Abstract
Imaging of the male genital tract (MGT) has assumed growing importance in andrological evaluation, playing a key role in specific issues. In particular, color-Doppler ultrasound (CDUS) is widely used to assess MGT abnormalities, providing useful information on three main andrological topics: infertility, testicular malignancy, and male accessory gland infection. Scrotal CDUS is very useful in assessing (1) scrotal organs and abnormalities when physical examination is unreliable; (2) signs of testicular dysgenesis, often related to sperm abnormalities and to a higher risk of cancer, and testicular lesions suggestive of malignancy; (3) scrotal pain, signs of inflammation (including epididymo-orchitis), and andrological emergencies (including testicular torsion); (4) varicocele; and (5) congenital absence of vas deferens (along with transrectal CDUS). Transrectal CDUS is useful in detecting signs suggestive of (1) MGT obstruction, including ejaculatory duct abnormalities, prostate median cysts, or SV enlargement/emptying impairment and (2) prostate and SV inflammation. However, MGT-CDUS still suffers from a lack of standardization, which is advisable. Along with CDUS, new imaging techniques, such as contrast-enhanced ultrasound, elastography, and magnetic resonance, have been proposed for improving MGT imaging. Even if they are promising, currently, there is not enough evidence for their routine use, with the exception of some specific indications.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Scrotal and transrectal imaging/color-Doppler ultrasound
- Male genital tract
- Male infertility
- Testicular malignancy
- Male accessory glands infection/inflammation
Introduction
Imaging of the male genital tract (MGT) has progressively expanded, playing a seminal role in andrological examination. In particular, ultrasound (US), which employs high-frequency sound waves to capture live images of organs inside the body, is widely used to assess MGT abnormalities (Ammar et al. 2012; Raza and Jhaveri 2012; Lotti and Maggi 2015). Gray-scale and color-Doppler US (CDUS) can provide useful information on three main andrological topics: infertility (Lotti and Maggi 2015), testicular malignancy (Woodward et al. 2002; Isidori and Lenzi 2008), and male accessory gland infection (MAGI) (La Vignera et al. 2012a). While scrotal CDUS has been used in medicine since a long time, only recently transrectal CDUS has assumed growing importance in MGT imaging, extending the examination to the prostate, seminal vesicles (SV), and deferential ampullas (Ammar et al. 2012; Lotti et al. 2012b; Raza and Jhaveri 2012). Recently, new imaging techniques, such as contrast-enhanced ultrasound (CEUS) (Cantisani et al. 2015) and elastography (Huang and Sidhu 2012), along with magnetic resonance (MRI) (Kim et al. 2007) have been proposed for improving the imaging of MGT abnormalities.
We previously systematically analyzed (Lotti and Maggi 2015) and recapitulate in this chapter the diagnostic impact of CDUS on the MGT in relation to infertility, testicular lesions, and MAGI. The role of the new imaging techniques is also scrutinized (see below, “New Imaging Techniques for the Evaluation of Testis and Prostate Abnormalities”).
Embryological Development of the Male Genital Tract
Testis and Epididymal Head
In week 6 of embryonic life, the human primordial germ cells (PGC), of endodermal origin, migrate from the yolk sac and invade the genital ridges, which proliferate from the coelomic epithelium into the underlying mesenchyme, forming the primary sex cords (Sadler 2011). The undifferentiated gonad develops into the testis toward week 7, modulated by Y chromosome genes. Sex cords, compartmentalized by the developing tunica albuginea, become the seminiferous tubules (ST), which give the tubuli recti that converge in the rete testis. At this stage, ST consist of spermatogonia, derived from the PGC, and two different types of somatic cells, the Sertoli cells, derived from the germinal epithelium, and the peritubular cells (myofibroblasts), included in the lamina propria covering the mature ST. ST become progressively separated by mesenchyme, which gives the interstitial Leydig cells. The developing testis lies adjacent to the mesonephros (embryonal transitory kidney), that, toward the end of month 2, begins to regress, with glomerular disappearance. Only 15–20 tubules, the efferent ducts, persist, link the rete testis, and connect the genital gland with the mesonephric duct. The efferent ducts constitute the future epididymal head, while the mesonephric duct evolves in the epididymis and vas deferens (Christensen and Dogra 2007; Sadler 2011). The strict link between the efferent ducts (future epididymal head) and the rete testis, and the different embryological development of different epididymal parts, may explain why, when epididymal agenesis occurs, part of the epididymal head is persisting (Sadler 2011), being detectable by US (see section “Agenesis”).
Testicular Descent
Between the month 3 of pregnancy and its end, the testes begins to descend from the lumbar region into the scrotum. Testicular descent includes two separate stages. The “transabdominal” stage (8–15 weeks of gestation) is characterized by gubernaculum testis enlargement (swelling reaction), modulated by insulin-like factor 3 (INSL3), leucine-rich repeat-containing G protein-coupled receptor 8 (LGR8) and anti-Müllerian hormone (AMH), and regression of the cranial suspensory ligament in response to testosterone (Christensen and Dogra 2007; Hutson et al. 2010, 2015; Virtanen and Toppari 2014). The “inguinoscrotal” stage (25–35 weeks of gestation) is characterized by gubernaculum and testis migration from the inguinal region into the scrotum, mainly regulated by androgens (Christensen and Dogra 2007; Garriga et al. 2009). Shortly afterward, the vaginal process, an evagination of the peritoneum which allows the abdominal fetal testis to reach the scrotum, obliterates with formation of the tunica vaginalis (Garriga et al. 2009).
Epididymis, Vas Deferens (VD), and Seminal Vesicles (SV)
The genital tracts have the same appearance in male and female embryos until week 7, consisting of two paramesonephric (Müllerian) and two mesonephric (Wolffian) ducts (Sajjad 2010). At the end of month 2 of pregnancy, androgen production by fetal testes induces regression of the Müllerian and differentiation (stabilization) of the Wolffian structures (Sajjad 2010). The prostatic utricle persists as a Müllerian remnant. The proximal Wolffian ducts give rise to the epididymis (body and tail) and the VD, while the distal part forms the deferential ampulla, SV, and ejaculatory ducts (Sajjad 2010; Sadler 2011; Shaw and Renfree 2014).
Prostate, Ureters, and Kidneys
At the end of week 5 of pregnancy, the ureteric bud arises from the mesonephric duct and blends with the metanephric blastema to become the primitive kidney, which ascends to the lumbar region at weeks 6–7. The ureteric bud becomes the ureter, which separates from the mesonephric duct, migrates cranially and opens into the bladder (Kim et al. 2009; Sadler 2011). Any alteration occurring during ureteric bud development may affect SV or VD formation (mesonephric duct origin). Accordingly, developmental anomalies of SV and VD are often associated with renal and/or ureteral anomalies. Due to embryological origin of the aforementioned organs, multiple developmental abnormalities of the genito-urinary tract may occur in the same individual (Sadler 2011) and must be investigated carefully, US playing a key role in their assessment (see section “Agenesis”).
The prostate develops from the urogenital sinus, by epithelial (entodermal) buds penetrating into the adjacent mesenchyme. Then, it encloses the ejaculatory ducts, the prostatic utricle, and the urethra (Kim et al. 2009; Sadler 2011).
Color-Doppler Ultrasound of the Male Genital Tract: Anatomy and Normal Patterns
Scrotal Region
The testes are normally located within the scrotum (Figs. 1 and 2). This external, superficial position allows their evaluation with a high-frequency small part transducer (7–15 MHz). Testes US examination is performed with the patient laying supine with the penis resting on the suprapubic region, applying abundant gel over the scrotal sac. The testes are examined in longitudinal, transverse, and oblique planes, and images are acquired in both gray-scale and color-Doppler modes, to assess testicular blood flow (Appelbaum et al. 2013; Ammar et al. 2012).
Schematic representation of the normal and pathologic features of the male genital tract (MGT) in relation to male reproductive health. Right side: normal anatomy of the MGT. Left side: pathologic features of the MGT suggesting obstructive or non-obstructive oligo-astheno-teratozoospermia (OAT)/azoospermia. Warning for malignancy is extensively discussed in the text (Adapted from Lotti and Maggi 2015, with permission)
Schematic representation of the scrotal organs and related arterial supply. The main structures of the testis, as well as epididymis and vas deferens, are shown in black and white. One testicular lobule is highlighted in green. The arterial supply of the scrotal organs is shown in red. The structure of the testis and the normal anatomy of the scrotal arterial supply are extensively discussed in the text (section Color-Doppler Ultrasound of the Male Genital Tract: Anatomy and Normal Patterns - Testis) (Adapted from Lotti and Maggi 2015, with permission)
Testis
Volume
Testis volume (TV) is clinically assessed by Prader’s orchidometer (PO) (Nieschlag and Behre 2010). Although orchidometry overestimates TV as compared to US (Behre et al. 1989; Lenz et al. 1993; Diamond et al. 2000; Goede et al. 2011; Rastrelli et al. 2013), Prader-derived TV may be considered a reliable surrogate of that measured by US, easier to perform and less expensive. In fact, PO- and US-derived TV are closely related (Rastrelli et al. 2013), both in young (Goede et al. 2011) and in adult eugonadal or hypogonadal subjects (Behre et al. 1989; Lenz et al. 1993; Diamond et al. 2000; Rastrelli et al. 2013). Nevertheless, US is more accurate in TV measurement than PO (Lenz et al. 1993; Sakamoto et al. 2007a, b). In particular, US plays an important role in evaluating TV when the physical examination is unreliable, such as in the case of large hydrocele, inguinal testis, epididymal enlargement/fibrosis, thickened scrotal skin (Behre et al. 1989; Sakamoto et al. 2006; Nijs et al. 2007; Behre and Zitzmann 2010), or small testis, in which the epididymis is large in comparison to the total TV (Goede et al. 2011).
TV varies with age. Prepubertal boys have a TV ≤3 ml, whereas a TV >3 ml represents the first sign of an ongoing puberty (Palmert and Dunkel 2012). During puberty, TV increases rapidly (Goede et al. 2011), up to tenfold between 10 and 15 years, reaching the maximum volume around the age of 20 (Béres et al. 1989). Reference growth curves for orchidometry-derived TV are available (Goede et al. 2011; Joustra et al. 2015). Some studies report a decrease of TV as a function of age (Stearns et al. 1974; Baker et al. 1976), while others did not (Harman and Tsitsouras 1980; Sparrow et al. 1980; Nieschlag et al. 1982). More recent studies suggest that a mild TV decline occurs starting from the 50–60 years on (Rastrelli et al. 2013; Pilatz et al. 2013a), although a significant TV reduction has been reported only in the eighth decade of life (Handelsman and Staraj 1985; Sartorius and Nieschlag 2010). In addition, malnutrition and illnesses seem to exert independent negative effects on TV (Handelsman and Staraj 1985). Due to differences in the nature of the populations studied – including geographic area, nourishment, ethnicity, and environmental factors (Diamond 1986; Takihara et al. 1987; Bahk et al. 2010) – so far there are no uniform TV reference values. Considering Europe, the mean PO-based TV reported in the general population is 20.0 ± 5.0 ml (Jørgensen et al. 2002; Jensen et al. 2004; Nordkap et al. 2016), whereas in infertile patients, it is 18.0 ± 5.0 ml (Nieschlag and Behre 2010). According to a recent study (Lotti et al. 2016a), there is a significant difference in mean TV, assessed both by Prader and US, comparing fertile and infertile subjects, TV decreasing as a function of severity of semen quality impairment.
At US, the adult testis appears as an oval-shaped organ of 30–50 mm length, 20–40 mm width, and 30 mm height (Appelbaum et al. 2013) (Fig. 3a). TV is usually calculated by applying the ellipsoid formula (length × width × height × 0.52), although the use of this formula is not universally accepted (Lin et al. 2009; Goede et al. 2011; Pilatz et al. 2013a). The mean difference in TV between PO and US is 4–5 ml (Carlsen et al. 2000; Sakamoto et al. 2007a, 2008a). Hence, assuming that a normal TV by PO is >14–15 ml (Takihara et al. 1986; Forti and Krausz 1998), the normal US-TV should be >10–11 ml (Lotti et al. 2012b). So far, testicular hypotrophy by US has been defined as a TV <12 ml (see Condorelli et al. 2013). Although US-derived TV values for boys aged 0–6 years (Kuijper et al. 2008) and 6 months to 18 years (Goede et al. 2011; Joustra et al. 2015) are available, normative values in the general adult population are lacking. As stated before, US-assessed TV varies according to the mathematical formula applied; however, it is similar among different ethnic groups (Lenz et al. 1993; Bahk et al. 2010; Pilatz et al. 2013a; Foresta et al. 2013). In a recent study (Pilatz et al. 2013a), TV is reported in the same subjects according to different mathematical formulas. Using the ellipsoid formula, an average TV of 14 ml was found in healthy German (Pilatz et al. 2013a), Danish (Lenz et al. 1993), and Italian (Foresta et al. 2013) men. Right TV has been reported larger than the left one by some (Béres et al. 1989; Lenz et al. 1993; Pilatz et al. 2013a), but not all (Bahk et al. 2010) authors. An average TV at US in infertile men ranges from ~10 (Lenz et al. 1994) to ~13.0 ± 5.0 ml (Sakamoto et al. 2007a), the latter study reporting that the mean TV measured by US was significantly lower than that measured by PO, with a mean difference of 5 ml, as previously reported (see above).
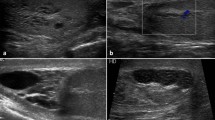
Normal color-Doppler ultrasound (CDUS) features of the organs of the male genital tract. a Testis of normal volume, homogeneity, and echogenicity with ellipsoid shape. Longitudinal (right figure) and transversal (left figure) scans of the testis, with length (D1), width (D2), and height (D3) measurements are reported. b Left figure: B-mode appearance of the spermatic cord (SC) and the upper pole of the testis (T) in longitudinal scan. Small, non-dilated venous vessels of the pampiniform plexus are difficult to differentiate from the other structures of the SC. Right figure: CDUS detection of the testicular artery (arrow) in the SC and recurrent ramus (^) of an intratesticular centripetal artery. Venous reflux at rest in the venous vessels is not detectable. c Normal epididymal head with triangular shape (dashed line) in longitudinal scan, homogeneous, with echogenicity comparable to that of the testis (T). Its length is measured from the top to the base of the triangle (dotted line). d Homogeneous epididymal body and tail and proximal vas deferens (pVD) in longitudinal scan. Their echogenicity is slightly hypoechoic compared to the testis and the epididymal head in (c). Their diameters are reported as dashed lines. Red dashed line indicates the end of the epididymal tail and the beginning of the pVD. The curve arrow indicates the epididymal-deferential handle. e Prostate of normal volume, homogeneity, and echogenicity in transversal scan. Peripheral and transitional zone (PZ and TZ) show a 3:1 ratio in young men. Right and left lobes (RL and LL, respectively) and periprostatic venous plexus (PVP) are indicated. Anterior-posterior and transverse diameters (“apd” and “td”, respectively) are reported. f Prostate of normal volume, homogeneity, and echogenicity in sagittal scan evaluated with “end fire” probe. Peripheral and transitional zone (PZ and TZ, respectively) and apex (A) are indicated, as well as bladder (B), urethra (U, yellow dotted line), ejaculatory duct (green dashed line), prostatic utricle (*), deferential ampulla (DA), and periprostatic venous plexus (pvp). The longitudinal diameter (“ld”) is reported and represented with a white dashed line. g Right and left seminal vesicles (rSV and lSV, respectively) with typical “bow-tie” appearance and, medial to them, right and left deferential ampullas (sDA and lDA, respectively) in transversal scan. h Seminal vesicle (SV) assessed by “end fire” probe in sagittal scan. Fundus and body are reported, as well as longitudinal and anterior-posterior diameters (“ld” and “apd” dashed lines, respectively). A schematic model of SV volume calculation is reported, using the “ellipsoid/prolate spheroid (d1 > d2 = d3)” (red ellipse) mathematical formula (d1 × d2 × d3 × 4/3 × π), with d1 = ld and d2 = apd, and d3 assumed = d2 (red dashed line) (According to Lotti et al. 2012a). i Distal vas deferens (dVD) and deferential ampulla (DA) beside a section of the seminal vesicle (SV) assessed by “end fire” probe in sagittal scan. j Ejaculatory duct (double green dashed lines) and prostatic utricle (*) assessed by “end fire” probe in sagittal scan. Deferential ampulla (DA), a section of the seminal vesicle (SV), urethral (U) course (dashed line), bladder (B) and prostate (Pr) are reported
Homogeneity and Echogenicity
The normal adult testis has a homogeneous fine echo-texture, made of medium level echoes with uniform distribution, similar to the echogenicity of the normal thyroid gland (Hamm and Fobbe 1995; Isidori and Lenzi 2008) (Fig. 3a). The testis is surrounded by a hyperechoic fibrous capsule, the tunica albuginea, which projects into the interior part with fibrous septa, dividing it into 200–400 lobules (Fig. 2). Each lobule contains interstitial Leydig cells and seminiferous tubules (Fig. 2), with germinal cells at different stages of maturation and somatic Sertoli cells. Seminiferous tubules account for ~85% of the entire TV (Takihara et al. 1987; Forti and Krausz 1998; Kollin et al. 2006). Septa radiating from the tunica albuginea (Fig. 2) may be seen as hypoechoic linear structures crossing the testis and converging into the hilum of the testis (mediastinum testis; Fig. 2), which appears as an eccentric hyperechoic line or triangle in longitudinal and transversal scans, respectively. Whereas the concept of echo-texture homogeneity is a relatively objective finding, that of normal echogenicity is more operator dependent. So far, two classifications are available for homogeneity (Lenz et al. 1993; Westlander et al. 2001) (Table 2). Echogenicity depends on the seminiferous tubules maturation and germ cell representation. Prepubertal testis is slightly more hypoechoic than the adult one, since seminiferous tubules have not developed a lumen yet. During puberty, testis echogenicity progressively increases, as a function of lumen development, up to adult level. Seminiferous tubule reduction and interstitium increase lead to a hypoechoic and/or inhomogeneous echo-texture (Béres et al. 1989; Loberant et al. 2010) (Fig. 4a, b, respectively).
