDa (Sfp-35) (Figs. 5 and 6; Hennebert et al., in prep.). Moreover, the bottom of the loading wells and the interface between the stacking and running gels were also stained by Coomassie blue, suggesting that some molecules present in the footprints may precipitate or be too large to enter the gel.
Sea star footprint proteins (Sfps) extracted from the adhesive material of Asterias rubens, separated on an 8% (w/v) polyacrylamide gel and stained using Coomassie blue. Numbers on the left side of the gel indicate the mass of the molecular weight markers (in kilodalton), and the names on the right, the sea star footprint proteins
Proteins and glycoproteins from the adhesive material of Asterias rubens identified by SDS-PAGE (a) and Western blot (b), respectively. a The sample was separated on an 8% polyacrylamide gel and stained using Coomassie blue after electrophoresis. Numbers on the left side of the gel indicate the mass of the molecular weight markers (in kilodalton), and the names on the right, the major sea star footprint proteins. b The samples were transferred onto PVDF membranes and labelled with biotinylated lectins followed by detection with streptavidin-HRP and an ECL kit. BLW bottom of loading wells, ISR interface between the stacking and running gels
Two of the Sfps, Sfp-290 and Sfp-210, were shown to be glycoproteins. Sfp-290 was strongly labelled by Con A, UEA I, and MALII, whilst a longer exposure time was needed to visualise labelling of this protein band by SJA. Sfp-210 was strongly labelled by UEA I, MAL II, RCA, GSL, DBA, and PNA and weakly by Con A and SJA (Fig. 6). In addition to these two Sfps, Con A, GSL, and DBA labelled other protein bands which were undetectable on gels stained with Coomassie blue (Fig. 6). Moreover, for Con A, UEA I, WGA, RCA, GSL, DBA, SJA, and PNA, a more or less intense reactivity was observed at the bottom of the loading wells, at the interface between the stacking and running gels or at both locations.
When Sfps were run on gels with different acrylamide concentrations, there was no difference in their apparent molecular weight, indicating that Sfp-290 and Sfp-210 would contain less than 10% carbohydrate (results not shown).
Discussion
Post-translational modifications of proteins have been demonstrated to play an important role in the adhesive mechanisms developed by a large diversity of organisms (Smith and Callow 2006). Among these modifications, hydroxylation and phosphorylation have been the most investigated. This comes with the fact that these modifications occur largely in the permanent adhesives of mussels and polychaetes, two well-studied organisms in terms of adhesion. Protein glycosylation, on the other hand, has been described sporadically in a wide range of organisms, using either permanent (mussels, barnacles, algal spores) or transitory adhesion (limpets, marsh periwinkle) (see Flammang 2006, for the description of the different types of adhesion). Our results demonstrate the involvement of glycoproteins in a third type of adhesion, the temporary adhesion of sea star tube feet.
The occurrence and nature of carbohydrate residues in the adhesive material produced by the sea star A. rubens was first investigated on tube foot sections. Among the 16 lectins tested, 11 labelled the disc epidermis at the level of the adhesive cells (Table 4). Con A labelled the cortex of spherical structures which could correspond to the secretory granules contained in adhesive cells. The ten other lectins (UEA I, WGA, SNA, MAL II, PHA-E, PHA-L, RCA, GSL I, DBA and SBA), on the other hand, showed labelling in the form of spots, which could also correspond to the granules. Among them, UEA I, SNA, MAL II, PHA-E, PHA-L, and DBA presented a very weak reactivity which was not successfully inhibited in the control reactions, indicating that it might not be specific or that the binding affinity of the lectins for the complex oligosaccharides found in tube foot sections is higher than for the monosaccharides used for inhibition (Debray et al. 1981). Contrary to the reactivity pattern of Con A which encompassed the whole disc epidermis, the localisation of the labelling was more limited for the other lectins, being restricted to specific areas of the adhesive epidermis. These patterns could correspond to differences in the accessibility of the different epitopes (i.e., the carbohydrate structures recognised by the lectins) according to the maturation of the adhesive material during its transport from the base to the apex of the adhesive cells (Flammang et al. 1994). Alternatively, they could result from fixation artefacts, different zones of the epidermis varying in their fixation state and therefore in their epitope availability (Hennebert and Flammang, unpubl. obs.).
The detection of specific carbohydrate structures at the level of the adhesive epidermis is not a sufficient argument to conclude with certainty that the labelled material corresponds to the adhesive secretion. Indeed, in addition to the adhesive cell secretory granules, many other spherical or ellipsoidal structures, such as nuclei or mucous vesicles, occur in the different cell types constituting the disc epidermis and may contain glycoconjugates. The lectins were therefore tested on the secreted adhesive material (i.e., on the adhesive footprints deposited by sea stars on glass slides) and on the proteins extracted from this material (i.e., the sea star footprint proteins, Sfps). However, it should be noted that footprints may contain intracellular proteins, cuticular material, and mucus in addition to the adhesive material (Flammang et al. 1998; Hennebert et al., in prep). In A. rubens, adhesive footprints are made up of a meshwork whose components are secreted by type 1 adhesive cells, deposited on a thin homogeneous layer produced by type 2 adhesive cells (Hennebert et al. 2008). All these components are secreted through the fuzzy coat, the outermost layer of the cuticle covering the disc epidermis, which remains incorporated in the footprints once the tube foot is detached. The observed lectin reactivity patterns reflect this footprint organisation: Con A and DBA labelled the whole footprints, in particular, the meshwork, whilst WGA and RCA labelled only the inner part of the meshes. On the other hand, a total of nine lectins bound to the proteins extracted from these footprints, especially two of the Sfps (Sfp-290 and Sfp-210; Table 4). Thus, of the 11 lectins labelling the tube foot disc epidermis, only four also reacted with footprints, and seven with the extracted footprint material (Table 4). On the contrary, two lectins binding extracted footprint material (SJA and PNA) did not label the disc epidermis or the footprints (Table 4). These discrepancies could be explained by the fact that the conformation, and thus the exposed epitopes of the glycoconjugates are different in the three tests. Glycan-containing molecules are indeed chemically fixed in histological sections, they occur in their native state in footprints, and they are separated under denaturing condition for electrophoresis. The comparative approach indicates that for the lectins which labelled the disc epidermis, but not the footprints or the Sfps (i.e., SNA, PHA-E, PHA-L, and SBA), the corresponding oligosaccharides probably do not enter in the composition of the adhesive material.
Two of the Sfps, Sfp-290 and Sfp-210, were shown to be glycoproteins. The oligosaccharides of glycoproteins can be classified into two groups: N-linked and O-linked oligosaccharides (Montreuil et al. 1986; Fukuda and Kobata 1993). Both types appear to be present in the adhesive material produced by A. rubens, as highlighted by the labelling with Con A which bind preferentially to N-linked oligosaccharides and with PNA which binds preferentially to O-linked oligosaccharides (Osawa and Tsuji 1987; Piller and Piller 1993). Sfp-290 therefore appears to bear mostly N-linked oligosaccharides, whilst Sfp-210 principally O-linked oligosaccharides. All N-linked oligosaccharides share the same pentasaccharide core, Manα1,3(Manα1,6)Manβ1,4GlcNACβ1,4GlcNAc (sugar abbreviations are defined in Table 1), linked to an asparagine residue, but differ in the carbohydrate chains linked to this core (Montreuil et al. 1986). The presence of this core associated with Man residues in Sfp-290, and to a much lesser extent in Sfp-210, is supported by the labelling with Con A (Kobata and Yamashita 1993). PSA and LCA present a specificity similar to that of Con A, but for these lectins, the presence of Fucα1,6 linked to GlcNAc is required for the binding (Debray et al. 1981). As no labelling of the adhesive cells, footprints, and Sfps was observed with these lectins, we can conclude that this disaccharide is not present in the glycan chains linked to the Sfps. The other lectins used essentially bind to the outer glycan chains of both types of oligosaccharides (Osawa and Tsuji 1987; Sueyoshi et al. 1988; Kobata and Yamashita 1993; Piller and Piller 1993). The labelling of the adhesive cells and of Sfp-290 and Sfp-210 with UEA I demonstrates that the oligosaccharides linked to these proteins contain terminal Fucα1,2 linked to Gal residues. Similarly, the binding of MAL II to both glycoproteins indicates the presence of NeuAcα 2,3 linked to GalNAc residues in their glycan chains (Yamamoto et al. 1997). Finally, terminal GalNac and Gal residues were detected in both Sfp-290 and Sfp-210, in various linkages, as inferred from RCA, GSL I, DBA, and SJA bindings (see Table 1; Wu et al. 1997).
