Abstract
Microglia are myeloid cells residing in the central nervous system that regulate innate and adaptive immune responses in development, infections, and neuropathological disorders. Clinical and experimental data show that microglia constitute the dominant immune infiltrate in malignant gliomas. The clinical significance of the mononuclear infiltrate in gliomas remains the subject of controversy. Despite accumulation of these immune cells in both low and high grade gliomas, the antitumor immune response is defective in glioblastomas. Some evidence shows the immunosuppressive and pro-invasive action of glioblastoma-infiltrating microglia. Molecular mechanisms responsible for dual and likely opposite role of these cells are being recently unraveled. This chapter summarizes the latest findings on the heterogeneity of glioma-infiltrating microglia/macrophages, functional characterization of their phenotype, and contribution to glioma pathology. Recent attempts to determine a profile of cytokine/chemokine production and gene expression profiling in CD11b+ cells isolated from patients or rodent gliomas revealed their similarity to alternatively activated, the M2-type macrophages observed in other tissues. Glioma-infiltrating microglia/macrophages acquire the alternative, pro-invasive phenotype in which their phagocytic, trophic, and tissue remodeling functions are enhanced. Cell culture and organotypic brain slice culture studies demonstrated the pro-invasive activity of microglia, and their polarization into tumor supportive cells. This is supported by studies of rodent experimental gliomas which reproduce glioblastoma pathology. Genetic or pharmacological ablation of microglia/macrophages impairs glioma growth, extends survival, and in some cases restores to some extent antitumor immune responses. The potential for targeting interactions between glioma and infiltrating microglia/macrophages in therapeutic interventions is discussed. Small molecule inhibitors of mitogen-activated protein kinase (MAPK)-signaling with immunosuppressive or anti-inflammatory properties, such as cyclosporine A and minocycline, were shown to block infiltration and activation of microglia in vitro and in organotypic brain slices. These molecules reduce infiltration of microglia/macrophages, angiogenesis, and tumor growth of experimental gliomas providing a rationale for blocking pro-invasive functions of microglia/macrophages as a new therapeutic strategy in glioblastomas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glioma
- Infiltrating microglia/macrophages
- M1/M2 phenotype
- Gene expression
- Pro/anti-inflammatory cytokines
- Tumor angiogenesis
1 Introduction
Microglia are an abundant portion of the central nervous system (CNS) cell population comprising 5–20 % of the total glial cell population. Microglial cells, in contrast to other CNS cell types, are of myeloid origin. The consensus phenotypic profile for the microglial cell is: CD68+, CD45low, CD11b+, CD11chigh, MHC class II+, CD14 (Guillemin and Brew 2004). Apart from resident microglia, extraparenchymal brain macrophages normally exist in the CNS as perivascular, choroid plexus-associated, and meningeal macrophages; therefore, the term “brain macrophage” is used to encompass all types of macrophages infiltrating the brain. Neurons control microglial function by physical contact or by releasing neurotransmitters, peptides, and/or growth factors including gamma-aminobutyric acid, glutamate, catecholamines, CD22, CCL21, fraktalkine (CX3CL1), which act on receptors present on microglia membrane. The surface molecule CD200 widely expressed on neurons, astrocytes, and oligodendrocytes via its receptor CD200R (exclusively expressed on microglia) leads to inactivation of microglia and keeps them in a resting state (Cardona et al. 2006; Kierdorf and Prinz 2013). Under physiological conditions, microglia perform tissue surveillance in the nervous system and produce neurotrophic factors supporting cell survival, regeneration, and neurogenesis (Neumann et al. 2009). These cells can respond to changes in sensory activity and can influence neuronal activity. During development and neurogenesis, microglia interactions with neurons help to shape the final patterns of neural circuits. Activated microglia can remove damaged cells as well as dysfunctional synapses, in a process termed “synaptic stripping” (Kettenmann et al. 2013).
One of the most remarkable features of microglia is their ability of morphological and functional plasticity in response to activating stimuli. In vivo imaging studies of transgenic mice, in which all microglia are fluorescently labeled after replacing the Cx3cr1 gene with the gene encoding enhanced green fluorescent protein, have revealed that in the brain, microglia are highly dynamic, moving constantly to actively survey the brain parenchyma. Transcranial time-lapse two-photon imaging of GFP-labeled microglia demonstrates that microglial cells display a ramified morphology with microglial processes highly dynamic in the intact mouse cortex. However, under number of pathological conditions, ramified microglia will activate and engage a series of morphological alterations that leads to a hypertrophy of microglial cell body and a retraction of their ramifications (Nimmerjahn et al. 2005; Hanisch and Kettenmann 2007). Fully activated (reactive) microglia harbor an amoeboid morphology similar to an activated macrophage. Microglial processes are highly motile and respond to chemoattractant molecules released by damaged or apoptotic cells (“find-me” signals) such as fractalkine and extracellular nucleotides (ATP, UDP). An engulfment synapse is formed between microglial receptors and their ligands in the membrane of the apoptotic cell (“eat-me” signals), leading to the tethering and engulfing of the apoptotic cell in a phagosome which matures by fusing with lysosomes. The apoptotic cell is fully degraded in the phagolysosome in less than 2 h (Sierra et al. 2013).
Detection of pathogen-associated molecular patterns (PAMPS) is mediated through scavenger receptors, Toll-like receptors (TLRs) such as the CD14/TLR4 complex, or receptors of the immunoglobulin superfamily (e.g., c-type lectins). Detection of apoptotic cells, in particular exposure of phosphatidylserine in the outer leaflet of the cell membrane, is mediated directly by several receptors, including brain-specific angiogenesis inhibitor 1—BAI-1 (Armstrong and Ravichandran 2011), and by bridging molecules such as milk fat globule-epidermal growth factor (MFG-E8). Another receptor is triggering receptor expressed on myeloid cells-2 (TREM2), whose loss of function prevents microglial phagocytosis (Takahashi et al. 2007). Anionic oligosaccharides such as bacterial lipopolysaccharides and heat shock protein 60 (Hsp60) exposed in the surface of apoptotic cells have been proposed as TREM2 ligands (Stefano et al. 2009). Antibodies (IgG) and proteins of the complement system such as C3b bind to Fc receptors and complement receptor 3 (CR3) on microglia, and mediate phagocytosis (Goodridge et al. 2012). Apoptotic cell clearance is generally an immunologically silent process and a common feature of microglial phagocytosis via TREM-2 or phophatidylserine receptors is a release of anti-inflammatory cytokines such as interleukin (IL)-10 and transforming growth factor beta (TGFβ) (Ravichandran 2010).
Microglial cells rapidly respond to pathological insults, becoming activated to induce a variety of effects that may contribute to both pathogenesis, or to confer neuronal protection. Once activated, they become immune effector cells mediating both innate and adaptive responses (Graeber and Streit 2010; Yang et al. 2010). Proper antigen presentation is critical in the generation of specific, durable responses by the adaptive immune system, and requires interaction between the T cell receptor and processed antigen peptide presented on major histocompatibility complex (MHC) molecules by the antigen presenting cells (APC).
Gliomas are the most frequent primary CNS tumors. According to the WHO classification gliomas are divided in low-grade (grades I and II) and high-grade (grades III and IV) tumors. Low-grade tumors are well-differentiated, slow-growing lesions. Grade I tumors are well restricted and surgically curable, whereas grade II tumors are diffuse, infiltrating lesions with a potential for progression towards a high-grade tumor. Pilocytic astrocytomas (grade I) also differ from diffuse astrocytomas in their altered and increased expression of immune response genes (Huang et al. 2005). The WHO classification divides the malignant gliomas of adults into astrocytic tumors—the most malignant of which is termed glioblastoma—as well as oligodendrogliomas and oligoastrocytomas. Glioblastoma is considered to be one of the most difficult human malignancies to manage, due to frequent dysfunctions of tumor suppressors and oncogenes. These highly infiltrative tumors often invade into normal brain tissues preventing surgical resection. Glioblastoma develops several mechanisms that mediate tumor escape from immune surveillance and produce immunosuppressive factors which results in T-cell anergy or apoptosis (Prins and Liau 2004; Albesiano et al. 2010). The mean survival of patients with glioblastoma has only increased slightly and generally ranges between 1 and 2 years in spite of recent therapeutic advancements.
Histopathologic studies of glioma tissue have shown consistently high levels of infiltrating microglia. Though, microglia could play a role in the immune defense against gliomas, their unharmed growth and highly malignant behavior indicates that microglial immune defense mechanisms do not function properly in these tumors (Graeber et al. 2002; Hussain et al. 2006a, b). In fact, recent evidence indicates that glioma-infiltrating microglia/macrophages promote glioma growth, invasion, and facilitate immunosuppression (Charles et al. 2012). In this chapter I will review microglial origin, phenotype, and activities in gliomas, and provide a plausible explanation for their dual role in these tumors.
