Abstract
Glioblastoma is a malignant tumor of astrocytic origin that is highly invasive, proliferative and angiogenic. Despite current advances in multimodal therapies, such as surgery, radio- and chemotherapy, the outcome for patients with glioblastoma is nearly always fatal. The glioblastoma microenvironment has a tremendous influence over the tumor growth and spread. Microglia and macrophages are abundant cells in the tumor mass. Increasing evidence indicates that glioblastoma recruits these cell populations and signals in a way that microglia and macrophages are subverted to promote tumor progression. In this chapter, we discuss some aspects of the interaction between microglia and glioblastoma, consequences of this interaction for tumor progression and the possibility of microglial cells being used as therapeutic vectors, which opens up new alternatives for the development of GBM therapies targeting microglia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Tumor Microenvironment and the Microglia
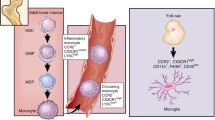
The central nervous system (CNS) is composed of several cell types, including neurons, astrocytes, oligodendrocytes, ependymal cells and microglia. Each cell type has distinct and essential roles for the optimal functioning of the CNS. As discussed in Chapters “Glial Cells and Integrity of the Nervous System,” “Microglia Function in the Normal Brain” and “Purine Signaling and Microglial Wrapping,” microglia are the resident immune cells in the CNS, but they are increasingly recognized to play diverse roles. Their embryonic origin is mesodermal, unlike other CNS cells, which have ectodermal origin. It appears that microglia progenitors come from the yolk sac early in development (Ginhoux et al. 2010). Phagocytosis of microorganisms, antigen presentation to lymphocytes, phagocytosis of cell debris, transient or aberrant axons and apoptotic cells during development, and secretion of neurotrophic factors are some of microglia functions in CNS (Vilhardt 2005; Mallat et al. 2005; Lima et al. 2010). Following lesions, microglia become active and assume an amoeboid phenotype and a high metabolic rate, synthesizing and secreting several cytokines, such as interleukin (IL)6, IL1β and tumor necrosis factor α (TNFα) (Vilhardt 2005; Yang et al. 2010).
Among all CNS pathologies, one of the deadliest is glioblastoma (GBM) . This malignant tumor of astrocytic origin is highly invasive, proliferative and angiogenic. Its invasive nature explains the high recurrence even after surgical resection (Lima et al. 2012). Survival is commonly about 14 months despite all efforts (Stupp et al. 2005). The GBM microenvironment has a tremendous influence over the tumor growth and spread. In a still not completely defined way, GBM subverts cells to act in its favor. Astrocytes were shown to have an increase in MMP-2 (matrix metalloproteinase-2) expression and to convert pro-MMP-2 to active form only in the presence of glioma cells (Le et al. 2003; Gagliano et al. 2009), suggesting a pro-tumor role of astrocytes. Besides, GBM cells produce VEGF (vascular endothelial growth factor) and DLL4 (delta-like ligand 4), which stimulate the angiogenesis that sustains tumor survival and growth (Bao et al. 2006; Li et al. 2007).
Microglia and macrophages are abundant cells in the tumor mass. GBM recruits these cell populations (Fig. 1) and signals in a way that microglia and macrophages are subverted to promote tumor progression. Moreover, GBM establishes an immunosuppressed niche, favoring even more its survival, growth, and spread (da Fonseca and Badie 2013). A current and important topic regarding these glioma-associated microglia and macrophages is the existence of two phenotypically distinct cell populations classified as M1 and M2. M1 macrophages are classically activated, developing an anti-tumor response through the activation of the immune system and production of reactive oxygen species, nitric oxide (NO) and proinflammatory cytokines, such as TNF. M2 macrophages are alternatively activated, performing immunosuppressive roles, such as release of IL10 and tumor promotion, as well as inducing metastatic processes by promoting angiogenesis and extracellular matrix degradation (Sica et al. 2008; Yang et al. 2010; Albesiano et al. 2010; Herrera et al. 2013). Indeed, the escape of tumor cells from the immune system has been related to a change of M1 to M2 phenotype during tumor progression (Schmieder et al. 2012). Similar to macrophages, glioma-associated microglia also present the M2 phenotype (Komohara et al. 2008; Fonseca et al. 2012; Gabrusiewicz et al. 2011).
Xenotransplanted tumor produced from human GBM cells injected into the caudate putamen of mouse brain. In this study, we used the human tumor cell line GBM95, established in our lab (Faria et al. 2006). After 15 days, the brains were perfused with fixative 4 % paraformaldehyde, cut into slices and immunostained with rabbit anti-mouse Iba1 (green) antibody (Confocal Microscope/Leica TCS-SP5), a marker of microglia /macrophages cells. Nuclei were stained with DAPI (blue). a Tumor mass. b Contralateral hemisphere, without tumor cells. Note the presence of activated microglia/macrophages in the tumor mass (a) and ramified resident microglia cells in the contralateral hemisphere (b). Bar 40 μm. This study was approved by the Ethics Committee of the Health Sciences Center at the Federal University of Rio de Janeiro (Protocol no. DAHEICB 015) and by the Brazilian Ministry of Health Ethics Committee (CONEP no. 2340). The “Principles of laboratory animal care” (NIH publication no. 85‐23, revised 1996) guidelines as well as The Code of Ethics of EU Directive 2010/63/EU were strictly followed for all experiments
Microglia -Glioblastoma Interaction
Malignant gliomas, particularly GBMs, the most aggressive astrocytoma, contain high levels of microglia infiltrates; about 30 % of tumor mass is composed of glioma-associated microglia and macrophages which has led to the hypothesis that microglia may have a role in GBM immunology (Badie and Schartner 2000; da Fonseca and Badie 2013; Yang et al. 2010). Indeed, evidence strongly suggests that microglia contribute to the immunosuppressive environment of GBMs and may promote tumor growth (Schartner et al. 2005; Yi et al. 2011). In this context, the accumulation of microglia in GBM tissue is due to local production of growth factors and chemoattractants, such as CCL2, by GBM cells (Prat et al. 2000). CCL2, also recognized as MCP-1 (macrophage chemoattractant protein 1), is one of the most highly expressed chemokines in many CNS injuries and exerts its biological function by binding to its high affinity receptor CCR2, which is expressed by microglia, astrocytes and brain microvascular endothelial cells (Yao and Tsirka 2014). The interaction between CCL2 and CCR2 triggers IL6 release by microglia, which is associated with GBM aggressiveness (Li et al. 2010; Rolhion et al. 2001). IL6 is implicated in many aspects of tumorigenesis, and it is identified as a growth factor for glioma stem cells (Wang et al. 2009). In addition, IL6 has been found to increase microglia production of MMP-2, facilitating tumor migration (Li et al. 2010). Markovic et al. (2005), using cultured brain slices where microglia were previously depleted with clodronate-filled liposomes, showed that injected glioma cells had decreased infiltrative capacity compared with glioma cells injected into control slices, possibly because of the decrease in MMP-2 levels, produced by microglia . Moreover, Platten et al. (2003), using a rat model of intracerebral glioma cell line implant, demonstrated that the glioma cell line that recruited more microglia resulted in a larger tumor mass, and they attributed this effect to CCL2/CCR2 pathways. Another important factor during GBM progression is TNFα, a proinflammatory cytokine widely secreted by microglia (Rivest 2009), which stimulates the secretion of several molecules including CCL2, IL6, IL1β, and NO (Allan and Rothwell 2001; D’Mello et al. 2009; Nadeau and Rivest 2000). On TNF receptor 1 (TNFR1) activation, IκBa, a protein that blocks NFκB signaling in resting cells, gets phosphorylated and degraded, leading to p65/p50 nuclear translocation and transcriptional activation of NFκB target genes, including TNFα itself (Baker et al. 2008; Tchoghandjian et al. 2013). The constitutively activated NFκB has been associated with invasive behavior and malignancy of GBM (Raychaudhuri et al. 2007; Tsunoda et al. 2005). NFκB activation triggers transcriptional activation of pro-migratory genes, like CXC chemokines, urokinase-type plasminogen activator (uPA) and matrix metalloproteinases, contributing to invasiveness of GBM (Tchoghandjian et al. 2013; Wu and Zhou 2010).
In addition to the factors released in the tumor microenvironment, proteins constitutively expressed by microglia may be implicated in GBM maintenance. Stress-inducible protein 1 (STI1) is a 66 kDa protein described as a co-chaperone that binds to both Hsp70 and Hsp90 and regulates their activities (Chen and Smith 1998; Song and Masison 2005). Our group has shown that STI1 released by microglia promotes tumor proliferation, modulates MMP-9 activity and stimulates the migration of human GBM cell lines in vitro (Fonseca et al. 2012). We demonstrated that microglial conditioned medium (MG CM) stimulated proliferation and migration of the GBM cell lines, and this effect was reversed when the anti-STI1 antibody was added to the MG CM or STI1 was removed by immunodepletion from the MG CM. Furthermore, the addition of STI1 antibody to MG CM significantly decreased MMP-9 activity (Fonseca et al. 2012). These data suggest that STI1 is an important factor for glioma progression. Using a glioma model of intracranial and subcutaneous implant of GL261, a murine glioma cell line, Fonseca et al. (2012) have also shown that STI1 expression increased with tumor progression, and was also upregulated in glioma-associated microglia and macrophages and infiltrating lymphocytes. On the other hand, STI1 expression did not significantly change in circulating leukocytes, and even decreased in leukocytes that infiltrated tumors propagated in the subcutaneous tissue. Therefore, these results demonstrated that STI1 expression is modulated by the brain tumor microenvironment, and for the first time correlated STI1 expression and glioma progression (Carvalho da Fonseca et al. 2014). Altogether, we conclude that microglia-GBM interaction determine the degree of GBM invasion, opening the way for the development of new therapeutic approaches.
Microglial cells are substantial producers of MMPs and inducers of GBM invasiveness (Alves et al. 2011; Hu et al. 2014). Hu et al. (2014) showed that MMP-9 levels were expressed by Iba1+ cells in human tumor samples, indicating that glioma-associated microglia were responsible for MMP-9 local production. Also, they demonstrated that GBM cells released soluble factors that induced the MMP-9 expression in glioma-associated microglia via Myeloid Differentiation Primary Response 88/Toll-like receptor 8 (MYD88/TLR8) signaling pathway. Interestingly, in this study, when microglial cells were treated with their inhibitor minocycline, levels of MMP-9 and TLR2 (Toll-like receptor 2) in glioma-associated microglia decreased, and consequently tumor invasion declined (Hu et al. 2014). A previous study had shown that GBM cells induced the expression of membrane-type-1 MMP (MT1-MMP) in glioma-associated microglia, promoting tumor invasion via the TLR2 signaling pathway. In this work, GL261 murine glioma cells were injected into TLR2 knockout mice, resulting in smaller tumors and reduced MT1-MMP levels in glioma-associated microglia (Vinnakota et al. 2013). Thus, it seems important to explore the role of MMPs and TLRs in microglial cells, since they can stimulate tumor progression.
