Abstract
Nanostructuring of thermosetting systems using the concept of templating and taking advantage of the self-assembling capability of block copolymers is an exciting way for designing new materials for nanotechnological applications. In this first part of the work, reactive blends based on stoichiometric amounts of a diglycidylether of bisphenol-A epoxy resin and 4,4′-diaminodiphenylmethane cure agent modified with three poly(ethylene oxide)-co-poly(propylene oxide)-co-poly(ethylene oxide) block copolymers were studied. Cure advancement of these systems was analyzed by differential scanning calorimetry. The experimental results show a delay of cure rate, which increases as copolymer content and PEO molar ratio in the block copolymer rise. Infrared spectroscopy shows that PEO block is mainly responsible of physical interactions between the hydroxyl groups of growing epoxy thermoset and ether bonds of block copolymer. These interactions are mainly responsible for the delaying of cure kinetics. The molar ratio between blocks also has a critical influence on the delaying of the cure rate. A mechanistic approach of cure kinetics allows us to relate the delay of cure as a consequence of block copolymer adding to physical interactions between components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanostructuring of thermosetting systems using the concept of templating and taking advantage of self-assembling capability of block copolymers is an exciting way for designing new nanotechnological applications based on nanoporous or nanostructured materials.
A large number of investigations were performed in the last decades to get a better understanding of the cure behavior of thermosetting systems. The kinetics of cure determines the final network, which influences physical and mechanical properties of cured systems. Many authors have studied the modification of thermosets with rubbers [1–3] or termoplastics [4–6] analyzing their influence on obtained morphologies. Kinetic studies of these systems were widely carried out.
The use of block copolymers as modifiers of epoxy resins was recently widely analyzed [7–13]. In recent years, the importance of control of microstructures has increased, especially in the nanometer scale. Many parameters govern the final morphology: cure cycle, volume fraction of each block, blend composition, and block/block and copolymer/epoxy interactions. Kinetics, together with thermodynamics, is a key factor for controlling the phase separation process, and for a better understanding of the micro- or nanoseparated morphologies generated under different cure conditions.
Poly(ethylene oxide)-co-poly(propylene oxide)-co-poly(ethylene oxide) (PEO–PPO–PEO) block copolymer was already employed as modifier for epoxy and phenolic resins [14–18]. Different final structures were obtained as a function of copolymer content and cure cycle [14–16]. The cure kinetics of an epoxy system modified with a PEO–PPO–PEO block copolymer with PEO:PPO block molar ratio of 0.8:1 was previously reported [17, 18].
The purpose of the present work is to analyze the influence of both cure kinetics and thermodynamics on nanostructuring epoxy systems modified with several PEO–PPO–PEO block copolymers. In this first part of the study, the influence of PEO:PPO ratio in the block copolymer on cure kinetics of PEO–PPO–PEO-modified epoxy systems is investigated. The influence of the ratio between blocks on mechanistic model constants is analyzed by comparison with previously studied EP-0.8:1 modified systems [17, 18]. In the second part of the study, the final properties of these systems will be related to their morphological (macro- or microphase separated) features, which are dependent on both cure conditions and PEO:PPO block molar ratio and content in the mixtures.
Experimental
The epoxy prepolymer used was a diglycidylether of bisphenol-A (DGEBA), DER-332, supplied by Dow Chemical. The hardener was an aromatic diamine 4,4′-diaminodiphenylmethane (DDM), HT-972, supplied by Ciba. The modifiers used were different hydroxyl-terminated PEO–PPO–PEO block copolymers from PolySciences and PEO and PPO homopolymers, from Sigma-Aldrich. Characteristics and structures of these materials are listed in Table 1.
PEO–PPO–PEO and homopolymers modified epoxy blends were prepared in the following way. Firstly, PEO–PPO–PEO was added to DGEBA resin at 80 °C and stirred for mixing. Then DDM was added in a stoichiometric amine/epoxy ratio with continual stirring in an oil bath at 80 °C for approximately 5 min until a homogeneous blend was achieved.
Differential scanning calorimetry (DSC) measurements were carried out in a Perkin-Elmer DSC-7 (calibrated with high purity indium). All experiments were conducted under nitrogen flow of 20 cm3 min−1, working with 5–7 mg of samples in open aluminum pans. Kinetic studies were performed isothermally at several cure temperatures (80, 100, 120, 140, 150, 160, and 170 °C). After thermograms leveled off to the baseline, all samples were rapidly cooled. Then, dynamical scans from 35 to 250 °C at 10 °C/min were performed to determine the residual heat of reaction (ΔH res). Conversion was calculated by dividing the enthalpy recorded in the isothermal scan at each cure time and the total enthalpy obtained from the sum of isothermal (ΔH iso) and the residual enthalpy.
Glass transition temperatures (T g) were measured by DSC dynamical scans from −100 to 50 °C at 20 °C/min.
Cloud point temperatures, T cp, of DGEBA/DDM/PEO/PPO/PEO blends were determined in a transmission optical microscope, Olympus BHT-M, equipped with a photoelectric cell, by dynamical scans from 35 to 250 °C at 10 °C/min. Cloud point temperatures were taken as the temperature at which a decrease in the transmitted light intensity was recorded.
Fourier transform infrared (FTIR) spectroscopic analysis was performed in a Perkin-Elmer 1600 PC spectrometer. The solid analyte was mixed with KBr. The spectra were taken with 2 cm−1 resolution and 20 scans were carried out from 4,000 to 400 cm−1. These measurements were used to investigate variations on free and associated OH groups in the analyzed mixtures.
Results and discussion
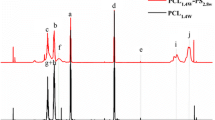
Initial miscibility of these uncured epoxy/block copolymers systems was studied by DSC. Figure 1 shows DSC thermograms of the individual components and systems modified with 20wt% of different copolymers. All the block copolymers, independent of their PEO:PPO ratio, show a single T g, which appears at around −72 °C for EP-0.33:1, −71 °C for EP-0.8:1, and −70 °C for EP-3:1 block copolymer due to the similar values of T g of both blocks, and a melting peak around 14, 18, and 56 °C, respectively, corresponding to PEO block. In addition, a crystallization peak around −46 and −47 °C can be observed for EP-0.33:1 and EP-0.8:1, respectively. PPO is an amorphous polymer with a T g of −73 °C. Uncured DGEBA/DDM system presents a T g of −16 °C. The blends modified with 20wt% EP-0.8:1, EP-0.33:1, and EP-3:1 block copolymers show a single T g at around −27, −26, and −25 °C, respectively, which are intermediate between those of neat block copolymers and uncured DGEBA/DDM system and also close to the value of system modified with PPO. This fact suggests complete miscibility between block copolymers and epoxy prepolymer. Indeed, assuming the same T g for both blocks in the block copolymers and using the T g values for PPO homopolymer and epoxy resin measured by DSC, the theoretical T g values for these blends obtained from Fox equation [19] are around −29 °C in all cases, which are similar to experimental values.
Cure kinetics
Cure kinetics was analyzed by DSC. Figure 2a presents dynamic thermograms for a neat epoxy system and its blends containing several amounts of EP-0.33:1 block copolymer. The cure reaction is kinetically affected by the copolymer content. The exothermic polymerization starts and finishes later as copolymer content increases, thus indicating that cure is delayed. Similar curves were observed for EP-0.8:1 [17] and EP-3:1 modified systems (not shown here).
Table 2 displays the cure enthalpy from dynamic measurements, ΔH T. The values are dependent upon copolymer content but apparently the reaction pathway of epoxy matrix polymerization is not modified because ΔH T decreases proportionally to the copolymer content for the three block copolymers. Exothermic peak values, T p, are also shown. They moved to higher values as the concentration of modifier increases, thus confirming the delaying of cure reaction by increasing the copolymer content. As can be seen in Fig. 2b, the displacement of exothermic peak at the same composition was the highest for EP-3:1. As expected, in the case of DGEBA/DDM/EP-3:1, T p appears at clearly higher temperatures than for blends modified with the other block copolymers. Other interesting features have to be noted in Fig. 2b. For EP-0.33:1 and EP-0.8:1 modified systems, a big shoulder appears, which is indicated by an arrow, that can be attributed to phase separation as it was previously shown for EP-0.8:1 modified blends and other epoxy blends [17, 20, 21]. Cloud point temperature appears in both blends in a similar temperature range than that for the beginning of the shoulder, T sh, observed by DSC analysis. However, in the case of the system modified with EP-3:1, this shoulder does not appear, which agrees with the transparency of blends for all EP-3:1 contents.
Figure 3 shows cure kinetics curves obtained for blends with different amounts of copolymer at 140 °C for each block copolymer. Solid and dashed lines in Fig. 3b correspond to kinetic modeled curves for neat and 20wt% EP-0.8:1 and theoretical curve obtained by modeling dilution effect for 20wt% EP-0.8:1. As expected, as block copolymer content increases, the cure reactions are shifted to longer times.
Cure kinetics curves for epoxy systems cured at 140 °C containing: 0wt% (■), 10wt% (○), 20wt% (▲), and 30wt% (□) of a EP-0.33:1, b EP-0.8:1, and c EP-3:1. In subpanel b, solid lines show predicted curves from kinetic model for neat and 20wt% EP-0.8:1 while dashed lines shows a theoretical curve obtained by modeling dilution effect for 20wt% EP-0.8:1
As Fig. 4 shows, for the same cure temperature and content, the reaction time increases as the molar ratio of PEO block in the modifier does. The observed delay does not seem to be only attributable to dilution effects. As previously reported for the EP-0.8:1 modified system [17, 22], specific interactions of epoxy matrix with the block copolymer exist. However, if these interactions were the same for both blocks of the copolymer, systems modified with copolymer with different block molar ratios had to show the same delay. Thus, physical interactions occur between the epoxy and PEO block, the cure rate of the system modified with EP-3:1 (higher PEO content) being the slowest. This fact is confirmed below by FTIR.
Figure 5a shows the FTIR spectra for mixtures with different contents of block copolymers cured at 80 °C and postcured at 190 °C. The bands centered at 3,427 cm−1 (broad band) and 3,559 cm−1 are attributed to associated and free hydroxyl groups, respectively [23–27]. At the same block copolymer content, as the PEO molar ratio in the block copolymer increases, the associated hydroxyl group band appears at lower wave numbers. Moreover, the intensity ratio between associated and free hydroxyl bands also increases. With respect to the effect of copolymer content, a clear lowering on wave number of the band of associated hydroxyl groups is observed for EP-0.8:1 and EP-3:1 modified systems as block copolymer content increases. However, for EP-0.33:1 modified system, this band shifts only slightly to lower wave numbers while a slight increase of the intensity ratio between associated and free hydroxyl bands is observed. In Fig. 5b, it can be seen that the ratio of intensity between associated and free hydroxyl bands increases more strongly with increasing conversion for EP-3:1 modified systems than for the systems modified with EP-0.8:1; in both cases, the ratio being higher than for the unmodified system. This fact can be attributed [17] to physical interactions between the OH groups initially existing or developed through cure reactions and the ether groups of the block copolymer [23]. These hydroxyl–ether interactions were not quantitatively determined due to both broad absorption band and the disappearance of free hydroxyl band. Anyway, it is clear that they modify the autocatalytic process, delaying the cure process. The presence of the methyl group in PPO block hinders physical interactions with hydroxyl groups [14]. Indeed, the ether groups of PEO blocks are mainly responsible for these physical interactions. Therefore, cure reactions become slower as PEO content in the block copolymer is higher. As a consequence, the cure process for the system modified with EP-3:1 is more delayed than for the system modified with EP-0.8:1.
Cure kinetics of EP-0.33:1 and EP-0.8:1 modified systems was also modeled until cloud point to know the influence of physical interactions between components of these blends. The mechanistic approach used for the analysis of cure kinetics considers the following cure steps: epoxy activation by hydrogen bonding with hydroxyl groups in the preequilibrium to form an epoxy-hydroxyl complex (Eq. 1); uncatalyzed addition of reactions of primary and secondary amine hydrogens with epoxy groups (Eqs. 2 and 3); and parallel, autocatalyzed reactions (Eqs. 4 and 5), which take place because of the initially existing OH groups and of those developed through cure reactions [28].





By defining K=ke 0 (dimensionless), \(K\prime _{1} = k\prime _{1} e_{0} ,\;K_{1} = k_{1} e_{0} ,\;C_{0} = \frac{{OH_{0} }} {{e_{0} }},\;and\;y = \frac{{{\left( {e - OH} \right)}}} {{e_{0} }}\) where e 0 and e are the concentrations of epoxy at time 0 and t, respectively; a 1 is the concentration of primary amino hydrogens and r is the ratio of secondary to primary amino-hydrogen rate constants, taken as 0.65 [29]; OH0 is the initial concentration of hydroxyl groups; and e-OH is the epoxy hydroxyl complex concentration. The cure kinetics is thus determined by the following equations:
where
and
The complex formation is represented with a dimensionless equilibrium constant K and the uncatalyzed addition and autocatalyzed reactions with dimensionless kinetic constants K′1 and K 1, respectively.
Figure 3b shows predicted curves for both neat system and system modified with 20wt% EP-0.8:1 (solid lines). Taking into account that although we show the curves for full conversion range, the modelization was done by taking experimental values only up to cloud point as examples. Similar to that observed previously [18], there is a good agreement between experimental and theoretical curves up to cloud point.
Table 3 shows the kinetic constants of DGEBA/DDM/EP-0.8:1 and EP-0.33:1 systems compared to those for the neat matrix obtained as the average values in the 80–170 °C range. In the case of EP-0.33:1 modified systems, the ratio between the different constants differs from the ratio between initial concentrations of epoxy equivalents. It seems that in a similar way to that observed for EP-0.8:1 block copolymer [18], in addition to dilution effect, the interactions between hydroxyl groups and block copolymer also affect the cure kinetics. Moreover, Fig. 3b shows both predicted curve from kinetic model (solid line) and theoretical curve obtained by modeling dilution effect (dashed line) for 20wt% EP-0.8:1 modified system. It can be clearly observed that reactions occur at a lower rate than that only considering dilution effect, thus confirming that in addition to such dilution effect, there are interactions between epoxy and block copolymers that affect reaction rate. As shown by K and K 1 values, the formation of epoxy–hydroxyl complex and catalytic processes are less reduced for EP-0.33:1 modified system than for EP-0.8:1 modified system. This fact corroborates that EP-0.33:1 interacts less with epoxy system due to its lower PEO block content. On the other hand, the increase of K′1 in the modified systems can also be observed. At the earlier stages of cure, as a consequence of physical interactions with PEO blocks, less epoxy groups interact with hydroxyl groups when block copolymer content is increased. Therefore, more epoxy groups are available for direct reaction with amine. This fact is more evident in the case of EP-0.8:1 modified system due to its higher PEO content.
Table 4 presents activation energies of neat system and EP-0.8:1 and EP-0.33:1 modified systems. While the activation energy values are similar for all systems, a slight variation in frequency factors is observed. Taking into account that the K 1 ratio decreases with copolymer content, the decrease of A 1 can be attributed to the slowing down of autocatalytic process as a consequence of physical interactions between the components, while the increase of A′1 is probably related to the increase of epoxy groups, which can directly react with the hardener in both cases. According to these results, the change in frequency factor values is higher for EP-0.8:1 than for EP-0.33:1 modified systems due to the interactions between PEO and epoxy matrix.
Conclusions
This study arises with the cure kinetics of an epoxy resin modified with three PEO–PPO–PEO block copolymers. The effect of PEO content and molar ratio in the block copolymer was investigated. DSC measurements showed initial miscibility for all systems. Macrophase separation occurs in the case of EP-0.33:1 and EP-0.8:1 modified systems during cure process but not for the block copolymer with a high PEO content.
Infrared spectroscopy showed that physical interactions between the block copolymers and epoxy system occur. The PEO block is mainly responsible for the physical interactions between the hydroxyl groups of growing epoxy thermoset and ether bonds of block copolymer. Kinetic analysis shows that the systems modified with copolymers with different block molar ratio present a different delay and the cure process is shifted to longer times as block copolymer content increases. It was proven that the delaying of cure kinetics is mainly due to these interactions. A mechanistic approach of cure kinetics allows us to relate, by means of the variations of kinetic constants, the delay of cure as a consequence of the addition of a block copolymer to physical interactions between components. The molar ratio between blocks has a high influence on the delaying of cure.
References
Bussi P, Ishida H (1994) J Appl Polym Sci 53:441
Chen D, Pascault JP, Sautereau H (1993) Polym Int 32:361
Verchere D, Sautereau JP, Pascault JP (1990) J Appl Polym Sci 41:467
Ritzenthaler S, Girard-Reydet E, Pascault JP (2000) Polymer 41:6375
Barral L, Cano J, López J, López-Bueno I, Nogueira P, Abad MJ, Ramírez C (2000) Polymer 41:2657
Su CC, Woo EM (1995) Polymer 36:2883
Grubbs RB, Dean JM, Broz ME, Bates FS (2000) Macromolecules 33:9522
Lipic PM, Bates FS, Hillmyer MA (1998) J Am Chem Soc 120:8963
Hillmyer MA, Lipic PM, Hajduk DA, Almdal W, Bates FS (1997) J Am Chem Soc 119:2749
Guo Q, Thomann R, Gronski W, Staneva R, Ivanova R, Stühn B (2003) Macromolecules 36:3635
Ritzenthaler S, Court F, David L, Girard-Reydet E, Leibler L, Pascault JP (2002) Macromolecules 35:6245
Ritzenthaler S, Court F, Girard-Reydet E, Leibler L, Pascault JP (2003) Macromolecules 36:118
Ruzette AV, Leibler L (2005) Nature Materials 4:19
Konosen H, Ruokolainen J, Torkkeli M, Serimaa R, Nyholm P, Ikkala O (2002) Macromol Chem Phys 203:388
Mijovic J, Shen M, Sy JW, Mondragon I (2000) Macromolecules 33:5235
Guo Q, Thomann R, Gronski W, Thurn-Albrecht T (2002) Macromolecules 35:3133
Larrañaga M, Martín MD, Gabilondo N, Kortaberria G, Corcuera MA, Riccardi CC, Mondragon I (2004) Polym Int 53:1495
Larrañaga M, Gabilondo N, Kortaberria G, Serrano E, Remiro PM, Riccardi CC, Mondragon I (2005) Polymer 46:7082
Fox TG (1956) Bull Am Phys Soc 1:123
Martinez I, Martin MD, Eceiza A, Oyanguren P, Mondragon I (2000) Polymer 41:1027
Remiro PM, Riccardi CC, Corcuera MA, Mondragon I (1999) J Appl Polym Sci 74:772
Jenninger W, Schawe JEW, Alig I (2000) Polymer 41:157
Guo Q, Harrats C, Groeninckx G, Koch MHJ (2001) Polymer 42:4127
Rocco AM, Moreira DP, Pereira RP (2003) Eur Polym J 39:1925
Luo X, Zheng S, Zhang N, Ma D (1994) Polymer 35:2619
Hu L, Lü H, Zheng S (2004) J Polym Sci Part B Polym Phys 42:2567
Zheng H, Zheng S, Guo Q (1997) J Polym Sci Part B Polym Phys 35:3169
Riccardi CC, Fraga F, Dupuy J, Williams RJJ (2001) J Appl Polym Sci 82:2319
Gyrard-Reydet E, Riccardi CC, Sautereau H, Pascault JP (1995) Macromolecules 28:7599
Acknowledgements
Funding for this work was provided by Ministerio de Ciencia y Tecnología (Spain) grants MAT1998-0656, MAT2000-0293, and MAT2001-0714. M. Larrañaga acknowledges financial support (grant for Ph.D.) from the Ministerio de Ciencia y Tecnología. C. C. Riccardi also thanks Gobierno Vasco/Eusko Jaurlaritza for the financial support for a sabbatical year.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larrañaga, M., Martin, M.D., Gabilondo, N. et al. Toward microphase separation in epoxy systems containing PEO–PPO–PEO block copolymers by controlling cure conditions and molar ratios between blocks. Colloid Polym Sci 284, 1403–1410 (2006). https://doi.org/10.1007/s00396-006-1512-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-006-1512-9