Abstract
The ubiquitously present glyoxalase pathway consists of two enzymes, Glyoxalase I and Glyoxalase II, which act in a stepwise manner and catalyze the detoxification of a highly cytotoxic metabolite methylglyoxal to d-lactate with the help of glutathione. Methylglyoxal (MG) is generated endogenously through different enzymatic and nonenzymatic reactions and is a potent glycating agent. It inhibits cell division and forms various degrees of irreversible adducts with cellular macromolecules such as nucleic acids, lipids, and proteins. MG along with reactive oxygen species (ROS) has been shown to accumulate in plant cells in response to various abiotic stresses including drought and their accumulation results in an imbalance in different cellular metabolic processes. Plants being sessile organisms have evolved various mechanisms that permit them to cope with and withstand various degrees of stress. The glyoxalase pathway is one such mechanism which acts to control excessive accumulation of MG and ROS in the system, either directly or in cooperation with other pathways involved in stress response. In response to drought, transcript and protein levels of glyoxalases are altered which is suggestive of their involvement in stress response. MG has also been shown to induce stress-responsive signaling cascades related to drought and even regulates stomatal movements. Here, we discuss the role of the plant glyoxalase pathway with respect to drought stress adaptation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glyoxalase (GLY)
- Methylglyoxal (MG)
- Reactive oxygen species (ROS)
- Reduced glutathione (GSH)
- Drought stress
16.1 Introduction
Crop plants are constantly exposed to a broad range of environmental stresses. Of which, drought is the most devastating one that barriers agroecosystem productivity (Lambers et al. 2008; Farooq et al. 2011). It adversely affects plant metabolism, growth, development, and survival, and thus, is a constraint for plant productivity worldwide (Ahuja et al. 2010; Hasanuzzaman and Fujita 2011; Hasanuzzaman et al. 2012). In addition, climate prediction models indicate more severe and frequent droughts in future, thereby drastically impacting global crop production (IPCC 2008; Manavalan et al. 2009). Being sessile and sensitive organisms, plants have evolved a wide range of molecular programs to readily sense, respond, and cope with changing environments in order to protect themselves from these unforeseen variations (Ahuja et al. 2010). Response mechanisms to drought stress involve changes at morphological, physiological, and biochemical levels (Zhu 2001). Re-programming in gene expression occurs under stress conditions causing alterations in plant biochemical, transcriptomic, and proteomic machinery (Cohen et al. 2010; Ahuja et al. 2010). In such situations, tolerance to stress can be achieved through modulation of several genes or by organizing the action of different genes from various cellular biochemical pathways (Sasaki-Sekimoto et al. 2005).
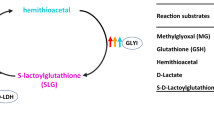
As a common phenomenon, stress leads to excessive production of certain deleterious chemical entities such as reactive oxygen species (ROS) and methylglyoxal (MG) in plants (Yadav et al. 2007; Hossain and Fujita 2010; Hossain et al. 2011a). MG is a ubiquitous metabolite generated as a concomitant of intracellular metabolism and, therefore, exists in all cells during normal physiological growth and development conditions and accumulates to higher concentrations under many environmental stresses (Yadav et al. 2008). It is responsible for oxidative stress either through increased production of ROS or by forming advanced glycation end products (AGEs) with macromolecules (Kalapos 2008; Sousa Silva et al. 2013). As it accumulates at higher concentrations under stress conditions, plants have evolved several detoxification mechanisms to combat the so-called dicarbonyl and oxidative stress caused by MG. The primary route for MG detoxification is the thiol-dependent glyoxalase system which catalyzes the conversion of cytotoxic MG (2-oxopropanal) to d-lactic acid via S-D-lactoylglutathione (SLG ) (Fig. 16.1). The presence of the glyoxalase pathway has been reported in several plant species and involves two enzymes, GLY I and GLY II, which have been purified as well as physiologically and biochemically characterized and functionally validated from various plant species (Yadav et al. 2007; Hoque et al. 2007; Hasanuzzaman and Fujita 2011; Hossain et al. 2014). The efficient role of this pathway in stress management has been extensively studied in various living organisms, including prokaryotes to eukaryotes, and has been shown to be associated with abiotic stress adaptation (Kaur et al. 2014a). Here, we discuss basic molecular programs suggested to confer tolerance to drought stress alongside their envisaged approaches. Special emphasis will be given on molecular mechanisms of glyoxalase pathway mediated drought stress tolerance in plants.
Different routes of methylglyoxal formation and detoxification system in plants. Nonenzymatic generation of MG through β-elimination of phosphate group from enediolate phosphate intermediate is the central route of MG synthesis in plants. Besides, metabolism of amino acids, fatty acids, and ketone bodies contribute to MG formation; first enzyme GLY I converts hemithioacetal formed from spontaneous combination of MG and GSH into S-d-lactoylglutathione which is then converted to d-lactate by GLY II, regenerating GSH in the system. DHAP dihydroxyacetone phosphate, GAP d-glyceraldehyde-3-phosphate
16.2 Effects of Drought on Plant Health
Drought is harmful for the plant growth and development with varying effects based on the severity of the stress. The plants also display a variety of responses on exposure to drought conditions causing alterations at both morphological and molecular levels (Farooq et al. 2009). Drought condition in plants results in alterations in relative water content , water and nutrient relations, photosynthesis, assimilate partitioning and respiration thereby, limiting economic yield (Farooq et al. 2009). Siddique et al. (2001) reported that the relative water content, transpiration rate of wheat and rice under drought stress was lower than control ones. Nutrient contents such as P and PO4 3− in the plant tissue decreased significantly under drought conditions, because of lowered PO4 3− mobility as a result of lower water availability (Peuke and Rennenberg 2004). Drought negatively affects plant photosynthetic efficiency caused by a reduction in leaf expansion, hampered photosynthetic machinery, and early leaf senescence (Wahid and Rasul 2005). The metabolism of carbohydrate, concentration of sucrose in leaves and their export rate decreased due to an increase in the acid invertase activity caused by drought stress (Kim et al. 2000). Liu and Li (2005) observed that the biomass of shoot and root, photosynthesis, and respiration rate of root reduced sharply in wheat exposed to severe drought conditions. Drought-induced yield reduction has been reported in pigeon pea also where a 40–55 % decrease in seed yield was observed at the flowering stage (Nam et al. 2001).
Environmental factors activate a variety of plant responses to drought stress, from altered gene expression and cellular metabolism to adjustment in proper growth and development, thus enabling them to survive under such conditions (Yamaguchi-Shinozaki and Shinozaki 2006; Rampino et al. 2006; Perera et al. 2008; Oh et al. 2009; Wilson et al. 2009). Under drought conditions, gene expression related to various processes such as signaling which includes transcription factors (like NAC family genes, basic leucine zippers, MYB-type transcription factors, zinc fingers, and ethylene-responsive factors) and protein kinases (like calcium-dependent protein kinase and CBL-interacting protein kinase); osmolyte biosynthesis (e.g., trehalose biosynthesis); accumulation of antioxidants (like Mn-superoxide dismutase); and several other processes are known to be affected (Sahoo et al. 2013). It has been reported that the severity as well as duration of the drought stress is determinate for economic yield reduction in many commercial field crop species (Table 16.1). In order to survive under stressful conditions plants must upregulate MG and ROS detoxification processes to avoid cellular damage and also to maintain steady state in different plant physiological processes. In this article we shall discuss the effect of drought stress at biochemical and molecular levels only.