2 Microglia/Macrophage Accumulation in Gliomas
2.1 Evaluation of Microglia/Macrophage Content in Gliomas by Immunohistochemistry
Although the presence of leukocytes within tumors has been recognized for over 100 years, advancements in histological techniques and flow cytometry allowed estimation of the extent of tumor infiltration by myeloid cells. In one of the first studies, the authors have analyzed 47 CNS (11 glioblastomas, 9 meningiomas, 3 medulloblastomas, 12 primary neural tumors, and 12 brain metastases) for their content of macrophages. Cell suspensions were prepared by enzymatic digestion and macrophages were determined by IgGEAC rosette assay (Morantz et al. 1979). The 11 glioblastomas had a mean macrophage content of 45 % (from 8 % to 78 %), the 9 meningiomas had 44 % (from 5 % to 81 %), the 3 medulloblastomas 6 % (from 2 % to 15 %), and the metastatic tumors 24 % (range: 4 % to 70 %) (Morantz et al. 1979). Immunohistochemical studies by Morimura et al. (1990) using macrophage markers: Fc-gamma (Fcγ) and complement receptors found in average 20–30 % positive cells of all cells present in 12 gliomas. Microglial cells were recognized by a restricted panel of macrophage markers (anti-Fcγ receptors 1, 2, 3, complement receptor CR3, HLA-DR, common leukocyte antigen CD45, and the monocyte marker RM3/1). Only low numbers of T cells were detected in the tumors. In peritumoral tissue mainly dendritic, microglia-like cells were present, with decreased expression of antigens CD4, RM3/1, and Fcγ receptors in comparison to those in gliomas (Morimura et al. 1990).
Morris and Esiri (1991) used monoclonal antibodies Mac387, KP1, and the lectin RCA-1 to stain for macrophages and microglia in 27 gliomas. They found RCA-1 to be the superior tool for detecting most macrophages and microglia, and detected more macrophages and microglia in high-grade gliomas than in low-grade (Morris and Esiri 1991). A study by Wierzba-Bobrowicz et al. (1994) using lectin RCA-1 on a panel of 40 gliomas demonstrated the presence of both the ramified and amoeboid microglia in the protoplasmic and fibrillary astrocytomas, while in glioblastoma and anaplastic astrocytomas the greatest number of amoeboid microglia and very rarely ramified microglial cells were found. In the non-anaplastic tumors such as gemistocytic astrocytomas, numerous but mostly ramified microglia have been observed.
Further studies of 72 brain tumors of different types using the monocyte/macrophage lineage markers (Ki-M1P, HLA-DR, KP1, My4, My7, Ki-M1, Ki-M6, EBM 11) showed the largest number of ramified and ameboid microglia as well as macrophages among glioblastoma and anaplastic gliomas (Roggendorf et al. 1996). Fewer, predominantly amoeboid, microglia cells were found in glial tumors of low malignancy. Neuronal tumors showed only a mild increase of microglia suggesting some type of specificity for microglia–glioma interactions.
Gemistocytic astrocytomas contain unusually high numbers of microglial cells and aberrant MHC Class II expression by tumor cells correlates with loss of immune-competent microglia. Although these tumors are graded as WHO grade II astrocytomas and their proliferative potential is low, they behave aggressively. Their poor prognosis could be due to pro-invasive role of microglia (Klein and Roggendorf 2001). An immunohistochemical double-labeling study of pilocytic astrocytomas and astrocytomas WHO grade II–IV using the antibodies Ki67 (as proliferation-marker) and CD68 (as microglia marker) demonstrated that microglial cells in astrocytic brain tumors proliferate, with the highest rates of proliferating microglia especially in pilocytic astrocytomas. The proliferation indices of microglia were lowest in fibrillary astrocytoma (Klein and Roggendorf 2001).
Our unpublished studies using HLA-DP, DQ, DR immunohistochemistry on a panel of brain tumors confirmed the strong immunoreactivity reflecting microglia/macrophage accumulation in glial tumors. Positive cells were localized diffusely throughout the tumor, around vessels; we did not observe the presence of positive cells in the areas of palisading necrosis. Malignant embryonal tumors such as PNET (primitive neuroectodermal tumors) or medulloblastoma did not show HLA-DP, DQ, DR immunoreactivity (Fig. 9.1). In adult gliomas we found the significant correlation between HLA-DP, DQ, DR immunoreactivity and tumor grade. Such correlation was not observed among pediatric tumors. Interestingly, we found numerous, but mostly hypertrophic HLA-DP, DQ, DR positive cells in pilocytic astrocytomas, while most HLA-DP, DQ, DR positive cells were amoeboid in glioblastoma. A meta-analysis of five microarray datasets characterizing the expression profiles of immune-defense associated and inflammatory genes in gliomas of different grades showed the reduced expression of many immune response and TLR signaling pathway genes in glioblastomas (unpublished). It suggests a dual role of glioma-infiltrating microglia/macrophages which in low-grade gliomas may exert effective antitumor responses.
Accumulation of microglia/macrophages in human brain tumors of different grades. The abundance of microglia/macrophages was determined immunohistochemically by evaluating HLA-DP, DQ, DR expression on paraffin-embedded sections of glioma biopsies from juvenile pilocytic astrocytoma (a), pleomorphic xanthoastrocytoma (b), primitive neuroectodermal tumor—PNET (c), and glioblastoma multiforme (d). Intense positive staining (brown) is present (magnification: ×100, inset: ×200) intratumorally and around vessels. Note the ramified morphology of HLA-DP, DQ, DR immunoreactive cells in anaplastic astrocytoma (b) and more amoeboid morphology of HLA-DP, DQ, DR immunoreactive cells in glioblastoma (d). No positive staining was detected in the areas of palisading necrosis (N) in glioblastomas
Several studies reported different accumulation of macrophages/microglial cells in tumors of different grades. The number of CD68+ cells was higher in glioblastoma (glioblastoma multiforme, GBM) than that in grade II or III gliomas and positively correlated with vascular density (Nishie et al. 1999). Infiltrating CD68+ cells were positively stained for heme oxygenase (HO)-1 that indicated HO-1 expression in infiltrating microglia/macrophages in gliomas. Deininger et al. (2000) reported that the number of macrophages/microglial cells, expressing cyclooxygenase (COX)-1, was significantly lower in oligodendrogliomas than in anaplastic oligodendrogliomas (Deininger et al. 2000). A study using another microglial marker, glucose transporter 5 (GLUT5), indicated that the number of GLUT5-positive microglia/macrophages was significantly higher in astrocytic tumors than in oligodendroglial tumors (Sasaki et al. 2004).
Infiltration experimental gliomas with microglia/macrophages have been demonstrated in rodent animal glioma models using immunohistochemical staining for Iba1 or CD68 antigens. Mouse glioma 261 (GL261) cells that carry point mutations in the K-ras and p53 genes are frequently used as a model of experimental glioblastoma. Transplanted GL261 cells develop both subcutaneous and intracranial tumors in immunocompetent C57BL/6 mice (Szatmári et al. 2006; Maes and Van Gool 2011). Microglia/macrophages accumulation was detected by Iba1 staining in experimental GL261 gliomas (Färber et al. 2008; Markovic et al. 2009). Histological detection of Iba1 antigen and flow cytometry studies show accumulation of microglia and macrophages in intracranial GL261 gliomas (Gabrusiewicz et al. 2011; Sielska et al. 2013).
The experimental rat RG-2 gliomas in rats were heavily infiltrated with microglia/macrophages, and infiltrating cells were positive for MHC Class II (Ia) antigens (Morioka et al. 1992a). Activated cells expressing CR3 complement receptor and MHC class II (Ia) antigen were found throughout the tumor and with increased density along the tumor periphery (Morioka et al. 1992b). Cells expressing the ED2 epitope (considered to be macrophages) were almost exclusively of the perivascular type and did not show distribution similar to MHC class II (Ia) expressing cells. The ED2 epitope was found sporadically on ramified microglial cells. The results showed glioma infiltration with microglia and blood mononuclear cells, and no evidence of tumor destruction (Morioka et al. 1992b).
Another glioma model is a transgenic rat expressing v-erbB (a viral, oncogenic form of the epidermal growth factor receptor) under transcriptional regulation by the S100-β promoter that develops a brain tumor. A recent study that assessed Iba1 staining for microglia/macrophages in brain tumors in transgenic rats (five malignant glioma, four anaplastic oligodendroglioma, four astrocytoma) demonstrates Iba1+ cells in the tumor core of malignant gliomas that were more activated than Iba1+ microglia of non-neoplastic brain tissue and intraparenchymal anaplastic oligodendrogliomas. Iba1 expression was positively correlated to Ki-67 expression in all the gliomas that suggests ongoing microglial proliferation. Most Iba1+ cells showed no or little expression of putative M2 phenotype markers, CD163 or CD204, in transgenic rats (Sasaki et al. 2013).
A new glioma model (ALTS1C1) derived from primary astrocytes transformed by SV40 large T antigen, followed by serial subcutaneous and intracranial passages in syngeneic C57BL/6J mice, was recently introduced. It recapitulates numerous neuropathological features of human high-grade gliomas and shows accumulation of microglia/macrophages in ALTS1C1 tumor-bearing brains (Wang et al. 2012). The authors noticed different expression levels of F4/80 and CD68 markers: in the primary tumor core both markers were expressed almost equally and overlapped, while in the tumor margin and the infiltration islands there were higher levels of F4/80 than CD68, and there was a subset of F4/80+/CD68− tumor-infiltrating cells which were rarely found in the primary tumor core (Wang et al. 2012).
Several studies using CD11b-TK mice in which a treatment with ganciclovir (GCV) results in ablation of microglia/macrophages (CD11b+, CD68+, Iba1+, CD45low cells) addressed the question of a role of these cells in glioma progression. Although, a preliminary study using this model has shown that depletion of microglia/macrophages in CD11b-TKmt-30 mice via GCV systemic injection resulted in the increased tumor size (Galarneau et al. 2007), a recent study using the same model showed that local ablation of microglia/macrophages in CD11b-TKmt-30 mice decreased tumor size and improved survival (Zhai et al. 2011). Studies of Neurofibromatosis-1 (NF1) genetically engineered mice that develop optic gliomas demonstrated the role of microglia in optic glioma proliferation. GCV treatment of CD11b-TK mice reduced Nf1 optic gliomas proliferation during tumor development and progression (Simmons et al. 2011). Altogether, findings from clinical and animal studies strongly suggest that malignant gliomas recruit microglia/macrophages, and induce production of tumor survival and invasion promoting factors, which in turn facilitates glioma growth and malignancy.
