Abstract
Bacteriocins are the subset of antimicrobial peptides (AMPs) produced by bacteria. They are small amphipathic peptides that interact with bacterial membranes leading to cell death. Most of the best known are produced by lactic acid bacteria used as food fermentation starters, because of their potential use as food preservatives. Bacteriocins are divided into two groups: lantibiotics that present posttranslational condensation rings and unmodified peptides. The first are subdivided into elongated versus globular lantibiotics, while four subgroups are recognized among unmodified bacteriocins. The genetic organization is in clusters that may reside into plasmids or transposons, formed by the structural gene, the export and immunity determinants, the quorum sensing governing production and any modification genes. Bacteriocins are active at extremely low concentrations (nM range) due to a dual mode of action: (a) binding to the membrane phospholipids and (b) specific recognition of surface components, both of which collaborate in pore formation. Development of resistance to bacteriocins is very infrequent due to the presence of two targets and is usually due to unspecific modifications of the cell envelope. Bacteriocins are used as food preservatives, either after total or partial purification or as extracts of producing bacteria. In situ production is also used, with the advantage of producing early lysis of the starter bacteria and ripening acceleration of the fermented product. They may also form part of hurdle technologies and be incorporated into packaging systems to allow extended liberation. Medical and veterinary applications are in their infancy but good results have been obtained against infection by Gram-positive bacteria and Helicobacter pylori.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lactic Acid Bacterium
- Bacteriocin Production
- Cheddar Cheese
- Wall Teichoic Acid
- Phosphotransferase Transport System
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Bacteriocins are the subset of antimicrobial peptides (AMPs) produced by bacteria. AMPs are synthesized by virtually all cellular organisms (the defensins generated by fungi, plants, insects, and vertebrates in general and the cathelicidins typical of mammals belong to this group). All of them have in common their size (usually less than 10 kDa.) and general structure, amphipathic molecules with a positive net charge (Nes et al. 1996; Perez et al. 2014). This promotes their interaction with plasma membranes, where they bind to the negatively charged phospholipids, sink into the fatty acid moiety and polymerize to form pores that abolish the membrane potential and allows leakage of cytoplasm solutes, leading to cell death (see below). AMPs from multicellular organisms tend to have a wide spectrum of action that comprises bacteria, fungi, protozoa and enveloped viruses, thus being a substantial component of their innate immunity. However, bacteriocins are usually only active against bacteria related to the producer (for example, those produced by Escherichia coli are specific for Enterobacteriaceae) although some, usually produced by Gram positive bacteria, may affect a wide range of other Gram-positive organisms and even inhibit spore germination. This selective toxicity may facilitate bacteriocin use for treatment of infectious diseases, especially those produced by antibiotic multiresistant bacteria (Smith and Hillman 2008; Hassan et al. 2012; Cotter et al. 2013).
Out of the 177 sequenced bacteriocins included in the specialized BACTIBASE web page (http://bactibase.pfba-lab-tun.org/main.php), 156 are produced by Gram-positive organisms, 18 by Gram-negative bacteria, and 3 by halophile archaea (i.e., they are not bacteriocins but illustrate on the universality of AMPs production). Out of the 156 Gram-positive bacteriocins, 113 are synthesized by lactic acid bacteria (LAB), a heterogeneous group of low G + C anaerobic organisms that generate lactic acid as the main end product of sugar fermentation. This may not reflect a higher capacity of synthesizing bacteriocins but, rather, a more profound search for them because LAB constitute the main source of fermented food starters, where in situ produced bacteriocins are believed to contribute to extend the shelf life of food products. Thus, research on bacteriocin biology has been mostly focused on LAB bacteriocins.
2 Bacteriocin Classification
Bacteriocins are divided into two major groups; class I is composed of small molecules (less than 5 kDa.) that present posttranslational modifications, while those of class II are made up of peptides with unmodified amino acids (Perez et al. 2014). The unusual residues present in class I bacteriocins arise from dehydration of serine and threonine to give 2,3-didehydroalanine and 2,3 didehydrobutyrine respectively. Frequently, thioether linkages with the sulfhydryl groups of neighboring cysteines are formed. The residues arising from this condensation are called lanthionine and 3-methyl-lanthionine respectively, this being the reason why they are collectively termed as lantibiotics (Asaduzzaman and Sonomoto 2009; Bierbaum and Sahl 2009). Bacteriocins of classes I and II can be divided into different subgroups depending on the secondary structure of the molecule, the number of peptides that form the active antimicrobial, and even their spectrum of susceptible bacteria.
Lantibiotics constitute about one-third of all bacteriocins and are classified into two main types, A and B. The first comprises peptides that have an elongated secondary structure in at least part of the molecule. Those of type B are globular, thus presenting a compacted arrangement. The type A lantibiotics are further subdivided into subtype A(I), made out of molecules with a more or less lineal structure, while subtype A(II) members have an elongated end, the rest being globular (Asaduzzaman and Sonomoto 2009; Bierbaum and Sahl 2009). Nisin A and subtilin are examples of subtype A(I), lacticin 481 and plantaricin C belong to subtype A(II) and mersacidin is a typical type B lantibiotic. This classification may need some modification, first because subtype A(II) and type B lantibiotics present just one gene that codifies for the dehydration and cyclization of the precursor peptide while the subtype A(I) has two separate genes. In addition, there are lantibiotics whose final structure does not conform to any of those defined by types A and B. Examples include sublancin and the two-component lantibiotics, made out by two peptides, both of which are needed for their biological activity (lacticin 3147).
Class II bacteriocins are traditionally subdivided into four subclasses: pediocin-like (class IIa), two component (class IIb), circular (class IIc), and miscellaneous (class IId). Class IIa is composed of a series of peptides initially grouped by their ability to inhibit Listeria monocytogenes. Since all of them present the YGNGV/L conserved peptide toward their NH2-end, it was postulated that this was responsible for their antimicrobial activity. However, it is now clear that other amino acids, forming a “cationic patch” in the NH2-terminal moiety, are also needed for the initial electrostatic interaction with the membrane phospholipids. The less conserved amphiphilic C-terminal part recognizes the susceptible bacteria and penetrates the hydrophobic membrane core, thanks to a hinge that is placed between both bacteriocin regions. Pediocin PA1/AcH, produced by Pediococcus acidilactici, is the model bacteriocin of this group (Ennahar et al. 2000; Drider et al. 2006).
Class IIb bacteriocins are composed by two different peptides, associated in equimolecular proportion. Each peptide may (thermophilin 13) or may not (lactococcin G and lactacin F) have antimicrobial activity, but they show a synergistic effect when acting together and, sometimes, their spectrum of susceptible bacteria changes with respect to that of the individual peptides (Nissen-Meyer et al. 2009; Perez et al. 2014).
Class IIc comprises the so-called circular bacteriocins. They are quite large (between 58 and 70 amino acids) and, although being synthesized as lineal peptides, become circularized by covalent binding between the first and last residues. This conformation makes them very resistant to heat (some retain their activity after treatment at 121 °C for 15 min), pH variation, and proteolytic digestion. Two subgroups have been recognized based on their physicochemical characteristics; the first comprises cationic peptides with isoelectric points close to 10, its model bacteriocin being AS-48, while the second has isoelectric points close to neutrality or clearly in the acid range (gassericin A and butyrivibriocin A respectively) (Sánchez-Hidalgo et al. 2011; Gabrielsen et al. 2014).
Finally, class IId is made out by all bacteriocins that cannot be included in any of the first three classes. It is a miscellaneous group that includes: (i) lineal peptides that do not present the YGNGV/L motif of pediocins, such as lactococcin A, (ii) bacteriocins that do not have a dedicated export system but use the general secretory mechanism of the cell; enterocin P and lactococcin 972 are well-known representatives of this group (Cintas et al. 1997; Martínez et al. 1999) (enterocin P presents the YGNGV/L motif of pediocins, so it may be classified as a class IIa bacteriocin as well) and (iii) leaderless bacteriocins, such as lacticin Q, which, significantly, has a formylated methionine as its first residue (Fujita et al. 2007).
3 Genetics and Regulation of Bacteriocin Synthesis
The genes involved in bacteriocin production are usually clustered and, frequently, form part of mobile genetic elements such as conjugative transposons (nisin) and plasmids (lactococcin 972) (Lubelski et al. 2008; Martínez et al. 1999). This facilitates their spread and explains, for example, the ability of P. pentosaceus and Lactobacillus plantarum strains to synthesize pediocin PA1/AcH (Miller et al. 2005). The clusters may also be located into the chromosome (subtilin) (Asaduzzaman and Sonomoto 2009).
