Abstract
Methylglyoxal (MG) is a cytotoxic metabolite inevitably produced as a side product of primary metabolic pathways via both enzymatic and non-enzymatic reactions. In plants, spontaneous generation of MG through breakdown of triose sugars (dihydroxyacetone phosphate and glyceraldehyde 3-phosphate) is believed to be the major route for MG formation. MG is maintained at basal levels in plants under normal conditions that accumulate to higher concentrations under various stresses, probably as a general consequence of all abiotic stresses. The toxic effects of MG is due to its ability to induce oxidative stress in cells, either directly through increased generation of reactive oxygen species (ROS) or indirectly via advanced glycation end product (AGE) formation. Thus, elevated MG levels have implications in inhibition of growth and development in plants. To keep MG levels in check, the two-step glyoxalase pathway comprising glyoxalase I (GLYI) and glyoxalase II (GLYII) enzymes has evolved as the major MG-scavenging detoxification system that converts MG to d-lactate using glutathione as a cofactor in this process. Over-expression of glyoxalase pathway has been shown to confer tolerance to multiple stresses that works by controlling MG levels and maintaining glutathione homeostasis in plants. Moreover, increased activity of triose phosphate isomerase under different stresses that use up triose sugars via glycolysis further prevents MG levels from accumulating in the system along with increasing the energy status of plants. Considering the fact that MG levels are maintained at a threshold concentration in plants even under physiological conditions and also observed MG-dependent induction in expression of triose phosphate isomerase, a role for MG in signaling pathways is suggested. Here, we provide an insight to the role of MG and glyoxalases in plant stress response with special mention about the possible involvement of MG in signaling pathway.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glyoxalase I
- Glyoxalase II
- Glutathione
- Methylglyoxal
- Triose phosphate isomerase
- Abiotic stress response
- Heavy metal stress
- Salinity
- Stress tolerance
1 Introduction
Abiotic stresses negatively impact plant growth and development resulting in extensive losses to agricultural production worldwide (Boyer 1982). Perception of stress followed by signal transmission to turn on the adaptive responses is the key step leading to plant stress tolerance. However, tolerance or sensitivity to stresses is a very complex event, with more than one stress simultaneously affecting the plant and that too at multiple stages of development. Collectively as a common consequence of stress, various metabolic pathways are affected in living systems and the intricate nature of these pathways poses a challenge for the identification of key regulatory components involved in abiotic stress response (Fraire-Velázquez and Balderas-Hernández 2013). Also associated with stress, is the increased generation of deleterious chemical entities, namely, reactive oxygen species (ROS) and methylglyoxal (MG), that are otherwise constantly produced as by-products of metabolic processes and scavenged by plant antioxidative defense system to maintain at certain steady-state levels. Accumulation of these toxic molecules disrupts the delicate balance leading to oxidative stress, which is ultimately responsible for alteration in metabolic behavior observed during stress. Though implications of ROS toxicity and signaling in plants have been well investigated by various research groups but the role of MG in abiotic stress response and signaling is not fully known.
The generation of MG in living systems is from the triose sugars via dissociable intermediate of the reaction catalyzed by triose phosphate isomerase (TPI) in glycolysis (Phillips and Thornalley 1993; Richard 1993). MG levels have been reported to elevate during various abiotic stress conditions and are responsible, to some extent, for the damage that occurs to cellular machinery under stress (Yadav et al. 2005a; Hoque et al. 2012a). The toxicity of MG is believed to be due to its ability to interact with and modify protein and nucleotide moieties leading to loss of cell viability culminating in cell death (Thornalley 1998, 2008). In order to counter the toxic effects of MG, the ubiquitous glyoxalase pathway has evolved that converts MG to d-lactate, thereby alleviating ill-effects of MG from the system (Thornalley 1993). In this regard, transgenic plants over-expressing glyoxalase genes exhibited improved tolerance to various stresses by resisting an increase in the levels of MG and maintaining redox homeostasis (Singla-Pareek et al. 2003, 2006). However, the relevance of indirect route of MG generation via the glycolytic enzyme TPI in stress response is largely undiscovered. We have reported that MG can induce TPI expression and activity, suggesting an involvement of this enzyme in stress response (Sharma et al. 2012). This, in corroboration to previously reported steady-state levels of MG maintained at some threshold concentration even in glyoxalase over-expressing plants, indicates towards involvement of MG as a signaling molecule even in the plant system. In this chapter, the implications of MG generating and scavenging pathways in abiotic stress response and the possible role of MG as a signal molecule are presented.
2 How Methylglyoxal Brings About Toxicity in Cells?
Methylglyoxal was first prepared by von Pechmann in 1887 on warming isonitrosoacetone with dilute sulfuric acid (von Pechmann 1887). It is a well-known reactive α-oxoaldehyde that is both a mutagen and genotoxic agent. The toxic effects of MG arise due to the ability of this compound to modify both proteins and nucleotides through the two functional groups, aldehyde and ketone, present in MG (Thornalley 2008). It is, however, the aldehydic group that is more prone to attack by any other functional groups than the ketonic group, elucidating the mode of action of MG in biological systems (Leoncini 1979). MG along with glyoxal is the most potent glycating agent, modifying amino groups of proteins. Generally, guanidine groups of arginine are susceptible for modifications, resulting in the formation of advanced glycation end products (AGEs) that are the mediators of MG-induced toxicity in biological systems (Thornalley 2008). MG reacts with arginine residues to form hydroimidazolone derivate (MG-H, with three related structural isomers), argpyrimidine, and THP (tetrahydropyrimidine) (Thornalley et al. 2003; Ahmed et al. 2002, 2005). In addition, the cross-linking between lysine residues and MG leads to the formation of CEL [Nε-(carboxyethyl)lysine] and MOLDs (methylglyoxal–lysine dimers) (Ahmed and Thornalley 2002; Ahmed et al. 2002). However, quantitative analysis of AGE revealed hydroimidazolone MG-H1 to be the major glycation adduct formed with argpyrimidine. In this context, a “dicarbonyl proteome” has been defined, which consists of proteins susceptible to modification by MG and undergo functional impairment as a consequence of these modifications (Rabbani and Thornalley 2012). The component proteins are linked to mitochondrial dysfunction in diabetes and ageing, oxidative stress, dyslipidemia, cell detachment, and anoikis and apoptosis and include albumin, hemoglobin, transcription factors, mitochondrial proteins, extracellular matrix proteins, and lens crystallins. Further, activity of several NADPH-generating enzymes is also reported to be reduced on exposure to MG (Morgan et al. 2013). This is due to the irreversible modification of arginine residues, which otherwise form an essential component of active sites and are required for NADP+ binding.
Besides proteins, nucleic acids and basic phospholipids can also be irreversibly modified by MG-mediated glycation reactions at the amino groups (Brown et al. 2005; Thornalley et al. 2010). Deoxyguanosine is the most reactive nucleotide susceptible to MG modification in physiological conditions. In vivo, the major MG-derived nucleotide AGEs are the imidazopurinone derivatives, which have been shown to be responsible for loss in genomic integrity associated with genotoxic effects (Thornalley et al. 2010).