2.2 Evaluation of Microglia and Macrophage Content in Gliomas by Flow Cytometry
Flow cytometry is used to identify microglial cells within the central nervous system. A flow cytometric phenotype for ramified microglia isolated from adult CNS was defined (CD45low CD11b/c+) in the Lewis and Brown Norway rats and clearly distinguished these cells from all blood-derived leukocytes, the latter being CD45high. Isolated microglia cells were mostly MHC class II positive (Sedgwick et al. 1991, 1993). Highly purified populations of CD45lowCD11b/c+ microglia and CD45highCD11b/c+ macrophages have been obtained from the adult CNS (Dick et al. 1995).
Badie and Schartner (2000) determined infiltration of immune cells into experimental gliomas (intracranially implanted rat C6, 9L, and RG-2 tumor cells) based on detection of CD11b/c, CD45, and CD8a antigens by flow cytometry. The extent of microglia (CD11bhigh, CD45low), macrophage (CD11bhigh, CD45high), and lymphocyte (CD11bnegative, CD45high) infiltration into tumors, tumor periphery, and contralateral tumor-free hemispheres was measured for each glioma type. They found that microglia account for 13–34 % of viable cells and were present in the tumors, tumor periphery, and contralateral tumor-free hemispheres. In contrast, macrophages were less prominent within the tumors and tumor periphery (4.2–12 %) and were rare in the contralateral tumor-free hemispheres (0.9–1.1 %). Among the tumor types, RG-2 gliomas had the least microglia/macrophage infiltration. The distribution pattern of lymphocytes varied among tumor models: whereas lymphocytes accounted for more than one-third of the cells in C6 and 9L tumors; they represented only 1 % of cells in RG-2 gliomas.
A recent study using immunomagnetic sorting of CD11b+ cells from intracranial murine EGFP-GL261 gliomas followed by detection of CD45 antigens by flow cytometry, demonstrated an early accumulation of activated microglia (8 days postimplantation) followed by accumulation of peripheral macrophages in gliomas at day 15th. Quantification of glioma-infiltrating microglia/macrophages revealed a ~2.6-fold increase in the number of microglia and a ~30-fold increase in the number of macrophages 15 days after tumor implantation (Gabrusiewicz et al. 2011). The Iba1 staining of tissue sections showed the strong increase in Iba1-positive cells at day 8 after tumor implantation. At the day 15 the tumor was heavily infiltrated with amoeboid, Iba1-positive cells that accumulated within and around the implanted glioma cells. Interestingly, Iba1-positive cells in the close vicinity of the tumor were evidently more activated and amoeboid than the cells located distantly (Fig. 9.2). Ramified microglia, with thin branching processes and a small cell body, were detected in the tumor-free parenchyma (Gabrusiewicz et al. 2011).
Accumulation of activated microglia/macrophages in murine experimental gliomas. (a) Representative confocal images of brain sections 15 days after implantation of pEGFP-N1 GL261 cells into the striatum of C57BL/6 mice. Note the infiltration and morphological transformation of glioma-infiltrating Iba1+ cells (white arrows). Scale bar: left image—1,000 μm, right image—20 μm. (b) Microglia and macrophages were separated using a magnetic-bead-conjugated anti-CD11b antibody and stained with CD45 PerCP-Cy5.5 and CD11b PE prior to FACS acquisition. Representative dot plots for microglia (Gate R4, CD11b+/CD45low) and macrophages (Gate R5, CD11b+/CD45high) from tumor-bearing hemispheres. (c) Quantification of glioma infiltrating microglia, macrophages, and T lymphocytes 8, 15, and 25 days after tumor cell implantation
Altogether, histological and flow cytometry studies show accumulation of microglia and macrophages in human gliomas and rodent experimental gliomas. Intratumoral microglia/macrophage density increases during glioma progression and correlates with the grade of malignancy. Neuronal or embryonal CNS tumors do not exhibit accumulation of microglia and macrophages within tumors.
3 Origin of Myeloid Cells Infiltrating Gliomas
To date, no single microglia-specific marker that does not also label peripheral macrophages or extraparenchymal brain macrophages has been identified; therefore the question remains whether all CD11b+ cells are CNS resident microglia or are at least in part from other sources such as bone marrow. Flow cytometry studies, based on evaluation of CD11b and CD45 antigens, show accumulation of both microglia and macrophages in human gliomas and rodent experimental gliomas.
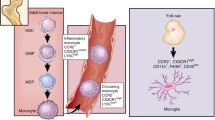
Microglial progenitors are yolk sac-derived, invade the brain during early embryonic development, and then locally proliferate in the brain (Ginhoux et al. 2010; Schulz et al. 2012). In contrast to other yolk sac-derived macrophages, they are not replaced during the postnatal period and later life by liver- or bone marrow (BM)-derived macrophages (Hoeffel et al. 2012). Results from irradiation/bone marrow reconstitution studies indicated that microglia in the brains of chimeric mice are slowly replenished by BM-derived cells and microglial turnover could accelerate under neurological conditions. There are also interspecies differences: reactive microglia in rats originate from resident microglia, whereas they have a mixed BM-derived and resident origin in mice, depending on the severity of ischemic tissue damage (Lambertsen et al. 2011). Recent works questioned the use of irradiation/reconstitution experiments and re-evaluation of microglia origin in the CNS suggests that postnatal hematopoietic progenitors do not significantly contribute to microglia population in the adult brain in mice (Davoust et al. 2008). In vivo lineage tracing studies established that adult microglia originate from primitive hematopoietic progenitors that arise before embryonic day 8 (Ginhoux et al. 2010).
Our studies of glioma-infiltrating microglia/macrophages in chimeric mice with the reconstituted bone marrow from GFP transgenic mice showed the significant infiltration of peripheral macrophages to the experimental murine DsRed-GL261 gliomas. Using flow cytometry we found that the infiltrating macrophages (CD11b+CD45hi) consist 60 % of all GFP cells found in the tumor-bearing hemisphere (unpublished). These results suggest that myeloid cells infiltrating malignant gliomas consist of a heterogeneous population that may arise from different origin (brain or blood and bone marrow derived) and exhibits different functionalities. Even if irradiation/bone marrow reconstitution studies exaggerate the extent of contribution of peripheral or BM-derived monocytes/macrophages to a pool of glioma-infiltrating myeloid cells, together with flow cytometry studies, it shows considerable accumulation of microglia and macrophages in glioblastoma. The lack of appropriate animal models and flow cytometry data from human low-grade gliomas do not allow estimating the contribution of peripheral or BM-derived monocytes/macrophages to pathobiology of these tumors.
4 Defining a Phenotype of Glioma-Infiltrating Microglia/Macrophages
4.1 Functional and Molecular Definition of Microglia/Macrophage Phenotype in Cocultures and Animal Glioma Models
4.1.1 Immune Responses of Microglia/Macrophages Exposed to Glioma
Although glioblastoma is immunogenic, immune-mediated eradication does not occur efficiently, and attempts at immunotherapy directed against brain tumors have been minimally successful (Grauer et al. 2009; Rolle et al. 2012). There are numerous impairments in glioma immunity which include: low peripheral lymphocyte counts, reduced hypersensitivity reactions to recall antigens, impaired mitogen-induced blastogenic responses by peripheral blood mononuclear cells, and increased CD8+ suppressor T cells (reviewed in Hussain and Heimberger 2005). Adaptive immune responses are remarkably deficient, with diminished responsiveness of peripheral T cells due to impaired transmembrane signaling through the T-cell receptor/CD3 complex (Morford et al. 1997). Soluble factors derived from glioblastoma cell lines increased chemotaxis of Treg, compared with conventional T cells, Treg infiltration, proliferation, and survival (Crane et al. 2012).
Activation of adaptive immune responses that are essential for tumor eradication/suppression is triggered by activation of innate immunity (Akira et al. 2001). Rodent studies have shown that microglia play a critical effector role in rapid responses to injury, autoimmune stimuli, and viral infections, and initiate CNS innate and adaptive immune responses through multiple TLRs (Olson and Miller 2004). The phenotype and function of microglia isolated from resected human glioma tissues by sequential Percoll density gradients have been characterized. Those studies demonstrated that glioma-infiltrating microglia/macrophages express Toll-like receptors (TLR1, 2, 3, 4) and the adaptor protein CD14, were capable of phagocytosis, however did not produce pro-inflammatory cytokines: IL-6, IFNα, and TNFα. Microglia isolated from normal brain were more efficient at tumor cytotoxicity than those derived from tumors (Hussain et al. 2006a).
Further studies revealed that despite surface MCH class II expression, glioma-infiltrating microglia/macrophages lack expression of the co-stimulatory molecules CD86, CD80, and CD40 critical for T-cell activation. Glioma patients demonstrate lack of effector/activated T cells (glioma-infiltrating CD8+ lymphocytes were CD8+CD25−) and an increased population of regulatory CD4 T cells (CD4+CD25+FOXP3+) infiltrating the tumor. Overall, glioma-infiltrating microglia/macrophages may have a few innate immune functions intact, but their capacity to secrete cytokines, to upregulate co-stimulatory molecules, and subsequently to activate antitumor effector T cells could not be sufficient to initiate immune responses (Hussain et al. 2006a).
