Abstract
This chapter deals with the study of the cure kinetics of epoxy/block copolymer blends in order to give a comprehensive account about the effect of adding this kind of modifier on the reaction rate of the network formation. Non-isothermal runs at constant heating rates and isothermal runs at constant temperature were carried out in order to determine the total heats of reaction released during curing for the epoxy blends modified with different contents of block copolymers. It was found a clearly delay of cure kinetics with the increase of block copolymer content. In order to understand the parameters affecting epoxy curing kinetics, the influence of block copolymer blocks chemical structure, and the molar ratio between blocks on the curing rate was also analyzed. Fourier transform infrared spectroscopy was used for this purpose. The experimental curves of isothermal curing were fitted to a phenomenological autocatalytic model and also to mechanistic model. Kinetics parameters were calculated from the previous models. The increase observed in activation energy values with the increase of block copolymer content corroborated that the physical interactions between the block copolymer and the epoxy significantly affect the curing behavior, agreeing with the observed delay.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
During the curing process of a modified epoxy system, the cross-linking reactions involve a number of chemical and physical changes, while the material turns from a viscous liquid to a solid. The comprehension of the cure kinetic behavior related to the network formation permits a clear analysis of the structure/property/processing relationships that will determine the proper set of process parameters for the development of high-performance blends and composites with the best structural and morphological properties. Differential scanning calorimetry (DSC) has been widely employed to study the curing process of epoxy systems. This technique is very effective for monitoring the network formation since it permits the measurement of the amount of heat that is either absorbed or evolved during the course of polymerization reactions (i.e., epoxy–amine systems are well known as exothermic reactions). Several works have been published about DSC studies, both isothermal and non-isothermal heating mode, to determine the reaction rate curves and kinetic parameters for epoxy/amine systems (Grillet et al. 1989; Verchere et al. 1990; Cole 1991; Serier et al. 1991; Deng and Martin 1994; Girard-Reydet et al. 1995; Vyazovkin and Sbirrazzuoli 1996; Ghaemy and Khandani 1998; Karkanas and Partridge 2000; Vinnik and Roznyatovsky 2006) and theirs blends with thermoplastics (Bonnet et al. 1999; Varley et al. 2000; Bonnaud et al. 2000; Swier and Van Mele 2003a; Swier et al. 2005; Bejoy et al. 2006; Varley 2007; Zhang et al. 2009), rubbers (Kim and Kim 1994; Calabrese and Valenza 2003; Raju et al. 2007), and block copolymers (Swier and Van Mele 2003b, c; Larrañaga et al. 2004, 2005; Kim et al. 2005; Larrañaga et al. 2006a; George et al. 2012, 2014; Cano et al. 2014; Hu et al. 2015), among others.
Diverse mathematical models have been also applied in order to obtain a comprehensive description and simulation of the experimental cure profiles taking into account the catalytic effects and the influence of the diffusion phenomena. Modeling of the curing behavior of epoxy–amine systems can be approached both mechanistically (Mijovic et al. 1992; Mijovic and Wijaya 1994; Blanco et al. 2005; Zvetkov 2005) and phenomenologically (Ryan and Dutta 1979; Barton 1985; Roşu et al. 2002; Du et al. 2004; Cai et al. 2008). Mechanistic models consider a complete scheme of consecutive and competitive reactions that take place during the curing process. As the cross-linking reaction of epoxy systems is very complex due to the close relationship between the chemical kinetics and changes in their physical properties, it is difficult to derive an accurate mechanistic model. Moreover, phenomena such as autocatalysis in the early stages or the effect of diffusion on the kinetic rate constants at later stages can further complicate modeling. Consequently, phenomenological approaches are preferred to study the curing kinetics of these thermosetting polymers. Phenomenological models are based on empirical or semiempirical equations which explain the autocatalytic behavior of the epoxy–amine reaction. It should be pointed out that the unmodified epoxy–amine curing reaction is well known as an autocatalytic mechanism (Smith 1961; Riccardi et al. 1984; Xu et al. 1994). The autocatalysis in epoxy–amine reaction is attributed to a termolecular intermediate with hydroxyl groups produced during curing. On the other hand, the referred phenomenological models have been widely employed to study the cure kinetics because they are simple and fit experimental data with relative success.
The blending of an epoxy resin with block copolymers consisted of an epoxy phobic block and another epoxy phylic, and/or reactive blocks that are capable to control self-assembling at the nanometer scale in the uncured and cured state have been widely explored due to their excellent properties that can be tailored after the complete network formation, such as good mechanical behavior. As it is well known, the final self-assembled morphology of epoxy/block copolymer blends depends principally on both kinetics and thermodynamic factors, such as the curing rate, the change of the viscosity during the phase separation, as well as the modifier concentration, volume fraction of each block, architecture, and molecular weight of the blocks (Lipic et al. 1998; Girard-Reydet et al. 1999, 2002; Mijovic et al. 2000; Grubbs et al. 2000; Ritzenthaler et al. 2002, 2003, Rebizant et al. 2004; Dean et al. 2003; ; Serrano et al. 2005, 2006, 2007; Meng et al. 2006; Larrañaga et al. 2006a, b; Tercjak et al. 2006; Maiez-Tribut et al. 2007; Hermel-Davidock et al. 2007; Ruiz-Pérez et al. 2008; Liu et al. 2008, 2010; Ocando et al. 2008, 2013; Garate et al. 2011, 2013; Wu et al. 2013; Liu 2013; Xu et al. 2015).
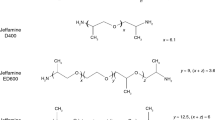
In particular, the effect of blending an epoxy–amine system with different amounts of amphiphilic block copolymers, consisted on poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEP–PPO–PEO) with differing volume fractions of PEO block, on the cure kinetics during the network formation has been systematically studied (Larrañaga et al. 2004, 2005, 2006a). It is possible to point out that epoxy–amine systems blended with poly(ethylene oxide) homopolymer (PEO) lead to a miscible material owing to the fact that the OH groups, developed during the network formation of the growing epoxy matrix, interact by hydrogen bonding with the ether oxygen of PEO avoiding phase separation (Larrañaga et al. 2007), whereas the poly(propylene oxide) (PPO) tends to phase separate from the forming epoxy network as the molecular weight is increased by curing reaction. Nevertheless, as the miscibility between polymers is also governed by the temperature of blending and composition, the control of both the reaction and the phase separation rate for a given modifier through the selection of an appropriate curing cycle (temperature vs. time) permits a fine-tune of the self-assembled morphologies (Liu 2013). Concerning this last argument, it was found that the phase separation in epoxy/PEO–PPO–PEO block copolymer blends at micro- or nanoscale depends mainly on the PEO content and curing temperature (Larrañaga et al. 2005). In addition, the cure kinetics curves obtained from DSC experiments of epoxy/PEP–PPO–PEO block copolymer blends were successfully fitted to an autocatalytic (Larrañaga et al. 2004) and mechanistic kinetic model (Larrañaga et al. 2005, 2006a). It was found that PEO–PPO–PEO slows the reaction rate both by acting as a diluent and by interfering with the autocatalytic process. This delay was related to a preferential hydrogen bonding interaction between the hydroxyl groups of the growing epoxy network and the PEO oxygens, which inhibit the autocatalysis process. Another finding was that the curing rate decreases with increasing block copolymer content in the epoxy blend as well as increasing PEO content in the block copolymer. This last fact also proved that the delaying of cure kinetics is mainly due to physical interactions between components (Larrañaga et al. 2006a).