In the case of lantibiotics, two genetic organizations are found. In both cases, the structural gene (lanA) codes for a prebacteriocin with no antimicrobial activity due to the presence of a NH2-extension of between 23 and 59 amino acids. This leader peptide acts as a scaffold for the posttranslational modification enzymes and serves as an export signal for the mature lantibiotic (Fig. 1).
Scheme of the lantibiotic nisin biosynthesis. The P nisI and P nisR promoters are constitutive and produce NisI that protects the cell and NisR-K that senses the presence of external nisin. Upon nisin-driven successive phosphorylation of NisK-NisR, this last protein induces expression of P nisA and P nisF and synthesis of prenisin occurs. This is recognized by the dehydratase NisB, folded by NisC, exported through NisT and the leader removed by NisP, to produce the active bacteriocin. NisI and the group NisE-F-G interact with mature nisin to avoid contact with the lipid II and the plasma membrane
The differences between type A(I) and type A(II)/class B lantibiotic synthesis clusters start with the genes that encode the posttranslational modification enzymes. In the first case, separate genes encode the Ser and Thr dehydratase (lanB) and the cyclase (lanC) that catalyzes their condensation with Cys to give the lanthionine residues, while, in the second, the product of a single determinant (lanM) fulfills both functions. LanM presents some sequence identity with LanC but no relation is perceived with LanB. In some cases, further modifications may be introduced by enzymes encoded by lanD genes; for instance, the C-terminal cysteine of mersacidin and mutacin 1140 (a type A(I) bacteriocin) is decarboxylated and converted into S-amino vinyl-D-cysteine (Asaduzzaman and Sonomoto 2009).
Once the posttranslational modification is completed, the lantibiotic is exported and the leader peptide eliminated. For the type A(I) molecules, secretion is mediated by the ABC transporter LanT and processing is performed by the extracellular protease LanP. LanT appears to recognize the leader peptide and, within it, the oligopeptide FNLD, located between positions −20 and −15 [this oligopeptide is a docking motive for LanB and LanC as well; Khusainov et al. (2011)] and a proline in position −2. Consequently, prepeptides and partially modified bacteriocins are secreted as well. In contrast, LanP only eliminates the leader peptide from totally modified molecules, this being the activation step of the bacteriocin. In the case of nisin, it appears that all intracellular biosynthesis steps are performed in multimeric complexes, bound to the internal layer of the membrane and formed by NisB, NisC and NisT dimers (Fig. 1) (Lubelski et al. 2008; Khusainov et al. 2011). Type A(II) and class B lantibiotics present a single gene (lanT) whose product fulfills both secretion and processing of the peptide, thanks to an extra NH2-terminal peptidase domain that recognizes a double GG/GA motif close to the cleavage site (Altena et al. 2000; Asaduzzaman and Sonomoto 2009).
Bacteriocin activation occurs outside but in the vicinity of the plasma membrane. It becomes thus necessary to build a system that protects the producing cell. In the case of lantibiotics, this is fulfilled by LanI (for immunity) a lipoprotein located on the external layer of the membrane that binds the antimicrobial peptide and prevents interaction with its targets. Additionally, a complex formed by the proteins LanEFG has the capacity to expel the lantibiotic from the membrane surface. Both systems appear to work synergistically, each one producing only 5–20 % of the full immunity level provided by both (Ra et al. 1999).
Production of bacteriocins in general and of lantibiotics in particular, is subjected to quorum sensing. The best characterized system is that of nisin (Fig. 1). In its biosynthetic cluster there are two contiguous genes, nisR-nisK, that form a constitutive operon and encode a response regulator and a histidine kinase, respectively. NisK protrudes though the membrane and recognizes extracellular nisin but not its precursors. Binding results in activation of the kinase activity, autophosphorilation of a His residue and subsequent transfer of the phosphate to NisR. NisR-P is a transcriptional activator that recognizes operator sequences located in the promoters of the main nisin operon, which governs expression of the nisA-B-T-C-I-P genes, and of nisF-E-G. Immediately behind nisA there is a transcription termination signal that is not absolute, thus allowing synthesis of high and moderate amounts of the prepeptide and the other proteins respectively. The rational of this is clear; nisin will be exported and diluted into the medium while the other proteins will remain associated to the cell. The increase of nisin concentration is paralleled with enhanced production of NisI and biosynthesis of the NisEFG proteins (Lubelski et al. 2008).
The genetic organizations for production of class IIa, IIb, and some IId bacteriocins are very similar. Two possibilities exist: a single operon that comprises all relevant genes or three clustered operons, one of which may have divergent transcription with respect to the other two (Ennahar et al. 2000). In the first case, the structural gene (or genes for class IIb) is/are followed by the immunity determinant, the ABC transporter that also eliminates the leader peptide (usually at a GG/GA motif) and a gene that encodes an accessory protein that might be involved in the immunity/export of the bacteriocin. This is the case for pediocin PA-1/AcH (class IIa) and lactococcin G (class IIb). In the case of the three-operon bacteriocins, the first includes the structural and immunity genes; the second, the ABC transporter/protease and the accessory protein determinants and the third carries the genes involved in regulation of bacteriocin production. This last operon is composed of three genes, the first of which encodes a pheromone peptide that structurally resembles a bacteriocin (small, cationic, amphipathic, heat-resistant, synthesized as a prepeptide that shares the processing signals) but without significant antimicrobial activity. The other two genes encode a membrane-associated histidine kinase and a response regulator, similar to those described for nisin (see above). Examples of this genetic organization are provided by sakacin P (class IIa) and plantaricin E/F (class IIb) (Brurberg et al. 1997; Nissen-Meyer et al. 2010).
The genetic organization of circular bacteriocin (class IIc) biosynthesis is not well understood. Between 5 and 7 genes appear to be needed, although some others, clustered with them, might play a role as well (Maqueda et al. 2008; Gabrielsen et al. 2014). Most proteins are hydrophobic, thus suggesting that bacteriocin synthesis is membrane associated. The structural gene encodes a prepeptide with a leader that may be as short as two amino acid residues. A second, cationic and hydrophobic, peptide is involved in immunity. The system also includes an integral membrane protein that presents a DUF 95-like transport domain, which might be associated to an ATPase also present in the cluster and to several other proteins, all of which might participate in bacteriocin export. Complementarily, most clusters have in their vicinity an accessory operon encoding a putative ABC transport complex that might enhance bacteriocin production and/or immunity (Diaz et al. 2003).
4 Mode of Action
Bacteriocins are highly potent bactericidal agents. As mentioned before, some of them have a narrow spectrum of activity targeting only close related species but many, particularly those produced by lactic acid bacteria, are active against a wider panel of Gram positive bacteria. Remarkably, antibiotic and bacteriocin resistance are independent and, consequently, no cross-resistance has been recorded so far. Gram-negative bacteria are intrinsically resistant to peptide bacteriocins produced by Gram-positive organisms due to the protective role of their outer membrane that prevents reaching of their target, i.e., the plasma membrane. Consistently with this, Gram-negative bacteria may become susceptible to wide spectrum bacteriocins such as nisin, when combined with EDTA or organic acids that disrupt the outer membrane.
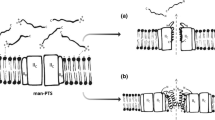
Pioneering studies on the mode of action of bacteriocins have been carried out with nisin, a lantibiotic produced by Lactococcus lactis, and with class IIa bacteriocins. Interaction with the plasma membrane and the formation of pores is a common theme among most LAB bacteriocins. However, in-depth structure–function studies have revealed that bacteriocins are not just mere pore formers but they may also interact with receptors or docking molecules, abrogating essential cell pathways which account for their potency. Moreover, non-membrane permeabilizing bacteriocins have also been described. A schematic representation of the mode of action of bacteriocins is depicted in Fig. 2. Unveiling the mode of action of bacteriocins is important to anticipate possible mechanisms of resistance as well as for a rational exploitation of their antimicrobial activity.
Bacteriocin mode of action. a Upon interaction with the negatively charged cell envelope, pore-forming positively charged bacteriocins insert into the plasma membrane when present at high concentration (µM range). b Lipid II or the membrane components of mannose-PTS may act as receptors or docking molecules to promote pore formation. c Binding to the cell wall precursor lipid II inhibits the synthesis of peptidoglycan, which may be combined with pore formation (nisin and other lantibiotics) or not (Lcn972, mersacidin). Hydrolysis of the peptidoglycan is accomplished by activation of endogenous autolysins
4.1 Pore Formation
The ability of many bacteriocins to interact with the plasma membrane resides on their physicochemical and structural properties. Pore-forming bacteriocins are unstructured in water but adopt an amphipilic α-helical structure in membrane mimicking environments that enables pore formation when present at high concentrations, i.e., in the micromolar range.
Based on their cationic character, pore formation begins with electrostatic interactions between the peptide and the negatively charged bacterial surface. Using model membranes, nisin has been shown, for example, to initially interact with the anionic lipids by electrostatic forces, followed by peptide aggregation. Translocation of the C-terminus of nisin across the membrane phospholipids is favored by the negative membrane potential and a wedge-like transient pore is formed (Fig. 2) (Driessen et al. 1995). In class IIa pediocin-like bacteriocins, their conserved positively charged N-terminus interacts with the negative cell envelope constituents and folds into a three-stranded antiparallel ß-sheet connected by a hinge region to an α-helix and a C-terminal tail in a hairpin structure. Pore formation is achieved by insertion of this hydrophobic C-terminal domain into the cytoplasmic membrane, likely forming a barrel stave like arrangement (Drider et al. 2006; Nissen-Meyer et al. 2009). Two-peptide non-modified bacteriocins (class IIb) also adopt a helical structure in a hydrophobic environment, with the peculiarity that further structuring occurs upon interaction with their complementary peptides that finally results in insertion into the membrane. The two peptides are thought to interact through helix–helix interactions involving the GXXG motifs present in both peptides (Nissen-Meyer et al. 2009). Bacteriocins of class IIc, such as the circular enterocin AS-48, have a compact globular structure with 5 α-helixes whose conformation also changes in hydrophobic environments, facilitating membrane disruption (Sánchez-Barrena et al. 2003).
Alteration of the membrane permeability barrier leads to a total or partial dissipation of the proton motive force, ultimately causing energy exhaustion and cell death. The specificity of the pores varies, with some bacteriocins producing leakage of just monovalent cations and others that mediate the efflux of ions, amino acids, and ATP. Other factors, such as membrane lipid composition and a certain membrane potential threshold, may also be required to determine the fate of the pore (Moll et al. 1999).
4.2 Docking Molecules or Receptors Involved in Bacteriocin Activity
When compared to the eukaryotic antimicrobial peptides, one striking feature of pore-forming bacteriocins is their strong in vivo potency (nanomolar vs. micromolar range) and their narrower spectrum of activity. This is due to the formation of target-mediated pores, i.e., disruption of membrane permeability occurs upon high affinity interaction of the peptides with docking molecules or receptors present in the cell envelope (Fig. 2).
The cell wall precursor lipid II is a prominent docking molecule linked to the mode of action of several LAB lantibiotics including nisin, plantaricin C, lacticin 3147, nukacin ISK-1 and lacticin 481, among others (Breukink and de Kruijff 2006). Lipid II is an essential precursor of cell wall biosynthesis. Thereby, binding of lipid II by pore-forming lantibiotics results in the combination of two modes of action in the same molecule: inhibition of cell wall biosynthesis and pore formation (Wiedemann et al. 2001). In the case of nisin, it has been shown that lipid II stabilizes the pore and is part of the pore itself in a lipid II:nisin 4:8 stoichiometry (Hasper et al. 2004). Interaction of nisin with lipid II also implies that the cell wall precursor is delocalized from the sites where it is needed, interfering with normal cell growth and division (Hasper et al. 2006). It is worth mentioning the example of the lantibiotic lacticin 3147: in this case, one of its composing peptides, LtnA1, binds lipid II and the complex recruits LtnA2, promoting insertion into the membrane and generation of the pore (Wiedemann et al. 2006b). Pore formation upon lipid II binding seems to occur in a species-specific fashion in the case of more globular lantibiotics such as plantaricin C, highlighting the complex interactions of bacteriocins and their targets (Wiedemann et al. 2006a).
Lcn972 is so far the only non-lantibiotic bacteriocin known to interact with lipid II (Martínez et al. 2008a). Contrary to others, Lcn972 does not form pores on susceptible cells. Instead, it inhibits cell wall biosynthesis at the division septum. As a consequence, cells elongate and, finally, die. Lcn972 has a very narrow spectrum of activity, targeting lactococci exclusively, suggesting that it might require other receptor(s) that determine its specificity. Inhibition of septum formation during cell division has also been suggested for garvicin A (Maldonado-Barragán et al. 2013).
Several class II bacteriocins use mannose phosphotransferase transport systems (man-PTS) as receptors (Fig. 2). Lactococcin A, lactococcin B and several class IIa bacteriocins are membrane-disrupting peptides that form complexes with the man-PTS components IIC and IID, the two membrane-associated proteins of this sugar transporter (Diep et al. 2007). Further experiments demonstrated that class IIa bacteriocins that share a similar spectrum of activity specifically interact with a 40-residues N-terminal external loop of the IIC component, whereas lactococcin A requires further interactions with the man-PTS, explaining its spectrum of activity restricted to lactococci (Kjos et al. 2011). It is speculated that the conserved N-terminal β-sheet domain of these bacteriocins is involved in the initial recognition of the man-PTS receptor that further enables the C-terminal domain to insert into the membrane to promote helix–helix interactions between the bacteriocin and the membrane proteins IIC/IID. It is not clear, however, how the integrity of the membrane is disrupted. Pores might be formed exclusively by bacteriocin molecules that assemble upon interaction with the man-PTS or, alternatively, the presence of the bacteriocin might force the transporter to remain in a permanent open-conformation (Kjos et al. 2011). Interestingly, the cognate immunity protein is part of the man-PTS-bacteriocin complex in the producer strains, suggesting that it may provide immunity by blocking efflux of intracellular solutes through the man-PTS.
Other putative bacteriocin receptors that have been recently proposed are the maltose ABC transporter for the circular peptide garvicin ML, a membrane metallopeptidase for the leaderless bacteriocin LsbB, and UppP, a membrane protein involved in cell wall biosynthesis, for the class IIb lactococcin G.
A large deletion encompassing several starch and maltose metabolic genes was identified in several independent L. lactis garvicin ML resistant mutants. Complementation with malEFG genes coding for the maltose ABC transporter restored sensitivity in L. lactis in a concentration-dependent fashion to wild-type levels, supporting its role in bacteriocin activity (Gabrielsen et al. 2012). The L. lactis Zn-dependent metalopeptidase YvjB was identified as the putative LsbB receptor using a cosmid library of a susceptible L. lactis strain. A resistant derivative transformed with this library was screened for susceptibility to LsbB and yvjB was identified as the only gene involved in restoration of the sensitive phenotype (Uzelac et al. 2013). Moreover, several independent resistant mutants were shown to carry mutations in the same gene and its heterologous expression in otherwise naturally resistant bacteria rendered them susceptible to LsbB. In this case, the last C-terminal 8 amino acids appears to be responsible of the interaction of LsbB and its putative receptor, as only peptides bearing these residues were able to block the inhibitory activity of LsbB (Ovchinnikov et al. 2014). Similarly, spontaneous L. lactis resistant mutants to lactococcin G mapped in uppP, which encodes a dispensable undecaprenyl pyrophosphate phosphatase involved in the synthesis of cell wall precursors. When this gene was expressed in the intrinsically resistant Streptococcus pneumoniae, it became susceptible to the bacteriocin (Kjos et al. 2014).
Physical interaction between these membrane proteins and the bacteriocins has not been experimentally proven yet. As described, their role as receptors is based on genetic and phenotypic evidences. Namely, knocking out the corresponding coding gene makes sensitive cells resistant, while its expression, even in heterologous hosts, is required for a sensitivity phenotype.
4.3 Other Modes of Action and Additional Killing Activities
The lantibiotics nisin, subtilin and bovicin HC5 inhibit spore outgrowth. The formation of lipid II targeted pores by nisin halts the establishment of the membrane potential, and consequently, inhibits metabolism in germinating spores which cannot develop further (Gut et al. 2011).