There is no data on the innate immune phenotype and function of microglia isolated from low-grade gliomas. However, gene expression studies in pilocytic and diffusely infiltrating grade II astrocytomas shed some light on differences in immune defense responses in various tumors. Comparison of gene expression profiles in WHO grade I benign pilocytic astrocytoma, diffusely infiltrating grade II astrocytomas and oligodendrogliomas revealed that the number of immune system-related genes (such as HLA-DRα, HLA-DPB1, HLA-DQB1, IgG3, IgGK, FCER1G, A2M, FCRN, IFI-56K, and DAP12) were upregulated in all tested tumors relative to normal cerebellum. However, immune-defense genes such as HLA-DRalpha, HLA-DPA1, HLA-DPB1, HLA-DQB1, A2M, TIMP1, TIMP2, CDKN1A, and SOCS3 were expressed at higher levels in pilocytic astrocytomas than diffuse astrocytomas and oligodendrogliomas (Huang et al. 2005). The results suggest that a pattern of immune gene expression in pilocytic astrocytomas differs from those of diffusely infiltrating low-grade gliomas and that their benign behavior may be related to upregulation of immune defense-associated genes.
The importance of ability to upregulate immune defense-associated genes and to launch effective antitumor responses by tumor infiltrating microglia/macrophages is strengthened by recent gene expression profiling data of human gliomas. Ten epidemiologic studies indicated that allergies appear to reduce glioma risk. In a meta-analysis of 12 studies evaluating allergy and risk of gliomas, a significant negative association (a protective effect) was found between allergy and glioma (Chen et al. 2011). The systematic analysis of 919 allergy- and inflammation-related genes in 142 glioblastoma tissue samples revealed downregulation of the majority of allergy- and inflammation-related genes in glioblastoma (Schwartzbaum et al. 2010). Moreover, 69 % of these genes were found negatively correlated with the expression of a glioma stem-like cell marker CD133, including allergy-related (e.g., IL-4R-α) and immunoregulatory genes (e.g., TGFβ1). A recent study evaluating gene expression microarray profiles of high grade gliomas from long-term survivors showed that increased immune gene expression and immune cell infiltration in tumors distinguished long-term from short-term survivors (Donson et al. 2012). Using immune cell-specific gene classifiers, both T cell-associated and myeloid linage-associated genes were shown to be enriched in high grade gliomas from long-term versus short-term survivors.
Taking into consideration previous results on the role of glioma initiating cells in tropism of microglia/macrophages to gliomas (Yi et al. 2011) and their polarization into immunosuppressive cells (Wu et al. 2010), we can postulate a critical role of polarized microglia/macrophages in shaping immune microenvironment of malignant human gliomas and the lack of effective antitumor defense against these tumors.
4.1.2 Phagocytic Properties of Microglia/Macrophages Exposed to Glioma
Microglia from human glioblastoma patients have the capability of phagocytosing latex microbeads ex vivo (Hussain et al. 2006a). Cocultures of human microglial cells (microglial CHME5 cell line) grown on collagen beads or on coverslips and placed on monolayer of C6 cells showed decreased bead phagocytosis after a transient increase (Voisin et al. 2010). Cell motility and viability were not affected in such cocultures. In contrast, a recent study shows enhancement of phagocytosis by primary rat microglial cultures exposed to rat C6 glioma cells or glioma-conditioned medium (Ellert-Miklaszewska et al. 2013). The same study shows the strong increase of microglial motility and sustained proliferation under such conditions. This discrepancy could arise from the use of an immortalized microglia CHME5 cell line which has different properties than primary microglial cultures. Studies of cyclodextrin-based nanoparticle (CDP-NP) uptake shows that CDP-NPs were preferentially taken up by BV2 and N9 microglial cells compared with GL261 glioma cells (Alizadeh et al. 2010). Fluorescent microscopy and flow cytometry analysis of intracranial GL261 gliomas demonstrated a predominant CDP-NP uptake by macrophages and microglia, within and around the tumor site (Alizadeh et al. 2010).
Tumor cells are known to evade phagocytosis by deficient “eat-me” signals on their membrane. Microglia phagocytose tumor cells induced to apoptosis with the cytotoxic agent etoposide (Chang et al. 2000). Normal human astrocytes, glioma cells, and microglia all phagocytosed apoptotic U-251 MG glioma cells; however, microglia had phagocytose fourfold more than did other cells. Binding of annexin-V to phosphatidylserine on apoptotic glioma cell membranes inhibited microglial phagocytosis by 90 % (Chang et al. 2000). Furthermore, the ability of microglia to phagocytose undamaged or rat 9L gliosarcoma cells damaged by alloreactive cytotoxic T lymphocytes (aCTL) has been demonstrated. In vitro, 5.5 ± 0.9 % of microglial cells isolated from tumor-bearing rat brains phagocytosed aCTL-damaged 9L cells. At 3 days following intracranial 9L cell infusion, 17.5 ± 0.1 % of the microglia phagocytosed CFSE-labeled aCTL-damaged 9L tumor cells within glioma-bearing brain (Kulprathipanja and Kruse 2004).
4.2 Characterization of Microglia/Macrophage Phenotype in Human Gliomas
Activated macrophages can be broadly classified in two main groups: classical, inflammatory macrophages (M1 type), activated by interferon gamma (IFNγ) and lipopolysaccharide (LPS), and alternatively activated macrophages (M2 type). The latter could be further subdivided depending on stimuli. In general, M1 type macrophages exhibit potent microbiocidal properties and promote strong IL-12-mediated Th1 responses, while M2 support Th2-associated effector functions. M2 polarized macrophages play a role in resolution of inflammation through high endocytic clearance capacities, trophic factor synthesis, accompanied by reduced pro-inflammatory cytokine secretion (Martinez et al. 2008; Solinas et al. 2009). Hallmarks of M2 macrophages are IL-10high IL-12low IL-1rahigh IL-1 decoyRhigh production, CCL17 and CCL22 secretion, high expression of mannose, scavenger and galactose-type receptors, poor antigen-presenting capability, enhanced phagocytic and wound-healing activities. M2 macrophages express specific changes in some metabolic pathways: arginine metabolism is oriented toward the production of ornitine and polyamine instead of citrulline and NO. Arginase-1 (Arg1) is upregulated in alternatively activated macrophages and, due to its higher affinity for arginine, competes with inducible nitric oxide synthase (iNOS), which metabolizes arginine. Tumor-associated macrophages (TAMs) resemble M2-polarized cells and play a pivotal role in tumor growth and progression. TAMs promote angiogenesis, remodeling, and repair of tissues. Activated M2 cells control the inflammatory response by downregulating M1-mediated functions (Solinas et al. 2009). Monocyte differentiation could be influenced by soluble factors in tumor microenvironment. In tumors, there is an established gradient of IL-10 that can switch monocyte differentiation toward macrophages rather than to dendritic cells (Li and Flavell 2008).
Compelling evidence suggests that glioma-associated microglia/macrophages resemble M2-polarized cells and support tumor growth and progression. The immune functions of CD11b/c+CD45+ glioma-infiltrating microglia/macrophages from postoperative tissue specimens of glioma patients have been reduced, as they did not produce pro-inflammatory cytokines (TNFα, interleukin 1β, or interleukin 6), and do not mediate T-cell proliferation. They express MHC class II, but they lacked expression of the co-stimulatory molecules CD86, CD80, and CD40 that are critical for T-cell activation. The presence of regulatory T cells may also contribute to the lack of effective immune activation against malignant human gliomas (Hussain et al. 2006a).
Macrophage scavenger receptors CD163 and CD204 are believed to be markers for “M2 type” macrophages. CD163 and CD204 positive cells were detected in gliomas. The ratio of CD163 and CD204 positive cells among glioma-infiltrating microglia/macrophages correlates with the histological grade of glioma (Komohara et al. 2008). Significant increases in arginase activity and G-CSF levels were observed in plasma specimens obtained from patients with glioblastoma (Raychaudhuri et al. 2011). Further, myeloid-derived suppressor cell (MDSC)-like cells have been detected in glioma patients (Rodrigues et al. 2010). Patients with glioblastoma (GBM) have increased MDSC counts (CD33+HLADR−) in their blood that are composed of neutrophilic (CD15+; >60 %), lineage-negative (CD15−CD14−; 31 %), and monocytic (CD14+; 6 %) subsets. T cells from patients with glioblastoma had suppressed IFN-γ production after stimulation and removal of MDSCs by pre-incubation with anti-CD33/CD15-coated beads significantly restored T cell function.
Increased tumor infiltration by MSDC has been reported in rat glioma models (Prins et al. 2002; Graf et al. 2005). Animal studies on GFAP-V(12)HA-ras mouse astrocytomas demonstrated that immune infiltration at the tumor site in mice is dominated by immunosuppressive cells from the early stages of tumor development, even at very early asymptomatic stages (Tran Thang et al. 2010). Phenotypical characterization of MSDC in GL261 murine glioma suggests that tumor-infiltrating MSDC are pleiotropic monocytes/macrophages that bear M1- and M2-type characteristics. Over 90 % were of the CD11b+F4/80+ monocyte/macrophage lineage, displayed a CD11b/c+Gr-1lowIL-4Rα+ phenotype, and suppressed the proliferation of activated splenic CD8+ T cells. These MDSCs expressed both M1 and M2 activation markers: CD206, CXCL10, IL-1β, TGF-β, and TNF-α mRNAs and CXCL10, CD206 proteins. In addition, the cells expressed CX3CR1 and CCR2 which are the markers of an inflammatory monocyte (Umemura et al. 2008).