Similar results about the delay on the cross-linking reaction of epoxy groups were obtained for epoxy/poly(styrene)-b-poly(ethylene oxide) (PS–PEO) block copolymer blends (Leonardi et al. 2015a). It is possible to emphasize that in these blends, the PS block was phase separated at low conversions, while the PEO block remained miscible up to very high conversions (Leonardi et al. 2015b). The lowering on reaction rate produced by block copolymer addition, illustrated in Fig. 1, was explained as a dilution effect by the large amount of miscible PEO block present in this copolymer as well as by a partial segregation of reactive monomers and short oligomers to the PS-rich phase. Regarding this last fact, it was established that a differential segregation of reactive components in both epoxy–amine-rich and block-rich phase can occur (Williams et al. 1997).
Conversion of epoxy groups at 135 °C for blends containing 0, 40, and 60 wt% BCP (Reprinted with permission Leonardi et al. (2015a))
Interestingly, it was published a study about the cure kinetics of epoxy/poly (butadiene-co-acrylonitrile) (PS-PAN) blends . This study took into account the contribution of diffusion phenomena on the reaction rate after gelation and crosslinking of epoxy-amine systems in order to fit the experimental data near to vitrification using the autocatalytic model (Kim et al. 2005).
Finally, the kinetics of curing of an epoxy system and their blends with epoxidized poly(styrene)-b-poly(butadiene)-b-poly(styrene) (eSBS) was studied using isothermal and non-isothermal DSC analyses (George et al. 2012, 2014). The experimental cure kinetics curves were phenomenologically modeled with success. It is possible to emphasize that the eSBS used for this study was a block copolymer with high degree of epoxidation (eSBS 47 mol%) to ensure the nanostructuring of these blends (Ocando et al. 2008).
The most relevant results about the effect of block copolymer addition on cure behavior of epoxy–amine blends by DSC analyses, as well as a comprehensive understanding of the kinetic parameters by applying mathematical models to describe the obtained experimental data, will be addressed in this chapter.
Non-isothermal DSC Analyses
In general, the heat released during the network formation determined by non-isothermal or dynamic DSC measurements is assumed to be directly proportional to the extent of consumption of the reactive groups in epoxy–amine systems. Non-isothermal runs at constant heating rates were carried out in order to determine the total heats of reaction (ΔH T ) released during dynamic curing for the epoxy blends modified with 10, 20, and 30 wt% of PEO–PPO–PEO (EP) block copolymer (M EO = 1088 and M PO = 1794 g mol−1) with a molar ratio between blocks, PEO:PPO, of 0.8:1 (EP-0.8:1) (Larrañaga et al. 2004). The epoxy–amine system used for this study was a diglycidylether of bisphenol A (DGEBA)/4,4-diaminodiphenylmethane (DDM) system . From the dynamic curing profiles (Fig. 2), it was concluded that the curing reaction for the studied systems was kinetically affected by the modifier content. This fact was proved by a displacement of exothermic polymerization temperature peaks (T p) to higher values as the concentration of block copolymer in the blend increases. Regarding the ΔH T , it was observed that the presence of block copolymer did not affect the reaction pathway due to this value decrease in proportion to the block copolymer content in the blend (Table 1). In addition, as can be seen from dynamic curing profiles (Fig. 2), a shoulder appeared (T sh) after the exothermic polymerization peak for the epoxy blends. This last behavior was attributed to the phase separation of the block copolymer, and it was corroborated by light transmission dynamic scan by the same authors. Table 1 summarizes the obtained values of ΔH T , T p, and T sh determined by DSC as well as the cloud point temperature (T cp) determined by transmission optical microscopy (TOM), a temperature where the phase separation occurs, and it was in agreement with T sh.
DSC dynamic scans carried out at a rate of 10 °C min−1, for epoxy mixtures containing various PEO-PPO-PEO contents (Reprinted with permission Larrañaga et al. (2004))
The observed delaying behavior on curing reaction was related to dilution effects, due to a reduction in the density of the reactive groups as the block copolymer content increased. Nevertheless, the authors also pointed out that in this epoxy/PEO–PPO–PEO blend, the delay on curing reaction was higher than the one observed in other epoxy blends containing other modifiers (Jenninger et al. 2000). Therefore, this observed behavior could not be explained only by a dilution effect. This fact suggested that the OH groups (developed in the cure reactions) interact through hydrogen bonding with ether oxygen of PEO, so decreasing the autocatalytic process, and therefore delaying the curing process (Larrañaga et al. 2007). Fourier transform infrared spectroscopy (FTIR) analyses (Fig. 3a) confirmed this hypothesis, revealing that the associated hydroxyl group bands shifted to lower wave numbers. In addition, from Fig. 3b, it was noticed that the intensity ratio between the associated hydroxyl band and the free hydroxyl band around 3570 cm−1 in the modified system increased with the increment of the conversion, and this increment is more significant compared with the unmodified systems (Larrañaga et al. 2004).
FTIR spectra for (a) all cured samples with various contents of modifier and (b) for neat epoxy and a 20 wt% PEO-PPO-PEO-modified mixture at different conversions (Reprinted with permission Larrañaga et al. (2004))
The influence on the delaying of cure rate with the molar ratio between blocks of diverse PEO–PPO–PEO (EP-0.33:1, EP-0.8:1, and EP-3:1) block copolymers was also demonstrated by DSC analyses (Larrañaga et al. 2006a). Figure 4b shows the dynamic thermograms for a neat epoxy–amine system and its blends containing 30 wt% of EP-0.33:1, EP-0.8:1, and EP-3:1. It was found that the displacement of exothermic peak was higher for the modified epoxy blend with high PEO content. This behavior was attributed to the occurrence of more physical interactions between the epoxy and PEO block in the blend modified with EP-3:1. The occurrence of more physical interactions was also confirmed by FTIR analyses. In addition, the shoulder in the dynamic heating profiles attributed to the macrophase separation process was observed in EP-0.33:1 and EP-0.8:1 modified epoxy blends but not for the block copolymer with high PEO content.
DSC dynamic scans for (a) epoxy systems with various EP-0.33:1 contents and (b) neat epoxy and 30 wt% PEO-PPO-PEO-modified systems (Reprinted with permission Larrañaga et al. (2006a))
A recent work was published about the effect of the addition of different amounts of eSBS block copolymer with 47 mol% of polybutadiene block epoxidation (eSBS47) on the cure kinetics of epoxy blends (George et al. 2012). The epoxy–amine system used in this study was DGEBA/DDM . Dynamic DSC experiments were carried out at different heating rates for the epoxy systems modified with 0, 10, 20, and 30 wt% of eSBS. It was observed that the exothermic peak maximum (Tpeak) undergoes a displacement to higher temperatures when heating rate was increased. This behavior was also observed at different blend composition. The average of enthalpy values obtained at different heating rate was used to estimate the total heat of the reaction (Table 2). From these results, it was noticed that the minimum in the exothermic curve corresponding to the maximum heat flow of the epoxy–amine reaction increases as the weight percentage of eSBS in the epoxy blend increases. This behavior was related to a plasticization effect and interactions between the epoxidized PB segments with the epoxy resin that causes retardation in cure reaction. FTIR studies carried out by the same authors corroborated the presence of interactions between the hydroxyl group of the growing epoxy network and oxirane groups of the epoxidized polybutadiene. The schematic representation of hydrogen bonding interaction between the epoxy resin and eSBS is illustrated in Fig. 5.
Schematic representation of hydrogen bonding interaction between hydroxyl hydrogen in DGEBA and epoxy oxygen atom of epoxidized SBS (1,4- and 1,2-epoxidized PB) (Reprinted with permission George et al. (2012))
In a subsequent work, the same authors studied the evolution of the total heats of reaction of an epoxy blend modified with 10 wt% of eSBS47 at different cure times by dynamic DSC measurements. For the kinetic studies, the epoxy blends were cured in an air oven for different periods at 90 °C prior to DSC analyses. From this study, it was observed that the evolution of the total heats of reaction for the 10 wt% eSBS47-modified epoxy blend decreased as a function of cure time, as a result of the epoxy–amine reaction, and essentially no exothermic reaction was observed when the cure time reached 90 min. This behavior was related to the fact that the mobility of some reactive sites could be frozen, causing an ending of the polymerization (George et al. 2014).
Interestingly, a recent study about the curing behavior of a cyanate ester/epoxy system and its blends containing PEO–PPO–PEO with molecular weight Mw = 8600 (F68) was published (Hu et al. 2015). For this purpose, the authors developed a series of mixtures varying the matrix composition (cyanate ester/epoxy ratio) and the block copolymer contents (up to 20 wt%). The matrix composition was varied, and some differences about the hydrogen bonding interactions and chemical reaction resulting from the cross-linked network structures were observed. DSC analyses (Fig. 6) revealed an exothermic polymerization peak at lower temperature that was related to homopolymerization reactions of cyanate ester groups. On the other hand, the exothermic polymerization peak observed at higher temperature was related to the formation of oxazolidinone groups (reaction between epoxy–cyanate ester). On the contrary to the results described above, this study indicated that cure reaction was accelerated by the incorporation of a small amount of block copolymer. In addition, the exothermic polymerization peak at lower temperature was less notorious in the case of higher block copolymer contents. Therefore, it was concluded that the presence of hydroxyl groups in block copolymers had a significant catalytic effect on the curing of cyanate ester/epoxy resins. This catalytic behavior was also corroborated by FTIR analyses, where it was clearly observed that the disappearing rate of both cyanate and epoxy groups was faster for the modified system than that of the neat resin.
Differential scanning calorimetry dynamic scans for a cyanate ester/epoxy resins with various F68 contents (Reprinted with permission (Hu et al. (2015))
Isothermal DSC Analyses
The curing rate of different contents of PEO–PPO–PEO block copolymer with 30wt% of PEO (EPE) blended with a DGEBA/m-xylylenediamine system was studied using DSC under isothermal conditions (Cano et al. 2014). The curing temperature for this purpose was chosen taking into account the lower critical solution temperature (LCST) behavior of this block copolymer. In this sense, isothermal runs at 25 °C were performed to the epoxy blends, and the resulting thermograms are shown in Fig. 7. The curing reaction between the epoxy resin and the amine was indicated as the drop in heat flow, and the reaction was considered complete when the isothermal DSC traces leveled off to the baseline. In agreement with the non-isothermal studies previously shown in this chapter, from these results, a clear delay in the curing reaction with the increment of the block copolymer content was also found. This behavior on curing reaction time was again related to the dilution effect in the blends that makes the reaction between the epoxy and the amine groups more difficult. In addition, it was also pointed out that the presence of more physical interactions between the epoxy resin and the PEO–PPO–PEO block copolymer by an increment on the amount of modifier, from 5 to 50 wt%, can be also responsible for the delay on curing rate.
Isothermal DSC thermograms of the neat epoxy system and all EPE/epoxy systems at 25 °C (Reprinted with permission Cano et al. (2014))
Similar results about the curing rate of epoxy blends depending on the amount of PEO–PPO–PEO block copolymer were obtained at an isothermal cure temperature of 120 °C (Larrañaga et al. 2004). It was observed from the reaction rate curves (dX/dt vs. time) that the peak for the maximum reaction rate decreased and the time at which this maximum takes places increase with the block copolymer content (Fig. 8). In addition, the authors related the shoulder observed in the reaction rate trace of the modified system with 30 wt% block copolymer with the occurrence of a macroseparation at the curing temperature of 120 °C. This last behavior was corroborated by the measurement of the cloud point by light transmission analysis at 120 °C.
Reaction rate curves of the epoxy mixtures containing various amounts of block copolymer cured at 120 °C (Reprinted with permission Larrañaga et al. (2004))
Interestingly, the influence of the curing temperature and block copolymer content on cure kinetics of epoxy/PEO–PPO–PEO block copolymer blends analyzed by isothermal DSC experiments was correctly fitted to a phenomenological autocatalytic model assuming equal reactivity of primary and secondary amino hydrogens with the epoxy groups (Larrañaga et al. 2004). The epoxy–amine system used for this study was a diglycidylether of bisphenol A (DGEBA)/4,4-diaminodiphenylmethane (DDM) system . This phenomenological autocatalytic approach employs empirical or semiempirical equations (Kamal and Sourour 1973; Sourour and Kamal 1976). Explained in a more detailed way, this model takes into account the reactions of the oxirane groups with the primary and secondary amines, as well as catalytic and autocatalytic effects. The generalized equation for this autocatalytic model is described by (Eq. 1):
where k 1 and k 2 correspond to the rate constant for the reaction catalyzed by proton donors initially present in the system (e.g., α-glycols present in the epoxy monomer) and proton donors that are produced during cure, respectively; X denotes the conversion of the epoxy groups, m and n are the kinetic exponents of the reactions, and m + n is the overall reaction order. The rate constants k 1 and k 2 are dependent on the temperature with an Arrhenius-type relationship: k 1 = A 1 exp (−E a1/RT) and k 2 = A 2 exp (E a2/RT), where A i denotes the collision frequency or Arrhenius frequency factor, E ai its corresponding activation energy, R the gas constant, and T the absolute temperature. In order to obtain accurate values of Eq. 1 parameters from isothermal curve data, a simple iterative method can be utilized until an apparent convergence of the m and n values is obtained, and Eq. 1 can be rewritten as
This equation takes into account the autocatalytic nature of the curing process with the term k 2 X m, while the uncatalyzed process is represented by k 1 . The values of E a1 and E a2 were obtained by plotting lnk 1 and lnk 2, respectively, versus 1/T. Therefore, Arrhenius plots of the rate constants from isothermal runs were characterized for straight lines where the slope is E a1/RT and E a2/RT and the intercept is lnA1and lnA 2, respectively. The summarized kinetics parameters, reaction constants, and activation energy, obtained for the epoxy/PEO–PPO–PEO blends, are shown in Table 3. From these results, it was pointed out that the reaction orders did not change very much with the content of block copolymer in the blend as well as with the cure temperature. On the other hand, k 2 decreased at all temperatures as the block copolymer content increases. This reduction in k 2was related to the decrease in the autocatalytic effect by specific interactions between the hydroxyl groups and the block copolymer. As discussed before these interactions were demonstrated by FTIR analyses (Fig. 3).
