Abstract
The macronutrient phosphorus is critical to the physiological ecology of eukaryotic microalgae and cyanobacteria. What are the forms of phosphorus produced and used by these groups? This chapter reviews the distribution and processing of phosphorus in eukaryotic microalgae and cyanobacteria with a focus on how new so-called “omics” methods, advances in chemical analyses, and cell-specific approaches have driven new discoveries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Phosphorus
- Algae
- Cyanobacteria
- Phosphate
- Phosphonate
- Phosphite
- Organic phosphorus
- Redox cycling
- Phosphorus stress
1 Introduction
Phosphorus is fundamental to life, serving an integral role in aspects of cellular metabolism ranging from energy storage, to cellular structure, to the very genetic material that encodes all life on the planet. Weathering of phosphorus rich rocks is the major source of new phosphorus into aquatic environments (Benitez-Nelson 2000; Paytan and McLaughlin 2007). This phosphorus is utilized and transformed by cyanobacteria and eukaryotic algae driving complex metabolic and biogeochemical dynamics. For reviews on the biogeochemical dynamics of phosphorus see (Benitez-Nelson 2000; Paytan and McLaughlin 2007). Dissolved organic phosphorus and its cycling in marine systems is comprehensively reviewed in (Karl and Björkman 2002; Karl 2014), and in Karl 2014 there are recent summaries of marine cellular phosphorus dynamics, stress responses, and interactions with the marine phosphorus cycle (Karl 2014).
This chapter focuses on phosphorus physiology in microalgae including cyanobacteria and eukaryotic groups. Many of the examples come from studies with marine species, so care should be applied when extrapolating to freshwater taxa, although many of the responses and underlying themes are consistent. This chapter also does not focus on phosphorus in macroalgae. There are many reviews focused on phosphorus physiology or metabolism in eukaryotic algae, and cyanobacteria which should be referred to for additional details on all of the topics highlighted in the following sections (Grossman 2000; Beardall et al. 2001; Grossman and Takahashi 2001; Dyhrman et al. 2007; White and Metcalf 2007; Dyhrman 2008; Scanlan et al. 2009; Villarreal-Chiu et al. 2012; McGrath et al. 2013; White and Dyhrman 2013).
Knowledge about cellular phosphorus dynamics in microalgae has been rapidly advancing with new methods and more sensitive approaches. This chapter builds upon the rich literature highlighted above with a primary focus on findings leveraged from technical developments in cell sorting, molecular ‘omic tools, and advances in 31P NMR, and mass spectrometry. The chapter focuses on how these advances have expanded understanding in the following sections; (2) Phosphorus in the cell, (3) Inorganic phosphorus utilization, (4) Organic phosphorus utilization, (5) Phosphorus stress responses, (6) Methodological advances, and (7) Emerging themes and ongoing challenges.
2 Phosphorus in the Cell
Phosphorus is of course a critical nutrient, and required for all cyanobacteria and eukaryotic algae for growth (Fig. 1). Phosphorus is used as an energy currency in signaling and driving reactions, and it is also a building block in biochemicals as critical to life as nucleic acids and lipid membranes (Merchant and Helmann 2012). There are two primary ways to think about phosphorus in the cell; the presence of phosphorus–rich biochemicals and the type of phosphorus bond in phosphorus-containing compounds.
An overview of the roles phosphorus plays in algae and known sparing or recycling mechanisms (Adapted from: Merchant and Helmann 2012)
2.1 Phosphorus Biochemicals
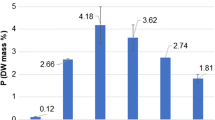
The major biochemical pools in a typical cell are: protein 52 %, polysaccharide 17 %, RNA 16 %, lipid 9.4 %, DNA 3.2 %, other <3 % (Karl 2014). This composition can vary considerably between taxa, and as a function of physiology. Of these pools, the largest phosphorus sink is nucleic acids (Fig. 1), making phosphorus essential for the storage expression of genetic information (Merchant and Helmann 2012). In fact, Van Mooy and Devol 2008 found that RNA synthesis was the largest biochemical sink for phosphate, accounting for about half of the total phosphate uptake, in North Pacific plankton communities dominated by Prochlorococcus (Van Mooy and Devol 2008). In this system phospholipids synthesis accounted for about, 20 % of the phosphate uptake, with the remainder (30 %) of phosphate uptake likely being accounted for by DNA, phosphorus biochemicals (e.g. ATP), and or abiotic adsorption (Van Mooy and Devol 2008). Other studies have also shown that synthesis of genomic DNA may compose as much as half of the total phosphorus demand for picocyanobacteria (Bertilsson et al. 2003). These contributions may not be consistent across all taxa, or physiological states, nevertheless they underscore the importance of phosphorus to the cellular lipid and nucleic acid pools of algae. It is worth noting that in a typical eukaryotic cell the RNA pool consists of about 80–85 % ribosomal RNA (28S, 18S, 5S), while 10–20 % is made up of a variety of a low molecular weight species (tRNAs, mRNA etc.). Thus there is a large demand for phosphorus associated with rRNA, that can be modulated to recycle phosphorus in some cases (Fig. 1).
Although the pool may be small, phosphorus is a major component in nucleoside triphosphates (Fig. 1) like ATP and GTP (Merchant and Helmann 2012). These critical biochemicals serve as the universal energy currency in the cell with many biosynthetic processes fueled directly or indirectly by their hydrolysis (Merchant and Helmann 2012). In this context the critical role that phosphorus plays in driving cellular metabolism in algae cannot be over stated. While measurements of specific phosphorus containing biochemicals like RNA or ATP are feasible, and valuable (both for cellular modeling studies and understanding phosphorus physiology and phosphorus cycling in field populations) direct measurements of these biochemicals in cultures or field populations of cyanobacteria and eukaryotic algae are uncommon (Karl 2014).
2.2 Phosphorus Bond Classes
Phosphorus in algae can be characterized by bond form, often utilizing 31P NMR to assess the chemical shift made by different bond forms to diagnose their presence and concentration in samples from eukaryotic algae and cyanobacteria. These bond forms include, phosphomonoester (P-O-C), phosphodiester (C-O-P-O-C), phosphonate (C-P), and polyphosphate (P-O-P-O-P) (Fig. 2). Surveys of cyanobacteria and algae suggest that the relative percentage of phosphorus in different bond forms is intrinsically variable between taxa, and can also vary with physiology and growth conditions. There is also some likely variability derived from the screening method used, as some forms do not extract well, and there can be biases around the specific biochemicals the bond type is contained in (Cade-Menun et al. 2005). Further, NMR approaches are not equally sensitive for all bond classes and materials, and sample processing could release enzymes or induce other alterations in the cellular phosphorus composition (Cade-Menun et al. 2005). A typical algal cell appears to be dominated by phosphate, phosphoester (monoester and diester) and polyphosphate (Clark et al. 1999; Dyhrman et al. 2009; Cade-Menun and Paytan 2010). The detection of phosphonate in eukaryotic algae or cyanobacteria is rare, although it may be present as trace amounts, or higher in some cases. For example, some studies have identified the presence of low amounts of phosphonate (<0.5 % total particulate phosphorus) using biochemical assays (Kittredge et al. 1969; Kittredge and Roberts 1969; Clark et al. 1999), and strains of the cyanobacterium Trichodesmium erythraeum Footnote 1 had a peak consistent with the presence of phosphonate which was detected using 31P NMR (Dyhrman et al. 2009). In the following sections, the properties, metabolic function and synthesis of polyphosphate, as well as phosphoester, and phosphonate bound organic matter are highlighted.
2.2.1 Polyphosphate
Polyphosphate compounds consist of three to thousands of orthophosphate groups linked together in a chain by phosphoanhydride (P-O-P) bonds (Fig. 2). Cellular polyphosphate can be found in many forms such as highly condensed storage granules, nucleotides such as adenosine triphosphate (ATP), and in inorganic chains (tripolyphosphate), which can range in length from three to thousands of residues long (Kornberg et al. 1999). The form of polyphosphate may differ in how it can be detected. For example, storage granules can be imaged through microscopy and staining techniques, and there are fluorometric methods for dissolved and particulate measurements of polyphosphate (Diaz and Ingall 2010; Mazard et al. 2012; Martin and Van Mooy 2013). Polyphosphate has also been detected in eukaryotic algae and cyanobacteria with 31P NMR (Dyhrman et al. 2009; Orchard et al. 2010b). Some polyphosphate containing biochemicals can be detected directly like ATP (Björkman and Karl 2001). There is no single perfect method for polyphosphate detection, because they are all biased and or prone to matrix effects and background to some degree. For example, polyphosphate chain length can bias the fluorometric method (Diaz and Ingall 2010), and there can be interference depending on the extraction procedure (Martin and Van Mooy 2013; Martin et al. 2014). Polyphosphate is intensively studied in the context of waste water treatment, but these methodological constraints have in part constrained studies of polyphosphate dynamics and metabolism in cyanobacteria and eukaryotic algae.
Polyphosphate is found in all major groups of life, but its function is varied, and in many regards remains unclear (Kornberg et al. 1999). With regards to the eukaryotic algae and cyanobacteria, polyphosphate has been examined in Chlamydomonas, Skeletonema, Thalassiosira, Synechocystis, Nostoc, Calothrix, Synechococcus and Trichodesmium among others (Romans et al. 1994; Capone et al. 1997; Gomez-Garcia et al. 2003; Mateo et al. 2006; Nishikawa et al. 2006, 2009; Diaz et al. 2008; Orchard et al. 2010b; Mazard et al. 2012). With its common presence, polyphosphate is largely thought to be ubiquitous (Raven and Knoll 2010). With this broad distribution, the metabolic functions of polyphosphate are variable and highly diverse. Cellular polyphosphate has been variously attributed to a stationary phase adaptation, an energy storage compound, a metal chelator, an osmotic regulator, a buffer against alkali conditions, a factor in DNA competency (as part of a DNA channel), and in phosphorus homeostasis (as a phosphate storage compound) among other potential functions (Kornberg 1995; Kornberg et al. 1999). It is largely this link between polyphosphate and phosphate storage that has driven research on polyphosphate dynamics as a function of phosphorus physiology in algae (see below). However, studies of polyphosphate function and biosynthesis are further complicated by the many varied factors that appear to influence polyphosphate concentrations and granule formation within cells; polyphosphate can accumulate in microbes as a function of growth phase, phosphorus supply, cation and metal concentrations, pH, or temperature (Kornberg 1995; Kornberg et al. 1999).
The production of polyphosphate in cyanobacteria is typically controlled by the ppK gene encoding a polyphosphate kinase (ppK) that reversibly adds phosphate to the end of the polyphosphate chain. For example, this enzyme would act to add or remove the gamma phosphate of ATP. The gene is common to all cyanobacteria that have been examined (Scanlan et al. 2009). The gene encoding a polyphosphate polymerase (Vtc4) has only recently been identified (Hothorn et al. 2009) in eukaryotes. This gene encodes a protein that interacts with the vacuole membrane and generates polyphosphate from the gamma phosphate in ATP in a phosphotransfer reaction to form polyphosphate chains. Polyphosphate polymerases and specific homologs of Vtc4 are present in the diatom Thalassiosira pseudonana (Hothorn et al. 2009), the pelagophyte Aureococcus anophagefferens (Wurch et al. 2011b), and the coccolithophore Emiliania huxleyi (Dyhrman et al. 2006b), but its distribution in other algae is not well known. Studies leveraging gene expression to examine trends in polyphosphate biosynthesis are hampered by the reversible nature of the biosynthesis enzymes like the polyphosphate kinase. However, upregulation of the Vtc4 polyphosphate polymerases has been observed in a number of studies (Dyhrman et al. 2006b, 2012). This may be to mobilize polyphosphate stores, but the expression of the genes does not appear to be linked to a dramatic reduction in cellular polyphosphate (Dyhrman et al. 2012).
There are two basic processes significant to polyphosphate dynamics related to phosphorus supply or phosphorus physiology (Eixler et al. 2006). The first, termed luxury uptake, is the storage of excess phosphate as polyphosphate when phosphorus is abundant. Luxury uptake of phosphorus has been documented in culture experiments with marine and freshwater algae (Bertilsson et al. 2003; White et al. 2006; Diaz et al. 2008). Luxury uptake has also been extensively studied in waste water treatment scenarios, where phosphorus is removed from activated sludge through luxury uptake and stored as polyphosphate in microbes (Pauli and Kaitala 1997; Crocetti et al. 2000). In algae, luxury uptake is likely to occur where phosphate is in excess relative to other resources like nitrogen, and algae are able to store this excess phosphate as polyphosphate for future utilization. Luxury uptake could thus drive the accumulation of polyphosphate in systems or areas where phosphate is in excess, like the coastal zone. Diaz et al. (2008) measured polyphosphate concentrations of ~7 % in coastal diatoms (Skeletonema spp.) under nutrient replete conditions and hypothesized a luxury uptake response. However, it is worth noting that for most algae the process of so called luxury uptake and formation of polyphosphate is counter intuitive as only a small fraction of a cell’s ATP requirement could be met by phosphorylation of ADP, even with a large “luxury” polyphosphate store. Polyphosphate takes up less volume than phosphate, so there may be size dependent influence over a given species’ production of polyphosphate when phosphorus is in excess. Raven and Knoll 2010 suggests this may be an important consideration for small cyanobacteria, but arguably less so when cells are larger (Raven and Knoll 2010). Another consideration is the potential for polyphosphate to increase cell density. The ballasting effect of polyphosphate in causing cells to sink is calculated to be greater if orthophosphate is stored as polyphosphate (Raven and Knoll 2010). Formation of polyphosphate under conditions of environmental excess, may be driven by factors other than phosphorus storage, which could in part explain the variability seen in cellular polyphosphate cycling.
The other process significant to polyphosphate dynamics is the overplus response, where phosphorus deplete cells accumulate polyphosphate in response to an increase in phosphate supply to levels greater than needed to meet phosphorus demand (Jacobson and Halmann 1982; Bolier et al. 1992). The overplus response is not particularly well studied in algae, but it has been hypothesized that overplus could drive the cellular accumulation of polyphosphate in low phosphorus systems where phytoplankton are phosphorus deficient, but experience variations in their phosphate environment (Karl and Björkman 2001, 2002). In fact, a large fraction of cellular phosphorus is found as polyphosphate in cyanobacteria from the genus Trichodesmium collected from the low phosphorus (<15 nM) Sargasso Sea. In this study, Orchard et al. 2010b hypothesized that this large allocation of phosphorus to polyphosphate could be the result of an overplus-like response (Orchard et al. 2010b). Clearly, the dynamics of cellular polyphosphate production is variable in both cultures and field populations. These polyphosphate dynamics clearly warrant further study to fully appreciate the role that polyphosphate plays in algal metabolism, phosphorus homeostasis, and influence over phosphorus biogeochemistry in different systems.
2.2.2 Phosphoester
Phosphorus containing organic matter with an ester bond is some of the most commonly observed, because phosphomonoester bonds (P-O-C), and phosphodiester (C-O-P-O-C) bonds are present in a number of important phosphorus-rich cellular biochemicals; these bonds are common in DNA, RNA, ATP, and lipids to name a few (Fig. 2). In studies utilizing 31P NMR to identify bond class, ester bond phosphorus is typically the major pool of organic phosphorus in algae (Dyhrman et al. 2009; Cade-Menun and Paytan 2010). Cade-Menun and Paytan 2010 showed that phosphoester in several algal species averaged across numerous control cultures was ~108 μmol g−1, representing about 25 % of the total cellular phosphorus in this bond class alone (Cade-Menun and Paytan 2010). The importance and abundance of ester bond phosphorus in cells is also reflected in the dissolved organic matter pool. For example, in typical marine systems high molecular weight dissolved organic phosphorus (DOP) is ~75 % (Clark et al. 1998) and in the larger fraction of DOP observed by Young and Ingall 2010, 80–85 % was phosphoester (Young and Ingall 2010).
Because this bond type is present in such diverse biochemicals, there is not a single specific gene or pathway that controls phosphoester biosynthesis. Rather, these pathways are as diverse as the biochemicals that contain ester bonds. The specific dynamics of ester bond organic matter have also not been studied in a comprehensive manner. Cade-Menun and Paytan 2010 examined the average phosphoester content in specific categories based on chemical shift with 31P NMR in suite of algae. Although there were subtle shifts in phosphoester composition as a function of light, temperature and phosphorus concentration, these were highly averaged responses (Cade-Menun and Paytan 2010). The 31P NMR approach could mask what are substantial intracellular rearrangements between different biochemicals, that do not resolve as a difference in the bulk phosphoester pool. More work is required to examine the dynamics of this bond class in algae.
2.2.3 Phosphonate
Phosphonate bond organic matter has a direct C-P linkage (Fig. 2), and unlike esters, phosphonates are not found in required cellular biochemicals like ATP, or nucleic acids. As a result, phosphonates were often considered a relic of a prebiotic age where oxygen concentrations were likely low, and organophosphonates, rather than organophosphoesters might have predominated (McGrath et al. 2013). For example in the late 1960s phosphonates were detected in the Murchison meteorite, suggesting a prebiotic origin (McGrath et al. 2013). However, increased scrutiny in recent years has built on early observations of phosphonates in microbes and invertebrates (Kittredge and Roberts 1969; White and Metcalf 2007), highlighting the potential significance of phosphonate in algae, and the importance of phosphonate cycling in aquatic systems (Karl 2014). Biochemicals with a phosphonate bond produced by microbes include 2-aminoethylphosphonate (2-AEP), phosphonoacetic acid, fosfomycin and methylphosphonate (Metcalf et al. 2012; Villarreal-Chiu et al. 2012). Only 2-AEP has been specifically detected in eukaryotic algae, including dinoflagellates and coccolithophores (Kittredge et al. 1969). Screening for phosphonate using 31P NMR, which requires additional analyses to identify specific compounds, has rarely detected phosphonate in cyanobacteria or eukaryotic algae (Cade-Menun et al. 2005; Cade-Menun and Paytan 2010). The major exception to date, is the presence of an apparent phosphonate chemical shift in 31P NMR profiles of T. erythraeum strains (Dyhrman et al. 2009). The phosphonate bond can be present in a diverse set of cellular biomolecules including lipids, proteins and antibiotics (McGrath et al. 2013). However, the presence of specific phosphonate biomolecules has not been examined in algae, and this warrants further investigation.
The metabolic roles of phosphonates in algae have not been directly examined, and of course depend on the biomolecules which contain the carbon phosphorus bond. In microbes, phosphonates like fosfomycin are antibiotics, and phosphonates like 2-AEP can be found as side groups on exopolysaccharides or glycoproteins, or in the polar head groups of membrane phosphonolipids (McGrath et al. 2013 and references therein). 2-AEP is similar to non phosphonate containing ethanolamine phosphate and is likely present in phosphonolipids (McGrath et al. 2013 and references therein). It has been suggested that phosphonolipids increase structural rigidity or protect against enzymatic degradation, relative to their ester bond counterparts, since the C–P bond is stronger, and not subject to hydrolysis by phosphatases (McGrath et al. 2013 and references therein). Characteristically, phosphonates are more resistant to chemical hydrolysis, thermal decomposition, enzymatic degradation and photolysis than similar compounds that contain phosphoester linkages (McGrath et al. 2013).
The initial step of most phosphonate biosynthesis is thought to begin with the reversible interconversion of phosphoenolpyruvate (PEP) or carboxyphosphoenolpyruvate (CPEP) to phosphonopyruvate or carboxyphosphonopyruvate via a PEP Mutase (Seidel et al. 1988) or CPEP Mutase (Hidaka et al. 1990) respectively. PEP Mutase typically acts with a phosphonopyruvate decarboxylase to catalyze the C-P bond formation, while the mechanism, or other enzymes, coupled to the CPEP Mutase are unknown (White and Metcalf 2007). One known exception to the PEP or CPEP Mutase biosynthesis pathways is the biosynthesis of methylphosphonate by the bacterium Nitrosopumilus maritimus (Metcalf et al. 2012). Whether phosphonate production in algae is mediated by these enzymes is unknown, as none of these enzymes have been specifically examined or characterized in cyanobacteria or eukaryotic algae.
Given the dearth of phosphonate biosynthesis and characterization studies in algae, the dynamics or regulation of phosphonate production is equally poorly understood. Some of these studies are constrained by the challenges in tracking low concentrations of the phosphonate bond, or specific phosphonate biochemicals like 2-AEP. There are no detailed studies of how cellular 2-AEP varies with growth phase, nutritional physiology or other factors. Using 31P NMR, Dyhrman et al. (2009) showed that phosphonate equivalents derived from an 18 ppm chemical shift co-varied at a roughly constant proportion (10 %) of the total cellular phosphorus. This suggests that phosphonate bond organic matter cycling within the cell was not related to phosphorus physiology (Dyhrman et al. 2009). However, there can be chemical interference with this chemical shift, and the relatively constant percentage could mask considerable rearrangement or cycling of phosphonate into different biomolecules. With the many recent discoveries regarding phosphonates in algae, studies of phosphonate compounds in algae are likely to continue to advance our understanding of their role in algal phosphorus physiology.
3 Inorganic Phosphorus Utilization
Phosphate is widely accepted to be the preferred form of phosphorus for growth, and is largely considered the only inorganic phosphorus source, although that view is changing. In the classical Monod model, growth rate would depend on the external concentration of phosphate (see Morel 1987). This model was altered to allow for internal storage, for example inorganic polyphosphate accumulation (Droop 1973). In the Droop model growth rate increases with increasing cell quota, so that growth rate is dependent on previous nutrient uptake as well as phosphate concentration in the environment. Many studies have illustrated the complexities involved in understanding nutrient uptake and growth (Morel 1987), as quotas are variable as a function of physiology, and that physiology can result in different spectrums of bioavailable phosphorus. Even more recently, the realization that phosphate is not likely the sole bioavailable inorganic phosphorus source to algae is driving renewed focus on this topic. This section focuses on phosphate, polyphosphate and phosphite utilization by eukaryotic algae and cyanobacteria.
3.1 Phosphate Uptake
Phosphate uptake is controlled by transporters in the cell membrane, and their form and abundance influences the kinetics of that uptake. Eukaryotic algae typically have multiple phosphate transporters, which has been observed in genome studies (Gobler et al. 2011; Read et al. 2013). The coccolithophore E. huxleyi, is one of only a few eukaryotic algae to have multiple strains sequenced, in this case the different isolates have different copy numbers of phosphate transporters (Read et al. 2013) which hints at the potential role of phosphorus physiology in driving genome differentiation. Although the eukaryotic algae have genes with clear homology to phosphate transporters, which have been studied in detail (Li et al. 2006, 2012), it is rare for them to be functionally characterized.
Phosphate uptake in the cyanobacteria is also controlled by phosphate transporters, which drive the affinity and rate of phosphate uptake. Some cyanobacterial genomes including strains of Crocosphaera watsonii (=Cyanobium waterburyi) and a single strain of Synechococcus (RS9916) appear to carry homologs of the E. coli pitA low affinity phosphate transporter (Dyhrman and Haley 2006; Scanlan et al. 2009; Bench et al. 2013). In E. coli this transporter mediates phosphate uptake in high phosphorus environments. In C. watsonii WH 8501 this gene does not appear to be regulated by phosphorus supply in contrast to the pstS component the pstSCAB phosphate uptake system (see Sect. 5.3) which is regulated by phosphorus supply (Dyhrman and Haley 2006). The pitA gene is rarely found in Synechococcus and is not apparently present in the Prochlorococcus genomes studied to date (Scanlan et al. 2009). It has been hypothesized that these groups may use the pstSCAB, high affinity phosphate uptake system, to mediate phosphate uptake regardless of phosphate concentration (Scanlan et al. 2009).
The kinetics of phosphate uptake typically behave with Michaelis-Menten kinetics where they have been observed in both pure culture and field populations, often using 33P or 32P radiotracers (Perry 1976; Casey et al. 2009; Laws et al. 2011). Specific uptake kinetic values and patterns of uptake include both monophasic and multiphasic uptake patterns (Chisholm and Stross 1976). Monophasic uptake suggests the presence of one transport system, and multiphasic kinetics the presence of more than one. For example, in the diatom T. weisflogii both Vmax (maximal uptake rate) and Km (affinity) increased with decreasing phosphate availability indicating the potential induction of a high affinity phosphate transport system and multiphasic kinetics (Donald et al. 1997). Similarly, Euglena gracilis exhibited multiphase phosphate uptake (Chisholm and Stross 1976). In Chlamydomonas, Vmax increases with phosphorus starvation, with the apparent presence of both low and high affinity phosphate transport systems. The high affinity systems control phosphate uptake at low phosphorus (Grossman and Takahashi 2001).
The kinetics of phosphate uptake have also been extensively studied in cultures and field populations of cyanobacteria. In culture, studies of phosphate uptake suggest that there is considerable variability in Km and Vmax between even strains of the same species (Fu et al. 2005). There is also considerable variability in these kinetic parameters as a function of phosphorus physiology. These observations are dependent on the types of transport systems each strain carries, and how their expression is modulated. For example, in the colony forming cyanobacterium Trichodesmium, Km does not appear to change in response to phosphorus physiology, however Vmax increases with decreasing phosphate availability (Fu et al. 2005), and higher Vmax was observed in Trichodesmium collected from low phosphorus systems relative to higher phosphorus systems (Orchard et al. 2010a). This could indicate that the same transport system is used regardless of phosphorus physiology, but that the number of transporters increases when cells are in a low phosphate environment. In Synechococcus WH7803 Km and Vmax both increased when phosphate was lowered indicating the potential induction of a high affinity phosphate transport system (Donald et al. 1997). Synechococcus PCC6803 has two complete pstSCAB systems one with low affinity and high velocity, the other with high affinity and low velocity, thus mediating different maximum phosphate uptake rates (Vmax) and half saturation constants (Pitt et al. 2010). In a last example, Prochlorococcus MED4 has a small cell-specific Vmax in culture (Krumhardt et al. 2013), and this value ranges from <0.02 in the Sargasso Sea (Casey et al. 2009) to between 5 and 20 amol P cell−1 day−1 in the higher phosphorus North Pacific (Duhamel et al. 2012). It can still compete in these environments however, because culture studies with Prochlorococcus MED4 indicate it has a high specific affinity for phosphate (Krumhardt et al. 2013). Measurements of uptake kinetics are valuable for modeling field populations. However, the detection of specific Vmax, Km, and phases is dependent on a number of factors including (1) the energy steps during transport like the use of ATP, (2) phosphorus bioavailability per cell, (3) the nutritional history of the cell, (4) the cell quota (which can shift based on physiology), and (5) the experimental substrate among other potential factors (Jansson 1988), and so specific patterns and values are not always directly comparable.
3.2 Polyphosphate Utilization
The bioavailability of polyphosphate to eukaryotic algae and cyanobacteria has not been studied in great detail. Inorganic polyphosphate is likely bioavailable if it is dissolved and in forms that are hydrolyzable by surface associated enzymes, or small enough to be taken up directly. There are limited studies on the bioavailability of pyrophosphate or polyphosphate in the eukaryotic algae, but ongoing work suggests that inorganic polyphosphate in chain lengths up to 120 residues is readily assimilated by a diversity of eukaryotic taxa (Diaz et al. 2015). The gene pathways mediating polyphosphate bioavailability are not well known.
Short (three residue) polyphosphate appears to be bioavailable to representative cyanobacteria, including Synechococcus and Prochlorococcus (Moore et al. 2005). Whether longer chain polyphosphate is bioavailable is not well known. In whole water samples from station ALOHA in the North Pacific that are likely dominated by cyanobacteria, polyphosphate was similar to glycerol phosphate, and other DOP compounds in its bioavailability (Björkman and Karl 1994). The mechanisms controlling extracellular polyphosphate metabolism in the cyanobacteria are not well understood. The genes for polyphosphate metabolism including a ppK and ppX, and the pyrophosphatase ppA can all act to break down polyphosphate into shorter chain lengths, but it is unclear if they are acting on exogenous polyphosphate. The ppA and ppK genes have been shown to be regulated by phosphorus physiology in Synechocystis strain PCC 6803 (Gomez-Garcia et al. 2003), but this is not the case for Synechococcus WH8102, or Microcystis aeruginosa (Tetu et al. 2009; Harke and Gobler 2013), suggesting that this pattern is not strongly consistent over different cyanobacterial groups.
3.3 Phosphite Metabolism
There is no evidence to date that any of the eukaryotic algae can use phosphite as a sole phosphorus source. However, a striking finding in recent years, is the discovery that Prochlorococcus strains can use phosphite as a sole phosphorus source (Feingersch et al. 2012; Martinez et al. 2012). One gene cluster implicated in phosphite metabolism is encoded by transport related genes (ptxABC) and a NAD-dependent phosphite dehydrogenase (ptxD). This gene cluster appears to be present in several cyanobacterial genomes including, Prochlorococcus MIT9301, MIT9303, Cyanothece sp. ATCC51142, and Trichodesmium erythraeum ISM101 Cyanothece CCY0110, Nostoc sp. PCC7120, Nostoc punctiforme PCC73102 and Nodularia spumigena CCY9414 (Martinez et al. 2012). Martinez et al. (2012) showed that Prochlorococcus MIT9301 can use phosphite as a sole phosphorus source, and that the ptxD gene complements E. coli phosphite utilization mutants in vivo. Martinez et al. (2012) also utilize metagenome and metatranscriptome data to show that phosphite utilization genes are expressed in low phosphorus waters and that their overall abundance is elevated in low phosphorus environments relative to high phosphorus environments. Heterotrophic bacteria can generate energy through the reduction of phosphite, which suggests that the prevailing concept of a lack of a phosphorus redox cycle in nature should be revisited (White and Metcalf 2007). Last, the apparent inability of the eukaryotic algae to metabolize phosphite, suggests this phosphorus source could drive niche separation between the eukaryotes and the cyanobacteria, at least in low phosphorus systems.
4 Organic Phosphorus Utilization
While inorganic phosphate is generally regarded as the most bioavailable form of phosphorus, it is increasingly recognized that organic phosphorus is a critical phosphorus source in aquatic environments. Although surprisingly little is known about the distribution and concentration of specific chemical constituents of the DOP pool in aquatic environments (see Karl and Björkman 2002), understanding of the bioavailability of specific forms of DOP has benefited dramatically from developments in genomics in particular. These studies have highlighted the diversity of bioavailable compounds as well as how variability in the gene distribution between strains and taxa could implicate phosphorus as an important driver of microbial niche partitioning. This section will review the presence and distribution of enzymes for the utilization of (1) phosphoester and (2) phosphonate bond organic matter. There are a number of sources that review the distribution and bioavailability of these bond classes (Karl and Björkman 2002; Dyhrman et al. 2007; White and Metcalf 2007; Villarreal-Chiu et al. 2012; Karl 2014).
4.1 Phosphoesterases
The concentration of phosphoester in the DOP pool is likely to be higher than phosphate in many systems such as the Sargasso Sea, because the DOP dominates dissolved inorganic phosphate, and phosphoester is the largest fraction of DOP (Jakuba et al. 2008; Young and Ingall 2010). As such, the presence and distribution of phosphoesterases likely plays a significant role in meeting algal phosphorus demand across many aquatic systems. Phosphoesterases are also likely important in phosphorus cycling and recycling in the cell as they hydrolyze phosphate from lipids, nucleic acids, and ATP among other biochemicals. Three important classes of phosphoesterases highlighted in the subsequent sections are (1) alkaline phosphatase, (2) phosphodiesterase, and (3) 5′ nucleotidase.
4.1.1 Alkaline Phosphatase
Alkaline phosphatase is arguably the most well-studied of the enzymes used by algae to hydrolyze ester form DOP. The enzyme is commonly present in eukaryotic algae and cyanobacteria where it hydrolyzes phosphate from phosphomonoesters for assimilation by the cell (Fig. 3). The enzyme is typically regulated by low phosphorus (Cembella et al. 1984a, b; Quisel et al. 1996; Lin et al. 2013). Although a primary inverse relationship to environmental phosphate is often observed, alkaline phosphatase activity can be detected in relatively high phosphate environments, and has even been observed to be induced by cyanobacterial toxins like cylindrospermopsin (Bar-Yosef et al. 2010), and cell-cell signaling (see Sect. 7.1.2) among other potential factors. Alkaline phosphatases are diverse and encoded by many different genes, often with many per genome, and the concurrent expression of many putative alkaline phosphatases in the transcriptome (Scanlan et al. 2009; Dyhrman et al. 2012; Read et al. 2013). It is possible that the multiple genes encode enzymes with different substrate specificities, metal co-factors or regulation patterns. For example, some cyanobacteria appear to carry both the phoX and phoA type genes (Orchard et al. 2009). Although homologs of the canonical E. coli phoA gene (Zn dependent) are present in some cyanobacteria and upregulated under low phosphorus conditions (Fuszard et al. 2010), recent research suggests that the phoX (thought to be Ca dependent) type alkaline phosphatase may be much more prevalent, at least in the marine cyanobacteria (Sebastian and Ammerman 2009; Kathuria and Martiny 2011). This finding was attributed to the fact that Zn concentrations in the aquatic systems like the ocean are low, whereas Ca is plentiful. However a recent study by Yong et al. (2014) determined that the PhoX enzyme has a novel Fe-Ca cofactor. Given that Fe is low in many regions of the ocean, Fe could limit the ability of marine cyanobacteria to hydrolyze phosphomonoesters in regions where phosphate was also low, like the North Pacific Subtropical Gyre. With Zn and Fe both at low concentrations in the ocean it is unclear why the Fe requiring phoX is more broadly distributed. The phoX type alkaline phosphatase is present in a diverse suite of marine and freshwater cyanobacteria, including the genera Trichodesmium, Synechococcus, Prochlorococcus, and Cylindrospermopsis (Orchard et al. 2009; Scanlan et al. 2009; Kathuria and Martiny 2011; Sinha et al. 2014). The phoX gene is upregulated by low phosphate conditions, in the cyanobacteria in which it has been examined (Orchard et al. 2009; Kathuria and Martiny 2011). Although the prevalence and importance of phoX is increasingly widely accepted, the study of these alkaline phosphatases is evolving as new tools (e.g. mass spectrometry proteomics) become available. For example, a recent study focused at the protein level found that the PhoA protein was much more abundant than the PhoX protein in phosphorus-starved Synechococcus WH8102 (Cox and Saito 2013).
A schematic of the common phosphohydrolytic enzymes and the reactions they catalyze. (a) Key enzymes for the hydrolysis of phosphoester found in eukaryotic algae and cyanobacteria. (b) Critical enzymes for the hydrolysis of phosphonate, some of which may be present in cyanobacteria. Many of the transcripts for many of these enzymes are induced by phosphorus stress in eukaryotic algae or cyanobacteria
Since the alkaline phosphatase encoding genes are not well characterized in the eukaryotic algae, the extent to which different alkaline phosphatases present in the eukaryotic algal genomes have different substrates, regulation patterns or co-factors is not comprehensively understood. Three examples from the eukaryotic algae, where the alkaline phosphatase protein has been purified and characterized include the green alga Chlamydomonas reinhardtii (Quisel et al. 1996), the dinoflagellate Prorocentrum minimum (=Prorocentrum cordatum) (Dyhrman and Palenik 1997), and the coccolithophore E. huxleyi (Xu et al. 2006). Putative alkaline phosphatase encoding genes have been identified in studies of the diatom T. pseudonana (Dyhrman et al. 2012), and the pelagophyte A. anophagefferens (Wurch et al. 2011b), where – like with the cyanobacteria – they are upregulated by low phosphate. This suggests that the enzyme is transcriptionally regulated in at least in this subset of examples. The metal co-factors associated with the alkaline phosphatases of the eukaryotic algae have not been widely studied. However, the coccolithophore E. huxleyi has an alkaline phosphatase that is sensitive to Zn, and possibly Co availability (Xu et al. 2006; Jakuba et al. 2008). Further, the diatom Phaeodactylum tricornutum has a phosphorus-regulated alkaline phosphatase that is not Zn dependent, but Ca activated more akin to the cyanobacteria (Lin et al. 2013).
Alkaline phosphatase activity is particularly well studied in algae because there are many substrate analogs for field studies (Dyhrman 2005). Phosphatase activity has been shown to be broadly present in both marine and freshwater environments (Duhamel et al. 2010 and references therein), where the activity is often (although not always) inversely related to the phosphate or total dissolved phosphorus concentration (Cembella et al. 1984a, b; Jansson et al. 1988; Karl and Björkman 2002; Karl 2014). There is also a fluorogenic substrate for use in cell-specific enzyme labeled fluorescence (ELF) assays (González-Gil et al. 1998). This substrate will tag cells with the enzyme activity with a fluorescent product (see below). Many studies have used this tool in both marine and freshwater systems to examine the distribution of alkaline phosphatase activity in both eukaryotic algae and cyanobacteria (González-Gil et al. 1998; Dyhrman and Palenik 1999; Rengefors et al. 2001, 2003; Dyhrman et al. 2002; Nedoma et al. 2003; Lomas et al. 2004; Ruttenberg and Dyhrman 2005; Dyhrman and Ruttenberg 2006; Nicholson et al. 2006; Hynes et al. 2009; Ranhofer et al. 2009; Duhamel et al. 2010; Mackey et al. 2012; Girault et al. 2013; McLaughlin et al. 2013). These studies suggest that alkaline phosphatase is widely present in field populations of algae, underscoring the importance of this enzyme in algal metabolism of phosphomonoesters.
4.1.2 Phosphodiesterase
The phosphodiesterases are not as well studied as alkaline phosphatases, however, the phosphodiesterase enzyme is also likely important for the metabolism of both exogenous and intracellular phosphodiesters. Phosphodiesterases act to break phosphodiester bonds in compounds like DNA, RNA, cyclic nucleotides, and lipids among others (Fig. 3). The genes encoding enzymes with phosphodiesterase activity are not well characterized relative to alkaline phosphatase, and there are likely many present in algae with a range of substrate specificities. For example cyclic nucleotide phosphodiesterase activity is related to nucleotide metabolism and broadly present in cyanobacteria including Synechocystis PCC6803, Arthrospira platensis, and Anabaena cylindrica (Sakamoto et al. 1991). General phosphodiesterase activity assayed with fluorometric substrates is also widespread among the eukaryotic algae including several species of Chaetoceros, Dytilum brightwellii, T. pseudonana, and the raphidophyte Heterosigma akashiwo (Yamaguchi et al. 2014). A phosphodiesterase was also partially purified and characterized as cell-surface associated in the diatom P. tricornutum (Flynn et al. 1986).
Phosphodiesterases may be regulated by a number of different factors, depending on their specificity and role in the cell. In the eukaryotic algae this activity appears to be increased when cells are starved for phosphate (Yamaguchi et al. 2014), and work in the diatom T. pseudonana suggests this enzyme is transcriptionally controlled (Dyhrman et al. 2012). Some eukaryotic algae, including diatoms and raphidophytes, have been shown to grow on phosphodiester as a sole phosphorus source, suggesting that the enzyme may act on exogenous sources (Yamaguchi et al. 2014). Culture studies have additionally identified the bioavailability of phosphodiesters such as cAMP in certain strains of Prochlorococcus and Synechococcus (Moore et al. 2005). This has been corroborated in field studies where the community is dominated by Prochlorococcus; here phsophodiester was a broadly available phosphorus source to the community (Björkman and Karl 1994). It is also thought that phosphorus-regulated phosphodiesterases could be involved in the breakdown of phospholipids seen in both marine algae and cyanobacteria under low phosphorus (Dyhrman et al. 2012).
Environmental measurements of phosphodiesterase activity are not common, but a recent study with samples spanning the North and South Pacific Oceans identified enhanced phosphodiesterase activity at the lowest (<10 nM) phosphate concentrations, and phosphodiesterase activity in the dissolved fraction was even higher than that of alkaline phosphatase activity (Sato et al. 2013). These results are consistent with culture studies and further emphasize the likely importance of this enzyme to DOP utilization by algae.
4.1.3 5′ Nucleotidase
The enzyme 5′ nucleotidase is also broadly present in algae where it hydrolyzes phosphate form 5′ nucleotides like ATP (Fig. 3). The genes encoding this enzyme are not well characterized, but putative 5′ nucleotidases are common in algae and detected in the genomes of the cyanobacteria and eukaryotic algae examined to date. 5′ nucleotidases are increasingly identified in transcriptome profiling studies, particularly with the eukaryotic algae like the pelagophyte A. anophagefferens, and the diatom, T. pseudonana among others (Dyhrman et al. 2006b, 2012; Wurch et al. 2011b). In these two cases, genes encoding 5′ nucleotidases were upregulated in transcriptomes from phosphorus-starved cells relative to replete, suggesting a transcriptional level regulation by phosphate. This differs from the lack of phosphorus regulation typically observed in heterotrophic bacteria (Ammerman and Azam 1985). A putative 5′nucleotidase transcript was also upregulated in phosphorus-depleted Synechococcus WH8102 (Tetu et al. 2009). The enzyme was partially purified and characterized as cell-surface associated in the diatom P. tricornutum (Flynn et al. 1986) and the coccolithophore E. huxleyi (Dyhrman and Palenik 2003). In both cases the enzyme activity was increased when cells were phosphorus- depleted (Flynn et al. 1986; Dyhrman and Palenik 2003). Last, a 5′ nucleotidase protein was also more abundant in a phosphorus-stressed proteome relative to a replete proteome in A. anophagefferens (Wurch et al. 2011a). Clearly this enzyme is broadly present and serves an important role in the metabolism of phosphorus, particularly when phosphorus is low.
It is common in algae to be able to grow on nucleotides as a sole phosphorus source, and the bioavailability and uptake phosphorus from exogenous ATP and AMP is well documented in cultures (Krumhardt et al. 2013) and field populations (Ammerman and Azam 1991; Björkman et al. 2012). The extent to which nucleotides are processed outside the cell versus taken up and then hydrolyzed inside the cell, is not well understood, but the studies available to date suggest that at least some nucleotidase activity is localized to the cell surface in eukaryotic algae (Flynn et al. 1986; Grossman and Takahashi 2001; Dyhrman and Palenik 2003; Wurch et al. 2011a), while cyanobacteria may be able to take up nucleotides directly.
Field measurements of bulk 5′ nucleotidase activity are rare, but do not appear to vary as a function of phosphate concentration, perhaps reflecting a lack of phosphorus regulation in heterotrophic bacteria (Ammerman and Azam 1985, 1991). Studies of ATP uptake and hydrolysis are increasingly common on flow sorted cyanobacterial and even small eukaryote populations. These studies suggest that utilization of ATP can potentially meet a large fraction of phosphorus demand in field populations of Prochlorococcus, Synechococcus, Trichodesmium, and picoeukaryotes, particularly when inorganic phosphate is low (Casey et al. 2009; Orchard et al. 2010a; Björkman et al. 2012; Duhamel et al. 2012).
4.2 Phosphonate
Phosphonates were generally considered to be an unavailable form of phosphorus for algal growth until the release of the marine cyanobacterial genomes revealed genes putatively involved in phosphonate metabolism (Palenik et al. 2003; Dyhrman et al. 2006a). These early observations have led to an expansion of work in this area, which collectively is demonstrating that many cyanobacteria have the ability to metabolize phosphonates through a diverse suite of enzyme systems (Scanlan et al. 2009; Martinez et al. 2010). Enzyme systems for the hydrolysis of phosphonates include substrate-specific enzymes like phosphonoacetaldehyde hydrolase, as well as the broad specificity C-P lyase (White and Metcalf 2007; Villarreal-Chiu et al. 2012; McGrath et al. 2013) (Fig. 3). Notably, clear pathways for phosphonate metabolism have not been identified in the eukaryotic phytoplankton, nor is there direct evidence from culture studies. If this finding is borne out by further scrutiny, a potentially significant component of the DOP pool is unavailable to the eukaryotes and may drive community composition changes where DOP is an important phosphorus source.
4.2.1 Substrate-Specific Phosphonate Hydrolases
Many of the phosphonate hydrolases are well characterized in heterotrophic bacteria, and their presence, distribution, and regulation increasingly so for the cyanobacteria. For comprehensive reviews see the following syntheses (White and Metcalf 2007; Villarreal-Chiu et al. 2012; McGrath et al. 2013). Substrate-specific enzymes include phosphonopyruvate hydrolase, phosphonoacetate hydrolase, and phosphonoacetaldehyde hydrolase (phosphonatase) among potential other less well-characterized enzymes (McGrath et al. 2013) (Fig. 3).
Phosphonoacetate hydrolase is encoded by the phnA gene (Villarreal-Chiu et al. 2012). It is a Zn metalloenzyme that hydrolyzes phosphonoacetate to form acetate and phosphate (McGrath et al. 2013) (Fig. 3). This enzyme would be a potential route for 2-AEP metabolism. Phosphonopyruvate hydrolase is encoded by palA and is also a metalloenzyme (Fig. 3). The latter is often encoded together with genes related to phosphonate transporter, but is not always regulated by phosphate (McGrath et al. 2013). Screens of ocean metagenomic data suggest that both genes are present, although phnA is much more abundant; present in ~11 % of genomes sampled in the Global Ocean Survey relative to ~0.1 % for palA (Villarreal-Chiu et al. 2012). Their oceanic distribution hints at the importance of these pathways of phosphonate metabolism in marine systems, however the distribution of these genes in cyanobacteria has not been established.
Phosphonoacetaldehyde hydrolase (phosphonatase) is encoded by phnX, and in the degradation of 2-AEP is linked to a 2-AEP pyruvate aminotransferase encoded by phnW (Fig. 3). These are present in some cyanobacteria including freshwater Synechococcus strain OS-B′ (Adams et al. 2008) and marine Synechococcus WH8102 (Su et al. 2003). The activity encoded by these genes can be induced by phosphorus deficiency (Villarreal-Chiu et al. 2012), or alternatively be substrate inducible (Adams et al. 2008), and further work is required to confirm their presence and regulation more broadly in the cyanobacteria.
Ongoing work in this area is contributing to a rapidly changing understanding of the phosphonate hydrolayses. Martinez et al. (2010) identified the presence of a 2-oxoglutarate dioxygenase, phnY, and a possible phosphonohydrolase, phnZ, in Prochlorococcus strains (MIT9303, MIT9301), which were sufficient to allow utilization of 2-AEP as the sole phosphorus source in E. coli. Interestingly, the frequency of the Prochlorococcus phnY and phnZ genes was significantly higher in the phosphorus-depleted surface waters of the Sargasso Sea compared with the North Pacific subtropical gyre (Coleman and Chisholm 2010; Martinez et al. 2010). However, the presence of these genes did not clearly confer the ability for Prochlorococcus MIT9301 to grow on 2-AEP as a sole phosphorus source, and the genes may be related to phosphite metabolism (Martinez et al. 2012). It is also important to note that evidence for phosphonate metabolism in the cyanobacteria has been identified in the absence of characterized gene pathways, suggesting there are other potential enzymes yet to be identified (Gomez-Garcia et al. 2011). In short, new pathways for phosphonate metabolism are still being identified and work remains in order to characterize these enzymes and the role they serve in cellular phosphorus metabolism for algae.
4.2.2 Broad Specificity C-P Lyase
In contrast to the substrate-specific phosphonate hydrolayses, there is a broad specificity enzyme complex called a C-P lyase, which can hydrolyze a diverse suite of phosphonate compounds (Fig. 3). The C-P lyase is encoded by a suite of genes denoted phnGHIJKLM (White and Metcalf 2004b, 2007). These genes are often linked to those for phosphonate transport denoted phnCDE (White and Metcalf 2007). The transport genes are broadly present in both marine and freshwater cyanobacteria (Scanlan et al. 2009; Bench et al. 2013; Harke and Gobler 2013). Conversely the C-P lyase encoding genes are less common (Dyhrman et al. 2006a; Scanlan et al. 2009). The phnJ gene is typically used as a marker for the C-P lyase enzyme, and it is present in Synechococcus sp. isolated from microbial mats (Adams et al. 2008), Trichodesmium (Dyhrman et al. 2006a), Cylindrospermopsis (Sinha et al. 2014), Nostoc PCC7120 (Dyhrman et al. 2006a), and Nodularia spumigena (Voss et al. 2013) to name a few. This gene set has not been observed in the marine picocyanobacteria like Prochlorococcus and Synechococcus to date (Scanlan et al. 2009). It is worth emphasizing that all of the C-P lyase containing cyanobacteria identified here are brackish, or freshwater except Trichodesmium. As such, Trichodesmium appears to occupy a unique niche with regard to phosphorus metabolism among the other marine cyanobacteria, which may explain why Trichodesmium is so successful in low phosphorus environments.
Expression of the C-P lyase genes is typically phosphorus controlled in E. coli and expression studies in the cyanobacteria suggest this is the case (Dyhrman et al. 2006a; Adams et al. 2008). Further, the expression of these genes has been seen in both marine and freshwater field populations (Dyhrman et al. 2006a; Gomez-Garcia et al. 2011). Although there are not fluorogenic substrates available for assaying C-P lyase activity, the enzyme activity can be tracked by the evolution of methane in the presence of methylphosphonate (Beversdorf et al. 2010). Using this type of assay, the Trichodesmium C-P lyase activity has been measured in both cultures and field populations, substantiating the gene expression results (Beversdorf et al. 2010). The composition of phosphonates is largely unknown, but the presence of a broad specificity enzyme may confer an advantage for growth on a broader spectrum of DOP in a select few cyanobacteria.
5 Phosphorus Stress Responses
Phosphorus deficiency has long been recognized as an important driver of algal physiological ecology in freshwater systems (Schindler 1977), and is increasingly recognized as a major driver of marine ecosystems (Karl 2014), influencing microbial genetic diversity (Coleman and Chisholm 2010) and global oceanic primary production (Benitez-Nelson 2000). For example, there is growing evidence that phosphorus limits marine primary production in the subtropical North Atlantic (Mather et al. 2008; Lomas et al. 2010), and other major ocean systems (Paytan and McLaughlin 2007), thus influencing the magnitude and rate of phosphorus and carbon export over modern and geological time-scales (Benitez-Nelson 2000; Paytan and McLaughlin 2007; Diaz et al. 2008). Often phosphorus deficiency, starvation, stress, and limitation are used interchangeably, but there are many subtleties to how these terms may be interpreted. Here the term phosphorus stress is used to mean a physiological response to low phosphorus (distinct from a stress response to high phosphorus), the extent to which the phosphorus stress response is able to recover phosphorus for the cell will dictate whether cellular growth is limited, or arrested by phosphorus. To cope with low phosphorus in nature, both cyanobacteria and eukaryotic algae have evolved an inducible, sophisticated, and multi-faceted, phosphorus stress response involving the following major strategies. These phosphorus stress responses include (1) robust sensor response control of phosphorus stress induced transcription, (2) phosphorus sparing or recycling (3) high affinity or increased phosphate transport, and (4) a switch to the utilization of alternative phosphorus forms.
5.1 Phosphorus Stress Signaling
In cyanobacteria, phosphorus stress responses are controlled by a sensor response system that results in the transcription of the set of genes cells need to respond to phosphorus deficiency. The genes making up this phosphorus stress response are often referred to as the pho regulon, after the term described for E. coli (Torriani-Gorini 1987; Wanner 1996). Transcription of the cyanobacterial pho regulon is thought to be controlled by a two component sensor response system (phoR, phoB), where PhoR senses phosphorus availability and activates the transcriptional regulator PhoB (Fig. 4). PhoB binds to specific regions of DNA upstream of pho regulon genes, called pho boxes. Pho boxes have been identified upstream of a number of putative pho regulon genes in marine cyanobacteria like Prochlorococcus, Synechococcus and Trichodesmium (Su et al. 2007). For example there is a putative pho box upstream of the phoX gene in Trichodesmium, which encodes an alkaline phosphatase induced by phosphorus stress (Orchard et al. 2009), as well as pho boxes present upstream of other phosphorus-regulated genes in Trichodesmium and Crocosphaera (Dyhrman et al. 2006a; Dyhrman and Haley 2006; Su et al. 2007; Orchard et al. 2009). This canonical phoB/phoR model is supported by studies in Synechococcus strain WH8102 where expression analyses of phoB/phoR mutants confirmed that these genes either directly or indirectly controlled transcription of pho regulon genes (Tetu et al. 2009). The similar sphS/R system in Synechocystis also controls the phosphorus stress response (Suzuki et al. 2004). In the cyanobacteria, there are also additional signaling genes to consider in the phosphorus stress response (Fig. 4). For example, strains of Synechococcus and Prochlorococcus have the ptrA gene, a paralog of the global nitrogen regulator ntcA (Scanlan et al. 2009). This gene is upregulated in response to phosphorus stress in Prochlorococcus strain MED4 along with phoR and phoB (Martiny et al. 2006; Reistetter et al. 2013), and Synechococcus strain WH8102 ptrA mutants have reduced inducible alkaline phosphatase activity relative to the wild type (Ostrowski et al. 2010).
The putative systems that control sensing and responding to phosphorus in algae. (a) The sensor response system for yeast involves the kinase (Pho81) which controls the phosphorylation or dephosphorylation of Pho4, which in turn controls transcription of the Pho genes (Lenburg and O’Shea 1996). The degree of phosphorylation on Pho4 can control the degree of transcription (Springer et al. 2003). In Chlamydomonas, a putative phosphorus regulatory protein Psr1, is also involved in regulating the transcription of phosphorus-responsive genes (Grossman and Takahashi 2001). The extent to which this model is broadly applicable in eukaryotic algae is poorly understood. (b) The putative sensor response system in cyanobacteria, is thought to be similar to E. coli, where PhoR is activated by low phosphorus, phosphorylating PhoB. PhoB controls transcription of the Pho regulon genes, with some exceptions (Su et al. 2007). In cyanobacteria like Synechococcus the PtrA protein has also been shown to have a regulatory role on sensing and responding to phosphorus stress (Ostrowski et al. 2010). Green indicates genes or proteins that have been observed to increase with phosphorus stress in the eukaryotic algae and cyanobacteria
The threshold for when this signal transduction cascade would occur is not well defined. It likely differs between isolates, and would potentially be a function of both exogenous phosphorus supply and intracellular phosphorus pools. In some cyanobacteria there is evidence of some pho regulon genes (e.g. fast and slow pstSCAB sets) being induced before others (Pitt et al. 2010), and it may be that there are components of the phosphorus stress response that are controlled by a different signaling cascade, or by changes in the phoB/phoR encoded mechanism that allow a graded response. For example, in Synechococcus WH 8102 there appears to be two controllers, with PhoB-dependent induction of high-affinity phosphate transporters, followed by the PtrA-dependent induction of phosphatases (Ostrowski et al. 2010).
The mechanisms controlling the phosphorus stress signaling cascade have been examined in Chlamydomonas (Grossman and Takahashi 2001), but are not well explored in many of the other eukaryotic algae. The Chlamydomonas signaling response appears to be controlled in part by the Psr1 protein (Fig. 4), which functions as a transcription regulator that influences transcription of the phosphorus stress response genes (Wykoff et al. 1999; Grossman 2000). Psr1 is the first regulator of phosphorus metabolism in eukaryotic algae to be identified, and it is related to regulators in Arabadopsis, not yeast (Wykoff et al. 1999). These findings suggest that phosphorus metabolism in Chlamydomonas and possibly other algae is regulated in a way that is different from that of nonphotosynthetic eukaryotes. However, recent analyses of phosphorus stress transcriptomes suggest that phosphorus stress signaling in some groups could be more akin to what is observed in yeast (Fig. 4). In yeast, the cyclin kinase system encoded by pho85 and pho80 acts to phosphorylate and block transcriptional activation of the phosphorus stress genes by pho4, a transcriptional activator (Lenburg and O’Shea 1996). The degree of phosphorylation may be tuned to the degree of phosphorus stress, allowing yeast to finely tune their stress responses (Komeili and O’Shea 1999; Springer et al. 2003). In low phosphorus conditions pho81 is upregulated and inhibits phosphorylation of pho4, allowing transcription of the phosphorus stress response genes (Komeili and O’Shea 1999; Wykoff and O’Shea 2001). In the pelagophyte A. anophagefferens putative pho81 and pho4 are upregulated under phosphorus stress (Frischkorn et al. 2014). Although certainly not definitive, this observation is suggestive of possible differences in phosphorus stress signaling between algal lineages, and this warrants closer scrutiny.
5.2 Phosphorus Sparing or Recycling
It has been widely observed that cyanobacteria and eukaryotic algae have the ability to modulate their phosphorus requirement (quota) thus reducing cellular phosphorus (Krauk et al. 2006). The mechanisms driving this phosphorus sparing are thought to generally fall into three main areas, reduction of phosphorus rich biochemicals, substitution of phosphorus rich biochemicals, and bypasses of phosphorus rich metabolic reactions.
5.2.1 RNA Recycling
Nucleic acids and lipids both represent major phosphorus reservoirs in phosphorus replete algae (Fig. 1). The cellular phosphorus found as RNA typically accounts for at least 50 % of the non-storage phosphorus in algae and plants (Raven 2013). It has been hypothesized (the growth rate hypothesis) that sustained rapid growth requires high concentrations of ribosomes. Since ribosomes are rich in phosphorus, this would predict growth rate and phosphorus content to be positively correlated (Flynn et al. 2010). Although the applicability of this hypothesis to algae is debatable (Flynn et al. 2010), this concept is consistent with a reduction in ribosomes and rRNA when phosphorus is deficient. When phosphorus is depleted studies have observed a decrease in RNA per cell (Grossman 2000) (Figs. 1 and 5), probably largely reflected in a decline in rRNA as protein translation slows, allowing this source of phosphorus to be recycled. In Chlamydomonas there is a reduction of the number of ribosomes in phosphorus-limited cells (Grossman 2000). In addition, global transcriptomic and proteomic studies in both cyanobacteria and eukaryotic algae have observed a down regulation of transcripts and ribosomal proteins in phosphorus-stressed cultures relative to replete controls (Tetu et al. 2009; Dyhrman et al. 2012). Although some of these responses may be common in any stressor that reduces growth rate, a reduction in the cellular rRNA pool would conserve this phosphorus for other uses in the cell.
A cell model illustrating common phosphorus stress responses in algae. Proteins in blue are found in both eukaryotic algae and cyanobacteria, while orange proteins are to date only found in cyanobacteria. Localization of the depicted proteins is for clarity and is not meant to represent actual cellular localization. Bars indicate changes in phosphorus containing biochemical pools or cellular inventories. ACP Acid phosphatase, APA Alkaline phosphatase (phosphomonoesterase), APR Adenosine-5′-phosphosulfate reductase, ASR Arsenate reductase, ATA Arsenite translocating ATPase, CPL Phosphonate (C-P) lyase, FGS Ferredoxin-dependent glutamate synthase, GST Glutathione S Transferase, NTD 5′ Nucleotidase, PDA Phosphate diesterase, PEP Phosphoenolpyruvate, PG Phospholipid, PNL Generic phosphonate lyase, PNT Phosphonate transporter, PolyP Polyphosphate, PPP Polyphosphate polymerase, PST P sugar transporter, PTA Phosphate transporter, PYK Pyruvate kinase, rRNA Ribosomal RNA, SQ Sulfolipid, SQD SQD1 (sulfolipid biosynthesis protein 1), SUP Sulfate permease, SUR Sulfate reductase, TPP Total particulate phosphate, UDPG Uridine diphosphate glucose
5.2.2 Phospholipid Substitution
Phospholipids are also a major phosphorus reservoir in algae. There is an increasingly rich literature spanning both cyanobacteria and eukaryotic algae that indicates many groups can substitute the non-phosphorus containing sulfolipid sulphoquinovosyldiacylglycerol (SQDG) for the phosphorus-rich phospholipid phosphatidylglercerol (PG) (Van Mooy et al. 2009; Merchant and Helmann 2012) (Fig. 5). This substitution is common in the marine cyanobacteria like Synechococcus and Trichodesmium, and eukaryotic algae including diatoms and coccolithophores (Van Mooy et al. 2009). For example, in Chlamydomonas, phosphorus deficiency reduces the phospholipids phosphatidylglercerol (PG) roughly 50 % in concert with an increase in sulfolipids (Merchant and Helmann 2012). In phosphorus-stressed cultures of eukaryotic algae Van Mooy et al. 2009 also observed that non-phosphorus containing ‘betaine’ lipids were substituted for phosphorus containing phosphatidylcholine. Van Mooy et al. (2009), suggesting that remodeling of the lipid membrane may be common but there are many subtleties to the lipids that are modulated when phosphorus is depleted. Studies in a representative diatom suggest this lipid substitution happens rapidly upon phosphorus stress, and the ratio of SQDG:PG also quickly reverts if stressed cells are refed with phosphorus (Martin et al. 2011). This is increasingly recognized as an important phosphorus sparing mechanism, with a reduction in phospholipids sparing between 10 % and 30 % of the phosphorus quota for model diatoms and coccolithophores (Van Mooy et al. 2009; Martin et al. 2011).
A protein putatively involved in sulfoplipid biosynthesis (a UDP-sulfoquinovose synthesis protein (encoded by sqdB)), was identified as upregulated in low phosphorus transcriptomes or proteomes of both A. anophagefferens and T. pseudonana, which both shift their SQDG:PG ratio under phosphorus stress (Wurch et al. 2011a; Dyhrman et al. 2012). However, in marine Prochlorococcus MED4 the sqdB transcript was not upregulated by P stress (Reistetter et al. 2013), nor was it upregulated in freshwater Microcystis aeruginosa (Harke and Gobler 2013). The production of betaine lipids is controlled by BTA1, a betaine lipid synthase, in Chlamydomonas (Riekhof et al. 2005), but this gene has either not been examined or detected in most of the other genomes from eukaryotic algae. For example, there is not a clear homolog of BTA1 in the T. pseudonana genome, or phosphorus-stressed transcriptomes and proteomes, even though this diatom is known to produce betaine lipids in response to phosphorus deficiency (Van Mooy et al. 2009; Dyhrman et al. 2012). Although substitution of phospholipids in a common phosphorus sparing mechanism, in many cases linking the substitution to the dynamics of specific genes has not been examined in detail. Collectively, these findings underscore the importance of phospholipid substitution but also emphasize that the molecular underpinnings of these responses are not fully understood.
5.2.3 Polyphosphate Dynamics
As discussed previously (Sect. 3.2), polyphosphate can serve as a storage compound that accumulates during luxury uptake in phosphorus-replete environments, and can be mobilized for growth in phosphorus deplete environments when cyanobacteria or eukaryotic algae experience phosphorus stress (Fig. 5). With this canonical understanding of polyphosphate, the cellular polyphosphate pool would be expected to decrease in phosphorus-stressed cyanobacteria and eukaryotic algae, although this is increasingly being shown to be more complicated than this canonical view.
It has been hypothesized that cells inducing a phosphorus stress response could experience a temporary excess of phosphorus that could repress continued phosphorus uptake. The upregulation of a polyphosphate polymerase or polyphosphate kinase to produce polyphosphate during phosphorus stress conditions could enable the creation of a sink of readily accessible phosphorus while also circumventing repression of phosphorus scavenging (Ogawa et al. 2000). In brief, it is thought that an increase in polyphosphate, or at least an increase in the ratio of polyphosphate to total particulate phosphate (polyP:TPP) may avoid transient phosphate accumulation and the down regulation of the phosphorus stress response. There is some evidence to support this hypothesis in algae. For example, the Vtc4 polyphosphate polymerase in E. huxleyi, T. pseudonana, and A. anophagefferens (Dyhrman et al. 2006b, 2012; Wurch et al. 2011b), and the ppK gene in Synechocystis PCC6803 (Gomez-Garcia et al. 2003), are all upregulated in phosphorus-stressed cultures relative to replete phosphorus controls. This observation is supported by an increase in polyP:TPP in phosphorus-stressed cultures of T. pseudonana, T. erythraeum, and Synechococcus WH8102 (Orchard et al. 2010b; Dyhrman et al. 2012; Martin et al. 2014). Although there could be an absolute decrease in polyphosphate with phosphorus stress, this pool may not decrease very much, such that the polyP:TTP ratio increases dramatically with phosphorus-stress.
Recent field work in the low phosphorus Sargasso Sea supports this observation. Martin et al. (2014) observed higher polyP:TPP in particulate matter dominated by cyanobacteria in the Sargasso Sea relative to the higher phosphorus regions (Martin et al. 2014). The supporting data implied that this observation was not necessarily the result of a strictly defined overplus scenario, but rather was the result of chronically low phosphorus and the phosphorus physiology of cyanobacteria in this region (Martin et al. 2014). This study, focused on a total cellular polyphosphate size fraction likely dominated by cyanobacteria, but the results are also consistent with taxon-specific measurements. Orchard et al. (2010a) saw similar increases in the ratio of polyphosphate to total particulate phosphorus in Sargasso Sea populations of the cyanobacterium Trichodesmium, higher than phosphorus replete culture controls (Orchard et al. 2010b). Further, trends in the picocyanobacterium Synechococcus from the same system, were also consistent with this trend (Martin et al. 2014).
In freshwater, lowering of phosphate in a eutrophic river, did not result in a lowering of algal community polyphosphate despite other evidence of phosphorus stress (Bolier et al. 1992). Taken together these observations emphasize the complicated dynamics of polyphosphate, and that the typical view that polyphosphate would be mobilized and largely drawn down by cells under phosphorus starvation is not necessarily the case. Polyphosphate may be drawn down, and or extensively cycled under phosphorus stress, but to date the reduction of total particulate phosphorus appears to largely be driven by changes in other phosphorus biochemicals (Fig. 5).
5.2.4 Phosphorus Bypasses
A last example of potential phosphorus sparing in algae involves bypassing of phosphorus rich metabolic reactions (Fig. 5). One example of a phosphorus rich metabolic pathway is glycolysis, where the conversion of one molecule of glucose into two molecules of pyruvate, requires two molecules of phosphate. Glycolysis in higher plants can be modulated by phosphorus stress in order to bypass those reactions that demand phosphate (Plaxton 1996). For example phosphoenolpyruvate carboxylase (PEPC) can serve as a glycolytic bypass enzyme by diverting phosphoenolpyruvate (PEP) to oxaloacetate (OAA) and releasing phosphate. OAA can then be converted to malate through the activity of malate dehydrogenase and eventually to pyruvate through a malic enzyme, thus completing the bypass of the ADP-requiring step of converting PEP directly to pyruvate catalyzed by pyruvate kinase (Plaxton 1996). Some evidence suggests that algae have this bypass (Theodorou et al. 1991; Wurch et al. 2011a; Dyhrman et al. 2012). Wurch et al. (2011a) identified a possible glycolytic bypass in A. anophagefferens using data from a low phosphorus proteome, and Dyhrman et al. (2012) found evidence of a glycolytic bypass in the diatom T. pseudonana, also from a low phosphorus proteome. However the extent to which this is true a phosphorus conservation strategy, and its presence in algae from other groups not highlighted here is largely unknown.
5.3 High Affinity or Increased Phosphate Transport
A common feature of the phosphorus stress response across both cyanobacteria and eukaryotic algae is the upregulation of phosphate transport systems when cells are phosphorus-stressed (Fig. 5). In some cases high affinity transporters are induced which would result in a decrease in Km and a change in the type or number of transporters would change Vmax. These potential shifts can be seen in culture studies or in the kinetic patterns for specific taxa as a function of phosphorus in the field (see Sect. 3.1). For example, phosphorus-stressed cultures of two different Trichodesmium strains had up to six times higher maximum phosphate uptake rates (Vmax) than the rates observed in phosphorus replete cultures (Fu et al. 2005).
In cyanobacteria, high affinity phosphate transport is controlled by pstSCAB, which includes a high affinity binding protein (PstS) and an ATP-driven transport complex (PstCAB) (Scanlan et al. 2009). These genes are common in all the cyanobacteria examined to date from both marine (Scanlan et al. 2009) and freshwater systems (Harke et al. 2012; Sinha et al. 2014). The regulation of this system by phosphorus stress is variable between species and related isolates (Martiny et al. 2006; Fuszard et al. 2010), although many studies have observed phosphorus stress upregulation of at least one copy of pstS (Martiny et al. 2006; Orchard et al. 2009; Harke et al. 2012). In some cases the full pstSCAB gene cassette is upregulated under phosphorus stress (Martiny et al. 2006), in others it appears that the pstCAB is constitutively expressed and only certain copies of pstS or certain pstSCAB sets are upregulated (Pitt et al. 2010). There are also examples of one copy of pstSCAB being an early responder to low phosphorus, while a second copy of the gene group is only induced later under extreme phosphorus stress (Pitt et al. 2010). In particular, the dynamics of pstS appear to track with aspects of P biogeochemistry. For example, Prochlorococcus pstS is overrepresented in genomes from the low phosphorus Sargasso Sea relative to the comparatively higher phosphorus North Pacific Subtropical Gyre (Coleman and Chisholm 2010), and both Synechococcus and Prochlorococcus PstS was detected in a metaproteome from the Sargasso Sea (Sowell et al. 2009). In summary it is common for cyanobacteria to modulate phosphate uptake as a function of phosphorus stress, and this is largely controlled through differential expression of pstS and or pstSCAB.
The upregulation of phosphate transporters is a common feature of studies in eukaryotic algae under phosphorus stress (Chung et al. 2003; Dyhrman et al. 2006b, 2012; Wurch et al. 2014). In Tetraselmis, there is a high affinity phosphate transporter that is upregulated when phosphorus is depleted (Chung et al. 2003), and this has been observed in the transcriptomes of a diverse array of other algae including two strains of A. anophagefferens (Wurch et al. 2011b; Frischkorn et al. 2014), the coccolithophore E. huxleyi (Dyhrman et al. 2006b), the prymnesiophyte Prymnesium parvum (Beszteri et al. 2012) and the diatom T. pseudonana (Dyhrman et al. 2012) among others. Many of these are Na+-dependent phosphate transporters, such as those characterized in plants (Dyhrman et al. 2012; Rubio et al. 2004). This pattern of high-affinity phosphate transporters being induced when phosphorus is low is also evident in changes in protein abundance (Dyhrman et al. 2012), and uptake kinetics (Perry 1976). In A. anophagefferens strain 1984, the transcription of phosphate transporter (PTA) is tightly controlled by phosphorus, as it is induced when exogenous phosphate is depleted, and the transcript signal is rapidly lost within just 2 h of phosphorus being re-supplied to phosphorus-stressed cells, the transcript is not even detectable within 24 h (Wurch et al. 2011a). Notably the protein does not turn over as quickly, making the transcript a potentially better indicator of instantaneous phosphorus stress, and the protein data suggesting that phosphorus stress induced changes in phosphate uptake, may extend a division or more after the cell is no longer phosphate deplete (Wurch et al. 2011a). The timing and turnover of transcript, protein and activity are important considerations when screening for these different signals in field populations.
5.4 Utilization of Alternative Phosphorus Forms
Arguably the most important and commonly observed phosphorus stress responses in cyanobacteria and eukaryotic algae is the induction of enzymes and transporters for the metabolism of alternative phosphorus forms (Fig. 5). These alternative phosphorus forms are typically organically complexed with either an ester, diester, or phosphonate bond (see Sect. 4). Other forms of phosphorus that appear to be bioavailable are polyphosphate, and phosphite (see Sects. 3.2 and 3.3). The specific substrates, genes, and enzymes involved in the utilization of alternative phosphorus forms is area of intense scrutiny and novel and surprising findings in recent years (see Sects. 3.2, 3.3, and 4). Much of the details of these pathways are discussed in other sections of this chapter. As was emphasized in those sections the majority of enzymes, transporters and metabolic pathways that control the utilization of alternative phosphorus forms, are upregulated when phosphorus drops below critical threshold levels, the presence of the substrate does not typically cause upregulation of the enzyme or pathways.
With their phosphorus stress induced regulation, the enzymes involved in the utilization of alternative phosphorus forms are often used as biomarkers of phosphorus physiology (Dyhrman 2008), the most common of which is the enzyme alkaline phosphatase. This marker of phosphorus stress can be extremely useful, but it is best employed when there is a comprehensive understanding of its activity, regulation, the turnover of the marker in question (e.g. activity, protein, or transcript), as well as the species or community under study (Dyhrman 2008).
A striking feature of phosphorus metabolism research in the last decade is the expansion of the alternative phosphorus forms known to be bioavailable. The suite of known phosphorus compounds that will support the growth of algae is growing rapidly, and is likely to continue to do so. In some cases knowledge about the environmental chemistry of particular substrates is lagging behind bioavailability studies (e.g. phosphite), and interdisciplinary studies that track specific compounds in the environment as well as their metabolism by algae will be important areas of future work.
6 Methodological Advances
A number of recent advances and approaches have developed which are allowing phosphorus metabolism, and phosphorus stress responses in the eukaryotic algae and cyanobacteria to be studied in new and powerful ways (Beardall et al. 2001; Dyhrman 2008; McLean 2013; Wagner et al. 2013). In the context of phosphorus metabolism, the new methodologies that have arguably had the most impact fall into two broad categories; (1) Cell or taxon-specific approaches and (2) Molecular ‘omic methods (Table 1).
6.1 Cell or Taxon-Specific Approaches
The value of screening physiology and cellular metabolism at a microbially-relevant scale is increasingly recognized (Azam and Malfatti 2007). Bulk approaches can miss subtlety in physiological responses to phosphorus availability, and how individual taxa compete for phosphorus among other variables. At a single-cell level, the cell-specific enzyme labeled fluorescence (ELF) substrate (Paragas et al. 1997) for detecting alkaline phosphatase activity has been widely applied (Dignum et al. 2004). This substrate is colorless and soluble when unreacted but when the enzyme hydrolyzes phosphomonoester the resulting reaction product is both fluorescent and insoluble; the product precipitating at the site of the enzyme activity. There have been many studies utilizing this substrate to examine enzyme localization (Dyhrman and Palenik 1999; Dyhrman et al. 2012), culture activity and physiology (González-Gil et al. 1998), and perhaps most commonly to look at the activity within or between species in field populations (Dyhrman 2008). The tool has been broadly applied to algae in both marine and freshwater environments to examine alkaline phosphatase activity and its regulation (see Sect. 4.1.1). One of the striking features of many of these studies is the cell to cell variability in the detection of enzyme activity from the same environment. This variability has been confirmed to not be an artifact through direct antibody labeling of the enzyme in the dinoflagellate P. minimum (Dyhrman and Palenik 2001), and could indicate the importance of small scale interactions between algae and their geochemical microenvironment, or their interactions with other cells (see Sect. 7.1.2). In summary, this methodological advance has been instrumental in expanding the study of both the enzyme, and algal physiological ecology at a microbially relevant spatial scale.
Single-cell genomics is of course a powerful tool to link metabolic capacity to specific, and typically uncultured, cells from the environment (Macaulay and Voet 2014). As was highlighted above, application of this tool is also serving to emphasize the diversity of both genotypes and the importance of the microenvironment. There are still few examples of its application in eukaryotic algae or cyanobacteria, but work by Kashtan et al. (2014) found that Prochlorococcus ecotypes are really a group of unique sub-populations of different genotypes consisting of “core” genes, and a set of “flexible” genes that are sometimes present. Notably, cells from different subpopulations carry similar flexible phosphonate related gene sets (Kashtan et al. 2014), suggesting that the metabolism of phosphonate can act as a niche defining feature. Here again a single-cell methodological advance is emphasizing the importance of phosphorus metabolism to the evolution of cyanobacterial genotypes at a fundamental level.
Flow cytometers capable of high fidelity and rapid sorting of cyanobacteria and picoeukaryotes take advantage of these groups’ intrinsic properties to sort populations based on size and pigmentation. This technology has been used to examine the uptake of radiolabeled phosphorus compounds and phosphate into specific populations (Björkman and Karl 1994; Casey et al. 2009; Duhamel et al. 2012). For example Casey et al. (2009) incubated Sargasso Sea populations with 33phosphate and 33ATP, then sorted cells into the picoeukaryote, Synechococcus and Prochlorococcus fractions with the flow cytometer and assayed isotope incorporation into the target populations (Casey et al. 2009). This general approach has been used in several recent studies (Casey et al. 2009; Björkman et al. 2012; Duhamel et al. 2012) to look at phosphorus uptake kinetics, competition between groups, and in particular to assay DOP utilization with proxy compounds like ATP. This major advance is allowing researchers to investigate how different populations of eukaryotic algae and cyanobacteria compete for phosphorus, and to what extent different phosphorus compounds are used to meet phosphorus demand.
6.2 Molecular ‘omic Approaches
Classic approaches to algal nutrition have most often used either cultured isolates or community level assays to examine nutrient uptake (Kudela and Cochlan 2000), nutrient ratios (Anderson et al. 2002 and references therein), and enzyme activity (Mulholland et al. 2003), among other approaches. These types of studies are very valuable, providing a basic understanding of phosphorus metabolism, much of which has been outlined above. However, advances in molecular approaches (Table 1) are expanding our understanding of how algae metabolize phosphorus in both cultures and field populations (Dyhrman 2008). In short, we are able to move beyond the outward responses of the cells to nutrients (e.g. changes in elemental composition or growth) to the underlying mechanisms that drive those responses. This level of molecular detail not only allows the promise of better predictive power with regards to a species’ response to phosphorus, but also provides the technological capability to specifically monitor that response in field populations. This section focuses on how (1) genomics and metagenomics, (2) transcriptomics and metatranscriptomics, (3) proteomics and metaproteomics, and (4) other molecular approaches (Table 1) have led to new insight into phosphorus metabolism and the role of phosphorus in the evolution and ecology of algae.
6.2.1 Genomics and Metagenomics
Genome sequencing was initially done in the cyanobacteria (Palenik et al. 2003; Rocap et al. 2003), and has now resulted in a handful of genomes for the eukaryotic algae (Rynearson and Palenik 2011). This has been one of the most dramatic advances driving research into the physiology of these groups in several decades (Table 1). Genomes can be screened for homologs to phosphorus-related genes in other organisms, and the genome sequence can serve as a framework for the development of other molecular applications like transcriptome profiling. The availability of genome sequences has resulted in the identification of genes that encode aspects of phosphorus physiology that were not considered with growth studies or other approaches, particularly with regard to studying microalgae metabolism of dissolved organic phosphorus. This was the case for the discovery of the C-P lyase gene complex in the Trichodesmium IMS101 genome (Dyhrman et al. 2006a). Phosphonate bound organic matter was largely thought to be refractory and not bioavailable to cyanobacteria until these genome observations. Now, these genome observations have resulted in a growing literature focused on the pathways, regulation, and bioavailability of phosphonates to cyanobacteria (Villarreal-Chiu et al. 2012). In one other example, analysis of the genomes for several strain of the coccolithophore E. huxleyi, found surprising variability in gene content with a set of core genes found in all strains, and a set of variable genes that were distributed unevenly across even closely related strains from the same environment (Read et al. 2013). Core genes included multiple copies of phosphate transporters and a high-efficiency alkaline phosphatase (Read et al. 2013), which underscores the importance of phosphorus acquisition to the ecophysiology and evolution of this organism. However, the numbers of phosphate transporters and alkaline phosphatases varied between strains, which is suggestive of the role genome variability plays in this organism’s capacity to thrive in a wide range of phosphorus regimes (Read et al. 2013). In brief, genome sequencing is dramatically changing views about phosphorus metabolism and the role of phosphorus in the selection and evolution of genotypes.
With the smaller genome sizes of cyanobacteria metagenome sequencing of field populations is also an emerging tool in studying the distribution of phosphorus metabolism genes in the field (Gilbert et al. 2011) (Table 1). Metagenome sequencing has been highly informative at the two oceanographic time-series sites; Station ALOHA in the subtropical North Pacific Gyre and the BATS Station in the Sargasso Sea region of the western North Atlantic. Comparing metagenome profiles of Prochlorococcus between the two sites Coleman and Chisholm (2010) discovered a number of phosphorus metabolism or scavenging genes were more abundant in the low phosphorus Sargasso Sea than in the higher phosphorus North Pacific subtropical Gyre. The authors suggest that this differential presence of key phosphorus genes is reflective of the selective role the environment plays in the evolutionary processes driving genome content. However, metagenome sequencing is still rarely used for gene discovery in the eukaryotic algae because of their large genomes and non-coding regions. Metagenome sequencing was recently used to do a partial reconstruction of an uncultured, flow-sorted haptophyte (Cuvelier et al. 2010). Although this and related studies have not used this approach to identify metabolic genes related to phosphorus, the approach does hold promise for future applications, particularly for important taxa that are not in culture.
6.2.2 Transcriptomics and Metatranscriptomics
Transcriptome profiling of a cell’s mRNA (Table 1) continues to evolve as a powerful approach for examining the responses of algae to phosphorus availability, and thus also in identifying the mechanistic drivers of phosphorus metabolism and scavenging. Particularly with the availability of genomes for model species, microarrays or high throughput RNA sequencing (e.g. RNA-seq) have been used to survey aspects of phosphorus metabolism in algae. This is even more critical for eukaryotic algae where the sequencing of nuclear eukaryotic algal genomes is challenging and they are still reasonably expensive to assemble, and annotate. Researchers have increasingly been leveraging transcriptome sequencing, assembly and annotation as an alternative for deriving information on metabolic capacity and even genes expression patterns in the algae particularly for the eukaryotes. Transcriptomics allows the rapid and efficient characterization of the expressed genes without the intergenic regions, introns, and repetitive DNA common to eukaryotes. This powerful tool is increasingly used for gene discovery in the eukaryotic algae, and for identifying the regulation pattern of those transcripts in both cyanobacteria and eukaryotic algae.
Gene discovery is poised to make extraordinary progress with the ongoing sequencing and analysis of marine eukaryotic algae through the MMETSP (Marine Microbial Eukaryote Transcriptome Sequencing Project – http://marinemicroeukaryotes.org/). The MMETSP initiated and funded by the Gordon and Betty Moore Foundation, will result in roughly 700 transcriptomes from ~17 Phyla. Although this is dramatically expanding our understanding of the molecular underpinnings driving algal physiology, there are some caveats to note about this dataset. One caveat is that the dataset still represents a relatively select group that is dominated by cultured isolates, many of which are from brackish or coastal environments. Furthermore, many of the samples were run on a single culture condition (typically in replete medium) or if there were conditions, they rarely included a phosphorus stress treatment. Many transcripts involved in the phosphorus stress response are expressed at very low levels (<5 reads per million) in replete, and are only abundant (~5,000 reads per million) when cells are phosphorus-starved (Dyhrman et al. 2012). It is possible that for some organisms, the transcriptome data does not include some of the phosphorus metabolism genes, because phosphorus-responsive transcripts were at such low expression levels they were not assembled in a typical replete transcriptome. Regardless of these caveats, the transcriptome data is advancing understanding of the molecular level pathways driving these cell’s physiological responses to phosphorus (Frischkorn et al. 2014). Recent assembly and low phosphorus profiling of the A. anophagefferens strain CCMP 1850 transcriptome, identified the transcripts putatively controlling phosphate uptake, organic phosphorus metabolism, and arsenate detoxification; determining important aspects of this organisms’ phosphorus stress response (Frischkorn et al. 2014). Further, transcriptome assembly resulted in the identification of the Vtc4 polyphosphate polymerase which was missed in the modeling of the strain CCMP 1984 genome (Frischkorn et al. 2014), highlighting the value of these approaches for gene discovery.
Beyond gene discovery, transcriptome profiling with microarrays or sequencing-based approaches have examined the transcripts differentially abundant in eukaryotic algae and cyanobacteria that are phosphorus-stressed (Dyhrman et al. 2006b, 2012; Erdner and Anderson 2006; Tetu et al. 2009; Wurch et al. 2011b, 2014; Harke and Gobler 2013). Although qRT-PCR based screening of genes of interest is quite valuable (Wurch et al. 2014), these global approaches are useful for identifying novel aspects of the phosphorus stress response without a priori knowledge about the most interesting targets. For example, a transcriptome study in T. pseudonana identified candidate genes that may be involved in the restructuring of the lipidome (see Sect. 5.2.2) that occurs under phosphorus stress (Dyhrman et al. 2012). This study also identified a number of phosphorus-responsive transcripts mapping to the genome where no gene model was predicted (Dyhrman et al. 2012). In this latter case, such global screening can highlight the regulation patterns of genes of unknown function, and genes that were not predicted in the gene modeling.
There is also a growing literature focused on profiling global (all transcripts) responses of algae in the field, so called metatranscriptomics (Table 1). This is most typically approached with either high throughput sequencing (Dyhrman et al. 2012) or arrays (Shilova et al. 2014). Using a sequencing based-approach to sample bacterioplankton from the coastal waters of the southeastern United States, Gifford et al. (2011) used an internal standard to make absolute (per liter) estimates of transcript numbers (Gifford et al. 2011). In the two coastal samples tested, the expression of genes encoding high affinity phosphate transport (pstSCAB), polyphosphate kinase (ppK), and low affinity phosphate transport (pitA) were all detected (Gifford et al. 2011). Notably, alkaline phosphatase (phoX, phoA), phosphonate enzymes (phnWXYZ) including genes for the C-P lyase (phnJ) were not consistently observed over the detection limit in coastal waters, perhaps underscoring that organic phosphorus cycling is not prevalent in this system (Gifford et al. 2011). In a related approach, undertaken in a low phosphorus lake (Vila-Costa et al. 2013), the authors observed expression of genes related to the metabolism of different DOP forms, pointing to a major role for DOP in controlling this ecosystem. Although these approaches were not directed to cyanobacteria specifically, they point to the utility of the approach in the context of understanding key aspects of phosphorus metabolism in situ. Metatranscriptome profiling in the eukaryotic algae has not been frequently employed nor used to examine aspects of phosphorus metabolism (Marchetti et al. 2012). This is likely to change soon as data from the MMETSP allows for more robust mapping and identification of sequence reads across a greater range of both species and environments.
6.2.3 Proteomics and Metaproteomics
Proteomics involves the identification of all of the proteins and their abundance in the cell (Table 1). Advances in mass spectrometry and the availability of increasing sequence databases from genome and transcriptome studies, is making proteomics an important new tool for examining phosphorus metabolism in algae. Queries made at the protein level have the major advantage of examining the molecules actually mediating phosphate transport etc., but assaying proteins of course comes with associated caveats in that, the method is not as comprehensive as assaying transcripts, some proteins do not extract well, and their identification is heavily reliant on gene modeling and sequence databases. In the context of phosphorus metabolism, global protein profiling has been used to study how the proteome is modulated by fluctuations in phosphate supply (Wurch et al. 2011a), and by phosphorus stress (Dyhrman et al. 2012) in eukaryotic algae with genomes. Recent work has also used proteomics to examine phosphorus stress responses and metal phosphorus interactions in isolates of Synechococcus (Mazard et al. 2012; Cox and Saito 2013). Here, Cox and Saito (2013) found that zinc levels modulated the proteins associated with the phosphorus stress response, suggesting that Zn and phosphorus metabolisms are linked in this strain. Interestingly, PhoX (Ca requiring – see Sect. 4.1.1) was less abundant than PhoA (Zn requiring – see Sect. 4.1.1), which goes against the prevailing thought that PhoX is most important in marine systems (Cox and Saito 2013).
Like proteomics, metaproteomics is a powerful approach for screening for the presence and abundance of proteins in the environment (Table 1). This tool will likely gain increasing utility for cyanobacteria and eukaryotic algae with the MMETSP, and increased numbers of genomes facilitating protein identification. Metatranscriptome profiling has been applied in both the South Atlantic (Morris et al. 2010) and the Sargasso Sea (Sowell et al. 2009), where both studies observed a dominance of transport related proteins. Prochlorococcus and Synechococcus phosphate transport-related proteins were identified in the Sargasso Sea (Sowell et al. 2009), but not in the South Atlantic (Morris et al. 2010), suggestive of the fact that lower phosphate concentrations in the Sargasso Sea are influencing the physiology of cyanobacteria in this system. Moving forward, these types of studies are likely to be expanded to other systems and the eukaryotic populations of algae.
6.2.4 Other Molecular Approaches
There are of course many molecular level approaches for tracking algal phosphorus metabolism in powerful ways. Targeted approaches will continue to be valuable for robust quantification of the transcripts and proteins that drive the cycling of phosphorus both inside and outside the cell. For example, Scanlan and Wilson (1999) purified a Synechococcus protein identified as the phosphate-binding protein PstS, assayed its regulation pattern using antibody probes, which then could also be used to trace the abundance of the protein across gradients in phosphorus concentration in the field. Additional examples are plentiful from both studies with the cyanobacteria (Orchard et al. 2009) and the eukaryotic algae (Dyhrman and Palenik 2001; Wurch et al. 2014).
Two additional global approaches that are gaining prominence are studies tracking global lipid composition (the lipidome) and global metabolite abundances (the metabolome) (Table 1). The lipidome is receiving intense scrutiny in the context of everything from viral susceptibility to phosphorus metabolism (Van Mooy et al. 2009; Martin et al. 2011; Fulton et al. 2014) and lipid screening methods have been instrumental in understanding how eukaryotic algae and cyanobacteria are able to modulate their lipid pool to reduce phosphorus demand (see Sect. 5.2.3). For example, lipid screening in both groups identified that betaine lipids, which are nitrogen containing can substitute for phospholipids in the eukaryotic algae (Van Mooy et al. 2009). As the authors point out, this is significant because there is then an increased demand for nitrogen, the so called nitrogen penalty (Van Mooy et al. 2009). In this way, the cycling and metabolism of nitrogen and phosphorus are linked.
Metabolomics is still in the early stages of being applied to the study of algae in culture or the environment (Kujawinski 2011). Although this tool is not as yet frequently employed to the study of phosphorus metabolism it is increasingly common. For example, studies have used metabolomic approaches to examine the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii (Renberg et al. 2010) and questions related to algal-based biofuel development (Ito et al. 2013). In the end, there is an ever growing suite of molecular approaches that will continue to transform understanding of phosphorus metabolism.
7 Emerging Themes and Ongoing Challenges
New discoveries in algal phosphorus metabolism are pointing to several themes that likely will be the focus of future work and new developments in our understanding of phosphorus in both algae and aquatic systems. These themes revolve around (1) metabolism of reduced phosphorus, and (2) the importance of cell-cell interactions. There are also ongoing challenges associated with advancing research on algal phosphorus metabolism, including but not limited to (1) a dearth of methods for assaying utilization of alternative phosphorus forms and (2) a lack of tools for quantifying specific phosphorus bonds and biochemicals, among others.
7.1 Emerging Themes
7.1.1 Metabolism of Reduced Phosphorus
One of the most striking features of recent phosphorus research in algae are the diversity of phosphorus forms and reduced valence states that appear to be utilized by the cyanobacteria in particular. In addition to serving as a phosphorus source, the oxidation of reduced phosphorus compounds could serve as an energy source – a phosphorus redox cycle in nature. Phosphorus is found in chemical forms with a range of oxidation states. Compounds with a valence of +5 include phosphate, and phosphoesters like phosphoethanolamine. Compounds with a valence of +3 include phosphite, and phosphonates like 2-AEP, as discussed in previous sections these are all potentially bioavailable to cyanobacteria. The bioavailability of compounds with a valence of +1 like hypophosphite, and phosphinates like the antibiotic phosphinothricin are less well understood, but hypophosphite is known to be bioavailable to some microbes and can be metabolized by the htxA operon in Pseudomonas stutzeri (White and Metcalf 2004a, 2007). In short, there is increasing evidence of cyanobacterial metabolism of compounds with a range of oxidation states. In P. stutzeri, a phosphite dehydrogenase is coupled to NADH formation suggestive of the potential for phosphorus metabolism to be linked to the energy released during oxidation (Costas et al. 2001). The long standing view that phosphorus occurs in a fully oxidized state (phosphate, Pi = +5), and that there is no redox cycle for phosphorus in the environment is increasingly being questioned as the diversity of oxidation states and compounds that cyanobacteria can metabolize is expanded and reduced phosphorus compounds are detected in nature (Pasek et al. 2014). A direct link to energy dynamics has not been established for the cyanobacteria, but the bioavailability of such a range of valence states is suggestive of a phosphorus redox cycle in nature (Karl 2014). If this is borne out with further investigation, this theme would truly reshape our understanding of algal phosphorus metabolism and their role in cycling phosphorus in the environment.
7.1.2 Cell-Cell Interactions
The central paradigm in algal phosphorus stress responses, and how algae modulate their phosphorus metabolism, is that this is controlled by cellular responses to phosphorus concentration or supply. This central tenet is what drives our conceptual modeling of how algae interact with the phosphorus cycle. This paradigm is broadly supported by observations. For example, on broad scales patterns in phosphorus-sparing lipid substitutions and alkaline phosphatase activities typically inversely correlate with inorganic phosphate concentration (Van Mooy et al. 2009; Martin et al. 2014). Despite these broad patterns there are a number of studies where there is clear variability within a species, even within the same system (Dyhrman and Palenik 1999). What drives this variability and what it means for understanding and tracking algal phosphorus metabolism is a new important theme in algal phosphorus research. One of the factors that may circumvent the known patterns and responses to phosphorus geochemistry is interactions between cells. Studies on this theme are sparse, but work by Van Mooy et al. (2012) showed that cell-cell communication via quorum sensing molecules had a major effect on Trichodesmium colony alkaline phosphatase activities. This study added the quorum sensing molecule to Trichodesmium colonies isolated from the low phosphorus Sargasso Sea and from the comparatively high phosphorus North Pacific subtropical gyre and found that the addition caused a doubling of alkaline phosphatase activity (Van Mooy et al. 2012). In short, causing cells to communicate modulated the activity of a critical enzyme, in a manner independent of the phosphorus chemistry. This is just one example, but it highlights the nuanced factors that can drive aspects of algal phosphorus metabolism, and will likely be an area of increased research in the future.
7.2 Ongoing Challenges
7.2.1 Assaying Metabolism of Alternative Phosphorus Forms
Despite many years of study, and renewed interest in aquatic phosphorus cycling, a number of ongoing challenges still impinge on our understanding of fundamental aspects of phosphorus acquisition and metabolism in algae. One such challenge involves the very isotopic composition of phosphorus. Phosphorus has at least 23 isotopes, but only one stable isotope 31P. This inherently limits studies on phosphorus relative to nitrogen or carbon where there are more than one stable isotope for fractionation studies. Some progress has been made on circumventing this issue by using the oxygen isotopes of phosphate (Blake et al. 2005; Colman et al. 2005; McLaughlin et al. 2006, 2013). However the fractionation by different enzymes is not yet well understood, complicating interpretation of these signals (Liang and Blake 2009).
Of the radioactive isotopes, only 32P and 33P have half-lives appropriate for their use as tracers in uptake or bioavailability studies. Typically phosphate and nucleotides like ATP are the only radiolabeled phosphorus compounds commercially available. With the increasingly recognized importance of organic phosphorus metabolism, and the metabolism of phosphite, an ongoing challenge is the lack of suitable substrates for tracer studies. This is particularly critical for assaying the kinetic values that could be used for better modeling of algal community dynamics, which largely neglect utilization of phosphate alternatives. Prioritizing synthesis of radiolabeled phosphonate substrates in particular would greatly facilitate research in this area.
Just as radiolabeled substrates are lacking, so too are colormetric or fluorometric substrates for studying enzymes other than the esters and diesters. If alkaline phosphatase is taken as an example, substantial insight can result as the number of assay tools increases. Imagine if an enzyme labeled fluorescence substrates could be developed for a phosphonate – this would dramatically expand understanding of algal phosphonate metabolism and phosphonate cycling in the environment.
7.2.2 Quantifying Phosphorus Biochemicals and Bond Classes
Analytical issues in the detection of specific phosphorus molecules, biochemical pools (polyphosphate), and bond forms (phosphonate), make tracing phosphorus mass balance in cells challenging. For example, the cellular compounds containing phosphonate bonds are present (as determined with 31P NMR) but largely unknown in many systems, and phosphonate does not appear to extract well in the few studies that have tried to study this bond class in algal samples (Cade-Menun et al. 2005). In the case of polyphosphate, there are chain length biases, among other issues like signal interference from DNA, in the detection of this critical pool (Diaz and Ingall 2010; Martin and Van Mooy 2013; Martin et al. 2014). This can result in over estimation of polyphosphate, and it is not uncommon to detect polyphosphate in amounts greater than the total cellular phosphorus as a result. Improved methodologies for the characterization of phosphorus containing biochemicals are needed to more fully ascertain cellular phosphorus dynamics.
7.2.3 Heterogeneity in Capabilities and Activities
For every example highlighted herein, there seems to be a counter and our understanding of this heterogeneity in capability and activity is only increasing. Even at the strain level there are vast differences in metabolic capacity. This is observed in the variable presence of phosphorus metabolic genes in single genomes of Prochlorococcus (Kashtan et al. 2014). It is also seen in the genomes of multiple E. huxleyi strains where the number of phosphate transporter genes per genome varied by up to a factor of 5 between strains (Read et al. 2013). This level of heterogeneity may confer substantial variability in the rates and kinetics of phosphate uptake in this species, presenting an ongoing challenge in extrapolating culture findings to the field among other issues. Another example related to apparent heterogeneity is the variability in the distribution of phosphorus into different bond forms, detected with 31P NMR. Here, even different species of the same genus can have very different profiles. For example, even when grown under the same conditions, T. erythraeum strains have a chemical shift at 18 ppm in the region of phosphonate, whereas T. theibautii does not (Dyhrman et al. 2009). There is no easy answer for how to deal with this heterogeneity, but one step forward would be the further development of the cell or taxon specific approaches outlined in earlier sections. Future work will need to contextualize this heterogeneity so that variability can appropriately be accounted for in modeling studies and those aimed at predictive understanding of phosphorus algae interactions.
8 Conclusions
Phosphorus plays a critical role in the metabolism, growth, ecophysiology and evolution of eukaryotic algae and cyanobacteria. Knowledge about cellular phosphorus dynamics in microalgae has been rapidly advancing with new methods and more sensitive approaches derived from flow cytometry, genomics, and mass-spectrometry among others. The chapter focused on how these advances have expanded understanding on fundamental aspects of phosphorus metabolism, and how these advances increasingly point to the molecular level underpinnings driving that metabolism. As these methods continue to evolve, new discoveries will build upon those highlighted herein.
Notes
- 1.
Wherever possible the currently accepted names for species are used. The name used in the paper cited is also indicated. For details of names see chapter “Systematics, Taxonomy and Species Names: Do They Matter?” of this book (Borowitzka 2016).
References
Adams MM, Gomez-Garcia MR, Grossman AR, Bhaya D (2008) Phosphorus deprivation responses and phosphonate utilization in a thermophilic Synechococcus sp. from microbial mats. J Bacteriol 190:8171–8184
Ammerman J, Azam F (1985) Bacterial 5′ nucleotidase in aquatic ecosystems: a novel mechanism of phosphorus regeneration. Science 227:1338–1340
Ammerman JW, Azam F (1991) Bacterial 5′-nucleotidase activity in estuarine and coastal marine waters – role in phosphorus regeneration. Limnol Oceanogr 36:1437–1447
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition and consequences. Estuaries 25:704–726
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou SG, Allen AE, Apt KE, Bechner M, Brzezinski MA, Chaal BK, Chiovitti A, Davis AK, Demarest MS, Detter JC, Glavina T, Goodstein D, Hadi MZ, Hellsten U, Hildebrand M, Jenkins BD, Jurka J, Kapitonov VV, Kroger N, Lau WWY, Lane TW, Larimer FW, Lippmeier JC, Lucas S, Medina M, Montsant A, Obornik M, Parker MS, Palenik B, Pazour GJ, Richardson PM, Rynearson TA, Saito MA, Schwartz DC, Thamatrakoln K, Valentin K, Vardi A, Wilkerson FP, Rokhsar DS (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86
Azam F, Malfatti F (2007) Microbial structuring of marine ecosystems. Nat Rev Microbiol 5:782–791
Bar-Yosef Y, Sukenik A, Hadas O, Viner-Mozzinim Y, Kaplan A (2010) Enslavement in the water body by toxic Aphanizomenon ovalisporum, inducing alkaline phosphatase in phytoplanktons. Curr Biol 20(17):1557–1561
Beardall J, Young E, Roberts S (2001) Approaches for determining phytoplankton nutrient limitation. Aquat Sci 63:44–69
Bench SR, Heller P, Frank I, Arciniega M, Shilova IN, Zehr JP (2013) Whole genome comparison of six Crocosphaera watsonii strains with differing phenotypes. J Phycol 49:786–801
Benitez-Nelson CR (2000) The biogeochemical cycling of phosphorus in marine systems. Earth Sci Rev 51:109–135
Bertilsson S, Berglund O, Karl D, Chisholm SW (2003) Elemental composition of marine Prochlorococcus and Synechococcus: implications for the ecological stoichiometry of the sea. Limnol Oceanogr 48:1721–1731
Beszteri S, Yang I, Jaeckisch N, Tillmann U, Frickenhaus S, Glockner G, Cembella A, John U (2012) Transcriptomic response of the toxic prymnesiophyte Prymnesium parvum (N. Carter) to phosphorus and nitrogen starvation. Harmful Algae 18:1–15
Beversdorf LJ, White AE, Björkman KM, Letelier RM, Karl DM (2010) Phosphonate metabolism of Trichodesmium IMS101 and the production of greenhouse gases. Limnol Oceanogr 55:1768–1778
Björkman K, Karl DM (1994) Bioavailability of inorganic and organic phosphorus-compounds to natural assemblages of microorganisms in Hawaiian coastal waters. Mar Ecol Prog Ser 111:265–273
Björkman KM, Karl DM (2001) A novel method for the measurement of dissolved adenosine and guanosine triphosphate in aquatic habitats: applications to marine microbial ecology. J Microbiol Meth 47:159–167
Björkman K, Duhamel S, Karl DM (2012) Microbial group specific uptake kinetics of inorganic phosphate and adenosine-5′-triphosphate (ATP) in the north pacific subtropical gyre. Front Microbiol 3:189. doi:10.3389/fmicb.2012.00189
Blake RE, O’Neil JR, Surkov AV (2005) Biogeochemical cycling of phosphorus: insights from oxygen isotope effects of phosphoenzymes. Am J Sci 305:596–620
Bolier G, de Koningh CJ, Schmale JC, Donze M (1992) Differential luxury phosphate response of planktonic algae to phosphorus removal. Hydrobiologia 243/244:113–118
Borowitzka MA (2016) Systematics, taxonomy and species names: do they matter? In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 655–681
Cade-Menun BJ, Paytan A (2010) Nutrient temperature and light stress alter phosphorus and carbon forms in culture-grown algae. Mar Chem 121:27–36
Cade-Menun BJ, Benitez-Nelson CR, Pellechia P, Paytan A (2005) Refining 31P nuclear magnetic resonance spectroscopy for marine particulate samples: storage conditions and extraction recovery. Mar Chem 97:293–306
Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter E (1997) Trichodesmium, a globally significant marine cyanobacterium. Science 276:1221–1229
Casey JR, Lomas MW, Michelou VK, Dyhrman ST, Orchard ED, Ammerman JW, Sylvan JB (2009) Phytoplankton taxon-specific orthophosphate (Pi) and ATP utilization in the western subtropical North Atlantic. Aquat Microb Ecol 58:31–44
Cembella AD, Antia NJ, Harrison PJ (1984a) The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: Part I. CRC Crit Rev Microbiol 10:317–391
Cembella AD, Antia NJ, Harrison PJ (1984b) The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: Part II. CRC Crit Rev Microbiol 11:13–81
Chisholm SW, Stross RG (1976) Phosphate uptake kinetics in Euglena gracilis (Euglenophyceae) grown on light/dark cycles. I. Synchronized batch cultures. J Phycol 12:210–217
Chung CC, Hwang SPL, Chang J (2003) Identification of a high-affinity phosphate transporter gene in a prasinophyte alga, Tetraselmis chui, and its expression under nutrient limitation. Appl Environ Microbiol 69:754–759
Clark LL, Ingall ED, Benner R (1998) Marine phosphorus is selectively remineralized. Nature 393:426
Clark LL, Ingall ED, Benner R (1999) Marine organic phosphorus cycling: novel insights from nuclear magnetic resonance. Am J Sci 299:724–737
Coleman ML, Chisholm SW (2010) Ecosystem-specific selection pressures revealed through comparative population genomics. Proc Natl Acad Sci U S A 107:18634–18639
Colman AS, Blake RE, Karl DM, Fogel ML, Turekian KK (2005) Marine phosphate oxygen isotopes and organic matter remineralization in the oceans. Proc Natl Acad Sci U S A 102:13023–13028
Costas AMG, White AK, Metcalf WW (2001) Purification and characterization of a novel phosphorus-oxidizing enzyme from Pseudomonas stutzeri WM88. J Biol Chem 276:17429–17436
Cox AD, Saito MA (2013) Proteomic responses of oceanic Synechococcus WH8102 to phosphate and zinc scarcity and cadmium additions. Front Microbiol 4:387. doi:10.3389/fmicb.2013.00387
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantification. Appl Environ Microbiol 66:1175–1182
Cuvelier ML, Allen AE, Monier A, McCrow JP, Messie M, Tringe SG, Woyke T, Welsh RM, Ishoey T, Lee JH, Binder BJ, DuPont CL, Latasa M, Guigand C, Buck KR, Hilton J, Thiagarajan M, Caler E, Read B, Lasken RS, Chavez FP, Worden AZ (2010) Targeted metagenomics and ecology of globally important uncultured eukaryotic phytoplankton. Proc Natl Acad Sci U S A 107:14679–14684
Diaz JM, Ingall ED (2010) Fluorometric quantification of natural inorganic polyphosphate. Environ Sci Tech 44:4665–4671
Diaz J, Ingall E, Benitez-Nelson C, Paterson D, de Jonge MD, McNulty I, Brandes JA (2008) Marine polyphosphate: a key player in geologic phosphorus sequestration. Science 320:652–655
Diaz JM, Björkman KM, Haley ST, Ingall ED, Karl DM, Longo AF, Dyhrman ST (2015) Polyphosphate dynamics at Station ALOHA, North Pacific subtropical gyre. Limnol. Oceanogr. doi:10.1002/lno.10206
Dignum M, Hoogveld HL, Matthijs HCP, Laanbroek HJ, Pel R (2004) Detecting the phosphate status of phytoplankton by enzyme-labeled fluorescence and flow cytometry. FEMS Microbiol Ecol 48:29–38
Donald KM, Scanlan DJ, Carr NG, Mann NH, Joint I (1997) Comparative phosphorus nutrition of the marine cyanobacterium Synechococcus WH7803 and the marine diatom Thalassiosira weisflogii. J Plankton Res 19:1793–1813
Droop MR (1973) Some thoughts on nutrient limitation in algae. J Phycol 9:264–272
Duhamel S, Dyhrman ST, Karl DM (2010) Alkaline phosphatase activity and regulation in the north pacific subtropical gyre. Limnol Oceanogr 55:1414–1425
Duhamel S, Björkman KM, Karl DM (2012) Light dependence of phosphorus uptake by microorganisms in the subtropical North and South Pacific ocean. Aquat Microb Ecol 67:225–238
Dyhrman ST (2005) Ectoenzymes in Prorocentrum minimum. Harmful Algae 4:619–627
Dyhrman ST (2008) Molecular approaches to diagnosing nutritional physiology in harmful algae: implications for studying the effects of eutrophication. Harmful Algae 8:167–174
Dyhrman ST, Haley ST (2006) Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl Environ Microbiol 72:1452–1458
Dyhrman ST, Palenik BP (1997) The identification and purification of a cell-surface alkaline phosphatase from the dinoflagellate Prorocentrum minimum (Dinophyceae). J Phycol 33:602–612
Dyhrman ST, Palenik BP (1999) Phosphate stress in cultures and field populations of the dinoflagellate Prorocentrum minimum detected by a single-cell alkaline phosphatase assay. Appl Environ Microbiol 65:3205–3212
Dyhrman ST, Palenik BP (2001) A single-cell immunoassay for phosphate stress in the dinoflagellate Prorocentrum minimum (Dinophyceae). J Phycol 37:400–410
Dyhrman ST, Palenik BP (2003) Characterization of ectoenzyme activity and phosphate-regulated proteins in the coccolithophorid Emiliania huxleyi. J Plankton Res 25:1215–1225
Dyhrman ST, Ruttenberg KC (2006) Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: implications for dissolved organic phosphorus remineralization. Limnol Oceanogr 51:1381–1390
Dyhrman ST, Webb E, Anderson DM, Moffett J, Waterbury J (2002) Cell-specific detection of phosphorus stress in Trichodesmium from the western North Atlantic. Limnol Oceanogr 47:1823–1836
Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury JB, Webb EA (2006a) Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439:68–71
Dyhrman ST, Haley ST, Birkeland SR, Wurch LL, Cipriano MJ, McArthur AG (2006b) Long serial analysis of gene expression for gene discovery and transcriptome profiling in the widespread marine coccolithophore Emiliania huxleyi. Appl Environ Microbiol 72:252–260
Dyhrman ST, Ammerman JW, Van Mooy BAS (2007) Microbes and the marine phosphorus cycle. Oceanography 20:110–116
Dyhrman ST, Benitez-Nelson CR, Orchard ED, Haley ST, Pellechia PJ (2009) A microbial source of phosphonates in oligotrophic marine systems. Nat Geosci 2:696–699
Dyhrman ST, Jenkins BD, Rynearson TA, Saito MA, Mercier ML, Alexander H, Whitney LP, Drzewianowski A, Bulygin VV, Bertrand EM, Wu ZJ, Benitez-Nelson C, Heithoff A (2012) The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS One 7:e33768. doi:10.1371/journal.pone.0033768
Eixler S, Karsten U, Selig U (2006) Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cell and its dependence on phosphate supply. Phycologia 45:53–60
Erdner DL, Anderson DM (2006) Global transcriptional profiling of the toxic dinoflagellate Alexandrium fundyense using massively parallel signature sequencing. BMC Genomics 7:88. doi:10.1186/1471-2164-7-88
Feingersch R, Philosof A, Mejuch T, Glaser F, Alalouf O, Shoham Y, Beja O (2012) Potential for phosphite and phosphonate utilization by Prochlorococcus. ISME J 6:827–834
Flynn KJ, Oepik H, Syrett PJ (1986) Localization of the alkaline phosphatase and 5′-nucleotidase activities of the diatom Phaeodactylum tricornutum. J Gen Microbiol 132:289–298
Flynn KJ, Raven JA, Rees TA, Finkel Z, Quigg A, Beardall J (2010) Is the growth rate hypothesis applicable to microalgae? J Phycol 46:1–12
Frischkorn K, Harke K, Gobler CJ, Dyhrman ST (2014) De novo assembly of Aureococcus anophagefferens transcriptomes reveals diverse responses to the low nutrient and low light conditions present during blooms. Front Microbiol 5:375. doi:10.3389/fmicb.2014.00375
Fu FX, Zhang YH, Bell PRF, Hutchins DA (2005) Phosphate uptake and growth kinetics of Trichodesmium (cyanobacteria) isolates from the North Atlantic ocean and the great barrier reef, Australia. J Phycol 41:62–73
Fulton JM, Fredricks HF, Bidle KD, Vardi A, Kendrick BJ, DiTullio GR, Van Mooy BAS (2014) Novel molecular determinants of viral susceptibility and resistance in the lipidome of Emiliania huxleyi. Environ Microbiol 16:1137–1149
Fuszard MA, Wright PC, Biggs CA (2010) Cellular acclimation strategies of a minimal picocyanobacterium to phosphate stress. FEMS Microbiol Lett 306:127–134
Gifford SM, Sharma S, Rinta-Kanto JM, Moran MA (2011) Quantitative analysis of a deeply sequenced marine microbial metatranscriptome. ISME J 5:461–472
Gilbert JA, Laverock B, Temperton B, Thomas S, Muhling M, Hughes M (2011) Metagenomics. In: Kwon YM (ed) High-throughput next generation sequencing: methods and application, 1st edn. Springer, New York, pp 173–183
Girault M, Arakawa H, Hashihama F (2013) Phosphorus stress of microphytoplankton community in the western subtropical North Pacific. J Plankton Res 35:146–157
Gobler CJ, Berry DL, Dyhrman ST, Wilhelm SW et al (2011) Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proc Natl Acad Sci U S A 108:4352–4357
Gomez-Garcia MR, Losada M, Serrano A (2003) Concurrent transcriptional activation of ppA and ppX genes by phosphate deprivation in the cyanobacterium Synechocystis sp. strain PCC 6803. Biochem Biophys Res Commun 302:601–609
Gomez-Garcia MR, Davison M, Blain-Hartnung M, Grossman AR, Bhaya D (2011) Alternative pathways for phosphonate metabolism in thermophilic cyanobacteria from microbial mats. ISME J 5:141–149
González-Gil S, Keafer B, Jovine RVM, Anderson DM (1998) Detection and quantification of alkaline phosphatase in single cells of phosphorus-limited marine phytoplankton. Mar Ecol Prog Ser 164:21–35
Grossman A (2000) Acclimation of Chlamydomonas reinhardtii to its nutrient environment. Protist 151:201–224
Grossman A, Takahashi H (2001) Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Annu Rev Plant Physiol Plant Mol Biol 52:163–210
Harke MJ, Gobler CJ (2013) Global transcriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLoS One 8:e69834. doi:10.1371/journal.pone.0069834
Harke MJ, Berry DL, Ammerman JW, Gobler CJ (2012) Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation. Microb Ecol 63:188–198
Hidaka T, Imai S, Hara O, Anzai H, Murakami T, Nagaoka K, Seto H (1990) Carboxyphosphonoenolpyruvate phosphonomutase, a novel enzyme catalyzing C-P bond formation. J Bacteriol 172:3066–3072
Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, Mayer A (2009) Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 324:513–516
Hynes AM, Chappell PD, Dyhrman ST, Doney SC, Webb EA (2009) Cross-basin comparison of phosphorus stress and nitrogen fixation in Trichodesmium. Limnol Oceanogr 54:1438–1448
Ito T, Tanaka M, Shinkawa H, Nakada T, Ano Y, Kurano N, Soga T, Tomita M (2013) Metabolic and morphological changes of an oil accumulating trebouxiophycean alga in nitrogen-deficient conditions. Metabolomics 9:S178–S187
Jacobson L, Halmann M (1982) Polyphosphate metabolism in the blue-green alga Microcystis aeruginosa. J Plankton Res 4:481–488
Jakuba RW, Moffett JW, Dyhrman ST (2008) Evidence for the linked biogeochemical cycling of zinc, cobalt, and phosphorus in the western North Atlantic ocean. Global Biogeochem Cycles 22:GB4012. doi:10.1029/2007GB003119
Jansson M (1988) Phosphate-uptake and utilization by bacteria and algae. Hydrobiologia 170:177–189
Jansson M, Olsson L, Pettersson K (1988) Phosphatases: origin, characteristics and function in lakes. Hydrobiologia 170:157–175
Karl DM (2014) Microbially mediated transformations of phosphorus in the sea: new views of an old cycle. Annu Rev Mar Sci 6:279–337
Karl D, Björkman KM (2001) Phosphorus cycle in seawater: dissolved and particulate pool inventories and selected phosphorus fluxes. In: Paul JH (ed) Marine microbiology. Academic, San Diego, pp 249–366
Karl DM, Björkman KM (2002) Dynamics of DOP. In: Hansell D, Carlson C (eds) Biogeochemistry of marine dissolved organic matter. Elsevier Science, Boston, pp 249–366
Kashtan N, Roggensack SE, Rodrigue S, Thompson JW, Biller SJ, Coe A, Ding H, Marttinen P, Malmstron R, Stocker R, Follows MJ, Stepanauskas R, Chisholm SW (2014) Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science 344:416–420
Kathuria S, Martiny AC (2011) Prevalence of a calcium-based alkaline phosphatase associated with the marine cyanobacterium Prochlorococcus and other ocean bacteria. Environ Microbiol 13:74–83
Kittredge JS, Roberts EA (1969) A carbon-phosphorus bond in nature. Science 164:37–42
Kittredge JS, Horiguchi M, Williams PM (1969) Aminophosphonic acids: biosynthesis by marine phytoplankton. Comp Biochem Physiol 29:859–863
Komeili A, O’shea EK (1999) Roles of phosphorylation sites in regulating activity of the transcription factor pho4. Science 284:977–980
Kornberg A (1995) Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol 177:491–496
Kornberg A, Rao N, Ault-Riche D (1999) Inorganic polyphosphate: a molecule of many functions. Ann Rev Biochem 68:89–125
Krauk JM, Villareal TA, Sohm JA, Montoya JP, Capone DG (2006) Plasticity of N: P ratios in laboratory and field populations of Trichodesmium spp. Aquat Microb Ecol 42:243–253
Krumhardt KM, Callnan K, Roache-Johnson K, Swett T, Robinson D, Reistetter EN, Saunders JK, Rocap G, Moore LR (2013) Effects of phosphorus starvation versus limitation on the marine cyanobacterium Prochlorococcus MED4 I: uptake physiology. Environ Microbiol 15:2114–2128
Kudela RM, Cochlan WP (2000) Nitrogen and carbon uptake kinetics and the influence of irradiance for a red tide bloom off southern California. Aquat Microb Ecol 21:31–47
Kujawinski EB (2011) The impact of microbial metabolism on marine dissolved organic matter. Ann Rev Mar Sci 3:567–599
Kujawinski EB, Longnecker K, Blough NV, Del Vecchio R, Finlay L, Kitner JB, Giovannoni SJ (2009) Identification of possible source markers in marine dissolved organic matter using ultrahigh resolution mass spectrometry. Geochim Cosmochim Acta 73:4384–4399
Laws EA, Pei SF, Bienfang P, Grant S (2011) Phosphate-limited growth and uptake kinetics of the marine prasinophyte Tetraselmis suecica. Aquaculture 322:117–121
Lenburg ME, O’Shea EK (1996) Signaling phosphate starvation. Trends Biochem Sci 21:383–387
Li QY, Gao XS, Sun Y, Zhang QQ, Song RT, Xu ZK (2006) Isolation and characterization of a sodium-dependent phosphate transporter gene in Dunaliella viridis. Biochem Biophys Res Commun 340:95–104
Li SH, Xia BB, Zhang C, Cao J, Bai LH (2012) Cloning and characterization of a phosphate transporter gene in Dunaliella salina. J Basic Microbiol 52:429–436
Liang YH, Blake RE (2009) Compound- and enzyme-specific phosphodiester hydrolysis mechanisms revealed by δ18O of dissolved inorganic phosphate: implications for marine P cycling. Geochim Cosmochim Acta 73:3782–3794
Lin HY, Shih CY, Liu HC, Chang J, Chen YL, Chen YR, Lin HT, Chang YY, Hsu CH, Lin HJ (2013) Identification and characterization of an extracellular alkaline phosphatase in the marine diatom Phaeodactylum tricornutum. Mar Biotechnol 15:425–436
Lomas MW, Swain A, Shelton R, Ammerman JW (2004) Taxonomic variability of phosphorus stress in Sargasso Sea phytoplankton. Limnol Oceanogr 49:2303-2309
Lomas MW, Burke AL, Lomas DA, Bell DW, Shen C, Dyhrman ST, Ammerman JW (2010) Sargasso sea phosphorus biogeochemistry: an important role for dissolved organic phosphorus (DOP). Biogeosciences 7:695–710
Macaulay IC, Voet T (2014) Single cell genomics: advances and future perspectives. PLoS Genet. doi:10.1371/journal.pgen.1004126
Mackey KRM, Mioni CE, Ryan JP, Paytan A (2012) Phosphorus cycling in the red tide incubator region of Monterey Bay in response to upwelling. Front Microbiol 3:33. doi:10.3389/fmicb.2012.00033
Marchetti A, Schruth DM, Durkin CA, Parker MS, Kodner RB, Berthiaume CT, Morales R, Allen AE, Armbrust EV (2012) Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. Proc Natl Acad Sci U S A 109:E317–E325
Martin P, Van Mooy BAS (2013) Fluorometric quantification of polyphosphate in environmental plankton samples: extraction protocols, matrix effects, and nucleic acid interference. Appl Environ Microbiol 79:273–281
Martin P, Van Mooy BAS, Heithoff A, Dyhrman ST (2011) Phosphorus supply drives rapid turnover of membrane phospholipids in the diatom Thalassiosira pseudonana. ISME J 5:1057–1060
Martin P, Dyhrman ST, Lomas ML, Poulton NJ, Van Mooy BAS (2014) Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc Natl Acad Sci U S A 111:8089–8094
Martinez A, Tyson GW, DeLong EF (2010) Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ Microbiol 12:222–238
Martinez A, Osburne MS, Sharma AK, DeLong EF, Chisholm SW (2012) Phosphite utilization by the marine picocyanobacterium Prochlorococcus MIT9301. Environ Microbiol 14:1363–1377
Martiny AC, Coleman ML, Chisholm SW (2006) Phosphate acquisition genes in Prochlorococcus ecotypes: evidence for genome-wide adaptation. Proc Natl Acad Sci U S A 103:12552–12557
Mateo P, Douterelo I, Berrendero E, Perona E (2006) Physiological differences between two species of cyanobacteria in relation to phosphorus limitation. J Phycol 42:61–66
Mather RL, Reynolds SE, Wolff GA, Williams RG, Torres-Valdes S, Woodsward EMS, Landolfi A, Pan X, Sanders R, Achterberg EP (2008) Phosphorus cycling in the North and South Atlantic ocean subtropical gyres. Nat Geosci 1:439–443
Mazard S, Wilson WH, Scanlan DJ (2012) Dissecting the physiological response to phosphorus stress in marine Synechococcus isolates (cyanophyceae). J Phycol 48:94–105
McGrath JW, Chin JP, Quinn JP (2013) Organophosphonates revealed: new insights into the microbial metabolism of ancient molecules. Nat Rev Microbiol 11:412–419
McLaughlin K, Kendall C, Silva SR, Young M, Paytan A (2006) Phosphate oxygen isotope ratios as a tracer for sources and cycling of phosphate in north San Francisco Bay, California. J Geophys Res Biogeosci 111:G3. doi:10.1029/2005JG000079
McLaughlin K, Sohm JA, Cutter GA, Lomas MW, Paytan A (2013) Phosphorus cycling in the Sargasso Sea: investigation using the oxygen isotopic composition of phosphate, enzyme-labeled fluorescence, and turnover times. Global Biogeochem Cycles 27:375–387
McLean TI (2013) “Eco-omics”: a review of the application of genomics, transcriptomics, and proteomics for the study of the ecology of harmful algae. Microb Ecol 65:901–915
Merchant SS, Helmann JD (2012) Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Adv Microb Phys 60:91–210
Metcalf WW, Griffin BM, Cicchillo RM, Gao JT, Janga SC, Cooke HA, Circello BT, Evans BS, Martens-Habbena W, Stahl DA, van der Donk WA (2012) Synthesis of methylphosphonic acid by marine microbes: a source for methane in the aerobic ocean. Science 337:1104–1107
Moore LR, Ostrowski M, Scanlan DJ, Feren K, Sweetsir T (2005) Ecotypic variation in phosphorus acquisition mechanisms within marine picocyanobacteria. Aquat Microb Ecol 39:257–269
Morel FMM (1987) Kinetics of nutrient uptake and growth in phytoplankton. J Phycol 23:137–150
Morris RM, Nunn BL, Frazar C, Goodlett DR, Ting YS, Rocap G (2010) Comparative metaproteomics reveals ocean-scale shifts in microbial nutrient utilization and energy transduction. ISME J 4:673–685
Mulholland MR, Lee C, Glibert PM (2003) Extracellular enzyme activity and uptake of carbon and nitrogen along an estuarine salinity and nutrient gradients. Mar Ecol Prog Ser 258:3–17
Nedoma J, Strojsová A, Vrba J, Komárková J, Simek K (2003) Extracellular phosphatase activity of natural plankton studied with ELF-97 phosphate: fluorescence quantification and labeling kinetics. Environ Microbiol 5:462–472
Nicholson D, Dyhrman S, Chavez F, Paytan A (2006) Alkaline phosphatase activity in the phytoplankton communities of Monterey Bay and San Francisco Bay. Limnol Oceanogr 51:874–883
Nishikawa K, Machida H, Yamakoshi Y, Ohtomo R, Saito K, Saito M, Tominaga N (2006) Polyphosphate metabolism in an acidophilic alga Chlamydomonas acidophila KT-1 (Chlorophyta) under phosphate stress. Plant Sci 170:307–313
Nishikawa K, Tominaga N, Uchino T, Oikawa A, and Tokunaga H (2009) Polyphosphate contributes to Cd tolerance in Chlamydomonas acidophila KT-1. In: Hagen KN (ed) Algae: nutrition, pollution control and energy sources, Nova Science Publishers, pp 13–21
Ogawa N, DeRisi J, Brown PO (2000) New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell 11:4309–4321
Orchard ED, Webb EA, Dyhrman ST (2009) Molecular analysis of the phosphorus starvation response in Trichodesmium spp. Environ Microbiol 11:2400–2411
Orchard ED, Ammerman JW, Lomas MW, Dyhrman ST (2010a) Dissolved inorganic and organic phosphorus uptake in Trichodesmium and the microbial community: the importance of phosphorus ester in the Sargasso Sea. Limnol Oceanogr 55:1390–1399
Orchard ED, Benitez-Nelson CR, Pellechia PJ, Lomas MW, Dyhrman ST (2010b) Polyphosphate in Trichodesmium from the low-phosphorus Sargasso Sea. Limnol Oceanogr 55:2161–2169
Ostrowski M, Mazard S, Tetu SG, Phillippy K, Johnson A, Palenik B, Paulsen IT, Scanlan DJ (2010) PtrA is required for coordinate regulation of gene expression during phosphate stress in a marine Synechococcus. ISME J 4:908–921
Palenik B, Brahamsha B, Larimer FW, Land M, Hauser L, Chain P, Lamerdin J, Regala W, Allen EE, McCarren J, Paulsen I, Dufresne A, Partensky F, Webb EA, Waterbury J (2003) The genome of a motile marine Synechococcus. Nature 424:1037–1042
Paragas VB, Zhang Y, Haughland P, Singer VL (1997) The EL-97 alkaline phosphatase substrate provides a bright, photostable, fluorescent signal amplification method for fish. J Histochem Cytochem 45:345–357
Pasek MA, Sampson JM, Atlas Z (2014) Redox chemistry in the phosphorus biogeochemical cycle. Proc Natl Acad Sci U S A 111:15468–15473
Pauli AL, Kaitala S (1997) Phosphate uptake kinetics by Acinetobacter isolates. Biotechnol Bioeng 53:304–309
Paytan A, McLaughlin K (2007) The oceanic phosphorus cycle. Chem Rev 107:563–576
Perry MJ (1976) Phosphate utilization by an oceanic diatom in phosphorus-limited chemostat culture and in oligotrophic waters of central North-Pacific. Limnol Oceanogr 21:88–107
Pitt FD, Mazard S, Humphreys L, Scanlan DJ (2010) Functional characterization of Synechocystis sp. strain PCC 6803 pst1 and pst2 gene clusters reveals a novel strategy for phosphate uptake in a freshwater cyanobacterium. J Bacteriol 192:3512–3523
Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47:185–214
Quisel JD, Wykoff DD, Grossman AR (1996) Biochemical characterization of the extracellular phosphatases produced by phosphorus-deprived Chlamydomonas reinhardtii. Plant Physiol 111:839–848
Ranhofer ML, Lawrenz E, Pinckney JL, Benitez-Nelson CR, Richardson TL (2009) Cell-specific alkaline phosphatase expression by phytoplankton from Winyah Bay, South Carolina, USA. Estuar Coasts 32:943–957
Raven JA (2013) RNA function and phosphorus use by photosynthetic organisms. Front Plant Sci 4:536. doi:10.3389/fpls.2013.00536
Raven JA, Knoll AH (2010) Non-skeletal biomineralization by eukaryotes: matters of moment and gravity. Geomicrobiol J 27:572–584
Read BA, Kegel J, Klute MJ, Kuo A, Lefebvre SC, Maumus F, Mayer C, Miller J, Monier A, Salamov A, Young J, Aguilar M, Claverie JM, Frickenhaus S, Gonzalez K, Herman EK, Lin YC, Napier J, Ogata H, Sarno AF, Shmutz J, Schroeder D, de Vargas C, Verret F, von Dassow P, Valentin K, Van de Peer Y, Wheeler G, Dacks JB, Delwiche CF, Dyhrman ST, Glockner G, John U, Richards T, Worden AZ, Zhang XY, Grigoriev IV et al (2013) Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 499:209–213
Reistetter EN, Krumhardt K, Callnan K, Roache-Johnson K, Saunders JK, Moore LR, Rocap G (2013) Effects of phosphorus starvation versus limitation on the marine cyanobacterium Prochlorococcus MED4 II: Gene expression. Environ Microbiol 15:2129–2143
Renberg L, Johansson AI, Shutova T, Stenlund H, Aksmann A, Raven JA, Gardestrom P, Moritz T, Samuelsson G (2010) A metabolomic approach to study major metabolite changes during acclimation to limiting CO2 in Chlamydomonas reinhardtii. Plant Physiol 154:187–196
Rengefors K, Pettersson K, Blenckner T, Anderson DM (2001) Species-specific alkaline phosphatase activity in freshwater spring phytoplankton: application of a novel method. J Plankton Res 23:435–443
Rengefors K, Ruttenberg KC, Haupert C, Taylor C, Howes BL, Anderson DM (2003) Experimental investigation of taxon-specific response of alkaline phosphatase activity in natural freshwater phytoplankton. Limnol Oceanogr 48:1167–1175
Riekhof WR, Sears BB, Benning C (2005) Annotation of genes involved in glycerolipid biosynthesis in Chlamydomonas reinhardtii: discovery of the betaine lipid synthase BTA1. Eukaryot Cell 4:242–252
Rocap G, Larimer FW, Lamerdin J, Malfatti S, Chain P, Ahlgren NA, Arellano A, Coleman M, Hauser L, Hess WR, Johnson ZI, Land M, Lindell D, Post AF, Regala W, Shah M, Shaw SL, Steglich C, Sullivan MB, Ting CS, Tolonen A, Webb EA, Zinser ER, Chisholm SW (2003) Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424:1042–1047
Romans KM, Carpenter EJ, Bergman B (1994) Buoyancy regulation in the colonial diazotrophic cyanobacterium Trichodesmium tenue: ultrastructure and storage of carbohydrate, polyphosphate, and nitrogen. J Phycol 30:935–942
Rubio L, Linares-Rueda A, García-Sánchez MJ, Fernández JA (2004) Physiological evidence for a sodium-dependent high affinity phosphate and nitrate transport at the plasma membrane of leaf and root cells of Zostera marina L. J Exp Bot 56:613–622
Ruttenberg KC, Dyhrman ST (2005) Temporal and spatial variability of dissolved organic and inorganic phosphorus, and metrics of phosphorus bioavailability in an upwelling-dominated coastal system. J Geophys Res Oceans 110:C10S13. doi:10.1029/2004JC002837
Rynearson TA, Palenik B (2011) Learning to read the oceans: genomics of marine phytoplankton. Adv Mar Biol 60:1–39
Sakamoto T, Murata N, Ohmori M (1991) The concentration of cyclic AMP and adenylate cyclase activity in cyanobacteria. Plant Cell Physiol 32:581–584
Sato M, Sakuraba R, Hashihama F (2013) Phosphate monoesterase and diesterase activities in the North and South Pacific ocean. Biogeosciences 10:7677–7688
Scanlan DJ, Wilson WH (1999) Application of molecular techniques to addressing the role of P as a key effector in marine ecosystems. Hydrobiologia 401:149–175
Scanlan DJ, Ostrowski M, Mazard S, Dufresne A, Garczarek L, Hess WR, Post AF, Hagemann M, Paulsen I, Partensky F (2009) Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev 73:249–299
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 195:260–262
Sebastian M, Ammerman JW (2009) The alkaline phosphatase phoX is more widely distributed in marine bacteria than the classical phoA. ISME J 3:563–572
Seidel HM, Freeman S, Seto H, Knowles JR (1988) Phosphonate biosynthesis – isolation of the enzyme responsible for the formation of a carbon phosphorus bond. Nature 335:457–458
Shilova IN, Robidart JC, Tripp HJ, Turck-Kubo K, Wwrik B, Post AF, Thompson AW, Ward BB, Hollibaugh JT, Millard A, Ostrowski M, Scanlan DJ, Paerl HW, Stuart R, Zehr JP (2014) A microarray for assessing transcription from pelagic marine microbial taxa. ISME J 8:1476–1491
Sinha R, Pearson LA, Davis TW, Muenchhoff J, Pratama R, Jex A, Burford MA, Neilan BA (2014) Comparative genomics of Cylindrospermopsis raciborskii strains with differential toxicities. BMC Genomics 15:83 doi:10.1186/1471-2164-15-83
Sowell SM, Wilhelm LJ, Norbeck AD, Lipton MS, Nicora CD, Barofsky DF, Carlson CA, Smith RD, Giovanonni SJ (2009) Transport functions dominate the SAR11 metaproteome at low-nutrient extremes in the Sargasso Sea. ISME J 3:93–105
Springer M, Wykoff DD, Miller N, O’Shea EK (2003) Partially phosphorylated pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol 1:261–270
Su Z, Dam P, Chen X, Olman V, Jiang T, Palenik B, Xu Y (2003) Computational inference of regulatory pathways in microbes: an application to phosphorus assimilation pathways in Synechococcus WH8102. Genome Inform 14:3–13
Su ZC, Olman V, Xu Y (2007) Computational prediction of pho regulons in cyanobacteria. BMC Genomics 8:156. doi:10.1186/1471-2164-8-156
Suzuki S, Ferjani A, Suzuki I, Murata N (2004) The sphS-sphR two component system is the exclusive sensor for the induction of gene expression in response to phosphate limitation in Synechocystis. J Biol Chem 279:13234–13240
Tetu SG, Brahamsha B, Johnson DA, Tai V, Phillippy K, Palenik B, Paulsen IT (2009) Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp. WH8102. ISME J 3:835–849
Theodorou ME, Elrifi IR, Turpin DH, Plaxton WC (1991) Effects of phosphorus limitation on respiratory metabolism in the green-alga Selenastrum minutum. Plant Physiol 95:1089–1095
Torriani-Gorini A (1987) The birth and death of the pho regulon. In: Torriani-Gorini A, Rothman FG, Silver S, Wright A, Yagil E (eds) Phosphate metabolism and cellular regulation in microorganisms. ASM Press, Washington, DC, pp 3–11
Van Mooy BAS, Devol AH (2008) Assessing nutrient limitation of Prochlorococcus in the North Pacific subtropical gyre by using an RNA capture method. Limnol Oceanogr 53:78–88
Van Mooy BAS, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblizek M, Lomas ML, Mincer TJ, Moore LR, Moutin T, Rappe MS (2009) Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458:69–72
Van Mooy BAS, Hmelo LR, Sofen LE, Campagna SR, May AL, Dyhrman ST, Heithoff A, Webb EA, Momper L, Mincer TJ (2012) Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J 6:422–429
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu DY, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Vila-Costa M, Sharma S, Moran MA, Casamayor EO (2013) Diel gene expression profiles of a phosphorus limited mountain lake using metatranscriptomics. Environ Microbiol 15:1190–1203
Villarreal-Chiu JF, Quinn JP, McGrath JW (2012) The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front Microbiol 3:19. doi:10.3389/fmicb.2012.00019
Voss B, Bolhuis H, Fewer DP, Kopf M, Moke F, Haas F, El-Shehawy R, Hayes P, Bergman B, Sivonen K, Dittmann E, Scanlan DJ, Hagemann M, Stal LJ, Hess WR (2013) Insights into the physiology and ecology of the brackish-water-adapted cyanobacterium Nodularia spumigena CYY9414 based on a genome-transcriptome analysis. PLoS One 8:e60224. doi:10.1371/journal.pone.0060224
Wagner ND, Hillebrand H, Wacker A, Frost PC (2013) Nutritional indicators and their uses in ecology. Ecol Lett 16:535–544
Wanner BL (1996) Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt FC (ed) Escherichia coli and salmonella cellular and molecular biology. ASM Press, Washington, DC, pp 1357–1381
White A, Dyhrman S (2013) The marine phosphorus cycle. Front Microbiol 4:105. doi:10.3389/fmicb.2013.00105
White AK, Metcalf WW (2004a) The htx and ptx operons of Pseudomonas stutzeri WM88 are new members of the pho regulon. J Bacteriol 186:5876–5882
White AK, Metcalf WW (2004b) Two c-p lyase operons in pseudomonas stutzeri and their roles in the oxidation of phosphonates, phosphite, and hypophosphite. J Bacteriol 186:4730–4739
White AK, Metcalf WW (2007) Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol 61:379–400
White AK, Spitz Y, Karl DM, Letelier R (2006) Flexible elemental stoichiometry in Trichodesmium spp. and its ecological implications. Limnol Oceanogr 51:1777–1790
Wurch LL, Bertrand EM, Saito MA, Van Mooy BAS, Dyhrman ST (2011a) Proteome changes driven by phosphorus deficiency and recovery in the brown tide-forming alga Aureococcus anophagefferens. PLoS One 6:e28949. doi:10.1371/journal.pone.0028949
Wurch LL, Haley ST, Orchard ED, Gobler CJ, Dyhrman ST (2011b) Nutrient-regulated transcriptional responses in the brown tide forming alga Aureococcus anophagefferens. Environ Microbiol 13:468–481
Wurch LL, Gobler CJ, Dyhrman ST (2014) Expression of a xanthine permease and phosphate transporter in cultures and field populations of the harmful alga Aureococcus anophagefferens: tracking nutritional deficiency during brown tides. Environ Microbiol 16:2444–2457
Wykoff DD, O’shea EK (2001) Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159:1491–1499
Wykoff DD, Grossman AR, Weeks DP, Usuda H, Shimogawara K (1999) Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc Natl Acad Sci U S A 96:15336–15341
Xu Y, Wahlund TM, Feng L, Shaked Y, Morel FMM (2006) A novel alkaline phosphatase in the coccolithophore Emiliania huxleyi (Prymnesiophyceae) and its regulation by phosphorus. J Phycol 42:835–844
Yamaguchi H, Arisaka H, Otsuka N, Tomaru Y (2014) Utilization of phosphate diesters by phosphodiesterase-producing marine diatoms. J Plankton Res 36:281–285
Yong SC, Roversi P, Lillington J, Rodriguez F, Krehenbrink M, Zeldin OB, Garman EF, Lea SM, Berks BC (2014) A complex iron-calcium cofactor catalyzing phosphotransfer chemistry. Science 345:1170–1173
Young CL, Ingall ED (2010) Marine dissolved organic phosphorus composition: insights from samples recovered using combined electrodialysis/reverse osmosis. Aquat Geochem 16:563–574
Acknowledgements
This work was in part supported by NSF OCE 13-16036, the Center for Microbial Oceanography: Research and Education, and the Woods Hole Oceanographic Institution (WHOI) Coastal Ocean Institute. The author thanks Sheean Haley for editorial assistance, Kathleen Ruttenberg for helpful discussions, and Kyle Frischkorn and the WHOI Graphics Department for assistance with figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dyhrman, S.T. (2016). Nutrients and Their Acquisition: Phosphorus Physiology in Microalgae. In: Borowitzka, M., Beardall, J., Raven, J. (eds) The Physiology of Microalgae. Developments in Applied Phycology, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-319-24945-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-24945-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24943-8
Online ISBN: 978-3-319-24945-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)