Soluble factors secreted by glioblastoma cells have been shown to stimulate cultured peripheral blood monocytes to differentiate into the M2-like or MSDC-like cells, characterized by increased CD163 and CD204 staining, reduced IL-12 and TNFα production (Komohara et al. 2008), and upregulation of IL-10 and TGF-β (Rodrigues et al. 2010). Peripheral blood monocytes cocultured with glioblastoma cells (U87 and U251) acquired immunosuppressive, MDSC-like features, including reduced CD14 (but not CD11b) expression, increased immunosuppressive IL-10, TGF-β, and B7-H1 expression, decreased phagocytic ability, and increased ability to induce apoptosis in activated lymphocytes. Direct contact between monocytes and glioblastoma cells was necessary for complete induction of such phenotype (Rodrigues et al. 2010). These results support a hypothesis that normal human monocytes exposed to malignant glioma cells may adopt an MDSC-like phenotype in glioma microenvironment. It means that many immunologically active cell types are present in malignant glioma patients and may contribute to immunosuppression. Interestingly, a recent study has demonstrated immunosuppressive properties of glioma cancer stem cells which produce soluble CSF-1, TGF-β1, and macrophage inhibitory cytokine (MIC)-1, thus inducing recruitment and polarization of macrophages/microglia into immunosuppressive cells (Wu et al. 2010).
Studies on cultured microglial cells demonstrate remarkable plasticity and ability to adapt different fate or differentiation route depending on a stimulus. Rat primary microglial cultures exposed to glioma-conditioned medium (G-CM) undergo morphological alterations and become motile and highly phagocytic. Global gene expression profiling of LPS- or G-CM-stimulated microglial cultures followed by computational analysis of gene expression pattern in differentially stimulated microglia revealed activation of alternative genetic programs. Most genes characteristic for innate and inflammatory immune responses, which are commonly upregulated during classical inflammation, were not induced by G-CM stimulation. The analysis of signaling pathways activated in microglia after G-CM demonstrates defective NFκB and STAT 1 activation, resulting in failure to mount production of inflammation mediators (IL-1β, iNOS, Cox2) and polarize to the classical inflammatory phenotype. Furthermore, the increased expression/production of many M2-like factors (Arg1, MT1-MMP, IL-10, TGF-β) in G-CM activated microglial cultures could be responsible for sustained proliferation and acquisition of the glioma-associated phenotype (Ellert-Miklaszewska et al. 2013). In particular, upregulation of genes coding for CD69—a C-type lectin R family member, a negative regulator of the immune response; CD86—the co-stimulatory molecule implicated in dendritic cell maturation; CCL2 (MCP-1, monocyte chemoattractant protein-1), CCL5 (RANTES, regulated on activation, normal T cell expressed and secreted), CXCL chemokines CXCL1, CXCL2, CXCL7, and CXCL14 was detected (Ellert-Miklaszewska et al. 2013). Glioma secretome-exposed microglia express the microglial fractalkine receptor CX3CR1 that could be important in the regulation of myeloid cell trafficking and polarization. These findings are summarized in Fig. 9.3.
Graphical summary of the classical, inflammatory, and pro-tumorigenic phenotypes of activated microglia. Primary rat microglial cultures exposed to lipopolysaccharide (LPS) or glioma secreted factors adapt different fates and polarize into pro-inflammatory or alternatively activated cells. Glioma-derived factors increase focal adhesion kinase and PI-3K/Akt signaling and activate ERK and p38 MAPK but not JNK signaling. In contrast to LPS stimulation, glioma secretome do not activate pro-inflammatory Stat1 and NFκB signaling in microglial cells. The genetic program induced by glioma secretome include: transcription factors c-Myc and Id1/3 (inhibitors of differentiation), and numerous coding for chemokines and cytokines regulating immune cell recruitment and trafficking. Glioma-induced activation is associated with increased TGF-β and IL-10 production, while LPS-induced cells produce inflammation mediators
Recent data support the notion that microglia/macrophage accumulation in diffuse glial tumors reflects participation of these cells in supporting the invasive potential of gliomas. Microglia and macrophages can secrete various cytokines, growth factors, and enzymes, including extracellular matrix proteases which directly or indirectly may influence tumor migration/invasiveness and proliferation (Watters et al. 2005; Li and Graeber 2012).
Experimental studies using brain organotypic slices, microglia-glioma cocultures, and genetic models, in which microglial cells are ablated in the tumor, strongly support pro-invasive role of glioma-infiltrating microglia (Markovic et al. 2005, 2009; Sliwa et al. 2007). The invasion of GFP-labeled GL261 glioblastoma cells in organotypic brain slices that were depleted of microglia by treatment with clodronate-filled liposomes was significantly decreased. Inoculation of exogenous microglia together with glioma cells into cultured brain slices increased the infiltrative behavior of the tumor depending on the microglia/glioma cell ratio and increased activity of metalloproteinase-2 (MMP-2). It has been shown that soluble factors released from glioma cells strongly stimulate MMP-2 activity in microglia (Markovic et al. 2009). Membrane bound MT-MMPs, in particular membrane-type MT1- and MT2-MMP, play a major role in activating MMP-2. Newly synthesized MMP-2 is secreted as an inactive pro-enzyme, which is cleaved on the cell surface by membrane-type MT1-MMP complexed with TIMP-2. Metalloproteinase MT1-MMP secreted by glioma-exposed microglia activates pro-MMP-2 in glioma cells that promotes tumor invasion, as was shown using brain slices from MT1-MMP-deficient mice and in a microglia depletion model (Markovic et al. 2009). Glioma-released factors induce the expression and activity of MT1-MMP via microglial toll-like receptors and the p38 MAPK pathway, as deletion of the toll-like receptor adapter protein MyD88 or p38 inhibition prevented MT1-MMP expression and activity in cultured microglial cells. Microglial MT1-MMP in turn activates glioma-derived pro-MMP-2 and promotes glioma expansion, as shown in an ex vivo model using MT1-MMP-deficient brain tissue and in microglia depleted mice (Markovic et al. 2009).
Activated microglia release cytokines which enhance tumor cell invasion (Wesolowska et al. 2008). Microglial cells exposed to glioblastoma cells secrete active TGF-β1, which through a paracrine loop stimulates glioblastoma invasion. Plasmid-transcribed small hairpin RNAs (shRNAs) which downregulate the TGF-β type II receptor (Tβ IIR) expression, effectively inhibited TGF-β-dependent signaling pathways and transcriptional responses in glioblastoma cells. Microglia strongly enhanced glioma invasiveness in the coculture system, but this activity was lost in glioma cells depleted of TGF-β type II receptor indicating an important role of microglia-derived TGF-β in tumor invasion. Moreover, tumorigenicity of glioblastoma cells depleted of TGF-β IIR in nude mice was reduced by 50 %.
Fas (CD95/APO-1) is a cell surface “death receptor” that mediates apoptosis upon engagement by its ligand—FasL but can also promote tumor invasion when apoptosis is compromised. Kleber and coworkers (Kleber et al. 2008) demonstrated that interaction of glioma cells with the surrounding brain tissue induces expression of FasL in both tumor and host cells. FasL modulated glioma invasion via PI-3K/MMP-dependent mechanism and neutralization of Fas activity blocked migration of glioma cells in a mouse syngenic model of intracranial glioblastoma. We demonstrated that a recombinant FasL Interfering Protein (FIP), which interferes with Fas signaling in C6 glioma cells, impaired cell motility and invasiveness of glioma cells in vitro. A blockade of Fas signaling reduced MMP-2 activity in glioma cells. Interestingly, reduction of MMP-2 activity was not due to downregulation of mmp-2 and mt1-mmp expression but was dependent on modulation of timp-2 mRNA and TIMP-2 protein levels by Fas signaling (Wisniewski et al. 2010). FasL expression is higher in many glioblastoma cell lines in comparison to non-transformed astrocytes and is upregulated in microglia exposed to glioma-conditioned medium (Wisniewski et al. 2010). Microglia accumulating within tumors account for half of the FasL expression in the murine intracranial tumors (Badie et al. 2001).
5 Molecules and Mechanisms Responsible for Microglia/Macrophage Accumulation in Gliomas
Signals, signaling pathways, and molecular mechanisms underlying “switch” or “reeducation” of glioma-infiltrating microglia and macrophages into pro-invasive cells are poorly known. Glioma cells produce several factors with a chemotactic activity which attract microglia, including monocyte chemotactic protein (MCP)-3 (Okada et al. 2009) and hepatocyte growth factor/scatter factor (HGF/SF), as shown by in vitro and in vivo studies (Wang et al. 2012). Macrophage proliferation, differentiation, and chemotaxis can be regulated by several factors, including macrophage colony stimulating factor (M-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), interleukin-34, and the chemokine CCL2 (Hibbs et al. 2007; Hamilton 2008; Pollard 2009). Increased M-CSF expression has been associated with a poor prognosis and increased angiogenesis in various non-brain tumors (Sapi and Kacinski 1999; Lin et al. 2002; Mroczko et al. 2007; Zhu et al. 2008; Mantovani and Sica 2010; Davies et al. 2011). Overexpression of M-CSF accelerates tumor progression and increases pulmonary metastasis in murine experimental breast cancers (Lin et al. 2001). RNAi-mediated silencing of M-CSF or its receptor c-FMS suppresses the growth of mammary tumor xenografts in mice and reduces macrophage infiltration, metalloproteinase expression, and angiogenesis (Aharinejad et al. 2004; Lin et al. 2006). Osteopetrotic (Csf1op/Csf1op) mice harbor a mutation in the Csf1 gene that causes an M-CSF deficiency (Wiktor-Jedrzejczak et al. 1990; Yoshida et al. 1990), affects myeloid lineage development, and reduces the number of monocytes/macrophages in many tissues (Wiktor-Jedrzejczak et al. 1992; Cecchini et al. 1994). Experimental breast cancer progression was delayed and lung metastases were reduced in op/op mice (Nowicki et al. 1996; Qian et al. 2009).
