Abstract
The dominant phosphorus compound classes were characterized in marine samples using a new, high recovery method for isolating and concentrating bulk dissolved organic matter (DOM) called combined electrodialysis + reverse osmosis (ED/RO). In contrast to earlier studies that use ultrafiltration (UF) to recover only the high molecular weight DOM, ED/RO is capable of isolating both low molecular weight (LMW) and high molecular weight (HMW) DOM. Samples were collected from a broad range of marine environments: along a transect incorporating coastal and offshore waters off the Southeastern United States, in Effingham Inlet, a Pacific fjord located on Vancouver Island, British Columbia and in the Amundsen Sea, Antarctica. Results from phosphorus nuclear magnetic resonance (31P NMR) analysis reveal a similar abundance of P compound classes among samples, phosphate esters (80–85%), phosphonates (5–10%) and polyphosphates (8–13%). These samples contain significantly higher proportions of polyphosphate P and P esters and lower proportions of phosphonates than measured in previous studies using the UF method. The much higher levels of polyphosphate detected in our samples suggests that polyphosphate is present mainly in the LMW dissolved matter fraction. Polyphosphates in dissolved matter may be present as (or derived from) dissolved nucleotides or organismal polyphosphate bodies, or both. Low molecular weight P esters are possibly composed of phosphoamino acids and small carbohydrates, like simple sugar phosphates and/or dissolved nucleotides. Phosphonates in DOM are more prevalent as HMW phosphonate compounds, which suggests that LMW phosphonates are more readily utilized in marine ecosystems. Overall, the investigation of DOM across a size spectrum that includes both the HMW and the LMW fractions reveals a new picture of phosphorus distribution, cycling and bioavailability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus is an essential nutrient for life, and the availability of phosphorus in ocean surface waters is an important control on ocean productivity. In surface waters of vast oligotrophic ocean regions, dissolved organic phosphorus (DOP) typically comprises the major fraction (~75–80%) of the total dissolved P (TDP) pool (Karl and Bjorkman 2002; Benitez-Nelson 2000; Karl and Yanagi 1997; Smith et al. 1986; Jackson and Williams 1985; Orrett and Karl 1987; Bjorkman and Karl 1994). Consequently, regeneration of P from DOP (Fig. 1) has been shown to play a significant role in supplying the P necessary for biological production (e.g. Karl and Bjorkman 2001; Dyhrman et al. 2006; Karl et al. 2008). Many P studies have focused on identifying specific individual biomolecules, such as adenosine triphosphate (ATP), that typically comprise a small percentage of total P in the ocean (Karl and Bjorkman 2002; Benitez-Nelson 2000). Other studies have investigated the bulk distribution of different P compound classes (e.g. phosphonates, P esters) in samples covering a specific molecular weight range that represent about 20–40% of the total P in the ocean (Clark et al. 1998, 1999; Kolowith et al. 2001; Sannigrahi et al. 2006). Because these studies have focused on relatively small fractions of the DOP pool, the bulk composition of marine DOP remains largely unknown.
The dissolved P cycle. Distribution of P in DOP either reflects direct production by organisms, selective decomposition of DOP compounds during production of DIP or selective assimilation of DOP compounds by organisms. The DOP pool includes P esters, phosphonates and both inorganic and organic polyphosphates. Decomposition includes such processes as alteration of compounds by ectoenzymes and photochemical degradation, the later of which only occurs in the photic zone
Uncertainty in DOP composition is a major impediment in our attempts to understand not only the global P cycle but also the coupled cycles of many other elements associated with marine production. DOM plays a number of central roles in oceanic biogeochemical cycles. It acts as both a conduit and a storehouse for nutrients and reduced carbon. Yet despite a great deal of research conducted over the past two decades into DOM composition and cycling, many questions remain unanswered. Most of these questions, such as the identities and sources of labile LMW dissolved molecules interacting with bacterial and photoautotroph populations, have remained difficult to examine because we lacked the methods necessary to concentrate such ephemeral compounds up to the levels at which they can be examined by modern analytical techniques.
However, a new technique called combined electrodialysis + reverse osmosis (ED/RO) recovers two to three times more DOM from seawater than earlier techniques, thereby allowing us to investigate DOM across a much larger molecular size spectrum than previously possible (Vetter et al. 2007; Gurtler et al. 2008; Koprivnjak et al. 2006). We demonstrate in this paper that the P composition of this more complete DOM isolate is quite different from that observed in previous studies of UF-extracted DOM, and it provides exciting new possibilities for rewriting our understanding of DOM sources, dynamics and interactions with other organic and inorganic pools.
