Abstract
The success of antiretroviral therapy in preventing progression of human immunodeficiency virus (HIV) infection to full-blown Acquired Immune Deficiency Syndrome (AIDS) and extending the life span of people infected with HIV has led to a large number of persons aging with HIV (PAWH). This cohort is experiencing high rates of cardiovascular disease, cancer, and other serious, often chronic illnesses resulting in premature multi-morbidity, polypharmacy and functional decline. It is unclear whether chronic HIV infection, its treatment and associated side effects (e.g. lipodystrophy), or other risk factors prominent in PAWH (e.g. smoking, drug use, social isolation/stress) are responsible for this early onset of disease and functional decline, but there is no doubt that rates of geriatric syndromes like frailty, falls and cognitive impairment occur in 55–60 year old PAWH at a rate equivalent to that seen in 70+ year old HIV-uninfected persons. It is still unclear whether HIV-associated ‘aging’ is truly due to acceleration of the aging process or whether HIV is a risk factor for multiple diseases leading to the “aged phenotype” at a younger age. Uncovering the critical processes which drive age-related changes and identifying therapeutic strategies to ameliorate them will be important for management of PAWH.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Why is there a chapter on HIV in a translational science textbook on aging? Effective antiretroviral therapy (ART) has resulted in many people with HIV infection living far beyond what was thought possible just a few years ago [1, 2]. It is estimated that over half of all U.S. HIV-infected persons will be >50 years by 2015 [3, 4], and the success and availability of ART is even leading to an aging HIV-infected population in developing nations [1, 5, 6], emphasizing the need for aging-related research in those countries where HIV burden of illness is greatest [7].
There is marked debate as to whether HIV accelerates aging itself or is an added risk factor for a number of diseases and conditions that lead to an “aged phenotype.” Of course, there is no single pathway that defines “aging” – in fact, two recent, excellent reviews [8, 9] emphasize a number of “hallmarks” of aging – biologic changes that accompany aging, but none is clearly “the” causal pathway. Cardiovascular disease (CVD) and many other diseases increase with age, and advancing age is the leading risk factor for CVD. But we generally don’t consider CVD risk factors (e.g. hypercholesterolemia) to be conditions that accelerate aging itself. However aging with HIV is different than aging with hypercholesterolemia; a much broader array of illnesses occurs with greater frequency in people aging with HIV (PAWH). This leads not only to prematurity of a single disease, but multiple diseases, as well as decreased physiologic reserve and increased vulnerability to catastrophic illness, hospitalization and death. Functional decline – physical and/or cognitive – often accompanies multi-morbidity or may occur independently, but in either case functional decline is the strongest risk factor for disability and loss of independence, particularly when social and family support structures are lacking. This state of multi-morbidity, vulnerability, functional decline and loss of independence is what we usually view as “old” – or the aged phenotype – and there is no doubt that this phenotype occurs earlier in PAWH when compared to HIV-uninfected persons [10–14]. In this chapter, we will briefly summarize a few examples of age-related serious non-AIDS events (SNAEs) such as CVD and cancer – and geriatric syndromes (functional decline/frailty and multi-morbidity) to highlight the clinical relevance and translational opportunities to link mechanisms to clinical outcomes in PAWH.
2 Increased Prevalence of Age-Related, Serious Non-AIDS Events (SNAEs) in PAWH
While life expectancy has increased markedly for PAWH, this group experiences a greater frequency of age-associated comorbid conditions, such as CVD, non-AIDS-defining cancers (liver, lung, anal), osteoporosis/osteopenia/bone fractures, metabolic syndrome, and neurocognitive dysfunction. These events are termed SNAEs and increasingly robust data suggest they are very common in PAWH, even those well controlled on ART [15]. CVD and cancer have the most robust database and are therefore examined in greater detail in the following paragraphs.
CVD risk factors and rates of acute coronary syndromes and heart failure are markedly increased in HIV-infected vs. age matched control subjects [16–20], and coronary artery “age” is accelerated on average by about 15 years in treated HIV-infected persons (median duration of ART ~11 years) as assessed by coronary artery calcium (CAC) score comparing PAWH to age-defined norms established in the MultiEthnic Study on Aging (MESA) cohort [21]. Higher levels of C-reactive protein, interleukin-6, and D-dimer have been shown to be significantly associated with an increased risk of all-cause mortality in HIV-infected individuals not on ART, and much of this is cardiovascular mortality [22]. Specific ART drugs also may be causally associated with early heart disease, even after controlling for age and traditional cardiovascular risk factors [23, 24]. Further, lipodystrophy and metabolic syndrome (altered body fat, hyperlipidemia and insulin resistance) are common in HIV-infected patients receiving ART [25, 26]. The redistribution of fat mass and progression to metabolic syndrome (12/100 patient-years) typically occurs within 3 years after the initiation of ART [27], when weight gain is often substantial, thus increasing cardiovascular disease risk. Enhanced cardiovascular “aging” is not limited to coronary artery disease. Left ventricular diastolic dysfunction and increased vascular stiffness [28–30] are more common in HIV-infected subjects versus uninfected, age-matched controls even after controlling for hypertension and other risk factors. Heart failure and atrial fibrillation, typically seen in older adults, is increasingly being reported in younger PAWH [18–20].
As ART use has become widespread, AIDS-defining cancers (Kaposi’s Sarcoma, lymphomas) have become less common in this population, but increased survival and perhaps decreased competing causes of AIDS-defining cancer deaths have led to increased numbers of non-AIDS-Defining Cancers (NADC) [31]. A number of NADC occur more frequently in PAWH than age-matched control cohorts [32] and NADC are increasingly a cause of death in PAWH [15]. Initial reports suggested the age of onset of many NADC was much earlier than in those without HIV, but most of this appears to be a cohort effect. PAWH are a younger cohort than the general population [1] so colon, lung or other cancers may appear to only be occurring in younger adults, but there aren’t many 70+ year old PAWH so this is often a false impression. As control groups and age-adjustments have been refined, it appears NADC are only minimally “accelerated” with regard to age at diagnosis – perhaps 3–5 years [33] (Table 1). It is important to note that some NADC that are most strongly related to age – breast cancer in women and prostate cancer in men – do not appear to be increased in those with HIV [33, 34], though data are sure to evolve as persons continue to age with HIV infection.
Another way to examine the question of whether HIV directly “ages” individuals or acts in parallel is to assess whether age remains an independent risk factor for SNAEs in PAWH. Within cohorts of PAWH, increased age is an independent predictor of stroke, myocardial infarction, fractures, osteoporosis, diabetes, and non-AIDS associated cancers, while controlling for CD4 count, viral load, intravenous drug use, smoking, and duration of HIV infection [35].
3 Geriatric Syndromes in PAWH
3.1 Multi-morbidity
Despite the success of ART, extensive evidence suggests HIV-infected persons are more likely than their HIV-uninfected counterparts to have multiple comorbidities at a young age. This is perhaps not surprising for illnesses with overlapping risk factors (i.e. hepatitis C, human papillomavirus [HPV]-related cancers), but it is also true across organ systems where intersecting risks are not so clear; early-onset of disease in individual organ systems in PAWH has been observed (e.g. coronary artery disease, arterial stiffness, cerebral blood flow, and bone fractures) [21, 36–39]. Chronic liver and renal diseases are also more common in PAWH compared to HIV uninfected populations [40]. Although behavioral factors such as smoking and illicit drug use are more prevalent in populations of PAWH, controlled studies have shown that these factors do not fully explain the increased risk for age-related conditions such as cardiovascular and liver disease [35, 41, 42]. Where aggressive ART is widely available, 58 % of HIV-infected subjects aged 51–60 have one or more of the following: renal failure, diabetes mellitus, bone fracture, hypertension or overt cardiovascular disease vs. only 35 % of HIV-uninfected controls [10, 35]. The rate of multi-morbidity (> one major chronic illness) at age >50 years is about 2.5 times higher in HIV-infected subjects vs. HIV-uninfected controls [10, 35, 40].
On average, PAWH aged 50 and older have up to three chronic illnesses, in addition to HIV [43] (Fig. 1). Depending on the population, studies have demonstrated increased prevalence of specific comorbidities. The onset of multi-morbidity appears to be accelerated 12–15 years in those with HIV infection [10]. Further, multi-morbidity risk assessments such as the Veterans Aging Cohort Study (VACS) Index derived and validated in HIV-infected subjects correlates with mortality risk and hospitalization [44, 45]. Importantly, the VACS index has now been validated to predict mortality in HIV-uninfected populations [45] demonstrating the generalizability of this integrated measure of cumulative damage to the hematopoietic, immunologic, hepatic and renal systems.
Prevalence of comorbidity burden, HIV-infected persons age > 50 (Data derived from [10])
3.2 Polypharmacy
In the setting of multi-morbidity, PAWH have increased risks of developing both HIV-associated non-AIDS (HANA) and non-HIV related conditions. Consequently, polypharmacy and increased complexity of care are becoming commonplace in the health management of PAWH, noting that the disease courses may be altered depending on the patient’s state of virologic suppression [46]. PAWH who are aged 50 and older are more likely to have at least one medication (in addition to ART) compared to PAWH younger than age 50 [47]. Specifically, older PAWH are more likely to take concurrent cardiovascular, gastrointestinal, and hormonal medications than younger patients [47].
The inherent complexity of polypharmacy translates into potential harm for older patients. In older adults without HIV, polypharmacy is a known risk factor for falls, adverse drug events (ADE) including drug-drug interactions (DDI), morbidity, and mortality [48]. These associations remain true for PAWH but may occur at younger ages compared to people without HIV infection [47–49]. At baseline, older patients are at increased risk for ADE, compared to younger patients. In addition to direct toxicity for the patient, ADE and DDI can mean decreased efficacy of therapy, both for HIV and other comorbidities, especially in the case of protease inhibitor (PI)-based ART [49]. The list of potential DDI is extensive and includes virtually every class of therapeutics, including cardiovascular, gastrointestinal, hematologic, anti-neoplastic, antimicrobial, psychiatric, and endocrine (including inhaled steroids which aren’t typically considered to have systemic effects) [47–50]. Predicting DDI is more challenging due to changes in drug metabolism that occur with normal aging, a process which may be accelerated or accentuated in PAWH. The known increased prevalence of liver and kidney disease in PAWH further complicates prediction and prevention of DDI and ADE [48, 49].
Adherence to ART is extremely important for PAWH and is a predictor of morbidity and mortality for these patients, but a significant challenge for many [49, 51–53]. Similar principles regarding consistent medication use can apply to other chronic illnesses, including the common HANA and non-HIV associated comorbidities which are so prevalent in this population. Recent data suggest that lower pill burden is an important factor in improving adherence and virologic suppression, making awareness (and avoidance if possible) of polypharmacy even more salient [54].
3.3 Frailty
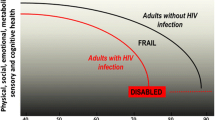
Frailty has been defined and various measures validated in older HIV-uninfected adults, but it is generally agreed to represent increased risk and decreased ability to recuperate from illness and injury. Frailty is increased in HIV-infected vs. age-matched HIV-uninfected controls [13, 55–61]. In those PAWH, there is a high correlation between various measures of frailty validated in seniors, though definitions vary from study to study and the reader should be cautious to assess frailty definitions, cohort effects, and control group definitions when comparing individual rates of frailty between studies [62]. Early research measured the prevalence of frailty using the frailty-related phenotype (FRP) in 55 year old men with HIV infection (infected for less than or equal to 4 years) as equivalent to the prevalence of FRP in men 65 years of age or older without HIV [12]. Onen et al. measured a prevalence of 9 % for frailty in an outpatient HIV clinic (mean age of 41.7 years), which was comparable to the prevalence of frailty in Caucasian Europeans aged 65 years and older [13]. In the same study, investigators measured patient-level characteristics; frailty was associated with socioeconomic status, multi-morbidity, lower education level, longer period of HIV infection, history of opportunistic infection, as well as an increased risk of hospitalization, number of hospitalizations, and inpatient length of stay [13]. Much of the early data suggested frailty in PAWH was associated with uncontrolled HIV/weight loss/wasting, but more recent data suggest frailty in HIV has been associated with obesity and intramuscular adiposity, as seen in HIV-uninfected older persons [59, 61, 63].
