Abstract
Clinical features, images, complications, treatments, and prognosis of 10 children with congenital portosystemic shunt (CPSS) were reviewed. Nine children were diagnosed with intrahepatic shunts while one presented with extrahepatic shunt. CPSS was detected by prenatal ultrasonography in four infants. Three infants presented with galactosemia without an enzyme deficiency. Two children presented with mental retardation and attention deficit hyperactivity disorder. Pulmonary hypertension developed in two patients. Spontaneous closure occurred in four infants with intrahepatic shunts including patent ductus venosus. The shunts were closed using transcatheter embolizations in four patients with intrahepatic shunts. Conclusion: Intrahepatic shunts may close spontaneously. Transcatheter embolization is effective for the treatment of symptomatic intrahepatic shunts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital portosystemic shunt (CPSS) is a rare disorder that may cause intrauterine growth retardation (IUGR) [1], galactosemia [5, 7, 10, 15], neonatal cholestasis [15], and hepatic encephalopathy [14]. CPSS has potentials to develop hepatic tumors, hepatopulmonary syndrome [4], and pulmonary hypertension [12]. Ultrasonography (US) imaging allows for the detection of CPSS prenatally [1] and postnatally. Early detection and appropriate management are important for a good prognosis. Spontaneous closure of an intrahepatic shunt sometimes occurs [19]. Conservative therapy, surgery, and transcatheter embolization have been used for the treatment of CPSS. The aim of this study is to investigate clinical features and outcomes of 10 children with CPSS.
Materials and methods
We reviewed clinical features, laboratory data, imaging results, complications, therapies, and outcomes with regards to 10 children (seven males and three females) with CPSS who were admitted to Seoul National University Children’s Hospital between 1998 and 2010. The activities of galactokinase, galactose-1-phophate uridytranferase, and UDP-galactose-4-epimerase were measured in infants with galactosemia by newborn screening.
The diagnoses of CPSS were based on US or computed tomography (CT). Shunts arising from the main portal vein to inferior vena cava (IVC) were classified as extrahepatic shunts. Abnormal intrahepatic connections between a branch of the portal vein and hepatic veins or the IVC, including the patent ductus venosus were classified as intrahepatic shunts [16]. All patients were followed up every 3–6 months depending on the severity of their symptoms. US was performed every 2 months until the age of 1 year and then once a year. This study was conducted in accordance with the Helsinki Declaration and approved by Ethics Committee at Seoul National University Hospital.
Results
Clinical features and complications
The ages at diagnosis of CPSS ranged from 2 days to 7 years. Seven patients were diagnosed at an age younger than 1 month, and three were diagnosed when older than 2 years. There were one extrahepatic shunt and nine intrahepatic shunts including three patent ductus venosus. The clinical features, types of shunt, complications, treatments, and outcomes for the patients are summarized in Table 1.
Four neonates were referred for abnormal vascular communications between the portal and systemic venous system as detected by prenatal US. Neonatal cholestasis developed in one neonate with intrauterine diagnosis. Three infants presented with galactosemia detected during newborn screening. Blood galactose concentration was 16.6 to 25.0 mg/dL (normal <8 mg/dL). Enzyme activities were normal, and galactosemia was improved through use of lactose-free formula. Neonatal cholestasis was discovered in two infants with galactosemia. IUGR was noted in two infants. Hyperammonemia was found in eight patients. Two children, aged 3 and 4 years, presented with mental retardation and attention deficit hyperactivity disorder, respectively. Brain magnetic resonance imaging (MRI) in both patients showed a high signal on T1-weighted images in the basal ganglia. One child presented with splenomegaly at an age of 2 years. Echocardiography was performed in six patients. Three patients had an atrial septal defect, and one had mild left pulmonary artery stenosis. One had a hypoplastic intrahepatic IVC with prominent azygos and hemiazygos vein. Two patients developed pulmonary hypertension at the time of diagnosis or during the follow-up. Cardiac catheterizations showed that mean pulmonary artery pressure was 36 and 31 mmHg in case 6 and 10, respectively. These patients were treated with sildenafil and bosentan.
Of the five patients who underwent brain MRI, case 6 had a severely hypoplastic right internal carotid artery that was communicating with the right vertebral artery via the proatlantal intersegmental artery. One patient had cutaneous hemangioma in the right thigh.
Treatments and prognosis
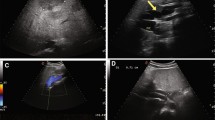
Spontaneous closure occurred in four patients with intrahepatic shunts including one patent ductus venosus between the ages of 2 and 9 months. Case 3 had an intrahepatic shunt between bifurcation of the portal vein and IVC. Portal hypertension and the hypoplastic right portal vein were noted with temporary occlusion test. Spontaneous closure of the shunt occurred at the age of 9 months (Fig. 1).
Spontaneous closure of an intrahepatic shunt in case 3. a US shows a direct communication between the portal vein bifurcation and the IVC (arrow); b IVC venogram demonstrates a shunt between the IVC and the portal vein (arrowheads). The hypoplastic right portal vein was noted (arrow); c color Doppler US imaging shows disappeared communication between the portal vein and the IVC
In four patients, the CPSS was closed using endovascular methods with coils, vascular plugs, or umbrella devices (Fig. 2). Early in our study, transcatheter embolizations were performed in two neonates with persistent hyperammonemia. Endovascular closure of symptomatic CPSS was successful in two children who displayed pulmonary hypertension and hepatic encephalopathy at ages between 5 and 8 years. In case 10, cognitive function improved and pulmonary hypertension regressed 1 year after successful shunt closure.
Transcatheter embolization of a patent ductus venosus in case 5. a Contrast-enhanced CT demonstrates shows a communication channel between umbilical segment of the left portal vein and the IVC suggesting patent ductus venosus (arrow); b retrograde portal venogram obtained via patent ductus venosus demonstrates patent portal venous system; c CT scan obtained 6 months after endovascular closure demonstrates complete closure of the shunt
Case 6 had an intrahepatic shunt between the right portal vein and the IVC. Catheter embolization of the shunt was attempted using a vascular plug at an age of 10 years. With the occlusion of the shunt, severe abdominal pain developed and intrahepatic portal system was not visualized. The vascular plug was removed immediately. Case 8 had a short and wide extrahepatic shunt between the main portal vein and the IVC, which was considered difficult to close by endovascular methods. Staged surgical closure was planned in these patients.
Discussion
In this study, CPSS was the cause of galactosemia in three neonates as detected by mass screening. It has been reported that hypergalactosemia without an enzyme deficiency can be caused by CPSS [5, 7, 10]. In these cases, galactose is not effectively extracted from portal blood flow in the liver because of mesenteric and splenic venous blood flow directly into the systemic veins via a CPSS. Hepatic imaging to detect CPSS should be included during evaluation of hypergalactosemia without an enzyme deficiency. In the absence of other complications, no treatment other than galactose elimination diet is indicated [13].
In addition to hypergalactosemia, prenatal US is an effective method for the recognition of CPSS [1]. Four patients in our series had a history of anomalous communications between the portal and systemic venous systems as discovered by prenatal US. It is important for the gynecologist to be aware of the significance of this anomaly. When CPSS is diagnosed by prenatal US, follow-up imaging studies should be performed after birth. Two infants in this study displayed IUGR, which may be caused by decreased liver perfusion in fetuses with CPSS [1].
CPSSs have been classified as extrahepatic and intrahepatic [16]. Extrahepatic shunts are end-to-side shunts in which the portal vein terminates in the IVC or side to side shunts where there is a venous communication between a patent portal vein and the IVC. Intrahepatic shunts are abnormal intrahepatic connections between branches of the portal vein and the hepatic vein/IVC or a persistent patent ductus venosus. Small intrahepatic shunts sometimes disappear spontaneously by the age of 1 or 2 years [4, 19]. The large shunts as well as the extrahepatic shunts persist throughout life and carry risks of complications [4].
US, CT, and MRI are useful for the diagnosis of CPSS. Color Doppler US has been reported to be the modality of choice for the diagnosis of CPSS [11]. Doppler US has been used to detect the abnormal communication between the portal vein and systemic venous system, as well as to plan the treatment by determining the shunt ratio [19]. US can detect the shunt anatomy but cannot assess the morphology of intrahepatic portal veins, suggesting that CT or MRI may be required [7, 8]. Owing to the rarity and lack of obvious symptoms of CPSS, incidental findings in imaging studies are important for the diagnosis of this disorder. Unless this disease is not taken into account by radiologists, CPSS with or without complications may remain unrecognized.
Because portal blood bypasses the liver through the abnormal vessel, this shunting can induce hyperammonemia with or without hepatic encephalopathy. Among our eight patients with hyperammonemia, two children presented with signs of hepatic encephalopathy, including mental retardation and attention deficit hyperactivity disorder. In one of our children, cognitive function improved and abnormal signals in brain MRI images disappeared after occlusion treatment. Disturbance of consciousness, tremors, disorientation, mental retardation, and learning difficulties are caused by portosystemic shunt [3, 18]. CPSS can also cause neonatal cholestasis, benign or malignant liver tumor, congestive heart failure, and hepatopulmonary syndrome [4, 8, 9]. Ohno et al. [12] reported that thromboembolitic pulmonary arterial hypertension was a critical complication in CPSS. Pulmonary hypertension was identified in two of our patients by cardiac catheterization. Pulmonary hypertension diminished in one patient with successful occlusion of the shunt.
In this study, one patient had a severely hypoplastic right internal carotid artery and the other had a hypoplastic intrahepatic IVC. Vascular anomalies associated with CPSS included interruption of IVC, splenic artery aneurysm [8], and coarctation of aorta [4]. Three children in this series had an atrial septal defect and one had a cutaneous hemangioma. Congenital heart disease was reported in 30% of CPSS cases [4] and cutaneous hemangioma [13] was associated with CPSS.
The management of CPSS is controversial. Although spontaneous closure of CPSS can be expected to occur until about the age of 2 years, close observation is necessary for the detection of neuropsychiatric symptoms and pulmonary hypertension. Closure of the shunt persisting after age 2 years should be considered because CPSS has risks of severe complications in children [6]. For symptomatic CPSS, surgical ligation or transcatheter embolization is a therapeutic option. Transcatheter embolization is safer and less invasive than surgery. Retrograde transcaval obliteration is recommended as the first choice of treatment for symptomatic CPSS [18]. However, portal hypertension and dislodgement of coils into the systemic circulation are potential complications of transcatheter coil embolization. Compared with embolization coils, vascular plugs can be positioned more precisely, repositioned, and removed if necessary [2]. Catheter embolization was attempted in five patients with intrahepatic shunts, and occlusion was successful without complication in four. Although early in our study transcatheter embolization was successful in two neonates, close observations until the age of 2 years may be a more appropriate choice. In this study, a vascular plug was removed due to abdominal pain and hypoplastic intrahepatic portal systems. Complete occlusion of the shunt in patients with a severely hypoplastic portal vein could result in acute portal hypertension and may lead to edema or necrosis of the colon [17]. Balloon occlusion test is useful before the closure of the shunt by measuring portal pressure while transiently occluding the shunt [6]. Extrahepatic shunts arising from the main portal vein are short and wide in diameter, making endovascular closure unsuccessful and require staged surgical closure [9]. Banding of the shunt might result in increased intrahepatic portal branches [6]. If the portal pressure is too high to allow closure in one step, temporary surgical banding of the shunt allows sufficient intrahepatic portal expansion to permit definite closure after verifying that intrahepatic portal flows have developed satisfactorily [4, 6].
In summary, spontaneous closure of intrahepatic shunts may occur and CPSS can lead to neuropsychiatric symptoms and pulmonary hypertension in childhood. Transcatheter embolization is effective for the treatment of symptomatic intrahepatic shunts.
References
Delle Chiaie L, Neuberger P, Von Kalle T (2008) Congenital intrahepatic portosystemic shunt: prenatal diagnosis and possible influence on fetal growth. Ultrasound Obstet Gynecol 32:233–235
Evans WN, Galindo A, Acherman RJ, Rothman A, Berthoty DP (2009) Congenital portosystemic shunts and AMPLATZER vascular plug occlusion in newborns. Pediatr Cardiol 30:1083–1088
Ferrero GB, Porta F, Biamino E, Mussa A, Garelli E, Chiappe F, Veltri A, Silengo MC, Gennari F (2010) Remittent hyperammonemia in congenital portosystemic shunt. Eur J Pediatr 169:369–372
Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY, Pariente D, Gauthier F, Jacquemin E, Bernard O (2010) Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr 51:322–330
Gitzelmann R, Forster I, Willi UV (1997) Hypergalactosaemia in a newborn: self-limiting intrahepatic portosystemic venous shunt. Eur J Pediatr 156:719–722
Kamata S, Kitayama Y, Usui N, Kuroda S, Nose K, Sawai T, Okada A (2000) Patent ductus venosus with a hypoplastic intrahepatic portal system presenting intrapulmonary shunt: a case treated with banding of the ductus venosus. J Pediatr Surg 35:655–657
Kono T, Hiki T, Kuwashima S, Hashimoto T, Kaji Y (2009) Hypergalactosemia in early infancy: diagnostic strategy with an emphasis on imaging. Pediatr Int 51:276–282
Konstas AA, Digumarthy SR, Avery LL, Wallace KL, Lisovsky M, Misdraji J, Hahn PF (2010) Congenital portosystemic shunts: imaging findings and clinical presentations in 11 patients. Eur J Radiol [Epub ahead of print]
Lautz TB, Tantemsapya N, Rowell E, Superina RA (2011) Management and classification of type II congenital portosystemic shunts. J Pediatr Surg 46:308–314
Nishimura Y, Tajima G, Dwi Bahagia A, Sakamoto A, Ono H, Sakura N, Naito K, Hamakawa M, Yoshii C, Kubota M, Kobayashi K, Saheki T (2004) Differential diagnosis of neonatal mild hypergalactosaemia detected by mass screening: clinical significance of portal vein imaging. J Inherit Metab Dis 27:11–18
Oguz B, Akata D, Balkanci F, Akhan O (2003) Intrahepatic portosystemic venous shunt: diagnosis by colour/power Doppler imaging and three-dimensional ultrasound. Br J Radiol 76:487–490
Ohno T, Muneuchi J, Ihara K, Yuge T, Kanaya Y, Yamaki S, Hara T (2008) Pulmonary hypertension in patients with congenital portosystemic venous shunt: a previously unrecognized association. Pediatrics 121:e892–899
Ono H, Mawatari H, Mizoguchi N, Eguchi T, Sakura N (1998) Clinical features and outcome of eight infants with intrahepatic porto-venous shunts detected in neonatal screening for galactosaemia. Acta Paediatr 87:631–634
Park JH, Cha SH, Han JK, Han MC (1990) Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol 155:527–528
Sakura N, Mizoguchi N, Eguchi T, Ono H, Mawatari H, Naitou K, Ito K (1997) Elevated plasma bile acids in hypergalactosaemic neonates: a diagnostic clue to portosystemic shunts. Eur J Pediatr 156:716–718
Stringer MD (2008) The clinical anatomy of congenital portosystemic venous shunts. Clin Anat 21:147–157
Takehara Y, Mori K, Edagawa T, Sugimoto M, Takehara H, Ito M, Kuroda Y (2004) Presumed hypoplastic intrahepatic portal system due to patent ductus venosus: importance of direct occlusion test of ductus venosus under open laparotomy. Pediatr Int 46:484–486
Tanoue S, Kiyosue H, Komatsu E, Hori Y, Maeda T, Mori H (2003) Symptomatic intrahepatic portosystemic venous shunt: embolization with an alternative approach. AJR Am J Roentgenol 181:71–78
Uchino T, Matsuda I, Endo F (1999) The long-term prognosis of congenital portosystemic venous shunt. J Pediatr 135:254–256
Acknowledgments
This study was supported by grant no. 04–20071070 from the Seoul National University Hospital Research Fund.
Conflict of interest
There was no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.J., Ko, J.S., Seo, J.K. et al. Clinical features of congenital portosystemic shunt in children. Eur J Pediatr 171, 395–400 (2012). https://doi.org/10.1007/s00431-011-1564-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-011-1564-9