Abstract
Neutron activation analysis (NAA) is an isotope specific analytical technique for the qualitative and quantitative measurement of chemical elements. The method is based upon the conversion of stable atomic nuclei into radioactive nuclei by irradiation with neutrons and the subsequent detection of the radiation emitted during the decay of these radioactive nuclei. All of the stable elements have properties suitable for production of radioactive isotopes albeit at strongly different reaction rates. Each radionuclide is uniquely characterized by its decay constant—the probability for the nuclear decay in unit time- and the type and energy of the emitted radiation. Amongst the several types of radiation that can be emitted, gamma-radiation offers the best characteristics for the selective and simultaneous detection of radionuclides and thus of elements.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Neutron Activation Analysis
- Hair Sample
- Research Reactor
- Test Portion
- Radiochemical Neutron Activation Analysis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 History
The discovery of the artificial radioactivity by Irene Curie and Frederic Joliot in 1934 motivated many physicists and chemists to perform experiments where stabile nuclides convert into radioactive after irradiation with alpha particles, protons and the recent discovered neutron (1932). In (1936) George de Hevesy and Hilde Levi used for first time nuclear reactions after irradiation with neutrons for elemental analysis. They worked at that time in the by Niels Bohr headed Institute for Theoretical Physics at the University of Copenhagen. The idea of George de Hevesy was very simple: he used the correlation between the stable initial nuclides and the produced amount of radioactive nuclides in order to obtain qualitative and quantitative analysis of the chemical elements which belong to the initial products.
The available neutron fluxes were small at that time and the identification of the obtained radionuclides was possible due to their different half-life times only. In spite of this the first example showed successful analysis of 1 % Dysprosium in Yttrium sample after bombardment with neutrons.
After World War II nuclear reactors with neutron flux densities in order of magnitude of 1012 cm−2 s−1 became available which allowed for achieving a detection limit for certain elements below 1 µg. This opened new research fields in solid state physics where the properties of the materials as a function of their impurity were investigated.
The development of the methodological procedures and high resolution gamma-spectrometry detectors enable nowadays to analyze simultaneously up to 50–60 elements in a single sample.
2 Theory
The activation of samples will result in a mixture of activities, which can be analysed for individual contributions by two approaches:
-
i.
The resulting radioactive sample is decomposed, and through chemical separations it is divided into fractions with a few elements each: Destructive or Radiochemical Neutron Activation Analysis.
-
ii.
The resulting radioactive sample is kept intact, and the elements are determined by taking advantage of the differences in decay rates via measurements at different decay intervals utilizing equipment with a high energy resolution: Non-destructive or Instrumental Neutron Activation Analysis (INAA, Greenberg et al. 2011).
2.1 Activation
The activation with neutrons is, upon preparation of the test portion, the first stage in an INAA procedure. Each atomic nucleus can capture a neutron during irradiation, resulting in a nuclear reaction in which often the nuclear mass changes; immediately (typically 10−14 s) after the capture (‘promptly’) excess energy in the form of photons and/or particles will be emitted. If the newly formed nucleus is unstable, it starts already during the activation decaying to a stable state by the emission of radiation through one or more of the following processes: α-decay, β–decay, electron capture, β+-decay, or internal transition decay. In most cases γ- and X-radiation will be emitted too, Fig. 10.1.
Neutrons are produced in:
-
Isotopic neutron sources, like 226Ra(Be), 124Sb(Be), 241Am(Be), 252Cf.
-
Particle accelerators or neutron generators. The most common types are based on the acceleration of deuterium ions towards a target containing either deuterium or tritium, resulting in the reactions 2H(2H,n)3He and 3H(2H,n)4He, respectively.
-
Nuclear research reactors. These are the strongest sources of neutrons. The neutron output of research reactors is often quoted as neutron flux in an irradiation facility and varies, depending on reactor design and reactor power, between 1011 and 1014 cm−2 s−1.
