Abstract
Brassicaceae are blessed with secondary metabolites called glucosinolates which form the defense arsenal of these plants. Glucosinolates and its degradation products are also proved to be beneficial in agriculture and human health even though some are known to be detrimental. The type of glucosinolates and its content displays huge diversity across different species. The glucosinolate diversity is primarily genetically controlled. The profile of glucosinolates also varies depending on the growth stages and external environment of the plant. The environmental factors include type of pest/pathogen attack, nutrient status of the plant, and other abiotic stress factors. The glucosinolate pathway is also linked to other major metabolic and signaling pathways resulting in a complex mechanism of regulation. Even though the regulatory mechanism is not completely understood, the current chapter integrates the knowledge available from the model plant Arabidopsis and related Brassica crops.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Brassica
- Glucosinolates
- Regulation
- Variability
- Differential accumulation
- Plant defense
- Sulfur deficiency
- Metabolic cross talk
1 Introduction
Brassicaceae is a large family of plants which consists of >3000 species in 370 genera [1]. Brassicaceae, often referred as Cruciferae, includes agriculturally important mustards, cabbages, broccoli, turnips, cresses, and their many relatives. The genus Brassica which comes under this family is remarkable for containing the largest number of agriculturally and horticulturally important crops than any other genus. Crops under the genus Brassica are cultivated since ancient times as vegetables (Brassica oleracea), oilseeds (Brassica napus, Brassica rapa, and Brassica juncea), and condiments (Brassica nigra, Brassica carinata, and B. juncea). The oldest references for the cultivation of Brassica crops come from India, China, and Japan. Six economically important Brassica species (B. rapa, B. nigra, B. oleracea, B. juncea, B. napus, and B. carinata) share three major genomes (A, B, and C). B. napus (2n = 38, AACC), B. juncea (2n = 36, AABB), and B. carinata (2n = 34, BBCC) are amphidiploid species resulted from combining genome sets of the low chromosome number species which are B. rapa (2n = 20, AA), B. nigra (2n = 16, BB), and B. oleracea (2n = 18, CC) [2, 3].
Plants are equipped with a diverse array of natural chemicals to cope up with environmental challenges either biotic or abiotic to balance their sessile nature. Among such compounds, glucosinolates (GSL) are one of the widely studied plant secondary metabolites that contribute toward plant fitness. Glucosinolates (mustard oil glucosides) are a diverse group of nitrogen and sulfur-rich anionic secondary metabolites derived from amino acids. Chemical structure of glucosinolates consists of a β-d-glucopyranose residue linked via a sulfur atom to a (Z)-N-hydroximinosulfate ester and a variable R group derived from amino acids (Fig. 1). Glucosinolates in intact form are known to be inactive in nature. Hydrolysis of glucosinolates by β-thioglucoside glucohydrolase (EC 3.2.3.1, myrosinases) yields a variety of products that are responsible for the biological activities of these compounds. In plants, myrosinases are located in specialized cells known as myrosin cells/idioblast cells of the phloem parenchyma [4], whereas the glucosinolates are held separately in the vacuoles or S-cells of most plant tissues. The compartmentalization prevents hydrolysis in natural conditions. This system has been called as “mustard oil bomb” [5] and constitutes a defense system against pests and diseases.
Exist as defense mechanism of plants [6–8], glucosinolates also impart pungency to Brassica cultivars and hence find its use in culinary purpose from time immemorial. However, some glucosinolates and their degradation products have anti-nutritional activities. Hence, development of Brassica varieties low in certain glucosinolates is a key thrust in Brassica breeding program [9, 10]. A major breakthrough in glucosinolate research was the discovery that degradation product of glucosinolate, glucoraphanin, i.e., sulforaphane, is a nutritional giant with immense healing properties including anticarcinogenic and antimicrobial activities [11, 12]. Because of its multitude of biological activities, glucosinolate biosynthesis and regulation is an active area of research worldwide. Since the model plant Arabidopsis thaliana belongs to Brassicaceae, a great extent of information furnished in this chapter is based on Arabidopsis.
2 Biosynthesis of Glucosinolates
Glucosinolate biosynthesis is known to be highly complex, and the use of model plant Arabidopsis has largely assisted toward our understanding of various steps involved in this biosynthesis [6, 13].
Glucosinolates can be broadly classified based on their biosynthetic precursor amino acid into aliphatic (derived from Ala, Val, Ile, Met, or Val), indolic (derived from Trp), and benzyl (derived from Phe and Tyr) glucosinolates. Till date, more than 130 glucosinolate structures have been identified across various members of Brassicaceae family [14], and Arabidopsis itself has about 40 types of glucosinolates, mainly derived from Met and Trp [15]. Table 1 enlists some of the glucosinolates which are commonly present in model plant Arabidopsis and cultivated Brassica species. In general, the series of reactions involved in glucosinolate biosynthesis can be further divided into three distinct phases, viz., chain elongation of the precursor amino acids, core glucosinolate structure formation, and side-chain modification reactions of the glucosinolate core. However, there is no side-chain elongation that occurs for indolic glucosinolate biosynthesis.
Step I: Side-chain elongation of precursor amino acids: Biosynthesis of glucosinolates initiates in cytosol with the deamination of the specific amino acid to corresponding α-keto acid /2-keto acid/2-oxo acid by branched-chain aminotransferases (BCATs) [6]. For instance, in the case of methionine-derived glucosinolates, the BCAT4 catalyzes the initial step of chain elongation by converting Met to 4-methylthio-2-oxobutanoic acid (MTOB) (Scheme 1a). The initial evidence of BCATs in deamination of Met was suggested by Diebold et al. [16] based on yeast complementation studies. In a more recent study [17], Arabidopsis bcat4 mutant showed increased accumulation of free Met pool and decreased biosynthesis of aliphatic glucosinolates, suggesting the role of BCAT4 in the first deamination step. After deamination, the resulting α-keto acids are further metabolized in a condensation reaction with acetyl-CoA, catalyzed by methylthioalkyl malate synthase (MAM1, MAM2, and MAM3 localized in GSL-ELONG locus) present inside chloroplasts to form substituted 2-malate derivatives [18–20]. MAM1 is involved in the production of glucosinolates derived from 1 to 3 chain elongation cycles (i.e., C3–C5 GSL), whereas MAM2 catalyzes the production of glucosinolates derived from single chain elongation cycle (C3-glucosinolate only). Besides MAM1/2 (At5g23010), the Arabidopsis GS-ELONG locus also harbors a tandemly duplicated gene (At5g23020) which encodes another enzyme MAM3 (formerly known as MAM-L), which contributes to the production of all aliphatic glucosinolates, although having higher specificity for longer chain lengths, C5 to C9 [20].
The glucosinolate biosynthesis pathway. (a) Chain elongation of precursor amino acid. (b) Formation of glucosinolate core. (c) Side-chain modification reactions. The reactions on the left panel show formation of aliphatic glucosinolates and those on right (of b and c) shows formation of indolic glucosinolates. Enzymes involved in the respective steps are given in red color font. (d) General scheme of chemical reactions involved in each step. 1 precursor amino acid, 2 chain-elongated amino acid, 3 glucosinolate core formation, 4 R group undergoing secondary modification reactions
The 2-malate derivative is then isomerized into 3-malate derivative which is further converted to homoketo acid by oxidative decarboxylation adding an extra carbon in the side chain than the starting compound (Scheme 1b). The isopropylmalate isomerases (IPMI1 and IPMI2) and isopropylmalate dehydrogenase (IPMDH1) are known to catalyze these reactions [21, 22]. The resulting homoketo acids are now chain elongated by a single methylene group. This molecule can either be transaminated by BCAT to yield homoMet and enter the core glucosinolate pathway or again can pass through additional elongation cycles resulting in homoketo acids with increased side-chain length up to nine methylene groups [6, 13, 23]. Based on knockdown assay in Arabidopsis, BCAT3 was identified as the chloroplast localized enzyme involved in the transamination of 2-oxoacids derived from Met [24]. Thus, BCATs are capable of both deamination and transamination reactions. These reactions generate a variety of chain-elongated derivatives of methionine.
Step II: Formation of glucosinolate core structure: The first chain elongation step of glucosinolate biosynthesis starts in cytosol [17], and later steps occur in chloroplast [6]. However, the core structure formation takes place in the cytosol. This implicates the need for a transporter which can shuttle between the cytoplasm and chloroplast membranes. Recently, Gigolashvili et al. [25] have shown that the bile acid transporter, BAT5, is involved in the transport of MTOB and chain-elongated keto acids across the chloroplast membrane before, during, and after side-chain elongation of keto acids.
Biosynthesis of glucosinolate core structure initiates with the conversion of precursor amino acids (chain elongated in the case of aliphatic) to aldoximes by cytochrome P450 monooxygenases of the CYP79 family (Scheme 1c). The CYP79B2 and CYP79B3 enzymes catalyze the formation of indolic-3-acetaldoxime (IAOx) from Trp [26], whereas CYP79F1 and CYP79F2 are specific to chain-elongated Met substrates [27]. CYP79A2 catalyzes this particular step in the case of benzyl glucosinolates [28]. The aldoximes are subsequently oxidized into aci-nitro compounds or nitrile oxides by enzymes of the CYP83 family. In Arabidopsis, two nonredundant enzymes, CYP83A1 and CYP83B1, oxidize aliphatic and indolic aldoximes to their corresponding aci-nitro compounds [29]. These compounds are strong electrophiles and hence can spontaneously react with thiols to form S-alkylthiohydroximate conjugates [23]. Cysteine was suggested to be the thiol donor for the reaction in vivo; however, it is not clear whether this conjugation is enzyme mediated [30]. More recently, Schlaeppi et al. [31] have shown glutathione as a potential sulfur donor for the reaction. The C-S bond of S-alkylthiohydroximates is then cleaved by a C-S lyase to yield thiohydroximates, pyruvate, and ammonia. Using bioinformatics approach, Mikkelsen et al. [32] have identified SUR1 as the C-S lyase involved in the glucosinolate biosynthesis. From mutant studies, it is also suggested that C-S lyase is a single gene family which lacks side-chain specificity in glucosinolate biosynthesis. Hence, formed thiohydroximates undergo a glucosylation reaction catalyzed by UGT74B1 glucosyltransferase [33] to generate desulfoglucosinolates . Analysis of ugt74b1 knockout mutant in Arabidopsis showed considerably decreased but not completely abolished level of indolic and aliphatic glucosinolates, thus indicating that other UGT activities may also be present in Arabidopsis plants. UGT74C1 has been suggested as a candidate gene having UGT activities involved in glucosinolate biosynthesis and seems to be more specific for Met-derived thiohydroximates [6, 13]. In the last stage of core structure synthesis, desulfoglucosinolates are sulfated by the sulfotransferases (SOT) to form the final glucosinolate core structure [13]. The sulfur donor for the reaction is suggested to be 3′-phosphoadenosine-5′-phosphosulfate (PAPS). Three Arabidopsis sulfotransferases , AtST5a, AtST5c, and AtST5b (SOT16, SOT17 and, SOT18, respectively), have been shown to catalyze this reaction. The AtST5a metabolizes tryptophan- and phenylalanine-derived desulfoglucosinolates, whereas AtST5b and AtST5c are specific to aliphatic glucosinolates [34].
Step III: Secondary modifications of glucosinolates: The final stage in glucosinolate biosynthesis is the secondary modifications of the side chain. The side chain undergoes various modification(s) such as acylation, oxidation, alkylation, and/or esterification reactions. Side-chain modifications of the glucosinolates occur through stepwise oxidation of methylthioalkyl moieties to methylsulfinylalkyl and then to alkenyl moieties (Scheme 1c). A subclade of flavin monooxygenase (FMOGS-OX1–5) was characterized which catalyze the conversion of methylthioalkyl glucosinolates into methylsulfinylalkyl glucosinolates [35, 36]. Two α-ketoglutarate-dependent dioxygenases (AOP2 and AOP3) are demonstrated to control the conversion of methylsulfinylalkyl to alkenyl- and hydroxyalkyl glucosinolates, respectively [37, 38]. A 2-oxo acid-dependent dioxygenase, GS-OH, is demonstrated to convert 3-butenyl to 2-hydroxy-but-3-enyl glucosinolate in Arabidopsis [39]. The side-chain elongation and side-chain modification reactions contribute to the vast diversity of glucosinolate structures which is described in detail in Sect. 3.2. It has been shown that esterification of Met-derived hydroxyl glucosinolates with benzyl CoA give rise to benzyloxy glucosinolates or sinapoylated to corresponding sinapoyloxypropyl or sinapoyloxybutyl glucosinolates [40]. Using genetic mapping and T-DNA insertion lines in Arabidopsis, benzyl oxidase (BZO1) has been identified to possess the benzoyl-CoA ligase activity [41]. Later, it has been proposed that BZO1 is involved in the synthesis of benzoate precursor cinnamoyl-CoA, rather than to generate benzoyl-CoA [42].
3 Regulation of Glucosinolate Biosynthesis
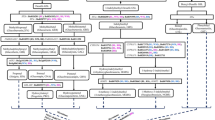
Genome sequencing and various genetic and functional screens in Arabidopsis and related Brassica crops have led to the identification of almost all the genes involved in every stage of the glucosinolate biosynthesis [6, 13, 23, 43, 44]. The genetic and molecular regulation of glucosinolate biosynthesis is highly complex (or quantitative) and not yet completely understood. In recent years, various functional studies in model plant Arabidopsis have led to the identification and functional analysis of key genes toward regulating the glucosinolate content and profile during plant growth and developmental stages and in response to various stress and elicitor treatments [45]. Our understanding of molecular regulation of glucosinolate biosynthesis in polyploid Brassica crops is still impeding owing to inherent polyploidy and complex genetic architecture of these crops. Homologs of some of the glucosinolate candidate genes are reported from some of the recently sequenced Brassica genomes [43, 44, 46, 47]; however, only a handful of these Brassica homologs has been functionally characterized till date [48–56]. Based on the recent information available from Arabidopsis and other Brassica crops, we have redrawn an updated scheme for the regulation of glucosinolate metabolism in Brassicaceae species, which is described in detail in the coming sections (Scheme 2).
Regulation of glucosinolate biosynthesis based on current literature. Molecules on green oval ( ) represent transcriptional regulators. Pathway genes having regulatory role are given in hexagons. Blue lines indicate direct regulation, whereas red lines with arrows show glucosinolate regulation under prolonged
sulfur deficiency. Dotted lines (both blue and black) indicate interaction either positive or negative but not yet fully understood. Other metabolic pathways having cross talk with indolic glucosinolate pathway are also shown. (
) represent transcriptional regulators. Pathway genes having regulatory role are given in hexagons. Blue lines indicate direct regulation, whereas red lines with arrows show glucosinolate regulation under prolonged
sulfur deficiency. Dotted lines (both blue and black) indicate interaction either positive or negative but not yet fully understood. Other metabolic pathways having cross talk with indolic glucosinolate pathway are also shown. ( ) indicate repression. (
) indicate repression. ( ) represent protein-protein interaction. Regulation among the major MYB transcription factors is also shown
) represent protein-protein interaction. Regulation among the major MYB transcription factors is also shown
3.1 Direct Transcriptional Regulators of Glucosinolate Biosynthesis
There are several transcription factors identified which regulate the biosynthesis of glucosinolates in model plant Arabidopsis and other related Brassica species, including ATR1, IQD1, AtDof1.1, and different members of subgroup-12 of R2R3-MYB superfamily.
One of the initial studies using molecular-genetic approach, IQD1 , a calmodulin-binding nuclear protein, was shown to regulate aliphatic and indolic glucosinolate biosynthesis in Arabidopsis [57]. Analysis of steady-state messenger RNA levels of glucosinolate biosynthesis genes in both gain- and loss-of-function mutants in Arabidopsis indicated that IQD1 regulates the expression of multiple genes of both aliphatic and indolic glucosinolate metabolism in a differential manner.
IQD1 was observed to be induced by mechanical stimuli, and hence with the help of previous literature it may be concluded that IQD1 might integrate early wound and pathogen/elicitor-induced changes in cytoplasmic Ca2+ concentrations and thus stimulate a wide array of coordinated defense responses, including the upregulation of glucosinolate biosynthesis [58]. Another central transcriptional regulatory component of glucosinolate biosynthesis proposed is SLIM1 -(Sulfur LIMitation), which represses the biosynthesis of glucosinolates in response to sulfate deficiency through the activation of the enzymes catabolizing glucosinolates and hence regulating the sulfur (S) assimilatory pathways [59]. It was observed that the slim1 mutation altered the expression of metabolic and regulatory genes of glucosinolate biosynthetic pathways including the initial step Met chain elongation enzymes, CYP79F1/F2, CYP79B2/B3, and CYP83B1, and the indolic transcription factor MYB34 (ATR1). Recently, SLIM1 was shown to be the upstream regulatory component to all other transcriptional regulators under sulfur deficiency [60, 61]. Skirycz et al. [62] identified transcription factor OBP2, also called as AtDof1.1 ( DNA-binding-with-one-finger), as a positive regulator of indolic glucosinolate biosynthesis in Arabidopsis. OPB2 was identified initially based on its homology to OPB1 in Arabidopsis [63]. It was observed in wild-type Arabidopsis plants that expression of OBP2 was stimulated by wounding, methyl jasmonic acid (MeJA ) treatment, and in response to insect feeding which are also known to induce expression of glucosinolate biosynthetic genes and a subsequent accumulation of glucosinolates. Also, overexpression of OBP2 in transgenic plants resulted in the upregulation of CYP79B2/B3 and CYP83B1 genes involved in the glucosinolate biosynthetic pathway and an increase in indolic glucosinolates. These findings further confirm that OBP2 is involved in the regulatory network controlling indolic glucosinolate biosynthesis in Arabidopsis [62].
In recent years, MYB class of transcription factors have been discovered as one of the major regulators of glucosinolate biosynthesis. MYB proteins represent a large family of proteins that include the conserved MYB DNA-binding domain. Out of the three MYB subfamilies, the R2R3-MYB subfamily is largely found in plants which are involved in a variety of plant functions including developmental processes and defense responses [64]. The R2R3-MYB family proteins are further classified into subgroups depending on specific functions they perform. Members of subgroup-12 R2R3-MYBs are transcription factors, which include MYB34, MYB51, MYB122, MYB28, MYB29, and MYB76, and are shown to be important regulators of glucosinolate biosynthesis. MYB34, MYB51, and MYB122 are regulators of indolic glucosinolates , whereas MYB28, MYB29, and MYB76 are involved in the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis [65]. Recently two other members of R2R3-MYB transcription factors, MYB115 and MYB118 are shown to control benzyloxy glucosinolate pathway in Arabidopsis [66].
Bender and Fink [67] initially identified the R2R3-MYB transcription factor, ALTERED TRYPTOPHAN REGULATION 1 (ATR1/MYB34) as a positive regulator of Trp biosynthesis from Arabidopsis. In Brassicaceae, indolic glucosinolates are derived from the amino acid Trp which is also the precursor for the plant hormone IAA. Later, Celenza et al. [68] have shown that ATR1 (MYB34) is a key regulator of indolic glucosinolate biosynthesis. Even though overexpression of ATR1 caused increase in IAA levels, more prominent elevation was observed for indolic glucosinolate levels, along with an upregulation of biosynthetic genes CYP79B2/B3 which are also involved in Trp metabolism. Furthermore, the atr1-defective mutation suppressed Trp gene deregulation in a cyp83B1 mutant background confirming the role of ATR1 as a key homeostatic regulator of Trp metabolism and hence indolic glucosinolates.
Analysis of transcript levels of genes located near the site of insertion of transposon in the activation-tagged HIG1-1D (an activation-tagged line high in indol-3-ylmethyl glucosinolate; I3M) mutant showed high level of overexpression of At1g18570 compared to the wild type. The product of this gene, a R2R3-MYB transcription factor, was later coined as HIGH INDOLIC GLUCOSINOLATE 1 (HIG1 or MYB51 ) [69]. To examine the role of HIG1 in glucosinolate biosynthesis, the glucosinolate accumulation pattern of HIG1-1D mutant and HIG1/MYB51 overexpression lines in Arabidopsis was analyzed. The HIG1-1D mutant accumulated up to six-fold higher levels of I3M compared to the wild type. Similarly, the overexpression lines also showed an enhancement of I3M levels up to eight-fold compared with the wild type. In addition, there was also an increase in the other indolic glucosinolates like 4MOI3M and 1MOI3M in these lines compared to the wild type. When transcript profiling of the candidate genes of the indolic glucosinolate and Trp biosynthesis was carried out, it was observed that the expression of the genes DHS1, TSB1, CYP79B2, CYP79B3, CYP83B1, and AtST5a was activated by HIG1/MYB51 in trans [69]. The level of major aliphatic glucosinolate 4MSOB was found to be lower in HIG1-1D and in HIG1/MYB51 overexpression lines compared to the wild-type plants which indicate that HIG1/MYB51 could exert opposite effects on the biosynthetic pathways of indolic and aliphatic glucosinolates. Expression of HIG1/MYB51 was shown to respond to mechanical stimuli like touch or wounding, and overexpression of HIG1/MYB51 resulted in increased plant resistance against generalist herbivores. Thus, these findings provide functional evidence that HIG1/MYB51 is a transcriptional regulator of indolic glucosinolate biosynthesis genes in A. thaliana.
Another close homolog of MYB51 in Arabidopsis which is involved in the regulation of indolic glucosinolates is MYB122 . Ectopic overexpression of HIG2/MYB122 in wild-type Col-0 background resulted in high levels of I3M accumulation but not that of other indolic glucosinolates [69]. Attempts to restore the low I3M level of the hig1-1 knockout mutant by overexpression of HIG2/MYB122 showed no restoration of function. However, the expression of some Trp pathway genes like ASA1, TSB1, CYP79B2, CYP79B3, and CYP83B1 was found to be enhanced along with elevated auxin levels. These results demonstrate that HIG2/MYB122 has the potential to upregulate indolic glucosinolate biosynthesis but only in the presence of a functional HIG1/MYB51 [65].
It was later confirmed that MYB34, MYB51, and MYB122 have distinct but overlapping functions in the regulation of indolic glucosinolate biosynthesis based on the expression analysis of these transcription factors in knockout mutants and overexpression lines. Expression levels of MYB122 was significantly increased in the myb34 mutant, and expression of both MYB34 and MYB122 was downregulated in myb51 mutant. However, expression levels of both MYB34 and MYB51 were unaffected in the myb122 double knockout mutant. In MYB51 overexpression lines, MYB34 and MYB122 transcript levels significantly increased, whereas the transcript level of MYB51 was marginally activated by MYB34 or MYB122 overexpression. The results are conclusive of the fact that the role of MYB34 and MYB122 partially overlap but not of MYB34 and MYB51 [70]. It can be hence inferred that MYB51 might be the major regulator of indolic glucosinolate biosynthesis - (Scheme 2).
In Arabidopsis, other members of the subgroup 12 of R2R3-MYB transcription factors, MYB28, MYB29, and MYB76, have been identified as positive regulators of aliphatic glucosinolate biosynthesis using an omics-based methodology [71], a trans-activation approach [72, 73], and a quantitative systems biology approach [74]. MYB28 or HIGH ALIPHATIC GLUCOSINOLATE 1 (HAG1) is shown to be the dominant regulator of aliphatic glucosinolate biosynthesis controlling the expression of almost all genes of the pathway [13]. The aliphatic glucosinolate content as well as transcript levels of aliphatic glucosinolate biosynthetic genes were elevated in gain-of-function mutants and decreased in RNAi knockdown mutants of HAG1 in Arabidopsis [69, 71, 74]. Strong MYB28/HAG1 promoter activity was observed in reproductive organs and mature leaves of the plants, the main sites of aliphatic glucosinolate accumulation. Arabidopsis transgenic lines overexpressing MYB28 showed reduced feeding preference of the generalist lepidopteran pest Spodoptera exigua . MYB28 expression was also found to be induced by glucose both in Arabidopsis and B. juncea [72, 75], suggesting the existence of transcriptional regulation for integrating carbohydrate availability and defense compound allocation upon biotic stress.
It was also demonstrated that other two members of subgroup 12 of R2R3-MYB transcription factor, namely, HAG2/MYB76 and HAG3/MYB29, were also involved in the biosynthesis of Met-derived glucosinolates [73]. MYB29 plays only a minor role in the regulation of aliphatic glucosinolates. There was no significant change in the expression of glucosinolate biosynthesis genes or glucosinolate content in myb29 gene knockdown plants suggesting that MYB29 may not be essential for basal level glucosinolate biosynthesis. However, the double knockout mutant myb28/myb29 abolished the synthesis of aliphatic glucosinolates, indicating the primary importance of both MYB28 and MYB29 in the regulation of aliphatic glucosinolates [13, 74]. In Arabidopsis, it was observed that on treatment with MeJA , the expression of MYB29 was induced but not that of MYB28. Expression of the aliphatic glucosinolate biosynthesis genes CYP79F1, CYP79F2, and CYP83A1 was also upregulated with the induction of MYB29. These results suggest that MYB28 is essential for basal level synthesis of aliphatic glucosinolates and MYB29 probably has a role in the induction of aliphatic glucosinolate biosynthesis genes in response to MeJA signaling [71].
Later, Sonderby et al. [13] proposed a new model for the regulation of aliphatic glucosinolates based on genotypic and systems analysis techniques, which suggests that MYB28 is the major transcriptional regulator involved in the regulation of both short- and long-chain aliphatic glucosinolates, whereas MYB29 and MYB76 mainly act toward short-chain aliphatic glucosinolates. But under certain conditions, MYB29 and MYB76 can also induce long-chain aliphatic glucosinolates in the absence of MYB28. The model also propose the presence of additional factors which can interact with both MYB29 and MYB76, located probably outside of the tissue, which is needed for the induction of long-chain aliphatic glucosinolates. The findings also show that MYB76 is independent of MYB28 and MYB29 in aliphatic glucosinolate biosynthesis regulation and it plays a role in determining the spatial distribution of aliphatic glucosinolates within the leaf (Scheme 2). Since there is a complex interplay that exists between these three transcription factors in glucosinolate regulation, understanding their individual role is challenging. Therefore, Li et al. [76] generated transgenic lines expressing each aliphatic MYB gene driven by its own promoter in the myb28myb29 mutant background to get a better picture. It was observed that the three MYB genes work differentially in regulating aliphatic glucosinolate profiles in a tissue-specific manner. Orthologs of MYB transcription factors have been identified from other Brassica crops, B. rapa ssp. pekinensis [43, 47, 77], B. oleracea var acephala [78], B. oleracea var italica [44, 79], and other related B. oleracea varieties including kale and kohlrabi [80] and B. juncea [81].
Recently, Arabidopsis basic helix-loop-helix transcription factors MYC2 , MYC3 , and MYC4 were identified as direct transcriptional regulators of glucosinolate biosynthesis [82]. These transcription factors were well documented previously for their potential to interact with JAZ repressors to control JA mediated responses, including defense against herbivory [83]. A study by Dombrecht et al. [84] using genome-wide transcriptional profiling of wild-type and jasmonate signal transduction mutant jasmonate-insensitive 1 (jin1/myc2) plants followed by functional analysis revealed that MYC2 negatively regulates indolic glucosinolate biosynthesis in response to jasmonic acid (JA) signaling. Later, it was observed that the triple mutant, myc2 myc3 myc4 (myc234), almost completely abolished glucosinolate biosynthesis and expression of glucosinolate biosynthesis genes was significantly reduced. However, the expression of known positive regulators MYB28 and MYB29 remained unaltered which shows that this regulation is direct and independent of MYB factors. The pest status of the mutant plants were also altered compared to the wild-type plants. Chromatin immunoprecipitation, yeast two-hybrid, and pulldown experiments showed that MYC2 binds directly to the promoter of several glucosinolate biosynthesis genes and MYC2/MYC3/MYC4 can interact with MYBs involved in both aliphatic and indolic glucosinolate biosynthesis (Scheme 2). The study concludes that MYC2, MYC3, and MYC4 are essential for constitutive and insect-inducible glucosinolate biosynthesis [82].
3.2 Biosynthetic Genes Controlling Glucosinolate Variability
There are over 130 glucosinolate structures documented so far [14], of which Arabidopsis posses about 40 types across different accessions [15]. The huge variability in the glucosinolate structures follows a complex regulatory mechanism. The existence of the different glucosinolate structures is also controlled by variability of individual gene functions, especially the genes involved in initial elongation and side-chain modification reactions (Scheme 2). In addition, the molecular function of genes varies depending on the plant species, accession, allelic condition, and the polymorphic state of the regulatory network controlling it. The huge variation in glucosinolates is believed to be evolved as a counter mechanism against variety of pests and diseases and other life-threatening challenges encountered by the plant.
Based on the available literature, there are four major loci that control variation in aliphatic glucosinolate accumulation namely, GS-ELONG , GS-OX , GS-AOP (contains QTLs GS-ALK a nd GS-OHP ), and GS-OH [37, 38, 85–87]. GS-ELONG is responsible for the variation in chain length of glucosinolates and is composed of three genes, MAM1, MAM2, and MAM3. Analysis of glucosinolate profiles and knockout studies showed that MAM1 catalyzes the production of aliphatic glucosinolates with two carbon chains and MAM2 plays an important role in the production of glucosinolates with a single carbon chain. MAM3 catalyzes the formation of all glucosinolate chain lengths especially those derived from one, five, and six elongation cycles, making this enzyme as the major player of glucosinolate chain length diversity across Brassicaceae [18, 20]. Moreover, MAM1 and MAM2 never exist together in the same accession except for Sorbo of Arabidopsis [19]. This gene duplication and neo-functionalization in MAM genes hence plays important role toward the diversification of glucosinolate profiles in Brassicaceae.
Through performing analysis on phylogeny and synteny relationships across 13 sequenced Brassicaceae species, a recent study has investigated the evolution of MAM genes, which underwent frequent tandem duplications [88]. The syntenic loci of MAM genes showed two independent lineage-specific evolution routes and were driven by positive selection after the divergence from Aethionema arabicum, an early branching sister group to the core Brassicaceae group. In the lineage I species (Capsella rubella, Camelina sativa, A. lyrata, and A. thaliana), the MAM loci have evolved three tandem genes encoding enzymes (MAM1, MAM2, and MAM3) responsible for the biosynthesis of aliphatic glucosinolates with different carbon chain lengths. In contrast, among the species belonging to lineage II (B. rapa, B. oleracea, Thellungiella salsuginea, T. halophila, Schrenkiella parvula, Sisymbrium irio, and Raphanus sativus), the MAM loci encode enzymes responsible for the biosynthesis of short-chain aliphatic glucosinolates only (MAM1/MAM2). Further biochemical studies of MAM enzyme variants from Brassicaceae species could be undertaken in the future to understand the biochemical basis of glucosinolate side-chain variability present in this economically important plant lineage.
Another set of loci that determine glucosinolate variability is involved in side-chain modification reactions. Genetic studies in B. napus and Arabidopsis identified important loci controlling the conversion of methylthioalkyl glucosinolate to methylsulfinylalkyl glucosinolate [87]. Later, this locus was designated as GS-OX [38]. Biochemical knowledge combined with the transcriptome co-regulation database analysis in Arabidopsis led to the identification of a flavin monooxygenase (FMOGS-OX1), which was mapped to GS-OX locus, and catalyzes the conversion of methylthioalkyl glucosinolates to methylsulfinylalkyl glucosinolates [35, 38]. Bacterial overexpression of the recombinant FMOGS-OX protein and analysis of the glucosinolate content in overexpression and knockout mutants of FMOGS-OX1 in Arabidopsis further confirmed FMOGS-OX1 as a major enzyme controlling the conversion of methylthioalkyl glucosinolates in the wild-type plant [35]. Phylogenetic analysis identified a crucifer specific subclade of FMO genes, named FMO GS-OX1–5, which was involved in the conversion of methylthioalkyl to methylsulfinylalkyl glucosinolates [36]. The study also showed that like FMOGS-OX1, FMOGS-OX2 to FMOGS-OX4 are also capable of catalyzing the S-oxygenation independent of chain length and FMOGS-OX5 is specific for 8-methylthiooctyl (8-MTO) glucosinolates [36].
QTL GS-AOP is yet another important locus modulating glucosinolate variability and content [37, 86]. GSL-AOP is the collective name for two tightly linked loci GS-ALK and GS-OHP. Fine scale mapping identified three AOP genes localized with these loci, namely, AOP1, AOP2, and AOP3. AOP2 and AOP3 are believed to be derived from the ancestral gene AOP1 by gene duplication [37, 89]. Both AOP2 and AOP3 encode a 2-oxoglutarate-dependent dioxygenase which is involved in the conversion of methylsulfinylalkyl glucosinolate to methylalkenyl glucosinolate and hydroxylalkyl glucosinolate, respectively. AOP2 is localized within GS-ALK whereas AOP3 is localized within GS-OHP l ocus [37]. Hence the presence, absence, or the allelic variations of these genes determine the type of glucosinolate accumulated in a particular plant species. For example, sulforaphane, an isothiocyanate derived by the hydrolysis of glucoraphanin, a methylsulfinylalkyl glucosinolate, has immense curing properties in humans including cancer prevention. However, the occurrence of glucoraphanin is limited to few Brassica species, owing to the non-functional AOP2 gene. In broccoli, a nonfunctional AOP2 homolog has been identified which is associated with high glucoraphanin accumulation. In B. rapa and B. juncea, three and four AOP2 gene homologs have been identified, respectively, which were found functional and hence explain the reason for the absence of glucoraphanin and presence of high amounts of alkenyl glucosinolates (sinigrin, gluconapin) in these Brassica crops [44, 46, 48, 50, 55].
It has been shown that, other than its catalysis function, AOP2 genes are also involved in the regulation of total aliphatic glucosinolate content as well [38, 90, 91]. Evidence for this feedback regulation mechanism was initially proposed based on the analysis of Arabidopsis Ler X Cvi recombinant inbred lines which showed an enhancement of aliphatic glucosinolates up to three-fold in the GS-ALK variant compared to the GS-OHP or the GS-AOP-null variant which concluded that GS-AOP has a significant role in controlling the total aliphatic glucosinolate concentration in the leaf. Similarly, overexpression of AOP2 gene from B. oleracea in Arabidopsis ecotype Col-0 which is null for both AOP2 and AOP3 resulted in the enhancement of aliphatic glucosinolates by increasing the expression of almost all biosynthetic genes and the major transcriptional regulators such as MYB28 and MYB29 [48, 91, 92]. In an effort to enhance glucoraphanin content in oilseed B. juncea through the downregulation of BjuAOP2 gene family, it was observed that total glucosinolate content was also significantly reduced. This was further substantiated by the downregulation of BjuMYB28 transcription factor gene, the major positive regulator of aliphatic glucosinolate biosynthesis in B. juncea [55]. All these findings corroborate the regulatory role of AOP2 in aliphatic glucosinolate accumulation. However, the specific role of AOP3 in controlling aliphatic glucosinolate accumulation is less understood. Recently, using QTL mapping and phenotypic analysis of F2 mapping population expressing different AOP3 transgenes confirmed the regulatory role of AOP3 in aliphatic glucosinolate biosynthesis [15]. It was found that AOP3 interact with GSL-ELONG loci to control glucosinolate accumulation.
The GS-OH locus is involved in the biosynthesis of hydroxylated alkenyl glucosinolate (2-hydroxybut-3-enyl glucosinolate) [85, 86, 93]. This glucosinolate produced by the oxidation of 3-butenyl glucosinolate show wide variation among different Brassica species indicating a variable control mechanism of biosynthesis [39, 94]. Hansen et al. [39] identified a 2-oxoacid-dependent dioxygenase (2-ODD) required for the formation of 2-hydroxybut-3-enyl glucosinolate from the precursor 3-butenyl glucosinolate using the existing natural variation as well as T-DNA mutations available within Arabidopsis populations. It was further suggested that 2-ODD could also determine the total content of aliphatic glucosinolates in a particular plant species.
3.3 Tissue-Specific Modulation of Glucosinolate Accumulation
We have seen that huge diversity of glucosinolates exists among Brassicaceae, primarily controlled by the presence, absence, or allelic variations in regulatory and biosynthetic genes. Accumulation of glucosinolates is also regulated both developmentally in various organs and tissues and with the age of the plant [95]. This developmental regulation has important consequences on the plant’s defense strategy. In most of the Brassicaceae plants, aliphatic glucosinolates are the predominant glucosinolates found in the aerial tissues, whereas indolic glucosinolates dominates the below ground tissues, i.e., roots. Roots possess higher levels of benzyl glucosinolate 2-phenylethyl (2-PE), compared to shoots possibly for rendering greater effectiveness and toxicity of its hydrolysis products in soil against the microbial invaders. Among the indolic glucosinolates, I3M is predominant in shoots, whereas roots majorly contain methoxy derivatives of indol-3-yl glucosinolate [96].
HPLC analysis of glucosinolate content in various organs of the model plant Arabidopsis ecotype Col-0 shows that dormant and germinating seeds contain the highest concentration of glucosinolates, followed by inflorescences, siliques, leaves, and roots. Glucosinolate content in leaf tissues also decline with age of the tissue [97]. The higher accumulation of glucosinolate content in reproductive tissues was later supported by the strong activity of the regulatory gene MYB28 in the inflorescence as evident by promoter GUS analysis. The GUS activity was found declining with senescence of leaves [72]. Promoter activity of indolic glucosinolate regulators MYB34 and MYB51 showed a different expression profile in different tissue types of Arabidopsis when analyzed histochemically. In general, MYB34 showed highest expression in meristematic tissues and young flowers, which are the main site of indolic glucosinolate biosynthesis and accumulation, whereas MYB51 promoter activity was highest in the vegetative parts of the plants, especially in mature rosette leaves. MYB51 expression was also observed in roots, but not in mature flowers or siliques [69].
Tissue-specific expression of transcription factors involved in glucosinolate biosynthesis has been studied in B. rapa ssp. pekinensis (Chinese cabbage) [77]. Homologs of Dof1.1 (Dof1.1-1), MYB34 (MYB34-1, 2, 3), and MYB51 (MYB51-1, 2, 3) showed highest expression in seeds. MYB28-1 showed highest expression in flowers whereas MYB28-2, MYB28-3, and MYB29 showed peak expression in stem. The other transcriptional regulators IQD1.1-1 and MYB122 showed maximum expression in the young leaves. Expression of IQD1.1-2 and MYB122-1 was found high in roots. Another study conducted by Baskar and Park [98] in Chinese cabbage showed an overlapping expression pattern between BrMYBs and their downstream genes during different developmental stages. BrMYB28.3 and BrMYB29.1 gene homologs were found expressed throughout the developmental stages with highest expression in seedlings and roots, respectively. In allotetraploid B. juncea, four homologs of MYB28 have been identified as the major transcriptional regulator of aliphatic glucosinolate which exhibited a differential expression pattern in different tissue types [81]. All four BjuMYB28 genes showed expression throughout leaf development but had a lower expression during the younger stages. Among the homologs, two BjuMYB28 genes, namely, BjuBMYB28-1 and −2, had relatively higher transcript accumulation in the primary and young leaves with the onset of the reproductive phase. A significantly higher expression of all four BjuMYB28 was observed in the mature and inflorescence leaves. The expression of all the four BjuMYB28 genes was highest in siliques. In addition sub-functionalization of homologs was also observed within leaves. The expression partitioning of regulators can be correlated with differential accumulation of glucosinolates, but not always true.
3.4 Environmental Regulation of Glucosinolate Biosynthesis
As glucosinolates play a vital role in plant fitness, the induction of glucosinolates is certainly regulated by plant-environment interactions. Both biotic and abiotic factors can contribute toward the selective activation of glucosinolates leading to its altered accumulation, in the plant organs, depending on the external stimuli. Plants are often exposed to multiple challenges simultaneously during their lifecycle. The defense mechanism is also regulated differentially when the plant is under a particular stress or a combination of stress factors. These factors result in a complex and interdependent mechanism of regulation of defense chemicals to cope up with such situations (Scheme 2).
It is well established that incidence of pest and diseases affect glucosinolate accumulation [7, 8, 99–103]. Profiles of glucosinolates also vary greatly with the type of pests and pathogens. According to literature, indolic glucosinolates are known to be more important for inducible resistance even though the roles of aliphatic glucosinolates are also undebatable [100, 103]. Although a large number of studies have been carried out in this direction, only a few which describe the molecular basis of glucosinolates-pests-pathogens interaction is focused in this section. A study correlating the signaling pathways modulating Arabidopsis glucosinolate accumulation and plant responses to insect attack using two phloem-feeding aphids Myzus persicae (generalist) and Brevicoryne brassicae (specialist) and two lepidopteran pests S. exigua Hubner (generalist) and Pieris rapae (specialist) found that insect feeding causes increase in short-chain aliphatic methylsulfinyl glucosinolates in all the first three cases, whereas P. rapae did not alter aliphatic glucosinolate content, but indolic glucosinolates increased slightly. However, gene expression associated with aliphatic glucosinolate biosynthesis increased after feeding by all species, indicating that glucosinolate accumulation is not always regulated at the level of these gene transcripts even though glucosinolate levels were altered during herbivory [104, 105].
It has been demonstrated that infestation of M. persicae (green peach aphid) on Arabidopsis causes an overall decrease in the glucosinolate content. However, production of indolic glucosinolate like 4-methoxyindol-3-ylmethyl glucosinolate is detected at the site of infection [100]. Kus´nierczyk et al. [106] studied the changes in gene expression levels of three A. thaliana ecotypes, Landsberg erecta (Ler), Wassilewskija (Ws), and Cape Verde Islands (Cvi) differing in their glucosinolate profiles, in response to feeding by aphids M. persicae and B. brassicae using oligonucleotide microarrays and qRT-PCR analysis. In all three ecotypes, indolic glucosinolate genes were upregulated up on both generalist and specialist feeding. The upregulation of the indolic glucosinolate biosynthesis pathway genes was highest in Ler, suggesting a different defense strategy. A concomitant increase in the indolic glucosinolates was also observed. In another case study in Arabidopsis, glucosinolate mutants with two important pest of Brassicaceae, the diamond back moth Plutella xylostella (specialist utilizing glucosinolate for host recognition) and Heliothis armigera (generalist pest), glucosinolate induction was observed. P. xylostella feeding induced both aliphatic and indolic glucosinolates, in most of the plant lines whereas feeding H. armigera mostly induced indolic glucosinolates [107]. Appel et al. [108] observed that infestation of S. exigua caterpillars on Arabidopsis elicited expression of genes involved in aliphatic glucosinolate biosynthesis, including MAMs, UGT74B1, UGT74C1, CYP79F1, CYP83A1, and AOP2 and indolic glucosinolate genes like CYP79B1, CYP79B2, and CYP79B3. The expression of myrosinase-encoding genes, thioglucoside glucohydrolase 1 and 2 (TGG1, TGG2), was found downregulated. In a recent study, it was found that infestation by cabbage moth Mamestra brassicae increased the levels of indol-3-yl-methyl, 1-methoxy-indol-3-yl-methyl, and total glucosinolates in both wild-type B. napus and MINELESS (lacking myrosin cells) seedlings. The mRNA transcript levels of the B. napus homologs of CYP79B2, CYP79B3, CYP83B1, GSTF9, and SUR1, key genes involved in indolic glucosinolate biosynthesis showed significant upregulation in MINELESS seedlings fed with M. brassicae [109]. These studies support the existence of differential regulation of glucosinolate biosynthesis and accumulation in different Brassica species which is also influenced by the species of pest.
Glucosinolate profiles are altered during pathogen attack. Schlaeppi et al. [110] have analyzed the involvement of Trp, indolic glucosinolates, and camalexin in the resistance against Phytophthora brassicae of Arabidopsis. Transcript profiling revealed that genes involved in the biosynthetic pathway of all the three compounds were coordinately induced in by P. brassicae infection. In a study conducted by Bednarek et al. [101], it was observed that MYB51 upregulates indolic glucosinolate biosynthesis in response to Flg22 , a microbe-associated molecular pattern (MAMP) molecule (these molecules play important role in plant resistance to pathogens), as evident by the significant downregulation of indolic biosynthetic genes like CYP79B2, CYP79B3, CYP83B1, SUR1, SUR2, UGT74B1, and AtST5a in myb51 mutant upon Flg22 induction in contrast with ATR1/MYB34. Two genes, PEN2 and PEN3, were found necessary for glucosinolate induction. Many other studies also showed the upregulation of indolic glucosinolate biosynthetic genes and transcriptional factors during pathogen attack [111–113]. Reports on induction of aliphatic glucosinolates by pathogens are limiting. One study suggests that in B. rapa, infection of Xanthomonas campestris pv. campestris (Xcc) induces the production of aliphatic glucosinolate gluconapin, even though its involvement in resistance is not clear [102, 114]. On the other hand, overexpression of BnMAM1, BnCYP83A1, and BnUGT74B1 in B. napus conferred greater resistance to fungal pathogens S. sclerotiorum and Botrytis cinerea [115] which shows the involvement of these genes in pathogen resistance.
The glucosinolate-mediated defense response is coordinated by various signaling molecules like jasmonic acid (JA), salicylic acid (SA), or ethylene (ET). These plant hormones have a well established role in eliciting plant defense response up on pest and pathogen attack. Apart from these hormones, there are other signaling molecules like glucose and mechanical injury/wounding that can also induce glucosinolate biosynthesis. Kiddle et al. [116] was among the pioneer in demonstrating the induction of glucosinolates in response to SA application. Soil application of SA induced glucosinolates, especially the benzyl glucosinolate 2-PE, in the leaves of oilseed rape (B. napus). Following this, next report came in the subsequent year, showing that spraying of B. napus plants with methyl jasmonate overaccumulated indolic glucosinolates especially 3-indolyl-methyl and 1-methoxy-3-indolyl-methyl-glucosinolates in a dose-dependent manner [117]. Brader et al. [118] demonstrated that jasmonate signaling is required for the induction of indolic glucosinolate biosynthesis in Arabidopsis through the activation of biosynthetic pathway genes in response to attack by the bacterial pathogen Erwinia carotovora. Pathogenic elicitors increased indolic glucosinolate content, particularly 3-indolyl-methyl-glucosinolate in the infected plants. The response is suggested to be mediated by JA as evident by the lack of induction in the jasmonate-insensitive mutant coi1-1. However, in this study, ET and SA could not elicit such a remarkable induction. A classic study by Mikkelsen et al. [119] describes the elicitation of glucosinolates in response to JA and SA. Wild-type Arabidopsis plants were treated with MeJA and 2,6-dichloro-isonicotinic acid (an SA analog), and the glucosinolate profiles were analyzed. The results showed that after MeJA treatment, the content of indolic glucosinolates was increased by three- to four-fold; particularly, the N-methoxy-indol-3-ylmethyl glucosinolate accumulated up to ten-fold in response to MeJA treatment. Upon SA treatment, 4-methoxy-indol-3-ylmethyl glucosinolate was also found to be overaccumulated. In contrast, among aliphatic glucosinolates, only 8-methylthiooctyl glucosinolate and 8-methylsulfinyloctyl glucosinolate levels were elevated. There was a corresponding transcriptional activation of indolic glucosinolate biosynthetic genes like CYP79B2 and CYP79B3 in response to jasmonate and SA. The study draws the conclusion that since different indolic glucosinolate biosynthesis genes are induced by jasmonate and SA, indolic glucosinolates might be responsible for an induced defense response, and the aliphatic glucosinolates might be primarily genetically regulated and not by biotic elicitors.
Cross talk between the signaling molecules and glucosinolate biosynthesis is very complex and can depend on the feeding behavior of the pest. It has been previously reported that JA and SA possess antagonistic roles [119, 120]. Most of the studies describe the induction using exogenously applied signaling molecules. Mewis et al. [104] evaluated the impact of constitutive and insect-induced changes in glucosinolates following attack by two phloem-feeding aphids (M. persicae and B. brassicae) and a chewing insect S. exigua using mutant and transgenic plants having changes in the JA (coi1), SA (npr1, non-expressor of PR genes 1), and ET (etr1, ethylene receptor 1) signaling pathways. Blocking of JA signaling in coi1 reduced constitutive glucosinolate concentrations. However, blocking of SA signaling at the mediator protein npr1 mutant increased glucosinolate content while enhanced SA signaling in hrl1 (hypersensitive response-like) mutants reduced constitutive glucosinolates. There was no significant impact on constitutive glucosinolate contents on blocking ET signaling (ET Resistant, etr1) and reducing SA concentrations in nahG transgene. It was hence proposed that increased glucosinolate accumulation in response to insect feeding require functional NPR1 and ETR1 but not COI1 or SA. NPR is suggested to be central regulatory protein in this complex interaction.
Transcriptional regulation of glucosinolates in response to JA, SA, and ACC (the ethylene precursor) is reported from Arabidopsis. Promoter GUS lines of the major aliphatic regulator MYB28 was generated, and the seedlings were treated with the abovementioned signaling molecules [72]. However, none of these molecules could induce MYB28 activity. Interestingly, MYB28 was found negatively regulated by SA. In another study, the aliphatic glucosinolate regulator MYB29 was found to be induced by JA signaling [71]. Recent update on cross talk between indolic glucosinolate regulators and the signaling molecules in Arabidopsis suggests that MYB51 is the major regulator of indolic glucosinolate biosynthesis upon SA and ET signaling and MYB34 is the key regulator upon ABA and JA signaling. The role of MYB122 is rather minor toward JA/ET-induced glucosinolate biosynthesis. However, all the three MYB factors are necessary for the induction of indolic glucosinolates in association with signaling molecules during plant defense [70]. Response of IQD1 was also studied in conjunction with these signaling molecules JA, SA, and ACC, the results of which showed no significant effect on transcript levels of this regulator [57]. However, AtDof1.1 was induced by JA [62].
Research studies have also suggested that glucose is an important signaling molecule that can induce glucosinolate transcriptional regulatory networks, integrating carbohydrate availability and hormone action during biotic stress [72]. It might be due to the fact that biotic stress, wounding, or infection by pathogens can alter the carbohydrate status of plant tissues. An extracellular invertase is suggested to be involved in carbon partitioning and integrating sugar, stress, and hormone signals [121]. Using promoter GUS lines in Arabidopsis, a significant induction of the native MYB28 promoter activity up on glucose treatment was observed [72]. Microarray data in Arabidopsis also shows that glucose induction can upregulate MYB28 [122]. Miao et al. [123] studied the effect of glucose on aliphatic glucosinolate biosynthesis in A. thaliana using mutants related to aliphatic glucosinolate biosynthesis and regulation, as well as glucose signaling. The study showed a significant enhancement in the individual and total glucosinolate contents up on glucose treatment, which was further explained by profound upregulation of MYB28 and MYB29, the two key transcriptional regulators of aliphatic glucosinolate biosynthesis. There was no enhancement observed for the double mutant myb28myb29. Moreover, the glucose-insensitive mutant (gin2-1) and the ABA-insensitive 5 mutant (abi5-7) showed substantial decrease in the content of total aliphatic glucosinolates. Concomitantly, the expression of MYB28 and MYB29 was also downregulated compared to the wild type in these lines. Another sugar-insensitive RGS1 (regulator of G-protein signaling) mutant (rgs1-2) also showed reduced accumulation of total aliphatic glucosinolates compared to the wild type. The results are conclusive of the fact that both HXK1- and RGS1-mediated sugar signaling regulate aliphatic glucosinolate biosynthesis through the involvement of transcription factors, MYB28, MYB29, and ABI5.
Interactions between JA, SA, and glucose in the regulation of glucosinolate biosynthesis genes were also reported. A recent report in Arabidopsis demonstrated that exogenous application of JA could enhance the glucose-induced glucosinolate biosynthesis, whereas the synergistic effect of SA and glucose on glucosinolate induction was comparably less [124]. The enhancement in the glucosinolate accumulation was supported by the upregulation of glucosinolate biosynthesis genes and transcription factors. Among the candidate genes, the expression of CYP79B2, CYP79B3, CYP79F1, CYP79F2, CYP83A1, CYP83B1, UGT74B1, and UGT74C1 was upregulated. The expression of transcriptional regulators, MYB28, MYB29, MYB34, and MYB122, also showed enhancement. Induction of indolic and aliphatic glucosinolates after treatment with JA and Glu in JA-insensitive mutants (coi1, jar1, and jin1) and glucose-insensitive mutants (rgs1-2 and abi5-7) was reduced. These results suggest that COI1, JAR1, JIN1, RGS1, and ABI5 are key components involved in the regulation of glucosinolate accumulation in response to JA and glucose treatments.
Among other phytohormones, brassinosteroids also affect glucosinolate biosynthesis [125]. 24-Epibrassinolide (EBR) has been shown to decrease glucosinolate biosynthesis in Arabidopsis. EBR significantly decreased the contents of both aliphatic glucosinolates including glucoiberin, glucoraphanin, and glucoerucin, and the indolic glucosinolates glucobrassicin and neoglucobrassicin. Glucosinolate estimation in BR-deficient mutant cpd showed significant enhancement in glucosinolate content, whereas the DWF4-ox transgenic plants overexpressing the BR biosynthetic gene DWF4 showed drastic reduction. It has been also shown that two major components in BR signal transduction BZR1 and BES1 are responsible for BR-mediated glucosinolate downregulation possibly by downregulating transcriptional regulators MYB28, MYB34, and MYB122 [125]. Mechanical damage or wounding is another stimulus which can regulate glucosinolate accumulation. Wounding mimic herbivory, even though few studies defend that, in herbivory, subsequent cascade of reactions may vary as insects probably have evolved strategies for counteracting the plants defense mechanism [126]. In Arabidopsis, it has been shown that mechanical stimuli such as touch or wounding can also induce the expression of MYB28 and MYB51 but not MYB34 or MYB122 [69, 72]. In Arabidopsis, expression of IQD1 and AtDof1.1 was also found wound inducible [57, 62].
Other than Arabidopsis, effect of elicitors has been reported from other Brassica species [96], with variable results. It was shown in B. napus that wounding can induce indolic glucosinolates [127]. Treatment of pak choi plants (B. ssp. chinensis) with different signaling molecules such as MeJA, JA, and methyl salicylate showed induction of glucosinolates only under JA [128]. Among the glucosinolates, only indolic glucosinolates were induced, with a dramatic enhancement of 1-methoxy-indol-3-ylmethyl glucosinolate. The molecular basis of the elevated glucosinolates were studied by the expression analysis of Arabidopsis orthologs of some of the candidate genes and major transcriptional regulators of indolic glucosinolates such as CYP79B2/B3, ST5a, MYB34, MYB51, and MYB122. The high glucosinolate phenotype was well correlated with the enhanced expression of these genes [128]. Similarly, JA and sugars have been found to enhance total glucosinolate levels in sprouting B. oleracea (broccoli), B. napus (rutabaga cabbage), B. rapa (turnip), and Raphanus sativus (China rose radish) and red radish [129]. In a recent study, effect of biotic elicitors on glucosinolate accumulation was studied in polyploid B. juncea [75]. Effects of JA, SA, glucose, and wounding were studied in a time course experiment. Total glucosinolates were enhanced under these stress conditions with an optimum accumulation observed 24 h post treatment. In general, all the aliphatic glucosinolates were enhanced mostly by glucose treatment, whereas indolic glucosinolates were highly induced under all the treatments except for glucose. Among the pool of glucosinolates, it was found that the indolic glucosinolates have more profound effect following the elicitor treatments. Indolic glucosinolates like 1-methoxy-indol-3-ylmethyl and its precursor indol-3-methyl glucosinolates showed strongest induction. Among the signaling molecules, JA was found to be the strongest inducer of indolic glucosinolates. Mechanical injury had the most pronounced effect on total glucosinolates accumulation. Expression profiling of multiple homologs of MYB transcriptional regulators of aliphatic and indolic glucosinolate biosynthesis showed differential expression patterns, suggesting a complex, coordinated, and overlapping effects toward glucosinolate accumulation in allotetraploid B. juncea.
3.5 Minerals and Other Abiotic Determinants of Glucosinolate Metabolism
The availability of macro- and micronutrients is vital for crop production. Nutrient status of the soil not only affects primary metabolism but also the secondary metabolism. Nutritional status also affects the feed value of the plants by invaders. Among the major nutrients, NPK (nitrogen, phosphorus, and potassium), effect of N and P can be both beneficial and detrimental with respect to plant defense [130]. Deficiency of potassium (K) makes the plant more susceptible to pest attack, as K deficiency increases the concentrations of sugars and amino acid which can attract the invaders [131]. The effect of other macro- and micronutrients are also depicted. Among all these, the well-studied nutrients with respect to glucosinolate regulation are described in this section.
Since glucosinolates are derived from amino acids, N status can play a major role in regulation of glucosinolate biosynthesis. MicroRNA profiling under nitrogen starvation showed that miR826 was induced under N deficiency. Interestingly, this microRNA was found to target the AOP2 gene which has a role in aliphatic glucosinolate regulation [132]. Later, the same group [133] identified another miRNA (miR5090) from the complementary transcript of the MIR826 gene, evolved from the inverse duplication of their common target gene, AOP2. Transcript levels of miR826, miR5090, and AOP2 transcripts in 10-day-old Arabidopsis (accession Cvi having functional AOP2) seedlings grown in N-deficient medium showed enhanced expression of both miR826 and miR5090, whereas the expression of AOP2 showed a decline, suggesting that AOP2 transcript level was negatively correlated with miR826 and miR5090 under N starvation. As expected, glucosinolate content was also declined. Most glucosinolate biosynthesis-associated genes were also downregulated under N starvation conditions. This nutrient-mediated regulation of glucosinolate candidate genes might be important for conserving the available N for vital metabolism of plants under stress conditions.
K is an important mineral having effect on glucosinolate accumulation. K deficiency induced JA and both indolic and aliphatic glucosinolates in Arabidopsis through COI1-dependent and COI1-independent pathways, respectively [134]. Transcript analysis of K-starved plants showed induction of genes involved in the biosynthesis of glucosinolates and JA signaling. Transcript and glucosinolate profiles of K-deficient plants were similar to herbivore attacked plants. The results suggest that the under K deficiency, JA pathway is induced as a means of fitness strategy of the plant by elevating glucosinolate content, thereby enhancing defense potential of the plant against insect pests.
Glucosinolates are S-rich secondary metabolites. Due to their high sulfur content and role in defense, glucosinolates are hypothesized to accumulate less under S-limiting condition, as plants utilize the available S for protein synthesis and essential functions instead of synthesizing secondary metabolites [135]. Hence, glucosinolates can also be considered as S reserve of Brassicaceae plants [30]. Induction of glucosinolates under S application is well documented in many cruciferous plants [136, 137]. Zhao et al. [138] showed that S deficiency reduced the accumulation of the alkenyl glucosinolates compared to that of indolic glucosinolates in B. napus. The condition was reversed up on application of S, in which alkenyl glucosinolates were largely induced. The authors suggest that higher induction of alkenyl glucosinolates in response to S might be due to the requirement of Met for their biosynthesis, as compared with the indolic glucosinolates which are derived from Trp. Similar study in turnip also showed that aliphatic glucosinolates, particularly 3-butenyl and 5-pentenyl, were strongly regulated on S application [139]. In broccoli (B. oleracea var. italica), differential accumulation pattern of glucosinolates under S-rich condition is reported, wherein aliphatic glucosinolates showed higher level of induction compared to indolic glucosinolates [140]. Since it is very clear that S plays an important role in glucosinolate regulation, much research was focused on discovering molecular basis of this interaction. Transcriptional regulation of glucosinolate biosynthesis genes have been reported under S starvation [136, 141].
Combining metabolomics and transcriptomics data of Arabidopsis grown under S deficiency, sulfotransferase genes clustering together with known glucosinolate biosynthesis genes were identified as candidate genes involved in glucosinolate biosynthesis [142]. Coexpression analysis under S deficiency also identified MYB28 as the major transcriptional regulator of aliphatic glucosinolate biosynthesis in Arabidopsis. It has been shown that almost all the genes including the transcriptional regulators MYB28, MYB29, and MYB34 were downregulated under S starvation [71]. S deficiency reduced the expression of glucosinolate biosynthesis genes such as BCAT4, MAM1, and MAM3 in SLIM1-dependant manner which suggests the possibility of a negative interaction between both pathways. However, the effect on the expression of MYB28, MYB29, and MYB76 was unclear [59]. Later, it has been shown that all the six MYB transcription factors involved in glucosinolate biosynthesis discovered to date (including MYB28, MYB29, MYB76, MYB34, MYB51, and MYB122) control biosynthesis genes involved in S assimilation such as APK, APK1, and APK [143]. SLIM1 was identified as a major transcriptional regulator of plant sulfur response, upstream to the MYB factors, affecting glucosinolate metabolism [60]. Another study in Arabidopsis [76] demonstrated that expression of MYB28, MYB29, and MYB76 responded to S stress in a differential manner in which the expression of MYB29 and MYB76 was repressed, whereas that of MYB28 was induced, which is somewhat in contrast with the previous observation [71]. A recent study revealed that the decrease in glucosinolate content under S deficiency was not coinciding with the transcript levels of these MYB regulators. Even though the glucosinolate content were declined, the transcript level of MYB34, MYB51, MYB122, and MYB28 almost remained unaltered during early time points. Interestingly with time, the transcript levels of these transcription factors increased. However, there was a correlation between the transcript levels of MYB29 and MYB76 and glucosinolate content. They hypothesized that negative effect of SLIM1 on glucosinolate regulatory genes can be overridden by a low glucosinolate signal inducing the transcription of MYBs in a feedback regulatory loop, up on continued S starvation (Scheme 2) [61].
In addition, there are other minerals which also affect glucosinolate biosynthesis. This includes calcium [144], molybdenum [145], zinc, boron, selenium, and cadmium. It has been shown that selenium fortification increases glucosinolate content in broccoli [146, 147]. Boron is shown to increase content of indolic glucosinolates [148]. Heavy metals like cadmium and zinc are known to enhance biosynthesis of glucosinolates in a dose-dependent manner in white cabbage plants [149]. Apart from minerals, there are other abiotic factors affecting glucosinolate metabolism [150]. These include temperature [151, 152], light conditions [153, 154], CO2 supply [155, 156], drought [157, 158], salinity [159, 160], and soil pH [161].
3.6 Cross Talk of Glucosinolate Biosynthesis Pathway with Other Metabolic Pathways
Cross talk between glucosinolates with other metabolic pathways has been elucidated based on the mutant phenotyping studies. Indolic glucosinolate biosynthesis is established to be linked with auxin biosynthesis/phenylpropanoid pathway /shikimic acid pathway (Scheme 2). Since indolic glucosinolate and auxin biosynthesis utilizes the common amino acid precursor Trp, the link between indolic glucosinolates and auxin has been largely investigated. Loss-of-function mutations in, BUS/SPS/CYP79F1, SUR1/C-S lyase1, SUR2/CYP83B1, and UGT74B1, the genes involved in glucosinolate core structure synthesis results in auxin-related phenotypes [23]. In bus, the knockdown mutant of CYP79F1 in Arabidopsis, a decrease in aliphatic glucosinolate was observed, whereas indolic glucosinolate and IAA levels were elevated [162]. It has been proposed that the level of IAA is regulated by the flux of indole-3-acetaldoxime through a cytochrome P450, CYP83B1, which serves as a metabolic branch point of auxin and indolic glucosinolate biosynthesis in Arabidopsis [163]. Disruption of function of SUR/C-S lyase1 and UGT74B1 also resulted in high auxin phenotype possibly due to the diversion of IAOx to IAA synthesis [164]. IAOx also serves as branch point in the synthesis of phytoalexin, camalexin [165]. It was shown that inhibition of camalexin biosynthesis from IAOx results in reduced accumulation of indolic glucosinolates and increased levels of IAA in Arabidopsis [23].
Characterization of ref2 mutant (REF2 encodes CYP83A1 involved in oxidation of aliphatic aldoximes) revealed a metabolic link between the glucosinolate biosynthesis and phenylpropanoid pathway [166]. The study suggests that a block at CYP83A1 activity can lead to phenylpropanoid and glucosinolate phenotypes having decreased COMT and caffeoyl-CoA O-methyltransferase activity as a result of overaccumulation of Met-derived aldoximes and IAA. A very recent study using ref5 (REF5 encoding CYP83B1) mutant showed reduced levels of indolic glucosinolate and enhanced accumulation of indole-3-acetaldoxime (IAOx). The ref5 mutants also showed an altered phenylpropanoid metabolism. Triple mutant, cyp83b1cyp79b2cyp79b3, showed no IAOx accumulation while showing an enhanced sinapoylmalate, indicating that levels of IAOx or a subsequent metabolite negatively influence phenylpropanoid accumulation. Analysis of double mutants, ref5ref2, showed a synergistic effect on the phenylpropanoid pathway [167].
Integrating transcriptomics and metabolomics data, it has been demonstrated that MYB51 and MYB122 have a positive correlation with the proximal network genes (downstream to Trp) that are involved in indolic glucosinolate biosynthesis, camalexin, and IAA biosynthesis. MYB29 and MYB76 showed negative correlation to genes that are involved in IAA and camalexin biosynthesis. Even though very weak, MYB28 and MYB34 showed positive correlation to the proximal network genes that are involved in indolic glucosinolate biosynthesis and negative correlation to genes that are involved in IAA and camalexin biosynthesis [168]. Recently, a novel microRNA, miR10515 has been identified to enhance IAA biosynthesis through the IAOx pathway, inhibiting both indolic glucosinolate and camalexin biosynthetic pathways [169]. However, the cross talk between these molecules is highly complex and not completely understood and needs further investigation.
3.7 Regulation of Glucosinolate Transport and Degradation
Glucosinolates are synthesized in leaves and are transported through phloem to developing seeds [97, 170]. Recently, Nour-Eldin et al. [171] have identified and characterized two members of the nitrate/peptide transporter family, GTR1 and GTR2, as high-affinity proton-dependent glucosinolate-specific transporters. The gtr1 and gtr2 double mutant in A. thaliana abolished glucosinolate accumulation in seeds and showed a concomitant overaccumulation in leaves and silique walls by about ten-fold, indicating that both transporters are essential for long-distance transport of glucosinolates. They have also suggested that GTR1 and GTR2 control the loading of glucosinolates from the apoplasm into the phloem. The regulatory mechanism of glucosinolate transport is yet unknown.
Glucosinolates are inactive in their native form. During tissue damage, glucosinolates stored in S-cells [172] get mixed with β-glucosidases called myrosinases (thioglucoside glucohydrolases, TGGs) which are compartmentalized in idioblasts or myrosin cells [173]. There are six members of TGGs identified in Arabidopsis [174], whereas B. napus have more than 20 myrosinases [175]. These have distinct organ- and tissue-specific activities [176]. Hydrolysis of thioglucoside linkage of glucosinolates results in the formation of glucose and an unstable aglycone moiety [6] which can spontaneously rearrange to form isothiocyanates or catalyzed by an epithiospecifier protein (ESP) to nitriles, epithionitriles [30, 177], depending on the biochemical properties of the specifier protein and the structure of the glucosinolate side chain, pH, and cofactors such as ferrous ion [173]. For example, if the glucosinolate side chain contains a hydroxyl group at carbon 3, oxazoline-2-thione is formed. At low pH, the formation of nitrile is favored, whereas neutral or high pH favors the formation of isothiocyanates. Other proteins like thiocyanate-forming protein (TFP) and nitrile-specifier proteins (NSPs) [173, 178] are also important in determining the type of degradation products. PEN2, another β-glucosidase, is also demonstrated to take part in indolic glucosinolate hydrolysis [101]. However, regulatory mechanism of glucosinolate degradation at molecular level is largely unexplored.
4 Conclusions and Future Prospects
We have seen that glucosinolate biosynthesis, diversity, and distribution are controlled by multiple factors which primarily include direct transcriptional regulators and polymorphism of genetic loci harboring candidate genes for chain elongation and side-chain modifications. Since glucosinolates serve as defense metabolites, the accumulation is highly affected by biotic challenges. The defense response is largely mediated through signaling molecules such as JA, SA, and ET. Since glucosinolates are S- and N-rich compounds, status of these nutrients also plays a major role in controlling glucosinolate metabolism. Availability of metabolites common to both primary metabolism and glucosinolate biosynthesis also plays a role in glucosinolate regulation leading to homoeostasis. There are abiotic factors which also influence glucosinolate metabolism in plants. There might be other possible routes of regulation such as post-transcriptional modifications and those involved in transport and regulation. All this interconnected interactions make the clear understanding of glucosinolate regulation in Brassica crops very complex. Integrating genomics and metabolomics data, the understanding of this complex regulatory mechanism is unraveled in Arabidopsis to a larger extent. However, the information from other Brassica crops is highly limiting. More efforts should be made in this direction as Brassicaceae contains large number of economically important crops. Fine tuning of the glucosinolate metabolism can lead to the improvement of nutritional quality of Brassicaceae crops by enhancing the beneficial glucosinolates and reducing those with anti-nutritional effects. Customizing the pathway can also improve the defense strategy of the plant against microbial and insect invaders.
Abbreviations
- ABA:
-
Abscisic acid
- BCAT:
-
Branched-chain aminotransferases
- CYP:
-
Cytochrome P450
- ET:
-
Ethylene
- FMO:
-
Flavin monooxygenase
- Glc:
-
Glucose
- GSL:
-
Glucosinolate
- GUS:
-
β-Glucuronidase
- HPLC:
-
High-performance liquid chromatography
- IPM:
-
Isopropylmalate
- JA:
-
Jasmonic acid
- MAM:
-
Methylthioalkyl malate synthase
- MeJA:
-
Methyl jasmonic acid
- Met:
-
Methionine
- QTL:
-
Quantitative trait loci
- Trp:
-
Tryptophan
References
Beilstein MA, Al-Shehbaz IA, Kellogg EA (2006) Brassicaceae phylogeny and trichome evolution. Am J Bot 93:607–619
Johnston JS, Pepper AE, Hall AE, Hodnett ZJCG, Drabek J, Lopez R, Price HJ (2005) Evolution of genome size in Brassicaceae. Ann Bot 95:229–235
Augustine R, Arya GC, Nambiar DM, Kumar R, Bisht NC (2014) Translational genomics in Brassica crops: challenges, progress, and future prospects. Plant Biotechnol Rep 8:65–81
Thangstad OP, Gilde B, Chadchawan S, Seem M, Husebye H, Bradley D, Bones AM (2004) Cell specific, cross-species expression of myrosinases in Brassica napus, Arabidopsis thaliana and Nicotiana tabacum. Plant Mol Biol 54:597–611
Luthy B, Matile P (1984) The mustard oil bomb – rectified analysis of the subcellular organization of the myrosinase system. Boichem Physiol Pfl 179:5–12
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Clay NK, Adio AM, Carine C, Jander G, Ausubel FM (2009) GSL metabolites required for an Arabidopsis innate immune response. Science 323:95–101
Hopkins RJ, Van Dam NM, Van Loon JJA (2009) Role of glucosinolates in insect plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83
Cartea ME, Velasco P (2008) Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochem Rev 7:213–229
Augustine R, Mukhopadhyay A, Bisht NC (2013) Targeted silencing of BjMYB28 transcription factor gene directs development of low glucosinolate lines in oilseed Brassica juncea. Plant Biotechnol J 11:855–886
Yanaka FJW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M (2009) Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori infected mice and humans. Cancer Prev Res 2:353–360
Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P (2012) Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res 5:603–611
Sonderby IE, Geu-Flores F, Halkier BA (2010) Biosynthesis of glucosinolates-gene discovery and beyond. Trends Plant Sci 15:283–290
Agerbirk N, Olsen CE (2012) Glucosinolate structures in evolution. Phytochemistry 77:16–45
Jensen LM, Kliebenstein DJ, Burow M (2015) Investigation of the multifunctional gene AOP3 expands the regulatory network fine-tuning glucosinolate production in Arabidopsis. Front Plant Sci 6:762
Diebold R, Schuster J, Daschner K, Binder S (2002) The branched-chain amino acid transaminase gene family in Arabidopsis encodes plastid and mitochondrial proteins. Plant Physiol 129:540–550
Schuster J, Knill T, Reichelt M, Gershenzon J, Binder S (2006) Branched-chain aminotransferase 4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell 18:2664–2679
Kroymann J, Textor S, Tokuhisa JG, Falk KL, Bartram S, Gershenzon J, Mitchell-Olds T (2001) A gene controlling variation in Arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol 127:1077–1088
Benderoth M, Textor S, Windsor AJ, Mitchell-Olds T, Gershenzon J, Kroymann J (2006) Positive selection driving diversification in plant secondary metabolism. Proc Natl Acad Sci U S A 103:9118–9123
Textor S, de Kraker JW, Hause B, Gershenzon J, Tokuhisa JG (2007) MAM3 catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiol 144:60–71
He Y, Mawhinney TP, Preuss ML, Schroeder AC, Chen B, Abraham L, Jez JM, Chen S (2009) A redox-active isopropylmalate dehydrogenase functions in the biosynthesis of glucosinolates and leucine in Arabidopsis. Plant J 60:679–690
Sawada Y, Kuwahara A, Nagano M, Narisawa T, Sakata A, Saito K, Hirai MY (2009) Omics-based approaches to methionine side chain elongation in Arabidopsis: characterization of gene encoding methylthioalkylmalate isomerase and methylthioalkylmalate dehydrogenase. Plant Cell Physiol 50:1180–1190
Grubb CD, Abel S (2006) Glucosinolate metabolism and its control. Trends Plant Sci 11:89–100
Knill T, Schuster J, Reichelt M, Gershenzon J, Binder S (2008) Arabidopsis branched-chain aminotransferase 3 functions in both amino acid and glucosinolate biosynthesis. Plant Physiol 146:1028–1039
Gigolashvili T, Yatusevich R, Rollwitz I, Humphry M, Gershenzon J, Flugge UI (2009) The plastidic bile acid transporter 5 is required for the biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana. Plant Cell 21:1813–1829
Hull AK, Vij R, Celenza JL (2000) Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci U S A 97:2379–2384
Chen S, Glawischning E, Jorgensen K, Naur P, Jorgensen B, Olsen CE, Hansen CH, Rasmussen H, Pickett JA, Halkier BA (2003) CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J 33:923–937
Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of Lphenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J Biol Chem 275:14659–14666
Naur P, Petersen BL, Mikkelsen MD, Bak S, Rasmussen H, Olsen CE, Halkier BA (2003) CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol 133:63–72
Wittstock U, Halkier BA (2002) Glucosinolate research in the Arabidopsis era. Trends Plant Sci 6:263–270
Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P (2008) The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J 55:774–786
Mikkelsen MD, Naur P, Halkier BA (2004) Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J 37:770–777
Grubb CD, Zipp BJ, Ludwig-Muller J, Masuno MN, Molinski TF, Abel S (2004) Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J 40:893–908
Klein M, Reichelt M, Gershenzon J, Papenbrock J (2006) The three desulfoglucosinolate sulfotransferase proteins in Arabidopsis have different substrate specificities and are differentially expressed. FEBS J 273:122–136
Hansen BG, Kliebenstein DJ, Halkier BA (2007) Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J 50:902–910
Li J, Hansen BG, Ober JA, Kliebenstein DJ, Halkier BA (2008) Subclade of flavin-monooxygenases involved in aliphatic glucosinolate biosynthesis. Plant Physiol 148:1721–1733
Kliebenstein DJ, Lambrix VM, Reichelt M, Gershenzon J, Mitchell-Olds T (2001) Gene duplication in the diversification of secondary metabolism: tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13:681–693
Kliebenstein DJ, Kroymann J, Brown P, Figuth A, Pedersen D, Gershenzon J, Mitchell-Olds T (2001) Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol 126:811–825
Hansen BG, Kerwin RE, Ober JA, Lambrix VM, Mitchell-Olds T, Gershenzon J, Halkier BA, Kliebenstein DJ (2008) A Novel 2-Oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol 148:2096–2108
Reichelt M, Brown PD, Schneider B, Oldham NJ, Stauber E, Tokuhisa J, Kliebenstein DJ, Mitchell-Olds T, Gershenzon J (2002) Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 9(6):663–671
Kliebenstein DJ, D’Auria J, Behere A, Kim J, Gunderson K, Breen J, Lee G, Gershenzon J, Last R, Jander G (2007) Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana. Plant J 51:1062–1076
Lee S, Kaminaga Y, Cooper B, Pichersky E, Dudareva N, Chapple C (2012) Benzoylation and sinapoylation of glucosinolate R-groups in Arabidopsis. Plant J 72:411–422
Zang YX, Kim HU, Kim JA, Lim MH, Jin M, Lee SC, Kwon SJ, Lee SI, Hong JK, Park TH, Mun JH, Seol YJ, Hong SB, Park BS (2009) Genome-wide identification of glucosinolate synthesis genes in Brassica rapa. FEBS J 276:3559–3574
Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin I, Zhao M, Yu J, Huang S, Wang X, Yue Z et al (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5:3930
Yan X, Chen S (2007) Regulation of plant glucosinolate metabolism. Planta 226:1343–1352
Bisht NC, Gupta V, Ramchiary N, Sodhi YS, Mukhopadhyay A, Arumugam N, Pental D, Pradhan AK (2009) Fine mapping of loci involved with glucosinolate biosynthesis in oilseed mustard (Brassica juncea) using genomic information from allied species. Theor Appl Genet 118:413–421
Wang H, Wu J, Sun S, Liu B, Cheng F, Sun R, Wang X (2011) Glucosinolate biosynthetic genes in Brassica rapa. Gene 487:135–142
Li G, Quiros CF (2003) In planta side-chain glucosinolate modification in Arabidopsis by introduction of dioxygenase Brassica homolog BoGSLALK. Theor Appl Genet 106:1116–1121
Liu Z, Hammerlindl J, Keller W, McVetty PBE, Daayf F, Quiros CF, Li G (2011) MAM gene silencing leads to the induction of C3 and reduction of C4 and C5 side-chain aliphatic glucosinolates in Brassica napus. Mol Breed 27:467–478
Liu Z, Hirani AH, McVetty PBE, Daayf F, Quiros CF, Li G (2012) Reducing progoitrin and enriching glucoraphanin in Brassica napus seeds through silencing of the GSL-ALK gene family. Plant Mol Biol 79:179–189
Zhu B, Wang Z, Yang J, Zhu Z, Wang H (2012) Isolation and expression of glucosinolate synthesis genes CYP83A1 and CYP83B1 in Pak Choi (Brassica rapa L. ssp. chinensis var. communis (N. Tsen & S.H. Lee) Hanelt). Int J Mol Sci 13(5):5832–5843
Meenu AR, Majee M, Pradhan AK, Bisht NC (2015) Genomic origin, expression differentiation and regulation of multiple genes encoding CYP83A1, a key enzyme for core glucosinolate biosynthesis, from the allotetraploid Brassica juncea. Planta 241:651–665
Wiesner M, Schreiner M, Zrenner R (2014) Functional identification of genes responsible for the biosynthesis of 1-methoxy-indol-3-ylmethyl-glucosinolate in Brassica rapa ssp. chinensis. BMC Plant Biol 8:114–124
Qu CM, Li SM, Duan XJ, Fan JH, Jia LD, Zhao HY, Lu K, Li JN, Xu XF, Wang R (2015) Identification of candidate genes for seed glucosinolate content using association mapping in Brassica napus L. Genes (Basel) 6(4):1215–1229
Augustine R, Bisht NC (2015) Biofortification of oilseed Brassica juncea with the anti-cancer compound glucoraphanin by suppressing the GSL-ALK gene family. Sci Rep 5:18005
Guo L, Yang R, Gu Z (2016) Cloning of genes related to aliphatic glucosinolate metabolism and the mechanism of sulforaphane accumulation in broccoli sprouts under jasmonic acid treatment. J Sci Food Agric. doi:10.1002/jsfa.7629
Levy M, Wang Q, Kaspi R, Parrella MP, Abel S (2005) Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J 43:79–96
Blume B, Nurnberger T, Nass N, Scheel D (2000) Receptor mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12:1425–1440
Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H (2006) Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18:3235–3251
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Frerigmann H, Gigolashvili T (2014) Update on the role of R2R3-MYBs in the regulation of glucosinolates upon sulfur deficiency. Front Plant Sci 5:626
Skirycz A, Reichelt M, Burow M, Birkemeyer C, Rolcik J, Kopka J, Zanor MI, Gershenzon J, Strnad M, Szopa J, Mueller-Roeber B, Witt I (2006) DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J 47:10–24
Kang HG, Singh KB (2000) Characterization of salicylic acid-responsive, Arabidopsis DOF domain proteins: over-expression of OBP3 leads to growth defects. Plant J 21:329–339
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010) MYB transcription factors in Arabidopsis. Trends Plant Sci 10:573–581
Gigolashvili T, Berger B, Flugge UI (2009) Specific and coordinated control of indole and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem Rev 8:3–13
Zhang Y, Li B, Huai D, Zhou Y, Kliebenstein DJ (2015) The conserved transcription factors, MYB115 and MYB118, control expression of the newly evolved benzoyloxy glucosinolate pathway in Arabidopsis thaliana. Front Plant Sci 6:343
Bender J, Fink GR (1998) A Myb homologue, ATR1, activates tryptophan gene expression in Arabidopsis. Proc Natl Acad Sci U S A 95:5655–5660
Celenza JL, Quiel JA, Smolen GA, Merrikh H, Silvestro AR, Normanly J, Bender J (2005) The Arabidopsis ATR1Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol 137:253–262
Gigolashvili T, Berger B, Mock HP, Muller C, Weisshaar B, Flugge UI (2007) The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 50:886–901
Frerigmann H, Gigolashvili T (2014) MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol Plant 7:814–828
Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuk A, Araki R, Sakurai N, Suzuki H, Aoki K, Goda H, Nishizawa OI, Shibata D, Saito K (2007) Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic GSL biosynthesis. Proc Natl Acad Sci U S A 104:6478–6483
Gigolashvili T, Yatusevich R, Berger B, Muller C, Flugge UI (2007) The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J 51:247–261
Gigolashvili T, Engqvist M, Yatusevich R, Muller C, Flugge UI (2008) HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol 177:627–642
Sønderby IE, Hansen BG, Bjarnholt N, Ticconi C, Halkier BA, Kliebenstein DJ (2007) A systems biology approach identifies a R2R3MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolate. PLoS One 2, e1322
Augustine R, Bisht NC (2015) Biotic elicitors and mechanical damage modulate glucosinolate accumulation by co-ordinated interplay of glucosinolate biosynthesis regulators in polyploid Brassica juncea. Phytochemistry 117:43–50
Li Y, Sawada Y, Hirai A, Sato M, Kuwahara A, Yan X, Hirai MY (2013) Novel insights into the function of Arabidopsis R2R3-Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Plant Cell Physiol 54(8):1335–1344
Kim B, Li X, Kim SJ, Kim HH, Lee J, Kim HR, Park SU (2013) MYB Transcription factors regulate glucosinolate biosynthesis in different organs of Chinese Cabbage (Brassica rapa ssp. pekinensis). Molecules 18:8682–8695
Araki R, Hasumi A, Nishizawa OI, Sasaki K, Kuwahara A, Sawada Y, Totoki Y, Toyoda A, Sakaki Y, Li Y, Saito K, Ogawa T, Hirai MY (2013) Novel bioresources for studies of Brassica oleracea: identification of a kale MYB transcription factor responsible for glucosinolate production. Plant Biotechnol J 11:1017–1027
Hsu FC, Wirtz M, Heppel SC, Bogs J, Krämer U, Khan MS, Bub A, Hell R, Rausch T (2011) Generation of Se-fortified broccoli as functional food: impact of Se fertilization on S metabolism. Plant Cell Environ 34:192–207
Yi GE, Robin AHK, Yang K, Park JI, Kang JG, Yang TJ, Nou IS (2015) Identification and Expression analysis of glucosinolate biosynthetic genes and estimation of glucosinolate contents in edible organs of Brassica oleracea subspecies. Molecules 20:13089–13111
Augustine R, Majee M, Gershenzon J, Bisht NC (2013) Four genes encoding MYB28, a major transcriptional regulator of aliphatic glucosinolate pathway, are differentially expressed in the allopolyploid Brassica juncea. J Exp Bot 64:4907–4921
Schweizer F, Fernández-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymonda P (2013) Arabidopsis basic Helix-Loop-Helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis insect performance, and feeding behavior. Plant Cell 25:3117–3132
Fernández-Calvo P, Chini A, Fernández-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, Pauwels L, Witters E, Puga MI, Paz-Ares J, Goossens A, Reymond P, De Jaeger G, Solano R (2011) The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23:701–715
Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, Kazan K (2007) MYC2 differentially modulates diverse jasmonate dependent functions in Arabidopsis. Plant Cell 19:2225–2245
Parkin I, Magrath R, Keith D, Sharpe A, Mithen R, Lydiate D (1994) Genetics of aliphatic glucosinolates. II. Hydroxylation of alkenyl glucosinolates in Brassica napus. Heredity 72:594–598
Mithen RF, Clarke J, Lister C, Dean C (1995) Genetics of aliphatic glucosinolates. III. Side chain structure of aliphatic glucosinolates in Arabidopsis thaliana. Heredity 74:210–215
Giamoustaris A, Mithen R (1996) Genetics of aliphatic glucosinolates IV Side-chain modification in Brassica oleracea. Theor Appl Genet 93:1006–1010
Zhang J, Wang X, Cheng F, Wu J, Liang J, Yang W, Wang X (2015) Lineage-specific evolution of Methylthioalkylmalate synthases (MAMs) involved in glucosinolates biosynthesis. Front Plant Sci 6:18
Neal CS, Fredericks DP, Griffiths CA, Neale AD (2010) The characterization of AOP2: a gene associated with biosynthesis of aliphatic alkenyl glucosinolates in Arabidopsis thaliana. BMC Plant Biol 10:170–186
Rohr F, Ulrichs C, Schreiner M, Zrenner R, Mewis I (2012) Responses of Arabidopsis thaliana plant lines differing in hydroxylation of aliphatic glucosinolate side chains to feeding of a generalist and specialist caterpillar. Plant Physiol Biochem 55:52–59
Burow M, Atwell S, Francisco M, Kerwin RE, Halkier BA, Kliebenstein DJ (2015) The Glucosinolate biosynthetic gene AOP2 mediates feed-back regulation of jasmonic acid signaling in Arabidopsis. Mol Plant 8:1201–1212
Wentzell AM, Rowe HC, Hansen BG, Ticconi C, Halkier BA, Kliebenstein DJ (2007) Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet 3, e162
Kliebenstein DJ, Gershenzon J, Mitchell-Olds T (2001) Comparative quantitative trait loci mapping of aliphatic, indole and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics 159:359–370
van Doorn HE, van der Kruk GC, van Holst GJ, Raaijmakers-Ruijs NCME, Postma E, Groeneweg B, Jongen WHF (1998) The glucosinolates sinigrin and progoitrin are important determinants for taste preference and bitterness of Brussels sprouts. J Sci Food Agric 78:30–38
Wentzell AM, Kliebenstein DJ (2008) Genotype, age, tissue, and environment regulate the structural outcome of glucosinolate activation. Plant Physiol 147:415–428
van Dam NM, Tytgat TOG, Kirkegaard JA (2009) Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem Rev 8:171–186
Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62:471–481
Baskar V, Park SW (2015) Molecular characterization of BrMYB28 and BrMYB29 paralogous transcription factors involved in the regulation of aliphatic glucosinolate profiles in Brassica rapa ssp. pekinensis. C R Bio 338(7):434–442
Tierens KF, Thomma BP, Brouwer M, Schmidt J, Kistner K, Porzel A, Mauch-Mani B, Cammue BP, Broekaert WF (2001) Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol 125:1688–1699
Kim JH, Jander G (2007) Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J 49:1008–1019
Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323:101–106
Sotelo T, Lema M, Soengas P, Cartea ME, Velasco P (2015) In vitro activity of glucosinolates and their degradation products against Brassica-pathogenic bacteria and fungi. Appl Environ Microbiol 81:432–440
Lankau RA, Kliebenstein DJ (2009) Competition, herbivory and genetics interact to determine the accumulation and fitness consequences of a defense metabolite. J Ecol 97:78–88
Mewis I, Appel HM, Hom A, Raina R, Schultz JC (2005) Major signalling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol 138:1149–1162
Mewis I, Tokuhisa JG, Schultz JC, Appel HM, Ulrichs C, Gershenzon J (2006) Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 67(22):2450–2462
Kusnierczyk A, Winge P, Midelfart H, Armbruster WS, Rossiter JT, Bones AM (2007) Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles after attack by polyphagous Myzus persicae and oligophagous Brevicoryne brassicae. J Exp Bot 58(10):2537–2552
Badenes-Perez FR, Reichelt M, Gershenzon J, Heckel DG (2013) Interaction of glucosinolate content of Arabidopsis thaliana mutant lines and feeding and oviposition by generalist and specialist lepidopterans. Phytochemistry 86:36–43
Appel HM, Maqbool SB, Raina S, Jagadeeswaran G, Acharya BR, Hanley JC Jr, Miller KP, Hearnes L, Jones AD, Raina R, Schultz JC (2014) Transcriptional and metabolic signatures of Arabidopsis responses to chewing damage by an insect herbivore and bacterial infection and the consequences of their interaction. Front Plant Sci 5:441
Ahuja I, van Dam NM, Winge P, Traelnes M, Heydarova A, Rohloff J, Langaas M, Bones AM (2015) Plant defence responses in oilseed rape MINELESS plants after attack by the cabbage moth Mamestra brassicae. J Exp Bot 66:579–592
Schlaeppi K, Abou-Mansour E, Buchala A, Mauch F (2010) Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J 62(5):840–851
Iven T, König S, Singh S, Braus-Stromeyer SA, Bischoff M, Tietze LF, Braus GH, Lipka V, Feussner I, Dröge-Laser W (2012) Transcriptional activation and production of tryptophan-derived secondary metabolites in Arabidopsis roots contributes to the defense against the fungal vascular pathogen Verticillium longisporum. Mol Plant 5(6):1389–1402
van de Mortel JE, de Vos RC, Dekkers E, Pineda A, Guillod L, Bouwmeester K, van Loon JJ, Dicke M, Raaijmakers JM (2012) Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol 160(4):2173–2188
Wei L, Jian H, Lu K, Filardo F, Yin N, Liu L, Qu C, Li W, Du H, Li J (2015) Genome-wide association analysis and differential expression analysis of resistance to Sclerotinia stem rot in Brassica napus. Plant Biotechnol J. doi:10.1111/pbi.12501
Velasco P, Lema M, Francisco M, Soengas P, Cartea ME (2013) In vivo and in vitro effects of secondary metabolites against Xanthomonas campestris pv. campestris. Molecules 18(9):11131–11143
Zhang Y, Huai D, Yang Q, Cheng Y, Ma M, Kliebenstein DJ, Zhou Y (2015) Overexpression of three glucosinolate biosynthesis genes in Brassica napus identifies enhanced resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS One 10(10), e0140491
Kiddle GA, Doughty KJ, Wallsgrove RM (1994) Salicylic acid induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J Exp Bot 45:1343–1346
Doughty KJ, Porter AJR, Morton AM, Kiddle G, Bock CH, Wallsgrove R (1991) Variation in the glucosinolate content of oilseed rape (Brassica napus L). Ann Appl Biol 118:469–477
Brader G, Tas E, Palva ET (2001) Jasmonate-dependent induction of indole glucosinolates in Arabidopsis by culture filtrates of the non specific pathogen Erwinia carotovora. Plant Physiol 126:849–860
Mikkelsen MD, Petersen BL, Glawischnig E, Jensen AB, Andreasson E, Halkier BA (2003) Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol 131:298–308
Glazebrook J, Chen W, Esters B, Chang HS, Nawrath C, Metraux JP, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34:217–228
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5:293
Li YH, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW (2006) Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a relevance vector machine. Genome Res 16:414–427
Miao HY, Wei J, Zhao YT, Yan HZ, Sun B, Huang JR, Wang Q (2013) Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. J Exp Bot 64:1097–1109
Guo R, Shen W, Qian H, Zhang M, Liu L, Wang Q (2013) Jasmonic acid and glucose synergistically modulate the accumulation of glucosinolates in Arabidopsis thaliana. J Exp Bot 64:5707–5719
Guo R, Qian H, Shen W, Liu L, Zhang M, Cai C, Zhao Y, Qiao J, Wang Q (2013) BZR1 and BES1 participate in regulation of glucosinolate biosynthesis by brassinosteroids in Arabidopsis. J Exp Bot 64(8):2401–2412
Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12:707–719
Bodnaryk RP (1992) Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry 31:2671–2677
Wiesner M, Hanschen FS, Schreiner M, Glatt H, Zrenner R (2013) Induced production of 1-methoxy-indol-3-ylmethyl glucosinolate by jasmonic acid and methyl jasmonate in sprouts and leaves of pak choi (Brassica rapa ssp. chinensis). Int J Mol Sci 14:14996–15016
Baenas N, Garcia-Viguera C, Moreno DA (2014) Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J Agric Food Chem 62:1881–1889
Fagard M, Launay A, Clément G, Courtial J, Dellagi A, Farjad M, Krapp A, Soulié M-C, Masclaux-Daubresse C (2014) Nitrogen metabolism meets phytopathology. J Exp Bot 65:5643–5656
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plantarum 133:682–691
Liang G, He H, Yu D (2012) miR826, a newly identified N-starvation-induced miRNA, was found to target the AOP2 gene identification of nitrogen starvation-responsive MicroRNAs in Arabidopsis thaliana. PLoS One 7(11):e48951
He H, Liang G, Li Y, Wang F, Yu D (2014) Two young MicroRNAs originating from target duplication mediate nitrogen starvation adaptation via regulation of glucosinolate synthesis in Arabidopsis thaliana. Plant Physiol 164(2):853–865
Troufflard S, Mullen W, Larson TR, Graham IA, Crozier A, Amtmann A, Armengaud P (2010) Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol 10:172
Falk KL, Tokuhisa JG, Gershenzon J (2007) The effect of sulfur nutrition on plant glucosinolate content: physiology and molecular mechanisms. Plant Biol 9(5):573–581
Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R (2003) Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J 33:633–650
Schonhof I, Blankenburg D, Muller S, Krumbein A (2007) Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J Plant Nutr Soil Sci 170:65–72
Zhao F, Evans EJ, Bilsborrow PE, Syers JK (1994) Influence of nitrogen and sulphur on the glucosinolate profile of rapeseed (Brassica napus L). J Sci Food Agri 64:295–304
Kim SJ, Matsuo T, Watannabe M, Watannabe Y (2002) Effect of nitrogen and sulphur application on the glucosinolate concentration in vegetable turnip rape (Brassica rapa L). Soil Sci Plant Nutr 48:43–49
Omirou MD, Papadopoulou KK, Papastylianou I, Constantinou M, Karpouzas DG, Asimakopoulos I, Ehaliotis C (2009) Impact of nitrogen and sulfur fertilization on the composition of glucosinolates in relation to sulfur assimilation in different plant organs of broccoli. J Agric Food Chem 57(20):9408–9417
Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yamaya T, Takahashi H (2003) Transcriptome profiling of sulfur-responsive genes in Arabidopsis reveals global effects of sulfur nutrition on multiple metabolic pathways. Plant Physiol 132:597–605
Hirai MY, Klein M, Fujikawa Y, Yano M, Goodenowe DB, Yamazaki Y, Kanaya S, Nakamura Y, Kitayama M, Suzuki H, Sakurai N, Shibata D, Tokuhisa J, Reichelt M, Gershenzon J, Papenbrock J, Saito K (2005) Elucidation of gene-to-gene and metabolite-to-gene networks in Arabidopsis by integration of metabolomics and transcriptomics. J Biol Chem 280:25590–25595
Yatusevich R, Mugford SG, Matthewman C, Gigolashvili T, Frerigmann H, Delaney S et al (2009) Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J 62:1–11
Vadassery J, Reichelt M, Hause B, Gershenzon J, Boland W, Mithöfer A (2012) CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol 159:1159–1175
Ide Y, Kusano M, Oikawa A, Fukushima A, Tomatsu H, Saito K, Hirai MY, Fujiwara T (2011) Effects of molybdenum deficiency and defects in molybdate transporter MOT1 on transcript accumulation andnitrogen/sulphur metabolism in Arabidopsis thaliana. J Exp Bot 62(4):1483–1497
Kim HS, Juvik JA (2011) Effect of selenium fertilization and methyl jasmonate treatment on glucosinolate accumulation in broccoli florets. J Am Soc Hort Sci 136:239–246
Avila FW, Yang Y, Faquin V, Ramos SJ, Guilherme LR, Thannhauser TW, Li L (2014) Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem 165:578–586
Shelp BJ, Shattuck VI, McLellan D, Liu L (1992) Boron nutrition and the composition of glucosinolates and soluble nitrogen compounds in two broccoli (Brassica oleracea var. italica) cultivars. Can J Plant Sci 72:889–899
Kusznierewicz B, Bączek-Kwinta R, Bartoszek A, Piekarska A, Huk A, Manikowska A, Antonkiewicz J, Namieśnik J, Konieczka P (2012) The dose-dependent influence of zinc and cadmium contamination of soil on their uptake and glucosinolatecontent in white cabbage (Brassica oleracea var. capitata f. alba). Environ Toxicol Chem 31(11):2482–2489
Martínez-Ballesta MC, Moreno DA, Carvajal M (2013) The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int J Mol Sci 14:11607–11625
Justen VL, Fritz VA (2013) Temperature-induced glucosinolate accumulation is associated with expression of BrMYB transcription factors. Hortscience 48:47–52
Johansen TJ, Hagen SF, Bengtsson GB, Mølmann JA (2016) Growth temperature affects sensory quality and contents of glucosinolates, vitamin C and sugars in swede roots (Brassica napus L. ssp. rapifera Metzg.). Food Chem 1(196):228–235
Huseby S, Koprivova A, Lee BR, Saha S, Mithen R, Wold AB, Bengtsson GB, Kopriva S (2013) Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. J Exp Bot 64(4):1039–1048
Kim YB, Chun JH, Kim HR, Kim SJ, Lim YP, Park SU (2014) Variation of glucosinolate accumulation and gene expression of transcription factors at different stages of Chinese cabbage seedlings under light and dark conditions. Nat Prod Commun 9(4):533–537
Schonhof I, Klaring HP, Krumbein A, Schreiner M (2007) Interaction between atmospheric CO2 and glucosinolates in broccoli. J Chem Ecol 33:105–114
La GX, Fang P, Teng YB, Li YJ, Lin XY (2009) Effect of CO2 enrichment on the glucosinolate contents under different nitrogen levels in bolting stem of Chinese kale (Brassica alboglabra L.). J Zhejiang Univ Sci B 10(6):454–464
Radovich TJK, Kleinhenz MD, Streeter JG (2005) Irrigation timing relative to head development influences yield components, sugar levels, and glucosinolate concentrations in cabbage. J Am Soc Hortic Sci 130:943–949
Schreiner M, Beyene B, Krumbein A, Stutzel H (2009) Ontogenetic changes of 2-propenyl and 3-indolylmethyl glucosinolates in Brassica carinata leaves as affected by water supply. J Agric Food Chem 57:7259–7263
Steinbrenner AD, Agerbirk N, Orians CM, Chew FS (2012) Transient abiotic stresses lead to latent defense and reproductive responses over the Brassica rapa life cycle. Chemoecology 22:239–250
Guo RF, Yuan GF, Wang QM (2013) Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J Zhejiang Univ Sci B 14(2):124–131
Velasco P, Cartea ME, Gonzalez C, Vilar M, Ordas A (2007) Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J Agric Food Chem 55:955–962
Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, Godde M, Uhl R, Pame K (2001) bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell 13:351–367
Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R (2001) CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13:101–111
Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Kamiya Y, Kasahara H (2009) Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc Natl Acad Sci U S A 106(13):5430–5435
Glawischnig E, Hansen BG, Olsen CE, Halkier BA (2004) Camalexin is synthesized from indole-3-acetaidoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc Natl Acad Sci U S A 101:8245–8250
Hemm MR, Ruegger MO, Chapple C (2003) The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 15(1):179–194
Kim JI, Dolan WL, Anderson NA, Chapple C (2015) Indole glucosinolate biosynthesis limits phenylpropanoid accumulation in Arabidopsis thaliana. Plant Cell 27(5):1529–1546
Malitsky S, Blum E, Less H, Venger I, Elbaz M, Morin S, Eshed Y, Aharoni A (2008) The transcript and metabolite networks affected by the two clades of Arabidopsis glucosinolate biosynthesis regulators. Plant Physiol 148:2021–2049
Kong W, Li Y, Zhang M, Jin F, Li J (2015) A novel Arabidopsis microRNA promotes IAA biosynthesis via the indole-3-acetaldoxime pathway by suppressing superroot1. Plant Cell Physiol 56(4):715–726
Chen S, Petersen BL, Olsen CE, Schulz A, Halkier BA (2001) Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiol 127:194–201
Nour-Eldin HH, Andersen TG, Burow M, Madsen SR, Jørgensen ME, Olsen CE, Dreyer I, Hedrich R, Geiger D, Halkier BA (2012) NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature 488(7412):531–534
Koroleva OA, Gibson TM, Cramer R, Stain C (2010) Glucosinolate-accumulating S-cells in Arabidopsis leaves and flower stalks undergo programmed cell death at early stages of differentiation. Plant J 64(3):456–469
Wittstock U, Burow M (2010) Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. Arabidopsis Book 8, e0134
Xu Z, Escamilla-Trevino LL, Zeng L, Lalgondar M, Bevan DR, Winkel BSJ, Mohamed A, Cheng C-L, Shih M-C, Poulton JE, Esen A (2004) Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol Biol 55:343–367
Rask L, Andreasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol 42:93–113
Lenman M, Falk A, Roedin J, Hoeglund A-S, Ek B, Rask L (1993) Differential expression of myrosinase gene families. Plant Physiol 103:703–711
Kissen R, Hyldbakk E, Wang CWV, Sormo CG, Rossiter JT, Bones AM (2012) Ecotype dependent expression and alternative splicing of epithiospecifier protein (ESP) in Arabidopsis thaliana. Plant Mol Biol 78:361–375
Kissen R, Bones AM (2009) Nitrile-specifier proteins involved in glucosinolate hydrolysis in Arabidopsis thaliana. J Biol Chem 284:12057–12070
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this entry
Cite this entry
Augustine, R., Bisht, N.C. (2017). Regulation of Glucosinolate Metabolism: From Model Plant Arabidopsis thaliana to Brassica Crops. In: Mérillon, JM., Ramawat, K. (eds) Glucosinolates. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-25462-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-25462-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25461-6
Online ISBN: 978-3-319-25462-3
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics