Abstract
The relationship between vitamin D and heart structure and function is of crucial importance in chronic kidney disease (CKD) because cardiovascular events including sudden cardiac death are, beside cancer, the major cause of premature mortality in these patients. The vitamin D receptor (VDR) is expressed in the heart and the vessels, and experimental studies have documented various molecular effects of vitamin D that may protect against heart diseases. Epidemiological studies in CKD patients identified vitamin D deficiency as a risk marker for adverse cardiovascular outcomes. Randomized controlled trials (RCT) have shown that vitamin D treatment exerts beneficial effects on some cardiovascular risk factors such as parathyroid hormone and proteinuria, but there was no effect on myocardial hypertrophy. Whether vitamin D treatment can significantly reduce cardiovascular events in CKD patients is still unclear because available data are based on very few and relatively small RCTs. It should, however, be noted that some RCTs including one meta-analysis suggest that patients on active vitamin D treatment experience fewer cardiovascular events. Regarding clinical use of vitamin D in CKD patients, it must therefore be stressed that although patients on active vitamin D treatment are at increased risk of hypercalcemia, there is no clear indication that this potential adverse effect translates into higher cardiovascular risk. These safety considerations are of great importance when considering the clinical use of active vitamin D treatment, but further large RCTs are urgently needed to better characterize the cardiovascular effects of vitamin D treatment in CKD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cardiovascular events including sudden cardiac death are, beside cancer, the major causes of mortality in chronic kidney disease (CKD) patients [1–3]. There exists a complex interplay between cardiovascular diseases and CKD–mineral and bone disorders (CKD–MBD) which are of great importance for monitoring, treatment, and outcome of CKD patients [4]. In this context, it is classically known that vitamin D effects are crucial for the regulation of calcium and phosphate homeostasis, but accumulating evidence from experimental studies indicates that vitamin D may also participate in the pathogenesis of cardiovascular diseases. In line with this, vitamin D deficiency has also been identified as a risk marker for an adverse cardiovascular risk profile and for increased cardiovascular events and mortality [5, 6]. In this book chapter, we will give an overview on the existing knowledge on vitamin D and cardiovascular disease in CKD with a particular focus on heart structure and function. In addition, we will also briefly summarize data on the relationship between vitamin D and vessel diseases as well as cardiovascular risk factors because heart diseases are frequently the final consequence of these pathologies. Our work is based on a Pubmed literature search until October 2015 using the search terms “vitamin D AND kidney AND cardiovascular” and “vitamin D AND kidney AND heart”. We also checked the reference lists from the identified articles for further relevant literature. We aimed to restrict our work to data in the setting of CKD because general data on vitamin D and cardiovascular diseases have already been well reviewed elsewhere, and vitamin D metabolism in CKD differs significantly from individuals with normal kidney function [7–13]. We refer to the existing literature and to other chapters of this book with regard to basic vitamin D knowledge in CKD patients.
2 Experimental Studies
2.1 VDR Activation and Heart Structure and Function
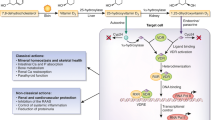
Expression of the vitamin D receptor (VDR) as well as of 1α-hydroxylase has been identified in the heart and the vessels, i.e. in cardiomyocytes, endothelial cells and vascular smooth muscle cells (VSMC) [14–17]. Main mechanisms for direct vitamin D effects on the myocardium are shown in Fig. 19.1. Knock-out mice for either VDR or 1α-hydroxylase are hypertensive with cardiac hypertrophy that is at least partially induced by overexpression of renin with increased activity of the renin angiotensin aldosterone system (RAAS). Even mice with a cardiomyocyte-specific VDR-knockout yield myocardial hypertrophy [18]. It is well documented in experimental studies that VDR activation suppresses cardiomyocyte hypertrophy, and reduces cell proliferation and atrial natriuretic peptide (ANP) gene expression [19–21]. Consistent with this, it has also been shown that rats on a low vitamin D diet develop myocardial hypertrophy and that treatment with VDR agonists exerts antihypertrophic effects [15, 22]. Interestingly, it has also been reported that calcitriol increases the expression and the activity of the type a natriuretic peptide receptor and may thus exert beneficial effects on cardiovascular health by e.g. increasing the excretion of sodium [23, 24].
VDR activation modulates myocardial contractility probably by regulating calcium flux in the myocardium [5, 25]. Regarding the vitamin D effects on myocardial function, it has been observed that 1α-hydroxylase knockout mice suffer from reduced systolic function that can be restored by calcitriol treatment [26]. Interestingly, VDR knockout mice showed accelerated rates of myocardial contraction and relaxation [27]. The effects of VDR activation on myocardial contractility are thus still puzzling but considering that calcitriol was also able to induce accelerated relaxation of cardiomyocytes, it is conceivable that VDR activation may be important for diastolic function [27]. This is supported by data in 5/6 nephrectomized rats showing that the VDR agonist VS-105 improves, beside ejection fraction and fractional shortening, also the E/A ratio, a routine echocardiographic parameter that is used to classify diastolic function [28].
VDR activation has also been shown to regulate myocardial extracellular matrix (ECM) turnover by effects on the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [29]. Whether vitamin D induced regulation of ECM turnover may protect against myocardial fibrosis is, however, still not entirely clear because experimental studies were not fully consistent. While studies in nephrectomized and uremic rats reported on antifibrotic effects of calcitriol and paricalcitol, the opposite, i.e. aggravated fibrosis, has been observed in rats with renal insufficiency that were treated with paricalcitol [30–32]. In murine models of cardiac steatosis, an increase in interstitial fibrosis is, however, observed in VDR knockout mice [33]. Furthermore, it was demonstrated that calcitriol prevents transforming growth factor β1 (TGFβ1) mediated pro-fibrotic changes in primary cardiac fibroblasts [34]. In diabetic rats, calcitriol reduced fibrosis and exerted beneficial effects on cardiac function [35, 36]. Survival rate and cardiac function after experimental myocardial infarction were also reduced in VDR knockout compared to wild-type mice [37]. Moreover, VDR activation protected against myocardial reperfusion injury in mice by reducing oxidative stress, and by inhibition of apoptosis and modulation of autophagy [38]. Apart from this, data from stress-exposed mice suggest that vitamin D signaling may protect against stress-induced deteriorating effects on the heart [39]. We can therefore conclude from these experimental data that VDR signaling is indeed important for the maintenance of a physiologic heart structure and function, but we must be aware that cell culture and animal studies may not adequately reflect the pathophysiology in CKD patients.
3 VDR Activation and Vessels
Excess as well as deficiency of vitamin D can lead to vascular calcification. Historically, it is well known for almost a century that vitamin D intoxication induces hypercalcemia with vascular calcification [7, 40]. High dose calcitriol treatment in subtotally nephrectomized rats leads to an increased aortic calcium and phosphate content and induces an osteoblastic phenotype in VSMC with an up-regulation of proteins regulating mineralization and calcium transport, and of osteogenic transcription factors [7, 41]. VDR activation induced vascular calcification may be induced by rather a systemic than a local effect because calcitriol induced aortic calcification in uremic rats did not differ between VDR knockout and VDR wild-type aortic allografts [42]. It is also important to note that calcitriol induced vascular calcification in rats is reversible after withdrawal of calcitriol [43, 44]. High serum phosphate levels seem to be critical for calcitriol induced vascular calcification because lowering phosphate levels can prevent vascular calcification in klotho knock-out mice, which are characterized by both high calcium and high calcitriol levels [43, 44]. In contrast to calcitriol induced vascular calcification it has also been reported that mice treated with a low vitamin D diet had more aortic calcification and higher expressions of osteogenic key factors than mice fed with recommended amounts of vitamin D [45, 46]. In a mouse model of CKD, calcitriol and paricalcitol protected against aortic calcification at dosages sufficient to correct secondary hyperparathyroidism, whereas higher dosages induced aortic calcification [47]. A molecular effect of VDR activation that may protect against vascular calcification is an increased expression of the anticalcification factor osteopontin as shown in aortic medial cells [48]. The osteogenic process of VSMC mineralisation induced by phosphate and tumor necrosis factor-α (TNF- α) could also be abrogated by VDR agonists [49].
Several experimental studies have, by the majority, shown that vitamin D may protect against endothelial dysfunction and atherosclerosis. VDR agonists improved endothelial function in 5/6 nephrectomized rats and in diabetic rats with early-stage nephropathy [50–52]. Mechanistically, it has been demonstrated that VDR signaling increases NO synthesis and reduces expression of cyclooxygenase-2 (COX-2) and thromboxane-prostanoid receptors [53, 54]. Expression of endothelial adhesion molecules, e.g. ICAM-1 (intercellular adhesion molecule 1) and VCAM-1 (vascular cell adhesion molecule 1), is suppressed by VDR activation, and knock-down of the VDR in endothelial cells was associated with endothelial activation characterized by increased leukocyte-endothelial cell interactions [55]. VSMC are also target cells for vitamin D and it has been reported that VDR knockout mice have an increased production of angiotensin-II and superoxide anions leading to premature senescence of VSMC [56]. VDR agonists may also suppress VSMC proliferation, but this has not been consistently reported in all studies [57, 58]. Moreover, calcitriol has been shown to inhibit foam cell formation and cholesterol uptake in macrophages of patients with type 2 diabetes mellitus [59]. Another anti-atherosclerotic effect of VDR activation is mediated by regulation of cholesterol efflux and macrophage polarization as shown in hypercholesterolemic swine [60]. Experimental data also indicate that VDR activation may promote vascular repair [61]
4 VDR Activation and Cardiovascular Risk Factors
Numerous experimental studies have shown that vitamin D may protect against a variety of classic and emerging risk factors. In this context, it must be stressed that the suppression of parathyroid hormone (PTH) is an important cardiovascular-protective effect of VDR activation when considering that PTH itself exerts several harmful effects on the heart and the vessels [62, 63]. Suppression of renin expression and the RAAS by vitamin D has been shown to prevent hypertension, atherosclerosis and heart diseases in experimental studies [14, 64–66]. Several other effects of VDR activation on cardiovascular risk factors such as inflammation, diabetes mellitus, arterial hypertension, blood lipids, coagulation, and renal diseases have been extensively reviewed elsewhere [7–11, 67].
5 Observational Studies
5.1 25-Hydroxyvitamin D and Cardiovascular Disease
Most, albeit not all, epidemiological studies in CKD patients showed that low levels of 25-hydroxyvitamin D (25[OH]D) are associated with an increased risk of cardiovascular disease and mortality including sudden cardiac death [68–80]. In small clinical studies, low serum 25(OH)D concentrations were partially associated with myocardial hypertrophy in CKD patients [81, 82]. Low serum 25(OH)D concentrations were associated with vascular calcification in some but not all studies in CKD patients [83–86]. Several studied in CKD patients showed, however, a significant association between vitamin D deficiency and endothelial dysfunction as well as clinical measures of atherosclerosis [85, 87–89]. It has been largely, but not consistently, observed that low serum 25(OH)D concentrations are associated with albuminuria, and decline of glomerular filtration rate (GFR) including progression to end-stage renal disease [77, 90–95].
6 Calcitriol and Cardiovascular Disease
Epidemiological studies in CKD patients have largely shown that low serum concentrations of calcitriol are associated with an increased risk of mortality and cardiovascular events [61, 66, 70, 88]. In CKD patients with and without dialysis, serum calcitriol levels were either inversely or not associated with vascular calcification and atherosclerosis [76, 78, 89, 90]. Moreover, in patients with advanced kidney disease, low serum calcitriol levels were predictive for initiation of long-term dialysis treatment [78].
7 Vitamin D Genetics and Cardiovascular Disease
In 182 dialysis patients there was a significant association between the Bsml VDR polymorphism and left ventricular mass index [99]. Similar associations have been confirmed in non-dialysis patients [100]. There was, however, no significant association between VDR polymorphisms and end-stage renal disease in a meta-analysis including 1510 patients and 1812 controls [101].
8 Vitamin D Treatment and Cardiovascular Diseases
8.1 Natural Vitamin D Treatment: Observational and Uncontrolled Studies
Some observational studies report on use of natural vitamin D supplements and cardiovascular diseases in CKD [102–113]. It has been observed that ergocalciferol treatment was associated with reduced cardiovascular events in 126 older men with CKD stages 3 and 4 [104]. An observational study in hemodialysis patients reported on significant improvement in left ventricular function (i.e. decreased end-diastolic and end-systolic diameters) in five patients treated with 100 μg 25(OH)D for 8 months when compared to five patients without vitamin D supplementation [105]. A 1-year prospective study in hemodialysis patients showed that oral cholecalciferol supplementation was associated with significantly reduced brain natriuretic peptide (BNP) levels and left ventricular mass index [106]. Another observational study in 30 hemodialysis patients confirmed that oral cholecalciferol supplementation over 6 months was associated with significantly decreased left ventricular mass index, and similar results were also obtained in a further observational study in dialysis patients [107, 108]. Moreover, it has been observed in 15 patients with IgA nephropathy, that parameters of cardiac autonomic tone were significantly improved when comparing values at baseline and 28 days after daily vitamin D supplementation with 10,000 International Units (IU) [109]. In 26 patients with CKD stage 3 and 4 it was reported that after cholecalciferol supplementation of 300,000 IU at baseline and after 8 weeks there was a significant decrease in E-Selectin, ICAM-1, and VCAM-1 at week 16 [110]. A study in 213 hemodialysis patients reported that ergocalciferol treatment is associated with significantly reduced frequency of vascular access dysfunction [111]. Natural vitamin D supplementation was also associated with reductions in inflammatory parameters in several, but not all, observational studies [106–108, 110, 112, 113].
8.2 Natural Vitamin D Treatment: Randomized Controlled Trials
Some randomized controlled trials (RCTs) have already been performed to study cardiovascular effects of natural vitamin D supplementation in CKD patients. A RCT in 84 dialysis patients receiving either 50,000 IU vitamin D per week for 8–12 weeks or placebo failed to show a significant effect on circulating pro-B-type natriuretic peptide (pro-BNP) concentration [114]. In 38 vitamin D deficient patients with CKD stage 3 and 4 who were randomized to either 50,000 IU vitamin D weekly for the first month and then monthly or placebo for an overall study period of 6 months, there were significant improvements in endothelium dependent microcirculatory vasodilatation and pulse pressure, and a reduction in tissue advanced glycation end products in the vitamin D compared to the placebo group [115]. Another RCT was performed in 60 hemodialysis patients who were randomly allocated to 50,000 IU vitamin D or placebo, once weekly for 8 weeks and then monthly for 4 months [116]. There was, however, no significant effect on pulse wave velocity (PWV) in that study [116]. There was also no significant vitamin D effect on left ventricular systolic function, left ventricular diastolic function, BNP, PWV, central blood pressure, 24-h blood pressure, and augmentation index in a RCT in 50 dialysis patients randomized to 3000 IU cholecalciferol daily or placebo for 6 months [117]. A further RCT was performed in 105 hemodialysis patients who received either ergocalciferol 50,000 IU weekly, 50,000 IU monthly or placebo for 1 year [118]. There were, apart from an increase in 25(OH)D, no significant effects on parameters of mineral metabolism or hospitalizations, but there was a non-significant trend towards reduced mortality in patients allocated to ergocalciferol with a hazard ratio (95 % confidence interval) of 0.28 (0.07–1.19). Moreover, there was no effect on arterio-vein access maturation in a cholecalciferol RCT in 52 hemodialysis patients [119]. In 96 hemodialysis patients awaiting transplantation it was evaluated in a RCT whether 50,000 IU cholecalciferol weekly for 1 year prevents alloreactive T-cell memory formation, but there were was no significant effect [120]. Another RCT in 38 hemodialysis patients did also not report on any vitamin D effect on cytokines (CRP and TNF-α), Th1 and Th2 lymphocyte frequencies and monocyte subset cell counts [121]. Regarding effects of vitamin D on vascular calcification in RCTs in CKD patients, it can be summarized that there were no relevant adverse effects but also no significant benefits, and the overall conclusion is that natural vitamin D supplementation is relatively safe in these patients [122, 123]. While it is logical that vitamin D supplementation is effective in increasing serum 25(OH)D levels across all stages of CKD, it has also been shown that PTH can also be suppressed by natural vitamin D treatment, albeit this has not been consistently confirmed in all RCTs [122–128]. Vitamin D RCT data in CKD patients on other cardiovascular risk factors such as e.g. glucose metabolism or blood pressure are sparse and did largely show no consistent and significant effect [102, 103]. Most studies performed in study cohorts without CKD did also fail to prove a significant blood pressure reduction by natural vitamin D supplementation [129, 130].
9 Active Vitamin D Treatment: Observational and Uncontrolled Studies
Since there are several RCTs published on active vitamin D treatment and cardiovascular diseases and its risk factors we just briefly mention some of the epidemiological studies on this topic. In this context, several observational studies have, by the majority, reported that the use of active vitamin D and its analogues is associated with significantly reduced risk of cardiovascular events and mortality [68, 96, 98, 131–150]. Some, albeit not all, observational or uncontrolled studies in CKD patients revealed that active vitamin D treatment is associated with improved left ventricular function, regression of myocardial hypertrophy, and reduction of QTc interval and dispersion [141–148]. Data on active vitamin D treatment and vascular calcification are sparse, but one study in 36 dialysis patients comparing low-dose versus high-dose calcitriol treatment for 1 year did not find any significant difference in vascular calcification [149]. Other studies, however, showed that prescription of active vitamin D treatment is associated with increased vascular calcification and vascular stiffness [150–152]. In contrast, active vitamin D treatment was associated with beneficial effects on parameters related to calcification and may thereby possibly protect against vascular damage [153–155]. Regarding observational studies on cardiovascular risk factors, it should be noted that active vitamin D treatment is, beyond its suppressive effects on PTH, associated with reduced proteinuria and anti-inflammatory effects in some, but not all, investigations [156–158]. It is also important to note that active vitamin D treatment causes an increase in creatinine generation without affecting glomerular filtration rate (GFR) [159].
9.1 Active Vitamin D Treatment: Randomized Controlled Trials
Two meta-analyses of RCTs have already addressed the research question on whether active vitamin D treatment has an effect on cardiovascular outcomes [160, 161]. Li et al. investigated the question whether active vitamin D analogues in predialysis CKD patients have an effect on cardiovascular events [161]. Five RCTs in 715 patients who experienced 35 cardiovascular events during a follow-up time of 16 weeks to 52 weeks were included into the meta-analysis [161]. As the main outcome, active vitamin D treatment was associated with a significantly reduced relative risk (RR) of 0.27 (95 % confidence interval [CI]: 0.13–0.59; p = 0.001) for cardiovascular events. In the same meta-analysis, active vitamin D treatment was also associated with reduced proteinuria, but there was no effect on left ventricular mass index, left ventricular systolic function, and systolic and diastolic blood pressure. There was, however, a significantly increased risk of hypercalcemia (i.e. serum calcium concentrations above 11.0 mg/dL [2.75 mmol/L]) associated with paricalcitol treatment with a RR (95 % CI) of 7.85 (2.92–21.1; p < 0.001). While the main outcome of this meta-analysis, i.e. the significant reduction of cardiovascular events, suggests cardiovascular benefits of active vitamin D analogues, it must be acknowledged that this study is clearly limited by the relatively low number of events [161]. Another meta-analysis of RCTs by Mann et al. addressed the question whether vitamin D treatment (with either active or natural vitamin D) in patients with CKD has an effect on all-cause mortality, cardiovascular mortality, and serious adverse cardiovascular events [160]. For the main outcome all-cause mortality, 13 trials in 1469 patients with 41 fatal events and a follow-up of 3–104 weeks were included, with the vast majority of patients in RCTs on active vitamin D treatment. There was no significant effect of vitamin D treatment on all-cause mortality with a RR (95 % CI) of 0.84 (0.47–1.52). Data on cardiovascular mortality were identified in 6 RCTs in 937 patients with 8 events and a RR (95 % CI) of 0.79 (0.26–2.28). Data on serious adverse cardiovascular events were identified in 8 RCTs in 1217 patients with 15 events and a RR (95 % CI) of 1.20 (0.49–2.99). The meta-analysis by Mann et al. was thus based on fewer cardiovascular events compared to the more recent meta-analysis by Li et al. Regarding the conclusions drawn from these meta-analyses it should be noted that the RCTs included were, in general, not a priori designed to evaluate cardiovascular events and that the low event rate is a clear limitation. It was thus concluded by Mann et al. that “the current state of the literature is unfit to systematically quantify any effect of vitamin D therapy on mortality and cardiovascular events” [160].
Specific effects of paricalcitol on myocardial structure and function have been evaluated in the PRIMO (Paricalcitol Capsule Benefits in Renal Failure-induced cardiac Morbidity) trial [162, 163]. In that RCT, 227 patients with CKD (GFR: 15–60 mL/min/1.73 m2) and preserved left ventricular ejection fraction with mild to moderate left ventricular hypertrophy, were randomly assigned to receive paricalcitol 2 μg daily (n = 115) or placebo (n = 112). The primary end point was change in left ventricular mass index at 48 weeks and secondary end points included measures of left ventricular diastolic and systolic function, cardiac volume indexes, cardiovascular events and cardiac biomarkers. The main outcome of the PRIMO trial was that paricalcitol did not reduce left ventricular mass index. Considering that the CI was narrow and that there was a marked decrease in PTH, suggesting a strong physiologic effect of paricalcitol, the authors concluded that even a larger sample size would have yielded similar results. While there were also no meaningful effects on most secondary outcomes, there was a significantly reduced risk of cardiovascular hospitalizations in the paricalcitol (n = 1) versus the placebo group (n = 8) (p = 0.04). In a post-hoc analysis of the PRIMO trial restricted to 196 patients with available echocardiographic data, it has been shown that left atrial volume index, a measure of diastolic dysfunction severity that indicates a higher cardiovascular risk, was significantly reduced after 48 weeks in the paricalcitol group (−2.97 mL/m2, 95 % CI: −4.00 to −1.59 mL/m2) compared to the placebo group (−0.70 mL/m2; 95 % CI: −1.93 to 0.53 mL/m2; p = 0.002) [163]. The rise in BNP throughout the PRIMO trial was also significantly attenuated in the paricalcitol (+8.4 pg/mL) versus the placebo group (+18.5 pg/mL; p = 0.02). These effects of paricalcitol on left atrial volume index and BNP are remarkable when considering that there was a similar blood pressure control in both groups and that RAAS inhibitor use was 80 %. It should also be noted that the effect of paricalcitol was homogeneous across all subgroups and that the changes in left atrial volume index paralleled the attenuation in BNP. Another RCT, the OPERA trial, on paricalcitol and left ventricular mass index as the primary outcome has been performed in 60 patients with CKD stage 3–5 and left ventricular hypertrophy [164]. Thirty patients were randomized to paricalcitol 1 μg daily and 30 patients to placebo. After 52 weeks, there was no significant difference in left ventricular mass index in the paricalcitol compared to the placebo group. Secondary outcome measures of left ventricular systolic and diastolic function did also not differ between the groups. Therefore, the results of the RCT by Wang et al. confirm the findings from the PRIMO trial by showing no effect of active vitamin D treatment on myocardial hypertrophy in a cohort with more severe CKD and secondary hyperparathyroidism when compared to the PRIMO study cohort. Interestingly, Wang et al. recorded two patients with hosptialization in the paricalcitol, and ten patients who were hospitalized in the placebo group (p = 0.02). Notably, no patient in the paricalcitol group had a cardiovascular-related hospitalization, whereas there were five patients with such an event in the placebo group.
Two further RCTs showed some beneficial effects of paricalcitol treatment on endothelial function [165, 166]. Zoccali et al. evaluated in a RCT in 88 patients with CKD stage 3–4 and a PTH greater than 65 pg/mL, the effect of paricalcitol 2 μg daily for 12 weeks on endothelium-dependent and endothelium-independent vasodilatation [166]. After 12 weeks, flow-mediated dilatation was significantly better in the paricalcitol compared to the placebo group, but there was no significant difference for endothelium-independent vasodilatation. A further RCT was conducted in 36 non-diabetic patients with CKD stage 3–4 who were randomly allocated to paricalcitol 2 μg daily (n = 12), paricalcitol 1 μg daily (n = 12), or placebo (n = 12) for 3 months [165]. Outcome measures were parameters of sympathetic activation, macro- and microvascular functions. While most outcome measures were not affected by treatment, there was a significant decline in endothelial function in all groups, except the 2 μg paricalcitol group.
Several trials evaluated the effects of active vitamin D treatment on proteinuria [167–173]. The meta-analyses in this field found that active vitamin D treatment decreases proteinuria significantly. These findings have been well reviewed elsewhere [168–173].
Some other studies have also investigated the impact of active vitamin D therapy on glucose metabolism but the results were mixed. Regarding other cardiovascular risk factors it should be noted that there were e.g. no consistent and relevant effects on blood pressure [174].
The beneficial effects of active vitamin D treatment on PTH and some parameters of bone and mineral metabolism have also been evaluated in RCTs, and it has been clearly shown that active vitamin D treatment suppresses PTH and some bone markers such as bone alkaline phosphatase [171, 175, 176]. The clinical relevance of the interaction between vitamin D and fibroblast growth factor 23 (FGF23) as well as the significance of FGF23 in the pathogenesis of cardiovascular diseases still need to be further studies [13].
Several studies, on active as well as on natural vitamin D supplementation, are still ongoing in CKD patients and will hopefully help to clarify the role of vitamin D treatment for heart structure and function [176–181].
10 Conclusions
While there is compelling evidence from experimental and observational studies indicating that vitamin D may exert beneficial effects on myocardial structure and function, there are only very few and limited data addressing these issues in RCTs in CKD patients. Based on the available evidence it is thus premature to draw firm and definite conclusions on the effects of vitamin D treatment on heart structure and function in CKD. In particular, RCT data on natural vitamin D treatment in CKD are sparse, whereas there are already some RCT data available on active vitamin D treatment. Results derived from the PRIMO study on improvements in left atrial volume index and serum BNP levels by paricalcitol treatment are promising regarding potential beneficial effects of active vitamin D treatment on heart structure and function, but these findings need further confirmation in future RCTs. It should, however, be noted that although data on hard cardiovascular endpoints are sparse and limited by low event rates, some RCTs including one meta-analysis of RCTs suggest that patients on active vitamin D treatment experience fewer cardiovascular events. Further large RCTs are therefore needed to address the question whether vitamin D treatment is clinically indicated to prevent and treat cardiovascular diseases in CKD patients. The currently available data suggesting reduced cardiovascular events in CKD patients on active vitamin D treatment (i.e. paricalcitol) are a scientifically sound rationale for further RCTs addressing the impact of active vitamin D treatment on cardiovascular events as a primary outcome. Regarding the current relatively widespread use of vitamin D in CKD patients, it must also be stressed that although patients on active vitamin D treatment are at increased risk of hypercalcemia, there is no clear indication from RCTs that this adverse effect of active vitamin D treatment translates into higher cardiovascular risk since the available literature suggests that cardiovascular events and mortality are rather reduced than increased with active vitamin D treatment. Therefore, while proposed beneficial effects of active vitamin D treatment on heart structure and function still need to be further evaluated, the evidence from RCTs is quite convincing that active vitamin D treatment, at doses commonly used in clinical practice, is not harmful for the heart. These safety considerations are of great importance when considering the use of active vitamin D treatment in CKD patients.
References
Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Nally Jr JV. Cause-specific deaths in non-dialysis-dependent CKD. J Am Soc Nephrol. 2015;26:2512–20.
Charytan DM, Lewis EF, Desai AS, Weinrauch LA, Ivanovich P, Toto RD, et al. Cause of death in patients with diabetic CKD enrolled in the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am J Kidney Dis. 2015;66:429–40.
Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20:259–72.
Kidney-Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int Suppl. 2009;113:S1–130.
Pilz S, Tomaschitz A, Drechsler C, Dekker JM, März W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010;54:1103–13.
Pilz S, Tomaschitz A, Drechsler C, de Boer RA. Vitamin D deficiency and heart disease. Kideny Int Suppl. 2011;1:111–5.
Zittermann A. Vitamin D, and cardiovascular disease. Anticancer Res. 2014;34:4641–8.
Pilz S, Gaksch M, O’Hartaigh B, Tomaschitz A, März W. The role of vitamin D deficiency in cardiovascular disease: where do we stand in 2013? Arch Toxicol. 2013;87:2083–103.
Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int. 2013;24:2167–80.
Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M, RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–55.
Carvalho LS, Sposito AC. Vitamin D for the prevention of cardiovascular disease: are we ready for that? Atherosclerosis. 2015;241:729–40.
Negri AL, Brandenburg VM. Calcitriol resistance in hemodialysis patients with secondary hyperparathyroidism. Int Urol Nephrol. 2014;46:1145–51.
Negri AL. Fibroblast growth factor 23: associations with cardiovascular disease and mortality in chronic kidney disease. Int Urol Nephrol. 2014;46:9–17.
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76.
Gardner DG, Chen S, Glenn DJ. Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol. 2013;305:R969–77.
Schnatz PF, Nudy M, O’Sullivan DM, Jiang X, Cline JM, Kaplan JR, et al. The quantification of vitamin D receptors in coronary arteries and their association with atherosclerosis. Maturitas. 2012;73:143–7.
Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, et al. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–12.
Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–47.
Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–7.
Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–88.
Panizo S, Barrio-Vázquez S, Naves-Díaz M, Carrillo-López N, Rodríguez I, Fernández-Vázquez A, et al. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant. 2013;28:2735–44.
Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258:E134–42.
Chen S, Ni XP, Humphreys MH, Gardner DG. 1,25 dihydroxyvitamin d amplifies type a natriuretic peptide receptor expression and activity in target cells. J Am Soc Nephrol. 2005;16:329–39.
Chen S, Olsen K, Grigsby C, Gardner DG. Vitamin D activates type A natriuretic peptide receptor gene transcription in inner medullary collecting duct cells. Kidney Int. 2007;72:300–6.
Choudhury S, Bae S, Ke Q, Lee JY, Singh SS, St-Arnaud R, et al. Abnormal calcium handling and exaggerated cardiac dysfunction in mice with defective vitamin d signaling. PLoS One. 2014;9:e108382.
Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–9.
Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149:558–64.
Wu-Wong JR, Chen YW, Wessale JL. Vitamin D receptor agonist VS-105 improves cardiac function in the presence of enalapril in 5/6 nephrectomized rats. Am J Physiol Renal Physiol. 2015;308:F309–19.
Weber KT, Weglicki WB, Simpson RU. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res. 2009;81:500–8.
Koleganova N, Piecha G, Ritz E, Gross ML. Calcitriol ameliorates capillary deficit and fibrosis of the heart in subtotally nephrectomized rats. Nephrol Dial Transplant. 2009;24:778–87.
Mizobuchi M, Nakamura H, Tokumoto M, Finch J, Morrissey J, Liapis H, et al. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol. 2010;121:188–92.
Repo JM, Rantala IS, Honkanen TT, Mustonen JT, Kööbi P, Tahvanainen AM, et al. Paricalcitol aggravates perivascular fibrosis in rats with renal insufficiency and low calcitriol. Kidney Int. 2007;72:977–84.
Glenn DJ, Cardema MC, Gardner DG. Amplification of lipotoxic cardiomyopathy in the VDR gene knockout mouse. J Steroid Biochem Mol Biol. 2015. doi:10.1016/j.jsbmb.2015.09.034. pii: S0960-0760(15)30092-3.
Meredith A, Boroomand S, Carthy J, Luo Z, McManus B. 1,25 dihydroxyvitamin D3 inhibits TGFβ1-mediated primary human cardiac myofibroblast activation. PLoS One. 2015;10(6):e0128655.
Lee TW, Kao YH, Lee TI, Chang CJ, Lien GS, Chen YJ. Calcitriol modulates receptor for advanced glycation end products (RAGE) in diabetic hearts. Int J Cardiol. 2014;173:236–41.
Lee TI, Kao YH, Chen YC, Tsai WC, Chung CC, Chen YJ. Cardiac metabolism, inflammation, and peroxisome proliferator-activated receptors modulated by 1,25-dihydroxyvitamin D3 in diabetic rats. Int J Cardiol. 2014;176:151–7.
Bae S, Singh SS, Yu H, Lee JY, Cho BR, Kang PM. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J Appl Physiol (1985). 2013;114:979–87.
Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong H, et al. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal. 2015;22:633–50.
Jiang P, Zhang WY, Li HD, Cai HL, Liu YP, Chen LY. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology. 2013;38:2091–8.
Mallick NP, Berlyne GM. Arterial calcification after vitamin-D therapy in hyperphosphatemic renal failure. Lancet. 1968;2:1316–20.
Zebger-Gong H, Müller D, Diercke M, Haffner D, Hocher B, Verberckmoes S, et al. 1,25-Dihydroxyvitamin D3-induced aortic calcifications in experimental uremia: up-regulation of osteoblast markers, calcium-transporting proteins and osterix. J Hypertens. 2011;29:339–48.
Lomashvili KA, Wang X, O’Neill WC. Role of local versus systemic vitamin D receptors in vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:146–51.
Bas A, Lopez I, Perez J, Rodriguez M, Aguilera-Tejero E. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res. 2006;21:484–90.
Razzaque MS. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011;79:708–14.
Schmidt N, Brandsch C, Kühne H, Thiele A, Hirche F, Stangl GI. Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLoS One. 2012;7:e35316.
Schmidt N, Brandsch C, Schutkowski A, Hirche F, Stangl GI. Dietary vitamin D inadequacy accelerates calcification and osteoblast-like cell formation in the vascular system of LDL receptor knockout and wild-type mice. J Nutr. 2014;144:638–46.
Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509–19.
Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261–70.
Aoshima Y, Mizobuchi M, Ogata H, Kumata C, Nakazawa A, Kondo F, et al. Vitamin D receptor activators inhibit vascular smooth muscle cell mineralization induced by phosphate and TNF-α. Nephrol Dial Transplant. 2012;27:1800–6.
Hirata M, Serizawa K, Aizawa K, Yogo K, Tashiro Y, Takeda S, et al. 22-Oxacalcitriol prevents progression of endothelial dysfunction through antioxidative effects in rats with type 2 diabetes and early-stage nephropathy. Nephrol Dial Transplant. 2013;28:1166–74.
Wu-Wong JR, Li X, Chen YW. Different vitamin D receptor agonists exhibit differential effects on endothelial function and aortic gene expression in 5/6 nephrectomized rats. J Steroid Biochem Mol Biol. 2015;148:202–9.
Wu-Wong JR, Noonan W, Nakane M, Brooks KA, Segreti JA, Polakowski JS, et al. Vitamin d receptor activation mitigates the impact of uremia on endothelial function in the 5/6 nephrectomized rats. Int J Endocrinol. 2010;2010:625852.
Dong J, Wong SL, Lau CW, Liu J, Wang YX, Dan He Z, et al. Calcitriol restores renovascular function in estrogen-deficient rats through downregulation of cyclooxygenase-2 and the thromboxane-prostanoid receptor. Kidney Int. 2013;84:54–63.
Andrukhova O, Slavic S, Zeitz U, Riesen SC, Heppelmann MS, Ambrisko TD, et al. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol. 2014;28:53–64.
Bozic M, Álvarez Á, de Pablo C, Sanchez-Niño MD, Ortiz A, Dolcet X, et al. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: implications for atherosclerosis development. PLoS One. 2015;10:e0136863.
Valcheva P, Cardus A, Panizo S, Parisi E, Bozic M, Lopez Novoa JM, et al. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis. 2014;235:247–55.
Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J Steroid Biochem Mol Biol. 2010;118:135–41.
Cardús A, Parisi E, Gallego C, Aldea M, Fernández E, Valdivielso JM. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006;69:1377–84.
Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98.
Yin K, You Y, Swier V, Tang L, Radwan MM, Pandya AN, et al. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015. pii: ATVBAHA.115.306132.
Wong MS, Leisegang MS, Kruse C, Vogel J, Schürmann C, Dehne N, et al. Vitamin D promotes vascular regeneration. Circulation. 2014;130:976–86.
Fitzpatrick LA, Bilezikian JP, Silverberg SJ. Parathyroid hormone and the cardiovascular system. Curr Osteoporos Rep. 2008;6:77–83.
Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63:20–31.
Li YC. Vitamin D: roles in renal and cardiovascular protection. Curr Opin Nephrol Hypertens. 2012;21:72–9.
Szeto FL, Reardon CA, Yoon D, Wang Y, Wong KE, Chen Y, et al. Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol Endocrinol. 2012;26:1091–101.
Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61:450–8.
Pilz S, Kienreich K, Rutters F, de Jongh R, van Ballegooijen AJ, Grübler M, et al. Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr Diab Rep. 2013;13:261–70.
Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–13.
Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87:1631–8.
Chonchol M, Cigolini M, Targher G. Association between 25-hydroxyvitamin D deficiency and cardiovascular disease in type 2 diabetic patients with mild kidney dysfunction. Nephrol Dial Transplant. 2008;23:269–74.
Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2011;26:1024–32.
Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–61.
Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, et al. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603–9.
Kramer H, Sempos C, Cao G, Luke A, Shoham D, Cooper R, et al. Mortality rates across 25-hydroxyvitamin D (25[OH]D) levels among adults with and without estimated glomerular filtration rate <60 ml/min/1.73 m2: the third national health and nutrition examination survey. PLoS One. 2012;7:e47458.
Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95.
Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukroun G, et al. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1128–35.
Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, et al. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. Am J Kidney Dis. 2012;60:567–75.
Marcén R, Jimenez S, Fernández-Rodriguez A, Galeano C, Villafruela JJ, Gomis A, et al. Are low levels of 25-hydroxyvitamin D a risk factor for cardiovascular diseases or malignancies in renal transplantation? Nephrol Dial Transplant. 2012;27 Suppl 4:iv47–52.
Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–83.
Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–82.
Patange AR, Valentini RP, Gothe MP, Du W, Pettersen MD. Vitamin D deficiency is associated with increased left ventricular mass and diastolic dysfunction in children with chronic kidney disease. Pediatr Cardiol. 2013;34:536–42.
Lai S, Coppola B, Dimko M, Galani A, Innico G, Frassetti N, et al. Vitamin D deficiency, insulin resistance, and ventricular hypertrophy in the early stages of chronic kidney disease. Ren Fail. 2014;36:58–64.
de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–12.
García-Canton C, Bosch E, Ramírez A, Gonzalez Y, Auyanet I, Guerra R, et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant. 2011;26:2250–6.
London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–20.
Fusaro M, Gallieni M, Rebora P, Rizzo MA, Luise MC, Riva H, et al. Atrial fibrillation and low vitamin D levels are associated with severe vascular calcifications in hemodialysis patients. J Nephrol. 2016;29:419–26.
Yadav AK, Banerjee D, Lal A, Jha V. Vitamin D deficiency, CD4+CD28null cells and accelerated atherosclerosis in chronic kidney disease. Nephrology (Carlton). 2012;17:575–81.
Zhang QY, Jiang CM, Sun C, Tang TF, Jin B, Cao DW, et al. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J Nephrol. 2015;28:471–6.
Chitalia N, Recio-Mayoral A, Kaski JC, Banerjee D. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis. 2012;220:265–8.
Shroff R, Aitkenhead H, Costa N, Trivelli A, Litwin M, Picca S, et al. Normal 25-hydroxyvitamin D levels are associated with less proteinuria and attenuate renal failure progression in children with CKD. J Am Soc Nephrol. 2015. pii: ASN.2014090947.
Fernández-Juárez G, Luño J, Barrio V, de Vinuesa SG, Praga M, Goicoechea M, et al. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clin J Am Soc Nephrol. 2013;8:1870–6.
de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6:2141–9.
Sahin I, Gungor B, Can MM, Avci II, Guler GB, Okuyan E, et al. Lower blood vitamin D levels are associated with an increased incidence of contrast-induced nephropathy in patients undergoing coronary angiography. Can J Cardiol. 2014;30:428–33.
Lee DR, Kong JM, Cho KI, Chan L. Impact of vitamin D on proteinuria, insulin resistance, and cardiovascular parameters in kidney transplant recipients. Transplant Proc. 2011;43:3723–9.
de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2007;50:69–77.
Inaguma D, Nagaya H, Hara K, Tatematsu M, Shinjo H, Suzuki S, et al. Relationship between serum 1,25-dihydroxyvitamin D and mortality in patients with pre-dialysis chronic kidney disease. Clin Exp Nephrol. 2008;12:126–31.
Andrade J, Er L, Ignaszewski A, Levin A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin J Am Soc Nephrol. 2008;3:1800–6.
Ogawa T, Ishida H, Akamatsu M, Matsuda N, Fujiu A, Ito K, et al. Relation of oral 1alpha-hydroxy vitamin D3 to the progression of aortic arch calcification in hemodialysis patients. Heart Vessels. 2010;25:1–6.
Testa A, Mallamaci F, Benedetto FA, Pisano A, Tripepi G, Malatino L, et al. Vitamin D receptor (VDR) gene polymorphism is associated with left ventricular (LV) mass and predicts left ventricular hypertrophy (LVH) progression in end-stage renal disease (ESRD) patients. J Bone Miner Res. 2010;25:313–9.
Santoro D, Gagliostro G, Alibrandi A, Ientile R, Bellinghieri G, Savica V, et al. Vitamin D receptor gene polymorphism and left ventricular hypertrophy in chronic kidney disease. Nutrients. 2014;6:1029–37.
Yang L, Wu L, Fan Y, Ma J. Associations among four polymorphisms (BsmI, FokI, TaqI and ApaI) of vitamin D receptor gene and end-stage renal disease: a meta-analysis. Arch Med Res. 2015;746:1–7.
Kandula P, Dobre M, Schold JD, Schreiber Jr MJ, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62.
Alvarez J, Wasse H, Tangpricha V. Vitamin D supplementation in pre-dialysis chronic kidney disease: a systematic review. Dermatoendocrinol. 2012;4:118–27.
Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. Treatment of 25-OH vitamin D deficiency in older men with chronic kidney disease stages 3 and 4 is associated with reduction in cardiovascular events. Am J Ther. 2013;20:480–6.
Coratelli P, Petrarulo F, Buongiorno E, Giannattasio M, Antonelli G, Amerio A. Improvement in left ventricular function during treatment of hemodialysis patients with 25-OHD3. Contrib Nephrol. 1984;41:433–7.
Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–11.
Bucharles S, Barberato SH, Stinghen AE, Gruber B, Piekala L, Dambiski AC, et al. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22:284–91.
Kidir V, Ersoy I, Altuntas A, Gultekin F, Inal S, Dagdeviren BH, et al. Effect of cholecalciferol replacement on vascular calcification and left ventricular mass index in dialysis patients. Ren Fail. 2015;37:635–9.
Mann MC, Hemmelgarn BR, Exner DV, Hanley DA, Turin TC, Wheeler DC, et al. Vitamin D supplementation is associated with stabilization of cardiac autonomic tone in IgA nephropathy. Hypertension. 2015;66:e4–6.
Chitalia N, Ismail T, Tooth L, Boa F, Hampson G, Goldsmith D, et al. Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS One. 2014;9:e91363.
Agarwal G, Vasquez K, Penagaluru N, Gelfond J, Qunibi WY. Treatment of vitamin D deficiency/insufficiency with ergocalciferol is associated with reduced vascular access dysfunction in chronic hemodialysis patients. Hemodial Int. 2015;19:499–508.
Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–61.
Jean G, Souberbielle JC, Chazot C. Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant. 2009;24:3799–805.
Seirafian S, Haghdarsaheli Y, Mortazavi M, Hosseini M, Moeinzadeh F. The effect of oral vitamin D on serum level of N-terminal pro-B-type natriuretic peptide. Adv Biomed Res. 2014;3:261.
Dreyer G, Tucker AT, Harwood SM, Pearse RM, Raftery MJ, Yaqoob MM. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: an exploratory, double blind, randomised controlled trial. PLoS One. 2014;9:e99461.
Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:1143–9.
Mose FH, Vase H, Larsen T, Kancir AS, Kosierkiewic R, Jonczy B, et al. Cardiovascular effects of cholecalciferol treatment in dialysis patients – a randomized controlled trial. BMC Nephrol. 2014;15:50.
Bhan I, Dobens D, Tamez H, Deferio JJ, Li YC, Warren HS, et al. Nutritional vitamin D supplementation in dialysis: a randomized trial. Clin J Am Soc Nephrol. 2015;10:611–9.
Wasse H, Huang R, Long Q, Zhao Y, Singapuri S, McKinnon W, et al. Very high-dose cholecalciferol and arteriovenous fistula maturation in ESRD: a randomized, double-blind, placebo-controlled pilot study. J Vasc Access. 2014;15(2):88–94.
Li L, Lin M, Krassilnikova M, Ostrow K, Bader A, Radbill B, et al. Effect of cholecalciferol supplementation on inflammation and cellular alloimmunity in hemodialysis patients: data from a randomized controlled pilot trial. PLoS One. 2014;9:e109998.
Seibert E, Heine GH, Ulrich C, Seiler S, Köhler H, Girndt M. Influence of cholecalciferol supplementation in hemodialysis patients on monocyte subsets: a randomized, double-blind, placebo-controlled clinical trial. Nephron Clin Pract. 2013;123:209–19.
Delanaye P, Weekers L, Warling X, Moonen M, Smelten N, Médart L, et al. Cholecalciferol in haemodialysis patients: a randomized, double-blind, proof-of-concept and safety study. Nephrol Dial Transplant. 2013;28:1779–86.
Massart A, Debelle FD, Racapé J, Gervy C, Husson C, Dhaene M, et al. Biochemical parameters after cholecalciferol repletion in hemodialysis: results from the VitaDial randomized trial. Am J Kidney Dis. 2014;64:696–705.
Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg EM, Sidelmann JJ, Jespersen J, et al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant. 2012;27:3523–31.
Sprague SM, Silva AL, Al-Saghir F, Damle R, Tabash SP, Petkovich M, et al. Modified-release calcifediol effectively controls secondary hyperparathyroidism associated with vitamin D insufficiency in chronic kidney disease. Am J Nephrol. 2014;40:535–45.
Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis. 2009;53:408–16.
Armas LA, Andukuri R, Barger-Lux J, Heaney RP, Lund R. 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2012;7:1428–34.
Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V. Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. Am J Clin Nutr. 2012;95:522–8.
Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745–54.
Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner-Pammer A, et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension. 2015;65:1195–201.
Zheng Z, Shi H, Jia J, Li D, Lin S. Vitamin D supplementation and mortality risk in chronic kidney disease: a meta-analysis of 20 observational studies. BMC Nephrol. 2013;14:199.
Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37:239–48.
Naves-Díaz M, Alvarez-Hernández D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–8.
Sugiura S, Inaguma D, Kitagawa A, Murata M, Kamimura Y, Sendo S, et al. Administration of alfacalcidol for patients with predialysis chronic kidney disease may reduce cardiovascular disease events. Clin Exp Nephrol. 2010;14:43–50.
Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–84.
Shoji T, Marubayashi S, Shigematsu T, Iseki K, Tsubakihara Y, Committee of Renal Data Registry, Japanese Society for Dialysis Therapy. Use of vitamin d receptor activator, incident cardiovascular disease and death in a cohort of hemodialysis patients. Ther Apher Dial. 2015;19:235–44.
Miller JE, Molnar MZ, Kovesdy CP, Zaritsky JJ, Streja E, Salusky I, et al. Administered paricalcitol dose and survival in hemodialysis patients: a marginal structural model analysis. Pharmacoepidemiol Drug Saf. 2012;21:1232–9.
Cozzolino M, Brancaccio D, Cannella G, Messa P, Gesualdo L, Marangella M, et al. VDRA therapy is associated with improved survival in dialysis patients with serum intact PTH ≤ 150 pg/mL: results of the Italian FARO Survey. Nephrol Dial Transplant. 2012;27:3588–94.
Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–56.
Bover J, Dasilva I, Furlano M, Lloret MJ, Diaz-Encarnacion MM, Ballarin J, et al. Clinical uses of 1,25-dihydroxy-19-nor-vitamin D(2) (paricalcitol). Curr Vasc Pharmacol. 2014;12:313–23.
Lemmilä S, Saha H, Virtanen V, Ala-Houhala I, Pasternack A. Effect of intravenous calcitriol on cardiac systolic and diastolic function in patients on hemodialysis. Am J Nephrol. 1998;18:404–10.
Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81.
Ivarsen P, Povlsen JV, Christensen KL. Effect of alfacalcidol on cardiac function in patients with chronic kidney disease stage 4 and secondary hyperparathyroidism: a pilot study. Scand J Urol Nephrol. 2012;46:381–8.
McGonigle RJ, Timmis AD, Keenan J, Jewitt DE, Weston MJ, Parsons V. The influence of 1 alpha-hydroxycholecalciferol on left ventricular function in end-stage renal failure. Proc Eur Dial Transplant Assoc. 1981;18:579–85.
McGonigle RJ, Fowler MB, Timmis AB, Weston MJ, Parsons V. Uremic cardiomyopathy: potential role of vitamin D and parathyroid hormone. Nephron. 1984;36:94–100.
Singh NP, Sahni V, Garg D, Nair M. Effect of pharmacological suppression of secondary hyperparathyroidism on cardiovascular hemodynamics in predialysis CKD patients: a preliminary observation. Hemodial Int. 2007;11:417–23.
Kim HW, Park CW, Shin YS, Kim YS, Shin SJ, Kim YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102:c21–9.
Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104:16810–5.
Morosetti M, Jankovic L, Palombo G, Cipriani S, Dominijanni S, Balducci A, et al. High-dose calcitriol therapy and progression of cardiac vascular calcifications. J Nephrol. 2008;21:603–8.
Charitaki E, Davenport A. Aortic pulse wave velocity in haemodialysis patients is associated with the prescription of active vitamin D analogues. J Nephrol. 2014;27:431–7.
Briese S, Wiesner S, Will JC, Lembcke A, Opgen-Rhein B, Nissel R, et al. Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease-impact of calcium and vitamin D therapy. Nephrol Dial Transplant. 2006;21:1906–14.
Drüeke TB, Massy ZA. Role of vitamin D in vascular calcification: bad guy or good guy? Nephrol Dial Transplant. 2012;27:1704–7.
Hansen D, Rasmussen K, Rasmussen LM, Bruunsgaard H, Brandi L. The influence of vitamin D analogs on calcification modulators. N-terminal pro-B-type natriuretic peptide and inflammatory markers in hemodialysis patients: a randomized crossover study. MC Nephrol. 2014;15:130.
Cianciolo G, La Manna G, Della Bella E, Cappuccilli ML, Angelini ML, Dormi A, et al. Effect of vitamin D receptor activator therapy on vitamin D receptor and osteocalcin expression in circulating endothelial progenitor cells of hemodialysis patients. Blood Purif. 2013;35:187–95.
Hansen D, Rasmussen K, Rasmussen LM, Bruunsgaard H, Brandi L. The influence of vitamin D analogs on calcification modulators. N-terminal pro-B-type natriuretic peptide and inflammatory markers in hemodialysis patients: a randomized crossover study. BMC Nephrol. 2014;15:130.
Trillini M, Cortinovis M, Ruggenenti P, Reyes Loaeza J, Courville K, Ferrer-Siles C, et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J Am Soc Nephrol. 2015;26:1205–14.
De Nicola L, Conte G, Russo D, Gorini A, Minutolo R. Antiproteinuric effect of add-on paricalcitol in CKD patients under maximal tolerated inhibition of renin-angiotensin system: a prospective observational study. BMC Nephrol. 2012;13:150.
Lucisano S, Arena A, Stassi G, Iannello D, Montalto G, Romeo A, et al. Role of paricalcitol in modulating the immune response in patients with renal disease. Int J Endocrinol. 2015;2015:765364.
Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD. Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int. 2011;80:1073–9.
Mann MC, Hobbs AJ, Hemmelgarn BR, Roberts DJ, Ahmed SB, Rabi DM. Effect of oral vitamin D analogs on mortality and cardiovascular outcomes among adults with chronic kidney disease: a meta-analysis. Clin Kidney J. 2015;8:41–8.
Li XH, Feng L, Yang ZH, Liao YH. The effect of active vitamin D on cardiovascular outcomes in predialysis chronic kidney diseases: a systematic review and meta-analysis. Nephrology (Carlton). 2015. doi:10.1111/nep.12505.
Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–84.
Tamez H, Zoccali C, Packham D, Wenger J, Bhan I, Appelbaum E, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164:902–9.
Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD – the OPERA trial. J Am Soc Nephrol. 2014;25:175–86.
Lundwall K, Jörneskog G, Jacobson SH, Spaak J. Paricalcitol, microvascular and endothelial function in non-diabetic chronic kidney disease: a randomized trial. Am J Nephrol. 2015;42:265–73.
Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64:1005–11.
de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–51.
Cheng J, Zhang W, Zhang X, Li X, Chen J. Efficacy and safety of paricalcitol therapy for chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2012;7:391–400.
de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol. 2013;24:1863–71.
Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, et al. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e61387.
Han T, Rong G, Quan D, Shu Y, Liang Z, She N, et al. Meta-analysis: the efficacy and safety of paricalcitol for the treatment of secondary hyperparathyroidism and proteinuria in chronic kidney disease. Biomed Res Int. 2013;2013:320560.
Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–8.
Zhao J, Dong J, Wang H, Shang H, Zhang D, Liao L. Efficacy and safety of vitamin D3 in patients with diabetic nephropathy: a meta-analysis of randomized controlled trials. Chin Med J (Engl). 2014;127:2837–43.
de Boer IH, Sachs M, Hoofnagle AN, Utzschneider KM, Kahn SE, Kestenbaum B, et al. Paricalcitol does not improve glucose metabolism in patients with stage 3–4 chronic kidney disease. Kidney Int. 2013;83:323–30.
Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3–4 CKD. Clin J Am Soc Nephrol. 2014;9:1620–6.
Coyne DW, Andress DL, Amdahl MJ, Ritz E, de Zeeuw D. Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant. 2013;28:2260–8.
Courbebaisse M, Alberti C, Colas S, Prié D, Souberbielle JC, Treluyer JM, et al. VITamin D supplementation in renAL transplant recipients (VITALE): a prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials. 2014;15:430.
Levin A, Perry T, De Zoysa P, Sigrist MK, Humphries K, Tang M, et al. A randomized control trial to assess the impact of vitamin D supplementation compared to placebo on vascular stiffness in chronic kidney disease patients. BMC Cardiovasc Disord. 2014;14:156.
Mann MC, Exner DV, Hemmelgarn BR, Hanley DA, Turin TC, MacRae JM, et al. The VITAH trial VITamin D supplementation and cardiac Autonomic tone in Hemodialysis: a blinded, randomized controlled trial. BMC Nephrol. 2014;15:129.
Keyzer CA, de Jong MA, Fenna van Breda G, Vervloet MG, Laverman GD, Hemmelder M, et al. Vitamin D receptor activator and dietary sodium restriction to reduce residual urinary albumin excretion in chronic kidney disease (ViRTUE study): rationale and study protocol. Nephrol Dial Transplant. 2015. pii: gfv033. [Epub ahead of print] Review.
Mehrotra A, Leung WY, Joson T. Nutritional vitamin D supplementation and health-related outcomes in hemodialysis patients: a protocol for a systematic review and meta-analysis. Syst Rev. 2015;4:13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pilz, S., Brandenburg, V., Ureña Torres, P.A. (2016). Vitamin D and Heart Structure and Function in Chronic Kidney Disease. In: Ureña Torres, P., Cozzolino, M., Vervloet, M. (eds) Vitamin D in Chronic Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-32507-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-32507-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32505-7
Online ISBN: 978-3-319-32507-1
eBook Packages: MedicineMedicine (R0)