In addition to Sfp-290 and Sfp-210, several lectins labelled areas corresponding to the bottom of the loading wells and to the interface between the stacking and running gels (Table 4), two zones also stained by Coomassie blue in the gels. Because most of the lectins reacting with these interfaces are those labelling Sfp-290 and Sfp-210 (i.e., Con A, RCA, DBA, and PNA), it is possible that these labelled zones represent protein aggregates comprising the two glycoproteins. However, they were also specifically labelled by one lectin (WGA) that did not bind the Sfps, suggesting the presence of other very large glycoconjugates. The binding of WGA on these molecules, on tube foot sections at the level of the adhesive cells and of the cuticle, and on fresh footprints together with the absence of labelling with sWGA indicate the presence of sialic acids (NeuAc) in the adhesive material. Indeed, WGA and sWGA share the same specificity for chitobiose (GlcNACβ1,4GlcNAc), but WGA also binds sialylated glycoconjugates whereas sWGA does not (Monsigny et al. 1980). This lectin binding could correspond to the labelling of proteoglycans (Ravindranath and Basilrose 2005). This is in agreement with Flammang et al. (1998) who showed that sea star footprint material contains uronic acids and sulphates, also suggestive of the presence of proteoglycans. As discussed by these authors, these proteoglycans may come either from the adhesive cells and/or from the cuticle. In this study, WGA was the only lectin which labelled the cuticle in addition to the adhesive cells, whilst the binding of the other lectins was restricted to the disc epidermis.
All the results taken together demonstrate that the footprints deposited by sea star tube feet contain proteins, glycoproteins, and larger molecules such as proteoglycans. Although all these molecules appear to be secreted by the adhesive cells, the latter may also be derived from the cuticle. The occurrence of carbohydrates in marine adhesives has been reported in several invertebrate groups (see “Introduction”). Some of them are linked to proteins but, in some groups, a large part is also present in the form of free polysaccharides. It is the case for the extracellular polymeric substance (EPS) secreted by bacteria and diatoms, in which the carbohydrate component comprises anionic polysaccharides with sulphate ester, uronic acids, hexoses, pentoses, and hexosamines (Bhaskar and Bhosle 2005; Molino and Wetherbee 2008). In their adhesives, the carbohydrate fraction may improve adhesion through electrostatic interactions between the polar and hydrogen-bonding functional groups of glycan chains (such as ethers, amides, hydroxyls, and carboxylates) and underwater surfaces (Haag 2006). These interactions could be direct or could require the presence of calcium and magnesium ions (Chiovitti et al. 2008). These divalent cations could also contribute to the glue cohesive strength by cross-linking the negatively charged glycoconjugates, as it is the case for the high-molecular-mass sulphated glycoproteins of diatoms (Chiovitti et al. 2008). Glycoproteins also appear as major components of the adhesives produced by terrestrial organisms, and they have been reported in organisms as diverse as fungi, insects, and frogs (Epstein and Nicholson 2006; Graham et al. 2006; Li et al. 2008). In the spores of some fungi, attachment to the substratum involves a glue composed of glycoproteins and/or proteoglycans, of which the carbohydrate moieties are important for adhesion. Indeed, experimental evidence, including inhibition of adhesion with the lectin Con A, indicates that mannose residues are involved in the adhesive mechanism (Epstein and Nicholson 2006). All these examples emphasise that glycoproteins are important components of many biological adhesives to which they impart a potential for both cohesive and adhesive strengths. The glycosylated Sfps could therefore be key components of the sea star temporary adhesive.
References
Ameye L, De Becker G, Killian C, Wilt F, Kemps R, Kuypers S, Dubois P (2001) Proteins and saccharides of the sea urchin organic matrix of mineralization: characterization and localization in the spine skeleton. J Struct Biol 134:56–66
Bhaskar PV, Bhosle NV (2005) Microbial extrapolymeric substances in marine biogeochemical processes. Curr Sci India 88:45–53
Caldwell GS, Pagett HE (2010) Marine glycobiology: current status and future perspectives. Mar Biotechnol 12:241–252
Carlsson SR (1993) Isolation and characterization of glycoproteins. In: Fukuda M, Kobata A (eds) Glycobiology: a practical approach. Oxford University Press, New York, pp 1–26
Chiovitti A, Heraud P, Dugdale TM, Hodson OM, Curtain RCA, Dagastine RR, Wood BR, Wetherbee R (2008) Divalent cations stabilize the aggregation of sulphated glycoproteins in the adhesive nanofibers of the biofouling diatom Toxarium undulatum. Soft Matter 4:811–820
Cummings RD (1993) Structural characterization of N-glycans obtained from metabolically-radiolabelled glycoproteins. In: Fukuda M, Kobata A (eds) Glycobiology: a practical approach. Oxford University Press, New York, pp 243–289
Cummings RD, Kornfeld S (1982) Characterization of the structural determinants required for the high affinity interaction of asparagines-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem 257:11230–11234
Debray H, Decout D, Strecker G, Spik G, Montreuil J (1981) Specificity of twelve lectins toward oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem 117:41–55
De Moor S, Waite JH, Jangoux M, Flammang P (2003) Characterization of the adhesive from Cuvierian tubules of the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea). Mar Biotechnol 5:45–57
Epstein L, Nicholson RL (2006) Adhesion and adhesives of fungi and oomycetes. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 41–62
Flammang P (2006) Adhesive secretions in echinoderms: an overview. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 183–206
Flammang P, Demeulenaere S, Jangoux M (1994) The role of podial secretions in adhesion in two species of sea stars (Echinodermata). Biol Bull 187:35–47
Flammang P, Michel A, Van Cauwenberge A, Alexandre H, Jangoux M (1998) A study of the temporary adhesion of the podia in the sea star Asterias rubens (Echinodermata, Asteroidea) through their footprints. J Exp Biol 201:2383–2395
Flammang P, Lambert A, Bailly P, Hennebert E (2009) Polyphosphoprotein-containing marine adhesives. J Adhes 85:447–464
Fukuda M, Kobata A (1993) Glycobiology: a practical approach. Oxford University Press, New York
Gabe M (1968) Techniques histologiques. Masson, Paris
Graham LD, Glattauer V, Peng YY, Vaughan PR, Wermeister JA, Tyler MJ, Ramshaw JAM (2006) An adhesive secreted by Australian frogs of the genus Notaden. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 207–223
Gravel P, Golaz O (1996) Identification of glycoproteins on nitrocellulose membranes using lectin blotting. In: Walker JM (ed) The protein protocols handbook. Humana, Totowa, pp 603–617
Haag P (2006) Mechanical properties of bacterial exopolymeric adhesives and their commercial development. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 1–19
Hennebert E, Viville P, Lazzaroni R, Flammang P (2008) Micro-and nanostructure of the adhesive material secreted by the tube feet of the sea star Asterias rubens. J Struct Biol 164:108–118
Kamino K (2006) Barnacle underwater attachment. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 145–166
Kamino K (2008) Underwater adhesive of marine organisms as the vital link between biological science and material science. Mar Biotechnol 10:111–121
Kobata A, Yamashita K (1993) Fractionation of oligosaccharides by serial affinity chromatography with use of immobilized lectin columns. In: Fukuda M, Kobata A (eds) Glycobiology: a practical approach. Oxford University Press, New York, pp 103–125
Leathem AJC, Atkins J (1983) Lectin binding to paraffin sections. In: Bullock GR, Petrusz P (eds) Techniques in immunocytochemistry (vol II). Academic, London, pp 39–70
Lee BP, Dalsin JL, Messersmith PB (2006) Biomimetic adhesive polymers based on mussel adhesive proteins. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 257–278
Li D, Huson GH, Graham LD (2008) Proteinaceous adhesive secretions from insects, and in particular the egg attachment glue of Opodiphthera sp. moths. Arch Insect Biochem 69:85–105
Michael TS (2009) Glycoconjugate organization of Enteromorpha (=Ulva) flexuosa and Ulva fasciata (Chlorophyta) zoospores. J Phycol 45:660–677
Molino PJ, Wetherbee R (2008) The biology of biofouling diatoms and their role in the development of microbial slimes. Biofouling 24:365–379
Monsigny M, Roche A, Sene C, Maget-Dana R, Delmotte F (1980) Sugar–lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem 104:147–153
Montreuil J, Bouquelet S, Debray H, Fournet B, Spik G, Strecker G (1986) Glycoproteins. In: Chaplin MS, Kennedy JF (eds) Carbohydrate analysis: a practical approach. Academic, Oxford, pp 143–204
Ohkawa K, Nishida A, Yamamoto H, Waite JH (2004) A glycosylated byssal precursor protein from the green mussel Perna viridis with modified Dopa side-chains. Biofouling 20:101–115
Osawa T, Tsuji T (1987) Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu Rev Biochem 56:21–42
Piller F, Piller V (1993) Structural characterization of mucin-type O-linked oligosaccharides. In: Fukuda M, Kobata A (eds) Glycobiology: a practical approach. Oxford University Press, New York, pp 491–328
Ravindranath RMH, Basilrose SRM (2005) Localization of sulfated sialic acids in the dentinal tubules during tooth formation in mice. Acta Histochem 107:43–56
Sagert J, Sun C, Waite JH (2006) Chemical subtleties of mussel and polychaete holdfasts. In: Smith AM, Callow JA (eds) Biological adhesives. Springer, Berlin, pp 125–143
Santos R, Hennebert E, Varela Coelho A, Flammang P (2009) The echinoderm tube foot and its role in temporary underwater adhesion. In: Gorb S (ed) Functional surfaces in biology. Springer, Nertherlands, pp 9–42
Segrest JP, Jackson RL (1972) Molecular weight determination of glycoproteins by polyacrylamide gel electrophoresis in sodium dodecyl sulphate. In: Ginsburg V (ed) Methods in enzymology vol 28B V. Academic, New York, pp 54–63
Silverman HG, Roberto FF (2007) Understanding marine mussel adhesion. Mar Biotechnol 9:661–681
Smith AM, Callow JA (2006) Biological adhesives. Springer, Berlin
Smith AM, Morin MC (2002) Biochemical differences between trail mucus and adhesive mucus from marsh periwinkle snails. Biol Bull 203:338–346
Smith AM, Quick TJ, St Peter RLS (1999) Differences in the composition of adhesive and non-adhesive mucus from the limpet Lottia limatula. Biol Bull 196:34–44
Stanley MS, Callow ME, Callow JA (1999) Monoclonal antibodies to adhesive cell coat glycoproteins secreted by zoospores of the green alga Enteromorpha. Planta 210:61–71
Stewart RJ, Weaver JC, Morse DE, Waite JH (2004) The tube cement of Phragmatopoma californica: a solid foam. J Exp Biol 207:4727–4734
Sueyoshi S, Tsuji T, Osawa T (1988) Carbohydrate-binding specificities of five lectins that bind to O-glycosyl-linked carbohydrate chains. Quantitative analysis by frontal-affinity chromatography. Carbohydr Res 178:213–224
Sun C, Fantner GE, Adams J, Hansma PK, Waite JH (2007) The role of calcium and magnesium in the concrete tubes of the sandcastle worm. J Exp Biol 210:1481–1488
Sun C, Shrivastava A, Reifert JR, Waite JH (2009) Halogenated DOPA in a marine adhesive protein. J Adhes 85:126–138
Thomas LA, Hermans CO (1985) Adhesive interactions between the tube feet of a starfish, Leptasterias hexactis, and substrata. Biol Bull 169:675–688
Waite JH (1987) Nature’s underwater adhesive specialist. Int J Adhes Adhes 7:9–14
Waite JH, Qin X (2001) Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 40:2887–2893
Waite JH, Andersen NH, Jewhurst S, Sun C (2005) Mussel adhesion: finding the tricks worth mimicking. J Adhes 81:297–317
Walker G (1987) Marine organisms and their adhesion. In: Wake WC (ed) Synthetic adhesives and sealants. Wiley, Chichester, pp 112–135
Wiegemann M (2005) Adhesion in blue mussels (Mytilus edulis) and barnacles (genus Balanus): mechanisms and technical applications. Aquat Sci 67:166–176
Wu AM, Song SC, Sugii S, Herp A (1997) Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J Biochem Biophys 34:61–71
Yamamoto K, Konami Y, Irimura T (1997) Sialic acid-binding motif of Maackia amurensis lectins. J Biochem 121:756–761
Zhao H, Waite JH (2006) Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus. J Biol Chem 281:26150–26158
Zhao H, Sun C, Stewart RJ, Waite JH (2005) Cement proteins of the tube-building polychaete Phragmatopoma californica. J Biol Chem 280:42938–42944
Zhao H, Sagert J, Hwang DS, Waite JH (2009) Glycosylated hydroxytryptophan in a mussel adhesive protein from Perna viridis. J Biol Chem 284:23344–23352
Acknowledgements
Work supported by a FRIA doctoral grant to E.H., by a FRFC Grant no 2.4532.07, by the “Service Public de Wallonie—Programme Winnomat 2” and by the “Communauté française de Belgique—Actions de Recherche Concertées”. P.F. is Senior Research Associate of the Fund for Scientific Research of Belgium (F.R.S.-FNRS). This study is a contribution of the “Centre Interuniversitaire de Biologie Marine” (CIBIM; http://www.ulb.ac.be/sciences/biomar/).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hennebert, E., Wattiez, R. & Flammang, P. Characterisation of the Carbohydrate Fraction of the Temporary Adhesive Secreted by the Tube Feet of the Sea Star Asterias rubens . Mar Biotechnol 13, 484–495 (2011). https://doi.org/10.1007/s10126-010-9319-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-010-9319-6