Figure 9 presents a comparison between the autocatalytic model and the experimental data for epoxy blends modified with 20 wt% of PEO–PPO–PEO. It is possible to emphasize that the experimental conversion values at a given temperature were defined as the ratio between the enthalpy of reaction at time t, (ΔHiso)t, and the sum of the total enthalpy from the isothermal and residual scans ((ΔHiso) + (ΔHres)). As can be seen, the model fitted quite well with the experimental conversion curves; small variations between experimental and theoretical values were related to vitrification effects (Larrañaga et al. 2004). Similar results about cure kinetics studies of epoxy blends, by the use of isothermal DSC analyses and a modified autocatalytic kinetic model, were obtained for the same group when an epoxy resin was modified with different amounts of two kinds of thermoplastics polymers (Fernández et al. 2001). It is possible to point out that the conversion increases in the initial stages of reaction, and then the cure reaction rate decreases at later stages because the blends became vitrified. This decrease in the cure reaction rate is related to the cross-linking density. In addition, the maximum in reaction rate against time plot is typical of autocatalytic mechanism. Therefore, it was concluded that the presence of block copolymer in the epoxy blends does not affect the autocatalytic nature of the reaction.
Comparisons between the autocatalytic model and experimental data for the mixture modified with a 20 wt% block copolymer at various cure temperatures (Reprinted with permission Larrañaga et al. (2004))
In a later work, the same authors (Larrañaga et al. 2005) used a mechanistic model to fit the experimental results obtained by DSC for the DGEBA/DDM system modified with PEO–PPO–PEO block copolymer. The employed mechanistic kinetic model considers a scheme of consecutive and competitive reactions that can take place during the curing process (Riccardi et al. 2001). As mentioned before, the curing process of epoxy–amine systems is a very complex procedure due to the chemical reactions and changes in the physical properties which are closely related; in this sense it is difficult to obtain an accurate mechanistic model (Riccardi and Williams 1986a, b; Mijovic et al. 1992; Mijovic and Wijaya 1994; Urbaczewski et al. 1990; Blanco et al. 2005; Zvetkov 2005). Nevertheless, Riccardi et al. (2001) introduced a simple mechanistic model that encloses an equilibrium reaction that produces an epoxy–hydroxyl complex established as the only intermediate species as well as two possible mechanisms for the use of amine hydrogens. It was found that this simple mechanism model provided a good fitting with respect to the experimental results obtained by DSC for the DGEBA/ethylenediamine (EDA) system, under both isothermal and constant heating rate conditions. It is possible to emphasize that the main advantage of this mechanistic model with respect to the phenomenological model is that the mechanistic model offers more predictive capability due to the results obtained from its equations which can be extrapolated to account for variations in the initial formulations (Chiao 1990; Chiao and Lyon 1990). In addition, this model can give an insight about the network structure. As a result, the evolution of different statistical parameters during the network formation can be predicted (Riccardi and Williams 1986b).
In this sense, following the work presented by Riccardi et al. about epoxy–amine systems (Riccardi et al. 2001), Larrañaga et al. proposed a mechanistic approach that includes the following alternatives for the curing reaction steps (Larrañaga et al. 2005): (1) epoxy activation by hydrogen bonding with hydroxyl groups in the pre-equilibrium to form an epoxy–hydroxyl complex, (2) uncatalyzed addition reactions of primary and secondary amine hydrogens with epoxy groups, (3) and parallely, autocatalyzed reactions by the OH groups produced during curing reactions. In this sense, the mechanistic kinetic model, assuming different reactivities of primary and secondary amine hydrogens, was defined by the following equations:
where
and
where e 0 and e denote the concentration of epoxy at time 0 and t, respectively, a 1 is the concentration of primary amino hydrogens, and r is the ratio of secondary to primary amino-hydrogen rate constants. The epoxy–hydroxyl complex formation is represented with the dimensionless equilibrium constants K. The autocatalyzed and uncatalyzed reactions are represented by the dimensionless kinetic constants K 1 and K′1, respectively; e-OH is the epoxy–hydroxyl complex concentration. C0 is OH-equivalent/epoxy-equivalent and y is e-OH/e0.
Figure 10 shows the fit of experimental curves with this mechanistic model for the epoxy system modified with 10 wt% PEO–PPO–PEO block copolymer at different curing temperatures. As can be seen, this model presented a satisfactory match with the experimental curves.
Comparison between the mechanistic model
(—) and experimental data at (□) 80 °C, (●) 100 °C, (Δ) 120 °C, (▼) 140 °C, (○) 150 °C ( ), 160 °C, and (
), 160 °C, and ( ) 170 °C for the blend modified with a 10 wt% block copolymer (Reprinted with permission Larrañaga et al. (2005))
) 170 °C for the blend modified with a 10 wt% block copolymer (Reprinted with permission Larrañaga et al. (2005))
From the analyses of the kinetic parameters sumarized in Table 4, it was found a hindering in the formation of epoxy–hydroxyl complex and the autocatalytic process by blending compared with neat epoxy. On the contrary, it was observed that the constant for the uncatalyzed reaction of epoxy with amine increases with copolymer content. The diminution in K and K 1 values was ascribed to the fact that the iterations between the OH groups formed during curing with the block copolymer are more favored. In the case of K′1, its increment was related to the fact that at early stages of reaction, less oxirane groups can interact with OH groups as the block copolymer content increases. In this sense, more oxirane groups are available for direct reaction with the amine. Therefore, from these results it was concluded that the influence of K and K 1 kinetics constants on curing rate is higher than the influence of K′1 because even though the uncaltalyzed process was favored, the block copolymer delayed the curing reaction.
On the other hand, the effect of the interactions between the epoxy–amine system and the PEO–PPO–PEO block copolymers on the reaction rate depending on the molar ratio between blocks was also proved by isothermal DSC analyses (Larrañaga et al. 2006). Figure 11 shows conversion of epoxy groups vs. time curves obtained for the epoxy blends containing different amounts of EP-0.33:1, EP-0.8:1, and EP-3:1block copolymers cured at 140 °C. From these curves, it was noticed that the epoxy blends modified with copolymers containing different block molar ratio present a different delay even at the same content of modifier. This last fact proved that the delaying of cure kinetics is mainly due to physical interactions between the epoxy (OH groups initially existing and developed during the network formation) and PEO block (ether groups) than a dilution effect due to the content of modifier in the growing network system. As a result, the system that presented the higher delay on the curing reaction was the system modified with the block copolymer with higher PEO content. FTIR analyses corroborated these results because it was observed that when the PEO molar ratio in the block copolymer is higher, the associated hydroxyl group band appears at lower wave numbers. In addition, the intensity ratio between associated and free hydroxyl bands also increases. It was also pointed out that the hydroxyl–ether interactions modified the autocatalytic process causing a delay on the curing process. Figure 11b also shows the predicted curve from the kinetic model and the theoretical curve obtained taking into account the dilution effect for the epoxy system modified with 20 wt% of EP-0.8:1, an appropriate agreement was pointed out. In addition, it was also noticed that the curing reactions occur at a lower rate than when just the dilution effect was taken into account for the estimation. This last fact also corroborated the hypothesis that the interactions between the block copolymer and the epoxy significantly affect the curing behavior.
Cure kinetics curves for epoxy systems cured at 140 °C containing 0 wt% (■), 10 wt% (○), 20 wt% (▲), and 30 wt% (□) of (a) EP-0.33:1, (b) EP-0.8:1, and (c) EP-3:1. In subpanel (b), solid lines show predicted curves from kinetic model for neat and 20 wt% EP-0.8:1, while dashed lines show a theoretical curve obtained by modeling dilution effect for 20 wt% EP-0.8:1 (Reprinted with permission Larrañaga et al. (2006a))
The kinetics constants obtained by the use of this mechanistic approach, as average values in the 80–170 °C range, for epoxy blends with EP-0.8:1 and EP-0.33:1 compared with the corresponding epoxy system are summarized in Table 5. From these results, it was pointed out that in addition to the dilution effect, the interactions between components play an important role. This finding was ascribed to the fact that the ratio between the kinetic constants of the blend and the neat epoxy is different if they are compared with the ratio between the initial concentration of epoxy equivalents of the blend and the neat epoxy. From K and K1 values, it was concluded that the formation of epoxy–hydroxyl complex and the autocatalytic processes are less restricted for the epoxy blends containing EP-0.33:1 than for the epoxy blend containing EP-0.8:1 when they are compared with the neat epoxy system. This behavior corroborated the fewer occurrences of physical interactions between components presented on the epoxy blend modified with the block copolymer containing lower PEO block content. Another finding was the increment of uncatalyzed kinetic parameter, K′1, in the modified systems, and it is more evident in the case of the epoxy system modified with EP-0.8:1 due to its higher PEO content.
The activation energy and frequency factor values of the epoxy system modified with EP-0.8:1 and EP-0.33:1 compared with the corresponding epoxy system are summarized in Table 6. It was noticed that the activation energy values yield similar values for all the systems. On the contrary, the frequency factors presented a slight variation, being higher for EP-0.8:1 than for EP-0.33:1 modified systems. In addition, the observed decrease of A1 values was attributed to the delay of the autocatalytic process as a consequence of physical interactions between the components, while the increase of A′1 values was related to the increase of epoxy groups that can react with the amine (Larrañaga et al. 2006).
The cure kinetic parameters of an epoxy system blended with ATPEI (amine terminated polyetherimide)–CTBN (carboxyl terminated poly(butadiene-co-acrylonitrile)) block copolymer, denoted as AB, were evaluated using isothermal DSC measurements and an autocatalytic model (Kim et al. 2005). Similar results to those discussed above about the effect on heat of reaction and final conversion of epoxy groups with the content of block copolymer were observed. Interestingly, this work proposed to take into account a rate equation with a diffusion control factor to explain the delay on curing process in the later stages of the reaction and to obtain a better match between the experimental values and the theoretical ones. The diffusion control factor f(X) is represented by the next equation:
where C denotes the parameter of diffusion control and X c is the critical value of cure conversion where reaction becomes controlled by diffusion. Therefore the final rate equation was rewritten as follow:
Table 7 summarized the kinetic parameters calculated using this autocatalytic model including the diffusion phenomena. It was noticed that the values of n increased as the reaction rate decreased. This behavior was explained by the fact that for the epoxy blends, the reaction can be hindered by the phase separation process. On the other hand, the values of m oscillated around 1.0 even though when the block copolymer content was increased. This last fact revealed that neither the curing reaction nor the vitrification process has a significant effect on the autocatalytic behavior.
Figure 12 depicts the conversion versus reaction time at 160 °C, 175 °C, and 190 °C of the epoxy blends containing different amounts of AB. The calculated solid and dashed traces were obtained from Eqs. 1 and 10 with and without the diffusion control factor, respectively. From this study, it was noticed that the model including the diffusion control factor fitted quite well with the experimental data at the later stage of reaction, while the differences between the predicted values without the diffusion control factor and the experimental data were pronounced. This result indicated that, at the later stage of reaction, the diffusion control factor in the rate expression should be considered. It was also observed that the differences became more pronounced when the curing temperature used is low.
Degree of conversion vs. time plot for the cure process of epoxy/DDS/AB systems; experimental data (symbols) and calculated data (lines) at various isothermal conditions, at 160 °C (a), 175 °C (b), and 190 °C (c) (Reprinted with permission Kim et al. (2005))
Finally, the curing kinetics studies for the epoxy system and its corresponding blends with 10, 20, and 30 wt% of eSBS (47 mol%) were conducted at different isothermal temperatures. The corresponding plot of conversion rate versus time is shown in Fig. 13. Similar results to those observed with the PEO–PPO–PEO block copolymer about the reaction rate delay with the increment of the block copolymer content in the blends were observed. This delay in this case was also attributed to a differential distribution of the epoxy–amine components in the epoxy-rich phase and in the block copolymer-rich phase, as a result of the phase separation process. The observed behavior on the extent of reaction with the time corroborated that the autocatalytic nature of the curing process was not affected by the inclusion of the block copolymer.
Conversion rate vs. time curve for DGEBA/eSBS (47 mol%)/DDM (0, 10, 20, and 30 wt%) blends at 110 °C (Reprinted with permission George et al. (2012))
The kinetics parameters were calculated by the autocatalytic model (Eq. 1). The activation energy values and frequency factors are summarized in Table 8. It was found that the activation energy values increased with the amount of eSBS. This last fact was pointed out as another sign of cure reaction delay of epoxy system by the addition of eSBS. In addition, the same behavior of k 1and k 2 with the content of block copolymer and temperature than that observed in Table 2 for the epoxy system modified with PEO–PPO–PEO was found.