Besides binding to lipid II, nisin has been shown to bind to lipid-bound precursors of the wall teichoic acid (WTA) biosynthesis pathway and promote pore formation in artificial liposomes (Müller et al. 2012). Whether this binding blocks WTA biosynthesis and contributes to nisin killing in vivo is unknown so far.
Pore-forming bacteriocins may have other effects on target cells that contribute to killing (Fig. 2). Nisin and other lantibiotics have been shown to separate endogenous autolysins from their natural inhibitors, the anionic cell envelope polymers, by a cation exchange-like process, thus inducing cell lysis (Bierbaum and Sahl 2009). Accumulation of highly reactive hydroxyl radicals also contribute to the antimicrobial activity of the pore-forming lacticin Q. As described for some antibiotics, lacticin Q increased the levels of hydroxyl radicals and cell viability of lacticin Q-treated cells could be restored by radical scavengers (Li et al. 2013).
4.4 Mechanisms of Resistance
Bacteriocin resistance can be achieved by nonspecific and specific mechanisms. The first mostly alter the properties of the cell envelope to reduce, or even prevent, the interaction of these antimicrobials with the plasma membrane. Bacteriocin-specific mechanisms protect the cells against unique or a group of related bacteriocins; for example, as a consequence of the loss of a common receptor (Table 1).
One of the general strategies adopted by sensitive bacteria to reduce their susceptibility toward bacteriocins is to increase the number of D-alanine esters into the teichoic acids to reduce the net negative charge of the cell envelope. In this way the initial electrostatic interactions are weakened and the positively charged bacteriocins bind less efficiently to the cells (Peschel et al. 1999). The formation of stable pores can be further prevented by changes in the composition of membrane phospholipids that may alter its fluidity (Kaur et al. 2011). Production of exopolysaccharides, reduced surface hydrophobicity, and a thicker cell wall due to the increased activity of penicillin-binding proteins also contribute to prevent the interaction of bacteriocins with the cytoplasmic membrane. All these changes on the cell envelope are often orchestrated by two-component systems that detect the stress caused by the bacteriocin and coordinate the expression of the genes involved in the phenotypes described above (Cotter et al. 2002; Martínez et al. 2007).
Multidrug resistance transporters and/or ABC transporters have been linked to resistance to nisin and other antimicrobials in several species (Majchrzykiewicz et al. 2010; Collins et al. 2010). These transporters provide protection by actively removing bacteriocin molecules out of the membrane, reducing their local concentration at the cell surface. In fact, immunity to several lantibiotics is often provided by such pumping devices in concurrence with small immunity proteins. Interestingly, bacteria carrying functional homologues of the ABC transporters involved in immunity have been detected in non-bacteriocin producers. This so-called immunity mimicry mechanism might be regarded as a specific bacteriocin resistance mechanism as it provides cross-resistance to bacteriocins that share similar immunity systems. Homologues to LtnFE, the ABC transporter involved in immunity to lacticin 3147, have been identified in non-lacticin 3147 producers (Enterococcus, Bacillus) and provide protection against this bacteriocin (Draper et al. 2009). These LtnFE homologues constitute a reservoir of bacteriocin resistance genes which may spread within lacticin 3147-sensitive populations.
Among bacteriocin-specific mechanisms, high resistance levels (over 1000 times) to all class IIa and some class IId bacteriocins have been linked to downregulation of the man-PTS genes. Inactivating mutations have been mapped in rpoN that specifies the sigma factor σ54 and in the gene coding for ManR, a σ54-dependent transcriptional regulator required for activation of the man-PTS genes (Kjos et al. 2011). A specific nisin-degrading enzyme, generally known as Nisin Resistance Protein (Nsr), has been detected in non-nisin producing L. lactis, Bacillus and several streptococcal species. L. lactis Nsr has been recently characterized as a lipoprotein with protease activity that specifically removes the last C-terminal amino acids of nisin (Sun et al. 2009).
Finally, several comparative genomic, transcriptomic, and phenomic studies of resistant strains have uncovered a possible role of global regulators on resistance to bacteriocins. This is the case, for example, of a putative regulator of an extracytoplasmic function sigma factor, which protected lactococcal cells against the cell wall-active bacteriocin Lcn972 (Roces et al. 2012a).
It is worth mentioning that resistance to bacteriocins usually implies energy costs that compromise the fitness of resistant mutants. In fact, despite of the wide use of nisin as a food preservative, resistance is not an issue for concern so far. Nevertheless, development of resistance to bacteriocins should be closely monitored as well as their impact in the physiology of the target bacteria. For example, development of bacteriocin resistance in S. aureus and L. monocytogenes inhibited infection of these food pathogens by specific bacteriophages, which are proposed as alternative preservatives in food (Martínez et al. 2008b; Tessema et al. 2011). In this scenario, the use of combined food preservation technologies (bacteriocins + phages) might become jeopardized.
5 Applications of Bacteriocins
Immediately after its discovery, nisin was applied to inhibit late blowing in cheeses caused by the gas formed by germinating Clostridium tyrobutyricum spores. The success of this approach along with the consumer’s demands for foods without chemically synthesized preservatives encouraged the search for other bacteriocins to be applied as food preservatives. Furthermore, the current knowledge on bacteriocin biology is also paving the way for innovative strategies exploiting bacteriocins in other fields, from health to biotechnological processes (Fig. 3).
5.1 Preservation of Food Products
Bacteriocins produced by LAB have several attributes that make them suitable as food biopreservatives: ability to inhibit Gram-positive pathogenic and spoilage bacteria (L. monocytogenes, S. aureus, Bacillus cereus, Clostridium botulinum, C. tyrobutyricum, etc.); susceptibility to digestive proteases; stability in a wide range of temperature and pH values; no alteration of the organoleptic properties of food as they are taste-, odor-, and colorless; no toxicity to eukaryotic cells and simplicity to scale-up production.
Bacteriocins may be added to food as pure preparations with the advantage of knowing exactly the doses being added, which gives a complete control of their action. However, this increases the production costs and requires approval by regulatory authorities. So far, nisin is the only bacteriocin approved worldwide as a food additive and is identified in the European Union as E234. A semi-purified preparation of Nisin A (Nisaplin® and Chrisin) is manufactured by Dupont Nutrition Biosciences ApS and Christian Hansen A/S, respectively. As a food additive, nisin shows high efficacy in protecting processed cheeses by controlling the outgrowth of spores of C. botulinum and C. tyrobutyricum (5–15 mg/kg), the development of L. monocytogenes in Riccotta cheese (2.5–5 mg/kg), also being used in stirred yogurt (0.5–1.25 mg/kg) to avoid overacidification through the shelf life (Delves-Broughton 2007). Nisin is less effective in meat products due to its adsorption to fat and enzymatic inactivation by gluthatione-S-transferases present in fresh beef muscle (Rose et al. 2002). However, nisin is effective in inhibiting the growth of lactic acid bacteria in vacuum-packed cooked ham stored for 60 days (Kalschne et al. 2014). Nisin and pediocins are also able to reduce L. monocytogenes in fresh-cut lettuce when used as a substitute of chemical disinfection in the washing solution (Allende et al. 2007).
In some countries, such as the US and Canada, it is allowed the use of fermentates as food ingredients, which may contain bacteriocins along with organic acids. They are produced by the fermentation of food grade substrates by bacteriocin-producing bacteria. Some already commercialized are: ALTATM 2431 (pediocin PA-1/AcH, Quest International); MICROGARDTM (with a Propionibacterium bacteriocin; Dupont Nutrition Biosciences ApS); and Bactoferm F-LC (a mix of a pediocin and a sakacin; manufactured by Christian Hansen A/S).
Bacteriocins can also be applied in food by in situ production. This requires bacteriocinogenic strains (protective cultures) well adapted to the particular food matrix (e.g., milk) where they grow and produce bacteriocin under the particular processing, ripening, and storage conditions (Gálvez et al. 2007). This system is cost-effective and is not affected by legal regulations. Nisin-producing L. lactis inhibit L. monocytogenes in Camembert cheese (Maisnier-Patin et al. 1992), S. aureus in Afuega’l Pitu cheese (Rilla et al. 2004), Listeria innocua in semi-hard cheese made with raw milk (Rodríguez et al. 2000) and Cl. tyrobutyricum, a late blowing agent, in semi-hard Vidiago cheese (Rilla et al. 2003). Protection against late blowing was also provided by the bacteriocin producer Lactobacillus gasseri K7 (Bogovic Matijasic et al. 2007). Spraying lacticin 3147 producers on the surface of smear and cottage cheeses inhibited L. monocytogenes development (O’Sullivan et al. 2006; McAuliffe et al. 1999). These bacteriocinogenic starters are also able to control cheese quality by suppressing growth of nonstarter lactic acid bacteria (NSLAB) populations during ripening. In this way, the risk of flavor defects in low-fat Cheddar cheese was reduced (Fenelon et al. 1999). Likewise, bacteriocin-producing cultures accelerate cheese ripening by increasing the lysis of the starter strains with the consequent release of intracellular enzymes to the cheese matrix. This is the case of the strain L. lactis DPC 3286 (lactococcins A, B, M producer) in Cheddar cheese (Morgan et al. 2002) or L. lactis INIA 415 (lacticin 481 and nisin Z producer) in Hispánico cheese (Ávila et al. 2005).
Bacteriocinogenic enterococci have been used as adjunct cultures in both cheeses and dairy fermented beverages. Enterococcus faecalis AS 48-32, the AS-48 producer, clearly inhibited Bacillus cereus growth in nonfat hard cheese throughout ripening (Muñoz et al. 2004). The enterocin A producer E. faecium MMRA, as protective adjunct culture, halted the growth of L. monocytogenes but not its viability during cold storage of Rayeb (Rehaiem et al. 2012).
Bacteriocins can be used in combination with other methods of preservation (heat, chelating agents, carbon dioxide, other biocides, etc.) as part of hurdle technology to enhance their efficacy. There are some examples compiled in the literature, most of which aimed to broaden the inhibition spectrum, to reduce the concentration needed of each preservative or to control the proliferation of sublethally injured cells (Gálvez et al. 2007). Pediocin PA-1/AcH combined with a CO2 atmosphere showed a synergistic effect against foodborne pathogens (Nilsson et al. 2000), while nisin activity against C. sporogenes was enhanced by nitrites (Rayman et al. 1981). Inactivation of this microorganism in milk was also enhanced by combining nisin and high pressure (Arqués et al. 2005). In addition, nisin was combined with pulse electric fields to inactivate S. aureus in milk (Sobrino-López and Martín-Belloso 2006), L. innocua in whey (Gallo et al. 2007) and L. monocytogenes in skim milk (Calderón-Miranda et al. 1999). Enhanced bactericidal activity against L. monocytogenes was shown by enterocin AS-48 in combination with chemical preservatives, essential oils and natural bioactive compounds (Cobo-Molinos et al. 2009).
The incorporation of bacteriocins in packaging systems allows slow release on the food products, thereby extending their shelf life. The antimicrobial efficacy may even be higher than that resulting from the addition of bacteriocins to the food matrix. In this regard, low-density polyethylene (LDPE) films coated with nisin inhibited Micrococcus luteus in raw milk (Mauriello et al. 2005), while enterocin 416K1 showed antilisterial activity on cheeses during cold storage (Issepi et al. 2008). Soy-based films impregnated with nisin and lauric acid reduced up to 6 log units the L. monocytogenes concentration on turkey bologna after 21 days at 4 °C (Dawson et al. 2002), while the immobilization of nisin on polyamide and cellulose lowered S. aureus concentration in Cheddar cheese (Scannell et al. 2000a) and in cheese slices under cold conditions for 3 months (Scannell et al. 2000b). Going further in the antimicrobial active packaging concept, nisin has also been incorporated (1–2 %) into polypropylene/montmorillonite nanocomposites to make packaging food material and inhibited L. monocytogenes, S. aureus and C. perfringens when tested on skimmed milk agar plates, thereby having a great potential to reduce post-process growth of food pathogens (Meira et al. 2014).
5.2 Medical and Veterinary Applications
The potency of bacteriocins in vitro, their low toxicity, and no cross-resistance with antibiotics supports their use as antimicrobials in clinical settings. Narrow spectrum bacteriocins can be used to fight against targeted pathogens without disturbing commensal populations. By contrast, broad-spectrum bacteriocins might be effective against unidentified infections produced by Gram-positive bacteria.
Lantibiotics such as nisin and lacticin 3147 exhibit in vitro activity against S. pneumoniae, methicillin-resistant S. aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile (Piper et al. 2009). The combination of nisin with ramoplanin, an antibiotic that inhibits peptidoglycan biosynthesis, exerts a synergistic effect on MRSA and VRE (Brumfitt et al. 2002). Topic formulations containing bacteriocins active against Propionibacterium acnes have also been tested for the treatment of acne (Kang et al. 2009). Lacticin 3147 was effective in vivo to treat a systemic infection by S. aureus Xen 29 in a murine model by subcutaneal treatment (Piper et al. 2012).
Nisin and lacticin 3147 have also been tested against clinically significant Mycobacteria (Mycobacterium tuberculosis, M. avium, M. kansasii). Lacticin appeared to be more effective than nisin (MIC90 7.5 vs. 60 mg/L) (Carroll et al. 2010).
Nisin appears to be an efficient alternative to antibiotics for treatment of mastitis treatment when applied as a topic solution (6 µg/mL) according to the results obtained in a clinical trial with the participation of eight women with clinical signs of staphylococcal mastitis (Fernández et al. 2008). In addition, an intramammary infusion containing nisin (MasOut®) is being developed as an alternative treatment for mastitis in lactating cows. The combination of lacticin 3147 with a commercial teat seal resulted in increasing protection against the mastitis-causing pathogen Streptococcus dysgalactiae (Ryan et al. 1998).
Another medical application of bacteriocins under exploration is their use against the etiological agent of gastroduodenal ulcer Helicobacter pylori, based on its in vitro susceptibility to nisin, pediocin O2, leucocin K, lacticins A164, BH5, JW3, and NK24 (Kim et al. 2003). Previously, a combination of nisin with glycerol monolaurate has been patented by Applied Microbiology Inc, as a method for treatment H. pylori infection and eradication of its colonization (Blackburn et al. 1997).
Currently, a few healthcare products containing bacteriocins are commercially available, including an edible toothpaste in Japan (www.neonisin.com) and a topical cream for treatment of skin infections (Biosynexus Inc, MA, USA). Nevertheless, some chemical properties of bacteriocins, in particular of lantibiotics, such as their low solubility, low activity at high pH, rapid degradation by intestinal enzymes and interaction with blood components should be improved to promote their use in health care.
5.3 Role of Bacteriocins in Probiosis
Bacteriocin production by probiotic bacterial strains may be regarded as an important trait in strain selection due to the putative advantage in the competitive dynamics established between bacteriocin producers and closely related species within very complex microbial communities as those of the gastrointestinal tract. The mechanisms behind the probiotic effect of bacteriocin production in vivo are still obscure. The contribution of bacteriocins to a positive effect on host health might be attributed to: (i) their action as colonizing peptides that allow probiotics competition with the indigenous microbiota; (ii) the elimination of pathogens by virtue of their antimicrobial activity; (iii) their role as signaling peptides that recruit other bacteria and/or the immune system to eliminate infectious microorganisms (Dobson et al. 2012).
Up to 56 species of Lactobacillus have been found as part of the intestinal microbiota. Most of them produced bacteriocins in vitro, and some also in vivo (Gillor et al. 2005), with activity against both Gram positive and Gram negative pathogens. L. johnsonii LA1 (Gotteland and Cruchet 2003) and L. acidophilus LB (Coconnier et al. 1998) inhibited H. pylori bound to intestinal epithelial cells. Another interesting study demonstrated that L. salivarius UC118 produces in vivo the potent bacteriocin Abp118 that protected mice from L. monocytogenes infection (Corr et al. 2007). Similarly, L. casei L26 LAFTI inhibited enterohemorrhagic E. coli and L. monocytogenes in mice (Su et al. 2007). Moreover, it has been reported that bacteriocin production by probiotic lactobacilli inhibited pathogens (e.g., Gardnerella vaginalis) in the human vagina (Aroutcheva et al. 2001).
Probiotic supplements containing bacteriocins administered to broiler chickens resulted in an increased rate survival when they were challenged with Salmonella pullorum (Audisio et al. 2000). Bacteriocin production is also one of the factors associated to the antibacterial effect of probiotics used as biocontrol agents in aquaculture (Verschuere et al. 2000). They are used as food supplements to improve the performance of aquatic microorganisms, being effective as growth promoters and pathogen inhibitors.
5.4 Expanding the Biotechnological Potential of Bacteriocins
The primary metabolite nature of bacteriocins along with a rather simple biosynthetic pathway facilitates the design of novel molecules through gene-based peptide engineering and, consequently, may expand their use as antimicrobials (Perez et al. 2014). The spectrum of inhibition, stability, interaction with food matrices or specific activity of bacteriocins could be modified by genetic engineering. For instance, a single amino acid change in nisin Z (the methionine at position 21 was replaced by lysine) resulted in a fivefold increase of its solubility at pH 8, which was accompanied by inhibition of Gram-negative bacteria (Yuan et al. 2004). Similarly, a nisin A derivative (glycine at position 29 was replaced by serine) had an extended inhibitory spectrum toward foodborne pathogens such as Cronobacter sakazakii, E. coli and Salmonella enterica serovar Tiphymurium (Field et al. 2012).
It has been also possible to create bacterial strains that produce several bacteriocins by conjugation of multiple bacteriocinogenic plasmids. In this way, the performance of commercial dairy starters can be enhanced as exemplified by transferring the lacticin 3147-encoding conjugative plasmid pMRC01 to Cheddar cheese starter strains (Coakley et al. 1997).
Genes involved in bacteriocin biosynthesis have been used to develop tools for genetic engineering, including regulated gene expression in Gram-positive bacteria. An important property of the nisin-inducible promoters is that the degree of activation depends on the intensity of the stimulus, i.e., the concentration of the extracellular nisin. This has been used for designing the NICE system, made out by cells that express nisR-K and vectors with multicloning sites behind the nisA promoter, where protein determinants can be inserted. Production of these proteins will be a function of the concentration of nisin added to the cell culture (Mierau and Kleerebezem 2005). The immunity determinants have also been exploited as food-grade markers in cloning vectors that make use of bacteriocins as selecting agents and, more recently, the introduction of full bacteriocin operons into plasmids has demonstrated to prevent plasmid segregation under non-selecting conditions (Takala and Saris 2002 ; Campelo et al. 2014). The development of these plasmids helps to reduce the use of antibiotics in biotechnological processes with genetically modified bacteria. Other strategies encompassed the use of the nisin biosynthetic machinery to introduce thioether rings into foreign peptides to improve their stability under harsh conditions (Kluskens et al. 2005).
Finally, bacteriocins may be very useful tools to uncover the molecular mechanisms and the physiology behind the stress response in susceptible bacteria. For example, the use of the cell wall-active bacteriocin Lcn972 has been instrumental to define the core response of L. lactis to cell envelope stress and to identify biomarkers for strain robustness (Roces et al. 2012b).
References
Allende A, Martínez B, Selma V, Gil MI, Suárez JE, Rodríguez A (2007) Growth and bacteriocin production by lactic acid bacteria in vegetable broth and their effectiveness at reducing Listeria monocytogenes in vitro and in fresh-cut lettuce. Food Microbiol 24:759–766
Altena K, Guder A, Cramer C, Bierbaum G (2000) Biosynthesis of the lantibiotic mersacidin: organization of a type B lantibiotic gene cluster. Appl Environ Microbiol 66:2565–2571
Aroutcheva AA, Simoes JA, Faro S (2001) Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect Dis Obstet Gynecol 9:33–39
Arqués JL, Rodríguez E, Gaya P, Medina M, Núñez M (2005) Effect of combinations of high-pressure treatment and bacteriocin-producing lactic acid bacteria on the survival of Listeria monocytogenes in raw milk cheese. Int Dairy J 15:893–900
Asaduzzaman SM, Sonomoto K (2009) Lantibiotics: diverse activities and unique modes of action. J Biosci Bioeng 107:475–487
Audisio MC, Oliver G, Apella MC (2000) Protective effect of Enterococcus faecium J96, a potential probiotic strain, on chicks infected with Salmonella pullorum. J Food Prot 63:1333–1337
Ávila M, Garde S, Gaya P, Medina M, Núñez M (2005) Influence of a bacteriocin-producing lactic culture on proteolysis and texture of Hispánico cheese. Int Dairy J 15:145–153
Bierbaum G, Sahl HG (2009) Lantibiotics: mode of action, biosynthesis and bioengineering. Curr Pharm Biotechnol 10(1):2–18
Blackburn P, Goldstein BP, Cook DJ (1997) Patent No WO1997025055 A1. Nisin in combination with glycerol monolaurate active against Helicobacter (Applicant Applied Microbiology Inc)
Boakes S, Wadman S (2008) The therapeutic potential of lantibiotics. Innov Pharm Technol 27:22–25
Bogovic Matijasic B, Koman Rajsp M, Perko B, Rogelj I (2007) Inhibition of Clostridium tyrobutyricum in cheese by Lactobacillus gasseri. Int Dairy J 17:157–166
Breukink E, de Kruijff B (2006) Lipid II as a target for antibiotics. Nat Rev Drug Discov 5(4):321–332
Brumfitt W, Salton MRJ, Hamilton-Miller JMT (2002) Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J Antimicrob Chemother 50:31–734
Brurberg MB, Nes IF, Eijsink VG (1997) Pheromone-induced production of antimicrobial peptides in Lactobacillus. Mol Microbiol 26:347–360
Calderón-Miranda ML, Barbosa-Cánovas GV, Swanson BG (1999) Transmission of electron microscopy of Listeria innocua treated by electric pulse fields and nisin in skimmed milk. Int J Food Microbiol 51:31–38
Campelo AB, Roces C, Mohedano ML, López P, Rodríguez A, Martínez B (2014) A bacteriocin gene cluster able to enhance plasmid maintenance in Lactococcus lactis. Microb Cell Fact 13:77
Carroll J, Draper LA, O’Connor PM, Coffey A, Hill C, Ross RP, Cotter PD, O’Mahony J (2010) Comparison of the activities of the lantibioticsnisin and lacticin 3147 against clinically significant mycobacteria. Int J Antimicrob Agents 36:132–136
Cintas LM, Casaus P, Håvarstein LS, Hernández PE, Nes IF (1997) Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol 63:4321–4330
Coakley M, Fitzgerald G, Ross RP (1997) Application and evaluation of the phage resistance- and bacteriocin-encoding plasmid pMRC01 for the improvement of dairy starter cultures. Appl Environ Microbiol 63:1434–1440
Cobo-Molinos A, Abriouel H, Lucas López R, Ben Omar N, Valdivia E, Gálvez A (2009) Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds, and chemical preservatives against Listeria monocytogenes in ready-to-eat salads. Food Chem Toxicol 47:2216–2223
Coconnier MH, Lievin V, Hemery E, Servin AL (1998) Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol 64:4573–4580
Collins B, Curtis N, Cotter PD, Hill C, Ross RP (2010) The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various beta-lactam antibiotics. Antimicrob Agents Chemother 54(10):4416–4423
Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Grahan CGM (2007) Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivariusUCC118. PNAS 104:7617–7621
Cotter PD, Guinane CM, Hill C (2002) The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob Agents Chemother 46(9):2784–2790
Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105
Dawson PL, Carl GD, Acton JC, Han IY (2002) Effect of lauric acid and nisin-impregnated soy-based films on the growth of Listeria monocytogenes on turkey bologna. Poultry Sci 81:721–726
Delves-Broughton J (2007) Nisin as biopreservative. Food Aust 57:525–527
Diaz M, Valdivia E, Martínez-Bueno M, Fernández M, Soler-González AS, Ramírez-Rodrigo H, Maqueda M (2003) Characterization of a new operon, as-48EFGH, from the as-48 gene cluster involved in immunity to enterocin AS-48. Appl Environ Microbiol 69:1229–1236
Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF (2007) Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci U S A 104(7):2384–2389
Dobson A, Cotter PD, Ross RP, Hill C (2012) Bacteriocin production: a probiotic trait? Appl Environ Microbiol 78:1–6
Draper LA, Grainger K, Deegan LH, Cotter PD, Hill C, Ross RP (2009) Cross-immunity and immune mimicry as mechanisms of resistance to the lantibiotic lacticin 3147. Mol Microbiol 71(4):1043–1054
Drider D, Fimland G, Héchard Y, McMullen LM, Prévost H (2006) The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev 70(2):564–582
Driessen AJ, van den Hooven HW, Kuiper W, van de Kamp M, Sahl HG, Konings RN, Konings WN (1995) Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry 34(5):1606–1614
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106
Fenelon MA, Ryan MP, Rea MC, Guinee TP, Ross RP, Hill C, Harrington D (1999) Elevated temperature ripening of reduced fat cheddar made with or without lacticin 3147-producing starter culture. J Dairy Sci 82:10–22
Fernández L, Delgado S, Herrero H, Maldonado A, Rodríguez JM (2008) The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J Hum Lact 24:311–316
Field D, Begley M, O´Connor PM, Hugenholtz F, Cotter PD, Hill C, Ross RP (2012) Bioengineered nisin A derivatives with enhanced activity against Gram positive and Gram negative pathogens. PLoS ONE 7:e46884
Fujita K, Ichimasa S, Zendo T, Koga S, Yoneyama F, Nakayama J, Sonomoto K (2007) Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl Environ Microbiol 73:2871–2877
Gabrielsen C, Brede DA, Hernández PE, Nes IF, Diep DB (2012) The maltose ABC transporter in Lactococcus lactis facilitates high-level sensitivity to the circular bacteriocin garvicin ML. Antimicrob Agents Chemother 56(6):2908–2915
Gabrielsen C, Brede DA, Nes IF, Diep DB (2014) Circular bacteriocins: biosynthesis and mode of action. Appl Environ Microbiol 80:6854–6862
Gallo LI, Pilosof AMR, Jagus RJ (2007) Effect of the sequence of nisin and pulsed electric fields treatments and mechanisms involved in the inactivation of Listeria innocua in whey. J Food Eng 79:188–193
Gálvez A, Abriouel H, López RL, Omar NB (2007) Bacteriocin based strategies for food biopreservation. Int J Food Microbiol 120:51–70
Gotteland M, Cruchet S (2003) Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J Antimicrob Chemother 51:1317–1319
Gillor O, Nigro LM, Riley MA (2005) Genetically engineered bacteriocins and their potential as the next generation of antimicrobials. Curr Pharm Des 11:1067–1075
Gut IM, Blanke SR, Van Der Donk WA (2011) Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem Biol 6(7):744–752
Hasper HE, de Kruijff B, Breukink E (2004) Assembly and stability of nisin-lipid II pores. Biochemistry 43(36):11567–11575
Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E (2006) An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313(5793):1636–1637
Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113:723–736
Issepi R, Pilati F, Marini M, Toselli M, de Niederhäusern S, Guerrieri E, Sabia Messi P, Manacardi G, Anacarso I, Bondi M (2008) Anti-listerial activity of a polymeric film coated with hybrid coatings doped with Enterocin 416K1 for use as bioactive food packaging. Int J Food Microbiol 123:281–287
Kalschne DL, Geitenes S, Veit MR, Sarmento CMP, Colla E (2014) Growth inhibition of lactic acid bacteria in ham by nisin: a model approach. Meat Sci 98:744–752
Kang BS, Seo JG, Lee GS, Kim JH, Kim SY, Han YW, Kang H, Kim HO, Rhee JH, Chung MJ, Park YM (2009) Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol 47:101–109
Kaur G, Malik RK, Mishra SK, Singh TP, Bhardwaj A, Singroha G, Vij S, Kumar N (2011) Nisin and class IIa bacteriocin resistance among Listeria and other foodborne pathogens and spoilage bacteria. Microb Drug Resist 17(2):197–205
Khusainov R, Heils R, Lubelski J, Moll GN, Kuipers OP (2011) Determining sites of interaction between prenisin and its modification enzymes NisB and NisC. Mol Microbiol 82:706–718
Kim TS, Hur JW, Yu MA, Cheigh C, Kim KN, Hwang JK, Pyun YR (2003) Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J Food Prot 66:3–12
Kjos M, Borrero J, Opsata M, Birri DJ, Holo H, Cintas LM, Snipen L, Hernández PE, Nes IF, Diep DB (2011) Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology 157(Pt 12):3256–3267
Kjos M, Oppegård C, Diep DB, Nes IF, Veening JW, Nissen-Meyer J, Kristensen T (2014) Sensitivity to the two-peptide bacteriocin lactococcin G is dependent on UppP, an enzyme involved in cell-wall synthesis. Mol Microbiol 92(6):1177–1187
Kluskens LD, Kuipers A, Rink R, de Boef E, Fekken S, Driessen AJ, Kuipers OP, Moll GN (2005) Post-translational modification of therapeutic peptides by NisB, the dehydratase of the lantibiotic nisin. Biochemistry 44(38):12827–12834
Li M, Yoneyama F, Toshimitsu N, Zendo T, Nakayama J, Sonomoto K (2013) Lethal hydroxyl radical accumulation by a lactococcal bacteriocin, lacticin Q. Antimicrob Agents Chemother 57(8):3897–3902
Lubelski J, Rink R, Khusainov R, Moll GN, Kuipers OP (2008) Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell Mol Life Sci 65:455–476
Maisnier-Patin S, Deschamps N, Tatini SR, Richard J (1992) Inhibition of Listeria monocytogenes in Camembert cheese made with a nisin-producing starter. Lait 72:249–263
Majchrzykiewicz JA, Kuipers OP, Bijlsma JJ (2010) Generic and specific adaptive responses of Streptococcus pneumoniae to challenge with three distinct antimicrobial peptides, bacitracin, LL-37, and nisin. Antimicrob Agents Chemother 54(1):440–451
Maldonado-Barragán A, Cárdenas N, Martínez B, Ruiz-Barba JL, Fernández-Garayzabal JF, Rodríguez JM, Gibello A (2013) Garvicin A, a novel class IId bacteriocin from Lactococcus garvieae that inhibits septum formation in L. garvieae strains. Appl Environ Microbiol 79(14):4336–4346
Maqueda M, Sánchez-Hidalgo M, Fernández M, Montalbán-López M, Valdivia E, Martínez-Bueno M (2008) Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol Rev 32:2–22
Martínez B, Fernández M, Suárez JE, Rodríguez A (1999) Synthesis of lactococcin 972, a bacteriocin produced by Lactococcus lactis IPLA 972, depends on the expression of a plasmid-encoded bicistronic operon. Microbiology 145:3155–3161
Martínez B, Zomer AL, Rodríguez A, Kok J, Kuipers OP (2007) Cell envelope stress induced by the bacteriocin Lcn972 is sensed by the lactococcal two-component system CesSR. Mol Microbiol 64(2):473–486
Martínez B, Böttiger T, Schneider T, Rodríguez A, Sahl HG, Wiedemann I (2008a) Specific interaction of the unmodified bacteriocin Lactococcin 972 with the cell wall precursor lipid II. Appl Environ Microbiol 74(15):4666–4670
Martínez B, Obeso JM, Rodríguez A, García P (2008b) Nisin-bacteriophage crossresistance in Staphylococcus aureus. Int J Food Microbiol 122(3):253–258
Mauriello G, De Luca E, La Storia A, Villani F, Ercolini D (2005) Antimicrobial activity of a nisin-activated plastic film for food packaging. Let Appl Microbiol 41:464–469
McAuliffe O, Hill C, Ross RP (1999) Inhibition of Listeria monocytogenes in cottage cheese manufactured with lacticin 3147producing starter culture. J Appl Microbiol 86:251–256
Meira SMM, Zehetmeyer G, Jardim AI, Scheibel JM, Bof de Oliveira RV, Brandelli A (2014) Polypropylene/montmorillonitenanocomposites containing nisin as antimicrobial food packaging. Food Bioprocess Techn 7:3349–3357
Mierau I, Kleerebezem M (2005) 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:705–717
Miller KW, Ray P, Steinmetz T, Hanekamp T, Ray B (2005) Gene organization and sequences of pediocin AcH/PA-1 production operons in Pediococcus and Lactobacillus plasmids. Lett Appl Microbiol 40:56–62
Moll GN, Konings WN, Driessen AJ (1999) Bacteriocins: mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 76(1–4):185–198
Morgan SM, O’Sullivan L, Ross RP, Hill C (2002) The design of three strain starter system for Cheddar cheese manufacture exploiting bacteriocin-induced starter lysis. Int Dairy J 17:760–769
Müller A, Ulm H, Reder-Christ K, Sahl HG, Schneider T (2012) Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors. Microb Drug Resist 18(3):261–270
Muñoz A, Maqueda M, Rodríguez A, Valdivia E (2004) Control of psychrotrophic Bacillus cereus in a non-fat hard type cheese by an enterococcal strain producing enterococin AS-48. J Food Prot 67:1517–1521
Nes IF, Diep DB, Håvarstein LS, Brurberg MB, Eijsink V, Holo H (1996) Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70:113–128
Nilsson L, Chen Y, Chikindas ML, Huss HH, Gram L, Montville TJ (2000) Carbon dioxide and nisin act synergistically on Listeria monocytogenes. Appl Environ Microbiol 66:769–774
Nissen-Meyer J, Rogne P, Öppegard C, Haugen HS, Kristiansen PE (2009) Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by gram-positive bacteria. Curr Pharm Biotechnol 10(1):19–37
Nissen-Meyer J, Oppegård C, Rogne P, Haugen HS, Kristiansen PE (2010) Structure and mode-of-action of the two-peptide (Class-IIb) bacteriocins. Probiotics Antimicrob Proteins 2:52–60
O’Sullivan L, O’Connor EB, Ross RP, Hill C (2006) Evaluation of live-culture-producing lacticin 3147 as a treatment for the control of Listeria monocytogenes on the surface of smear-ripened cheese. J Appl Microbiol 100:135–143
Ovchinnikov KV, Kristiansen PE, Uzelac G, Topisirovic L, Kojic M, Nissen-Meyer J, Nes IF, Diep DB (2014) Defining the structure and receptor binding domain of the leaderless bacteriocin LsbB. J Biol Chem 289(34):23838–23845
Perez RH, Zendo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 13 (Supl 1):S2
Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F (1999) Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274(13):8405–8410
Piper C, Cotter PD, Paul Ross RP, Hill C (2009) Discovery of medically significant lantibiotics. Curr Drug Discov Technol 6:1–18
Piper C, Cassey PG, Hill C, Cotter PD, Ross RP (2012) The lantibiotic Lacticin 3147 prevents systemic spread of Staphylococcus aureus in a murine infection model. Int J Microbiol 2012:e806230
Ra R, Beerthuyzen MM, de Vos WM, Saris PE, Kuipers OP (1999) Effects of gene disruptions in the nisin gene cluster of Lactococcus lactis on nisin production and producer immunity. Microbiology 145:1227–1233
Rayman MK, Aris B, Hurst A (1981) Nisin: a possible alternative or adjunct to nitrite in the preservation of meats. Appl Environ Microbiol 41:375–380
Rehaiem A, Martínez B, Manai M, Rodríguez A (2012) Technological performance of the enterocin A producer Enterococcus faecium MMRA as a protective adjunct culture to enhance hygienic and sensory attributes of traditional fermented milk ‘Rayeb’. Food Bioprocess Technol 5:2140–2150
Rilla N, Martínez B, Delgado T, Rodríguez A (2003) Inhibition of Clostridium tyrobutyricum in Vidiago cheese by Lactococcus lactis ssp. lactis IPLA 729, a nisin Z producer. Int J Food Microbiol 85:22–33
Rilla N, Martínez B, Rodríguez A (2004) Inhibition of a methicillin-resistant Staphylococcus aureus strain in Afuega’l Pitu cheese by the nisin Z producing strain Lactococcus lactis subsp. lactis IPLA 729. J Food Prot 67:928–933
Roces C, Pérez V, Campelo AB, Blanco D, Kok J, Kuipers OP, Rodríguez A, Martínez B (2012a) The putative lactococcal extracytoplasmic function anti-sigma factor llmg2447 determines resistance to the cell wall-active bacteriocin lcn972. Antimicrob Agents Chemother 56(11):5520–5527
Roces C, Rodríguez A, Martínez B (2012b) Cell wall-active bacteriocins and their applications beyond antibiotic activity. Probiotics Antimicrob Proteins 4:259–272
Rodríguez E, Arqués JL, Gaya P, Tomillo J, Núñez M, Medina M (2000) Behaviour of Staphylococcus aureus in semihard cheese made from raw milk with nisin-producing starter cultures. Milchwissenschaft 55:633–635
Rose NL, Palcic MM, Sporns P, McMullen LM (2002) Nisin: a novel substrate for glutathione S-transferase isolated from fresh beef. J Food Sci 67:2288–2293
Ryan MP, Meaney WJ, Ross RP, Hill C (1998) Evaluation of Lacticin 3147 and a teat seal containing this bacteriocin for inhibition of mastitis pathogens. Appl Environ Microbiol 64:2287–2290
Sánchez-Barrena MJ, Martínez-Ripoll M, Gálvez A, Valdivia E, Maqueda M, Cruz V, Albert A (2003) Structure of bacteriocin AS-48: from soluble state to membrane bound state. J Mol Biol 334(3):541–549
Sánchez-Hidalgo M, Montalbán-López M, Cebrián R, Valdivia E, Martínez-Bueno M, Maqueda M (2011) AS-48 bacteriocin: close to perfection. Cell Mol Life Sci 68:2845–2857
Scannell AGM, Hill C, Ross RP, Marx S, Hartmeier W, Arendt EK (2000a) Continuous production of lacticin 3147 and nisin using cells immobilized in calcium alginate. J Appl Microbiol 89:573–579
Scannell AGM, Hill C, Ross RP, Marx S, Hartmeier W, Arendt EK (2000b) Development of bioactive food packaging materials using immobilised bacteriocins Lacticin 3147 and Nisaplin®. Int J Food Microbiol 60:241–249
Smith L, Hillman J (2008) Therapeutic potential of type A (I) lantibiotics, a group of cationic peptide antibiotics. Curr Opin Microbiol 11:401–408
Sobrino-Lopez A, Martin-Belloso O (2006) Enhancing inactivation of Staphylococcus aureus in skim milk by combining high-intensity pulsed electric fields and nisin. J Food Prot 69:345–353
Su P, Henriksson A, Mitchell H (2007) Survival and retention of the probiotic Lactobacillus casei LAFTI®L26 in the gastrointestinal tract of the mouse. Let Appl Microbiol 44:120–125
Sun Z, Zhong J, Liang X, Liu J, Chen X, Huan L (2009) Novel mechanism for nisin resistance via proteolytic degradation of nisin by the nisin resistance protein NSR. Antimicrob Agents Chemother 53(5):1964–1973
Takala TM, Saris PE (2002) A food-grade cloning vector for lactic acid bacteria based on the nisin immunity gene nisI. Appl Microbiol Biotechnol 59(4–5):467–471
Tessema GT, Moretro T, Snipen L, Axelsson L, Naterstad K (2011) Global transcriptional analysis of spontaneous sakacin P-resistant mutant strains of Listeria monocytogenes during growth on different sugars. PLoS ONE 6(1):e16192
Uzelac G, Kojic M, Lozo J, Aleksandrzak-Piekarczyk T, Gabrielsen C, Kristensen T, Nes IF, Diep DB, Topisirovic L (2013) A Zn-dependent metallopeptidase is responsible for sensitivity to LsbB, a class II leaderless bacteriocin of Lactococcus lactis subsp. lactis BGMN1-5. J Bacteriol 195(24):5614–5621
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol R 64:655–671
Wiedemann I, Breukink E, van Kraaij C, Kuipers OP, Bierbaum G, de Kruijff B, Sahl HG (2001) Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J Biol Chem 276(3):1772–1779
Wiedemann I, Böttiger T, Bonelli RR, Schneider T, Sahl HG, Martínez B (2006a) Lipid II-based antimicrobial activity of the lantibiotic plantaricin C. Appl Environ Microbiol 72(4):2809–2814
Wiedemann I, Böttiger T, Bonelli RR, Wiese A, Hagge SO, Gutsmann T, Seydel U, Deegan L, Hill C, Ross P, Sahl HG (2006b) The mode of action of the lantibiotic lacticin 3147–a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol Microbiol 61(2):285–296
Yuan J, Zhang ZZ, Chen XZ, Yang W, Huan LD (2004) Site-directed mutagenesis of the hinge region of nisin Z and properties of nisin Z mutants. Appl Microbiol Biotechnol 64:806–815
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Martínez, B., Rodríguez, A., Suárez, E. (2016). Antimicrobial Peptides Produced by Bacteria: The Bacteriocins. In: Villa, T., Vinas, M. (eds) New Weapons to Control Bacterial Growth. Springer, Cham. https://doi.org/10.1007/978-3-319-28368-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-28368-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28366-1
Online ISBN: 978-3-319-28368-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)