Abstract
Semiflexible polyesters prepared by an alternating arrangement of biphenyl mesogenic units and aliphatic spacers represent an ideal model for studying the behavior of main-chain liquid crystalline polymers (MCLCP). The transition temperatures, type of mesophase and rate of mesophase–crystal transformation can be tailored by the utilization of a suitable flexible spacer, so that polymers with a stable mesophase at room temperature can be prepared. Furthermore, if the structure of the spacer is adequately chosen, the liquid-crystallization can be slowed down, and sometimes it is possible to quench the amorphous state by cooling the isotropic melt at not very high rates. Following these strategies a rich variety of glass forming liquid crystalline polymers can be prepared. The dynamical behavior of a MCLCP is closely related to the phase present. The segmental dynamics (α relaxation) is the result of cooperative motions near the glass transition of repeating units of the chains built in the structure. The α process behaves differently in the amorphous than in the liquid crystalline (or crystalline) states. Here, the preparation of the polymers and the analysis of their mesomorphic behavior by differential scanning calorimetry and X-ray diffraction are reviewed. The discussion of mechanical/electrical relaxations related to different phases is also included. Eventually, the link between molecular composition, structure and dynamics of the MCLCPs will be established.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Thermotropic polyesters
- Smectic mesophases
- Amorphous glass
- Liquid-crystalline glass
- Mechanical/electrical relaxations
15.1 Introduction
Aromatic main-chain polyesters have been extensively investigated and represent the most important class of liquid crystalline polymers (LCPs). They have been of interest as engineering plastics due to the combination of some properties such as their low density, chemical resistance, dimensional stability, exceptional mechanical properties and low gas permeability. However, all-aromatic polyesters present the inconvenience of poor solubilities and very high transition temperatures, often in the range of polymer degradation. Therefore, in the last decades considerable effort was made to improve the processability of these polymers by modification of their chemical structure and/or molecular architecture. A very usual strategy is the incorporation of flexible spacer segments between mesogenic units. In this way, thermotropic semiflexible polymers with rather accessible transition temperatures can be built by an alternating arrangement of flexible spacers and rigid mesogenic groups. In general, the liquid crystalline behavior of these semiflexible polymers hinges on the combination of the inherent anisotropic molecular interaction of the mesogenic units and the relative position of these units in the macromolecular chain, which is dictated by the chemical structure and the even/odd character of the flexible spacers connecting them.
The influence of the nature of the spacer on the phase behavior of semiflexible polymers with biphenyl mesogenic groups has been subject of many investigations (Meurisse et al. 1981; Jackson and Morris 1990; Pérez et al. 1992, 1997, 2000, 2003a, b; Watanabe et al. 1992, 1997; Fernández-Blázquez et al. 2007b; Loman et al. 1995; Tokita et al. 1998; Osada et al. 2004; Bello et al. 1990, 2001b; Martínez-Gómez et al. 2004, 2008, 2010; Ezquerra et al. 2005; Encinar et al. 2012) showing the ability of these polymers to exhibit low-ordered smectic mesophases (SmA, SmCA or SmC), which may be briefly described as lamellar structures with a disordered lateral packing of the molecules. As depicted in Fig. 15.1, both mesogens and molecular axes are perpendicular to the smectic layer planes in the SmA mesophase (orthogonal mesophase). However, although the average molecular axes are also perpendicular to the smectic planes in the SmCA structure, the mesogens are inclined in relation to the normal to the smectic planes, with opposite tilt direction for mesogens located in adjacent layers. On the other hand, the SmC structure is a tilted mesophase. The angle of inclination between the molecular axes and the normal to the smectic plane is temperature-dependent.
It has been proved that the transition temperatures, mesophase structure and rate of crystallization (mesophase–crystal transformation) can be controlled with suitable changes in the structure of the flexible spacer, so that polymers with stable mesophases at room temperature can be prepared. Furthermore, if the structure of the flexible spacer is adequately chosen, the liquid–crystallization can be slowed down, and sometimes it is possible to quench the amorphous state by cooling the isotropic melt at not very high rates. These facts, tailored phase behavior and possibility of freezing the glassy states of both the amorphous and the liquid crystalline phases, makes these polymers ideal model systems to study the general behavior of main-chain liquid crystalline polymers (MCLCP).
In this chapter, the preparation and thermotropic behavior of main-chain semiflexible polyesters based on biphenyl mesogenic units are reviewed. The influence of the polymer chemical structure on the mesomorphic properties is analyzed and some relationships between chemical, structure and liquid crystalline properties are established.
15.2 Preparation of Main-Chain Semiflexible Polyesters with Biphenyl Mesogenic Units
The representative structures of main-chain semiflexible polyesters derived from the biphenyl mesogenic unit are shown in Table 15.1. The preparation of these polymers can be performed by a two-step melt polycondensation in the presence of titanium (IV) isopropoxide as catalyst. The first step is the transesterification at around 200 °C for 20–24 h under nitrogen atmosphere. The second polycondensation reaction is performed at temperatures up to 250 °C under vacuum, to provide a high molar mass polymer. Polyesters, commonly called polybibenzoates, and copolyesters can be directly prepared from the commercially available dimethyl-4,4′-biphenyl-dicarboxylate diester and a glycol. On the other hand, polyetheresters, where ester and ether alternate as linking groups between the mesogen and the spacer along the polymeric chain, are prepared from a precursor containing the biphenyl mesogenic group. This precursor is previously synthesized by reaction of ethyl-4-hidroxybiphenyl-4′-carboxylate with a bromoalkanol in the presence of potassium carbonate (Nakata and Watanabe 1994) or by reaction with a glycol under Mitsunobu conditions (Martínez-Gómez et al. 2006; Fernández-Blázquez et al. 2004; del Campo et al. 2002). In the latter, two different precursors, with link sequence ester-ether or ester-ether-ether-ester, can be obtained depending on the stoichiometry (Fig. 15.2b).
15.2.1 Diols Used as Flexible Spacers
Several glycols with different chemical structures and lengths have been employed as flexible spacers. Some of them are commercially available, such as all-methylene glycols, diethylene glycol, triethylene glycol and glycols with methyl substituents as for example 1-methyl-1,4-butanediol, 2-methyl-1,3-propanediol and 3-methyl-1,5-pentanediol. Non-commercial glycols bearing ether groups can be synthesized by protonic acid catalyzed ring-opening reaction of a cyclic ether (oxirane or oxetane) with a glycol (Bello et al. 2001b; Martínez-Gómez et al. 2004, 2008; Encinar et al. 2012). The polymerization of the cyclic ether is suppressed by using a large excess of glycol and maintaining a low instantaneous concentration of oxirane or oxetane in the reaction mixture. Under these conditions, the reaction of the protonated monomer with the glycol is greatly favored and, therefore, the desired ether-glycol dimer is mainly obtained. Particularly interesting are those glycols bearing an ether group and a methyl substituent both in an asymmetric position, leading to random copolymer structures with liquid crystalline phases extending over wide temperature regions.
The stabilization of the mesophase is more noticeable when a mixture of isomer glycols is used as spacer. Figure 15.3 shows, as an example, the chemical structures and proportions of the isomer glycols obtained in the reaction of propylene oxide with 1,4-butanediol. Because propylene oxide is an asymmetrically substituted oxirane, two pathways in the opening of the ring are possible, and therefore, a mixture of the isomer ether-glycols I and II are obtained (Martínez-Gómez et al. 2008). The composition of this mixture is I = 46 %, II = 54 %, which indicates no significant preference for either of the two directions of the ring-opening. The carbon-oxygen bonds are considerably polarized in the protonated oxirane and two pseudo-carbocations can be formulated. Although the reaction with the unsubstituted carbon atom is the more favorable steric situation, the inductive effect of the methyl group stabilizes the partial positive charge on the substituted carbon, counteracting the steric hindrance.
In the case of ether glycols obtained by reaction of oxetane with a branched glycol, owing to the differences in reactivity of the primary versus secondary alcoholic groups, isomer mixtures enriched in the product derived from the addition of the more reactive primary alcoholic group are obtained (Bello et al. 2001b) (Fig. 15.3c).
15.3 Phase Behavior of Semiflexible Polyesters with Biphenyl Mesogenic Units
15.3.1 Techniques for the Analysis of the Phase Behavior
The analysis of the phase behavior of LCPs is sometimes complicated because these polymers usually exhibit a sequence of mesophases with different degree of order (polymesomorphism) and/or the mesophase is transformed into a three-dimensional crystal (polymorphism). The combination of differential scanning calorimetry (DSC) and X-ray diffraction techniques is very useful for the study of the thermotropic behavior of LCPs. Moreover, taking advantage of the high intensity of synchrotron radiation, very short acquisition times can be used, so that the experiments can be carried out under real time conditions, and temperature programs comparable to those employed in DSC can be imposed to the samples. Therefore, both aspects, thermal transitions and phase structure, can be determined by real-time variable-temperature diffraction experiments using synchrotron radiation (Pérez et al. 2009).
Additional information about the phase behavior can be achieved by polarized optical microscopy. Unfortunately, microscopic observations, which are so revealing in low molar mass liquid crystal, are not always helpful in dealing with thermotropic main chain polymers. Polymers with high or relatively high molecular weights typically develop textures that are not easily identifiable.
15.3.2 Polybibenzoates
Many works have been published about the phase behavior of semiflexible polybibenzoates, showing that the thermotropic behavior is strongly conditioned by the nature (chemical structure and even/odd character) of the flexible spacer. In these studies, different aliphatic spacers of diverse structures and lengths have been used, which can be classified in three main categories: linear all-methylene, oxyalkylene and branched spacers. Examples of structures of flexible spacers in polybibenzoates and the relevant phase behavior data are collected in Table 15.2. Most polybibenzoates reported in the literature exhibit a low-ordered smectic mesophase (SmA, SmCA or SmC) and only some branched polymers derived from poly(tetramethylene glycol p,p′-bibenzoate), PB4, have been reported to be nematic. Notice that the liquid crystalline state of polybibenzoates often transforms into a crystalline phase upon cooling.
As usual in main-chain liquid crystalline semiflexible polymers (Chiellini and Laus 1998), the melting (Tm) and isotropization (Ti) temperatures of polybibenzoates decrease significantly with increasing length of the flexible spacer, and Ti normally decreases in a zig-zag fashion in homologous series in which the spacer length regularly increases (Pérez et al. 1997; Watanabe et al. 1997). This is the so-called odd-even effect, where Ti tends to be higher for the polymers with an even spacer, but this oscillation is attenuated on ascending the series. This odd-even oscillation is also found for the transition entropies and for the spacing of the smectic layer. The explanation given for this effect is based on the differences in the packing arrangement of the polymer chains between even and odd members. Thus, a fairly extended conformation is produced, with the mesogenic groups approximately parallel to the chain axis, for even members. On the contrary, the valence angles for the odd members force the chain to adopt a less extended arrangement, and the mesogens form an angle of about 30° with respect to the chain axis direction (about 60° between two successive mesogens). In a real system, however, it may be expected that more than a single conformation could intervene in the formation of the smectic layers compatible with the relatively low requirements of the mesophase order. Those different packing arrangements are also responsible for the different type of mesophase structure formed: polybibenzoates with an even spacer show a smectic A mesophase while for odd members a smectic CA mesophase is found.
For polybibenzoates incorporating linear all-methylenic spacers (CH2)m, the mesophase is only stable at temperatures just below its formation and a rapid transformation into a three-dimensional crystalline structure is produced on cooling. The melting of this crystal is monotropic for those polymers with m ≥7, while for the lower members the melting is enantiotropic and the mesophase is also observable on heating because the crystal is transformed into the mesophase prior to its isotropization.
The insertion of side methyl groups in the spacer results in a decrease of the structural and geometrical symmetry of the macromolecules, and a lowering of the interchain interactions. As a result, the tendency of the elongated LCP chains to build a supramolecular structure is reduced. Thus, a diminution of the transition temperatures with respect to the unsubstituted polymer is usually observed, accompanied by a decrease in the tendency to form smectic mesophases. As it can be observed in Table 15.2, polybibenzoates with methyl groups in the spacer display transition temperatures considerably lower than those found for their analogous unsubstituted polymers: a decrease of 100, 136, 63 and 86° is produced by branching a methyl group in poly(trimethylene glycol p,p′-bibenzoate), poly(tetramethylene glycol p,p′-bibenzoate), poly(pentamethylene glycol p,p′-bibenzoate) and poly(hexamethylene glycol p,p′-bibenzoate) polymers, respectively. Furthermore, it is noteworthy that when only one methyl group is present in the spacer, the formation of the smectic mesophase is preserved in all the polymers investigated. However, for polymers with two methyl groups in the spacer, as is the case of BB4(2,3-diMe) or BB4(2,2-diMe), the smectic mesophase is replaced by the nematic mesophase. Thus, the effective accommodation of two lateral methyl groups into the mesophase structure is attained by packing the molecules in a nematic structure, which has only orientational order. Another general behavior of branched polybibenzoates is their tendency to alter the orthogonal SmA to the tilted SmC. The SmC mesophase is especially interesting in those cases where the branched spacer incorporates chiral carbons, since it may develop ferroelectric properties.
The steric hindrance effect of methyl groups is more pronounced in the packing efficiency for crystal structures, and, consequently, polymers with a very low, if any, melting temperature are observed, thus leading to mesophases extending over wide temperature regions. Polybibenzoates with short branched spacers, as BB32, BB4(1-Me) and BB5(3-Me), develop a stable mesophase. However, for polymers with longer spacers, as is the case of P6MeB, the accommodation of the side methyl groups is easier and a highly ordered structure can be obtained.
Comparing with the analogous polymers containing polymethylene spacers, two major effects are observed when oxyalkylene spacers are used: the reduction of the transition temperatures and a considerable decrease of the transformation of the mesophase into a more ordered phase. Thus, the mesophase of these polymers is often stable for a considerable time at room temperature, although the crystallization can be reached by annealing the polymer for long times at temperatures above the glass transition. The reduction of the transition temperatures increases as the number of ether groups present in the spacer increases. For instance, the isotropization temperature changes from 202 °C for poly(octamethylene glycol p,p′-bibenzoate), P8MB, to 160 °C for polybibenzoate P3O4B which has only one ether group in the spacer, and to only 115 °C for PTEB, with two ether groups. Regarding the type of mesophase, the parity of the spacer, as it happens with polybibenzoates with all-methylene spacers, is clearly reflected on the structure of the mesophase formed, being of the type SmCA for odd spacers (PDEB, PDTMB, PDETB) and SmA for even spacer (P3O4B, PTEB). Moreover, the presence of the ether groups in the even spacers seems to favor the transformation from the orthogonal SmA mesophase to the tilted SmC mesophase.
Asymmetric spacers bearing both an ether group and a methyl substituent are particularly interesting because they lead to copolymer structures forming stable mesophases and, hence, problems associated with the formation of crystalline structures are completely avoided. A more irregular macromolecular structure can be obtained by using a mixture of isomer spacers, so that the liquid-crystallization is also slowed down, making possible the obtainment of the amorphous glass state for these systems. One example is the polybibenzoate PPO4B. Its irregular chemical structure, together with the vicinity of the isotropization temperature, Ti = 85 °C, and the amorphous glass transition, Tg = 32 °C, allows the freezing of the amorphous state by a rapid quenching of the isotropic melt in liquid nitrogen. However, when the amorphous sample is heated just above the glass transition, the liquid crystalline state is rapidly developed.
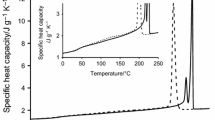
Another interesting polybibenzoate of this type is PTEMeB. This polymer has the advantage that the amorphous glass can be easily quenched from the melt. In fact, the liquid crystalline state is formed at rather low rates, in such a way that it is not observed at the usual scanning rates in the calorimeter. Therefore, the DSC curves during a cooling-heating cycle show only the change in the specific heat associated with the amorphous glass transition (at around 25 °C on heating). However, when the sample is annealed above the glass transition, a low-ordered smectic mesophase is developed as deduced from DSC and X-ray diffraction experiments. As it is seen in Fig. 15.4, the extent of the amorphous-mesophase transformation achieved during the annealing of PTEMeB is reflected in the glass transition region. Two separated steps, centred at 15 °C and 25 °C, are differentiated in the DSC curves, which intensities (increment of specific heat) depend on the annealing time. At annealing time equal to zero the glass transition at 25 °C, associated with the amorphous phase of PTEMeB, is only observed. The intensity of this step diminishes with annealing time, in other words, when the content of the mesophase formed increases. At the same time, a new step at 15 °C appears and grows to be the only one observed at high annealing times, together with an endotherm at 54 °C corresponding to the isotropization of the mesophase.
15.3.3 Copolyesters
Copolymerization is a widely used strategy to extend the structure-property spectrum of polymers, besides to reduce costs. Moreover, it is another method of lowering the transition temperatures. Several studies have been published on copolyesters of bibenzoate with non-mesogenic units. For example, it has been reported that the incorporation of bibenzoate groups to poly(ethylene terephthalate) enhanced the glass transition temperature, and the mechanical and gas barrier properties. However, no evidence of liquid crystallinity was found in these copolymers, although a “frustrated” liquid crystalline structure was proposed (Ma et al. 2002; Liu et al. 2003; Schiraldi et al. 2001). When larger spacers are employed, liquid crystalline structures are more easily developed. Accordingly, poly(diethylene isophthalate-co-4,4′-bibenzoate) copolymers with 20 mol % isophthalate or less exhibit liquid crystalline character (Hu et al. 2004). However, the nonlinear isophthalate comonomer is rather effective in destroying the liquid crystalline order. Less disrupting are the terephthalate units, and when heptamethylene spacer is used, a considerable amount (up to around 40–50 mol %) of terephthalate groups can be copolymerized with bibenzoate units, yet exhibiting liquid crystalline properties (Pérez-Manzano et al. 2006).
More recently, the incorporation of non-mesogenic 2,6-napthalate units to poly(triethylene glycol p,p′-bibenzoate), PTEB, has been investigated (Martínez-Gómez et al. 2011). Copolymers with up to 30 % naphthalate content have the ability to exhibit smectic liquid crystalline phases, which are very stable and do not transform into crystalline structures. Moreover, the isotropization temperatures of these copolyesters of naphthalate are easily accessible (100–70 °C) and the phase behavior can be tailored by an appropriate selection of the comonomer composition. The sequence of phases isotropic-SmA-SmC is observed in polymers with naphthalate content below about 10 mol %, while the transition SmA-SmC is lost for comonomer contents above 10 %, and only the SmA mesophase is found for compositions between 10 and 30 mol %. Moreover, the formation of the liquid crystalline state is considerably slowed down as the naphthalate content increases, so that the copolymer with 30 % content can be easily quenched down into the amorphous glass.
Another family of random copolyesters based on the biphenyl group is that obtained by polymerization of bibenzoate with two different alkane diols (Watanabe et al. 1997). It has been found that the incorporation of all-methylene spacers with the same parity, even or odd, does not significantly disrupt the structure of the mesophase formed, SmA or SmCA, respectively. The isotropization temperature of the smectic mesophase falls on a smooth curve close to that representing the arithmetic average of the homopolymer temperatures. Moreover, the crystal-mesophase transition temperature decreases, so that the mesophase is exhibited in a wide temperature region. However, the smectic phase becomes unstable and alters to the nematic phase when it is forced to accommodate flexible spacers of very different lengths. On the other hand, when spacers of comparable length but different even/odd nature are copolymerized, as for example hexamethylene and pentamethylene segments, the smectic mesophase is observed in the entire composition range. However, a eutectic behavior is found for the isotropization temperatures of the mesophases. In these systems, the two repeating units differing in the parity are not compliant with the packing requirements of the crystalline state, thus a depression of the crystallization tendency is observed when the second component is added.
Copolymers with two different spacers sequenced in a regularly alternate fashion may develop very interesting smectic structures (Nakata and Watanabe 1997). If the two spacers are sterically incompatible, and there is sufficient lateral attraction between identical spacers of adjacent polymer chains, segregation into a bilayer smectic phase may occur. When two odd-numbered spacers are used, this bilayer structure is especially interesting since it would be ferroelectric even in non-chiral systems.
15.3.4 Polyetheresters
Polyetheresters, where both ester and ether groups are used as linking units between the biphenyl mesogens and the spacers, represent an alternative class of semiflexible main-chain LC polymers based on the biphenyl group. However, these systems have been less investigated, probably because their preparation involves a more complicated synthetic route, and only a few examples are described in the literature (Nakata and Watanabe 1994; Martínez-Gómez et al. 2006; Fernández-Blázquez et al. 2004; del Campo et al. 2002). Then, although it is not possible to establish conclusive correlations between the spacer structure and the thermotropic properties of these systems, the following statements can be concluded. All the polyetheresters reported in the literature develop low-ordered smectic mesophases. The ether linkage produces similar odd-even effect to the ester in polyester. When the spacer is a linear all-methylene segment, as it happens with polybibenzoates, the mesophase is rapidly transformed into a more ordered phase upon cooling (Nakata and Watanabe 1994). This transformation is inhibited in polyetheresters with methyl-substituted trimethylene spacers. Indeed, the rate of mesophase formation is also slowed down in such a way that the isotropic melt can be easily quenched into the glassy amorphous state (Fernández-Blázquez et al. 2004; del Campo et al. 2002).
15.4 Dynamic Mechanical Behavior in Liquid Crystal Polymers with Biphenyl Mesogens
Dynamic mechanical analysis (DMA) is a mechanical test referring to the response of a material as it is subjected to a periodic force. The study of this response leads to know the viscoelastic behavior of the material, because the elastic response can be separated from the viscous response through their respective moduli: storage (E′) and loss (E″), as well as the damping factor (tan δ) that is the ratio between both moduli (tan δ = E″/E′). This fact has caused that DMA was a technique widely used in the study of molecular relaxation processes taking place in polymers (Duncan 2008). The most accurate type of measurement in DMA is to heat the sample in a temperature scan at different frequencies, because the relaxation processes are function of the frequency. The range of frequencies, despite being narrow (around four decades), is sufficient for studying the shifting of damping maxima to higher temperatures with increasing frequencies. The secondary relaxations obey the Arrhenius law for frequency-temperature shift.
where ΔH is the apparent activation energy of the motional process. The main relaxation (glass transition) is ruled by the Williams, Landel, and Ferry relationship.
Most of the main chain liquid crystal polymers with biphenyl mesogens analyzed by DMA are polybibenzoates with all-methylene (Pereña et al. 1991; Pérez et al. 1994) or oxymethylene spacers (Benavente et al. 1993), copolymers combining both kind of spacers (Benavente et al. 1996), and copolymers with non-mesogenic aromatic moieties as isophthalate (Hu et al. 2004), but in all cases without lateral groups. In general, these polymers display three mechanical relaxations, called α, β and γ in order of decreasing temperature (Pérez et al. 1997).
In all of these MCLCP, α relaxation is the main relaxation and is considered the glass transition owing to the high tan δ maximum value of the relaxation and the corresponding sharp decrease of the storage modulus. The apparent activation energy of the process is higher than 400 kJ mol−1, confirming the assignation of this relaxation as the glass transition of these polymers (Pérez et al. 1997). Therefore the α relaxation temperature depends on the flexibility of the polymer chain, and in the case of MCLCP is directly dependent on spacer flexibility. Particularly in polybibenzoates with all-methylene (Pereña et al. 1991) or oxymethylene spacers (Benavente et al. 1993), longer spacers lead to lower temperatures, but the odd-even effect also has to be considered (Pérez et al. 1997). The presence of ether groups in the spacer, not only slows down the crystallization from the mesophase, but also decreases the α relaxation temperature (as it is observed in the case of PB8 and PTEB (Benavente et al. 1996) whose temperatures are 45 and 0 °C respectively), since spacer flexibility is increased. Moreover, the intensity of the α peak, considering tan δ values, increases with the length of the spacer.
The β relaxation for MCLCP takes place in the temperature interval between −80 and −50 °C. This relaxation has a complex origin, is characteristic of polyesters, and originates from movements of several groups as phenyl and carboxyl groups. In fact, this relaxation in aromatic polyester has been reported to be composed of two overlapped peaks (Diaz-Calleja et al. 1986, 1989). Similarly to the α relaxation, the temperature location of β relaxation for polybibenzoates depends on the spacer length, decreasing with the length increase. This fact confirms the complex character of this relaxation and the possibility of different steric hindrances of the reorganizational motions of the carboxyl and phenylene groups depending on the spacer length, so that the apparent activation energy of this relaxation increases slightly with the length of the spacer in values around 100 kJ mol−1 (Pérez et al. 1997).
At the lowest temperatures the γ relaxation is found, overlapping with β relaxation, and coinciding in location and activation energy with the typical γ relaxation of polyethylene (Heaton et al. 1996). The γ relaxation in polyethylene was firstly attributed to crankshaft movements of polymethylenic chains (Schatzki 1966). Though a lot of work concerning this relaxation in polyethylene has been done, there remains no clear consensus regarding the details of the underlying motional process (Boyd 1985). There is, however, a body of opinions which support one or more of the various models for restricted conformational transitions as kink formation, inversion and migration (Schatzki 1966; Boyd 1975; Boyd and Breitling 1974). Molecular dynamics simulations have been a powerful tool to corroborate the just mentioned nature of these conformational motions underlying this relaxation (Heaton et al. 1996; Boyd et al. 1994; Jin and Boyd 1998). This type of motion requires chains containing sequences of three or more methylenic units. Regardless of the length and even nature, in case of oxyethylene spacers, there is no significant variation in location, intensity, and activation energy of the relaxation. This fact suggests the same type of motion for all-methylene or oxymethylene spacers (Pérez et al. 1997).
As it was mentioned above, most of the MCLCP analyzed by DMA are polybibenzoates with linear spacers, spite of the important influence of substituent groups in the spacer. Previous sections show that branched spacers reduces the crystal formation as well as the crystallization or liquid crystallization rate. Combining this effect with short spacers (three methylenes) in a polyetherester, as was described in Sect. 15.3.4, a system is obtained with high glass transition temperature (90 °C) that develops a low order mesophase (SmCA) with very slow formation rate (Fernández-Blázquez et al. 2004). These thermal and structural properties let this polymer, named PH31B32 (Fig. 15.5a), be easily prepared in either the pure isotropic amorphous state or in a low ordered smectic mesophase, and thus to analyze the corresponding viscoelastic relaxations in both pure phases.
Figure 15.5b shows the storage modulus for samples exhibiting pure liquid crystal, pure amorphous and mixture of the two phases. The magnitude of the modulus depends on the percentage of the liquid crystal phase, increasing about 21 % at −140 °C and around 28 % at room temperature (Fernández-Blázquez et al. 2005). The localization of α relaxation is also clearly dependent on the amount of liquid crystal phase, being at higher temperature for amorphous samples as it was observed in Fig. 15.4 for PTEMeB. This fact is also observed in Fig. 15.5c for the loss modulus. Taking into account that α relaxation is associated to glass transition, in turn, is related to the freezing of segmental motions, and the liquid crystalline phases retain some mobility around the longitudinal axes of the mesogen. It seems reasonable to expect a glass transition of the liquid crystalline phase at the temperature at which the minimum free volume required for the rotations is approached (Fernández-Blázquez et al. 2004). The glass transition temperature of polymers is closely related to the flexibility of the chains in the sense that a high value of Tg is generally assumed to be connected with relatively high barriers of bond rotations. These barriers depend not only on the type of bond, but also on the intermolecular constraint and therefore on the supramolecular arrangement of the chains. For this reason the glass transition temperature of the liquid crystalline phase can differ from that of the amorphous phase.
Secondary relaxations β and γ are observed in Fig. 15.5c and are similar in both cases, and just a small temperature shift of 7 °C higher for amorphous sample is observed, but with similar activation energy, around 70 kJ mol−1. The γ relaxation appears as a shoulder of β relaxation without any differences among the three samples. In summary, there are clear differences between amorphous and liquid crystal state, although those differences are much smaller than the ones found between a classical amorphous polymer and its semicrystalline counterpart.
DMA experiments have been also performed to study one of the most reported phenomena of liquid crystal polymers in the last two decades as is the polymer chain orientation in fibers (Osada et al. 2004; Bello et al. 2001a; Martínez-Gómez et al. 2003; Fernández-Blázquez et al. 2007a; Rodriguez-Amor et al. 2008). Two distinct orientations are reported in fibers. In the most usual case, the molecules are oriented parallel to the stretching direction: if the fiber is obtained from isotropic melt or isotropic liquid crystal phase at low temperature and high strain rate the molecules are in parallel orientation mainly. On the contrary, at high temperature and low strain rate, quasi-equilibrium conditions are attained, and the macromolecules are disposed with an anomalous perpendicular orientation in relation to the stretching. This unusual feature is characteristic of liquid crystal polymers with only low order mesophases and unable to crystallize (or crystallizable with quite low crystallization rate, like PDEB) (Rodriguez-Amor et al. 2008).
DMA experiments were carried out on PPO4B samples which were drawn at different strain rates and temperatures, in such a way that either parallel or perpendicular orientation, or a mixture of them, was obtained (Martínez-Gómez et al. 2003). Later, strips cut from these oriented sheets in three directions, 0°, 45°, and 90° in relation to the drawn direction, were tested. All specimens, independently on fiber and cut direction, showed the three relaxations described above in the same temperature locations and without differences in activation energy. The interesting differences were found in the value of storage modulus, as it is observed in Fig. 15.6. The physical properties of polymers depend critically on their molecular orientation. Taking into account that the orientation of the chains is a function of the experimental condition, the best mechanical properties are not always in the fiber direction. It is generally observed that high degrees of molecular orientation lead to increased strength and stiffness along the alignment direction, but from the data shown in Fig. 15.6 this is not always the case: for anomalous orientation the worse mechanical properties coincide with the fiber direction, and the best are in the transverse direction.
Fibers of BB5(3-Me) with the smectic layers perpendicular and parallel to the fiber axis were also analyzed by DMA to study the micro-Brownian motions of the polymer (Osada et al. 2004). Time-temperature superposition was applied to the α relaxation, therefore the frequency of the micro-Brownian motion in the SmCA phase can be estimated despite the narrow frequency range in DMA. No significant different were found between both directions at temperatures below the glass transition. However, at smectic temperatures (above glass transition) the frequency of the motions along the layer normal direction is 2.5 times faster than that of the motions along the layer, suggesting that the micro-Brownian motions in the SmCA phase are decoupled between the two directions parallel and perpendicular to the smectic layer.
15.5 Dielectric Relaxation in Polybibenzoates
Dielectric spectroscopy is a useful technique to follow the dynamics of glass forming systems (Kremer and Schönhals 2002). Dielectric relaxation in polymers is the consequence of the collective response of disordered polar chains under an external electrical field of tunable frequency. The molecular mechanism responsible of the dynamic glass transition in the amorphous phase, the α relaxation process, is related to the cooperative segmental motion of chains near the glass transition. The molecular origin of the α relaxation observed near the glass transition of mesophases is not yet completely known. Nevertheless, the dielectric relaxation of liquid crystalline polymers is an excellent tool to study their phase transitions and its related dynamics.
Dielectric studies of main chain liquid crystalline polymers are rather scarce and most of them are focused on polyesters (Ezquerra et al. 2005; Encinar et al. 2012; García-Bernabé et al. 2004). The comparative study of the polybibenzoates PTEMeB and PTEB (see Table 15.2) is particularly interesting. The absence or presence of the small methyl group as lateral substituent is responsible of very different behavior in terms of phase map, kinetics and dynamics. On one hand, PTEMeB can be easily quenched to an amorphous state and it has a rather slow mesophase formation (see Fig. 15.4). On the other hand, PTEB quickly forms on cooling a liquid crystal phase of the type SmA, which is further transformed into SmC (see Table 15.2), these two processes being reversible on heating. Both transition phenomena can be followed by dielectric spectroscopy.
Figure 15.7a shows the dielectric loss curves monitored during the isothermal formation of the liquid crystal of PTEMeB. It involves an intensity exchange between relaxation peaks. Initially, only one peak is present: the αIso process, related to the dynamic glass transition of the amorphous phase. At higher times, this mode decreases, and a new peak appears at higher frequencies, which is related to the αLC relaxation of the liquid crystal glass. From the qualitative behavior of the loss curves of Fig. 15.7a, we can infer two stages in the kinetics. The first 6 h (fast stage) represent a relatively fast decay of the αIso mode and the corresponding increase of the αLC mode. Given the overlapping of the curves at long times, the second stage consists of a slow intensity exchange between the two peaks. Simultaneous dielectric and X-ray diffraction experiments of a similar polybibenzoate, C31DTB, have shown that the first stage (fast) is related to the smectic layer formation and the second one to the increase of order within smectic domains (Ezquerra et al. 2005). Two different relaxation frequencies are observed: the α relaxation of the isotropic phase is slower than the α relaxation of the liquid crystal state. This is consistent with the observed lower glass transition temperature in the mesophase compared to the amorphous state (see Fig. 15.4). It suggests that, in the liquid crystal glass, the molecular origin of the dynamic glass transition is other than the cooperative segmental motion of the chains, characteristic of amorphous polymers. Moreover, it has been proposed that the molecular mechanisms of the mesophase glass transition are the rotational and translational motions of the elongated chains that require less free volume (Ahumada et al. 1996). The dielectric intensity of both peaks is slightly higher in the mesophase, probably related to a better alignment of the dipolar vectors within the orientationally ordered phase. Another feature of this dynamics exchange process is the acceleration of the transient modes during the phase transition. Finally, it has been shown that the fast stage of the mesophase formation follows an athermal 1D crystallization Avrami kinetics (Encinar et al. 2012).
(a) Real time dielectric loss during the isothermal transformation of PTEMeB: isotropic liquid to liquid crystal. The temperature is 30 °C and the curves are 20 min apart. The arrows indicate the time evolution of the α relaxation of both phases. The symbols highlight the first 6 h of curves. Reprinted with permission from ref Encinar et al. (2012). Copyright 2012 American Chemistry Society. (b) Isothermal dielectric loss curves of PTEB (from left to right: 18 °C to 58 °C, at 4 °C steps). The arrows indicate the evolution of the α relaxation in SmC and SmA mesophases; observed, respectively, at low and high temperatures
Figure 15.7b shows the dielectric loss curves measured at different increasing temperature steps of the liquid crystal of PTEB. The α relaxation peak shifts monotonically to higher frequencies as the temperature increases. Below the phase transition temperature (approx. 46 °C on heating) the maximum intensity of the αSmC peaks, related to the tilted SmC mesophase, remains constant. At higher temperatures the peak intensity decreases leading into the αSmA process, related to the orthogonal SmA mesophase. A comprehensive analysis by fitting Havriliak Negami functions (Havriliak and Havriliak 1997) to the loss curves confirms that also the dielectric strength, i.e. the peak area, decreases when the phase transition takes place (Fig. 15.8). The dielectric strength is proportional to the dipole density and dipole-dipole vector correlations. The net dipolar moment of the PTEB molecule is due to the carbonyl groups (C=O) normal to the main chain adjacent to the biphenyl mesogens. If we consider that the dipole density is the same in both smectic phases (invoking the mass conservation), the dielectric strength decrease on the SmA phase can be related to a loss of dipolar pair correlation. The abrupt fall of dielectric strength resembles the also abrupt change of the correlation length parameter when the SmC to SmA transition takes place (Martínez-Gómez et al. 2010). Indeed, the transition to the orthogonal mesophase is accompanied by both an increase of the interlayer correlation length, i.e. longitudinal order between smectic layers, and by the loss of intralayer correlations of the local tilt directions in the SmC mesophase. Therefore, the pair dipolar correlations can be assigned mainly to short range intralayer correlations instead of long range interlayer correlations. On the other hand, since the tilting angle and layer thickness vary linearly with temperature in both phases (steeper in the SmC phase), it is unlikely to relate the dielectric strength fall to the smooth temperature dependence of layer thickness. During the SmC to SmA transition no apparent change on the relaxational frequency temperature dependence is detected (see Fig. 15.8). Additionally, only one static glass transition is detected in the thermogram. For this reasons, in the present system, the molecular origin of the smectic dynamic glass transition seems to be independent of the orientational state.
15.6 Conclusions and Future Perspective
The results summarized in this chapter clearly demonstrate that it is possible with simple chemical and structural modifications in the flexible spacer to tailor the thermotropic behavior of semiflexible main-chain polyesters based on biphenyl mesogens. Following such synthetic strategies, the thermal transitions, mesophase structure, mesophase stability and rate of liquid crystalline formation can be controlled. Furthermore, it has been shown that the election of an appropriate spacer allows the design of polymers in which both the glassy amorphous state and the glassy smectic state can be separately obtained and, consequently, both glassy states can be independently analyzed.
In recent years, the interest on LCPs has been renewed as precursors of liquid crystalline elastomers, which exhibit remarkable thermomechanical properties due to the combination of polymer network elasticity with the anisotropic structure of the liquid crystalline state (Brand and Finkelmann 1998; Ohm et al. 2010, 2012; Burke and Mather 2010). Because of the stronger coupling of liquid crystalline order to macroscopic network deformation, elastomers based on smectic main-chain LC polymers are expected to show unique thermomechanical properties. Hence, networks prepared from semiflexible polybibenzoates may have a great potential as smectic elastomers.
In this context, biphenyl-containing polymers may find a valuable application as smectic liquid crystalline elastomers. Although, there have been a few investigations on the deformation behavior of networks based on biphenyl mesogens (Ishige et al. 2008a, b; Hiraoka et al. 2009), there is much work still to be done to better understand the behavior of these exciting materials from both scientific and practical application perspectives. In fact, we have already synthesized some of these network systems, with the biphenyl group as mesogen, and the preliminary characterization shows outstanding shape-memory ability, with both excellent shape recovery and shape fixing.
References
Ahumada O, Ezquerra TA, Nogales A, Baltà-Calleja FJ, Zachmann HG (1996) Influence of liquid crystalline order on the dielectric. Relaxation of random copolyesters of PET, PEN, and PHB. Macromolecules 29:5002–5009
Bello A, Pérez E, Marugán MM, Pereña JM (1990) Liquid-crystalline poly[oxybis(trimethylene) p, p′-bibenzoate]: effect of the central ether group. Macromolecules 23:905–907
Bello P, Bello A, Lorenzo V (2001a) Anomalous orientation of poly(trimethylene oxy-2 methyl trimethylene p, p′-bibenzoate) as revealed by means of tensile tests. Polymer 42:4449–4452
Bello P, Bello A, Riande E, Heaton NJ (2001b) Thermotropic polyesters with flexible spacers bearing ether bonds in asymmetric position. Macromolecules 34:181–186
Benavente R, Pereña JM, Pérez E, Bello A (1993) Relaxation processes in thermotropic polydibenzoates with oxyethylene spacers in the main chain. Polymer 34:2344–2347
Benavente R, Zhu Z, Pereña JM, Bello A, Pérez E (1996) Dynamic mechanical relaxations of liquid crystalline copolyesters derived from bibenzoic acid. Polymer 37:2379–2384
Boyd R (1975) Energetics of kinks in polyethylene. J Polym Sci Polym Phys 13:2345–2355
Boyd R (1985) Relaxation processes in crystalline polymers: molecular interpretation. A review. Polymer 26:1123–1133
Boyd R, Breitling R (1974) Conformational analysis of crankshaft motions in polyethylene. Macromolecules 7:855–862
Boyd R, Gee R, Han J, Jin Y (1994) Conformational dynamics in bulk polyethylene: a molecular-dynamics simulation study. J Chem Phys 101:788–797
Brand HR, Finkelmann H (1998) Physical properties of liquid crystalline elastomers. In: Demus D, Goodby J, Gray GW, Spiess H-W, Vill V (eds) Handbook of liquid crystals, vol 3. Wiley-VCH, Weinheim, pp 277–302
Burke KA, Mather PT (2010) Soft shape memory in main-chain liquid crystalline elastomers. J Mater Chem 20:3449–3457
Chiellini E, Laus M (1998) Main chain liquid crystalline semiflexible polymers. In: Demus D, Goodby J, Gray GW, Spiess H-W, Vill V (eds) Handbook of liquid crystals, vol 3. Wiley-VCH, Weinheim, pp 26–51
del Campo A, Bello A, Pérez E, García-Bernabé A, Diaz-Calleja R (2002) Amorphous-smectic glassy main-chain LCPs, 1. Poly(ether esters) derived from hydroxybibenzoic acid and (R, S)- and (R)-2-methylpropane-1,3-diol. Macromol Chem Phys 203:2508–2515
Diaz-Calleja R, Riande E, Guzman J (1986) Influence of static strain on dynamic mechanical-behavior of amorphous networks prepared from aromatic polyesters. J Polym Sci Polym Phys 24:337–344
Diaz-Calleja R, Riande E, Guzman J (1989) Conformational and relaxation studies on polyesters derived from terephthalic acid and propylene and dipropylene glycol. Macromolecules 22:3654–3659
Duncan J (2008) Principles and applications of mechanical thermal analysis. In: Gabbott P (ed) Principles and applications of thermal analysis. Blackwell Publishing, Oxford
Encinar M, Martínez-Gómez A, Rubio RG, Pérez E, Bello A, Prolongo MG (2012) X-ray diffraction, calorimetric, and dielectric relaxation study of the amorphous and smectic states of a main chain liquid crystalline polymer. J Phys Chem B 116:9846–9859
Ezquerra TA, Martínez-Gómez A, Álvarez C, Alonso E, Sanz A, Bello A, Pérez E, Funari SS, Dommach M (2005) Structure-dynamics relationship during the amorphous to smectic transition of a main chain liquid crystalline polymer. J Non-Cryst Solids 351:2768–2772
Fernández-Blázquez JP, Bello A, Pérez E (2004) Observation of two glass transitions in a thermotropic liquid-crystalline polymer. Macromolecules 37:9018–9026
Fernández-Blázquez JP, Bello A, Pérez E (2005) Dynamic mechanical analysis of the two glass transitions in a thermotropic polymer. Polymer 46:10004–10010
Fernández-Blázquez JP, Bello A, Pérez E (2007a) Parallel and perpendicular orientation in a thermotropic main-chain liquid-crystalline polymer. Macromolecules 40:703–709
Fernández-Blázquez JP, Bello A, Pérez E (2007b) Synthesis, phase behaviour and mechanical properties of poly(2-methyl-1,3-propanediol-4,4′-bibenzoate). Macromol Chem Phys 208:2611–2620
García-Bernabé A, Diaz-Calleja R, Sanchis MJ, del Campo A, Bello A, Pérez E (2004) Amorphous-smectic glassy main chain LCPs. II. Dielectric study of the glass transition. Polymer 45:1533–1543
Havriliak S Jr, Havriliak SJ (1997) Dielectric and mechanical relaxation in materials: analysis, interpretation, and application to polymers. Hanser, New York
Heaton NJ, Benavente R, Perez E, Bello A, Pereña JM (1996) The gamma relaxation in polymers containing ether linkages: conformational dynamics in the amorphous phase for a series of polybibenzoates containing oxyethylene spacers. Polymer 37:3791–3798
Hiraoka K, Tashiro T, Tokita M, Watanabe J (2009) Spontaneous deformation of main-chain liquid-crystalline elastomer composed of smectic polyesters. Liq Cryst 36:115–122
Hu YS, Liu RYF, Schiraldi DA, Hiltner A, Baer E (2004) Solid-state structure of copolyesters containing a mesogenic monomer. Macromolecules 37:2128–2135
Ishige R, Osada K, Tagawa H, Niwano H, Tokita M, Watanabe J (2008a) Elongation behavior of a main-chain smectic liquid crystalline elastomer. Macromolecules 41:7566–7570
Ishige R, Tokita M, Naito Y, Zhang CY, Watanabe J (2008b) Unusual formation of a smectic A structure in cross-linked monodomain elastomer of main-chain LC polyester with 3-methylpentane spacer. Macromolecules 41:2671–2676
Jackson WJ Jr, Morris JC (1990) Polyesters of 4,4′-biphenyldicarboxylic acid and aliphatic glycols for high-performance plastics. ACS Symp Ser 435:16–32
Jin Y, Boyd R (1998) Subglass chain dynamics and relaxation in polyethylene: a molecular dynamics simulation study. J Chem Phys 108:9912–9923
Kremer F, Schönhals A (2002) Broadband dielectric spectroscopy. Springer, Berlin
Liu RYF, Hu YS, Hibbs MR, Collard DM, Schiraldi DA, Hiltner A, Baer E (2003) Comparison of statistical and blocky copolymers of ethylene terephthalate and ethylene 4,4′-bibenzoate based on thermal behavior and oxygen transport properties. J Polym Sci Polym Phys 41:289–307
Loman AJB, Van Der Does L, Bantjes A, Vulic I (1995) Effect of methyl groups on the thermal properties of polyesters from methyl substituted 1,4-butanediols and 4,4′-biphenyldicarboxylic acid. J Polym Sci Polym Chem 33:493–504
Ma H, Hibbs M, Collard DM, Kumar S, Schiraldi DA (2002) Fiber spinning, structure, and properties of poly(ethylene terephthalate-co-4,4′-bibenzoate) copolyesters. Macromolecules 35:5123–5130
Martínez-Gómez A, Pereña JM, Lorenzo V, Bello A, Pérez E (2003) Mechanical properties of drawn smectic mesophases. Poly(tetramethylenoxypropylene p, p′-bibenzoate). Macromolecules 36:5798–5803
Martínez-Gómez A, Bello A, Pérez E (2004) Thermotropic behavior of a liquid crystalline polybibenzoate with an asymmetric oxymethylenic spacer. Macromolecules 37:8634–8640
Martínez-Gómez A, Pérez E, Bello A (2006) Phase behavior of a liquid crystalline polyetherester derived from 4′-hydroxy-1,1′-biphenyl-4-carboxylic acid and the ether-diol 4-(3-hydroxypropoxy)butan-1-ol. Macromolecules 47:2080–2090
Martínez-Gómez A, Bello A, Pérez E (2008) Synthesis and structural studies of poly(tetramethyleneoxypropylene p, p′-bibenzoate). e-Polymers 69:1–26
Martínez-Gómez A, Pérez E, Bello A (2010) Polymesomorphism and orientation in liquid-crystalline poly(triethylene glycol p, p′-bibenzoate). Colloid Polym Sci 288:859–867
Martínez-Gómez A, Pérez E, Bello A (2011) Tailoring the phase behavior in thermotropic copolyesters. Macromol Chem Phys 212:1971–1980
Meurisse P, Noel C, Monnerie L, Fayolle B (1981) Polymers with mesogenic elements and flexible spacers in the main chain: aromatic-aliphatic polyesters. Br Polym J 13:55–63
Nakata Y, Watanabe J (1994) A new type of main-chain liquid-crystal polymer derived from 4′-hydroxybiphenyl-4-carboxylic acid and its smectic mesophase behaviour. J Mater Chem 4:1699–1703
Nakata Y, Watanabe J (1997) Frustrated smectic phase with unusual density modulation along layer observed in main chain type of polymers. Polym J 29:193–197
Ohm C, Brehmer M, Zentel R (2010) Liquid crystalline elastomers as actuators and sensors. Adv Mater 22:3366–3387
Ohm C, Brehmer M, Zentel R (2012) Applications of liquid crystalline elastomers. Adv Polym Sci 250:49–94
Osada K, Koike M, Tagawa H, Tokita M, Watanabe J (2004) Thermotropic liquid crystals of main-chain polyesters having a mesogenic 4,4′-biphenyldicarboxylate unit. Anisotropic micro-Brownian motions of polymer in smectic CA phase. Macromol Chem Phys 205:1051–1057
Pereña JM, Marugán MM, Bello A, Pérez E (1991) Viscoelastic relaxations in thermotropic polybibenzoates. J Non-Cryst Solids 131:891–893
Pérez E, Riande E, Bello A, Benavente R, Pereña JM (1992) Thermotropic properties and conformational studies on poly(triethylene glycol p, p′-bibenzoate) and poly(octamethylene p, p′-bibenzoate). Macromolecules 25:605–610
Pérez E, Zhen Z, Bello A, Benavente R, Pereña JM (1994) Phase-transitions in liquid-crystalline poly(octamethylene p, p′-dibenzoate). Polymer 35:4794–4798
Pérez E, Pereña JM, Benavente R, Bello A (1997) Characterization and properties of thermotropic polybibenzoates. In: Cheremisinoff NP (ed) Handbook of engineering polymeric materials. Marcel Dekker, New York, pp 383–397
Pérez E, del Campo A, Bello A, Benavente R (2000) Synchrotron X-ray study of the phase transitions in liquid crystal polyesters derived from p, p′-bibenzoic acid and racemic- and (R)-3-methyl-1,6-hexanediol. Macromolecules 33:3023–3030
Pérez E, Benavente R, Cerrada ML, Bello A, Pereña JM (2003a) Synchrotron X-ray and DSC studies of the phase behaviour of poly(diethylene glycol p, p′-bibenzoate). Macromol Chem Phys 204:2155–2162
Pérez E, Todorova G, Krasteva M, Pereña JM, Bello A, Marugán MM, Shlouf M (2003b) Structure and phase transitions of poly(heptamethylene p, p′-bibenzoate): time-resolved synchrotron WAXS and DSC studies. Macromol Chem Phys 204:1791–1799
Pérez E, Fernández-Blázquez JP, Martínez-Gómez A, Bello A, Cerrada ML, Benavente R, Pereña JM (2009) Applications of synchrotron X-ray diffraction to the study of the phase behavior in liquid crystalline polymers. Lect Notes Phys 776:157–182
Pérez-Manzano J, Fernández-Blázquez JP, Bello A, Pérez E (2006) Liquid-crystalline copolymers of bibenzoate and terephthalate units. Polym Bull 56:571–577
Rodriguez-Amor V, Fernández-Blázquez JP, Bello A, Pérez E, Cerrada ML (2008) Molecular weight effect on the obtainment of parallel and perpendicular orientation in thermotropic poly(diethylene glycol p, p′-bibenzoate). Polym Bull 60:89–96
Schatzki Y (1966) Molecular interpretation of gamma-transition in polyethylene and related compounds. J Polym Sci Polym Symp 14:139–140
Schiraldi DA, Occelli ML, Gould SAC (2001) Atomic force microscopy (AFM) study of poly(ethylene terephthalate-co-4,4′-bibenzoate): a polymer of intermediate structure. J Appl Polym Sci 82:2616–2623
Tokita M, Osada K, Watanabe J (1998) Thermotropic liquid crystals of main-chain polyesters having a mesogenic 4,4′-biphenyldicarboxylate unit XI. Smectic liquid crystalline glass. Polym J 30:589–595
Watanabe J, Hayashi M, Kinoshita S, Niori T (1992) Thermotropic liquid crystals of polyesters having mesogenic p, p′-bibenzoate unit IV. Mesophase behavior of polyester with branched spacers. Polym J 24:597–601
Watanabe J, Hayashi M, Nakata Y, Niori T, Tokita M (1997) Smectic liquid crystals in main-chain polymers. Prog Polym Sci 22:1053–1087
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Martínez-Gómez, A., Encinar, M., Fernández-Blázquez, J.P., Rubio, R.G., Pérez, E. (2016). Relationship Between Composition, Structure and Dynamics of Main-Chain Liquid Crystalline Polymers with Biphenyl Mesogens. In: Thakur, V., Kessler, M. (eds) Liquid Crystalline Polymers. Springer, Cham. https://doi.org/10.1007/978-3-319-22894-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-22894-5_15
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22893-8
Online ISBN: 978-3-319-22894-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)