Abnormal color-Doppler ultrasound (CDUS) features of the organs of the male genital tract. a Testis with low volume and hypoechoic echo-texture, detected in a man with a history of cryptorchidism. b Testis with echo-texture inhomogeneity in sagittal scan. c Testicular microlithiasis with “starry sky” appearance. d CDUS evaluation of dilated veins of the pampiniform plexus with colored signal (left), showing continuous reflux at rest (***), increasing with Valsalva (arrow), identifying a severe, sonographic-defined, varicocele, according to different classifications (see Table 3). e Dilated and inhomogeneous epididymal body and tail and proximal vas deferens (pVD), with irregularly shaped mass (*) in the epididymal tail region, detected in sagittal scan. f Left figure: section of a dilated deferential ampulla (DA) beside a dilated seminal vesicle (SV) with areas of endocapsulation (*) and thick septa (arrow) detected by “end fire” probe in sagittal scan. Right figure: dilated, inhomogeneous epididymal tail and proximal vas deferens (pVD), with coarse calcifications (arrow). g Left figure, dilated (>12 mm), inhomogeneous, hypoechoic epididymal head; right figure, abrupt interruption of the proximal vas deferens (pVD) in a man with congenital bilateral absence of vas deferens. Epididymal body and tail are also visualized in sagittal scan. h Prostate with hyperemia end elevated arterial peak systolic velocity. i Midline prostatic cyst (*) in transversal (left) and sagittal (right) scan. The prostatic utricle is indicated with an arrow. P prostate, B bladder. j Upper figure, ejaculatory duct dilation (arrow) and microcalcification (short arrow), and seminal vesicle cyst (#), assessed by “end fire” probe in sagittal scan. Lower figure, ejaculatory duct cyst (*). SV seminal vesicle, Pr prostate, U urethra, B bladder
Vascularization
Scrotal perfusion is ensured by three main arteries: the testicular artery (TA), which supplies the testis; the deferential artery, which perfuses the epididymis and vas deferens; and the cremasteric artery, which supplies the tissues around the testis and the scrotal wall (Horstman et al. 1991a; Isidori and Lenzi 2008) (Figs. 1 and 2). TA arises from the aorta, below the renal arteries, while the deferential and the cremasteric arteries are branches of the vesicular artery and of the inferior epigastric artery, respectively (Horstman et al. 1991a) (Fig. 2). TA enters the spermatic cord at the deep inguinal ring and reaches the upper pole of the testis (Horstman et al. 1991a) (Figs. 2 and 3b). From this point, TA penetrates the tunica albuginea in the posterior side of the testis and runs along the fibrous capsule brunching the capsular and the transmediastinal arteries (Fig. 2).
The capsular arteries run beneath the tunica albuginea in a layer called tunica vasculosa, over the surface of seminiferous tubules (Schlegel and Li-Ming 1997) (Fig. 2). They supply centripetal arteries that enter the testicular parenchyma and flow toward the hilum (Horstman et al. 1991a) penetrating between the septa dividing the testicular lobules (Schlegel and Li-Ming 1997). As they approach the mediastinum, the centripetal arteries arborize into recurrent rami that branch back in the opposite direction, carrying blood from the mediastinum into the testis (Middleton et al. 1989; Migaleddu et al. 2012) (Fig. 2).
The transmediastinal artery is a centrifugal artery present in about half of the men. It enters at the mediastinum and runs across the testicular parenchyma with a straight course, to form capsular branches on the opposite side (Horstman et al. 1991a; Pais et al. 2004) (Fig. 2).
Pampiniform Plexus
Normal pampiniform plexus (Fig. 1) is scarcely assessed by physical examination as well as by gray-scale US, because it is difficult to differentiate it from the other structures of the spermatic cord (Fig. 3b). Color or power Doppler, which usually help in appreciating vascular flow, should not detect any venous reflux. In normal conditions, US detects a complex network of small vessels <2 mm (Dogra et al. 2003; Cina et al. 2006) converging into the spermatic veins. The right spermatic vein enters into the inferior vena cava with an acute angle, whereas the left one enters perpendicular into the left renal vein. For this reason, the latter is characterized by a higher blood hydrostatic pressure. When the pressure becomes excessive and/or venous valve impairment occurs, venous reflux and dilation may occur, leading to varicocele (Gat et al. 2008) (see section “Varicocele”).
Epididymis and Proximal Vas Deferens
The normal epididymis is a soft organ adjacent to the testis, arbitrarily divided into three parts: head, body, and tail (Figs. 1 and 2). The head consists of 10–15 efferent ductules originating from the rete testis and converging in a single, convoluted tube in the distal portion (Fig. 2). At US, the epididymis is usually detected posterior and lateral with respect to the testis, with the head at the upper pole and the tail at the lower pole of the male gonad. The normal head is triangular, with isoechoic to slightly hyperechoic texture with respect to the testis (Dogra et al. 2003; Lee et al. 2008) (Fig. 3c). It is usually measured in a longitudinal scan from the top to the base of the triangle (Pezzella et al. 2013; Pilatz et al. 2013a) (Fig. 3c). The body and tail diameters are measured in a longitudinal scan considering the anterior-posterior diameters (Pezzella et al. 2013; Vicari 1999) (Fig. 3d). A head of 5–12 mm (Behre et al. 1995; Vicari 1999; Dogra et al. 2003; Lee et al. 2008; Pezzella et al. 2013; Pilatz et al. 2013a), a body of 2–4 mm (Behre et al. 1995; Dogra et al. 2003; Lee et al. 2008), and a tail of 2–6 mm (Lee et al. 2008; Dogra et al. 2003; Vicari 1999) have been proposed as normal. Blood flow is detectable by CDUS in discrete vascular spots in all tracts of the epididymis (Keener et al. 1997).
The vas deferens (VD) is a straight tense cord which runs along the spermatic cord (Figs. 1 and 2). VD absence, with or without epididymal agenesis, is often difficult to be evaluated by physical examination, and US is useful to confirm this finding. At scrotal US, the proximal VD appears as a straight duct, slightly hypoechoic compared to the epididymis, extending from the epididymal tail (Fig. 3d) toward the inguinal channel (Isidori and Lenzi 2008).
Prostate-Vesicular Region
The prostate-vesicular region can be studied by transabdominal or transrectal US (TRUS) (Huang Foen Chung et al. 2004; Stravodimos et al. 2009). Although some authors revealed no significant difference between the two US to measure prostate volume (Huang Foen Chung et al. 2004), other authors reported that TRUS is more accurate in predicting adenoma volume in BPH patients (Stravodimos et al. 2009). In addition, in our opinion, TRUS has higher accuracy in detecting echo-texture and vascular parameters. Hence, in this chapter, we will focus on TRUS.
TRUS is performed using a transrectal biplanar probe (linear and convex transducer, 6.5–7.5 MHz) and/or an “end fire” probe (6.5 MHz, field of view 50–200°), with the patient placed in the left lateral decubitus, scanning the organs in transverse, longitudinal, and oblique ways (Behre et al. 1995; Older and Watson 1996; Vicari 1999; Lotti et al. 2011a, b; Lotti et al. 2012a, c).
Prostate
The prostate is an exocrine gland, which surrounds the urethra (Fig. 1) just below the neck of the bladder. It produces prostatic fluid, an acidic secretion that makes up ~30% of the total ejaculate (Cooper 2010).
At TRUS, the normal prostate appears different according to age, with a triangular or pear shape in younger and older subjects, respectively (Older and Watson 1996; Raza and Jhaveri 2012). Its base lies at the bladder neck, at the beginning of the urethra, detectable in a longitudinal scan as a hypoechoic duct curving toward the prostatic apex. TRUS identifies a peripheral zone (PZ), which extends laterally and posteriorly from the apex to the base, and a transitional zone (TZ), centrally located and slightly hypoechoic (Fig. 3e). PZ and TZ show a 3:1 ratio in young men (Doble and Carter 1989; Jin et al. 2001). A central zone has been also described (Berger et al. 2006; Jin et al. 2001). Prostate volume (PV) is often measured using a planimetric method (Behre et al. 1995; Vicari 1999; Lotti et al. 2011a, b). It is calculated measuring three diameters (anterior-posterior and transverse in the transversal scan, longitudinal in the sagittal one; Fig. 3e, f) using the mathematical formula of the ellipsoid (Collins et al. 1995; St Sauver et al. 2006; Lotti et al. 2011a, 2013d). “TZ volume” is similarly calculated (St Sauver et al. 2006; Lotti et al. 2013d). A PV of 20–25 ml has been proposed as normal in young men (Raza and Jhaveri 2012). The normal adult prostate shows thin, densely packed, and homogeneously deployed echoes. Periprostatic venous plexus is detectable as a slightly hypoechoic system of vessels (Fig. 3e, f). Intraprostatic arteries are grouped in central/periurethral and peripheral/capsular arteries, supplying the TZ and PZ, respectively (Older and Watson 1996).
Seminal Vesicles, Deferential Ampullas, and Ejaculatory Ducts
Seminal vesicles (SV) are paired and saccular structures, which lie superior and posterior to the prostate between the bladder and the rectum (Fig. 1) (Ramchandani et al. 1993; Kim and Lipshultz 1996; Kim et al. 2009). They produce an alkaline fluid contributing 50–80% of the ejaculate volume (Ramchandani et al. 1993; Kim et al. 2009).
At TRUS, SV have a typical “bow-tie” appearance in transversal scans and a tennis-racket shape in longitudinal scans (Fig. 3g, h, respectively). SV echo-texture is characterized by homogenous fine echoes and is slightly less echogenic than the prostate (Ramchandani et al. 1993; Kim and Lipshultz 1996). In relatively young subjects, SV volume is negatively associated with age (Lotti et al. 2012a) and tends to shrink after the fifth decade, showing a significant reduction in the eighth compared to the fourth decade (Terasaki et al. 1993). SV volume increases with sexual abstinence (Lotti et al. 2012a), whereas it decreases in current smokers as a function of smoking habit and of lifetime exposure to cigarette smoking (Lotti et al. 2015). SV volume is also positively affected by testosterone (Sasagawa et al. 1989, 1990), prolactin (Lotti et al. 2013a), and free triiodothyronine (fT3; Lotti et al. 2016b) levels. While most of the available studies assessed SV diameters, we recently proposed to calculate SV volume by measuring the maximum longitudinal and anterior-posterior diameters, using the “ellipsoid/prolate spheroid” mathematical formula (Lotti et al. 2012a) (Fig. 3h). SV volume varies with ejaculation and is positively related to the ejaculate volume, but not with sperm parameters (Lotti et al. 2012a). SV emptying with ejaculation is positively related to fT3 levels (Lotti et al. 2016b), and subjects with subclinical hyperthyroidism show a higher reduction of SV longitudinal diameters after ejaculation as compared with eu- and hypothyroid men (Lotti et al. 2016b).
The deferential ampullas (Fig. 1) appear at TRUS as oval structures medial to the SV in transversal scans, cephalic to the prostate, or as distal VD enlargements in longitudinal scans (Fig. 3g, i, respectively). They have an echo-texture similar to that of SV.
The ejaculatory ducts (Fig. 1) appear at TRUS as fine and hypoechoic, with a normal caliber <2 mm. They are detectable in longitudinal scans crossing the prostate up to the urethra (Kim and Lipshultz 1996; Raza and Jhaveri 2012) (Fig. 3j).
Color-Doppler Ultrasound in Pathological Conditions
Testis
Volume
Low TV (Fig. 1) at Prader evaluation is associated with worse sperm parameters, including lower total sperm count (Handelsman et al. 1984; Bujan et al. 1989; Arai et al. 1998; Jørgensen et al. 2002; Sakamoto et al. 2008a), motility (Bujan et al. 1989; Arai et al. 1998; Sakamoto et al. 2008a), and normal morphology (Jørgensen et al. 2002). In addition, a low TV is related to abnormalities in sexual hormones, including low testosterone and increased gonadotropin levels (Sakamoto et al. 2008a; Rastrelli et al. 2013). Finally, TV correlates with fatherhood (Fisher et al. 2012). Accordingly, TV measured by US is positively related to total sperm count (Lenz et al. 1993, 1994; Sakamoto et al. 2008a; Cooper 2010), motility (Sakamoto et al. 2008a), normal morphology (Lenz et al. 1993), and testosterone levels and negatively to gonadotropins (Sakamoto et al. 2008a; Sakamoto and Ogawa 2009). In addition, a reduced US-TV is associated with worse nonconventional sperm parameters, including higher sperm DNA fragmentation – particularly seminal levels of PIdimmer population (Lotti et al. 2016c) and M540 bodies (Lotti et al. 2012d) – as well as higher chromatin compactness, mitochondrial membrane potential, and phosphatidylserine externalization (Condorelli et al. 2013).
On the other hand, oversize testes are considered abnormal, this finding being defined “macroorchidism ” or “megalotestes ” (Lachiewicz and Dawson 1994; Meschede et al. 1995). However, a clear cutoff indicative of macroorchidism, both at PO or US, is lacking. One report defined macroorchidism as a TV >95th percentile of the standard testicular curves (Lachiewicz and Dawson 1994). A TV ≥4 ml at Prader evaluation in infants and children up to 8 years old (see Lachiewicz and Dawson 1994) and a TV >25–30 ml in adults have been suggested as indicative of macroorchidism (Nielsen et al. 1982; Meschede et al. 1995). The majority of the studies on megalotestes have been performed on men with fragile X syndrome or mental retardation, but limited reports described macroorchidism also in men with different diseases (see Lachiewicz and Dawson 1994). Slightly enlarged TV may, however, be a normal variant in adult men (Nielsen et al. 1982). In addition, any definition of abnormal enlarged testes should refer to the ethnic group, age, and anthropometric parameters (Nielsen et al. 1982). Interestingly, limited studies report that large male gonads show normal function (Cantu et al. 1976; Berkovitz et al. 1986; Meschede et al. 1995).
Echo-Pattern Abnormalities
The main echo-pattern abnormality of the testis is inhomogeneity (Figs. 1 and 4a, b). So far, testicular inhomogeneity (TI) has been classified on 5-point scale by Lenz et al. (1993) and Westlander et al. (2001) (Table 2). TI is detected by US as overexpressed hypoechoic striae, giving a mottled, scratched, or netting appearance defined as “striated” (Loberant et al. 2010) (Fig. 4b), suggesting atrophy and fibrosis (Loberant et al. 2010). In fact, the hypoechoic striae mirror the thickening of the interlobular septa, deriving from a decrease in the parenchymal component of the testis and an increase of the interstitium (Loberant et al. 2010). TI builds up with age (Lenz et al. 1993), and although it is frequent in elderly men and considered normal, in young subjects, it is considered an abnormal finding and is associated with several pathological conditions (see Table 2). TI has been associated with testicular dysfunction (Lenz et al. 1993; Behre et al. 1995), abnormal sperm morphology (Lenz et al. 1993; Lotti et al. 2013), and history of cryptorchidism (Lenz et al. 1993). TI has been associated also with testis malignancy (see below and Table 1), in particular with a high risk of carcinoma in situ in subjects with a history of cryptorchidism (Lenz et al. 1987; Lenz 1991) or with testicular microlithiasis (Elzinga-Tinke et al. 2010). At present, US follow-up is suggested if severe TI is detected, especially if additional risk factors for testicular malignancy (see section “Testicular Lesions”) are present. Conversely, a diagnostic testicular biopsy is not recommended (Lotti and Maggi 2015). Finally, diffuse TI could be related to massive tumors or lymphoma, the latter occurring later in life (50–70 years) and hard at physical examination, usually highly vascularized at CDUS (Woodward et al. 2002; Isidori and Lenzi 2008).
Vascularization
The detection of the testis vascular perfusion plays a key role in the differential diagnosis among testicular torsion (absent), infarction (absent or peripheral), epididymo-orchitis, or some malignant conditions (i.e., leukemia, lymphoma) (enhanced), supporting suspicions arising from clinical abnormal findings (Isidori and Lenzi 2008) (see Table 1). This is relevant, because these conditions may exert a definitive negative effect on male reproductive and general health (Tekgül et al. 2008; Sharp et al. 2013; Grabe et al. 2013; Jungwirth et al. 2016). In addition, the knowledge of testicular vascularization (see section “Vascularization” and Fig. 2) is relevant to perform testis surgery. In fact, centripetal arteries (Fig. 2) are end arteries, and their damage during testicular sperm extraction (Schlegel and Li-Ming 1997; Ron-El et al. 1998) and, rarely, percutaneous fine needle sperm aspiration (Friedler et al. 1997) may lead to devascularization of one or more lobules. Testicular open or percutaneous biopsy may also lead to intratesticular or extratunical hematoma formation (Schlegel and Li-Ming 1997; Friedler et al. 1997) and to inflammatory changes affecting spermatogenesis (Schlegel and Li-Ming 1997). Interestingly, specific vascular parameters such as arterial resistivity or pulsatility index, measured both in the testicular artery or in intratesticular vessels, represent a new frontier of research in fertility evaluation, however not yet used in the clinical practice (see section “Testis CDUS and Surgical Sperm Retrieval in Azoospermic Subjects”).
Testicular Findings and Lesions
Several abnormal CDUS testicular findings may be associated with male reproductive or general health problems (see Tables 1 and 2).
Calcifications
Calcifications are calcium deposits within the seminiferous tubules (Richenberg and Brejt 2012). Solitary parenchymal calcifications may be due to a prior trauma, orchitis, infarction, torsion, or chemo−/radiotherapy or may be associated with testicular atrophy, maldescended testis, or, rarely, a burnt-out tumor (Mihmanli and Kantarci 2009; Raza and Jhaveri 2012; Appelbaum et al. 2013).
-
Microcalcifications are nonshadowing bright echogenic foci measuring 1–3 mm randomly scattered throughout the testicular parenchyma.