In plants, adverse effect of MG on barley seed germination was demonstrated where growth inhibition was shown to be proportional to the concentration of MG (Mankikar and Rangekar 1974). Inhibition in response to low concentration of MG was recoverable to some extent but at higher than 1 μM MG concentration, the damage was extensive and irreversible. Addition of cysteine or methionine counteracted the detrimental effects of MG at 0.1 μM concentrations and less, thereby implicating the involvement of sulfhydryl groups of key enzymes (Mankikar and Rangekar 1974). Other growth processes, apart from seed germination, such as root elongation and chlorosis are also affected by MG (Hoque et al. 2012a). Hoque and coworkers have shown that 0.1 mM MG delays root elongation in Arabidopsis, whereas a week-long exposure to 1 mM MG inhibited germination by 21 % and also repressed root elongation along with inducing chlorosis. Further, MG is also shown to inhibit activities of various enzymes involved in antioxidant defense such as glutathione S-transferase (GST) activity, which can be reversed by the exogenous application of glutathione (Hoque et al. 2010). Also, MG can inhibit activity of cytosolic ascorbate peroxidase (APX), an enzyme playing a key role in the protection of cells from oxidative damage by scavenging ROS (Hoque et al. 2012b). The authors suggested that inhibition of GST and APX activities was mainly due to their modification by MG, which thereby lowers the affinity of the enzymes for their respective substrates. In spinach, addition of MG to chloroplasts is known to stimulate photosynthetic electron transport in thylakoid membranes (Saito et al. 2011). MG has been identified as a Hill oxidant that catalyses the photoreduction of O2 at photosystem I, leading to the production of O2 - thereby enhancing oxidative stress, which ultimately disrupts photosynthesis (Saito et al. 2011).
3 Synthesis and Turnover of Methylglyoxal
Methylglyoxal being a ubiquitous product of cellular metabolism is produced as a result of both enzymatic and non-enzymatic reactions. Its production is inevitable being tightly coupled to glycolysis. However, the rate of production varies depending upon the organism, tissue, cell metabolism, and physiological niche.
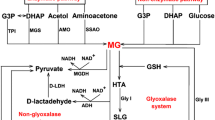
Undoubtedly, the major pathway for MG synthesis in biological systems is via its spontaneous generation in a glycolytic bypass, as a result of decomposition of triose sugars, GAP and DHAP (glyceraldehydes 3-phosphate and dihydroxyacetone phosphate, respectively) (Richard 1991; Phillips and Thornalley 1993). This reaction was initially reported in mid-1930s by Meyerhof and Lohmann (1934), but was ignored by referring to it as a mere experimental artifact. Later, Richard (1993) who investigated the mechanism of formation of MG from triose phosphates showed the physiological significance of this reaction. At the physiological pH, there is a high tendency for loss of α-carbonyl proton from the triose phosphates, producing an enediolate phosphate intermediate possessing low energy barrier for phosphate group elimination (Richard 1984). It is thus the deprotonation followed by spontaneous β-elimination of phosphate group of triose phosphates that leads to the formation of MG as the by-product of glycolysis (Richard 1993). However, TPI catalyzing the reversible interconversion of triose phosphates, DHAP and GAP, avoids the spontaneous degradation of the transition state intermediate into MG by stabilizing the enzyme-bound enediolate phosphate intermediate. In fact, the enzyme-bound enediolate phosphate intermediate is protonated 106-fold faster compared to the rate at which phosphate group is expelled (Richard 1991). Even then, the reaction catalyzed by TPI is not perfect and the enediolate intermediate may leak from the active site, producing MG in a side reaction. Thus, MG formation from triose sugars is believed to be the major route of its production under physiological conditions. Other minor routes of non-catalyzed MG generation include metabolism of acetone and aminoacetone (Kalapos 1999). MG metabolism through various pathways in plants is depicted in Fig. 13.1.
Metabolism of MG in plants. MG is generated spontaneously as a side product of glucose, lipid, and protein metabolic pathways. Detoxification of MG occurs majorly via glyoxalase pathway comprising GLYI and GLYII enzymes. However, GSH-independent GLYIII, aldose reductase, and dehydrogenase are also capable of reducing MG
The enzyme-catalyzed production of MG has been reported only in animals and bacteria and not in plants. These include oxidation of aminoacetone in the catabolism of l-threonine, catalyzed by the enzyme semicarbazide-sensitive amine oxidase (Lyles and Chalmers 1992), the oxidation of ketone bodies by myeloperoxidase (Aleksandrovskii 1992), and the oxidation of acetone by cytochrome P450 (Casazza et al. 1984; Koop and Casazza 1985). In addition, under pathological conditions such as ketosis or diabetic ketoacidosis, the oxidation of ketone bodies also seems to be an important source of MG (Turk et al. 2006). Unlike the above-described pathways where MG is generated as a side product, MG synthase is the only known enzyme, which specifically catalyzes MG synthesis and for this it uses the triose sugar DHAP as the substrate (Hopper and Cooper 1971, 1972). Interestingly, MG synthase is co-operatively inhibited by inorganic phosphate (Pi), and this regulation controls the glycolytic flux depending on the availability of Pi (Cooper 1984). It has been reported in many organisms such as bacteria (Hopper and Cooper 1971, 1972), yeast (Babel and Hofmann 1981; Murata et al. 1985), goat liver (Ray and Ray 1981), but no such activity has been yet reported from plants.
The breakdown of MG is regarded as an important detoxification mechanism, which protects the system from its detrimental effects. The primary route for MG detoxification is through the two-step glyoxalase pathway comprising glyoxalase I (GLYI; S-D lactoylglutathione lyase) and glyoxalase II (GLYII; hydroxyacylglutathione hydrolase) enzymes, which act sequentially to convert MG into d-lactate (Fig. 13.1). The first enzyme, GLYI isomerizes hemithioacetal formed from the spontaneous combination of MG and GSH to S-D lactoylglutathione, which is then hydrolyzed by the GLYII enzyme to yield d-lactate, regenerating GSH in the process (Racker 1951; Crook and Law 1952). The result of glyoxalase system was earlier believed to be a dead-end product until the determination of d-lactate dehydrogenase activity in the biological systems through which d-lactate is converted to pyruvate (Long and Kaplan 1968; Pratt et al. 1979). Like MG, glyoxalase pathway is also ubiquitously present in the biological systems, highlighting the fundamental importance of this MG-scavenging mechanism in plants.
Apart from glyoxalases, other enzymes such as aldo-keto reductases can also consume MG thereby reducing it to the corresponding alcohol (Ko et al. 2005; Simpson et al. 2009; Narawongsanont et al. 2012). Over-expression of MG detoxifying enzymes, both glyoxalases (Veena and Sopory 1999; Singla-Pareek et al. 2003, 2006, 2008; Saxena et al. 2011; Alvarez Viveros et al. 2013) and aldo-keto reductases (Hegedüs et al. 2004; Simpson et al. 2009; Turóczy et al. 2011) has resulted in transgenic plants with improved tolerance to abiotic stresses, which keep MG levels in check and thereby contribute to proper growth and sustenance of plants under stress conditions.
