Abstract
Male genital tract ultrasound (MGT-US) represents an essential diagnostic tool in andrology. Scrotal US has shown a relevant impact on reproductive and general male health, assessing features related to reproductive health, scrotal pain, masses, and trauma. Transrectal US has assumed growing relevance in infertility and chronic pelvic pain assessment. Penile US is widely used to investigate erectile dysfunction, structural penile abnormalities, and priapism. Finally, US can assess male breast abnormalities such as gynecomastia, lipomastia, and lesions.
Until very recent years, MGT-US lacked standardization. Recently, thanks to international societies including the European Academy of Andrology, several standards in US have been achieved. We report here normal MGT-US anatomy and analyze MGT-US abnormalities in relation to male reproductive and general health. In addition, we report here the standards in andrological US as derived from recent guidelines and evidence-based studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
To date, imaging of the male genital tract (MGT) represents an essential diagnostic tool in andrology, allowing physicians to complete the diagnostic workup of the andrologic patient, especially when anamnesis, physical, and biochemical examinations do not provide sufficient information for adequate patient management. In particular, ultrasound (US) represents the gold standard method for scrotal investigation, and a useful tool to evaluate the prostate–vesicular region (Lotti and Maggi 2015; Lotti et al. 2021a), and the vascular and structural characteristics of the penis and male breast tissue. Using high-frequency sound waves, US is a simple, rapid, and harmless diagnostic tool able to provide live images of the MGT organs and, among imaging techniques, is the least expensive. The high-resolution gray scale mode associated with color- and power Doppler examination allows sonographers to investigate size, echotexture, and vascular features of the scrotal and prostate–vesicular organs, as well as penile characteristics and male breast tissue, and to detect their abnormalities (Lotti and Maggi 2015). More recently, the use of contrast-enhanced US (CEUS) and sonoelastography (SE) have led to further improvements especially in the differential diagnosis of scrotal diseases (Sidhu et al. 2018; Săftoiu et al. 2019). However, in specific cases, other imaging techniques, such as magnetic resonance imaging (MRI), can help to better characterize some equivocal findings at US, as in the case of ambiguous testicular lesions or suspected prostate cancer.
So far, scrotal US has shown a relevant impact both on reproductive and on general male health (Lotti and Maggi 2015; Sidhu et al. 2018; Săftoiu et al. 2019; Lotti et al. 2021a), assessing scrotal features related to reproductive health, scrotal pain, masses, and trauma. In addition, transrectal US (TRUS) application has assumed a growing relevance especially in infertility and chronic pelvic pain assessment. Furthermore, penile color Doppler US (PCDU) is widely used to investigate erectile dysfunction, structural penile abnormalities (including fibrosis, trauma, and tumors), dorsal vein thrombosis, and priapism. Finally, US can be used to assess male breast abnormalities, including gynecomastia, lipomastia, and male breast lesions.
Although US has been widely used to explore the MGT organs, until very recently the method used to assess several qualitative and quantitative US parameters had not been standardized, and normative parameters and thresholds to distinguish normal and pathologic features were often not evidence-based (Lotti and Maggi 2015). Recently, thanks to the efforts of different radiological, urological, and andrological societies, including the European Academy of Andrology (EAA) (Lotti et al. 2020; Lotti et al. 2021b), several standards in US have been achieved. We report here concepts of normal US anatomy of the MGT and critically analyze MGT-US abnormalities in relation to male reproductive and general health. In addition, we report here the standards in andrological US as derived from recent guidelines and evidence-based studies.
2 Scrotal US
2.1 Indications
The indications for scrotal US are reported in Table 6.1.
2.2 Methodological Standards
2.2.1 Scrotal Color Doppler Ultrasonography
The standardization of the methodology used to perform scrotal color Doppler ultrasonography (CDUS) is relatively new. Practical recommendations for performing scrotal CDUS have been reported by the SIU/SIEUN collaboration in 2014 (Martino et al. 2014) and in the AIUM Practice Guideline in 2015 (AIUM Practice Guideline for the Performance of Scrotal Ultrasound Examinations 2015). In 2015, Lotti and Maggi published a systematic review dealing with the measurement and assessment (as well as clinical significance) of MGT quantitative and qualitative parameters, respectively. In particular, the authors reported how each organ/segment (e.g., testis, epididymal head, body, tail, vas deferens pampiniform plexus) of the scrotal sac had been measured, and the classifications used to stratify (e.g., testis inhomogeneity) severity. In addition, the authors reported the thresholds suggested in previous studies to distinguish normal from pathologic features, in an effort to align them. However, the authors concluded that, for several parameters, sonographic imaging of male genital tract was suffering from a lack of standardization, often leading to subjective and vague diagnoses. For this reason, the EAA promoted an international multicenter study (see at https://www.andrologyacademy.net/eaa-studies) aimed at defining the male genital tract-CDUS reference ranges and characteristics as derived from a cohort of healthy, fertile men. The development and methodology of the “EAA US study” were reported in a 2020 study (Lotti et al. 2020). A detailed description of the Standard Operating Procedures (SOPs) to evaluate scrotal quantitative and qualitative parameters, and assessment of the CDUS intra- and inter-operator comparability, has been reported in a further study (Lotti et al. 2021b). In our opinion, following the CDUS SOPs proposed by the EAA US consortium in clinical practice will help to reduce operator-dependent differences among sonographers. The EAA-proposed SOPs to assess scrotal CDUS have been reported elsewhere (Lotti et al. 2021a, 2021b) (see https://www.andrologyacademy.net/eaa-studies).
2.2.2 Contrasted-Enhanced US
The methodological standards for the clinical practice of contrasted-enhanced US (CEUS) in nonhepatic applications, including scrotum investigation, have been reported by the EFSUMB Guidelines and in the 2017 updated version (Sidhu et al. 2018). As a result, the assessment of some pathological conditions using CEUS has improved (Sidhu et al. 2018). Using time–intensity curves, evaluating the wash-in and wash-out curves may help to distinguish malignant from benign tumors, although CEUS analyses still overlap between different histological types. In addition, CEUS can discriminate nonviable regions in testicular trauma and can identify segmental testicular infarction (Sidhu et al. 2018).
2.2.3 Sonoelastography
The methodological standards for the clinical practice of sonoelastography (SE) in nonhepatic applications, including testicular investigation, have been reported by the EFSUMB Guidelines and Recommendations in the updated version (Săftoiu et al. 2019). So far, strain elastography and shear wave elastography, which includes acoustic radiation force impulse-based techniques, and transient elastography are available. The basic principles of SE have been extensively described in previous EFSUMB guidelines (Bamber et al. 2013), while methodological standardization for different organs, including the testis, is reported in the updated EFSUMB guidelines (Săftoiu et al. 2019). From a methodological point of view, the use of SE to investigate focal testicular lesions can only be recommended in conjunction with other US techniques, as there is overlap between benign and malignant neoplasms (Săftoiu et al. 2019). SE assessing overall background parenchyma has been used to investigate infertility, TML, and undescended testis (Săftoiu et al. 2019). However, currently, these specific applications are restricted to research.
2.3 US Anatomy, Normal and Abnormal Patterns, Clinical Utility, and US Standards
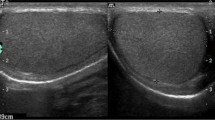
Clinical utility and standards in scrotal US, including CDUS, CEUS, and SE, are discussed below. Table 6.2 shows normal values and cutoff of the main scrotal US parameters as well as US classifications used previously and currently in evaluating scrotal organs at gray scale and color Doppler US. Table 6.3 shows scrotal US clinical utility and impact on male reproductive health management. Figure 6.1 shows a schematic representation of the normal and pathologic features of the scrotal organs in relation to male reproductive health. Figure 6.2 shows some examples of normal and abnormal US features of the scrotal organs. Normal and abnormal scrotal US patterns are discussed below.
Schematic representation of the normal and pathologic features of the male genital tract (MGT) in relation to male reproductive health. Left side: Normal anatomy of the MGT. Right side: pathologic features of the MGT suggesting obstructive or nonobstructive oligo-astheno-teratozoospermia (OAT)/azoospermia. Warning for malignancy is extensively discussed in the text. Adapted from Lotti and Maggi 2015
Normal (left side) and abnormal (right side) color Doppler ultrasound (CDUS) features of the scrotal organs. Panel (a), Testis of normal volume, homogeneity, and echogenicity with ellipsoid shape. Longitudinal (right figure) and transversal (left figure) scans of the testis, with length (D1), width (D2), and height (D3) measurements reported. Panel (b), Left figure: B-mode appearance of the spermatic cord (SC) and the upper pole of the testis (T) in longitudinal scan. Small, nondilated venous vessels of the pampiniform plexus are difficult to differentiate from the other structures of the SC. Right figure: CDUS detection of the testicular artery (arrow) in the SC and recurrent ramus (^) of an intratesticular centripetal artery. Venous reflux at rest in the venous vessels is not detectable. Panel (c), normal epididymal head with triangular shape (dashed line) in longitudinal scan, homogeneous, with echogenicity comparable to that of the testis (T). Its length is measured from the top to the base of the triangle (dotted line). Panel (d), homogeneous epididymal body and tail and proximal vas deferens (pVD) in longitudinal scan. Their echogenicity is slightly hypoechoic compared to the testis and the epididymal head in panel C. Their diameters are reported as dashed lines. Red dashed line indicates the end of the epididymal tail and the beginning of the pVD. The curve arrow indicates the epididymal–deferential handle. Panel (e), normal pampiniform plexus. Venous vessels are not dilated (<3 mm). Panel (f), testis with low volume and hypoechoic echotexture, detected in a man with a history of cryptorchidism. Panel (g), testis with echotexture inhomogeneity in sagittal scan. Panel (h), testicular microlithiasis with “starry sky” appearance. Panel (i), dilated, and inhomogeneous epididymal body and tail and proximal vas deferens (pVD), with irregularly shaped mass (*) in the epididymal tail region, detected in sagittal scan. Panel (j), CDUS evaluation of dilated veins of the pampiniform plexus with colored signal (left), showing continuous reflux at rest (***), increasing with Valsalva (arrow), identifying a severe, sonographic-defined, varicocele, according to different classifications
So far, scrotal US has shown a relevant impact on both reproductive and general male health. In fact, US has been used to assess scrotal features related to (i) reproductive health, (ii) scrotal pain, (iii) scrotal masses, and (iv) scrotal trauma (Isidori and Lenzi 2008; Lotti and Maggi 2015; Richenberg et al. 2015; Sidhu et al. 2018; Săftoiu et al. 2019; Freeman et al. 2020; Lotti et al. 2021a). (i) Regarding reproductive health, US can detect alterations in size, echotexture, and vascularization of the testes associated with sperm abnormalities and, eventually, with low testosterone levels. In addition, scrotal US provides information on epididymal and deferential abnormalities, possibly associated with semen quality impairment, or on their bilateral absence, causing obstructive azoospermia. Finally, scrotal color Doppler US (CDUS) is able to detect and stage varicocele, which may exert a negative role on sperm parameters. (ii) Regarding scrotal pain, CDUS can detect testicular or epididymal size and echopattern abnormalities as well as hypervascularization, suggesting inflammation (i.e., orchitis and epididymitis), or an absent vascularization, indicating spermatic cord/testicular torsion. Furthermore, CDUS can assess severe varicocele or inguinal/scrotal hernias, eventually associated with mild discomfort and even overt pain. (iii) Moreover, US plays a key role in investigating testicular or extratesticular masses, characterizing them as benign or malignant with fair accuracy, although without providing diagnostic certainty. In addition, US can assess testicular microlithiasis (TML), which, when associated additional risk factors (see below), might underlie a coexisting or developing testicular malignancy. (iv) Finally, US plays a crucial role in the evaluation of scrotal trauma.
2.3.1 Testis
2.3.1.1 US Anatomy
Testicles are pair organs normally located in the scrotal sac. This peculiar position requires the use of a high-spatial resolution transducer dedicated to the study of soft parts (7–15 MHz) (Lotti and Maggi 2015). Usually, the patient lies supine with the penis resting on the suprapubic region and gel applied to the scrotum, supported by a towel placed between the thighs. The testes are examined in transverse, oblique, and longitudinal planes, and images are acquired in both gray scale and color Doppler modes, to assess testicular blood flow. At US, normal adult testicles appear as ellipsoid organs of 3–5 cm length, 2–4 cm width, and 3 cm anterior-posterior size (Lotti and Maggi 2015). They are surrounded by an echoic fibrous capsule, the tunica albuginea, which projects into the interior of the testis with fibrous septa. Septa may be seen as delicate linear hypoechoic striae, converging to form the mediastinum testis, which appears in a longitudinal scan as a hyperechoic line, eccentrically located. Septa divide the testis into 200–400 lobules. Each lobule contains interstitial Leydig cells and seminiferous tubules, with differentiating germinal cells and somatic Sertoli cells. It is estimated that seminiferous tubules account for ~85% of the entire testicular volume (Lotti and Maggi 2015), while Leydig cells for ~3%. Hence, changes in testicular volume are mainly related to variations of the testicular parenchyma.
The normal adult testis is characterized at US by a homogeneous granular echotexture, made up of uniformly distributed medium-level echoes resembling the echogenicity of the normal thyroid gland (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015) (see paragraph “Testicular homogeneity and echogenicity”). Scrotal arterial perfusion has been well described and depicted elsewhere (Lotti and Maggi 2015). The testes are mainly perfused by the testicular arteries, which arise from the aorta, distal to the renal arteries, enter the spermatic cord at the deep inguinal ring, and reach the upper testicular pole. Each testicular artery lies in the spermatic cord with the ipsilateral cremasteric artery (a branch of the inferior epigastric artery) and the deferential artery (a branch of the vesicular artery). Although there are anastomoses between these vessels, i) the testicular artery primarily supplies the testis, and one of its branches also the epididymal head, ii) the deferential artery perfuses the epididymis (mainly body and tail) and vas deferens, and iii) the cremasteric artery supplies the peritesticular tissues and the scrotal wall. After entering the scrotum, the testicular artery runs along the posterior aspect of the testis and penetrates the tunica albuginea, supplying two sets of arteries, the capsular and the transmediastinal arteries. The capsular arteries have a superficial course beneath the tunica albuginea in a layer called tunica vasculosa, over the surface of seminiferous tubules. They branch centripetal arteries that enter the testicular parenchyma and flow toward the mediastinum penetrating between the septa separating the seminiferous tubules. As they approach the mediastinum, the centripetal arteries arborize into recurrent rami that branch back in the opposite direction, carrying blood from the mediastinum into the testis. In some men, a large branch of the testicular artery, the centrifugal transmediastinal artery, enters at the mediastinum and runs across the testicular parenchyma with a straight course, to form capsular branches on the opposite side.
2.3.1.2 US Normal and Abnormal Patterns
2.3.1.2.1 Testicular Volume
Testicular volume (TV) is an essential parameter in clinical practice, reflecting not only the seminal and hormonal male status but also the presence of previous or current testicular or systemic disorders (Lotti and Maggi 2015; Lotti and Maggi 2018). TV is usually assessed clinically by Prader’s orchidometer. However, orchidometry overestimates TV when compared to US, and US offers a greater accuracy in TV measurement than physical examination (Behre et al. 1989; Lotti and Maggi 2015; Lotti et al. 2021a). Prader’s orchidometer- and US-derived TV are closely related, both in boys and in adult eugonadal or hypogonadal subjects (Lotti and Maggi 2015). Hence, in clinical practice, Prader’s orchidometer-derived TV may be considered a reliable surrogate of US-measured TV, easier to perform, and not costly. Nevertheless, US maintains a role in TV assessment when physical examination is unreliable, such in case of large hydrocele or large varicoceles, inguinal testis, epididymis enlargement or fibrosis, thickened scrotal skin, obesity (see Table 6.1).
Previous studies reported that US-estimated TV was positively related to total sperm count, sperm motility, normal sperm morphology, and testosterone levels and negatively with LH and FSH levels (Isidori and Lenzi 2008; Lotti and Maggi 2015; Lotti et al. 2021a, b). A negative correlation between US-TV and nonconventional sperm parameters (sperm DNA fragmentation, percentage of spermatozoa with low mitochondrial membrane potential, phosphatidylserine externalization, or chromatin compactness) has been also reported.
TV US estimation varies according to the mathematical formula applied. In the last decade, no consensus on the best mathematical model was achieved. Previous studies, as discussed in comprehensive articles (Lotti and Maggi 2015), reported US-assessed TV using ellipsoid formular (length x height x width x 0.52), Lambert’s (length x height x width x 0.71), or Hansen’s (length × width × 0.52), making comparisons between different studies complicated. The most commonly applied formula is the ellipsoid formula. However, in studies investigating the difference between US and “real” TV by water displacement, the empirical Lambert’s formula was reported to be superior. In 2014, SIU/SIEUN recommendations (Martino et al. 2014) supported the use of the ellipsoid formula, whereas in 2015 AIUM guidelines (AIUM Practice Guideline for the Performance of Scrotal Ultrasound Examinations 2015) reported that, in pediatric patients, TV could be provided using Lambert’s or the ellipsoid formula.
Using the ellipsoid formula, healthy German and Danish men showed a median TV of ~14 ml, young Italian and South Korean men a mean TV of ~15 ml and ~ 18 ml, respectively, and fertile Italian men a mean TV of ~19 ml (see Table 6.2) (Lotti and Maggi 2015; Lotti et al. 2021a, b). The relatively wide range of a “normal” average TV could depend on the lack of international US SOPs standardization, on the difference between “mean” and “median” TV values, on the diverse age range of the subjects studied and on differences between populations studied, belonging to different countries. Although TV difference among ethnic groups and TV variations with age in adult men seem to be modest, available studies on these topics are relatively scanty and, in some cases, conflicting, representing possible confounders. A TV <12 ml has been proposed by previous studies to indicate testicular hypotrophy at US, using the ellipsoid formula or irrespective of the mathematical formula used, however, without any evidence-base (Lotti et al. 2021a) (Table 6.2). In addition, a TV <10 ml using Lambert’s formula was reported to be associated with testicular dysfunction, however, only in two studies assessing Japanese men with infertility (Lotti et al. 2021a).
Recently, the ESUR guidelines on varicocele (Freeman et al. 2020) supported the use of Lambert’s formula to calculate TV at US, considered the most accurate according to previous studies, however, without a “strong” consensus. Most recently, the EAA US consortium, evaluating a cohort of 248 healthy, fertile men (Lotti et al. 2020), reported the US reference range of testicular diameters and TV according to the ellipsoid, Lambert’s and Hansen’s mathematical formulas, providing evidence-based normative parameters (Lotti et al. 2021b). In the EAA study, the US-TV calculated with the ellipsoid formula showed the most accurate correlation with the Prader orchidometer-assessed TV, while Lambert’s formula overestimated orchidometry (Lotti et al. 2021b). Hence, EAA supports the use of the ellipsoid formula, considering that it fits better into clinical (and not experimental) reality (Lotti et al. 2021b). In addition, the ellipsoid formula is easier to use in clinical practice, since it is automatically calculated by most US devices. Using the ellipsoid formula, the EAA US consortium reported a mean TV of ~17 ml in healthy, fertile men (Table 6.2). Since the EAA study is an international multicenter one, the aforementioned value can be considered the “normal” mean TV of the European adult population of reproductive age. The multicenter nature of the study and the limited age range (23–53 years) of the subjects investigated avoid confounders related to nationality/ethnicity and aging. The EAA US-TV lowest reference limit for right and left testis is 12 and 11 ml, respectively, defining, in an evidence-based manner, “testicular hypotrophy” as being below these thresholds (Lotti et al. 2021b).
Regarding the evaluation of TV in the pediatric population (Lotti et al. 2021a), reference curves for mean US-TV are available for boys aged 0 to 6 years, 0.5 months to 18 years, and 6 months to 19 years. Of note, the last study also reported the distribution of TV within the Tanner stages of pubic hair development. However, all these studies were performed in the Netherlands; hence, their results might not apply to different ethnic groups. As a corollary, a Korean study also evaluated changes and ranges of pediatric TV in 0- to 10-year-old boys, however, without reporting clear reference curves.
2.3.1.3 Testicular Homogeneity and Echogenicity
Since the eighties, the normal adult testis has been described as characterized by a homogeneous granular echotexture, made up of uniformly distributed medium-level echoes (i.e., homogeneous and normoechoic) resembling the echogenicity of the normal thyroid gland (Isidori and Lenzi 2008; Lotti and Maggi 2015). The occurrence of testicular structural abnormalities is associated with an alteration of echo distribution, leading to inhomogenicity and abnormal echogenicity (see below).
The image resolution and clinical significance of testicular inhomogeneity (TI) have changed over time. In the past, the US image quality and resolution were significantly lower than today. Hence, at first, severe TI was considered as a warning for the presence of a possible testicular malignancy. In this setting, the reported association of very irregular testicular patterns and carcinoma in situ in cryptorchid men or testicular tumor by Lenz et al. (1993) are paradigmatic. Lenz et al. reported the first classification of TI, proposing a five-point “testicular echotexture score” ranging from 0 (regular pattern) to 5 (tumor suspected) (Table 6.2), which correlated positively with the presence of a carcinoma in situ. In addition, the TI score negatively correlated with normal sperm morphology, sperm count, and US-TV. In 1996, the “striated testis” was first described, a specific US pattern characterized by the presence of hypoechoic striae within the testicular parenchyma, resembling the black stripes of a zebra’s coat (Lotti and Maggi 2015; Lotti et al. 2021a). In the aforementioned study, the striated US pattern was associated with testicular fibrosis and not malignancy. Subsequently, the hypoechoic striae were associated with an exaggeration of the normally inapparent interlobular septa due to seminiferous tubule reduction and interstitial proliferation, and the inhomogeneous, striated testicular pattern was associated with atrophy and fibrosis. Hence, over time, the clinical significance of TI has shifted from a risk factor for the presence of a testicular tumor to an US abnormal pattern associated with other pathological conditions and/or testicular function impairment (see below). In parallel, due to the advancing US technology, “suspected tumors” have been described as “nodular lesions” and no more as “echotexture abnormalities.” In agreement, in 2001 Westlander et al. proposed a four-point scale TI classification, a semiqualitative score modified from that of Lenz et al. not considering suspected tumors. The classification was modified soon after in a five-point scale (Table 6.2) introducing a further category, “intratesticular lesions observed after testicular sperm aspiration,” which resolved after 6–9 months of follow-up.
Whereas TI is often observed in the elderly and considered normal, in young subjects it has been associated with several pathological conditions, including ischemia, orchitis, trauma, torsion, exposure to physical or chemical agents, chemo- and radiotherapy or alcohol abuse, Klinefelter, and metabolic syndrome (Lotti and Maggi 2015; Lotti et al. 2021a). In addition, TI has been associated with testicular function impairment including hypogonadism, abnormal sperm morphology, impaired sperm quality, and azoospermia. Recently, Pozza et al. (2020) developed, in a cohort of 2230 men, a semiquantitative, multiparametric (including bilateral US-TV, echotexture, echogenicity, and microlithiasis) score, ranging from 0 to 7 and named “testicular ultrasound (TU) score,” that has proved significantly more accurate than the Lenz score (Abdulwahed et al. 2013) in predicting impaired spermatogenesis, and able to predict hypogonadism. In the TU score (Pozza et al. 2020), the parameter testicular “homogeneity” has been considered dichotomously (0. homogeneous; 1. inhomogeneous). Since subjects with a homogeneous testis showed better sperm and hormonal parameters compared with the rest of the sample, testis homogeneity can be considered “normal.”
In 2021, the EAA US consortium (Lotti et al. 2021b) proposed a new, four-point scale classification of TI, easy to use in clinical practice and avoiding the term “suspected tumors” (see Table 6.2). In a cohort of healthy, fertile men, only a very few subjects had TI, always of a mild degree (grade 1). Those subjects showed lower sperm vitality compared with the rest of the sample. Hence, it is possible to define “normal” as the presence of a homogeneous, or at least slightly inhomogeneous (mild TI), testicular pattern, especially from a reproductive point of view.
Regarding testicular echogenicity, no standardized classification has been published in the past, although the description of normoechoic, hypoechoic, or hyperechoic testis has been previously reported (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015). Echogenicity depends on the seminiferous tubules’ maturation and germ cell representation. Prepubertal testis has been described as slightly more hypoechoic than the adult one, since seminiferous tubules have not yet developed a lumen. During puberty, as a function of lumen development, testis echogenicity progressively increases, up to average adult level. The normal adult testis appears homogeneous at US (see above). In case of testicular damage (congenital or acquired), a reduction of testicular parenchyma and/or an increase in interstitium occur, often leading to a hypoechoic echotexture. Detection of focal abnormalities may underly several conditions, including tumors, while a diffuse hypoechogenicity may be related to widespread malignancies (see below, in the paragraph “Testicular lesions”). However, more often, diffuse hypoechogenicity is observed in damaged testes (e.g., undescended and/or hypotrophic testes; see below) and can be associated to TI at US, indicating parenchymal reduction and interstitial proliferation. In line with the latter issue, testis hypoechogenicity, as well as TI, have been also associated with increased levels of M540 bodies, round anucleated elements detected by flow cytometry in the semen, considered as markers of testicular apoptosis/spermatogenesis derangement. With reference to the echotexture of the whole testis (and not to focal lesions), the EAA US consortium (Lotti et al. 2021b) has recently classified testicular echogenicity on a three-point Likert scale (see Table 6.2).
2.3.1.4 Testicular Vascularization
The vascular anatomy of the testis has been described in detail elsewhere (Lotti and Maggi 2015). Pictures of the flow characteristics of the main testicular arteries and their branches have been also reported elsewhere (Bertolotto and Trombetta 2012).
Testis vascularization plays a critical role in the differential diagnosis of orchitis (see below), orchi-epididymitis, or some malignant conditions (i.e., leukemia, lymphoma (enhanced), testicular torsion (absent), infarction (absent or peripheral)) (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015; Lotti et al. 2021a). This is also true for other pathological conditions resulting in testicular ischemia, such as tension hydroceles (Lotti et al. 2021a), as well as in the assessment of scrotal trauma, when considered along with other clinical and US features (Ramanathan et al. 2021). In the setting of acute scrotal pain, a normal scrotal content and testis vascularity should prompt extension of the US investigation to the abdomen, to exclude nonscrotal causes of acute pain. In addition, the vascular pattern of intratesticular lesions may suggest their nature as benign or malignant. This is important, in particular, in patients with lymphomas or hematological malignancies, in patients with bilateral synchronous tumors, and in patients with multiple, synchronous lesions in the same testis, in which the therapeutic approach is determined by the most aggressive histotype (Lotti et al. 2021a). However, so far, the assessment of testis vascularization with CDUS is still qualitative, with no clear quantitative cutoff distinguishing the aforementioned conditions. CEUS and SE (see below) have improved the capacity to investigate pathological processes, however, with no diagnostic certainty.
Until 2021, the reference range of testis vascular parameters was lacking. Only one study (Pilatz et al. 2013) reported the reference range of a single parameter, the peak systolic velocity of the testicular artery, evaluating 306 healthy Caucasian men aged 18–88 years. Other available studies investigated testis vascularization to assess different pathological conditions qualitatively (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015; Lotti et al. 2021a). In addition, some testis vascular parameters have been associated with sperm quality or have been suggested to be useful in discriminating obstructive and nonobstructive azoospermia or residual spermatogenic areas in nonobstructive azoospermia. However, at present, they have been investigated only for research purposes, with no impact on the clinical management of azoospermic men (see below). Finally, CDUS could be used to evaluate possible damage occurring during testicular sperm extraction (Lotti and Maggi 2015).
Recently, the EAA US study (Lotti et al. 2021b) reported a standardization of the measurement of testis vascular parameters (see https://www.andrologyacademy.net/eaa-studies) and their reference ranges in healthy, fertile subjects (see Table 6.2). In addition, a recent meta-analysis demonstrated that CDUS represents an effective imaging modality for diagnosing testicular torsion in adult patients with acute scrotal pain (Lotti et al. 2021a). On the other hand, recently the ESUR published a position statement on imaging in scrotal trauma (Ramanathan et al. 2021), clearly defining the role of CDUS evaluation of testicular/scrotal vascularization in different traumatic conditions, and indicating CEUS and SE as advanced techniques useful as problem-solving modalities in equivocal cases. Brief statements on this topic were also reported in the 2020 AUA urotrauma guidelines (Morey et al. 2021).
2.3.1.5 Orchitis
CDUS plays a key role in identifying orchitis, detecting enhanced testis vascularization, along with diffuse testicular enlargement, inhomogeneous, mainly hypoechoic, testicular echotexture, and reactive hydrocele (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Pilatz et al. 2013; Lotti and Maggi 2015). Post-orchitis testis may present with inhomogeneous echotexture, mainly hypo- or hyperechoic, with normal or reduced vascularization and micro- or macrocalcifications. Although it has been reported that, in the case of testicular involvement, chronic inflammation may result in testicular atrophy, a study suggests that, under conservative treatment, no testicular atrophy occurs after acute epididymo-orchitis (Pilatz et al. 2013).
2.3.1.6 Testicular Microlithiasis
Testicular microlithiasis (TML) is an US diagnosis. TML is related to the presence of microcalcifications, which are bright echogenic nonshadowing foci less than 3 mm (Lotti and Maggi 2015; Richenberg et al. 2015). They are made of microcalcium deposits with surrounding fibrosis. They do not cause symptoms and are impalpable. Microcalcifications must be distinguished from macrocalcifications, which have never worried sonographers or clinicians, and which have been associated with a prior testicular insult (trauma, orchitis, infarction, torsion, chemo/radiotherapy), testicular atrophy, or maldescended testis (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015). However, they can be rarely related to a burnt-out tumor. In these aforementioned cases, the associated pathological condition, and not the presence of the macrocalcification itself, could play a negative role on general and/or reproductive male health.
The first sonographic identification of TML was described in 1987 as “innumerable tiny bright echoes diffusely and uniformly scattered throughout in the substance of testes” (Lotti et al. 2021a). Since then, a large number of varying definitions have been used in the sonographic literature on this topic. However, the two main definitions for TML proposed in the past were as follows: ≥ 5 microcalcifications in the whole testis or ≥ 5 microcalcifications per field of view (Richenberg et al. 2015) (Table 6.2). So far, the ESUR defined TML as the presence of ≥5 microcalcifications per field of view, leading to a worldwide accepted definition (Table 6.2).
TML association with testicular tumors and infertility has been widely debated. Recent meta-analyses support a significant association between TML and testicular cancer (Lotti et al. 2021a). No meta-analyses have evaluated the association between TML and sperm parameters, while recent reviews reported that the relationship between TML and male infertility is still debated.
According to a previous version (Albers et al. 2013) of the EAU guidelines, the presence of TML with no associated risk factors (see below) did not require scrotal US follow-up or biopsy, while that with associated risk factors (infertility, bilateral TML, atrophic testes, history of cryptorchidism or of testicular cancer) was considered an indication for regular scrotal US follow-up, and, eventually, testicular biopsy. Current EAU guidelines (Salonia et al. 2021; Laguna et al. 2021) on TML management have not changed significantly in the last decade. So far, it is still a critical issue that in men with “TML and additional risk factors” either US follow-up or biopsy is advised, leading to possible different and nonstandardized managements of the patient. In addition, the timing of the testicular US follow-up has not been suggested. However, in 2015, the ESUR published guidelines (Richenberg et al. 2015) on TML imaging and follow-up, suggesting that annual follow-up is advised only in patients with “TML and additional risk factors” (personal/family history of testicular tumor, maldescent testis, orchidopexy, testicular atrophy) up to age 55, and, eventually, in men with no risk factors but diffuse (“starry sky”) TML. Annual US follow-up is suggested also for children/adolescents with maldescent testis/post-orchiopexy or with testicular atrophy. In addition, recommendations for men with genetic disorders (including Klinefelter and McCune-Albright syndromes) have been reported and are the same as for the general population. Finally, ESUR indication for biopsy in TML men is very limited. In particular, in men who, at orchiectomy for a germ cell tumor, show TML or atrophy of the contralateral testis, a testicular biopsy may be indicated to look for carcinoma in situ.
Regarding the possible relationship between TML and male infertility, several studies have reported that the prevalence of TML in infertile men is higher than in fertile men (Lotti et al. 2021a). In addition, in a relatively large cohort of men with fertility intention, those with TML showed worse semen parameters than the rest of the sample. More recently, in a larger cohort of males of infertile couples, men with TML showed lower mean testis volume and sperm concentration and higher FSH levels than those with limited (<5 hyperechogenic spots per sonogram) or no microcalcifications. TML appears linked to infertility as an indicator/part of testicular dysgenesis syndrome (Lotti and Maggi 2015; Lotti et al. 2021a). As a corollary, so far, in infertile men TML is not considered a risk factor for the production of antisperm antibodies.
2.3.1.7 Testicular Lesions
Since the first application of gray scale US to investigate scrotal content, the main interest of physicians has been to explore scrotal masses. Over time, technical US skills have improved, focusing to date on testicular lesions with different approaches, including CDUS, CEUS, and SE (Lotti and Maggi 2015; Bertolotto et al. 2018; Huang et al. 2020). However, US is still not accurate enough to define the origin of several lesions, and histology remains the only certain diagnostic tool (Lotti and Maggi 2015; Lotti et al. 2021a).
Until the advent of CEUS, CDUS was the only way to evaluate testicular and extratesticular lesions. Sonologists mainly described the characteristics of the lesion, including size, homogeneity, echogenicity, margins, and vascularization (Lotti et al. 2021a). However, especially for large lesions, surgery was mandatory. Differential diagnosis was difficult, particularly when, at US, severe TI was detected. The difference between anechoic or solid lesions was detectable, allowing clinicians to distinguish intratesticular benign cysts from possible malignant lesions. However, large solid lesions were considered as likely neoplasms. In addition, differential diagnosis between hypoechoic areas, underlying segmental infarction, post-traumatic or post-inflammatory outcomes, intratesticular hematomas, or possible tumors was difficult. The finding of large lesions required compulsory surgery, while that of millimetric lesions was managed with strict follow-up, however requiring surgery in the event of unstable characteristics over time.
So far, with the improvement of US devices’ resolution and vascular assessment, gray scale US with power/color Doppler is able to evaluate testicular lesions quite well, suggesting, in some cases, specific diagnosis. Clinical and CDUS patterns of testicular and extratesticular lesions have been described in detail elsewhere (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012). Table 6.4 summarizes the CDUS characteristics of the main malignant and benign testicular lesions. In addition, new US imaging techniques, such as CEUS and SE, have improved the characterization of testicular abnormalities, both in adult and in pediatric patients (Bertolotto et al. 2018; Huang et al. 2020). According to EFSUMB recommendations (Sidhu et al. 2018), CEUS can distinguish vascularized from nonvascularized focal testicular lesions, helping to exclude malignancy. In addition, CEUS can discriminate nonviable regions in testicular trauma and can identify segmental infarction. Finally, CEUS can identify abscess formation and infarction in severe epididymo-orchitis. As a corollary, recently ESUR published its position statements on imaging in scrotal trauma (Ramanathan et al. 2021), reporting standardization, methodology, and information derived from CDUS/CEUS/SE application. Regarding SE, according to EFSUMB recommendations (Săftoiu et al. 2019), its use for the evaluation of focal testicular lesions can only be recommended in conjunction with other US techniques, as there is overlap between benign and malignant neoplasms.
Regarding methodology and indications, palpation should be the first step of an US investigation of the scrotal content and, in selected cases, can help to identify scrotal lesions that are not immediately seen at US (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015). According to recent EAU guidelines (Laguna et al. 2021), high-frequency (>10 MHz) testicular US should be used to confirm a testicular tumor even in the presence of a clinically evident testicular lesion. The use of testicular US can (i) determine whether a mass is intra- or extra-testicular; (ii) determine the volume and anatomical location of the testicular lesion; (iii) be used to characterize the contralateral testicle to exclude other lesions and identify risk factors for carcinoma in situ (Laguna et al. 2021). Testicular US is also recommended for all men with retroperitoneal or visceral masses and/or without elevated tumor markers in the absence of a palpable testicular mass and for fertility workup evaluation (Laguna et al. 2021).
According to the EAU guidelines, every subject with a suspected testicular mass must undergo surgical exploration, with orchiectomy if a malignant tumor is found or testicular biopsy with histological examination if the diagnosis is not clear. Regarding large nodule management, US should be performed even in the presence of a clinically evident testicular mass (Lotti and Maggi 2015; Laguna et al. 2021). In this scenario, US frequently plays an adjuvant role, sometimes allowing differential diagnosis among different clinical conditions (i.e., malignancy, inflammation, cysts) and evaluating the contralateral testis. However, currently, the real challenge is represented by imaging and management of small (millimetric) lesions. Small hypoechoic areas, especially when not vascularized, may be related to spermatoceles, cysts, focal Leydig cell hyperplasia, fibrosis, and focal inhomogeneity due to previous pathologic conditions (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015). However, they may also indicate small tumors. Hence, they require careful evaluation and follow-up, with periodic US examination, especially if additional risk factors for malignancy are present (i.e., infertility, bilateral TML, cryptorchidism, testicular atrophy, inhomogeneous parenchyma, history of testicular tumor, contralateral tumor, and age <50 years) (Salonia et al. 2021; Laguna et al. 2021). If a small nodule grows, or additional risk factors for malignancy are present, testicular biopsy/surgery should be considered.
Recently, ESUR published recommendations (Rocher et al. 2016) on incidentally US-detected nonpalpable testicular lesions in adults. According to the ESUR, characterization of testicular lesions is primarily based on US examination. Most small nonpalpable testicular lesions seen on US are benign simple cysts and require neither follow-up nor surgery. Nonpalpable single sporadic solid nodules <5 mm without any microliths are benign in up to 80% of cases, with Leydig cell tumors being the most frequent. US follow-up can be an alternative to orchiectomy in young and/or infertile men if tumor markers are negative. Large (>1 cm), multiple, mixed cystic, heterogeneous or solid vascularized nodules, irregular margins, associated microliths, or hypoechoic regions may indicate malignancy. CEUS optimizes enhancement in lesions which are apparently avascular at color Doppler. The rate of the wash-in and the wash-out of the contrast agent may help to differentiate malignant from benign tumors. Leydig cell tumors have been reported to demonstrate a prolonged wash-out in one study, and a shorter filling time than germ cell tumors in another. The role of CEUS is evolving; however, only a few studies are available to date, limiting the recommendations for the routine use of CEUS for managing incidental testicular masses. Conversely, the role of SE in differentiating between malignant and benign testicular nodules is still unclear (Săftoiu et al. 2019). Accordingly, a recent study reported that strain ratio measurement offers no improvement over elastographic qualitative assessment of testicular lesions, and that SE may support conventional US in identifying non-neoplastic lesions when findings are controversial, but its added value in clinical practice has yet to be proven (Lotti et al. 2021a). More recently, the same authors prospectively evaluated a large cohort of patients with Leydig cell tumors, reporting a good oncological prognosis when recognized early. The authors indicated that tissue-sparing enucleation is curative and should replace orchiectomy and that conservative surgery or active surveillance in compliant patients through clinical and radiological follow-up can be considered safe options.
2.3.1.8 Cryptorchidism
The term cryptorchidism is derived from the Greek words kryptos and orchis, literally meaning “hidden testis.” Cryptorchidism, or undescended testis, is the absence of at least one testicle in the scrotum (Lotti and Maggi 2015; Leslie et al. 2021). The undescended testis is commonly unilateral, being bilateral in one out of ten cases. It is associated with an increased risk of infertility and testicular malignancy. Cancer commonly occurs in the undescended testis; however, one out of five tumors occur in the contralateral descended testis. About 80% of undescended testes are located within the inguinal canal, 5–16% high in the abdomen, while rarely the testis can be ectopic from the path of descent or absent/vanishing. The AUA Guidelines report that in pediatric patients, more than 70% of cryptorchid testes are palpable by physical examination and need no imaging. In the remaining 30% of cases with a nonpalpable testis, the challenge is to confirm absence or presence of the testis and to identify its location (Leslie et al. 2021). The role of US in the pediatric preoperative planning before orchiopexy is controversial and has changed over time (Leslie et al. 2021). On the other hand, US is useful in adult men with a history of cryptorchidism/orchiopexy.
Until the last decade, some pediatricians used to require US to locate a nonpalpable undescended testis. In fact, a nonpalpable testis may be present in the inguinal-scrotal region or within the abdominal cavity, or it may be absent (Tasian and Copp 2011; Leslie et al. 2021). Surgical exploration is mandatory to localize the testis when present or confirm an absent testis. However, accurate presurgical diagnosis of an absent testis would spare a child unnecessary surgery, while the correct localization of the testis could limit the extent of surgery. Observational studies performed in pediatric patients until 2011 revealed conflicting diagnostic performance features and did not rigorously evaluate the clinical utility of US in localizing nonpalpable testes.
In 2011, a systematic review and meta-analysis (Tasian and Copp 2011) reported that US does not reliably localize nonpalpable testes or rule out an intra-abdominal testis in pediatric patients. Hence, all recent guidelines recommend against the use of US in pediatric patients since it does not change management nor add diagnostic accuracy, except in selective cases (e.g., suspicion of sexual development disorders) (Leslie et al. 2021).
On the other hand, guidelines dealing with US in adult men with a history of cryptorchidism are not available. However, it is well recognized that in adult men who have undergone orchiopexy US plays a key role in cancer detection or in the follow-up of the cryptorchid and contralateral testis (Lotti and Maggi 2015). Recommendations on the follow-up timing and duration in men with a history of undescended testis/orchiopexy are not available. Considering that cryptorchidism is a risk factor for testicular cancer more relevant than TML, recommendations given by the ESUR for the follow-up of “TML with additional risk factors” (Richenberg et al. 2015) could be suggested in principle, i.e., annual follow-up up to age 55. However, cryptorchid men should be educated on prevention with frequent self-examination of the testes, especially in the age range (15–34 years) (Lotti and Maggi 2015) associated with the highest occurrence of testicular malignancy, to identify a lump possibly underlying a growing tumor early.
As a corollary, some men may present to clinicians with a nonpalpable testis. Since some authors reported that US can reliably identify a cryptorchid testis lying below the level of the internal inguinal ring, US may be suggested to identify the undescended testis at the high scrotal level or in the inguinal canal (Lotti and Maggi 2015). However, if US was unreliable, other imaging investigations or surgical exploration should be suggested.
At US, the cryptorchid testis is often hypotrophic, inhomogeneous, hypoechoic, with or without macro- or microcalcifications and with normal or reduced vascularization (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015; Lotti et al. 2021a). However, especially in cases of early orchidopexy, it could show normal volume and echotexture. Echotexture inhomogeneity can be associated with one or more hypoechoic micronodular lesions, often resulting from cryptorchidism- or postoperative-related damage. Nodular lesions should be managed as reported above (see the paragraph “Testicular lesions”) and even more carefully considering the cryptorchidism-related risk of cancer. CEUS can distinguish vascularized from nonvascularized focal testicular lesions, helping to exclude malignancy (Sidhu et al. 2018).
2.3.2 Epididymis and Vas Deferens
2.3.2.1 US Anatomy
The normal epididymis is a soft organ that lies along the superior margin of the testis and is classically divided into three segments: head, body, and tail. At US, the epididymis is usually detected posterior-laterally to the testis, with the head and tail at the upper and lower pole of the testis, respectively (Lotti and Maggi 2015). The normal epididymal head is triangular, with echogenicity comparable to that of the testis and usually slightly more echogenic than the body and tail. Blood flow is detectable by CDUS in discrete vascular spots in all tracts of the epididymis (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015; Lotti et al. 2021a).
The vas deferens is a straight tense cord which extends along the spermatic cord. Vas deferens absence, with or without epididymal agenesis, is often a difficult palpatory diagnosis, to be confirmed by US. The vas deferens appears at US as a straight duct, slightly hypoechoic compared with the epididymis, originating from the epididymal tail and extending, along the spermatic cord, toward the inguinal channel (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015).
Until 2021, values indicating epididymal normal and abnormal size have been only suggested empirically and were lacking for the vas deferens. In addition, normal or enhanced epididymal vascularization was qualitative and operator-dependent concepts. Recently, the EAA US study (Lotti et al. 2021b) reported a standardization of the measurement of the epididymal-deferential parameters and led to the identification of reference ranges and normative thresholds for epididymal segments and vas deferens size and vascular parameters (see Table 6.2). Normal epididymal head, body, tail, and vas deferens size have been defined in an evidence-based way as <11.5, 5, 6, and 4.5 mm, respectively. In addition, normal values of different epididymal (and testicular) vascular parameters have been reported.
2.3.2.2 US Abnormal Patterns
CDUS plays a key role in investigating abnormalities of epididymal size, echopattern, and vascularization, which, alone or combined, can suggest different diagnoses.
2.3.2.3 Acute and Chronic Epididymitis
In subjects with scrotal pain or prostatitis-like symptoms, the dilation of the whole epididymis or one of its segments (especially head or tail) associated with hypervascularization suggests inflammation (Pilatz et al. 2013; Lotti and Maggi 2015; Lotti et al. 2021a). In the acute form, an enlarged hypoechoic inhomogeneous epididymis with enhanced vascularization is often detected, while in the chronic form the epididymis is often dilated and may appear hyperechoic and vascularization is only slightly increased (see below). A dilated epididymis associated with echopattern abnormalities (including calcifications) may also represent the outcome of a past infection/inflammation, currently asymptomatic. Acute epididymitis usually presents with a painful hemiscrotum, epididymal swelling, and fever. CDUS plays a key role in identifying diagnostic features, revealing mild-to-severe hyperemia and epididymal enlargement, mainly of the tail or both the tail and head, along with inhomogeneous echotexture, often hypoechoic with scattered hyperechoic foci, and reactive hydrocele with skin thickening. Concomitant orchitis, revealed in almost half of all the cases, is associated with hydrocele, testicular enlargement, hyperperfusion, and pain (Pilatz et al. 2013; Lotti and Maggi 2015). Under conservative treatment, epididymal CDUS parameters normalize (Pilatz et al. 2013). Hence, along with clinical characteristics, CDUS may play a role in the follow-up of acute epididymitis. Chronic epididymitis often involves the tail, with coarse calcifications in a variably enlarged hypo- or hyperechoic epididymis. The organ has an irregular profile, inhomogeneous, sometimes with hard irregularly shaped masses or indenting the testicular parenchyma, mimicking a primary testicular mass. Hydrocele and tunica albuginea thickening are commonly associated, the latter sometimes so severe as to be called “fibrous pseudotumor.” Acute and chronic epididymitis may result in abnormal sperm parameters and the occurrence of antisperm antibodies (Lotti and Maggi 2015; Lotti et al. 2021a). As a corollary, US evaluation of the epididymis after a scrotal trauma shows features mimicking epididymitis (Ramanathan et al. 2021; Morey et al. 2021).
2.3.2.4 Epididymal Nodules/Cysts
US allows the assessment of epididymal nodules, often perceived at physical examination, frequently represented by cysts or spermatoceles, but possibly underlying benign (including tuberculosis-related granulomatous masses) or, very rarely, malignant lesions (Isidori and Lenzi 2008; Bertolotto and Trombetta 2012; Lotti and Maggi 2015). At clinical evaluation, cysts and spermatoceles are detected as tense-elastic spherical formations within the epididymis, mainly located in the head. Epididymal cysts and spermatoceles are detectable in one out of four men and appear at US as anechoic avascular and slightly hypoechoic inhomogeneous formations, respectively. Their clinical significance and association with male infertility have not been yet defined, since their involvement in complete epididymal obstruction and obstructive azoospermia has never been proven. Conversely, epididymal injury secondary to excision surgery, mainly performed for large and painful lesions, may lead to epididymal obstruction. Hence, epididymal cyst surgery is not suggested for restoring fertility.
2.3.2.5 Epididymal and Deferential Dilation
In subjects with obstructive azoo- or oligospermia, the detection of epididymal enlargement may suggest post-testicular obstruction, which could be (i) at the epididymal level (especially when the downstream vas deferens shows a normal size), (ii) at the vas deferens level, especially in men treated by epididymovasostomy, after vasectomy, or with absence of vas deferens, or (iii) at the prostatic level, the latter to be further investigated extending US to the prostate-vesicular region (Lotti and Maggi 2015; Lotti et al. 2021a).
In subjects with distal obstruction, a dilated, inhomogeneous proximal vas deferens may be seen. These characteristics can be seen also in patients with vasectomy as well as chronic inflammation or diabetes which may cause luminal or parietal calcifications, respectively. An epididymal tail >6 mm and/or head >12 mm have been proposed as suggestive of epididymal inflammation, while a head >~11 mm as indicative of obstruction. After vasectomy, an epididymal head >15 mm or >2 cm has been reported. To date, according to the EAA US study (Lotti et al. 2021b), epididymal head, body, tail, and vas deferens size may be defined in an evidence-based way as >11.5, 5, 6, and 4.5 mm, respectively (Table 6.2).
2.3.2.6 Congenital Uni- or Bilateral Absence of Vas Deferens
Vas deferens, epididymis, and seminal vesicles embryologically originate from the Wolffian duct. Hence, when vas deferens agenesis occurs, it may be associated with agenesis/abnormalities of these structures. Subsequently, if vas deferens agenesis is detected at scrotal US, the examination should be extended to the prostate-vesicular region by TRUS. Interestingly, when complete vas deferens and epididymis agenesis occurs, epididymal head persists and is detectable by US (Lotti and Maggi 2015). Congenital bilateral absence of vas deferens (CBAVD) accounts for 1–2% of infertile men, 4–17% of azoospermic men, and up to 25% of those with obstructive azoospermia (Lotti and Maggi 2015). CBAVD may be isolated or associated with cystic fibrosis. Almost all men with cystic fibrosis also have CBAVD. About 80% of CBAVD subjects have at least one CFTR mutation. CBAVD is associated with bilateral seminal vesicle agenesis in about half of the patients and usually presents with normal kidneys. A few cases of CBAVD may present with kidney agenesis and are usually not related to CFTR gene mutations. Conversely, when kidney agenesis occurs, CFTR gene mutations are rarely involved. In subjects with CBAVD, testes are usually normal in volume and function. Hence, CBAVD investigation by US is essential in the diagnosis of obstructive azoospermia and for clinical decision-making, since surgical sperm retrieval is virtually always positive.
Approximately 1% of men have congenital unilateral absence of vas deferens (CUAVD (Lotti and Maggi 2015). CUAVD is associated with ipsilateral and contralateral seminal vesicles agenesis in 90 and 20% of patients, respectively, and with renal agenesis in 80% of cases. Subjects with CUAVD are usually fertile, but at high risk for infertility, having a single patent vas deferens. In addition, those with CUAVD and contralateral SV agenesis may have contralateral deferential ampulla atresia. Therefore, a subset of men with CUAVD may have abnormal semen parameters or azoospermia. Similar problems may be present in subjects with CUAVD and contralateral testis damage.
2.3.3 Pampiniform Plexus and Varicocele
2.3.3.1 US Anatomy
Normal pampiniform plexus is scarcely appreciable by physical examination, while CDUS is able to examine it with great accuracy (Lotti and Maggi 2015). In normal conditions, the pampiniform plexus appears as a complex network of small vessels converging into the spermatic veins. The right spermatic vein enters obliquely into the inferior vena cava, whereas the left one enters perpendicular into the left renal vein and is therefore burdened by higher blood hydrostatic pressure. When the pressure becomes excessive and/or the venous valvular mechanism is impaired, venous reflux and dilation may occur, leading to varicocele (see below).
2.3.3.2 US Abnormal Patterns: Varicocele
An abnormal dilatation of the pampiniform plexus characterized by retrograde venous flow indicates the presence of a varicocele (Lotti and Maggi 2015). According to the aforementioned anatomical considerations (see above), varicocele is mainly (90%) detected on the left side. The prevalence of varicocele, virtually absent in boys aged <11 years, increases with age, up to 15% in the general adult population. It has been estimated that the prevalence of varicocele in adult men with primary infertility is about 35% (Lotti and Maggi 2015). Most studies, but not all, report worse sperm parameters in subjects with varicocele. However, 75% of subjects with varicocele have normal semen parameters. In addition, one out of three fertile men has a varicocele (Lotti et al. 2020). Hence, the impact of varicocele on couple infertility is still under debate.
Varicocele is clinically classified into three grades: (1) palpated during Valsalva maneuver, (2) palpated without Valsalva maneuver, and (3) visible. While the clinical classification of varicocele has been universally accepted since 1970, the diagnosis and classification of varicocele with CDUS is one of the most debated topics in andrology/urology (Lotti and Maggi 2015; Freeman et al. 2020; Lotti et al. 2021a; b).
Several classifications have been proposed over time, with differences mainly related to the cutoff diameter to indicate a dilated vein, the indication or not of the vein’s extension in the scrotal sac for grading varicocele, duration of the venous reflux, and the presence or not of testicular hypotrophy in the most severe grade (Lotti and Maggi 2015; Freeman et al. 2020). In a 2015 systematic review (Lotti and Maggi 2015), Lotti and Maggi attempted to align the classifications available until then, supporting for the severe form the presence of a continuous venous reflux at rest, increasing or not during a Valsalva maneuver. In the aforementioned review, previous varicocele classifications have been described in detail. Table 6.5 shows the available CDUS classifications of varicocele severity.
In 2020, the ESUR published guidelines (Freeman et al. 2020) for detection, classification, and grading of varicocele. ESUR reported methodological recommendations, supporting a standardized protocol for varicocele US examination. According to ESUR guidelines, a gray scale and color Doppler examination, with spectral Doppler analysis, should be performed bilaterally with the patient supine and standing, during spontaneous breathing and during the Valsalva maneuver. Measurement of the largest vein, irrespective of location, with the patient in the upright position and during the Valsalva maneuver is recommended. A maximum venous diameter ≥3 mm can be considered diagnostic for a varicocele, grading varicocele according to Sarteschi classification. A reflux in the testicular veins lasting >2 seconds with the patient standing and during the Valsalva maneuver should be considered to be abnormal. TV should be measured in all cases. In patients with subclinical varicoceles imaging follow-up is recommended. After varicocele repair, US can be used to identify early postoperative complications. Sperm analysis forms the basis of follow-up after varicocele repair, without the routine use of US (Gravas et al. 2021). The recommendation against the routine use of US after varicocele repair did not gain a strong consensus (Gravas et al. 2021). The latter point depends on the fact that some members of the ESUR-Scrotal and Penile Imaging Working Group (SPIWG) involved in the guidelines’ production supported the idea that US after varicocele repair is necessary, in order to identify varicocele persistence or recurrence in the short term. This issue is relevant to understand if possible changes in sperm analysis after varicocele repair depends on the treatment itself or on casual oscillation in sperm parameters when there is no evidence of varicocele correction.
More recently, the EAA US study (Lotti et al. 2021b) assessed reference ranges for pampiniform plexus CDUS parameters in healthy, fertile men (Lotti et al. 2020). The EAA US consortium reported SOPs for varicocele evaluation, welcoming most, but not all, ESUR recommendations. In particular, the EAA consortium supported the measurement of the largest vein with the patient standing, at rest (and not during Valsalva maneuver) in order to avoid the possible confounder of a variable intraabdominal pressure increase with Valsalva, recommending Valsalva maneuver be used for varicocele grading, to be done according to Sarteschi et al./Liguori et al. classifications (essentially overlapping) (Lotti and Maggi 2015). In addition, the EAA consortium also suggested the evaluation of the maximum diameter of the internal spermatic vein between the inguinal ligament and upper pole of the testis (Lotti and Maggi 2015) besides the assessment of the convoluted vessels below, supporting the 3 cm threshold to define vein dilation. Finally, the evaluation of TV using the ellipsoid instead of Lambert’s formula was suggested (Lotti et al. 2021b). Of note, the EAA consortium defined “severe” varicocele as venous vessel dilation (>3 mm) characterized by a continuous (long-lasting, without reporting duration cutoff) venous reflux at rest, increasing, or not during a Valsalva maneuver (Lotti et al. 2021a; Lotti et al. 2021b), consistent with grade 4 and 5 varicocele according to Sarteschi et al./Liguori et al. classifications. Table 6.5 reports the varicocele classification proposed by the EAA US consortium.
The EAA study reported a varicocele prevalence of ~37% in fertile men (with a severe form in almost one out of five men), similar to that reported in primary infertile men (Lotti et al. 2021b). These data suggest that varicocele may exert a scanty effect on male fertility and that its surgical correction should be limited to highly selected populations (Lotti et al. 2020; Lotti et al. 2021b). Accordingly, current EAU Guidelines on male infertility (Salonia et al. 2021) support very specific indications for varicocele treatment both in adults and in adolescents.
3 Prostate and Seminal Vesicles US
The prostate–vesicular region can be studied by transabdominal or transrectal US (TRUS). Although some authors revealed no significant difference between the two US modalities to measure prostate volume, other authors reported that TRUS is more accurate in predicting adenoma volume in BPH patients (Lotti and Maggi 2015). In addition, in our opinion, TRUS has higher accuracy in detecting echotexture and vascular parameters. Hence, in this chapter we will focus on TRUS. TRUS is performed using a transrectal biplanar probe (linear and convex transducer, 6.5–7.5 MHz) and/or an “end fire” probe (6.5 MHz, field of view 50–200°), with the patient placed in the left lateral decubitus, scanning the organs in transverse, longitudinal, and oblique ways (Lotti and Maggi 2015).
3.1 Indications
Although EAU guidelines suggest a very limited value of TRUS in different fields (Gravas et al. 2021; Engeler et al. 2021; Salonia et al. 2021), with clear utility only in evaluating obstructive azoospermia, according to other recent publications TRUS can be useful for several aims (see below). Accordingly, possible TRUS indications are reported in Table 6.1.
3.2 Methodological Standards
As reported above for scrotal US, the EAA US study (Lotti et al. 2020) recently reported SOPs to assess TRUS according to a multicentric consensus and to previous studies and guidelines. A detailed description of the SOPs to evaluate TRUS quantitative and qualitative parameters has been reported on the EAA website (https://www.andrologyacademy.net/eaa-studies).
3.3 Anatomy, Normal and Abnormal Patterns, Clinical Utility, and Standards
Table 6.2 shows normal values and cutoff of the main US parameters of the prostate and seminal vesicles. Figure 6.3 shows a schematic representation of the normal and pathologic features of the organs of the prostate–vesicular region in relation to male reproductive health. Figure 6.3 shows some examples of normal and abnormal US features of the prostate-vesicular region. Normal and abnormal TRUS patterns are discussed below.
Normal (left side) and abnormal (right side) color Doppler ultrasound (CDUS) features of the prostate-vesicular region. Panel (a), prostate of normal volume, homogeneity, and echogenicity in transversal scan. Peripheral and transitional zones (PZ and TZ) show a 3:1 ratio in young men. Right and left lobes (RL and LL, respectively) and periprostatic venous plexus (PVP) are indicated. Anterior-posterior and transverse diameters (“apd” and “td,” respectively) are reported. Panel (b), prostate of normal volume, homogeneity, and echogenicity in sagittal scan evaluated with “end fire” probe. Peripheral and transitional zone (PZ and TZ, respectively) and apex (A) are indicated, as well as bladder (B), urethra (U, yellow dotted line), ejaculatory duct (green dashed line), prostatic utricle (*), deferential ampulla (DA), and periprostatic venous plexus (pvp). The longitudinal diameter (“ld”) is reported and represented with a white dashed line. Panel (c), right and left seminal vesicles (rSV and lSV, respectively) with typical “bow-tie” appearance and, medial to them, right and left deferential ampullas (sDA and lDA, respectively) in transversal scan. Panel (d), seminal vesicle (SV) assessed by “end fire” probe in sagittal scan. Fundus and body are reported, as well as longitudinal and anterior-posterior diameters (“ld” and “apd” dashed lines, respectively). A schematic model of SV volume calculation is reported, using the “ellipsoid/prolate spheroid (d1 >d2 = d3) ” (red ellipse) mathematical formula (d1 × d2 × d3 × 4/3 × π), with d1 = ld and d2 = apd, and d3 assumed = d2 (red dashed line) (according to Lotti et al. 2012). Panel (e), distal vas deferens (dVD) and deferential ampulla (DA) beside a section of the seminal vesicle (SV) assessed by “end fire” probe in sagittal scan. Bladder (B) and prostate (Pr) are visible. Panel (f), left figure: section of a dilated deferential ampulla (DA) beside a dilated seminal vesicle (SV) with areas of endocapsulation (*) and thick septa (arrow) detected by “end fire” probe in sagittal scan. Right figure: dilated, inhomogeneous epididymal tail and proximal vas deferens (pVD), with coarse calcifications (arrow). Panel (g), left figure: dilated (>12 mm), inhomogeneous, hypoechoic epididymal head; right figure: abrupt interruption of the proximal vas deferens (pVD) in a man with congenital bilateral absence of vas deferens. Epididymal body and tail are also visualized in sagittal scan. Panel (h), prostate with hyperemia end elevated arterial peak systolic velocity. Panel (i), midline prostatic cyst (*) in transversal (left) and sagittal (right) scan. The prostatic utricle is indicated with an arrow. P, prostate; B, bladder. Panel (j), upper figure, ejaculatory duct dilation (arrow) and microcalcification (short arrow), and seminal vesicle cyst (#), assessed by “end fire” probe in sagittal scan. Lower figure, ejaculatory duct cyst (*). SV, seminal vesicle; Pr, prostate; U, urethra; B, bladder
TRUS clinical utility and impact on male reproductive health management are reported in Table 6.3. So far, TRUS has shown a relevant impact on both reproductive and general male health (Lotti and Maggi 2015) (Table 6.1). In fact (i) TRUS shows a key role in obstructive azoospermia; (ii) TRUS is useful in evaluating prostate inflammation and related pelvic pain or premature ejaculation; (iii) TRUS is useful in evaluating prostate volume in relation to LUTS. In addition, (iv) TRUS can offer indirect information on male androgenization evaluating prostate and seminal vesicles volume, which are reduced in hypogonadal subjects. (v) However, TRUS is not useful in assessing prostate cancer.
3.3.1 Prostate
3.3.1.1 US Anatomy
The prostate is an exocrine gland, which surrounds the urethra just below the neck of the bladder. It produces prostatic fluid, an acidic secretion that makes up ~20% of the total ejaculate (Lotti and Maggi 2015). At TRUS, the normal prostate appears different according to age, with a triangular or pear shape in younger and older subjects, respectively. Its base lies at the bladder neck, at the beginning of the urethra, detectable in a longitudinal scan as a hypoechoic duct curving toward the prostatic apex. TRUS identifies a peripheral zone, which extends laterally and posteriorly from the apex to the base, and a transitional zone, centrally located and slightly hypoechoic. Peripheral and transitional zones show a 3:1 ratio in young men. A central zone has also been described. Prostate volume (PV) is often measured using a planimetric method (Behre et al. 1995; Lotti and Maggi 2015; Lotti et al. 2014a). It is calculated by measuring three diameters (anterior-posterior and transverse in the transversal scan, longitudinal in the sagittal one; using the mathematical formula of the ellipsoid. Transitional zone or adenoma volumes are similarly calculated. A PV of 20–25 ml has been previously proposed as “normal” in young men. However, recently, the EAA US study reported as “normal” a prostate volume between 15 ml and 35 ml (Table 6.2). The normal adult prostate shows thin, densely packed, and homogeneously deployed echoes. Periprostatic venous plexus is detectable as a slightly hypoechoic system of vessels. Intraprostatic arteries are grouped in central/periurethral and peripheral/capsular arteries, supplying the transitional and peripheral zones, respectively. The ejaculatory ducts (EDs) appear at TRUS as fine and hypoechoic, with a normal diameter <2 mm. They are detectable in longitudinal scans crossing the prostate up to the urethra (Lotti and Maggi 2015).
3.3.1.2 US Normal and Abnormal Patterns
3.3.1.2.1 Prostate Volume
Detection of prostate volume (PV) by TRUS is important in subjects with LUTS, since it can predict LUTS progression and the risk of complications (Gravas et al. 2021), while it has a low impact in the workup of male infertility (Lotti and Maggi 2015) (Table 6.3). A reduced PV suggests hypogonadism, because the prostate is an androgen-dependent gland (Lotti and Maggi 2015). An increased PV is related to benign prostatic enlargement (BPE). A PV >30 ml has been previously suggested as indicative for initial gland enlargement (Lotti and Maggi 2015). However, according to the EAA US study, prostate enlargement should be defined as >35 ml (Table 6.2). A PV >60 ml has been suggested as indicative for a severe increase (Lotti and Maggi 2015). BPE has a continuum spectrum of TRUS abnormalities ranging from larger transitional zone to a well-defined adenoma. The typical TRUS characteristics of BPE are echotexture inhomogeneity, occasional cysts, well- and poorly defined nodules, and calcifications, especially at the “surgical capsule” (Lotti and Maggi 2015). Interestingly, BPE has been recently associated with overweight/obesity and metabolic syndrome (Lotti et al. 2014a).
3.3.1.2.2 Prostate Inflammation
Several TRUS features are considered suggestive of chronic prostate inflammation, including glandular asymmetry, hypo- or hyperechogenicity with calcifications and periprostatic venous dilation (Lotti and Maggi 2015). However, the aforementioned criteria are observational and not evidence-based, qualitative in some cases (hypo- or hyperechogenicity), lacking thresholds identifying abnormal patterns in others (no cutoff for glandular asymmetry or periprostatic venous dilation), or “static” (the presence of prostate calcifications is long-lasting and detectable lifelong in the same subject), suggesting chronic/past but not acute/subacute inflammation. Subsequently, increasing evidence suggested a TRUS role in identifying current prostate inflammation by evaluating “dynamic” CDUS findings such as hyperemia and high peak systolic velocity (PSV) detected in prostatic arteries. Cho et al. suggested ≥15 parenchymal Doppler spots as indicative of prostate hyperemia (Lotti and Maggi 2015). Berger et al. suggested a PSV >15 cm/s, evaluated in the arteries of the transitional zone, to define prostatic inflammation in men with age >45 years and benign prostatic hyperplasia (Lotti and Maggi 2015). Lotti et al. reported, in an evidence-based way, that a prostatic artery PSV >11 cm/s could identify prostatitis-like symptoms in relatively young subjects (males of infertile couples) (Lotti et al. 2014b) (see Table 6.3). Accordingly, in healthy fertile men, the recent EAA US study found a prostatic artery PSV <11 cm/s (Table 6.2). As a corollary, some authors demonstrated that prostatic arterial PSV was positively associated with a score related to premature ejaculation severity, confirming from an US point of view that clinical prostate inflammation is an organic cause of premature ejaculation (Lotti and Maggi 2015).
3.3.1.3 Ejaculatory Duct (ED) Obstruction/Abnormalities
Ejaculatory duct obstruction (EDO) affects 1–5% of infertile men and may be congenital or acquired. Congenital causes include EDs atresia/stenosis, midline prostatic cysts, or ED congenital cysts (Lotti and Maggi 2015; Singh et al. 2012). Acquired causes may be secondary to infection/inflammation, post-infective/inflammatory midline prostatic cysts, calcifications, or iatrogenic. Detection of bilateral EDO by TRUS is useful in defining the diagnosis of obstructive azoospermia and its clinical management, considering surgical treatments if specific abnormalities are found (see below and Table 6.3). Subjects with congenital or noninfectious causes of EDO, or with partial EDO, have better improvements in semen parameters after treatment than those with infectious causes or complete EDO. TRUS findings in suspected EDO include midline prostate cysts and ED dilation, calcifications, or cysts (Lotti and Maggi 2015). Dilated SV (anterior-posterior diameter >15 mm) and enlarged deferential ampulla (diameter >6 mm) have also been previously suggested as EDO-related findings (see below). As a corollary, Lotti et al. (2012) proposed a new parameter related to the SV emptying capacity, “SV ejection fraction,” reporting a cutoff (21.6%) suggestive of complete or partial EDO. However, further studies are needed to assess the clinical relevance of this parameter.
Intraprostatic cysts can be classified as congenital or acquired, or, based on their position within the prostate, as midline, paramedian, and lateral cysts (Singh et al. 2012; Lotti and Maggi 2015). Midline cysts affect 1–5% of men, with a higher frequency in infertile men. They may cause partial or complete EDO, with reduced sperm count or obstructive azoospermia, respectively, often associated with SV obstruction/dilation, reduced ejaculate volume, and pH. At TRUS, they appear as roundish or pear/oval-shaped anechoic formations in transversal and longitudinal scans, respectively. According to previous studies, two main different cystic entities have been recognized. The first, Müllerian cyst, is thought to arise from a regression failure of the Müllerian ducts, causing a focal saccular dilation. This cyst is located at midline or slightly lateral to midline, is large and may extend above the base of the prostate, does not communicate with the urethra or contain spermatozoa, and may be associated with various genitourinary abnormalities. However, it eventually may erode ED and include sperm. The second, utricular cyst, is thought to derive from dilation of the prostatic utricle, is strictly midline, smaller than the former, and confined to the prostate; it communicates with the urethra and usually contains spermatozoa. Both midline cysts may cause EDO by deviating or compressing ED. Midline cyst-related EDO may be diagnosed only after TRUS-guided cyst aspiration (Singh et al. 2012; Lotti and Maggi 2015; Lotti et al. 2018), which will allow cyst reduction and restore semen emission. This is of clinical relevance, since aspiration of large cysts in subjects with obstructive azoospermia may lead to semen parameter improvement (Table 6.3). However, after this procedure, midline cysts may enlarge and lead to EDO and azoospermia again, after variable times. In this case, TRUS should be considered to evaluate cyst recurrence (Table 6.3). Various complications may be associated with prostate cysts, besides infertility, such as urinary tract infection, pain, recurrent epididymitis or prostatitis, and hemospermia (Singh et al. 2012). A recent study (Lotti et al. 2018) performed on a large series of males of infertile and fertile couples reported that infertile men with a midline prostatic cyst (MPC) showed a lower seminal volume and sperm count and a higher prevalence of azoospermia than the rest of the infertile sample or fertile men, and a higher frequency of US signs suggestive of EDO. A midline prostatic cyst with a volume >0.117 ml or a transversal diameter >5 mm identified subjects with severe oligo- or azoospermia with an overall accuracy of ~75%. Accordingly, in fertile men, the highest MPC volume was 0.117 ml, suggesting it as a biological threshold not compromising semen quality. Eleven men with infertility, semen abnormalities, and large MPC (>0.250 ml) underwent TRUS-guided cyst aspiration, which led to sperm count improvement in all patients and natural pregnancy in some cases. Finally, the authors proposed an algorithm, based on semen parameters, useful in identifying a MPC in males of infertile couples. Hence, midline prostatic cyst size cutoffs for complete or partial EDO are now available, and large cysts must be considered as a possible easily treatable causes of obstructive infertility. In fact, TRUS-guided cyst aspiration can be suggested for a cyst volume >0.250 ml (and, eventually, for a volume >0.117 ml) in subjects with suspected obstructive severe oligo- or azoospermia, especially with FSH <8 U/l, to obtain seminal parameters improvement and eventually natural pregnancy.
Ejaculatory duct (paramedian) cysts may be congenital, originating from Wolffian ducts, or acquired, and may be related or not to cystic fibrosis (Singh et al. 2012). Uni- or bilateral, they may lead to obstructive azoospermia. In this case, cyst detection by TRUS is useful in clinical management (Table 6.3), their surgical treatment often restoring semen emission (Lotti and Maggi 2015). Ejaculatory duct dilation has been defined as an ED diameter >2 mm (Lotti and Maggi 2015; Singh et al. 2012) and may be related to inflammatory distal stenosis, which is often difficult to detect. Ejaculatory duct calcifications may be associated with EDO, but are not a reliable indicator of it (Lotti and Maggi 2015). They have also been associated with hemospermia and prostatitis-like symptoms (Lotti et al. 2014b; Lotti and Maggi 2015). Accordingly, EDO may be associated with hemospermia, prostatitis, and painful ejaculation. In select cases, transurethral resection of EDs resulted in marked improvement in semen parameters, and pregnancies have been achieved (Lotti and Maggi 2015).
3.3.1.4 Prostate Cancer
Prostate cancer is usually suspected on the basis of digito-rectal examination and/or increased PSA levels. At TRUS, prostate cancer is often seen as a hypoechoic lesion in the peripheral zone of the gland; however, it can be isoechoic or hyperechoic, and it is not always anatomically well defined (Sarkar and Das 2016). Definitive diagnosis depends on histopathological verification of cancer in prostate biopsy cores or specimens from transurethral resection of the prostate or prostatectomy for benign prostatic enlargement (Mottet et al. 2021). According to EAU guidelines, gray scale TRUS is not reliable in detecting prostate cancer (Mottet et al. 2021). Thus, there is no evidence that US-targeted biopsies can replace systematic ones and there is not enough evidence for TRUS routine use in prostate cancer assessment. Nowadays, multiparametric MRI (mpMRI) is considered the best imaging tool to evaluate prostate cancer with high aggressiveness (Mottet et al. 2021). Adherence to PI-RADS guidelines for mpMRI acquisition and interpretation is strongly recommended.
3.3.2 Seminal Vesicles and Deferential Ampullas
3.3.2.1 US Anatomy
Seminal vesicles (SV) are paired and saccular structures, which lie superior and posterior to the prostate between the bladder and the rectum. They produce an alkaline fluid contributing ~80% of the ejaculate volume. At TRUS, SV have a typical “bow-tie” appearance in transversal scans, and a tennis-racket shape in longitudinal scans (Lotti et al. 2012; Lotti and Maggi 2015). SV echotexture is characterized by homogenous fine echoes and is slightly less echogenic than the prostate. In relatively young subjects, SV volume is negatively associated with age and tends to shrink after the fifth decade, showing a significant reduction in the eighth compared to the fourth decade. SV volume increases with sexual abstinence, whereas it decreases in current smokers as a function of smoking habit and of lifetime exposure to cigarette smoking. SV volume is also positively regulated by testosterone (Lotti and Maggi 2015), prolactin (Lotti et al. 2013), and free triiodothyronine (fT3) levels (Lotti et al. 2016). While most of the available studies assessed SV diameters, Lotti et al. (2012) proposed to calculate SV volume by measuring the maximum longitudinal and anterior–posterior diameters, using the “ellipsoid/prolate spheroid” mathematical formula. SV volume varies with ejaculation and is positively related to the ejaculate volume, but not with sperm parameters. SV emptying with ejaculation is positively related to fT3 levels, and subjects with subclinical hyperthyroidism show a higher reduction of SV longitudinal diameters after ejaculation as compared with eu- and hypothyroid men (Lotti et al. 2016). The deferential ampullas appear at TRUS as oval structures medial to the SV in transversal scans, cephalic to the prostate, or as distal VD enlargements in longitudinal scans (Lotti and Maggi 2015). They have an echotexture similar to that of SV.
3.3.2.2 US Normal and Abnormal Patterns
3.3.2.2.1 Volume
SV volume abnormalities include dilation and hypoplasia. SV dilation has been defined, based on SV diameters, as a SV anterior–posterior diameter >14 mm or 15 mm, suggestive of EDO (Lotti and Maggi 2015). Lotti et al. (2012) proposed an algorithm calculating SV volume. So far, a volumetric cutoff for SV dilation is lacking. However, the EAA US study recently reported the reference ranges of SV diameters and volume in healthy, fertile men, suggesting the higher SV reference values evaluated after ejaculation as possible cutoffs distinguishing normal and dilated SV (Table 6.2). A higher post-ejaculatory SV volume has been associated with a higher prevalence of SV abnormalities (see below), a higher prostate volume, and detection of a prostatic midline cyst (Lotti et al. 2012), causing partial or complete EDO (Lotti et al. 2018), as well as signs suggestive of upstream MGT dilation, such as higher deferential and epididymal tail diameters (Lotti et al. 2012).
SV hypoplasia has been defined as a SV anterior–posterior diameter <5 mm or <7 mm or as SV longitudinal diameter <25 mm (Lotti and Maggi 2015). The term mainly refers to congenitally small SV, although an acquired form may be associated with testosterone deficiency. So far, a SV volume cutoff for SV hypoplasia is lacking. Similarly, to as reported above for SV dilation, the EAA US study suggested the lower reference values of SV diameters and volume evaluated after ejaculation as possible cutoffs distinguishing normal and hypoplastic SV (Table 6.2).
3.3.2.3 Echopattern Abnormalities
Several US features are suggestive of SV abnormalities and have been associated with inflammation or stasis. In particular, “SV areas of endocapsulation,” that should be assessed after ejaculation (Lotti et al. 2012; Lotti et al. 2018), are considered a feature suggestive of EDO (Lotti and Maggi 2015).
3.3.2.4 Obstruction-Related Findings
Enlarged SV anterior-posterior diameter, as well as “SV areas of endocapsulation” (see above), has been related to partial EDO (Lotti and Maggi 2015). Diagrams showing partial EDO percentage probability in function of SV anterior-posterior diameter variation have been reported. Reduced “SV ejection fraction” (<21.6%) is suggestive of impaired SV emptying and partial EDO and is associated with higher prevalence of SV giant cysts and ED abnormalities (dilation, calcifications, or cysts) (Lotti et al. 2012).
Reduced or absent contraction of SV during ejaculation without a clear obstructive cause has been defined as “functional SV atony.” Signs suggestive of SV atony have been reported in subjects with type 2 diabetes mellitus with or without diabetic neuropathy (Lotti and Maggi 2015).
3.3.2.5 SV Agenesis, Hypoplasia, and Cysts
SV congenital abnormalities include defects in number (agenesis, fusion), maturation (hypoplasia), and canalization (cysts) of the glands (Lotti and Maggi 2015). Their detection is clinically relevant, because they are often associated with abnormal development of other mesonephric/metanephric derivatives, such as the vas deferens, ureter, and kidney, which should be evaluated by US. Unilateral SV agenesis arises if an insult occurs before the seventh week of gestation, when the ureteric bud arises from the mesonephric duct (Kim et al. 2009; Lotti and Maggi 2015). It is often associated with ipsilateral renal agenesis (~80%) or other renal abnormalities (Kim et al. 2009). Bilateral SV agenesis is associated with CFTR mutations in ~70% of cases, with CBAVD in half of the cases and with normal kidneys (Kim et al. 2009). Congenital SV hypoplasia may be isolated or associated with other congenital genitourinary anomalies (Kim et al. 2009). SV cysts are rare and may be congenital or acquired (Lotti and Maggi 2015). Congenital SV cyst may be isolated or, more frequently, associated with other genitourinary anomalies. They are mainly secondary to EDO caused by development abnormalities of the distal portion of the mesonephric duct. They are associated with ipsilateral renal agenesis (Zinner syndrome) or dysgenesis in two-thirds of cases (Lotti and Maggi 2015). Ectopic ureteral insertion into the SV, ED, vas deferens, or prostatic urethra or vas deferens agenesis may also be present (Kim et al. 2009). Bilateral SV cysts have been reported to occur in about half of patients with autosomal-dominant polycystic kidney disease (Lotti and Maggi 2015). Hence, detection of SV cysts by TRUS is clinically relevant, leading to careful evaluation of the urinary tract by US. Acquired cysts are usually unilateral and associated with inflammation-related EDO (Lotti and Maggi 2015). Cystic SV dilatation has been associated with perineal pain.
4 Specific Applications of Scrotal and Transrectal US
4.1 Sensitivity and Specificity in Discriminating Obstructive and Nonobstructive Azoospermia
Du et al. (2010) reported that performing simultaneous scrotal and transrectal US, assessing TV and genital tract obstruction-related findings, respectively, discriminates obstructive and nonobstructive azoospermia (OA and NOA, respectively) with 95% sensitivity and 97% specificity and may be of help in evaluating OA etiology. Abdulwahed et al. (2013) reported that scrotal US is more sensitive for OA and specific for NOA detection, while TRUS showed the opposite trend. Both imaging examinations had greater specificity than sensitivity for OA, indicating that US is better able to exclude, more than to diagnose, OA. However, US is still unlikely to replace testicular biopsy.
4.2 Testis US and Surgical Sperm Retrieval in Azoospermic Subjects
Sperm retrieval by testicular surgery has been reported in 50–60% of men with NOA and in almost all with OA (Lotti and Maggi 2015). Among the different CDUS characteristics investigated as predictors of successful sperm retrieval by testicular biopsy in azoospermic men, testicular parameters such as TV and vascularization have shown increasing evidence in the last few years, although still controversial and with limited clinical utility (see below). Azoospermic men with normal FSH levels and positive sperm retrieval have higher TV compared to those with a negative harvesting (Lotti and Maggi 2015). TV in OA has been reported as higher than in NOA patients (Du et al. 2010). Subjects with CBAVD-related OA usually have testes with normal volume and function (Singh et al. 2012) and sperm retrieval by testicular biopsy is virtually certain. In NOA subjects, most, but not all, studies reported higher TV in subjects with a positive sperm retrieval (Lotti and Maggi 2015). TV has been reported as an independent parameter related to testicular biopsy outcome. A total TV of 16 ml or a mean TV of 9.5 ml have been proposed as a cutoff for a positive sperm retrieval (Lotti and Maggi 2015). However, other authors report that TV is not a useful parameter for sperm retrieval prediction (Lotti and Maggi 2015). In fact, sperm can be retrieved by surgery even in men with very small testis, such as those with Klinefelter’s syndrome (Lotti and Maggi 2015). On the other hand, subjects with spermatogenic arrest, usually showing normal TV and FSH levels, are characterized by a poor surgical outlook. Hence, even if US has some prognostic value in surgical sperm retrieval outcomes, it is limited, playing a limited role in the workup of the infertile male. Interestingly, some vascular parameters detected by testicular CDUS have been associated with sperm quality (Lotti and Maggi 2015; Lotti et al. 2021a), suggested as useful in discriminating OA and NOA or residual spermatogenic areas in NOA. However, at present, they have been evaluated only for research purposes, with no impact on the clinical management of the azoospermic men.
4.3 Scrotal and Transrectal US and Hormonal Treatments
The US assessment of testis, prostate, and SV characteristics before the beginning and/or during hormonal treatment represents an effective tool in evaluating the response of target organs and in monitoring suspicious findings. Baseline US-assessed TV represents one of the main determinants of gonadotropin responsiveness in subjects with hypogonadotropic hypogonadism (HH). In fact, a better response in terms of sperm output and ongoing pregnancy has been observed for basal TV >4 ml, although a subsequent meta-analysis failed to find any significant association between TV and appearance of spermatozoa in the semen upon gonadotropin therapy. In addition, US has been performed by some authors to evaluate TV increment during hormonal treatment. GnRH or gonadotropin treatments in HH subjects were associated with a TV increase. A 12-week treatment with FSH in men with idiopathic infertility demonstrated a TV increase of 5 ml compared to baseline (Lotti and Maggi 2015). Considering that among infertile and azoospermic men, the risk of testicular malignancy is higher, scrotal US could be performed with prevention purpose in azoospermic and/or HH subjects unresponsive to hormonal treatment (Lotti and Maggi 2015). However, at present, no agreement on US testis surveillance in these subjects is available. TRUS is a useful tool in evaluating prostate response to hormonal treatment in HH subjects under gonadotropin, GnRH, or T treatment (Behre et al. 1995; Lotti and Maggi 2015), monitoring their possible effect on volume increment. In addition, SV volume shows changes at US during hormonal supplementation of hypogonadal men or hypogonadic subjects with Klinefelter syndrome (Lotti and Maggi 2015).
5 Penile US
5.1 Penile US: Indications
Indications for performing penile US are reported in Table 6.1.
5.2 Penile US: Methodological Standards
Although penile US assessment is not fully standardized, efforts toward methodological standardization and interpretation of findings have been made (Aversa and Sarteschi 2007; Patel et al. 2012; Sikka et al. 2013). In particular, standard operating procedures (SOPs) for the vascular assessment of ED have been reported (Sikka et al. 2013). An isolated, quiet room with dim light is required. The patient must be lying on his back and a 7–15 MHz linear probe must be used and located at the peno-scrotal junction with the penis resting on the soprapubic region. The position of the sample volume should be at the peno-scrotal junction, because measurements at more distal sites are influenced by the intracavernosal pressure degree, leading to lower PSV detection. The sampling of the main cavernous artery must be verified to avoid secondary vessel or a penetrating branch from the dorsal artery. The angle of insonation should be between 40° and 60°, preferably 60°. The sample volume and angle correction should follow the direction of vascular flow. In our opinion, vascular parameters should be evaluated in the first linear part of the cavernosal artery, immediately after the end of the cavernous artery bending in the cavernous body, avoiding ascents and descents of “corkscrew” arteries characterized by flow turbulence. Although some authors (Corona et al. 2008; Corona et al. 2010; Rastrelli et al. 2014) report that flaccid PCDU could be performed alone or before dynamic evaluation, the universal consensus is to perform dynamic PCDU under pharmacologically induced erection (Sikka et al. 2013; Salonia et al. 2021). For this purpose, an intracavernosal injection (ICI) of a single or combination of vasoactive agents (e.g., prostaglandin E1 [PGE1], phentolamine, and/or papaverine) is administered. ICI with 10 mcg PGE1 is frequently used in clinical practice and in most of the available studies. After vasodilating agent injection, Doppler measurements of vascular parameters should be monitored for at least 20 (Aversa and Sarteschi 2007) - 30 (Sikka et al. 2013) minutes. In fact, the highest PSV develops after 5–6 minutes from the vasodilating agent injection in four out of five subjects, while about 20% of patients have a greater latency period (range of 1–18 min). Patient’s anxiety should be avoided or reported in the medical report, since its related sympathetic stimulation can lead to vasoconstriction and false low PSV measurement in healthy men (Aversa and Sarteschi 2007; Patel et al. 2012; Sikka et al. 2013). The most frequently used vascular parameters considered to evaluate ED in dynamic conditions are peak systolic velocity (PSV) and end-diastolic velocity (EDV). In most of the studies, a PSV <35 cm/s is considered suggestive of arterial insufficiency (Aversa and Sarteschi 2007; Sikka et al. 2013; Patel et al. 2012), while a PSV <25 cm/s is indicative of “severe” arterial insufficiency. An EDV >5 cm/s is suggestive of veno-occlusive dysfunction (VOD) (Aversa and Sarteschi 2007). Although the aforementioned cutoffs are commonly used, the EAU suggests a PSV <30 cm/s and EDV >3 cm/s as indicative of arterial insufficiency and VOD, respectively (Salonia et al. 2021). Attention should be paid to diagnosis of VOD. Primary VOD is in fact relatively rare, usually due to tunica albuginea degenerative changes (e.g., in Peyronie’s disease, diabetes, elderly men) or traumatic injury (penile fracture), structural alterations in the fibroelastic components of the trabeculae, cavernous smooth muscle, and endothelium (e.g., in Peyronie’s disease), or acquired venous shunts (e.g., after surgical correction of priapism) in presence of normal arterial supply. More frequently, VOD is secondary to arterial insufficiency (Sikka et al. 2013). In this case, VOD does not represent the cause of ED, but a PCDU finding associated with low arterial flow. In fact, reduced arterial flow cannot guarantee blood supply enough to allow a complete swelling of the corpora cavernosa, leading to low or absent compression of peripheral veins against the tunica albuginea and venous leakage (Patel et al. 2012). Finally, in flaccid conditions, a PSV <13 cm/s is considered indicative of arterial insufficiency (Corona et al. 2008), while a FPA <1.17 m/s (Lotti et al. 2021a) is suggestive of severe arterial insufficiency and is considered a predictor of forthcoming MACE (Rastrelli et al. 2014).
5.3 Penile US: Anatomy, Normal and Abnormal Patterns, Clinical Utility, and US Standards
Figure 6.4 shows some normal and abnormal vascular and structural patterns of the penis.
Normal and abnormal US patterns of the penis. Panels (a–e): Vascular anatomy end evaluation of erectile dysfunction. Panel (a). Corpora cavernosa (cc) and corpus spongiosum (cs) of the penis in transversal scan. Cavernosal arteries are revealed by CDUS as red dots in the center of the corpora cavernosa. Panel (b). Normal cavernosal artery detected in sagittal scan. Arrows indicated the right position to locate the sample volume to evaluate vascular CDUS parameters. Panel (c). The sample volume and angle correction (preferably 60°) should follow the vascular flow direction. A normal waveform at dynamic PCDU is shown, characterized by a rapid ascent (steep rise, culminating in a sharp peak) and a relative rapid descent, with a normal peak systolic velocity (>35 cm/s) and end-diastolic velocity (<5 cm/s). Panel (d). A normal waveform at flaccid PCDU is shown, characterized by a rapid ascent (steep rise, culminating in a sharp peak) and a relative rapid descent, resembling a “mountain,” with a normal peak systolic velocity (>13 cm/s) and acceleration. Panel (e). An abnormal waveform at flaccid PCDU is shown, characterized by a slow ascent and slow descent, resembling a “hill,” with an abnormal peak systolic velocity (<13 cm/s) and acceleration (<1.17 m/s2) in a subject with erectile dysfunction and type 2 diabetes. Panels (f–j): US characteristics of Peyronie’s disease. Panel (f). Abnormal/thickened (left side) and normal (<2 mm) tunica albuginea (transversal scan). Panel (g). Thickened, hyperechoic, intercavernosal septum (transversal scan). Panel (h). Thickened intercavernosal septum with a calcification and periseptal fibrosis (slightly hyperechoic regions of the corpora cavernosa) (transversal scan). Panel (i). Hypoechoic plaque of the tunica albuginea in a subject with recent onset of Peyronie’s disease (longitudinal scan). Panel (j). Hyperechoic, calcified plaque under the tunica albuginea, involving the corpora cavernosa, in a subject with long-lasting Peyronie’s disease (longitudinal scan)
5.3.1 US Anatomy and Normal Patterns
A detailed description of penile US anatomy is available in previous studies (Patel et al. 2012; Jung et al. 2018). The penis consists of two paired corpora cavernosa, which are the main erectile bodies, and the corpus spongiosum, which contains the urethra. The corpora cavernosa are wrapped in the tunica albuginea, a resistant, inextensible, connective envelope, and are separated by the intracavernosal septum. The intercavernous septum is perforated, allowing for communication of blood (and injected pharmacological agents) across the midline. Proceeding from the inside out of the penis, Buck fascia and dartos fascia are located below the skin. Between the tunica albuginea and the Buck fascia, in the dorsal portion of the penis, two dorsal penile arteries run, the deep dorsal vein and paired neurovascular bundles. The superficial dorsal veins are contained between the Buck fascia and the outer dartos fascia. The inner portion of the corpora consists of interconnected sinusoids separated by smooth muscle trabeculae and is crossed by cavernosal arteries in the central part. The primary source of blood supply to the penis is usually through the internal pudendal artery, which arises from the anterior division of the internal iliac artery. The internal pudendal artery becomes the common penile artery after giving off a branch to the perineum. The branches of the penile artery are the dorsal artery, the bulbourethral artery, and the cavernosal artery. During erection, the cavernosal artery is responsible for the tumescence of the corpus cavernosum during erection, and the dorsal artery and the bulbourethral artery are responsible for engorgement of the glans penis. Distally, three branches of penile arteries join to form a vascular anastomotic ring near the glans. Variants in arterial anatomy exist in up to 20% of men, but their relevance to clinical practice is not fully established. The cavernosal artery gives off many helicine arteries, which in turn flow into the sinusoids via multiple arterioles supplying the sinusoids and the trabecular erectile tissue. The helicine arteries are contracted and tortuous in the flaccid state and become dilated and straight during erection. Venous drainage from the three corpora originates in the tiny venules beneath the tunica albuginea, forming the subtunical venular plexus before exiting as the emissary veins. Outside the tunica albuginea, a prominent deep dorsal vein provides the main venous drainage via the spongiosal, circumflex, and cavernosal veins. Then, a single deep dorsal vein runs upward behind the symphysis pubis to join the periprostatic venous plexus.
PCDU dynamic “phases” and related changes in the waveform have been well described elsewhere (Aversa and Sarteschi 2007; Patel et al. 2012). In the flaccid penis, the resting smooth muscle tonicity of the cavernosal arterioles and sinusoids is elevated, leading to a high-resistance vascular bed with resultant low volume inflow and outflow. When stimulated to erection, relaxation of the smooth muscle occurs through increased parasympathetic drive from the sacral nervous plexus. This relaxation results in increased cavernosal artery flow, leading to engorgement of the cavernosal sinusoids and penile lengthening and tumescence. The engorging sinusoids compress the exiting venules and emissary veins, leading to passive limitation of venous outflow. The high inflow and restricted outflow rapidly increase intracorporal pressure leading to erected penis rigidity. When intracorporal pressure nears systolic pressure, reduction in inflow will also normally occur. When intracorporal pressure exceeds systolic pressure for a long time, as in the case of ischemic priapism, reduction in inflow occurs.
At US, in sagittal scans, the corpora cavernosa are depicted as longitudinally orientated vascular beds of mixed echogenicity, with the tunica albuginea visualized as a thin echogenic envelope, usually <2–3 mm thick (Patel et al. 2012; Jung et al. 2018). In the absence of cavernosal fibrosis, the interface between the tunica and the underlying cavernosal tissue is distinct. The corpus spongiosum is visualized on the ventral surface and is of slightly higher reflectivity than the corpora cavernosa. The cavernosal arteries can be identified within the corpora cavernosa at US as parallel hypoechoic lines inside a hyperechoic binary. In transversal scan, the two corpora cavernosa are displayed as two circles of mixed echogenicity, with the surrounding tunica albuginea and a hypoechoic central spot with a thin hyperechoic labrum (section of the cavernosal artery), while the corpus spongiosum appears as a smaller median circle between and below their ventral and median surfaces. At rest, the normal cavernosal artery has a diameter of 0.3–0.7 mm (Patel et al. 2012), and an intima-media thickness (IMT) <0.3 mm (Caretta et al. 2019). At color Doppler, in longitudinal scan, a colored (red) line can be observed running across each corpora cavernosa, representing the cavernosal artery, which, in transversal scan, appears as a red dot in the center of the corpora cavernosa circular section 2 (Patel et al. 2012; Jung et al. 2018). During erection, the normal cavernosal artery diameter can increase up to 1 mm, and its ramifications can be well identified. At rest, sampling the cavernosal arteries, the normal PSV is >13 cm/s (Corona et al. 2008), while during erection the normal PSV and EDV are >35 cm/s (or 30 cm/s according to EAU guidelines) and <5 cm/s (or 3 cm/s according to EAU guidelines), respectively (Aversa and Sarteschi 2007; Patel et al. 2012).
5.3.2 US Abnormal Patterns
5.3.2.1 Erectile Dysfunction (ED)
As reported above, PCDU is considered a second-level procedure to assess ED (Sikka et al. 2013; Salonia et al. 2021). PCDU can be performed in flaccid or dynamic conditions. According to international guidelines, dynamic PCDU, performed after ICI (usually of PGE1, 10 mcg), is considered the gold standard method to assess vasculogenic ED (Sikka et al. 2013; Salonia et al. 2021). At rest (flaccid PCDU), ED due to arterial insufficiency is defined for a PSV <13 cm/s (Corona et al. 2008) and/or a FPA <1.17 m/s (Rastrelli et al. 2014). The aforementioned thresholds are also considered predictive of forthcoming MACE (Corona et al. 2008; Rastrelli et al. 2014) (see above). After ICI (dynamic PCDU), arterial insufficiency is defined for a PSV <35 cm/s (or 30 cm/s according to EAU guidelines), while “severe” arterial insufficiency is defined for a PSV <25 cm/s (Aversa and Sarteschi 2007; Corona et al. 2010; Patel et al. 2012). A PSV <25 cm/s is considered predictive of forthcoming MACE (see above). In addition, recently, some authors reported that a cavernosal artery IMT >0.3 mm, evaluated in dynamic state, can be considered as an additional parameter indicating an “unhealthy” artery, predictive of forthcoming MACE (Caretta et al. 2019). In fact, vasculogenic ED can manifest earlier than coronary, carotid, or femoral artery diseases because the smaller penile arteries reach critical narrowing, with insufficient blood flow, earlier than larger vessels (“artery size hypothesis”) (Corona et al. 2010). Accordingly, a previous study reported, in a limited number of subjects, that men with positive ischemic heart disease (IHD) at stress electrocardiography show lower PSV and a significant higher prevalence of PSV <25 cm/s than those with negative IHD (Shamloul et al. 2004). Accordingly evaluating 60 men (25 with IHD and ED, 25 with IHD alone and 10 with ED alone) with invasive angiography for coronary arteries and aorto-ilio-pudendal arteries and dynamic PCDU, some authors reported that men with ED alone had a higher rate of peripheral lesions than patients with coronary heart disease alone and that 10% of patients with ED alone had coronary heart disease (Sanad et al. 2020). In addition, men with penile arterial insufficiency show carotid and/or lower limb US abnormalities in 75% of cases (Vicari et al. 2005), and subjects with higher cavernous artery IMT show higher IMT in common carotid and femoral arteries (Caretta et al. 2019).
As a corollary, other parameters can be considered suggestive of arterial insufficiency at PCDU. In a qualitative analysis, both at rest and in dynamic conditions, a normal waveform resembles a “mountain,” with a rapid ascent (steep rise, culminating in a sharp peak) and a relative rapid descent, while an abnormal one resembles a “hill.” In addition, at dynamic PCDU, when diffuse arteriolar damage is present, cavernosal arteries ramifications are reduced (Aversa and Sarteschi 2007; Patel et al. 2012).
Arterial insufficiency can be associated with “secondary” veno-occlusive dysfunction (VOD), which can be defined only during dynamic PCDU when EDV is >5 cm/s (or >3 cm/s according to EAU guidelines) beside low PSV (secondary VOD) (Aversa and Sarteschi 2007; Patel et al. 2012). Conversely, “primary” VOD can occur in specific cases (Aversa and Sarteschi 2007; Bertolotto et al. 2009; Patel et al. 2012), including tunica albuginea degenerative changes (e.g., in Peyronie’s disease, diabetes, old men) or traumatic injury (penile fracture), structural alterations in the fibroelastic components of the corpora cavernosa (e.g., in Peyronie’s disease) or acquired venous shunts (e.g., after surgical correction of priapism), leading to lack of complete compression of exiting venules and emissary veins against the tunica albuginea (see above “methodological standards”). In these cases, there is a normal arterial supply (normal PSV) but an elevated EDV, suggesting venous outflow as the primary cause of vasculogenic ED.
5.3.2.2 Peyronie’s Disease
Peyronie’s disease (PD) is a connective tissue disorder characterized by the occurrence of fibrous plaques in the tunica albuginea surrounding the corpora cavernosa, possibly associated with localized partial fibrosis of the corpora cavernosa (Bertolotto et al. 2009; McCauley and Dean 2020). PD is characterized by pain with erection (especially at PD onset), palpable penile nodules/plaques, abnormal curvature of the penis and/or penile loss of girth, shortening or deformity/indentation, evident in the erected penis, and can lead to ED (see below). Pharmacologically induced erection is the gold standard to evaluate PD plaques as well as to evaluate the curvature, severity, and morphological abnormalities of the penis when a sufficient erection is achieved after ICI (Bertolotto et al. 2009; McCauley and Dean 2020). However, in our opinion, plaques and other US abnormalities (see below) can be evaluated even in the flaccid state, and penile curvature or abnormalities can be assessed by photographic documentation performed during erection induced with autoeroticism by the patient at home. PD plaques are identified as localized or diffuse areas in which the tunica albuginea is thickened (>2 mm) (Bertolotto et al. 2009; McCauley and Dean 2020). The plaques are more often located on the dorsal part of the penis, but they can also be found ventrally or laterally (Bertolotto et al. 2009). Some authors report the identification of hypoechoic plaques at PD onset (acute phase); however, a normoechoic or slightly hyperechoic plaque (tunica albuginea thickness) is often observed both in acute and in chronic PD phases (Bertolotto et al. 2009; McCauley and Dean 2020). Along with tunica albuginea thickness, other abnormalities can be observed in PD, including micro- or macrocalcifications within the tunica albuginea, the intercavernosal septum or the corpora cavernosa; septal fibrosis/thickness; intracavernous diffuse or partial fibrosis, characterized by slightly hyperechoic areas within the corpora cavernosa; tunica albuginea partial or extended (up to circular) deformity leading to an objectively localized indentation of the penis or to hourglass/bottleneck morphology, respectively (Bertolotto et al. 2009; McCauley and Dean 2020). The aforementioned abnormalities can sometimes be detected alone, representing US findings of PD with no clear tunica albuginea thickness/plaques. When severe tunica albuginea degenerative changes or intracavernous diffuse fibrosis (extending to the smooth muscle of the corpora) occur, a patient with normal cavernosal arterial supply can experience ED due to primary VOD (Aversa and Sarteschi 2007; Patel et al. 2012) (see above). However, rarely, fibrosis can involve the media of penile arteries leading to PD-related vasculogenic ED (McCauley and Dean 2020). More often, ED in patients with PD can be a consequence of aging and/or other comorbidities (McCauley and Dean 2020), and a mixed ED (including both arterial insufficiency and VOD) can be observed.
5.3.2.3 Penile Mondor’s Disease (Superficial Dorsal Vein Thrombophlebitis)
Penile Mondor’s disease (PMD) is the thrombophlebitis of the superficial dorsal vein of the penis (Bertolotto et al. 2009). It usually presents with a sudden hard “rope” on the penile dorsal surface, in the location of the dorsal vein, usually associated with pain, especially during erection (Avery and Scheinfeld 2013). PCDU represents an important tool in diagnosing the condition and monitoring patients (Bertolotto et al. 2009; Avery and Scheinfeld 2013). A lack of blood flow and noncompressibility of the dorsal vein indicate thrombosis. PMD can be self-limited; however, anticoagulative agents or low-molecular-weight heparin can be used to treat the thrombophlebitis. Resolution leads to flow restoration in the dorsal vein. Sometimes outcomes of a thrombus can remain visible as hyperechoic linear findings lying on the dorsal venous wall.
5.3.2.4 Priapism
PCDU can be useful in evaluating priapism. Priapism is defined as a persistent (>4 h) painful erection not related to sexual desire (Silberman et al. 2021). It can be distinguished into two forms, “low-flow” and “high-flow” (Silberman et al. 2021). Low-flow or ischemic priapism is the more frequent form, characterized by a disorder of venous blood outflow and a full painful erection. It is characterized by a very low-velocity high-resistance flow, with low PSV (resembling that observed at PCDU in flaccid condition) and negative EDV (Bertolotto et al. 2009; Avery and Scheinfeld 2013), similar to a long-lasting severe phase 5 of the dynamic PCDU phases (Patel et al. 2012) (see above). The specific waveform observed depends on the fact that intracorporal pressure exceeds systolic pressure; hence, no venous outflow is possible (similar to what is observed during a full, normal, rigid erection) and arterial inflow is low (reduced respect to a full, normal, rigid erection). Low-flow priapism is a true emergency. In fact, prolonged obstruction of venous outflow causing sustained high cavernous pressures and arterial ischemia can lead to irreversible ischemic changes and permanent ED (Avery and Scheinfeld 2013; Silberman et al. 2021).
High-flow priapism is the less frequent form, characterized by unregulated arterial inflow and a partial, incomplete erection (Silberman et al. 2021). Its etiology includes pelvic or penile trauma or surgery, often leading to a cavernosal artery tear or a cavernosal artery-lacunar fistula. A fistula, characterized by vascular turbulence, can be often observed at PCDU, as well as cavernous hematoma, appearing as a hypoechoic area within the corpora cavernosa. A low-resistance flow is present, with high PSV and positive EDV (Bertolotto et al. 2009; Avery and Scheinfeld 2013), similar to a phase 2 of the dynamic PCDU phases (Patel et al. 2012) (see above).
5.3.2.5 Penile Trauma
Penile injury may result from penetrating or blunt trauma (Bertolotto et al. 2009; Avery and Scheinfeld 2013). Patients with penile injury usually present with painful swelling and deformity of the shaft, either diffuse or circumscribed. Imaging may be required to evaluate the extent of the penile injury, locate hematomas, identify involvement of the penile urethra, or rule out albugineal disruption (Bertolotto et al. 2009; Avery and Scheinfeld 2013). US allows differentiation of cavernosal, septal, and extra-albugineal hematomas, which appear as hypoechoic areas; recognition of associated vascular injury, which may be seen as a cavernosal artery-lacunar fistula with flow turbulence (see also above, “high flow priapism”); identification of a tunical tear as an interruption in the echogenic interface of the normally appearing albuginea (Bertolotto et al. 2009; Avery and Scheinfeld 2013). MRI is more accurate in location of penile hematoma and evaluation of tunical disruption (Bertolotto et al. 2009; Avery and Scheinfeld 2013). CT can be requested in polytrauma patients, in whom urethral injuries are associated with pelvic bone fractures (Bertolotto et al. 2009; Avery and Scheinfeld 2013).
5.3.2.6 Penile Tumors
Penile tumors may be primary or secondary (Bertolotto et al. 2009; Avery and Scheinfeld 2013). Primary penile tumor usually manifest as an area of induration or a warty exophytic growth (Bertolotto et al. 2009; Avery and Scheinfeld 2013). In patients with primary penile tumors, imaging is indicated for staging purposes, and the relationships between the tumor and adjacent structures are better evaluated in the erected penis (after ICI) (Bertolotto et al. 2009; Avery and Scheinfeld 2013). At US (Bertolotto et al. 2009; Avery and Scheinfeld 2013), primary penile tumors usually have variable echogenicity and poor vascularization. Inflamed tumors may demonstrate increased vascularity and may be painful. When there is initial infiltration of the tunica albuginea, the latter may be in contact with the lesion and focally thickened with decreased echogenicity, showing no interruption. When infiltration of the corpora cavernosa occurs, an interruption of the interface of the tunica albuginea can be detected. Secondary tumors depend on metastatic invasion of the corpora cavernosa by a malignant neoplasm and can manifest as painful penile induration, either circumscribed or diffuse (Bertolotto et al. 2009; Avery and Scheinfeld 2013). At US, metastatic lesions may appear as circumscribed tumor nodules within the corpora cavernosa, diffuse infiltration, or both. Venous stasis and thrombosis can be associated due to infiltration of the normal venous drainage (Bertolotto et al. 2009; Avery and Scheinfeld 2013). Direct infiltration of the tunica albuginea can be identified as a tunical interruption. Although US is more accurate than clinical examination for measuring the extent of the tumor, MRI is the standard imaging modality for staging penile malignancies (Bertolotto et al. 2009; Avery and Scheinfeld 2013). Usually, US has a role in tumor staging when there are contraindications to MRI.
6 Male Breast US
6.1 Male Breast US: Indications
When clinical examination is equivocal, male breast US can be useful to distinguish gynecomastia and lipomastia (Niell et al. 2018; Kanakis et al. 2019) (Table 6.1). In addition, if there is clinical suspicion of breast malignancy, US can help to characterize the clinical finding (Niell et al. 2018; Kanakis et al. 2019) (Table 6.1).
6.2 Male Breast US: Methodological Standards
Male breast US is not completely standardized (Chau et al. 2016; Kim and Kim 2019; Swerdloff and Ng 2019; Önder et al. 2020). Breast US is performed with the patient supine. Most authors define gynecomastia as the presence of increased breast tissue, without specifying any cutoff (Chau et al. 2016; Kim and Kim 2019; Önder et al. 2020). Some authors have proposed to define gynecomastia as the presence of breast tissue with an anteroposterior depth >1 cm measured from the skin surface under the nipple to the anterior surface of the pectoralis muscle (Dialani et al. 2010). Pure lipomastia is defined at US as the presence of increased subcutaneous adipose tissue with no evidence of increased male breast tissue (Dialani et al. 2010; Chau et al. 2016; Kim and Kim 2019; Önder et al. 2020). Sometimes gynecomastia and lipomastia can be observed together in the same subject.
6.3 Male Breast US Anatomy, Normal and Abnormal Patterns, Clinical Utility, and US Standards
Figure 6.5 shows some normal and abnormal US patterns of the male breast.
6.3.1 Male Breast US Anatomy and Normal Pattern
Male breast anatomy before and after puberty has been well described elsewhere (Swerdloff and Ng 2019). US anatomy of the normal male breast consists mainly of subcutaneous fat and a modest subareolar button of breast tissue, slightly hypoechoic ducts (Chau et al. 2016; Kim and Kim 2019; Önder et al. 2020). Although normal breast tissue is virtually absent (small breast button) and asymptomatic in males, various abnormalities can affect the male breast, including benign findings, such as gynecomastia or lipomastia, or, rarely, malignancy.
6.3.2 Male Breast US Abnormal Patterns
The most recent guidelines on male breast abnormalities support breast imaging only when clinical examination is equivocal (Niell et al. 2018; Kanakis et al. 2019). Breast clinical examination represents the gold standard to evaluate possible male breast abnormalities, confirming the presence of palpable glandular tissue to discriminate gynecomastia from lipomastia (pseudogynecomastia) and ruling out the suspicion of malignant breast tumor (Niell et al. 2018; Kanakis et al. 2019).
6.3.3 Gynecomastia
Gynecomastia is defined clinically as generalized enlargement of male breast tissue possibly accompanied by discomfort or pain (especially at onset) (Swerdloff and Ng 2019). It can occur normally during three phases of life: after birth, at puberty, and in elderly men (Swerdloff and Ng 2019). The mechanisms underlying the “physiologic” development of gynecomastia, reported above, as well as diseases and drugs associated with gynecomastia have been described elsewhere (Swerdloff and Ng 2019). When gynecomastia is found, a testicular exam is essential, and if a testicular mass is suspected, testicular US is mandatory to investigate possible estrogen-secreting neoplasms, especially Leydig-, Sertoli-, and granulosa-cell tumors (Kanakis et al. 2019; Swerdloff and Ng 2019).
Clinically, gynecomastia is defined as subareolar breast tissue of ≥2 cm in diameter (Swerdloff and Ng 2019). Histologically, there are three phases of proliferative changes described in gynecomastia, including “florid,” “intermediate/transition,” and “fibrous/inactive” gynecomastia, well described elsewhere (Chau et al. 2016; Kim and Kim 2019; Swerdloff and Ng 2019). At US, gynecomastia is defined by some authors as the presence of increased breast tissue described as anteroposterior depth >1 cm from the skin surface under the nipple to the anterior surface of the pectoralis muscle (Dialani et al. 2010). Three patterns of gynecomastia can be observed: nodular, dendritic, and diffuse, reflecting underlying histologic changes within the male breast (Dialani et al. 2010; Chau et al. 2016; Kim and Kim 2019; Önder et al. 2020). “Nodular” gynecomastia occurs in the early (florid) phase of gynecomastia and is indicative of the early phase of ductal and stromal proliferation. It typically correlates with symptomatic duration of <1 year. US demonstrates a subareolar fan- or disk-shaped area of hypoechoic tissue that may be slightly hypervascular due to the proliferation of stroma. “Dendritic” gynecomastia occurs with long-standing gynecomastia that has been present for 1 year or longer, is quiescent and fibrotic, and has been associated with stromal fibrosis and dilated ducts. It typically presents as a flame-shaped, serpiginous hypoechoic area with finger-like projections in the subareolar region. Due to stromal fibrosis, this subtype is typically irreversible. “Diffuse” gynecomastia is usually seen in the setting of high-dose estrogen therapy or exogenous hormone use. It presents with increased volume and echogenicity of breast tissue, identical to female breasts.
In nodular gynecomastia of recent onset (less than 6 months), under medical treatment responders usually improve with reduced pain within 1 month (Swerdloff and Ng 2019), and a reduction in breast tissue can be observed at US after 3–6 months. However, if gynecomastia is established since a long time (dendritic form), medical treatment often fails to produce breast regression, and surgery can be considered (Kanakis et al. 2019; Swerdloff and Ng 2019).
6.3.4 Pseudogynecomastia/Lipomastia
Pseudogynecomastia is characterized by increased subareolar fat without enlargement of the breast glandular component. It is a frequent finding in obese men. US is typically not necessary, but if performed due to clinical concern, it will demonstrate subcutaneous adipose tissue.
6.3.5 Male Breast Cancer
Male breast cancer is very rare and is suspected if an immobile firm mass, usually painless, is found on physical examination. At US, most invasive carcinomas appear as solid, hypoechoic, irregularly shaped masses with spiculated or microlobulated margins in the subareolar region, often eccentric to the nipple (Chau et al. 2016; Kim and Kim 2019; Önder et al. 2020).
6.3.6 Specific Use of Male Breast US
Male breast US can be used to evaluate suspected gynecomastia in men with advanced prostate cancer under androgen deprivation treatment (Swerdloff and Ng 2019). In addition, male breast US can be used to evaluate breasts in transgender subjects (Phillips et al. 2014). In transgender men, US can be useful to evaluate postmastectomy complications, including hematoma, seroma, and abscess formation. In addition, breast cancer may occur in residual breast tissue of those patients who underwent bilateral mastectomies (Phillips et al. 2014). In transgender women, breast tissue will increase over time under hormonal therapy, reaching maturity by 2–3 years with a more pronounced nipple-areola complex (Phillips et al. 2014). The breast tissue that develops should not be considered as gynecomastia, since it contains lobules and resembles breast tissue seen in natal women. Breast cysts and fibroadenoma can develop, and breast cancer, although rare, can occur in transgender women (Phillips et al. 2014). In addition, breast augmentation with implants is commonly used as part of sex reassignment surgery. Hence, training as female breast sonographers is suggested for sonographists evaluating transgender subjects.
Key Points
-
Scrotal US is useful to assess reproductive health, scrotal pain, masses, and trauma.
-
Regarding reproductive health, scrotal US can detect testicular location, hypotrophy, inhomogeneity, microlithiasis, lesions, and varicocele as well as epididymal abnormalities and congenital bilateral absence of vas deferens.
-
Regarding scrotal pain, scrotal US can detect testicular or epididymal inflammation (hyperemia), torsion (absent vascularization), lesions, and other abnormalities.
-
Contrast-enhanced US can improve the differential diagnosis of scrotal diseases, especially focal testicular lesions.
-
Sonoelastography can improve the differential diagnosis of focal testicular lesions in conjunction with other US techniques.
-
Transrectal US is useful to assess obstructive azoo-/oligospermia, especially through detection of congenital bilateral absence of vas deferens, midline prostatic cysts, or ejaculatory duct obstruction.
-
Transrectal US has assumed a growing relevance in chronic pelvic pain assessment, detecting signs of chronic or current prostate inflammation.
-
Penile US, performed in flaccid or dynamic state, can be used to investigate erectile dysfunction, structural penile abnormalities (fibrosis, trauma, tumors), dorsal vein thrombosis, and priapism.
-
Breast US can assess male breast abnormalities, including gynecomastia, lipomastia, and lesions.
-
Thanks to the efforts of different international societies, including the European Academy of Andrology, several standards and reference ranges in US have been achieved.
References
Abdulwahed SR, Mohamed EE, Taha EA, Saleh MA, Abdelsalam YM, ElGanainy EO (2013) Sensitivity and specificity of ultrasonography in predicting etiology of azoospermia. Urology 81:967–971
AIUM (2015) Practice guideline for the performance of scrotal ultrasound examinations. J Ultrasound Med 34:1–5. https://doi.org/10.7863/ultra.34.8.15.13.0006
Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP (2013) Guidelines on testicular cancer. European Association of Urology Guidelines. EAU Guidelines Office, Arnhem
Aversa A, Sarteschi LM (2007) The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J Sex Med 4:1437–1447
Avery LL, Scheinfeld MH (2013) Imaging of penile and scrotal emergencies ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 34(2):169–184
Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 34(2):169–84.
Behre HM, Nashan D, Nieschlag E (1989) Objective measurement of testicular volume by ultrasonography: evaluation of the technique and comparison with orchidometer estimates. Int J Androl 12:395–403
Behre HM, Kliesch S, Schädel F, Nieschlag E (1995) Clinical relevance of scrotal and transrectal ultrasonography in andrological patients. Int J Androl 18:27–31
Bertolotto M, Trombetta C (2012) Scrotal pathology. Springer-Verlag, Berlin Heidelberg
Bertolotto M, Pavlica P, Serafini G, Quaia E, Zappetti R (2009) Painful penile induration: imaging findings and management. Radiographics 29(2):477–493
Bertolotto M, Muça M, Currò F, Bucci S, Rocher L, Cova MA (2018) Multiparametric US for scrotal diseases. Abdom Radiol (NY) 43(4):899–917
Bertolotto M, Freeman S, Richenberg J, Belfield J, Dogra V, Huang DY, Lotti F, Markiet K, Nikolic O, Ramanathan S, Ramchandani P, Rocher L, Secil M, Sidhu PS, Skrobisz K, Studniarek M, Tsili A, Turgut AT, Pavlica P, Derchi LE, Members of the ESUR-SPIWG WG (2020) Ultrasound evaluation of varicoceles: systematic literature review and rationale of the ESUR-SPIWG Guidelines and Recommendations. J Ultrasound 23(4):487–507
Caretta N, De Rocco PM, Minicuci N, Palego P, Valente U, Garolla A, Ferlin A, Foresta C (2019) Penile doppler ultrasound predicts cardiovascular events in men with erectile dysfunction. Andrology 7(1):82–87
Chau A, Jafarian N, Rosa M (2016) Male breast: clinical and imaging evaluations of benign and malignant entities with histologic correlation. Am J Med 129(8):776–791
Condorelli R, Calogero AE, La Vignera S (2013) Relationship between Testicular Volume and Conventional or Nonconventional Sperm Parameters. Int J Endocrinol 145792. https://doi.org/10.1155/2013/145792. Epub 2013 Sep 5. PMID: 24089610; PMCID: PMC3780703
Cornud F, Belin X, Amar E, Delafontaine D, Hélénon O, Moreau JF (1999) Varicocele: strategies in diagnosis and treatment. Eur Radiol 9:536–545
Corona G, Fagioli G, Mannucci E, Romeo A, Rossi M, Lotti F, Sforza A, Morittu S, Chiarini V, Casella G, Di Pasquale G, Bandini E, Forti G, Maggi M (2008) Penile doppler ultrasound in patients with erectile dysfunction (ED): role of peak systolic velocity measured in the flaccid state in predicting arteriogenic ED and silent coronary artery disease. J Sex Med 5(11):2623–2634
Corona G, Monami M, Boddi V, Cameron-Smith M, Lotti F, de Vita G, Melani C, Balzi D, Sforza A, Forti G, Mannucci E, Maggi M (2010) Male sexuality and cardiovascular risk. A cohort study in patients with erectile dysfunction. J Sex Med 7(5):1918–1927
Dhabuwala CB, Kumar AB, Kerkar PD, Bhutawala A, Pierce J (1989) Patterns of Doppler recordings and its relationship to varicocele in infertile men. Int J Androl 12:430–438
Dialani V, Baum J, Mehta TS (2010) Sonographic features of gynecomastia. J Ultrasound Med 29(4):539–547
Du J, Li FH, Guo YF, Yang LM, Zheng JF, Chen B, Zhu JS, Liu Q (2010) Differential diagnosis of azoospermia and etiologic classification of obstructive azoospermia: role of scrotal and transrectal US. Radiology 256:493–503
Dubin L, Amelar RD (1970) Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril 21:606–609
Engeler D, Baranowski AP, Berghmans B, Borovicka J, Cottrell AM, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, de Williams ACC, Parsons B, Goonewardene S, Abreu-Mendes P, Zumstein VV (2021) Chronic pelvic pain. European Association of Urology Guidelines https://uroweborg/guideline/chronic-pelvic-pain/#4 Accessed April 2021
Freeman S, Bertolotto M, Richenberg J, Belfield J, Dogra V, Huang DY, Lotti F, Markiet K, Nikolic O, Ramanathan S, Ramchandani P, Rocher L, Secil M, Sidhu PS, Skrobisz K, Studniarek M, Tsili A, Tuncay Turgut A, Pavlica P, Derchi LE, members of the ESUR-SPIWG WG (2020) Ultrasound evaluation of varicoceles: guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for detection, classification, and grading. Eur Radiol 30(1):11–25
Gravas S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, Rieken M, Speakman MJ, Tikkinen KAO, Guidelines Associates: M. Karavitakis, I. Kyriazis, S. Malde, V. Sakalis, R. Umbach (2021) Management of non-neurogenic male LUTS. European Association of Urology Guidelines https://uroweborg/guideline/treatment-of-non-neurogenic-male-luts/#4 Accessed April 2021
Guideline developed in collaboration with the American College of Radiology; Society for Pediatric Radiology; Society of Radiologists in Ultrasound (2015) AIUM practice guideline for the performance of scrotal ultrasound examinations. J Ultrasound Med 34:1–5. https://doi.org/10.7863/ultra.34.8.15.13.0006
Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP (1980) The Doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Br J Urol 52:50–56
Hoekstra T, Witt MA (1995) The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol 153:82–84
Huang DY, Pesapane F, Rafailidis V, Deganello A, Sellars ME, Sidhu PS (2020) The role of multiparametric ultrasound in the diagnosis of paediatric scrotal pathology. Br J Radiol 93(1110):20200063
Iosa G, Lazzarini D (2013) Hemodynamic classification of varicoceles in men: our experience. J Ultrasound 16:57–63
Isidori AM, Lenzi A (2008) Scrotal CDU: morphological and functional atlas. Forum Service Editore s.r.l, Genova
Jung DC, Park SY, Lee JY (2018) Penile doppler ultrasonography revisited. Ultrasonography 37(1):16–24
Kanakis GA, Nordkap L, Bang AK, Calogero AE, Bártfai G, Corona G, Forti G, Toppari J, Goulis DG, Jørgensen N (2019) EAA clinical practice guidelines-gynecomastia evaluation and management. Andrology 7(6):778–793
Kim SH, Kim YS (2019) Ultrasonographic and mammographic findings of male breast disease. J Ultrasound Med 38(1):243–252
Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP, King BF Jr (2009) Imaging of the seminal vesicle and vas deferens. Radiographics 29:1105–1121
Laguna MP, Albers P, Algaba F, Bokemeyer C, Boormans JL, Fischer S, Fizazi K, Gremmels H, Leão R, Nicol D, Nicolai N, Oldenburg J, Tandstad T (2021) Testicular cancer. European Association of Urology Guidelines https://uroweb.org/guideline/testicular-cancer/. Accessed March 2021
Lenz S, Giwercman A, Elsborg A, Cohr KH, Jelnes JE, Carlsen E, Skakkebaek NE (1993) Ultrasonic testicular texture and size in 444 men from the general population: correlation to semen quality. Eur Urol 24:231–238
Leslie SW, Sajjad H, Villanueva CA (2021) Cryptorchidism. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL); PMID: 29261861
Liguori G, Trombetta C, Garaffa G, Bucci S, Gattuccio I, Salame` L, Belgrano E (2004) Color Doppler ultrasound investigation of varicocele. World J Urol 22:378–381
Lotti F, Maggi M (2015) Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update 21(1):56–83. https://doi.org/10.1093/humupd/dmu042. Epub 2014 Jul 19. PMID: 25038770
Lotti F, Maggi M (2018) Sexual dysfunction and male infertility. Nat Rev Urol 15:287–307
Lotti F, Corona G, Colpi GM, Filimberti E, Innocenti SD, Mancini M, Baldi E, Noci I, Forti G, Maggi M (2012) Seminal vesicles ultrasound features in a cohort of infertility patients. Hum Reprod 27(4):974–982
Lotti F, Corona G, Maseroli E, Rossi M, Silverii A, Degl'innocenti S, Rastrelli G, Forti G, Maggi M (2013) Clinical implications of measuring prolactin levels in males of infertile couples. Andrology 1(5):764–771
Lotti F, Corona G, Vignozzi L, Rossi M, Maseroli E, Cipriani S, Gacci M, Forti G, Maggi M (2014a) Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl 16(2):295–304
Lotti F, Corona G, Mondaini N, Maseroli E, Rossi M, Filimberti E, Noci I, Forti G, Maggi M (2014b) Seminal, clinical and colour-doppler ultrasound correlations of prostatitis-like symptoms in males of infertile couples. Andrology 2(1):30–41
Lotti F, Maseroli E, Fralassi N, Degl'Innocenti S, Boni L, Baldi E, Maggi M (2016) Is thyroid hormones evaluation of clinical value in the work-up of males of infertile couples? Hum Reprod 31(3):518–529
Lotti F, Corona G, Cocci A, Cipriani S, Baldi E, Degl'Innocenti S, Franco PN, Gacci M, Maggi M (2018) The prevalence of midline prostatic cysts and the relationship between cyst size and semen parameters among infertile and fertile men. Hum Reprod 33:2023–2034
Lotti F, Frizza F, Balercia G, Barbonetti A, Behre HM, Calogero AE, Cremers JF, Francavilla F, Isidori AM, Kliesch S, La Vignera S, Lenzi A, Marcou M, Pilatz A, Poolamets O, Punab M, Peraza Godoy MF, Rajmil O, Salvio G, Shaeer O, Weidner W, Maseroli E, Cipriani S, Baldi E, Degl'Innocenti S, Danza G, Caldini AL, Terreni A, Boni L, Krausz C, Maggi M (2020) The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: clinical, seminal and biochemical characteristics. Andrology 8(5):1005–1020
Lotti F, Bertolotto M, Maggi M (2021a, 2021) Historical trends for the standards in scrotal ultrasonography: what was, what is and what will be normal. Andrology. https://doi.org/10.1111/andr.13062. Epub ahead of print
Lotti F, Frizza F, Balercia G, Barbonetti A, Behre HM, Calogero AE, Cremers JF, Francavilla F, Isidori AM, Kliesch S, La Vignera S, Lenzi A, Marcou M, Pilatz A, Poolamets O, Punab M, Peraza Godoy MF, Rajmil O, Salvio G, Shaeer O, Weidner W, Maseroli E, Cipriani S, Baldi E, Degl'Innocenti S, Danza G, Caldini AL, Terreni A, Boni L, Krausz C, Maggi M (2021b) The European Academy of Andrology (EAA) ultrasound study on healthy, fertile men: scrotal ultrasound reference ranges and associations with clinical, seminal, and biochemical characteristics. Andrology 9(2):559–576
Martino P, Galosi AB, Bitelli M, Consonni P, Fiorini F, Granata A, Gunelli R, Liguori G, Palazzo S, Pavan N, Scattoni V, Virgili G, Imaging Working Group-Societa Italiana Urologia (SIU); Società Italiana Ecografia Urologica Andrologica Nefrologica (SIEUN) (2014) Practical recommendations for performing ultrasound scanning in the urological and andrological fields. Arch Ital Urol Androl 86(1):56–78
McCauley JF, Dean RC (2020) Diagnostic utility of penile ultrasound in Peyronie’s disease. World J Urol 38(2):263–268
Morey AF, Broghammer JA, Hollowell CMP, McKibben MJ, Souter L (2021) Urotrauma guideline 2020: AUA guideline. J Urol 205(1):30–35
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, van der Kwast TH, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, van der Poel HG, Rouvière O, Schoots IG, Tilki D, Wiegel T, Willemse PM, Cornford P (2021) Prostate cancer. European Association of Urology Guidelines https://uroweb.org/guideline/prostate-cancer/Accessed April 2021
Niell BL, Lourenco AP, Moy L, Baron P, Didwania AD, diFlorio-Alexander RM, Heller SL, Holbrook AI, Le-Petross HT, Lewin AA, Mehta TS, Slanetz PJ, Stuckey AR, Tuscano DS, Ulaner GA, Vincoff NS, Weinstein SP, Newell MS (2018) ACR appropriateness criteria® evaluation of the symptomatic male breast. J Am Coll Radiol 15(11S):S313–S320
Önder Ö, Azizova A, Durhan G, Elibol FD, Akpınar MG, Demirkazık F (2020) Imaging findings and classification of the common and uncommon male breast diseases. Insights Imaging 11(1):27
Oyen RH (2002) Scrotal ultrasound. Eur Radiol 12:19–34
Patel DV, Halls J, Patel U (2012) Investigation of erectile dysfunction. Br J Radiol 85 Spec No 1(Spec Iss 1):S69–S78
Pauroso S, Di Leo N, Fulle I, Di Segni M, Alessi S, Maggini E (2011) Varicocele: Ultrasonographic assessment in daily clinical practice. J Ultrasound 14(4):199–204
Pedersen MR, Osther PJS, Rafaelsen SR (2018) Ultrasound Evaluation of Testicular Volume in Patients with Testicular Microlithiasis. Ultrasound Int Open 4(3):E99–E103. https://doi.org/10.1055/a-0643-4524. Epub 2018 Sep 12. PMID: 30250943; PMCID: PMC6143373
Phillips J, Fein-Zachary VJ, Mehta TS, Littlehale N, Venkataraman S, Slanetz PJ (2014) Breast imaging in the transgender patient. AJR Am J Roentgenol 202(5):1149–1156
Pilatz A, Wagenlehner F, Bschleipfer T, Schuppe HC, Diemer T, Linn T, Weidner W, Altinkilic B (2013) Acute epididymitis in ultrasound: results of a prospective study with baseline and follow-up investigations in 134 patients. Eur J Radiol 82:e762–e768
Pozza C, Kanakis G, Carlomagno F, Lemma A, Pofi R, Tenuta M, Minnetti M, Tarsitano MG, Sesti F, Paoli D, Anzuini A, Lenzi A, Isidori AM, Gianfrilli D (2020) Testicular ultrasound score: a new proposal for a scoring system to predict testicular function. Andrology 8:1051–1063
Ramanathan S, Bertolotto M, Freeman S, Belfield J, Derchi LE, Huang DY, Lotti F, Markiet K, Nikolic O, Ramchandani P, Richenberg J, Rocher L, Sidhu PS, Skrobisz K, Tsili A, De Visschere P, Campo I, Kozak O, Dogra V (2021) Imaging in scrotal trauma: a European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) position statement. Eur Radiol 31(7):4918–4928
Rastrelli G, Corona G, Lotti F, Aversa A, Bartolini M, Mancini M, Mannucci E, Maggi M (2014) Flaccid penile acceleration as a marker of cardiovascular risk in men without classical risk factors. J Sex Med 11(1):173–186
Richenberg J, Belfield J, Ramchandani P, Rocher L, Freeman S, Tsili AC, Cuthbert F, Studniarek M, Bertolotto M, Turgut AT, Dogra V, Derchi LE (2015) Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol 25(2):323–330
Rocher L, Ramchandani P, Belfield J, Bertolotto M, Derchi LE, Correas JM, Oyen R, Tsili AC, Turgut AT, Dogra V, Fizazi K, Freeman S, Richenberg J (2016) Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: impact of radiological findings on management radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur Radiol 26:2268–2278
Săftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, Amy D, Bachmann-Nielsen M, Bob F, Bojunga J, Brock M, Calliada F, Clevert DA, Correas JM, D'Onofrio M, Ewertsen C, Farrokh A, Fodor D, Fusaroli P, Havre RF, Hocke M, Ignee A, Jenssen C, Klauser AS, Kollmann C, Radzina M, Ramnarine KV, Sconfienza LM, Solomon C, Sporea I, Ştefănescu H, Tanter M, Vilmann P (2019) The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: update 2018. Ultraschall Med 40(4):425–453
Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, Martinez-Salamanca I, Minhas S, Serefoğlu EC, Verze P (2021) Sexual and reproductive health. European Association of Urology Guidelines https://uroweb.org/guideline/sexual-and-reproductive-health/Accessed March 2021
Sanad AM, Younis SE, Oraby MA, Hegazy H, El-Sakka AI (2020) Relation between severity of coronary artery disease and aorto-ilio-pudendal artery disease in patients with ischemic heart disease-associated vascular erectile dysfunction. J Sex Med 17(6):1086–1093
Sarkar S, Das S (2016) A review of imaging methods for prostate cancer detection. Biomed Eng Comput Biol 7(Suppl 1):1–15
Sarteschi LM, Paoli R, Bianchini M, Menchini Fabris GF (1993) Lo studio del varicocele con eco-color Doppler. G Ital Ultrasonologia 4:43–49
Shamloul R, Ghanem HM, Salem A, Elnashaar A, Elnaggar W, Darwish H, Mousa AA (2004) Correlation between penile duplex findings and stress electrocardiography in men with erectile dysfunction. Int J Impot Res 16(3):235–237
Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H (2018) The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (Long Version). Ultraschall Med 39(2):e2–e44
Sikka SC, Hellstrom WJ, Brock G, Morales AM (2013) Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med 10(1):120–129
Silberman M, Stormont G, Hu EW (2021) Priapism. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL; 2021 PMID: 29083574
Singh R, Hamada AJ, Bukavina L, Agarwal A (2012) Physical deformities relevant to male infertility. Nat Rev Urol 9:156–174
Swerdloff RS, Ng CM (2019) Gynecomastia: etiology, diagnosis, and treatment. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. MDText.com, Inc., South Dartmouth (MA); 2000–. PMID: 25905330
Tasian GE, Copp HL (2011) Diagnostic performance of ultrasound in nonpalpable cryptorchidism: a systematic review and meta-analysis. Pediatrics 127(1):119–128
Vicari E, Arcidiacono G, Di Pino L, Signorelli S, Arancio A, Sorrentino F, Battiato C, D'Agata R, Calogero AE (2005) Incidence of extragenital vascular disease in patients with erectile dysfunction of arterial origin. Int J Impot Res 17(3):277–282
Westlander G, Ekerhovd E, Granberg S, Lycke N, Nilsson L, Werner C, Bergh C (2001) Serial ultrasonography, hormonal profile and antisperm antibody response after testicular sperm aspiration. Hum Reprod 16:2621–2627
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lotti, F., Zitzmann, M., Behre, H.M. (2023). Ultrasound Imaging in Andrology. In: Nieschlag, E., Behre, H.M., Kliesch, S., Nieschlag, S. (eds) Andrology. Springer, Cham. https://doi.org/10.1007/978-3-031-31574-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-31574-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31573-2
Online ISBN: 978-3-031-31574-9
eBook Packages: MedicineMedicine (R0)