Abstract
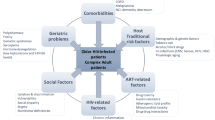
Older and frail patients with human immunodeficiency virus (HIV) constitute a treatment challenge in terms of the cumulative effects of aging and antiretroviral therapy (ART).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Key Points-
Older and frail patients with human immunodeficiency virus (HIV) constitute a treatment challenge in terms of the cumulative effects of aging and antiretroviral therapy (ART).
-
ART is recommended for patients >50 years of age, regardless of CD4 cell count, in consideration that the risk of non-AIDS-related complications may increase and the immunologic response to ART may be reduced in older patients with HIV.
-
Recommended antiretroviral (ARV) drug choices for the elderly are the same as for the general population. However, due to age related comorbidities, specific drug toxicities should be considered when choosing drug regimens.
-
Current guidelines do not recommend age or frailty as criteria for ARV switching. Three major issues frequently coexisting in this special population may indicate ARV switch: comorbidities, greater medication use and age-related changes in pharmacokinetics, and pharmacodynamics.
Older and frail patients with HIV constitute a treatment challenge in terms of the cumulative effects of aging and ART. There are currently no specific treatment guidelines available that focus on the older population, mainly due to the limited information on the efficacy and safety of selected ARV regimens for older patients. Most recently, regulatory agencies such as the Food and Drug Administration (FDA) and European Medicine Agency (EMA) request age stratification (above or under 50 years of age) in the analysis of the results for registrational clinical trials. However, due to the exclusion of patients with comorbidities, the included elderly population may not be representative. Furthermore, ‘frailty’ as the phenotype of biological aging is not considered in the development of treatment guidelines.
HIV experts and primary care providers should work together to optimize the medical care of older and frail patients with HIV and complex comorbidities, in addition to preventing secondary transmission of HIV among older adults.
General issues regarding ARV toxicity (cardiovascular, renal, bone, polypharmacy, and drug–drug interactions), although improved, are still a matter of concern when managing ARVs, particularly so in the elderly population.
10.1 When to Start Antiretroviral Therapy
Current Department of Health and Human Services guidelines [1] recommend starting ARV therapy in patients with HIV aged >50 years, regardless of CD4+ cell count. This is due to increased rates of progression of untreated HIV disease, immune senescence, comorbidities that are exacerbated by CD4+ cell loss, and inferior immune reconstitution after the initiation of therapy [2–12].
International Antiviral Society guidelines base their recommendations on the principle of ‘treatment as prevention’ and reinforce this level of recommendation in the elderly population [13]. European AIDS Clinical Society (EACS) guidelines, on the other hand, do mention age categories to suggest an intensified screening for comorbidities in the elderly population but do not suggest any age category for starting ARV in the adult population [14].
Several studies have documented that CD4 T cell recovery after starting ART is generally less robust in older patients than in younger patients [1, 11, 15–18]. This incomplete immune recovery occurs both in patients with CD4+ cell counts below and above 350 cells/mm3 [11, 16–22]. These inferior clinical outcomes in older patients with HIV are not explained by poor virological results; older patients have an improved adherence to therapy [23, 24], more often achieve virological control of HIV replication, and subsequently develop virological breakthrough less often than younger patients [23, 25–28].
A systematic review and meta-analysis of 12 studies that included adherence data showed that older age was associated with a 27 % reduced risk of non-adherence to ART (relative risk 0.72; 95 % CI 0.64–0.82), including both short-term and long-term adherence [29].
10.2 What to Start
Recommended ARV drug choices for the elderly are the same as for the general population. However, in consideration of age-related comorbidities, specific drug toxicities should be taken into account when choosing drug regimens. Table 10.1 summarizes ART-associated common and/or severe adverse effects to be considered in the treatment of the elderly population at higher risk of comorbidities [30].
Particular attention should be paid to postmenopausal women or women entering menopause. Menopause is a physiological time frame where rapid hormonal, biochemical, anthropometric, and psychological changes occur, making women more vulnerable to ARV toxicities. The most relevant comorbidity in this category is osteoporosis.
Disentangling the effects of menopause in comorbidity onset requires well-designed studies with adequate numbers of HIV-infected and HIV-uninfected women. Future studies should follow women from premenopause through menopause, using both surveys and laboratory measurements for menopause diagnosis, and control for confounders related to normal aging processes, in order to inform optimal clinical management for menopausal women living with HIV.
Altogether, we may say that randomized clinical trials able to inform ARV choice in patients with comorbidities are still limited, but a changing landscape is appearing.
A study presented at the 2015 Conference on Retroviruses and Opportunistic Infections assessed the safety, tolerability, and efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) single-tablet regimen (STR) in treatment-naïve and treatment-experienced HIV-positive adult participants with estimated glomerular filtration rate (eGFR) between 30 and 69 mL/min, in which the median baseline age was 58 years [31]. Switching to E/C/F/TAF was associated with no change in actual GFR, reductions in proteinuria and markers of proximal renal tubular function, and improvements in hip and spine bone mineral density (BMD). Adverse events, grades, and frequencies were similar in patients with a baseline eGFR <50 and ≥50 mL/min. These 48-week data support the virological efficacy and renal and bone safety of once daily, single-tablet E/C/F/TAF therapy for patients with HIV and mild to moderate renal impairment (eGFR 30–69 mL/min). This particular drug combination has a 15-times lower concentration in serum than tenofovir, is 7-times more concentrated in the intracellular lymphocyte cytoplasm, appears to have a limited bone and kidney toxicity, and qualifies to be studied as the nucleoside reverse transcriptase inhibitor (NRTI) backbone of the aging patient [32–34].
10.3 What to Change
Switching a successful ARV regimen should be done carefully and only when the potential benefits of the change outweigh the potential complications of altering treatment. The fundamental principle of regimen switching is to maintain viral suppression. Before any treatment switch is implemented, it is critical to review the following:
-
the patient’s medical and full ARV history (including prior virologic responses);
-
resistance test results;
-
viral tropism (when maraviroc [MVC] is being considered);
-
human leukocyte antigen (HLA) B*5701 status (when abacavir [ABC] is being considered);
-
comorbidities;
-
adherence history;
-
concomitant medications and supplements (and their potential for drug interactions); and
-
prior intolerances to any ARV drug.
Current guidelines do not recommend age or frailty as criteria for ARV switching. Nevertheless, three major issues frequently coexisting in this special population may suggest the need for an ARV switch, and are discussed in the following sections.
10.3.1 Comorbid Conditions
Bone, kidney, metabolic, and cardiovascular health impairment, for example, are more frequent in older adults with HIV and should be closely monitored in such patients. In the past 15 years, the availability of new and less toxic drugs have allowed a progressive switch from a reactive strategy, based on ARV switch in patients with clinical comorbidities, into a more aggressive pre-emptive strategy in which the switch is made to prevent the risk of functional decline of organ reserve. Table 10.2 summarizes switch strategies to cope with specific adverse event risk, providing a rationale for ARV choice [30].
10.3.2 Greater Medication Use
Polypharmacy in both the general older population and in patients with HIV infection is very common. Recent data from the Swiss HIV Cohort demonstrate that among patients 65 years of age and older, 14 % received medications from four or more classes of non-HIV medications, and lipid-lowering agents were the most commonly prescribed non-ART medication [35]. These data are consistent with findings from the Veteran’s Administration Cohort Study (VACS), wherein among those 50 years of age and older, 55 % were on five or more daily medications [36]. These studies report prescription medication only and therefore likely underestimate the prevalence of polypharmacy.
Use of prescription and over the counter medication, herbal supplements, and even recreational drugs increase the possibility of complex and unpredictable drug–drug interactions (DDIs) [37–39]. Polypharmacy has also been associated with increased risk of adverse drug reactions, increased hospitalizations, poor adherence, falls, and fractures [40–45].
In this context, elevated pill burden and risk of severe DDIs appear to give an advantage to INSTI-highly active antiretroviral therapy (HAART) (regimen with particular regards to fixed-dose combination and/or drugs with no ritonavir or cobicistat boosting). The new NNRTI, rilpivirine, appears to have limited DDIs. DDI occurs more commonly with boosted PI regimens.
10.3.3 Age-Related Changes in Pharmacokinetics and Pharmacodynamics
Pharmacokinetic (PK) changes associated with aging include a reduction in renal and hepatic clearance and an increase in volume of distribution of lipid soluble drugs (leading to a prolongation of elimination half-life). Pharmacodynamic (PD) changes involve altered (usually increased) sensitivity to several classes of drugs, such as anticoagulants, cardiovascular, and psychotropic drugs [46] (Table 10.3).
Aging is associated with a decrease in renal tubular secretion, glomerular filtration, and decreased functioning of the hepatic CYP450 [38, 48, 49]. A better understanding of the effects of aging on the clinical pharmacology of therapeutic agents would enhance the quality of prescribing.
In this context, the age-related changes in drug handling (PK) and response (PD) appear to provide advantages and disadvantages to the different ARV classes:
-
Integrase inhibitor therapy offers the advantages of a favorable safety profile, good tolerability, and absence of significant DDIs.
-
Fusion inhibitors have a null impact on PK and PD.
-
NNRTIs and PIs may act as inducers or inhibitors of CYP metabolism and permeability glycoprotein transporters. NRTIs may enhance some metabolic toxicities, in particular interfering with mitochondrial function.
The general principles (mentioned above) that guide the choice for ART use in day-to-day management of ARV in older patients with HIV are summarized in Table 10.4.
Frailty has not been considered as a factor in choosing specific ARV regimens either in treatment-naïve or in treatment-experienced patients with HIV.
10.3.4 Clinical Trials
A recent analysis from the Modena HIV Metabolic Clinic (MHMC) aimed to describe patterns of ARV use in relation to age, gender, and frailty (Figs. 10.1 and 10.2) [50]. In the retrospective review of data from 1240 participants with undetectable viral load and CD4 cell count ≥500 cells/μL (providing 5024 annual study visits) frailty was retrospectively quantified via 37-item frailty index, based on the cumulative deficits model. Frailty index variables excluded markers of HIV severity or immune depletion [50].
Age and frailty spectrum in different antiretroviral drug classes in patients currently exposed to treatment [50]
Age and frailty spectrum in different antiretroviral regimens in patients currently exposed to treatment [50]
Regardless of the retrospective observational study design, which does not allow for any causative correlation, differences in ARV use were observed in relation to frailty and age. Patients on INSTI, NRTI-sparing regimens, or dual therapy were more likely to have a higher frailty index, independent of age. This may be the result of a clinical attitude towards ARV selection in frail patients, which could be the proof of principle for future randomized clinical trials, using the frailty index as a primary end point. Alternatively, the frailty index may be used as an inclusion criterion to stratify overall health of patients.
It is premature to state that INSTI regimens, NRTI-sparing regimens (usually dual therapy), and mono-PI therapy should be preferred in frail patients, nevertheless, in consideration of a progressive day-to-day use of these regimens in elderly and frail patients, the following sections summarize clinical studies using these treatment options.
Regardless of the small sample size in many of these studies the overall number of patients who have been enrolled in total is considerably high and it may be argued that a significant amount of clinical experience already exists with these regimens.
10.3.4.1 INSTI-Based Regimens
Integrase inhibitor (INI) therapy offers the advantages of a favorable safety profile, good tolerability, and absence of significant DDIs, in addition to high efficacy.
Messiaen et al. [51] conducted a review and meta-analysis of studies that used INIs in ARV-naïve and treatment-experienced patients with either virological failure or were switching while virologically suppressed. Combination treatment with INIs and dual NRTIs showed to be more beneficial for treatment-naïve patients compared to other currently used treatment strategies. In treatment-experienced patients with virological failure, use of INSTIs also proved to be beneficial. However, in patients with a history of therapy failure, switching a high genetic barrier drug towards an INI was not supported.
A summary of studies investigating INSTI therapy in treatment-naïve and treatment-experienced patients can be found in Tables 10.5 and 10.6, respectively.
10.3.4.2 NRTI-Sparing Regimens
One of the most important arguments in favor of the assessment of NRTI-sparing regimens has been a growing understanding of the long-term toxicity profile of the newer, relatively safe NRTIs in common use currently, such as tenofovir and abacavir. Newer drugs with high potency and often with a more favorable safety profile are becoming available, leading to an interest in NRTI-sparing regimens.
A review by Acchra and Boyd [60] examined studies of NRTI-sparing regimens in adult patients with HIV (Tables 10.7, 10.8, and 10.9). They found that an NRTI-sparing strategy (with an INI and boosted PI) was supported in the treatment of experienced patients on a failing regimen. In naïve patients, or in those switching from virologically suppressive regimens, evidence was sparse and largely came from small exploratory trials or observational studies. In such cases caution should be exercised in choosing the right patient and new ARV regimen, in order to avoid virological failure. Another concern was the residual toxicity of the ritonavir boost in PI-containing NRTI-sparing regimens. Additional research is needed to understand the true potential of NRTI-sparing regimens and how to minimize adverse effects associated with ritonavir-boosting.
10.3.4.3 Mono-PI Therapy
The potential benefits from switching from a triple drug regimen to ritonavir-boosted PI (PI/r) monotherapy is currently a topic of some interest (Tables 10.10 and 10.11), as it allows reduction of NRTI-related toxicity (lipoatrophy, renal disease, BMD loss) and reduced cost versus dual or triple therapy, while retaining other classes as future treatment options. There is an increased risk of low level viremia with monotherapy, but this has been shown to be reversible with NRTI re-introduction. The emergence of PI resistance has been rare on PI/r monotherapy and re-introduction of NRTI remains possible in such cases.
Such a strategy can only be considered in stable, long-term virologically suppressed patients who have demonstrated good adherence to ART, have no history of PI failure, do not have chronic hepatitis B, do not have HIV-associated neurocognitive disorders, and who are able to tolerate low dose ritonavir [101].
The recent PIVOT study [99] (Table 10.11) randomized 587 UK patients, who already had an undetectable viral load on stable treatment, to either PI/r monotherapy or triple therapy. It included 4 years of follow-up. Approximately 80 % in the boosted PI monotherapy arm used darunavir/r (DRV/r), 14 % lopinavir/r (LPV/r), and 7 % another PI. Viral load rebounded much more frequently in the monotherapy group (35 %) compared to the triple therapy group (3.2 %). All rebounds on monotherapy re-suppressed either spontaneously or with NRTI re-introduction. The PI arm was non-inferior on the primary outcome of loss of future drug options. There were no significant differences in serious disease complications, adverse events, or neurocognitive function between the arms [99].
The PROTEA trial [93] (Table 10.11) randomized 273 patients with HIV-1 RNA <50 copies/mL for over 24 weeks on current ARVs. Patients were switched to DRV/r 800/100 mg once-daily, either as monotherapy or with two NRTIs. Monotherapy showed lower efficacy (86 %) versus triple ART at week 48 (95 %). However, this lower efficacy was seen mainly in patients with CD4 nadir levels below 200 cells/μL. There was no development of PI resistance [93].
The European AIDS Clinical Society guidelines state that PI/r monotherapy with either DRV/r or LPV/r, twice-daily, might represent an option in persons with intolerance to NRTIs or for treatment simplification in substance abusers with documented frequent interruption of combination ART. Such a strategy should only be applied to persons without a history of failure on prior PI-based therapy, who have had a HIV-viral load <50 copies/mL in at least the past 6 months, and who do not have chronic hepatitis B infection [14].
10.4 Future Perspectives
The success of HAART has significantly changed the pattern of HIV infection in developed countries, with the ‘graying’ of the HIV-infected population as an important testament to its success. This has provided new challenges relating to the care of older patients, particularly with regard to the management of comorbidities and ART toxicity, which scientists and physicians are addressing through the refinement of existing ART, the development of new agents, and a growing focus on a more holistic approach to care (involving the integration of concepts from general medicine and geriatrics into HIV care).
It is evident that the management of ART in patients aging with HIV implies a detailed knowledge of patient health in both physical and psychological domains in order to tailor the most appropriate ARV regimen. Therefore, the choice of regimen goes beyond the goal of achieving HIV viral load below the limit of detection and contributes to the overall management of the health profile.
Future research should investigate the association between frailty and ARV choice in people living with HIV. In this context frailty may be useful either as inclusion criteria or as a clinical end point for randomized clinical trials comparing ARV treatment regimens in elderly patients with HIV. This knowledge and experience will likely be extremely useful in the care of older patients.
References
AIDSinfo: guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. Accessed 21 Aug 2015.
CASCADE Collaboration. Differences in CD4 cell counts at seroconversion and decline among 5739 HIV-1-infected individuals with well-estimated dates of seroconversion. J Acquir Immune Defic Syndr. 2003;34:76–83.
Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355:1131–7.
Phillips A, Pezzotti P. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS. 2004;18:51–8.
Phillips AN, Lee CA, Elford J, et al. More rapid progression to AIDS in older HIV-infected people: the role of CD4+ T-cell counts. J Acquir Immune Defic Syndr. 1991;4:970–5.
Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41.
Lodwick RK, Sabin CA, Porter K, et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–5.
Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–601.
Monforte AD, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–53.
Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–73.
Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS. 2001;15:1576–9.
Khanna N, Opravil M, Furrer H, et al. CD4+ T cell count recovery in HIV type 1–infected patients is independent of class of antiretroviral therapy. Clin Infect Dis. 2008;47:1093–101.
Günthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society – USA Panel. JAMA. 2014;312:410–25.
European AIDS Clinical Socity: EACS guidelines 7.1. 2014. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed 21 Aug 2015.
Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58:1312–21.
Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–72.
Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4:255–62.
Baker JV, Peng G, Rapkin J, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541–6.
Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43.
Li X, Margolick JB, Jamieson BD, Rinaldo CR, Phair JP, Jacobson LP. CD4(+) T-cell counts and plasma HIV-1 RNA levels beyond 5 years of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;57:421–8.
Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–18.
Phillips AN, Gazzard B, Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–21.
Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, Quesenberry Jr CP. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167:684–91.
Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18 (Suppl 1):S19–25.
Grabar S, Kousignian I, Sobel A, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18:2029–38.
Porter K, Walker S, Hill T, et al. Changes in outcome of persons initiating highly active antiretroviral therapy at a CD4 count less than 50 Cells/mm3. J Acquir Immune Defic Syndr. 2008;47:202–5.
Mussini C, Manzardo C, Johnson M, et al. Patients presenting with AIDS in the HAART era: a collaborative cohort analysis. AIDS. 2008;22:2461–9.
Lampe FC, Gatell JM, Staszewski S, et al. Changes over time in risk of initial virological failure of combination antiretroviral therapy: a multicohort analysis, 1996 to 2002. Arch Intern Med. 2006;166:521–8.
Ghidei L, Simone MJ, Salow MJ, et al. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs Aging. 2013;30:809–19.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2015. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 21 Aug 2015.
Pozniak A, Arribas J, Gupta SK, et al. Safety of tenofovir alafenamide in renal impairment. Conference on retroviruses and opportunistic infections (CROI), 23–26 Feb 2015, Seattle.
Lee WA, He GX, Eisenberg E, et al. Selective Intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49:1898–906.
Birkus G, Wang R, Liu X, et al. Cathepsin A Is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob Agents Chemother. 2007;51:543–50.
Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm. 2013;10:459–66.
Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–9.
Justice A. Overview of ageing: a. how can we optimize care in the context of multimorbidity? Yale University; 2011. http://docslide.us/documents/overview-of-ageing-a-how-can-we-optimize-care-in-the-context-of-multimorbidity-amy-c-justice-md-phd-professor-yale-university-schools-of-medicine.html. Accessed 21 Aug 2015.
Marzolini C, Back D, Weber R, et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011;66:2107–11.
Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82:87–96.
Tseng A, Szadkowski L, Walmsley S, Salit I, Raboud J. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother. 2013;47:1429–39.
Barat I, Andreasen F, Damsgaard EM. Drug therapy in the elderly: what doctors believe and patients actually do. Br J Clin Pharmacol. 2001;51:615–22.
Franceschi M, Scarcelli C, Niro V, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf. 2008;31:545–56.
Gnjidic D, Le Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28:237–53.
Lin CF, Wang CY, Bai CH. Polypharmacy, aging and potential drug-drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28:219–25.
Secoli SR, Figueras A, Lebrão ML, de Lima FD, Santos JL. Risk of potential drug-drug interactions among Brazilian elderly: a population-based, cross-sectional study. Drugs Aging. 2010;27:759–70.
Tamura BK, Bell CL, Inaba M, Masaki KH. Outcomes of polypharmacy in nursing home residents. Clin Geriatr Med. 2012;28:217–36.
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14.
Schoen JC, Erlandson KM, Anderson PL. Clinical pharmacokinetics of antiretroviral drugs in older persons. Expert Opin Drug Metab Toxicol. 2013;9:573–88.
Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61:331–9.
Ramsay LE, Tucker GT. Clinical pharmacology: drugs and the elderly. Br Med J (Clin Res Ed). 1981;282:125–7.
Guaraldi G, Brothers TD, Zona S, et al. Frailty and age are independently associated with patterns of HIV antiretroviral use in a clinical setting. 5th international workshop on HIV and aging, 21–22 Oct 2014, Baltimore.
Messiaen P, Wensing AM, Fun A, Nijhuis M, Brusselaers N, Vandekerckhove L. Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis. PLoS One. 2013;8:e52562.
Cotte L, Durant J, Brochier C, et al. Safety and efficacy of a maraviroc-raltegravir combination following a 6 month induction with maraviroc-raltegravir-tenofovir-emtricitabine in naïve HIV-1 infected patients with CCR5 Virus: interim analysis of the No Nuc No Boost study. 7th international AIDS Society conference on HIV pathogenesis treatment and prevention, June 30–July 3 2013, Kuala Lumpur.
Dolutegravir-lamivudine as dual therapy in naive HIV-infected patients: a pilot study (PADDLE). 2015. https://clinicaltrials.gov/ct2/show/NCT02211482. Accessed 21 Aug 2015.
Reliquet V, Chirouze C, Allavena C, et al. Nevirapine-raltegravir combination, an NRTI and PI/r sparing regimen, as maintenance antiretroviral therapy in virologically suppressed HIV-1-infected patients. Antivir Ther. 2014;19:117–23.
Katlama C, Assoumou L, Valantin MA, et al. Maraviroc plus raltegravir dual therapy in aviremic HIV infected patients with lipodystrophy: results from the ROCnRAL ANRS 157 Study. 20th conference on retroviruses and opportunistic infections, 3–6 Mar 2013, Atlanta.
Switch to Maraviroc + Integrase inhibitor. 2014. https://clinicaltrials.gov/ct2/show/NCT01896921. Accessed 21 Aug 2015.
Safety & efficacy of dual therapy with raltegravir/lamivudine (RALAM). 2014. https://clinicaltrials.gov/ct2/show/NCT02284035. Accessed 21 Aug 2015.
Monteiro P, Perez I, Laguno M, et al. Dual therapy with etravirine plus raltegravir for virologically suppressed HIV-infected patients: a pilot study. J Antimicrob Chemother. 2014;69:742–8.
Calin R, Valantin MA, Simon A, et al. Raltegravir/etravirine dual therapy as a virologically safe treatment option in suppressed HIV-1-infected patients without previous NNRTI failure. 7th international AIDS Society conference on HIV pathogenesis treatment and prevention, 30 June–3 July 2013, Kuala Lumpur.
Achhra AC, Boyd MA. Antiretroviral regimens sparing agents from the nucleoside(tide) reverse transcriptase inhibitor class: a review of the recent literature. AIDS Res Ther. 2013;10:33.
Mills A, Mildvan D, Podzamczer D, et al. Maraviroc once-daily nucleoside analog-sparing egimen in treatment-naive patients: randomized, open-label pilot study. J Acquir Immune Defic Syndr. 2013;62:164–70.
Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2013;29:256–65.
Kozal MJ, Lupo S, DeJesus E, et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naïve HIV-infected patients: SPARTAN study results. HIV Clin Trials. 2012;13:119–30.
Taiwo B, Swindells S, Berzins B, et al. Week 48 results of the Maraviroc Plus Darunavir/ritonavir Study (MIDAS) for treatment-naive patients infected with R5-tropic HIV-1. 19th international AIDS conference, 22–27 July 2012, Washington, DC.
Taiwo B, Acosta EP, Ryscavage P, et al. Virologic response, early HIV-1 decay, and maraviroc pharmacokinetics with the nucleos(t)ide-free regimen of maraviroc plus darunavir/ritonavir in a pilot study. J Acquir Immune Defic Syndr. 2013;64:167–73.
Bedimo R, Drechsler H, Turner D, et al. RADAR study: raltegravir combined with boosted darunavir has similar safety and antiviral efficacy as tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. 6th international AIDS Society conference on HIV pathogenesis, treatment and prevention, 17–20 July 2011, Rome.
Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS. 2011;25:2113–22.
Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106.
Boyd MA, Kumarasamy N, Moore CL, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet. 2013;381:2091–9.
Hoy J, Martin A, Moore C, et al. Changes in bone mineral density over 48 weeks among participants randomised to either lopinavir/ritonavir (LPV/r) + 2–3 N(t)RTI or LPV/r + raltegravir as second-line therapy: a sub-study of the SECONDLINE trial. 7th IAS conference on HIV pathogenesis, treatment and prevention, 30 June–3 July 2013, Kuala Lumpar.
Paton N, Kityo C, Hoppe A. A pragmatic randomised controlled strategy trial of three second-line treatment options for use in public health rollout programme settings: the Europe-Africa Research Network for Evaluation of Second-line Therapy (EARNEST) Trial. 7th international AIDS conference on HIV pathogenesis, treatment and prevention, 30 June–3 July 2013, Kuala Lumpur.
Tashima K, Smeaton L, Andrade A, et al. Omitting NRTI from ARV regimens is not inferior to adding NRTI in treatment-experienced HIV+ subjects failing a protease inhibitor regimen: the ACTG OPTIONS study. 20th conference on retroviruses and opportunistic infections, 3–6 Mar 2013, Atlanta.
Ruane P, Brinson C, Kumar P, et al. Intelence aNd pRezista Once A Day Study (INROADS): a multicenter, single-arm, open-label study of once daily combination of etravirine (ETR) and darunavir/ritonavir (DRV/r) as dual therapy in early treatment-experienced subjects. 7th international AIDS conference on HIV pathogenesis, treatment and prevention, 30 June–3 July, 2013, Kuala Lumpur.
Imaz A, Llibre JM, Mora M, et al. Efficacy and safety of nucleoside reverse transcriptase inhibitor-sparing salvage therapy for multidrug-resistant HIV-1 infection based on new-class and new-generation antiretrovirals [Erratum appears in]. J Antimicrob Chemother. 2011;66:358–62.
Nozza S, Galli L, Bigoloni A, et al. Durability and safety of a novel salvage therapy in R5-tropic HIV-infected patients: maraviroc, raltegravir, etravirine. J Acquir Immune Defic Syndr. 2011;56:e113–5.
Florence E, De Wit S, Castagna A, et al. HIV RNA suppression rates after 24 weeks of treatment with etravirine, darunavir/ritonavir and raltegravir in the etravirine early access programme. Int J STD AIDS. 2010;21:224–5.
Ward DJ, O’Neill DF. Nucleoside-sparing antiretroviral regimens in clinical practice. The 53rd interscience conference on antimicrobial agents and chemotherapy (ICAAC), 10–13 Sept 2013, Denver.
Burgos J, Crespo M, Falcó V, et al. Simplification to dual antiretroviral therapy including a ritonavir-boosted protease inhibitor in treatment-experienced HIV-1-infected patients. J Antimicrob Chemother. 2012;67:2479–86.
Ofotokun I, Sheth AN, Sanford SE, et al. A switch in therapy to a reverse transcriptase inhibitor sparing combination of lopinavir/ritonavir and raltegravir in virologically suppressed HIV-infected patients: a pilot randomized trial to assess efficacy and safety profile: the KITE study. AIDS Res Hum Retroviruses. 2012;28:1196–206.
Carey D, Pett SL, Bloch M, et al. A randomized study of pharmacokinetics, efficacy, and safety of 2 raltegravir plus atazanavir strategies in ART-treated adults. J Acquir Immune Defic Syndr. 2012;60:143–9.
Cordery DV, Hesse K, Amin J, Cooper DA. Raltegravir and unboosted atazanavir dual therapy in virologically suppressed antiretroviral treatment-experienced HIV patients. Antivir Ther. 2010;15:1035–8.
Allavena C, Mounoury O, Rodallec A, Reliquet V, Billaud E, Raffi F. Efficacy and safety of an NRTI-sparing dual regimen of raltegravir and ritonavir-boosted protease inhibitor in a triple antiretroviral class-experienced population. HIV Clin Trials. 2009;10:337–40.
Fischl MA, Collier AC, Mukherjee AL, et al. Randomized open-label trial of two simplified, class-sparing regimens following a first suppressive three or four-drug regimen. AIDS. 2007;21:325–33.
Ghosn J, Flandre P, Cohen-Codar I, et al. Long-term (96-week) follow-up of antiretroviral-naïve HIV-infected patients treated with first-line lopinavir/ritonavir monotherapy in the MONARK trial. HIV Med. 2010;11:137–42.
Delaugerre C, Flandre P, Chaix ML, et al. Protease inhibitor resistance analysis in the MONARK trial comparing first-line lopinavir-ritonavir monotherapy to lopinavir-ritonavir plus zidovudine and lamivudine triple therapy. Antimicrob Agents Chemother. 2009;53:2934–9.
