Abstract
The interest of consumers and government organizations on improving the quality of food and its impact on human health is growing in recent years. Consuming low-calorie foodstuffs are increasingly demanded, and the presence of artificial sweeteners plays an important role not only in food but also in waste reaching the environment. Analysis of these compounds is important in assessing food safety and quality. This chapter reviews the analytical approaches for the extraction and reliable identification and quantification of most commonly used artificial sweeteners in food, pharmaceutical, and environmental related matrices. The advantages and disadvantages of determination techniques used are described, with special emphasis on liquid chromatography coupled to mass spectrometry with different approaches, including applications of high resolution mass spectrometry. The possibility of using new materials for efficient extraction, miniaturized extraction techniques, and the potential for quantification of LC-MS/MS techniques are highlighted as future prospects, based on achievements.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
Sweetener is a food additive used to impart a sweet taste to foods or in table-top sweeteners, included in the Regulation 1333/2008 [1]; it serves for one or more of the following purposes: replacing sugars for the production of energy-reduced food, noncariogenic food, or food with no added sugars and replacing sugars where this permits an increase in the shelf-life of the food or producing food intended for particular nutritional uses.
These additives are known as low-calories or nonnutritive sweeteners. Each provides little or no energy, in most cases because it activates sweet taste receptors at very low concentrations relative to sugar, with estimates of the potency of artificial sweeteners (SWTs) ranging from about 200 times to up to 20,000 times the sweetness of sucrose. They are of plant origin or obtained by chemical synthesis and are used in the food industry for their sweetening power [2].
It is important to balance the calories you consume with the calories you burn by maintaining a sensible, balanced diet combined with regular physical activity. Energy density (kcal g−1) of foods is an important determinant of energy intake in a meal or over the course of the day. By substituting sugar for low calorie sweeteners, it is possible to lower the energy density of foods and drinks offering an easy method of reducing calories while maintaining the palatability of the diet. As such, low calories sweeteners can play a helpful role in assisting the achievement of weight maintenance or weight loss, as part of a balanced diet [3].
The low-calorie sweeteners should have the following characteristics: have a similar taste profile as sucrose, noncaloric at normal usage levels, noncariogenic, safe, natural in origin, commercially available at a competitive price, easy to use, stable under a range of processing and usage conditions, inert and compatible with a wide range of food ingredients, stable on storage, provide some bulking effect and mouthfeel, biodegradable, etc.
The intense sweeteners currently authorized in Europe comprise 11 compounds of various chemical natures [4–7], while nine high-intensity sweeteners are FDA-approved as food additives in the United States [8]. They are used in the formulation of foods , beverages, and as excipients in pharmaceutical industry, essentially for their sweetening role but also for their technological properties (stabilizers, texturizers) (Tables 1 and 2).
Regulation 1129/2011 [4–6] includes the Union list of food additives approved for use in foods and conditions of use:
-
The name of the food additive and its E number. The “E number” refers to Europe and shows that the additive is regarded as safe in Europe. In effect, the E is a guarantee of safety. Food additives must be included either by name or by an E number in the ingredient list.
-
The foods to which the food additive may be added (at the maximum intended use level or the maximum permitted level): dairy products, edible ice, fruit and vegetables, confectionery, cereals, meat, fish, table-top sweeteners, beverages, etc.
-
The conditions under which the food additive may be used.
-
Restrictions on the sale of the food additive directly to the final consumer.
Each low sweeteners used in food and drink production has its own unique taste profile, technical characteristics, and benefits. Low sweeteners can be used alone or in combination with each other as a blend. It is possible to set the taste of sweetness to the demands of a products and consumer taste, while taking into account factors such as stability and cost.
The Acceptable Daily Intake (ADI) is a guideline quantity that represents the amount of low calorie sweetener that can be safely consumed on a daily basis throughout a person’s lifetime without any health problems. Joint FAO (Food and Agricultural Organization, on de UN) WHO (World Health Organization) Expert Committee of Food Additives (JECFA) introduced the concept of the ADI for the safety regulation of all food and drink additives in 1961. Other international scientific authorities such as US-FDA (Drug and Food Administration of United States), EFSA (European Food Safety Authority), and ISA (International Society of Automation) use the same method of deriving the ADI independently, guaranteeing consistency of food safety worldwide.
ADI “not specified” is applicable to a food substance of very low toxicity in which, on the basis of the available data (chemical, biochemical, toxicological, and other), the total dietary intake of the substance arising from its use at the levels necessary to achieve the desired effect and from its acceptable background in food does not represent a hazard to health.
GRAS (Generally Recognized As Safe) notices have been submitted to FDA for three types of high-intensity sweeteners, thaumatin (extracted from the arils of the fruit of Thaumatococcus daniellii), certain steviol glycosides obtained from the leaves of the stevia plant (Stevia rebaudiana Bertoni), and extracts obtained from Siraitia grosvenorii (Swingle fruit), also known as Luo Han Guo or monk fruit [8, 9], on the priority list of substances proposed for evaluation.
Furthermore, EFSA is reevaluating all food additives by 2020. Over the past few decades the European Union (EU) has put in place a robust food safety system that helps to ensure that consumers are protected from possible food-related risks. Many sweeteners were approved more recently and are scheduled for review after 2015. EFSA can also reprioritize a food additive in light of new information; for example, safety of advantame [10], the deadline for the artificial sweetener aspartame was brought forward from 2013 to 2020 due to concerns raised regarding recent studies [11]. The reevaluation of all approved sweeteners listed in Directive 94/35/EC [12] shall be completed by 31 December 2020 [13].
The metabolic and physiological properties and stability of low-calorie sweeteners are shown in Table 2 [3, 9, 14, 15].
The influence of low-calorie sweeteners on hunger, satiety, and energy intake has been addressed in many laboratory studies and reviews [3, 16]. While the use of low calorie sweeteners does not, in itself, result in a rapid weight loss, it may promote long-term dietary compliance by improving the diversity, variety, and the overall palatability of a reduced energy diet. Several studies have examined the acute effects of low sweeteners on hunger and food intakes. They concluded that replacing sucrose by low calorie sweeteners in foods or drinks does not increase food intake or hunger in children nor has shown to increase food intake in normal weight or overweight men and women. Studies with adults have reported either unchanged or reduced motivation to eat, regardless or whether the low calorie sweetener was delivered in a solid or liquid medium. Mattes and Popkin [17] indicate that the substitution of low-calorie sweeteners for a nutritive sweetener generally elicits incomplete energy compensation, but evidence of long-term efficacy for weight management is not available. The addition of low-calorie sweeteners to diets poses no benefit for weight loss or reduced weight gain without energy restriction. They have concluded that the available evidence either refuted or was insufficient to refute or support each of these potential mechanisms or hypotheses for low-calorie sweeteners increasing appetite, hunger, or energy intake. The Academy of Nutrition and Dietetics updates its position low calorie sweeteners allow a more versatile approach to weight management and may even encourage compliance with a diet [18]. Even modest amounts of weight loss have been shown to contribute significantly to a reduction in risk associated with obesity and overweight, such as diabetes and heart disease. In 2012 the American Heart Association (AHA) and the American Diabetes Association (ADA) [19] issued a joint scientific statement on low calorie sweeteners and their potential usefulness in helping people achieve and maintain a healthy body weight and help people with diabetes to control their glucose level. Other authors [20] indicate that they do not raise blood glucose levels and can be used to control weight and to treat hypoglycemia. If they are used excessively, they can increase weight, promote obesity, and can cause impairment of normal metabolic responses. However, conclude that artificial sweeteners should be used in a limited amount. Moreover, use of natural sweeteners should be increased.
The opinion of the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) [21] on the assessment of the nutritional benefits and risks related to intense sweeteners indicates there is sufficient scientific information to support the claims that intense sweeteners as all sugar replacers lead to a lower rise in blood sugar levels after meals and maintain tooth mineralization by decreasing tooth demineralization again if consumed instead of sugars.
However, EFSA’s experts [22] could find no clear cause and effect relationship to justify the claims that intense sweeteners when replacing sugars maintain normal blood sugar levels or maintain/achieve a normal body weight. After an analysis of all of the scientific literature, it appears that, despite a large number of studies, the data are insufficient to determine any long-term nutritional benefits related to the consumption of products containing low-calorie sweeteners as sugar substitutes. ANSES and Bellisle [16, 21] particularly stress the lack of relevant conclusive studies on the supposed benefits of artificial sweeteners, in the context of their broad, long-standing use in nutrition, although the available data do not show any risks related with their consumption.
On the other hand, it seems that the abuse of artificial sweeteners is not convenient, especially in children, because it could persistently alter sweet preferences, leading to enhanced intake of sugars throughout adulthood. Also it could interfere with learning of basic relations between sweet tastes and the delivery of calories, which in turn could negatively affect regulation of metabolic processes and could alter the composition of the gut microbiota, which in turn can contribute to metabolic dysregulation [23–25]. Artificial sweeteners should be used in a limited amount; their excessive use can increase weight, promote obesity, and cause impairment of normal metabolic responses [20].
The determination of low-calorie sweeteners is of great importance not only to identify and quantify their content in foods but also because due to increase consumption; they can be found in the environment and are considered emerging pollutants. They are predominately used in the food industry for the production of sugar-free low calorie foodstuffs, to replace sugar, and to enhance the taste of some personal care products, such as toothpaste as well as drugs and sanitary products. Thus, there are a variety of beverages, foods, and food supplements in which artificial sweeteners are present, so it is necessary to provide validated analytical methodology for their detection and quantification. The need to monitor the use and consumption in different countries is required to know if intake levels frequently exceed the advised acceptable daily intake (ADI).
Artificial sweeteners are highly consumed with increasing trends in consumption. Excretion after human consumption (not metabolized by the human body and excreted unchanged (Table 2)) is undoubtedly a major source of artificial sweeteners in the environment, but it is surely not the only one. Sweeteners have been detected worldwide in a variety of environmental media; nevertheless, monitoring of their presence is still not required by any existing regulations [26]. Its current presence in wastewater has made that some sweeteners are regarded as high-priority emerging contaminants.
Acesulfame , saccharin , cyclamic acid, and sucralose are artificial sweeteners with very limited metabolism in the human digestive system [3, 14, 27]. Therefore, they pass through the human body virtually unchanged, ending up in wastewaters. Artificial sweeteners were ubiquitously present in wastewater-contaminated surface waters. As very water-soluble and relatively persistent compounds they can also persist through conventional water treatment, thus ending up in drinking water.
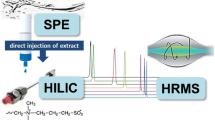
In recent years, several reviews were published in relation to presence of artificial sweeteners in foodstuffs [28] and as recognized class of emerging environmental contaminants [26, 29]. To avoid major overlap with previously published reviews, we focus our attention on the analytical strategies from the point of view of their presence in foods, pharmaceuticals, and environmental matrices published in the last years, with special emphasis on discussion of the different aspects about their determination. The main objective of this chapter is to present the advances in sample preparation methods used for liquid chromatography-mass spectrometry (LC-MS) analysis of artificial sweeteners in foodstuffs, environmental matrices, and pharmaceuticals. A schematic diagram of sample preparation steps and analytical techniques used in artificial sweeteners analysis is shown in Fig. 1.
2 Artificial Sweeteners Trace Analysis Techniques
Environmental samples and foods are complex mixtures of different components contained in varying amounts, making analysis a challenging task. As a consequence, the analysis of sweeteners is important in the evaluation of food safety [12, 30], including the potential detrimental effects on human health. Therefore, reliable analytical methods are needed to control the levels of sweetener in several foodstuff matrices, pharmaceuticals, and aquatic environment samples [28, 29]. Due to the complexity of the food-based or sewage matrices, sample preparation is a previous step before the final analysis. Solid phase extraction (SPE) has proven to be a useful and common sample preparation technique for the determination of artificial sweeteners. It is compatible with many chromatographic techniques, allowing preconcentration and clean-up of samples. Liquid chromatography is the most widely used technique for the determination, combined with mass or tandem mass spectrometry detection (MS or MS/MS).