Frailty is potentially mediated more by inflammation and body composition than by HIV infection itself. This is compounded by the fact that optimal immune function may be hindered by age-related changes that are independent of virologic suppression [46, 64]. In PAWH, frailty is associated with central obesity, sarcopenia, and increased muscle fat density for age [65]. Oursler et al. showed that, despite ART, physical function in PAWH aged 50 years and older was worse compared to HIV-uninfected people [60]. Regardless of age, HIV-infected patients with chronic pulmonary disease had worse physical function compared to HIV-uninfected people, such that a 50 year old person with HIV and chronic obstructive pulmonary disease (COPD) had functional measurements approximating a 68 year old person with COPD, but without HIV [60]. Within populations of PAWH, the prevalence of frailty is increased in people who also use intravenous drugs [43]. Not surprisingly, frail PAWH have a high prevalence of comorbidities, including hypertension, COPD, viral hepatitis, dementia, and cancer; this pattern of multi-morbidity mirrors trends seen in the larger population of PAWH [11].
Beyond the effects that frailty may have on physical health and mental well-being, this phenotype has implications for healthcare delivery and models of care. Guideline-driven care may not be practical or universally applicable to PAWH if their risks of various conditions change at different age breakpoints or based on factors other than what has been measured in the foundational studies. Use of more tailored prediction tools such as the VACS Index may be more applicable due to incorporation of multiple biomarkers [46].
3.4 Neurocognitive Impairment
A full examination of the neurologic manifestations of HIV and even discussion limited to cognitive impairment is beyond the scope of this review. Briefly, 50 % of PAWH will develop an HIV-associated neurocognitive disorder (HAND) [43, 66]. HAND is a spectrum of clinical conditions ranging from asymptomatic neurocognitive impairment (ANI – least severe) to HIV-associated dementia (HAD – most severe, previously known as “AIDS Dementia Complex”) [67] (Fig. 2). The symptoms can be largely reversed with ART, but the incidence of HAND is associated with worse adherence. The impact of HAND is marked with HAND being associated with decreased ability to complete daily functions, poorer quality of life, and shorter survival. While the incidence and prevalence of HAND are decreasing due to ART, the incidence and prevalence of ANI and mild neurocognitive disorder (MND) are stable to increasing, spurring a recommendation for universal neurocognitive screening of PAWH [67, 68]. Furthermore, HIV itself may alter brain structure, despite ART, thus, the full expression of HIV-related cognitive disorders may require time to become apparent [69].
3.5 Quality of Life and Mental Health
Compared to HIV-uninfected people, PAWH (ages ≥ 50 years) are not as happy, optimistic about aging, or resilient [43, 70]. They also experience more perceived stress, anxiety about the future, and lower quality of physical and mental health [70]. Social isolation, a common occurrence in older adults regardless of HIV status, is associated with increased risk for hospitalization and all-cause mortality. The social networks for older PAWH may shrink due to common age-related factors (e.g. age-related deaths, limited transportation, geographic isolation) and/or more HIV-specific factors: loss of peers earlier in the HIV/AIDS epidemic, stigma, marginalization in the current make-up of the epidemic [43, 71, 72]. While both HIV-infected and HIV-uninfected older adults may experience social isolation to some degree, HIV infection alone is associated with increased risk and prevalence of social isolation [71].
4 Potential Mechanisms Linking Chronic HIV Infection with Age-Related Conditions
4.1 Immunological Similarities Between HIV Infection and Healthy Aging
The overlapping burden of morbidities and SNAEs in PAWH and aged individuals has led to the hypothesis that similar pathogenic mechanisms are driving the development of these diseases in both populations. Indeed there are many immunological parallels between chronic HIV infection and healthy aging which are summarized in Table 2 and discussed in detail below.
4.1.1 Adaptive Immune Changes
The reduced number of naïve T cells and reduced CD4:CD8 T cell ratio observed in the aged is a hallmark of T cell immunosenescence [73, 74] and also occurs in HIV-infected individuals due in part to thymic involution and reduced regenerative capacity [75, 76]. Low CD4:CD8 T cell ratio is an independent predictor of non-AIDS mortality [77] and cardiovascular disease risk [78, 79] in HIV-infected individuals, suggesting T cell immunosenescence has important clinical implications in the context of PAWH. Importantly, the majority of HIV-infected individuals on long term ART fail to normalize the CD4:CD8 T cell ratio, despite restoration of CD4+ T cell levels [80]. Elderly HIV-uninfected and HIV-infected individuals also exhibit increased T cell activation (as measured by expression of the activation markers HLA-DR and CD38) [76, 81], an increased susceptibility to spontaneous apoptosis [82, 83] and an expansion of ‘senescent’ memory CD8+ T cells which lack expression of the co-stimulatory molecule CD28, contain shortened telomeres and exhibit a reduced proliferative potential [84–86]. Expansion of this cell population is thought to be largely driven by chronic antigenic stimulation by cytomegalovirus (CMV), with a large proportion of CD8+ T cells in both HIV-infected and aged individuals being specific for CMV epitopes (discussed further below). However, there are phenotypic differences in the T cells expanded due to HIV infection and those observed in CMV+ HIV seronegative individuals, in that the former show an increased number of transitional memory cells and a reduced proportion of CD28-cells expressing CD57 (a marker of reduced proliferative capacity), with low levels of this population being associated with increased risk of mortality [87]. These observations suggest that although there are many phenotypic similarities between HIV infection and aging, the mechanistic drivers, and thus immunological consequences of, senescent T cell expansion in HIV and aging may be subtly different.
While the above mentioned immunological alterations due to HIV are significantly improved by ART, they typically fail to normalize, and defects including reduced naïve T cell proportions, inverted CD4:CD8 T cell ratios and increased T cell activation largely persist in virologically suppressed HIV-infected individuals (reviewed in [88]). Furthermore, aging appears to impact negatively on the immunological benefit of ART and associated reductions in immune activation, with older HIV-infected individuals exhibiting muted naïve T cell regeneration following ART initiation [89], suggesting immunological aging may heighten HIV-related immune dysfunction in older HIV-infected individuals.
During aging, there is a reduction in the number of both total and memory B cells and defects emerge in class switching and antibody production which are thought to contribute to impaired vaccine response in the elderly [81, 90]. Viremic HIV infection is similarly associated with reduced total and memory B cell numbers together with hypergammaglobulinemia, increased cellular activation and increased susceptibility to apoptosis [91]. ART reverses many of these defects, although virologically suppressed HIV-infected individuals continue to show impaired antibody production, reduced vaccine responses and an incomplete restoration of memory B cells [92, 93]. Markers of HIV disease severity including viral load and immune activation are associated with an increased frequency of regulatory B cells (Bregs), which inhibit CD8+ T cell proliferation and function via a mechanism involving IL-10 and PD-1 [94], which may potentiate immune dysfunction in HIV. Bregs from HIV-infected individuals constitutively express higher levels of IL-6, IL-10 and cellular activation markers, suggesting increased Breg activation in vivo [95]. Interestingly, older HIV-infected individuals show an altered pattern of B cell restoration after ART initiation, including expansion of the naïve population to levels greater than those in uninfected individuals [96], suggesting that HIV and age may potentiate immune dysfunction in PAWH. These studies collectively indicate that HIV infection induces a phenotype within the adaptive immune system which resembles age-related immunosenescence and immune dysfunction, and viral suppression associated with ART only partially improves these parameters.
4.1.2 Innate Immune Changes
Immunological similarities are also seen between HIV infection and healthy aging within the innate immune system. Monocytes from both HIV-infected individuals and the elderly show impaired phagocytic function, increased TLR4-mediated production of pro-inflammatory cytokines and chemokines and an increase in phenotypic markers of activation, including an expansion of the inflammatory CD16+ monocyte subset [97–101]. Viral suppression associated with ART appears to normalize some of these changes, such as the proportions of CD16+ subsets, whilst other markers of monocyte dysfunction persist [97, 102]. Elevated levels of soluble plasma monocyte/innate immune activation markers including the chemoattractant CXCL10 (released from IFNγ-stimulated monocytes), neopterin and soluble(s) receptors CD14 and CD163 (shed from activated monocytes/macrophages) are elevated in both the elderly and HIV-infected individuals and although ART reduces the levels of these markers in HIV infection, they fail to normalize [97, 98, 103–107].
An increase in total NK cell number, due to expansion of the CD56dim population occurs during aging and in acute HIV infection [74, 108]. Aging is associated with a minimal impairment of NK cell cytotoxic function and cytokine production [109–111], whilst overall cytolytic activity is impaired in HIV infection (most prominently in viremic infection) which may be due in part to expansion of an anergic CD56neg population in HIV-infected individuals [108]. NK cells from both viremic and virologically suppressed HIV-infected individuals show heightened basal activation [112, 113] and spontaneous ADCC activity [112], whilst cytokine production is impaired [114]. The functionality of neutrophils is similarly impaired in HIV infection as in aging, as evidenced by impaired phagocytosis and oxidative burst but a heightened basal level of activation [115]. The impact of age on the number, activation state and function of dendritic cells remains unclear due to conflicting findings (reviewed in [116]), although chemotaxis and antigen uptake are impaired in aged humans [117, 118] whilst HIV infection is associated with impaired ex vivo response of plasmacytoid dendritic cells to TLR7 ligands [119]. Taken together, these data suggest that a signature of increased activation but dysregulated function is a common effect of both HIV infection and aging on innate immune cells, although much work is required to fully define the extent of these effects. It is important to note that many of the above mentioned age-related immunological changes have been observed in cross-sectional studies of HIV-infected individuals with varying degrees of immunosuppression both prior to and following ART initiation. Future longitudinal studies are required in cohorts of individuals who initiate ART early and maintain immunocompetence to adequately determine the impact of virologically suppressed HIV infection on age-related immune changes in PAWH.
4.2 Telomere Shortening
The presence of telomeres at the ends of chromosomes protects the DNA from damage and preserves the replicative potential of the cell. Telomere length progressively decreases with age and triggers replicative senescence, which contributes to immunosenescence and immune aging [120]. Telomere shortening is associated with risk of a range of age-related diseases including malignancies [121], cardiovascular/metabolic disease [122–124] and neurocognitive disease [125, 126] (summarized in Table 3 and reviewed in [195]) and has been linked with premature death in a large prospective study in Denmark [123]. HIV infection is associated with heightened telomere shortening within both T cells [85] and monocytes [97]. However, epidemiological links between shortened telomeres and HIV-related co-morbidities have received little investigation to date.
Telomere length is maintained within cells via the action of telomerase and premature telomere shortening in HIV infection may be due to reduced activity of this critical enzyme. The HIV proteins Vpr [196] and Tat [197] have been shown to inhibit telomerase in vitro whilst HIV-infected individuals appear to have an impaired ability to upregulate telomerase in response to cell stimulation [198]. Antiretroviral therapy may also contribute to premature telomere shortening as the nucleos(t)ide reverse transcriptase inhibitor (NRTI) drugs can inhibit the telomerase reverse transcriptase (TERT) component of human telomerase. In vitro studies have shown that even modern, relatively non-toxic NRTIs such as tenofovir and emtricitabine show inhibitory effects on human TERT [199, 200], and can accelerate telomere loss in cultured cells [199] whilst a small cross-sectional study found telomeres from individuals on NRTI-containing regimens were shorter than HIV negative controls and HIV-infected individuals taking non-NRTI containing regimens [200]. NRTIs remain the backbone of ART regimens throughout the world, but the accumulated consequences of decades of NRTI-treatment on oxidative stress and telomere shortening remain to be defined.
4.3 Oxidative Stress
An imbalance between levels of oxidants and anti-oxidants occurs during aging, resulting in increased plasma markers of oxidative stress in the elderly [201, 202] which contribute to immunosenescence and inflamm-aging (reviewed in [203]). HIV infection is also associated with increased levels of oxidative stress, with decreased plasma levels of anti oxidant factors such as glutathione and increased levels of the oxidative stress marker malondialdehyde found in both viremic and virologically suppressed HIV-infected individuals [204, 205]. High intracellular levels of the antioxidant factors N-acetylcysteine and glutathione inhibit HIV replication in infected cells [206] whilst low levels of these factors are associated with increased NF-kB-mediated transcription of HIV and a heightened ability of the pro-inflammatory cytokine TNF to activate HIV transcription [207], suggesting a positive feedback loop between inflammation and HIV replication. The mechanism responsible for decreased anti oxidant levels in HIV may involve the HIV Tat protein, which has been shown in mouse models to decrease production of anti oxidants and induce mitochondrial damage [208]. Certain antiretroviral (ARV) drugs including PIs and NRTIs increase the production of reactive oxygen from cells treated in vitro [209]. Consistent with this, one study reported higher levels of oxidative stress in ART-treated individuals as compared to both untreated HIV-infected and uninfected individuals, however the HIV-infected individuals in this study had significantly higher levels of a number of confounding factors including concurrent hepatitis C infection [210]. More data from virologically suppressed HIV-infected cohorts with adequate control of variables which may potentially influence oxidative stress are required to determine the impact of oxidative stress on immune aging in the modern ART era.
