Abstract
Purpose
Congenital portosystemic shunts (CPSS) with portal venous hypoplasia cause hyperammonemia. Acute shunt closure results in portal hypertension. A transcatheter method of staged shunt reduction to afford growth of portal vessels followed by shunt closure is reported.
Methods
Pressure measurements and angiography in the CPSS or superior mesenteric artery (SMA) during temporary occlusion of the shunt were performed. If vessels were diminutive and the pressure was above 18 mmHg, a staged approach was performed, which included implantation of a tailored reducing stent to reduce shunt diameter by ~50 %. Recatheterization was performed approximately 3 months later. If the portal pressure was below 18 mmHg and vessels had developed, the shunt was closed with a device.
Results
Six patients (5 boys, 1 girl) with a median age of 3.3 (range 0.5–13) years had CPSS portal venous hypoplasia and hyperammonemia. Five patients underwent staged closure. One patient tolerated acute closure. One patient required surgical shunt banding because a reducing stent could not be positioned. At median follow-up of 3.8 (range 2.2–8.4) years, a total of 21 procedures (20 transcatheter, 1 surgical) were performed. In all patients, the shunt was closed with a significant reduction in portal pressure (27.7 ± 11.3 to 10.8 ± 1.8 mmHg; p = 0.016), significant growth of the portal vessels (0.8 ± 0.5 to 4.0 ± 2.4 mm; p = 0.037), and normalization of ammonia levels (202.1 ± 53.6 to 65.7 ± 9.6 μmol/L; p = 0.002) with no complications.
Conclusion
Staged CPSS closure is effective in causing portal vessel growth and treating hyperammonemia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abnormalities of the developing vitelline venous fetal circulation can result in a congenital portosystemic shunt (CPSS), also referred to as the Abernethy malformation [1, 2]. This bypass of the liver causes postprandial hyperammonemia [3, 4] and other disturbances of liver function including hepatitis, nodular hyperplasia, hepatopulmonary syndrome, and malignancy [1, 3–10]. CPSS is often associated with diminished flow to the fetal portal system, and the resultant portal venous hypoplasia (PVH) of these vessels prohibits acute transcatheter and surgical closure of the shunt because this would lead to portal hypertension [1, 2, 7–9]. These patients usually require a surgical reduction of the shunt or a liver transplant [11–17].
We report on our methods and results in a series of consecutive patients with CPSS PVH who underwent percutaneous transcatheter reduction of the shunt to afford gradual growth of the portal venous vessels followed by complete closure of the shunt.
Materials and Methods
Patients
All patients with a diagnosis of CPSS PVH who underwent an attempt at transcatheter closure at our institution were identified from the cardiac catheterization/interventional radiology database. Patient data, procedural characteristics, hemodynamic and angiographic findings, and clinical status were recorded from the patient records. All patients were included in the study. The retrospective study was approved by the institutional review board. Informed written consent was obtained before the procedure.
Catheterization
The procedure was performed under general anesthesia as a result of patient age. Sheaths (4–6F, Cordis, Roden, The Netherlands) were placed percutaneously to the femoral vessels and the right internal jugular vein; access to the latter was to afford an easier approach to the often acutely angled shunt or hepatic vessels. After heparinization (100 IU/kg), hemodynamic and angiographic evaluations were performed. Angiography was performed directly in the CPSS, or a selective digitally subtracted angiogram was performed in the superior mesenteric artery to demonstrate the venous drainage of the gut. A catheter (typically a 5F Marker pigtail; Merit Medical, South Jordan, UT) was then advanced in to the shunt, which was occluded temporarily by a compliant balloon (Tyshak; NuMED, Hopkinton, NY) at the site of common drainage. This catheter was used to assess portal pressure before and during balloon occlusion and also to perform power angiography during occlusion of the shunt to identify and measure the portal vessels. If the pressure did not rise above a mean of 18 mmHg and normal portal vessels were identified, the shunt was closed. If diminutive vessels were present and the pressure rose above 18 mmHg, a staged approach was adopted.
Staged Reduction Method
At the site of common drainage, a tailor-made reducing stent with a diameter ~50–60 % of the CPSS, depending on the degree of PVH, was implanted, reducing stent formation. The largest diameter of the shunt was measured with a calibrated catheter or sizing balloon. A large-diameter bare metal stent (Genesis; Cordis, Roden, The Netherlands) or PTFE-covered stent (CP; NuMED, Hopkinton, NY) was expanded on a balloon outside the patient. A 2-0 Prolene or silk suture was stitched through the struts in the midportion of the stent. The stent was then crimped onto an inflated high-pressure balloon of a diameter ~50–60 % of the shunt, and a knot was tied and the extra suture tissue removed. This stent was then crimped onto a low-pressure balloon of a diameter similar to the shunt. An 11F long sheath (Mullins, Cook, Bloomington, IN) was advanced into the shunt from the right internal jugular vein. The stent was advanced through the sheath and when in position was gradually expanded with an inflation device so that the edges of the stent apposed the shunt vessel walls while the center remained narrow. Care was taken not to overinflate the balloon and tear the suture knot (Fig. 1). The position and the effect of the reducing stent on flow were assessed by angiography, and the portal pressure was recorded. If diminutive portal vessels were present and the pressure distal to the reducing stent rose above 18 mmHg, a staged approach was adopted. The patients were followed with blood tests for postprandial ammonia levels and duplex ultrasound of the portal vessels. Once a significant change was noted, the patient was recatheterized and the procedure was repeated. If the portal pressure was less than 18 mmHg during balloon occlusion, then the shunt was closed by placing an Amplatzer occluder (AGA Medical, Golden Valley, MN) in the narrowed stent. If the portal pressure was high, then the patient was either followed and recatheterized at a later date or a second reducing stent was implanted in the first.
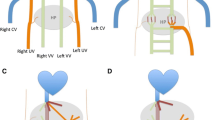
A Injection of contrast in the Abernethy malformation; no portal vessels are seen. B Power angiography during balloon occlusion demonstrates small portal vessels. C Reducing stent expanded in Abernethy malformation, lateral view. D Injection of contrast after reducing stent shows mild fill of the small portal vessels. E Angiography without occlusion 5 months later demonstrates a normal portal system with significant portal vessel growth as compared to A and B
Results
Between February 2002 and July 2010, six patients (Table 1) of median age 3.3 (range 0.5–13.1) years underwent transcatheter evaluation and treatment for CPSS PVH and hyperammonemia. Five of these patients had direct connections between the common portal vein and the inferior vena cava (Fig. 1), one of whom had two smaller additional connections. One patient had hepatopulmonary syndrome. One infant had a large congenital splenorenal shunt (Fig. 2). Initial postprandial ammonia levels were 202.1 ± 53.6 μmol/L. Temporary balloon occlusion of the CPSS was achieved in all patients with a mean portal pressure of 27.7 ± 11.3 mmHg. The mean initial central portal vein diameter was 0.8 ± 0.5 mm (Table 2).
A Catheter advanced from the right internal jugular vein to the inferior vena cava and the left renal vein. Contrast injection in the splenorenal shunt demonstrates the splenic vein, and no portal venous system is seen. B Balloon occlusion of the renal vein and splenorenal shunt with injection of contrast in the shunt demonstrates an extremely hypoplastic portal venous system. C Late phase of digital subtraction angiography in the superior mesenteric artery demonstrates a normal portal venous system 4 months after banding of the shunt
A reducing stent was implanted in four patients, and the CPSS was completely closed in one with an Amplatzer PDA device because the portal pressure was 18 mmHg. The infant with multiple portal–inferior vena cava connections initially underwent closure of the largest connection after test balloon occlusion. Mean fluoroscopy time was 25.6 min.
The infant with the splenorenal CPSS required surgical banding of the shunt because it was too short to position a reducing stent.
Complications
There were no major or minor complications.
Follow-up
At median follow-up of 3.8 (range 2.2–8.4) years, a total of 21 procedures (20 transcatheter, 1 surgical) were performed in these patients. In all six patients, the shunt was closed with a significant reduction in portal pressure (10.8 ± 1.8 mmHg; p = 0.016). All patients had significant growth of the portal vessels (4.0 ± 2.4 mm; p = 0.037), normalization of ammonia levels (65.7 ± 9.6 μmol/L; p = 0.002), and no complications. The child with hepatopulmonary syndrome experienced complete correction of the arterial saturation to 96 % within 5 months of the initial procedure.
Discussion
Here we describe our experience with transcatheter staged closure of CPSS to redirect flow in to the hypoplastic portal vessels to promote their gradual growth. PVH occurs in CPSS when the normal fetal circulatory function of the ductus venosus is bypassed or absent [7–10]. The ductus venosus acts as a resistor-diverter to the umbilical venous return directing a portion of the flow to the developing portal system while the remainder of the oxygenated blood is deflected to the fetal heart. We proposed that the reestablishment of this lost fetal mechanism for portal venous growth can be imitated by a flow-reducing stent without causing prohibitive portal hypertension and its sequelae.
The applicability of this approach is clearly demonstrated in the child with severe hypoxemia from hepatopulmonary syndrome resulting from an Abernethy malformation and PVH. Similar cases have been described; the treatment at that time was liver transplantation [14, 15]. Our patient was a poor candidate for transplantation because of severe cyanosis, with an oxygen saturation of 72 %. Temporary balloon occlusion of the shunt caused the portal pressure to rise over 30 mmHg. The reducing stent caused a rise in portal mean pressure from 8 to 16 mmHg. Three weeks after implantation, the oxygen saturation rose to 88 %, indicating reduction of the intrapulmonary shunt, and a duplex ultrasound of the liver revealed increased portal venous flow. Three months after implantation, catheterization revealed normal portal vessels and a pressure of 12 mmHg when the CPSS was temporarily occluded. The shunt was closed with an Amplatzer PDA device, and the patient had a normal saturation at follow-up 3 years later.
A similar approach of staged closure was recently reported in one case, which used a more complex methodology that included closure of the CPSS and a restrictive transjugular intrahepatic portosystemic shunt procedure with a satisfactory result of portal vessel growth [16]. Other staged closure methods with coils and surgery have been performed in cats and dogs, in which CPSS are more common [18, 19]. A recent review reported on five cases of CPSS PVH that were treated by surgical reduction of the shunt by surgical banding techniques [17].
The method of stent reduction used in our report was based on a previous case report by Moore and Murphy on the closure of a large venous connection [20]. Commercial reducing stents for flow reduction in transjugular intrahepatic portosystemic shunt in adults are available in Europe, and the use of ePTFE-covered stents has been described for this purpose [21, 22]. The specific anatomy of the CPSS, especially in small children, does not afford the use of off-the-shelf equipment, which is why a tailor-made approach had to be adopted. One report described the use of tailor-made flow reducing stents in four children with portosystemic shunts, two with postsurgical splenorenal shunts and two with Abernethy malformations [23]. The children had a combination of complications of shunt overflow and intrahepatic or extrahepatic portal vein hypoplasia, with two having undergone previous liver transplantation. Reducing stent implantation in three shunts led to a reversal of symptoms, increased portal flow, and increase in portal vein diameter; in one of these patients, the shunt was closed with an Amplatzer device. The Amplatzer Occluder, as well as the more recently described Vascular Plug, are ideal for CPSS closure because they provide excellent occlusive properties while being relatively short in length [24].
The anatomy and several classifications for CPSS have been described previously in the literature [7–10, 12] and are beyond the purpose and scope of this report. However, we wish to focus on the differentiation between malformations that relate to the complete absence or hypoplasia of the portal venous system. In four of our patients, CPSS venography or digital subtraction angiography in the superior mesenteric artery failed to demonstrate any portal venous branches (Fig. 1A). Our technique of balloon occlusion of the CPSS during shunt venography demonstrated portal vessels (Fig. 1B), albeit diminutive, in all patients, which led directly to the successful transcatheter interventions that we describe here. We therefore believe that all patients diagnosed with absence of portal vessels, Abernethy type 1, by other means of imaging (MR venography, CT venography) have to be referred for balloon occlusion venography before a final diagnosis of true absence of the portal vessels can be established. Preprocedure imaging by CT or MR venography can be useful in determining the anatomy of the CPSS and aid in planning the route of intervention and size and length of stent that will be required.
The plasticity of the native portal venous system is clearly demonstrated in our current experience when the hypoplasia caused by low fetal flow is reversed, even after a number of years, as flow is redirected in to the vessels. Flow restriction, redirection, and subsequent CPSS closure can be safely and effectively achieved by staged transcatheter techniques in amenable anatomy while avoiding the development of portal hypertension. This approach can avoid the need for liver transplantation in these challenging cases.
References
Witters P, Maleux G, George C et al (2008) Congenital veno-venous malformations of the liver: widely variable clinical presentations. J Gastroenterol Hepatol 23:e390–e394
Stringer MD (2008) The clinical anatomy of congenital portosystemic venous shunts. Clin Anat 21:147–157
Ferrero GB, Porta F, Biamino E et al (2010) Remittent hyperammonemia in congenital portosystemic shunt. Eur J Pediatr 169:369–372
Akahoshi T, Nishizaki T, Wakasugi K et al (2000) Portal-systemic encephalopathy due to a congenital extrahepatic portosystemic shunt: three cases and literature review. Hepatogastroenterology 47:1113–1116
Dhalluin-Venier V, Fabre M, Jacquemin E et al (2008) Liver cell adenomas and portosystemic shunt. Gastroenterol Clin Biol 32:164–166
Alvarez AE, Ribeiro AF, Hessel G et al (2002) Abernethy malformation: one of the etiologies of hepatopulmonary syndrome. Pediatr Pulmonol 34:391–394
Lisovsky M, Konstas AA, Misdraji J (2011) Congenital extrahepatic portosystemic shunts (Abernethy malformation): a histopathologic evaluation. Am J Surg Pathol 35:1381–1390
Morgan G, Superina R (1994) Congenital absence of the portal vein: two cases and a proposed classification system for portosystemic vascular anomalies. J Pediatr Surg 29:1239–1241
Howard ER, Davenport M (1997) Congenital extrahepatic portocaval shunts—the Abernethy malformation. J Pediatr Surg 32:494–497
Murray CP, Yoo SJ, Babyn PS (2003) Congenital extrahepatic portosystemic shunts. Pediatr Radiol 33:614–620
Tercier S, Delarue A, Rouault F et al (2006) Congenital portocaval fistula associated with hepatopulmonary syndrome: ligation vs liver transplantation. J Pediatr Surg 41:e1–e3
Lautz TB, Tantemsapya N, Rowell E, Superina RA (2011) Management and classification of type II congenital portosystemic shunts. J Pediatr Surg 46:308–314
Morikawa N, Honna T, Kuroda T et al (2008) Resolution of hepatopulmonary syndrome after ligation of a portosystemic shunt in a pediatric patient with an Abernethy malformation. J Pediatr Surg 43:e35–e38
Singhal A, Srivastava A, Goyal N et al (2009) Successful living donor liver transplant in a child with Abernethy malformation with biliary atresia, ventricular septal defect and intrapulmonary shunting. Pediatr Transpl 13:1041–1047
Emre S, Arnon R, Cohen E et al (2007) Resolution of hepatopulmonary syndrome after auxiliary partial orthotopic liver transplantation in Abernethy malformation. A case report. Liver Transpl 13:1662–1668
Kuo MD, Miller FJ, Lavine JE et al (2010) Exploiting phenotypic plasticity for the treatment of hepatopulmonary shunting in Abernethy malformation. J Vasc Interv Radiol 21:917–922
Franchi-Abella S, Branchereau S, Lambert V et al (2010) Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr 51:322–330
Wolschrijn CF, Mahapokai W, Rothuizen J et al (2000) Gauged attenuation of congenital portosystemic shunts: results in 160 dogs and 15 cats. Vet Q 22:94–98
Partington BP, Partington CR, Biller DS, Toshach K (1993) Transvenous coil embolization for treatment of patent ductus venosus in a dog. J Am Vet Med Assoc 202:281–284
Moore JW, Murphy JD (2000) Use of a bow tie stent occluder for transcatheter closure of a large anomalous vein. Catheter Cardiovasc Interv 49:437–440
Fanelli F, Salvatori FM, Rabuffi P et al (2009) Management of refractory hepatic encephalopathy after insertion of TIPS: long-term results of shunt reduction with hourglass-shaped balloon-expandable stent-graft. Am J Roentgenol 193:1696–1702
Kroma G, Lopera J, Cura M et al (2009) Transjugular intrahepatic portosystemic shunt flow reduction with adjustable polytetrafluoroethylene-covered balloon-expandable stents. J Vasc Interv Radiol 20:981–986
Stewart JK, Kuo WT, Hovsepian DM et al (2011) Portal venous remodeling after endovascular reduction of pediatric autogenous portosystemic shunts. J Vasc Interv Radiol 22:1199–1205
Passalacqua M, Lie KT, Yarmohammadi H (2012) Congenital extrahepatic portosystemic shunt (Abernethy malformation) treated endovascularly with vascular plug shunt closure. Pediatr Surg Int 28:79–83
Conflict of interest
Bruckheimer is a paid consultant for Atrium Medical Corp., Hudson, NH. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruckheimer, E., Dagan, T., Atar, E. et al. Staged Transcatheter Treatment of Portal Hypoplasia and Congenital Portosystemic Shunts in Children. Cardiovasc Intervent Radiol 36, 1580–1585 (2013). https://doi.org/10.1007/s00270-013-0581-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0581-7