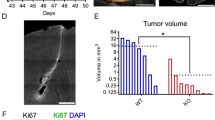
We found upregulation of GM-CSF levels in glioma-bearing brains and increased expression of Csf2, but not Csf1, in murine GL261 glioma cells compared with non-transformed astrocytes (Gabrusiewicz et al. 2011). Knockdown of GM-CSF expression in GL261 glioma cells strongly reduced microglia-dependent invasion in organotypical brain slices. The number of infiltrating microglia/macrophages (Iba1+ cells) and intratumoral angiogenesis were reduced in murine gliomas depleted of GM-CSF. It resulted in reduction of tumor growth and extended animal survival. M1/M2 gene profiling in sorted microglia/macrophages suggests impairment of their pro-invasive activation in GM-CSF-depleted gliomas (Sielska et al. 2013). Deficiency of M-CSF did not affect glioma growth in op/op mice in vivo and the accumulation of Iba1+ cells. This suggests that glioma-derived GM-CSF is responsible for the recruitment/activation of microglia/macrophages and contributes to tumor progression. The increased expression of CSF2 and inverse correlation with patient survival was found in human glioblastoma (Sielska et al. 2013). The elevated expression of CSF2 in human glioblastoma and the autocrine or paracrine action of GM-CSF contributing to glioma proliferation in vitro have been recently demonstrated (Revoltella et al. 2012).
The chemokine receptor CX3CR1 and its ligand CX3CL1 (fractalkine) are known to be involved in immune responses, and influence migration of macrophages, microglia, and lymphocytes in vivo (Combadiere et al. 2007). CX3CR1 was localized on Iba1 and CD11b/c positive glioma-infiltrating microglia/macrophages (GIMs). Cultured human GIMs responded to CX3CL1-triggered activation of CX3CR1 with adhesion and migration in vitro (Held-Feindt et al. 2010). However, formation of GL261 glioma and animal survival rates were not affected in CX3CR1-deficient C57BL/6 mice. Tumor-bearing CX3CR1−/− mice had similar numbers of infiltrating microglia and CD4+, CD8+, FoxP3+, or Ly49G2+ lymphocytes as control mice. These data indicate that CX3CR1 has little or no effects on glioma formation or the migration of microglia and lymphocytes into GL261 tumors (Liu et al. 2008).
The experimental ALTS1C1 gliomas expressed a relatively high level of CXCL12 (stromal cell-derived factor-1, SDF-1) in vitro and in vivo. Inhibition of CXCL12 production reduced tumor invasiveness and the resulting tumors had well-defined borders and were lacking infiltration tracts. Inhibition of CXCL12 production reduced tropism of microglia/macrophages (determined by confocal imaging using CD68 and F4/80 markers) toward hypoxia (Wang et al. 2012). The authors postulate that a CXCL12-concentration gradient could be the driving force for tropism microglia/macrophages toward hypoxia.
Glial cell line-derived neurotrophic factor (GDNF) is expressed in gliomas, Gl261, and human glioma cell lines, and this factor has been shown as a strong chemoattractant for cultured microglia. Knockdown of GDNF expression in mouse GL261 glioma cells by shRNA diminished accumulation of microglia in tumors, while overexpression of GDNF in fibroblasts seeded into hollow fibers implanted to the brain promoted microglia attraction. GDNF release from human or mouse glioma had a strong effect on microglia accumulation; glioma-induced astrogliosis was not affected. Injection of GDNF depleted GL261 glioma cells into mouse brains resulted in reduced tumor expansion and improved survival (Ku et al. 2013).
6 Targeting Glioma-Infiltrating Microglia/Macrophages as a Innovative Therapeutic Strategy
Recent studies from our laboratory demonstrated that pharmacological inhibition of infiltration and activation of brain resident microglia and peripheral macrophages effectively impairs tumor growth in mice (Sliwa et al. 2007; Gabrusiewicz et al. 2011). Cyclosporine A (CsA), a widely used immunosuppressive drug and atypical MAPK signaling inhibitor (Zawadzka et al. 2012), reduced amoeboid transformation of microglial cells, and inhibited microglia-dependent invasion of glioma cells in vitro and in organotypic brain slices. A systemically applied CsA inhibited microglia/macrophages infiltration (as determined by flow cytometry and immunofluorescence) and blocked expression/activity of proteolytic enzymes (MMP-2, MT1-MMP) and production of cytokines (IL-10 and GM-CSF) (Gabrusiewicz et al. 2011). Our data demonstrate that blockade of microglia/macrophage infiltration and inhibition of their pro-invasive behavior reduce glioma growth in mice.
Minocycline, a semisynthetic antibiotic of the tetracycline family, has emerged as a potent anti-inflammatory and microglia targeting drug, beneficial in animal models of several CNS disorders. For the reason of the good tolerance and penetration into the brain, minocycline has been clinically tested for stroke, multiple sclerosis, spinal cord injury, and some neurodegenerative diseases (Kim and Suh 2009). The treatment with minocycline reduced the expression of MT1-MMP in glioma-infiltrating microglia in vitro and in organotypic brain slices. This reduction is dependent on the presence of microglia. Glioma growth in an experimental mouse model was strongly reduced by the addition of minocycline to drinking water, compared to untreated controls. Coherently, MT1-MMP was abundantly expressed in glioma-associated microglia in controls, but was strongly attenuated in tumors of minocycline treated animals (Markovic et al. 2011). This suggests that the clinically approved antibiotic minocycline is a promising new candidate for adjuvant therapy against malignant gliomas. However, minocycline and CsA treatments have some drawbacks because while blocking tumor-associated activation of microglia, they exert immunosuppressive action and inactivate many components of the immune system. Minocycline represses MHC II expression in microglia (an event requisite for T cell reactivation) in an experimental model of autoimmune diseases via the inhibition of transcription factor CIITA expression (Nikodemova et al. 2007).
Recent studies defined more specific, intracellular signaling pathways which could be targeted. Signal transducer and activator of transcription 3 (STAT 3) plays a suppressive role in antitumor immunity. STAT 3 was activated in immortalized N9 microglial cells exposed to GL261 glioma-conditioned medium that resulted in upregulation of IL-10 and IL-6, and downregulation of IL1-β. Inhibition of STAT 3 by CPA-7 (the platinum (IV)-based anticancer drug) or specific siRNA reversed glioma-induced cytokine expression profile in N9 cells. Furthermore, inactivation of STAT 3 in intracranial GL261 gliomas by siRNA resulted in inhibition of tumor growth (Zhang et al. 2009).
A novel small molecule inhibitor of STAT3—WP1066, which can penetrate the CNS in mice, reversed tolerance in immune cells isolated from GBM patients. Specifically, WP1066 induced the expression of co-stimulatory molecules on peripheral macrophages and glioma-infiltrating microglia, stimulated the production of the immune-stimulatory cytokines interleukin (IL)-2, IL-4, IL-12, and IL-15, and induced proliferation of effector T cells from GBM patients that are refractory to CD3 stimulation (Hussain et al. 2007).
Glioblastoma is heterogeneous and (as many other tumors) contains glioma stem cells or glioma-initiating cells, a subpopulation of cells that possess the capacity for self-renewal and forming neurospheres in vitro, are capable of pluripotent differentiation, and could initiate tumors in vivo. Glioma-initiating cells have been shown to suppress adaptive immunity by affecting capacity to inhibit T-cell proliferation, triggering T-cell apoptosis, and induction of FoxP3(+) regulatory T cells (Wei et al. 2010). A recent study demonstrates that glioma cancer stem cells produce sCSF-1, TGF-β1, and MIC-1, cytokines known to recruit and polarize the macrophages/microglia to immunosuppressive phenotype. Glioma-initiating cells polarized human macrophages/microglia to an M2-like phenotype, inhibited phagocytosis, induced the secretion of the immunosuppressive cytokines IL-10 and TGF-β1, and enhanced their capacity to inhibit T-cell proliferation. The inhibition of phagocytosis and the secretion of IL-10 were reversed when the STAT 3 pathway in glioma stem cells was blocked with a pharmacological inhibitor WP1066 or specific STAT shRNA (Wei et al. 2010). However, such approaches are in infancy, these findings illustrate that interactions between glioma and brain macrophages are potential, promising targets in glioma therapy.
There are also approaches based on local TLR stimulation to induce antitumor immunity. TLR9 is overexpressed in human and murine glioma cell lines. Injection of CpG-oligonucleotides—CpG-ODN (TLR9 inducers) into C57BL/6 mice implanted with GL261 glioma prolonged the survival of mice with tumors. CpG-ODN induced TLR9 downregulation and apoptosis of GL261 cells in vitro as well as in vivo. Furthermore, the CpG stimulation enhanced the antigen presenting capacity of microglia, shifted the immune response toward CD8+ T cells, and decreased the number of regulatory T cells (El Andaloussi et al. 2006). Intratumoral injection of TLR1/2 (Pam3Cys-SK4) or TLR7 (R848) agonist produced a significant survival benefit, whereas agonists of TLR3 (poly(I:C)) or TLR4 (purified LPS) alone were not effective. Studies using wild-type and TLR9(−/−) knockout mice revealed that the efficacy of CpG-ODN treatment required TLR9 expression on nontumor cells (Grauer et al. 2008).
Concluding Remarks
A simple response to a question whether microglia in gliomas are friends or foes proved to be difficult. Histopathological studies of human and rodent glioma tissues have consistently shown high levels of infiltrating microglia/macrophages. The abundance of amoeboid, activated microglia/macrophages is higher in diffusive and invasive gliomas than in benign tumors. The presence of activated microglia/macrophages does not reflect activation of immune defense response in malignant gliomas; on the contrary unharmed growth and highly malignant behavior indicates that microglial immune defense mechanisms do not function properly in these tumors. Moreover, these cells are responsible for local and generalized immunosuppression. Glioma-infiltrating microglia/macrophages acquire the alternative, pro-invasive phenotype in which phagocytic, trophic, and tissue remodeling functions of these cells are enhanced. Cell culture and organotypic brain slice culture studies demonstrated the pro-invasive activity of microglia and their polarization into tumor supportive cells. Several mechanisms underlying the pro-invasive activity of microglia have been demonstrated such as TGFβ1 and FasL production, MT1-MMP-dependent activation of MMP-2. This is well supported by the results of animal glioma studies because most of them represent malignant gliomas. Genetic or pharmacological ablation of microglia/macrophages impairs glioma growth, extends survival, and in some cases restores to some extent antitumor immune responses.
On the other hand, low-grade gliomas are characterized by the presence of activated, hypertrophic, or ramified microglia/macrophages, non-diffusive growth and effective antitumor responses. Unfortunately, due to a lack of proper cellular and animal models, there is no conclusive data if microglia/macrophages infiltrating low-grade gliomas are “friends” which have the classical, inflammatory phenotype and perform protective functions, typical for microglia under physiological conditions.
Since no single microglia-specific marker exists that does not also label peripheral macrophages or extraparenchymal brain macrophages, it is difficult to estimate proportion and contribution of different populations of myeloid cells to glioma pathobiology. Flow cytometry studies of human and rodent glioma tissues clearly demonstrated the presence of both microglia and peripheral macrophages among immune cells recruited to gliomas. It is possible that the myeloid-derived infiltrating cells are functionally distinct from the resident microglia and peripheral macrophages undergo specific “education” in glioma microenvironment. Some faint and fragmentary evidence support this idea, f.e. IL-10 is produced mainly by CD11b+CD45low cells isolated from GL261 gliomas (Gabrusiewicz et al. 2011). Studies on the role of myeloid cell populations under neurodegenerative conditions suggest that peripheral macrophages infiltrating CNS are regulatory and can perform immune-resolving functions (London et al. 2013). As proposed by Michal Schwartz (Schwartz 2010), the blood-derived macrophages are similar to “alternatively activated” macrophages (also known as tissue repairing or M2). They are not spontaneously recruited but their infiltration to CNS could be enhanced by glioma-activated microglia. These blood-derived, immunosuppressive, wound healing macrophages should reinforce repair mechanisms but instead they help tumor to grow.
Understanding the mechanisms responsible for high invasiveness of glioblastoma cells—which is even accelerated by interactions of the brain tumor with surrounding cells—may lead to identification of specific targets for a future treatment. A better understanding of microglia–glioma interaction may provide better methods to manipulate the glioma microenvironment to allow the generation of a specific and lasting anti-glioma immunity. Therapies that will effectively target invasive glioblastoma cells may significantly improve therapeutic outcome. Thus, counteracting accumulation and activation of brain macrophages should be taken into account when considering the development of more effective therapy against malignant gliomas.
Abbreviations
- BM:
-
Bone marrow
- G-CM:
-
Glioma-conditioned medium
- GDNF:
-
Glial cell-derived neurotrophic factor
- GFP:
-
Green fluorescent protein
- GLUT5:
-
Glucose transporter 5
- GM-CSF:
-
Granulocyte-macrophage colony stimulating factor
- HO:
-
Heme oxygenase
- IFN:
-
Interferon
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase (ERK1 and ERK2)
- MCP-1:
-
Monocyte chemoattractant protein-1 (CCL2)
- M-CSF:
-
Macrophage colony stimulating factor
- MMP:
-
Matrix metalloproteinase
- MT1-MMP:
-
Membrane type 1-MMP (=MMP14)
- RANTES:
-
Regulated on activation normal T cell expressed and secreted, CCL5
- TGF:
-
Transforming growth factor
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
References
Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schäfer R, Stanley ER, Abraham D (2004) Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res 64(15):5378–5384
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2(8):675–680
Albesiano E, Han JE, Lim M (2010) Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am 21(1):17–29
Alizadeh D, Zhang L, Hwang J, Schluep T, Badie B (2010) Tumor-associated macrophages are predominant carriers of cyclodextrin-based nanoparticles into gliomas. Nanomedicine 6(2):382–390
Armstrong A, Ravichandran KS (2011) Phosphatidylserine receptors: what is the new RAGE? EMBO Rep 12(4):287–288
Badie B, Schartner JM (2000) Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery 46(4):957–961, discussion 961–952
Badie B, Schartner J, Prabakaran S, Paul J, Vorpahl J (2001) Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol 120(1–2):19–24
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924
Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER (1994) Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120(6):1357–1372
Chang GH, Barbaro NM, Pieper RO (2000) Phosphatidylserine-dependent phagocytosis of apoptotic glioma cells by normal human microglia, astrocytes, and glioma cells. Neuro Oncol 2(3):174–183
Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H (2012) The brain tumor microenvironment. Glia 60(3):502–514
Chen C, Xu T, Chen J, Zhou J, Yan Y, Lu Y, Wu S (2011) Allergy and risk of glioma: a meta-analysis. Eur J Neurol 18(3):387–395
Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F (2007) CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 117(10):2920–2928
Crane CA, Ahn BJ, Han SJ, Parsa AT (2012) Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol 14(5):584–595
Davies LC, Rosas M, Smith PJ, Fraser DJ, Jones SA, Taylor PR (2011) A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur J Immunol 41(8):2155–2164
Davoust N, Vuaillat C, Androdias G, Nataf S (2008) From bone marrow to microglia: barriers and avenues. Trends Immunol 29(5):227–234
Deininger MH, Meyermann R, Trautmann K, Morgalla M, Duffner F, Grote EH, Wickboldt J, Schluesener HJ (2000) Cyclooxygenase (COX)-1 expressing macrophages/microglial cells and COX-2 expressing astrocytes accumulate during oligodendroglioma progression. Brain Res 885(1):111–116
Dick AD, Ford AL, Forrester JV, Sedgwick JD (1995) Flow cytometric identification of a minority population of MHC class II positive cells in the normal rat retina distinct from CD45lowCD11b/c+ CD4low parenchymal microglia. Br J Ophthalmol 79(9):834–840
Donson AM, Birks DK, Schittone SA, Kleinschmidt-DeMasters BK, Sun DY, Hemenway MF, Handler MH, Waziri AE, Wang M, Foreman NK (2012) Increased immune gene expression and immune cell infiltration in high-grade astrocytoma distinguish long-term from short-term survivors. J Immunol 189(4):1920–1927
El Andaloussi A, Sonabend AM, Han Y, Lesniak MS (2006) Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia 54(6):526–535
Ellert-Miklaszewska A, Dabrowski M, Lipko M, Sliwa M, Maleszewska M, Kaminska B (2013) Molecular definition of the pro-tumorigenic phenotype of glioma-activated microglia. Glia 61(7):1178–1190
Färber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N, Kettenmann H (2008) An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci 39(4):579–585
Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B (2011) Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One 6(8):e23902
Galarneau H, Villeneuve J, Gowing G, Julien JP, Vallières L (2007) Increased glioma growth in mice depleted of macrophages. Cancer Res 67(18):8874–8881
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845
Goodridge HS, Underhill DM, Touret N (2012) Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 13(8):1062–1071
Graeber MB, Streit WJ (2010) Microglia: biology and pathology. Acta Neuropathol 119(1):89–105
Graeber MB, Scheithauer BW, Kreutzberg GW (2002) Microglia in brain tumors. Glia 40(2):252–259
Graf MR, Sauer JT, Merchant RE (2005) Tumor infiltration by myeloid suppressor cells in response to T cell activation in rat gliomas. J Neurooncol 73(1):29–36
Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, Adema GJ (2008) TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol 181(10):6720–6729
Grauer OM, Wesseling P, Adema GJ (2009) Immunotherapy of diffuse gliomas: biological background, current status and future developments. Brain Pathol 19(4):674–693
Guillemin GJ, Brew BJ (2004) Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 75(3):388–397
Hamilton JA (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8(7):533–544
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394
Held-Feindt J, Hattermann K, Muerkoster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, Mehdorn HM, Mentlein R (2010) CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exp Cell Res 316(9):1553–1566
Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR (2007) Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol 178(10):6435–6443
Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F (2012) Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209(6):1167–1181
Huang H, Hara A, Homma T, Yonekawa Y, Ohgaki H (2005) Altered expression of immune defense genes in pilocytic astrocytomas. J Neuropathol Exp Neurol 64(10):891–901
Hussain SF, Heimberger AB (2005) Immunotherapy for human glioma: innovative approaches and recent results. Expert Rev Anticancer Ther 5(5):777–790
Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB (2006a) The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol 8(3):261–279
Hussain SF, Yang D, Suki D, Grimm E, Heimberger AB (2006b) Innate immune functions of microglia isolated from human glioma patients. J Transl Med 4:15
Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, Priebe W, Heimberger AB (2007) A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res 67(20):9630–9636
Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77(1):10–18
Kierdorf K, Prinz M (2013) Factors regulating microglia activation. Front Cell Neurosci 7:44
Kim HS, Suh YH (2009) Minocycline and neurodegenerative diseases. Behav Brain Res 196(2):168–179
Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, Schreglmann N, Letellier E, Zuliani C, Klussmann S, Teodorczyk M, Gröne HJ, Ganten TM, Sültmann H, Tüttenberg J, von Deimling A, Regnier-Vigouroux A, Herold-Mende C, Martin-Villalba A (2008) Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 13(3):235–248
Klein R, Roggendorf W (2001) Increased microglia proliferation separates pilocytic astrocytomas from diffuse astrocytomas: a double labeling study. Acta Neuropathol 101(3):245–248
Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216(1):15–24
Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Synowitz M, Glass R, Kettenmann H (2013) GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol 125(4):609–620
Kulprathipanja NV, Kruse CA (2004) Microglia phagocytose alloreactive CTL-damaged 9L gliosarcoma cells. J Neuroimmunol 153(1–2):76–82
Lambertsen KL, Deierborg T, Gregersen R, Clausen BH, Wirenfeldt M, Nielsen HH, Dalmau I, Diemer NH, Dagnaes-Hansen F, Johansen FF, Keating A, Finsen B (2011) Differences in origin of reactive microglia in bone marrow chimeric mouse and rat after transient global ischemia. J Neuropathol Exp Neurol 70(6):481–494
Li MO, Flavell RA (2008) Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 28(4):468–476
Li W, Graeber MB (2012) The molecular profile of microglia under the influence of glioma. Neuro Oncol 14(8):958–978
Lin EY, Nguyen AV, Russell RG, Pollard JW (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193(6):727–740
Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW (2002) The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia 7(2):147–162
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66(23):11238–11246
Liu C, Luo D, Streit WJ, Harrison JK (2008) CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol 198(1–2):98–105
London A, Cohen M, Schwartz M (2013) Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci 7:34
Maes W, Van Gool SW (2011) Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother 60(2):153–160
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22(2):231–237
Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H (2005) Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol 64(9):754–762
Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, van Rooijen N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H (2009) Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A 106(30):12530–12535
Markovic DS, Vinnakota K, van Rooijen N, Kiwit J, Synowitz M, Glass R, Kettenmann H (2011) Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun 25(4):624–628
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Morantz RA, Wood GW, Foster M, Clark M, Gollahon K (1979) Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg 50(3):305–311
Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL (1997) T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol 159(9):4415–4425
Morimura T, Neuchrist C, Kitz K, Budka H, Scheiner O, Kraft D, Lassmann H (1990) Monocyte subpopulations in human gliomas: expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathol 80(3):287–294
Morioka T, Baba T, Black KL, Streit WJ (1992a) Immunophenotypic analysis of infiltrating leukocytes and microglia in an experimental rat glioma. Acta Neuropathol 83(6):590–597
Morioka T, Baba T, Black KL, Streit WJ (1992b) Inflammatory cell infiltrates vary in experimental primary and metastatic brain tumors. Neurosurgery 30(6):891–896
Morris CS, Esiri MM (1991) Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 1. Gliomas. J Neurol Sci 101(1):47–58
Mroczko B, Groblewska M, Wereszczyńska-Siemiatkowska U, Okulczyk B, Kedra B, Łaszewicz W, Dabrowski A, Szmitkowski M (2007) Serum macrophage-colony stimulating factor levels in colorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin Chim Acta 380(1–2):208–212
Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132(Pt 2):288–295
Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID (2007) Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem 282(20):15208–15216
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M (1999) Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 5(5):1107–1113
Nowicki A, Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski AA, Maruszynski M, Aukerman SL, Wiktor-Jedrzejczak W (1996) Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer 65(1):112–119
Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, Iwama T, Takami T (2009) Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol 34(6):1621–1627
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173(6):3916–3924
Pollard JW (2009) Trophic macrophages in development and disease. Nat Rev Immunol 9(4):259–270
Prins RM, Liau LM (2004) Cellular immunity and immunotherapy of brain tumors. Front Biosci 9:3124–3136
Prins RM, Scott GP, Merchant RE, Graf MR (2002) Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer Immunol Immunother 51(4):190–199
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4(8):e6562
Ravichandran KS (2010) Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med 207(9):1807–1817
Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J (2011) Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol 13(6):591–599
Revoltella RP, Menicagli M, Campani D (2012) Granulocyte-macrophage colony-stimulating factor as an autocrine survival-growth factor in human gliomas. Cytokine 57(3):347–359
Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF (2010) Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol 12(4):351–365
Roggendorf W, Strupp S, Paulus W (1996) Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol 92(3):288–293
Rolle CE, Sengupta S, Lesniak MS (2012) Mechanisms of immune evasion by gliomas. Adv Exp Med Biol 746:53–76
Sapi E, Kacinski BM (1999) The role of CSF-1 in normal and neoplastic breast physiology. Proc Soc Exp Biol Med 220(1):1–8
Sasaki A, Yamaguchi H, Horikoshi Y, Tanaka G, Nakazato Y (2004) Expression of glucose transporter 5 by microglia in human gliomas. Neuropathol Appl Neurobiol 30(5):447–455
Sasaki A, Yokoo H, Tanaka Y, Homma T, Nakazato Y, Ohgaki H (2013) Characterization of microglia/macrophages in gliomas developed in S-100β-v-erbB transgenic rats. Neuropathology 33(5):505–514
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336(6077):86–90
Schwartz M (2010) “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: from skin-activated macrophages to infiltrating blood-derived cells? Brain Behav Immun 24(7):1054–1057
Schwartzbaum JA, Huang K, Lawler S, Ding B, Yu J, Chiocca EA (2010) Allergy and inflammatory transcriptome is predominantly negatively correlated with CD133 expression in glioblastoma. Neuro Oncol 12(4):320–327
Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 88(16):7438–7442
Sedgwick JD, Schwender S, Gregersen R, Dörries R, ter Meulen V (1993) Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med 177(4):1145–1152
Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J, Vinnakota K, Kettenmann H, Kotulska K, Grajkowska W, Kaminska B (2013) Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol 230(3):310–321
Sierra A, Abiega O, Shahraz A, Neumann H (2013) Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci 7:6
Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, Rodriguez FJ, Gutmann DH (2011) Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol 70(1):51–62
Sliwa M, Markovic D, Gabrusiewicz K, Synowitz M, Glass R, Zawadzka M, Wesolowska A, Kettenmann H, Kaminska B (2007) The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain 130(Pt 2):476–489
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073
Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, Meldolesi J (2009) The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem 110(1):284–294
Szatmári T, Lumniczky K, Désaknai S, Trajcevski S, Hídvégi EJ, Hamada H, Sáfrány G (2006) Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci 97(6):546–553
Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H (2007) TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med 4(4):e124
Tran Thang NN, Derouazi M, Philippin G, Arcidiaco S, Di Berardino-Besson W, Masson F, Hoepner S, Riccadonna C, Burkhardt K, Guha A, Dietrich PY, Walker PR (2010) Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res 70(12):4829–4839
Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T (2008) Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol 83(5):1136–1144
Voisin P, Bouchaud V, Merle M, Diolez P, Duffy L, Flint K, Franconi JM, Bouzier-Sore AK (2010) Microglia in close vicinity of glioma cells: correlation between phenotype and metabolic alterations. Front Neuroenergetics 2:131
Wang SC, Hong JH, Hsueh C, Chiang CS (2012) Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab Invest 92(1):151–162
Watters JJ, Schartner JM, Badie B (2005) Microglia function in brain tumors. J Neurosci Res 81(3):447–455
Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, Sawaya R, Lang FF, Heimberger AB (2010) Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther 9(1):67–78
Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, Franciszkiewicz K, Chouaib S, Kaminska B (2008) Microglia-derived TGF-beta as an important regulator of glioblastoma invasion–an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene 27(7):918–930
Wierzba-Bobrowicz T, Kuchna I, Matyja E (1994) Reaction of microglial cells in human astrocytomas (preliminary report). Folia Neuropathol 32(4):251–252
Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER (1990) Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A 87(12):4828–4832
Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell KW, Ahmed-Ansari A, Ostertag W (1992) CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol 20(8):1004–1010
Wisniewski P, Ellert-Miklaszewska A, Kwiatkowska A, Kaminska B (2010) Non-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFkappaB-TIMP-2 pathway. Cell Signal 22(2):212–220
Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB (2010) Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol 12(11):1113–1125
Yang I, Han SJ, Kaur G, Crane C, Parsa AT (2010) The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci 17(1):6–10
Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H (2011) Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol 232(1–2):75–82
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345(6274):442–444
Zawadzka M, Dabrowski M, Gozdz A, Szadujkis B, Sliwa M, Lipko M, Kaminska B (2012) Early steps of microglial activation are directly affected by neuroprotectant FK506 in both in vitro inflammation and in rat model of stroke. J Mol Med (Berl) 90(12):1459–1471
Zhai H, Heppner FL, Tsirka SE (2011) Microglia/macrophages promote glioma progression. Glia 59(3):472–485
Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B (2009) Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia 57(13):1458–1467
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26(16):2707–2716
Acknowledgments
I would like to thank Dr. Aleksandra Ellert-Miklaszewska and Malgorzata Sielska for reading and help with editing of the manuscript. This work was supported by a grant 2012/04/A/NZ3/00630 from The National Science Centre Poland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Kaminska, B. (2014). Microglia in Gliomas: Friend or Foe?. In: Sedo, A., Mentlein, R. (eds) Glioma Cell Biology. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1431-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1431-5_9
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1430-8
Online ISBN: 978-3-7091-1431-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)