16.3 Methylglyoxal Synthesis, Toxicity, and Accumulation Under Drought Conditions
MG is unavoidably produced during metabolism even under normal physiological conditions (Yadav et al. 2005; Hossain et al. 2009). The generation rate of MG varies depending upon the organism, tissue, cell, and physiological conditions (Yadav et al. 2005) and is formed via different nonenzymatic and enzymatic pathways (Richard 1993). In plants, spontaneous synthesis of MG by nonenzymatic mechanisms is considered to be the central route for its generation under normal and stress circumstances (Fig. 16.2). The nonenzymatic formation of MG occurs via removal of phosphoryl group through β-elimination from 1,2-enediolate of triose sugars, dihydroxyacetone phosphate (DHAP) and d-glyceraldehyde-3-phosphate (GAP) , during glycolysis (Phillips and Thornalley 1993; Richard 1993). Under stress, in order to maintain metabolic homeostasis, the glycolysis increases resulting in disproportion in the pathway. As a result, excessive MG is inevitably produced as a byproduct of glycolysis during such conditions. Apart from glycolysis, several other sources for MG generation have also been reported and include oxidation of aminoacetone (Lyles and Chalmers 1992), ketone bodies (Aleksandrovskii 1992), and acetone (Casazza et al. 1984; Koop and Casazza 1985) (Fig. 16.1). Besides these, Maillard (Thornalley et al. 1999) and lipoperoxidation (Esterbauer et al. 1982) reactions also contribute to nonenzymatic sources of MG.
Correlation between MG and ROS generation and their effects on cellular functions during drought stress in plants. MG exhibits direct inhibitory effects on proteins, lipids, and nucleic acids, resulting in carbonyl stress. Generation of ROS and depletion of glutathione is an indirect effect, causing cell damage or death and has been referred to as oxidative stress
Excessive MG is toxic to the cell inhibiting cell proliferation (Ray et al. 1994). It can easily react with amine groups of proteins, nucleic acids, and lipids in an irreversible manner and form methylglyoxal-derived Advance Glycation End Products (MAGE) . MG forms hydroimidazolone derivate (three related structural isomers; MG-H1, MG-H2 and MG-H3), argpyrimidine and tetrahydropyrimidine (THP) with arginine residues (Gomes et al. 2006) and also with lysine residues forming CEL [Nε-(carboxyethyl)lysine] and MOLDs (methylglyoxal–lysine dimers) (Gomes et al. 2006), and upon reaction with nucleic acids it generates MGdG {3-(2-deoxyribosyl)-6,7-dihydro-6,7-dihydroxy-6-methylimidazo-[2,3-b]purine-9(8)-one} and CEdG [N2-(1-carboxyethyl)-deoxyguanosine] adducts (Thornalley 2003a). In addition, amine-containing basic phospholipids (phosphatidylethanolamine and phosphatidylserine) react with MG and form lipid linked AGEs (carboxymethylethanolamine) (Brown et al. 2005). Furthermore, MG has also been shown to induce ROS formation and apoptosis by activation of signal-regulating kinase (ASK1) (Du et al. 2001). The toxicity of MG is also evident from its ability to cause increased sister chromatin exchange, endoreduplication, DNA strand breaks as well as inducing point mutations (Chaplen 1998). Moreover, it is associated with inhibition of normal growth and development (Hoque et al. 2012c) and results in a number of diverse detrimental effects including the formation of advanced glycation end products (AGEs ) and influencing the antioxidant defense system (Wu and Juurlink 2002; Hoque et al. 2010). MG levels rise to toxic concentrations in plants on exposure to drought stress. In rice, MG concentration at physiological conditions is about 27.5 ± 1.2 and 62.3 ± 3.2 μmol/g fresh weight in root and shoot, respectively, which increase two- to sixfold in response to drought (Yadav et al. 2005). In another study, MG concentration is reported to increase 1.63-fold as compared to control condition after 24 h of drought stress in pumpkin seedlings (Hossain et al. 2009).
16.4 Methylglyoxal Detoxification Pathways
Methylglyoxal (MG) is a physiological highly reactive genotoxic and cytogenic α-oxoaldehyde compound. Due to highly reactive properties of MG, its concentrations must be kept below the threshold levels to sustain cellular homeostasis. Whatever route through which MG is produced, it is primarily detoxified by the ubiquitous glyoxalase pathway (Thornalley 1993). Recent investigations in plants have demonstrated the involvement of the glyoxalase system in drought stress tolerance (Hossain et al. 2009; Hasanuzzaman and Fujita 2011). Apart from glyoxalase pathway, there are other enzymes involved in the detoxification process as well (Kalapos 1999).
16.4.1 Glyoxalase Pathway
The glyoxalase pathway is a ubiquitous mechanism for cellular metabolism of MG in the living systems and operates in the cytoplasm of cells in both prokaryotes and eukaryotes. At the time of its discovery in 1913 (Neuberg 1913; Dakin and Dudley 1913), it was believed to be a single enzyme. Later in 1951, involvement of two enzymes for MG detoxification was reported (Racker 1951). The thiol-dependent glyoxalase system comprises two enzymes, glyoxalase I (GLY I; S-D lactoylglutathione lyase; EC 4.4.1.5) and glyoxalase II (GLY II; hydroxyacylglutathione hydrolase; EC 3.1.2.6). The first enzyme of the pathway, GLY I, catalyzes the conversion of MG to S-d-lactoylglutathione with the help of reduced glutathione (GSH), while the second enzyme, GLY II, converts S-d-lactoylglutathione to d-lactic acid and regenerates GSH back to the system (Racker 1951) (Fig. 16.1). MG detoxification is highly dependent on the availability and concentration of endogenous GSH and thus, insufficiency of cellular GSH leads to the accumulation of MG . The overexpression studies of glyoxalase enzymes have demonstrated that glyoxalases can prevent excessive accumulation of MG in plants under stress conditions, acting primarily by maintaining intracellular antioxidant pools (Singla-Pareek et al. 2003; Hoque et al. 2007; Hasanuzzaman and Fujita 2011; Hasanuzzaman et al. 2011; El-Shabrawi et al. 2010; Ghosh et al. 2014). Additional information on the biological function of glyoxalase system comes from the molecular engineering studies of the corresponding genes. Several investigations provide a potential framework for understanding the physiological roles of the glyoxalase system in higher plants in response to various stresses. However, underexpression of glyoxalase I in tobacco showed increased levels of MG leading to cytotoxicity resulting in failure of seed germination (Yadav et al. 2005). In addition, it was reported that glyoxalase enzymes increased the tolerance of plants to drought-induced oxidative damage by maintaining the GSH/GSSG ratio (Hasanuzzaman and Fujita 2011). Further, upregulation of GLY I and GLY II can confer stress tolerance to plants. It was reported that drought stress enhanced GLY II transcript expression in Brassica and rice (Yadav et al. 2007; Saxena et al. 2005).