2 Methods
2.1 Sampling Locations
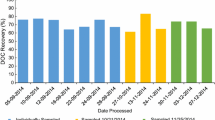
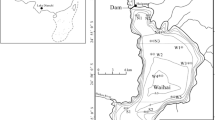
With our small-scale prototype ED/RO equipment, sample processing can be quite lengthy. Therefore, we were only able to process a limited number of samples for this study. In order to gain a preliminary view of the potential range in marine P composition, samples were collected from five sites in four very different marine environments, ranging from oligotrophic Gulf Stream waters to the eutrophic coastal environments of Georgia and Vancouver Island to highly productive waters of the Antarctic Amundsen Sea (Table 1). The Gulf Stream sampling site is located off the coast of Savannah, Georgia. The Gulf Stream is a powerful current on the western boundary of the North Atlantic subtropical gyre and is characterized by warm, oligotrophic waters. Coastal samples were taken from Effingham Inlet and Savannah, Georgia at Skidaway Institute of Oceanography. Effingham Inlet is a Pacific fjord, located on the west coast of Vancouver Island in Barkley Sound. The Amundsen Sea is located ~1,100 km west of the Antarctic Peninsula and was partially covered by ice during sampling. Latitudes and longitudes for all sampling sites are presented in Table 1.
2.2 Sample Collection
Open ocean samples were collected from a Niskin bottle rosette sampler. A 200 l Gulf Stream sample was collected aboard the R/V Savannah in July 2008. Effingham Inlet samples were taken in April and July 2007 aboard the R/V Barnes. Two large volume samples of 50 and 120 l were collected from Effingham Inlet at depths of 61 and 78 m, respectively. A 200 l sample was collected near Savannah, Georgia by direct pumping from the Skidaway Institute of Oceanography Dock. A 200 l Antarctic sample was collected and processed in the Amundsen Sea in December 2008 aboard the Icebreaker Oden.
2.3 Electrodialysis/Reverse Osmosis Technique
Coupled ED/RO extraction of DOM from seawater samples was performed generally following the protocols described in Vetter et al. (2007). Briefly, all samples and rinse solutions were passed through a 0.45-μm filter. The system was cleaned for the Effingham samples by circulating a minimum 50 l of sample through both the ED and RO systems for 1 h. For all other samples, at least two, 1-h long, 50 l system rinses were performed. ED is used to remove ions from the seawater sample. In ED, the seawater sample (to be deionized) is contacted with a concentrate flow (to receive ions) across many pairs of cation and anion-exchange membranes. A DC electrical current is directed through the membrane stack. Only ionized dissolved components are targeted by ED. The ion-exchange membranes are non-porous above the size of the interstitial spaces between the polymer chains in the cross-linked hydrated ion-exchange resin. The process results in a concentrate solution, which is discarded, and a deionized solution (diluate). The diluate solution is subsequently concentrated via RO in order to isolate dissolved molecules for analysis.
Concentrated, desalted samples were transferred into clean 10-l HDPE bottles and frozen immediately. Both the RO and ED systems were rinsed with 0.01 M NaOH to recover adsorbed fractions of the DOM in a sample. System mass balances were calculated to determine DOM recoveries using the protocols described in Koprivnjak et al. (2009). DOP measurements on the whole seawater samples are not available, thus recoveries could not be calculated in terms of P. However, previous studies have shown that P recoveries are similar to C recoveries (Kolowith et al. 2001; Clark et al. 1999; Sannigrahi et al. 2006). Therefore, recoveries reported in Table 1 are calculated based on DOC concentrations in the initial whole seawater sample compared to DOC concentrations in the final ED/RO concentrate. The current applied to the system was constantly monitored to make sure that it remains below the limiting current density determined for the specific system geometry and flow characteristics (Vetter et al. 2007).
2.4 31P NMR
After samples were freeze-dried and ground, bulk P composition was determined with the use of a solid-state 31P NMR instrument at the NMR center in the School of Chemistry and Biochemistry at the Georgia Institute of Technology. The instrument is a Bruker DSX 400 spectrophotometer and uses cross-polarization–magic-angle spinning (CP-MAS) at a 31P frequency of 161 MHz. About 90 mg of powdered sample was packed into 4-mm-diameter cylindrical zirconia rotor fitted with Kel-F cap and spun at 10,000 ± 10 Hz in a Bruker magic-angle spinning probe (Sannigrahi and Ingall 2005).
For all samples, a cross-polarization sequence, optimized to obtain semiquantitative data, was used with a 1.0 ms contact time and a pulse delay of 4 s (Sannigrahi et al. 2005). For each sample, at least 8,000 transients were collected. After the spectra were obtained, peak areas were integrated off-line using the Spinworks software package. Areas were determined by dropping vertical lines to the baseline in a similar fashion as that of Hedges et al. (2002). Gaussian fits and other more complicated spectral deconvolution techniques were not employed, because little of the necessary information on shapes and positions of peaks in natural samples is available.
Functional groups within the sample were recognized by comparing chemical shift values relative to an external phosphoric acid standard. Chemical shift values are dimensionless and are given in parts per million (ppm) relative to the phosphoric acid standard, which is set at 0 ppm. These values are essentially variations in resonance frequency of different compound classes compared to a standard. To determine the percentage of sample belonging to each phosphorus functional group, the spectra were integrated over the following chemical shifts in ppm (Fig. 2): −15 to −25 (polyphosphate); 7 to −15 (phosphate esters); 30–15 (phosphonate); 69–47 and −55 to −77 (spinning side bands for phosphate esters). Spinning side bands are an artifact of magic-angle spinning and are added to the appropriate associated peak area. The analytical error of 31P NMR as determined from previous studies is ±10% of the measured value (Sannigrahi and Ingall 2005; Hedges et al. 2002; Sannigrahi et al. 2005).
The 31P NMR chemical shifts corresponding to different P compound classes superimposed on the 31P NMR spectrum of the Effingham Inlet sample taken at 78 m (Table 1). Asterisks denote spinning side bands
3 Results and Discussion
A comparison of the relative abundances of three P compound classes (polyphosphates, P esters and phosphonates) between samples collected with ED/RO and samples collected with UF in previous studies yields three key observations (Table 1): (1) Polyphosphates are not readily observed in samples collected using UF but are observed in all samples collected by ED/RO. (2) P esters comprise the largest fraction of the DOP pool for both collection techniques. (3) Phosphonates make up a smaller portion of DOP in ED/RO samples when compared to UF samples.
The dominance of P esters in the DOM pool is consistent with previous investigations of the HMW fraction recovered by UF; however, differences in the relative abundance of other P compounds such as phosphonates and polyphosphates to P esters between ED/RO and UF samples are undoubtedly related in part to the different molecular size fractions recovered by the two techniques.
Although differences abound between samples recovered using ED/RO compared to UF samples from the literature in similar locations, perhaps the most intriguing finding is the rather limited compositional variation in ED/RO samples between the vastly different sites sampled so far. This finding is in close agreement with the results of Kolowith et al. (2001), in which no variation was found in the relative proportions of P compound classes in HMW DOM recovered from several oligotrophic ocean regions and at all depths. Furthermore, our results show striking similarities between very oceanographically different regions—eutrophic and oligotrophic. These observations seem to indicate that DOP is relatively the same compositionally throughout the world ocean.
It is perplexing that regions with varying types of organisms and nutrient levels would exhibit such a small variance in abundance of P compound classes, but these findings are consistent with the small variance seen in studies of other DOP size classes (Clark et al. 1998, 1999; Kolowith et al. 2001, Sannigrahi et al. 2006). Phosphorus is cycled rapidly in marine environments (Smith et al. 1985; Karl and Bjorkman 2002; Benitez-Nelson 2000; Benitez-Nelson and Buesseler 1999). Thus, labile forms of P are quickly removed, leaving a pool of less labile material with a slow turnover time (Clark et al. 1998, 1999; Kolowith et al. 2001; Karl and Bjorkman 2002). Although each environment contains different organisms, similar molecules must be preferentially selected for decomposition and produced during decomposition processes, which leads to the P distribution observed.
3.1 Polyphosphate
Polyphosphates have been identified in marine particulate organic matter (Paytan et al. 2003), but polyphosphates were only recently recognized in marine dissolved matter (Diaz et al. 2008). In keeping with the nomenclature used in the field, we will simply be referring to polyphosphate as part of the DOP fraction recovered using ED/RO. However, we emphasize that polyphosphate bonds contain no C, and therefore molecules containing polyphosphate bonds are inorganic unless attached to organic moieties. Ultrafiltration samples contain only the HMW dissolved polyphosphate fraction, but samples recovered using ED/RO contains both HMW and LMW fractions. Because polyphosphate can exist in a range of molecular sizes depending on the length of the linear polyphosphate polymer, we expected to find dissolved polyphosphates in a continuum of molecular weights. However, polyphosphates were only present in the LMW. For this reason, we suspect that the amount of HMW polyphosphate recovered by conventional UF techniques is not zero as previously reported but that it is too low to be detected. Comparison of UF samples to ED/RO samples indicates that polyphosphates are most prevalent in the LMW fractions of DOM. The presence of polyphosphates in LMW DOM is surprising especially in nutrient-poor open ocean environments, such as the Gulf Stream, where such P-rich molecules would be a valuable potential resource for microorganisms (Moore et al. 2005). The presence of LMW polyphosphates in such environments suggests that (1) LMW polyphosphates are in forms that are difficult for organisms to utilize; or (2) LMW polyphosphates are released into the water column more rapidly than they are utilized and are thus allowed to build up to detectable levels (Fig. 1).
A polyphosphate compound consists of three or more orthophosphate groups linked together in a chain by phosphoanhydride (P–O–P) bonds. According to the current understanding of polyphosphates in cells, organisms respond to nutrient fluctuations by accumulating polyphosphates through two different mechanisms: polyphosphate overplus and luxury uptake (Karl and Bjorkman 2002). Polyphosphate overplus is the process by which cells in P-starved environments assimilate and store large quantities of P in the form of polyphosphates when P becomes temporarily available. Luxury uptake allows organisms to form polyphosphates when another nutrient other than P limits growth, thereby permitting the organism to accumulate excess P reserves that would become highly valuable under future potential P starvation. One form in which organisms can store excess P is by constructing chains of phosphate, which are encapsulated into granules for later use. Biogenic polyphosphate granules are complexed with charge-balancing cations, principally divalent cations such as calcium, and are stored within the cell cytoplasm until the organism requires it. When the cell lyses, the polyphosphate granules can be released back into the water column, where the granules may remain stable until they are used by other organisms.
Cellular polyphosphate can be found in many forms. Here, we will focus on two forms of polyphosphate found inside cells: as storage granules and as nucleotides such as adenosine triphosphate (ATP). The larger-sized granules should be recovered by both UF and ED/RO. However, the largest percentage of dissolved polyphosphate in seawater appears within the LMW portion, and this LMW portion can only be recovered by ED/RO. While these LMW polyphosphates may be primarily comprised of small (<1,000 Da) storage granules, the nucleotide interpretation of LMW dissolved polyphosphate is also consistent with the molecular nature of these compounds. For example, ATP has an atomic mass of ~500 Da. The cutoff for UF is 1,000 Da, making nucleotides impossible to recover unless ED/RO is used.
Given the rapid cycling of LMW polyphosphates in numerous cellular biochemical reactions, it might be presumed that nucleotides should be highly bioreactive and, therefore, unstable in the water column. However, Bjorkman and Karl (2005) were able to measure concentrations of ATP and GTP (guanosine triphosphate) at Station Aloha in the North Pacific subtropical gyre, near Hawaii, and found these nucleotides to decrease in concentration with depth. Therefore, despite the fact that the nucleotides are reactive, they are allowed to build up to some quantifiable extent. To this extent, perhaps the utilization of dissolved nucleotides by pelagic organisms is uptake-limited.
If we assume typical surface DOP concentrations of 0.1–0.2 μM (Karl and Bjorkman 2002), and use our average polyphosphate content of 11%, a rough calculation indicates that polyphosphate concentrations should be in the nano-molar range. The dissolved nucleotide concentrations measured by Bjorkman and Karl (2005) are on the order of pico-molar, which are too small to account for the dissolved polyphosphate we observe. To reconcile the data, it is crucial to understand what is actually being detected by the methods used. The firefly bioluminescence assay employed by Bjorkman and Karl (2005) is only able to detect specific nucleotides, but NMR is sensitive to all trinucleotides. Any molecule with three or more phosphate groups in a chain (not only ATP and GTP) is designated a polyphosphate by NMR. One possibility is that LMW polyphosphate is derived from degraded nucleotides, which may be less bioavailable than the original nucleotides and can no longer be characterized as such.
Ultimately, the oceanographic importance of LMW dissolved polyphosphate depends on how easily organisms may utilize it. Although we might hypothesize that the structure of this polyphosphate is much like that of ATP, which is readily used by organisms, the most important control on bioavailability may be the size of the molecules. For example, ATP with an atomic mass of approximately 500 Da is small enough to be taken up directly into the cell. Once inside, ATPases can break down the phosphoanhydride bonds within the ATP molecule in order to utilize phosphorus, or the molecule may be kept intact to be used in metabolic processes. However, every organism has a limit on the size of molecule that it can take up directly. For example, Weiss et al. (1991) found that the outer membrane of Gram-negative bacteria can only transport molecules up to about 600 Da. Therefore, if we consider granules as a source, it is possible that these polyphosphates could be too large to cross cell membranes without first being broken up by extracellular enzymes. This would imply that the bioavailability of these compounds depends on the prevalence of organisms that can manufacture the appropriate enzymes. The structure and size of dissolved polyphosphates remain uncertain and will be the focus of future research.
3.2 Phosphate Esters
In all ED/RO samples, we observe greater amounts of phosphate esters compared to samples recovered by UF (Table 1). Ultrafiltration only recovers molecules from the HMW (>1,000 Da) fraction of DOM, while ED/RO isolates both HMW and LMW compounds. Comparison of UF samples to ED/RO samples suggests that the LMW fraction is enriched in P esters. LMW P ester molecules, such as glucose phosphate and fructose phosphate, are important to key universal cellular metabolic processes, such as glycolysis. Determining the types of molecules that likely compose these LMW P esters can help provide insights as to the reactivity and oceanographic significance of this compound class.
Phosphate esters are synthesized by all living organisms and are incorporated into numerous common biomolecules such as phosphoamino acids, phosphosugars and phospholipids. For this reason, it is not surprising that P esters comprise the most abundant compound class in marine DOP. Because most of the organic matter in the ocean is released by phytoplankton, the dominance of P esters in DOM recovered by ED/RO is undoubtedly related to the abundance of these compounds in plankton cells.
An organism will typically prefer to directly take up dissolved inorganic phosphorus (DIP) from seawater, rather than use a DOP molecule. This is because the use of DOP requires the organism to manufacture an enzyme to break the phosphate out of the organic compound before it can be utilized (Fig. 1). All marine organisms share the ability to produce enzymes, such as alkaline phosphatase, which are capable of breaking the C–O–P bond of a phosphate ester (Karl and Bjorkman 2002). Laboratory studies have shown, however, that marine bacteria begin to produce alkaline phosphatase even when there are already high levels of DIP in the water column (Hassan and Pratt 1977). The ubiquity of alkaline phosphatase and other ester hydrolyzing enzymes in ocean waters leads to large fluxes of DIP derived from ester molecules into the water column.
Upon examining possible associations of biomolecules with P in HMW DOM samples, Sannigrahi et al. (2006) found a strong positive correlation (R 2 > 0.80) between P content and amino acids and carbohydrates, but no correlation between P and lipid contents. If similar trends extend to the LMW fraction, a correlation between amino acids and carbohydrates might also be expected. Indeed, the addition of phosphate groups to amino acids by a P ester linkage forms phosphoamino acids, which are very small molecules that play an important role in the regulation of cellular metabolism. In bacteria, the amino acids histidine, aspartic acid and glutamic acid are usually favored for phosphorylation (Sannigrahi et al. 2006; Yan et al. 1998).
It is also possible that many of these LMW P esters are associated with carbohydrates. Carbohydrates comprise 10–70% of the plankton cell (Romankevich 1984) and are the largest fraction of organic matter in the ocean, accounting for 20–30% in surface waters (Pakulski and Benner 1994). Studies have shown that carbohydrates account for up to 80–90% of total extracellular release by phytoplankton (Myklestad 1995) and that most carbohydrates in the water column come from cells (Biersmith and Benner 1998; Romankevich 1984). Therefore, phytoplankton provides a sizable flux of carbohydrates to the water column.
Of these carbohydrates, it is probable that LMW aldoses and nucleotides constitute a significant portion of LMW P esters. Aldoses play an important role in cellular function and are likely the bulk of LMW carbohydrates in cells. A study revealed that the largest percentage of aldoses (68%) is found in the LMW fraction of DOM (Skoog and Benner 1997). Because glucose is the dominant aldose in phytoplankton cellular material (Biersmith and Benner 1998), glucose phosphate is likely to make up a large portion of LMW P esters. In fact, Skoog and Benner (1997) found glucose to be the most abundant aldose in LMW DOM. However, the same study reports only about 7–20% of carbohydrates have been identified as aldoses, when measuring aldoses using HPAE-PAD (high performance anion-exchange chromatography with pulsed amperometric detection) separation/detection process and total carbohydrate using MBTH (Skoog and Benner 1997). This may be due to the limitations of methods used to measure aldoses in seawater that arise when the compound has been altered. If an aldose has been degraded, it may not be measured by the HPAE-PAD separation/detection technique, but may still be recognized as an aldose by NMR or MBTH if it still contains the functional groups.
Nucleotides contain one P ester bond and a three-member phosphate chain. If the LMW polyphosphate compounds we measured are mainly composed of nucleotides that still have a P ester group attached, another contributor to LMW P ester compounds would be nucleotides. The presence of nucleotides in a sample will lead to corresponding increases in the relative abundances of both polyphosphates and P esters, when compared to abundances of UF samples from the literature. This is indeed what we observe, and may be evidence that the LMW P ester abundance has a large contribution from nucleotides.
Many LMW P ester compounds are small enough to be readily utilized and be transported directly across the cell membrane. Just as the presence of nucleotides in DOM is enigmatic, so is the existence of these small P esters. The extracellular enzyme, alkaline phosphatase, is needed to utilize these compounds and is ubiquitous in seawater. Therefore, LMW P esters would be expected to be generally reactive. However, they are present in abundance in DOM. Phytoplankton cells provide a large flux to the water column, which may allow the compounds to build up to some extent. An alternate explanation for the presence of LMW P esters is that they are altered to a form that few organisms can utilize, yet may contain the functional groups detectable by NMR.
3.3 Phosphonates
When comparing our data to the three previous studies (Table 1), phosphonate abundance in samples processed using ED/RO is about half of that in samples processed by UF. Lower phosphonate abundances in ED/RO samples indicate that (1) LMW phosphonates are not as common as HMW phosphonates in marine organisms; or (2) the distribution reflects the end result of cycling P processes. If the distribution reflects cycling processes, then LMW phosphonates are removed from DOM more readily than HMW phosphonates.
Phosphonates are a group of organic molecules characterized by a direct C–P bond, rather than the more prevalent C–O–P ester linkage found in most phosphorus-containing biomolecules. There are not many direct studies of the occurrence of specific phosphonate compounds in marine settings. The occurrence as detected by NMR is widespread as indicated by the results presented here and in several studies (Kolowith et al. 2001; Sannigrahi et al. 2006; Clark et al. 1999). Phosphonate compounds have been identified in many marine organisms (Kolowith et al. 2001) but typically comprise a small percentage of the total phosphorus. In fact, biochemical pathways that cycle phosphonates in the ocean are highly unusual, given the low prevalence of phosphonates in marine systems, and may be artifacts of ancient metabolic processes (Pasek 2008). Most organisms do not produce the enzymes required to break the C–P bond, resulting in a buildup of phosphonates in the environment. However, research has shown that nitrogen-fixing bacteria, such as trichodesmium, living in oligotrophic waters have the ability to utilize phosphonates (Dyhrman et al. 2006). Bacteria may also represent an important source of phosphonates in marine systems (Kolowith et al. 2001).
The specific biomolecules and structures containing phosphonates in marine organisms are not as well defined as polyphosphates and P esters. It has been speculated that at least some of the P in HMW fractions is in the form of phosphonolipids. These phosphonolipids may increase the resistance to degradation of cellular membranes (Kennedy and Thompson 1970; Rosenburg 1973). This chemical resistance may manifest itself in the relative preservation and abundance of these compounds in the HMW P pool. On the other hand, Sannigrahi et al. (2006) did not find a correlation between lipid and P contents in DOM, which may rule out the phosphonolipids as a key contributor to the phosphonates.
It is possible that LMW phosphonate compounds may be equally or more common in cells than HMW phosphonate compounds. Thus, decrease in phosphonate abundance in the ED/RO material relative to UF-recovered DOM suggests that LMW phosphonate compounds may be more bioavailable than HMW phosphonate. There have been few direct studies in marine environments, but studies in soils and wastewater treatment systems reveal the utilization of several LMW phosphonate compounds, such as aminoethylphosphonate and methylphosphonate, in cells (Cook et al. 1978; Daughton et al. 1979). If many of the LMW compounds are small enough to travel across cell membranes, their utilization may in fact be more energetically favorable (Rittenberg and Hespell 1975). Indeed, LMW phosphonate compounds, such as methylphosphonate, are bioavailable to certain organisms, and utilization of these compounds is important on a global level (Ingall 2008; Karl et al. 2008). Thus, the distribution of phosphonates in DOM recovered by ED/RO more likely reflects the aggregate of a number of P cycling processes.
4 Conclusions
Use of the ED/RO technique has yielded new insights into marine DOP composition and cycling. Samples recovered using ED/RO and analyzed by 31P NMR reveal similar relative proportions of P esters (80–85%), phosphonates (5–10%) and polyphosphates (8–13%) in marine settings ranging from oligotrophic to eutrophic. We hypothesize these striking similarities between different sites are a result of similar decomposition processes operating in all marine ecosystems, causing the accumulation of similar, less bioavailable P compounds. Of particular interest is the LMW pool, which past methods of isolating DOM have been inadequate at recovering. Virtually all polyphosphates and a sizable portion of P esters in DOM occur in this LMW fraction. Ultimately, the oceanographic importance of the LMW pool will depend on the bioavailability of the mixture of LMW DOP compounds that compose it. Future research will be necessary to better define the distribution and bioavailability of LMW DOP compounds in the ocean.
References
Benitez-Nelson CR (2000) The biogeochemical cycling of phosphorus in marine systems. Earth-Sci Rev 51:109–135
Benitez-Nelson CR, Buesseler KO (1999) Variability of inorganic and organic phosphorus turnover rates in the coastal ocean. Nature 6727:502–505
Biersmith A, Benner R (1998) Carbohydrates in phytoplankton and freshly produced dissolved organic matter. Mar Chem 63(1–2):131–144
Bjorkman K, Karl DM (1994) Bioavailability of inorganic and organic phosphorus compounds to natural assemblages of microorganisms in Hawaiian coastal waters. Mar Ecol Prog Ser 111:265–273
Bjorkman K, Karl DM (2005) Presence of dissolved nucleotides in the North Pacific Subtropical Gyre and their role in cycling of dissolved organic phosphorus. Aquat Microb Ecol 39:193–203
Clark LL, Ingall ED, Benner R (1998) Marine phosphorus is selectively remineralized. Nature 393:426
Clark LL, Ingall ED, Benner R (1999) Marine organic phosphorus cycling: novel insights from nuclear magnetic resonance. Am J Sci 299:724–737
Cook AM, Daughton CG, Alexander M (1978) Phosphate utilization by bacteria. J Bacteriol 133(1):85–90
Daughton CG, Cook AM, Alexander M (1979) Bacterial conversion of alkylphosphonates to natural products via carbon-phosphorus bond cleavage. J Agric Food Chem 27(6):1375–1382
Diaz J, Ingall E, Benitez-Nelson C, Paterson D, de Jonge MD, McNulty I, Brandes JA (2008) Marine polyphosphate: a key player in geologic phosphorus sequestration. Science 320:652–655
Dyhrman ST, Chappell PD, Haley ST, Moffet JW, Orchard ED, Waterbury JB, Webb EA (2006) Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 439:68–71
Gurtler BK, Vetter TA, Perdue EM, Ingall E, Koprivnjak JK, Pfromm PH (2008) Combining reverse osmosis and pulsed electrical current electrodialysis for improved recovery of dissolved organic matter from seawater. J Membr Sci 323:328–336
Hassan HM, Pratt D (1977) Biochemical and physiological properties of alkaline-phosphatases in 5 isolates of marine-bacteria. J Bacteriol 129:1607–1612
Hedges JI, Baldock JA, Gelinas Y, Lee C, Peterson ML, Wakeham SG (2002) The biochemical and elemental compositions of marine plankton: a NMR perspective. Mar Chem 78:47–63
Ingall ED (2008) Oceanography: making methane. Nat Geosci 1(7):419–420
Jackson GA, Williams PM (1985) Importance of dissolved organic nitrogen and phosphorus to biological nutrient cycling. Deep Sea Res 32:223–235
Karl DM, Bjorkman K (2001) Phosphorus cycle in seawater: dissolved and particulate pool inventories and selected phosphorus fluxes. Methods Microbiol 30:239–270
Karl DM, Bjorkman K (2002) Dynamics of DOP. In: Hansell D, Carlson C (eds) Biochemistry of marine dissolved organic matter. Elsevier, USA, pp 246–366
Karl DM, Yanagi K (1997) Partial characterization of the dissolved organic phosphorus pool in the oligotrophic North Pacific Ocean. Limnol Oceanogr 42:1398–1405
Karl DM, Beversdorf L, Bjorkman K, Church M, Martinez A, DeLong EF (2008) Aerobic production of methane in the sea. Nat Geosci 1(7):473–478
Kennedy KE, Thompson GA (1970) Phosphonolipids—localization in surface membranes of tetrahymena. Science 168(3934):989–991
Kolowith LC, Ingall ED, Benner R (2001) Composition and cycling of marine organic phosphorus. Limnol Oceanogr 46(2):309–320