4.4 Chronic Inflammation and Immune Activation
Increased inflammation is one of the cornerstones of immunological aging and geroscience, and appears to be potentiated by HIV infection. Indeed, chronic inflammation and related immune activation likely has the greatest impact on morbidity and mortality in PAWH in the post-ART era. Inflammaging is a well-documented state of chronic, low-grade inflammation occurring progressively with age and is associated with the development of many age-related morbidities and functional decline in the elderly [211]. Markers of inflammation including IL-6, TNFα and high-sensitivity C-reactive protein (hsCRP) are elevated in both HIV-infected individuals and the elderly [212, 213] and are associated with increased risk of SNAEs including CVD, frailty, malignancies, bone disease and neurocognitive decline. Inflammation is intrinsically linked with cellular activation, and biomarkers of immune activation and inflammation are increasingly being recognised as risk predictors of inflammatory diseases in HIV infection, as they are in the aged (see Table 3). Biomarkers of monocyte/macrophage activation including plasma levels of sCD163 and sCD14 are predictive of age-related diseases including neurocognitive impairment/dementia [141, 176–178], malignancies [165] and also mortality [214] in HIV infection (see Table 3). Chronic monocyte/macrophage activation appears to be particularly relevant for the development of CVD in HIV infection; biomarkers of monocyte activation including the proportion of inflammatory CD16+ monocytes, the expression of monocyte activation markers (i.e. CD11b) and the soluble activation markers mentioned above are associated with atherosclerosis and its progression [158, 159, 215], arterial inflammation [170], coronary calcium score [167] and the presence of non-calcified carotid plaques [171] in HIV-infected individuals. Importantly, these associations have been made in cohorts of primarily virologically suppressed individuals, suggesting mechanisms other than overt HIV viremia are involved. Indeed, in the post-ART era, markers of inflammation and/or immune activation are emerging as more relevant predictors of disease outcome and death in virologically suppressed individuals than traditional HIV biomarkers such as viral load and CD4+ T cells count [157, 216]. Recent data reporting an association between sCD163 levels and telomere length [217] provide a direct link between monocyte/macrophage activation and potentiation of immunological aging. Given chronic inflammation/immune activation and resultant disease burden are similar between HIV-infected individuals and the aged, the question arises to what extent the mechanisms driving these phenomena are similar in both populations and what contributing factors may be unique to HIV infection.
5 Factors Potentiating Age-Related Changes and Morbidity in HIV-Infected Individuals
The development of SNAEs in PAWH is multifactorial, and typically results from the combined effects of traditional risk factors, HIV-specific effects, and a potentiation of age-related changes (see Fig. 3).
5.1 Traditional Risk Factors
Traditional risk factors for disease development are highly relevant for the aging HIV-infected population, not only as they are often more readily modifiable but also because they may potentiate HIV-specific factors. Many cohort studies report a higher prevalence of smoking amongst HIV-infected participants [218–220], and whilst illicit drug use is higher within certain high risk HIV-infected populations, this variable is often not adequately assessed or controlled for in HIV cohort studies. Relevant to the development of cardiovascular disease, HIV infection is associated with dyslipidemia and metabolic alterations, which are discussed further below.
5.2 Metabolic Alterations
Hyperglycemia occurs in up to 17 % of HIV-infected individuals receiving ART and diabetes mellitus is more common in HIV infected vs seronegative people [221], with some studies reporting up to a fourfold increased risk due to HIV [222]. Insulin resistance in ART-treated HIV infection is largely associated with the use of protease inhibitor antiretroviral drugs, which act to inhibit the glucose transporter Glut-4 [223], although hepatitis C virus (HCV) co-infection, inflammation and immunodeficiency also contribute to insulin resistance and diabetes in HIV infection [221]. High glucose levels have been shown to increase the susceptibility of CD4+ T cells in HIV infection in vitro by upregulating the expression of the HIV co-receptor CXCR4 [224], whilst increased expression of Glut-1 on T cells from HIV-infected individuals (irrespective of ART) is associated with T cell activation and immunodeficiency [169]. Taken together, these data suggest that metabolic alterations due to both HIV and its treatment not only increase the risk of co-morbidities such as diabetes, but may also perpetuate HIV replication and immune activation to further drive immune exhaustion and senescence in PAWH.
HIV-related lipodystrophy syndrome is common in HIV infection, and includes lipoatrophy (loss of subcutaneous fat) and dyslipidemia. Lipoatrophy appears to be largely due to PI and NRTI use, particularly the NRTIs stavudine and zidovudine (reviewed in [225]). Whilst HIV infection per se is associated with lipid alterations including high triglyceride and low HDL levels (thought to be due to the effect of inflammation on lipid peroxidation, reactive oxygen species production and vascular changes [226, 227]), the majority of dyslipidemia observed in the post-ART era is due to the specific effects of antiretroviral drugs.
5.3 Antiretroviral Drugs
Although highly effective in inhibiting HIV replication and maintaining immune health, many antiretroviral drugs, particularly the NRTIs, have some degree of toxicity which is at least partially attributable to effects on the mitochondria. The ability of NRTIs to inhibit HIV reverse transcription is due to structural similarities between NRTIs and endogenous nucleos(t)ides, and whilst nuclear DNA polymerases are not significantly affected by NRTIs, the mitochondrial replicase pol γ is inhibited by NRTIs at physiologically relevant levels, resulting in depletion of mitochondrial DNA and increased oxidative stress (reviewed in [208]). Specifically, zidovudine and stavudine have been shown to increase oxidative stress in a number of cell types including adipocytes and macrophages [228]. As discussed above, NRTIs are also able to inhibit the RT component of cellular telomerase and may potentially contribute to premature telomere shortening. Interestingly, certain NRTIs have recently been shown to be able to inhibit NLRP3 inflammasome-mediated activation of caspase-1 and subsequent production of the pro-inflammatory cytokines IL-1β and IL-18 [229], suggesting NRTIs may have an unexpected influence on cytokine production in HIV-infected individuals receiving these drugs.
HIV-infected individuals treated with ART have a relative risk of CVD of 1.52 (95 % CI 1.35–1.70) compared to untreated individuals [230], suggesting ART may contribute to the pathogenesis of CVD. Indeed, recent use of certain PIs and the NRTIs abacavir and didanosine has been associated with increased risk of myocardial infarction [231, 232] although the association with abacavir was not reproduced in a randomised control trial and remains controversial [233]. The increased risk attributable to PIs is largely due to an effect on lipid levels, as 70–80 % of HIV-infected individuals receiving PI-containing ART regimens show elevated lipid levels [226]. Most PIs (with the possible exception of atazanavir) have been shown to induce dyslipidemia involving increased plasma concentrations of triglycerides, total cholesterol and LDL [234], all of which are known risk factors for cardiovascular disease. The mechanism involves a direct effect of PIs on adipocyte differentiation and also an ability of these drugs to inhibit factors involved in lipid transport and metabolism [227].
Untreated HIV infection results in loss of bone mineral density which contributes to increased fracture risk and osteoporosis (as discussed above), but ART-initiation potentiates this effect and results in a further loss of bone mineral density of approximately 2–6 % within the first 2 years of ART initiation. This effect is thought to be due to disruption of the delicate immunological balance in the bone marrow which governs osteogenesis, and specific antiretroviral drugs including the NRTI tenofovir [235] and the protease inhibitor class of drugs have been shown to potentiate bone loss in ART-treated individuals [236].
The relatively recent introduction of ART, combined with the lengthy and multifactorial pathogenesis of many HIV-related co-morbidities, means that significant associations between specific ARVs and disease outcomes are continuing to emerge. HIV-infected individuals initiating therapy in the early days largely did so with low CD4 counts and received ARVs which have since been phased out due to side effects and toxicities. Thus, ongoing and future longitudinal studies will be critical for evaluating the long term effects of current ARVs on immune changes and the development of age-related diseases in HIV-infected individuals who avoid significant immunological damage by initiating ART at higher CD4 T cell counts.
6 Mechanisms That May Contribute to Chronic Inflammation and Immune Activation in HIV
6.1 Microbial Translocation and Endotoxemia
HIV infection is associated with increased permeability of the gut to microbial products, which translocate across the gut epithelium and eventually into the bloodstream, resulting in increased plasma levels of the bacterial endotoxin lipopolysaccharide (LPS) and bacterial DNA in HIV-infected individuals [237]. The cause of increased gut barrier permeability in HIV infection is due to immunodeficiency and structural defects within the gut-associated lymphoid tissue (GALT) resulting from HIV-mediated T cell depletion [238]. The majority of lymphocytes in the body are contained in GALT, which is an important site for both pathogenesis and persistence of HIV. CD4+ T cells are rapidly depleted from the GALT during primary HIV infection and remain depleted into chronic infection. Studies in Simian Immunodeficiency Virus (SIV)-infected macaques (a pathogenic animal model of HIV infection) have revealed that peak infection of CD4+ T cells in the lamina propria of the gut occurs within 10 days of infection, at which point 93 % of target CD4+ memory T cells are infected [239]. While effective ART suppresses viral replication and restores peripheral CD4+ T cells, gut-associated CD4+ T cells remain depleted years after ART initiation [240]. Interestingly, a subset of HIV-infected individuals who maintain high CD4+ T cells counts and low/undetectable viral loads in the absence of ART (known as long term non-progressors) maintain normal CD4+ T cell levels in the GALT [241], suggesting the importance of this compartment for disease pathogenesis. The mechanism of increased gut permeability in HIV involves epithelial disruption and decreased production of tight junction proteins in the distal portions of the colon [242] which is consistent with increased levels of intestinal fatty acid binding protein (I-FABP; a marker of enterocyte damage) and zonulin-1 (a regulator of tight junction permeability) in the plasma of HIV-infected individuals [157, 243]. The inability to fully restore GALT structure and function despite effective restoration of peripheral T cells by ART means that chronic endotoxemia (elevated levels of LPS in the blood) persists in virologically suppressed HIV-infected individuals. Lipopolysaccharide (LPS) is a potent immune activator which is recognised by toll-like receptor (TLR)-4 expressing cells such as monocytes/macrophages in an immune complex consisting of LPS-binding protein (LBP), the adaptor protein MD2 and either soluble or cell-bound CD14. LPS signalling stimulates the production of pro-inflammatory cytokines including IL-6, TNF and type I interferons. Microbial translocation is considered a significant driver of both HIV disease and related co-morbidities, with gut translocation markers such as LPS, the LPS binding protein LBP and I-FABP/zonulin-1 associated with immune activation and HIV disease progression [237, 244, 245], cardiovascular and metabolic disease [159, 160], neurocognitive impairment [141] and mortality [157, 216].
In contrast to HIV, relatively little is known regarding the effect of age on the integrity of the gut epithelium in humans [246], however work in Drosophila has demonstrated that loss of intestinal barrier integrity occurs with aging and is a better predictor of age-related morbidity and death than chronological age [247]. Increased plasma levels of LPS [98] and LBP [248] in the elderly indicate microbial translocation, may also increase during aging and the inverse association between LBP levels and physical function in the aged [248] suggests it may also contribute to morbidity in this population, although this requires further investigation.
6.1.1 Alterations to the Gut Microbiome
Within the GALT, cytokines including IL-17 and IL-22 play a critical role in maintaining gut integrity and orchestrating the mucosal immune responses to gut pathogens. Depletion of CD4+ T cells from the gut during HIV infection reduces the production of these cytokines and disrupts the delicate mucosal immunological balance. The gut microbiome interacts intimately with mucosal immunity and helps educate and regulate immune cells. Significant alterations are observed in the gut microbiome of HIV-infected individuals, with sequence analysis of bacterial communities from stool/gut mucosa samples revealing an overall increase in genetic diversity, an expansion of Prevotella and potentially pathogenic bacteria and a reduced proportion of Bacteroidia species [249–252]. Importantly, these changes in microbial communities are associated with inflammation, innate and adaptive immune activation and markers of disease progression in HIV-infected individuals. ART appears to only partially normalize the bacterial composition of the microbiome in a proportion of treated individuals [251]. A higher proportion of bacteria from the order Lactobacillales (lactic acid-producing bacteria) in the distal gut of ART naïve individuals has been associated with more favorable immunological parameters including higher pre-ART CD4+ T cells counts and CD4:CD8 T cells ratio but lower viral loads and sCD14 levels [253]. The complex interplay between the gut microbiome, GALT immunity and systemic inflammation/immune activation continues to be elucidated but may reveal an important mechanism of persistent immune dysfunction in HIV which can be targeted therapeutically.
6.1.2 Cytomegalovirus (CMV) and Latent Viral Infections
Accumulative immune stimulation by pathogens and subsequent immune exhaustion is an integral mechanism of immune aging and heightened pathogen burden due to concurrent and reactivated viral infections may hasten this process in PAWH. While CMV-seropositivity rates vary considerably between different countries (ranging from 40 to >90 %), there is a consistent trend of increasing seropositivity with age [254] and CMV is recognized as a significant driver of immunosenescence [255, 256]. CMV infection profoundly shifts the lymphocyte subset proportions towards a differentiated memory T cell phenotype [257, 258]. In aged individuals, the proportion of CD8+ T cells specific for a small number of CMV epitopes can represent up to 27 % of the total CD8+ pool [259], with these cells typically being dysfunctional and exhibiting an immunosenescent phenotype [260]. CMV seropositivity has also been associated with an increased risk of age-related diseases such as cardiovascular disease [261].
CMV disease is a significant cause of morbidity and mortality in HIV-infected individuals with AIDS and/or severe immunodeficiency [262], while asymptomatic CMV infection also appears to potentiate immunosenescence in HIV-infected individuals. CMV infection is almost ubiquitous in the HIV-infected population with seropositivity rates of approximately 95 % [263] and the presence of IgM antibodies suggests viral reactivation/reinfection commonly occurs [264]. Levels of CMV-specific CD8+ T cells are up to twice as high in HIV-infected as in uninfected individuals and persist in ART-treated individuals despite long term virological suppression [265], which is consistent with reactivation and impaired immune control [263]. HIV-infected/CMV seronegative subjects show higher CD4:CD8 T cells ratios and less phenotypic evidence of immunosenescence than HIV/CMV seropositive individuals [266] whilst serum CMV IgG levels, which are increased in HIV-infected individuals, correlate with inflammatory markers [267]. Taken together, these observations suggest that CMV seropositivity may potentiate HIV-related immunosenescence and inflammation and hasten the aging process.
Although ART reduces HIV viral load to near undetectable levels in the plasma, residual HIV replication (up to 20 copies/mL) can be detected in the plasma of the majority of virologically suppressed individuals using ultra-sensitive assays [268]. In addition, ongoing HIV replication may persist at higher levels within anatomical sites such as lymphoid tissue where antiretroviral drugs may fail to penetrate to effective therapeutic concentrations. Reactivation/replication of other latent viruses including Epstein–Barr virus (EBV) and Herpes Simplex Viruses (HSV) also appears to be heightened in HIV-infected individuals, likely due to increased immune activation. HSV-2 reactivation occurs frequently in HIV-infected individuals, is positively associated with HIV viral load [269] and is shed more frequently in HIV-infected vs seronegative individuals [270]. EBV viral loads in HIV-infected individuals are reportedly greater than those in EBV+ HIV-uninfected individuals [271].
Human endogenous retroviruses (HERVs) are a family of replication defective viral elements which comprise up to 8 % of the human genome. Although thought to be largely silent, increased transcription of HML-2 RNA (a member of the HERV-K family) has been demonstrated in PMBCs from HIV-infected individuals [272] and has also been detected at increased levels in plasma in some [273] but not all [272] studies. Increased HERV transcription may be due to heightened immune activation and/or the ability of the HIV Tat protein to activate endogenous retroviral transcriptional elements [274]. Although cause and effect are difficult to delineate, it is clear that heightened inflammation/immune activation and reactivation of latent viral infections may constitute a self-perpetuating cycle contributing to immune exhaustion and immunosenescence in many PAWH.
6.1.3 Concurrent Infections
The development of age-related morbidities in HIV-infected individuals can be influenced by concurrent infection with a range of pathogens. Co-infection with HCV can be up to 90 % in certain high risk HIV+ groups, and is associated with an increased risk of coronary heart disease [275], osteoporotic fracture [276], and neurocognitive impairment [277], suggesting hepatitis C infection may potentiate the pathogenesis of these conditions. The mechanism of this is unclear, although a potentiation of inflammation and immune activation is likely, and increased levels of pro-inflammatory factors such as IL-6 have been demonstrated in HIV/HCV co-infected, as compared to mono or uninfected individuals [278]. Active HCV infection is also associated with shorter leukocyte telomere length in those with HIV [279]. Taken together, these data suggest that HCV co-infection may further heighten inflammation/immune activation and associated immunosenescence in HIV-infected individuals and potentiate the development of age-related diseases.
HIV-infected individuals co-infected with tuberculosis (TB) have significantly increased pro-inflammatory cytokine production [280] and ART initiation in highly immunocompromised HIV+/TB+ individuals often results in TB-associated immune reconstitution inflammatory syndrome, which results in significant pro-inflammatory cytokine production [281]. Heightened CD4+ T cell activation and pro-inflammatory cytokine production also occurs in malaria co-infection [282]. These observations suggest concurrent infections may further potentiate inflammation due to HIV and aging in co-infected individuals, however further studies are required to elucidate the full impact of these effects on age-related disease outcomes.
7 Potential Treatments/Interventions to Alleviate the Effects of HIV on Aging/SNAEs
The immunological similarities between HIV infection and aging (particularly chronic inflammation and its consequences) suggest that addressing mechanism of aging may alleviate premature aging and disease pathogenesis in HIV-infected individuals. A large number of preliminary trials are underway to address immune activation, inflammation, microbial translocation and other mechanisms of enhanced aging in PAWH, but none has yet demonstrated efficacy in definitive clinical trials [283–287]. If this is accomplished in PAWH, it will have vast implications for aging in general and may be applicable to a much broader population.
8 Concluding Remarks
The success of antiretroviral therapy in preventing AIDS and extending the life span of HIV-infected people has revealed unexpected parallels between the impact of HIV infection and aging on immune function. Current research is only beginning to uncover how HIV may be potentiating age-related changes and the consequences of this for premature aging and increased risk of age-related comorbidities in those living and aging with HIV. It is still unclear whether HIV-associated ‘aging’ is the result of chronic infection, or whether those infected with HIV at an older age may experience similar effects. Furthermore, the impact of long-term ARV drug use on age-related process remains to be fully elucidated. HIV infection further complicates the many health challenges experienced by aging individuals including multi-morbidity, polypharmacy, impaired physical and mental health and reduced quality of life. Uncovering the critical processes which drive age-related changes and identifying therapeutic strategies to ameliorate the residual effects of HIV will be important for ongoing management of the increasingly aging HIV-infected population.
References
Costagliola D (2014) Demographics of HIV and aging. Curr Opin HIV AIDS 9(4):294–301. doi:10.1097/coh.0000000000000076
High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR Jr, Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P, Aging OARWGoHa (2012) HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 60 Suppl 1:S1–S18. doi:10.1097/QAI.0b013e31825a3668
Kirk JB, Goetz MB (2009) Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc 57(11):2129–2138. doi:10.1111/j.1532-5415.2009.02494.x
Luther VP, Wilkin AM (2007) HIV infection in older adults. Clin Geriatr Med 23(3):567–583, vii. doi:10.1016/j.cger.2ar007.02.004
Mills EJ, Barnighausen T, Negin J (2012) HIV and aging – preparing for the challenges ahead. N Engl J Med 366(14):1270–1273. doi:10.1056/NEJMp1113643
Bloomfield GS, Khazanie P, Morris A, Rabadan-Diehl C, Benjamin LA, Murdoch D, Radcliff VS, Velazquez EJ, Hicks C (2014) HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr 67 Suppl 1:S40–S53. doi:10.1097/qai.0000000000000257
Narayan KM, Miotti PG, Anand NP, Kline LM, Harmston C, Gulakowski R 3rd, Vermund SH (2014) HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low- and middle-income country settings. J Acquir Immune Defic Syndr 67 Suppl 1:S2–S7. doi:10.1097/qai.0000000000000267
Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, Khalsa PS, Kohanski RA, Li XL, Macchiarini F, Niederehe G, Oh YS, Pawlyk AC, Rodriguez H, Rowland JH, Shen GL, Sierra F, Wise BC (2014) Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S1–S3. doi:10.1093/gerona/glu041
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. doi:10.1016/j.cell.2013.05.039
Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F (2011) Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 53(11):1120–1126. doi:10.1093/cid/cir627
Ruiz M, Cefalu C (2011) Characteristics of frail patients in a geriatric-HIV program: the experience of an Urban Academic Center at one year follow-up. J Int Assoc Phys AIDS Care 10(3):138–143. doi:10.1177/1545109711399658
Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB, Study MAC (2007) HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 62(11):1279–1286
Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET (2009) Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 59(5):346–352. doi:10.1016/j.jinf.2009.08.008
Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB (2014) Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep 11(3):279–290. doi:10.1007/s11904-014-0215-y
Miller CJ, Baker JV, Bormann AM, Erlandson KM, Huppler Hullsiek K, Justice AC, Neuhaus J, Paredes R, Petoumenos K, Wentworth D, Winston A, Wolfson J, Neaton JD (2014) Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 9(4), e95061. doi:10.1371/journal.pone.0095061
Triant VA, Lee H, Hadigan C, Grinspoon SK (2007) Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 92(7):2506–2512. doi:10.1210/jc.2006-2190
Hall AM, Hendry BM, Nitsch D, Connolly JO (2011) Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis 57(5):773–780. doi:10.1053/j.ajkd.2011.01.022
Cannillo M, D’Ascenzo F, Grosso Marra W, Cerrato E, Calcagno A, Omede P, Bonora S, Mancone M, Vizza D, DiNicolantonio JJ, Pianelli M, Barbero U, Gili S, Annone U, Raviola A, Salera D, Mistretta E, Vilardi I, Colaci C, Abbate A, Zoccai GB, Moretti C, Gaita F (2014) Heart failure in patients with human immunodeficiency virus: a review of the literature. J Cardiovasc Med. doi:10.2459/jcm.0000000000000168
Triant VA (2014) Epidemiology of coronary heart disease in patients with human immunodeficiency virus. Rev Cardiovasc Med 15(Suppl 1):S1–S8
Remick J, Georgiopoulou V, Marti C, Ofotokun I, Kalogeropoulos A, Lewis W, Butler J (2014) Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 129(17):1781–1789. doi:10.1161/circulationaha.113.004574
Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena MG, Esposito R, Palella F, Raggi P (2009) Coronary aging in HIV-infected patients. Clin Infect Dis 49(11):1756–1762. doi:10.1086/648080
Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5(10), e203. doi:10.1371/journal.pmed.0050203
Deeks SG, Phillips AN (2009) HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 338:a3172. doi:10.1136/bmj.a3172
Gedela K, Vibhuti M, Pozniak A, Ward B, Boffito M (2014) Pharmacological management of cardiovascular conditions and diabetes in older adults with HIV infection. HIV Med 15(5):257–268. doi:10.1111/hiv.12116
Martinez E, Mocroft A, Garcia-Viejo MA, Perez-Cuevas JB, Blanco JL, Mallolas J, Bianchi L, Conget I, Blanch J, Phillips A, Gatell JM (2001) Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet 357(9256):592–598. doi:10.1016/s0140-6736(00)04056-3
Paula AA, Falcao MC, Pacheco AG (2013) Metabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspects. AIDS Res Ther 10(1):32. doi:10.1186/1742-6405-10-32
Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, Cooper DA, Emery S (2007) Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS 21(18):2445–2453. doi:10.1097/QAD.0b013e3282efad32
Cerrato E, D’Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, Castagno D, Omede P, Quadri G, Sciuto F, Presutti D, Frati G, Bonora S, Moretti C, Gaita F (2013) Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J 34(19):1432–1436. doi:10.1093/eurheartj/ehs471
Papita A, Albu A, Fodor D, Itu C, Carstina D (2011) Arterial stiffness and carotid intima-media thickness in HIV infected patients. Med Ultrason 13(2):127–134
Seaberg EC, Benning L, Sharrett AR, Lazar JM, Hodis HN, Mack WJ, Siedner MJ, Phair JP, Kingsley LA, Kaplan RC (2010) Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke 41(10):2163–2170. doi:10.1161/strokeaha.110.583856
Cobucci RN, Lima PH, de Souza PC, Costa VV, Cornetta MD, Fernandes JV, Goncalves AK (2014) Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: a systematic review. J Infect Public Health. doi:10.1016/j.jiph.2014.08.003
Burgi A, Brodine S, Wegner S, Milazzo M, Wallace MR, Spooner K, Blazes DL, Agan BK, Armstrong A, Fraser S, Crum NF (2005) Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer 104(7):1505–1511. doi:10.1002/cncr.21334
Shiels MS, Pfeiffer RM, Engels EA (2010) Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med 153(7):452–460. doi:10.7326/0003-4819-153-7-201010050-00008
Shiels MS, Goedert JJ, Moore RD, Platz EA, Engels EA (2010) Reduced risk of prostate cancer in U.S. men with AIDS. Cancer Epidemiol Biomarkers Prev 19(11):2910–2915. doi:10.1158/1055-9965.epi-10-0741
Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, Bertisch B, Bernasconi E, Weber R, Swiss HIVCS (2011) Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 53(11):1130–1139. doi:10.1093/cid/cir626
Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ (2010) HIV infection and aging independently affect brain function as measured by. J Infect Dis 201(3):336–340. doi:10.1086/649899
Womack JA, Goulet JL, Gibert C, Brandt CA, Skanderson M, Gulanski B, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC (2013) Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 56(10):1498–1504. doi:10.1093/cid/cit056
Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC (2011) Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One 6(2), e17217. doi:10.1371/journal.pone.0017217
Chan W, Dart AM (2011) Vascular stiffness and aging in HIV. Sex Health 8(4):474–484. doi:10.1071/sh10160
Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, Bryant K, Justice AC (2007) Aging and infectious diseases: do patterns of comorbidity vary by HIV status. Clin Infect Dis 45(12):1593–1601. doi:10.1086/523577
El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C (2006) CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 355(22):2283–2296. doi:10.1056/NEJMoa062360
Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC (2013) HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 173(8):614–622. doi:10.1001/jamainternmed.2013.3728
Rueda S, Law S, Rourke SB (2014) Psychosocial, mental health, and behavioral issues of aging with HIV. Curr Opin HIV AIDS 9(4):325–331. doi:10.1097/coh.0000000000000071
Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, Rimland D, Rodriguez-Barradas MC, Oursler KK, Brown ST, Braithwaite RS, May M, Covinsky KE, Roberts MS, Fultz SL, Bryant KJ (2010) Towards a combined prognostic index for survival in HIV infection: the role of “non-HIV” biomarkers. HIV Med 11(2):143–151. doi:10.1111/j.1468-1293.2009.00757.x
Akgun KM, Gordon K, Pisani M, Fried T, McGinnis KA, Tate JP, Butt AA, Gibert CL, Huang L, Rodriguez-Barradas MC, Rimland D, Justice AC, Crothers K (2013) Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected Veterans. J Acquir Immune Defic Syndr 62(1):52–59. doi:10.1097/QAI.0b013e318278f3fa
Justice AC, Braithwaite RS (2012) Lessons learned from the first wave of aging with HIV. AIDS 26 Suppl 1:S11–S18. doi:10.1097/QAD.0b013e3283558500
Marzolini C, Back D, Weber R, Furrer H, Cavassini M, Calmy A, Vernazza P, Bernasconi E, Khoo S, Battegay M, Elzi L (2011) Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother 66(9):2107–2111. doi:10.1093/jac/dkr248
Gleason LJ, Luque AE, Shah K (2013) Polypharmacy in the HIV-infected older adult population. Clin Interv Aging 8:749–763. doi:10.2147/cia.s37738
Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA (2012) Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS 26 Suppl 1:S39–S53. doi:10.1097/QAD.0b013e32835584ea
Frankel JK, Packer CD (2011) Cushing’s syndrome due to antiretroviral-budesonide interaction. Ann Pharmacother 45(6):823–824. doi:10.1345/aph.1P731
Ingle SM, May MT, Gill MJ, Mugavero MJ, Lewden C, Abgrall S, Fatkenheuer G, Reiss P, Saag MS, Manzardo C, Grabar S, Bruyand M, Moore D, Mocroft A, Sterling TR, D’Arminio Monforte A, Hernando V, Teira R, Guest J, Cavassini M, Crane HM, Sterne JA, Antiretroviral Therapy Cohort C (2014) Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis 59(2):287–297. doi:10.1093/cid/ciu261
Park WB, Choe PG, Kim SH, Jo JH, Bang JH, Kim HB, Kim NJ, Oh M, Choe KW (2007) One-year adherence to clinic visits after highly active antiretroviral therapy: a predictor of clinical progress in HIV patients. J Intern Med 261(3):268–275. doi:10.1111/j.1365-2796.2006.01762.x
Giordano TP, Gifford AL, White AC Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, Backus LI, Mole LA, Morgan RO (2007) Retention in care: a challenge to survival with HIV infection. Clin Infect Dis 44(11):1493–1499. doi:10.1086/516778
Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, Gallant JE, Mugavero MJ, Mills EJ, Giordano TP (2014) Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis 58(9):1297–1307. doi:10.1093/cid/ciu046
Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB (2011) A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci 66(9):1030–1038. doi:10.1093/gerona/glr097
Önen NF, Overton ET (2011) A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr Aging Sci 4(1):33–41
Terzian AS, Holman S, Nathwani N, Robison E, Weber K, Young M, Greenblatt RM, Gange SJ (2009) Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health 18(12):1965–1974. doi:10.1089/jwh.2008.1090
Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, Jacobson LP (2009) Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr 50(3):299–306
Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ (2012) A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc 60(3):545–549. doi:10.1111/j.1532-5415.2011.03819.x
Oursler KK, Goulet JL, Crystal S, Justice AC, Crothers K, Butt AA, Rodriguez-Barradas MC, Favors K, Leaf D, Katzel LI, Sorkin JD (2011) Association of Age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS 25(1):13–20. doi:10.1089/apc.2010.0242
Bauer LO, Wu Z, Wolfson LI (2011) An obese body mass increases the adverse effects of HIV/AIDS on balance and gait. Phys Ther 91(7):1063–1071. doi:10.2522/ptj.20100292
Erlandson KM, Allshouse AA, Jankowski CM, Duong S, Mawhinney S, Kohrt WM, Campbell TB (2012) Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 13(6):324–334. doi:10.1310/hct1306-324
Houston DK, Ding J, Nicklas BJ, Harris TB, Lee JS, Nevitt MC, Rubin SM, Tylavsky FA, Kritchevsky SB, Health ABCS (2009) Overweight and obesity over the adult life course and incident mobility limitation in older adults: the health, aging and body composition study. Am J Epidemiol 169(8):927–936. doi:10.1093/aje/kwp007
Ruiz M, Cefalu C (2011) Frailty syndrome in patients with HIV infection. Clin Geriatr 19(2):46–49
Pathai S, Bajillan H, Landay A, High K (2014) Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci 69(7):833–842
Valcour V, Paul R, Chiao S, Wendelken LA, Miller B (2011) Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis 53(8):836–842. doi:10.1093/cid/cir524
Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program (2013) Clin Infect Dis 56(7):1004–1017. doi:10.1093/cid/cis975
Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, America IDSo (2014) Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis 58(1):e1–e34. doi:10.1093/cid/cit665
Ances BM, Ortega M, Vaida F, Heaps J, Paul R (2012) Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 59(5):469–477. doi:10.1097/QAI.0b013e318249db17
Moore RC, Moore DJ, Thompson WK, Vahia IV, Grant I, Jeste DV (2013) A case-controlled study of successful aging in older HIV-infected adults. J Clin Psychiatry 74(5):e417–e423. doi:10.4088/JCP.12m08100
Greysen SR, Horwitz LI, Covinsky KE, Gordon K, Ohl ME, Justice AC (2013) Does social isolation predict hospitalization and mortality among HIV+ and uninfected older veterans? J Am Geriatr Soc 61(9):1456–1463. doi:10.1111/jgs.12410
Shippy RA, Karpiak SE (2005) The aging HIV/AIDS population: fragile social networks. Aging Ment Health 9(3):246–254. doi:10.1080/13607860412331336850
Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K (1992) Differential age-change in the numbers of CD4 + CD45RA+ and CD4 + CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev 63(1):57–68
Xu X, Beckman I, Ahern M, Bradley J (1993) A comprehensive analysis of peripheral blood lymphocytes in healthy aged humans by flow cytometry. Immunol Cell Biol 71(Pt 6):549–557. doi:10.1038/icb.1993.61
Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA (1995) CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest 95(5):2061–2066. doi:10.1172/JCI117892
Kalayjian RC, Landay A, Pollard RB, Taub DD, Gross BH, Francis IR, Sevin A, Pu M, Spritzler J, Chernoff M, Namkung A, Fox L, Martinez A, Waterman K, Fiscus SA, Sha B, Johnson D, Slater S, Rousseau F, Lederman MM (2003) Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis 187(12):1924–1933. doi:10.1086/375372
Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A, Perez-Molina JA, Sainz T, Navas E, Hermida JM, Quereda C, Moreno S (2014) Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 9(1), e85798. doi:10.1371/journal.pone.0085798
Menozzi M, Zona S, Santoro A, Carli F, Stentarelli C, Mussini C, Guaraldi G (2014) CD4/CD8 ratio is not predictive of multi-morbidity prevalence in HIV-infected patients but identify patients with higher CVD risk. J Int AIDS Soc 17(4 Suppl 3):19709. doi:10.7448/IAS.17.4.19709
Bernal E, Serrano J, Perez A, Valero S, Garcia E, Marin I, Munoz A, Verdu JM, Vera C, Cano A (2014) The CD4:CD8 ratio is associated with IMT progression in HIV-infected patients on antiretroviral treatment. J Int AIDS Soc 17(4 Suppl 3):19723. doi:10.7448/IAS.17.4.19723
Saracino A, Bruno G, Scudeller L, Volpe A, Caricato P, Ladisa N, Monno L, Angarano G (2014) Chronic inflammation in a long-term cohort of HIV-infected patients according to the normalization of the CD4:CD8 ratio. AIDS Res Hum Retroviruses 30(12):1178–1184. doi:10.1089/aid.2014.0080
Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, Marcato A, Passeri G, Ortolani C, Forti E et al (1993) Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood 82(9):2767–2773
Phelouzat MA, Arbogast A, Laforge T, Quadri RA, Proust JJ (1996) Excessive apoptosis of mature T lymphocytes is a characteristic feature of human immune senescence. Mech Ageing Dev 88(1–2):25–38
de Oliveira Pinto LM, Garcia S, Lecoeur H, Rapp C, Gougeon ML (2002) Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1)- and TNFR2-mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood 99(5):1666–1675
Effros RB (1997) Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol 21(6):471–478
Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV (1996) Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS (London, England) 10(8):F17–F22
Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM (1999) Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190(2):157–167
Lee SA, Sinclair E, Hatano H, Hsue PY, Epling L, Hecht FM, Bangsberg DR, Martin JN, McCune JM, Deeks SG, Hunt PW (2014) Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 9(2), e89444. doi:10.1371/journal.pone.0089444
Deeks SG (2011) HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 62:141–155. doi:10.1146/annurev-med-042909-093756
Kalayjian RC, Spritzler J, Pu M, Landay A, Pollard RB, Stocker V, Harthi LA, Gross BH, Francis IR, Fiscus SA, Tebas P, Bosch RJ, Valcour V, Lederman MM, Adult ACTG, Study T (2005) Distinct mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J Infect Dis 192(9):1577–1587. doi:10.1086/466527
Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB (2011) Age effects on B cells and humoral immunity in humans. Ageing Res Rev 10(3):330–335. doi:10.1016/j.arr.2010.08.004
Moir S, Fauci AS (2009) B cells in HIV infection and disease. Nat Rev 9(4):235–245. doi:10.1038/nri2524
Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, Wilson R, Gotch F, Gazzard B, Kelleher P (2007) Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol 178(12):8212–8220
De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sonnerborg A, Chiodi F (2004) Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103(6):2180–2186. doi:10.1182/blood-2003-07-2375
Siewe B, Stapleton JT, Martinson J, Keshavarzian A, Kazmi N, Demarais PM, French AL, Landay A (2013) Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8(+) T cell function in vitro. J Leukoc Biol 93(5):811–818. doi:10.1189/jlb.0912436
Siewe B, Keshavarzian A, French A, Demarais P, Landay A (2013) A role for TLR signaling during B cell activation in antiretroviral-treated HIV individuals. AIDS Res Hum Retroviruses 29(10):1353–1360. doi:10.1089/AID.2013.0115
Van Epps P, Matining RM, Tassiopoulos K, Anthony DD, Landay A, Kalayjian RC, Canaday DH (2014) Older age is associated with peripheral blood expansion of naive B cells in HIV-infected subjects on antiretroviral therapy. PLoS One 9(9), e107064. doi:10.1371/journal.pone.0107064
Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM (2012) HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS (London, England) 26(7):843–853. doi:10.1097/QAD.0b013e328351f756
Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM (2012) Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11(5):867–875. doi:10.1111/j.1474-9726.2012.00851.x
Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Cheng WJ, Paukovics G, Palmer CS, Novak RM, Jaworowski A, Landay AL, Crowe SM (2013) Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One 8(1), e55279. doi:10.1371/journal.pone.0055279
Clark JA, Peterson TC (1994) Cytokine production and aging: overproduction of IL-8 in elderly males in response to lipopolysaccharide. Mech Ageing Dev 77(2):127–139. doi:0047-6374(94)90020-5 [pii]
Delpedro AD, Barjavel MJ, Mamdouh Z, Faure S, Bakouche O (1998) Signal transduction in LPS-activated aged and young monocytes. J Interferon Cytokine Res 18(6):429–437
Jaworowski A, Ellery P, Maslin CL, Naim E, Heinlein AC, Ryan CE, Paukovics G, Hocking J, Sonza S, Crowe SM (2006) Normal CD16 expression and phagocytosis of Mycobacterium avium complex by monocytes from a current cohort of HIV-1-infected patients. J Infect Dis 193(5):693–697
Spencer ME, Jain A, Matteini A, Beamer BA, Wang NY, Leng SX, Punjabi NM, Walston JD, Fedarko NS (2010) Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J Gerontol A Biol Sci Med Sci 65(8):858–865. doi:10.1093/gerona/glq066, glq066 [pii]
Antonelli A, Rotondi M, Fallahi P, Ferrari SM, Paolicchi A, Romagnani P, Serio M, Ferrannini E (2006) Increase of CXC chemokine CXCL10 and CC chemokine CCL2 serum levels in normal ageing. Cytokine 34(1–2):32–38. doi:10.1016/j.cyto.2006.03.012, S1043-4666(06)00132-3 [pii]
Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC (2011) Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 204(1):154–163. doi:10.1093/infdis/jir214, jir214 [pii]
Kamat A, Misra V, Cassol E, Ancuta P, Yan Z, Li C, Morgello S, Gabuzda D (2012) A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS One 7(2), e30881. doi:10.1371/journal.pone.0030881
Méndez-Lagares G, Romero-Sánchez MC, Ruiz-Mateos E, Genebat M, Ferrando-Martínez S, Muñoz-Fernández MÁ, Pacheco YM, Leal M (2013) Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J Infect Dis 207(8):1221–1225. doi:10.1093/infdis/jit025
Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M (2005) Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106(10):3366–3369
Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R (1999) NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol 34(2):253–265. doi:S0531-5565(98)00076-X [pii]
Ogata K, Yokose N, Tamura H, An E, Nakamura K, Dan K, Nomura T (1997) Natural killer cells in the late decades of human life. Clin Immunol Immunopathol 84(3):269–275
Fernandes G, Gupta S (1981) Natural killing and antibody-dependent cytotoxicity by lymphocyte subpopulations in young and aging humans. J Clin Immunol 1(3):141–148
Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, Kramski M, Hearps AC, Cameron PU, Lewin SR, Crowe SM, Jaworowski A (2012) Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol 189(3):1491–1499. doi:10.4049/jimmunol.1200458
Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, Moretta L, Moretta A, De Maria A (2004) Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol 34(8):2313–2321. doi:10.1002/eji.200425251
Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, Ondercin J, Perussia B, Montaner LJ (2002) Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol 168(11):5764–5770
Moroni F, Di Paolo ML, Rigo A, Cipriano C, Giacconi R, Recchioni R, Marcheselli F, Malavolta M, Mocchegiani E (2005) Interrelationship among neutrophil efficiency, inflammation, antioxidant activity and zinc pool in very old age. Biogerontology 6(4):271–281. doi:10.1007/s10522-005-2625-0
Agrawal A, Agrawal S, Tay J, Gupta S (2008) Biology of dendritic cells in aging. J Clin Immunol 28(1):14–20. doi:10.1007/s10875-007-9127-6
Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S (2007) Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol 178(11):6912–6922
Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ (2004) Aging and innate immune cells. J Leukoc Biol 76(2):291–299. doi:10.1189/jlb.1103592
Martinson JA, Roman-Gonzalez A, Tenorio AR, Montoya CJ, Gichinga CN, Rugeles MT, Tomai M, Krieg AM, Ghanekar S, Baum LL, Landay AL (2007) Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol 250(1–2):75–84
Kaszubowska L (2008) Telomere shortening and ageing of the immune system. J Physiol Pharmacol 59(Suppl 9):169–186
Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, Ronnett BM, De Marzo AM (2004) Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res 10(10):3317–3326. doi:10.1158/1078-0432.CCR-0984-03
Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A (2007) Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165(1):14–21. doi:10.1093/aje/kwj346
Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Nordestgaard BG (2012) Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol 32(3):822–829. doi:10.1161/ATVBAHA.111.237271
Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S (2010) Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 30(8):1649–1656. doi:10.1161/ATVBAHA.110.205492
Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R (2012) Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol 69(10):1332–1339. doi:10.1001/archneurol.2012.1541
Hochstrasser T, Marksteiner J, Humpel C (2012) Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp Gerontol 47(2):160–163. doi:10.1016/j.exger.2011.11.012
Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton NI, Prineas RJ, Neaton JD (2012) Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 7(9), e44454. doi:10.1371/journal.pone.0044454
De Luca A, de Gaetano DK, Colafigli M, Cozzi-Lepri A, De Curtis A, Gori A, Sighinolfi L, Giacometti A, Capobianchi MR, D’Avino A, Iacoviello L, Cauda R, D’Arminio MA (2013) The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: a nested case--control study. BMC Infect Dis 13(1):414. doi:10.1186/1471-2334-13-414
Koethe JR, Dee K, Bian A, Shintani A, Turner M, Bebawy S, Sterling TR, Hulgan T (2013) Circulating interleukin-6, soluble CD14, and other inflammation biomarker levels differ between obese and nonobese HIV-infected adults on antiretroviral therapy. AIDS Res Hum Retroviruses 29(7):1019–1025. doi:10.1089/aid.2013.0016
Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, Defilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd-Jones D (2013) Inflammation and sudden cardiac death in a community-based population of older adults: The Cardiovascular Health Study. Heart Rhythm 10(10):1425–1432. doi:10.1016/j.hrthm.2013.07.004
Empana JP, Jouven X, Canoui-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P (2010) C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arterioscler Thromb Vasc Biol 30(10):2047–2052. doi:10.1161/atvbaha.110.208785
Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, Sarnak MJ, Newman AB (2012) Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci 67(9):970–976. doi:10.1093/gerona/glr261
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101(15):1767–1772
Jenny NS, Tracy RP, Ogg MS, le Luong A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE (2002) In the elderly, interleukin-6 plasma levels and the -174G > C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol 22(12):2066–2071
Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, Maka K, Martin JN, Ganz P, Deeks SG (2012) Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc 1(2). pii: jah3-e000422. doi:10.1161/jaha.111.000422
Biron A, Bobin-Dubigeon C, Volteau C, Piroth L, Perre P, Leport C, Prazuck T, Jovelin T, Billard M, Sebille V, Bard JM, Raffi F, Biron C (2012) Metabolic syndrome in French HIV-infected patients: prevalence and predictive factors after 3 years of antiretroviral therapy. AIDS Res Hum Retroviruses 28(12):1672–1678. doi:10.1089/aid.2012.0048
Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA (2010) Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 33(10):2244–2249. doi:10.2337/dc10-0633
Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF Jr, Wang TJ, Murabito JM, Vasan RS, Benjamin EJ (2013) Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol 33(7):1728–1733. doi:10.1161/atvbaha.112.301174
Parkner T, Sorensen LP, Nielsen AR, Fischer CP, Bibby BM, Nielsen S, Pedersen BK, Moller HJ (2012) Soluble CD163: a biomarker linking macrophages and insulin resistance. Diabetologia 55(6):1856–1862. doi:10.1007/s00125-012-2533-1
Shikuma CM, Barbour JD, Ndhlovu LC, Keating SM, Norris PJ, Budoff M, Parikh N, Seto T, Gangcuangco LM, Ogata-Arakaki D, Chow D (2013) Plasma monocyte chemoattractant protein-1 and tumor necrosis factor-alpha levels predict the presence of coronary artery calcium in HIV-infected individuals independent of traditional cardiovascular risk factors. AIDS Res Hum Retroviruses. doi:10.1089/aid.2013.0183
Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D (2008) Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 3(6), e2516. doi:10.1371/journal.pone.0002516
Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE (2002) Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59(3):371–378
Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA (2010) Association of C-reactive protein with cognitive impairment. Arch Neurol 67(1):87–92. doi:10.1001/archneurol.2009.308
Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, Ratanasuwan W, Gendelman HE, Swindells S (2001) Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis 184(6):699–706. doi:10.1086/323036
Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM (2004) Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 61(5):668–672. doi:10.1001/archneur.61.5.668
Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK (1999) A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci 54(7):M357–M364
Borges AH, Silverberg MJ, Wentworth D, Grulich AE, Fatkenheuer G, Mitsuyasu R, Tambussi G, Sabin CA, Neaton JD, Lundgren JD (2013) Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS (London, England) 27(9):1433–1441. doi:10.1097/QAD.0b013e32835f6b0c
Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB (2005) Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev 14(10):2413–2418. doi:10.1158/1055-9965.EPI-05-0316
de Pablo P, Cooper MS, Buckley CD (2012) Association between bone mineral density and C-reactive protein in a large population-based sample. Arthritis Rheum 64(8):2624–2631. doi:10.1002/art.34474
Eriksson AL, Moverare-Skrtic S, Ljunggren O, Karlsson M, Mellstrom D, Ohlsson C (2013) High sensitive CRP is an independent risk factor for all fractures and vertebral fractures in elderly men: The MrOS Sweden study. J Bone Min Res. doi:10.1002/jbmr.2037
Ding C, Parameswaran V, Udayan R, Burgess J, Jones G (2008) Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab 93(5):1952–1958. doi:10.1210/jc.2007-2325
Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, Pfeilschifter J (2001) Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab 86(5):2032–2042
Margolick J, Jacobson L, Lopez J (2012) Frailty and circulating concentrations of proinflammatory cytokines and chemokines in HIV-infected and -uninfected men in the Multicenter AIDS Cohort Study (MACS). Paper presented at the 3rd international workshop on HIV and aging, Baltimore, 5–6 Nov 2012
Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, Wilson CC, MaWhinney S, Kohrt WM, Campbell TB (2013) Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 208(2):249–259. doi:10.1093/infdis/jit147
Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ (1999) Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47(6):639–646
Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP (2002) Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162(20):2333–2341
Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM (2014) Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 210(8):1228–1238. doi:10.1093/infdis/jiu238
Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS (2012) Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 206(10):1558–1567
Blodget E, Shen C, Aldrovandi G, Rollie A, Gupta SK, Stein JH, Dube MP (2012) Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One [Electronic Resource] 7(8), e42624
Pedersen KK, Pedersen M, Troseid M, Gaardbo JC, Lund TT, Thomsen C, Gerstoft J, Kvale D, Nielsen SD (2013) Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr. doi:10.1097/QAI.0b013e31829f919d
Manner IW, Baekken M, Kvale D, Oektedalen O, Pedersen M, Nielsen SD, Nowak P, Os I, Trøseid M (2013) Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med 14(6):354–361. doi:10.1111/hiv.12015
Lassenius MI, Pietilainen KH, Kaartinen K, Pussinen PJ, Syrjanen J, Forsblom C, Porsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M (2011) Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34(8):1809–1815. doi:10.2337/dc10-2197
Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F (2013) Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One 8(1), e54600. doi:10.1371/journal.pone.0054600
Vassallo M, Dunais B, Durant J, Carsenti-Dellamonica H, Harvey-Langton A, Cottalorda J, Ticchioni M, Laffon M, Lebrun-Frenay C, Dellamonica P, Pradier C (2013) Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: the Neuradapt study. J Neurovirol 19(4):376–382. doi:10.1007/s13365-013-0181-y
Marks MA, Rabkin CS, Engels EA, Busch E, Kopp W, Rager H, Goedert JJ, Chaturvedi AK (2013) Markers of microbial translocation and risk of AIDS-related lymphoma. AIDS (London, England) 27(3):469–474. doi:10.1097/QAD.0b013e32835c1333
Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, Bai F, D’Eril GV, Monforte AD, Marchetti G (2012) T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 7(9), e46073. doi:10.1371/journal.pone.0046073
Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, Lederman MM, Storer N, Labbato DE, McComsey GA (2014) Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS (London, England) 28(7):969–977. doi:10.1097/QAD.0000000000000158
Palmer CS, Ostrowski M, Gouillou M, Tsai L, Yu D, Zhou J, Henstridge DC, Maisa A, Hearps AC, Lewin SR, Landay A, Jaworowski A, McCune JM, Crowe SM (2013) Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS (London, England). doi:10.1097/QAD.0000000000000128
Reiner AP, Lange EM, Jenny NS, Chaves PHM, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP (2013) Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 33(1):158–164. doi:10.1161/atvbaha.112.300421
Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK (2012) Arterial inflammation in patients with HIV. JAMA 308(4):379–386. doi:10.1001/jama.2012.6698
Burdo TH, Lo J, Abbara S, Wei J, Delelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S (2011) Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 204(8):1227–1236. doi:10.1093/infdis/jir520, jir520 [pii]
Aristoteli LP, Moller HJ, Bailey B, Moestrup SK, Kritharides L (2006) The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis 184(2):342–347. doi:10.1016/j.atherosclerosis.2005.05.004, S0021-9150(05)00339-4 [pii]
Fjeldborg K, Christiansen T, Bennetzen M, J Møller H, Pedersen SB, Richelsen BR (2013) The macrophage-specific serum marker, soluble CD163, is increased in obesity and reduced after dietary-induced weight loss. Obesity (Silver Spring). doi:10.1002/oby.20376
Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK (2012) Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf) 77(3):385–390. doi:10.1111/j.1365-2265.2011.04284.x
Moller HJ, Frikke-Schmidt R, Moestrup SK, Nordestgaard BG, Tybjaerg-Hansen A (2011) Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin Chem 57(2):291–297. doi:10.1373/clinchem.2010.154724, clinchem.2010.154724 [pii]
Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, Morgello S, Gabuzda D (2011) Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 57(5):371–379. doi:10.1097/QAI.1090b1013e3182237e3182254
Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D (2012) Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 60(3):234–243. doi:10.1097/QAI.1090b1013e318256f318253bc
Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS (London, England) 27(9):1387–1395. doi:10.1097/QAD.0b013e32836010bd
Blasko I, Knaus G, Weiss E, Kemmler G, Winkler C, Falkensammer G, Griesmacher A, Wurzner R, Marksteiner J, Fuchs D (2007) Cognitive deterioration in Alzheimer’s disease is accompanied by increase of plasma neopterin. J Psychiatr Res 41(8):694–701. doi:10.1016/j.jpsychires.2006.02.001
Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A, Walston JD, Fedarko NS (2011) IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing 40(4):475–481. doi:10.1093/ageing/afr047, afr047 [pii]
Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt P, Karim R, Kern DM, Hodis HN, Deeks SG (2011) T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 203(4):452–463
Longenecker C, Funderburg N, Jiang Y, Debanne S, Storer N, Labbato D, Lederman M, McComsey G (2013) Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. doi:10.1111/hiv.12013
Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Parrinello CM, Hunt P, Deeks SG, Hodis HN (2011) T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis 217(1):207–213
Gazzola L, Bellistri GM, Tincati C, Ierardi V, Savoldi A, del Dole A, Tagliabue L, d’Arminio Monforte A, Marchetti G (2013) Association between peripheral T-Lymphocyte activation and impaired bone mineral density in HIV-infected patients. J Transl Med 11:51. doi:10.1186/1479-5876-11-51
Lee SA, Sinclair E, Jain V, Huang Y, Epling L, Van Natta M, Meinert CL, Martin JN, McCune JM, Deeks SG, Lederman MM, Hecht FM, Hunt PW (2014) Low proportions of CD28–CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis 210(3):374–382. doi:10.1093/infdis/jiu109
Sanders JL, Fitzpatrick AL, Boudreau RM, Arnold AM, Aviv A, Kimura M, Fried LF, Harris TB, Newman AB (2012) Leukocyte telomere length is associated with noninvasively measured age-related disease: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 67(4):409–416. doi:10.1093/gerona/glr173
Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA (2006) Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 29(2):283–289
Mu Y, Zhang Q, Mei L, Liu X, Yang W, Yu J (2012) Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol 29(2):893–898. doi:10.1007/s12032-011-9866-3
Chen S, de Craen AJ, Raz Y, Derhovanessian E, Vossen AC, Westendorp RG, Pawelec G, Maier AB (2012) Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study. Immun Ageing 9(1):18. doi:10.1186/1742-4933-9-18
Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, Anderson JL (2000) Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation 102(16):1917–1923
Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, Deeks SG (2006) Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS (London, England) 20(18):2275–2283. doi:10.1097/QAD.0b013e3280108704
Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt PW, Deeks SG, Hodis HN, Kaplan RC (2012) Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 205(12):1788–1796. doi:10.1093/infdis/jis276
Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP (2010) Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 171(10):1144–1152. doi:10.1093/aje/kwq062
Savva GM, Pachnio A, Kaul B, Morgan K, Huppert FA, Brayne C, Moss PA (2013) Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell 12(3):381–387. doi:10.1111/acel.12059
Kong CM, Lee XW, Wang X (2013) Telomere shortening in human diseases. FEBS J 280(14):3180–3193. doi:10.1111/febs.12326
Wang X, Singh S, Jung HY, Yang G, Jun S, Sastry KJ, Park JI (2013) HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex. J Biol Chem 288(22):15474–15480. doi:10.1074/jbc.M112.416735
Comandini A, Naro C, Adamo R, Akbar AN, Lanna A, Bonmassar E, Franzese O (2013) Molecular mechanisms involved in HIV-1-Tat mediated inhibition of telomerase activity in human CD4(+) T lymphocytes. Mol Immunol 54(2):181–192. doi:10.1016/j.molimm.2012.12.003
Reynoso R, Laufer N, Bolcic F, Quarleri J (2010) Telomerase activity in peripheral blood mononuclear cells from HIV and HIV-HCV coinfected patients. Virus Res 147(2):284–287. doi:10.1016/j.virusres.2009.11.006
Hukezalie KR, Thumati NR, Cote HC, Wong JM (2012) In vitro and ex vivo inhibition of human telomerase by anti-HIV nucleoside reverse transcriptase inhibitors (NRTIs) but not by non-NRTIs. PLoS One 7(11), e47505. doi:10.1371/journal.pone.0047505
Leeansyah E, Cameron PU, Solomon A, Tennakoon S, Velayudham P, Gouillou M, Spelman T, Hearps A, Fairley C, de Smit V, Pierce AB, Armishaw J, Crowe SM, Cooper DA, Koelsch KK, Liu JP, Chuah J, Lewin SR (2013) Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: a potential factor contributing to HIV-associated accelerated aging. J Infect Dis 207(7):1157–1165. doi:10.1093/infdis/jit006
Mutlu-Turkoglu U, Ilhan E, Oztezcan S, Kuru A, Aykac-Toker G, Uysal M (2003) Age-related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects. Clin Biochem 36(5):397–400
Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, Grune T (2006) Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res 40(5):495–505. doi:10.1080/10715760600592962
Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L (2011) Oxidative stress, inflamm-aging and immunosenescence. J Proteomics 74(11):2313–2323. doi:10.1016/j.jprot.2011.06.005
Gil L, Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS (2003) Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res 47(3):217–224. doi:S1043661802003201 [pii]
Kalinowska M, Bazdar DA, Lederman MM, Funderburg N, Sieg SF (2013) Decreased IL-7 responsiveness is related to oxidative stress in HIV disease. PLoS One 8(3), e58764. doi:10.1371/journal.pone.0058764
Mihm S, Ennen J, Pessara U, Kurth R, Droge W (1991) Inhibition of HIV-1 replication and NF-kappa B activity by cysteine and cysteine derivatives. AIDS (London, England) 5(5):497–503
Staal FJ, Roederer M, Herzenberg LA, Herzenberg LA (1990) Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A 87(24):9943–9947
Torres RA, Lewis W (2014) Aging and HIV/AIDS: pathogenetic role of therapeutic side effects. Lab Invest 94(2):120–128. doi:10.1038/labinvest.2013.142
Lagathu C, Eustace B, Prot M, Frantz D, Gu Y, Bastard JP, Maachi M, Azoulay S, Briggs M, Caron M, Capeau J (2007) Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther 12(4):489–500
Mandas A, Iorio EL, Congiu MG, Balestrieri C, Mereu A, Cau D, Dessi S, Curreli N (2009) Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol 2009:749575. doi:10.1155/2009/749575
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128(1):92–105
Deeks SG, Tracy R, Douek DC (2013) Systemic effects of inflammation on health during chronic HIV infection. Immunity 39(4):633–645. doi:10.1016/j.immuni.2013.10.001
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC (2011) Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203(3):780–790. doi:10.1093/infdis/jiq118, jiq118 [pii]
Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, Hearps AC, Cheng W, Trevillyan J, Lewin SR, Sviridov D, Elliott JH, Jaworowski A, Dart AM, Crowe SM (2013) Associations between blood monocyte markers and carotid atherosclerosis in HIV-positive patients. Immunol Cell Biol 92(2):133–138. doi:10.1038/icb.2013.84
Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL (2014) Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 210(8):1248–1259. doi:10.1093/infdis/jiu254
Srinivasa S, Fitch KV, Petrow E, Burdo TH, Williams KC, Lo J, Cote HC, Grinspoon SK (2014) Soluble CD163 is associated with shortened telomere length in HIV-infected patients. J Acquir Immune Defic Syndr 67(4):414–418. doi:10.1097/QAI.0000000000000329
Chew D, Steinberg MB, Thomas P, Swaminathan S, Hodder SL (2014) Evaluation of a smoking cessation program for HIV infected individuals in an urban HIV clinic: challenges and lessons learned. AIDS Res Treat 2014:237834. doi:10.1155/2014/237834
Tron L, Lert F, Spire B, Dray-Spira R, Group AN-Vs (2014) Tobacco smoking in HIV-infected versus general population in France: heterogeneity across the various groups of people living with HIV. PLoS One 9(9), e107451. doi:10.1371/journal.pone.0107451
Browning KK, Wewers ME, Ferketich AK, Diaz P (2013) Tobacco use and cessation in HIV-infected individuals. Clin Chest Med 34(2):181–190. doi:10.1016/j.ccm.2013.01.005
Kalra S, Agrawal N (2013) Diabetes and HIV: current understanding and future perspectives. Curr Diab Rep 13(3):419–427. doi:10.1007/s11892-013-0369-9
Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS (2005) Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 165(10):1179–1184. doi:10.1001/archinte.165.10.1179
Dube MP (2000) Disorders of glucose metabolism in patients infected with human immunodeficiency virus. Clin Infect Dis 31(6):1467–1475. doi:10.1086/317491
Lan X, Cheng K, Chandel N, Lederman R, Jhaveri A, Husain M, Malhotra A, Singhal PC (2013) High glucose enhances HIV entry into T cells through upregulation of CXCR4. J Leukoc Biol 94(4):769–777. doi:10.1189/jlb.0313142
de Waal R, Cohen K, Maartens G (2013) Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction. PLoS One 8(5), e63623. doi:10.1371/journal.pone.0063623
Souza SJ, Luzia LA, Santos SS, Rondo PH (2013) Lipid profile of HIV-infected patients in relation to antiretroviral therapy: a review. Rev Assoc Med Bras 59(2):186–198. doi:10.1016/j.ramb.2012.11.003
Galescu O, Bhangoo A, Ten S (2013) Insulin resistance, lipodystrophy and cardiometabolic syndrome in HIV/AIDS. Rev Endocr Metab Disord 14(2):133–140. doi:10.1007/s11154-013-9247-7
Caron M, Auclairt M, Vissian A, Vigouroux C, Capeau J (2008) Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir Ther 13(1):27–38
Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, Amarnath S, Fowler DH, Radwan M, Young MT, Pittman K, Kubes P, Agarwal HK, Parang K, Hinton DR, Bastos-Carvalho A, Li S, Yasuma T, Mizutani T, Yasuma R, Wright C, Ambati J (2014) Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 346(6212):1000–1003. doi:10.1126/science.1261754
Islam FM, Wu J, Jansson J, Wilson DP (2012) Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 13(8):453–468. doi:10.1111/j.1468-1293.2012.00996.x
Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, D’Arminio Monforte A, Friis-Moller N, Kirk O, Pradier C, Weller I, Phillips AN, Lundgren JD (2008) Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 371(9622):1417–1426. doi:10.1016/S0140-6736(08)60423-7
Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, Monforte AD, Friis-Moller N, Kirk O, Fontas E, Weller I, Phillips A, Lundgren J (2010) Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 201(3):318–330. doi:10.1086/649897
Bavinger C, Bendavid E, Niehaus K, Olshen RA, Olkin I, Sundaram V, Wein N, Holodniy M, Hou N, Owens DK, Desai M (2013) Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One 8(3), e59551. doi:10.1371/journal.pone.0059551
Wohl DA, McComsey G, Tebas P, Brown TT, Glesby MJ, Reeds D, Shikuma C, Mulligan K, Dube M, Wininger D, Huang J, Revuelta M, Currier J, Swindells S, Fichtenbaum C, Basar M, Tungsiripat M, Meyer W, Weihe J, Wanke C (2006) Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis 43(5):645–653. doi:10.1086/507333
Yin MT, Zhang CA, McMahon DJ, Ferris DC, Irani D, Colon I, Cremers S, Shane E (2012) Higher rates of bone loss in postmenopausal HIV-infected women: a longitudinal study. J Clin Endocrinol Metab 97(2):554–562. doi:10.1210/jc.2011-2197
Hoy J (2011) Bone, fracture and frailty. Curr Opin HIV AIDS 6(4):309–314. doi:10.1097/COH.0b013e3283478741
Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12(12):1365–1371
Douek D (2007) HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med 15(4):114–117
Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT (2005) Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434(7037):1148–1152. doi:10.1038/nature03513
Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S (2003) Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 77(21):11708–11717
Ciccone EJ, Greenwald JH, Lee PI, Biancotto A, Read SW, Yao MA, Hodge JN, Thompson WL, Kovacs SB, Chairez CL, Migueles SA, Kovacs JA, Margolis LB, Sereti I (2011) CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J Virol 85(12):5880–5888. doi:10.1128/JVI.02643-10
Chung CY, Alden SL, Funderburg NT, Fu P, Levine AD (2014) Progressive proximal-to-distal reduction in expression of the tight junction complex in colonic epithelium of virally-suppressed HIV+ individuals. PLoS Pathog 10(6), e1004198. doi:10.1371/journal.ppat.1004198
Vesterbacka J, Nowak P, Barqasho B, Abdurahman S, Nystrom J, Nilsson S, Funaoka H, Kanda T, Andersson LM, Gisslen M, Sonnerborg A (2013) Kinetics of microbial translocation markers in patients on efavirenz or lopinavir/r based antiretroviral therapy. PLoS One 8(1), e55038. doi:10.1371/journal.pone.0055038
Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, Verucchi G, Antinori A, Costantini A, Giacometti A, di Caro A, D’Arminio Monforte A (2011) Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS 25(11):1385–1394. doi:10.1097/QAD.0b013e3283471d10
Nowroozalizadeh S, Mansson F, da Silva Z, Repits J, Dabo B, Pereira C, Biague A, Albert J, Nielsen J, Aaby P, Fenyo EM, Norrgren H, Holmgren B, Jansson M (2010) Microbial translocation correlates with the severity of both HIV-1 and HIV-2 infections. J Infect Dis 201(8):1150–1154. doi:10.1086/651430
Man AL, Gicheva N, Nicoletti C (2014) The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol 289(1–2):112–118. doi:10.1016/j.cellimm.2014.04.001
Rera M, Clark RI, Walker DW (2012) Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A 109(52):21528–21533. doi:10.1073/pnas.1215849110
Stehle JR Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP (2012) Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol A Biol Sci Med Sci 67(11):1212–1218. doi:10.1093/gerona/gls178
Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A (2014) A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 10(2), e1003829. doi:10.1371/journal.ppat.1003829
Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC (2014) An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 7(4):983–994. doi:10.1038/mi.2013.116
Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, Deeks SG, Hunt PW, Lynch SV, McCune JM (2013) Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 5(193):193ra191. doi:10.1126/scitranslmed.3006438
Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE (2013) Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14(3):329–339. doi:10.1016/j.chom.2013.08.006
Perez-Santiago J, Gianella S, Massanella M, Spina CA, Karris MY, Var SR, Patel D, Jordan PS, Young JA, Little SJ, Richman DD, Smith DM (2013) Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS (London, England) 27(12):1921–1931
Cannon MJ, Schmid DS, Hyde TB (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20(4):202–213. doi:10.1002/rmv.655
Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A (2009) Cytomegalovirus and human immunosenescence. Rev Med Virol 19(1):47–56
Derhovanessian E, Larbi A, Pawelec G (2009) Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol 21(4):440–445
Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P (2009) Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol 155(3):423–432. doi:10.1111/j.1365-2249.2008.03785.x
Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G (2011) Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol 92(Pt 12):2746–2756. doi:10.1099/vir.0.036004-0
Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G (2003) Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol 23(4):247–257
Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G (2004) Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol 39(4):607–613. doi:10.1016/j.exger.2003.11.016
Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE (2011) Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6(2), e16103. doi:10.1371/journal.pone.0016103
Arama V, Mihailescu R, Radulescu M, Arama SS, Streinu-Cercel A, Youle M, Group C-HS (2014) Clinical relevance of the plasma load of cytomegalovirus in patients infected with HIV-A survival analysis. J Med Virol 86(11):1821–1827. doi:10.1002/jmv.24027
Stone SF, Price P, French MA (2006) Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J Antimicrob Chemother 57(4):585–588. doi:10.1093/jac/dkl049
Anuradha B, Mane Pratibha M, Vijayadurga S (2011) The reactivation of the cytomegalovirus (CMV) infection in HIV infected patients. J Clin Diagn Res 5(4):749–751
Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG (2010) Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 5(1), e8886. doi:10.1371/journal.pone.0008886
Barrett L, Stapleton SN, Fudge NJ, Grant MD (2014) Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS (London, England) 28(14):2045–2049. doi:10.1097/QAD.0000000000000405
Solana R, Tarazona R, Aiello AE, Akbar AN, Appay V, Beswick M, Bosch JA, Campos C, Cantisan S, Cicin-Sain L, Derhovanessian E, Ferrando-Martinez S, Frasca D, Fulop T, Govind S, Grubeck-Loebenstein B, Hill A, Hurme M, Kern F, Larbi A, Lopez-Botet M, Maier AB, McElhaney JE, Moss P, Naumova E, Nikolich-Zugich J, Pera A, Rector JL, Riddell N, Sanchez-Correa B, Sansoni P, Sauce D, van Lier R, Wang GC, Wills MR, Zielinski M, Pawelec G (2012) CMV and immunosenescence: from basics to clinics. Immun Ageing 9(1):23. doi:10.1186/1742-4933-9-23
Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS (2008) Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 105(10):3879–3884
Schacker T, Zeh J, Hu HL, Hill E, Corey L (1998) Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis 178(6):1616–1622
Kim HN, Meier A, Huang ML, Kuntz S, Selke S, Celum C, Corey L, Wald A (2006) Oral herpes simplex virus type 2 reactivation in HIV-positive and -negative men. J Infect Dis 194(4):420–427. doi:10.1086/505879
Dehee A, Asselot C, Piolot T, Jacomet C, Rozenbaum W, Vidaud M, Garbarg-Chenon A, Nicolas JC (2001) Quantification of Epstein-Barr virus load in peripheral blood of human immunodeficiency virus-infected patients using real-time PCR. J Med Virol 65(3):543–552
Bhardwaj N, Maldarelli F, Mellors J, Coffin JM (2014) HIV-1 infection leads to increased transcription of human endogenous retrovirus HERV-K (HML-2) proviruses in vivo but not to increased virion production. J Virol 88(19):11108–11120. doi:10.1128/JVI.01623-14
Contreras-Galindo R, Kaplan MH, Markovitz DM, Lorenzo E, Yamamura Y (2006) Detection of HERV-K(HML-2) viral RNA in plasma of HIV type 1-infected individuals. AIDS Res Hum Retroviruses 22(10):979–984. doi:10.1089/aid.2006.22.979
Gonzalez-Hernandez MJ, Swanson MD, Contreras-Galindo R, Cookinham S, King SR, Noel RJ Jr, Kaplan MH, Markovitz DM (2012) Expression of human endogenous retrovirus type K (HML-2) is activated by the Tat protein of HIV-1. J Virol 86(15):7790–7805. doi:10.1128/JVI.07215-11
Freiberg MS, Chang CC, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, Rimland D, Goetz MB, Butt AA, Rodriguez Barradas MC, Gibert C, Leaf D, Brown ST, Samet J, Kazis L, Bryant K, Justice AC, Veterans Aging Cohort S (2011) The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes 4(4):425–432. doi:10.1161/CIRCOUTCOMES.110.957415
Maalouf NM, Zhang S, Drechsler H, Brown GR, Tebas P, Bedimo R (2013) Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Min Res 28(12):2577–2583. doi:10.1002/jbmr.1988
Richardson JL, Nowicki M, Danley K, Martin EM, Cohen MH, Gonzalez R, Vassileva J, Levine AM (2005) Neuropsychological functioning in a cohort of HIV- and hepatitis C virus-infected women. AIDS (London, England) 19(15):1659–1667
Salter ML, Lau B, Mehta SH, Go VF, Leng S, Kirk GD (2013) Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr 64(5):488–495. doi:10.1097/QAI.0b013e3182a7ee2e
Zanet DL, Thorne A, Singer J, Maan EJ, Sattha B, Le Campion A, Soudeyns H, Pick N, Murray M, Money DM, Cote HC, Therapy CETGoH, Aging C (2014) Association between short leukocyte telomere length and HIV infection in a cohort study: No evidence of a relationship with antiretroviral therapy. Clin Infect Dis 58(9):1322–1332. doi:10.1093/cid/ciu051
Bentwich Z, Maartens G, Torten D, Lal AA, Lal RB (2000) Concurrent infections and HIV pathogenesis. AIDS (London, England) 14(14):2071–2081
Chang CC, Crane M, Zhou J, Mina M, Post JJ, Cameron BA, Lloyd AR, Jaworowski A, French MA, Lewin SR (2013) HIV and co-infections. Immunol Rev 254(1):114–142. doi:10.1111/imr.12063
Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W (2013) Effect of malaria on HIV/AIDS transmission and progression. Parasites & Vectors 6:18. doi:10.1186/1756-3305-6-18
Calza L, Trapani F, Bartoletti M, Manfredi R, Colangeli V, Borderi M, Grossi G, Motta R, Viale P (2012) Statin therapy decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-α in HIV-infected patients treated with ritonavir-boosted protease inhibitors. HIV Clin Trials 13(3):153–161. doi:10.1310/hct1303-153
Tenorio AR, Chan ES, Bosch RJ, Macatangay BJ, Read SW, Yesmin S, Taiwo B, Margolis DM, Jacobson JM, Landay AL, Wilson CC, for the AT (2014) Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune Non-responders to antiretroviral therapy – ACTG A5286. J Infect Dis. doi:10.1093/infdis/jiu515
Paton Ni GRLDDT et al (2012) Effects of Hydroxychloroquine on immune activation and disease progression among hiv-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA 308(4):353–361. doi:10.1001/jama.2012.6936
Baker JV, Huppler Hullsiek K, Prosser R, Duprez D, Grimm R, Tracy RP, Rhame F, Henry K, Neaton JD (2012) Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One 7(10), e46894. doi:10.1371/journal.pone.0046894
Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, Fine DM, Coombs RW, Jacobson JM, Landay AL, Douek DC, Tressler R, Read SW, Wilson CC, Deeks SG, Lederman MM, Gandhi RT, Team ACTGA (2014) Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis 210(10):1549–1554. doi:10.1093/infdis/jiu305
Acknowledgements
Support for KH and AL from NIA 1R24AG044325.
Editor
Robin Huebner, National Institute of Allergy and Infectious Diseases (NIAID), NIH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing
About this chapter
Cite this chapter
Hearps, A., Schafer, K., High, K., Landay, A. (2016). HIV and Aging: Parallels and Synergistic Mechanisms Leading to Premature Disease and Functional Decline. In: Sierra, F., Kohanski, R. (eds) Advances in Geroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-23246-1_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-23246-1_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23245-4
Online ISBN: 978-3-319-23246-1
eBook Packages: MedicineMedicine (R0)