16.4.2 Non-glyoxalase Pathways
In addition to glyoxalases, there are other ways in which MG can be detoxified in the plant system. Since MG contains both ketone and aldehyde groups, it can readily undergo oxidation or reduction reactions (Kalapos 1999; Yadav et al. 2008). Consequently, the enzymes which are involved in oxido-reduction can catalyze the conversion of MG to either acetol or lactaldehyde. Enzymes such as aldo-reductases and dehydrogenases catalyze such reactions (Fig. 16.1). ALR1 (Alcohol; NADP-oxido-reductase, EC. 1.1.1.2), ALR2 (alditol: NAD poxido-reductase, EC. 1.1.1.21), and ALR3 (carbonyl reductase; EC. 1.1.1.184) are representatives of reductase family involved in MG detoxification. These ALRs have been shown to possess broad substrate specificity and are potentially involved in MG detoxification in the plants. Overexpression of aldose/aldehyde reductase (ALR) in tobacco plants has been shown to confer tolerance against drought stress. The transgenic plants exhibited reduced loss of photosynthetic efficiency and decreased lipid peroxidation, thiobarbituric acid reactive species (TBRS) and H2O2 accumulation as compared to non-transgenic plants (Hideg et al. 2003). Further, pyruvate dehydrogenases are found in abundance in plants and have also been shown to catalyze MG detoxification (Baggetto and Lehninger 1987). Therefore, efficient detoxification of MG might be a sustainable strategy for tolerance against various stresses (Hasanuzzaman and Fujita 2011).
16.5 Correlation Between MG and ROS Production
In plants, stress is generally associated with increased levels of MG and ROS such as superoxide radical (O2 –), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radical (OH·) (Van Breusegem et al. 2001; Chaves et al. 2003; Reddy et al. 2004). Being a potent and highly reactive glycating agent, it accelerates inactivation of antioxidant defense mechanism (Martins et al. 2001; Thornalley 2003b). MG is interlinked with ROS, as evident from the generation of ROS during both formation and decomposition of MG in different cellular reactions (Table 16.2). In plants, MG hampers normal physiological metabolic functions either directly or indirectly through generation of ROS in cells (Hoque et al. 2012a, c) (Fig. 16.2). ROS can readily react with various biologically important macromolecules such as proteins, lipids, and DNA, resulting in oxidative damage and impedes the normal cellular metabolic functions (Apel and Hirt 2004; Foyer and Noctor 2005). Prolonged drought stress accelerates overproduction of MG as well as ROS resulting in oxidative damage (Yadav et al. 2005; Smirnoff 1993). Thus, excessive accumulation of ROS can overcome the antioxidant defense system and results in alteration in metabolic processes, reduction in photosynthesis, interruptions in cellular coordination leading to growth retardation, reduced fertility, causes premature senescence and death of plants (Hossain et al. 2011b; Saito et al. 2011; Krasensky and Jonak 2012). Therefore, ROS should be regulated in plants through the synchronization of ROS production and ROS scavenging systems to withstand oxidative damage by homeostatic regulation of signaling events (Foyer and Noctor 2005).
16.6 Impairment of Cellular Functions by MG and ROS
MG and ROS are generated during the course of metabolism in vivo and are highly reactive glycation agents. The probable involvement of ROS in reactions between MG and macromolecules was first reported by Szent-Györgyi in the 1960s (Szent-Györgyi 1968). Later in the 1970s, the interaction between protein amines and MG was investigated (Kon and Szent-Györgyi 1973). Moreover, the ROS generating ability of MG was also reported (Kalapos et al. 1993). Currently, little information is available regarding the role of MG and ROS under drought stress conditions in plants. However, relationship of MG and ROS with cellular macromolecules had been studied over the course of time and can be utilized to study the emerging role of glyoxalase in plant drought tolerance. This section concentrates on understanding the mechanism of MG and ROS toxicity in the plants.
16.6.1 MG-Mediated Disruption in Cellular Functioning
Being a strong electrophile, MG can readily modify functional groups of various macromolecules and thus, influences their biological activity (Kalapos 1994). It disturbs cellular metabolism upon excessive accumulation and is directly involved in imposing carbonyl stress (Fig. 16.2), when MG levels supersede detoxification capability of glyoxalase I and other related enzymes, then carbonyls bind to protein, lipids, and other macromolecules, thereby leading to ROS generation and that advanced to apoptosis or to malfunction (Kalapos 2008). It is also inhibiting the activity of various important cellular enzymes, including glycolytic enzymes, intra-mitochondrial enzymes, Na+-K+-ATPase, transport proteins and enzymes participating in cell defense (Leoncini et al. 1980; Kun 1950; Mira et al. 1991; Ferguson et al. 1998; Vander Jagt et al. 1997; Amicarelli et al. 2003). Further, it is also capable of reacting with nucleic acids, and is suggested to be a carcinogenic, mutagenic, and teratogenic agent (Hasegawa et al. 1995; Sugimura and Sato 1983; Chaplen 1998; Brambilla et al. 1985). GSH is a well-known intracellular antioxidant agent involved in the protection of cells from oxidative stress (Sen 1997). It may be trapped as S-2-hydroxyacylglutathione at excessive accumulation of MG and subsequently causing GSH depletion (Kalapos et al. 1992). However, MG can act as directly as cytotoxic agent affecting various cellular machineries or it can reduce GSH concentration under stress condition. It is reported that a significant decrease in GSH levels occur in the presence of various concentrations of MG (Kalapos et al. 1992). Additionally, MG also decreased the thiol containing proteins level in isolated mitochondria (Kun 1950). Finally, MG inhibits the activity of several enzymes (Kalapos 1994) and also depletes GSH levels both in vivo and in vitro (Amicarelli et al. 2003).
16.6.2 ROS-Mediated Disruption in Cellular Functioning
Despite their toxic nature, ROS actually have a double role in vivo depending on their concentration, duration and site of action, preceding encounter to stress, etc. (Miller et al. 2010). In general, low doses are treated as signals that mediate at least some part of the responses towards stress while at certain levels of phytotoxicity, they cause a great threat that may in due course lead to programmed cell death (Gechev and Hille 2005). When the cellular ROS concentration exceeds beyond the threshold levels, then living systems can be said to be in a state of “oxidative stress” (Fig. 16.2). Abiotic stress such as drought leads to excessive accumulation of ROS due to imbalance in cellular homeostasis (Sharma and Dubey 2005). They can pose cellular damage by triggering oxidation of proteins, peroxidation of lipids, damage to nucleic acids, inhibition of enzyme activities, activation of programmed cell death (PCD ) eventually leading to death of the cells (Reddy et al. 2004; Sharma and Dubey 2005; de Carvalho 2008; Ahuja et al. 2010; Karuppanapandian et al. 2011).
16.7 Drought Induced Alteration in Expression of Glyoxalase Genes
The role of glyoxalase genes has been demonstrated under abiotic stress conditions through various transcriptomic studies. Stress-induced alterations in glyoxalase gene expression clearly suggest a direct role of glyoxalase genes in stress adaptation and acclimation pathway. Upon mannitol treatment, a two- to threefold upregulation in GLY I expression has been observed in different tissues such roots, stems, and leaves (Espartero et al. 1995). GLY I preferentially accumulates in the phloem sieve elements as revealed through immunohistochemical localization analysis. Further, a dose-dependent GLY I transcript analysis has also been performed in Brassica juncea in response to salt, drought, and heavy metal stresses (Veena and Sopory 1999). A significant two- to threefold enhancement in the level of GLY I transcript was observed in response to 400 mM mannitol. In order to identify novel genes involved in desiccation tolerance in the foliage of the grass Sporobolus stapfianus, Blomstedt et al. 1998 prepared a cDNA library from the desiccated leaf tissue. After differential screening, six clones including GLY I have been identified that show increased transcript abundance and thus might be associated with desiccation tolerance. Northern blot analysis showed a threefold increase in GLY I transcript in response to dehydration as compared to the fully hydrated tissue and a twofold increase in response to subsequent drying. In S. stapfianus , GLY I transcripts are also induced by 1.6-fold after treatment with ABA. Moreover, microarray analysis of transgenic plants overexpressing NAC transcription factor genes shows upregulation of several stress-inducible genes including GLY I and resulting transgenic plants show significant tolerance towards drought stress (Tran et al. 2004). Further, a sharp fourfold upregulation in GLY I expression has been observed after transcriptome profiling of wild type and co-suppressed MSI1 (chromatin assembly factor 1) Arabidopsis lines (Alexandre et al. 2009). Apart from activation of GLY I transcripts, co-suppressed MSI1 plants have increased levels of free proline and showed enhanced tolerance towards drought. A noticeable increase in the GLY I transcript was also observed in pumpkin seedlings in response to different stresses including drought, salinity, heavy metal, and heat (Hossain et al. 2009). Moreover, genome wide expression analysis of Arabidopsis and rice using microarray data identified several glyoxalase members with altered expression in response to drought stress (Mustafiz et al. 2011). An upregulation in expression of AtGLYI3, AtGLYI6, and AtGLYI7 genes occurs in a time-dependent manner under drought conditions in Arabidopsis seedlings, whereas AtGLYI2, AtGLYI4, and AtGLYI9 are downregulated under such conditions. Similarly, rice glyoxalase genes , OsGLYI2, OsGLYI6, and OsGLYI11, are induced, but OsGLYI5 and OsGLYI10 are downregulated in response to drought stress in the rice seedlings. Expression of rice GLY I transcripts were further analyzed in the 2 weeks rice seedlings in response to different abiotic stresses such as heat, cold, dehydration, wounding, MG, salt, and oxidative stress by qRT-PCR (Kaur et al. 2013). A 4.5-fold upregulation in OsGLYI-11.2 expression was observed, followed by OsGLYI-7.1 under drought conditions; while other members OsGLYI-2, OsGLYI-8, and OsGLYI-11.3 showed sharp decline in gene expression. Furthermore, differential gene expression studies in soybean leaf tissues revealed upregulation of GLY I family members along with other regulatory and functional genes under drought stress (Le et al. 2012).
Like GLY I, expression of GLY II transcript was also found to vary under different stresses. Expression of rice GLY II gene was analyzed in response to various abiotic stresses such as desiccation, salinity, heat, cold, and ABA and SA treatment (Yadav et al. 2005). Significant accumulation of GLY II transcript was found in response to all stress agents. Desiccation stress resulted in the accumulation of GLY II transcript in a short duration of 15 min followed by gradual increase in accumulation with time till 2 h (Yadav et al. 2007). Genome wide transcript analysis of rice GLY II transcripts showed strong induction of all GLY II members in response to drought stress (Mustafiz et al. 2011). Amongst the Arabidopsis GLY II genes, the expression of AtGLYII1 and AtGLYII2 was found to be highly upregulated in response to drought stress in both shoot and root tissues (Mustafiz et al. 2011). However AtGLYII3, AtGLYII4, and AtGLYII5 were downregulated in response to drought stress in both shoot and root tissues in Arabidopsis.
16.8 Drought Induced Alteration in Levels of Glyoxalase Proteins
Proteins are vital components of living organisms that are directly involved in various physiological and metabolic pathways of cells. Hence, studying variations in levels of glyoxalase proteins or their enzyme activities will give more precision in understanding the role of these enzymes in stress adaptation and in efficient monitoring of the stress response. Activity of glyoxalase has been monitored by various research groups under different environmental stimuli. Initial reports have revealed an increase in GLY I activity during cell division (Deswal et al. 1993) and proliferative callus cultures of groundnut (Arachis hypogaea L.cv. JL24) (Jain et al. 2002). To identify the altered proteins during drought stress, functional proteome studies have been performed and have secured an important place in the era of comparative and functional genomics. To investigate the mechanism of plants’ osmotic stress response , rice protein profiles were monitored from mannitol-treated plants using proteomics approach (Zang and Komatsu 2007). Proteins from the basal part of leaf sheaths showed strong induction in levels of GLY I protein in response to stress. To study the changes in wheat grain proteome in response to drought, two-dimensional gel electrophoresis among three wheat genotypes with different genetic background was performed under well-watered and drought conditions (Hajheidari et al. 2007). The overall effect of drought was highly significant and about 650 spots were reproducibly detected and analyzed. Mass spectrometry analysis using MALDITOF/TOF led to the identification of 57 proteins with significant alteration. A significant downregulation (twofold) in GLY I protein levels was observed in the susceptible genotypes , with no or insignificant changes in the tolerant counterpart.
Further, GLY I protein was also identified in a two-dimensional gel electrophoresis experiment carried out in two distinct sunflower genotypes in response to drought (Castillejo et al. 2008). The susceptible genotype showed a decrease in the intensity of the 17 spots out of 28 altered proteins. The proteins that showed a decline in their levels included a GLY I protein, along with some other important proteins such as photosystem II oxygen-evolving complex protein 1 , carbonic anhydrase , RubisCO large and small subunits , ferredoxin-NADP+ reductase, phosphoglycerate kinase, glyceraldehyde-3-phosphate dehydrogenase, aldolase and superoxide dismutase. Furthermore, comparative proteomic analysis of differentially expressed chickpea and rice extracellular matrix proteins also led to the identification of a GLY I protein during dehydration stress (Bhushan et al. 2007; Pandey et al. 2010). GLY I protein was also found to be significantly upregulated in the nuclear fraction of chickpea in response to dehydration stress (Pandey et al. 2008). In addition, analysis of drought responsive leaf proteome of a C3 xerophyte, Citrullus lanatus also revealed alteration in levels of GLY I protein (Akashi et al. 2011).
Significant increase in levels of GLY I protein and GLY I activity was observed in onion bulb in response to various stress treatments (Hossain et al. 2007). An induction of 1.3- to 1.4-fold was observed in both the levels of GLY I protein and activity in response to drought stress. A sharp increase in GLY I activity (1.27-fold) was observed after 24 h of drought stress in pumpkin seedlings (Hossain et al. 2009). A similar pattern of induction was observed in GLY II enzyme activity in response to drought. The potential role of various chemical compounds in increasing drought tolerance by enhancing glyoxalase enzyme activity has been determined by different studies (Hasanuzzaman and Fujita 2011; Alam et al. 2013). For instance, drought stress induced oxidative damage of rapeseed seedlings could be reversed by the pretreatment of selenium that enhances the activities of antioxidant and MG detoxifying enzymes (Hasanuzzaman and Fujita 2011). Selenium pretreated rapeseed seedlings exposed to various degrees of drought stress showed a sharp rise in their ascorbic acid level, reduced glutathione content, and maintained a high GSH/GSSG ratio as compared with the drought-stressed plants without selenium treatment. It has been reported that pretreatment with 25 mM of selenium resulted in a 23 % increase in GLY I activity and also a significant increase in GLY II activity in rapeseed seedlings as compared to control. A similar study showed that exogenous addition of salicylic acid in mustard seedlings mediates short-term tolerance against drought stress by upregulating the antioxidant defense and glyoxalase pathway (Alam et al. 2013). Drought stress resulted in a sharp decline in the level of ascorbate, relative water content, and chlorophyll content in the mustard seedlings, but increased their proline, malondialdehyde, and H2O2 levels. However, salicylic acid supplementation in the drought stressed seedlings enhanced ascorbate, reduced glutathione, chlorophyll, and relative water content, as well as decreased the GSSG level to maintain the ratio of GSH/GSSG. Salicylic acid supplemented drought stressed seedlings also enhanced the enzyme activities of GLY I, GLY II, and different antioxidant enzymes as compared to drought-stressed plants without salicylic acid supplementation. Moreover, temperature (either heat or cold)-shock positively modulates the oxidative protection in salinity and drought stressed mustard (Brassica campestris L.) seedlings in a very similar mechanism by increasing glyoxalase activity (Hossain et al. 2013a, b). Seedlings pre-exposed to either heat-shock or cold-shock conditions positively modulate the activities of GLY I and GLY II, and maintain lower levels of GSSG, H2O2, and malondialdehyde as compared to the control as well as non-treated drought stressed seedlings.
16.9 Signaling Roles of MG in Regulation of Stomatal Closure and Stress Responsive Gene Expression
Despite having inhibitory effects on cell growth, MG has been shown to possess signaling roles in bacteria (Campbell et al. 2007), humans (Kang et al. 1996; Akhland et al. 2001), and yeast (Maeta et al. 2005; Takatsume et al. 2006). However in plants, role of MG in signal transduction is less studied. Nonetheless, it has been reported that MG induces ROS formation (Hoque et al. 2012a) and that ROS mediates abscisic acid (ABA) and methyl jasmonate (MeJA) signaling pathways in guard cells related to stomatal regulation (Munemasa et al. 2007). Hoque and coworkers have shown that MG induces stomatal closure in a reversible manner and also induces generation of ROS in Arabidopsis (Hoque et al. 2012a). It was found that MG induced significant accumulation of ROS and also increased cytosolic Ca2+ oscillations in the guard cells which were suppressed by pretreatment with SHAM (salicylhydroxamic acid). SHAM-sensitive peroxidases diffuse extracellular oxidative burst into the intracellular space contributing to intracellular ROS accumulation in the guard cells and trigger stomatal closure via a Ca2+-dependent pathway (Hoque et al. 2012a). Additionally, it was also observed that MG was also engaged in inhibiting light-induced stomatal opening via the modification of C-terminal region of KAT1, an inward-rectifying potassium channel thereby inhibiting K+ influx into the guard cells (Hoque et al. 2012b). The involvement of MG in regulation of stomatal movements indicates towards its role in signal transduction pathways in drought stress adaptation. Because of closure of stomata is the primary response of almost all plants to drought to prevent transpirational water loss (Mansfield and Atkinson 1990). Regulation of stomata may result in response to decrease in leaf turgor or low humidity atmosphere (Ludlow and Muchow 1990; Maroco et al. 1997). In response to drought, MG levels have been reported to increase up to sixfold depending upon the crop species (Yadav et al. 2005).
Further, MG is capable of altering expression of genes known to be involved in drought stress adaptation. For instance, MG was found to affect the transcript levels of ABA-dependent genes, RD29B and RAB18, which are generally induced in response to dehydration. MG could significantly induce RD29B (fivefold) and RAB18 (threefold) gene expression that too in a concentration-dependent manner (Hoque et al. 2012c). In addition, MG has also been shown to enhance expression of triose phosphate isomerase (OscTPI) and OsETHE1 in rice (Sharma et al. 2012; Kaur et al. 2014b). Moreover, global gene expression profiles in rice in response to exogenous MG showed its involvement in signal transduction. MG affected the expression of various genes involved in stress-induced signal transduction cascades such as protein kinases (mitogen-activated protein kinase, calcium/calmodulin-dependent protein kinases, Ser/Thr protein kinase, histidine kinase, and receptor-like kinase) and transcription factors (bZIP, AP2 domain-containing protein, NAM, WRKY, and zinc finger proteins), which were significantly represented in the perturbed transcriptomes, indicating an interlink between MG and stress-responsive signal transduction pathways (Kaur et al. 2015). Collectively, MG plays a significant role in signal transduction possibly acting as a stress signal molecule in plants, where it conveys signals to the cellular machinery to maintain the cellular homeostasis towards adaptation in drought stress.
16.10 Conclusion and Future Perspective
The pathways involved in drought stress adaptation in plants are regulated at both physiological and molecular levels. Molecular information of response and tolerance mechanisms is likely to pave way for engineering plants that could make them withstand drought stress. Many achievements have been made over the last few years in understanding the protective role of glyoxalases in MG detoxification under drought conditions (Fig. 16.3). Drought stress leads to increased accumulation of MG and MG-derived ROS. It is now well known that MG has deleterious effects on plant growth and development and that glyoxalase pathway serves an important detoxification role in the living systems. Several transcriptome and proteome studies carried out to identify genes involved in drought stress response have revealed a link between glyoxalases and drought stress adaptation indicating glyoxalase pathway to be a crucial intracellular component of plant stress response. Further, MG transmits signals to the cellular machinery for inducing changes in plant transcriptome, transcription factors, protein kinase as well as regulation of stomatal movements for adaptation to drought stress conditions. However, the specific role of MG as a signal molecule itself or as a component in signaling cascade in plants needs further investigation for deeper understanding of its role in stress response and tolerance.
Role of glyoxalase pathway in drought stress adaptation. During drought, MG and ROS levels increase which then impair the redox balance of cell. MG levels also induce ROS generation through the formation of AGEs, resulting in ROS-mediated cellular injury and death (a). Increase in glyoxalase activity through overexpression helps in maintaining cellular redox homoeostasis under drought stress by reducing MG levels and regenerating GSH back into the system, thereby decreasing ROS generation which leads to improved drought tolerance (b)
References
Ahuja I, de Vos RCH, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trends Plant Sci. 2010;12:664–74.
Akashi K, Yoshida K, Kuwano M, Kajikawa M, Yoshimura K, Hoshiyasu S, Inagaki N, Yokota A. Dynamic changes in the leaf proteome of a C3 xerophyte, Citrullus lanatus (wild watermelon), in response to water deficit. Planta. 2011;233:947–60.
Akhland AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, Kurokawa K, Nakashima I. Glyoxal and methylglyoxal trigger distinct signals for MAP family kinases and caspase activation in human endothelial cells. Free Radic Biol Med. 2001;31:20–30.
Akram M. Growth and yield components of wheat under water stress of different growth stages. Bangladesh J Agric Res. 2011;36:455–68.
Alam MM, Hasanuzzaman M, Nahar K, Fujita M. Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2013;7:1053–63.
Aleksandrovskii YA. Antithrombin III, C1 inhibitor, methylglyoxal, and polymorphonuclear leukocytes in the development of vascular complications in diabetes mellitus. Thromb Res. 1992;67:179–89.
Alexandre C, Möller-Steinbach Y, Schönrock N, Gruissem W, Hennig L. Arabidopsis MSI1 is required for negative regulation of the response to drought stress. Mol Plant. 2009;2(4):675–87.
Amicarelli F, Colafarina S, Cattani F, Cimini A, Di Ilio C, Ceru MP, Miranda M. Scavenging system efficiency is crucial for cell resistance to ROS mediated methylglyoxal injury. Free Radic Biol Med. 2003;35:856–71.
Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99.
Baggetto LG, Lehninger AL. Isolated tumoral pyruvate dehydrogenase can synthesize acetone which inhibits pyruvate oxidation as well as other aldehydes. Biochem Biophys Res Commun. 1987;145:153–9.
Basnayake J, Fukai S, Ouk M. Contribution of potential yield, drought tolerance and escape to adaptation of 15 rice varieties in rainfed lowlands in Cambodia. Proceedings of the Australian Agronomy Conference. Brisbane: Australian Society of Agronomy; 2006.
Bhushan D, Pandey A, Choudhary MK, Datta A, Chakraborty S, Chakraborty N. Comparative proteomics analysis of differentially expressed proteins in chickpea extracellular matrix during dehydration stress. Mol. Cell Proteom. 2007;6:1868–84.
Blomstedt CK, Gianello RD, Hamill JD, Neale AD, Gaff DF. Drought-stimulated genes correlated with desiccation tolerance of the resurrection grass Sporobolus stapfianus. Plant Growth Regul. 1998;24:153–61.
Brambilla G, Sciaa L, Faggin P, Finollo R, Bassi AM, Ferro M, Marinari UM. Methylglyoxal-induced DNA-protein cross-links and cytotoxicity in Chinese hamster ovary cells. Carcinogenesis. 1985;6:683–6.
Brown BE, Dean RT, Davies MJ. Glycation of low-density lipoproteins by methylglyoxal and glycolaldehyde gives rise to the in vitro formation of lipid-laden cells. Diabetologia. 2005;48:361–9.
Campbell AK, Naseem R, Holland IB, Matthews SB, Wann KT. Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ transients and inhibit growth in E. coli. Arch Biochem Biophys. 2007;468:107–13.
Casazza JP, Felver ME, Veech RL. The metabolism of acetone in rat. J Biol Chem. 1984;259:231–6.
Castillejo MA, Maldonado AM, Ogueta S, Jorrın JV. Proteomic analysis of responses to drought stress in sunflower (Helianthus annuus) leaves by 2DE gel electrophoresis and mass spectrometry. Open Proteom J. 2008;1:59–71.
Chaplen FWR. Incidence and potential implications of the toxic metabolite methylglyoxal in cell culture: A review. Cytotechnology. 1998;26:173–83.
Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol. 2003;30:239–64.
Cohen D, Bogeat-Triboulot MB, Tisserant E, Balzergue S, Martin-Magniette ML, Lelandais G, Ningre N, Renou JP, Tamby JP, Le Thiec D, Hummel I. Comparative transcriptomics of drought responses in Populus: a meta-analysis of genome-wide expression profiling in mature leaves and root apices across two genotypes. BMC Genomics. 2010;11:630.
Dakin HD, Dudley HW. An enzyme concerned with the formation of hydroxyl acids from ketonic aldehydes. J Biol Chem. 1913;14:155–7.
de Carvalho MHC. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008;3:156–65.
Deswal R, Chakravarty TN, Sopory SK. The glyoxalase system in higher plants: regulation in growth and differentiation. Biochem Soc Trans. 1993;21:527–30.
Du J, Suzuki H, Nagase F, Akhand AA, Ma XY, Yokoyama T, Miyata T, Nakashima I. Superoxide-mediated early oxidation and activation of ASK1 are important for initiating methylglyoxal-induced apoptosis process. Free Radic Biol Med. 2001;31:469–78.
Dutra F, Knudsen FS, Curi D, Bechara EJ. Aerobic oxidation of aminoacetone, a threonine catabolite: Iron catalysis and coupled iron release from ferritin. Chem Res Toxicol. 2001;14:1323–9.
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma. 2010;245:85–96.
Espartero J, Sanchez-Aguayo I, Pardo JM. Molecular characterization of glyoxalase-I from a higher plant: upregulation by stress. Plant Mol Biol. 1995;29:1223–33.
Esterbauer H, Cheeseman KH, Dianzani MU, Poli G, Slater TF. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982;208:129–40.
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron Sustain Dev. 2009;29(1):185–212.
Farooq M, Bramley H, Palta JA, Siddique KHM. Heat stress in wheat during reproductive and grain filling phases. Crit Rev Plant Sci. 2011;30:491–507.
Ferguson GP, Tötemeyer V, MacLean MJ, Booth IR. Methylglyoxal production in bacteria: suicide or survival? Arch Microbiol. 1998;170:209–19.
Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75.
Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol. 2005;168(1):17–20.
Ghosh A, Pareek A, Sopory SK, Singla-Pareek SL. A glutathione responsive rice glyoxalase II, OsGLYII-2, functions in salinity adaptation by maintaining better photosynthesis efficiency and anti-oxidant pool. Plant J. 2014;80(1):93–105.
Gomes RA, Vicente Miranda H, Silva MS, Graca G, Coelho AV, Ferreira AE, Cordeiro C, Freire AP. Yeast protein glycation in vivo by methylglyoxal. Molecular modification of glycolytic enzymes and heat shock proteins. FEBS J. 2006;273:5273–87.
Hajheidari M, Eivazi A, Buchanan BB, Wong JH, Majidi I, Salekdeh GH. Proteomics uncovers a role for redox in drought tolerance in wheat. J Proteome Res. 2007;6:1451–60.
Hasanuzzaman M, Fujita M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res. 2011;143:1758–76.
Hasanuzzaman M, Hossain MA, Fujita M. Selenium induced upregulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res. 2011;143:1704–21.
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M. Plant Responses and tolerance to abiotic oxidative stress: Antioxidant defenses is a key factors. In: Bandi V, Shanker AK, Shanker C, Mandapaka M, editors. Crop stress and its management: Perspectives and strategies. Berlin: Springer; 2012. p. 261–316.
Hasegawa R, Ogiso T, Imaida K, Shirai T, Ito N. Analysis of the potential carcinogenicity of coffee and its related compounds in a medium-term liver bioassay of rats. Food Chem Toxicol. 1995;33:15–20.
Hideg E, Nagy T, Oberschall A, Dudits D, Vass I. Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280-320) stresses. Plant Cell Environ. 2003;26:513–22.
Hoque MA, Banu MN, Nakamura Y, Shimoishi Y, Murata Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl induced damage in cultured tobacco cells. J Plant Physiol. 2007;165:813–24.
Hoque MA, Uraji M, Banu MNA, Mori IC, Nakamura Y, Murata Y. The effect of methylglyoxal on glutathione S-transferase from Nicotiana tabacum. Biosci Biotechnol Biochem. 2010;74:2124–6.
Hoque TS, Uraji M, Ye W, Hossain MA, Nakamura Y, Murata Y. Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J Plant Physiol. 2012a;169:979–86.
Hoque TS, Okuma E, Uraji M, Furuichi T, Sasaki T, Hoque MA, Nakamura Y, Murata Y. Inhibitory effects of methylglyoxal on light-induced stomatal opening and inward K+ channel activity in Arabidopsis. Biosci Biotechnol Biochem. 2012b;76:617–9.
Hoque TS, Uraji M, Tuya A, Nakamura Y, Murata Y. Methylglyoxal inhibits seed germination and root elongation and up-regulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biol. 2012c;14:854–8.
Hossain MA, Fujita M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol Mol Biol Plants. 2010;16:19–29.
Hossain MD, Rohman MM, Fujita M. Comparative investigation of glutathione S-transferases, glyoxalase I and allinase activities in different vegetable crops. J Crop Sci Biotechnol. 2007;10:21–8.
Hossain MA, Hossain MZ, Fujita M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci. 2009;3:53–64.
Hossain MA, Hasanuzzaman M, Fujita M. Coordinate induction of antioxidant defense and glyoxalase system by exogenous proline and glycinebetaine is correlated with salt tolerance in mung bean. Front Agric China. 2011a;1:1–14.
Hossain MA, Teixeira da Silva JA, Fujita M. Glyoxalase system and reactive oxygen species detoxification system in plant abiotic stress response and tolerance: An intimate relationship. In: Shanker AK, Venkateswarlu B, editors. Abiotic Stress/Book 1. Croatia: INTECH-Open Access; 2011b. p. 235–66.
Hossain MA, Mostofa MG, Fujita M. Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed. 2013a;4:50–70.
Hossain MA, Mostofa MG, Fujita M. Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (Brassica campestris L.) seedlings. J Plant Sci Mol Breed. 2013b;2:2.
Hossain MA, Mostofa MG, Burritt DJ, Fujita M. Modulation of reactive oxygen species and methylglyoxal detoxification systems by exogenous glycinebetaine and proline improves drought tolerance in mustard (Brassica juncea L.). Int J Plant Biol Res. 2014;2(2):1014.
Jain M, Choudhary D, Kale RK, Bhalla-Sarin N. Salt and glyphosphate-induced increase in glyoxalase I activity in cell lines of groundnut (Arachis hypogaea). Physiol Plant. 2002;114:499–505.
Johnson JM, Halsall HB, Heinemen WR. Redox activation of galactose oxidase: thin-layer electrochemical study. Biochemistry. 1985;24:1579–85.
Kalapos MP. Methylglyoxal toxicity in mammals. Toxicol Lett. 1994;73:3–24.
Kalapos MP. Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol Lett. 1999;110:145–75.
Kalapos MP. The tandem of free radicals and methylglyoxal. Chem Biol Interact. 2008;171(3):251–71.
Kalapos MP, Garzó T, Antoni F, Mandl J. Accumulation of S-D-lactoylglutathione and transient decrease of glutathione level caused by methylglyoxal load in isolated hepatocytes. Biochim Biophys Acta. 1992;1135:159–64.
Kalapos MP, Littauer A, de Groot H. Has reactive oxygen a role in methylglyoxal toxicity? A study on cultured rat hepatocytes. Arch Toxicol. 1993;67:369–72.
Kang Y, Edwards LG, Thornalley PJ. Effect of methylglyoxal on human leukaemia 60 cell growth: modification of DNA, G1 growth arrest and induction of apoptosis. Leuk Res. 1996;5:397–405.
Karuppanapandian T, Wang HW, Prabakaran N, Jeyalakshmi K, Kwon M, Manoharan K, Kim W. 2,4-dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol Biochem. 2011;49:168–77.
Kaur C, Vishnoi A, Ariyadasa TU, Bhattacharya A, Singla-Pareek SL, Sopory SK. Episodes of horizontal gene-transfer and gene-fusion led to co-existence of different metal-ion specific glyoxalase I. Sci Rep. 2013;3:3076.
Kaur C, Ghosh A, Pareek A, Sopory SK, Singla-Pareek SL. Glyoxalases and stress tolerance in plants. Biochem Soc Trans. 2014a;42(2):485–90.
Kaur C, Mustafiz A, Sarkar A, Ariyadasa TU, Singla-Pareek SL, Sopory SK. Expression of abiotic stress inducible ETHE1-like protein from rice is higher in roots and is regulated by calcium. Physiol Plant. 2014b;152(1):1–16. doi:10.1111/ppl.12147.
Kaur C, Kushwaha HR, Mustafiz A, Pareek A, Sopory SK, Singla-Pareek SL. Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front Plant Sci. 2015;6:682.
Kersten PJ, Kirk TK. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987;169:2195–201.
Kim JY, Mahé A, Brangeon J, Prioul JL. A maize vacuolur invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000;124:71–84.
Kon H, Szent-Györgyi A. Charge transfer between amine and carbonyl. Proc Natl Acad Sci U S A. 1973;70:3139–40.
Koop DR, Casazza JP. Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J Biol Chem. 1985;260:13607–12.
Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot. 2012;63:1593–608.
Kun K. Inhibition of succinic dehydrogenase by methylglyoxal. J Biol Chem. 1950;187:289–97.
IPCC. Kundzewicz ZW, Palutikof J, Wu S, editors. Climate change and water. Technical paper of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2008.
Lafitte HR, Yongsheng G, Yan S, Lil ZK. Whole plant responses, key processes, and adaptation to drought stress: the case of rice. J Exp Bot. 2007;58:169–75.
Lambers H, Chapin FS, Pons TL. Plant physiological ecology. 2nd ed. New York: Springer; 2008.
Le DT, Nishiyama R, Watanabe Y, Tanaka M, Seki M, Ham LH, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP. Differential gene expression in soybean leaf tissues at late developmental stages under drought stress revealed by genome-wide transcriptome analysis. PLoS One. 2012;7, e49522.
Leoncini G, Maresca M, Bonsignore A. The effect of methylglyoxal on the glycolytic enzymes. FEBS Lett. 1980;117:17–8.
Liu HS, Li FM. Root respiration, photosynthesis and grain yield of two spring wheat in response to soil drying. Plant Growth Regul. 2005;46:233–40.
Ludlow MM, Muchow RC. A critical evaluation of traits for improving crop yields in water-limited environments. Adv Agron. 1990;43:107–53.
Lyles GA, Chalmers J. The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem Pharmacol. 1992;43:1409–14.
Maeta K, Izawa S, Inoue Y. Methylglyoxal, a metabolite derived from glycolysis, functions as signal initiator of the high osomolarity glycerol-miotgen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Sacchromyces cerevisiae. J Biol Chem. 2005;280:253–60.
Manavalan LP, Guttikonda SK, Tran LS. Nguyen HT (2009) Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009;50:1260–76.
Mansfield TJ, Atkinson CJ. Stomatal behaviour in water stressed plants. In: Alscher RG, Cumming JR, editors. Stress responses in plants: adaptation and acclimation mechanisms. New York: Wiley-Liss; 1990. p. 241–64.
Maroco JP, Pereira JS, Chaves MM. Stomatal responses to leaf-to-air vapour pressure deficit in Sahelian species. Aust J Plant Physiol. 1997;24:381–7.
Martins AMTBS, Cordeiro CAA, Freire AMJP. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 2001;499:41–4.
Mazahery-Laghab H, Nouri F, Abianeh HZ. Effects of the reduction of drought stress using supplementary irrigation for sunflower (Helianthus annuus) in dry farming conditions. Pajouheshva-Sazandegi Agron Hort. 2003;59:81–6.
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–67.
Mira ML, Martinho F, Azevedo MS, Manso CF. Oxidative inhibition of red blood cell ATPases by glyceraldehyde. Biochim Biophys Acta. 1991;1060:257–61.
Monneveux P, Sánchez C, Beck D, Edmeades GO. Drought tolerance improvement in tropical maize source populations: evidence of progress. Crop Sci. 2006;46:180–91.
Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 2007;143:1398–407.
Mustafiz A, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL. Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct Integr Genomics. 2011;11(2):293–305.
Nam NH, Chauhan YS, Johansen C. Effect of timing of drought stress on growth and grain yield of extra-short-duration pigeonpea lines. J Agric Sci. 2001;136:179–89.
Nayyar H, Kaur S, Singh S, Upadhyaya HD. Differential sensitivity of Desi (small-seeded) and Kabuli (large-seeded) chickpea genotypes to water stress during seed filling: effects on accumulation of seed reserves and yield. J Sci Food Agr. 2006;86:2076–82.
Neuberg C. The destruction of lactic aldehyde and methylglyoxal by animal organs. Biochem J. 1913;49:502–6.
Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK. Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol. 2009;150(3):1368–79.
Pandey A, Chakraborty S, Datta A, Chakraborty N. Proteomics approach to identify dehydration responsive nuclear proteins from chickpea (Cicer arietinum L.). Mol. Cell Proteom. 2008;7:88–107.
Pandey A, Rajamani U, Verma J, Subba P, Chakraborty N, Datta A, Chakraborty S, Chakraborty N. Identification of extracellular matrix proteins of rice (Oryza sativa L.) involved in dehydration-responsive network: a proteomic approach. J Proteome Res. 2010;9:3443–64.
Perera IY, Hung CY, Moore CD, Stevenson-Paulik J, Boss WF. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell. 2008;20(10):2876–93.
Peuke AD, Rennenberg H. Carbon, nitrogen, phosphorus, and sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): phosphorus most affected by drought. Trees. 2004;18:639–48.
Phillips SA, Thornalley PJ. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem. 1993;212:101–5.
Racker E. The mechanism of action of glyoxalase. J Biol Chem. 1951;190:685–96.
Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C. Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006;29(12):2143–52.
Ray S, Dutta S, Halder J, Ray M. Inhibition of electron flow through complex I of the mitochondrial respiratory chain of Ehrlich ascites carcinoma cells by methylglyoxal. Biochem J. 1994;303:69–72.
Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–202.
Richard JP. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem Soc Trans. 1993;21:549–53.
Sahoo KK, Tripathi AK, Pareek A, Singla-Pareek SL. Taming drought stress in rice through genetic engineering of transcription factors and protein kinases. Plant Stress. 2013;7(1):60–72.
Saito R, Yamamoto H, Makino A, Sugimoto T, Miyake C. Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O(2) at photosystem I: a symptom of plant diabetes. Plant Cell Environ. 2011;34:1454–64.
Samarah NH. Effects of drought stress on growth and yield of barley. Agron Sustain Dev. 2005;25:145–9.
Samarah NH, Mullen RE, Cianzio SR, Scott P. Dehydrin-like proteins in soybean seeds in response to drought stress during seed filling. Crop Sci. 2006;46:2141–50.
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hiraj MY, Noji M, Saito K, Masuda T, Takamiya KI, Shibata D, Ohta H. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005;44:563–668.
Saxena M, Bisht R, Roy DS, Sopory SK, Bhalla-Sarinn M. Cloning and characterization of a mitochondrial glyoxalase II from Brassica juncea that is upregulated by NaCl, Zn and ABA. Biochem Biophys Res Commun. 2005;336:813–9.
Sen CK. Nutritional biochemistry of cellular glutathione. Nutr Biochem. 1997;8:660–72.
Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005;46(3):209–21.
Sharma S, Mustafiz A, Singla-Pareek SL, Shankar Srivastava P, Sopory SK. Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal Behav. 2012;7:1337–45.
Siddique MRB, Hamid A, Islam MS. Drought stress effects on water relations of wheat. Bot Bull Acad Sin. 2001;41:35–9.
Singla-Pareek SL, Reddy MK, Sopory SK. Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci U S A. 2003;100:14672–7.
Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58.
Sousa Silva M, Gomes RA, Ferreira AE, Ponces Freire A, Cordeiro C. The glyoxalase pathway: the first hundred years… beyond. Biochem J. 2013;453(1):1–15.
Sugimura T, Sato S. Mutagens–carcinogens in foods. Cancer Res. 1983;43:2415–21.
Szent-Györgyi A. Bioelectronics. New York: Academic; 1968.
Takatsume Y, Izawa S, Inoue Y. Methylglyoxal as a signal initiator for activation of the stress-activated protein kinase cascade in the fission yeast Schizosaccharomyces pombe. J Biol Chem. 2006;281:9086–92.
Thornalley PJ. The glyoxalase system in health and disease. Mol Asp Med. 1993;14:287–371.
Thornalley PJ. Protecting the genome: defence against nucleotide glycation and emerging role of glyoxalase I overexpression in multidrug resistance in cancer chemotherapy. Biochem Soc Trans. 2003a;31:1372–7.
Thornalley PJ. Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003b;31:1343–8.
Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–16.
Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004;16:2481–98.
Van Breusegem F, Vranova E, Dat JF, Inze D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001;161:405–14.
Vander Jagt DL, Hunsaker LA, Vander Jagt TJ, Gomez MS, Gonzales DM, Deck LM, Royer RE. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem Pharmacol. 1997;53:1133–40.
Veena RVS, Sopory SK. Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J. 1999;17:385–95.
Wahid A, Rasul E. Photosynthesis in leaf, stem, flower and fruit. In: Pessarakli M, editor. Handbook of photosynthesis. 2nd ed. Boca Raton: CRC Press; 2005. p. 479–97.
Wilson PB, Estavillo GM, Field KJ, Pornsiriwong W, Carroll AJ, Howell KA, Woo NS, Lake JA, Smith SM, Harvey Millar A, von Caemmerer S, Pogson BJ. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009;58(2):299–317.
Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39(3):809–14.
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun. 2005;337:61–7.
Yadav SK, Singla-Pareek SL, Kumar M, Pareek A, Saxena M, Sarin NB, Sopory SK. Characterization and functional validation of glyoxalase II from rice. Protein Expr Purif. 2007;51(1):126–32.
Yadav SK, Singla-Pareek SL, Sopory SK. An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol Drug Interact. 2008;23(1-2):51–68.
Yamaguchi-Shinozaki K, Shinozaki K. Transcription regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803.
Yu PH, Wright S, Fan EH, Lun DZR, Gubisne-Harberle D. Physiological and pathological implications of semicarbazide sensitive amine oxidase. Biochim Biophys Acta. 2003;1647:193–9.
Zang X, Komatsu S. A proteomics approach for identifying osmotic-stress-related proteins in rice. Phytochemistry. 2007;68:426–37.
Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6(2):66e71.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hasan, M.R., Ghosh, A., Kaur, C., Pareek, A., Singla-Pareek, S.L. (2016). Glyoxalase Pathway and Drought Stress Tolerance in Plants. In: Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, LS. (eds) Drought Stress Tolerance in Plants, Vol 1. Springer, Cham. https://doi.org/10.1007/978-3-319-28899-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-28899-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28897-0
Online ISBN: 978-3-319-28899-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)