Another factor that contributes to microglial recruitment during tumor progression is the glial cell-line-derived neurotrophic factor (GDNF). GDNF is a neurotrophic factor involved in dopaminergic neuronal survival, but it can also contribute to tumor progression. High levels of GDNF have been observed in GBM cells (Ng et al. 2009; Wiesenhofer et al. 2000); however, little is known about the correlation of GDNF and the attraction of microglia during tumor progression. Ku et al. (2013) have recently demonstrated that GDNF is expressed in GBM cells and plays an important role in microglia recruitment during tumor progression They showed that microglia expressed both GDNF receptors, GFRa-1 and GFRa-2, and then they used transwell assays to understand how GDNF could modulate the migration of microglia . For this, they used GBM cell conditioned medium depleted of GDNF, by using a specific shRNA, and observed that there was reduced microglia migration. In addition, they injected GBM cells not expressing GDNF into mouse brain and, after 2 weeks, observed lower levels of Iba1+ microglia infiltration and a reduced tumor size. So, GDNF is indeed an important factor expressed by GBM cells for microglia attraction (Ku et al. 2013).
Epidermal growth factor receptor (EGFR) also plays a significant role during GBM invasion and aggressiveness (Ohgaki and Kleihues 2007; Sangar et al. 2014). It is possible that microglia cells stimulate GBM invasion through the EGFR signaling (Nolte et al. 1997). In particular, Coniglio et al. (2012) demonstrated in vitro that microglia secreted EGF, which, in turn, activated the EGFR on GBM cells and consequently induced tumor migration.
Change in the Microglial Profile May Be Useful
Studies by Penfield (1925) led to the suggestion that microglial cells fight tumors (for review see Charles et al. 2011; Li and Graeber 2012). However, other investigators continue to believe that microglial cells behave as expected for macrophages and are able to promote antigen presentation, release of cytokines and phagocytosis even in the presence of GBM.
Kren et al. (2010) observed the expression of HLA-G and HLA-E, immune-modulatory nonclassical molecules with anti-tumor activity, by glioma-associated microglia and macrophages in most cases of human GBM of 26 samples analyzed. The role of these molecules in GBM is not well described. Even so, their observations go against the hypothesis that microglial cells and macrophages may be attracted to the tumor site and promote tumor invasion through inhibition of the cytolysis by NK-cell and T-cell, once they consider that the detected expression of HLA-G and HLA-E in glioma-associated microglia and macrophages indicates a role in immune functions (Kren et al. 2010). In this sense, an in vitro study showed that the conditioned culture medium of microglia promoted apoptotic cell death of glioma cells; when microglial cells were previously treated with LPS or IFNγ (Interferon γ), this effect was more pronounced. Proteomic analysis was used to identify the secreted proteins, and several cathepsin proteases were found to be expressed, especially cathepsin B, as was NO, suggesting a microglial role in tumor cytotoxicity (Hwang et al. 2009). Despite these observations, much clinical evidence and many in vitro studies indicate that microglia and macrophages that infiltrate the brain tumor have pro-tumor functions, promoting cell growth and migration (Li and Graeber 2012; Alves et al. 2011; da Fonseca and Badie 2013). On the other hand, after treatment with polyinosinic-polycytidylic acid (poly [I:C]), an agonist for Toll-like receptor 3, glioma-associated microglia obtained from patients with GBM started to secrete toxic soluble factors when cocultured with different GBM cell lines. This was also true when they used the supernatant of glioma-associated microglia previously stimulated with poly (I:C). Interestingly, these factors had toxic effects only on tumor cells, since astrocytes and neurons cultures were not affected (Kees et al. 2012). Thus, Kees et al. (2012) demonstrated that it is possible to change the behavior of microglial cells from a tumor-supporting role to a tumor-suppressing function after poly (I:C) exposure. In other words, switching the M2 profile described for glioma-associated microglia, to an M1 profile led to gaining anti-tumor activities. In the same way, Chiu et al. (2011) proposed that in vitro microglial anti-tumor functions could be reestablished with treatment with IL12. Indeed, after IL12 stimulation, microglia increased the levels of TRAIL (TNF-related apoptosis inducing ligand) releasing and phagocytic activity.
In addition, Lisi et al. (2014) demonstrated that the inhibition of mTOR (mammalian target of rapamycin), which is activated in gliomas by many deregulated pathways, polarizes glioma-activated microglia to an M1 profile conferring cytotoxic functions upon microglial cells and preventing the M2 state that is involved in tumor establishment. In fact, iNOS was increased followed by a decrease in IL10 gene expression after treatment with rapamycin and its analog RAD001 in microglia (Lisi et al. 2014).
A recent in vivo study showed that MIF (macrophage migration inhibitory factor) is highly expressed on glioma cells, whereas its receptor, CD74, is expressed only in glioma-associate microglia . In this study, GBM cells and glioma-associate microglia were isolated from primary human tumors. A higher level of CD74-positive glioma-associate microglia was associated with increased patient survival, representing a positive prognostic parameter associated with the anti-tumor M1 profile (Zeiner et al. 2014).
In face of all these studies, we can conclude that microglia are a potential therapeutic target for the treatment of GBM. Certainly, the more we know about the interaction between microglia and GBM cells, the more we will know about the tumor biology. Data showing microglial cells promote tumors are substantial, especially from studies mentioned above that have attempted to manipulate the activation state of microglia, rescuing their M1 profile instead of M2 profile to fight against tumors.
Possible Therapies Using Microglia as a Therapeutic Target in the Fight Against Cancer
Currently, the state of the art therapy for GBM consists of surgical resection of the tumor, followed by chemotherapy with Temozolomide and radiotherapy. Despite the aggressive therapy, median survival remains only 14.6 months after diagnosis (Stupp et al. 2005). Many preclinical and clinical studies are trying to improve patient survival, but without success. Some strategies focus on the interaction of the tumor cells with the microenvironment; we will discuss some of the strategies that are being currently developed focusing on the microglia-glioma interaction.
The STAT 3 (signal transducer and activator of transcription 3) pathway is constitutively expressed in high-grade gliomas (Yu et al. 2007; Weissenberger et al. 2004) and has already been implicated in GBM pathogenesis, progression and immune evasion (Takeda et al. 1999; O’Farrell et al. 1998; Lang et al. 2002). STAT 3 is also upregulated in microglial cells under glioma influence (Zhang et al. 2009a), at least in part by interaction between glioma S100B and IL-6 with microglial RAGE (Receptor for Advanced Glycation End products) and IL6R (Bromberg and Wang 2009; Zhang et al. 2009b). The activation of this pathway inhibits macrophage activation and reduces expression of co-stimulatory molecules necessary for antigen presentation by naive T-cells; it increases the secretion of immunomodulatory cytokines IL6 and IL10, while reducing lymphocyte-stimulating cytokines (IL2, IL4, IL12 and IL15) (Cheng et al. 2003; Walker et al. 2003; Hussain et al. 2007). Consistent with this, STAT 3 inhibition in glioma cells using siRNAs reverses the cytokine expression profile, leading to microglia /macrophage activation and tumor growth inhibition in a mouse model (Zhang et al. 2009a). Pharmacological agents such as small STAT 3 inhibitors that penetrate the CNS have an anti-proliferative and proapoptotic effect on glioma cell lines (Takeda et al. 1999; O’Farrell et al. 1998; Lang et al. 2002; Iwamaru et al. 2007). Apart from that, these agents are capable of reversing the immune tolerant microenvironment by activating microglial cells, through production of lymphocyte-stimulating cytokines (IL2, IL4, IL12, IL15 and CXCL10) and upregulation of co-stimulatory molecules (CD80 and CD86), and also by stimulating T-cell proliferation and inducing a Th1-response (Iwamaru et al. 2007; Cheng et al. 2003; Hussain et al. 2007). There are on-going phase I and II clinical trials with STAT 3 and its more important activator IL6 for several malignancies for which this pathway is important, such as head and neck cancer, multiple myeloma and prostate cancer (Sansone and Bromberg 2012). These trials might be translated into a new therapeutic option for malignant gliomas, where this pathway was also recently shown to be important.
The role of the immune system in the treatment of the CNS tumors gained prominence with clinical trials using oligodeoxynucleotides containing CpG motifs (CpG-ODN) for GBM patients. CpG-ODNs are strongly immunostimulating agents, activating both innate and specific immunity. Biological effects of CpG-ODN are mediated by Toll-like receptor 9 (TLR9) (Klinman 2004; Krieg 2004), mainly expressed by B-lymphocytes and plasmacytoid dendritic cells in humans, and also by microglial and glioma cells (Ribes et al. 2010; El Andaloussi et al. 2006). In pre-clinical models, local treatment with CpG-ODN injections, either alone or combined with radiation therapy , reduced tumor size, with no toxicity to brain parenchyma (Carpentier et al. 2000; Auf et al. 2001; Meng et al. 2005). It was shown that tumor rejection was due not only to direct toxicity in tumor cells, but also to modulation of microglia/macrophages and induction of a Th1 response (El Andaloussi et al. 2006; Carpentier et al. 2000; Auf et al. 2001). This new therapy was so promising in preclinical models that it was rapidly translated into phase I and II clinical trials. After a promising phase I study, with the few side effects limited to transient worsening of neurological condition and fever (Carpentier et al. 2006), phase II trials presented at the 2009 American Society of Clincal Oncology (ASCO) annual meeting showed only modest activity in the 6-month progression-free survival (PFS) of the cohort, with only a few cases showing radiological response (Carpentier et al. 2010; Ursu et al. 2009). This trial did not define the clinical or molecular characteristics of patients who might have benefitted from this trial, and this therapy was not continued.
Apart from these innovative strategies, old medications with newly discovered functions are also being investigated. Some glioma drugs reduce tumor growth in preclinical models by modulating microglial activity. The first, minocycline, a semi-synthetic broad spectrum tetracycline antibiotic described as capable of counteracting microglial activation into a proinflammatory phenotype by p38-MAPK inhibition (Suk 2004), reduces tumor growth in vitro and in vivo by inhibiting microglial MT1-MMP expression (Markovic et al. 2011). Another is propentofylline, an atypical synthetic methylxanthine with CNS glial modulating and anti-inflammatory actions (Si et al. 1996, 1998), described as capable of decreasing tumor growth in preclinical GBM models by a direct effect on microglial cells (and not in tumor-infiltrating macrophages) through tumor necrosis factor receptor of mouse embryo (TROY/TNFRSF19) inhibition (Jacobs et al. 2012a, b). And, more recently, Sarkar et al. (2014) demonstrated that microglial cells derived from non-glioma human subjects can markedly reduce the sphere-forming capacity of glioma stem cells (GSCs) by inducing cell-cycle arrest, reducing proliferation and inducing differentiation, most likely through IL-8 and MCP-1. Apart from that, Amphotericin B (a polyene antifungal drug) stimulates glioma-associated microglia through TLR signaling, reducing GSC survival and sphere-forming capacity in a manner resembling the action of microglia from healthy subjects. Daily treatment of mice harboring intracranial GSCs with non-toxic doses of Amphotericin B also substantially prolongs mouse survival (Sarkar et al. 2014).
Aside from pharmacological approaches, strategies have also been developed using microglia as vehicles for gene therapy in conjunction with MRI tracking. Ribot et al. (2007) labeled microglial cells with MRI contrast agents to ascertain that the injected cells were migrating to the tumor mass. The labeled cells were also transfected with a thymidine kinase suicide gene, which causes cell death after administration of its substrate, gancyclovir (GCV). This system is suitable because it induces a bystander effect: first, monophosphorylated GCV passes through intercellular gap junctions and thereby triggers the death of cells that have not been transduced; second, apoptotic bodies released by dead cells are taken up by adjacent viable cells which then die, amplifying this phenomenon (Caruso et al. 1993; Qiao et al. 2000; Burrows et al. 2002). Thus, a small quantity of enzyme and a low level of transduction are sufficient to cause tumor regression (Spencer 2000) under pharmacological control, since intracellular signaling occurs only if GCV is administered. The investigators demonstrated that the injected microglial cells are attracted to the tumor mass and that suicide gene activation with GCV reduces tumor growth and prolongs survival in a preclinical model of human GBM in nude mice (Ribot et al. 2007; Caruso et al. 1993; Qiao et al. 2000; Ribot et al. 2011).
Recent knowledge of microglia’s effects on the microenvironment of malignant gliomas has led to the discovery of several pathways that are promising therapeutic targets and a new prognostic molecular marker. This time, instead of a mutation or protein expression in tumor cells, the prognostic marker is a polymorphism in a microglial chemokine receptor gene associated with cell migration: the CX3CR1-I249 allele. This allele variant is associated with prolonged mean survival of GBM patients (23.5 v 14.1 months; P < 0.0001) and with reduced tumor infiltration by microglia (Rodero et al. 2008). For the first time, a microglial marker has been characterized as an independent, favorable prognostic factor and might be useful in predicting survival in GBM patients.
Since the phase III trials of Temozolomide in 2005, many options have been studied for the treatment of malignant gliomas without much success, including anti-angiogenic therapy with Bevacizumab (Avastin®) (Chinot et al. 2014) and such other chemotherapy regimens as Procarbazine, Lomustine (CCNU), and Vincristine (PCV) (Brada et al. 2010). Also, many strategies that seemed promising in preclinical trials, such as the CpG-ODNs, are rather disappointing in clinical trials. Thus, understanding of the biology of the CNS tumors and of the microenvironment’s influence on tumor progression is becoming increasingly important for developing new therapeutic strategies for this deadly disease.
Concluding Remarks
GBMs are the most aggressive tumors of astrocytic lineage. Despite significant progress in cancer research, which has led to the development of more effective therapies for some types of solid tumors, there is no effective treatment for GBM. In this chapter, we discussed some relevant properties of microglia in contact with GBM. Better understanding of the interactions between the tumor and its microenvironment, particularly microglial cells, is important for combating GBM. In this sense, the development of new therapies targeting the microglia or the factors produced by them that are specifically related to tumor progression may be an effective alternative.
Abbreviations
- CNS:
-
Central nervous system
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- GBM:
-
Glioblastoma
- MMP:
-
Matrix metalloproteinase
- VEGF:
-
Vascular endothelial growth factor
- DLL4:
-
Delta-like ligand 4
- NO:
-
Nitric oxide
- MCP-1 (CCL2):
-
Macrophage chemoattractant protein 1
- CCR2:
-
CCL2 receptor
- TNFR1:
-
TNF receptor 1
- IkBa:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- NF-kB:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cells
- uPA:
-
Urokinase-type plasminogen activator
- STI1:
-
Stress-inducible protein 1
- Hsp:
-
Heat shock protein
- MG CM:
-
Microglial conditioned medium
- MYD88/TLR8:
-
Myeloid Differentiation Primary Response 88/Toll-like receptor 8
- TLR:
-
Toll-like receptor
- MT1-MMP:
-
Membrane-type-1 MMP
- GDNF:
-
Glial cell-line-derived neurotrophic factor
- GFRa:
-
GDNF receptor
- EGFR:
-
Epidermal growth factor receptor
- EGF:
-
Epidermal growth factor
- poly [I:C]:
-
Polyinosinic-polycytidylic acid
- TRAIL:
-
TNF-related apoptosis inducing ligand
- mTOR:
-
Mammalian target of rapamycin
- iNOS:
-
Inducible nitric oxide synthase
- MIF:
-
Macrophage migration inhibitory factor
- STAT:
-
Signal transducer and activator of transcription
- RAGE:
-
Receptor for Advanced Glycation End products
- S100B:
-
S100 calcium binding protein B
- CpG-ODN:
-
Oligodeoxynucleotides containing CpG motifs
- TROY/TNFRSF19:
-
Tumor necrosis factor receptor of mouse embryo
- GSC:
-
Glioma stem cell
- MRI:
-
Magnetic resonance imaging
- GCV:
-
Gancyclovir
References
Albesiano E, Han JE, Lim M (2010) Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am 21(1):17–29. doi:10.1016/j.nec.2009.08.008
Allan SM, Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2(10):734–744. doi:10.1038/35094583
Alves TR, Lima FR, Kahn SA, Lobo D, Dubois LG, Soletti R, Borges H, Neto VM (2011) Glioblastoma cells: a heterogeneous and fatal tumor interacting with the parenchyma. Life Sci 89(15–16):532–539. doi:10.1016/j.lfs.2011.04.022
Auf G, Carpentier AF, Chen L, Le Clanche C, Delattre JY (2001) Implication of macrophages in tumor rejection induced by CpG-oligodeoxynucleotides without antigen. Clin Cancer Res 7(11):3540–3543
Badie B, Schartner JM (2000) Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery 46(4):957–961; discussion 961–952
Baker BJ, Qin H, Benveniste EN (2008) Molecular basis of oncostatin M-induced SOCS-3 expression in astrocytes. Glia 56(11):1250–1262. doi:10.1002/glia.20694
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66(16):7843–7848. doi:10.1158/0008-5472.CAN-06-1010
Brada M, Stenning S, Gabe R, Thompson LC, Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K, Beall S, Collins VP, Lee SM (2010) Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol 28(30):4601–4608
Bromberg J, Wang TC (2009) Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 15(2):79–80
Burrows FJ, Gore M, Smiley WR, Kanemitsu MY, Jolly DJ, Read SB, Nicholas T, Kruse CA (2002) Purified herpes simplex virus thymidine kinase retroviral particles: III. Characterization of bystander killing mechanisms in transfected tumor cells. Cancer Gene Ther 9(1):87–95
Carpentier AF, Xie J, Mokhtari K, Delattre JY (2000) Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res 6(6):2469–2473
Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, Martin-Duverneuil N, Sanson M, Lacomblez L, Taillibert S, Puybasset L, Van Effenterre R, Delattre JY, Carpentier AF (2006) Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol 8(1):60–66
Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrié M, Meng Y, Richard M, Parizot C, Laigle-Donadey F, Gorochov G, Psimaras D, Sanson M, Tibi A, Chinot O, Carpentier AF (2010) Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol 12(4):401–408
Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann JL, Klatzmann D (1993) Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA 90(15):7024–7028
Carvalho da Fonseca AC, Wang H, Fan H, Chen X, Zhang I, Zhang L, Lima FR, Badie B (2014) Increased expression of stress inducible protein 1 in glioma-associated microglia/macrophages. J Neuroimmunol 274(1–2):71–77. doi:10.1016/j.jneuroim.2014.06.021
Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H (2011) The brain tumor microenvironment. Glia 59(8):1169–1180. doi:10.1002/glia.21136
Chen S, Smith DF (1998) Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem 273(52):35194–35200
Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, Yu H, Jove R, Sotomayor EM (2003) A critical role for Stat3 signaling in immune tolerance. Immunity 19(3):425–436
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Chiu TL, Peng CW, Wang MJ (2011) Enhanced anti-glioblastoma activity of microglia by AAV2-mediated IL-12 through TRAIL and phagocytosis in vitro. Oncol Rep 25(5):1373–1380. doi:10.3892/or.2011.1213
Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, Segall JE (2012) Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med 18:519–527. doi:10.2119/molmed.2011.00217
da Fonseca AC, Badie B (2013) Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol 2013:264124. doi:10.1155/2013/264124
D’Mello C, Le T, Swain MG (2009) Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factorα signaling during peripheral organ inflammation. J Neurosci 29(7):2089–2102. doi:10.1523/JNEUROSCI.3567-08.2009
El Andaloussi A, Sonabend AM, Han Y, Lesniak MS (2006) Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia 54(6):526–535
Faria J, Romao L, Martins S, Alves T, Mendes FA, de Faria GP, Hollanda R, Takiya C, Chimelli L, Morandi V, de Souza JM, Abreu JG, Moura Neto V (2006) Interactive properties of human glioblastoma cells with brain neurons in culture and neuronal modulation of glial laminin organization. Differentiation 74(9–10):562–572. doi:10.1111/j.1432-0436.2006.00090.x
Fonseca AC, Romao L, Amaral RF, Assad Kahn S, Lobo D, Martins S, Marcondes de Souza J, Moura-Neto V, Lima FR (2012) Microglial stress inducible protein 1 promotes proliferation and migration in human glioblastoma cells. Neuroscience 200:130–141. doi:10.1016/j.neuroscience.2011.10.025
Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B (2011) Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE 6(8):e23902. doi:10.1371/journal.pone.0023902
Gagliano N, Costa F, Cossetti C, Pettinari L, Bassi R, Chiriva-Internati M, Cobos E, Gioia M, Pluchino S (2009) Glioma-astrocyte interaction modifies the astrocyte phenotype in a co-culture experimental model. Oncol Rep 22(6):1349–1356
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845. doi:10.1126/science.1194637
Herrera M, Herrera A, Dominguez G, Silva J, Garcia V, Garcia JM, Gomez I, Soldevilla B, Munoz C, Provencio M, Campos-Martin Y, Garcia de Herreros A, Casal I, Bonilla F, Pena C (2013) Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci 104(4):437–444. doi:10.1111/cas.12096
Hu F, Ku MC, Markovic D, Od AD, Lehnardt S, Synowitz M, Wolf SA, Kettenmann H (2014) Glioma-associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline. Int J Cancer 135 (11):2569–2578. doi:10.1002/ijc.28908
Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, Priebe W, Heimberger AB (2007) A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res 67(20):9630–9636
Hwang SY, Yoo BC, Jung JW, Oh ES, Hwang JS, Shin JA, Kim SY, Cha SH, Han IO (2009) Induction of glioma apoptosis by microglia-secreted molecules: the role of nitric oxide and cathepsin B. Biochim Biophys Acta 1793(11):1656–1668. doi:10.1016/j.bbamcr.2009.08.011
Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y (2007) A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26(17):2435–2444
Jacobs VL, Landry RP, Liu Y, Romero-Sandoval EA, De Leo JA (2012a) Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro Oncol 14(2):119–131
Jacobs VL, Liu Y, De Leo JA (2012b) Propentofylline targets TROY, a novel microglial signaling pathway. PLoS ONE 7(5):e37955
Kees T, Lohr J, Noack J, Mora R, Gdynia G, Todt G, Ernst A, Radlwimmer B, Falk CS, Herold-Mende C, Regnier-Vigouroux A (2012) Microglia isolated from patients with glioma gain antitumor activities on poly (I:C) stimulation. Neuro Oncol 14(1):64–78. doi:10.1093/neuonc/nor182
Klinman DM (2004) Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 4(4):249–258
Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216(1):15–24. doi:10.1002/path.2370
Kren L, Muckova K, Lzicarova E, Sova M, Vybihal V, Svoboda T, Fadrus P, Smrcka M, Slaby O, Lakomy R, Vanhara P, Krenova Z, Michalek J (2010) Production of immune-modulatory nonclassical molecules HLA-G and HLA-E by tumor infiltrating ameboid microglia/macrophages in glioblastomas: a role in innate immunity? J Neuroimmunol 220(1–2):131–135. doi:10.1016/j.jneuroim.2010.01.014
Krieg AM (2004) Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep 6(2):88–95
Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Synowitz M, Glass R, Kettenmann H (2013) GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol 125(4):609–620. doi:10.1007/s00401-013-1079-8
Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ (2002) Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol 169(5):2253–2263
Le DM, Besson A, Fogg DK, Choi KS, Waisman DM, Goodyer CG, Rewcastle B, Yong VW (2003) Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci 23(10):4034–4043
Li W, Graeber MB (2012) The molecular profile of microglia under the influence of glioma. Neuro Oncol 14(8):958–978. doi:10.1093/neuonc/nos116
Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, Harris AL (2007) Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 67(23):11244–11253. doi:10.1158/0008-5472.CAN-07-0969
Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J (2010) IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep 23(6):1553–1559
Lima FRS, da Fonseca ACC, Faria GP, Dubois LGF, Alves TR, Faria J, Moura Neto V (2010) The origin of microglia and the development of the brain. In: Ulrich H (ed) Perspectives of stem cells: from tools for studying mechanisms of neuronal differentiation towards therapy. Springer, Netherlands. doi:10.1007/978-90-481-3375-8_12
Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC, Garcia C, Romao L, Brito J, Holanda-Afonso R, Faria J, Borges H, Moura-Neto V (2012) Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 1826(2):338–349. doi:10.1016/j.bbcan.2012.05.004
Lisi L, Laudati E, Navarra P, Dello Russo C (2014) The mTOR kinase inhibitors polarize glioma-activated microglia to express a M1 phenotype. J Neuroinflammation 11:125. doi:10.1186/1742-2094-11-125
Mallat M, Marin-Teva JL, Cheret C (2005) Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol 15(1):101–107. doi:10.1016/j.conb.2005.01.006
Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H (2005) Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol 64(9):754–762
Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, Kettenmann H (2011) Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun 25(4):5
Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, Delattre JY (2005) Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer 116(6):992–997
Nadeau S, Rivest S (2000) Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor κ B activity in the brain during endotoxemia. J Neurosci 20(9):3456–3468
Ng WH, Wan GQ, Peng ZN, Too HP (2009) Glial cell-line derived neurotrophic factor (GDNF) family of ligands confer chemoresistance in a ligand-specific fashion in malignant gliomas. J Clin Neurosci 16(3):427–436. doi:10.1016/j.jocn.2008.06.002
Nolte C, Kirchhoff F, Kettenmann H (1997) Epidermal growth factor is a motility factor for microglial cells in vitro: evidence for EGF receptor expression. Eur J Neurosci 9(8):1690–1698
O’Farrell AM, Liu Y, Moore KW, Mui AL (1998) IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat-3-dependent and -independent pathways. EMBO J 17(4):1006–1018
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170(5):1445–1453. doi:10.2353/ajpath.2007.070011
Penfield W (1925) Microglia and the process of phagocytosis in gliomas. Am J Pathol 1(1):77–90
Platten M, Kretz A, Naumann U, Aulwurm S, Egashira K, Isenmann S, Weller M (2003) Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol 54(3):388–392. doi:10.1002/ana.10679
Prat E, Baron P, Meda L, Scarpini E, Galimberti D, Ardolino G, Catania A, Scarlato G (2000) The human astrocytoma cell line U373MG produces monocyte chemotactic protein (MCP)-1 upon stimulation with beta-amyloid protein. Neurosci Lett 283(3):177–180
Qiao J, Black ME, Caruso M (2000) Enhanced ganciclovir killing and bystander effect of human tumor cells transduced with a retroviral vector carrying a herpes simplex virus thymidine kinase gene mutant. Hum Gene Ther 11(11):1569–1576
Raychaudhuri B, Han Y, Lu T, Vogelbaum MA (2007) Aberrant constitutive activation of nuclear factor κB in glioblastoma multiforme drives invasive phenotype. J Neurooncol 85(1):39–47. doi:10.1007/s11060-007-9390-7
Ribes S, Ebert S, Regen T, Agarwal A, Tauber SC, Czesnik D, Spreer A, Bunkowski S, Eiffert H, Hanisch UK, Hammerschmidt S, Nau R (2010) Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated streptococcus pneumoniae by murine microglia. Infect Immun 78(2):865–871
Ribot E, Bouzier-Sore AK, Bouchaud V, Miraux S, Delville MH, Franconi JM, Voisin P (2007) Microglia used as vehicles for both inducible thymidine kinase gene therapy and MRI contrast agents for glioma therapy. Cancer Gene Ther 14(8):724–737
Ribot EJ, Miraux S, Konsman JP, Bouchaud V, Pourtau L, Delville MH, Franconi JM, Thiaudière E, Voisin PJ (2011) In vivo MR tracking of therapeutic microglia to a human glioma model. NMR Biomed 24(10):1361–1368
Rivest S (2009) Regulation of innate immune responses in the brain. Nat Rev Immunol 9(6):429–439. doi:10.1038/nri2565
Rodero M, Marie Y, Coudert M, Blondet E, Mokhtari K, Rousseau A, Raoul W, Carpentier C, Sennlaub F, Deterre P, Delattre JY, Debré P, Sanson M, Combadière C (2008) Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients withglioblastoma. J Clin Oncol 26(36):5957–5964
Rolhion C, Penault-Llorca F, Kemeny JL, Lemaire JJ, Jullien C, Labit-Bouvier C, Finat-Duclos F, Verrelle P (2001) Interleukin-6 overexpression as a marker of malignancy in human gliomas. J Neurosurg 94(1):97–101. doi:10.3171/jns.2001.94.1.0097
Sangar V, Funk CC, Kusebauch U, Campbell DS, Moritz RL, Price ND (2014) Quantitative proteomic analysis reveals effects of EGFR on invasion-promoting proteins secreted by glioblastoma cells. Mol Cell Proteomics 13(10):2618–2631. doi:10.1074/mcp.M114.040428
Sansone P, Bromberg J (2012) Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 30(9):10
Sarkar S, Döring A, Zemp FJ, Silva C, Lun X, Wang X, Kelly J, Hader W, Hamilton M, Mercier P, Dunn JF, Kinniburgh D, van Rooijen N, Robbins S, Forsyth P, Cairncross G, Weiss S, Yong VW (2014) Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci 17(1):46–55
Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B (2005) Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia 51(4):279–285. doi:10.1002/glia.20201
Schmieder A, Michel J, Schonhaar K, Goerdt S, Schledzewski K (2012) Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol 22(4):289–297. doi:10.1016/j.semcancer.2012.02.002
Si QS, Nakamura Y, Schubert P, Rudolphi K, Kataoka K (1996) Adenosine and propentofylline inhibit the proliferation of cultured microglial cells. Exp Neurol 137(2):345–349
Si Q, Nakamura Y, Ogata T, Kataoka K, Schubert P (1998) Differential regulation of microglial activation by propentofylline via cAMP signaling. Brain Res 812(1–2):97–104
Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A (2008) Macrophage polarization in tumour progression. Semin Cancer Biol 18(5):349–355. doi:10.1016/j.semcancer.2008.03.004
Song Y, Masison DC (2005) Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Sti1 (Hop1). J Biol Chem 280(40):34178–34185. doi:10.1074/jbc.M505420200
Spencer DM (2000) Developments in suicide genes for preclinical and clinical applications. Curr Opin Mol Ther 2(4):433–440
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352 (10):987–996
Suk K (2004) Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett 366(2):167–171
Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S (1999) Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10(1):39–49
Tchoghandjian A, Jennewein C, Eckhardt I, Rajalingam K, Fulda S (2013) Identification of non-canonical NF-κB signaling as a critical mediator of Smac mimetic-stimulated migration and invasion of glioblastoma cells. Cell Death Dis 4:e564. doi:10.1038/cddis.2013.70
Tsunoda K, Kitange G, Anda T, Shabani HK, Kaminogo M, Shibata S, Nagata I (2005) Expression of the constitutively activated RelA/NF-κB in human astrocytic tumors and the in vitro implication in the regulation of urokinase-type plasminogen activator, migration, and invasion. Brain Tumor Pathol 22(2):79–87. doi:10.1007/s10014-005-0186-1
Ursu R, Carpentier A, Metellus P, Barrie M, Meng Y, Laigle-Donadey F, Tibi A, Chinot O, Carpentier AF (2009) Phase II trial of intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma. J Clin Oncol 27:15S
Vilhardt F (2005) Microglia: phagocyte and glia cell. Int J Biochem Cell Biol 37(1):17–21. doi:10.1016/j.biocel.2004.06.010
Vinnakota K, Hu F, Ku MC, Georgieva PB, Szulzewsky F, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Lehnardt S, Hanisch UK, Synowitz M, Markovic D, Wolf SA, Glass R, Kettenmann H (2013) Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neuro Oncol 15(11):1457–1468. doi:10.1093/neuonc/not115
Walker PR, Calzascia T, de Tribolet N, Dietrich PY (2003) T-cell immune responses in the brain and their relevance for cerebral malignancies. Brain Res Brain Res Rev 42(2):97–122
Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, MacSwords J, Cao Y, McLendon RE, Wang XF, Hjelmeland AB, Rich JN (2009) Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells 27(10):2393–2404. doi:10.1002/stem.188
Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J (2004) IL-6 is required for glioma development in a mouse model. Oncogene 23(19):3308–3316
Wiesenhofer B, Stockhammer G, Kostron H, Maier H, Hinterhuber H, Humpel C (2000) Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol 99(2):131–137
Wu Y, Zhou BP (2010) TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br J Cancer 102(4):639–644. doi:10.1038/sj.bjc.6605530
Yang I, Han SJ, Kaur G, Crane C, Parsa AT (2010) The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci 17(1):6–10. doi:10.1016/j.jocn.2009.05.006
Yao Y, Tsirka SE (2014) Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci 71(4):683–697. doi:10.1007/s00018-013-1459-1
Yi D, Hua TX, Lin HY, Kui CL, Ning LX, Wang ZZ (2011) Antitumor treatment efficacy by targeting epidermal growth factor receptor and vascular endothelial growth factor receptor-2 in an orthotopic human glioblastoma model. J Neurooncol 104(1):93–101. doi:10.1007/s11060-010-0479-z
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7(1):41–51
Zeiner PS, Preusse C, Blank AE, Zachskorn C, Baumgarten P, Caspary L, Braczynski AK, Weissenberger J, Bratzke H, Reiss S, Pennarz S, Winkelmann R, Senft C, Plate KH, Wischhusen J, Stenzel W, Harter PN, Mittelbronn M (2014) MIF receptor CD74 is restricted to microglia/macrophages, associated with a M1-polarized immune milieu, and prolonged patient survival in gliomas. Brain Pathol 25(4):491–504. doi:10.1111/bpa.12194
Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B (2009a) Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia 57(13):1458–1467
Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J, Badie SA, Badie B (2009b) S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia 59(3):486–498
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
da Fonseca, A.C.C., Amaral, R., Garcia, C., Geraldo, L.H., Matias, D., Lima, F.R.S. (2016). Microglia in Cancer: For Good or for Bad?. In: von Bernhardi, R. (eds) Glial Cells in Health and Disease of the CNS. Advances in Experimental Medicine and Biology, vol 949. Springer, Cham. https://doi.org/10.1007/978-3-319-40764-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-40764-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40762-3
Online ISBN: 978-3-319-40764-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)