Koprivnjak JF, Perdue EM, Pfromm PH (2006) Coupling reverse osmosis with electrodialysis to isolate natural organic matter from fresh waters. Water Res 40(19):3385–3392
Koprivnjak JF, Pfromm PH, Ingall E, Vetter TA, Schmitt-Kopplin P, Hertkorn N, Frommberger M, Knicker H, Perdue EM (2009) Chemical and spectroscopic characterization of marine dissolved organic matter isolated using coupled reverse osmosis—electrodialysis. Geochim Cosmochim Acta 73:4215–4231
Moore LR, Ostrowski M, Scanlan DJ, Feren K, Sweetsir T (2005) Ecotypic variation in phosphorus acquisition mechanisms within marine picocyanobacteria. Aquat Microb Ecol 39(3):257–269
Myklestad SM (1995) Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Sci Total Environ 165(1–3):155–164
Orrett K, Karl DM (1987) Dissolved organic phosphorus production in surface seawater. Limnol Oceanogr 32:383–395
Pakulski JD, Benner R (1994) Abundance and distribution of carbohydrates in the ocean. Limnol Oceanogr 39(4):930–940
Pasek MA (2008) Rethinking early Earth phosphorus geochemistry. Proc Natl Acad Sci USA 105:853–858
Paytan A, Cade-Menun BJ, McLaughlin K, Faul KL (2003) Selective phosphorus regeneration of sinking marine particles: evidence from 31P-NMR. Mar Chem 82:55–70
Rittenberg SC, Hespell RB (1975) Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol 121:1158–1165
Romankevich EA (1984) Geochemistry of organic matter in the ocean. Springer, New York, p 334
Rosenburg H (1973) Phosphonolipids. In: Ansell GB, Hawthorne JN, Dawson RMC (eds) Form and function of phosphonolipids. Elsevier, Amsterdam, pp 333–344
Sannigrahi P, Ingall E (2005) Polyphosphate as a source of enhanced P fluxes in marine sediments overlain by anoxic waters: Evidence from 31P NMR. Geochem Trans 6(3):52–59
Sannigrahi P, Ingall ED, Benner R (2005) Cycling of dissolved and particulate organic matter at station Aloha: insights from 13C NMR spectroscopy coupled with elemental, isotopic, and molecular analyses. Deep Sea Res I 52(8):1429–1444
Sannigrahi P, Ingall E, Benner R (2006) Nature and dynamics of phosphorus-containing components of marine dissolved and particulate organic matter. Geochim Cosmochim Acta 70(23):5868–5882
Skoog A, Benner R (1997) Aldoses in various size fractions of marine organic matter: implications for carbon cycling. Limnol Oceanogr 42(8):1803–1813
Smith REH, Harrison WG, Harris L (1985) Phosphorus exchange in marine microplankton communities near Hawaii. Mar Biol 86:74–75
Smith SV, Kimmerer J, Walsh TW (1986) Vertical flux and biochemical turnover regulate nutrient limitation of net organic production in the North Pacific Gyre. Limnol Oceanogr 31:161–167
Vetter TA, Perdue EM, Ingall E, Koprivnjak JF, Pfromm PH (2007) Combining reverse osmosis and electrodialysis for more complete recovery of dissolved organic matter from seawater. Sep Purif Technol 56:383–387
Weiss MS, Abele U, Weckesser J, Welte W, Schiltz E, Schultz GE (1991) Molecular architecture and electrostatic properties of a bacterial porin. Science 254:1627–1630
Yan JX, Packer NH, Gooley AA, William KL (1998) Protein phosphorylation: technologies for the identification of phosphoamino acids. J Chromatogr 80:23–41
Acknowledgments
This material is based upon work supported by the National Science Foundation under Grants 0526178 and 0849494. We thank Dr. Johannes Leisen and Dr. Les Gelbaum of the Georgia Institute of Technology NMR center for their help with the NMR analyses. The authors would also like to thank the crews of the R/V Barnes and the Icebreaker Oden for assistance with field sampling and Julia Diaz for help during field sampling and sample analysis. The authors greatly appreciate the welcoming base for our field studies provided by George Patterson and John Platenius of the Clayoquot Field Station in Tofino, British Columbia and Jay Brandes and Bill Savidge of the Skidaway Institute of Oceanography. We thank George Luther and two anonymous reviewers for helpful comments and Victoria Van Cappellen for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Young, C.L., Ingall, E.D. Marine Dissolved Organic Phosphorus Composition: Insights from Samples Recovered Using Combined Electrodialysis/Reverse Osmosis. Aquat Geochem 16, 563–574 (2010). https://doi.org/10.1007/s10498-009-9087-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-009-9087-y