Abstract
Background
Few studies have investigated measures to prevent small bowel injuries induced by aspirin. Our aim was to evaluate the effect of probiotic treatment on the small bowel injuries induced by chronic low-dose aspirin use.
Methods
Thirty-five patients who took low-dose enteric-coated aspirin 100 mg daily (for more than 3 months) plus omeprazole 20 mg daily and were diagnosed as having unexplained iron deficiency anemia participated in this prospective randomized controlled trial. We assigned the patients to receive probiotic treatment with Lactobacillus casei for 3 months (L. casei group) or not receive the probiotic (control group). Patients underwent capsule endoscopy (CE) before and after treatment.
Results
Twenty-five patients, including 13 in the L. casei group and 12 in the control group, underwent the full analysis. Significant decreases in the number of mucosal breaks and the CE score were observed at the 3-month evaluation in the L. casei group as compared with the results in the control group (P = 0.039). The change from the baseline in the median number of mucosal breaks in the L. casei group was −2, as compared with 0.5 in the control group. The change from the baseline in the median CE score in the L. casei group was −228 compared with −4 in the control group (P = 0.026).
Conclusions
Co-administration of L. casei is effective for the treatment of aspirin-associated small bowel injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-dose aspirin, commonly defined as 75–325 mg daily, is widely used in the clinical setting for the prevention of primary and secondary cardiovascular and cerebrovascular thrombotic events [1–3]. However, it is well-known that the use of low-dose aspirin is also associated with a risk of serious upper gastrointestinal complications, such as peptic ulceration and bleeding [4, 5]. Until recently, attention had mainly been focused on aspirin-induced damage of the stomach and duodenum; it had remained under debate as to whether low-dose aspirin might also be injurious to the small bowel, even though ‘full-dose’ aspirin taken as an anti-inflammatory and analgesic medication had been well known to exert intestinal toxicity.
There has been growing interest among gastroenterologists on the adverse effects of aspirin on the small bowel, especially as new endoscopic techniques, such as capsule endoscopy (CE) and double-balloon enteroscopy, have become available for the evaluation of small bowel lesions [6, 7]. In a preliminary CE study, we demonstrated that even short-term administration of low-dose aspirin induced mild mucosal inflammation of the small bowel [8]. In addition, recent clinical studies have revealed that chronic use of low-dose aspirin causes a variety of severe lesions in the small bowel, including erosions, ulcerations and diaphragm-like strictures [9, 10]. However, few studies have investigated measures to prevent small bowel injury induced by aspirin. The recommended treatment for small bowel injury in patients taking low-dose aspirin is withdrawal of aspirin; however, in the majority of patients, low-dose aspirin is used as an antiplatelet agent and can therefore not be discontinued on account of the increased risk of cardiovascular or cerebrovascular morbidity and mortality. Thus, novel means for the treatment of this enteropathy are urgently needed.
It has been suggested that aspirin causes gastric mucosal injury through the inhibition of cyclooxygenase (COX) and a topical irritant effect [11]. In regard to injuries of the small bowel, the same mechanisms are considered to be involved in increasing the intestinal permeability, which allows mucosal exposure to a variety of enterobacteria, with consequent bowel inflammation and injury. Inflammatory responses triggered by gram-negative bacteria have been reported to play a key role in nonsteroidal anti-inflammatory drug (NSAID)-induced enteropathy [12]. Therefore, we hypothesized that modulation of the intestinal flora might be useful as a protective measure against NSAID/aspirin-induced enteropathy.
Probiotics are living microorganisms that belong to the natural flora, and are important to the health and well-being of the host [13]. Probiotic bacteria have been demonstrated to have possible therapeutic effects against intestinal inflammation [14, 15]. Probiotic Lactobacillus strains have been reported to possess antimicrobial activity [16, 17]. Administration of Lactobacillus casei (L. casei) has been shown to prevent the development of experimental colitis [18]. Furthermore, recent observations also support the role of probiotics in the treatment of NSAID-induced small bowel mucosal inflammation [19, 20]. L. casei has been demonstrated to exhibit a preventive effect on indomethacin-induced small bowel injury in an animal experiment [19].
The aim of this study was to evaluate the effects of probiotic treatment (L. casei) on small bowel injury in chronic low-dose aspirin users.
Methods
Study design
This was a pilot, prospective, two-center, endoscopist-blinded, randomized, controlled study. All eligible patients from two hospitals who consented to participate in this study underwent CE at study entry (baseline CE). Eligible patients not meeting any of the exclusion criteria (see below for definitions of the exclusion criteria) were randomized at a 1:1 ratio to receive either probiotic treatment with L. casei (L. casei group) or not receive probiotic treatment (control group). Patients in the L. casei group received viable L. casei (BIOLACTIS® POWDER, Yakult Honsha, Tokyo, Japan) at doses of 45 × 108 to 63 × 109 colony-forming units (CFU) daily for 3 months; patients in the control group received no drugs. An independent clinician who was not part of the investigation conducted the allocation and block randomization according to a computer-generated schedule. Post-treatment CE was performed after 3 months of treatment. The data of patients who discontinued aspirin or probiotic use during the study period were excluded from the final analysis. This study was conducted in accordance with the Declaration of Helsinki. This two-center study was conducted with the approval of the ethics committee at both institutions (Yokohama City University Hospital and Yokohama Rosai Hospital). Written informed consent was obtained from all the patients. This trial is registered with the UMIN Clinical Trials Registry, no. UMIN000001550.
Patients
Patients taking low-dose enteric-coated aspirin 100 mg once daily (for more than 3 months) plus omeprazole 20 mg once daily, who were found to have unexplained iron deficiency anemia (decline in blood hemoglobin concentration to below 13 g/dl in men and 12 g/dl in women with iron deficiency) were eligible for inclusion in the study and for the baseline CE. All of the patients had undergone a total colonoscopy and gastroscopy prior to undergoing CE. Written informed consent for the CE procedure was obtained from all the patients. Patients were excluded from the study if they had known or suspected small bowel obstruction or stricture, swallowing disorders, an implanted pacemaker, pregnancy, history of surgical operation or radiation therapy for the abdomen, active gastrointestinal disease or inflammatory bowel disease, a history of overt gastrointestinal bleeding, positive stool cultures for any pathogens, or any serious disease of the central nervous system, liver or kidney. Patients who had taken NSAIDs, misoprostol, sulphasalazine, probiotics, prebiotics, synbiotics or antibiotics within 3 months prior to the study were also not eligible for participation in the study. The patients underwent a baseline CE examination at study entry, based on which further exclusion criteria were added, including failure to access the full length of the small bowel and the presence of small bowel lesions that could cause iron deficiency anemia, such as angioectasia and tumors.
Capsule endoscopy procedure and evaluation
All videos were reviewed using the PillCam SB and PillCam SB2 CE system (Given Imaging Ltd., Israel). CE was performed after a 12-h fasting period. No bowel preparations, such as polyethylene glycol solution or sodium phosphate, were used.
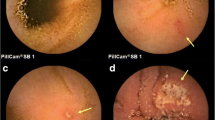
Two independent investigators (H.E. and T.H.) who were blinded to the allocation status of the subjects to the L. casei or control group separately reviewed each of the CE examinations. If the two investigators reported different findings for a particular lesion, a consensus was reached through discussion. The small bowel mucosal injury was classified into mucosal breaks or reddened lesions as follows [21]: mucosal breaks were defined as lesions with central pallor and surrounding erythema; neither the depth of the ulcers nor the size of the lesions was taken into consideration; reddened lesions were defined as reddish mucosal changes such as reddened folds, denuded areas and petechiae, all grouped into a single classification. Examples of typical mucosal breaks and reddened lesions found in this study are shown in Fig. 1. The numbers of mucosal breaks and reddened lesions of the small bowel were calculated for each patient and compared between before and after treatment, to evaluate the efficacy of probiotics in aspirin-associated enteropathy.
In addition, we also determined the CE score [22] for small bowel mucosal inflammatory changes to strengthen the validity of the results. This scoring index was based on three capsule endoscopic variables: villous appearance, ulceration and stenosis. The severity of the mucosal inflammatory changes was assessed by tertiles, dividing the small bowel transit time into three equal time allotments. The total score was the sum of the highest tertile score plus the stenosis score. The results were classified into three categories based on the final numerical score: normal or clinically insignificant change (<135), mild change (between 135 and 790), and moderate or severe change (≥790). This scoring system has been shown to be useful for evaluating aspirin-associated small bowel mucosal disease activity and for objectively scoring the small bowel inflammatory disease state [23].
Laboratory studies, including a complete blood count and blood chemistry, were performed at study entry and at the end of treatment.
The primary efficacy endpoint of this study was the changes in the numbers of small bowel lesions (mucosal breaks and reddened lesions) and of the CE score from the baseline CE to the post-treatment CE performed after 3 months of treatment. The percentage of patients with at least one mucosal break was also calculated in both groups. The secondary endpoints included the change from the baseline to the post-treatment assessment of the serum hemoglobin concentration.
Safety assessment
A safety assessment was carried out based on documentation of any adverse events that occurred during the study period.
Statistical analysis
The results were presented as the mean or median (± standard deviation or range) for quantitative data and as frequency (percentage) for the categorical data. Age and hemoglobin concentration were compared by Student’s t test. The duration of aspirin use, the number of mucosal breaks, reddened lesions and CE score were compared by the Mann-Whitney U test. The proportions of patients with mucosal breaks or reddened lesions were compared by Fisher’s exact test. The Wilcoxon’s signed rank test was used to compare the number of mucosal breaks/reddened lesions, CE score, and hemoglobin concentration at the baseline CE and with those at the post-treatment CE in each group. The changes from the baseline in the number of mucosal breaks/reddened lesions, CE score and hemoglobin concentration after 3 months’ treatment were compared between the L. casei and control groups by the Mann-Whitney U test. P values of <0.05 were considered to denote statistical significance.
Results
Patient characteristics
Between May 2009 and June 2010, 35 patients participated in this trial and underwent a baseline CE examination at study entry. Among the 35 patients, 29 were found to be eligible for this study; of these, 15 were randomly assigned to the L. casei group and 14 to the control group. Six patients were found to be ineligible based on our exclusion criteria: four patients were excluded because of the presence of small bowel angioectasia, and two because the capsule did not reach the cecum within the reading time. None of the patients developed permanent retention of the capsule and required endoscopic/surgical removal of the capsule. After the randomization, an additional three patients, comprising one from the L. casei group and two from the control group, were excluded from our final analysis because of the discontinuation of the aspirin treatment for medical reasons during the study period, and a further one patient from the L. casei group was excluded because of poor compliance with the study medication (L. casei). Follow-up CE was not performed in these four patients. Thus, post-treatment CE for analysis of the changes in the small bowel lesions was carried out in 13 patients of the L. casei group and 12 patients of the control group. A flow chart of the study is shown in Fig. 2. All the patients’ clinical data were followed for at least 3 months; however, none of the patients was newly diagnosed to have Crohn’s disease, Behçet’s disease or intestinal tuberculosis.
The characteristics of the patients are shown in Table 1. Of the 24 patients, 7 patients, including 3 of the L. casei group and 4 of the control group, were receiving aspirin in combination with another anticoagulant. There were no significant differences between the two groups at the baseline CE examination with regard to the patient characteristics, the CE findings or the CE scores. At entry, the baseline median number of mucosal breaks was 3 (range 0–41), the number of reddened lesions was 9 (range 3–37), and the baseline median CE score was 340 (range 112–1518) in the L. casei group, with corresponding results of 2.5 (0–91), 8 (0–16) and 348 (112–2,140), respectively, in the control group. The percentage of patients with at least one mucosal break was 84.6% in the L. casei group and 75.0% in the control group.
Efficacy assessment
Capsule endoscopy findings after probiotic treatment
As shown in Table 2, in the L. casei group, the number of mucosal breaks decreased significantly from a median of three in the baseline CE to a median of one in the post-treatment CE (P = 0.008). In the control group, no significant difference in the median number of mucosal breaks was observed between baseline and post-treatment CE (P = 0.859). A decrease in the percentage of patients with at least one mucosal break was observed in response to probiotic treatment in the L. casei group [84.6% (11/13) in the baseline CE versus 53.8% (7/13) in the post-treatment CE]; however, the difference did not reach statistical significance (P = 0.202). On the other hand, in the control group, the percentage of patients with mucosal breaks increased slightly during the study period; there was no significant difference within the group between these time points (P > 0.999).
Reddened lesions were found in all patients, regardless of probiotic treatment, at post-treatment CE. The difference in the median number of reddened lesions before and after treatment was statistically significant (P = 0.020) in the L. casei group, but not significant (P = 0.670) in the control group (Table 2).
In the primary efficacy analysis, the decrease in the number of mucosal breaks from the baseline CE to the post-treatment CE was significantly greater in the L. casei group than that in the control group (P = 0.039) (Table 3). The decrease in the number of reddened lesions from the baseline CE to the post-treatment CE was also significantly greater in the L. casei group than that in the control group (P = 0.005) (Table 3).
Capsule endoscopy score for small bowel mucosal inflammatory changes
As shown in Fig. 3a, L. casei significantly improved the median CE scores from 340 (range 112–1518) in the baseline CE to 143 (range 8–462) in the post-treatment CE (P = 0.004). Both the patients who were categorized as showing moderate or severe changes (score ≥ 790) at the baseline CE in the L. casei group converted to mild change (135 ≤ score < 790) after treatment. Furthermore, probiotic treatment changed the category of the CE score in five of the ten patients from mild change (135 ≤ score < 790) to normal or clinically insignificant change (score < 135). In the control group, the median CE scores were comparable between baseline (348; range 112–2140) and post-treatment CE (340; range 112–1,630); this difference was not statistically significant (P = 0.875) (Fig. 3b). Of the ten patients who were categorized as having mild change at the baseline CE in the control group, only one converted to normal or clinically insignificant change, while in one patient, the changes were found to have deteriorated to moderate or severe change at the post-treatment CE. In one patient, normal or clinically insignificant change at the baseline CE worsened to mild change at the post-treatment CE. These results suggest the probiotic co-therapy attenuated the severity of the aspirin-associated mucosal injury.
The decrease in the CE score from the baseline CE to the post-treatment CE was significantly greater in the L. casei group than in the control group (P = 0.026) (Table 3).
Distribution of small bowel lesions
In most patients, the mucosal breaks were multifocal and were evenly distributed in the small bowel (Table 4). However, reddened lesions showed a tendency to exist in the proximal part of the small bowel (Table 4).
Table 5 compares the distribution of small bowel injuries at the baseline and post-treatment CE to evaluate the correlation between the therapeutic effect of L. casei and the distribution of aspirin-induced small bowel injuries. In the first tertile, significant decreases in the percentage of the patients with mucosal breaks and the number of mucosal breaks were observed at the post-treatment CE compared with the results at the baseline CE (P = 0.047, P = 0.017, respectively). A decrease in the number of mucosal breaks in the third tertile was observed in response to probiotic treatment in the L. casei group; however, the difference did not reach statistical significance (P = 0.058).
Change in hemoglobin concentration
The hemoglobin concentration significantly increased after treatment in the L. casei group (median 11.1–12 g/dl; P = 0.002) (Fig. 4a). In the control group, on the other hand, no significant difference of the hemoglobin concentration was noted between the baseline and post-treatment CE (median 11.6–11.8 g/dl; P = 0.196) (Fig. 4b).
In the secondary efficacy analysis, the increase in the hemoglobin concentration from the baseline to post-treatment was greater in the L. casei group than in the control group; however, the difference did not reach statistical significance (P = 0.183) (Table 3).
Safety
No side effects or significant changes from the baseline values of any of the laboratory parameters examined were recorded in either group of patients.
Discussion
This is the first randomized controlled trial using CE performed to examine the efficacy of probiotic treatment on small bowel injury in chronic low-dose aspirin users. We found that patients treated with L. casei showed a significant decrease in the number of small bowel lesions associated with low-dose aspirin use. Moreover, this probiotic treatment was associated with a significant improvement in the CE scoring index. Thus, co-administration of L. casei decreased the incidence and severity of aspirin-associated small bowel injury.
The pathogenesis of NSAID/aspirin-induced enteropathy is likely to be multifactorial; however, the response to antibiotic treatment suggests a significant role for the enteric bacteria [24–27]. NSAID/aspirin ingestion may disrupt the homeostasis of the intestinal flora and induce overgrowth of gram-negative and anaerobic bacterial species [28]. Enterobacterial translocation into the mucosa represents the first step that sets in motion a series of events leading to gross lesion formation. In particular, gram-negative bacteria have been reported to play a key role in NSAID/aspirin-induced enteropathy [12]. The role exerted by enterobacteria in the pathogenesis of NSAID/aspirin-induced enteropathy is assumed to be similar to those of Crohn’s disease [29]. Therefore, based on the previous studies showing the efficacy of probiotics against inflammatory bowel disease, we decided to use a probiotic strain for this study. Probiotic Lactobacillus strains, including L. casei, have been reported to possess antimicrobial activity [16, 17]. Lactobacillus strains inhibit the growth of bacterial pathogens and can even have a bactericidal effect mediated by the production of metabolites, such as lactic acid, and the resultant lowering of the pH [30]. In particular, the efficacy of L. casei on intestinal inflammation has been demonstrated in various studies [31, 32]. L. casei has been proven effective in improving murine chronic inflammatory bowel diseases by inhibiting the expression of pro-inflammatory cytokines in lamina propria mononuclear cells. The potent pro-inflammatory cytokine tumor necrosis factor α (TNF-α) seems to be one of the key factors in the pathogenesis of intestinal inflammation in both Crohn’s disease and NSAID-induced enteropathy [33–35]. L. casei has been reported to modulate the production of TNF-α released by inflamed Crohn’s disease mucosa [35]. On this basis, we decided to use a single-strain probiotic bacterium, L. casei. Moreover, recent studies have supported the potential therapeutic role of probiotics in small bowel inflammation induced by NSAIDs or aspirin. Watanabe et al. [19] reported that the L. casei strain Shirota protects against indomethacin-induced small bowel injury in rats and that its probiotic effects may be mediated through the anti-inflammatory effects of lactic acid. More recently, Montalto et al. [20] showed that treatment with probiotic mixture (VSL#3) including L. casei significantly reduced the fecal calprotectin concentrations in healthy volunteers receiving indomethacin. However, the efficacy of probiotics has only been indirectly evaluated, and up till now, there have been no reports on low-dose aspirin-associated small bowel injury. In the present study, we directly evaluated the effect of L. casei using CE and found that the probiotic stimulated healing of aspirin-associated mucosal injuries.
As regards doses and time of administration, up till the present no clear data regarding the relationship between the amount of probiotic bacteria and the beneficial effects have been reported. In particular, no studies have appeared small bowel injury in chronic NSAIDs/aspirin users. Therefore, we based the therapeutic term (3 months) according to the previous studies [36, 37] in which the effect of the probiotic on small bowel inflammation was endoscopically evaluated in a similar manner to our study. As for the dose of L. casei, it would be desirable to use a high dosage probiotics containing a high concentration of live bacteria to ensure their survival with functional activity along the entire length of the intestine. It is unclear whether the dose of L. casei used in this study is optimal, but the dose of L. casei used in the present study (45 × 108 to 63 × 109 CFU daily) is higher compared with similar previous work. Thus, we designed our study based on the speculation that this dose of L. casei would be enough to prevent aspirin-induced small bowel injury. Further investigations are needed to confirm the optimal therapeutic term and doses of probiotic treatment on small bowel injury.
As for the prevention of NSAID-induced small bowel injury, several studies have already shown that omeprazole, a proton pump inhibitor, is not effective [38, 39], whereas misoprostol, a prostaglandin analog, effectively reduced the incidence of small bowel lesions induced by 2 weeks' administration of diclofenac [21]. Prostaglandin has been shown to reverse NSAID-induced changes in intestinal permeability [40], a local intestinal event that is considered to play a pivotal role in the development of inflammation and injury. The efficacy of this drug has also been reported in aspirin-induced enteropathy [41]. However, misoprostol is often poorly tolerated because of its side effects, such as diarrhea and abdominal pain. Indeed, in a pilot study to evaluate the efficacy of misoprostol on aspirin-induced enteropathy, 3 of the 11 patients who received misoprostol discontinued the drug owing to the development of severe watery diarrhea [41]. Therefore, development of an effective alternative agent against NSAID/aspirin-induced enteropathy is strongly desired.
Although our previous report showing the characteristics of small bowel injury in chronic low-dose aspirin users demonstrated that ulcers were observed mainly in the distal part of the small bowel [9], the mucosal breaks in this study did not show a similar tendency. A possible reason for this discrepancy is that the definition of the CE findings in this study abandoned the differentiation of mucosal breaks from other terms, such as erosions or ulcers, to simplify the evaluation of the efficacy of L. casei on aspirin-induced small bowel mucosal injury. Indeed, ulcers showed a tendency to exist in the distal part of the small bowel if we differentiated ulcers from mucosal breaks in this study (data not shown). Another possible reason is the interindividual differences among the patients who participated in the study. This study included asymptomatic patients with unexplained iron deficiency anemia, while our previous study included symptomatic patients with symptoms such as gastrointestinal bleeding or abdominal pain [9]. Furthermore, we compared the distribution of small bowel injuries at the baseline and post-treatment CE to evaluate the correlation between the therapeutic effect of L. casei and the distribution of aspirin-induced small bowel injuries. In the first tertile, significant decreases in the percentage of the patients with mucosal breaks and the number of mucosal breaks were observed at the post-treatment CE compared with the results at the baseline CE. A decrease in the number of mucosal breaks in the third tertile was also observed in response to probiotic treatment in the L. casei group; however, the difference did not reach statistical significance. These results may be influenced by the difference in the intestinal microbial flora between the proximal and the distal small bowel [42]. The luminal bacterial load increases from the proximal to the distal small bowel, and these changes may play a pathogenic role in NSAID/aspirin-induced injury. The efficacy of probiotic treatment in the distal part of the small bowel can be explained by the modulation of the abundant intestinal bacteria, thereby preventing enterobacteria from invading the small bowel mucosa. Although the intestinal bacterial flora is likely to be sparse in the proximal small bowel, low numbers of microorganisms, mainly consisting of acid-tolerant lactobacilli and streptococci, exist in the proximal part of the small bowel. The probiotics might have a great effect on these enterobacteria in the proximal small bowel because the concentration of ingested live bacteria with functional activity is higher in the proximal small bowel than in the distal small bowel. The actual mechanisms of probiotics against small bowel injuries remain poorly understood, and further investigations are needed.
CE has revealed numerous inflammatory lesions and has shed light on the small bowel mucosal injury induced by NSAIDs and aspirin. Despite these investigations, the clinical significance of NSAID/aspirin-associated mucosal injury is not yet clear. Almost all the patients taking low-dose aspirin have some degree of intestinal mucosal injuries, but it has not been investigated as to whether these lesions of the small bowel can actually explain the iron deficiency anemia of unknown source in patients on low-dose aspirin. Our results demonstrated that treatment with L. casei produced a significant improvement in serum hemoglobin concentration that was not observed in the control individuals. The hemoglobin concentration in the L. casei group changed in parallel with the small bowel mucosal injuries (the number of small bowel lesions and CE score), suggesting that these mucosal injuries might induce microbleeding and be the cause of the anemia of unknown source. On the other hand, the hemoglobin concentrations in a few patients in the control group increased without probiotic treatment. Further studies are needed to elucidate the correlation between the small bowel injuries and changes in the blood hemoglobin concentration in chronic low-dose aspirin users.
The present study had a number of limitations. The primary concern is the possibility that the CE findings might not be direct consequences of the low-dose aspirin administration. Follow-up CE examinations after aspirin withdrawal were not performed because the majority of the patients who take aspirin as an antiplatelet agent could not discontinue it. However, no patients had a new diagnosis of Crohn’s disease, Behçet’s disease, intestinal tuberculosis or other inflammatory bowel diseases. Moreover, the CE findings and scores in this study were consistent with those in other recent investigations that studied the characteristics of the small bowel injury in chronic low-dose aspirin users [9, 23, 41]. Thus, although most of the CE findings of this study are suggestive, they are still not definitive. Another limitation was the design of this study. Although our study was conducted as a randomized controlled trial, there was no placebo control group, and the study size was small. A placebo-controlled large-scale trial is needed to confirm our results. In addition, four patients were excluded from the current analysis after the randomization, and the follow-up CE was not performed in these patients. An intention-to-treat analysis would be desirable.
In conclusion, data from this pilot study suggest that probiotic treatment (L. casei) protects against aspirin-associated small bowel injury. Further larger scale studies are necessary to confirm the beneficial effect of probiotics.
References
Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–18.
Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–83.
Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ. 1995;310:827–30.
Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–94.
Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417.
Yamamoto H, Sekine Y, Sato Y, Higashiwaza T, Miyata T, Iino S, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–20.
Endo H, Hosono K, Inamori M, Kato S, Nozaki Y, Yoneda K, et al. Incidence of small bowel injury induced by low-dose aspirin: a crossover study using capsule endoscopy in healthy volunteers. Digestion. 2009;79:44–51.
Endo H, Hosono K, Inamori M, Nozaki Y, Yoneda K, Fujita K, et al. Characteristics of small bowel injury in symptomatic chronic low-dose aspirin users: the experience of two medical centers in capsule endoscopy. J Gastroenterol. 2009;44:544–9.
Matsumoto T, Kudo T, Esaki M, Yano T, Yamamoto H, Sakamoto C, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–6.
Graham DY, Smith JL. Aspirin and the stomach. Ann Intern Med. 1986;104:390–8.
Watanabe T, Higuchi K, Kobata A, Nichio H, Tanigawa T, Shiba M, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–7.
Fuller R. Probiotics in human medicine. Gut. 1991;32:439–42.
Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–9.
Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9.
Asahara T, Nomoto K, Watanuki M, Yokokura T. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother. 2001;45:1751–60.
Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, et al. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004;70:518–26.
Chung YW, Choi JH, Oh TY, Eun CS, Han DS. Lactobacillus casei prevents the development of dextran sulphate sodium-induced colitis in Toll-like receptor 4 mutant mice. Clin Exp Immunol. 2008;151:182–9.
Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol. 2009;297:G506–13.
Montalto M, Gallo A, Curigliano V, D’Onofrio F, Santoro L, Covino M, et al. Clinical trial: the effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy—a randomized, double-blind, cross-over, placebo-controlled study. Aliment Pharmacol Ther. 2010;32:209–14.
Fujimori S, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339–46.
Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–54.
Endo H, Hosono K, Higurashi T, Sakai E, Iida H, Sakamoto Y, et al. Quantitative of low-dose aspirin-associated small bowel injury using a capsule endoscopy scoring index. Dig Endosc. 2011;23:56–61.
Davies NM, Jamali F. Pharmacological protection of NSAID-induced intestinal permeability in the rat: effect of tempo and metronidazole as potential free radical scavengers. Hum Exp Toxicol. 1997;16:345–9.
Banerjee AK, Peters TJ. Experimental non-steroidal anti-inflammatory drug-induced enteropathy in the rat: similarities to inflammatory bowel disease and effect of thromboxane synthetase inhibitors. Gut. 1990;31:1358–64.
Koga H, Aoyagi K, Matsumoto T, Iida M, Fujishima M. Experimental enteropathy in athymic and euthymic rats: synergistic role of lipopolysaccharide and indomethacin. Am J Physiol. 1999;276:G576–82.
Bjarnason I, Hayllar J, Smethurst P, Price A, Gumpel MJ. Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropathy. Gut. 1992;33:1204–8.
Kent TH, Cardelli RM, Stamler FW. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol. 1969;54:237–49.
Scarpignato C. NSAID-induced intestinal damage: are luminal bacteria the therapeutic target? Gut. 2008;57:145–8.
Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol. 2006;100:1324–32.
Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N, et al. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140:417–26.
Ingrassia I, Leplingard A, Darfeuille-Michaud A. Lactobacillus casei DN-114001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn’s disease patients to adhere to and to invade intestinal epithelial cells. Appl Environ Microbiol. 2005;71:2880–7.
Bertrand V, Guimbaud R, Tulliez M, Mauprivez C, Sogni P, Couturier D, et al. Increased in tumor necrosis factor-alpha production linked to the toxicity of indomethacin for the rat small intestine. Br J Pharmacol. 1998;124:1385–94.
Saud B, Nandi J, Ong G, Finocchiaro S, Levine RA. Inhibition of TNF-alpha improves indomethacin-induced enteropathy in rats by modulating iNOS expression. Dig Dis Sci. 2005;50:1677–83.
Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, et al. Increased mucosal tumor necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–64.
Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F, et al. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn’s disease after ileo-caecal resection. Inflamm Bowel Dis. 2007;12:135–42.
Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther. 2003;17:509–15.
Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41.
Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Aisenberg J, Bhadra P, et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007;15:1211–22.
Bjarnason I, Smethurst P, Fenn CG, Lee CE, Menzies IS, Levi AJ. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1989;34:407–11.
Watanabe T, Sugimori S, Kameda N, Machida H, Okazaki H, Tanigawa T, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–82.
Fujimori S, Gudis K, Takahashi Y, Seo T, Yamada Y, Ehara A, et al. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur J Clin Invest. 2010;40:504–10.
Acknowledgments
This work was supported in part by Health and Labour Sciences Research Ministry of Health, Labour and Welfare of Japan to A.N.
Conflict of interest
The authors of the article have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endo, H., Higurashi, T., Hosono, K. et al. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. J Gastroenterol 46, 894–905 (2011). https://doi.org/10.1007/s00535-011-0410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0410-1