A novel type of MG utilizing glyoxalase has been reported in bacteria and recently in Arabidopsis and humans, which does not require GSH, and unlike the conventional glyoxalase pathway catalyzes the conversion of MG to d-lactate in a single step (Misra et al. 1995). In humans and Arabidopsis, they are known as DJ-1 proteins and are believed to be the genetic cause for the early onset of Parkinson’s disease in humans (Bonifati et al. 2003; Lee et al. 2012; Kwon et al. 2013). In Arabidopsis, six members in the DJ-1 family have been reported and the ectopic expression of one of the members (DJ-1d) has been shown to restore tolerance to MG in bacterial strain lacking GLYI and GLYIII genes (Kwon et al. 2013). The wild-type bacteria are resistance to 0.7 mM MG concentration whereas the mutant strain lacking GLYI and GLYIII cannot tolerate more than 0.5 mM MG. However, upon transformation with DJ-1d gene, mutant strain could successfully grow on 0.7 mM MG-supplemented medium, thereby reverting the growth defect of mutant cells (Kwon et al. 2013). Another member of the DJ-1 family from Arabidopsis, AtDJ1C, has also been studied and found to be required for viability. Though it is an atypical member of the DJ-1 family since it lacks a conserved cysteine residue required for enzymatic activity in other superfamily members, it is essential for proper chloroplast development and homozygous disruption of the AtDJ1C gene results in nonviable albino seedlings (Lin et al. 2011).
4 Methylglyoxal Levels Under Stress Conditions
Endogenous MG generation has been reported in all biological systems that rapidly increases under stress and disease conditions in bacteria, animals, mammals, and yeast (Thornalley 1990; Kalapos et al. 1992; Wu and Juurlink 2002). Similarly in plants, MG levels have been reported to increase in response to abiotic, biotic stresses as well as other stimuli such as white light, 2,4-D and ABA (Chen et al. 2004; Yadav et al. 2005a; Hossain et al. 2009). Under physiological conditions, MG concentration lies within 30–75 μM range in various plant species such as rice, Pennisetum, tobacco, and Brassica seedlings and is same in both leaves and roots except rice, where MG levels are lower in roots compared to leaves. However, a 2–6-fold increase in MG levels is observed in response to salinity, drought, and cold stress conditions (Yadav et al. 2005a). Noticeably under salt stress, MG levels increased to as high as 75–200 μM in these plants, suggesting accumulation under salinity stress being a universal response. These values are much higher than what has been reported in yeast and animals (Chaplen et al. 1998; Martins et al. 2001). It is possible that increased rate of glycolysis under stress leads to higher levels of MG in plants. However, another possibility which cannot be undermined is the spontaneous degradation of triosephosphates to MG during pre-analytical processing of samples. Further studies using newer and more sensitive detection methods are likely to resolve these inconsistencies in measurement.
Methylglyoxal levels are also reported to increase significantly in response to white light (2.21-fold) in pumpkin seedlings (Hossain et al. 2009), where treatment with exogenous MG, ABA, and salinity and drought stress also increases MG production manifold (which is in accordance with increased GLYI activity under these stresses). Also, infection with Aspergillus flavus increases MG production by 2.5-fold in a maize genotype, consequently resulting in the production of aflatoxin, a carcinogenic secondary metabolite (Chen et al. 2004). Banu et al. (2010) have also demonstrated a significant ~2-fold increase in MG levels in tobacco BY-2 cells in response to 200 mM NaCl stress. Taken together, it can be inferred that accumulation in MG under different stress conditions is unavoidable as it is probably a common consequence of both abiotic and biotic stresses in plants (Chen et al. 2004; Yadav et al. 2005a; Hossain et al. 2009).
5 Role of Glyoxalase Pathway in Plant Stress Physiology
Since generation of MG and increase in its concentration under stress conditions is an unavoidable phenomenon in biological systems, manipulation of glyoxalase pathway holds the potential for protection of plants against toxic effects of MG. Initial studies describing an upregulation in transcript levels of glyoxalase genes in response to various stress cues had opened the vistas for future of glyoxalase research in plant stress response. Espartero and coworkers (1995) showed a 2–3-fold increase in GLYI transcripts in roots, stems, and leaves of tomato plants treated with NaC1, mannitol, and ABA. Thereafter, a GLYI gene from Brassica juncea was transformed in tobacco in order to investigate the functional significance of glyoxalases in planta. Veena and Sopory (1999) reported that over-expression of GLYI gene is capable of inducing tolerance towards salinity stress and exogenous MG application in plants. This is achieved through controlling MG levels and maintaining reduced GSH pool. Similar results were also obtained when GLYI was transformed in Vigna mungo (Bhomkar et al. 2008). Further, over-expression of GLYII gene from rice has been carried out in different plants such as tobacco, rice, and recently in Brassica juncea, which imparts significant tolerance to high MG and salt treatments similar to GLYI gene (Singla-Pareek et al. 2003, 2008; Wani and Gosal 2011; Saxena et al. 2011). A balance was maintained in Na+/K+ ratio in the transgenic rice plants compared to wild-type (WT) in both shoot and root that correlated well with normal growth of these plants and formed the basis of minimizing Na+ toxicity under salt stress (Singla-Pareek et al. 2008). Also, the transgenic tobacco plants could grow, flower, and set normal viable seeds under continuous salinity stress conditions (Singla-Pareek et al. 2003). Likewise, the transgenic Brassica plants over-expressing OsGLYII gene also showed significant levels of salinity stress tolerance by delaying senescence (Saxena et al. 2011). Interestingly, the double-transgenic tobacco plants expressing the entire pathway (GLYI + GLYII) outperformed the single transgenic lines, expressing either GLYI or GLYII genes or also non-transformed WT plants under salinity and heavy metal stresses (Singla-Pareek et al. 2003, 2006). The transgenic plants could grow well in the presence of 5 mM ZnCl2 without any yield penalty and could tolerate toxic concentrations of other heavy metals as cadmium and lead. A reduction in MG levels along with maintaining higher levels of reduced GSH under salinity stress and increased phytochelatin production after zinc treatment is believed to confer stress tolerance in the transgenic plants (Yadav et al. 2005b; Singla-Pareek et al. 2006). Similarly, transgenic tomato plants expressing similar construct showed improved salinity stress tolerance by decreasing oxidative stress (Alvarez Viveros et al. 2013). In corroboration to observed heavy metal tolerance of plants expressing glyoxalase pathway, transformation of a wheat GLYI gene in tobacco also leads to increased tolerance to zinc when compared to untransformed control (Lin et al. 2010). Over-expression of sugar beet GLYI in tobacco also confers enhanced tolerance to MG, salt, mannitol, and H2O2 treatments (Wu et al. 2013).
Transcriptome and proteome analysis has also indicated the role of glyoxalase pathway in stress response. A recent genome-wide study in rice and Arabidopsis has indicated the multiple stress inducible nature of glyoxalases in these species, which also undergo developmental and tissue-specific variations (Mustafiz et al. 2011; Kaur et al. 2013). In Brassica juncea, GLYI transcript is upregulated in response to salinity, mannitol, and heavy metal stresses (Veena and Sopory 1999). Additionally, GLYI has been identified as dehydration-induced gene in foliage grass Sporobolus stapfianus (Blomstedt et al. 1998). In wheat, GLYI expression is induced in response to F. graminearium infection and NaCl and ZnCl2 treatments (Lin et al. 2010). The assessment of stress transcriptome and proteomes in stress-sensitive and stress-tolerant varieties has clearly indicated the role of glyoxalase genes in stress response with stress-tolerant species exhibiting higher expression of glyoxalases even under nonstress conditions (Chao et al. 2005; Witzel et al. 2009; Sun et al. 2010). A comparative transcriptome profiling of salt-tolerant wild tomato and a salt-sensitive tomato cultivar revealed two GLYI genes being salt stress inducible only in the wild tomato suggesting a more effective detoxification system in the tolerant species (Sun et al. 2010). The inspection of root proteome of barley genotypes with contrasting response towards salinity stress also revealed glyoxalase proteins to be either downregulated or present at low levels in sensitive varieties compared to constitutive or increased expression in tolerant varieties (Witzel et al. 2009). Moreover, glyoxalase activity is also induced under stress conditions as reported in onion bulbs and pumpkin seedlings, providing further conformity to the role of glyoxalases in stress response (Hossain et al. 2009; Hossain and Fujita 2009).