2.1 Sample Preparation Strategies
The presence of sweeteners in foods, environment matrices, and pharmaceuticals at different concentrations levels led to the development of numerous analytical procedures for their determination. Sample preparation technique depends on the matrix and must include the compounds extraction, clean-up, and concentration steps. In simple matrices, as drinks, the dilution of the sample and direct analysis are suitable. In matrices more complex as food or environmental, extraction and additional sample clean-up to eliminate matrix constitutes is usually necessary to obtain appropriate liquid chromatographic separation and tandem MS detection. Solid phase extraction (SPE) is a well-established technique for the preconcentration and clean-up of target compounds from aqueous samples. The target analytes are transferred from the matrix to a solid sorbent to be retained by different mechanisms in the retention stage and after selecting the suitable solvent they are eluted from the sorbent in the elution step.
The applicability of various SPE sorbents for isolation of sweeteners was investigated by Zygler et al. [31] using several types of LC–MS compatible buffers. Furthermore, in SPE selective highly extractions when molecularly imprinted polymers are used as sorbents can be achieved [32]. Critical evaluation of the procedures described in this chapter involves the matrix type and the more appropriate sample preparation method for the corresponding instrumental technique.
2.1.1 Isolation and Preconcentration of Artificial Sweeteners from Beverages and Processed Foods
Sweeteners are commonly used in various types of beverages (soft drinks, wines, fruit beverages, fermented milk drinks) and processed food as candies, chewing gum, yogurt products, jams, pickles, canned fruits, dried fruits, various sauces, dehydrated soups, jellies, and bakery products [28] for maintaining characteristics of aliment low-calorie and food quality.
Generally, the beverages contain considerable amount of different sweeteners which have good solubility in water. The carbonated drinks are degassed in an ultrasonic bath , in order to remove the carbon dioxide gas that might be present in the beverage, and then the samples are simply diluted 50- to 100-fold with deionized water [33–35], Milli-Q water [36, 37], methanol: water (8:92, v/v) [38] or (1: 1 v/v) [39], mobile phase [40] or 500 times with 0.1% aqueous formic acid [41]. Instant products as nectars, juice, instant pudding, and sauces were solubilized in water using an ultrasonic bath for 15 min and centrifuged to separate solids [36] or add trichloroacetic acid for the precipitation of proteins, before the centrifugation and dilution [41].
In matrices more complex containing milk or solids, the sample was purified by SPE using an Oasis HLB cartridge, after dilution [42]. A variety of methods involving pre-treatment by solvent extraction with diethyl ether and methanol to remove oil components were described [43]. Shah et al. [44] used 0.075% formic acid adjusted to pH 4.5 with N,N-diisopropylethylamine to dissolve yogurt samples that were shaken using a digital Vortex for 30 min. The resultant supernatant was cleaned-up using a 3 mL C18 cartridge conditioned with MeOH and extraction buffer. The isolated analytes were eluted with methanol. A liquid-liquid extraction method combined with SPE was developed for the determination of neotame in nonalcoholic beverages [45]. The sample was extracted with formic acid/triethylamine/ultrapure water (4:125:5000 v/v/v) in an ultrasonic bath and the supernatant was loaded into C18-SPE cartridge and NEO was eluted with methanol. Zygler et al. [46] determined nine high-intensity sweeteners in a variety of food samples (i.e., beverages, dairy, and fish products). The samples were extracted using a buffer composed of formic acid and N,N-diisopropylethylamine at pH 4.5 in ultrasonic bath during 10 min. The obtained extracts were cleaned-up using Strata-X 33 μm Polymeric SPE column.
The isolation of sweeteners from beverages was also performed using microextraction methods. Hashemi et al. [47] used a headspace single-drop microextraction (HS-SDME) method to determine cyclamate by gas chromatography. The procedure is based on the reaction of cyclamate with nitrite in slight acidic medium and extraction of cyclohexene formed in a microdrop for direct injection into the gas chromatograph. Several extracting solvents such as toluene, benzene, propanol, 1-butanol, benzylalcohol, xylene, and n-dodecane were investigated for extraction of cyclohexene. The use of 2.5 μL of n-dodecane gave the best extraction efficiency, integrating sample clean-up, preconcentration, and sample introduction into one step.
Solid phase microextraction (SPME) was used for the selective isolation of acesulfame. Moein et al. [48] developed a sol–gel based molecularly imprinted polymer nanofiber by electro-spinning technique on the surface of a stainless steel bar. The fiber was applied for online selective SPME and determination of acesulfame coupled with HPLC.
2.1.2 Isolation and Preconcentration of Artificial Sweeteners from Environmental Matrices
Since the first reports documenting the high production, consumption, and widespread occurrence of artificial sweeteners in the aquatic environment, they are considered as priority emerging contaminants [49, 50]. Analytical methods currently used for the determination of SWTs in food and environmental matrices have been reported, and several critical reviews have recently been published [28, 29]. The following three topics were focused: (1) overview of analytical methods for trace analysis, (2) occurrence in the aquatic environment, and (3) advanced treatment processes for the removal of artificial sweeteners. In addition to these reviews, other reviews have appeared describing certain aspects related to the presence of some particular sweetener such as sucralose [51] detected in municipal effluents and surface waters in the United States and Europe, epidemiological studies concerning the use aspartame [52] as low-calorie sweetener, and stevia [53] highlighting its remarkable potential as an intense high-potency sweetener.
2.1.2.1 Environmental Water and Wastewater
The concentrations of SWTS in environmental water and wastewater were reported in the order of μg L−1 which requires a method detection limit (MDL) at the ng L−1 level [28, 29]. Several methods for the analysis of environmental contaminants based con LC-MS/MS were developed by direct analysis using large-volume injection (LVI) or concentration by SPE [54]. LVI is an analytical technique that is performed by the direct-injection of a large sample volume onto the liquid chromatographic column and only requires centrifugation or filtration of water samples. Wu et al. [55] integrated the large volume injection approach (500 μL injection) with ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) for quantification of acesulfame and sucralose, with MDL of 0.2 and 5 ng L−1, respectively. Similarly, Berset et al. [56] developed a DI-HPLC–MS/MS method using an injected volume of 100 μL for quantitative determination of acesulfame, cyclamate, saccharin, sucralose, neohesperidine, neotame, aspartame, and its metabolite diketopiperazine in wastewater effluents, surface waters, groundwater, and tap water samples.
SPE is widely accepted as extraction and clean-up method to isolate sweeteners from waste water and surface water. The typical SPE phases used were evaluated in different publications [26, 57–61]. Scheurer et al. [57] tested different cartridges : BakerbondTM styrol-divinylbenzene (SDB 1), Isolute® ENV+, C18, Bond Elut® PPL, StrataTM X, StrataTM X-AW and and Oasis® HLB, WAX, MAX, and MCX. This work concluded that the styrol-divinylbenzene phase SPE columns were the most effective for removing the matrix effect. For five of seven analytes under investigation, method recoveries >75% were obtained for tap water (50 mL), adjusting the sample at pH 3 prior to loading, with polymeric sorbent SDB 1 cartridges, which were eluted using methanol. Zygler et al. [26] also investigated the applicability of several commercially available C18-bonded silica, phenyl-bonded silica, and polymeric SPE sorbents for isolation of nine intense sweeteners in relation to the composition and pH value of buffers used. In this study, very high recoveries (better than 92%) for all studied compounds were obtained using formic acid–N,N-diisopropylethylamine buffer adjusted to pH 4.5 and C18-bonded silica sorbents. Other polymeric sorbents as Strata-X polymeric RP and Oasis HLB were found to be suitable for quantitative extraction of sweeteners from aqueous solutions. But, in this work real aqueous matrices were not evaluated. Three different approaches of polymeric SPE sorbents (reversed-phase, weak and strong anion-exchange mixed-mode) have been compared for the determination of six artificial sweeteners in environmental waters [58]. The reversed-phase sorbents (Oasis® HLB and StrataTM X) showed the best performance. Oasis®HLB provided recoveries between 73% and 112% and limits of quantification of 0.01–0.5 μg L−1 when river water and effluent and influent wastewater were tested. Oasis® HLB cartridges (500 mg) also were used to extract seven SWTs from influent waters, both secondary and tertiary effluent and river samples [59, 60]. The SPE conditions were optimized to enable the direct injection of the organic extract into the hydrophilic interaction liquid chromatography (HILIC) system. The samples were adjusted to pH 3, loaded, and the phase was subjected to a washing step with H2O (pH = 3) to remove salts and highly polar compounds. Finally, the analytes were eluted with a mixture of NH4OH:MeOH:ACN (1:4:15). Contrary, when ten commercial SPE cartridges were tested by Gan et al. [62], satisfactory recoveries (77–99%) for seven SWTs were obtained using a Poly-Sery PWAX cartridge with 25 mM sodium acetate solution (pH 4) as wash buffer and methanol containing 1 mM tris (hydroxymethyl) amino methane (TRIS) as elution solvent versus relative lower recoveries obtainer with Waters Oasis® WAX. The reason maybe the difference in the structure of the sorbents, CNW® Poly-Sery PWAX is packed with amino functionalized styrene/divinylbenzene copolymer, a packing different to Oasis® WAX. This method was successfully applied to the analysis of wastewater, tap water, surface water, and groundwater [61]. Artificial SWTs were simultaneous determined with 24 pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) by SPE [63]. Five cartridges were tested for simultaneous extraction of all target analytes. Good recoveries (≥70%) were observed for all compounds when extraction was performed using Chromabonds® HR-X (500 mg, 6 mL) cartridges under acidic condition (pH 2).
Numerous studies have demonstrated the presence of artificial sweeteners in domestic wastewater, surface water, and groundwater [28, 64–66]; therefore, sweeteners are considered as ideal indicators for the investigation of wastewater contamination in water supplies. Oasis®WAX was the phase selected to investigate the use of two artificial sweeteners, cyclamate and acesulfame, as an indicator set for contamination by wastewater within the rural catchment of a karst spring [67]. The combination of rather persistent acesulfame with more degradable cyclamate can provide valuable additional information on the origin and/or the age of contamination. Acesulfame, saccharin, and cyclamate were selected for Roy et al. [68], and they were detected in leachate or leachate-impacted groundwater being saccharin the dominant sweetener and cyclamate was detected less frequently. Also Oasis®WAX was selected to extract sucralose , acesulfame, saccharin, and cyclamate from boreal lakes and rivers [27] finding concentrations similar to previously reported in European surface waters.
Alternatively, SPE is a technique that has the possibility to operate in mode online coupling to liquid chromatography. An online SPE system was used for the determination of sucralose in reclaimed and drinking waters [69, 70]. 10.0 mL of sample was injected into a 10.0 mL loop and then loaded onto a SPE column (HyperSep Retain PEP) by the loading LC pump, followed by a wash step to remove interferences. The sucralosa was retained in the SPE column and the matrix that is not retained during the extraction process was directed to waste. After 5.3 min, when the valve was switched to Inject Position, the solvent flows through the SPE column to chromatographic system. The use of an orbitrap HRMS detector in combination with a fast and robust online SPE preconcentration methodology that does not require sample pretreatment is proposed as an alternative for ultra-trace quantitation of sucralose in environmental aqueous samples [71]. The online SPE column was a Hypersep Retain PEP® (20 mm × 3 mm, 1.2 μm) presenting high sensitivity and selectivity with method detection limits (MDL, 1.4 ng L−1) which are lower than any MDL reported in the literature.