Conclusions
Differential scanning calorimetry, both non-isothermal and isothermal mode, has demonstrated to be a very effective technique to study the curing process of epoxy/block copolymer blends. The experimental cure kinetics curves of epoxy/block copolymer blends can be successfully fitted to both phenomenological autocatalytic and mechanistic kinetic models. It has been observed that the cure kinetics during the network formation of epoxy/block copolymer blends is affected by modifier concentration, volume fraction, chemical structure, and molecular weight of the blocks, among others. Both DSC modes demonstrated that curing rate decreases as block copolymer content increases. The delay in reaction rate of epoxy/block copolymer blends can be attributed to the dilution effect due to a reduction in the density of reactive groups as the block copolymer content increases, interactions between blocks and the growing epoxy network which inhibits autocatalytic process, as well as a partial segregation of reactive monomers and oligomers to the block copolymer-rich phase. The delay in cure rate attributed to interactions between epoxy and block copolymer blocks was corroborated by FTIR analyses. However, it was observed that the presence of hydroxyl groups in block copolymers had a significant effect on the curing reaction. Isothermal curing was successfully fitted to a phenomenological autocatalytic model based on a semiempirical equation which takes into account catalytic and autocatalytic effects and also to mechanistic model accounting an epoxy–hydroxyl complex, uncatalyzed and parallel catalyzed reactions. Phenomenological autocatalytic model fitted quite well with the experimental conversion curves, and the observed small deviations at high conversion values were related to vitrification effects. The autocatalytic model including diffusion phenomena fitted quite well with the experimental data in the later stage of the reaction. Mechanistic model corroborated that in addition to dilution effect, the physical interactions between components play an important role, and the observed delay is mainly due to these physical interactions than dilution effects. The kinetic parameters calculated from these mathematical models showed an increase in activation energies as block copolymer contents increase in agreement with the delay on curing rate observed.
References
Barton JM (1985) The application of differential scanning calorimetry (DSC) to the study of epoxy resin curing reactions. In: Epoxy resins and composites I. Springer, Berlin/Heidelberg, pp 111–154
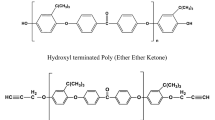
Bejoy F, Rao VL, Poel GV, Posada F, Groeninckx G, Ramaswamy R, Thomas S (2006) Cure kinetics, morphological and dynamic mechanical analysis of diglycidyl ether of bisphenol-A epoxy resin modified with hydroxyl terminated poly (ether ether ketone) containing pendent tertiary butyl groups. Polymer 47:5411–5419
Blanco M, Corcuera MA, Riccardi CC, Mondragon I (2005) Mechanistic kinetic model of an epoxy resin cured with a mixture of amines of different functionalities. Polymer 46:7989–8000
Bonnaud L, Pascault JP, Sautereau H (2000) Kinetic of a thermoplastic-modified epoxy-aromatic diamine formulation: modeling and influence of a trifunctional epoxy prepolymer. Eur Polym J 36:1313–1321
Bonnet A, Pascault JP, Sautereau H, Taha M, Camberlin Y (1999) Epoxy-diamine thermoset/thermoplastic blends. 1. Rates of reactions before and after phase separation. Macromolecules 32:8517–8523
Cai H, Li P, Sui G, Yu Y, Li G, Yang X, Ryu S (2008) Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochimica Acta 473:101–105
Calabrese L, Valenza A (2003) Effect of CTBN rubber inclusions on the curing kinetic of DGEBA-DGEBF epoxy resin. Eur Polym J 39:1355–1363
Cano L, Builes DH, Tercjak A (2014) Morphological and mechanical study of nanostructured epoxy systems modified with amphiphilic poly (ethylene oxide-b-propylene oxide-b-ethylene oxide) triblock copolymer. Polymer 55:738–745
Chiao L (1990) Mechanistic reaction kinetics of 4,4′-diaminodiphenyl sulfone cured tetraglycidyl-4, 4′-diaminodiphenylmethane epoxy resins. Macromolecules 23:1286–1290
Chiao L, Lyon RE (1990) A fundamental approach to resin cure kinetics. J Compos Mater 24:739–752
Cole KC (1991) A new approach to modeling the cure kinetics of epoxy/amine thermosetting resins. 1. Mathematical development. Macromolecules 24:3093–3097
Dean JM, Verghese NE, Pham HQ, Bates FS (2003) Nanostructure toughened epoxy resins. Macromolecules 36:9267–9270
Deng Y, Martin GC (1994) Diffusion and diffusion-controlled kinetics during epoxy-amine cure. Macromolecules 27:5147–5153
Du S, Guo ZS, Zhang B, Wu Z (2004) Cure kinetics of epoxy resin used for advanced composites. Polym Inter 53:1343–1347
Fernández B, Corcuera MA, Marieta C, Mondragon I (2001) Rheokinetic variations during curing of a tetrafunctional epoxy resin modified with two thermoplastics. Eur Polym J 37:1863–1869
Garate H, Mondragon I, Goyanes S, D’Accorso NB (2011) Controlled epoxidation of poly (styrene-b-isoprene-b-styrene) block copolymer for the development of nanostructured epoxy thermosets. J Polym Sci Part A: Polym Chem 49:4505–4513
Garate H, Mondragon I, D’Accorso NB, Goyanes S (2013) Exploring microphase separation behavior of epoxidized poly(styrene-b-isoprene-b-styrene) block copolymer inside thin epoxy coatings. Macromolecules 46:2182–2187
George SM, Puglia D, Kenny JM, Jyotishkumar P, Thomas S (2012) Cure kinetics and thermal stability of micro and nanostructured thermosetting blends of epoxy resin and epoxidized styrene-block-butadiene-block-styrene triblock copolymer systems. Polym Eng Sci 52:2336–2347
George SM, Puglia D, Kenny JM, Parameswaranpillai J, Thomas S (2014) Reaction-induced phase separation and thermomechanical properties in epoxidized styrene-block-butadiene-block-styrene triblock copolymer modified epoxy/DDM system. Ind Eng Chem Res 53:6941–6950
Ghaemy M, Khandani MH (1998) Kinetics of curing reaction of DGEBA with BF3-amine complexes using isothermal DSC technique. EurPolym J 34:477–486