6 Triose Phosphate Isomerase: Regulation Under Stress and Role in Maintaining Methylglyoxal Homeostasis
TPI, being an important component of glycolysis, has been well studied and is found to be highly conserved in nature, exhibiting roughly 50 % sequence conservation from bacteria to humans (Joseph-McCarthy et al. 1994). TPI adjusts the rapid equilibrium between DHAP and GAP, produced via aldolase during glycolysis and thereby serves an important physiological role which is also reflected through studies manifesting effects of TPI deficiency or loss in its activity in humans. TPI deficiency is a rare autosomal recessive multisystem genetic disease, characterized by reduced enzyme activity in all tissues leading to the elevation of DHAP levels in erythrocytes (Schneider 2000). It was initially described in humans in 1965 and is associated with a progressive and severe neurological disorder, characterized by chronic hemolytic anemia frequently leading to death in early childhood (Schneider et al. 1965). Although there is no indication that DHAP accumulation is toxic, but it is the spontaneous decomposition of DHAP to MG that results in extensive damage to the system (Phillips and Thornalley 1993) as it can readily modify both proteins and DNA molecules (Thornalley 2008).
In plants, different organelle-specific forms of TPI viz. cytosol and chloroplast localized forms have been reported, but much is still to be known about the role of these different isozymes (Pichersky and Gottlieb 1984). However, a plastid-localized TPI has been shown to be crucial for the transition from heterotrophic to autotrophic growth during post-germinative seedling establishment in Arabidopsis (Chen and Thelen 2010). It was found that the reduction in activity of the plastid-localized TPI leads to stunted growth and abnormal chloroplast development, probably due to MG toxicity developed as a result of DHAP and MG accumulation in the developing plastids. Further, the role of cytosolic TPI has also been investigated, where reduction in cTPI activity in roots of potato leads to significant changes in several pathways of carbon metabolism such as modifications in glycolysis, pentose phosphate pathway, amino acid pool, and lipid metabolism (Dorion et al. 2010).
TPI has also been reported to be involved in plant stress response and alteration in expression levels is observed under abiotic or biotic stress conditions (Riccardi et al. 1998; Morris and Djordjevic 2001). Transcript levels of cytosolic TPI from rice have been shown to rise gradually under submergence stress, reaching maxima at 24 h and are maintained thereafter till 48 h (Umeda and Uchimiya 1994). In fact after 20 h of submergence, a marked increase in TPI levels occurs in roots and culms in rice seedlings but no such change is observed in leaf tissues (Xu et al. 1994). Likewise, cTPI expression is also induced in response to water deficit conditions in maize (Riccardi et al. 1998) and iron deficiency in Arabidopsis (Thimm et al. 2001). Selective alterations with respect to TPI induction have also been reported in response to desiccation, salt, oxygen deprivation, and high temperature stress (Minhas and Grover 1991). Transcript levels increase under desiccation, salt, oxygen deprivation, and high temperature stresses in shoots and oxygen deprivation and high temperature treatment in roots (Minhas and Grover 1991). In addition, we have recently shown that there is an increase in OscTPI transcript, protein, and enzyme activity in rice in response to various abiotic stresses and MG treatment (Sharma et al. 2012). Interestingly, MG treatment led to a ~2-fold increase in OscTPI transcript levels and also induced corresponding activity in a concentration-dependent manner (Sharma et al. 2012). This may probably help in restoring the balance in the glycolytic cycle towards ATP generation and ultimately rescuing the plant from stress. Also, we checked the stress response of recombinant OscTPI in E. coli (Fig. 13.2). The bacterial cells over-expressing OscTPI displayed better survival ability in response to low MG concentration (0.5 mM) whereas at 5 mM MG, no significant differences in growth pattern of untransformed and OscTPI transformed cells could be noticed. This is because higher MG concentration may be lethal to the bacteria.
Taken together, it can be said that environmental stresses affect mitochondrial respiration and photosynthetic processes thereby impairing oxygen uptake, energy levels, and ATP content (Botha et al. 1984; Purvis and Shewfelt 1993; Grass and Burris 1995; Tezara et al. 1999). As a result, glycolysis becomes the primary mode of energy production in plant tissues under low oxygen conditions, leading to increased activity of most of the glycolytic enzymes under such hypoxic and anoxic conditions (Mustroph and Albrecht 2003).
7 Methylglyoxal as a Signaling Molecule in Biological Systems
Despite the ubiquitous presence of glyoxalase pathway in biological systems and existence of multiple forms of these enzymes in plants, MG is still maintained at threshold levels (30–75 μM) even in glyoxalase over-expressing plants (Yadav et al. 2005a). These findings suggest a role for MG, which is much beyond a mere toxic compound. MG-mediated protein modifications besides having implications in glycation have also been investigated in the context of stress-induced signaling in different species. Role of MG as a signaling molecule has been demonstrated in yeast and animals but not much work has been done in plants.
MG is known to initiate signal transduction through the high osmolarity glycerol-mitogen-activated protein kinase (HOG-MAPK) cascade in yeast (Maeta et al. 2005). It activates the Yap1 transcription factor, which is important for the oxidative-stress response in Saccharomyces cerevisiae, thereby facilitating its translocation to nucleus (Maeta et al. 2004). Further, osmotic stress or MG stimulation has been shown to phosphorylate Hog1 via pbs2 leading to its translocation to nucleus, where transcription factors Msn2/4 are then recruited to the promoter region of stress-responsive genes possessing stress response elements (STRE). Also, MG can activate the uptake of Ca2+ in yeast cells, thereby stimulating the calcineurin/Crz1-mediated Ca2+ signaling (Maeta et al. 2005). In Schizosaccharomyces pombe, Pap1 along with the Sty1/Spc1 stress-activated protein kinase (SAPK) pathway has been identified as respective homologues of Yap1 transcription factor and HOG-MAPK pathway of S. cerevisiae, which are involved in response to MG toxicity (Zuin et al. 2005; Takatsume et al. 2006). MG is also reported to attenuate the rate of overall protein synthesis in S. cerevisiae by activating the protein kinase Gcn2 to phosphorylate the alpha subunit of translation initiation factor 2 (eIF2α) (Nomura et al. 2008).
In animals, MG is shown to induce signals, which trigger processes such as apoptosis, and also have implications in vascular complications of diabetes, through two distinct signal cascades, protein tyrosine kinase (PTK)-dependent control of ERK1 and ERK2 (extracellular signal-regulated kinase) and PTK-independent redox-linked activation of JNK (c-Jun N-terminal kinase)/p38 MAPK and caspases (Akhand et al. 2001). It has been shown to induce oxidative stress-mediated apoptosis by facilitating the phosphorylation of p38 MAPK in nerve-derived Schwann cells of rat (Fukunaga et al. 2005). Recently, the phosphorylation of Akt1 (protein kinase B) by MG has been reported in adipose tissues, which thereby stimulates adipogenesis in obese Zucker rats (Jia et al. 2012).
In plants, until now very preliminary information is available regarding the role of MG in stress-induced signaling. The involvement of MG in inducing stomatal closure, ROS production, and cytosolic-free calcium concentration has been investigated in order to clarify its role in Arabidopsis guard cells (Hoque et al. 2012c). MG was found to reduce stomatal apertures in a dose-dependent manner with the process being reversible at only low MG concentration (1 mM). Further, it induced O2 − production in whole leaves and ROS accumulation in the guard cells, which was completely abolished by 1 mM salicylhydroxamic acid (SHAM). MG at 1 mM concentration could increase cytosolic Ca2+ oscillations in the guard cells that were suppressed by pretreatment with 1 mM SHAM. Collectively, this data indicated that MG-induced stomatal closure involves an extracellular oxidative burst, which diffuses into the intracellular space leading to intracellular ROS accumulation in guard cells, and this result in stomatal closure via Ca2+-dependent pathway (Hoque et al. 2012c). In addition, MG can also inhibit light-induced stomatal opening in Arabidopsis by inhibiting K+ influx into the guard cells, which most likely occurs via the modification of C-terminal region of the inward-rectifying potassium channel (Hoque et al. 2012d). The role of MG in closure of stomatal aperture is believed to be important to withstand extreme environmental conditions.
The stress-responsive gene expression in Arabidopsis has been investigated using an ABA-deficient mutant, aba2-2 in response to MG (Hoque et al. 2012a). For this purpose, the transcription of ABA-independent gene, RD29A- and ABA-dependent genes, RD29B and RAB18, was monitored in the presence and absence of MG in 2-week-old Arabidopsis wild type and aba2-2 mutant seedlings. The MG treatment did not affect transcript level of RD29A in either plant type, but significantly increased transcription levels of ABA-dependent genes, RD29B (fivefold at 1 mM MG) and RAB18 (threefold at 1 mM MG) in concentration-dependent manner in the wild-type seedlings. However in the aba2-2 mutant, MG did not induce RD29B or RAB18 transcription even at 1.0 mM concentration. This suggested that MG can act through an ABA-dependent pathway to affect transcription and also plant developmental processes in Arabidopsis.
Our studies have shown that MG can induce OscTPI expression, which in turn, increases protein and enzyme activity (Sharma et al. 2012). This shifts the reaction towards GAP formation, thereby decreasing DHAP levels and consequently lowering MG concentration by feedback mechanism (Fig. 13.3). MG-dependent regulation of OscTPI seems advantageous to the system as increased OscTPI expression, on the one hand, is likely to reduce MG-mediated toxicity and on the other hand, will increase the energy status of the cell by adjusting the equilibrium towards GAP formation. Moreover, we have also observed an upregulation in transcript levels of several glyoxalase genes in rice (Mustafiz et al. 2011) and also non-MG metabolism-related gene, OsETHE1, in response to exogenous MG application (Kaur et al. 2014). We believe that MG has profound effect on transcriptome and proteome of plant species, which is indeed quite possible since MG is perceived as a stress by the plant, like various abiotic stresses. This is even supported by our recent transcriptome studies in rice in response to MG treatment, which also indicates towards a massive alteration in cellular functioning of a cell after MG application (unpublished data). Collectively, this data gives strong indication towards an important role of MG as signal molecule in plants as well, being involved in diverse stress adaptation pathways.
MG, glyoxalase, and TPI interplay during stress. During stress, MG levels increase as a consequence of imbalance in metabolic pathways. Elevated MG levels increase TPI expression and activity, shifting the equilibrium towards completion of glycolytic cycle. Also, MG upregulates glyoxalase pathway genes thereby accelerating its detoxification through feedback mechanism and hence, keeping MG levels in check. “+” indicates activation and “−” indicates inhibition of the particular gene/pathway
8 Functional Genomics Perspective in Triose Phosphate Isomerase and Glyoxalase Research
Functional genomics approaches, including transcriptome, proteome, and metabolome-based studies, are being increasingly used for simultaneous analysis of large number of genes. Using these techniques, it has become easier to analyze the effect of hormonal, chemical, or environmental responses on various aspects of plant development at a large scale. In this context, stress transcriptomes and proteomes have been assessed for alteration in gene and protein expression profiles in order to identify the underlying mechanism of plant defense and stress adaptation, which can help in raising stress-tolerant plants.
A number of such studies have led to the identification of glyoxalases as stress-responsive genes with increased expression in response to stress. For instance, transcriptome studies describing the response of Arabidopsis seedlings to usual contaminants present in soil, such as heavy metals and xenobiotic compounds, have indicated an involvement of glyoxalases under such conditions. A threefold induction in GLYII transcript in response to the xenobiotic, 2,4,6-trinitrotoluene (TNT); increased expression of GLYI on application of herbicide atrazine; or upregulation in response to arsenite; suggest a role of fundamental importance for glyoxalases in stress and adaptive responses (Ekman et al. 2003; Ramel et al. 2007; Chakrabarty et al. 2009). Further, ABA treatment in shoots of rice seedlings, or application of high hydrostatic pressure in germinating rice seeds, has also been shown to increase abundance of GLYI transcripts (Lin et al. 2003; Liu et al. 2008). Even comparative transcriptome studies in stress-sensitive and stress-tolerant plant varieties highlight the significance of glyoxalases. Highly salt-tolerant rice variety, Nona Bokra, showed increase in transcript levels of two glyoxalase genes under salt stress. In contrast, the salt-sensitive rice variety IR28, exhibited a significantly different regulation pattern for these genes (Chao et al. 2005).
Similarly, several proteome studies have reported an upregulation in levels of TPI protein in response to stress. Though the direct role of metabolism-related proteins in defense is less known but their role in maintaining metabolite pool in order to drive the metabolic processes for countering stress cannot be undermined. For instance, levels of TPI protein along with other enzymes of glycolysis were induced in response to oxidative stress, in the green alga Haematococcus pluvialis (Wang et al. 2004); and likewise exposure to cadmium in poplar also enhanced levels of TPI (Kieffer et al. 2009). Moreover, proteomic analysis of salt stress-responsive proteins in rice roots led to identification of TPI as one of the upregulated proteins (Yan et al. 2005). Likewise, inspection of salt-stressed proteome of the halophyte C4 plant, Aeluropus lagopoides, revealed an induction in levels of both TPI and GLYI proteins (Sobhanian et al. 2010). Measuring metabolite content in shoots after salt treatment revealed a decrease in DHAP and corresponding increase in GAP levels, in correlation with the observed increase in TPI expression under salt stress. Also, reduced glutathione levels were found to be relatively lower under stress, which is logical since GSH serves an important role in maintaining redox homeostasis in cells, being used in several antioxidant reactions, such as one catalyzed by GLYI (Sobhanian et al. 2010). In addition, developmental events such as grain filling and seed maturation in barley using two-dimensional gel electrophoresis also revealed the involvement of glyoxalases in developmental processes. The glyoxalase protein was found to be present throughout the development process, but showed maximum accumulation at the desiccation stage, suggestive of its involvement in seed development. On the other hand, TPI was found to be expressed throughout the development and as multiple spots on the gel, probably serving a housekeeping role as a component of energy cycle (Finnie et al. 2002).
