Abstract
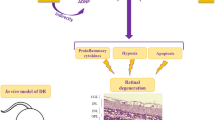
Pituitary adenylate cyclase activating polypeptide (PACAP) is a widespread neuropeptide that is well known for its general cytoprotective effects in different neuronal injuries, such as traumatic brain and spinal cord injury, models of neurodegenerative diseases, and cerebral ischemia. PACAP and its receptors also occur in the retina. In this review, we summarize the retinoprotective effects of PACAP. In vitro, PACAP is protective against glutamate, thapsigargin, anisomycin, oxidative stress, UV light, high glucose, inflammation, and anoxia. Both the neural retina and the pigment epithelial cells can be protected by PACAP in various experimental paradigms. In vivo, the protective effects of intravitreal PACAP treatment have been shown in the following models in rats and mice: excitotoxic injury induced by glutamate, N-methyl-d-aspartate (NMDA) or kainate, ischemic injury induced by carotid artery ligation and high intraocular pressure, degeneration caused by UV-A light, optic nerve transection, and streptozotocin-induced diabetic retinopathy as well as retinopathy of prematurity. Molecular biological methods have revealed that PACAP activates anti-apoptotic, while inhibits pro-apoptotic signaling pathways, and it also stimulates an anti-inflammatory environment in the retina. Altogether, PACAP is suggested to be a potential therapeutic retinoprotective agent in various retinal diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Pituitary Adenylate Cyclase Activating Polypeptide (PACAP)
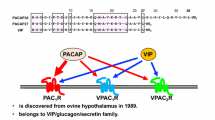
Pituitary adenylate cyclase activating polypeptide (PACAP) was isolated from ovine hypothalamus based on its stimulating effect on adenylate cyclase enzyme in anterior pituitary cells [1, 2]. PACAP has two isoforms: PACAP1-27 and PACAP1-38, containing 27 and 38 amino acids, respectively. PACAP is a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon superfamily [3]. Its receptors can be divided into two groups, PAC1 receptor and VPAC receptors (VPAC1 and VPAC2) [4]. The biological effects of PACAP are diverse [4], but one of the most studied actions of the peptide is its potent neuroprotective effect. Earlier studies have shown that PACAP protects neurons in vitro against various toxic agents (glutamate, 6-OHDA, oxidative stress, etc.). The neuroprotective role of PACAP has been also demonstrated in vivo in different animal models, such as cerebral ischemia, traumatic brain injury, spinal cord injury, and Parkinson’s disease. These neuroprotective effects have been summarized in several reviews by different authors [4–11].
PACAP is also protective in sensory organs, including the auditory and visual systems [12–14]. The retina is a special tissue, an extension of the central nervous system (CNS) containing different neurons, and it also provides an excellent model to measure neuronal degenerations. PACAP has been shown to protect the different neurons of the retina in numerous in vitro and in vivo models of retinal degenerations. The purpose of the present review is to summarize the current knowledge of the retinoprotective functions of PACAP. The retinoprotective effects of PACAP provide a new, exciting area of research, and several novel data have been published since the appearance of the first two extensive reviews on the retinoprotective effects of PACAP [12, 15]. Therefore, we briefly summarize retinoprotective effects until 2010 and discuss findings in the last 5 years more in detail.
Distribution of PACAP in the Retina
Several studies have described the presence of PACAP and PACAP receptors in the rat retina. PACAP immunopositive cell bodies were found in amacrine, horizontal cells in the inner nuclear layer (INL), in the ganglion cell layer (GCL) and also in the Muller glial cells. PACAP positive nerve fibers were also present in the nerve fiber layer (NFL), and also in the inner plexiform layer (IPL), but no PACAP positivity was found in the outer nuclear layer (ONL) or the retinal pigment epithelium (RPE) layer [16–20]. The presence of PACAP was also found in the mouse [21], teleost [22], turtle [23], and chicken retina [24]. PACAP appearance showed in a subset of retinal ganglion cells, which cells are photosensitive due to expression of melanopsin. The PACAP expression in this subset of ganglion cells is partly controlled by dopamine, while it is not affected by photoreceptor degeneration [25, 26]. Melanopsin-containing retinal ganglion cells are known to be more resistant in various types of degeneration, such as axonal injury [27], and it has been proven by numerous studies that these melanopsin-containing cells almost all co-store PACAP, which might contribute to the highly resistant nature of these cells [28–30]. PACAP- and melanopsin-containing retinal ganglion cells play an important role in several light-activated non-image-forming functions of the retina and the complimentary signaling due to PACAP neurotransmission has been shown to be important in addition to the glutamatergic signaling [31, 32].
PACAP immunopositivity was also found in the limboretinal pathway [33]. PACAP and its receptors were also described in the developing retina of mammals [34]. In developing chicken retina PACAP was expressed in the INL from embryonic day 8 (E8) [35]. In zebrafish during the pharyngula period, at 24 h of post fertilization PACAP-like immunoreactivity (PACAP-LI) was detected in the superficial layer of the retina, and during the hatching period, at 72 h of post fertilization in the GCL [36]. PACAP-LI was absent from the retina in the zebrafish at day 13 of larval development, suggesting that PACAP may have a role in the control of cell differentiation and proliferation [36, 37]. In the rat, PACAP mRNA was expressed in the GCL in the developing retina at E20 [38].
Distribution of PACAP Receptors in the Retina
Earlier studies showed the distribution and localization of PACAP receptors in the retina. Nilsson et al. [39] characterized the receptors of PACAP and their coupling to adenylate cyclase in albino rat eye, and D’Agata and Cavallaro also described the expression of PACAP/VIP receptor variants and their coupling to phospholipase C in the rat retina [16]. In chicken retina at early stage (E6) PAC1 receptor and its mRNA can be found [35]. The mRNA of PACAP receptors and the protein expression have also been shown in all the layers of the neonatal rat retina [40]. Seki and coworkers described the localization and strong expression of PAC1 receptor and its mRNA in different retinal layers (NFL, INL, GCL), weaker expression in the ONL, OPL, IPL, and the outer segments of the photoreceptors in the rat retina [41, 42]. Moreover, different PAC1 receptor mRNA variants (short, hop) were found in the cell bodies and the processes of ganglion and amacrine cells [41]. Finally, PAC1 receptor has been described in Muller glial cells [19, 43].
PACAP also plays a role in retinal development. The expression of the specific PAC1 receptor and VPAC receptors has been confirmed in the developing mammalian retina, including the retinal progenitor cells [44]. A shift in PAC1 receptor isoform expression (null, hip, hop1, hiphop1) during development might explain the different roles exerted during differentiation of retinal neural elements [45, 46].
PACAP controls the proliferation of retinal progenitor cells through the activation of all 3 receptors, and the cAMP/PKA/CREB pathway. This activation resulted in downregulation of cyclin D1 and the transcription factor Klf4, while p27kip1 protein content did not change [44, 47]. The overall effect of these changes was an antiproliferative effect.
Protective Effects of PACAP in the Retina In Vitro
The in vitro protective effects are summarized in Table 30.1.
In Vitro Protective Effects in the Neural Retina
The first in vitro studies presented that VIP, a related peptide to PACAP [48], or PACAP1-27 and PACAP1-38 [49] have retinoprotective effect against glutamate toxicity using cultured retinal neurons. PACAP1-27, PACAP1-38, and VIP increased cAMP, and the protective effects of VIP or PACAP isoforms were antagonized by VIP6-28 (VIP antagonist) or PACAP6-38 (PACAP antagonist), respectively. In addition, H-89 (PKA inhibitor) also decreased the protective effects of VIP or PACAP1-27, PACAP1-38.
Silveira et al. [40] found that anisomycin (1 μg/ml) inhibited protein synthesis and induced cell death in the neuroblastic layer of retinal explants from newborn rats. PACAP1-27 or PACAP1-38 dose-dependently prevented the anisomycin-induced cell death. One nanomolar PACAP1-27 or PACAP1-38 administration in parallel significantly reduced the number of dead cells with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) procedure and the number of pyknotic profiles per mm2 in the neuroblast layer (NBL). A similar retinoprotective effect was observed with PAC1 receptor agonist maxadilan, but not with glucagon. Neuroprotection by PACAP1-38 was also measured in photoreceptor cell death caused by thapsigargin in the ONL [40]. In the retinal explant model, PACAP6-38 and the specific PAC1 receptor antagonist Maxd.4 antagonized the neuroprotective effect. Molecular and immunohistochemical analysis demonstrated that the retinoprotective effects are most probably mediated by the PAC1 receptor, which is expressed in all the retinal layers of the neonatal retina. Treatment of PACAP1-38 induced CREB immunoreactivity in the NBL. PACAP1-38 administration also increased cAMP, but not IP3, and a cAMP-dependent protein kinase inhibitor Rp-cAMPS could block the retinoprotective effects of PACAP1-38.
In adult turtles, horizontal cells showed light responses 18, 22, 42, and 46 h after removal of the eyes. Rabl et al. [50] demonstrated that the amplitudes of light responses were larger in slices at all time-points in PACAP1-38-containing solution (0.165 μM PACAP) compared to control retinal slices in anoxic condition. These results provide evidence that PACAP1-38 has neuroprotective effects in hypoxic/anoxic conditions in the adult turtle retina in vitro.
In Vitro Protective Effects in the Retinal Pigment Epithelial Cells
Retinal pigment epithelial (RPE) cells play an essential role in photoreceptor survival and metabolism. Several diseases are associated with malfunction of the pigment cells. The first results concerning the effects of PACAP came from Zhang et al. [51]. Presence of mRNA for PAC1 and VPAC1 receptors was confirmed in unstimulated human RPE cells (ARPE19). VPAC2 mRNA was expressed in cells stimulated with IL-1β. PACAP treatment at 0.1–1 μM concentrations inhibited the IL-1 β-stimulated IL-6, IL-8, and MCP-1 mRNA and protein levels. The dense immunofluorescence of NF-kB in the nucleus after stimulation with IL-1β was decreased by PACAP [51]. Studies following these first results in pigment cells revealed several other effects of PACAP in these retinal cells.
ARPE19 cells exposed to PACAP1-38 could be rescued against oxidative stress induced by H2O2 in a dose-dependent manner [52]. Highest efficacy was observed at 100 nM PACAP concentration, but it was effective in a range 10 pM–1 μM, while not effective at 1 pM. Using flow cytometric and JC-1 assay, PACAP treatment was proven to reduce apoptotic cell death. PACAP1-38 and 6-38 alone had no effect on cell survival. The survival-promoting effect was inhibited by PI3K/Akt inhibitors, but not affected by MAPK inhibitors [52]. A subsequent study investigated the signaling pathways in oxidative stress-induced pigment epithelial cell damage [53]. Western blot results revealed that PACAP treatment could activate the protective ERK/CREB and Akt pathways, while it inhibited the phosphorylation of p38 MAPK and JNK. Several cytokines and other apoptotic markers were altered upon oxidative stress, as measured by cytokine and phospho-kinase arrays. PACAP administration counteracted several of these changes. 10 and 100 nM PACAP decreased the oxidative stress-induced increases of the proinflammatory and/or proapoptotic factors Bad; Bax; Trail R2 DR5; FADD; Fas TNFR; HIF-1α; HO-1; cIAP-1; HSP-27; SMAC/Diablo; p53; eNOS; Paxillin; PLCgamma-1; STAT4; RSK1/2. In contrast, PACAP could increase the protective Lyn; Yes; and Src.
A more recent study has reported that PACAP regulates semaphorin4A expression from RPE cells [54]. The semaphorin protein family has members that influence brain and retinal development, by providing neural guidance. Semaphorin4A is expressed in the GCL, INL and RPE cell during the developmental period of contact-building between photoreceptors and RPE cells. Human ARPE cells were cocultured with PC12 cells (prototype neuronal cells) based on a collagen vitrigel membrane. The authors found that in the presence of neural cells the protein and mRNA levels of semaphorin4A (but not other semaphorins) were increased, an effect mimicked by PACAP (but not VIP, NPY, or enkephalin). Furthermore, this effect was also associated with ERK phosphorylation (but not that of JNK or p38 MAPK). All these effects of PACAP were blocked by the PACAP antagonist PACAP6-38. Furthermore, PACAP6-38 downregulated the cytokine IL-6 expression, indicating that endogenous PACAP participates in the neural cell-induced IL-6 production (other cytokines out of the tested ones were not influenced by the neuronal co-culture). These results suggest that the PACAP secreted by neural cells regulates several factors secreted by RPE cells, important for retinal development and homeostasis [54]. The local secretion of PACAP necessary for these effects is supported by the high concentration used in this study (1 μM and 10 μM PACAP, lower concentrations were not reported), a concentration much higher than the ones reported to exert protective effects against oxidative stress [52, 53].
Another study investigated the potential of PACAP and VIP against the disruption of tight junctions in the retina, a major factor in macular edema developing in diabetic retinopathy [55]. ARPE19 cells were cultured either in normal glucose or in high glucose in combination with the pro-inflammatory IL-1β. Effects on permeability were evaluated by measuring both apical-to-basolateral movements of fluorescein isothiocyanate (FITC) dextran and transepithelial electrical resistance [55]. Results of these experiments demonstrated that normal glucose + IL-1β and, to a greater extent, high glucose + IL-1β significantly increased FITC-dextran diffusion, paralleled by decreased electrical resistance. PACAP or VIP reversed these effects. Furthermore, high glucose + IL-1β-induced reduction of claudin-1 and ZO-1 expression was reversed by PACAP and VIP. Occludin expression was not affected in any of the conditions tested. Altogether, these findings show that both peptides counteract high glucose + IL-1β-induced damage in ARPE19 cells, suggesting that they might be relevant to the maintenance of outer blood–retinal barrier function in edema accompanying diabetic retinopathy [55]. These results are in accordance with our earlier observations in an in vitro blood–brain barrier (BBB) model [56]. We found that PACAP treatment improved the barrier properties of the brain endothelium. PACAP induced an increase in the transendothelial electrical resistance, which is the most important marker of the tightness of the tight junctions. Moreover, PACAP had a protective role against glucose deprivation- and oxidative stress-induced junctional damage in microvascular brain endothelial cells [56].
Protective Effects of PACAP in the Retina In Vivo
The in vivo protective effects are summarized in Table 30.2.
Excitotoxic Injury in the Retina
Glutamate, a nonessential amino acid, acts as an excitatory neurotransmitter in the retina under normal conditions. However, glutamate in high concentrations can be toxic to the retinal cells by overstimulation of the glutamate receptors. Monosodium glutamate (MSG) administration given systemically passes the BBB of newborn rats and so it can, can injure the retina [57] and lead to loss of the entire inner retinal layers [58]. The excitotoxic damage of the retina is a main factor in retinal diseases, such as glaucoma and ischemia-induced retinopathy. We have previously shown in a number of studies that PACAP is protective against excitotoxicity induced by systemic monosodium-glutamate lesion. We showed that, three times intravitreal MSG treatment on postnatal days 1, 5 and 9 caused severe neurodegeneration in the inner retinal layers in newborn rat pups [58, 59]. Three weeks after the MSG administration the thickness of the total retina was decreased, the distance of the outer limiting membrane-inner limiting membrane (OLM-ILM) was only half of the normal retina. In addition, the IPL almost disappeared, and the fusion of the INL and GCL could be observed. The number of the cells per 100 μm in the GCL was also decreased [58–61]. Although PACAP1-38 is able to cross the BBB, systemic PACAP1-38 treatment caused insignificant improvement of the retinal morphology following MSG-induced excitotoxic injury [62]. In contrast, intravitreally injected PACAP1-38 (1–100 pmol) resulted in significant amelioration of this degeneration [58, 59, 61]. While 1 pmol PACAP1-38 was slightly effective, treatment with 100 pmol PACAP1-38 was able to significantly attenuate the MSG-induced toxicity and the entire retina seemed to be normal. Similar protection could be seen by PACAP1-27 treatment [60]. The application of the two widely used PACAP antagonists (PACAP6-38, PACAP6-27) led to a more pronounced degeneration, indicating that, endogenously present PACAP plays a key role against retinal toxicity [60]. A similar degree of retinoprotection was found using enriched environment, but combining these two neuroprotective strategies (PACAP and enriched environment) did not result in increased neuroprotection in excitotoxic retinal injury [63].
Cell-type specific effects of PACAP were shown in MSG-induced retinal degeneration by means of immunohistochemistry. In the MSG-treated retinas, the cell bodies and processes in the INL, IPL, and GCL layers displayed less immunoreactivity for vesicular glutamate transporter 1 (VGLUT1), tyrosine hydroxylase (TH), vesicular GABA transporter (VGAT), parvalbumin, and calretinin. After MSG treatment the calbindin-positive horizontal cells did not appear to be affected. In MSG + PACAP1-38 co-treated retinas, the immunoreactivity patterns were similar compared to the normal eyes. These data suggest that PACAP has a general, but not subtype-specific protective mechanism against glutamate-induced excitotoxic injury in the rat retina [64].
We also studied potential protective signaling mechanisms in MSG treated retinas. We found that intravitreal administration of PACAP preceding the MSG treatments induced significant increases in the phosphorylation, that is, the activation of ERK1/2 and its downstream target, CREB, 12 h after the treatment compared to the contralateral untreated eye during the first two treatments, with no further elevations 24 h after treatments. These results demonstrate that the degenerative effect of MSG and the protective effect of PACAP involve complex kinase signaling pathways and are related to cAMP/ERK/CREB activation [65]. Furthermore, after MSG treatment proapoptotic signaling proteins such as caspase-3, JNK, apoptosis inducing factor (AIF) and cytochrome-c increased, and some anti-apoptotic proteins (phospho-PKA, phospho-Bad, Bcl-xL, 14-3-3) decreased. Co-treatment with PACAP1-38 counteracted the MSG-induced apoptotic effects, the level of pro-apoptotic signals was significantly decreased, while the anti-apoptotic proteins were significantly increased [65–67]. A follow-up study confirmed the anti-apoptotic effect of PACAP in MSG-induced retinal lesion of the newborns by reinforcing that PACAP decreased caspase-3 levels and also demonstrating the decreased level of caspase-9 [68]. In addition, we showed that the PACAP antagonist PACAP6-38 increased cytochrome-c release, caspase-3, JNK activity and decreased phospho-Bad, phospho PKA, Bcl-xL, 14-3-3 protein activity [66, 67]. These in vivo observations, similarly to the results of Atlasz et al. [60], indicating that endogenously present PACAP has retinoprotective effects in glutamate-induced excitotoxicity.
We studied the short-term functional consequences of MSG treatment in the mouse retina. Spontaneous and light-evoked spikes of retinal ganglion cells (RGCs) from wild type mice were recorded using a 60-channel multielectrode array. MSG remarkably elevated the free intracellular calcium (Ca2+) concentration and also increased the spontaneous spiking 4–5 min after drug application. During this time, spike correlations between RGC pairs were reduced. However, after 10–15 min of MSG application, the spontaneous activity of most RGCs was dramatically reduced or totally eliminated. Pretreatment with PACAP1-38 prevented the MSG effects as indicated by little or no change in the spontaneous spiking patterns during the course of recordings (up to 60 min). Moreover, the Ca2+ influx was noticeably decreased by PACAP1-38. In addition, MSG blocked the light-evoked responses of all recorded cells. Based on these data, application of PACAP1-38, rescued RGCs from the short-term MSG-induced insults [69].
Kainic acid is a glutamate receptor agonist, which leads to excitotoxic cell death in the CNS, including the retina [43]. It was found that 5 nmol kainic acid caused an extensive loss of AMPA/kainate receptors expressing ganglion cells [70]. Previously we showed that microiontophoretically applied PACAP1-38 could block the excitatory effects of kainic acid in several brain areas in vivo [71]. In the rat retina, pretreatment with 10 pmol intravitreal PACAP1-38 was neuroprotective against kainic-acid induced neurotoxicity, as shown by the attenuated ganglion cell loss [43].
N-methyl-d-aspartate (NMDA) treatment also leads to similar lesions. A radioimmunoassay analysis showed that in contrast to VIP, retinal PACAP levels do not change after intravitreal NMDA injection, which might be caused by a compensatory upregulation [72]. A recent study has confirmed that PACAP-mediated pathways are protective in NMDA-induced retinal lesion. Cheng et al. [73] showed that intravitreal NMDA injection led to a decrease in light aversion, and decreases in the a and b-waves in the electroretinogram accompanied by a photopic negative response. These alterations were reversed by cyclic PACAP1-5 treatment. This treatment also resulted in decreased ganglion cell loss and apoptosis assessed by TUNEL-staining [73].
A similar protective effect was described in mice with PACAP1-38, at a concentration of 100 pmol. The PACAP-attenuated NMDA-induced retinal damage in mice was associated with modulation of the microglia/macrophage status into an acquired deactivation subtype [74]. NMDA injection led to an increase in microglia/macrophage number, while PACAP co-administration attenuated this effect in addition to the diminished ganglion cell loss. The co-injection of the antagonist PACAP6-38 counteracted these protective effects and the beneficial effects were not observed in IL-10 knockout mice. PACAP administration also led to elevated expression of the mRNA for the protective cytokines IL-10 and TGF-β [74]. In summary, these results show that acquired deactivation type of the microglia/macrophage cells favors neuroprotection in the retina and this is stimulated by PACAP.
The question was raised whether these apparent morphological improvements by PACAP administration also lead to functional amelioration in MSG-induced retinal damage [75]. Electroretinographic recordings revealed a marked decrease in both the b- and a-wave values after MSG treatment, reflecting the function of the inner retinal layers and the photoreceptors, respectively. In retinas receiving intravitreal PACAP treatment, these values were significantly increased. Thus, the functional outcome, although not parallel with the morphology, was significantly improved after PACAP treatment. These observations are important from the clinical point of view, showing, that PACAP treatment is able to improve the functional properties of the retina in excitotoxic damage [75].
Ischemic Injury in the Retina
Retina is one of the most sensitive tissues to hypoxia in the human body. Several diseases such as artery occlusion, diabetic retinopathy and high intraocular pressure cause hypoperfusion in the retina. Depending on the rat strain, permanent bilateral common carotid artery occlusion (BCCAO) leads to reduction in the cerebral blood flow, causes hypoperfusion-induced biochemical and behavioral changing and also produces retinal degeneration [76, 77]. Our research group showed in a series of experiments that intravitreal PACAP is highly protective in ischemic retinal lesion. We found that BCCAO caused severe damage in all layers of the retina. Two weeks after permanent carotid artery ligation, the thickness of the plexiform layers was markedly decreased, and as a consequence, the distance between the OLM-ILM was significantly less compared to the control retina. In several treated animals, in the IPL some dots of about 1 μm in diameter were seen. Based on the relatively even distribution, these dots seemed to be degenerating bipolar cell terminals. In addition, the outer segments of the photoreceptors became shorter, and their structure was also damaged after the insult [76]. Intravitreal PACAP1-38 treatment following BCCAO led to a nearly intact appearance of the retinal layers, which is well reflected by the morphometric analysis. The thickness of all retinal layers, but not the number of cells in the GCL, was preserved in PACAP1-38 treated animals. PACAP antagonist PACAP6-38 significantly attenuated the protective effects of PACAP1-38.
In 2010, we provided immunohistochemical description of several retinal cell types that were damaged by BCCAO and could be partially or fully rescued by intravitreal PACAP1-38 treatment. Using the cell type specific markers (VGLUT1, TH, VGAT, parvalbumin, calbindin, calretinin, PKCα, and GFAP) marked changes were detected after BCCAO, and the alterations observed in the immunostaining for all examined antisera were counteracted by intravitreal PACAP1-38 treatment [13]. These studies provided the basis for the promising therapeutic potential of PACAP in ischemic retinal diseases [78].
Subsequent studies confirmed these original observations. Seki et al. [79] showed that intravitreal PACAP treatment protected ganglion cells against a different kind of ischemic lesion induced by high intraocular pressure. In this model, PACAP exerted a bimodal protective effect with peaks at 10 fM and 10–100 pM. The protective effect was blocked by cAMP antagonist at both high and low protective doses, while a MAP kinase inhibitor only blocked the effects of PACAP at a low dose, suggesting that the protective effects of PACAP are mediated by different signaling at low and high doses [79].
In a series of studies, we confirmed these findings and compared the efficacy of several analogs and related peptides to PACAP1-38 [80, 81]. We investigated the effects of the following PACAP fragments: PACAP 4-13, 4-22, 6-10, 6-15, 11-15, and 20-31 and three related peptides (VIP, secretin, glucagon) in BCCAO-induced ischemic retinopathy. We confirmed that the most effective molecule is PACAP1-38, while the other fragments or related peptides did not lead to retinoprotection [81]. The only exception was VIP, which showed retinoprotection in a dose ten times higher than that of PACAP [80]. In addition, our preliminary results demonstrate that even in form of eye-drops, PACAP can lead to retinoprotective effects in ischemic retinopathy, a route of application with major possible clinical relevance [82].
In order to elucidate some of the molecular mechanisms, we studied MAP kinases and Akt expression as well as cytokines in BCCAO-induced retinal ischemia in rats [83]. We revealed that PACAP treatment alone did not influence the phosphorylation of Akt or the MAPKs, but decreased the hypoperfusion-induced activation of both p38MAPK and JNK and increased the activation of the protective Akt and ERK1/2 in hypoperfused retinas. The cytokine profile dramatically changed after BCCAO, with most cytokines and chemokines (such as CINC, CNTF, fractalkine, sICAM, IL-1, LIX, Selectin, MIP-1, RANTES, and TIMP-1) increased, a result attenuated by PACAP. In addition, PACAP increased the expression of vascular endothelial growth factor (VEGF) and thymus chemokine. These results provided further insight into the neuroprotective mechanism induced by PACAP in ischemic retinal injuries, showing that PACAP ameliorated hypoperfusion injury involving Akt, MAPK pathways and anti-inflammatory actions [83].
Whether the morphological amelioration is accompanied by functional improvement was tested using electroretinography (ERG) in another set of experiments [84]. Retinal damage and protective effects of PACAP were quantified by the changes in the waveforms and amplitudes. BCCAO-induced functional retinal degeneration was already observed on postoperative days 2 and lasted throughout the entire observation period. Intravitreal injection of PACAP immediately after BCCAO resulted in a more retained retinal function as assessed by average ERG waveforms compared to the BCCAO-operated groups on both postoperative 2nd and 14th days. ERGs of PACAP-treated ischemic eyes were similar to the intact controls in contrast to the ERGs of saline-treated BCCAO retinas. PACAP treatment significantly counteracted the ischemia-induced alterations in the amplitudes of both the “a” and “b” waves of the ERG on postoperative day 14. The elapse time of the major oscillatory potentials (OPs) was reduced in the BCCAO-ischemic group, but PACAP treatment led to significant protection. The results confirm that the previously described morphological protection induced by PACAP treatment is reflected in functional improvement in ischemic retinal lesions [84].
A recent study has revealed several metabolomics changes induced by PACAP in ex vivo retinal ischemia induced by sodium azide treatment for various periods of time [85]. PACAP induced several changes in addition to the reduction of ischemia-induced cell death, VEGF overexpression, and glutamate release. Apoptosis, as evaluated by TUNEL staining, was reduced in the ONL, INL, and GCL. Elevated levels of caspase-3 mRNA were also reversed by PACAP treatment. Glutamine, a precursor of glutamate, was significantly elevated in ischemic conditions, and reversed by PACAP administration. Ischemia is associated with oxidative injury and so the authors evaluated the oxidative status of the retinal explants. It was found that PACAP had a positive effect on glutathione while it reduced the oxidized glutathione levels, and counteracted the increased gamma-glutamyl cysteins levels with accompanying increase of cysteine. The accumulation of pro-inflammatory factors and peroxidized lipids was prevented by PACAP. Three nitric oxide metabolites were found to be upregulated in ischemic retinas: arginine, ornithine and citrulline. Treatment with PACAP reversed these changes. The significantly decreased level of cAMP was restored by PACAP treatment and glycolytic flues were normalized preventing the over-accumulation of lactate or promoting the downregulation of the glyoxalate antioxidant system. Upregulated inositol levels were also abolished. PACAP seemed to promote a shift towards pentose-phosphate pathway while maintaining higher levels of NADPH compared to ischemic controls. It also led to a downregulation of purine metabolism. This study concluded that peptidergic systems, such as PACAP, deserve attention in treating retinal ischemia [85].
Thanks to all these results, PACAP is now on the list of potential neuroprotective molecules in combating ischemic retinopathy [78, 86].
Optic Nerve Injury in the Retina
Optic nerve transection is a model of apoptotic neuronal cell death in the adult CNS [87]. Seki et al. [88] reported that optic nerve trauma resulted approximately 55 % loss of cells in the GCL. In this model, the number of cells in the GCL in animals treated before the operation with PACAP1-38 at 10 and 100 pmol in 3 μl saline (dose-dependent) intravitreally, was significantly increased compared with the control group on the 14th day after surgery [88], providing evidence that PACAP is protective in this injury model.
UV Radiation Injury in the Retina
We showed, for the first time, that PACAP protected against two different kinds (diffused and focused) of ultraviolet (UV)-A induced degenerations. Thickness of all layers as well as the number of cells in the ONL and INL and in the GCL was evaluated by standard morphological and morphometrical analyses. After diffuse UV-A irradiation, significant decrease of the number of the cells in the GCL was observed. In addition, severe degeneration in the ONL and INL was seen, and the IPL was swollen. PACAP1-38 treatment after the UV-A irradiation significantly ameliorated the light-induced degeneration, the ONL and INL thickness seemed to be normal, and the number of cells in INL, ONL, and GCL was significantly increased [89]. In a model of focused UV-A irradiation, the shape of the laser focus was observed on the retina, tissue gaps were found in the ONL and in the photoreceptor layer, and the IPL was reduced. The total thickness of the retina was significantly less than in unaffected areas. We observed protective effects of PACAP in all of the different retinal layers [90].
Succeeding studies confirmed this effect in several different models. In an in vitro model of UV-B irradiation, PACAP was shown to protect RGC-5 retinal ganglion cells expressing PAC1 receptors [73, 91]. This effect was mimicked also by a cyclic PACAP derivative, cyclic PACAP1-5 [73]. Furthermore, pretreatment with this short derivative effectively blocked apoptosis, maintained structural integrity, and decreased production of reactive oxygen species (ROS). Both PACAP and cyclic PACAP1-5 counteracted the UV-B-induced decrease in the protective Bcl-2 and the increase in the deleterious Bax protein levels [73].
Diabetic Retinopathy in the Retina
Our recent studies have shown that PACAP is also protective in diabetic retinopathy (DR), a major microangiopathy affecting the vision of diabetic patients. Our first results demonstrated that in the early stages of diabetic retinopathy, amacrine cells underwent characteristic degeneration, especially the dopaminergic amacrine cells. PACAP prevented the typical alterations induced in 1-month diabetes: the degeneration in arborization and synapses of tyrosine-hydroxylase positive dopaminergic amacrine cells [13]. In a rat model of streptozotocin-induced diabetic retinopathy, early signs do not include overall changes in thickness of retinal layers, but the decrease in the number of ganglion cells and the upregulation of GFAP as a sign of Müller glial cell activation, can be observed. These changes were counteracted by three times 100 pmol/5 μl intravitreal PACAP administration [92]. Furthermore, the degeneration of the photoreceptor outer segments and terminals was attenuated in PACAP-treated rats. PACAP increased the levels of PAC1-receptor and TH determined with molecular biological methods. These early results suggested a therapeutic potential in DR.
Subsequently, our research group and others confirmed these early findings [93]. Intraocular PACAP injection markedly attenuated diabetic retinal injury: increased levels of the anti-apoptotic p-Akt, p-ERK1, p-ERK2, PKC, Bcl-2, while decreased levels of the pro-apoptotic p-p38MAPK and activated caspases (8, 3, 12) were detected. The number of apoptotic cells increased in all nuclear layers of diabetic retinas, but significantly decreased after PACAP treatment. Our results clearly demonstrated that the protective effects of PACAP are mediated, at least partly, by attenuating apoptosis, including also that of the dopaminergic amacrine cells [93]. Our most recent study shed further light on this PACAP-induced retinoprotection. Electron microscopic analysis revealed that retinal pigment epithelium, the ribbon synapses and other synaptic profiles suffered alterations in diabetes. However, in PACAP-treated diabetic retinas more bipolar ribbon synapses were found intact in the inner plexiform layer than in DR animals. The ribbon synapse was marked with C-terminal binding protein 2 (CtBP2)/Bassoon and formed horseshoe-shape ribbons, which were more retained in PACAP-treated diabetic retinas than in DR rats. These results are supported by molecular biological data. The selective degeneration of related structures such as bipolar and ganglion cells could be ameliorated by PACAP treatment. In summary, intravitreal administration of PACAP may have therapeutic potential in streptozotocin-induced DR through maintaining synapse integrity in the vertical pathway [94].
D’Amico and coworkers [95] analyzed the expression of hypoxia-induced factors (HIF) in DR 3 weeks after the induction of diabetes with streptozotocin. They found that the expression of HIF-1α and HIF-2α significantly increased in diabetic rats, but not after 1 intravitreal PACAP treatment. Conversely, the expression of HIF-3α was significantly downregulated in retinas of diabetic rats, and increased after PACAP treatment. These results suggest that the retinoprotective effects of PACAP are partially mediated by interfering with the expression of HIFs that play an important role in pathological vasculogenesis occurring in DR [95].
These studies, together with the results demonstrating the effects of PACAP on the blood–retinal barrier properties in vitro (see above), show that PACAP has therapeutic potential in DR [96–98]. Several experiments support the efficacy of PACAP treatment in animal models of diabetes-related diseases, such as DR, another major microangiopathic complication of diabetes [99, 100]. Thus, PACAP is among the emerging molecules to fight diabetic complications, similarly to VEGF antagonists, antioxidants, anti-inflammatory agents, and other neuropeptides [96, 101, 102].
Retinopathy of Prematurity (ROP)
ROP is a major complication in premature newborns. It is a vasoproliferative disorder, the main features of which are well depicted in a rat model of oxygen-induced retinopathy. Our preliminary studies have shown that PACAP is able to reverse some of the deleterious effects of this retinopathy. Intravitreal PACAP1-38 treatment ameliorated the vascular extent of the retinopathic eyes without alterations in vessel morphology. Moreover, cytokine expression profiles indicate that PACAP may block the new vessel formation and have a positive influence on the remodeling of the tissue [103].
Studies in PACAP and Receptor Gene Modified Animals
We have described that although PACAP deficient mice have normal retinas at young age, aging signs appear earlier than in their wild-type mates [104]. As it has been shown in numerous models, PACAP knockout (KO) mice are more vulnerable to different types of tissue injuries from ischemia to oxidative stress and even trauma [105]. In our experiments, PACAP KO mice that underwent 10 min of BCCAO followed by 2-week reperfusion period had significantly greater retinal damage, as shown by the thickness of the whole retina, the morphometric analysis of the individual retinal layers, and the cell numbers in the INL and GCL. Exogenous PACAP administration could partially protect against this retinal degeneration in the KO mice. These results clearly show that endogenous PACAP reacts as a stress-response peptide that is necessary for endogenous protection against different retinal insults [106].
The higher vulnerability of PACAP deficient mice against lesions was confirmed by another study by Endo et al. [107]. The authors found, in accordance with our results, that under normal circumstances, there was no difference in the number of retinal ganglion cells in heterozygous PACAP deficient mice and wild-type counterparts [107]. However, when these mice were subjected to NMDA-induced toxicity, PACAP heterozygous mice reacted with increased loss of ganglion cells [107]. The number of apoptotic cells, as measured by TUNEL staining, peaked on day 1 in heterozygous mice, while the peak was on day 3 in wild types, suggesting that apoptotic cell death began earlier in mice deficient in PACAP. These effects were reversed by simultaneous injection of PACAP in heterozygous mice. These results confirm that endogenous PACAP is protective in the retina and prove that even partial loss of PACAP-driven neuroprotection decreases resistance against harmful effects [107]. Our recent, preliminary results also confirm that endogenous PACAP is protective in lipopolysaccharide (LPS)-induced retinal inflammation, as shown by the increased inflammatory status of PACAP KO. Twenty-four hours after LPS treatment several cytokines and chemokines showed significant increase in the PACAP knockout mice compared to the normal, wild types [108].
Little is known about retinal morphology or function in mice with PAC1 receptor abnormalities. Engelund and coworkers [109] described that pupillary light reflex was altered in PAC1 receptor knockout mice. The finding that PACAPergic signaling is essential for normal retinal development is also confirmed by studies with transgenic mice. It was described by Lang et al. [110] during development that overexpression of the PAC1 receptor led to an early exit from retinal proliferation, reduced signaling of GABAergic neurons. Furthermore, this led to decreased visual function [110]. These data show that a proper balance of PACAP signaling is required for appropriate retinogenesis and visual acuity.
Studies in Tumor Cells
PACAP can exert different effects in cells derived from various sources and especially from tumors. While in most cell types PACAP is protective, in some tumors it exerts toxic effects [111]. Furthermore, PACAP6-38 is in most cell types an antagonist, it can also behave as an agonist in some, tumorous and even non-tumorous, cells [112, 113]. Retinoblastoma is a malignant tumor of retinal progenitor cells. Human retinoblastoma cells are known to express PACAP receptors [114]. In Y79 retinoblastoma cells, PACAP1-38 did not influence cell survival at nanomolar concentrations, but at 1–5 μM concentrations, it reduced survival [115]. Interestingly, PACAP1-27 and maxadilan had negligible effects, while two membrane-penetrating analogs of PACAP also decreased cell viability. The authors also revealed that this cytotoxic effect might be via an interaction of p38, MEK1/2, and JNK signaling, because inhibitors of these pathways led to an even more expressed cytotoxicity [115]. These results suggest that high concentrations of PACAP1-38 might lead to a transmembrane penetration and lead to direct cytotoxicity at sites other than the conventional PACAP receptor-mediated signaling.
Abbreviations
- 6-OHDA:
-
6-hydroxydopamine
- AIF:
-
Apoptosis inducing factor
- AMPA receptor:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- Bad:
-
Bcl-2-associated death promoter
- Bax:
-
Bcl-2-associated X protein
- BBB:
-
Blood–brain barrier
- BCCAO:
-
Bilateral common carotid artery occlusion
- Bcl-2:
-
B-cell lymphoma 2
- Bcl-xl:
-
B-cell lymphoma-extra large
- cAMP/PKA/CREB:
-
Cyclic AMP/protein-kinase A/cAMP response element-binding protein
- CIAP-1:
-
Cellular inhibitor of apoptosis protein-1
- CINC:
-
Cytokine-induced neutrophil chemoattractant
- CNS:
-
Central nervous system
- CNTF:
-
Ciliary neurotrophic factor
- CtBP2:
-
C-terminal binding protein 2
- DR:
-
Diabetic retinopathy
- eNOS:
-
Endothelial nitric oxide synthase
- ERG:
-
Electroretinography
- ERK:
-
Extracellular signal-regulated kinase
- Fas TNFR:
-
Fas tumor necrosis factor receptor
- FITC:
-
Fluorescein isothiocyanate
- GCL:
-
Ganglion cell layer
- GFAP:
-
Glial fibrillary acidic protein
- HIF1α, 2α, 3α:
-
Hypoxia-inducible factor 1-alpha, 2-alpha, 3-alpha
- HO-1:
-
Heme oxygenase-1
- HSP-27:
-
Heat shock protein-27
- IL-1β:
-
Interleukin-1 beta
- INL:
-
Inner nuclear layer
- IP3:
-
Inositol trisphosphate
- IPL:
-
Inner plexiform layer
- JNK:
-
c-Jun N-terminal kinase
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MIP-1:
-
Macrophage inflammatory protein 1
- MSG:
-
Monosodium glutamate
- NBL:
-
Neuroblast layer
- NF-kB:
-
Nuclear factor-kB
- NFL:
-
Nerve fiber layer
- NMDA:
-
N-methyl-d-aspartate
- NPY:
-
Neuropeptide Y
- OLM-ILM:
-
Outer limiting membrane-inner limiting membrane
- ONL:
-
Outer nuclear layer
- OPL:
-
Outer plexiform layer
- Ops:
-
Oscillatory potentials
- PAC1:
-
PACAP receptor 1
- PACAP:
-
Pituitary adenylate cyclase activating polypeptide
- PACAP KO:
-
PACAP knockout
- PKCα:
-
Protein kinase C alpha
- PLC gamma-1:
-
Phospholipase C gamma-1
- RANTES:
-
Regulated on activation normal T cell expressed and secreted
- RGCs:
-
Retinal ganglion cells
- ROP:
-
Retinopathy of prematurity
- ROS:
-
Reactive oxygen species
- RPE:
-
Retinal pigment epithelial
- RSK1/2:
-
Ribosomal s6 kinase 1, 2
- sICAM:
-
Soluble intercellular adhesion molecule
- STAT4:
-
Signal transducer and activator of transcription 4
- TGF-β:
-
Transforming growth factor beta
- TH:
-
Tyrosine hydroxylase
- TIMP-1:
-
Tissue inhibitor metallopeptidase inhibitor-1
- Trail R2 DR5:
-
TRAIL receptor 2—death receptor 5
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UV-A, B:
-
Ultraviolet A, ultraviolet B
- VEGF:
-
Vascular endothelial growth factor
- VGAT:
-
Vesicular GABA transporter
- VGLUT1:
-
Vesicular glutamate transporter 1
- VIP:
-
Vasoactive intestinal peptide
- VPAC1:
-
Vasoactive intestinal peptide (VIP) receptor type 1
- VPAC2:
-
Vasoactive intestinal peptide (VIP) receptor type 2
- ZO-1:
-
Zona occludens protein 1
References
Arimura A. PACAP: the road to discovery. Peptides. 2007;28:1617–9.
Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74.
Arimura A, Shioda S. Pituitary adenylate cyclase polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interactions. Front Neuroendocrinol. 1995;16:53–8.
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357.
Ohtaki H, Nakamachi T, Dohi K, Shioda S. Role of PACAP in ischemic neural death. J Mol Neurosci. 2008;36:16–25.
Reglodi D, Kiss P, Lubics A, Tamas A. Review of the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–72.
Reglodi D, Renaud J, Tamas A, Tizabi Y, Socias B, Del-Bel E, et al. Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol. doi: 10.1016/j.pneurobio.2015.10.004. Accessed 2 Nov 2015.
Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, et al. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci. 2006;1070:550–60.
Shioda S, Nakamachi T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides. 2015;72:202–7.
Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Rev Curr Pharm Des. 2004;10:2861–89.
Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci. 2002;24:14–23.
Atlasz T, Szabadfi K, Kiss P, Racz B, Gallyas F, Tamas A, et al. Pituitary adenylate cyclase activating polypeptide in the retina: focus on the retinoprotective effects. Ann N Y Acad Sci. 2010;1200:128–39.
Atlasz T, Szabadfi K, Kiss P, Tamas A, Toth G, Reglodi D, et al. Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res Bull. 2010;81:497–504.
Nemeth A, Szabadfi K, Fulop B, Reglodi D, Kiss P, Farkas J, et al. Examination of calcium-binding protein expression in the inner ear of wild type, heterozygous and homozygous pituitary adenylate cyclase activating polypeptide (PACAP)-knockout mice in kanamycin-induced ototoxicity. Neurotox Res. 2014;25:57–67.
Nakamachi T, Matkovits A, Seki T, Shioda S. Distribution and protective function of pituitary adenylate cyclase-activating polypeptide in the retina. Front Endocrinol (Lausanne). 2012;3:145.
D’Agata V, Cavallaro S. Functional and molecular expression of PACAP/VIP receptors in the rat retina. Mol Brain Res. 1998;54:161–4.
Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004;316:99–113.
Izumi S, Seki T, Shioda S, Zhou CJ, Arimura A, Koide R. Ultrastructural localization of PACAP immunoreactivity in the rat retina. Ann NY Acad Sci. 2000;921:317–20.
Kubrusly RC, da Cunha MC, Reis RA, Soares H, Ventura AL, Kurtenbach E, et al. Expression of functional receptors and transmitter enzymes in cultured Muller cells. Brain Res. 2005;1038:141–9.
Seki T, Shioda S, Izumi S, Arimura A, Koide R. Electron microscopic observation of pituitary adenylate cyclase activating polypeptide (PACAP)-containing neurons in the rat retina. Peptides. 2000;21:109–13.
Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefe’s Arch Clin Exp Ophthalmol. 2007;245:267–75.
Grone BP, Zhao S, Chen CC, Fernald RD. Localization and diurnal expression of melanopsin, vertebrate ancient opsin, and pituitary adenylate cyclase activating peptide mRNA in a teleost retina. J Biol Rhythms. 2007;22:558–61.
Reglodi D, Somogyvari-Vigh A, Vigh J, Li M, Lengvari I, Arimura A. Pituitary adenylate cyclase activating polypeptide is highly abundant in the nervous system of anoxia-tolerant turtle, Pseudemys scripta elegans. Peptides. 2001;22:873–8.
Jozsa R, Somogyvari-Vigh A, Reglodi D, Hollosy T, Arimura A. Distribution and daily variations of PACAP in the chicken brain. Peptides. 2001;22:1371–7.
Sakamoto K, Liu C, Kasamatsu M, Pozdeyev NV, Iuvone PM, Tosini G. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–36.
Wan J, Zheng H, Hu BY, Xiao HL, She ZJ, Chen ZL, et al. Acute photoreceptor degeneration down-regulates melanopsin expression in adult rat retina. Neurosci Lett. 2006;400:48–52.
Perez de Sevilla Muller L, Sargoy A, Rodriguez AR, Brecha NC. Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina. PLoS One. 2014;9:e93274.
Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, Gamlin PD. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014;522:2231–48.
La Morgia C, Ross-Cisneros FN, Hannibal J, Montagna P, Sadun AA, Carelli V. Melanopsin-expressing retinal ganglion cells: implications for human diseases. Vision Res. 2011;51:296–302.
Langel JL, Smale L, Esquiva G, Hannibal J. Central melanopsin projections in the diurnal rodent. Arvicanthis niloticus. Front Neuroanat. 2015;9:93.
Delwig A, Majumdar S, Ahern K, LaVail MM, Edwards R, Hnaski TS, et al. Glutamatergic neurotransmission from melanopsin retinal ganglion cells is required for neonatal photoaversion but not adult pupillary light reflex. PLoS One. 2013;8:e83974.
Purrier N, Engeland WC, Kofuji P. Mice deficient of glutamatergic signaling from intrinsically photosensitive retinal ganglion cells exhibit abnormal circadian photoentrainment. PLoS One. 2014;9:e111449.
Vereczki V, Koves K, Csaki A, Grosz K, Hoffman GE, Fiskum G. Distribution of hypothalamic, hippocampal and other limbic peptidergic neuronal cell bodies giving rise to retinopetal fibers: anterograde and retrograde tracing and neuropeptide immunohistochemical studies. Neuroscience. 2006;140:1089–100.
Bagnoli P, Dal Monte M, Casini G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol Histopathol. 2003;18:1219–42.
Borba JC, Henze IP, Silveira MS, Kubrusly RC, Gardino PF, de Mello MC, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) can act as a determinant of the tyrosine hydroxylase phenotype of dopaminergic cells during retina development. Dev Brain Res. 2005;156:193–201.
Mathieu M, Girosi L, Vallarino M, Tagliafierro G. PACAP in developing sensory and peripheral organs of the zebrafish, Danio rerio. Eur J Histochem. 2005;49:167–78.
Mathieu M, Ciarlo M, Trucco N, Griffero F, Damonte G, Salis A, et al. Pituitary adenylate cyclase activating polypeptide in the brain, spinal cord and sensory organs of the zebrafish, Danio rerio, during development. Brain Res Dev Brain Res. 2004;151:169–85.
Skoglosa Y, Takei N, Lindholm D. Distribution of pituitary adenylate cyclase activating polypeptide mRNA in the developing rat brain. Mol Brain Res. 1999;65:1–13.
Nilsson SF, De Neef P, Robberecht P, Christophe J. Characterization of ocular receptors for pituitary adenylate cyclase activating polypeptide (PACAP) and their coupling to adenylate cyclase. Exp Eye Res. 1994;58:459–67.
Silveira MS, Costa MR, Bozza M, Linden R. Pituitary adenylate cyclase activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J Biol Chem. 2002;277:16075–80.
Seki T, Shioda S, Ogino D, Nakai Y, Arimura A, Koide R. Distribution and ultrastructural localization of a receptor for pituitary adenylate cyclase activating polypeptide and its mRNA in the rat retina. Neurosci Lett. 1997;238:127–30.
Seki T, Izumi S, Shioda S, Zhou CJ, Arimura A, Koide R. Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Ann N Y Acad Sci. 2000;921:366–9.
Seki T, Nakatani M, Taki C, Shinohara Y, Ozawa M, Nishimura S, et al. Neuroprotective effects of PACAP against kainic acid-induced neurotoxicity in rat retina. Ann N Y Acad Sci. 2006;1070:531–4.
Njaine B, Martins RA, Santiago MF, Linden R, Silveira MS. Pituitary adenylyl cyclase-activating polypeptide controls the proliferation of retinal progenitor cells through downregulation of cyclin D1. Eur J Neurosci. 2010;32:311–21.
Denes V, Czotter N, Lakk M, Berta G, Gabriel R. PAC1-expressing structures of neural retina alter their PAC1 isoform splicing during postnatal development. Cell Tissue Res. 2014;355:279–88.
Lakk M, Szabo B, Volgyi B, Gabriel R, Denes V. Development-related splicing regulates pituitary adenylate cyclase-activating polypeptide (PACAP) receptors in the retina. Invest Ophthalmol Vis Sci. 2012;53:7825–32.
Njaine B, Rocha-Martins M, Vieira-Vieira CH, De-Melo LD, Linden R, Braas K, et al. Pleiotropic functions of pituitary adenylyl cyclase-activating polypeptide on retinal ontogenesis: involvement of KLF4 in the control of progenitor cell proliferation. J Mol Neurosci. 2014;54:430–42.
Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H, et al. Protective effects of vasoactive intestinal peptide against delayed glutamate neurotoxicity in cultured retina. Brain Res. 1998;809:127–36.
Shoge K, Mishima HK, Saitoh T, Ishihara K, Tamura Y, Shiomi H, et al. Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Res. 1999;839:66–73.
Rabl K, Reglodi D, Banvolgyi T, Somogyvari-Vigh A, Lengvari I, Gabriel R, et al. PACAP inhibits anoxia-induced changes in physiological responses in horizontal cells in the turtle retina. Regul Pept. 2002;109:71–4.
Zhang XY, Hayasaka S, Chi ZL, Cui HS, Hayasaka Y. Effect of pituitary adenylate cyclase-activating polypeptide (PACAP) on IL-6, IL-8, and MCP-1 expression in human retinal pigment epithelial cell line. Curr Eye Res. 2005;30:1105–11.
Mester L, Kovacs K, Racz B, Solti I, Atlasz T, Szabadfi K, et al. Pituitary adenylate cyclase-activating polypeptide is protective against oxidative stress in human retinal pigment epithelial cells. J Mol Neurosci. 2011;43:35–43.
Fabian E, Reglodi D, Mester L, Szabo A, Szabadfi K, Tamas A, et al. Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J Mol Neurosci. 2012;48:493–500.
Ko JA, Hirata J, Yamane K, Sonoda KH, Kiuchi Y. Up-regulation of semaphorin 4A expression in human retinal pigment epithelial cells by PACAP released from cocultured neural cells. Cell Biochem Funct. 2015;33:29–36.
Scuderi S, D’Amico AG, Castorina A, Imbesi R, Carnazza ML, D’Agata V. Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides. 2013;39:119–24.
Wilhelm I, Fazakas C, Tamas A, Toth G, Reglodi D, Krizbai IA. PACAP enhances barrier properties of cerebral microvessels. J Mol Neurosci. 2014;54:469–76.
Szabadfi K, Atlasz T, Horvath G, Kiss P, Hamza L, Farkas J, et al. Early postnatal enriched environment decreases retinal degeneration induced by monosodium glutamate treatment. Brain Res. 2009;1259:107–12.
Babai N, Atlasz T, Tamas A, Reglodi D, Toth G, Kiss P, et al. Search for the optimal monosodium glutamate treatment schedule to study the neuroprotective effects of PACAP in the retina. Ann N Y Acad Sci. 2006;1070:149–55.
Babai N, Atlasz T, Tamas A, Reglodi D, Kiss P, Gabriel R. Degree of damage compensation by various PACAP treatments in monosodium glutamate-induced retina degeneration. Neurotox Res. 2005;8:227–33.
Atlasz T, Szabadfi K, Reglodi D, Kiss P, Tamas A, Toth G, et al. Effects of pituitary adenylate cyclase activating polypeptide (PACAP1-38) and its fragments on retinal degeneration induced by neonatal MSG treatment. Ann N Y Acad Sci. 2009;1163:348–52.
Tamas A, Gabriel R, Racz B, Denes V, Kiss P, Lubics A, et al. Effects of pituitary adenylate cyclase activating polypeptide in retinal degeneration induced by monosodium-glutamate. Neurosci Lett. 2004;372:110–3.
Kiss P, Tamas A, Lubics A, Lengvari I, Szalai M, Hauser D, et al. Effects of systemic PACAP treatment in monosodium glutamate-induced behavioral changes and retinal degeneration. Ann N Y Acad Sci. 2006;1070:365–70.
Kiss P, Atlasz T, Szabadfi K, Horvath G, Griecs M, Farkas J, et al. Comparison between PACAP- and enriched environment-induced retinal protection in MSG-treated newborn rats. Neurosci Lett. 2011;487:400–5.
Atlasz T, Szabadfi K, Kiss P, Babai N, Koszegi Z, Tamas A, et al. PACAP-mediated neuroprotection of neurochemically identified cell types in MSG-induced retinal regeneration. J Mol Neurosci. 2008;36:97–104.
Racz B, Tamas A, Kiss P, Toth G, Gasz B, Borsiczky B, et al. Involvement of ERK and CREB signaling pathways in the protective effect of PACAP in monosodium glutamate-induced retinal lesion. Ann N Y Acad Sci. 2006;1070:507–11.
Racz B, Gallyas Jr F, Kiss P, Toth G, Hegyi O, Gasz B, et al. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involves inhibition of proapoptotic signaling pathways. Regul Pept. 2006;137:20–6.
Racz B, Gallyas Jr F, Kiss P, Tamas A, Lubics A, Lengvari I, et al. Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotox Res. 2007;12:95–104.
Lakk M, Denes V, Gabriel R. Pituitary adenylate cyclase-activating polypeptide receptors signal via phospholipase C pathway to block apoptosis in newborn rat retina. Neurochem Res. 2015;40:1402–9.
Atlasz T, Balogh M, Werling D, Zhang Y, Reglodi D, Bloomfield S, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) prevents monosodium glutamate (MSG) induced functional disturbances in the mouse retina. Presented at 9th FENS Forum of Neuroscience July 5–9, 2014, Milan, Italy.
Zhang D, Sucher NJ, Lipton SA. Co-expression of AMPA/kainate receptor-operated channels with high and low Ca2+ permeability in single rat retinal ganglion cells. Neuroscience. 1995;67:177–88.
Atlasz T, Koszegi Z, Babai N, Tamas A, Reglodi D, Kovacs P, et al. Microiontophoretically applied PACAP blocks excitatory effects of kainic acid in vivo. Ann N Y Acad Sci. 2006;1070:143–8.
Teuchner B, Dimmer A, Humpel C, Amberger A, Fischer-Colbrie R, Nemeth J, et al. VIP, PACAP-38, BDNF and ADNP in NMDA-induced excitotoxicity in the rat retina. Acta Ophthalmol. 2011;89:670–5.
Cheng H, Ding Y, Yu R, Chen J, Wu C. Neuroprotection of a novel cyclopeptide C*HSDGIC* from the cyclization of PACAP (1-5) in cellular and rodent models of retinal ganglion cell apoptosis. PLoS One. 2014;9, e108090.
Wada Y, Nakamachi T, Endo K, Seki T, Ohtaki H, Tsuchikawa D, et al. PACAP attenuates NMDA-induced retinal damage in association wit modulation of the microglia/macrophage status into an acquired deactivation subtype. J Mol Neurosci. 2013;51:493–502.
Varga B, Szabadfi K, Kiss P, Fabian E, Tamas A, Griecs M, et al. PACAP improves functional outcome in excitotoxic retinal lesion: an electroretinographic study. J Mol Neurosci. 2011;43:44–50.
Atlasz T, Babai N, Kiss P, Reglodi D, Tamas A, Szabadfi K, et al. Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen Comp Endocrinol. 2007;153:108–14.
Mester L, Szabo A, Atlasz T, Szabadfi K, Reglodi D, Kiss P, et al. Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox Res. 2009;16:68–76.
Szabadfi K, Mester L, Reglodi D, Kiss P, Babai N, Racz B, et al. Novel neuroprotective strategies in ischemic retinal lesions. Int J Mol Sci. 2010;11:544–61.
Seki T, Itoh H, Nakamachi T, Endo K, Wada Y, Nakamura K, et al. Suppression of rat retinal ganglion cell death by PACAP following transient ischemia induced by high intraocular pressure. J Mol Neurosci. 2011;43:30–4.
Szabadfi K, Danyadi B, Kiss P, Tamas A, Fabian E, Gabriel R, et al. Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J Mol Neurosci. 2012;48:501–7.
Werling D, Reglodi D, Kiss P, Toth G, Szabadfi K, Tamas A, et al. Investigation of PACAP fragments and related peptides in chronic retinal hypoperfusion. J Ophthalmol. 2014:563812.
Werling D, Kvarik T, Varga R, Nagy N, Mayer F, Vaczy A, et al. Investigating the retinoprotective effects of PACAP eye-drop in ischemic retinopathy. Presented at 12th International Symposium on VIP, PACAP and Related Peptides, September 21–26, 2015, Cappadocia, Turkey.
Szabo A, Danyadi B, Bognar E, Szabadfi K, Fabian E, Kiss P, et al. Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci Lett. 2012;523:93–8.
Danyadi B, Szabadfi K, Reglodi D, Mihalik A, Danyadi T, Kovacs Zs, et al. PACAP application improves functional outcome of chronic retinal ischemic injury in rats—evidence from electroretinographic measurements. J Mol Neurosci. 2014;54:293–9.
D’Alessandro A, Cervia D, Catalani E, Gevi F, Zolla L, Casini G. Protective effects of the neuropeptides PACAP, substance P and the somatostatin analogue octreotide in retinal ischemia: a metabolomic analysis. Mol Biosyst. 2014;10:1290–304.
Cervia D, Casini G. The neuropeptide systems and their potential role in the treatment of mammalian retinal ischemia: a developing story. Curr Neuropharmacol. 2013;11:95–101.
Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–86.
Seki T, Itoh H, Nakamachi T, Shioda S. Suppression of ganglion cell death by PACAP following optic nerve transection in the rat. J Mol Neurosci. 2008;36:57–60.
Atlasz T, Szabadfi K, Kiss P, Marton Z, Griecs M, Hamza L, et al. Effects of PACAP in UV-A radiation-induced retinal degeneration models in rats. J Mol Neurosci. 2011;43:51–7.
Atlasz T, Szabadfi K, Molnar A, Kiss P, Reglodi D, Marton ZS, et al. PACAP protects rat retina from UV-A radiation-induced degeneration. Presented at the Satellite Symposium of the 9th International Symposium on VIP, PACAP and Related Peptides, October 2–3, 2009, Yakushima, Japan.
Ding Y, Cheng H, Yu R, Tang C, Liu X, Chen J. Effects of cyclopeptide C*HSDGIC* from the cyclization of PACAP (1-5) on the proliferation and UVB-induced apoptosis of the retinal ganglion cell line RGC-5. Peptides. 2012;36:280–5.
Szabadfi K, Atlasz T, Kiss P, Reglodi D, Szabo A, Kovacs K, et al. Protective effects of the neuropeptide PACAP in diabetic retinopathy. Cell Tissue Res. 2012;348:37–46.
Szabadfi K, Szabo A, Kiss P, Reglodi D, Setalo Jr G, Kovacs K, et al. PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem Int. 2014;64:84–91.
Szabadfi K, Reglodi D, Szabo A, Szalontai B, Valasek A, Setalo Gy Jr et al. Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: focus on the vertical information processing pathway. Neurotox Res. 2016;29:432–46.
D’Amico AG, Maugeri G, Reitano R, Bucolo C, Saccone S, Drago F, et al. PACAP modulates expression of hypoxia-inducible factors in streptozotocin-induced diabetic rat retina. J Mol Neurosci. 2015;57:501–9.
Gabriel R. Neuropeptides and diabetic retinopathy. Br J Clin Pharmacol. 2013;75:1189–201.
Marzagalli R, Scuderi S, Drago F, Waschek JA, Castorina A. Emerging role of PACAP as a new potential therapeutic target in major diabetes complications. Int J Endocrinol. 2015;2015:160928.
Szabadfi K, Pinter E, Reglodi D, Gabriel R. Neuropeptides, trophic factors, and other substances providing morphofunctional and metabolic protection in experimental models of diabetic retinopathy. Int Rev Cell Mol Biol. 2014;311:1–121.
Banki E, Degrell P, Kiss P, Kovacs K, Kemeny A, Csanaky K, et al. Effect of PACAP treatment on kidney morphology and cytokine expression in rat diabetic nephropathy. Peptides. 2013;42C:125–30.
Banki E, Kovacs K, Nagy D, Juhasz T, Degrell P, Csanaky K, et al. Molecular mechanisms underlying the nephroprotective effects of PACAP in diabetes. J Mol Neurosci. 2014;54:300–9.
Ola MS, Ahmed MM, Ahmad R, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J Mol Neurosci. 2015;56:440–8.
Scuderi S, D’Amico AG, Federico C, Saccone S, Magro G, Bucolo C, et al. Different retinal expression patterns of IL-1α, IL-1β, and their receptors in a rat model of type 1 STZ-induced diabetes. J Mol Neurosci. 2015;56:431–9.
Kvarik T, Mammel B, Reglodi D, Kovacs K, Werling D, Bede B, Vaczy A, Fabian E, Toth G, Kiss P, Tamas A, Ertl T, Gyarmati J, Atlasz T. PACAP is protective in a rat model of retinopathy of prematurity. J Mol Neurosci 2016 in press. DOI:10.1007/s12031-016-0797-5.
Szabadfi K, Kiss P, Reglodi D, Tamas A, Hashimoto H, Helyes ZS, et al. Neurochemical differences between wild type and PACAP KO mice in adult and aging retina. Presented at the 8th FENS Forum of Neuroscience, July 14–18, 2012, Barcelona, Spain.
Reglodi D, Kiss P, Szabadfi K, Atlasz T, Gabriel R, Horvath G, et al. PACAP is an endogenous protective factor—insights from PACAP deficient mice. J Mol Neurosci. 2012;48:482–92.
Szabadfi K, Atlasz T, Kiss P, Danyadi B, Tamas A, Helyes ZS, et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox Res. 2012;21:41–8.
Endo K, Nakamachi T, Seki T, Kagami N, Wada Y, Nakamura K, et al. Neuroprotective effect of PACAP against NMDA-induced retinal damage in the mouse. J Mol Neurosci. 2011;43:22–9.
Atlasz T, Váczy A, Kovács K, Lőkös E, Kvárik T, Werling D, et al. The protective role of the endogenous PACAP in LPS-induced inflammation in the retina. Presented at 12th International Symposium on VIP, PACAP and Related Peptides, September 21–26, 2015, Cappadocia, Turkey.
Engelund A, Fahrenkrug J, Harrison A, Luuk H, Hannibal J. Altered pupillary light reflex in PACAP receptor-1 deficient mice. Brain Res. 2012;1453:17–25.
Lang B, Zhao L, McKie L, Forrester JV, McCaig CD, Jackson IJ, et al. GABAergic amacrine cells and visual function are reduced in PAC1 transgenic mice. Neuropharmacology. 2010;58:215–25.
Brubel R, Boronkai A, Reglodi D, Racz B, Nemeth J, Kiss P, et al. Changes in the expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the human placenta during pregnancy and its effects on survival of JAR choriocarcinoma cells. J Mol Neurosci. 2010;42:450–8.
Juhasz T, Helgadottir SL, Reglodi D, Tamas A, Zakany R. Signalisation of VIP and PACAP in chondrogenesis and osteogenesis. Peptides. 2015;66:51–7.
Saghy E, Payrits M, Helyes ZS, Reglodi D, Banki E, Toth G, et al. Stimulatory effect of pituitary adenylate-cyclase activating polypeptide 6-38, M65 and vasoactive intestinal polypeptide 6-28 on trigeminal sensory neurons. Neuroscience. 2015;308:144–56.
Olianas MC, Ennas MG, Lampis G, Onali P. Presence of pituitary adenylate cyclase-activating polypeptide receptors in Y-79 human retinoblastoma cells. J Neurochem. 1996;67:1803–9.
Wojcieszak J, Zawilska JB. PACAP38 and PACAP6-38 exert cytotoxic activity against human retinoblastoma Y79 cells. J Mol Neurosci. 2014;54:463–8.
Acknowledgements
This study was supported by OTKA K104984, 119759 Arimura Foundation, TAMOP 4.2.4.A/2-11-1-2012-0001 “National Excellence Program,” MTA-PTE “Lendulet” Program, Bolyai Scholarship of the Hungarian Academy of Sciences, PTE AOK KA Research Grant, National Brain Research Programme KTIA_13_NAP-A-III/5 and New National Excellence Program (UNKP). This work is dedicated to the 650th anniversary of the University of Pecs.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Atlasz, T. et al. (2016). Protective Effects of PACAP in the Retina. In: Reglodi, D., Tamas, A. (eds) Pituitary Adenylate Cyclase Activating Polypeptide — PACAP. Current Topics in Neurotoxicity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-319-35135-3_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-35135-3_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-35133-9
Online ISBN: 978-3-319-35135-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)