2.1.2.2 Sludges
Sludge originates from the process of treatment of waste water. Due to the physical-chemical processes involved in the treatment, the sludge tends to concentrate heavy metals and poorly biodegradable trace organic compounds as well as potentially pathogenic organisms present in waste waters [72]. The Sewage Sludge Directive 86/278/EEC seeks to encourage the use of sewage sludge in agriculture and to regulate its use in such a way as to prevent harmful effects on soil, vegetation, animals, and man [73]. The artificial SWTs are trace organic which are present in sewage sludge as was reported for sucralose in the Swedish Screening Program 2007 [74, 75]. Sewage sludge samples were treated with diluted HCl, the suspension was centrifuged and the liquid phase collected to be processed by solid phase extraction on Oasis® HLB and the extract was cleaned by passing through a mixed-mode ion exchange SPE-cartridge (Isolute-MM). From the first data obtained, these studies concluded that sucralose was not significantly accumulated in sewage sludge. Pressurized liquid extraction (PLE) followed by liquid chromatography–tandem mass spectrometry was proposed for the simultaneous determination of sweeteners in sewage sludge [76, 77]. A procedure based on PLE using water followed by SPE was optimized for the determination of six artificial SWTs in sewage sludge [76]. The extraction cell was filled with the sample and sand and two cellulose filters placed on the bottom and the top. The extraction was carried out with aqueous formate buffer (pH 3.5) at 80 °C during a single static cycle of 21 min. Finally, the water extract was concentrated by SPE following a procedure for wastewater samples, previously described [58]. Acesulfame, cyclamate, saccharin, and sucralose were found in the samples at concentrations ranging from 17 to 628 ng g−1 dw. The PLE optimized conditions by Arbeláez et al. [77] were: MeOH:water (1:1 v/v) as the extraction solvent, 5 min preheating period, 40 °C, extraction pressure of 1500 psi, a static period of 5 min in one cycle, flush volume of 40% of the cell volume, and nitrogen purge time of 90 s. Due to the high matrix effect present in the sample, a clean-up with C18 in-cell was proposed and the extract obtained was subjected to another SPE with Oasis® HLB. The method was successfully applied and of the eight compounds, five were determined in all of the samples analyzed, with acesulfame and saccharin being recorded at the highest concentrations of up to 481 and 591 μg kg−1 (dw), respectively.
A study to evaluate the mass loadings, removal efficiencies, and environmental emission of sucralose, saccharin, aspartame, and acesulfame was based on the concentrations measured in wastewater influent, primary effluent, effluent, suspended particulate matter (SPM), and sludge collected from two wastewater treatment plants (WWTPs) in the Albany area of New York State [78]. The SPM and sludge samples were extracted with 6 mL of methanol/water mixture (5:3 v/v) using an ultrasonic bath. Extracts were centrifuged and purified by passage through Oasis®HLB cartridges. Aspartame and saccharin were significantly removed from wastewater treatment plants; however, sucralose and acesulfame were removed at <2.0%. This procedure to extract sludge samples was adapted for Subedi et al. [79] with the modification of the used cartridges, Sep-pak® Vac C18 to purify the extract. The media concentrations of SWTs in sludge from domestic WWTPD and mixed WWTPM (domestic plus industrial) were on the order of aspartame > saccharin > acesulfame > sucralose. These results were compared with those reported in the USA [78], which were sucralose > aspartame ≈ saccharin > acesulfame. The differences in the patterns of SWTs in sludge in the USA and Korea suggest differences in per-capita consumption of individual artificial SWTs and the removal in WWTP treatment processes. Soil samples and dust samples were extracted with 25 mL of Milli-Q water buffered at pH 4 [80] and then supernatant was concentrated using a SPE protocol as described above for water samples [62]. Saccharin, cyclamate, and acesulfame were the dominant artificial SWTs in both gas and particulate phase, with concentrations varying from 0.02 to 1940 pg m−3.
2.1.3 Isolation and Preconcentration of SWTs from Pharmaceuticals
Artificial sweeteners are used as excipients in the pharmaceutical industry in different pharmaceutical formulations [81]. They are added to chewable tablets and liquid preparations to mask the unpleasant taste of the medicament at concentrations regulated by FDA and by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) that attributed an Acceptable Daily Intake (ADI) for each SWT [82]. The development of analytical methods for the simultaneous quantification of active ingredient and excipients in pharmaceutical formulation is required for quality control purpose and also counterfeit drug control.
The presence of sweeteners and other substances in 73 pharmaceutical preparations of 35 medicines for oral administration were evaluated according to drug labeling information of the excipients [83]. No data were reported in relation to analytical methodology for sample dissolution. The artificial sweetener sodium saccharin was found in 38.3% of samples. Aspartame, cyclamate, acesulfame-K, and saccharin were determined in food products and pharmaceutical samples by CZE [84, 85] and diet supplements [86]. The liquid samples were appropriately diluted with water and the solid samples were weighed, solubilized, and diluted appropriately in water. The validated method was suitable to quantification of all analytes for the routine analysis.
Molecularly imprinted polymer (MIP) for aspartame has been prepared to selectively and specifically separate aspartame from aqueous solution and various pharmaceutical samples which contain aspartame as the major constituent [87]. The MIP was prepared using N-(2-ammonium-ethylpiperazinium) maleimidopropane sulfonate copolymer bearing zwitterionic centers along the backbone via a surface confined grafting procedure. The quantitative aspartame recovered from the sample was 88%.
2.2 Liquid Chromatography Mass-Spectrometry Methods for Quantification
There are analytical methodologies available to determine sweeteners in different matrices such as food, drinks, dietary products, drugs and sanitary products, and environmental samples. Analytical methods are faster, highly efficient, accurate, and sensitive for the identification and quantification of sweeteners in foodstuffs due to advances in the development of chromatographic instrumentation and mass spectrometric analyzers. High performance liquid chromatography (HPLC)–MS is a unique tool for reliable characterization of complex mixtures. Its excellent figures of merit are a consequence of the combination of the separative power of HPLC to the power of MS to identify molecular structure. HPLC is the most popular method using reversed phase (RP) columns based on C18 or C8 stationary phases [26]. Usually, the mobile phase combines water and (methanol and acetonitrile) using buffers such as ammonium acetate, acetic acid, or formic acid to search a compromise between chromatographic separation and electrospray ionization (ESI) sensitivity [26].
Chromatographic separations may be faster achieved by increasing the flow rate of the mobile phase, by decreasing the length of the column, reducing the stationary phase particle diameter, or increasing the temperature of analysis. The emergence of ultra high performance liquid chromatography (UHPLC) in 2004 (termed UPLC by Waters Corp., Milford, MA) makes possible to use columns packed with sub 2 μm particle size that allow good resolution and short analysis time [88]. Recent developments in special phases that promote polar compound retention are being evaluated, namely bridged ethylsiloxane/silica hybrid (BEH) C18 (1.7 μm) [42] and high strength silica (HSS) T3 C18 (1.8 μm) [27], both columns incorporated trifunctional ligand bonding chemistries on the particles, Kinetex® C18 (1.7 μm) based on core-shell silica support [38] and Hypersil GOLD™ C18 (1.9 μm) based on high purity silica technology with a proprietary bonding and endcapping procedure [36], were evaluated. The BEH chemistry utilizes new endcapping processes that ensure good peak shape for basic analytes. The HSS T3 C18 bonded phase was fabricated to retain and separate small water-soluble polar organic compounds. By nano-structuring technology, a durable, homogeneous porous shell is grown on a solid silica core to create a core-shell particle that produces less band broadening and high efficiencies compared to fully porous particles in RP separations. Hypersil GOLD columns provide outstanding peak symmetry.
The column temperature increase results in enhanced efficiency, mass transfer, and linear velocity [88]. However, high temperatures are not used routinely in the analysis of sweeteners. As an example, the analysis of sweeteners by LC-MS/MS in the range of 110–150 °C was proposed using a Shodex ETRP1 column (4 μm) [37].
Various traditional detectors are used in combination with liquid chromatography for sweeteners analysis but with complex matrices such as food and environmental samples, mass spectrometry has become the technique of choice in order to ensure selectivity and confirmation of target analytes. Several papers have demonstrated the utility of HPLC in combination with ultraviolet (UV) detection for acesulfame-K (ACS-K), aspartame (ASP), and saccharin (SAC) in commercial soft drinks [89] and mixtures of ASP and ACS-K in artificial sweeteners using partial least square (PLS-2) multivariate calibration [90]. Fast and high resolution LC methods were achieved by recent developments in UHPLC techniques with Diode Array Detector (DAD) and Rapid Resolution High Definition (RRHD) columns, such as the screening of food additives, including three sweeteners (ACS-K, ASP, and SAC) [91, 92]. Kailasam describes an application using a ZORBAX Eclipse Plus C18 (1.8 μm) column to separate nine additives in soft drinks and colas within a run time of 1 min even with 1 μL injection volume [91]. The separation of five additives in soft drinks and coffee sweeteners, described by Pedjie, was carried out using a Restek® Pinnacle® DB C18 (3 μm) column with a run time of 3.5 min and 4 μL injection volume [92]. Furthermore, the separation by UPLC-DAD of ACS-K, ASP, SAC, cyclamate (CYC), and neotame (NEO) from food was affected by the variables such as pH of the buffer solution, proportion of solvents in the mobile phase, flow rate, and column temperature during the optimization of chromatographic method [36]. For this reason, multivariate central composite design was used for the simultaneous optimization of 13 responses applying the Derringer and Suich desirability function. After optimization, the method was applied with good resolution (except for CYC) and low analysis time (11 min) for 25 samples from 9 food matrices (ready to drink tea, soft drink, nectar, juice, instant juice, instant pudding, jam, tomato sauce, and barbecue sauce). However, UV detection is not suitable for cyclamate (CYC) and sucralose (SCL) because they do not absorb in the UV/visible range due to a lack of chromophore group [28]. Another detector, which has been successfully combined with HPLC, was evaporative light scattering detector (ELSD). The eluent stream passes through a nebulizer into an evaporation chamber, where the solvent is evaporated to leave a mist of tiny sample particles. These scatter a light beam, and the extent of the light scattering is proportional to the amount of sample present. A simultaneous identification and quantification of nine sweeteners, i.e., ACS-K, ASP, CYC, SAC, sucralose (SCL), neohesperidine dihydrochalcone (NHDC), NEO, alitame (ALI), and dulcin (DUL), in beverages, canned or bottled fruits, and yogurts was performed by HPLC-ELSD [93]. ELSD can detect virtually any analyte without the need for chromophores or fluorophores but the analyte should be less volatile than the mobile phase. Separation of all sweeteners was obtained in less than 25 min using an aqueous buffer solution composed of formic acid and trimethylamine (pH = 4.5), with an injection volume of 8 μL.
All these detectors qualify substances based on retention time, and they are quantified based on the height or the area of the chromatographic signal. The resolution obtained is high, but the determination and precise quantification of substances can be difficult if multiple components elute at approximately the same time during simultaneous multianalyte analysis. However, LC-MS systems combine outstanding resolution liquid chromatography separation with outstanding capabilities qualitative mass spectrometry. Comparatively, mass spectrometry (MS) is a highly sensitive detection technique that ionizes sample components by various methods, separating the resulting ions in vacuum according to their mass-to-charge ratios and measures the intensity of each ion. The mass spectra obtained can indicate the level of concentration of ions that have a given mass, being very useful for qualitative analysis. When changing from single MS to tandem mass spectrometry (MS/MS), selectivity is greatly enhanced, and therefore improved detection and quantitation limits can be achieved.
It provides a wealth of structural information, and at the same time increases selectivity, which allows identification and quantitation of even co-eluting compounds. But the belief that the importance of chromatographic separation and the sample clean-up is less important because the mass spectrometry provides high selectivity and sensitivity is an error and should be considered during the validation process [94].
The most common soft ionization sources or atmospheric pressure ionization (API) are ESI and atmospheric pressure chemical ionization (APCI). In most of the published studies, LC-MS and LC-MS/MS methods are used for the determination of artificial sweeteners (Table 3). The instruments used were equipped with ESI source which uses electrical energy to assist the transfer of ions from solution into the gaseous phase before they are subjected to mass spectrometric analysis. Most of the studies used negative ESI mode for the mass-spectrometric detection [29]. Neutral and relatively nonpolar molecules, lacking a functional group capable of carrying charge, do not ionize well using ESI. The constant consumption of sample and the susceptibility to ion suppression effects are two important drawbacks that ESI presents. Some MS instruments are equipped with both ESI and APCI sources. The sensitivity of LC-MS analysis can be improved when APCI is used. APCI presents some advantages over ESI, in the ionization of thermally stable polar and nonpolar compounds and decreasing signal suppression for several compounds in different matrices. However, thermal decompositions of labile compounds in the heated nebulization or high demands on solvent purity are some of the weakness of APCI.
2.2.1 Methods Based on LC-MS
Single quadrupole (Q) mass analyzers were used in several applications for sweeteners analysis. A disadvantage of single quadrupole instruments is the high intensity of background signals produced from sample matrix. The matrix effect may either reduce (ionization suppression) or increase (ionization enhancement) the analyte signal. Different ways for ionization suppression/enhancement evaluation were reported due to the need to adequately compensate these effects and internal standard/matrix-matched/standard addition calibration methods were considered [95].
A HPLC/ESI-MS method was developed for the simultaneous determination of seven artificial sweeteners (ACS-K, ASP, CYC, SAC, SCL, NEO, ALI) and one natural sweetener, stevioside, in different food samples [96]. The compounds were quantified using selective ionization recording (SIR) or selected ion monitoring (SIM), where only one of the ions produced in the ion source is focused and detected. The cycle time for a given ratio m/z is very short and many cycles can be performed for a certain time. The m/z were 178, 397, 377, 293, 641, 312, 162, and 182 to CYC, SCL, NEO, ASP, stevioside (STV), ALI, ACS-K, and SAC, respectively, using warfarin sodium (SIR m/z 307) as an internal standard (IS). ESI was operated in negative ion mode to generate quasimolecular ions [M − H]−. The quantification was based on internal standard calibration to avoid matrix effect. It was observed, the ion suppression by the sample matrix increased significantly for the analytes eluted near the dead time of the column, implying that the strong polar components in the sample matrix intensively suppressed the ionization of the analytes. The limits of detection (LODs) were below 0.10 μg mL−1, whereas the limits of quantification (LOQs) were below 0.30 μg mL−1. The recoveries were in the range of 95.4–104.3%, with relative standard deviation of <10% for the analytes. Compared to the HPLC-ELSD method [94], the present method had a higher sensitivity and partial structural information.
Zygler et al. [46] proposed a HPLC/MS method for simultaneous determination of nine sweeteners, ACS-K, ASP, CYC, SAC, SCL, NHDC, NEO, ALI, and DUL, in food products (i.e., beverage, yogurt, fish product), including a clean-up step. Methyl derivative of cyclamic acid was used as IS. Quantification of all compounds was performed using SIM mode. ESI in negative ion mode was employed. The most intense signal is obtained from [M − H]− except for DUL which came from a formic acid–DUL adduct [DUL + HCOO−]−. ACS-K, SAC, and SCL presented nonlinear response in the considered concentration range. The LODs were below 0.25 μg mL−1, whereas the LOQs were below 2.5 μg mL−1. No matrix effect was observed and external calibration approach has been employed. The recoveries for all sweeteners were in the range of 84.2–106.7%, with relative standard deviation of <10%. Possibly, the decomposition of ASP and NEO dipeptides by proteolytic enzymes released from fish cells during homogenization of the sample, caused losses observed for these two sweeteners.
2.2.2 Methods Based on LC-MS/MS
Often more structured information is needed than has been generated by the ionization method used. This information can be obtained by coupling two analyzers separated by a collision cell (tandem mass spectrometers) [97]. Triple quadrupole (QqQ) are among the most common MS/MS systems operating as tandem in space analyzers. The first analyzer is used to select the compound of interest. Then, this ion goes to the collision cell normally pressurized with an inert gas such as argon. Fragmentation of this ion in the cell is produced by collision induced dissociation (CID). MS/MS acts as a mass filter to selectively monitor a specific molecular ion (selected reaction monitoring, SRM). For quantification, the acquisition of two states selected ion is performed. The first spectrometer is programmed to transmit the first ion of interest to the collision cell, and then one of the product ions is monitored after the second spectrometer. With the selection of a suitable internal standard, the mass spectrometer can be changed rapidly between the four ions (two precursor ions and two associated product ions) and the relative intensities of the product ions monitored. This technique is known as multiple reaction monitoring (MRM) and is able to achieve high levels of specificity. Analytical methodologies based on mass spectrometry for the determination of artificial sweeteners in environmental media were reviewed by Kokotou et al. [26]. Beam-type analyzers are used in tadem-in-space instruments, whereas trapping instruments are classified in tandem-in-time. In a QTrap system the third quadrupole can be switched between ion trap mode and quadrupole mode, so the instrument combines useful features of both triple quadrupole and ion trap analyzers. Ion trap mode allows to enhance sensitivity in product ion scanning and to induce additional fragmentation.
Most MS/MS systems are tandem-in-space instruments such as QqQ and quadrupole time-of-flight (QTOF). The third quadrupole of QqQ can be replaced by a TOF analyzer to produce a QTOF mass spectrometer. This system passes all ions in a pulse and separates them in time but does not scan, unlike QqQ. However, the advantage of the QTOF is the speed in which an MS/MS spectrum can be obtained. High-resolution mass spectrometry (HRMS) is based on full scan data giving the possibility of retrospective analysis of data based on an a posteriori hypothesis, unlike MS/MS. HRMS allows the elucidation of the elemental composition of analytes on the basis of accurate mass and isotopic standards. Recently, the increasing interest in the use of HRMS in the environmental field was observed using hybrid tandem mass instruments, for example, QTOF, QTrap, and the Orbitrap mass analyzer, which offer a combination of the characteristics of the two analyzers. The Orbitrap device consists of a small electrostatic device into which ion packets are injected at high energies to orbit around a central, spindle-shaped electrode. The image current of the axial motion of the ions is picked up by the detector and this signal is Fourier transformed to yield high resolution mass spectra.
2.2.2.1 Triple Quadrupole (QqQ) System
Recently, artificial sweeteners have been shown to play an important role as emerging contaminants in the aquatic environment, especially SCL. Many of the studies used QqQ MS and negative ESI mode and were summarized in a review [29]. One of them, organized by the European Commission’s Joint Research Centre (JRC) evaluated the occurrence of sucralose in European surface waters by LC–MS/MS using negative ESI and triple quadrupole [98]. The sucralose molecule shows the characteristic MRM transition [M − H]− → [M − H–Cl]− 395 → 359 under chlorine loss. To avoid strong ion suppression quantification was performed by isotope dilution with deuterated sucralose d6 internal standard. The recoveries obtained are not completely satisfactory varying from 62 ± 9% in tap water, ∼55% for the river water, to ∼26% for the wastewater. Since the sweeteners can be quite persistent in WWTPs, the suitability of ACS-K [64] or SCL [69] as chemical markers for domestic wastewater was investigated by HPLC–ESI-MS/MS. Quantification was made using peak area ratios relative to the internal standard and with standard addition to account for matrix effects [64]. SCL proved to be an indicator for the presence of conventional biologically treated municipal and domestic wastewater, septic system sources to water bodies [69], and finished drinking water [99] in the United States. Occurrence data for 85 trace organic compounds was examined using HPLC–ESI-MS/MS with negative and positive ESI modes and two based C18 columns. The sucralose ionization suppression could be largely corrected by dilution, using sucralose d6 isotope dilution, but the recovery for sucralose-d6 was greater than 50%. For this reason, gemfibrozil were used as IS and diluted tenfold before analysis to minimize matrix effects [69]. Minten et al. developed a LC-MS/MS method to assess the efficacy of treatment plants for removing SCL. A special MRM technique where the two quadrupoles monitor the same m/z was applied. The sodium adduct of SCL was used for quantification, because lower detection limits were obtained when it was compared to the sucralose quasi-molecular ion in negative ion mode [100].
APCI represents an alternative ionization source for species in LC analysis, such as SCL, that are difficult to ionize or tend to show low sensitivity in LC-MS/MS analysis. To this end, a (SPE)-LC-APCI/MS/MS was proposed and validated for the determination of SCL at low ng L−1 levels in drinking and reclaimed waters from South Florida, United States. [70]. APCI operated in the negative mode showed better sensitivity than ESI, where adducts ions with methanol and inorganic ions are found. The two SRM transitions monitored for SCL were 397 → 361 and 397 → 359 for quantitation and confirmation, respectively. For sucralose-d6 the transitions were 403 → 367 and 403 → 365 for quantitation and confirmation, respectively. Sucralose is frequently detected at significant levels in the aquatic environment, possibly due to its high resistance to photodegradation, minimum sorption, and high solubility. Experiments of the potential of sucralose photodegradation using multiple light sources and water matrices were tested. The findings are in agreement with previous results indicating that sucralose is a good tracer of anthropogenic pollution of waters [69, 99].
Many authors enhanced sweeteners analysis using stationary phases that reduce analysis time or alternatives to offset the matrix effect. Thus, the occurrence of four sweeteners in the aquatic environment from Switzerland was investigated by Buerge et al. [64] using a Gemini C18 (5 μm). ACS-K, CYC, SAC, SCL were determined in ∼17 min and Aspartame was not selected in this study because was assumed it is quickly biodegraded in WWTPs [64]. An increase in temperature can have beneficial effects. As the temperature increases, shorter run times can be achieved; the viscosity of the mobile phase decreased, resulting in lower pressures and lower mobile-phase viscosity also improves diffusion in the chromatographic system, giving narrower peaks. A development based on Zorbax Eclipse XDB-C8 (5 μm) at 40 °C with a flow rate of 0.8 mL min−1 was carried out for the separation of ACS-K, ASP, CYC, SAC, SCL, NHDC, and NEO [57]. However, no retention time data were provided. Also, Kubica et al. [40] presented a HPLC-ESI-MS/MS for the determination of ACS-K, ASP, CYC, SAC, SCL, ALI, NHDC, NEO and five common steviol glycosides in soft and alcoholic beverages. An Ascentis Express C18 column (2.7 μm) set at 40 °C allows the complete separation of analytes in 16 min. Sensitivity of ASP, ALI, and NEO was increased using positive ESI mode. Using considerable dilution of the analyzed samples, no matrix effects were observed. The demands to process hundreds of samples in a short period of time have resulted LC-MS/MS equipment using reverse phase columns with a particle size smaller. Different chromatographic columns were tested, i.e., Zorbax Eclipse XDB-C18 (1.8 and 5 μm), Kinetex C18 (2.6 μm), Ascentis Express C18 (2.7 μm), Ascentis Express HILIC (2.6 μm), and Ascentis Express RP-Amide (2.7 μm) in the determination of SAC, CYC, ASP-K, ACE, NHDC, SCL, STV, and GA in river water and wastewater [60]. The last column provided the best resolution for the eight sweeteners analyzed with a column temperature of 25 °C in less than 12 min. In another case where two types of chromatographic separation were compared, the reversed-phase provided better performance than hydrophilic interaction (HILIC) in sweeteners analysis of wastewater [58]. The MS/MS analysis showed that predominant precursor ions were [M − H]− when ESI− was applied, except for SCL and STV, since their adduct with Cl−[M + Cl35]−was the predominant precursor ion [60]. The matrix effect was evaluated with different clean-up solvents, elution sol-vents, sample volumes, and commercial SPE cartridges. Moreover, ion suppression could not be completely eliminated and two isotopically labeled standards (ASP-d3 and SCL-d6) as surrogates also eight-point matrix-matched calibration curves were used to the compensate the matrix effect and recoveries for each analyte.
The maximum permissible amount of sweeteners in food varies significantly due to their frequent synergistic use therein. Hence the analytical methodology, based on HPLC or UHPLC-MS/MS, has great interest for the control of food quality and application of the regulation. Based on green chromatography approach, high temperature liquid chromatography HTLC–MS/MS method which included a dual temperature and organic modifier gradient was used in the determination of nine sweeteners in drink samples [37]. Temperature in the range of 110–150 °C was applied, whereas the mobile phase is formed by water and percentages of ethanol varying from 3% to 20%. The separation was achieved in less than 20 min (23 min, including column re-equilibration). Also, the advantages of two different instrumental systems (PDA and MS/MS detection) using two different LC systems (UPLC and HPLC) were developed and compared for the control of the content of ACS-K, ASP, CYC, SAC, SCL, NEO, and NHDC in 66 beverage products available on the Spanish market from national and international industries [38]. A reduced analysis cost was obtained with UPLC-PDA that showed other advantages such as good resolution and high sensitivity in a run time of 3 min for five target sweeteners when a Kinetex C18 column (1.7 μm) was used. Also, no matrix effects are reported. LC-ESI-MS/MS method guaranteed the unequivocal determination of all the sweeteners in the same run and confirmed the results obtained by UPLC-PDA. Matrix matched with IS calibration curves were applied to correct matrix effect in MS/MS method. The validation parameters of UPLC-PDA method were successfully compared with other methods reported.
UPLC–MS/MS has demonstrated to be a powerful tool with the ability to transfer existing HPLC conditions directly provided fast chromatographic separations by increasing temperature of column such as in the investigation of the occurrence of ACS, SAC, CYC, and SCL in boreal rivers and lakes [27]. An Acquity UPLC HSS T3 (1.8 μm) set at 40 °C provided a separation in less than 2 min. The analytes were identified by retention times and MRM with specific transitions for each compound. SCL was quantified by means of a chlorine adduct precursor [M + Cl]−, m/z 433, which had a higher peak area than [M − H]− ion, m/z 395. Transition m/z 395 > 359 was used as a qualifier. Despite the matrix effect was corrected by the response of the mass-labeled surrogates in the IS calibration, low values of recoveries were obtained.
UHPLC-MS/MS using both negative and positive ESI modes was applied for the simultaneous analysis of ten compounds, namely ACS-K, ASP, CYC, SAC, SCL, NEO, GA, ALI, DUL, rebaudioside A (REB), and diketopiperazines (DKP) which is the main decomposition product of ASP, in various types of beverages sold in Japan [42]. All the compounds were ionized in negative ESI mode, except ALI and DUL, which were ionized in positive ESI mode. Several C18 columns were tested but ACQUITY UPLC BEH C18 column set at 40 °C showed better separation of all the components from highly polar ACS-K to weakly polar GA and excellent peak shapes in less than 7 min of elution time. The matrix effect was negligible because the samples were diluted 500 times. SCL showed the highest LOD and LOQ (0.56 and 1.9 μg mL−1, respectively). Multiple sweeteners (ACS-K, SCL and ASP) were detected simultaneously in two carbonated drinks. ACS-K and SCL were detected in two sport drinks. It is noted that four sweeteners (ACS-K, SCL, ASP, and REB) were added as sugar substituents to low-sugar and low-calorie products.
2.2.2.2 Hybrid Analyzers (QTrap and QTOF) and Orbitrap HRMS System
Several studies proposed analytical methodologies based on the use of QTrap. A development based on Zorbax Eclipse XDB-C8 (5 μm) at 40 °C with a flow rate of 0.8 mL min−1 was carried out for the separation of ACS-K, ASP, CYC, SAC, SCL, NHDC, and NEO [57]. However, no retention time data were provided. All sweeteners were quantified by external standard calibration, except sucralose, which was quantified by internal standard calibration (sucralose-d6) to correct matrix effect. The recoveries for six of the seven tested artificial sweeteners are lower due to ion suppression and not to losses during sample extraction. In order to increase the ionization yield 20 mM Tris (hydroxymethyl) amino methane was added post-column. This strong base facilitates deprotonation of the weakly acidic analytes. Furthermore, reliable information about sweeteners content in foodstuffs is a concern to both consumers and quality control agencies. In this regard, LC-MS/MS method was used for the simultaneous separation of ACS-K, ASP, CYC, DUL, NEO, NHDC, SAC, SCL STV, and glycyrrhizic acid (GA) on an analytical column, Luna Phenyl-Hexyl at 35 °C [34]. Various food matrices including 27 beverages (16 alcoholic and 11 nonalcoholic beverages) and 15 pickled foods (1 pickled pepper, 3 candies, and 11 candied fruits) were analyzed. Negative ESI mode was applied for all sweeteners except for DUL and NEO for which was used positive ESI mode. Matrix effect was corrected by matrix-matched calibration .
Studies of some sweeteners such as SCL or ACS in water used LC-MS/MS based on QTrap operating with negative electrospray ionization in MRM mode. For example, Mawhinney et al. quantified the amount of SCL present in select US drinking water systems [99]. Sucralose was quantified using the (M − H)− precursor ion at m/z 395.0 and the Cl product ion at m/z 35.0 and confirmed using the (M − H)− precursor ion at m/z 397.0 and the Cl− product ion at m/z 35.0. The sample integrity during long-term storage was performed on a set of sample extracts stored for 36–48 months at −80 °C. SCL showed to be a relatively inert and stable molecule, very soluble in MeOH, the storage solvent.
Another discussion was the fact that SCL has a complicated chlorine isotopic distribution and a difference in sensitivity about 400 times less than ACE. Therefore, different approaches were used with the aim of enhancing the SCL sensitivity. A study compared a LC-MS/MS with QqQ operating in MRM mode (in negative and positive ion mode) with an LC-QTOF-MS operating in MS mode (negative and positive ion mode) [101]. In the first system, SCL exhibited the precursor [M − H]− ion at m/z 395 using negative ESI mode. Under positive ESI, SCL ionized as the sodium adduct, at m/z 239 and m/z 221 MRM transitions, as expected from the results obtained in TOF analyses. In positive ion mode, SCL forms a strong sodium adduct that can be easily fragmented. In negative ion mode, the fragmentation is less favored, resulting in less sensitivity and selectivity when using MS-MS conditions. This is because they only include the loss and detection of a chlorine atom. LC-QTOF-MS provides high-resolution accurate mass determination. The most sensitive analytical methodology for the analysis of SCL in water samples was LC/MS-MS under positive mode of ionization providing low limits of detection of 15 ng L−1. This same problem was retaken by Wu et al. who used integrated large volume injection (500 μL) approach with UHPLC-MS/MS sensitive method to identify MRM transitions that can produce higher sensitivity signals for SCL [55]. First, the QTrap-MS analysis was at negative ESI MRM transition m/z 395–359 allowed to obtain sensitivity in the range of μg L−1. Using positive ESI, sodium adducts [M + Na]+ were adopted as the precursor ion but salt effects reduce ionization efficiency, and the sodium adducts of SCL do not provide reproducible signals for quantification [100]. For this reason, the study of Wu et al. delved about the chlorine adducts of SCL in negative ESI. The same was confirmed for SUC-d6. The source of Cl− for the formation of [M + Cl]− is probably due to the fragmentation of SCL in the ion source or the impurities in the SCL standards since no HCl is added in the mobile phase. Different precursor ions, i.e., [M − H]−, [M + Cl]−, and [M + HCOO]− were compared using the injection volume of 20 μL. The lower LOD of SCL was 0.22 μg L−1, for the ion transition of [M + HCOO]− m/z 441.0–395.0. Moreover, the LODs for ACE and SCL were reduced to 0.2 and 5 ng L−1, respectively, with the UHPLC-TOF-MS/MS method including an injection volume of 500 μL.
The orbitrap HRMS is an alternative that avoids detection by MS/MS but allows simultaneous quantitation of analytes and confirmation, through the use of high-to-power resolution mass spectrometry to detect the intact anion. This system combine very low maintenance costs (relative to other HRMS machines such as FT-ICRs which require cryogenic gases) with the high resolving power and virtually background-free detection associated with Fourier-transform mass spectrometers. Very high sensitivity and selectivity were obtained by adding a basic buffer to enhance negative-mode ionization of the complete SCL isotopic signature and its detection and quantitation at ultra-trace levels (LOD 1.4 ng L−1 and LOQ 5.7 ng L−1) lower than any limits reported before in the literature [71]. In a recent work, Wu et al. [55] recommended 0.1% formic acid as modifier for LC-MS/MS analysis of SCL. However, Batchu et al. [71] proposed the use of a basic mobile phase which enhances the deprotonation product minimizing the production of chloride and forming adducts that would decrease the yield of the analytical signal. The proposed LC-HRMS method was applied to a set of seawater and estuarine samples and WWTP.
2.3 Other Determination Techniques
The LC-MS and LC-MS/MS methods proved to be the best choice for environmental and food analysis of artificial sweeteners, as seen above, since low LODs can be achieved without the need of the time-consuming derivatization step. However, some chromatographic methods, which include derivatization, were used, as shown in the following developments and applications. Three chromatographic techniques were applied for the determination of SCL [102, 103] or CYC [104] using derivatization step. GC methods show high resolution, but they require derivatization steps prior to analysis. 70 μL of MSTFA–1% TMCS (N-methyl-N-trimethylsilytrifluoroacetamide w/1% trimethylchlorosilane) with 30 μL of pyridine was added after extraction of SCL and heated for 30 min at 70 °C, before GC with detection by ion trap mass spectrometer operated in scan mode from 60 to 650 m/z [102]. This study presented the first concentration data for SCL for North American coastal and open ocean waters. A method based on Gas Chromatography-Electron Capture Detector (GC-ECD) was developed, including the CYC conversion to N,N-dichlorocyclohexylamine with sodium hypochlorite for 5 min at room temperature [104]. The method presented several advantages such as the simplicity of the sample preparation steps, the high stability of the derivatization products, the high speed of the chromatographic separation, and the high selectivity of the detection. The LOD and LOQ for yellow wine and fruit juice were 0.05 and 0.2 mg L−1, respectively. The LOD and LOQ for cake and preserved fruit were 0.25 and 0.8 mg kg−1, respectively. A high-performance thin-layer chromatography (HPTLC) method was developed to analyze SCL in water [103]. Separation was performed in parallel on a HPTLC plate silica gel 60 F254 with a mixture of isopropyl acetate, MeOH, and water (15:3:1, v/v/v) within 15 min. Various postchromatographic derivatization reactions (with p-aminobenzoic, β-naphthol, and aniline diphenylamine o-phosphoric acid) were compared to selectively detect SCL in effluent and surface water matrices, due to the weak native UV absorption of SCL (≤200 nm). Thereby the last derivatization reagent was slightly preferred. The limit of quantification (LOQ) of SCL in drinking and surface water was 100 ng L−1 and the recovery was 80%. The comparison of the means obtained by HPTLC and the respective means of six laboratories, analyzed by HPLC–MS/MS or HPLC-TOF-MS with the use of mostly isotopically labeled standards, was not statistically significant.
The ecotoxicological impact of artificial sweeteners and its transformation products requires further research, as evidenced by the detection of SAC and CYC in leachate or leachate-impacted groundwater at levels comparable to those of untreated wastewater at 14 of 15 municipal landfill sites tested, including several closed for >50 years [68]. For this study Ion chromatography (IC) system coupled to QTrap-MS/MS operating in negative ESI mode was used. Two MRM transitions were monitored for both sweeteners.
Other spectroscopic methods were also described in the literature. Raman spectroscopy is a photonic high-resolution technique that provides, in few seconds, chemical and structural information. The Fourier Transform (FT) technique can detect all wavelengths at the same time improving the resolution, acquisition time spectrum, and signal-to-noise (S/R) ratio of conventional Raman spectroscopy. All this combined with chemometric tools, i.e., partial least squares (PLS), principal component regression (PCR), and counter-propagation artificial neural networks (CP-ANN) methods, offer the possibility of reliable quantification of ASP in commercial sweeteners [105]. ASP concentrations in the range of 17.46–35.93% were obtained in excipient formulations which contained, by weight, 55.8–76.9% lactose, 2.7–5.5% leucine, 2.9–4.7% CMCNa, and up to 0.6% magnesium stearate. Another spectroscopic technique used in quality control is based on Nuclear Magnetic Resonance (NMR) which is concerned with the magnetic properties of certain nuclei. High resolution 1H-NMR combined to principal component analysis (PCA) was used for the identification and the quantification of different soft drinks additives that included three sweeteners (ACS-K, CYC, and ASP) [106]. Moreover, the combination between NMR and chemometric data analysis was a suitable tool for cola drink authentication.
HPLC methods demand a large amount of organic solvents which is harmful to the operator. Additionally, both HPLC and GC require complicated and extensive sample preparation, which consumes time and effort. In this context, Capillary Electrophoresis (CE) is an analytical separation technique that generally offers shorter analysis time and relatively low consumption of consumables. Thus, several studies demonstrated the utility of CE for the analysis of artificial sweeteners. Capacitively coupled contactless conductivity detection (C4D) was applied for detection on CE of ASP, CYC, SAC, and ACS-K in commercial samples of soft drinks and tabletop sweetener formulations [35]. The pH of the separation buffer controls both the analyte charge and the level of electroosmotic flow (EOF). A relatively high pH value of 9.4 was necessary to use in order to render all sweeteners in the anionic charged form required for separation and detection. However, CE separation with EOF reversion demonstrated problems, especially with broadening of the SAC peak, due to interactions of the SAC with EOF inverters tested. Separations with good resolution can be obtained in less than 6 min. This analysis time is smaller than half of that required in the previous isotachophoresis method also using conductivity detection for determination of sweeteners in chewing gums and candies [107]. The LODs obtained with the CE-C4D method are lower (1.4–4.2 mg L−1) than those usually attained by CE with photometric detection. Several approaches were developed to enhance CE-C4D technique. The use of superimposed hydrodynamic pumping was found to be of great benefit in the determination of the same sweeteners in low calorie soft drinks, sweets, and a tabletop sweetener formulation by CE-C4D with analysis times of less than 1 min [108]. Band broadening was avoided by using capillaries of a narrow 10 μm internal diameter. The use of surface modification to eliminate or reverse the EOF was not necessary due to the superimposed bulk flow. The conditions for rapid separations not only led to higher limits of detection, but also a narrower dynamic range. On-line preconcentration such as field-amplified sample injection (FASI) was used to overcome the sensitivity CE-C4D limitations because it has enrichment factors of several 100-fold or higher [33]. The FASI-CE-C4D method demonstrated to be applicable for simultaneous determination of ACS-K, SAC, and CYC in beverages. The separation was achieved within 10 min using 20 mmol L−1 HAc (pH 3.3.) as running buffer. No EOF modifier is required to be added into the background electrolyte (BGE) so the problems of broadening of the analyte peaks are avoided.
On the other hand, a simple capillary zone electrophoresis (CZE) method with UV detection was developed for the determination of NEO in nonalcoholic beverage [45]. Both borate and phosphate were tested as the background electrolyte. The results showed that the borate buffer gave a better peak shape and shorter migration time (less than 5 min) than the phosphate buffer. Buffer pH 8 proved to be ideal on migration behavior. LOD was 0.118 μg mL−1. Excellent LODs (4.4 μg L−1 for ACS-K, 6.7 μg L−1 for SAC and 8.8 μg L−1 for CYC) were achieved. In most of the previous papers, separation time until 6–10 min was required to separate four sweeteners. A new sub-minute method based on CZE using indirect UV detection and short-end injection procedure (SEIP) was developed for the analysis of ASP, CYC, SAC, and ACE-K [86]. The BGE used in these measurements were buffered at pH around 9, to guarantee total ionization of the sweeteners in the anionic form, and ASP was also buffered at pH 1.4 to measure its mobility in the cationic form. The LOD and LOQ were lower than 6.5 and 21.5 mg L−1, respectively.
Finally, a modification of CE, micellar electrokinetic chromatographic (MEKC) method which is suitable for neutral and charged analytes to be separated in a single injection was able to separate 11 food additives simultaneously within 30 min [109]. Six sweeteners, i.e., ALI, ASP, SAC, NEO, STV, and ACS-K, were determined in beverage, yogurt, and candied fruit samples using MEKC. The separation buffer consisted of 20 mmol L−1 sodium tetraborate, 42 mmol L−1 boric acid (pH 8.83), and 100 mmol L−1 sodium deoxycholate. The detection wavelength was 214 nm and the LODs were in the ranges of 0.25–2.5 mg L−1. To demonstrate the accuracy of the proposed MEKC method, a FAPAS® proficiency test sample containing caffeine and SAC was successfully analyzed.
3 Conclusions and Futures Perspectives
This chapter has focused on the analytical strategies for the determination of artificial sweeteners. Monitoring foodstuffs for additives is an area of increasing concern and importance. First, depending the selected matrix, different strategies for sample preparation have been considered. Many of the methods of liquid environmental matrices are based on the SPE and microextraction techniques coupled with powerful analytical techniques (LC-MS and LC-MS/MS) which have obtained lower limits of detection and high selectivity in their determination. The trends in sample preparation include miniaturization, automation, high throughput performance, online coupling with analytical instruments, and low-cost operation using little or no solvent consumption [110]. Last years, high selective materials as MIPs have become an alternative to existing commercial sorbents for SPE. The investigation of smart materials [111] based on carbon nanotubes, nano inorganic oxides, ionic liquids, and MIP sol-gels will be explored in extraction and microextraction techniques (SPME and MEPS) of artificial sweeteners from complex matrices. To date, there are very few studies related to the presence of artificial sweeteners in sludge and sample preparation methods for their extraction. Further investigation is required to identify and quantify these compounds and the degradation products formed in wastewater and sludge.
After a detailed overview of the literature on the use of LC-MS methods in foodstuffs and environmental field, it seems clear that tandem MS hyphenated to UHPLC is still the reference technique for determination of sweeteners in complex matrices, mainly because of the resolution and sensitivity attainable by use of these instruments working in MRM mode. Often identification and quantification are both required. Most current LC based methods rely on QqQ-MS/MS. LC-MS/MS data on precursor and product ions under negative and positive ionization mode are often used. ASP, SAC, and SCL can be determined (individually or as sodium adducts) in both ionization modes, although higher sensitivity and more fragments were obtained only through the positive ionization mode. However, the only possible way to achieve simultaneous determination of artificial sweeteners by LC-MS and LC-MS/MS techniques is by running analysis under negative ESI. Throughout literature a large majority of methods referred to the determination of SCL but an advantage of LC-MS/MS is the capacity to the simultaneous analysis of a wide variety of sweeteners within a single analytical run with minimal incremental cost. This has the potential to simplify laboratory set and provide additional useful information (e.g., metabolite profiles). The internal standardization method was mainly used in quantification due to the need to adequately compensate the matrix effects. QTrap and TOF mass analyzers have enabled popularization of HRMS coupled to LC, leading to new perspectives in food quality control and regulation enforcement of these compounds considered as environmental pollutants. LC-HRMS provide significant advantage in the possibility of discrimination of ions due to high mass resolving power; however, the main drawback of LC-HRMS methods is in quantification. On the other hand, different stationary phases [88], some of them based on stimuli-responsive polymers, can offer advantages for chromatographic development related to higher specificity for retention and sequential elution of artificial sweeteners at cost-effective prices. These polymers can be integrated in the stationary phase in the form of cross-linked networks or grafted on to solid beads or inert surfaces.
In summary, the application of extraction approaches using specific sorbents, green solvents, and miniaturized analytical systems reducing labor consumption will contribute to the development of environment-friendly analytical techniques to determination of trace emerging pollutants such as artificial sweeteners. Furthermore, the advantages of chromatographic methodologies, i.e., the use of monolithic columns, the use of column temperature, and the use of sub-2 μm particle size column or fused-core column technologies, can be good alternatives for high-efficiency and fast LC separations.
Abbreviations
- ACS-K:
-
Acesulfame
- ADA:
-
American Diabetes Association
- ADI:
-
Acceptable daily intake
- AHA:
-
American Heart Association
- ALI:
-
Alitame
- ANSES:
-
Agency for Food Environmental and Occupational Health & Safety
- APCI:
-
Atmospheric pressure chemical ionization
- API:
-
Atmospheric pressure ionization
- ASP:
-
Aspartame
- C18:
-
Octadecylsilane
- CE:
-
Capillary electrophoresis
- CFR:
-
Code of Federal Regulations
- CID:
-
Collision induced dissociation
- CP-ANN:
-
Counter-propagation artificial neural networks
- CYC:
-
Cyclamate
- DAD:
-
Diode array detector
- DKP:
-
Diketopiperazine
- DUL:
-
Dulcin
- EDCs:
-
Endocrine disrupting chemicals
- EFSA:
-
European Food Safety Authority
- ELSD:
-
Evaporative light scattering detector
- EOF:
-
Electroosmotic flow
- ESI:
-
Electrospray ionization
- FAO:
-
Food and Agriculture Organization of the United Nations
- FASI:
-
Field-amplified sample injection
- FT:
-
Fourier Transform
- GA:
-
Glycyrrhizic acid
- GC-ECD:
-
Gas chromatography-electron capture detector
- GRAS:
-
Generally recognized as safe
- HILIC:
-
Hydrophilic interaction liquid chromatography
- HPTLC:
-
High-performance thin-layer chromatography
- HRMS:
-
High-resolution mass spectrometry
- HS-SDME:
-
Headspace single-drop microextraction
- IC:
-
Ion chromatography
- ISA:
-
International Society of Automation
- JECFA:
-
Joint Expert Committee on Food Additives
- JRC:
-
Joint Research Centre
- LODs:
-
Limits of detection
- LOQs:
-
Limits of quantification
- LVI:
-
Large-volume injection
- MDL:
-
Method detection limit
- MEKC:
-
Micellar electrokinetic chromatographic
- MeOH:
-
Methanol
- MIP:
-
Molecularly imprinted polymer
- MRM:
-
Multiple reaction monitoring
- MSTFA:
-
N-methyl-N-trimethylsilytrifluoroacetamide
- NEO:
-
Neotame
- NHDC:
-
Neohesperidin dihydrochalcone
- NMR:
-
Nuclear magnetic resonance
- PCR:
-
Principal component regression
- PLE:
-
Pressurized liquid extraction
- PLS:
-
Partial least squares
- PPCPs:
-
Pharmaceuticals and personal care products
- Q:
-
Single quadrupole
- QqQ:
-
Triple quadrupole
- QTOF:
-
Quadrupole time-of-flight
- RP:
-
Reversed phase
- RRHD:
-
Rapid resolution high definition
- SAC:
-
Saccharin
- SGFE:
-
Siraitia grosvenorii Swingle fruit extract
- SIM:
-
Selected ion monitoring
- SIR:
-
Selective ionization recording
- SPE:
-
Solid phase extraction
- SPM:
-
Suspended particulate matter
- SPME:
-
Solid phase microextraction
- STV:
-
Stevioside
- SUC:
-
Sucralose
- SWTs:
-
Sweeteners
- TMCS:
-
Trimethylchlorosilane
- TRIS:
-
Tris (hydroxymethyl) amino methane
- UHPLC-MS/MS:
-
Ultra-high performance liquid chromatography-tandem mass spectrometry
- WHO:
-
World Health Organization
- WWTPs:
-
Wastewater treatment plants
References
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off J Eur Union L 354:16–33
US FDA (United States Food and Drug Administration) (2014) http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397716.htm. Accessed 22 Jan 2016
ISA (International Sweeteners Association) (2014) Low calories sweeteners and you. http://www.sweeteners.org/en. Accessed 25 Jan 2016
Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off J Eur Union L 295:1–177
Commission Regulation (EU) No 1131/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with regard to steviol glycosides. Off J Eur Union L 295:205–211
Commission Regulation (EU) No 497/2014 of 14 May 2014 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as regards the use of advantame as a sweetener text with EEA relevance. Off J Eur Communities 143:6–13
EFSA (European Food SafetyAuthority) (2014) http://www.efsa.europa.eu/en/topics/topic/sweeteners. Accessed 22 Jan 2016
FDA (Food and Drug Administration) http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm397725.htm. Accessed 22 Jan 2016
Grembecka M (2015) Natural sweeteners in a human diet. Rocz Panstw Zakl Hig 66:195–202
EFSA (European Food Safety Authority) (2013) Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA J 11:3301–3369. doi:10.2903/j.efsa.2013.3301
EFSA (European Food Safety Authority) (2013) Scientific opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J 11:3496–3759. doi:10.2903/j.efsa.2013.3496
European Commission (2006) Directive 2006/52/EC of European Parliament and Council of 5 July 2006 amending Directive 95/2/EC on food additives other than colours and sweeteners and Directive 94/35/EC on sweeteners for use in foodstuffs. Off J Eur Union L 204:10–22. Brussels
Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re-evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. Off J Eur Communities 80:19–27
Spillane WJ (2006) Optimising sweet taste in food. CRC Press, Boca Raton
Kaneko R, Kitabatake N (2001) Sweetness of sweet protein thaumatin is more thermoresistant under acid conditions than under neutral or alkaline conditions. Biosci Biotech Bioch 65:409–413. doi:10.1271/bbb.65.409
Bellisle F (2015) Intense sweeteners, appetite for the sweeteners taste, and relationship to weight management. Curr Obes Rep 4:106–110. doi:10.1007/s13679-014-0133-08
Mattes RD, Popkin BM (2009) Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr 89:1–14. doi:10.3945/ajcn.2008.26792
Ficth C, Keim KS (2012) Position of the Academy of Nutrition and Dietetic: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 112:739–758
AHA (American Heart Association), ADA (American Diabetes Association) (2012) Nonnutritive sweeteners: current use and health perspectives: a scientific statement. Circulation 126:509–519. doi:10.1161/CIR0b013e31825c42ee
Ain Q, Khan SA (2015) Artificial sweeteners: safe or unsafe? Recent Adv Endocr 65:225–227
ANSES (French Agency for Food, Environmental and Occupational Health & Safety) (2015) OPINION of the French Agency for Food, Environmental and Occupational Health & Safety of 19 November 2014, revised on 9 January 2015 on the assessment of the nutritional benefits and risks related to intense sweeteners. www.anses.fr. ANSES Opinion Request no. 2011-SA-0161
EFSA (2011) Scientific opinion on the substantiation of health claims related to intense sweeteners and contribution to the maintenance or achievement of a normal body weight (ID 1136, 1444, 4299), reduction of post-prandial glycaemic responses (ID 4298), maintenance of normal blood glucose concentrations (ID 1221, 4298), and maintenance of tooth mineralisation by decreasing tooth demineralisation (ID 1134, 1167, 1283) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J 9:2229–2255
Pepino MY (2015) Metabolic effects of non-nutritive sweeteners. Physiol Behav 152:450–455. doi:10.1016/j.physbeh.2015.06.024
Swithers SE (2015) Artificial sweeteners are not the answer to childhood obesity. Appetite 93:85–90. doi:10.1016/j.appet.2015.03.027
Shankar P, Ahuja S, Sriram K (2013) Non-nutritive sweeteners: review and update. Nutrition 29:1293–1299. doi:10.1016/j.nut.2013.03.024
Kokotou MG, Asimakopoulos AG, Thomaidis NS (2012) Artificial sweeteners as emerging pollutants in the environment: analytical methodologies and environmental impact. Anal Methods 4:3057–3070. doi:10.1039/c2ay05950a
Perkola N, Sainio P (2014) Quantification of four artificial sweeteners in Finnish surface waters with isotope-dilution mass spectrometry. Environ Pollut 184:391–396. doi:10.1016/j.envpol.2013.09.017
Zygler A, Wasik A, Namieśnik J (2009) Analytical methodologies for determination of artificial sweeteners in foodstuffs. Trends Anal Chem 28:1082–1102. doi:10.1016/j.trac.2009.06.008
Lange FT, Scheurer M, Brauch HJ (2012) Artificial sweeteners a recently recognized class of emerging environmental contaminants: a review. Anal Bioanal Chem 403:2503–2518. doi:10.1007/s00216-012-5892-z
GRAS (Generally Recognized as Safe) (2013) Code of Federal Regulations Title 21, Parts 172 and 189. Food and Drug Administration (FDA). Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172 http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=189. Accessed 20 Mar 2016
Zygler A, Wasik A, Namiésnik J (2010) Retention behaviour of some high-intensity sweeteners on different SPE sorbents. Talanta 82:1742–1748. doi:10.1016/j.talanta.2010.07.070
Carro-Díaz AM, Lorenzo-Ferreira RA (2013) Molecularly imprinted polymers for sample preparation. In: Alvarez-Lorenzo C, Concheiro A (eds) Handbook of molecularly imprinted polymers. Smithers Rapra Technology, Shropshire. ISBN 978-1-84735-959-9
Yang L, Zhou S, Xiao Y, Tang Y, Xie T (2015) Sensitive simultaneous determination of three sulfanilamide artificial sweeters by capillary electrophoresis with on-line preconcentrationand contactless conductivity detection. Food Chem 188:446–451. doi:10.1016/j.foodchem.2015.04.060
Chang CS, Yeh TS (2014) Detection of 10 sweeteners in various foods by liquid chromatography/tandem mass spectrometry. J Food Drug Anal 22:318–328. doi:10.1016/j.jfda.2014.01.024
Bergamo AB, Fracassi da Silva JA, Pereira de Jesus D (2011) Simultaneous determination of aspartame, cyclamate, saccharin and acesulfame-Kin soft drinks and tabletop sweetener formulations by capillary electrophoresis with capacitively coupled contactless conductivity detection. Food Chem 124:1714–1717. doi:10.1016/j.foodchem.2010.07.107
Botelho Dias C, Dillenburg Meinhart A, Queiroz Pane D, Augusto Ballus C, Teixeira Godoy H (2015) Multivariate optimisation and validation of a method for the separation of five artificial sweeteners by UPLC-DAD in nine food matrices. Food Anal Methods 8:1824–1835. doi:10.1007/s12161-014-0056-8
Ordoñez EY, Rodil R, Quintana JB, Cela R (2015) Determination of artificial sweeteners in beverages with green mobile phases and high temperature liquid chromatography–tandem mass spectrometry. Food Chem 169:162–168. doi:10.1016/j.foodchem.2014.07.132
Lorenzo RA, Pena MT, Fernández P, González P, Carro AM (2015) Artificial sweeteners in beverages by ultra performance liquid chromatography with photodiode array and liquid chromatography tandem mass spectrometry. Food Control 47:43–52. doi:10.1016/j.foodcont.2014.06.035
Ma K, Li X-J, Wang H-F, Zhao M (2015) Rapid and sensitive method for the determination of eight food additives in red wine by ultra-performance liquid chromatography tandem mass spectrometry. Food Anal Methods 8:203–212. doi:10.1007/s12161-014-9893-8
Kubica P, Namieśnik J, Wasik A (2015) Determination of eight artificial sweeteners and common Stevia rebaudiana glycosides in non-alcoholic and alcoholic beverages by reversed-phase liquid chromatography coupled with tandem mass spectrometry. Anal Bioanal Chem 407:1505–1512. doi:10.1007/s00216-014-8355-x
de Queiroz Pane D, Botelho Dias C, Dillenburg Meinhart A, Augusto Ballus C, Teixeira Godoy H (2015) Evaluation of the sweetener content in diet/light/zero foods and drinks by HPLC-DAD. J Food Sci Technol 52:6900–6913. doi:10.1007/s13197-015-1816-1
Sakai H, Yamashita A, Tamura M, Uyama A, Mochizuki N (2015) Simultaneous determination of sweeteners in beverages by LC-MS/MS. Food Addit Contam Part A 32:808–816. doi:10.1080/19440049.2015.1018341
Ohtsuki T, Sato K, Abe Y, Sugimoto N, Akiyama H (2015) Quantification of acesulfame potassium in processed foods by quantitative 1H NMR. Talanta 131:712–718. doi:10.1016/j.talanta.2014.08.002
Shah R, Farris S, De Jager LS, Begley TH (2015) A novel method for the simultaneous determination of 14 sweeteners of regulatory interest using UHPLC-MS/MS. Food Addit Contam Part A 32:141–151. doi:10.1080/19440049.2014.994111
Hu F, Xu L, Luan F, Liu H, Gao Y (2013) Determination of neotame in non-alcoholic beverage by capillary zone electrophoresis. J Sci Food Agric 93:3334–3338. doi:10.1002/jsfa.6181
Zygler A, Wasik A, Kot-Wasik A, Namieśnik J (2011) Determination of nine high-intensity sweeteners in various foods by high-performance liquid chromatography with mass spectrometric detection. Anal Bioanal Chem 400:2159–2172. doi:10.1007/s00216-011-4937-z
Hashemi M, Habibi A, Jahanshahi N (2011) Determination of cyclamate in artificial sweeteners and beverages using headspace single-drop microextraction and gas chromatography flame-ionisation detection. Food Chem 124:1258–1263. doi:10.1016/j.foodchem.2010.07.057
Moein MM, Javanbakht M, Karimi M, Akbari-adergani B (2015) Molecularly imprinted sol–gel nanofibers based solid phase microextraction coupled on-line with high performance liquid chromatography for selective determination of acesulfame. Talanta 134:340–347. doi:10.1016/j.talanta.2014.11.011
Richardson SD (2010) Environmental mass spectrometry: emerging contaminants and current issues. Anal Chem 82:4742–4774. doi:10.1021/ac101102d
Richardson SD, Ternes TA (2014) Water analysis: emerging contaminants and current issues. Anal Chem 86:2813–2848. doi:10.1021/ac500508t
Tollefsen KE, Nizzetto L, Huggett DB (2012) Presence, fate and effects of the intense sweetener sucralose in the aquatic environment. Sci Total Environ 438:510–516. doi:10.1016/j.scitotenv.2012.08.060
Marinovich M, Galli CL, Bosetti C, Gallus S, La Vecchia C (2013) Aspartame, low-calorie sweeteners and disease: regulatory safety and epidemiological issues. Food Chem Toxicol 60:109–115. doi:10.1016/j.fct.2013.07.040
Lemus-Mondaca R, Vega-Gálvez A, Zura-Bravo L, Ah-Hen K (2012) Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects. Food Chem 132:1121–1132. doi:10.1016/j.foodchem.2011.11.140
Backe WJ, FieldIs JA (2012) SPE necessary for environmental analysis? A quantitative comparison of matrix effects from large-volume injection and solid-phase extraction based methods. Environ Sci Technol 46:6750–6758. doi:10.1021/es300235z
Wu M, Qian Y, Boyd JM, Hrudey SE, Le XC, Li XF (2014) Direct large volume injection ultra-high performance liquid chromatography-tandem mass spectrometry determination of artificial sweeteners sucralose and acesulfame in well water. J Chromatogr A 1359:156–161. doi:10.1016/j.chroma.2014.07.035
Berset JD, Ochsenbein N (2012) Stability considerations of aspartame in the direct analysis of artificial sweeteners in water samples using high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). Chemosphere 88:563–569. doi:10.1016/j.chemosphere.2012.03.030
Scheurer M, Brauch HJ, Lange FT (2009) Analysis and occurrence of seven artificial sweetenersnin German waste water and surface water and in soil aquifer treatment (SAT). Anal Bioanal Chem 394:1585–1594. doi:10.1007/s00216-009-2881-y
Ordoñez EY, Quintana JB, Rodil R, Cela R (2012) Determination of artificial sweeteners in water samples by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1256:197–205. doi:10.1016/j.chroma.2012.07.073
Salas D, Borrull F, Fontanals N, Marcé RM (2015) Hydrophilic interaction liquid chromatography coupled to high-resolution mass spectrometry to determine artificial sweeteners in environmental waters. Anal Bioanal Chem 407:4277–4285. doi:10.1007/s00216-014-8330-6
Arbeláez P, Borrull F, Pocurull E, Marcé RM (2015) Determination of high-intensity sweeteners in river water and wastewater by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1393:106–114. doi:10.1016/j.chroma.2015.03.035
Gan Z, Sun H, Feng B, Wang R, Zhang Y (2013) Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Res 47:4928–4937. doi:10.1016/j.watres.2013.05.038
Gan Z, Sun H, Wang R, Feng B (2013) A novel solid-phase extraction for the concentration of sweeteners in water and analysis by ion-pair liquid chromatography–triple quadrupole mass spectrometry. J Chromatogr A 1274:87–96. doi:10.1016/j.chroma.2012.11.081
Tran NH, Hu J, Ong SL (2013) Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC–MS/MS and isotope dilution. Talanta 113:82–92. doi:10.1016/j.talanta.2013.03.072
Buerge IJ, Buser HR, Kahle M, Muller MD, Poiger T (2009) Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environ Sci Technol 43:4381–4385. doi:10.1021/es900126x
Van Stempvoort RD, Roy JW, Brown SJ, Bickerton G (2011) Artificial sweeteners as potential tracers in groundwater in urban environments. J Hydrol 401:126–133. doi:10.1016/j.jhydrol.2011.02.013
Hillebrand O, Nödler K, Sauter M, Licha T (2015) Multitracer experiment to evaluate the attenuation of selected organic micropollutants in a karst aquifer. Sci Total Environ 506–507:338–343. doi:10.1016/j.scitotenv.2014.10.102
Zirlewagen J, Licha T, Schiperski F, Nödler K, Scheytt T (2016) Use of two artificial sweeteners, cyclamate and acesulfame, to identify and quantify wastewater contributions in a karst spring. Sci Total Environ 547:356–365. doi:10.1016/j.scitotenv.2015.12.112
Roy JW, Van Stempvoort DR, Bickerton G (2014) Artificial sweeteners as potential tracers of municipal landfill leachate. Environ Pollut 184:89–93. doi:10.1016/j.envpol.2013.08.021
Oppenheimer J, Eaton A, Badruzzaman M, Haghani AW, Jacangelo JG (2011) Occurrence and suitability of sucralose as an indicator compound of wastewater loading to surface waters in urbanized regions. Water Res 45:4019–4027. doi:10.1016/j.watres.2011.05.014
Batchu SR, Quinete N, Panditi VR, Gardinali PR (2013) Online solid phase extraction liquid chromatography tandem mass spectrometry (SPE-LC-MS/MS) method for the determination of sucralose in reclaimed and drinking waters and its photo degradation in natural waters from South Florida. Chem Cent J 7:141–149. doi:10.1186/1752-153X-7-141
Batchu SR, Ramirez CE, Gardinali PR (2015) Rapid ultra-trace analysis of sucralose in multiple-origin aqueous samples by online solid-phase extraction coupled to high-resolution mass spectrometry. Anal Bioanal Chem 407:3717–3725. doi:10.1007/s00216-015-8593-6
http://ec.europa.eu/environment/waste/sludge/index.htm. Accessed 15 Mar 2016
Council Directive (1986) Council Directive of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off J Eur Communities 181:0006–0012
Brorström-Lundén E, Svenson A, Viktor T, Woldegiorgis A, Remberger M, Kaj L, Dye C, Bjerke A, Schlabach M (2008) Measurements of sucralose in the Swedish Screening Program 2007. PART I. Sucralose in surface waters and STP samples. Swedish Environmental Research Institute, Stockholm
Brorström-Lundén E, Svenson A, Viktor T, Woldegiorgis A, Remberger M, Kaj L, Dye C, Bjerke A, Schlabach M (2008) Measurements of sucralose in the Swedish Screening Program 2007. PART II. Sucralose in biota samples and regional STP samples. Swedish Environmental Research Institute, Stockholm
Ordoñez EY, Quintana JB, Rodil R, Cela R (2013) Determination of artificial sweeteners in sewage sludge samples using pressurised liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1320:10–16. doi:10.1016/j.chroma.2013.10.049
Arbeláez P, Borrull F, Marcé RM, Pocurull E (2015) Trace-level determination of sweeteners in sewage sludge using selective pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1408:15–21. doi:10.1016/j.chroma.2015.07.001
Subedi B, Kannan K (2014) Fate of artificial sweeteners in wastewater treatment plants in New York State, U.S.A. Environ Sci Technol 48:13668–13674. doi:10.1021/es504769c
Subedi B, Lee S, Moon H-B, Kannan K (2014) Emission of artificial sweeteners, select pharmaceuticals, and personal care products through sewage sludge from wastewater treatment plants in Korea. Environ Int 68:33–40. doi:10.1016/j.envint.2014.03.006
Gan Z, Sun H, Yao Y, Zhao Y, Li Y, Zhang Y, Hu H, Wang R (2014) Distribution of artificial sweeteners in dust and soil in China and their seasonal variations in the environment of Tianjin. Sci Total Environ 488–489:168–175. doi:10.1016/j.scitotenv.2014.04.084
Rowe RC, Sheskey PJ, Cook WG, Fenton ME (2012) Handbook of pharmaceutical excipients, 7th edn. Pharmaceutical Press/American Pharmacists Association, Philadelphia. ISBN 978 0 85711 027 5
Pifferi G, Restani P (2003) The safety of pharmaceutical excipients. Il Farmaco 58:541–550. doi:10.1016/S0014-827X(03)00079-X
Balbani APS, Stelzer LB, Montovani JC (2006) Pharmaceutical excipients and the information on drug labels. Rev Bras Otorrinolaringol 72:400–406
Corrêa de Carvalho R, Pereira Netto AD, Ferreira de Carvalho Marques F (2014) Simultaneous determination of strontium ranelate and aspartame in pharmaceutical formulation for the treatment of postmenopausal osteoporosis by capillary zone electrophoresis. Microchem J 117:214–219. doi:10.1016/j.microc.2014.06.024
Pereira Vistuba J, Danielli Dolzana M, Vitali L, Leal de Oliveira MA, Micke GA (2015) Sub-minute method for simultaneous determination of aspartame, cyclamate, acesulfame-K and saccharin in food and pharmaceutical samples by capillary zone electrophoresis. J Chromatogr A 1396:148–152. doi:10.1016/j.chroma.2015.03.070
Grembecka M, Szefer P (2012) Simultaneous determination of caffeine and aspartame in diet supplements and non-alcoholic beverages using liquid-chromatography coupled to corona CAD and UV-DAD detectors. Food Anal Methods 5:1010–1017. doi:10.1007/s12161-011-9334-x
Singh M, Kumar A, Tarannum N (2013) Water-compatible ‘aspartame’-imprinted polymer grafted on silica surface for selective recognition in aqueous solution. Anal Bioanal Chem 405:4245–4252. doi:10.1007/s00216-013-6812-6
Núñez O, Gallart-Ayala H, Martins CPB, Lucci P (2012) New trends in fast liquid chromatography for food and environmental analysis. J Chromatogr A 1228:298–323. doi:10.1016/j.chroma.2011.10.091
Dossi N, Toniolo R, Susmel S, Pizzariello A, Bontempelli G (2006) Simultaneous RP-LC determination of additives in soft drinks. Chromatographia 63:557–562. doi:10.1365/s10337-006-0793-y
Cantarelli MA, Pellerano RG, Marchevsky EJ, Camiña JM (2009) Simultaneous determination of aspartame and acesulfame-K by molecular absorption spectrophotometry using multivariate calibration and validation by high performance liquid chromatography. Food Chem 115:1128–1132. doi:10.1016/j.foodchem.2008.12.101
Kailasam S (2010) Ultrafast and sensitive analysis of sweeteners, preservatives and flavorants in nonalcoholic beverages using the Agilent 1290 Infinity LC system Agilent Technologies, Inc. Publication Number 5990-5590EN. www.agilent.com/chem/1290
Pedjie N (2010) Analysis of common sweeteners and additives in beverages with the PerkinElmer Flexar FX-15 System equipped with a PDA Detector PerkinElmer, Inc. 009473_01. www.perkinelmer.com
Wasik A, McCourt J, Buchgraber M (2007) Simultaneous determination of nine intense sweeteners in foodstuffs by high performance liquid chromatography and evaporative light scattering detection – development and single-laboratory validation. J Chromatogr A 1157:187–196. doi:10.1016/j.chroma.2007.04.068
Kruve A, Rebane R, Kipper K, Oldekop ML, Evard H, Herodes K, Ravio P, Leito I (2015) Tutorial review on validation of liquid chromatography–mass spectrometry methods: part I. Anal Chim Acta 870:29–44. doi:10.1016/j.aca.2015.02.017
Kruve A, Rebane R, Kipper K, Oldekop ML, Evard H, Herodes K, Ravio P, Leito I (2015) Tutorial review on validation of liquid chromatography–mass spectrometry methods: part II. Anal Chim Acta 870:8–28. doi:10.1016/j.aca.2015.02.016
Yang DJ, Chen B (2009) Simultaneous determination of nonnutritive sweeteners in foods by HPLC/ESI-MS. J Agric Food Chem 57:3022–3027. doi:10.1021/jf803988u
Hird SJ, Lau BPY, Schuhmacher R, Krska R (2014) Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. Trends Anal Chem 59:59–72. doi:10.1016/j.trac.2014.04.005
Loos R, Gawlik BM, Boettcher K, Locoro G, Contini S, Bidoglio G (2009) Sucralose screening in European surface waters using a solid-phase extraction-liquid chromatography–triple quadrupole mass spectrometry method. J Chromatogr A 1216:1126–1131. doi:10.1016/j.chroma.2008.12.048
Mawhinney DB, Young RB, Vanderford BJ, Borch T, Snyder SA (2011) Artificial sweetener sucralose in U.S. drinking water systems. Environ Sci Technol 45:8716–8722. doi:10.1021/es202404c
Minten J, Adolfsson-Erici M, Björlenius B, Alsberg T (2011) A method for the analysis of sucralose with electrospray LC/MS in recipient waters and in sewage effluent subjected to tertiary treatment technologies. Int J Environ Anal Chem 91:357–366. doi:10.1080/03067310903582333
Ferrer I, Zweigenbaum JA, Thurman EM (2013) Analytical methodologies for the detection of sucralose in water. Anal Chem 85:9581–9587. doi:10.1021/ac4016984
Mead RN, Morgan JB, Avery GB Jr, Kieber RJ, Kirk AM, Skrabal SA, Willey JD (2009) Occurrence of the artificial sweetener sucralose in coastal and marine waters of the United States. Mar Chem 116:13–17. doi:10.1016/j.marchem.2009.09.005
Morlock GE, Schuele L, Grashorn S (2011) Development of a quantitative high-performance thin-layer chromatographic method for sucralose in sewage effluent, surface water, and drinking water. J Chromatogr A 1218:2745–2753. doi:10.1016/j.chroma.2010.11.063
Yu S, Zhu B, Lv F, Li S, Huang W (2012) Rapid analysis of cyclamate in foods and beverages by gas chromatography-electron capture detector (GC-ECD). Food Chem 134:2424–2429. doi:10.1016/j.foodchem.2012.04.028
Mazurek S, Szostak R (2011) Quantification of aspartame in commercial sweeteners by FT-Raman spectroscopy. Food Chem 125:1051–1057. doi:10.1016/j.foodchem.2010.09.075
Maes P, Monakhova YB, Kuballa T, Reusch H, Lachenmeier DW (2012) Qualitative and quantitative control of carbonated cola beverages using 1H NMR spectroscopy. J Agric Food Chem 60:2778–2784. doi:10.1021/jf204777m
Herrmannová M, Krivánková L, Bartos M, Vytras K (2006) Direct simultaneous determination of eight sweeteners in foods by capillary isotachophoresis. J Sep Sci 29:1132–1137. doi:10.1016/j.foodchem.2010.07.107
Stojkovic M, Mai TD, Hauser PC (2013) Determination of artificial sweeteners by capillary electrophoresis with contactless conductivity detection optimized by hydrodynamic pumping. Anal Chim Acta 787:254–259. doi:10.1016/j.aca.2013.05.039
Wu Y, Li J, Zhao S, Ding X (2016) Simultaneous determination of 11 food additives by micellar electrokinetic capillary chromatography. Food Anal Methods 9:589–595. doi:10.1007/s12161-015-0225-4
Moein MM, Abdel-Rehim A, Abdel-Rehim M (2015) Microextraction by packed sorbent (MEPS). Trends Anal Chem 67:34–44. doi:10.1016/j.trac.2014.12.003
Lorenzo RA, Carro AM, Concheiro A, Alvarez-Lorenzo C (2015) Stimuli-responsive materials in analytical separation. Anal Bioanal Chem 407:4927–4948. doi:10.1007/s00216-015-8679-1
Acknowledgments
The authors would like to thank the Spanish Ministry of Science and Innovation (Project AGL-2014-53647-R) and the Dirección General de Tráfico (Ministry of Interior, Spain), Project SPIP2015-01838, and FEDER for their financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this entry
Cite this entry
Lorenzo Ferreira, R.A., Lage Yusty, M.A., Carro Díaz, A.M. (2018). Analytical Strategies to Determine Artificial Sweeteners by Liquid Chromatography-Mass Spectrometry. In: Mérillon, JM., Ramawat, K. (eds) Sweeteners. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-27027-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-27027-2_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27026-5
Online ISBN: 978-3-319-27027-2
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics