Abstract
The management of complications of portal hypertension in children remains a challenge due to limited evidenced-based approaches and the marked heterogeneity of the relevant pediatric disorders. It is not necessarily appropriate to apply treatment paradigms developed for adults to children given the marked differences in the pathophysiology of the operant diseases. This review summarizes progress made in the diagnosis and management of pediatric portal hypertension since Baveno V, with a focus on issues related to varices in two disorders, extrahepatic portal venous obstruction (EHPVO) and biliary atresia (BA). Portal hypertension in both diseases presents in the context of relatively preserved hepatocellular function, which distinguishes this from experiences with adults. Approaches to secondary prophylaxis of variceal hemorrhage seem more secure than either screening for or primary prophylaxis of varices. Response to the Kasai hepatoportoenterostomy for BA, in particular the establishment of bile flow, has a potentially marked impact on progression of not only complications of portal hypertension but overall short-term prognosis and therefore the approach to the management of portal hypertension. The ability of the mesoRex bypass to “cure” EHPVO in some children has profound implications for optimal management of this disease. In pediatrics, generalized guidelines are difficult to establish, and a complete understanding of the natural history of a particular pediatric disorder along with knowledge of the pros and cons of potential interventions is critical for relatively informed clinical decision-making.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Portal hypertension remains a major clinical issue for children with chronic liver disease. This report updates progress in the understanding and management of portal hypertension in children since Baveno V [1, 2]. Approaches to the management of complications of portal hypertension in children are frequently driven by expert opinion and not fully evidence based [3]. Practical issues in the conduct of rigorous clinical trials of therapies in pediatric portal hypertension have impeded advances. For instance, it is estimated that ~35,000,000 pediatric lives (~½ of the children in the United States) would need to be accounted for in the catchment of a powered study of primary prophylaxis of variceal hemorrhage in children [4]. Despite these limitations, progress is being made in this very important field; highlights of that progress are summarized here.
Portal hypertension is well described in a wide range of pediatric disorders, many of which are fundamentally distinct from the diseases that afflict adults (Table 29.1). Those differences have profound implications for diagnosis and management. Two common causes of portal hypertension, biliary atresia, and extrahepatic portal vein obstruction (EHPVO, also known as portal vein thrombosis) have a myriad of critical differences from the common hepatocellular-based disorders that lead to portal hypertension in adults. Most notable is the fact that portal hypertension is an early manifestation of these disorders at a time when hepatic function is relatively intact. Technical issues arise related to the small size of many children with portal hypertension. The range of complicated diseases that lead to portal hypertension in children continues to expand. Interesting recent case series of Fontan-related liver disease and obliterative portal venopathy have been published since Baveno V [5, 6].
A variety of very important clinical sequelae arise from the consequences of portal hypertension (Table 29.2). Recent reports highlight the prevalence of some of these issues in children. Variceal hemorrhage is clearly an issue for children and can be a dramatic mode of presentation. A comprehensive literature review identified reports of bleeding from varices in a large number of children with congenital hepatic fibrosis [7]. When surveilled, esophageal varices are commonly found in congenital hepatic fibrosis in the setting of autosomal recessive polycystic kidney disease [8]. More than 50 % of children with EHPVO presented with variceal hemorrhage [9]. A multicenter cross-sectional analysis of children and young adults with biliary atresia identified a history of variceal hemorrhage in 20 % of those children [10]. This number likely underestimates the prevalence of this problem in biliary atresia as children who had undergone liver transplantation early in life were not captured in the analysis. The overlapping manifestations of portal hypertension in biliary atresia are nicely illustrated in a complicated Venn diagram from that cross-sectional investigation (Fig. 29.1). A similar analysis of children with alpha-1 antitrypsin deficiency identified portal hypertension as a major clinical issue [11]. In both of these studies, chronic ascites was not common. This complication is often a harbinger of advancing liver disease in children leading to considerations for liver transplantation. Hepatopulmonary syndrome may be relatively common in children with portal hypertension. The prevalence identified may be very much dependent upon the implementation of screening techniques. Transcutaneous oxygen saturation measurement in an upright position is easily employed although there is controversy about its sensitivity [12]. Arterial blood gas measurement is not straightforward in children and is even more difficult to accomplish in an upright position. Despite these limitations, Sari identified arterial hypoxemia in 9 of 40 children with portal hypertension [13]. Formal documentation of hepatopulmonary syndrome was made in four of these children. Portopulmonary hypertension has been described in children, although difficulties in its identification may limit our understanding of the scope of this issue in pediatrics [14, 15]. Quality of life in children with EHPVO is reduced and related to the degree of hypersplenism and failure to thrive [16]. All domains of quality of life including physical, emotional, social, and school function are affected. Variceal eradication and/or portosystemic shunt surgery does not necessarily resolve these quality-of-life issues. Overt hepatic encephalopathy is uncommon in children with chronic liver disease. In contrast, minimal hepatic encephalopathy may be underappreciated, although it is not easy to identify in children [17].
Features of portal hypertension in biliary atresia. Overlapping manifestations of portal hypertension in a cross-sectional multicenter investigation of children with biliary atresia (Reproduced with permission from the publisher, Shneider et al. [10])
Since Baveno V, progress has been made in deriving quality evidence to serve as the basis for clinical decision-making in Pediatric Hepatology. The scope of advancement has been quite variable with respect to fundamental aspects of the management of varices in children. It is interesting that the relative security in decision-making appears to increase as one moves from screen and primary prophylaxis to secondary prophylaxis of variceal hemorrhage. As is the case in the care of adults, endoscopic band ligation is clearly preferable to sclerotherapy for secondary prophylaxis of variceal hemorrhage [18]. In most recently reported case series, general anesthesia is required for the conduct of endoscopic management of varices in children. The ramifications of repeated general anesthesia in young children with chronic liver disease may not be fully realized [19, 20]. Anesthesia exposure in children less than 3 years of age may be associated with subsequent language and abstract reasoning deficits [21]. Unfortunately, size limitations may require injection sclerotherapy in children who are less than 10 or 15 kg. In a broad-ranging pediatric experience, 16 of 55 children required sclerotherapy for secondary prophylaxis for variceal hemorrhage [22]. In this cohort, there was ~90 % success in obliterating varices, although rebleeding occurred at a mean of 13 months from the initial hemorrhage. Focused efforts in biliary atresia, where bleeding can occur fairly early in life, necessitate a greater reliance on sclerotherapy (25 out of 30 children [23]). Four to five sessions of sclerotherapy were required for attempted variceal obliteration in these children with biliary atresia. Eradication was reported in 73 %, with relapse of varices in 45 % and rebleeding in 2 of 22 children. Nearly 50 % of these children went on to liver transplantation with 12 months of the initial bleeding episode. Treatment and secondary prophylaxis of gastric varices in children are not well described. Balloon-occluded retrograde transvenous obliteration and endoscopic cyanoacrylate injection have been successfully employed in a limited number of children [24–27]. Twenty-one children with gastric varices were successfully treated with endoscopic injections of ~0.3 ml of a 1:1 mixture of n-butyl-2-cyanoacrylate and lipiodol. Initial rates of hemostasis were high, 96 %, although rebleeding events occurred in nearly half of the children often within one year of treatment [27].
The use of nonselective ß-blockers (NSBB) in the management of portal hypertension in children remains quite controversial and poorly informed by solid evidence of optimal approaches and efficacy. Propranolol is the most widely used agent in pediatrics, even though it is not approved for use in children by the US Food and Drug Administration for any indication, let alone for portal hypertension. Variable basal heart rate during normal development and difficulties in accurate measurement of heart rate in younger children have hampered the use of a standard reduction in heart rate as a guide to pediatric NSBB dosing. Hepatic venous pressure gradient has been measured in a limited number of children with some technical issues and not in support of assessing the potential efficacy of NSBB [28, 29]. Propranolol was used in combination with endoscopic secondary prophylaxis of variceal bleeding in 25 of 43 children [30]. In this nonrandomized retrospective analysis of clinical practice, there did not appear to be a major benefit of adding NSBB to endoscopic therapy in terms of recurrence of either varices or variceal hemorrhage.
In light of the relatively intact hepatic function and lack of significant comorbidities in many children who bleed from varices, portosystemic shunt surgery may be an interesting and underutilized approach. Distal splenorenal shunts were performed in 20 children, ten of whom had intrinsic liver disease [31]. Children selected for this approach had compensated liver disease manifest by an absence of significant ascites, an average INR of 1.3, and direct bilirubin of 0.5. The average age at shunt procedure was 11 years and with a mean follow-up of 3.5 years shunt patency was 100 %. No overt hepatic encephalopathy was noted although specific testing for minimal hepatic encephalopathy was not performed. A long-term risk of pulmonary complications of portosystemic shunting may exist in these patients, and ongoing monitoring after a successful procedure is probably warranted. Portosystemic shunt surgery for EHPVO was associated with a nonstatistically significant increase in the prevalence of minimal hepatic encephalopathy [32]. Long-term patency of these shunts has been demonstrated in children with EHPVO [33]. With the advent of polytetrafluoroethylene-coated endografts, one also wonders about the utility of transjugular intrahepatic portosystemic shunting as a method of secondary prophylaxis [34–36]. In a cohort, primarily consisting of teenagers with chronic liver disease, there was 100 % success in shunt placement [34]. Pressure gradients fell as would be expected from ~16 to ~6 mmHg. Varices were coil embolized in five children. In midterm follow-up, patency was high at mean follow-up of 20 months. One child developed encephalopathy. No revisions of shunts were required and there was a small increase in platelet counts after the procedure.
Clinical decision-making related to secondary prophylaxis of variceal hemorrhage in children with EHPVO is unique due to the availability and success of the mesoRex bypass procedure [37]. In this interesting procedure, the extrahepatic portal vein thrombosis is typically bypassed using a jugular vein graft connecting the superior mesenteric vein to the intrahepatic left portal vein within the Rex recessus (Fig. 29.2). This is distinct from a shunting procedure as it restores normal blood flow to the liver and is not associated with portosystemic shunting. When successful, this procedure reverses many of the abnormalities associated with EHPVO. In a retrospective comparison of mesoRex bypass to distal splenorenal shunting, significantly better improvement in thrombocytopenia, coagulopathy, and hyperammonemia were observed in children who underwent the mesoRex procedure. In some cases, anastomotic stenosis requires endovascular dilatation [38]. Neurocognitive testing has been previously shown to be better after mesoRex compared to distal splenorenal shunting [39].
Diagram of mesoRex bypass diagram of the mesoRex bypass procedure (Reproduced with permission from the publisher, Emre et al. [31])
The response to mesoRex bypass procedures suggests a remarkable plasticity of the intrahepatic portal system. This plasticity is no more evident than in recent and fascinating clinical experiences with congenital portosystemic shunts. Congenital portosystemic shunts, also known as Abernethy malformation, are rare vascular malformations where there is direct shunt from the portal to the systemic circulation [40]. These malformations are likely the result of lack of appropriate developmental changes in fetal mesenteric vasculature. The clinical sequelae of these rare malformations are related to portosystemic shunting directly and not from intrinsic liver disease or portal hypertension per se. Hepatopulmonary and pulmonary hypertension are relatively frequent clinical manifestations of this disorder [41–44]. The development of liver tumors with potential for malignant transformation is an important complication of abnormal portal blood flow in these children. In many cases, there is no apparent extrahepatic portal vein – even when the congenital shunt is temporarily balloon occluded. Liver transplantation has been performed in some cases, and review of explanted liver may reveal an absence of intrahepatic portal vein structures [45]. It would be reasonable to presume that closure of these shunts would lead to intractable and severe portal hypertension. Surprisingly, this is not the case [41]. Staged closure of congenital portosystemic shunts is associated with development of intrahepatic portal blood flow [41, 46]. The staging typically includes interventional or operative shunt narrowing that is associated with a temporary increase in portal pressure. It is unclear if this increase in portal pressure is the key factor leading to remodeling of the portal vasculature. After a few months, with development of the intrahepatic portal venous system, complete occlusion can be undertaken. This approach can lead to resolution of sequelae of portosystemic shunting including decrease of liver tumor size, resolution of hepatopulmonary syndrome, and stabilization of pulmonary hypertension. This unique pediatric experience indicates a heretofore unappreciated plasticity of the portal vasculature in children.
There is a remarkable paucity of high-quality reported literature on the event of acute variceal hemorrhage in children. Endoscopic information has been presented, but details of clinical course and related morbidity are almost nonexistent in the pediatric literature. Mortality after variceal hemorrhage can be extracted from a number of published experiences, although strict application of Baveno definitions related to timing is not generally employed. This information is absolutely critical for informed decision-making related to primary prophylaxis, yet the data is primarily unavailable.
The utility of primary prophylaxis of variceal hemorrhage in children is controversial [23, 47, 48]. Surveys of clinical experts demonstrate this controversy [49]. There have been several recent reports of primary prophylaxis in pediatrics. A Finnish study focused on 47 children with biliary atresia [47]. The plan was to begin surveillance and intervention at 12 months of age. Six children had bled before primary prophylaxis could be initiated. In 16 children, endoscopic sclerotherapy was initiated – four subsequently had variceal hemorrhage. The risk of developing varices and bleeding from those varices was highly related to the response to the Kasai procedure performed for the underlying diagnosis of biliary atresia. In those whose jaundice did not clear, defined by a cutoff total bilirubin of 40 μM, the odds ratio of bleeding was 17. In a similar experience, 36 children with biliary atresia underwent primary endoscopic prophylaxis at a mean age of 22 month and weight of 11 kg [23]. Sclerotherapy was required in 21 of the children. Interestingly, the mean platelet count in these infants and young children with varices was 167,000. Four endoscopic treatments were required, with early rebleeding occurring in only two patients and rebleeding in only four. Varices relapsed in 13. Of great interest in this cohort was the finding that survival with native liver was nearly identical in those who underwent primary or secondary prophylaxis. For biliary atresia, one of the competing therapies is liver transplantation. Some suggest that liver transplantation is indicated for children with biliary atresia who have poor bile flow after the Kasai hepatoportoenterostomy [50]. This recognizes the relatively poor short-term prognosis for these children [51]. Many of the children who have required early primary prophylaxis for varices are those with biliary atresia and poor bile flow after Kasai hepatoportoenterostomy. One wonders if liver transplantation may be a better approach for these children [48]. A single-center experience from Kolkata has reported the use of NSBB for primary prophylaxis of variceal hemorrhage [52]. Sixty-two children with varices, 41 of whom had sinusoidal disease, were randomized to either propranolol or carvedilol. In a 2-year follow-up, only three children had variceal hemorrhage. No major difference in response to one therapy over another could be determined, although there may be theoretical and technical advantages to the use of carvedilol.
Significant efforts have led to advances in determining methods to predict the presence of and risk of bleeding from varices in children with portal hypertension. All of these investigations require surveillance endoscopy for the gold standard assessment of the presence or absence of esophageal varices. Interestingly, the number of these studies is much greater than reports of primary prophylaxis. Simple assessments like spleen size and platelet counts can be informative as a predictor of varices [53, 54]. Platelet count may not be informative in younger children for reasons that are not clear. Spleen size may be difficult to standardize as a measure and must be normalized to age-specific criteria. Clinical prediction rules have been developed to predict the presence of varices [55–57]. Parameters that are typically assessed include AST, platelet count, albumin, and spleen maximal linear dimension by sonography. In general, platelet count and spleen size measurements are fairly good predictors of varices. More complex predictor rules do not add a great deal to the predictive power. AUROCs for most of these parameters range between 0.70 and 0.84. Liver stiffness as measured by transient elastography has also been investigated for its utility to predict varices in children with biliary atresia [58–60]. Children with varices typically have liver stiffness in the range of 17–38 kPa, while those without were in the range of 8–12 kPa. Spleen stiffness is being investigated as an alternative assessment [61]. Endoscopic findings that predict risk of bleeding in children are not well described overall. Red markings, gastric varices along the cardia, and varix size are predictive of subsequent variceal hemorrhage in children with biliary atresia [62, 63]. In one study, large varices were defined by their response to insufflation, with large varices (grade II and III) being those that did not flatten in response to insufflation [62].
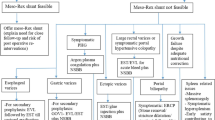
Despite significant progress since Baveno V, clinicians caring for children with portal hypertension face difficult clinical decision-making. Strict evidence-based decisions are difficult to derive. Numerous summaries have been written, and concerted efforts to provide expert pediatric-oriented opinion on Baveno IV and V have been published [3, 64, 65]. In light of the current available information, a personal biased set of recommendations for the approach to biliary atresia and extrahepatic portal vein obstruction is presented in Table 29.3. For each disease, there are critical clinical parameters that influence decisions. For biliary atresia, the early response to the Kasai hepatoportoenterostomy is critical. In children where the surgery has not worked, as manifest by poor bile drainage, near-term prognosis is poor and liver transplantation should be actively considered. In this case, there may not be a role for surveillance, and if possible, liver transplantation would serve as primary prophylaxis. Secondary prophylaxis would typically include endoscopic therapy with subsequent liver transplantation. In children with good bile flow after surgery, the decision-making is more complicated. My own personal bias is against surveillance and primary prophylaxis, although expert clinicians do both along the lines of recommendations for adults. Secondary prophylaxis would be predominantly endoscopic with consideration for the use of distal splenorenal shunting for those with intractable problems and good hepatic reserve. For EHPVO, a key issue is whether the intrahepatic portal vasculature is patent, i.e., favorable for mesoRex bypass. When there is favorable anatomy, strong consideration for early mesoRex bypass should be given. Surveillance in this case that reveals varices may be an indication for the surgery as primary prophylaxis. In the case of secondary prophylaxis when bleeding is the initial presenting problem, endoscopic therapy is typically a primary approach with mesoRex bypass as a definitive and favorable therapy. Decision-making in those with an unfavorable anatomy is more complicated. One of the amazing complexities of pediatrics is what to do with the myriad of other pediatric diseases that have their own special clinical issues. A complete understanding of the natural history of the particular disease along with understanding the pros and cons of potential interventions in children is critical for relatively informed decision-making.
References
de Franchis R (2010) Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 53:762–768
Ling S, Shneider B (2011) Portal hypertension in children. Current practice and the need for evidence. In: de Franchis R (ed) Proceedings of the fifth Baveno international consensus workshop. Wiley, Hoboken, pp 189–196
Shneider BL, Bosch J, de Franchis R, Emre SH, Groszmann RJ, Ling SC, Lorenz JM et al (2012) Portal hypertension in children: expert pediatric opinion on the report of the Baveno V Consensus Workshop on Methodology of Diagnosis and Therapy in Portal Hypertension. Pediatr Transplant 16:426–437
Ling SC, Walters T, McKiernan PJ, Schwarz KB, Garcia-Tsao G, Shneider BL (2011) Primary prophylaxis of variceal hemorrhage in children with portal hypertension: a framework for future research. J Pediatr Gastroenterol Nutr 52:254–261
Elder RW, McCabe NM, Hebson C, Veledar E, Romero R, Ford RM, Mahle WT et al (2013) Features of portal hypertension are associated with major adverse events in Fontan patients: the VAST study. Int J Cardiol 168:3764–3769
Franchi-Abella S, Fabre M, Mselati E, De Marsillac ME, Bayari M, Pariente D, Jacquemin E et al (2014) Obliterative portal venopathy: a study of 48 children. J Pediatr 165:190–193, e192
Srinath A, Shneider BL (2012) Congenital hepatic fibrosis and autosomal recessive polycystic kidney disease. J Pediatr Gastroenterol Nutr 54:580–587
Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, Daryanani KT et al (2013) Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology 144:112–121, e112
Ferri PM, Ferreira AR, Fagundes ED, Liu SM, Roquete ML, Penna FJ (2012) Portal vein thrombosis in children and adolescents: 20 years experience of a pediatric hepatology reference center. Arq Gastroenterol 49:69–76
Shneider BL, Abel B, Haber B, Karpen SJ, Magee JC, Romero R, Schwarz K et al (2012) Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 55:567–573
Teckman JH, Rosenthal P, Abel R, Bass LM, Michail S, Murray KF, Rudnick DA et al (2015) Baseline analysis of a young alpha-1-AT deficiency liver disease cohort reveals frequent portal hypertension. J Pediatr Gastroenterol Nutr 61(1):94–101
Hoerning A, Raub S, Neudorf U, Muntjes C, Kathemann S, Lainka E, Stehling F et al (2014) Pulse oximetry is insufficient for timely diagnosis of hepatopulmonary syndrome in children with liver cirrhosis. J Pediatr 164:546–552, e541–542
Sari S, Oguz D, Sucak T, Dalgic B, Atasever T (2012) Hepatopulmonary syndrome in children with cirrhotic and non-cirrhotic portal hypertension: a single-center experience. Dig Dis Sci 57:175–181
Ridaura-Sanz C, Mejia-Hernandez C, Lopez-Corella E (2009) Portopulmonary hypertension in children. A study in pediatric autopsies. Arch Med Res 40:635–639
Whitworth JR, Ivy DD, Gralla J, Narkewicz MR, Sokol RJ (2009) Pulmonary vascular complications in asymptomatic children with portal hypertension. J Pediatr Gastroenterol Nutr 49:607–612
Krishna YR, Yachha SK, Srivastava A, Negi D, Lal R, Poddar U (2010) Quality of life in children managed for extrahepatic portal venous obstruction. J Pediatr Gastroenterol Nutr 50:531–536
D’Antiga L, Dacchille P, Boniver C, Poledri S, Schiff S, Zancan L, Amodio P (2014) Clues for minimal hepatic encephalopathy in children with noncirrhotic portal hypertension. J Pediatr Gastroenterol Nutr 59:689–694
Zargar SA, Javid G, Khan BA, Yattoo GN, Shah AH, Gulzar GM, Singh J et al (2002) Endoscopic ligation compared with sclerotherapy for bleeding esophageal varices in children with extrahepatic portal venous obstruction. Hepatology 36:666–672
Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, Brady JE et al (2014) Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology 120:1319–1332
Olsen EA, Brambrink AM (2013) Anesthesia for the young child undergoing ambulatory procedures: current concerns regarding harm to the developing brain. Curr Opin Anaesthesiol 26:677–684
Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A et al (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130:e476–e485
Kim SJ, Oh SH, Jo JM, Kim KM (2013) Experiences with endoscopic interventions for variceal bleeding in children with portal hypertension: a single center study. Pediatr Gastroenterol Hepatol Nutr 16:248–253
Duche M, Ducot B, Ackermann O, Baujard C, Chevret L, Frank-Soltysiak M, Jacquemin E et al (2013) Experience with endoscopic management of high-risk gastroesophageal varices, with and without bleeding, in children with biliary atresia. Gastroenterology 145:801–807
Takahashi T, Yoshida H, Mamada Y, Taniai N, Tajiri T (2009) Balloon-occluded retrograde transvenous obliteration for gastric varices in a child with extrahepatic portal venous obstruction. J Nippon Med Sch 76:173–178
Rosen D, Chu J, Patel R, Moon J, Iyer K, Arnon R (2014) Balloon-occluded retrograde transvenous obliteration for recurrent fundal gastric variceal bleeding in an adolescent. Pediatr Transplant 18:E193–E196
Saad WE, Anderson CL, Patel RS, Schwaner S, Caldwell S, Pelletier S, Angle J et al (2015) Management of gastric varices in the pediatric population with balloon-occluded retrograde transvenous obliteration (BRTO) utilizing sodium tetradecyl sulfate foam sclerosis with or without partial splenic artery embolization. Cardiovasc Intervent Radiol 38:236–241
Oh SH, Kim SJ, Rhee KW, Kim KM (2015) Endoscopic cyanoacrylate injection for the treatment of gastric varices in children. World J Gastroenterol 21:2719–2724
Miraglia R, Luca A, Maruzzelli L, Spada M, Riva S, Caruso S, Maggiore G et al (2010) Measurement of hepatic vein pressure gradient in children with chronic liver diseases. J Hepatol 53:624–629
Woolfson J, John P, Kamath B, Ng VL, Ling SC (2013) Measurement of hepatic venous pressure gradient is feasible and safe in children. J Pediatr Gastroenterol Nutr 57:634–637
dos Santos JM, Ferreira AR, Fagundes ED, Ferreira AP, Ferreira LS, Magalhaes MC, Bittencourt PF et al (2013) Endoscopic and pharmacological secondary prophylaxis in children and adolescents with esophageal varices. J Pediatr Gastroenterol Nutr 56:93–98
Emre S, Dugan C, Frankenberg T, Hudgins LC, Gagliardi R, Artis AT, Rodriguez-Laiz G et al (2009) Surgical portosystemic shunts and the Rex bypass in children: a single-centre experience. HPB (Oxford) 11:252–257
Srivastava A, Yadav SK, Lal R, Yachha SK, Thomas MA, Saraswat VA, Gupta RK (2010) Effect of surgical portosystemic shunt on prevalence of minimal hepatic encephalopathy in children with extrahepatic portal venous obstruction: assessment by magnetic resonance imaging and psychometry. J Pediatr Gastroenterol Nutr 51:766–772
Sharma N, Bajpai M, Kumar A, Paul S, Jana M (2014) Portal hypertension: a critical appraisal of shunt procedures with emphasis on distal splenorenal shunt in children. J Indian Assoc Pediatr Surg 19:80–84
Vo NJ, Shivaram G, Andrews RT, Vaidya S, Healey PJ, Horslen SP (2012) Midterm follow-up of transjugular intrahepatic portosystemic shunts using polytetrafluoroethylene endografts in children. J Vasc Interv Radiol 23:919–924
Di Giorgio A, Agazzi R, Alberti D, Colledan M, D’Antiga L (2012) Feasibility and efficacy of transjugular intrahepatic portosystemic shunt (TIPS) in children. J Pediatr Gastroenterol Nutr 54:594–600
Zurera LJ, Espejo JJ, Lombardo S, Gilbert JJ, Canis M, Ruiz C (2015) Safety and efficacy of expanded polytetrafluoroethylene-covered transjugular intrahepatic portosystemic shunts in children with acute or recurring upper gastrointestinal bleeding. Pediatr Radiol 45:422–429
Lautz TB, Keys LA, Melvin JC, Ito J, Superina RA (2013) Advantages of the meso-Rex bypass compared with portosystemic shunts in the management of extrahepatic portal vein obstruction in children. J Am Coll Surg 216:83–89
Lautz TB, Kim ST, Donaldson JS, Superina RA (2012) Outcomes of percutaneous interventions for managing stenosis after meso-Rex bypass for extrahepatic portal vein obstruction. J Vasc Interv Radiol 23:377–383
Mack CL, Zelko FA, Lokar J, Superina R, Alonso EM, Blei AT, Whitington PF (2006) Surgically restoring portal blood flow to the liver in children with primary extrahepatic portal vein thrombosis improves fluid neurocognitive ability. Pediatrics 117:e405–e412
Stringer MD (2008) The clinical anatomy of congenital portosystemic venous shunts. Clin Anat 21:147–157
Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY et al (2010) Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr 51:322–330
Law YM, Mack CL, Sokol RJ, Rice M, Parsley L, Ivy D (2011) Cardiopulmonary manifestations of portovenous shunts from congenital absence of the portal vein: pulmonary hypertension and pulmonary vascular dilatation. Pediatr Transplant 15:E162–E168
Kim MJ, Ko JS, Seo JK, Yang HR, Chang JY, Kim GB, Cheon JE et al (2012) Clinical features of congenital portosystemic shunt in children. Eur J Pediatr 171:395–400
Sokollik C, Bandsma RH, Gana JC, van den Heuvel M, Ling SC (2013) Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr 56:675–681
Emre S, Arnon R, Cohen E, Morotti RA, Vaysman D, Shneider BL (2007) Resolution of hepatopulmonary syndrome after auxiliary partial orthotopic liver transplantation in Abernethy malformation. A case report. Liver Transpl 13:1662–1668
Bruckheimer E, Dagan T, Atar E, Schwartz M, Kachko L, Superina R, Amir G et al (2013) Staged transcatheter treatment of portal hypoplasia and congenital portosystemic shunts in children. Cardiovasc Intervent Radiol 36:1580–1585
Lampela H, Kosola S, Koivusalo A, Lauronen J, Jalanko H, Rintala R, Pakarinen MP (2012) Endoscopic surveillance and primary prophylaxis sclerotherapy of esophageal varices in biliary atresia. J Pediatr Gastroenterol Nutr 55:574–579
Molleston JP, Shneider BL (2013) Preventing variceal bleeding in infants and children: is less more? Gastroenterology 145:719–722
Gana JC, Valentino PL, Morinville V, O’Connor C, Ling SC (2011) Variation in care for children with esophageal varices: a study of physicians’, patients’, and families’ approaches and attitudes. J Pediatr Gastroenterol Nutr 52:751–755
Shneider BL, Mazariegos GV (2007) Biliary atresia: a transplant perspective. Liver Transpl 13:1482–1495
Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, Bezerra J et al (2006) A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 148:467–474
Samanta T, Purkait R, Sarkar M, Misra A, Ganguly S (2011) Effectiveness of beta blockers in primary prophylaxis of variceal bleeding in children with portal hypertension. Trop Gastroenterol 32:299–303
Mo YH, Chen HL, Hsu WM, Peng SS (2012) Less-invasive MR indices of clinically evident esophageal variceal bleeding in biliary atresia patients. J Formos Med Assoc 111:482–488
Alcantara RV, Yamada RM, De Tommaso AM, Bellomo-Brandao MA, Hessel G (2012) Non-invasive predictors of esophageal varices in children and adolescents with chronic liver disease or extrahepatic portal venous obstruction. J Pediatr (Rio J) 88:341–346
Gana JC, Turner D, Mieli-Vergani G, Davenport M, Miloh T, Avitzur Y, Yap J et al (2011) A clinical prediction rule and platelet count predict esophageal varices in children. Gastroenterology 141:2009–2016
Adami MR, Ferreira CT, Kieling CO, Hirakata V, Vieira SM (2013) Noninvasive methods for prediction of esophageal varices in pediatric patients with portal hypertension. World J Gastroenterol 19:2053–2059
Isted A, Grammatikopoulos T, Davenport M (2015) Prediction of esophageal varices in biliary atresia: derivation of the “varices prediction rule”, a novel noninvasive predictor. J Pediatr Surg [Epub ahead of print]
Chang HK, Park YJ, Koh H, Kim SM, Chung KS, Oh JT, Han SJ (2009) Hepatic fibrosis scan for liver stiffness score measurement: a useful preendoscopic screening test for the detection of varices in postoperative patients with biliary atresia. J Pediatr Gastroenterol Nutr 49:323–328
Colecchia A, Di Biase AR, Scaioli E, Predieri B, Iughetti L, Reggiani ML, Montrone L et al (2011) Non-invasive methods can predict oesophageal varices in patients with biliary atresia after a Kasai procedure. Dig Liver Dis 43:659–663
Chongsrisawat V, Vejapipat P, Siripon N, Poovorawan Y (2011) Transient elastography for predicting esophageal/gastric varices in children with biliary atresia. BMC Gastroenterol 11:41
Goldschmidt I, Brauch C, Poynard T, Baumann U (2014) Spleen stiffness measurement by transient elastography to diagnose portal hypertension in children. J Pediatr Gastroenterol Nutr 59:197–203
Duche M, Ducot B, Tournay E, Fabre M, Cohen J, Jacquemin E, Bernard O (2010) Prognostic value of endoscopy in children with biliary atresia at risk for early development of varices and bleeding. Gastroenterology 139:1952–1960
Wanty C, Helleputte T, Smets F, Sokal EM, Stephenne X (2013) Assessment of risk of bleeding from esophageal varices during management of biliary atresia in children. J Pediatr Gastroenterol Nutr 56:537–543
Shneider B, Emre S, Groszmann R, Karani J, McKiernan P, Sarin S, Shashidhar H et al (2006) Expert pediatric opinion on the Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. Pediatr Transplant 10:893–907
Superina R, Shneider B, Emre S, Sarin S, de Ville de Goyet J (2006) Surgical guidelines for the management of extra-hepatic portal vein obstruction. Pediatr Transplant 10:908–913
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Shneider, B.L. (2016). Portal Hypertension in Pediatrics: Controversies and Challenges 2015 Report. In: de Franchis, R. (eds) Portal Hypertension VI. Springer, Cham. https://doi.org/10.1007/978-3-319-23018-4_29
Download citation
DOI: https://doi.org/10.1007/978-3-319-23018-4_29
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23017-7
Online ISBN: 978-3-319-23018-4
eBook Packages: MedicineMedicine (R0)