Thus, with the advent of high-throughput techniques, our knowledge regarding plant stress response has considerably increased, and in fact, these techniques have helped us better understand the underlying mechanisms of plant responses to various stimuli.
9 Conclusions
Methylglyoxal is a potent cytotoxin even in plants, which needs to be removed from the system to protect it from the undesirable effects of MG. In response to stress conditions, MG levels increase in plants thereby conferring toxicity in the system mainly through the modifications of protein and nucleotide moieties. MG produced as a result of both enzymatic and non-enzymatic reactions is an unavoidable consequence of stress, and thus detoxification mechanisms play an important role in reducing the concentration of MG from the system, which is largely generated from the triose sugars as a dissociable intermediate of the reaction catalyzed by TPI. This probably indicates an involvement of this glycolytic enzyme in stress response where expression and activity of TPI in the physiological systems and the alteration in stress due to imbalance in metabolism, may in part determine the rate of MG generation. The glyoxalase pathway by scavenging MG has been shown to confer tolerance to multiple stresses mainly by reducing MG levels and maintaining redox homeostasis, thus playing a role of immense significance. Moreover, MG initiates apoptosis in animals, controls stomatal movements in plants, and also induces gene expression. Thus, it is possible that similar to ROS, MG might also be acting as an important messenger in the intricate signaling cascade.
References
Ahmed N, Thornalley PJ (2002) Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatisation with aminoquinolyl-N-hydroxysuccimidyl-carbamate and intrinsic fluorescence. Biochem J 364:15–24
Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ (2002) Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatisation by 6-aminoquinolyl-N-hydroxysuccimidyl-carbamate and application to Nε-carboxymethyl-lysine- and Nε-(1-carboxyethyl)lysine-modified albumin. Biochem J 364:1–14
Ahmed N, Babaei-Jadidi R, Howell SK, Beisswenger PJ, Thornalley PJ (2005) Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 48:1590–1603
Akhand AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, Kurokawa K, Nakashima I (2001) Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic Biol Med 31:20–30
Aleksandrovskii YA (1992) Antithrombin III, C1 inhibitor, methylglyoxal, and polymorphonuclear leukocytes in the development of vascular complications in diabetes mellitus. Thromb Res 67:179–189
Alvarez Viveros MF, Inostroza-Blancheteau C, Timmermann T, González M, Arce-Johnson P (2013) Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol Biol Rep 40:3281–3290
Babel W, Hofmann KH (1981) The conversion of triosephosphate via methylglyoxal, a bypass to the glycolytic sequence in methylotrophic yeasts? FEMS Microbiol Lett 10:133–136
Banu MN, Hoque MA, Watanabe-Sugimoto M, Islam MM, Uraji M, Matsuoka K, Nakamura Y, Murata Y (2010) Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Biosci Biotechnol Biochem 74:2043–2049
Bhomkar P, Upadhyay CP, Saxena M, Muthusamy A, Prakash NS, Poggin K, Hohn T, Sarin NB (2008) Salt stress alleviation in transgenic Vigna mungo L. Hepper (blackgram) by overexpression of the glyoxalase I gene using a novel Cestrum yellow leaf curling virus (CmYLCV) promoter. Mol Breed 22:169–181
Blomstedt CK, Gianello RD, Hamill JD, Neale AD, Gaff DF (1998) Drought-stimulated genes correlated with desiccation tolerance of the resurrection grass Sporobolus stapfianus. Plant Growth Regul 24:153–161
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299:256–259
Botha FC, Small JGC, Grobbelaar N (1984) The effect of water stress on the respiration and some aspects of respiratory metabolism of Citrullus lanatus seeds. Seed Sci Technol 12:585–595
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Brown BE, Dean RT, Davies MJ (2005) Glycation of low-density lipoproteins by methylglyoxal and glycolaldehyde gives rise to the in vitro formation of lipid-laden cells. Diabetologia 48:361–369
Casazza JP, Felver ME, Veech RL (1984) The metabolism of acetone in rat. J Biol Chem 259:231–236
Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, Tripathi RD, Tuli R (2009) Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 74:688–702
Chao DY, Luo YH, Shi M, Luo D, Lin HX (2005) Salt-responsive genes in rice revealed by cDNA microarray analysis. Cell Res 15:796–810
Chaplen FW, Fahl WE, Cameron DC (1998) Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A 95:5533–5538
Chen M, Thelen JJ (2010) The plastid isoform of triose phosphate isomerase is required for the postgerminative transition from heterotrophic to autotrophic growth in Arabidopsis. Plant Cell 22:77–90
Chen ZY, Brown RL, Damann KE, Cleveland TE (2004) Identification of a maize kernel stress-related protein and its effect on aflatoxin accumulation. Phytopathology 94:938–945
Cooper RA (1984) Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol 38:49–68
Crook EM, Law K (1952) Glyoxalase: the role of the components. Biochem J 52:492–499
Dorion S, Clendenning A, Jeukens J, Salas JJ, Parveen N, Haner AA, Law RD, Force EM, Rivoal J (2010) A large decrease of cytosolic triosephosphate isomerase in transgenic potato roots affects the distribution of carbon in primary metabolism. Planta 236:1177–1190
Ekman DR, Lorenz WW, Przybyla AE, Wolfe NL, Dean JF (2003) SAGE analysis of transcriptome responses in Arabidopsis roots exposed to 2,4,6-trinitrotoluene. Plant Physiol 133:1397–1406
Espartero J, Sanchez-Aguayo I, Pardo JM (1995) Molecular characterization of glyoxalase-I from a higher plant: upregulation by stress. Plant Mol Biol 29:1223–1233
Finnie C, Melchior S, Roepstorff P, Svensson B (2002) Proteome analysis of grain filling and seed maturation in barley. Plant Physiol 129:1308–1319
Fraire-Velázquez S, Balderas-Hernández VE (2013) Abiotic stress in plants and metabolic responses. In: Vahdati K, Leslie C (eds) Abiotic stress—plant responses and applications in agriculture. Intechopen, Rijeka. ISBN 978-953-51-1024-8
Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S, Kasuga M (2005) Methylglyoxal induces apoptosis through oxidative stress-mediated activation of p38 mitogen-activated protein kinase in rat Schwann cells. Ann N Y Acad Sci 1043:151–157
Grass L, Burris JS (1995) Effect of heat stress during seed development and maturation on wheat (Triticum durum) seed quality. II. Mitochondrial respiration and nucleotide pools during early germination. Can J Plant Sci 75:831–839
Hegedüs A, Erdei S, Janda T, Tóth E, Horváth G, Dudits D (2004) Transgenic tobacco plants overproducing alfalfa aldose/aldehydes reductase show higher tolerance to low temperature and cadmium stress. Plant Sci 166:1329–1333
Hopper DJ, Cooper RA (1971) The regulation of Escherichia coli methylglyoxal synthase: a new control site in glycolysis? FEBS Lett 13:213–216
Hopper DJ, Cooper RA (1972) The purification and properties of Escherichia coli methylglyoxal synthase. Biochem J 128:321–329
Hoque MA, Uraji M, Banu MNA, Mori IC, Nakamura Y, Murata Y (2010) The effect of methylglyoxal on glutathione S-transferase from Nicotiana tabacum. Biosci Biotechnol Biochem 74:2124–2126
Hoque MA, Uraji M, Torii A, Banu MN, Mori IC, Nakamura Y, Murata Y (2012a) Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum. J Biochem Mol Toxicol 26:315–321
Hoque TS, Okuma E, Uraji M, Furuichi T, Sasaki T, Hoque MA, Nakamura Y, Murata Y (2012b) Inhibitory effects of methylglyoxal on light-induced stomatal opening and inward K+ channel activity in Arabidopsis. Biosci Biotechnol Biochem 76:617–619
Hoque TS, Uraji M, Tuya A, Nakamura Y, Murata Y (2012c) Methylglyoxal inhibits seed germination and root elongation and up-regulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biol (Stuttg) 14:854–858
Hoque TS, Uraji M, Ye W, Hossain MA, Nakamura Y, Murata Y (2012d) Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J Plant Physiol 169:979–986
Hossain MA, Fujita M (2009) Purification of glyoxalase I from onion bulbs and molecular cloning of its cDNA. Biosci Biotechnol Biochem 73:2007–2013
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aus J Crop Sci 3:53–64
Jia X, Chang T, Wilson TW, Wu L (2012) Methylglyoxal mediates adipocyte proliferation by increasing phosphorylation of Akt1. PLoS One 7:e36610
Joseph-McCarthy D, Lolis E, Komives EA, Petsko GA (1994) Crystal structure of the K12M/G15A triosephosphate isomerase double mutant and electrostatic analysis of the active site. Biochemistry 33:2815–2823
Kalapos MP (1999) Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol Lett 110:145–175
Kalapos MP, Garzo T, Antoni F, Mandal J (1992) Accumulation of S-D lactoylglutathione and transient decrease of glutathione level caused by methylglyoxal load in isolated hepatocytes. Biochim Biophys Acta 113:159–64
Kaur C, Vishnoi A, Ariyadasa TU, Bhattacharya A, Singla-Pareek SL, Sopory SK (2013) Episodes of horizontal gene-transfer and gene-fusion led to co-existence of different metal-ion specific glyoxalase I. Sci Rep 3:3076
Kaur C, Mustafiz A, Sarkar A, Ariyadasa TU, Singla-Pareek SL, Sopory SK (2014) Expression of abiotic stress inducible ETHE1-like protein from rice is higher in roots and is regulated by calcium. Physiol Plant 152:1–16
Kieffer P, Schröder P, Dommes J, Hoffmann L, Renaut J, Hausman J-F (2009) Proteomic and enzymatic response of poplar to cadmium stress. J Proteomics 72:379–396
Ko J, Kim I, Yoo S, Min B, Kim K, Park C (2005) Conversion of methylglyoxal to acetol by Escherichia coli aldo-keto reductases. J Bacteriol 187:5782–5789
Koop DR, Casazza JP (1985) Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J Biol Chem 260:13607–13612
Kwon K, Choi D, Hyun JK, Jung HS, Baek K, Park C (2013) Novel glyoxalases from Arabidopsis thaliana. FEBS J 280:3328–3339
Lee JY, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, Park C (2012) Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Genet 21:3215–3225
Leoncini G (1979) The role of alpha-oxoaldehydes in biological systems. Ital J Biochem 28:285–294
Lin F, Xu SL, Ni WM, Chu ZQ, Xu ZH, Xue HW (2003) Identification of ABA-responsive genes in rice shoots via cDNA macroarray. Cell Res 13:59–68
Lin F, Xu J, Shi J, Li H, Li B (2010) Molecular cloning and characterization of a novel glyoxalase I gene TaGly I in wheat (Triticum aestivum L.). Mol Biol Rep 37:729–735
Lin J, Nazarenus TJ, Frey JL, Liang X, Wilson MA, Stone JM (2011) A plant DJ-1 homolog is essential for Arabidopsis thaliana chloroplast development. PLoS One 6:e23731
Liu X, Zhang M, Duan J, Wu K (2008) Gene expression analysis of germinating rice seeds responding to high hydrostatic pressure. J Plant Physiol 165:1855–1864
Long GL, Kaplan NO (1968) D-lactate specific pyridine nucleotide lactate dehydrogenase in animals. Science 162:685–686
Lyles GA, Chalmers J (1992) The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem Pharmacol 43:1409–1414
Maeta K, Izawa S, Okazaki S, Kuge S, Inoue Y (2004) Activity of the Yap1 transcription factor in Saccharomyces cerevisiae is modulated by methylglyoxal, a metabolite derived from glycolysis. Mol Cell Biol 24:8753–8764
Maeta K, Izawa S, Inoue Y (2005) Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J Biol Chem 280:253–260
Mankikar S, Rangekar P (1974) Effects of methylglyoxal on germination of barley. Fyton 32:9–16
Martins AM, Cordeiro CA, Ponces Freire AM (2001) In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett 499:41–44
Meyerhof O, Lohmann K (1934) Über die enzymatische Gleichgewichtsreaktion zwischen Hexosediphosphorsäure und Dioxyacetonphosphorsäure. Biochem Z 271:89–110
Minhas D, Grover A (1991) Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. Plant Sci 146:41–51
Misra K, Banerjee AB, Ray S, Ray M (1995) Glyoxalase III from Escherichia coli: a single novel enzyme for the conversion of methylglyoxal into D-lactate without reduced glutathione. Biochem J 305:999–1003
Morgan PE, Sheahan PJ, Pattison DI, Davies MJ (2013) Methylglyoxal-induced modification of arginine residues decreases the activity of NADPH-generating enzymes. Free Radic Biol Med 61C:229–242
Morris AC, Djordjevic MA (2001) Proteome analysis of cultivar-specific interactions between Rhizobium leguminosarum biovar trifolii and subterranean clover cultivar Woogenellup. Electrophoresis 22:586–598
Murata K, Fukuda Y, Watanabe K, Saikusa T, Shimosaka M, Kimura A (1985) Characterization of methylglyoxal synthase in Saccharomyces cerevisiae. Biochem Biophys Res Commun 131:190–198
Mustafiz A, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct Integr Genomics 11:293–305
Mustroph A, Albrecht G (2003) Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol Plant 117:508–520
Narawongsanont R, Kabinpong S, Auiyawong B, Tantitadapitak C (2012) Cloning and characterization of AKR4C14, a rice aldo-keto reductase, from Thai Jasmine rice. Protein J 31:35–42
Nomura W, Maeta K, Kita K, Izawa S, Inoue Y (2008) Role of Gcn4 for adaptation to methylglyoxal in Saccharomyces cerevisiae: Methylglyoxal attenuates protein synthesis through phosphorylation of eIF2alpha. Biochem Biophys Res Commun 376:738–742
Phillips SA, Thornalley PJ (1993) The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem 212:101–105
Pichersky E, Gottlieb LD (1984) Plant triose phosphate isomerase isozymes: purification, immunological and structural characterization, and partial amino Acid sequences. Plant Physiol 74:340–347
Pratt EA, Fung LW, Flowers JA, Ho C (1979) Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry 18:312–316
Purvis AC, Shewfelt RL (1993) Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant 88:712–718
Rabbani N, Thornalley PJ (2012) Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 42:1133–1142
Racker E (1951) The mechanism of action of glyoxalase. J Biol Chem 190:685–696
