Abstract
In secondary prevention—among survivors of a myocardial infarction (MI), occlusive stroke, transient ischemic attack, or CABG surgery or those with stable angina—aspirin significantly reduces the risk of subsequent MI, stroke, and vascular death. Furthermore, in patients suffering an acute MI, occlusive stroke, or unstable angina, aspirin significantly reduces the risks of MI, stroke, and vascular death. In primary prevention, however, the totality of evidence is insufficient upon which to make general guidelines for aspirin. In large-scale trials of primary prevention in men and women without established CVD, and subsequent meta-analyses, aspirin produced a significant reduction in the risk of a first MI, but not that of stroke or cardiovascular death. In addition, in primary prevention the absolute benefit is far lower, because the average 10-year absolute risk of CHD in apparently healthy individuals included in the trials of primary prevention was <5 %. Thus, a 20 % relative risk reduction in individuals with a 10-year risk of 5 % is about 1 %, whereas a 10 % relative risk reduction is about 0.5 %. Furthermore, if one assumes that the risk of a bleeding event in an individual receiving primary prevention is the same as that for a patient receiving secondary prevention, the benefits outweigh the risks only when the 10-year risk of CVD is >10 %. Finally, patients with prior gastrointestinal bleeding, as well as those taking nonsteroidal anti-inflammatory drugs, or with gastrointestinal symptoms due to ulcer disease are all at increased risk of developing major bleeding when taking aspirin.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cardiovascular disease (CVD), which includes coronary heart disease (CHD) and stroke, is by far the leading cause of death in most developed countries and is rapidly becoming the leading cause of death in the world. This alarming and potentially avoidable situation results mainly from major increases in cigarette smoking, obesity, and physical inactivity in both developed and developing countries [1]. In this context, the availability of an adjunctive drug therapy that is readily available at low cost represents a desirable possible option which requires a reliable totality of evidence that includes large-scale randomized trials designed to test the hypothesis.
The totality of evidence on aspirin in a very wide range of high-risk patients supports its routine prescription by health-care providers to decrease their risks of subsequent occlusive CVD events, but, in low-risk primary prevention subjects, the absolute benefits on occlusion may not outweigh the absolute risks on bleeding [2].
In this chapter, we review the benefits and risks of aspirin in high-risk secondary and primary prevention patients as well as moderate- and low-risk primary prevention subjects. We quantitate the relative and absolute benefits on various manifestations of occlusive vascular diseases as well as the relative and absolute risks on gastrointestinal and bleeding consequences. Although aspirin is available in many countries over the counter, its utilization to treat and prevent chronic conditions such as cardiovascular disease should be based on an individual judgment of the responsible clinician that weighs the absolute benefits against the absolute risks.
Aspirin and Its Beneficial Effects on Occlusive CVD Events
Who Does Need Aspirin?
How Much Is the Magnitude of the Benefit?
In secondary prevention of CVD, the Antithrombotic Trialist’s Collaboration performed the most comprehensive, worldwide meta-analysis of 195 randomized trials of antiplatelet therapy, principally with aspirin, among more than 135,000 high-risk patients with prior evidence of cardiovascular disease, including prior or acute myocardial infarction, prior or acute stroke, or transient ischemia attacks, and other high-risk groups such as unstable angina, chronic stable coronary disease, and peripheral artery disease, as well as patients with coronary artery bypass grafts or percutaneous coronary interventions [3]. Aspirin produced a statistically significant and clinically important 22 % reduction in risk of subsequent vascular event. In this wide range of patients with prior cardiovascular disease, there were absolute reductions of approximately 36 vascular events per 1000 patients with a prior myocardial infarction treated for a mean of 27 months, 36 events per 1000 patients with a previous stroke or transient ischemic attack treated for 29 months, and 22 events per 1000 patients with other high-risk conditions treated for 22 months. With respect to dose of aspirin, in indirect comparisons as well as direct comparisons in three trials testing this hypothesis, there were no significant differences in efficacy or safety between doses of 75–150 mg/day and 160–325 mg/day. In addition, the most plausible mechanisms of aspirin are on thrombosis and statins on atherosclerosis, suggesting that the benefits of both drugs used simultaneously would be additive [4]. Importantly, relevant information on this hypothesis was generated from a meta-analysis of randomized trials of statins in secondary prevention which aspirin was used in varying frequencies. In this meta-analysis, the combination of aspirin and statins conferred, at the very least, additive clinical benefits than either agent alone on myocardial infarction, occlusive stroke, and death from cardiovascular disease. In fact, the probability that the benefits were greater than just additive was 92 %. Finally, these benefits were apparent in the two largest individual trials, namely, LIPD and CARE, that comprised this meta-analysis [5].
With respect to aspirin given during acute myocardial infarction , the Second International Study of Infarct Survival (ISIS-2) randomized 17,187 patients within 24 h on onset of their symptoms of acute myocardial infarction in a 2 × 2 factorial design to aspirin (162.5 mg), streptokinase (SK) (1.5 million units), both active treatments, or both placebos [6]. At 35 days, the primary prespecified endpoints of total mortality were reduced to 23 % by aspirin, 25 % by SK, and 42 % by aspirin and SK together. For aspirin, the mortality benefits were similar regardless of whether administration was within 1 h or up to 24 h after onset of symptoms of acute MI. In contrast, those treated within 6 h with SK had a 30 % reduction in mortality and with SK and aspirin a 52 % reduction. Among those assigned at random to aspirin, there were statistically significant and clinically important reductions in vascular deaths of 23 %, nonfatal reinfarction of 49 %, and nonfatal stroke of 46 %. Major bleeds requiring transfusions were similar in the aspirin and placebo groups (0.4 %). After 35 days of treatment with aspirin, there were no excess risks of cerebral hemorrhages and only a slight increase in major bleeds. In terms of absolute risk reductions of vascular events, there was an avoidance of 38 events per 1000 patients with an acute myocardial infarction treated for one month [6].
In acute occlusive stroke , there are two landmark trials of aspirin. In each trial, occlusive stroke was initially diagnosed by the responsible clinician and subsequently confirmed by CT scanning. The International Stroke Trial (IST) randomized 19,435 patients to 300 mg aspirin daily or open control [7]. The Chinese Acute Stroke Trial (CAST) randomized about 20,000 patients to 160 mg aspirin daily or placebo [8]. Each showed benefits, and a meta-analysis showed a statistically significant and clinically important 11 % reduction in vascular events as well as nonfatal stroke and vascular deaths. In terms of absolute risk reductions, for every 1000 patients with acute occlusive stroke, treatment with aspirin avoided nine vascular events. Thus, for all patients with acute occlusive stroke, aspirin should be administered promptly and continued long term [7, 8].
As regards primary prevention , the Physician’s Health Study was the first to demonstrate a statistically significant benefit of aspirin on first myocardial infarction in 22,071 apparently healthy men [9, 10]. Since that time, there have been five additional major trials in men and women which comprise over 90,000 subjects. A comprehensive meta-analysis of these six major primary prevention trials using individual participant data provided more reliable comparison of the benefits and risks of aspirin in apparently healthy people. While all four of the proportional reductions in major coronary events and in ischemic stroke in the primary and in the secondary prevention trials were similar to each other, vascular mortality was not significantly reduced in the primary prevention trials. Since the numbers of fatal events were far smaller in the primary prevention trials, a proportional reduction comparable with that in the secondary prevention trials could not be excluded. Regardless of the similarities in proportional reductions, the absolute benefits of aspirin are far smaller in the primary than in the secondary prevention trials due to the far lower absolute risks of the apparently healthy subjects [11].
In the primary prevention trials, there were no significant modifications of the benefits of aspirin by age, smoking history, blood pressure, total cholesterol, body mass index, history of diabetes, or sex. In addition, an earlier suggestion that the beneficial effects of aspirin in primary prevention might differ between men and women has not been supported by the more robust data from the secondary prevention trials [12].
In primary prevention , aspirin reduces risk of a first myocardial infarction, but the data on stroke and vascular deaths remain inconclusive. In addition, the average absolute risk of subjects randomized in the primary prevention trials was so low that it is not possible to get reliable estimates of the benefit-to-risk ratio in primary prevention in subjects at moderate risk. Nonetheless, to maximize the benefit-to-risk ratio in primary prevention, most current guidelines recommend that aspirin be given to those above a certain level of absolute risk at baseline. These guidelines implicitly assume, perhaps erroneously, that risks of bleeding remain constant and that the GI risk in all individuals is similar. While the currently available trial results could well help inform appropriate individual clinical judgments on use of long-term aspirin, they do not seem to justify general guidelines advocating the routine use of aspirin in all apparently healthy individuals above a moderate level of risk of coronary heart disease. The ongoing trials in moderate- to high-risk primary prevention may facilitate a reliable benefit-to-risk ratio of aspirin in primary prevention among subjects at moderate risk [11]. Nobody would disagree that a nonfatal myocardial infarction or stroke is more likely to be disabling than a nonfatal bleed, but any judgment about the use of aspirin in primary prevention should be made on an individual clinical basis. Thus, in primary prevention, at present, the appropriate and judicious use of aspirin by clinicians based on individual clinical judgments that weigh the absolute benefits on first myocardial infarction against the absolute risks of the drug will avoid premature morbidity and possibly mortality from cardiovascular disease.
Aspirin and Risks of Bleeding in the Gastrointestinal Tract
Extent of the Damage to Upper and Lower GI Tract: How much Is the Risk? Who Is at Risk?
Due to its antiplatelet effect, aspirin increases the risk of bleeding in different organs and systems. A recent meta-analysis with data from 31 trials reporting on any bleeds showed a significantly increased risk of bleeding using low-dose aspirin compared with controls (OR, 1.54; 95 % CI, 1.36–1.74; P < .001). Seventy one individuals (95 % CI, 63–90) had to be treated to harm (NNH value) a patient with any bleed. The incidence rate difference was 8.1 (95 % CI, 4.0–12.2) per 1000 person-years. The authors also found an association between dose of aspirin and increased risks of bleeding (B coefficient, 0.60; 95 % CI, 0.18–1.02; P = .004) and also an increased risk for hemorrhagic strokes (92 vs. 56; Peto OR, 1.67; 95 % CI, 1.12–2.48; P = .008; I2 = 21 %) [13].
The most frequent site of significant bleeding events associated with aspirin treatment occurs in the GI tract. Aspirin induces a wide spectrum of adverse events in GI tract, including symptoms without lesions to bleeding from the upper and lower GI tract, although the most common cause is peptic ulcer bleeding. Upper GI symptoms include those related to gastroesophageal reflux and dyspepsia which can be present in up to 15–20 % of patients taking aspirin [14, 15]. These symptoms are associated with decreased adherence or even aspirin therapy discontinuation as high as 50 %, which is associated with a threefold increased risk of CV events. Clinical symptoms are not predictive of the presence of mucosal damage. Endoscopic-controlled studies have shown that most patients taking aspirin have gastroduodenal erosions. However, the incidence of ulcers is lower [16, 17]. A study of 187 patients taking aspirin without gastro-protectant drugs showed an ulcer point prevalence of 11 % (95 % CI 6.3–15.1 %) and projected a yearly ulcer incidence of 28 %. Only 20 % of patients with ulcer had dyspeptic symptoms, not significantly different from patients without ulcer [14].
It is estimated that aspirin use is associated with a two–fourfold increase in symptomatic or complicated ulcers [13, 18]. The estimated average excess risk is five cases per 1000 aspirin users per year [19]. A meta-analysis of 33 RCTs involving 87,581 individuals with 338,735 person-years of follow-up evaluation [13] found an increased risk of any GI bleeds with low-dose aspirin use (OR, 1.31; 95 % CI, 1.21–1.42; P < .001), translating into an NNH of 166 (95 % CI, 125–250; IRD, 2.1; 95 % CI, 0–4.7 per 1000 person-years). Neither fatal GI bleeds (OR, 0.94; 95 % CI, 0.47–1.87; P = .87) nor fatal hemorrhagic strokes (33 vs. 23; Peto OR, 1.42; 95 % CI, 0.84–2.41) were associated significantly with aspirin use. Observational studies have reported in general higher-risk estimates of upper GI bleeding [20, 21].
Another meta-analysis of individual participant data in six primary prevention trials (95,000 individuals at low average risk) and 16 secondary prevention trials (17,000 individuals at high average risk) [11, 22] found in primary prevention studies a small increase in hemorrhagic stroke with aspirin treatment vs. placebo (0.04 %vs. 0.03 %, p = 0.05), with no significant differences in vascular mortality (0.19 % vs. 0.19 % per year, p = 0.7). Aspirin allocation increased major gastrointestinal and extracranial bleeds (0.10 %vs. 0.07 % per year, p < 0.0001). In this study, the main risk factors for coronary disease were also risk factors for bleeding. In the secondary prevention trials, aspirin allocation was not associated with that increase in hemorrhagic stroke. Aspirin increased major gastrointestinal and other extracranial bleeds by about half in the primary prevention trials (0·10 % vs. 0.07 % per year; RR 1·54 [1.30–1·82], p < 0.0001). The excess risk was mainly due to nonfatal bleeds, perhaps by chance. In secondary prevention trials, there was an excess of major bleeds among aspirin-treated patients (RR 2·69 [1.25–5.76], p = 0.01) (Table 11.1).
The association of aspirin use with adverse effects on the lower GI tract is less documented. A systematic review found a small increase of fecal blood loss (0.5–1.5 mL per day) in aspirin users [23]. One study conducted in healthy volunteers showed that enteric-coated aspirin treatment was associated with small intestine mucosal damage in 50 % of volunteers; a few volunteers developed asymptomatic deep ulcers. These lesions could explain why some aspirin users develop bleeding of “unknown” source, iron deficiency anemia, or hypoproteinemia. A study in health professionals concluded that aspirin increases significantly the risk of diverticulitis and diverticular bleeding, reporting an HR = 1.25 (95 % CI 1.05–1.47) for diverticulitis and an HR = 1.70 (95 % CI 1.21–2.39) for diverticular bleeding [24]. Another Japanese study also found association of aspirin and other antiplatelet agents with diverticular bleeding [25]. A more recent study by Lanas et al. [26] quantified the relative risk of upper and lower GI bleeding associated with use of NSAIDs, antiplatelet drugs, and anticoagulants. NSAIDs, anticoagulants, aspirin, and nonaspirin antiplatelet agents were associated with both upper and lower GI bleeding. The adjusted relative risks of upper and lower GI bleeding for aspirin were 1.7 (95 % CI 1.2–2.6) and 2.7 (95 % IC 1.8–4.1), respectively, whereas for nonaspirin antiplatelets were 2.8 (95 % CI 1.4–25.8) and 2.8 (95 % IC 1–3.2), respectively.
Risk Factors
The risk of upper GI complications differs among aspirin users. Several risk factors for GI bleeding in patients treated with antiplatelet aspirin therapy have been reported and include history of peptic ulcer disease, older age, concomitant use of NSAIDs or other antiplatelet agents or anticoagulants, severe comorbidity, aspirin dose, and H. pylori infection. Other potential risk factors have been also mentioned (corticosteroids, alcohol use, and high body mass). Importantly, one of the most recent meta-analyses [11, 22] concluded that GI and CV risk factors were similar including age, male sex, diabetes, smoking, and high blood pressure. The relative risk of GI bleeding increases with the number of risk factors present in the patient.
History of complicated and uncomplicated peptic ulcer is the most important risk factor in aspirin users [27, 29]. The meta-analysis of serious vascular events and major bleeds in six primary prevention trials and 16 secondary prevention trials showed that age (per decade) was associated with an increased risk of major extracranial bleed (RR 2.15, CI 95 %, 1.93–2.39) [11].
Aspirin use is frequent among NSAID users (20–25 % in clinical trials), mainly in the elderly. The combination increases further two–threefold the risk compared to monotherapy with aspirin [27, 28, 30]. The GI benefits of selective COX-2 inhibitors over nonselective NSAIDs are reduced with the coadministration of aspirin, although a meta-analysis of all available trials that included patients treated with aspirin and nonselective or selective NSAIDs showed a lower risk of GI complications in patients taking a selective COX-2 inhibitors plus aspirin, compared with those taking nonselective NSAIDs plus aspirin [RR 0.72 (95 % CI 0.62–0.95)] [31]. It must be pointed out that these studies were nonrandomized trials and the data were obtained from indirect comparisons.
Dual antiplatelet therapy (aspirin plus other antiplatelet agents) is common in several clinical scenarios. Dual therapy increases the risk of GI bleeding to a higher degree (two- to threefold) than aspirin alone. The absolute risk increase was in the range of 0.6–2.0 % [32]. In the CHARISMA (clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance) trial, patients treated with the combination therapy had a higher risk of moderate to severe bleeding during the first year when compared to aspirin alone [33]. However, not all studies have confirmed these results [27, 34]. Anticoagulants do not affect directly the GI mucosa but have a high anti-hemostatic effect. Most data suggest that concomitant use of anticoagulant and aspirin increases the risk of GI bleeding to a higher degree than aspirin alone [13].
A recent systematic review evaluated the influence of H. pylori on upper GI bleeding risk in aspirin users. Authors concluded that current data do not allow performing meta-analyses and that no firm conclusion could be drawn on this issue [35]. We have recently performed the largest case-control study evaluating the interaction between H. pylori infection and aspirin or NSAID use. H. pylori infection, NSAID use, and aspirin treatment were independent risk factors for upper GI bleeding but found no interaction between aspirin and H. pylori infection [36]. In contrast, a cohort study showed that patients with peptic ulcer bleeding history treated with aspirin in whom H. pylori was eradicated had a similar recurrent bleeding rate not far different to those aspirin-treated patients with no risk factor [37]. Sub-analysis of this cohort showed however that in the presence of other concomitant gastrotoxic medication, the risk of bleeding was higher than in the average-risk cohort.
Iijima and colleagues evaluated the possible biphasic effects of H. pylori infection on aspirin-induced gastropathy depending on the gastric acid secretion level. They concluded that in the presence of sufficient amounts of gastric acid, H. pylori and aspirin could synergistically damage the gastric mucosa, while in the absence of sufficient gastric acid, the infection could even suppress the aspirin-induced gastropathy. These biphasic effects could explain the controversy data in the literature about the role of H. pylori infection in the GI risk in aspirin users [38].
Finally, aspirin dose is another important aspect to consider related to its GI risk of bleeding. Although studies are somehow conflicting, most data show that the GI risk with aspirin is dose dependent and that 75–100 mg daily is enough to obtain the CV benefits. Therefore, the current recommendation is to use the lowest possible aspirin dose (≤100 mg/day) for the prevention of CV event. The risk of GI complications with aspirin seems to be higher in the first month of treatment, whereas with longer durations, the risk decreases and then remains constant over time [21, 39, 40]. This effect has been explained as the consequence of gastric adaptation to aspirin [41]. The use of enteric-coated or enteric-buffered preparations does not reduce the risk of GI complications [42]. The reason for this is explained on the understanding that the main effect of aspirin on the gastric mucosa depends on the systemic effects rather than in the local “topical” effects of this compound.
Aspirin Beyond the CV System and the GI Tract
With respect to other effects of aspirin , randomized data suggest benefits of low dose in the prevention of migraine [43] and high dose in the treatment [44]. Observational data suggest that elderly individuals who self-select for aspirin have lower rates of loss of cognitive function, but this hypothesis requires direct testing in randomized trials of sufficient size and duration [45].
In addition, a recently reported meta-analysis of randomized trials, most of which were not designed a priori to test the hypothesis, suggests beneficial effects of aspirin on overall cancer mortality [46].
Thus, the randomized data on prevention and treatment of colon polyps as well as primary prevention of colon cancer are more reliable than the data from other shorter-term trials which, in turn, are more reliable than the observational data on other cancers
How Do I Identify the Actual Risk of My Patient?
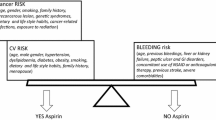
On the basis of the available evidence, the recommendation of aspirin in primary prevention depends on the accurate assessment of cardiovascular risk as part of the decision-making process . CV risk can be easily estimated today by using the Framingham’s 10-year risk estimations or the Systematic Coronary Risk Evaluation (SCORE) , a European cardiovascular disease risk assessment. Framingham’s tables for CV risk were based on the method reported on the paper published by Wilson and colleagues [47] for the prediction of cardiovascular heart disease (angina pectoris, recognized and unrecognized myocardial infarction, coronary insufficiency, and coronary heart disease death), and it is accessible online and in multiple apps. The risk is based on several key variables including age, gender, blood pressure, cholesterol levels, and smoking (http://www.framinghamheartstudy.org/ risk/coronary.html#tab3). The CV risk estimation with Framingham’s tables seems to apply better for American countries and the UK.
The SCORE system provides an estimation of the 10-year risk of fatal cardiovascular disease stratification in the primary prevention of CV disease [48]. The CV risk can be grouped into low, moderate, and high based on a six-step method that combines a calculation of the 10-year risk for coronary heart disease and for noncoronary cardiovascular disease, together with the presence of CV risk factors, such as smoking, cholesterol, and systolic blood pressure. CV risk is estimated to be low when that risk is <2 %, moderate when the risk is placed between 2 and 5 %, and high when >5 %. Diabetes and previous CV pathologies (acute myocardial infarction, angina, and ictus) are not included in these models since they are categorized directly in the high-CV-risk group (Table 11.2). There is sufficient evidence to be considered; these CV risk estimators are accurate and are widely accepted and used worldwide.
GI Risk Estimations
GI risk estimations are not as standardized as those described for the CV risk. Based on previous reports [49, 50], patients may be classified into three GI categories when taking NSAIDs (Table 11.3). That risk can be used for any patient who may receive potential gastrotoxic drug such as aspirin. Patients at low GI risk are considered patients without any of the risk factors mentioned in the previous section. Patients at moderate GI risk include those with at least one of the following GI risk factors: (1) age 60 or older, (2) concomitant use of NSAIDs, (3) concomitant use of corticosteroids, (4) concomitant use of other antiplatelet agents, (5) history of symptomatic peptic ulcer, and (6) history of dyspepsia. Patients at high GI risk were those with either a GI bleeding history or concomitant use of anticoagulants or the presence of three risk factors of those described for moderate GI risk. These levels of risk were based on the estimated incidence of events obtained from combining different risk factors that would put patients at a similar risk level to those with a history of bleeding peptic ulcers. In this way, in low-GI-risk patients, the expected rate of upper GI complications should not exceed 1.5 events per 100 patients/year, whereas for those at moderate GI risk, the rate should be between 1.5 and 10, and for high-GI-risk patients, the rate should be greater than ten events per 100 patients/year. Patients being treated with anticoagulants (warfarin or coumadin or NOACs) were considered high risk, because bleeding events in anticoagulated patients can be more severe, when taking other gastrotoxic drugs.
A recent study has provided an aspirin GI risk calculator tool [51] that may help physicians in assessing the actual risk of their patients and use appropriate therapy. The authors of this tool used data reported by Hernandez-Diaz and Garcia-Rodriguez [19] as a baseline for the construction of tables and algorithms. This study characterized aspirin users together with major gastrointestinal risk factors and provided incidence rates as well as excess risk of upper GI complications linked to low-dose aspirin. These estimations were based on data from the UK General Practice Research Database and systematic reviews of the literature. Based on those reports, the ASA Risk Calculator (available online at www.asariskcalculator.es) assumes an overall baseline upper GI bleeding incidence rate of 1 per 1000 person-years and then constructs absolute incidence rates within each risk subgroup based on pooled estimates and 95 % confidence interval reported from different meta-analyses. The calculator assumes that the pooled relative risks of upper GI bleeding was 2.0 for aspirin at doses <300 mg/day. The major risk factors for the development of upper GI bleeding were age, male gender, history of complicated peptic ulcer, history of uncomplicated peptic ulcer, and concomitant use of NSAIDs, anticoagulants, or clopidogrel. Based on those estimations, and as reported in their original article, the calculator shows that in patients with low CV risk, the use of aspirin induces more GI harm than CV benefits in almost all clinical scenarios. In patients with high CV risk, aspirin is recommended, but the GI harms may overcome sometimes the CV benefits in some patients. Eventually, the calculator provided recommendations that the use of PPI with/without H. pylori eradication can reduce the harm and increases the CV benefits of aspirin in most clinical scenarios. However, in patients with complicated peptic ulcer history and other risk factors, the CV benefits may still be offset by GI harm.
Can We Reduce the GI Risk?
Reducing the GI risk linked to aspirin treatment will increase the net beneficial CV effect and increase treatment compliance. There are several strategies to minimize the upper GI damage induced by cardiovascular aspirin: (1) reducing modifiable risk factors (including eradication of H. pylori infection), (2) using the most appropriate aspirin dose, and (3) using gastroprotective agents.
Reducing Modifiable Risk Factors
Avoidance of Concomitant Gastrotoxic Medication
Concomitant treatment of aspirin with NSAIDs (nonselective and COX-2 selective), other antiplatelet agents, anticoagulants, and to a lesser extent corticosteroids increases the risk of developing upper GI bleeding complications and probably lower GI bleeding as well. Guidelines strongly suggest avoiding the combination of NSAIDs and aspirin if possible. In addition, the concomitant use of aspirin with ibuprofen, and perhaps naproxen, should be avoided because these NSAIDs interfere with the antiplatelet effect of aspirin. This is due to competition between both drugs for a common docking site within the COX-1 channel. If these combinations were used, aspirin should be taken first and well before dosing with ibuprofen or naproxen, but still interaction is possible, especially if a patient takes enteric-coated aspirin where aspirin is being released slowly for several hours and the T max takes 4–5 h to occur [14–16].
Eradication of H. pylori Infection
H. pylori eradication is controversial in patients without history of peptic ulcer taking aspirin. This aspect has been commented above in the risk factors section. Several studies have evaluated the effect of H. pylori eradication in the prevention of peptic ulcer bleeding recurrence in aspirin users. The most important study was conducted by Chan and colleagues and compared long-term PPI treatment vs. H. pylori eradication in H. pylori-positive patient with a recent peptic ulcer GI bleeding event. The re-bleeding rate was similar in both groups at 6 months of follow-up [1.9 % vs. 0.9 %, respectively, (absolute difference 1 %; 95 % CI 1.9–3.9 %)] [55]. However, the small sample size and short time of follow-up could have prevented a different outcome and conclusion. The largest long-term prospective cohort study has been published recently [37]. Over nine hundred patients were divided into three cohorts and were followed up for 10 years or until death. The cohorts were (1) H. pylori-positive patients with bleeding ulcers in which H. pylori infection was eradicated, (2) H. pylori-negative patients with bleeding ulcers, and (3) new users of aspirin without prior peptic ulcer. None of them received regular PPI treatment. The incidence of upper GI bleeding was not significantly different between the H. pylori-eradicated cohort (1.09; 95 % CI 0.61–1.98) and the average-risk cohort of patients without history of peptic ulcer (0.67; 95 %CI 0.42–1.06). Sub-analysis of the H. pylori-related peptic ulcer bleeding cohort showed that in the presence of other risk factors, these patients had higher risk of ulcer bleeding compared to the non-peptic ulcer history cohort. Moreover, H. pylori-negative patients with bleeding ulcers had a high risk of recurrent bleeding. The impact of these findings is reduced because of the lack of direct comparisons and the clinical differences between the cohorts. The ongoing HEAT (Helicobacter Eradication Aspirin Trial) study aimed at the evaluation of the effect of H. pylori eradication on the incidence of upper GI bleeding in aspirin-treated patients can provide quality evidence on the role of eradication in primary CV prevention in aspirin users (ClinicalTrials.gov, NCT01506986), but this will take a few years to be known. In the meantime, guidelines recommend that H. pylori infection should be tested and treated in all patients with previous ulcer history. Still those at high risk should receive prevention therapy with antisecretory agents.
Aspirin Dose
As commented above, the GI bleeding risk is dose dependent , whereas the maximal CV beneficial effect can be obtained with 75–100 mg of aspirin daily. Based on these widely assumptions and evidence, today these low doses of aspirin are prescribed worldwide for this indication, and all risk-benefit balances are based on these doses that provide the best outcomes.
Gastroprotective Agents
Very few studies have evaluated the effect of misoprostol on aspirin-related GI injury. An endoscopic study showed that misoprostol significantly lowered the incidence of erosions in healthy volunteers taking LDA [56]. Misoprostol has shown also to reduce the incidence of small bowel erosions in aspirin users [57].
Data with H2 blockers are a bit more consistent. These drugs can suppress gastric acid production by up to 70 % over 24 h. Two prospective case-control studies developed by Lanas and colleagues showed conflictive data on the efficacy of H2 receptor antagonist. In the first study published in 2001, the risk of upper GI bleeding in patient taking LDA was not significantly reduced by H2 receptor antagonist use (OR 0. 5, 95 % CI 0.2–1.2) [28]. However, in the second study published in 2007, H2 receptor antagonists reduced significantly the risk of upper GI bleeding in LDA users (RR 0.40, 95 % CI 0.19–0.73) [58]. As in many other observational studies, confounding factors may have affected the outcome and explain the differences between these two studies.
The most important clinical trial with H2 blockers was conducted in Scotland and compared high-dose famotidine (20 mg/12 h) for 12 weeks vs. placebo in aspirin users without ulcers at baseline [59]. Patients treated with famotidine had a significantly lower incidence of ulcers than placebo group (3, 8 % vs. 23, 5 %, respectively). However, there were several relevant concerns to consider: (1) rate of H. pylori infection was higher in placebo group and (2) some patients of famotidine group did not have final endoscopy evaluation. In any case, due to the potential interaction of PPIs with clopidogrel, the use of famotidine in patients taking dual antiplatelet therapy has been recommended. However, most guidelines still recommend the use of PPI in high-risk patients taking aspirin [32].
PPIs are potent inhibitors of gastric acid secretion. Several studies have explored the impact of PPI on reducing endoscopic damage and the risk of GI complications in users of aspirin. Today, considerable evidence support that PPIs are more effective than H2 blockers as gastroprotective agents in antiplatelet users [60] by comparing directly PPI (pantoprazole 20 mg) with high dose of famotidine (40 mg twice) in the prevention of recurrence of uncomplicated or complicated peptic ulcer in aspirin users. Recurrent GI bleeding and uncomplicated ulcer were significantly more common in the famotidine group than in the pantoprazole group (7.7 % vs. 0 %, p < 0.05 and 12.3 % vs. 0 %, p < 0.05, respectively). A recent nested case-control study investigated the impact of different prevention strategies against GI complications in aspirin or NSAID users; 2049 cases and 20,000 controls were included [61]. The risk of upper GI bleeding associated with PPI use was 0.5 events per 100 patient-years among aspirin users, 0.18 among clopidogrel users, and 0.17 among dual antiplatelet therapy users. The corresponding estimates for H2 receptor antagonist tended to be smaller.
Many endoscopic studies have shown the high efficacy of different PPI compounds at standard doses in the prevention of upper gastrointestinal mucosal damage [58]. Sugano et al. [62] published this year the LAVENDER study. This was a double bind, randomized, placebo-controlled, and prospective trial that evaluated the efficacy of esomeprazole (20 mg once daily) for 72 weeks in the prevention of recurrent peptic ulcer in aspirin users. Authors concluded that esomeprazole 20 mg over 48 weeks prevented the recurrence of peptic ulcers. Interestingly 45 % of patients were H. pylori positive, which suggests that esomeprazole protected against ulcer recurrence irrespective of H. pylori status. The recent published PLANETARIUM study evaluated the efficacy , dose-response relationship (10 mg, 5 mg, and active control), and safety of rabeprazole for peptic ulcer recurrence over 24 weeks in Japanese patients on aspirin treatment. The cumulative recurrence rates of peptic ulcers were 1.4 and 2.8 % in rabeprazole groups (5 mg and 10 mg, respectively), significantly lower than in the active control group (21.7 %). In rabeprazole groups, there were not bleeding ulcers. Therefore, rabeprazole prevented recurrence of peptic ulcers without evidence of a major dose-response effect in patients on aspirin therapy [63].
The efficacy of PPI in the prevention of recurrence of ulcer complications has also been confirmed in several studies. Lai and colleagues [64] performed a RCT that compared lansoprazole (30 mg/day) with placebo in aspirin users with history of peptic ulcer and who had already received H. pylori eradication therapy. Patients were treated with lansoprazole or placebo for one year. Patients on lansoprazole had significantly less recurrence of ulcer complications than those treated with placebo (1.6 % vs. 14.8 %). This study suggested that H. pylori eradication was not sufficient to prevent ulcer bleeding recurrence in high-risk aspirin users. Combined treatment (H. pylori eradication plus PPI) seems the most adequate therapy for these patients.
As we commented above, dual antiplatelet therapy with aspirin and clopidogrel increases the risk of GI bleeding. The use of this therapy is increasing, especially in patients with coronary stents. The COGENT study [65] evaluated both the occurrence of CV and GI events in this clinical scenario. Patients receiving dual antiplatelet therapy with clopidogrel and aspirin were randomized to omeprazole or placebo. A total of 3873 patients were included and 51 patients had a GI event (bleeding, symptomatic ulcers, or erosions, obstruction, or perforation). In the omeprazole group, the event rate was 1.1 % compared with 2.9 % in placebo group (HR 0.34, 95 % CI, 0.18–0.63, p < 0.001). The rate of upper GI bleeding was also significantly lower in PPI group (HR 0.13, 95 % CI, 0.03–0.56). No differences in CV events were present at the end of the study between the two arms, which rejected the hypothesis that omeprazole and clopidogrel interaction could have a clinical impact on the occurrence of CV events in patients taking dual therapy plus a PPI [65].
Prevention of Lower GI Bleeding with Aspirin
Very few studies have focused on the prevention of lower damage associated with aspirin. Only a preliminary work has suggested that co-therapy with probiotics can reduce the risk of developing anemia in patients who take aspirin and PPI [66]. This approach is based on the growing perception that the microbiota has a role in the mucosal damage induced by NSAIDs and aspirin in the small bowel affecting its permeability. PPI co-therapy would change the microbiota profile making the environment more susceptible to damage by these compounds.
A Rational Therapeutic Approach to Common Clinical Scenarios
Secondary Prevention
During Occlusive CVD
The Second International Study of Infarct Survival (ISIS-2) randomized 17,187 patients within 24 h on onset of their symptoms of acute myocardial infarction in a 2 × 2 factorial design to aspirin (162.5 mg), streptokinase (SK) (1.5 million units), both active treatments, or both placebos [6]. At 35 days, the primary prespecified endpoints of total mortality were reduced to 23 % by aspirin, 25 % by SK, and 42 % by aspirin and SK together. For aspirin, the mortality benefits were similar regardless of whether administration was within 1 h or up to 24 h after onset of symptoms of acute MI. In contrast, those treated within 6 h with SK had a 30 % reduction in mortality and with SK and aspirin a 52 % reduction.
Among those assigned at random to aspirin, there were statistically significant and clinically important reductions in vascular deaths of 23 %, nonfatal reinfarction of 49 %, and nonfatal stroke of 46 %. Major bleeds requiring transfusions were similar in the aspirin and placebo groups (0.4 %). After 35 days of treatment with aspirin, there were no excess risks of cerebral hemorrhages and only a slight increase in major bleeds. In terms of absolute risk reductions of vascular events, there was an avoidance of 38 events per 1000 patients with an acute myocardial infarction treated for one month.
With respect to the benefit-to-risk ratio, aspirin given within 24 h of onset of symptoms of acute myocardial infarction avoided 23 deaths with no increase in cerebral hemorrhage. In contrast, SK given within 12 h avoided 30 deaths but caused three cerebral hemorrhages. As regards benefit to cost, the cost per life saved during acute MI is about $88,000 for tPA, $12,000 for SK, and $13 for aspirin [67].
There are two landmark trials of aspirin in acute occlusive stroke. In each trial, occlusive stroke was initially diagnosed by the responsible clinician and subsequently confirmed by CT scanning. The International Stroke Trial (IST) randomized 19,435 patients to 300 mg aspirin daily or open control (IST Lancet). The Chinese Acute Stroke Trial (CAST) randomized about 20,000 patients to 160 mg aspirin daily or placebo [8]. Each showed benefits and a meta-analysis showed a statistically significant and clinically important 11 % reduction in vascular events as well as nonfatal stroke and vascular deaths. In terms of absolute risk reductions, for every 1000 patients with acute occlusive stroke, treatment with aspirin avoided nine vascular events.
Thus, for all patients suffering acute myocardial infarction or acute occlusive stroke, aspirin should be administered promptly and continued long term [2].
Among Survivors of Occlusive CVD
The Antithrombotic Trialist’s Collaboration performed the most comprehensive, worldwide meta-analysis of 195 randomized trials of antiplatelet therapy, principally with aspirin, among more than 135,000 high-risk patients with prior evidence of cardiovascular disease, including prior or acute myocardial infarction, prior or acute stroke, or transient ischemia attacks, and other high-risk groups such as unstable angina, chronic stable coronary disease, and peripheral artery disease, as well as patients with coronary artery bypass grafts or percutaneous coronary interventions [3]. Aspirin produced a statistically significant and clinically important 22 % reduction in risk of subsequent vascular event. In this wide range of patients with prior cardiovascular disease, there were absolute reductions of approximately 36 vascular events per 1000 patients with a prior myocardial infarction treated for a mean of 27 months, 36 events per 1000 patients with a previous stroke or transient ischemic attack treated for 29 months, and 22 events per 1000 patients with other high-risk conditions treated for 22 months. With respect to dose of aspirin, in indirect comparisons as well as direct comparisons in three trials testing this hypothesis, there were no significant differences in efficacy or safety between doses of 75–150 mg/day and 160–325 mg/day.
During Occlusive CVD in At-Risk GI Patients
Patients who develop an occlusive CVD and are at risk of GI complications based on the presence of risk factors should receive GI prevention therapy. This approach would prevent a GI bleeding event that may jeopardize the success of the CV therapy. The most recommended approach in that scenario is the prescription of a PPI at standard doses p.o. or endovenously if no oral feeding is permitted. In that case, a loading dose of 40 mg of pantoprazole or any other available PPI is required followed by 40 mg/day till oral route is reintroduced. In patients receiving dual antiplatelet therapy with aspirin and clopidogrel, the potential metabolic interaction of PPI with clopidogrel has been a hot topic for debate, and different regulatory bodies have suggested to avoid PPI therapy, especially omeprazole and esomeprazole [68]. Available clinical evidence [65], however, speaks against these strong recommendations, and different guidelines suggest PPI (any) therapy when the GI risk is high. Famotidine is an alternative, but prescribers should be aware that its efficacy in the prevention of GI events is lower than PPIs. At discharge, patients should be maintained on GI prevention therapy as risk factors are present (see next section).
Among Survivors of Occlusive CVD in At-Risk GI Patients
Patients who have survived to an occlusive CVD are at high risk of new CV events, and therefore, they will be taking aspirin or any other antiplatelet agent or combination of them. In many cases, they are also at increased GI risk, even when prescribed aspirin at doses as low as 75 mg/day. Special caution should be taken in those over the age of 70 or those who had a previous ulcer history. The best therapeutic approach is to prescribe co-therapy with a PPI at standard doses (20 mg of omeprazole, 20 mg of pantoprazole, 20 mg of esomeprazole, 15 mg of lansoprazole, or 20 mg of rabeprazole). In patients who take clopidogrel concomitantly with aspirin, a PPI is also recommendable if the GI risk is high. As commented above, famotidine could be prescribed at high dose but in the understanding that its efficacy is lower than that observed, for example, with pantoprazole. Finally, in case of patients with ulcer history, H. pylori eradication should be carried out followed by PPI therapy. Use of other gastrotoxic drugs should be avoided [69].
High-Risk Primary Prevention
Patients with Metabolic Syndrome
Patients with metabolic syndrome , a constellation of obesity, dyslipidemia, hypertension, and insulin resistance leading to diabetes, have 10-year risks of a first CHD event of 16–18 %. In addition to therapeutic lifestyle changes, such patients are most likely to derive net benefits from the additive or more benefits from statins and aspirin.
Diabetics
In observational studies of diabetes of long duration, their risks of a first CHD event are comparable to those with a prior event so some guidelines recommend aspirin. These studies, however, are generally of diabetics of long duration so any decision to use aspirin, pending the outcome of ASCEND [70], should be an individual clinical judgment that weighs the absolute risk of occlusion against the absolute risk of bleeding.
Subjects 70 and older: It is well described that the elderly have higher risks of occlusion, but they also have higher risks of bleeding. Thus, any decision to use aspirin should be an individual clinical judgment [70].
Low- to Moderate-Risk Primary Prevention
Men under 50 and women under 60 H&W. In general, such apparently healthy men and women will have an absolute risk of a first CHD event that is lower than the absolute risk of bleeding. Thus, the clinician should make individual judgments that may be influenced by other risk factors such as obesity, physical inactivity, and family history of a premature CHD event which is generally considered to have been 55 or less in a male or 65 or less in a female first-degree relative.
Special Categories of Patients
On Aspirin Who Need NSAIDs
Patients on aspirin who need NSAIDs is a common clinical scenario, since patients who suffer from OA or AR among other rheumatic diseases often are older and have high CV risk or had suffered from a CV event [50]. Furthermore, ns-NSAIDs and coxibs are associated with increased CV risk. Only naproxen at 500 mg b.i.d. has been shown not to be associated with increased risk. In these last circumstances, NSAID (ns-NSAIDs or coxibs) use should be avoided. If there is high CV risk but patients had not suffered a previous CV event, NSAID treatment should be taken at the lowest possible dose and for the shorter period of time. Naproxen is the NSAID of choice since its CV risk is lower than any other NSAIDs or coxibs. Ibuprofen, and even probably naproxen, should be avoided if patients are taking aspirin since these drugs, especially ibuprofen, interact with the antiplatelet effect of aspirin. Taking these drugs before the aspirin dosing may not be sufficient, since interaction may still occur, especially if patients take enteric-coated aspirin. In these circumstances, the Tmax of aspirin lasts 4–5 h after dosing [54]. Recent guidelines have provided useful recommendation on what to do in these clinical scenarios (Fig. 11.1).
(a–c) Prescription recommendations on NSAID use in patients taking ASA according to GI and CV risk (Adapted from Lanas et al. Gastroenterol Hepatol 2013 [71])
Need Aspirin but at High GI Risk
As commented above for patients who receive aspirin in secondary prevention, patients who need aspirin in a primary prevention setting should be questioned for careful evaluation of the GI risk factors. When the CV benefits are outweighed by the GI risk, we recommend prescribing therapy with a PPI. Common risk factors are age 70 or older, ulcer history, concomitant treatment with NSAIDs, corticosteroids, or anticoagulants or other antiplatelet agents. There is no need for high dose of PPI; standard dose is enough. There are several guidelines instructing on this (refs), but also risk calculators are available online that will help the practitioner on the clinical decision process for individual cases (www.asariskcalculator.com) [51].
Need Aspirin but Develop a GI Complication
The occurrence of a gastrointestinal bleeding complication in patients treated with aspirin for cardiovascular prevention, especially secondary prevention, is a difficult clinical challenge, since discontinuation of platelet inhibition in patients may have fatal consequences and this need to be balanced against the potential fatal outcome of a severe GI bleeding event.
There are two different clinical scenarios: (1) patients treated with aspirin for CV prevention who develop upper GI bleeding and (2) hospitalized patients who have just undergone a stent placement and develop an acute GI bleeding event. Based on the accumulated evidence, current guidelines state that early reintroduction (within 3 days) of aspirin is highly recommended in patients taking aspirin for secondary cardiovascular prevention (Fig. 11.2). The first RCT [72] that specifically evaluated the effect of no interruption of aspirin in patients who presented an acute upper GI bleeding event while taking cardiovascular aspirin concluded that these patients had lower all-cause 8-week mortality rates when compared to those where aspirin was interrupted and not reinitiated (1.3 % vs. 12.9 %). The difference was mainly due to lower mortality attributable to CV complications (1.3 % vs. 10.3 %). The recurrent ulcer bleeding at 30 days was higher in the early aspirin group (10.3 % vs. 5.4 %). Other more recent studies have confirmed these results [73]. If aspirin was indicated for the primary prevention, a reconsideration of the actual indication of aspirin is warranted, and the time for aspirin reintroduction can be prolonged at least till hospital discharge and preferably till the ulcer is healed.
Clinical management in patients who develop an acute upper GI bleeding event when taking ASA for cardiovascular prevention (Adapted from reference Gralnek et al. [74])
In patients who have undergone a stent placement, the risk of thrombosis depends on the time interval between stent implantation and discontinuation of antiplatelet therapy. Dual antiplatelet therapy is recommended for at least 12 months after drug-eluting stents and at least 4 weeks after placement of a bare metal stent. Based on current evidence , and in agreement with expert consensus reports [32], patients with active ulcer bleeding should be treated endoscopically followed by high-dose PPI therapy. If endoscopy shows peptic ulcer with low-risk stigmata, aspirin should not be withdrawn. However, if endoscopy shows high-risk stigmata, aspirin should be stooped and reintroduced early, preferably within a 3–5-day window. If acute bleeding occurs soon after the placement of a coronary stent, the risk of thrombosis is very high. We believe that early endoscopy followed by a high dose of PPI is the best option and usually dual antiplatelet should be maintained, at least clopidogrel. These clinical decisions may be difficult, and a close collaboration between the gastroenterologist and cardiologist is required.
Future Perspectives
Aspirin use in primary prevention of cardiovascular disease is controversial, and as shown in this review, current evidence is insufficient upon which to make general guidelines for aspirin. One explanation can be that studies have included patients at low cardiovascular risk with estimated coronary event rates <1 % per person-years. The scientific community is expectant to see the outcomes of five ongoing clinical primary prevention trials that have enrolled patients at high CV risk. The safety and efficacy of daily aspirin in this setting is very important. These results will need to be balanced against the detrimental effect of bleeding in the GI tract and the CNS. However, comparisons should be fair, and perhaps the right balance in terms of benefits and risk should be made based on the number of deaths induced and the number of deaths avoided. In this equation, the number of cancer deaths avoided should be placed in the equation if ongoing studies show which populations benefit from aspirin use in this regard.
The exact impact of adverse events of aspirin treatment in the lower GI tract should be better defined and these affects also be added in the risk-benefit balance. At the same time, current and future therapies of GI prevention in both the upper GI tract should be further investigated. In this way, the modification of the intestinal microbiota should be explored, and well-designed trials should be put in place sooner than later.
The outcomes of new antiplatelet agents, or combinations of them, now in the market require further evaluation in terms of benefits and adverse events since current evidence is deficient.
The assessment of CV, GI, and cancer risk and the benefits of aspirin at individual level may be difficult to determine in clinical practice. In order to overcome this problem and provide appropriate therapeutic strategies in clinical practice, new electronic tools based on the most recent and contrasted evidence are needed. The prospect of a personalized choice for any antiplatelet therapy in the individual patient appears realistic in the near future.
References
Hennekens CH. Increasing global burden of cardiovascular disease; current knowledge and future directions for research on risk factors. Special report. Circulation. 1998;97:1095–102.
Hennekens CH, Dalen JE. Aspirin in the treatment and prevention of cardiovascular disease: Past and current perspectives and future directions. Am J Med. 2013;126(5):373–8.
Antithrombotic Trialists Collaboration, Barnett H, Bousser M-G, Boysen G, Breddin K, Britton M, Buring J, Cairns J, Canner P, Collins R, Cortellaro M, Daniels A, Deykin D, Elwood P, Elwin E-E, Eschwege E, Farrell B, Fields WS, Gent M, Gray R, Guiraud-Chaumeil B, Hennekens CH, Klimt C, Lewis HD, Loria Y, Lowenthal A, MacMahon C, Miller J, Peto R, Qizilbash N, Reuther R, Richards S, Rosen A, Sandercock P, Sorensen PS, Sweetnam D, Taylor W, Thibult N, van Gijn J, Vogel G, Warlow C, Yusuf S. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction and stroke in high-risk patients. Br Med J. 2002;24:71–86.
Hebert PR, Pfeffer MA, Hennekens CH. Use of statins and aspirin to reduce cardiovascular disease. J Cardiovasc Pharmacol Ther. 2002;7:77–80.
Hennekens CH, Sacks F, Tonkin A, Jukema W, Byington RP, Pitt B, Berry DA, Berry SM, Ford NF, Walker AJ, Nataragan K, Sheng-Lin C, Fiedorek FT, Belder R. Additive benefits of pravastatin and aspirin to decrease risks of cardiovascular disease: Randomized and observational comparisons of secondary prevention trials and their meta-analysis. Arch Intern Med. 2004;164(1):40–4.
ISIS-2 (Second International Study of Infarct Survival) Collaborative Group (Australia: Hunt D; Austria: Dienstl F; Belgium: De Backer G; Canada Cairns J; Denmark Pedersen F; Finland: Kala R; France: Boissel J-P; Germany: Schroder R; Ireland: Horgan J; New Zealand Fitzpatrick M; Norway: Kjekshus Collins R, Peto R, Sleight P; US: Hennekens CH: Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction. Lancet 1988; 2:349-360.
International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomized trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic stroke. Lancet. 1997;349:1569–81.
Chinese Acute Stroke Trial (CAST) Collaborative Group. CAST: randomized placebo-controlled trial of early aspirin use in 20,000 patients with acute ischemic stroke. Lancet. 1997;349:1641–9.
Steering Committee of the Physicians’ Health Study Research Group. Preliminary report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1988;318:262–4.
Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35.
Antithrombotic Trialists Collaboration Primary Prevention Writing Group , Baigent C, Blackwell L, Buring J, Collins R, Emberson J, Godwin J, Hennekens C, Kearney P, Meade T, Patrono C, Peto R, Roncaglioni R, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60.
Hennekens CH, Hollar D, Baigent C. Gender differences in response to aspirin in cardiovascular disease: An hypothesis formulated, but not tested. Nat Clin Pract Cardiovasc Med. 2006;3:4–5.
Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol. 2011;9(9):762–8.
Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Eriksson B, Appelman-Eszczuk S, Långström G, Naesdal J, Serrano P, Singh M, Skelly MM, Hawkey CJ. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther. 2005;22(9):795–801.
Cayla G, Collet JP, Silvain J, Thiefin G, Woimant F, Montalescot G. Prevalence and clinical impact of Upper Gastrointestinal Symptoms in subjects treated with low dose aspirin: the UGLA survey. Int J Cardiol. 2012;156(1):69–75. doi:10.1016/j.ijcard.2010.10.027. Epub 2010 Nov 18.
Muller P, Fuchs W, Simon B. Studies on protective effect of lansoprazole on human gastric mucosa against low-dose acetylsalicylic. An endoscopic controlled double-blind study. Arzneimittelforschung. 1997;47:758–60.
Moore A, Bjarnason I, Cryer B, Garcia-Rodriguez L, Goldkind L, Lanas A, et al. Evidence for endoscopic ulcers as meaningful surrogate endpoint for clinically significant upper gastrointestinal harm. Clin Gastroenterol Hepatol. 2009;7(11):1156.
Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ. 1995;310:827–30.
Hernández-Díaz S, García Rodríguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22.
Chan FK, To KF, Wu JC, Yung MY, Leung WK, Kwok T, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 2002;359(9300):9–13.
Lanas A, García-Rodríguez LA, Arroyo MT, Gomollón F, Feu F, González-Pérez A, Zapata E, Bástida G, Rodrigo L, Santolaria S, Güell M, de Argila CM, Quintero E, Borda F. Piqué JM; Asociación Española de Gastroenterología. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55(12):1731–8. Epub 2006 May 10.
Hennekens CH, Baigent C. Prevention. Aspirin in primary prevention--good news and bad news. Nat Rev Cardiol. 2012;9:262–3.
Moore RA, Derry S, McQuay HJ. Faecal blood loss with aspirin, nonsteroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors: systematic review of randomized trials using autologous chromium-labelled erythrocytes. Arthritis Res Ther. 2008;10(1):R7.
Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427–33.
Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K, Tanaka S, Watanabe K, Sakurai T, Yokoi C, Akiyama J, Yanase M, Mizokami M, Uemura N. Colonic diverticular hemorrhage associated with the use of nonsteroidal anti-inflammatory drugs, low-dose aspirin, antiplatelet drugs, and dual therapy. J Gastroenterol Hepatol. 2014;29(10):1786–93.
Lanas A, Carrera P, Arguedas Y, Garcia S, Bujanda L, Calvet X, Ponce J, Perez-Asisa A, Castro M, Muñoz M, Sostres C. and Garcia Rodrigez. Risk of Upper and Lower Gastrointestinal Bleeding in Patients Taking Non-steroidal Anti-inflammatory Drugs, Antiplatelet Agents, or Anticoagulants. Clin Gastroenterol Hepatol. 2015;13(5):906–12.
Cea Soriano L, Rodriguez LA. Risk of upper gastrointestinal bleeding in a cohort of new users of low-dose ASA for secondary prevention of cardiovascular outcomes. Front Pharmacol. 2010;1:126.
Lanas A, Bajador E, Serrano P, Fuentes J, Carreno S, Guardia J, et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med. 2000;343:834–9.
Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment Pharmacol Ther. 2002;16:779–86.
Sorensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, McLaughlin JK, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95:2218–24.
Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28.
Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502–17.
Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KA, Shao M, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation. 2010;121:2575–83.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
Fletcher EH, Johnston DE, Fisher CR, Koerner RJ, Newton JL, Gray CS. Systematic review: Helicobacter pylori and the risk of upper gastrointestinal bleeding risk in patients taking aspirin. Aliment Pharmacol Ther. 2010;32(7):831–9.
Sostres C, Carrera-Lasfuentes P, Benito R, Roncales P, Arruebo M, Arroyo MT, Bujanda L, García-Rodríguez LA, Lanas A. Peptic Ulcer Bleeding Risk. The Role of Helicobacter Pylori Infection in NSAID/Low-Dose Aspirin Users. Am J Gastroenterol. 2015;110(5):684–9.
Chan FK, Ching JY, Suen BY, Tse YK, Wu JC, Sung JJ. Effects of Helicobacter pylori infection on long-term risk of peptic ulcer bleeding in low-dose aspirin users. Gastroenterology. 2013;144(3):528–35.
Iijima K, Ara N, Abe Y, Koike T, Iwabuchi T, Shinkai H, Uno K, Endo H, Asano N, Shimosegawa T. Biphasic effects of H. pylori infection on low-dose aspirin-induced gastropathy depending on the gastric acid secretion level. J Gastroenterol. 2012;47(12):1290–7. doi:10.1007/s00535-012-0598-8.
Casado-Arroyo R, Gargallo C, Lanas Arbeloa A. Balancing the risk and benefits of low-dose aspirin in clinical practice. Best Pract Res Clin Gastroenterol. 2012;26:173–84.
Sugano K. Prevention of upper gastrointestinal ulcer and complications in low-dose aspirin users. Curr Pharm Des. 2015;21:5082–8.
Graham DY, Smith JL, Spjut HJ, Torres E. Gastric adaptation. Studies in humans during continuous aspirin administration. Gastroenterology. 1988;95:327–33.
de Abajo FJ, Garcia Rodriguez LA. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clin Pharmacol. 2001;1:1.
Buring JE, Peto R, Hennekens CH. Low-dose aspirin for migraine prophylaxis. JAMA. 1990;264:1711–3.
Diener HC, Lampl C, Reimnitz P, Voelker M. Aspiring in the treatment of acute migraine attacks. Expert Rev Neurother. 2006;6(4):563–73.
Stürmer T, Glynn RJ, Field TS, Taylor JO, Hennekens CH. Aspirin use and cognitive function in the elderly. Am J Epidemiol. 1996;143(7):683–91.
Rothwell P, Fowkes GR, Belch JFF, Ogawa H, Warlow C, Meade TW. Effect of daily aspirin on long term risk of death due to cancer: analysis of individual patient data from randomized trials. Lancet. 2011;377:31–41.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47.
Casado-Arroyo R, Bayrak F, Sarkozy A, Chierchia GB, de Asmundis C, Brugada P. Role of ASA in the primary and secondary prevention of cardiovascular events. Best Pract Res Clin Gastroenterol. 2012;26(2):113–23.
Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010;69(8):1453–8.
Lanas A, Garcia-Tell G, Armada B, Oteo-Alvaro A. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011;9:38.
Lanas A, Polo-Tomás M, Casado-Arroyo R. The aspirin cardiovascular/gastrointestinal risk calculator--a tool to aid clinicians in practice. Aliment Pharmacol Ther. 2013;37(7):738–48.
Capone ML, Sciulli MG, Tacconelli S, Grana M, Ricciotti E, Renda G, Di Gregorio P, Merciaro G, Patrignani P. Pharmaco-dynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol. 2005;45:1295–301.
Anzellotti P, Capone ML, Jeyam A, Tacconelli S, Bruno A, Tontodonati P, Di Francesco L, Grossi L, Renda G, Merciaro G, Di Gregorio P, Price TS, Garcia Rodriguez LA, Patrignani P. Low-dose naproxen interferes with the antiplatelet effects of aspirin in healthy subjects: recommendations to minimize the functional consequences. Arthritis Rheum. 2011;63:850–9.
Patrignani P, Tacconelli S, Piazuelo E, Di Francesco L, Dovizio M, Sostres C, Marcantoni E, Guillem-Llobat P, Del Boccio P, Zucchelli M, Patrono C, Lanas A. Reappraisal of the clinical pharmacology of low-dose aspirin by comparing novel direct and traditional indirect biomarkers of drug action. J Thromb Haemost. 2014;12(8):1320–30.
Chan FK, Chung SC, Suen BY, Lee YT, Leung WK, Leung VK, Wu JC, Lau JY, Hui Y, Lai MS, Chan HL, Sung JJ. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med. 2001;344(13):967–73.
Donnelly MT, Goddard AF, Filipowicz B, Morant SV, Shield MJ, Hawkey CJ. Low-dose misoprostol for the prevention of low-dose aspirin-induced gastroduodenal injury. Aliment Pharmacol Ther. 2000;14(5):529–34.
Watanabe T, Sugimori S, Kameda N, Machida H, Okazaki H, Tanigawa T, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6(11):1279–82.
Lanas A, García-Rodríguez LA, Arroyo MT, Bujanda L, Gomollón F, Forné M, Aleman S, Nicolas D, Feu F, González-Pérez A, Borda A, Castro M, Poveda MJ. Arenas J; Investigators of the Asociación Española de Gastroenterología (AEG). Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102(3):507–15.
Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomized, double-blind, placebo-controlled trial. Lancet. 2009;374(9684):119–25.
Ng FH, Wong SY, Lam KF, Chu WM, Chan P, Ling YH, et al. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology. 2010;138(1):82–8.
Lin KJ, Hernandez-Diaz S, Garcia Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141(1):71–9.
Sugano K, Choi MG, Lin JT, Goto S, Okada Y, Kinoshita Y, Miwa H, Chiang CE, Chiba T, Hori M, Fukushima Y, Kim HS, Chang CY, Date M, LAVENDER Study Group. Multinational, double-blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the LAVENDER study. Gut. 2014;63(7):1061–8. doi:10.1136/gutjnl-2013-304722. Epub 2013 Dec 10.
Iwakiri R, Higuchi K, Kato M, Fujishiro M, Kinoshita Y, Watanabe T, Takeuchi T, Yamauchi M, Sanomura M, Nakagawa H, Sugisaki N, Okada Y, Ogawa H, Arakawa T, Fujimoto K. Randomised clinical trial: prevention of recurrence of peptic ulcers by rabeprazole in patients taking low-dose aspirin. Aliment Pharmacol Ther. 2014;40(7):780–95. doi:10.1111/apt.12907. Epub 2014 Aug 6.
Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346(26):2033–8.
Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909–17.
Endo H, Higurashi T, Hosono K, Sakai E, Sekino Y, Iida H, Sakamoto Y, Koide T, Takahashi H, Yoneda M, Tokoro C, Inamori M, Abe Y, Nakajima A. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. J Gastroenterol. 2011;46(7):894–905.
Hennekens CH, Jonas MA, Buring JE. The benefits of aspirin in acute myocardial infarction. Still a well-kept secret in the United States. Arch Intern Med. 1994;154(1):37–9.
Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol. 2010;105(1):34–41.
Lanas A, Gargallo CJ. Management of low-dose aspirin and clopidogrel in clinical practice: a gastrointestinal perspective. J Gastroenterol. 2015;50(6):626–37.
Hennekens CH, DeMets DL. Prevention: Aspirin in primary prevention needs individual judgements. Nat Rev Cardiol. 2014;11(8):438–40.
Lanas A, Benito P, Alonso J, Hernández-Cruz B, Barón-Esquivias G, Perez-Aísa A, Calvet X, García-Llorente JF, Gobbo M, Gonzalez-Juanatey JR. Safe prescription recommendations for non steroidal anti-inflammatory drugs: consensus document elaborated by nominated experts of three scientific associations (SER-SEC-AEG). Gastroenterol Hepatol. 2014;37:107–27.
Sung JJ, Lau JY, Ching JY, Wu JC, Lee YT, Chiu PW, Leung VK, Wong VW, Chan FK. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152(1):1–9.
Derogar M, Sandblom G, Lundell L, Orsini N, Bottai M, Lu Y, Sadr-Azodi O. Discontinuation of low-dose aspirin therapy after peptic ulcer bleeding increases risk of death and acute cardiovascular events. Clin Gastroenterol Hepatol. 2013;11(1):38–42.
Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47(10):a1–46. doi:10.1055/s-0034-1393172.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Wood, S.K., Lanas, A., Hennekens, C.H. (2016). Aspirin in the Treatment and Prevention of Cardiovascular Disease: Need for Individual Clinical Judgments. In: Lanas, A. (eds) NSAIDs and Aspirin. Springer, Cham. https://doi.org/10.1007/978-3-319-33889-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-33889-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33887-3
Online ISBN: 978-3-319-33889-7
eBook Packages: MedicineMedicine (R0)