In the majority of INAA procedures reactor neutrons are used for the activation. Materials can be activated in any physical state, viz. solid, liquid or gaseous. There is no fundamental necessity to convert solid material into a solution prior to activation; INAA is essentially considered to be a non-destructive method although under certain conditions some material damage may occur due to thermal heating, radiolysis and radiation tracks by e.g., fission fragments and α-radiation emitting nuclei.
2.2 Decay
Radioactive decay is a statistically random process; the probability that a given nucleus will decay in a certain time interval depends only of the time of observation. It is not possible to predict when a given nucleus will decay, but the decay characteristics can be described by the physical laws of radioactive decay whereas documented decay schemes provide the details of the decay of radionuclides, such as, e.g., half-lives involved, types of radiation emitted and relative intensities.
2.3 Measurement
In NAA, nearly exclusively the (energy of the) gamma-radiation is measured because of the penetrating power of this type of radiation, and the selectivity that can be obtained from the distinct energies of the photons. The gamma-spectrum is analyzed to identify the radionuclides and their amounts of induced activity in order to derive the target elements from which they have been produced. The masses of the elements are derived from the net peak areas by comparison with the induced activity of the same neutron activation produced radionuclides from known amounts of the element of interest in the calibrator.
The combination of energy of emitted radiation, relative intensities if photons of different energies are emitted and the half-life of the radionuclide is unique for each radionuclide, and forms the basis of the qualitative information in NAA.
3 Experimental Procedures
3.1 Practical Execution of NAA
In the practice, a NAA procedure consists of the simultaneous irradiation of test portions of the unknown sample and a known amount of a comparator of the same element—serving as a calibrator—, and the sequential measurements of the activities. An INAA procedure has the following steps:
-
Preparation (e.g., drying) and weighing of test portions of sample and calibrator; no need for dissolution.
-
Encapsulation of the test portions in e.g., plastic foil, plastic capsules or quartz ampoules.
-
Activation via simultaneous irradiation of test portions and calibrators with neutrons.
-
No chemical separations, material remains intact.
-
Sequential measurements of the activities in each test portion and calibrator by gamma-ray spectrometry after one or more decay periods. Thus, complementary information on various elements can be obtained.
-
Interpretation of the gamma-ray spectra towards elements and their masses.
The turnaround time (time between initiation of analysis and reporting) is largely element and matrix dependent, as the signal of the radionuclide of interest has to be measured in the presence and intensity of the signals of the radionuclides produced of the other elements in the sample. It may be necessary to wait hours to weeks after the irradiation to attain the optimal measurement conditions by decaying the interfering activity. Results on elements that can be measured via short-half-life radionuclides (such as Al, Mg, Ti, V, Cl, Mn, Se, Ca, S) can be available within minutes to hours since the measurement follows immediately the irradiation. In other cases, the turnaround time may vary from 1 week to 3 weeks.
3.2 Analytical Characteristics
The analytical characteristics of INAA are:
-
Non-destructive analysis. The test-portion does not have to be converted into a solution, thus dissolution losses do not exist.
-
Bulk analysis. The penetrating power of neutrons and gamma-radiation allows for analysis of the bulk masses of test portions up to several kilograms in size.
-
The method is based upon processes in the atomic nucleus. The chemical form and physical state of the elements do not influence the activation and decay process.
-
The method can be applied in a self-validating manner. A unique combination exists of the gamma-ray energies, their intensity ratios and the half-lives of the radionuclides, which provides opportunities for consistency checking.
-
Many adjustable experimental parameters, sometimes over several orders of magnitude, allow for optimizing the experimental design and identifying potential problems.
-
Elements such as C, H, N, O and Si, often found as major components of many matrices, do not activate well and contribute little or no gamma-ray activity.
-
The method is suitable for measurement of masses in the order range of 10−6–10−9 g and less, depending on the element to be measured.
4 Areas of Application
The element of interest and sample matrix should have specific chemical properties, physical forms and physical characteristics for analysis by NAA. The activation rates, half-lives and energies of the gamma-ray emission of the radionuclides are decisive factors for selecting NAA. The very low Z elements (like H, He, B, Be, C, N, O) are not suitable for measurement by (thermal neutron) NAA, as are a few other elements like Tl, Pb and Bi.
Sample matrices of high density that contain significant fractions of high atomic number elements (both affecting gamma-ray self-attenuation) are not good candidates for NAA measurements. Similarly, matrices that have extremely high neutron absorbing properties are difficult to measure accurately due to neutron self-attenuation. Significant mass fractions of B, Li and U are undesirable because their neutron capture results in the emission of charged-particle radiation, which may cause excessive thermal heating during the irradiation.
Examples of measurements where the analytical characteristics of neutron activation analysis are employed at full advantage include:
-
Solid materials that are difficult to bring completely into a solution, such as soils, rocks, minerals, air particulate matter, new composite materials, and materials with C, H, N, O as major elements like biological material and plastics
-
Solid materials that are easy to contaminate during preparation of the test portion if digestion would be needed for a different analytical technique. Examples are ultrapure materials, ultra-small amounts and biological tissues and fluids
-
Solid materials that are unique and should keep their integrity, such as materials from forensic studies and archaeological, cultural and art objects
-
Solid materials of which the bulk composition must be determined and for which surface, or near-surface techniques such as X-ray fluorescence spectrometry and some solid-state spectrometric techniques are inadequate.
Such samples can be found within the following selected group of disciplines:
-
Archaeology—amber, bone, ceramics, coins, glasses, jewelry, metal artifacts and sculptures, paintings, pigments, pottery, soils and clays, stone artifacts.
-
Biomedicine—animal and human tissues, blood and blood components, bone, drugs and medicines, gallstones, hair, implant corrosion, kidney and—stones, medical plants and herbs, milk, nails, placenta, snake venom, teeth, dental enamel and—fillings, urine and—stones.
-
Environmental science and related fields—aerosols, atmospheric particulates, fossil fuels and their ashes, animals, birds, insects, fish, aquatic and marine biota, seaweed, algae, lichens, mosses, plants, trees, household and municipal waste, soils, sediments, sewage sludge.
-
Forensics—bomb debris, bullet lead, explosives, glass fragments, paint, hair, gunshot residue swabs, shotgun pellets.
-
Geology and geochemistry—asbestos, bore hole samples, bulk coals and coal products, coal and oil shale components, crude oils, cosmo-chemical samples, coral, diamonds, exploration and biogeochemistry, meteorites, ocean nodules, rocks, sediments, soils, glacial till, ores and separated minerals.
-
Industrial products—alloys, catalysts, ceramics, refractory materials, coatings, electronic materials, fertilizers, fissile and other safeguard materials, graphite, high purity and high-tech materials, integrated circuit packing materials, oil products, pharmaceutical products, plastics, semiconductors, pure silicon and silicon processing.
-
Nutrition—composite diets, foods, honey, seeds, spices, vegetables, milk and milk formulae, yeast.
5 Examples
The measurement by INAA of elements in wood as an indicator of metal-organic wood preservatives illustrates the unique capabilities of the technique. Such an assessment cannot be done using XRF since results would only indicate the contents at the surface, not what has penetrated. Other techniques such as AAS or ICP, requiring dissolution of the wood, are not reliable, because the chemical may form insoluble species in the wood; even if the chemical is soluble, it is still necessary to dissolve the whole piece of wood to be able to measure it. Second, they are too labour intensive and costly. A measurement by INAA can be done on a bulk sample of 20 × 20 × 4 mm3 without any sample preparation preceding the neutron irradiation.
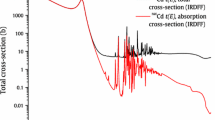
The use of potentially environmental hazardous elements like Cd in plastics is restricted by law in several countries. The recycling industry has to assess the Cd mass fraction of ‘input’ raw materials consisting of pieces of plastic of approximately 0.5 × 0.5 cm2, usually of different colours and composition (Fig. 10.2).
-
For conventional analysis such as by XRF, AAS or ICP, the pieces should be either dissolved, or reduced further in size and a thoroughly homogenized. The alternative is analysis by INAA where easily samples of several grams to kilograms can be analysed without the need to homogenization. Plastics contain mainly of the elements H, C, O and N, which upon neutron irradiation do not produce interfering activity. As such, the material become ‘transparent’ for the radiation emitted by the radionuclides produced in the bulk sample from the other elements in the material.
One of the famous examples of application of NAA for cultural heritage research is the investigation of hairs from Napoleon (Analytical Applications of Nuclear Techniques 2004; Clemenza et al. 2008; Lin et al. 2004).
The circumstances of Napoleon’s demise on 5 May 1821 have been shrouded by suspicion. The generally held belief was that he has fell victim to carcinoma of the stomach—the supposed cause of his father’s death in 1785. But an analysis of five samples of Napoleon’s hair taken after his death showed a largely enhanced level of arsenic. There was a hypothesis that he was poisoned. Therefore several hair samples taken from Napoleon at different stages of his life as well as from other persons living at this period (The King of Rome, Empress Josephine) were investigated by NAA method for determination of the Arsenic contamination with high precision. The Napoleonic hairs came from when he was a boy in Corsica, during his exile on the Island of Elba, on the day of his death (May 5, 1821) on Saint Helena and on the day after his death.
Samples taken from Napoleon’s son in 1812, 1816, 1821 and 1826 as well as samples from the Empress Josephine, collected upon her death in 1814, also were analyzed.
The hair samples were provided by the Glauco-Lombardi Museum in Parma (Italy), the Malmaison Museum in Paris and the Napoleonic Museum in Rome.
The samples were placed in capsules near the core of the reactor at the University of Pavia, Italy for neutron activation. The Arsenic concentration was determined from the 559 and 563 keV γ activity from the activation product 76As (Fig. 10.3).
The first result from the investigation was that the average Arsenic level was high but comparable with the typical level at that time. Modern persons show lower levels of Arsenic contamination. The level of arsenic in the hair samples from 200 years ago was found to be 100 times greater than the average level detected in samples from persons living today. In fact, the Emperor’s hair had an average arsenic level of around ten parts per one million, whereas the arsenic level in the hair samples from currently living persons was around one-tenth of a part per one million. In other words, at the beginning of the 19th century, people evidently ingested arsenic that was present in the environment in quantities that are currently considered dangerous (Fig. 10.4).
The second result was that there were no significant differences in arsenic levels between when Napoleon was a boy and during his final days in Saint Helena. According to the researchers, including toxicologists who participated in the study, it is evident that this was not a case of poisoning but instead the result of the constant absorption of arsenic.
6 Facilities
About 100–150 neutron activation analysis facilities exist worldwide, the majority directly affiliated within institutes of organizations governing a nuclear research reactor, such as national nuclear research establishments and/or universities. Other facilities have the irradiations carried our remotely in such a reactor and the neutron-activated samples transferred back for gamma-ray spectrometry and analysis at their premises.
The facilities differ mainly in analytical capabilities due to
-
Neutron flux available. This varies between 1012 cm−2 s−1 for the miniature research reactors to 1014 cm−2 s−1 for the high flux reactors.
-
Reactor operation schedule. Some research reactors operate for 100 h or more continuously (‘around the clock’); others have shorter operation schedules, in some cases only a few hours daily. Reactor schedule affects, to some degree, the analytical capacity (number of samples to be irradiated).
-
Availability of irradiation systems for analysis using very short half-life radionuclides (half-lives varying from 10 s to 10 min), essential for measuring elements like Al, Mg, Ti, V, Ca.
-
The type and number of gamma-ray spectrometers available for measurement of the induced activity. The gamma-ray spectrometers are equipped with semiconductor detectors. Well-type semiconductors offer the highest counting efficiency but are not available in all facilities.
-
Degree of automation. Automation in neutron activation analyses, especially in measurement of the activity, requires self-made sample changers and related software for automated data processing. Facilities are increasingly implementing this, but it is not universal available yet. The higher the degree of automation, the higher the throughput, analysis capacity and the shorter the reporting time.
-
Calibration methodology. Many facilities apply the ‘relative’ or ‘comparator’ method in which an unknown is analysed simultaneously with a sample of similar but known composition, like a certified reference material. This limits the number of elements that can be reported, Increasingly, facilities are implemented other calibration methods that allow for reporting of mass fractions and detection limits of over 50 elements, without the need for analysis of a reference material.
-
Degree of quality management implementation and laboratory accreditation (compliance with ISO/IEC17025:2005). Many NAA laboratories aim at such an accreditation, amongst others, to satisfy end-users. Accredited NAA laboratories are currently (2016) be found in The Netherlands, Belgium, Hungary, Romania, Slovenia, South Korea, Malaysia, Indonesia, Syria, Pakistan, Chile, Argentina, South Africa. This number of countries is increasing.
In the practice, there are only small differences (max. a factor of 5–10) in detection limits in different facilities. The differences in analysis throughput (number of samples to be analysed in a gven period) are larger, depending on the number of spectrometers available, the degree of automation and sometimes the operation schedule of the reactor.
The IAEA Research Reactor Data Base specifies, for each research reactor, if NAA facilities are available and operational. An overview of countries with NAA facilities may be found in given in Table 10.1, without claiming completeness. There are also countries without research reactors in which NAA is carried out using accelerator (neutron generator) based neutrons, or using isotopic neutron sources. These are not listed here.
References
Analytical Applications of Nuclear Techniques (2004) IAEA STI/PUB/1181, IAEA, Vienna. ISBN 92-0-114703-1, http://www-pub.iaea.org/books/IAEABooks/6878/Analytical-Applications-of-Nuclear-Techniques. Last visited 19 Feb 2016
Clemenza M et al (2008) Misure con attivazione neutronica sulla presenza di Arsenico nei capelli di Napoleone Bonaparte e di suoi famigliari. Il Nuovo Saggiatore 24:19–30
Greenberg RR, Bode P, De Nadai Fernandes EA (2011) Neutron activation analysis: a primary method of measurement. Spectrochim Acta B 66:193–241
Hevesy G, Levi H (1936) The action of neutrons on the rare earth elements. Det Kgl Danske Vidensk Selsk Mat-Fys Medd XIV, 5:3–34
IAEA Research Reactor Data Base. https://nucleus.iaea.org/RRDB/RR/ReactorSearch.aspx. Last visited 19 Feb 2016
IAEA Technical Document (TECDOC) 1215 (2001) Use of research reactors for neutron activation analysis, IAEA, Vienna, Austria. http://www-pub.iaea.org/books/IAEABooks/6171/Use-of-Research-Reactors-for-Neutron-Activation-Analysis. Last visited 19 Feb 2016
Lin X, Alber D, Henkelmann R (2004) Elemental contents in Napoleon’s hair cut before and after his death: did Napoleon die of arsenic poisoning? Anal Bioanal Chem 379:218–220
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bode, P. (2017). Neutron Activation Analysis (NAA). In: Kardjilov, N., Festa, G. (eds) Neutron Methods for Archaeology and Cultural Heritage. Neutron Scattering Applications and Techniques. Springer, Cham. https://doi.org/10.1007/978-3-319-33163-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-33163-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33161-4
Online ISBN: 978-3-319-33163-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)