Gathe Jr JC, Yeh RF, Mayberry C, et al. Single-agent therapy with lopinavir/ritonavir suppresses HIV-1 viral replication in ARV naïve patients: IMANI II – 96 week final results. 48th annual international conference on antimicrobial agents and chemotherapy (ICAAC), 25–28 Oct 2008, Washington, DC.
Cameron DW, da Silva BA, Arribas JR, et al. A 96-week comparison of lopinavir-ritonavir combination therapy followed by lopinavir-ritonavir monotherapy versus efavirenz combination therapy. J Infect Dis. 2008;198:234–40.
Arribas JR, Pulido F, Delgado R, et al. Lopinavir/ritonavir as single-drug therapy for maintenance of HIV-1 viral suppression: 48-week results of a randomized, controlled, open-label, proof-of-concept pilot clinical trial (OK Study). J Acquir Immune Defic Syndr. 2005;40:280–7.
Arribas JR, Delgado R, Arranz A, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and 2 nucleosides for maintenance therapy of HIV: 96-week analysis. J Acquir Immune Defic Syndr. 2009;51:147–52.
Nunes EP, Santini de Oliveira M, Merçon M, et al. Monotherapy with Lopinavir/Ritonavir as maintenance after HIV-1 viral suppression: results of a 96-week randomized, controlled, open-label, pilot trial (KalMo study). HIV Clin Trials. 2009;10:368–74.
Valantin MA, Lambert-Niclot S, Flandre P, et al. Long-term efficacy of darunavir/ritonavir monotherapy in patients with HIV-1 viral suppression: week 96 results from the MONOI ANRS 136 study. J Antimicrob Chemother. 2012;67:691–5.
Arribas JR, Clumeck N, Nelson M, Hill A, van Delft Y, Moecklinghoff C. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load <50 HIV-1 RNA copies/mL at baseline. HIV Med. 2012;13:398–405.
Antinori A, Arribas J, Fehr J, et al. The PROTEA trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV-1 RNA below 50 copies/mL. J Int AIDS Soc. 2014;17 (Suppl 3):19525.
Santos JR, Moltó J, Llibre JM, et al. Antiretroviral simplification with darunavir/ritonavir monotherapy in routine clinical practice: safety, effectiveness, and impact on lipid profile. PLoS One. 2012;7:e37442.
Wilkin TJ, McKinnon JE, DiRienzo AG, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy: final 48-week clinical and virologic outcomes. J Infect Dis. 2009;199:866–71.
Karlström O, Josephson F, Sönnerborg A. Early virologic rebound in a pilot trial of ritonavir-boosted atazanavir as maintenance monotherapy. J Acquir Immune Defic Syndr. 2007;44:417–22.
Vernazza P, Daneel S, Schiffer V, et al. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS. 2007;21:1309–15.
Castagna A, Spagnuolo V, Galli L, et al. Simplification to atazanavir/ritonavir monotherapy for HIV-1 treated individuals on virological suppression: 48-week efficacy and safety results. AIDS. 2014;28:2269–79.
Paton NI, Stöhr W, Arenas-Pinto A. Protease inhibitor monotherapy for long-term management of HIV infection: a randomised, controlled, open-label, non-inferiority trial. Lancet HIV. 2015;2:e417–26.
Goelz J, Wolf E, Moll A, Koegl C, Jaeger H. Single agent HAART with lopinavir/r (LPV/r) in ART-naive and pre-treated HIV-1-infected patients. XVI international AIDS conference, 13–18 Aug 2006,Toronto.
Arribas JR, Doroana M, Turner D, Vandekerckhove L, Streinu-Cercel A. Boosted protease inhibitor monotherapy in HIV-infected adults: outputs from a pan-European expert panel meeting. AIDS Res Ther. 2013;10:3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Guaraldi, G., Gomes, A.F., Silva, A.R. (2016). Antiretroviral Treatment in Older Patients. In: Guaraldi, G., Falutz, J., Mussi, C., Silva, A. (eds) Managing the Older Adult Patient with HIV. Adis, Cham. https://doi.org/10.1007/978-3-319-20131-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-20131-3_10
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-20130-6
Online ISBN: 978-3-319-20131-3
eBook Packages: MedicineMedicine (R0)