Girard-Reydet E, Riccardi CC, Sautereau H, Pasault JP (1995) Epoxy-aromatic diamine kinetics. Part 1. Modeling and influence of the diamine structure. Macromolecules 28:7599–7607
Girard-Reydet E, Sautereau H, Pascault JP (1999) Use of block copolymers to control the morphologies and properties of thermoplastic/thermoset blends. Polymer 40:1677–1687
Girard-Reydet E, Sévignon A, Pascault JP, Hoppe CE, Galante MJ, Oyanguren PA, Williams RJJ (2002) Influence of the addition of polystyrene-block-poly(methyl methacrylate) copolymer (PS-b-PMMA) on the morphologies generated by reaction-induced phase separation in PS/PMMA/epoxy blends. Macromol Chem Phys 203:947–952
Grillet AC, Galy J, Pascault JP, Bardin I (1989) Effects of the structure of the aromatic curing agent on the cure kinetics of epoxy networks. Polymer 30:2094–2103
Grubbs RB, Dean JM, Broz ME, Bates FS (2000) Reactive block copolymers for modification of thermosetting epoxy. Macromolecules 33:9522–9534
Hermel-Davidock TJ, Tang HS, Murray DJ, Hahn SF (2007) Control of the block copolymer morphology in templated epoxy thermosets. J Polym Sci Part B: Polym Phys 45:3338–3348
Hu C, Yu J, Huo J, Chen Y, Ma H (2015) Nanostructures and thermal-mechanical properties of cyanate ester/epoxy thermosets modified with poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer. Polym Adv Technol 26:907–916
Jenninger W, Schawe JEK, Alig I (2000) Calorimetric studies of isothermal curing of phase separating epoxy networks. Polymer 41:1577–1588
Kamal MR, Sourour S (1973) Kinetics and thermal characterization of thermoset cure. Polym Eng Sci 13:59–64
Karkanas PI, Partridge IK (2000) Cure modeling and monitoring of epoxy/amine resin systems. I. Cure kinetics modeling. J Appl Polym Sci 77:1419–1431
Kim DS, Kim SC (1994) Rubber modified epoxy resin. I: cure kinetics and chemorheology. Polym Eng Sci 34:625–631
Kim D, Beak JO, Choe Y, Kim W (2005) Cure kinetics and mechanical properties of the blend system of epoxy/diaminodiphenyl sulfone and amine terminated polyetherimide-carboxyl terminated poly (butadiene-co-acrylonitrile) block copolymer. Korean J Chem Eng 22:755–761
Larrañaga M, Martin MD, Gabilondo N, Kortaberria G, Corcuera MA, Riccardi CC, Mondragon I (2004) Cure kinetics of epoxy systems modified with block copolymers. Polym Int 53:1495–1502
Larrañaga M, Gabilondo N, Kortaberria G, Serrano E, Remiro P, Riccardi CC, Mondragon I (2005) Micro-or nanoseparated phases in thermoset blends of an epoxy resin and PEO-PPO-PEO triblock copolymer. Polymer 46:7082–7093
Larrañaga M, Martin MD, Gabilondo N, Kortaberria G, Eceiza A, Riccardi CC, Mondragon I (2006a) Toward microphase separation in epoxy systems containing PEO-PPO-PEO block copolymers by controlling cure conditions and molar ratios between blocks. Colloid Polym Sci 284:1403–1410
Larrañaga M, Arruti P, Serrano E, De la Caba K, Remiro P, Riccardi C, Mondragon I (2006b) Towards microphase separation in epoxy systems containing PEO/PPO/PEO block copolymers by controlling cure conditions and molar ratios between blocks. Part 2. Structural characterization. Colloid Polym Sci 284:1419–1430
Larrañaga M, Mondragon I, Riccardi CC (2007) Miscibility and mechanical properties of an amine-cured epoxy resin blended with poly (ethylene oxide). Polym Int 56:426–433
Leonardi AB, Zucchi IA, Williams RJ (2015a) The change in the environment of the immiscible block stabilizes an unexpected HPC phase in a cured block copolymer/epoxy blend. Eur Polym J 71:164–170
Leonardi AB, Zucchi IA, Williams RJ (2015b) Generation of large and locally aligned wormlike micelles in block copolymer/epoxy blends. Eur Polym J 65:202–208
Lipic PM, Bates FS, Hillmyer MA (1998) Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures. J Am Chem Soc 120:8963–8970
Liu Y (2013) Polymerization-induced phase separation and resulting thermomechanical properties of thermosetting/reactive nonlinear polymer blends: a review. J Appl Polym Sci 127:3279–3292
Liu J, Sue HJ, Thompson ZJ, Bates FS, Dettloff M, Jacob G, Verghese N, Pham H (2008) Nanocavitation in self-assembled amphiphilic block copolymer-modified epoxy. Macromolecules 41:7616–7624
Liu J, Thompson ZJ, Sue HJ, Bates FS, Hillmyer MA, Dettloff M, Jacob G, Verghese N, Pham H (2010) Toughening of epoxies with block copolymer micelles of wormlike morphology. Macromolecules 43:7238–7243
Maiez-Tribut S, Pascault JP, Soule ER, Borrajo J, Williams RJJ (2007) Nanostructured epoxies based on the self-assembly of block copolymers: a new miscible block that can be tailored to different epoxy formulations. Macromolecules 40:1268–1273
Meng F, Zheng S, Li H, Liang Q, Liu T (2006) Formation of ordered nanostructures in epoxy thermosets: a mechanism of reaction-induced microphase separation. Macromolecules 39:5072–5080
Mijovic J, Wijaya J (1994) Reaction kinetics of epoxy/amine model systems. The effect of electrophilicity of amine molecule. Macromolecules 27:7589–7600
Mijovic J, Fishbain A, Wijaya J (1992) Mechanistic modeling of epoxy-amine kinetics. 1. Model compound study. Macromolecules 25:979–985
Mijovic J, Shen M, Sy JW, Mondragon I (2000) Dynamics and morphology in nanostructured thermoset network/block copolymer blends during network formation. Macromolecules 33:5235–5244
Ocando C, Tercjak A, Serrano E, Ramos JA, Corona-Galván S, Parellada MD, Fernández-Berridi MJ, Mondragon I (2008) Micro- and macrophase separation of thermosetting systems modified with epoxidized styrene-block-butadiene-block-styrene linear triblock copolymers and their influence on final mechanical properties. Polym Int 57:1333–1342
Ocando C, Fernández R, Tercjak A, Mondragon I, Eceiza A (2013) Nanostructured thermoplastic elastomers based on SBS triblock copolymer stiffening with low contents of epoxy system. Morphological behavior and mechanical properties. Macromolecules 46:3444–3451
Raju T, Durix S, Sinturel C, Omonov T, Goossens S, Groeninckx G, Moldenaers P, Thomas S (2007) Cure kinetics, morphology and miscibility of modified DGEBA-based epoxy resin–effects of a liquid rubber inclusion. Polymer 48:1695–1710
Rebizant V, Venet AS, Tournilhac F, Girard-Reydet E, Navarro C, Pascault JP, Leibler L (2004) Chemistry and mechanical properties of epoxy-based thermosets reinforced by reactive and nonreactive SBMX block copolymers. Macromolecules 37:8017–8027