-
Testicular microlithiasis (TM) (Fig. 1) is defined as more than five echogenic foci in a single US image (Richenberg and Brejt 2012; Raza and Jhaveri 2012; Appelbaum et al. 2013). They can be limited, “clusters,” or diffuse (“starry sky” appearance; Fig. 4c) (Mihmanli and Kantarci 2009; Elzinga-Tinke et al. 2010). TM prevalence ranges from 0.6% to 9% (see Richenberg and Brejt 2012). However, some authors (Elzinga-Tinke et al. 2010) reported a prevalence of 43% considering less than five microcalcifications for US scan as TM. Interestingly, these authors (Elzinga-Tinke et al. 2010) reported detection of carcinoma in situ in ~25% of TM patients, particularly when “clusters” of TM or testicular inhomogeneity were present. Although most of previous studies reported an association between TM and testicular cancer, recent literature is debating this relationship (see Appelbaum et al. 2013 and Table 1), and a meta-analysis suggests no causal link between TM and testicular neoplasms (Richenberg and Brejt 2012). According to the European Association of Urology (EAU) guidelines (Albers et al. 2013), US follow-up of TM is recommended in subjects with additional risk factors for testicular malignancy (see section “Testicular Lesions”). Solely, the presence of TM is not considered an indication for regular scrotal US follow-up or biopsy (Albers et al. 2013). Conversely, testicular biopsy should be offered to men with TM and high-risk conditions (infertility and bilateral TM, atrophic testes, undescended testes, a history of testicular tumors, or contralateral TM) (Jungwirth et al. 2016).
The relationship between TM and infertility is under debate (Aizenstein et al. 1998; Miller and Sidhu 2002; Yee et al. 2011). In infertile men, asymptomatic TM is not considered as a risk factor for the production of antisperm antibodies (Clyne 2012; Jiang and Zhu 2013). Recent evidence supports TM as an additional feature of the “testicular dysgenesis syndrome” (see section “Cryptorchidism” and Fig. 1) suggesting a genetic background (Tan and Eng 2011). So far, the impact of TM in the management of male infertility is low (Table 1).
Orchitis
The inflammation of the testis is defined orchitis . Primary orchitis is mainly viral (mumps orchitis), occurring in 20%–30% of infected postpubertal men (Grabe et al. 2013). In almost half of the cases, its onset follows an epididymitis (Horstman et al. 1991b; Pilatz et al. 2013b), and in this case, its etiology is mainly bacterial. Orchitis is usually characterized by diffuse testis swelling and pain (Grabe et al. 2013). At CDUS, orchitis presents with testis hypervascularization (see Table 1), diffuse enlargement, inhomogeneous, mainly hypoechoic, testicular echo-texture, and reactive hydrocele (see in Dogra et al. 2003; Isidori and Lenzi 2008; Ammar et al. 2012; Pilatz et al. 2013b). Post-orchitis testis may present with inhomogeneous echo-texture, mainly hypo- or hyperechoic, with normal or reduced vascularization and micro- or macro-calcifications (Isidori and Lenzi 2008; Ammar et al. 2012). Although chronic inflammation may result in testicular atrophy (see Grabe et al. 2013), no atrophy occurs if an acute epididymo-orchitis is adequately treated (Pilatz et al. 2013b). However, orchitis often hesitates in sperm abnormalities (Isidori and Lenzi 2008; Ammar et al. 2012).
Testicular Lesions
Testicular lesions (Fig. 1) can be detected by US incidentally, especially during infertility assessment or when a patient is seeking medical care for scrotum discomfort, lump or painless swelling of the testis, or dull or heavy pain, the latter reported by 10%–20% of males with testis malignancy (Isidori and Lenzi 2008). Differential diagnosis is difficult, particularly when, at US, severe inhomogeneity is detected. Testicular lesions may be small (millimetric) or large. Small hypoechoic areas, especially when not vascularized, may be related to spermatoceles, cysts, focal Leydig cell hyperplasia, fibrosis, and focal inhomogeneity due to previous pathologic conditions (Isidori and Lenzi 2008). However, they may also indicate small tumors (Isidori and Lenzi 2008). Hence, they require careful evaluation and follow-up, with periodic US examination (see Table 1), especially if additional risk factors for malignancy are present (i.e., infertility, bilateral TM, cryptorchidism, testicular atrophy, inhomogeneous parenchyma, history of testicular tumor, contralateral tumor, and age < 50 years (van Casteren et al. 2009; Elzinga-Tinke et al. 2010; Albers et al. 2013; Jungwirth et al. 2016). If a small nodule grows (Dohle et al. 2012), or additional risk factors for malignancy are present, testicular biopsy/surgery should be considered (Jungwirth et al. 2016). Large nodules may be either benign or malignant. CDUS is not accurate enough to define the origin of the lesion, and histology remains the only certain diagnostic tool (Woodward et al. 2002). However, CDUS may detect some specific characteristic suggestive or not of malignancy. Clinical and CDUS patterns of testicular lesions have been described in detail elsewhere (Woodward et al. 2002; Isidori and Lenzi 2008). Table 3 summarizes the CDUS characteristics of the main malignant and benign testicular lesions. In addition, new imaging techniques, such as contrast-enhanced ultrasound, elastography, and MRI, have improved the characterization of testicular lesions (Huang and Sidhu 2012) (see section “New Imaging Techniques for the Evaluation of Testis and Prostate Abnormalities”).
According to the EAU guidelines (Albers et al. 2013), scrotal US is useful to confirm the presence of a testicular mass and to explore the contralateral testis. US discloses a testicular mass with a sensitivity of ~100% (Kim et al. 2007). US detects whether a mass is intra- or extratesticular (Kim et al. 2007), its features, and differentiates among different clinical conditions (i.e., malignancy, inflammation, cysts) (Montgomery and Bloom 2011; Woodward et al. 2002). US should be performed even when a testicular malignancy is clinically evident (Shaw 2008; Albers et al. 2013). According to the EAU guidelines (Albers et al. 2013), every subject with a suspected testicular mass must undergo surgical exploration, with orchiectomy if a malignant tumor is found, or testicular biopsy with histological examination if the diagnosis is not clear. In our opinion, US is useful in detection and follow-up of small lesions or of additional risk factors for malignancy, playing an adjuvant role in the management of large/hard lesions (Table 1).
Testicular tumors account for 4%–6% of all MGT neoplasms and 1%–2% of the male neoplasms and represent the most common malignancy in young men (15–34 years; Woodward et al. 2002). Testicular cancer is often associated with other testicular disorders, such as sperm abnormalities, male infertility , or maldescended testis. Malignancy-related sperm alterations depend on neoplasm volume and histology, general tumor effects, disease stage, previous testicular disorders, and orchiectomy (Trost and Brannigan 2012; Rives et al. 2012). Among seminal samples of oligozoospermic men cryopreserved for cancer, those from subjects with a testicular tumor show the worse basal semen quality and recovery after thawing (Degl’Innocenti et al. 2013; Hotaling et al. 2013) and motility recovery similar to that of noncancer samples (see Degl’Innocenti et al. 2013). Combined information on sperm concentration, age, and contralateral TV may predict the risk of contralateral carcinoma in situ in patients with unilateral testicular germ cell tumor (Rud et al. 2013).
Cryptorchidism
The term cryptorchidism is derived from the Greek words kryptos and orchis, literally meaning “hidden testis.” This affection includes abnormal testis development and/or failure of its descent into the scrotal sac (see Christensen and Dogra 2007). Cryptorchidism is the most common abnormality in newborn males, affecting 1%–6% of full-term neonates and ~0.8% of infants at 1 year of age, with a higher overall incidence for preterm infants (see Christensen and Dogra 2007). It is unilateral in ~90% of patients and bilateral in the remaining ~10%. Cryptorchidism is associated with an increased risk for infertility and testicular cancer (Fig. 1), the latter showing four to sevenfold higher prevalence than in the healthy population. In ~20% of cryptorchid patients, testis malignancy occurs in the contralateral descended testis, resulting more frequent when both testes are maldescended (see Christensen and Dogra 2007). Furthermore, cryptorchidism is associated with urinary tract abnormalities, including renal agenesis or ectopia, ureteral duplication, SV agenesis or cysts, and hypospadias (see Christensen and Dogra 2007). Some authors consider maldescended testis as one manifestation of a primitive generalized defect in genitourinary embryogenesis, also advocating a common origin for cryptorchidism, defective spermatogenesis, and testicular germ cell tumor (“testicular dysgenesis syndrome”; Skakkebaek and Jørgensen 2005). On the other hand, considering that almost 80% of maldescended testes are located within the inguinal canal, and 5%–16% within the abdomen, it has been suggested that a higher temperature of these sites with respect to that of the scrotal sac may lead to a secondary degeneration of the cryptorchid testis. Regarding infertility, germ cell failure seems not to be congenital, but rather acquired, beginning approximately at the age of 4 months and progressively decreasing thereafter, finally reaching gonadal atrophy at the age of 5. Hence, early surgical correction has been advocated to prevent infertility (see Christensen and Dogra 2007). Finally, undescended testes show an increased risk for testicular torsion (Schultz and Walker 1984).
At US, the cryptorchid testis is often characterized by a reduced volume, low echogenicity (Fig. 4a), and inhomogeneity with or without macro- or microcalcifications (Christensen and Dogra 2007; Ozden et al. 2012). The role of US in the setting of cryptorchidism and preoperative planning before orchiopexy is controversial (Christensen and Dogra 2007; Ozden et al. 2012). A recent systematic review and meta-analysis reports that US does not reliably localize nonpalpable testes or rule out an intra-abdominal testis (Tasian and Copp 2011). Hence, performing US does not change the clinical management of nonpalpable testes. However, US can reliably identify a cryptorchid testis lying below the level of the internal inguinal ring (Nijs et al. 2007). In conclusion, US plays a key role in cancer detection or in the follow-up of the cryptorchid and contralateral testes (see Table 1).
Varicocele
Varicoceles are abnormally dilated veins of the pampiniform plexus (Figs. 1 and 4d), characterized by retrograde venous flow (Forti and Krausz 1998; Forti et al. 2003; Zini and Boman 2009). According to the aforementioned anatomical considerations (see section “Pampiniform Plexus”), varicocele is detected on the left side in ~90% of cases (Fig. 1) (Sakamoto and Ogawa 2008; Zini and Boman 2009). Varicocele is virtually absent in prepubertal boys, and its prevalence increases with age, up to ~15% in the general adult population (Forti et al. 2003; Canales et al. 2005; Cayan and Woodhouse 2007). In the clinical practice, varicocele is classified into three grades: I, palpated during Valsalva maneuver; II, palpated without Valsalva maneuver; and III, visible (Dubin and Amelar 1970). While clinically assessed grade III varicoceles are easily diagnosed, detection of milder forms depends on the investigator experience. In fact, its detection may be distorted by cremasteric contraction, previous surgery, hydroceles, or maldescended testis (see Liguori et al. 2012). Pathophysiologic mechanisms leading to varicocele development and its clinical associations have been extensively described elsewhere (see Sakamoto and Ogawa 2008; Gat et al. 2008).
Venography of the internal spermatic vein is considered the gold standard for varicocele assessment (see Geatti et al. 1991; Lee et al. 2008; Liguori et al. 2012) since it is characterized by a high technical accuracy and interobserver concordance (see Lee et al. 2008). However, venography is time-consuming, invasive, and exposes to radiations (see Liguori et al. 2012). Hence, even if it is the most accurate technique for varicocele detection and often used for comparison with all the other diagnostic approaches in research studies, venography is currently indicated in clinical practice only in selected cases (see Liguori et al. 2012). So far, the American Urology Association/American Society for Reproductive Medicine (AUA/ASRM; see Practice Committee of American Society for Reproductive Medicine 2008) and the EAU Guidelines on Male Infertility (Jungwirth et al. 2016) suggest to diagnose a varicocele by clinical examination, because “only palpable varicocele have been documented as being associated with infertility” (AUA/ASRM; see Practice Committee of American Society for Reproductive Medicine 2008). However, the diagnosis should be confirmed by CDUS (Jungwirth et al. 2016), especially when physical examination is not conclusive (AUA/ASRM; see Practice Committee of American Society for Reproductive Medicine 2008). In particular, CDUS shows higher diagnostic accuracy than physical examination (see Lee et al. 2008), becoming precious when the latter is unreliable (Liguori et al. 2012), and it is considered the imaging modality of choice for detection and grading varicocele (Liguori et al. 2012), offering a more complete stratification of lower grades (Isidori and Lenzi 2008). In addition, CDUS may identify the so-called “false” clinical varicocele, referring to dilated vessels without venous reflux (Isidori and Lenzi 2008), and plays a key role in detecting postoperative recurrence/persistence (Lund et al. 2000; Tefekli et al. 2001; Isidori and Lenzi 2008). Compared to venography, physical examination has a 50%–70% sensitivity in varicocele detection, while CDUS 93% (see Lee et al. 2008; Zini and Boman 2009). Hence, CDUS has become the most widely accepted, as well as the most frequently used, modality for varicocele evaluation (see Lee et al. 2008). In conclusion, although physical examination remains the cornerstone of varicocele management, CDUS have a higher diagnostic accuracy (Table 1).
At CDUS, varicocele should be assessed with the patient lying down and standing. A palpable varicocele feels like a “bag of worms” and is easier to be detected in upright position. When a suspected varicocele is not clearly palpable, it should be examined, while the patient performs a Valsalva maneuver in a standing position (AUA/ASRM; see Practice Committee of American Society for Reproductive Medicine 2008). Some authors suggest evaluating the internal spermatic vein between the upper pole of the testis and the inguinal ligament, in order to assess a straight vein instead of the convoluted vessels below (Orda et al. 1987). The size and location of the varices, their number, basal intermittent or continuous reflux at CDUS, and changes during Valsalva maneuver should be considered.
The US gray-scale appearance of varicocele consists of multiple (>3), hypoechoic, serpiginous tubular structures of varying size, >2–3 mm, which could extent from the upper pole of the testis to the bottom of the scrotal sac (Dogra et al. 2003; Lee et al. 2008; Isidori and Lenzi 2008; Raheem 2013). Available CDUS classifications of varicocele severity are reported in Table 4, which attempts to harmonize the different classifications. In most of them, severe CDUS varicocele is defined by a continuous venous reflux at rest, increasing or not during Valsalva maneuver (Fig. 4d).
Most of the studies report a TV reduction in men with varicocele, with normal or slightly increased gonadotropin levels (Zini and Boman 2009; Raheem 2013). Conversely, the effect of varicocele on testosterone levels is under debate (Tanrikut et al. 2011). A recent meta-analysis showed an increase in testosterone levels after varicocele surgical correction (Li et al. 2012). Most studies, but not all, report worse sperm parameters in subjects with varicocele. However, these studies have been conducted in infertile subjects, while data in fertile men are contrasting. In addition, 75% of subjects with varicocele have normal semen parameters (see Zini and Boman 2009). The majority of the studies reported no difference in paternity comparing men with or without varicocele (see Zini and Boman 2009). The possibility of reverting infertility through varicocele treatment is under debate. According to the AUA/ASRM (see Practice Committee of American Society for Reproductive Medicine 2008), when varicocele is detected in men with couple infertility, surgery should be considered when all of the following conditions are met: (1) varicocele is clinically palpable; (2) the couple has known infertility; (3) the female partner has normal fertility or a potentially treatable cause of infertility; and (4) the male partner shows abnormality in semen parameters or sperm function tests. Varicocele treatment for infertility is not indicated in patients with either normal semen quality or a subclinical varicocele. A recent meta-analysis reported that varicocele surgery improves sperm parameters (count, total, and progressive motility), reduces sperm DNA damage and seminal oxidative stress, and improves sperm ultramorphology (Baazeem et al. 2011). Although there is no conclusive evidence that a varicocelectomy improves spontaneous pregnancy rates, a recent Cochrane review concluded that surgical or radiological treatment in subfertile men, with a clinically manifest varicocele and poor semen quality, may be of benefit, reporting one additional pregnancy for every seven men treated (Kroese et al. 2013). In addition, some men with scrotal pain, low testosterone, non-obstructive azoospermia, or at risk for testicular dysfunction may benefit from varicocele repair (Schlegel and Goldstein 2011). However, varicocelectomy may be associated with complications, such as hydrocele, inadvertent arterial ligation, testicular atrophy, vas deference occlusion, and epididymitis (Isidori and Lenzi 2008; Iaccarino and Venetucci 2012); hence its potential benefits must be carefully considered. These considerations are even more critical when subclinical varicocele is considered. Subclinical varicocele is a radiological entity defined as venous reflux detectable by CDUS but not at physical examination. A recent Cochrane review considers its treatment disputable, the number needed to treat to benefit being 17 (Kroese et al. 2012). However, treatment of subclinical varicocele with grade III reflux according to Cornud (1999), defined as permanent reflux, regardless of the size of the veins, resulted in changes similar to those seen after repair of palpable varicocele (Cornud et al. 1999; Lee et al. 2008). In addition, grade III reflux according to Cornud (1999) was found to be palpable in 60% of cases (Cornud et al. 1999; Lee et al. 2008). Hence, detection of “permanent reflux” by CDUS (see Table 4) could be an indication for varicocele treatment when physical examination is inconclusive, although, at present, this is not evidence based.
Finally, varicocele has also been suggested as a potential cause of intrapelvic venous congestion, prostate inflammation, and, eventually, prostatitis-related premature ejaculation (Lotti et al. 2009). Accordingly, a previous study reported that varicocele surgery leads to improvement of premature ejaculation (Ahmed et al. 2014).
Epididymis and Vas Deferens
CDUS Abnormalities
At physical examination, tense-elastic spherical findings detected within the epididymis, mainly in the head, may represent cysts or spermatoceles. Epididymal cysts and spermatoceles are present in ~25% of men and appear at US as anechoic avascular and slightly hypoechoic findings, respectively (Leung et al. 1984). Their clinical significance and association with male infertility has not been yet defined, since no study demonstrated their relationship with complete epididymal obstruction and obstructive azoospermia (see Singh et al. 2012). Hence, their detection by US has no impact on infertility decision-making (Table 1). Conversely, epididymal injury secondary to excision surgery, mainly performed for large and painful lesions, may lead to epididymal obstruction (see Singh et al. 2012). Hence, epididymal cyst removal is not suggested for fertility improvement (Table 1).
Epididymal US echo-texture abnormalities have been associated with acute or chronic inflammation (see Table 2; Woodward et al. 2003; Isidori and Lenzi 2008; Lotti et al. 2011a; Lotti and Maggi 2013). Acute epididymitis is characterized by a painful hemiscrotum, epididymal swelling, and fever (Grabe et al. 2013; Pilatz et al. 2013b). CDUS plays a key role in its detection, revealing epididymal hyperemia and dilation (see Table 1), mainly of the tail or both the tail and head (Pilatz et al. 2013b), along with inhomogeneous echo-texture, often hypoechoic with scattered hyperechoic foci, and reactive hydrocele with skin thickening (Horstman et al. 1991b; Woodward et al. 2003; Isidori and Lenzi 2008; Pilatz et al. 2013b). Concomitant orchitis, revealed in ~50% of men, is associated with hydrocele, testicular increase, hyperemia, and pain (Pilatz et al. 2013b). Under conservative treatment, epididymal CDUS parameters normalize (Pilatz et al. 2013b). Hence, along with clinical characteristics, CDUS may play a role in the follow-up of acute epididymitis (Table 1). Chronic epididymitis frequently affects the tail, with coarse calcifications in a dilated hypo- or hyperechoic epididymis (Fig. 4e, f). This process is characterized by US inhomogeneity, irregular profile, and hard irregularly shaped masses (Fig. 4e) or indenting the testicular parenchyma, mimicking a primary testicular mass (Woodward et al. 2003). Hydrocele and tunica albuginea thickening are commonly associated, the latter sometimes so severe as to be called “fibrous pseudotumor ” (Isidori and Lenzi 2008). Epididymitis impact on male reproductive function seems to be more relevant than inflammation/infection of the prostate and/or SV (Haidl et al. 2008; see sections “CDUS Abnormalities” and “Echo-Pattern Abnormalities”). Acute epididymitis may lead to transient semen impairment, although often persistent detrimental effects are observed (see Rusz et al. 2012). Chronic epididymitis may result in reduced sperm count and motility (Haidl et al. 2008).
Regarding VD, chronic inflammation and diabetes may be associated with luminal or parietal calcifications, respectively (Kim et al. 2009) (Fig. 4f). A dilated, inhomogeneous proximal VD may be seen in patients with chronic inflammation (Fig. 4e), distal VD, or ED obstruction – often showing deferential ampulla and SV dilation/echo-texture abnormalities (Fig. 4f) – or vasectomy (see also sections “Obstruction-Related Findings” and “Ejaculatory Ducts Obstruction/Abnormalities”). Hence, according to medical history, epididymal and VD dilation is indicative of a distal obstruction (Fig. 1), suggesting the extension of the US examination to the prostate-vesicular region by transrectal ultrasound (TRUS) (Table 1).
Obstruction-Related Findings
Primary obstruction may only be suggested, but not proven, by US (Table 1). Epididymal tail >6 mm (Vicari 1999) and head >11 (Pezzella et al. 2013) or >12 mm (Vicari 1999) have been proposed as suggestive of obstruction (Fig. 4e, g). Obstruction of the epididymal tail or of proximal VD has been demonstrated in blocked seminal tracts in subjects treated by epididymovasostomy (Matsuda et al. 1994). After vasectomy, an epididymal head >15 mm in vasectomized patients (Reddy et al. 2004) and an increment of 2 cm in epididymal head, with a higher frequency of cysts and inhomogeneity, have been reported (Jarvis and Dubbins 1989; Cho et al. 2011) (Fig. 4g). Abrupt tapering, tubular ectasia, enlargement (Fig. 1), with or without calcifications, or mass-like lesions, together with a normal VD caliber, suggest partial or complete epididymal obstruction (Moon et al. 2006). Similar findings have been described for secondary changes of the proximal VD (Donkol 2010). It has not yet been clearly demonstrated that MAGI results in complete epididymal or VD obstruction, with the exception of genital tuberculosis (Dohle 2003), which may present, at US, with enlarged hypoechoic epididymis with an irregular profile, calcifications, and firm granulomatous masses (Isidori and Lenzi 2008).
Agenesis
Congenital uni- or bilateral agenesis of vas deferens (CUAVD and CBAVD, respectively) (Fig. 4g) may be partial or complete, depending on the level of the Wolffian duct abnormality. Since VD, seminal vesicles (SV), ejaculatory ducts. and epididymis have a similar embryological origin from the Wolffian duct, VD agenesis may be associated with agenesis or abnormalities of these structures. Hence, if VD agenesis is detected at scrotal US, TRUS should be performed to extend the examination to the prostate-vesicular (Table 1). Interestingly, when VD and epididymis agenesis occurs, epididymal head is always present and detectable by US (Sadler 2011; Singh et al. 2012).
CBAVD accounts for 1%–2% of infertile men, 4%–17% of azoospermic men, and up to 25% of those with obstructive azoospermia (Singh et al. 2012). CBAVD may be isolated or associated with cystic fibrosis. Almost all men with cystic fibrosis also have CBAVD. Cystic fibrosis is common in Caucasian populations, but rare in others (see Yu et al. 2012). A recent meta-analysis reported that 78% of CBAVD subjects have at least one CFTR mutation (Yu et al. 2012), which may exhibit ethnic differences. CBAVD is associated with bilateral SV agenesis in ~50% of the patients and usually presents with normal kidneys (Schlegel et al. 1996). Almost ~20% of cases of CBAVD, ~20% of cases of unilateral SV agenesis, and ~70% of cases of unilateral SV giant cyst may present with kidney agenesis and are usually not related to CFTR gene mutations (Singh et al. 2012). Subjects with CBAVD usually show normal testes volume and function (Silber et al. 1990). Hence, CBAVD investigation by US is essential in the diagnosis of obstructive azoospermia and in its clinical decision-making, since surgical sperm retrieval is virtually always positive (see Table 1).
CUAVD is present in ~1% of men. CUAVD is associated with ipsilateral and contralateral SV agenesis in 90% and 20% of patients, respectively, and with renal agenesis in 79% of cases (Singh et al. 2012). Subjects with CUAVD are usually fertile, but at high risk for infertility, having a single patent VD. Furthermore, those with CUAVD and contralateral SV agenesis may have contralateral deferential ampulla atresia. Hence, a subset of men with CUAVD may have abnormal semen parameters or azoospermia (Singh et al. 2012). Similar problems may be present in subjects with CUAVD and contralateral testis damage.
Prostate
Volume
Detection of prostate volume (PV) by TRUS is important in subjects with LUTS, while it has a low impact in the work-up of male infertility (Table 1). A reduced PV suggests hypogonadism, because prostate is an androgen-dependent gland (Behre et al. 1995; Jin et al. 2001). An increased PV is related to benign prostatic enlargement (BPE). A PV >30 ml has been suggested as indicative for initial gland enlargement (Older and Watson 1996) and >60 ml for a severe increase (Gacci et al. 2012). BPE has a continuum spectrum of TRUS abnormalities ranging from larger transitional zone to a well-defined adenoma. The typical TRUS characteristics of BPE are echo-texture inhomogeneity, occasional cysts, well- and poorly defined nodules, and calcifications, especially at the “surgical capsule” (Older and Watson 1996). Interestingly, BPE has been recently associated with overweight/obesity (Lotti et al. 2011b) and metabolic syndrome (Lotti et al. 2013c).
CDUS Abnormalities
The impact of prostatitis on semen parameters is under debate (La Vignera et al. 2011a; Rusz et al. 2012, Lotti et al. 2013d). Accordingly, the relationship between prostate inflammation, CDUS-related abnormalities, and semen quality is controversial. Hence, the assessment of CDUS prostate abnormalities by TRUS has a low impact on male infertility management (Table 1). Conversely, several TRUS features have been considered suggestive of prostate inflammation (Table 2), and increasing evidence suggests a TRUS role in identification of dynamic CDUS findings related to prostatitis-like symptoms, such as hyperemia and higher peak systolic velocity in prostatic arteries (Fig. 4h) (Lotti et al. 2013d and Table 2). However, so far, EAU guidelines consider ultrasound as a technique with limited value in assessing chronic pelvic pain (Engeler et al. 2016).
Ejaculatory Ducts Obstruction/Abnormalities
-
Ejaculatory ducts obstruction (EDO) affects 1%–5% of infertile men and may be congenital or acquired (Singh et al. 2012). Congenital causes include ED atresia/stenosis, midline prostatic cysts, or ED congenital cysts (Fig. 1). Acquired causes may be secondary to infection/inflammation, calcifications, or iatrogenic (Fisch et al. 2002). Detection of bilateral EDO by TRUS is useful in defining the diagnosis of obstructive azoospermia and its clinical management, considering surgical treatments if specific abnormalities are found (see below and Table 1). Subjects with congenital or noninfectious causes of EDO, or with partial EDO, have better improvements in semen parameters after treatment than those with infectious causes or complete EDO (see Fisch et al. 2002; El-Assmy et al. 2012).
-
TRUS findings in suspected EDO include midline prostate cysts and ED dilation, calcifications, or cysts (Fig. 4i, j). Dilated SV (anterior-posterior diameter >15 mm) and enlarged deferential ampulla (diameter >6 mm) have also been previously suggested as EDO-related findings (Fig. 1) (Jarow 1993; Engin et al. 2000; Engin 2012; Jungwirth et al. 2016). We recently proposed a new parameter related to the SV emptying capacity, “SV ejection fraction,” reporting a cutoff suggestive for complete or partial EDO (Lotti et al. 2012a). However, further studies are needed to assess the clinical relevance of this parameter.
-
Intraprostatic cysts can be classified as congenital or acquired, or, based on their position within the prostate, as midline, paramedian, and lateral cysts Nghiem et al. 1990; Singh et al. 2012; Shebel et al. 2013). Midline cysts (Fig. 1) affect 1%–5% of men, with a higher frequency in infertile men. They may cause partial or complete EDO, with reduced sperm count or obstructive azoospermia, respectively, often associated with SV obstruction/dilation, reduced ejaculate volume, and pH (see Singh et al. 2012). At TRUS, they appear as roundish or pear-/oval-shaped anechoic formations in transversal and longitudinal scans, respectively (Fig. 4i). According to previous studies (see Nghiem et al. 1990; Singh et al. 2012; Shebel et al. 2013), two main different cystic entities have been recognized. The first, Müllerian cyst, is thought to arise from a regression failure of the Müllerian ducts, causing a focal saccular dilation. This cyst is located at midline or slightly lateral to midline, is large and may extend above the base of the prostate, does not communicate with the urethra or contain spermatozoa, and may be associated with various genitourinary abnormalities (see Nghiem et al. 1990; Singh et al. 2012; Shebel et al. 2013). However, it eventually may erode ED and include sperm (McDermott et al. 1995). The second, utricular cyst, is thought to derive from dilation of the prostatic utricle, is strictly midline, smaller than the former, and confined to the prostate; it communicates with the urethra and usually contains spermatozoa. Both midline cysts may cause EDO by deviating or compressing ED (see Nghiem et al. 1990; Singh et al. 2012; Shebel et al. 2013). However, even if detection of a midline cyst suggests EDO, conclusions concerning its functional significance cannot be drawn, and size cutoffs for complete EDO have not been reported (Table 1). Midline cyst-related EDO may be diagnosed only after TRUS-guided aspiration (Donkol 2010), which will allow cyst reduction and restore semen emission. This is of clinical relevance, since aspiration of large cysts in subjects with obstructive azoospermia may lead to semen parameter improvement (Table 1). However, after this procedure, midline cysts may enlarge and lead to EDO and azoospermia again, after variable times. In this case, TRUS should be considered to evaluate cyst recurrence (Table 1). Various complications may be associated with prostate cysts, besides infertility, such as urinary tract infection, pain, recurrent epididymitis or prostatitis, and hemospermia (Singh et al. 2012).
-
Ejaculatory duct (paramedian) cysts (Figs. 1 and 3j) may be congenital, originating from Wolffian ducts, or acquired and may be related or not to cystic fibrosis (Singh et al. 2012). Uni- or bilateral, they may lead to obstructive azoospermia. In this case, cyst detection by TRUS is useful in clinical management (Table 1), their surgical treatment often restoring semen emission (Engin 2012).
-
Ejaculatory duct dilation (Figs. 1 and 3j) has been defined as an ED diameter >2 mm (Fisch et al. 2002; Engin et al. 2000; Engin 2012) and may be related to inflammatory distal stenosis, which is often difficult to detect (Cornud et al. 1997).
-
Ejaculatory duct calcifications (Figs. 1 and 3j) may be associated with EDO but are not a reliable indicator of it (Jarow 1993; Fisch et al. 2002; Engin 2012). They have also been associated with hemospermia (Littrup et al. 1988) and prostatitis-like symptoms (Lotti et al. 2013d) (Table 2). Accordingly, EDO may be associated with hemospermia, prostatitis, and painful ejaculation.
In select cases, transurethral resection of ED results in marked improvement in semen parameters, and pregnancies have been achieved (Fisch et al. 2002; Donkol 2010; Engin 2012).
Prostate Cancer
Prostate cancer is usually suspected on the basis of digito-rectal examination and/or increased PSA levels. At TRUS, prostate cancer is often seen as a hypoechoic lesion in the peripheral zone of the gland; however it can be isoechoic or hyperechoic, and it is not always anatomically well defined (Sarkar and Das 2016). Definitive diagnosis depends on histopathological verification of cancer in prostate biopsy cores or specimens from transurethral resection of the prostate or prostatectomy for benign prostatic enlargement (Mottet et al. 2016). According to EAU guidelines, gray-scale TRUS is not reliable in detecting prostate cancer (Smeenge et al. 2012). Thus, there is no evidence that US-targeted biopsies can replace systematic ones and there is not enough evidence for TRUS routine use in prostate cancer assessment (Mottet et al. 2016).
Seminal Vesicles
Volume
SV volume abnormalities include dilation and hypoplasia. SV dilation (Fig. 1) has been defined, based on SV diameters, as a SV anterior-posterior diameter >14 (Vicari 1999) or 15 mm (Jarow 1993; Fisch et al. 2002; Engin et al. 2000, Engin 2012; Jungwirth et al. 2016), suggestive of EDO (Jungwirth et al. 2016). Recently, we proposed an algorithm calculating SV volume (Lotti et al. 2012a; see section “Seminal Vesicles, Deferential Ampullas, and Ejaculatory Ducts”). So far, a volumetric cutoff for SV dilation is lacking. However, a higher post-ejaculatory SV volume has been associated with a higher prevalence of SV abnormalities (see sections “Echo-Pattern Abnormalities,” “Obstruction-Related Findings,” and “SV Agenesis, Hypoplasia, and Cysts”), a higher prostate volume and detection of a prostatic midline cyst, supposed to cause partial or complete EDO, as well as signs suggestive of upstream MGT dilation, such as higher deferential and epididymal tail diameters (Lotti et al. 2012a) (Fig. 1).
SV hypoplasia has been defined as a SV anterior-posterior diameter <5 (Raviv et al. 2006) or <7 mm (Vicari 1999) or as SV longitudinal diameter <25 mm (Donkol 2010). So far, a SV volume cutoff is lacking. The term mainly refers to congenitally small SV (Kim et al. 2009), although an acquired form may be associated with testosterone deficiency (Sasagawa et al. 1989, 1990).
Echo-Pattern Abnormalities
Several US features are suggestive of SV abnormalities and have been associated with inflammation or stasis (see Table 2). Their possible negative impact on semen quality/quantity is controversial (La Vignera et al. 2011a; Rusz et al. 2012). In particular, “SV areas of endocapsulation,” which should be assessed after ejaculation (Lotti et al. 2012a and Table 2), is considered a feature suggestive of EDO (Colpi et al. 1997; Jungwirth et al. 2016), however with low impact on clinical decision-making (Table 1).
Obstruction-Related Findings
Enlarged SV anterior-posterior diameter has been related to partial EDO (Littrup et al. 1988; Kim and Lipshultz 1996; Colpi et al. 1997) as well as “SV areas of endocapsulation” (see section “Echo-Pattern Abnormalities”). Diagrams showing partial EDO percentage probability in function of SV anterior-posterior diameter variation have been reported (Colpi et al. 1997). Reduced “SV ejection fraction” (see section “Ejaculatory Ducts Obstruction/Abnormalities”) is suggestive of impaired SV emptying and partial EDO and is associated with higher prevalence of SV giant cysts and ED abnormalities (dilation, calcifications, or cysts) (Lotti et al. 2012a) (Fig. 1).
Reduced or absent contraction of SV during ejaculation without a clear obstructive cause has been defined as “functional SV atony” (La Vignera et al. 2011b, c, d). Signs suggestive of SV atony have been reported in subjects with type 2 diabetes mellitus with or without diabetic neuropathy (La Vignera et al. 2009, 2011b, c, d).
SV Agenesis, Hypoplasia, and Cysts
SV congenital abnormalities include defect in number (agenesis, fusion), maturation (hypoplasia), and canalization (cysts) of the glands (Vohra and Morgentaler 1997). Their detection is clinically relevant, because they are often associated with abnormal development of other mesonephric/metanephric derivatives, such as the VD, ureter, and kidney (Patel et al. 2002), which should be evaluated by US (Table 1).
Unilateral SV agenesis arises if an insult occurs before the seventh week of gestation, when the ureteric bud arises from the mesonephric duct (Kim et al. 2009). It is often associated with ipsilateral renal agenesis (79%) or other renal abnormalities (12%) (Kim et al. 2009).
Bilateral SV agenesis is associated with CFTR mutations in 64%–73% of cases, with CBAVD in half of the cases and with normal kidneys (Kim et al. 2009). SV abnormalities are observed in 50% of children and 90% of adults with cystic fibrosis, the latter showing bilateral agenesis in half of cases, supporting the hypothesis of a progression of the cystic fibrosis-related abnormalities (Carter et al. 1989; Cornud et al. 1997; Rathaus et al. 2006).
Congenital SV hypoplasia may be isolated or associated with other congenital genitourinary anomalies (Kim et al. 2009).
SV cysts (Figs. 1 and 3j) are rare and may be congenital or acquired. Congenital SV cyst may be isolated or, more frequently, associated with other genitourinary anomalies. They are mainly secondary to EDO caused by development abnormalities of the distal portion of the mesonephric duct (Patel et al. 2002). They are associated with ipsilateral renal agenesis (Zinner syndrome) or dysgenesis in two-thirds of cases (King et al. 1989). Ectopic ureteral insertion into the SV, ED, VD, or prostatic urethra or VD agenesis may also be present (Kim et al. 2009). Bilateral SV cysts have been reported to occur in 44%–60% of patients with autosomal dominant polycystic kidney disease (Danaci et al. 1998). Hence, detection of SV cysts by TRUS is clinically relevant, leading to evaluate carefully the urinary tract by US (Table 1). Acquired cysts are usually unilateral and associated with inflammation-related EDO (Patel et al. 2002). Cystic SV dilatation has been associated with perineal pain (Littrup et al. 1988).
Specific Applications of Scrotal and Transrectal Ultrasound
Sensitivity and Specificity in Discriminating Obstructive and Non-obstructive Azoospermia
Du et al. (2010) reported that performing scrotal and transrectal US, at the same time assessing TV and genital tract obstruction-related findings, respectively, discriminates obstructive and non-obstructive azoospermia (OA and NOA, respectively) with 95% sensitivity and 97% specificity and may be of help in evaluating OA etiology. More recently, Abdulwahed et al. (2013) reported that scrotal US is more sensitive for OA and specific for NOA detection, while TRUS showed the opposite trend. Both imaging examinations had greater specificity than sensitivity for OA, indicating that US is better able to exclude, more than to diagnose, OA. However, US is still unlikely to replace testicular biopsy (Abdulwahed et al. 2013).
Testis CDUS and Surgical Sperm Retrieval in Azoospermic Subjects
Sperm retrieval by testicular surgery has been reported in 50%–60% of men with NOA and in almost all with OA (Dohle et al. 2012). Among the different CDUS characteristics investigated as predictors of successful sperm retrieval by testicular biopsy in azoospermic men, testicular parameters such as TV and vascularization have shown increasing evidence in the last few years, although still controversial and with limited clinical utility (see below).
Azoospermic men with normal FSH levels and positive sperm retrieval have higher TV compared to those with a negative harvesting (Mitchell et al. 2011). TV in OA has been reported as higher than in NOA patients (Moon et al. 2006; Du et al. 2010). Subjects with CBAVD-related OA usually have testes with normal volume and function (Silber et al. 1990; Singh et al. 2012), and sperm retrieval by testicular biopsy is virtually certain. In NOA subjects, most (Ziaee et al. 2006; Ravizzini et al. 2008; Turunc et al. 2010; Boitrelle et al. 2011), but not all (Tournaye et al. 1997; Dohle et al. 2012), studies reported higher TV in subjects with a positive sperm retrieval. TV has been reported as an independent parameter related to testicular biopsy outcome (Boitrelle et al. 2011). A total TV of 16 ml (Boitrelle et al. 2011) or a mean TV of 9.5 ml (Ziaee et al. 2006) have been proposed as a cutoff for a positive sperm retrieval. However, other authors report that TV is not a useful parameter for sperm retrieval prediction (Tournaye et al. 1997; Dohle et al. 2012). In fact, sperms can be retrieved by surgery even in men with very small testis, such as those with Klinefelter’s syndrome (Dabaja and Schlegel 2013; Bryson et al. 2014). On the other hand, subjects with spermatogenic arrest, usually showing normal TV and FSH levels, are characterized by a poor surgical outlook (Hung et al. 2007). Hence, we conclude that even if US has some prognostic value in surgical sperm retrieval outcomes, it is limited, playing a limited role in the work-up of the infertile male (Table 1).
Interestingly, some vascular parameters detected by testicular CDUS have been associated with sperm quality (Herwig et al. 2007; Hillelsohn et al. 2013), suggested as useful in discriminating OA and NOA (Foresta et al. 1998; Battaglia et al. 2001; Biagiotti et al. 2002a, b; Schurich et al. 2009) or residual spermatogenic areas in NOA (Foresta et al. 1998). However, at present, they have been evaluated only for research purpose, with no impact on the clinical management of the azoospermic men (Table 1).
Scrotal and Transrectal Ultrasound and Hormonal Treatments
The sonographic assessment of testis, prostate, and SV characteristics before the beginning and/or during hormonal treatment represents an effective tool in evaluating the response of target organs and in monitoring the appearance of suspicious findings. Baseline US-assessed TV represents one of the main determinants of gonadotropin responsiveness in subjects with hypogonadotropic hypogonadism (HH). In fact, a better response in terms of sperm output and ongoing pregnancy has been observed for basal TV >4 ml (Liu et al. 2002), although a recent meta-analysis failed to find any significant association between TV and appearance of spermatozoa in the semen upon gonadotropin therapy (Rastrelli et al. 2014). In addition, US has been performed by some authors to evaluate TV increment during hormonal treatment. GnRH (Canale et al. 1990) or gonadotropin (Main et al. 2002; Miyagawa et al. 2005) treatment in HH subjects was associated with a TV increase up to 170%. A 12-week treatment with FSH in men with idiopathic infertility demonstrated a TV increase of 5 ml compared to baseline (Kamischke et al. 1998). Considering that among infertile (Jacobsen et al. 2000; Walsh et al. 2009) and azoospermic (Eisenberg et al. 2013) men the risk of testicular malignancy is higher, scrotal US should be performed with prevention purpose in azoospermic and/or HH subjects unresponsive to hormonal treatment. However, at present, no agreement on US testis surveillance in these subjects is available.
TRUS is a useful tool in evaluating prostate response to hormonal treatment in HH subjects under gonadotropin, GnRH, or T treatment (Canale et al. 1990; Behre et al. 1995), monitoring not only their possible effect on volume increment but also for cancer screening, along with palpation and PSA measurements (Behre et al. 1995). Also SV volume shows changes at US during hormonal supplementation of hypogonadal men with (Sasagawa et al. 1989) or without (Sasagawa et al. 1990) Klinefelter syndrome.
Color-Doppler Ultrasound Clinical Utility and Impact on Male Reproductive Health Management
Table 1 summarizes our view on the clinical utility of MGT-CDUS evaluation on clinical decision-making (ranking from mild to high relevance) in male infertility according to the different sites and types of abnormalities. In most cases, clinical, hormonal, and seminal parameters are informative enough for the management of infertile men (Krausz 2011; Lotti et al. 2012b). However, MGT-CDUS shows a critical role in specific conditions. Table 5 offers a provisional summary of seminal, US, and hormonal correlates of some recognized causes of male infertility. MGT-CDUS, particularly TRUS, shows a key role in obstructive azoospermia (Du et al. 2010; Abdulwahed et al. 2013), leading to operational decision-making, such as TRUS-guided cyst aspiration if a large prostatic cyst is found, surgical treatment if ejaculatory duct abnormalities are observed, and testicular biopsy if CBAVD is detected (Fisch et al. 2002; Donkol 2010; Engin 2012). In particular, EAU Guidelines on Male Infertility (Jungwirth et al. 2016) consider TRUS important to detect distal obstruction. SV enlargement (anterior-posterior diameter >15 mm), and SV roundish anechoic areas are TRUS abnormalities often associated with EDO, especially when semen volume is <1.5 ml (Jungwirth et al. 2016). Prostate midline and ejaculatory duct cysts or calcifications are anomalies often associated with obstructive azoospermia (Jungwirth et al. 2016). In addition, scrotal US is helpful in finding other signs of obstruction (e.g., dilatation of rete testis, enlarged epididymis) (Jungwirth et al. 2016).
Transrectal and scrotal US are useful in detecting CBAVD or CUAVD (EAU Guidelines on Male Infertility; Jungwirth et al. 2016), suggesting more specific examinations (CFTR gene evaluation, urinary tract evaluation by US, and surgical sperm extraction) (see Forti and Krausz 1998; Lotti et al. 2012b). TRUS may detect SV cysts, usually associated with other genito-urinary abnormalities (Patel et al. 2002) and should prompt other investigations.
Scrotal US offers a greater accuracy in TV evaluation than assessment by Prader orchidometer (Lenz et al. 1993; Sakamoto et al. 2007a, b), but Prader- and US-derived TV are closely related (Goede et al. 2011; Rastrelli et al. 2013); hence TV assessed clinically is sufficient for the management of infertile men in everyday clinical practice. However, scrotal US is useful in evaluating testicular characteristics when physical examination is unreliable (Behre et al. 1989; Sakamoto et al. 2006; Behre and Zitzmann 2010), such as in the case of large hydrocele, inguinal testis, epididymal enlargement/fibrosis, small testes, and thickened scrotal skin.
Scrotal US is able to detect findings suggestive of testicular damage in non-obstructive azoospermia (Abdulwahed et al. 2013); however, it is not predictive of sperm retrieval in spermatogenic arrest-associated non-obstructive azoospermia (Hung et al. 2007).
Scrotal US is useful in assessing or monitoring signs of testicular dysgenesis (i.e., inhomogeneous testicular echo-pattern, microcalcifications, cryptorchid testis, and suspected small lesions) (Christensen and Dogra 2007; Dohle et al. 2012; Jungwirth et al. 2016) or testicular abnormalities resembling malignancy (Isidori and Lenzi 2008; Albers et al. 2013).
CDUS is useful in localizing inguinal (Nijs et al. 2007) but not intra-abdominal testes (Tasian and Copp 2011), and its role is debated in preoperative planning (Christensen and Dogra 2007; Ozden et al. 2012).
MGT-CDUS shows poor utility in surgical sperm extraction decision-making, since the latter is performed even when small testes or karyotype abnormalities are found (Dabaja and Schlegel 2013; Bryson et al. 2014).
Physical examination may be considered sufficient for diagnosis and decision-making in varicocele treatment (AUA/ASRM; see Practice Committee of American Society for Reproductive Medicine 2008); however, EAU Guidelines on Male Infertility (Jungwirth et al. 2016) report that “the diagnosis of varicocele should be confirmed by CDUS.” In particular, scrotal CDUS plays a role when physical examination is unreliable, exploring persistent reflux in clinical and subclinical varicocele, and is useful in evaluating venous reflux recurrence/persistence after surgery (see Lee et al. 2008).
New Imaging Techniques for the Evaluation of Testis and Prostate Abnormalities
Contrast-Enhanced Ultrasound (CEUS)
Nowadays, contrast-enhanced ultrasonography (CEUS) is a significant advancement in imaging, although with a still limited clinical diffusion (Piscaglia et al. 2012). CEUS involves the use of microbubble contrast agents and specialized imaging techniques to show sensitive blood flow and tissue perfusion information. It provides an effective evaluation of microvascularization, a parameter that aids characterization of lesions and depiction of tumor angiogenesis (Cantisani et al. 2015). CEUS is a safe and easily performed technique with no requirement for ionizing radiation and no risk of nephrotoxicity. The main indication for CEUS in testicular pathology assessment is differential diagnosis of testicular lesions. Differential diagnosis between hypovascular and avascular lesions, for instance, may be difficult at CDUS assessment, especially for small lesions. CEUS is extremely effective in assessing the presence or lack of lesion perfusion (Cantisani et al. 2015). However, while CEUS is valuable to distinguish between vascularized and avascular testicular lesions, characterization of the former as benign or malignant is challenging. Only few studies are dealing with use of CEUS for characterization of testicular nodules. In most of cases, both benign and malignant tumors enhance strongly (Cantisani et al. 2015). Analysis of time-intensity curves after bolus microbubble injection may be of help. A prospective study, performed on 115 consecutive small nonpalpable lesions (<15 mm), reported that the rapidity of washin and washout was the parameter that better differentiated malignant from benign tumors, with a typical prolonged washout observed in Leydig cell tumors (Isidori et al. 2014). However, a recent multicenter study (Drudi et al. 2016), investigating 14 Leydig cell tumors and 17 seminomas, reported that Leydig cell tumors had earlier and higher enhancement compared to seminoma but washout characteristics did not differ significantly. Hence, although CEUS shows high accuracy in distinguishing vascularized and avascular testis lesions, further studies with larger series of patients are required to validate differentiation between benign and malignant tumors, particularly Leydig cell tumor and seminoma (Cantisani et al. 2015).
Other applications of CEUS in testis imaging are related to differential diagnosis of testicular torsion, areas of infarction and abscess, extent of hematomas, and hemorrhagic areas within the testis in patients with scrotal trauma (see, for review, Cantisani et al. 2015).
While there is an increasing interest in the use of CEUS in testicular pathology, its application in prostatic abnormalities seems less promising. Several studies investigated the potential of CEUS to identify prostate cancer (see Cantisani et al. 2015; Sano and Uemura 2015). Although the initial results were encouraging (Mitterberger et al. 2010), other studies did not confirm these results (Seitz et al. 2011; Taverna et al. 2011). Thus, in patients with prostate cancer or with possible recurrence after treatment, CEUS remains an interesting topic for research but cannot be recommended for routine clinical use (Cantisani et al. 2015). Other suggested uses of CEUS in prostate assessment include the study of hemodynamics in response to medical treatment in patients with BPH (Bertolotto et al. 2009), although its utility must be confirmed by further studies.
Sonographic Elastography (SE)
Sonographic elastography (SE) is a method to assess the mechanical properties of a tissue, by applying stress and detecting tissue displacement using US. There are several SE techniques used in clinical practice; strain (compression) SE is the most common technique that allows real-time visualization of the elastographic map on the screen. SE outputs include a color-coded representation (qualitative assessment) of the lesion, and a semiquantitative characterization by strain ratio, calculated as the ratio of the surrounding parenchyma to the lesions, providing a measure of lesion stiffness. A color scale from red to blue characterizes the color-coded elastograms. Red indicates the highest elastic strain (softest tissue), and blue indicates no strain (hardest tissue). Available scales have been published (Patel et al. 2012), including a 5-point scale by Itoh et al. (2006) at first used for qualitative assessment of breast and thyroid nodules.
SE has been successfully used in the evaluation of acute scrotal pathology (Patel et al. 2014; Yusuf et al. 2015). However, to date, only few retrospective (Schurich et al. 2009; Grasso et al. 2010; Aigner et al. 2012; Huang and Sidhu 2012; Patel et al. 2012) and one prospective (Goddi et al. 2012) study explored the role of SE in focal testicular lesions. In particular, only one retrospective study assessed the value of strain ratio measurements in assessing neoplastic testicular lesions (Pastore et al. 2014). A recent study (Pozza et al. 2016) evaluated prospectively the accuracy of qualitative and strain ratio SE in the differential diagnosis of 106 nonpalpable testicular lesions. The authors concluded that testicular SE may support conventional US in identifying nonneoplastic lesions when findings are controversial, but its additional benefit in clinical practice remains to be proven. In particular, SE could be helpful in differentiating nonpalpable testicular malignancies from nonneoplastic lesions in challenging cases, but it cannot be used to discriminate benign from malignant neoplasms (Pozza et al. 2016).
SE also provides additional information for detecting prostate cancer and guiding biopsies (Correas et al. 2013). SE has high sensitivity in prostate cancer detection and shows high negative predictive values, ensuring that only few cancers will be missed (Correas et al. 2013). SE should become an additional method of imaging the prostate, complementing the conventional TRUS and MRI. Currently there is not enough evidence for SE routine use (Mottet et al. 2016).
Magnetic Resonance Imaging (MRI)
MRI can be useful as a problem-solving tool when traditional US findings are equivocal. MRI allows characterization of scrotal masses as intratesticular or extratesticular and can demonstrate various types of lesions and tissue, including cysts or fluid, solid masses, fat, and fibrosis, as well as inflammatory or vascular abnormalities (Kim et al. 2007; Tsili et al. 2014). Gadolinium-enhanced MRI can help differentiating a benign cystic lesion and a cystic neoplasm. In addition, it can also be used to demonstrate areas of absent or reduced testicular perfusion, such as in segmental testicular infarct (Kim et al. 2007). Finally, MRI can demonstrate an intraabdominal undescended testis, which can be difficult to detect with US, and is superior to US in differentiation between an undescended testis and testicular agenesis (Kim et al. 2007).
MRI provides a precise imaging diagnosis of SV defect, which is considered superior to the TRUS examination for the patients with CAVD (Chiang et al. 2013).
The primary indication at undergoing MRI of the prostate is the evaluation of prostate cancer after a TRUS-guided prostate biopsy has confirmed cancer, in order to determine if there is extracapsular extension (Sala et al. 2006; Hricak et al. 2007). Both the American College of Radiology (ACR) and European Society of Uroradiology (ESUR) advocate the use of multiparametric MRI in prostate imaging (Quon et al. 2015). MRI can also be used to detect and localize cancer when the PSA is persistently elevated, but routine TRUS biopsy is negative (Roethke et al. 2012). Following radical prostatectomy, patients with elevated PSA should also be examined using MRI.
Conclusions
Nowadays, scrotal and transrectal imaging of the MGT have greatly helped in assessing anatomy and physiology of MGT and pathology such as male infertility, MGT lesions, and inflammation. However, MGT-CDUS still suffers from a lack of standardization and often tends to produce subjective and vague diagnoses. This is the main reason why the European Academy of Andrology promoted an ongoing multicenter study (see at http://www.andrologyacademy.net/studies.aspx) aimed at defining the anatomic and functional characteristic of healthy, fertile men, defined as partners of a pregnant woman in the second or third trimester of pregnancy or who fathered a child during the last year, following natural conception. Defining US parameters of a healthy and fertile cohort will be of great help in establishing criteria for its pathological counterpart. Finally, new imaging techniques, such as CEUS, elastography, and MRI, have improved the characterization of MGT lesions. However, currently, there is not enough evidence for their routine use, with the exception of some specific indications.
References
Abdulwahed SR, Mohamed EE, Taha EA, Saleh MA, Abdelsalam YM, ElGanainy EO. Sensitivity and specificity of ultrasonography in predicting etiology of azoospermia. Urology. 2013;81:967–71.
Aigner F, De Zordo T, Pallwein-Prettner L, Junker D, Schafer G, Pichler R, Leonhartsberger N, Pinggera G, Dogra VS, Frauscher F. Real-time sonoelastography for the evaluation of testicular lesions. Radiology. 2012;263:584–9.
Aizenstein RI, DiDomenico D, Wilbur AC, O'Neil HK. Testicular microlithiasis: association with male infertility. J Clin Ultrasound. 1998;26:195–8.
Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP. Guidelines on testicular cancer. European Association of Urology Guidelines. In: EAU Guidelines Office. The Netherlands: Arnhem; 2013.
American Institute of Ultrasound, Ahmed AF, Abdel-Aziz AS, Maarouf AM, Ali M, Emara AA, Gomaa A. Impact of varicocelectomy on premature ejaculation in varicocele patients. Andrologia. 2014 Mar 10.; Epub ahead of print.
Ammar T, Sidhu PS, Wilkins CJ. Male infertility: the role of imaging in diagnosis and management. Br J Radiol. 2012;85:S59–68.
Andersson AM, Petersen JH, Jørgensen N, Jensen TK, Skakkebaek NE. Serum inhibin B and follicle-stimulating hormone levels as tools in the evaluation of infertile men: significance of adequate reference values from proven fertile men. J Clin Endocrinol Metab. 2004;89:2873–9.
Appelbaum L, Gaitini D, Dogra VS. Scrotal ultrasound in adults. Semin Ultrasound CT MR. 2013;34:257–73.
Arai T, Kitahara S, Horiuchi S, Sumi S, Yoshida K. Relationship of testicular volume to semen profiles and serum hormone concentrations in infertile Japanese males. Int J Fertil Womens Med. 1998;43:40–7.
Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, Salonia A, Weidner W, Zini A. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808.
Bahk JY, Jung JH, Jin LM, Min SK. Cut-off value of testes volume in young adults and correlation among testes volume, body mass index, hormonal level, and seminal profiles. Urology. 2010;75:1318–23.
Baker HWG, Burger HG, de Kretser DM, Hudson B, O’Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC. Changes in the pituitary-testicular system with age. Clin Endocrinol. 1976;5:349–72.
Battaglia C, Giulini S, Regnani G, Madgar I, Facchinetti F, Volpe A. Intratesticular Doppler flow, seminal plasma nitrites/nitrates, and nonobstructive sperm extraction from patients with obstructive and nonobstructive azoospermia. Fertil Steril. 2001;75:1088–94.
Behre HM, Nashan D, Nieschlag E. Objective measurement of testicular volume by ultrasonography: evaluation of the technique and comparison with orchidometer estimates. Int J Androl. 1989;12:395–403.
Behre HM, Kliesch S, Schädel F, Nieschlag E. Clinical relevance of scrotal and transrectal ultrasonography in andrological patients. Int J Androl. 1995;18:27–31.
Behre HM, Zitzmann M. Imaging diagnostics. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology. Male reproductive health and dysfunction, 3rd Ed. Berlin: Springer Verlag; 2010. Chapter 6, p. 101–107.
Béres J, Papp G, Pazonyi I, Czeizel E. Testicular volume variations from 0 to 28 years of age. Int Urol Nephrol. 1989;21:159–67.
Berger AP, Horninger W, Bektic J, Pelzer A, Spranger R, Bartsch G, Frauscher F. Vascular resistance in the prostate evaluated by colour Doppler ultrasonography: is benign prostatic hyperplasia a vascular disease? BJU Int. 2006;98(3):587–90.
Berkovitz GD, Wilson DP, Carpenter NJ, Brown TR, Migeon CJ. Gonadal function in men with the Martin-Bell (fragile-X) syndrome. Am J Med Genet. 1986;23:227–39.
Bertolotto M, Trincia E, Zappetti R, Bernich R, Savoca G, Cova MA. Effect of Tadalafil on prostate haemodynamics: preliminary evaluation with contrastenhanced US. Radiat Med. 2009;114:1106–14.
Biagiotti G, Cavallini G, Modenini F, Vitali G, Gianaroli L. Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int. 2002a;90:903–8.
Biagiotti G, Vitali G, Cavallini G. Dyspermia and testicular artery peak systolic velocity. Arch Ital Urol Androl. 2002b;74:243–6.
Boitrelle F, Robin G, Marcelli F, Albert M, Leroy-Martin B, Dewailly D, Rigot JM, Mitchell V. A predictive score for testicular sperm extraction quality and surgical ICSI outcome in non-obstructive azoospermia: a retrospective study. Hum Reprod. 2011;26:3215–21.
Bryson CF, Ramasamy R, Sheehan M, Palermo GD, Rosenwaks Z, Schlegel PN. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol. 2014;191:175–8.
Bujan L, Mieusset R, Mansat A, Moatti JP, Mondinat C, Pontonnier F. Testicular size in infertile men: relationship to semen characteristics and hormonal blood levels. Br J Urol. 1989;64:632–7.
Canale D, Mais V, Turchi P, Andreini F, Melis GB, Menchini-Fabris GF. Ultrasound monitoring of testis and prostate maturation in hypogonadotropic hypogonadic males during gonadotropin-releasing hormone treatment. Fertil Steril. 1990;53:537–40.
Canales BK, Zapzalka DM, Ercole CJ, Carey P, Haus E, Aeppli D, Pryor JL. Prevalence and effect of varicoceles in an elderly population. Urology. 2005;66:627–31.
Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, Drudi FM, Malpassini F, Isidori A, Meloni FM, Calliada F, D'Ambrosio F. Growing indications for CEUS: the kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84:1675–84.
Cantu JM, Scaglia HE, Medina M, Gonzalez-Diddi M, Moranto T, Moreno ME, Perez-Palacios G. Inherited congenital normofunctional testicular hyperplasia and mental deficiency. Hum Genet. 1976;33:23–33.
Carlsen E, Andersen AG, Buchreitz L, Jørgensen N, Magnus O, Matulevicuus V, Nermoen I, Petersen JH, Punab M, Suominen J, Zilaitiene B, Giwercman A. Inter-observer variation in the results of the clinical andrological examination including estimation of testicular size. Int J Androl. 2000;23:248–53.
Carter SS, Shinohara K, Lipshultz LI. Transrectal ultrasonography in disorders of the seminal vesicles and ejaculatory ducts. Urol Clin N Am. 1989;16:773–90.
Cayan S, Woodhouse CR. The treatment of adolescents presenting with a varicocele. BJU Int. 2007;100:744–7.
Chiang HS, Lin YH, Wu YN, Wu CC, Liu MC, Lin CM. Advantages of magnetic resonance imaging (MRI) of the seminal vesicles and intra-abdominal vas deferens in patients with congenital absence of the vas deferens. Urology. 2013;82(2):345–51.
Cho IR, Keener TS, Nghiem HV, Winter T, Krieger JN. Prostate blood flow characteristics in the chronic prostatitis/pelvic pain syndrome. J Urol. 2000;163:1130–3.
Cho SH, Min SK, Lee ST. Associations of ultrasonographic features with scrotal pain after vasectomy. Korean J Urol. 2011;52:782–6.
Christiansen E, Purvis K. Diagnosis of chronic abacterial prostato-vesiculitis by rectal ultrasonography in relation to symptoms and findings. Br J Urol. 1991;67:173–6.
Christensen JD, Dogra VS. The undescended testis. Semin Ultrasound CT MR. 2007;28:307–16.
Cina A, Minnetti M, Pirronti T, Vittoria Spampinato M, Canadè A, Oliva G, Ribatti D, Bonomo L. Sonographic quantitative evaluation of scrotal veins in healthy subjects: normative values and implications for the diagnosis of varicocele. Eur Urol. 2006;50:345–50.
Clyne M. Male factor infertility: Asymptomatic testicular microlithiasis does not increase antisperm antibody production in infertile men. Nat Rev Urol. 2012. doi:10.1038/nrurol.2012.179. [Epub ahead of print].
Coley BD. Sonography of pediatric scrotal swelling. Semin Ultrasound CT MR. 2007;28(4):297–306.
Collins GN, Raab GM, Hehir M, King B, Garraway WM. Reproducibility and observer variability of transrectal ultrasound measurements of prostatic volume. Ultrasound Med Biol. 1995;21:1101–5.
Colpi GM, Negri L, Nappi RE, Chinea B. Is transrectal ultrasonography a reliable diagnostic approach in ejaculatory duct sub-obstruction? Hum Reprod. 1997;12:2186–91.
Condorelli R, Calogero AE, La Vignera S. Relationship between Testicular Volume and Conventional or Nonconventional Sperm Parameters. Int J Endocrinol. 2013;2013:145792. doi:10.1155/2013/145792. Epub 2013 Sep 5.
Cooper TG. Semen analysis. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology. Male reproductive health and dysfunction, 3rd Ed. Berlin: Springer Verlag. 2010; p. 125–138.
Cornud F, Belin X, Delafontaine D, Amar T, Hélénon O, Moreau JF. Imaging of obstructive azoospermia. Eur Radiol. 1997;7:1079–85.
Cornud F, Belin X, Amar E, Delafontaine D, Hélénon O, Moreau JF. Varicocele: strategies in diagnosis and treatment. Eur Radiol. 1999;9:536–45.
Correas JM, Tissier AM, Khairoune A, Khoury G, Eiss D, Hélénon O. Ultrasound elastography of the prostate: state of the art. Diagn Interv Imaging. 2013;94(5):551–60.
Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl. 2013;15:35–9.
Danaci M, Akpolat T, Baştemir M, Sarikaya S, Akan H, Selçuk MB, Cengiz K. The prevalence of seminal vesicle cysts in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1998;13:2825–8.
Degl’Innocenti S, Filimberti E, Magini A, Krausz C, Lombardi G, Fino MG, Rastrelli G, Maggi M, Baldi E. Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: prediction of post-thaw outcome using basal semen quality. Fertil Steril 10 Sep 2013. doi:10.1016/j.fertnstert.2013.08.005. [Epub ahead of print]
Dhabuwala CB, Kumar AB, Kerkar PD, Bhutawala A, Pierce J. Patterns of Doppler recordings and its relationship to varicocele in infertile men. Int J Androl. 1989;12:430–8.
Di Trapani D, Pavone C, Serretta V, Cavallo N, Costa G, Pavone-Macaluso M. Chronic prostatitis and prostatodynia: ultrasonographic alterations of the prostate, bladder neck, seminal vesicles and periprostatic venous plexus. Eur Urol. 1988;15:230–4.
Diamond JM. Ethnic differences. Variation in human testis size. Nature. 1986;320:488–9.
Diamond DA, Paltiel HJ, DiCanzio J, Zurakowski D, Bauer SB, Atala A, Ephraim PL, Grant R, Retik AB. Comparative assessment of pediatric testicular volume: orchidometer versus ultrasound. J Urol. 2000;164:1111–4.
Doble A, Carter SS. Ultrasonographic findings in prostatitis. Urol Clin N Am. 1989;16:763–72.
Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227:18–36.
Dohle GR. Inflammatory-associated obstructions of the male reproductive tract. Andrologia. 2003;35:321–4.
Dohle GR, Elzanaty S, van Casteren NJ. Testicular biopsy: clinical practice and interpretation. Asian J Androl. 2012;14:88–93.
Donkol RH. Imaging in male-factor obstructive infertility. World J Radiol. 2010;2:172–9.
Drudi FM, Valentino M, Bertolotto M, Malpassini F, Maghella F, Cantisani V, Liberatore M, De Felice C, D'Ambrosio F. CEUS time intensity curves in the differentiation between leydig cell carcinoma and seminoma: a multicenter study. Ultraschall Med. 2016;37(2):201–5.
Du J, Li FH, Guo YF, Yang LM, Zheng JF, Chen B, Zhu JS, Liu Q. Differential diagnosis of azoospermia and etiologic classification of obstructive azoospermia: role of scrotal and transrectal US. Radiology. 2010;256:493–503.
Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–9.
Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril. 2013;100:681–5.
Ekerhovd E, Westlander G. Testicular sonography in men with Klinefelter syndrome shows irregular echogenicity and blood flow of high resistance. J Assist Reprod Genet. 2002;19:517–22.
El-Assmy A, El-Tholoth H, Abouelkheir RT, Abou-El-Ghar ME. Transurethral resection of ejaculatory duct in infertile men: outcome and predictors of success. Int Urol Nephrol. 2012;44:1623–30.
Elzinga-Tinke JE, Sirre ME, Looijenga LH, van Casteren N, Wildhagen MF, Dohle GR. The predictive value of testicular ultrasound abnormalities for carcinoma in situ of the testis in men at risk for testicular cancer. Int J Androl. 2010;33:597–603.
Engeler D, Baranowski AP, Borovicka J, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, de C Williams AC, Guidelines Associates, Cottrell A, Goonewardene S. EAU guidelines on chronic pelvic pain. In: EAU Guidelines Office. The Netherlands: Arnhem; 2016.
Engin G. Transrectal US-guided seminal vesicle aspiration in the diagnosis of partial ejaculatory duct obstruction. Diagn Interv Radiol. 2012;18:488–95.
Engin G, Kadioğlu A, Orhan I, Akdöl S, Rozanes I. Transrectal US and endorectal MR imaging in partial and complete obstruction of the seminal duct system. A comparative study. Acta Radiol. 2000;41:288–95.
Fisch H, Kang YM, Johnson CW, Goluboff ET. Ejaculatory duct obstruction. Curr Opin Urol. 2002;12:509–15.
Fisher AD, Rastrelli G, Bandini E, Corona G, Balzi D, Melani C, Monami M, Matta V, Mannucci E, Maggi M. Metabolic and cardiovascular outcomes of fatherhood: results from a cohort of study in subjects with sexual dysfunction. J Sex Med. 2012;9:2785–94.
Foresta C, Garolla A, Bettella A, Ferlin A, Rossato M, Candiani F. Doppler ultrasound of the testis in azoospermic subjects as a parameter of testicular function. Hum Reprod. 1998;13:3090–3.
Foresta C, Garolla A, Frigo AC, Carraro U, Isidori AM, Lenzi A, Ferlin A. Anthropometric, penile and testis measures in post-pubertal Italian males. J Endocrinol Investig. 2013;36:287–92.
Forti G, Krausz C. Clinical review 100: evaluation and treatment of the infertile couple. J Clin Endocrinol Metab. 1998;83:4177–88.
Forti G, Krausz C, Cilotti A, Maggi M. Varicocele and infertility. J Endocrinol Investig. 2003;26:564–9.
Friedler S, Raziel A, Strassburger D, Soffer Y, Komarovsky D, Ron-El R. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia. Hum Reprod. 1997;12:1448–93.
Garriga V, Serrano A, Marin A, Medrano S, Roson N, Pruna X. US of the tunica vaginalis testis: anatomic relationships and pathologic conditions. Radiographics. 2009;29:2017–32.
Gat Y, Gornish M, Heiblum M, Joshua S. Reversal of benign prostate hyperplasia by selective occlusion of impaired venous drainage in the male reproductive system: novel mechanism, new treatment. Andrologia. 2008;40:273–81.
Geatti O, Gasparini D, Shapiro B. A comparison of scintigraphy, thermography, ultrasound and phlebography in grading of clinical varicocele. J Nucl Med. 1991;32:2092–7.
Goddi A, Sacchi A, Magistretti G, Almolla J, Salvadore M. Real-time tissue elastography for testicular lesion assessment. Eur Radiol. 2012;22:721–30.
Goede J, Hack WW, Sijstermans K, van der Voort-Doedens LM, Van der Ploeg T, Meij-de Vries A, Delemarre-van de Waal HA. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr. 2011;76:56–64.
Grabe M, Bjerklund-Johansen TE, Botto H, Çek M, Naber KG, Pickard RS, Tenke P, Wagenlehner F, Wullt B. Guidelines on urological infections, European Association of Urology Guidelines. In: EAU Guidelines Office. The Netherlands: Arnhem; 2013.
Grasso M, Blanco S, Raber M, Nespoli L. Elasto-sonography of the testis: preliminary experience. Arch Ital Urol Androl. 2010;82:160–3.
Haidl G, Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia. 2008;40:92–6.
Hamm B, Fobbe F. Maturation of the testis: ultrasound evaluation. Ultrasound Med Biol. 1995;21:143–7.
Handelsman DJ, Staraj S. Testicular size: the effects of aging, malnutrition, and illness. J Androl. 1985;6:144–51.
Handelsman DJ, Conway AJ, Boylan LM, Turtle JR. Testicular function in potential sperm donors: normal ranges and the effects of smoking and varicocele. Int J Androl. 1984;7:369–82.
Harman SM, Tsitsouras PD. Reproductive hormones in aging men: I. Measurement of sex steroids, basal luteinizing hormone and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab. 1980;51:35–40.
Herwig R, Tosun K, Schuster A, Rehder P, Glodny B, Wildt L, Illmensee K, Pinggera GM. Tissue perfusion-controlled guided biopsies are essential for the outcome of testicular sperm extraction. Fertil Steril. 2007;87:1071–6.
Hillelsohn JH, Chuang KW, Goldenberg E, Gilbert BR. Spectral Doppler sonography: a noninvasive method for predicting dyspermia. J Ultrasound Med. 2013;32:1427–32.
Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP. The Doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Br J Urol. 1980;52:50–6.
Hoekstra T, Witt MA. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol. 1995;153:82–4.
Horstman WG, Middleton WD, Melson GL, Siegel BA. Color Doppler US of the scrotum. Radiographics. 1991a;11:941–57.
Horstman WG, Middleton WD, Melson GL. Scrotal inflammatory disease: colorDoppler US findings. Radiology. 1991b;179:55–9.
Hotaling JM, Lopushnyan NA, Davenport M, Christensen H, Pagel ER, Muller CH, Walsh TJ. Raw and test-thaw semen parameters after cryopreservation among men with newly diagnosed cancer. Fertil Steril. 2013;99:464–9.
Hricak H, Choyke PL, Eberhardt SC, et al. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243(1):28–53.
Huang DY, Sidhu PS. Focal testicular lesions: colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis. Br J Radiol. 2012;85:S41–53.
Huang Foen Chung JW, de Vries SH, Raaijmakers R, Postma R, Bosch JL, van Mastrigt R. Prostate volume ultrasonography: the influence of transabdominal versus transrectal approach, device type and operator. Eur Urol. 2004;46(3):352–6.
Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178:608–12.
Hutson JM, Balic A, Nation T, Southwell B. Cryptorchidism. Semin Pediatr Surg. 2010;19:215–24.
Hutson JM, Li R, Southwell BR, Newgreen D, Cousinery M. Regulation of testicular descent. Pediatr Surg Int. 2015;31:317–25.
Iaccarino V, Venetucci P. Interventional radiology of male varicocele: current status. Cardiovasc Intervent Radiol. 2012;35:1263–80.
Iosa G, Lazzarini D. Hemodynamic classification of varicoceles in men: our experience. J Ultrasound. 2013;16:57–63.
Isidori AM, Lenzi A. Scrotal CDU: morphological and functional atlas. Genova: Forum Service Editore s.r.l; 2008.
Isidori AM, Pozza C, Gianfrilli D, Giannetta E, Lemma A, Pofi R, Barbagallo F, Manganaro L, Martino G, Lombardo F, Cantisani V, Franco G, Lenzi A. Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology. 2014;273(2):606–18.
Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50.
Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, Moller H. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–92.
Jarow JP. Transrectal ultrasonography of infertile men. Fertil Steril. 1993;60:1035–9.
Jarvis LJ, Dubbins PA. Changes in the epididymis after vasectomy: sonographic findings. AJR Am J Roentgenol. 1989;152:531–4.
Jensen TK, Jørgensen N, Punab M, Haugen TB, Suominen J, Zilaitiene B, Horte A, Andersen AG, Carlsen E, Magnus Ø, Matulevicius V, Nermoen I, Vierula M, Keiding N, Toppari J, Skakkebaek NE. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol. 2004;159:49–58.
Jiang H, Zhu WJ. Testicular microlithiasis is not a risk factor for the production of antisperm antibody in infertile males. Andrologia. 2013;45:305–9.
Jin B, Conway AJ, Handelsman DJ. Effects of androgen deficiency and replacement on prostate zonal volumes. Clin Endocrinol. 2001;54:437–45.
Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, Andersson AM, Haugen TB, Horte A, Jensen TK, Magnus Ø, Petersen JH, Vierula M, Toppari J, Skakkebaek NE. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod. 2002;17:2199–208.
Joustra SD, van der Plas EM, Goede J, Oostdijk W, Delemarre-van de Waal HA, Hack WW, van Buuren S, Wit JM. New reference charts for testicular volume in Dutch children and adolescents allow the calculation of standard deviation scores. Acta Paediatr. 2015;104(6):e271–8.
Jungwirth A, Diemer T, Dohle GR, Kopa Z, Krausz C, Tournaye H. EAU guidelines on male infertility. In: EAU Guidelines Office. The Netherlands: Arnhem; 2016.
Kamischke A, Behre HM, Bergmann M, Simoni M, Schäfer T, Nieschlag E. Recombinant human follicle stimulating hormone for treatment of male idiopathic infertility: a randomized, double-blind, placebo-controlled, clinical trial. Hum Reprod. 1998;13:596–603.
Kamoi K. Clinical usefulness of transrectal sonography and transperineal color Doppler flow imaging in the diagnosis of intrapelvic venous congestion syndrome. Nihon Hinyokika Gakkai Zasshi. 1996;87:1009–17. [Article in Japanese].
Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–9.
Keener TS, Winter TC, Nghiem HV, Schmiedl UP. Normal adult epididymis: evaluation with color Doppler US. Radiology. 1997;202:712–4.
Kim ED, Lipshultz LI. Role of ultrasound in the assessment of male infertility. J Clin Ultrasound. 1996;24:437–53.
Kim W, Rosen MA, Langer JE, et al. US-MR Imaging correlation in pathologic conditions of the scrotum. Radiographics. 2007;27:1239–53.
Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP, King Jr BF. Imaging of the seminal vesicle and vas deferens. Radiographics. 2009;29:1105–21.
King BF, Hattery RR, Lieber MM, Williamson Jr B, Hartman GW, Berquist TH. Seminal vesicle imaging. Radiographics. 1989;9:653–76.
Kollin C, Hesser U, Ritzen EM, Karpe B. Testicular growth from birth to two years of age, and the effect of orchidopexy at age nine months: a randomized, controlled study. Acta Paediatr. 2006;95:318–24.
Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25:271–85.
Kroese AC, de Lange NM, Collins JA, Evers JL. Varicocele surgery, new evidence. Hum Reprod Update. 2013;19:317.
Kuijper EA, van Kooten J, Verbeke JI, van Rooijen M, Lambalk CB. Ultrasonographically measured testicular volumes in 0- to 6-year-old boys. Hum Reprod. 2008;23:792–6.
La Vignera S. Seminal vesicles of infertile patients with male accessory gland infection: ultrasound evaluation after prolonged treatment with tadalafil, a selective phosphodiesterase-5 inhibitor. Andrologia. 2012. doi:10.1111/and.12027. [Epub ahead of print].
La Vignera S, Calogero AE, Arancio A, Castiglione R, De Grande G, Vicari E. Transrectal ultrasonography in infertile patients with persistently elevated bacteriospermia. Asian J Androl. 2008;10:731–40.
La Vignera S, Lanzafame F, Di Mauro M, Condorelli R, Vicari E. Spermatic and ultrasound characterization of young diabetic patients. Arch Ital Urol Androl. 2009;81:245–7.
La Vignera S, Condorelli RA, Di Mauro M, D’Agata R, Vicari E, Calogero AE. Seminal vesicles and diabetic neuropathy: ultrasound evaluation. J Androl. 2011a;32:478–83.
La Vignera S, Condorelli RA, Vicari E, D’Agata R, Calogero AE. Seminal vesicles and diabetic neuropathy: ultrasound evaluation in patients with couple infertility and different levels of glycaemic control. Asian J Androl. 2011b;13:872–6.
La Vignera S, Vicari E, Condorelli R, D'Agata R, Calogero AE. Hypertrophic-congestive and fibro-sclerotic ultrasound variants of male accessory gland infection have different sperm output. J Endocrinol Investig. 2011c;34:e330–5.
La Vignera S, Vicari E, Condorelli R, D’Agata R, Calogero AE. Ultrasound characterization of the seminal vesicles in infertile patients with type 2 diabetes mellitus. Eur J Radiol. 2011d;80:e64–7.
La Vignera S, Vicari E, Condorelli RA, D'Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review). Int J Androl. 2011e;34:e330–47.
Lachiewicz AM, Dawson DV. Do young boys with fragile X syndrome have macroorchidism? Pediatrics. 1994;93:992–5.
Lee JC, Bhatt S, Dogra VS. Imaging of the epididymis. Ultrasound Q. 2008;24:3–16.
Lenz S. Cancer of the testicle diagnosed by ultrasound and the ultrasonic appearance of the contralateral testicle. Scand J Urol Nephrol Suppl. 1991;137:135–8.
Lenz S, Giwercman A, Skakkebaek NE, Bruun E, Frimodt-Møller C. Ultrasound in detection of early neoplasia of the testis. Int J Androl. 1987;10:187–90.
Lenz S, Giwercman A, Elsborg A, Cohr KH, Jelnes JE, Carlsen E, Skakkebaek NE. Ultrasonic testicular texture and size in 444 men from the general population: correlation to semen quality. Eur Urol. 1993;24:231–8.
Lenz S, Thomsen JK, Giwercman A, Hertel NT, Hertz J, Skakkebaek NE. Ultrasonic texture and volume of testicles in infertile men. Hum Reprod. 1994;9:878–81.
Leung ML, Gooding GA, Williams RD. High-resolution sonography of scrotal contents in asymptomatic subjects. AJR Am J Roentgenol. 1984;143:161–4.
Li F, Yue H, Yamaguchi K, Okada K, Matsushita K, Ando M, Chiba K, Fujisawa M. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol. 2012;19:149–54.
Liguori G, Trombetta C, Garaffa G, Bucci S, Gattuccio I, Salamè L, Belgrano E. Color Doppler ultrasound investigation of varicocele. World J Urol. 2004;22:378–81.
Liguori G, Bucci S, Trombetta C, Boris B, Bertolotto M. Imaging the infertile male-1: varicocele. In: Bertolotto M, Trombetta C, editors. Scrotal pathology. Medical radiology. Diagnostic imaging. Berlin: Springer-Verlag; 2012. p. 261–74.
Lin CC, Huang WJ, Chen KK. Measurement of testicular volume in smaller testes: how accurate is the conventional orchidometer? J Androl. 2009;30:685–9.
Littrup PJ, Lee F, McLeary RD, Wu D, Lee A, Kumasaka GH. Transrectal US of the seminal vesicles and ejaculatory ducts: clinical correlation. Radiology. 1988;168:625–8.
Liu PY, Gebski VJ, Turner L, Conway AJ, Wishart SM, Handelsman DJ. Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Hum Reprod. 2002;17:625–33.
Loberant N, Bhatt S, McLennan GT, Dogra VS. Striated appearance of the testes. Ultrasound Q. 2010;26:37–44.
Lotti F, Maggi M. Interleukin 8 and the male genital tract. J Reprod Immunol. 2013;100(1):54–65. Review.
Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21:56–83.
Lotti F, Corona G, Colpi GM, Filimberti E, Degli Innocenti S, Mancini M, Baldi E, Noci I, Forti G, Adorini L, Maggi M. Elevated body mass index correlates with higher seminal plasma interleukin 8 levels and ultrasonographic abnormalities of the prostate in men attending an andrology clinic for infertility. J Endocrinol Investig. 2011a;34:e336–42.
Lotti F, Corona G, Mancini M, Filimberti E, Degli Innocenti S, Colpi GM, Baldi E, Noci I, Forti G, Adorini L, Maggi M. Ultrasonographic and clinical correlates of seminal plasma interleukin-8 levels in patients attending an andrology clinic for infertility. Int J Androl. 2011b;34:600–13.
Lotti F, Corona G, Colpi GM, Filimberti E, Innocenti SD, Mancini M, Baldi E, Noci I, Forti G, Maggi M. Seminal vesicles ultrasound features in a cohort of infertility patients. Hum Reprod. 2012a;27:974–82.
Lotti F, Corona G, Krausz C, Forti G, Maggi M. The infertile male-3: endocrinological evaluation. In: Bertolotto M, Trombetta C, editors. Scrotal pathology. Medical radiology. Diagnostic imaging. Berlin: Springer-Verlag; 2012b. p. 223–40.
Lotti F, Corona G, Rastrelli G, Forti G, Jannini EA, Maggi M. Clinical correlates of erectile dysfunction and premature ejaculation in men with couple infertility. J Sex Med. 2012c;9:2698–707.
Lotti F, Tamburrino L, Marchiani S, Muratori M, Corona G, Fino MG, Degl'Innocenti S, Forti G, Maggi M, Baldi E. Semen apoptotic M540 body levels correlate with testis abnormalities: a study in a cohort of infertile subjects. Hum Reprod. 2012d;27:3393–402.
Lotti F, Corona G, Degli Innocenti S, Filimberti E, Scognamiglio V, Vignozzi L, Forti G, Maggi M. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013a;1:229–39.
Lotti F, Corona G, Maseroli E, Rossi M, Silverii A, Degl'Innocenti S, Rastrelli G, Forti G, Maggi M. Clinical implications of measuring prolactin levels in males of infertile couples. Andrology. 2013b;1:764–71.
Lotti F, Corona G, Mondaini N, Maseroli E, Rossi M, Filimberti E, Noci I, Forti G, Maggi M. Seminal, clinical and color-Doppler ultrasound correlations of prostatitis-like symptoms in males of infertile couples. Andrology. 2013c;2(1):30–41.
Lotti F, Corona G, Vignozzi V, Rossi M, Maseroli E, Cipriani S, Gacci M, Forti G, Maggi M. Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl. 2013d;16(2):295–304.
Lotti F, Corona G, Vitale P, Maseroli E, Rossi M, Fino MG, Maggi M. Current smoking is associated with lower seminal vesicles and ejaculate volume, despite higher testosterone levels, in male subjects of infertile couples. Hum Reprod. 2015;30(3):590–602.
Lotti F, Corona G, Castellini G, Maseroli E, Fino MG, Cozzolino M, Maggi M. Semen quality impairment is associated with sexual dysfunction according to its severity. Hum Reprod. 2016a;31(12):2668–80.
Lotti F, Maseroli E, Fralassi N, Degl’Innocenti S, Boni L, Baldi E, Maggi M. Is thyroid hormones evaluation of clinical value in the work-up of males of infertile couples? Hum Reprod. 2016b;31(3):518–29.
Lotti F, Tamburrino L, Marchiani S, Maseroli E, Vitale P, Forti G, Muratori M, Maggi M, Baldi E. DNA fragmentation in two cytometric sperm populations: relationship with clinical and ultrasound characteristics of the male genital tract. Asian J Androl. 2016c.; [Epub ahead of print].
Lund L, Roebuck DJ, Lee KH, Sørensen HT, Yeung CK. Clinical assessment after varicocelectomy. Scand J Urol Nephrol. 2000;34:119–22.
Main KM, Schmidt IM, Toppari J, Skakkebaek NE. Early postnatal treatment of hypogonadotropic hypogonadism with recombinant human FSH and LH. Eur J Endocrinol. 2002;146:75–9.
Matsuda T, Horii Y, Yoshida O. Obstructive azoospermia of unknown origin: sites of obstruction and surgical outcomes. J Urol. 1994;151:1543–6.
McDermott VG, Meakem 3rd TJ, Stolpen AH, Schnall MD. Prostatic and periprostatic cysts: findings on MR imaging. Am J Roentgenol. 1995;164:123–7.
Meares Jr EM. Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974;4:560–6.
Meschede D, Behre HM, Nieschlag E. Endocrine and spermatological characteristics of 135 patients with bilateral megalotestis. Andrologia. 1995;27:207–12.
Middleton WD, Thorne DA, Melson GL. Color Doppler ultrasound of the normal testis. AJR Am J Roentgenol. 1989;152:293–7.
Migaleddu V, Virgilio G, Del Prato A, Bertolotto M. Sonographic scrotal anatomy. In: Bertolotto M, Trombetta C, editors. Scrotal pathology. Medical radiology. Diagnostic imaging. Berlin: Springer-Verlag; 2012. p. 41–54.
Mihmanli I, Kantarci F. Sonography of scrotal abnormalities in adults: an update. Diagn Interv Radiol. 2009;15:64–73.
Miller FN, Sidhu PS. Does testicular microlithiasis matter? A review. Clin Radiol. 2002;57:883–90.
Mitchell V, Robin G, Boitrelle F, Massart P, Marchetti C, Marcelli F, Rigot JM. Correlation between testicular sperm extraction outcomes and clinical, endocrine and testicular histology parameters in 120 azoospermic men with normal serum FSH levels. Int J Androl. 2011;34:299–305.
Mitterberger MJ, Aigner F, Horninger W, et al. Comparative efficiency of contrast-enhanced colour Doppler ultrasound targeted versus systematic biopsy for prostate cancer detection. Eur Radiol. 2010;20:2791–6.
Miyagawa Y, Tsujimura A, Matsumiya K, Takao T, Tohda A, Koga M, Takeyama M, Fujioka H, Takada S, Koide T, Okuyama A. Outcome of gonadotropin therapy for male hypogonadotropic hypogonadism at university affiliated male infertility centers: a 30-year retrospective study. J Urol. 2005;173:2072–5.
Montgomery JS, Bloom DA. The diagnosis and management of scrotal masses. Med Clin N Am. 2011;95:235–44.
Moon MH, Kim SH, Cho JY, Seo JT, Chun YK. Scrotal US for evaluation of infertile men with azoospermia. Radiology. 2006;239:168–73.
Mottet N et al., Guidelines on prostate cancer, European Association of Urology Guidelines, 2016.; https://uroweb.org/guideline/prostate-cancer/
Nghiem HT, Kellman GM, Sandberg SA, Craig BM. Cystic lesions of the prostate. Radiographics. 1990;10:635–50.
Nickel JC, Downey J, Hunter D, Clark J. Prevalence of PLS in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001;165:842–5.
Nielsen KB, Tommerup N, Dyggve HV, Schou C. Macroorchidism and fragile X in mentally retarded males. Clinical, cytogenetic, and some hormonal investigations in mentally retarded males, including two with the fragile site at Xq28, fra(X)(q28). Hum Genet. 1982;61:113–7.
Nieschlag E, Behre HM. Anamnesis and physical examination. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology. Male reproductive health and dysfunction, 3rd Ed. Berlin: Springer Verlag; 2010. Chapter 5, p. 93–100.
Nieschlag E, Lammers U, Freischem CW, Langer K, Wickings EJ. Reproductive functions in young fathers and grandfathers. Clin Endocrinol Metab. 1982;55:676–81.
Nijs SM, Eijsbouts SW, Madern GC, Leyman PM, Lequin MH, Hazebroek FW. Nonpalpable testes: is there a relationship between ultrasonographic and operative findings? Pediatr Radiol. 2007;37:374–9.
Nordkap L, Jensen TK, Hansen ÅM, Lassen TH, Bang AK, Joensen UN, Blomberg Jensen M, Skakkebæk NE, Jørgensen N. Psychological stress and testicular function: a cross-sectional study of 1,215 Danish men. Fertil Steril. 2016;105:174–87.e1–2.
Older RA, Watson LR. Ultrasound anatomy of the normal male reproductive tract. J Clin Ultrasound. 1996;24:389–404.
Orda R, Sayfan J, Manor H, Witz E, Sofer Y. Diagnosis of varicocele and postoperative evaluation using inguinal ultrasonography. Ann Surg. 1987;206:99–101.
Oyen RH. Scrotal ultrasound. Eur Radiol. 2002;12:19–34.
Ozden E, Turgut AR, Dogra VS. Imaging the undescended testis. In: Bertolotto M, Trombetta C, editors. Scrotal pathology. Medical radiology. Diagnostic imaging. Berlin: Springer-Verlag; 2012. p. 301–12.
Pais D, Fontoura P, Esperança-Pina JA. The transmediastinal arteries of the human testis: an anatomical study. Surg Radiol Anat. 2004;26:379–83.
Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366:443–53.
Pastore AL, Palleschi G, Maceroni P, Manfredonia G, Autieri D, Cacciotti J, Sardella B, Porta N, Petrozza V, Carbone A. Correlation between semiquantitative sonoelastography and immunohistochemistry in the evaluation of testicular focal lesions. Cancer Imaging. 2014;14:29.
Patel B, Gujral S, Jefferson K, Evans S, Persad R. Seminal vesicle cysts and associated anomalies. BJU Int. 2002;90:265–71.
Patel K, Sellars ME, Clarke JL, Sidhu PS. Features of testicular epidermoid cysts on contrast enhanced sonography and real-time tissue elastography. J Ultrasound Med. 2012;31:115–22.
Patel KV, Huang DY, Sidhu PS. Metachronous bilateral segmental testicular infarction: multiparametric ultrasound imaging with grey-scale ultrasound, Doppler ultrasound, contrast enhanced ultrasound (CEUS) and real-time tissue elastography (RTE). J Ultrasound. 2014;17:233–8.
Pauroso S, Di Leo N, Fulle I, Di Segni M, Alessi S, Maggini E. Varicocele. Ultrasonographic assessment in daily clinical practice. J Ultrasound. 2011;14:199–204.
Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51:524–33.
Pezzella A, Barbonetti A, Micillo A, D’Andrea S, Necozione S, Gandini L, Lenzi A, Francavilla F, Francavilla S. Ultrasonographic determination of caput epididymis diameter is strongly predictive of obstruction in the genital tract in azoospermic men with normal serum FSH. Andrology. 2013;1:133–8.
Pilatz A, Rusz A, Wagenlehner F, Weidner W, Altinkilic B. Reference values for testicular volume, epididymal head size and peak systolic velocity of the testicular artery in adult males measured by ultrasonography. Ultraschall Med. 2013a;34:349–54.
Pilatz A, Wagenlehner F, Bschleipfer T, Schuppe HC, Diemer T, Linn T, Weidner W, Altinkilic B. Acute epididymitis in ultrasound: results of a prospective study with baseline and follow-up investigations in 134 patients. Eur J Radiol. 8 Sept 2013b. doi:10.1016/j.ejrad.2013.08.050. [Epub ahead of print].
Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, Claudon M, Clevert DA, Correas JM, D’Onofrio M, Drudi FM, Eyding J, Giovannini M, Hocke M, Ignee A, Jung EM, Klauser AS, Lassau N, Leen E, Mathis G, Saftoiu A, Seidel G, Sidhu PS, ter Haar G, Timmerman D, Weskott HP. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33(1):33–59.
Pozza C, Gianfrilli D, Fattorini G, et al. Diagnostic value of qualitative and strain-ratio elastography in the differential diagnosis of testicular lesions. Andrology. 2016;6:1193–203.
Practice Committee of American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril. 2008;90:S247–9.
Purvis K, Christiansen E. Infection in the male reproductive tract. Impact, diagnosis and treatment in relation to male infertility. Int J Androl. 1993;16:1–13.
Quon J, Kielar AZ, Jain R, et al. Assessing the utilization of functional imaging in multiparametric prostate MRI in routine clinical practice. Clin Radiol. 2015;70(4):373–8.
Raheem OA. Surgical management of adolescent varicocele: systematic review of the world literature. Urol Ann. 2013;5:133–9.
Ramchandani P, Banner MP, Pollack HM. Imaging of the seminal vesicles. Semin Roentgenol. 1993;28:83–91.
Rastrelli G, Corona G, Lotti F, Boddi V, Mannucci E, Maggi M. Relationship of testis size and LH levels with incidence of major adverse cardiovascular events in older men with sexual dysfunction. J Sex Med. 2013;10:2761–73.
Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2:794–808.
Rathaus V, Werner M, Freud E, Mei-Zahav M, Mussaffi H, Blau H. Sonographic findings of the genital tract in boys with cystic fibrosis. Pediatr Radiol. 2006;36:162–6.
Raviv G, Mor Y, Levron J, Shefi S, Zilberman D, Ramon J, Madgar I. Role of transrectal ultrasonography in the evaluation of azoospermic men with low-volume ejaculate. J Ultrasound Med. 2006;25:825–9.
Ravizzini P, Carizza C, Abdelmassih V, Abdelmassih S, Azevedo M, Abdelmassih R. Microdissection testicular sperm extraction and IVF-ICSI outcome in nonobstructive azoospermia. Andrologia. 2008;40:219–26.
Raza SA, Jhaveri KS. Imaging in male infertility. Radiol Clin North Am. 2012;50:1183–200.
Reddy NM, Gerscovich EO, Jain KA, Le-Petross HT, Brock JM. Vasectomy-related changes on sonographic examination of the scrotum. J Clin Ultrasound. 2004;32:394–8.
Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors? Eur Radiol. 2012 Nov;22:2540–6.
Rives N, Perdrix A, Hennebicq S, Saïas-Magnan J, Melin MC, Berthaut I, Barthélémy C, Daudin M, Szerman E, Bresson JL, Brugnon F, Bujan L. The semen quality of 1158 men with testicular cancer at the time of cryopreservation: results of the French National CECOS Network. J Androl. 2012;33:1394–401.
Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30(2):213–8.
Ron-El R, Strauss S, Friedler S, Strassburger D, Komarovsky D, Raziel A. Serial sonography and colour flow Doppler imaging following testicular and epididymal sperm extraction. Hum Reprod. 1998;13:3390–3.
Rud CN, Daugaard G, Rajpert-De Meyts E, Skakkebæk NE, Petersen JH, Jørgensen N. Sperm concentration, testicular volume and age predict risk of carcinoma in situ in contralateral testis of men with testicular germ cell cancer. J Urol. 14 Jun 2013. doi:10.1016/j.juro.2013.06.023. [Epub ahead of print]
Rusz A, Pilatz A, Wagenlehner F, Linn T, Diemer T, Schuppe HC, Lohmeyer J, Hossain H, Weidner W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J Urol. 2012;30:23–30.
Sadler TW. Langman’s medical embriology. 12th ed. Philadelphia: Lippincott Williams & Wilkins; 2011.
Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J Obstet Gynaecol Res. 2010;36:929–37.
Sakamoto H, Ogawa Y. Is varicocele associated with underlying venous abnormalities? Varicocele and the prostatic venous plexus. J Urol. 2008;180:1427–31.
Sakamoto H, Ogawa Y. Does a clinical varicocele influence the relationship between testicular volume by ultrasound and testicular function in patients with infertility? Fertil Steril. 2009;92:1632–7.
Sakamoto H, Saito K, Shichizyo T, Ishikawa K, Igarashi A, Yoshida H. Color Doppler ultrasonography as a routine clinical examination in male infertility. Int J Urol. 2006;13:1073–8.
Sakamoto H, Saito K, Ogawa Y, Yoshida H. Testicular volume measurements using Prader orchidometer versus ultrasonography in patients with infertility. Urology. 2007a;69:158–62.
Sakamoto H, Saito K, Oohta M, Inoue K, Ogawa Y, Yoshida H. Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology. 2007b;69:152–7.
Sakamoto H, Ogawa Y, Yoshida H. Relationship between testicular volume and testicular function: comparison of the Prader orchidometric and ultrasonographic measurements in patients with infertility. Asian J Androl. 2008;10:319–24.
Sala E, Eberhardt SC, Akin O, et al. Endorectal MR imaging before salvage prostatectomy: tumor localization and staging. Radiology. 2006;238(1):176–83.
Sano F, Uemura H. The utility and limitations of contrast-enhanced ultrasound for the diagnosis and treatment of prostate cancer. Sensors (Basel). 2015;15(3):4947–57.
Sarkar S, Das S. A review of imaging methods for prostate cancer detection. Biomed Eng Comput Biol. 2016;7(Suppl 1):1–15.
Sarteschi LM, Paoli R, Bianchini M, Menchini Fabris GF. Lo studio del varicocele con eco-color Doppler. G Ital Ultrasonologia. 1993;4:43–9.
Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79.
Sasagawa I, Nakada T, Kazama T, Terada T, Katayama T. Testosterone replacement therapy and prostate/seminal vesicle volume in Klinefelter's syndrome. Arch Androl. 1989;22:245–9.
Sasagawa I, Nakada T, Kazama T, Satomi S, Terada T, Katayama T. Volume change of the prostate and seminal vesicles in male hypogonadism after androgen replacement therapy. Int Urol Nephrol. 1990;22:279–84.
Schlegel PN, Goldstein M. Alternate indications for varicocele repair: non-obstructive azoospermia, pain, androgen deficiency and progressive testicular dysfunction. Fertil Steril. 2011;96:1288–93.
Schlegel PN, Li-Ming S. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688–92.
Schlegel PN, Shin D, Goldstein M. Urogenital anomalies in men with congenital absence of the vas deferens. J Urol. 1996;155:1644–8.
Schultz KE, Walker J. Testicular torsion in undescended testes. Ann Emerg Med. 1984;13:567–9.
Schurich M, Aigner F, Frauscher F, Pallwein L. The role of ultrasound in assessment of male fertility. Eur J Obstet Gynecol Reprod Biol. 2009;144:S192–8.
Seitz M, Gratzke C, Schlenker B, Buchner A, Karl A, Roosen A, Singer BB, Bastian PJ, Ergün S, Stief CG, Reich O, Tilki D. Contrast-enhanced transrectal ultrasound (CE-TRUS) with cadence-contrast pulse sequence (CPS) technology for the identification of prostate cancer. Urol Oncol. 2011;29(3):295–301.
Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009;106:3443–8.
Sharp VJ, Kieran K, Arlen AM. Testicular torsion: diagnosis, evaluation, and management. Am Fam Physician. 2013;88:835–40.
Shaw J. Diagnosis and treatment of testicular cancer. Am Fam Physician. 2008;77:469–74.
Shaw G, Renfree MB. Wolffian duct development. Sex Dev. 2014;8(5):273–80.
Shebel HM, Farg HM, Kolokythas O, El-Diasty T. Cysts of the lower male genitourinary tract: embryologic and anatomic considerations and differential diagnosis. Radiographics. 2013;33:1125–43.
Shinbo H, Kurita Y, Takada S, Imanishi T, Otsuka A, Furuse H, Nakanishi T, Mugiya S, Ozono S. Resistive index as risk factor for acute urinary retention in patients with benign prostatic hyperplasia. Urology. 2010;76:1440–5.
Shoskes DA, Lee CT, Murphy D, Kefer J, Wood HM. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2007;70:235–8.
Silber SJ, Patrizio P, Asch RH. Quantitative evaluation of spermatogenesis by testicular histology in men with congenital absence of the vas deferens undergoing epididymal sperm aspiration. Hum Reprod. 1990;5:89–93.
Silveira LF, Latronico AC. Approach to the patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013;98:1781–8.
Simoni M, Nieschlag E. Endocrine laboratory diagnosis. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology. Male reproductive health and dysfunction. 3rd ed. Berlin: Springer Verlag; 2010. p. 109–18.
Singh R, Hamada AJ, Bukavina L, Agarwal A. Physical deformities relevant to male infertility. Nat Rev Urol. 2012;9:156–74.
Skakkebaek NE, Jørgensen N. Testicular dysgenesis and fertility. Andrologia. 2005;37:217–8.
Smeenge M, et al. Role of transrectal ultrasonography (TRUS) in focal therapy of prostate cancer: report from a consensus panel. BJU Int. 2012;110:942.
Sparrow D, Bosse R, Rowe JW. The influence of age, alcohol consumption, and body build on gonadal function in men. Clin Endocrinol Metab. 1980;51:508–12.
St Sauver JL, Jacobson DJ, Girman CJ, McGree ME, Lieber MM, et al. Correlations between longitudinal changes in transitional zone volume and measures of benign prostatic hyperplasia in a population-based cohort. Eur Urol. 2006;50:105–11.
Stearns EL, MacDonnell JA, Kaufman BJ, Padua R, Lucman TS, Winter JSD, Faiman C. Declining testicular function with age. Am J Med. 1974;57:761–6.
Stravodimos KG, Petrolekas A, Kapetanakis T, Vourekas S, Koritsiadis G, Adamakis I, Mitropoulos D, Constantinides C. TRUS versus transabdominal ultrasound as a predictor of enucleated adenoma weight in patients with BPH: a tool for standard preoperative work-up? Int Urol Nephrol. 2009;41(4):767–71.
Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8.
Takihara H, Cosentino MJ, Sakatoku J, Cockett AT. Significance of testicular size measurement in andrology. II. Correlation of testicular size with testicular function. J Urol. 1987;137:416–9.
Tan MH, Eng C. Testicular microlithiasis: recent advances in understanding and management. Nat Rev Urol. 2011;8:153–63.
Tanrikut C, McQuaid JW, Goldstein M. The impact of varicocele and varicocele repair on serum testosterone. Curr Opin Obstet Gynecol. 2011;23:227–31.
Tasian GE, Copp HL. Diagnostic performance of ultrasound in nonpalpable cryptorchidism: a systematic review and meta-analysis. Pediatrics. 2011;127:119–28.
Taverna G, Morandi G, Seveso M, Giusti G, Benetti A, Colombo P, Minuti F, Grizzi F, Graziotti P. Colour Doppler and microbubble contrast agent ultrasonography do not improve cancer detection rate in transrectal systematic prostate biopsy sampling. BJU Int. 2011;108(11):1723–7.
Tefekli A, Cayan S, Uluocak N, Poyanli A, Alp T, Kadioğlu A. Is selective internal spermatic venography necessary in detecting recurrent varicocele after surgical repair? Eur Urol. 2001;40:404–8.
Tekgül S, Riedmiller H, Dogan HS, Hoebeke P, Kocvara R, Nijman R, Chr R, Stein R. Guidelines on paediatric urology, European Association of Urology Guidelines. In: EAU Guidelines Office. The Netherlands: Arnhem; 2008.
Terasaki T, Watanabe H, Kamoi K, Naya Y. Seminal vesicle parameters at 10-year intervals measured by transrectal ultrasonography. J Urol. 1993;150:914–6.
Tournaye H, Verheyen G, Nagy P, Ubaldi F, Goossens A, Silber S, Van Steirteghem AC, Devroey P. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod. 1997;12:80–6.
Trost LW, Brannigan RE. Oncofertility and the male cancer patient. Curr Treat Options in Oncol. 2012;13:146–60.
Tsili AC, Giannakis D, Sylakos A, Ntorkou A, Sofikitis N, Argyropoulou MI. MR imaging of scrotum. Magn Reson Imaging Clin N Am. 2014;22(2):217–38.
Turunc T, Gul U, Haydardedeoglu B, Bal N, Kuzgunbay B, Peskircioglu L, Ozkardes H. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: a prospective comparative study. Fertil Steril. 2010;94:2157–60.
van Casteren NJ, Looijenga LH, Dohle GR. Testicular microlithiasis and carcinoma in situ overview and proposed clinical guideline. Int J Androl. 2009;32:279–87.
Vicari E. Seminal leukocyte concentration and related specific reactive oxygen species production in patients with male accessory gland infections. Hum Reprod. 1999;14:2025–30.
Virtanen HE, Toppari J. Embryology and physiology of testicular development and descent. Pediatr Endocrinol Rev. 2014;11(Suppl 2):206–13.
Vohra S, Morgentaler A. Congenital anomalies of the vas deferens, epididymis, and seminal vesicles. Urology. 1997;49:313–21.
Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6.
Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Impot Res. 2009;21:1–8.
Westlander G, Ekerhovd E, Granberg S, Lycke N, Nilsson L, Werner C, Bergh C. Serial ultrasonography, hormonal profile and antisperm antibody response after testicular sperm aspiration. Hum Reprod. 2001;16:2621–7.
Woodward PJ, Sohaey R, O'Donoghue MJ, Green DE. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiographics. 2002;22:189–216.
Woodward PJ, Schwab CM, Sesterhenn IA. From the archives of the AFIP: extratesticular scrotal masses: radiologic-pathologic correlation. Radiographics. 2003;23:215–40.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press; 2010.
Yee WS, Kim YS, Kim SJ, Choi JB, Kim SI, Ahn HS. Testicular microlithiasis: prevalence and clinical significance in a population referred for scrotal ultrasonography. Korean J Urol. 2011;52:172–7.
Yu J, Chen Z, Ni Y, Li Z. CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. Hum Reprod. 2012;27:25–35.
Yusuf G, Konstantatou E, Sellars ME, Huang DY, Sidhu PS. Multiparametric sonography of testicular hematomas: features on grayscale, color Doppler, and contrast-enhanced sonography and strain elastography. J Ultrasound Med. 2015;34:1319–28.
Zhao H, Luo J, Wang D, Lu J, Zhong W, Wei J, Chen W. The value of transrectal ultrasound in the diagnosis of hematospermia in a large cohort of patients. J Androl. 2012;33:897–903.
Ziaee SA, Ezzatnegad M, Nowroozi M, Jamshidian H, Abdi H, Hosseini Moghaddam SM. Prediction of successful sperm retrieval in patients with nonobstructive azoospermia. Urol J. 2006;3:92–6.
Zini A, Boman JM. Varicocele: red flag or red herring? Semin Reprod Med. 2009;27:171–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Lotti, F., Maggi, M. (2017). Color-Doppler Ultrasound and New Imaging Techniques in Andrological Examination. In: Simoni, M., Huhtaniemi, I. (eds) Endocrinology of the Testis and Male Reproduction. Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-44441-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-44441-3_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44440-6
Online ISBN: 978-3-319-44441-3
eBook Packages: MedicineReference Module Medicine