Ramel F, Sulmon C, Cabello-Hurtado F, Taconnat L, Martin-Magniette ML, Renou JP, El Amrani A, Couée I, Gouesbet G (2007) Genome-wide interacting effects of sucrose and herbicide-mediated stress in Arabidopsis thaliana: novel insights into atrazine toxicity and sucrose-induced tolerance. BMC Genomics 8:450
Ray S, Ray M (1981) Isolation of methylglyoxal synthase from goat liver. J Biol Chem 256:6230–6233
Riccardi F, Gazeau P, de Vienne D, Zivy M (1998) Protein changes in response to progressive water deficit in maize. Quantitative variation and polypeptide identification. Plant Physiol 117:1253–1263
Richard JP (1984) Acid–base catalysis of the elimination and isomerization-reactions of triose phosphates. J Am Chem Soc 106:4926–4936
Richard JP (1991) Kinetic parameters for the elimination reaction catalyzed by triosephosphate isomerase and an estimation of the reaction’s physiological significance. Biochemistry 30:4581–4585
Richard JP (1993) Mechanism for the formation of methylglyoxal from triosephosphates. Biochem Soc Trans 21:549–553
Saito R, Yamamoto H, Makino A, Sugimoto T, Miyake C (2011) Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O(2) at photosystem I: a symptom of plant diabetes. Plant Cell Environ 34:1454–1464
Saxena M, Roy SB, Singla-Pareek SL, Sopory SK, Bhalla-Sarin N (2011) Overexpression of the Glyoxalase II gene leads to enhanced salinity tolerance in Brassica juncea. Open Plant Sci J 5:23–28
Schneider AS (2000) Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Baillieres Best Pract Res Clin Haematol 13:119–140
Schneider AS, Valentine WN, Hattori M, Heins HL Jr (1965) Hereditary hemolytic anemia with triosephosphate isomerase deficiency. N Engl J Med 272:229–235
Sharma S, Mustafiz A, Singla-Pareek SL, Shankar Srivastava P, Sopory SK (2012) Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal Behav 7:1337–1345
Simpson PJ, Tantitadapitak C, Reed AM, Mather OC, Bunce CM, White SA, Ride JP (2009) Characterization of two novel aldo-keto reductases from Arabidopsis: expression patterns, broad substrate specificity, and an open active-site structure suggest a role in toxicant metabolism following stress. J Mol Biol 392:465–480
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci U S A 100:14672–14677
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 140:613–623
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17:171–180
Sobhanian H, Motamed N, Rastgar Jazii F, Nakamura T, Komatsu S (2010) Salt stress induced differential proteome and metabolome response in the shoots of Aeluropus lagopoides (Poaceae), a Halophyte C4 Plant. J Proteome Res 9:2882–2897
Sun W, Xu X, Zhu H, Liu A, Liu L, Li J, Hua X (2010) Comparative transcriptomic profiling of a salt-tolerant wild tomato species and a salt-sensitive tomato cultivar. Plant Cell Physiol 51:997–1006
Takatsume Y, Izawa S, Inoue Y (2006) Methylglyoxal as a signal initiator for activation of the stress-activated protein kinase cascade in the fission yeast Schizosaccharomyces pombe. J Biol Chem 281:9086–9092
Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Thimm O, Essigmann B, Kloska S, Altmann T, Buckhout TJ (2001) Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol 127:1030–1043
Thornalley PJ (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269:1–11
Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Aspects Med 14:287–371
Thornalley PJ (1998) Glutathione-dependent detoxification of α-oxoaldehyde by the glyoxalase system: involvement in disease mechanisms and antiproliferative activity of glyoxalase I inhibitors. Chem Biol Interact 111–112:137–151
Thornalley PJ (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems - role in ageing and disease. Drug Metabol Drug Interact 23:125–150
Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, Dawnay A (2003) Quantitative screening of advanced glycation end products in cellular and extracellular proteins by tandem mass spectrometry. Biochem J 375:581–592
Thornalley PJ, Waris S, Fleming T, Santarius T, Larkin SJ, Winklhofer-Roob BM, Stratton MR, Rabbani N (2010) Imidazopurinones are markers of physiological genomic damage linked to DNA instability and glyoxalase 1-associated tumour multidrug resistance. Nucleic Acids Res 38:5432–5442
Turk Z, Nemet I, Varga-Defteardarovic L, Car N (2006) Elevated level of methylglyoxal during diabetic ketoacidosis and its recovery phase. Diabetes Metab 32:176–180
Turóczy Z, Kis P, Török K, Cserháti M, Lendvai A, Dudits D, Horváth GV (2011) Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol Biol 75:399–412
Umeda M, Uchimiya H (1994) Differential transcript levels of genes associated with glycolysis and alcohol fermentation in rice plants (Oryza sativa L.) under submergence stress. Plant Physiol 106:1015–1022
Veena RVS, Sopory SK (1999) Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17:385–395
von Pechmann H (1887) Über die Spaltung der Nitrosoketone. Ber Dtsch Chem Gesells 20:3213–3214
Wang SB, Chen F, Sommerfeld M, Hu Q (2004) Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcus pluvialis (Chlorophyceae). Planta 220:17–29
Wani SH, Gosal SS (2011) Introduction of OsglyII gene into Oryza sativa for increasing salinity tolerance. Biol Plant 55:536–540
Witzel K, Weidner A, Surabhi GK, Börner A, Mock HP (2009) Salt stress-induced alterations in the root proteome of barley genotypes with contrasting response towards salinity. J Exp Bot 60:3545–3557
Wu L, Juurlink BH (2002) Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension 39:809–814
Wu C, Ma C, Pan Y, Gong S, Zhao C, Chen S, Li H (2013) Sugar beet M14 glyoxalase I gene can enhance plant tolerance to abiotic stresses. J Plant Res 126:415–425
Xu Y, Yu H, Hall TC (1994) Rice triosephosphate isomerase gene 5′ sequence directs [beta]-glucuronidase activity in transgenic tobacco but requires an intron for expression in rice. Plant Physiol 106:459–467
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005a) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337:61–67
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005b) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271
Yan S, Tang Z, Su W, Sun W (2005) Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics 5:235–244
Zuin A, Vivancos AP, Sansó M, Takatsume Y, Ayté J, Inoue Y, Hidalgo E (2005) The glycolytic metabolite methylglyoxal activates Pap1 and Sty1 stress responses in Schizosaccharomyces pombe. J Biol Chem 280:36708–36713
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kaur, C., Sharma, S., Singla-Pareek, S.L., Sopory, S.K. (2015). Methylglyoxal, Triose Phosphate Isomerase, and Glyoxalase Pathway: Implications in Abiotic Stress and Signaling in Plants. In: Pandey, G. (eds) Elucidation of Abiotic Stress Signaling in Plants. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2211-6_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2211-6_13
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2210-9
Online ISBN: 978-1-4939-2211-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)