Riccardi CC, Williams RJ (1986a) A kinetic scheme for an amine‐epoxy reaction with simultaneous etherification. J Appl Polym Sci 32:3445–3456
Riccardi CC, Williams RJ (1986b) Statistical structural model for the build-up of epoxy-amine networks with simultaneous etherification. Polymer 27:913–920
Riccardi CC, Adabbo HE, Williams RJJ (1984) Curing reaction of epoxy resins with diamines. J Appl Polym Sci 29:2481–2492
Riccardi CC, Fraga F, Dupuy J, Williams RJJ (2001) Cure kinetics of diglycidylether of bisphenol A-ethylenediamine revisited using a mechanistic model. J Appl Polym Sci 82:2319–2325
Ritzenthaler S, Court F, David L, Girard-Reydet E, Leibler L, Pascault JP (2002) ABC triblock copolymers/epoxy-diamine blends. 1. Keys to achieve nanostructured thermosets. Macromolecules 35:6245–6254
Ritzenthaler S, Court F, Girard-Reydet E, Leibler L, Pascault JP (2003) ABC triblock copolymers/epoxy-diamine blends. 2. Parameters controlling the morphologies and properties. Macromolecules 36:118–126
Roşu D, Caşcaval CN, Mustatǎ F, Ciobanu C (2002) Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochimica Acta 383:119–127
Ruiz-Pérez L, Royston GJ, Fairclough JPA, Ryan AJ (2008) Toughening by nanostructure. Polymer 49:4475–4488
Ryan ME, Dutta A (1979) Kinetics of epoxy cure: a rapid technique for kinetic parameter estimation. Polymer 20:203–206
Serier A, Pascault JP, Lam TM (1991) Reactions in aminosilane-epoxy prepolymer systems. I. Kinetics of epoxy-amine reactions. J Polym Sci Part A: Polym Chem 29:209–218
Serrano E, Martin MD, Tercjak A, Pomposo JA, Mecerreyes D, Mondragon I (2005) Nanostructured thermosetting systems from epoxidized styrene butadiene block copolymers. Macromol Rapid Commun 26:982–985
Serrano E, Tercjak A, Kortaberria G, Pomposo JA, Mecerreyes D, Zafeiropoulos NE, Stamm M, Mondragon I (2006) Nanostructured thermosetting systems by modification with epoxidized styrene-butadiene star block copolymers. Effect of epoxidation degree. Macromolecules 39:2254–2261
Serrano E, Tercjak A, Ocando C, Larrañaga M, Parellada MD, Corona-Galván S, Mecerreyes D, Zafeiropoulos NE, Stamm M, Mondragon I (2007) Curing behavior and final properties of nanostructured thermosetting systems modified with epoxidized styrene-butadiene linear diblock copolymers. Macrom Chem Phys 208:2281–2292
Smith IT (1961) The mechanism of the crosslinking of epoxide resins by amines. Polymer 2:95–108
Sourour S, Kamal MR (1976) Differential scanning calorimetry of epoxy cure: isothermal cure kinetics. Thermochim Acta 14:41–59
Swier S, Van Mele B (2003a) Mechanistic modeling of the epoxy-amine reaction in the presence of polymeric modifiers by means of modulated temperature DSC. Macromolecules 36:4424–4435
Swier S, Van Mele B (2003b) In situ monitoring of reaction-induced phase separation with modulated temperature DSC: comparison between high-Tg and low-Tg modifiers. Polymer 44:2689–2699
Swier S, Van Mele B (2003c) The heat capacity signal from modulated temperature DSC in non-isothermal conditions as a tool to obtain morphological information during reaction-induced phase separation. Polymer 44:6789–6806
Swier S, Van Assche G, Vuchelen W, Van Mele B (2005) Role of complex formation in the polymerization kinetics of modified epoxy-amine systems. Macromolecules 38:2281–2288
Tercjak A, Larrañaga M, Martin M, Mondragon I (2006) Thermally reversible nanostructured thermosetting blends modified with poly (ethylene-b-ethylene oxide) diblock copolymer. J Therm Anal Calorim 86:663–667
Urbaczewski E, Pascault JP, Sautereau H, Riccardi CC, Moschiar SS, Williams RJ (1990) Influence of the addition of an aliphatic epoxide as reactive diluent on the cure kinetics of epoxy/amine formulations. Die Makromolekulare Chemie 191:943–953
Varley RJ (2007) Reaction kinetics and phase transformations during cure of a thermoplastic-modified epoxy thermoset. Macromol Mater Eng 292:46–61
Varley RJ, Hodgkin JH, Hawthorne DG, Simon GP, McCulloch D (2000) Toughening of a trifunctional epoxy system Part III. Kinetic and morphological study of the thermoplastic modified cure process. Polymer 41:3425–3436
Verchere D, Sauterau H, Pascault JP (1990) Buildup of epoxy cycloaliphatic amine networks. Kinetics, vitrification and gelation. Macromolecules 23:725–731
Vinnik RM, Roznyatovsky VA (2006) Kinetic method by using calorimetry to mechanism of epoxy-amine cure reaction. J Therm Anal Calorim 85:455–461
Vyazovkin S, Sbirrazzuoli N (1996) Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 29:1867–1873
Williams RJJ, Rozenberg BA, Pascault JP (1997) Reaction-induced phase separation in modified thermosetting polymers. In: Polymer analysis polymer physics. Springer, Berlin/Heidelberg, pp 95–156
Wu S, Guo Q, Kraska M, Stühn B, Mai YW (2013) Toughening epoxy thermosets with block ionomers: the role of phase domain size. Macromolecules 46:8190–8202
Xu L, Fu JH, Schlup JR (1994) In situ near-infrared spectroscopic investigation of epoxy resin-aromatic amine cure mechanisms. J Am Chem Soc 116:2821–2826
Xu Q, Zhou Q, Shen K, Jiang D, Ni L (2015) Nanostructured epoxy thermoset templated by an amphiphilic PCL-b-PES-b-PCL triblock copolymer. J Polym Sci Part B: Polym Phys. doi:10.1002/polb.23917
Zhang J, Guo Q, Fox BL (2009) Study on thermoplastic-modified multifunctional epoxies: influence of heating rate on cure behaviour and phase separation. Comp Sci Tech 69:1172–1179
Zvetkov VL (2005) Mechanistic modeling of the epoxy-amine reaction: model derivations. Thermochimica Acta 435:71–84
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Ocando, C., Fernandez, R., Corcuera, M.A., Eceiza, A. (2017). Cure Kinetics of Epoxy/Block-Copolymer Blends. In: Parameswaranpillai, J., Hameed, N., Pionteck, J., Woo, E. (eds) Handbook of Epoxy Blends. Springer, Cham. https://doi.org/10.1007/978-3-319-40043-3_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-40043-3_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40041-9
Online ISBN: 978-3-319-40043-3
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics