Abstract
Two independent events—the identification of activating mutations of the RET proto-oncogene, a receptor tyrosine kinase, in medullary thyroid carcinoma, and the recognition that small organic molecules could bind to and inhibit phosphorylation of signaling molecules, thereby inactivating the pathway—led to the recognition that kinase inhibitors could be used to treat medullary thyroid carcinoma (MTC). The introduction of these compounds into clinical practice has transformed the treatment of metastatic MTC and provided insight into the mechanisms by which RET causes C-cell transformation. This chapter will review the progress in this field over the past 7 years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple endocrine neoplasia type 2
- Calcitonin
- Tyrosine kinase inhibitor
- Vandetanib
- Cabozantinib
- RET proto-oncogene
- Medullary thyroid carcinoma
- Metastasis
1 Introduction
The past few decades have been a remarkable period of discovery for cancer biology. Research on specific tumor types and the use of high-throughput DNA sequencing has identified a broad spectrum of signaling abnormalities in cancer. These defects fall into two broad categories: those that activate signaling molecules and regulate growth or prevent death, and a second category where a molecule that normally inhibits growth or promotes cell death is lost or inactivated.
Another concept that has emerged, particularly over the past decade, is the identification of mutant genes that drive accelerated growth, transformation, and metastasis—so-called driver mutations—that occur in the germ line or are acquired somatically. These are to be differentiated from so-called passenger mutations that are acquired during the transformation process and may contribute to transformation, but are not essential (Stratton et al. 2009).
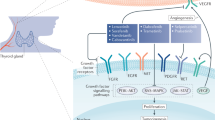
Medullary thyroid carcinoma (MTC) is a tumor derived from the calcitonin (CT)-producing parafollicular or C cells of the thyroid gland. This neuroendocrine cell type is thought to derive from the pharyngeal endoderm or neural crest (or both) and coalesces to form a structure called the ultimobranchial body, which subsequently migrates into the developing thyroid gland (see Chapter: “Thyroid C-cell biology and oncogenic transformation”). The parafollicular cell is the major production site of CT, a hormone that functions to modulate osteoclast-mediated bone resorption, and is used as a tumor marker to diagnose and monitor progression of MTC (Wells et al. 2015).
In 1961, the observation was made that MTC (along with parathyroid neoplasia and pheochromocytoma) is transmitted as an autosomal dominant trait in families as a part of a syndrome designated multiple endocrine neoplasia type 2 (MEN2) (Sipple 1961). This observation led subsequently to the identification of large multi-generational families with MEN2, mapping of the causative gene in the 1980s and the identification of the RET proto-oncogene mutations in 1993 (Donis-Keller et al. 1993; Mulligan et al. 1993).
RET is a member of the receptor tyrosine kinase family. It is a single-pass receptor that partners with one of the 4 extracellular proteins (GFRα1-4) to function as a receptor for one of the 4 ligands, artemin, glial-cell line-derived neurotrophic factor, neurturin, and persephin (PSPN). In the C or parafollicular cell, GFRα-4 partners with RET to form a receptor for PSPN. Activation of RET by PSPN leads to dimerization of the receptor, autophosphorylation of specific tyrosine residues within the receptor and activation of downstream signaling pathways (Ibanez 2013). The phosphorylation occurs by interaction of adenosine triphosphate (ATP) with the RET intracellular kinase domain and the subsequent transfer of phosphate to the receptor and downstream signaling molecules.
The mutations of RET associated with MEN2 fall into two broad categories: extracellular and intracellular. The extracellular mutations (codon 634 is the most common) promote dimerization of the receptor, autophosphorylation, and activation of downstream signaling pathways. The most common intracellular mutation (codon M918T) causes phosphorylation in the absence of dimerization and subsequent activation of downstream signaling pathways (Santoro and Carlomagno 2013). It was demonstrated subsequently that more than 50 % of sporadic MTCs have a RET activating mutation (Wohllk et al. 1996) and, more recently, HRAS and KRAS mutations have been identified in 15–30 % and BRAF mutations in 0–7 % of sporadic MTCs without identifiable RET mutations (Goutas et al. 2008; Boichard et al. 2012; Moura et al. 2011; Agrawal et al. 2013). That RET, RAS, and BRAF mutations are the likely “driver mutations” in MTC is evidenced by the fact that exomic sequencing of MTCs, both hereditary and sporadic, has identified RET, HRAS, KRAS, and BRAF mutations but few other consistent mutations. That is not to say that other mutations do not occur. In the largest reported study of exomic sequencing in sporadic MTC, 18 nonidentical mutations were identified; none were considered to be driver mutations (Agrawal et al. 2013). It also does not exclude the possibility of loss of genomic material or rearrangements.
2 How Does the RET Proto-Oncogene Cause Transformation of the C Cell—the Role of ATF4
The cancer genome atlas project has provided much insight into the molecular abnormalities identified in a broad spectrum of cancer. Included in the first 34 malignancies to be sequenced was papillary thyroid carcinoma. Perhaps the most remarkable finding of this analysis was the low frequency of gene mutation that occurs in papillary thyroid carcinoma. This contrasts with other malignancy types such as melanoma and lung cancer where there are a large number of molecular abnormalities observed. Preliminary exome sequence analysis suggests MTC will fall into the same category as papillary thyroid carcinoma with few mutations other than RET, RAS, and BRAF. While this does not exclude the possibility that there are other genetic abnormalities that contribute to transformation not yet been identified, we are left with the reality that mutation of a single gene (RET or RAS or BRAF) may drive transformation of the C cell.
Studies over the past 2 decades since RET was first identified as the cause for hereditary MTC have provided some insight into molecular pathogenesis. We know with some certainty that phosphorylation of RET residue tyrosine 1062 and others, through ligand activation or mutation of the receptor, results in activation of RAS/MAPK and JNK pathways. However, it is not clear how activation of these pathways leads to transformation. Is continuous activation of RET, as would be seen with an activating mutation of RET, sufficient for transformation or are additional molecular events (loss of genetic material or activation of other oncogenes through mutation) required for transformation to occur? There is currently no clear answer to these questions, although we know from several studies of MTC tumors that additional molecular events (loss of all or part of chromosomes 1p, 22q, 7q36.1, 12p13.31, 13q12.11, and 19p13.3–19p13.11) occur with some frequency. A question that has challenged the field is why a period of 4–10 years is required for C cells to evolve from normal to hyperplasia to MTC. Recent studies that implicate the transcription factor ATF4 in the downstream RET cascade may provide some insight into why C cells accumulate.
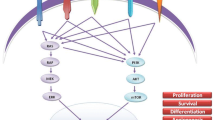
ATF4 is a member of the CREB family of transcription factors and also plays a central role in the regulation of the integrated stress response, a process that activates survival or death pathways when the cell is exposed to stress. Increased ATF4 expression causes increased NOXA and PUMA expression. These two proteins activate caspase pathways and promote entry into the apoptotic pathway. Recent studies have shown that upon activation (by ligand or mutation), RET is transported intact from the cell membrane to the nucleus where it is available to interact with nuclear proteins (Bagheri-Yarmand et al. 2015). RET joins a growing group of receptor tyrosine kinases capable of exerting direct nuclear effects (Hsu and Hung 2007; Lemmon et al. 2014). In the nucleus, RET interacts with and downregulates expression of ATF4 (Fig. 1). In another surprise, RET, which is known as a receptor tyrosine kinase, also phosphorylates ATF4 on threonine residues, thus functioning as a dual-specificity kinase (Bagheri-Yarmand et al. 2015). This interaction causes two effects: transcriptional downregulation of ATF4, and its ubiquitination and subsequent degradation. This causes downregulation of NOXA and PUMA leading to decreased apoptosis. The attendant reduction of cell death may be causative for the accumulation of C cells (C-cell hyperplasia) that is a precursor to MTC and occurs in the thyroid glands of children with germline-activating mutations of RET.
Role of ATF4 in regulation of apoptosis. Activation of RET by ligand activation or mutation results in its translocation to the nucleus, where it reduces transcription and promotes ubiquitination of the transcription factor ATF4. Reduced expression of ATF4 lowers expression of NOXA and PUMA, two factors that promote apoptosis. As a result, C-cell death is inhibited by RET activation and may be a factor in the development of C-cell hyperplasia
While much work is needed to place these recent observations in proper perspective, they are likely to have relevance to the mechanism by which RET-specific tyrosine kinases cause MTC cell death.
3 The Recognition that Tyrosine Kinase Inhibitors Have Efficacy in the Treatment of MTC—the Gastrointestinal Stromal Tumor Precedent
The dawn of tyrosine kinase inhibitor use in oncology occurred with the recognition that imatinib, a small organic ATP analogue that preferentially interacts with the ATP binding pocket of a kinase domain and inhibits ATP binding, was capable of reversing the hematologic abnormalities associated with chronic myelogenous leukemia. This led to its examination in solid tumors. In 2001, a case report documented a dramatic response of a gastrointestinal stromal tumor (GIST) to imatinib, a small organic molecule that inhibited ATP binding to the kit receptor (Joensuu et al. 2001). What is remarkable about this observation and the subsequent experience was the recognition that inhibition of signaling caused by a “driver mutation” not only prevented growth, but also triggered massive apoptotic death of tumor cells, thereby leading to substantial reductive effects on tumor mass. Indeed the term, “oncogene addiction” was coined in an attempt to explain why a small organic ATP analogue that targeted the kinase domain of a receptor could have such profound effects on a solid tumor in the context of a multitude of other genetic abnormalities (Pagliarini et al. 2015).
4 Approved Tyrosine Kinase Inhibitors for Treatment of MTC
It was a report from Carlomagno and colleagues in 2002 that first brought this into focus for MTC (Carlomagno et al. 2002). They demonstrated vandetanib, a tyrosine kinase inhibitor with known activity against the vascular endothelial growth factor receptor 2 (KDR) and the epidermal growth factor receptor (EGFR), also targeted the RET receptor and inhibited RET-mediated MTC transformation and growth. Although these observations prompted excitement in the MEN2 community, convincing the pharmaceutical manufacturer to invest in a clinical trial for a rare thyroid cancer required perseverance. A phase II trial led by Wells et al. in patients with metastatic MTC demonstrated that 20 % of the 30 treated patients experienced a 30 % or greater reduction of tumor size (Wells et al. 2010) and a second phase II trial with a lower dose performed by Robinson and colleagues showed substantial activity (Robinson et al. 2010). The results of these trials convinced the manufacturer to support the first phase III trial ever performed for MTC. This trial of vandetanib (300-mg starting dose) demonstrated a prolongation of progression-free survival of 11 months with a hazard ratio of 0.46 and an objective response rate of 45 % (Wells et al. 2012). The design of the study (patients on placebo were permitted to cross over to active drug if they had disease progression) will make a determination of overall survival difficult, although an analysis is planned. Serum CT and carcinoembryonic antigen (CEA) response rates (decline in serum CT and CEA) were 62 and 59 %, respectively, and were durable (Wells et al. 2012). Vandetanib was approved for treatment for metastatic MTC in April 2011 by the Food and Drug Administration (FDA) in the USA and in February 2012 by the European Medicine Agency (EMA). The speed of evaluation and approval was remarkable. From the time of treatment of the first patient in the phase II trial to approval was less than 7 years.
After the identification of vandetanib activity, additional kinase inhibitors were found to have activity against RET. Among these was cabozantinib, a multi-kinase agent with greater affinity for RET and KDR than vandetanib and activity against the MET oncogene (Table 1). In a phase I/II trial, 10 of 35 patients (29 %) with measureable MTC had a partial response (>30 % reduction in tumor diameter) and an additional 15 of 37 patients (41 %) had stable disease for an overall response rate of 68 % (Kurzrock et al. 2011). These findings led to a phase III study of cabozantinib that differed in several significant ways from the earlier phase III study for vandetanib. The first was the requirement that patients have evidence of progression during a 14-month period prior to entry (the vandetanib phase III study did not require disease progression). The second was a study design in which crossover from placebo to active drug was not permitted, making it possible to compare overall survival of the treatment and placebo groups. Thus, patients were randomized to either cabozantinib 140 mg or placebo. The estimated progression-free survival for cabozantinib was 11.2 months compared with 4 months for the placebo, a highly significant difference. The overall response rate was 28 % for cabozantinib and 0 % for the placebo group (Elisei et al. 2013). A recent analysis (a mean of 52.4 months after study initiation) showed a 5.5-month overall survival advantage of cabozantinib over placebo, although this did not reach statistical significance in the intention to treat analysis (Schlumberger et al. 2015). However, there was evidence of a 24.5-month survival advantage for patients with codon M918T mutations compared to placebo (44.3 vs. 18.9 months), a statistically significant improvement. Cabozantinib was approved for marketing November 2012 by the FDA and March 2014 by the EMA.
It is important to recognize that both US and European regulatory agencies have cautioned physicians that these agents should be used only in patients with progressive metastatic MTC. There is no evidence that these agents are curative and at the approved doses toxicity occurs commonly. Despite these concerns, there is the sense that these agents are altering clinical outcomes in a subset of patients with metastatic MTC as will be discussed later in this chapter.
At this time, there is no basis for choosing one of the approved therapies over another—there have been no direct comparison of these two agents. There may be legitimate reasons to consider one or the other. For example, it may be appropriate to consider cabozantinib over vandetanib in a patient who is on a pharmacological agent(s) that prolongs the QT interval; similarly, in a patient who develops palmar-plantar erythrodysesthesia on cabozantinib, it may be appropriate to consider switching the patient to vandetanib.
One of the remarkable but not highlighted findings in the phase II and III studies of both vandetanib and cabozantinib is the subset of the patients who had very substantial tumor responses within the first several months of treatment, suggesting a phenomenon similar to that observed for GIST tumors treated with imatinib. The rapidity of response (as evidenced by reductions of tumor size and rapid and sustained decreases in tumor markers) suggests a triggering of apoptotic cell death similar to that observed earlier in treatment of GIST tumors with imatinib. Alternatively, this could result from an inhibition of vascular flow. The finding that tyrosine kinase inhibition of RET activity downregulates ATF4 and through it enhances apoptosis (Bagheri-Yarmand et al. 2015), provides a plausible regulatory mechanism for the rapid effect of kinase inhibitors on tumor size, the so-called phenomenon of oncogene addiction. Another potential mechanism is the effects of vandetanib and cabozantinib on VEGFR2. There is clear evidence of increased VEGF and VEGFR2 expression by MTC and tumor vasculature. Some of the effects to cause rapid MTC death may be related to a reduction in blood flow to the tumor. The fact that each of the tyrosine kinase inhibitors with activity in MTC targets not only RET, but also VEGFR2 provides credence to this thought process.
5 What Constitutes an Appropriate Trial of Therapy?
How long should one continue therapy before determining that a particular therapy is or is not effective? There seem to be at least two different patterns of response. The first is characterized by rapid (within the first month or two) decreases in serum CT and CEA and radiographic evidence at first imaging (usually 3 months after initiation of therapy) of response. In this situation, continuation of therapy is axiomatic. Inevitably, the rate of response slows. A question for which there is no answer at present when this happens is whether to continue the therapeutic agent at the initial dose, with potential development of toxicity, or to consider a dose reduction. In most cases, if there is no toxicity, it is appropriate to continue the initial dose. If toxicity develops, a dose reduction will become mandatory. Dose reduction is usually associated with a plateauing or reversal of the response. A second pattern of positive response is defined by a slow but continuous decline in tumor markers and a reduction in the size of the lesion over a 6-, 9-, and 12-month period with continued declines over a 1- to 2-year period. Therapy should not be discontinued if there is no response at time of first radiographic assessment. This type of response is often durable for a number of years.
In a substantial minority of patients, after an initial response, there will be progression of disease, either at the site(s) of initial response or other sites. Unless there is a specific explanation for the progression, such as a drug holiday because of side effects, in most cases it will be appropriate to discontinue the therapeutic agent and consider the options discussed below.
6 Other Tyrosine Kinase Inhibitors with Activity in Medullary Thyroid Carcinoma
The recognition that multi-kinase inhibitors, particularly those that target RET and VEGFR2, have activity in MTC led to an examination of kinase inhibitors with similar target specificity in phase II MTC trials. None has been examined in a phase III trial, but the results of phase I/II trials for several are promising. These agents are summarized in Table 1 and are also highlighted in several recent reviews. Some of these agents, particularly sunitinib (Carr et al. 2010) and lenvatinib (Schlumberger et al. 2012), have significant activity and could be considered as potential third-line therapy in MTC. Other agents including axitinib, pazopanib (Bible et al. 2014), and sorafenib have lower levels of activity (Table 1). Another of these agents, motesanib (Sherman et al. 2008), despite comparable activity against RET and VEGFR2 in vitro, had little activity in a phase II MTC trial, presumably because of diarrhea-induced malabsorption of the agent leading to inadequate plasma concentrations (Schlumberger et al. 2009).
6.1 When Is It Appropriate to Consider second- or third-Line or Salvage Therapy?
There is no formal literature on the question of third-line or salvage therapy for MTC. However, a small experience exists for papillary thyroid carcinoma where about 40 % of patients treated with a second-line therapy will have a subsequent response (Dadu et al. 2014). This combined with anecdotal experience in clinical trials, demonstrating that a small but significant percentage of patients, after failing one therapy, will respond to a second therapy makes it reasonable to consider a second- or even third-line therapy. The choice of a second-line therapy would most commonly include an approved therapy (either vandetanib or cabozantinib); a third-line therapy could include sunitinib or lenvatinib, each approved for other indications and available. Several other kinase inhibitors, such as pazopanib, sorafenib, or axitinib, have lower activity, or there is less experience; therefore, these agents would fall to a lower level of consideration.
6.2 Other Agents or Combinatorial Therapy
The recognition that RET activates MAP kinase and JNK pathways has raised the question of whether agents that target PI3 K pathways (such as mTOR inhibitors) might have utility in the treatment of MTC. Preclinical literature indicates that mTOR inhibitors, either alone (Lyra et al. 2014) or in combination with tyrosine kinase inhibitors that target RET (Gild et al. 2013), have considerable activity in MTC model systems. There is a limited experience with the mTOR inhibitor, everolimus, in humans that shows activity (Faggiano et al. 2012). Clinical trials that combine RET-specific kinase inhibitors and mTOR inhibitors will undoubtedly be developed over the next several years. One issue that has emerged in combinatorial trials of targeted agents is the potential for not only greater activity but also accentuation of toxicity. For therapies taken for extended time periods, this is a significant issue that will have to be addressed in clinical trials.
Similarly, the aforementioned studies defining the role of ATF4 in the regulation of C-cell apoptosis provides another potential therapeutic target (Bagheri-Yarmand et al. 2015). Although there are preliminary in vitro data to suggest the utility of stabilizing ATF4 in MTC as a therapeutic strategy, either alone or in combination with RET-targeted agents, these studies have not yet reached the clinical arena.
7 Side Effects of Approved Tyrosine Kinase Inhibitors
The signaling molecules targeted with high affinity (RET, VEGFR, EGFR, and MET) by the two approved tyrosine kinase inhibitors have broad importance in human biology and regulate the neurologic, gastrointestinal, vascular, dermatologic and hepatic systems, and among others. It is therefore not surprising that multi-kinase inhibitors that target several of these receptor systems should have toxicity. Indeed, the term “targeted therapy” refers to an agent that targets a specific signaling pathway in a neoplastic process, but conveniently ignores that the fact that these same signaling systems are important for a broad spectrum of normal biologic processes. The challenge for the clinician is to balance the substantial positive effects of these agents (on-target effects) with their off-target toxicity.
Tables 2 and 3 list the most common side effects and laboratory abnormalities observed with the two approved agents from their phase III studies (Elisei et al. 2013; Wells et al. 2012). The range of toxicity for both agents is significant, particularly when prescribed at the approved starting dose of 300 mg for vandetanib and 140 mg for cabozantinib. There have been no trials reported that directly compare the approved starting doses to lower dose therapy, although an FDA-mandated trial comparing vandetanib 300–150 mg for treatment of MTC is currently underway. There have been no trials that directly compare vandetanib to cabozantinib.
Management of toxicity can be challenging, particularly for individuals who have trained in endocrinology and have less experience with the types of toxicity observed with cancer treatment. In contrast, oncologists are very familiar with toxicity, but have less experience managing other medical problems (hypothyroidism, hypoparathyroidism, and adrenal insufficiency following bilateral adrenalectomy) that occur in MEN2. In some oncologic centers, endocrinologists have become comfortable with management of toxicity associated with these agents and directly manage these patients; in others, close partnerships have developed between oncologists and endocrinologists to provide optimal management for these patients.
There are several issues related to toxicity that are important to address. The first relates to the “black box warning” and the risk evaluation mitigation strategy (REMS) program mandated by the FDA for vandetanib. This agent caused prolongation of the QT interval in approximately 14 % of treated patients in the phase III trial (Wells et al. 2012), and there was unexplained death in several patients who participated in the phase II and phase III trials. Physicians who prescribe vandetanib are required to participate in a risk evaluation and management program (REMS) that mandates periodic electrocardiographic evaluation and avoidance of other drugs that may cause prolongation of the QT interval. In practice, routine evaluation of electrocardiograms for QT prolongation before and during therapy and avoidance of other medications that prolong the QT interval will permit safe use of this compound. Other vandetanib effects that may contribute to QT prolongation include hypokalemia, hypocalcemia, hypothyroidism, and hypomagnesemia (Wells et al. 2012). These should be addressed promptly when recognized.
The risks of hypocalcemia, hypomagnesemia, and hypothyroidism are much greater in patients with MTC who have undergone thyroidectomy and who may have compromised parathyroid function. Both vandetanib and cabozantinib, and most other compounds described in Table 1 that target RET and VEGFR2, cause malabsorption and diarrhea. In patients who have compromised parathyroid function (common in patients with extensive surgical neck dissections), malabsorption of vitamin D, calcium, and magnesium can lead to symptomatic hypocalcemia. Malabsorption of thyroid hormone occurs with high frequency. Adjustment of calcium, vitamin D, and thyroid hormone dosages is required in most patients and many require magnesium supplementation. In a subset of these patients, the dose adjustments can be substantial.
The most troubling side effect of cabozantinib is hand–foot reaction or palmar-plantar erythrodysesthesia, a disabling, desquamating condition of the palms of the hands and the plantar surface of the feet that may be associated with considerable pain. Inevitably, these patients will require dermatologic evaluation and treatment with local and occasionally systemic corticosteroids and a likely dose reduction of the cabozantinib. The frequency of hand–foot reaction with cabozantinib is approximately 50 % (Elisei et al. 2013), and if a patient needs to continue use of hands or feet during work, this may be a dose-limiting toxicity.
As illustrated in Table 2, hypertension occurs in approximately one-third of patients treated with either cabozantinib or vandetanib. It is important to be vigilant and measure blood pressure regularly (or have it measured by the patient or patient’s primary physician) and to treat hypertension aggressively. Most commonly, the hypertension will respond to some combination of ACE inhibitor, angiotensin receptor blockade, α- or β-antagonists, or calcium channel inhibitors. Uncontrolled hypertension can necessitate stoppage of the drug related to development of intractable headache or renal or cardiac dysfunction.
8 The Decision to Initiate Therapy with a Tyrosine Kinase Inhibitor—When to Move from Watchful Waiting to Active Therapy—a Case-Based Approach
8.1 Watchful Waiting
The question of when to consider initiation of therapy is often a difficult one. MTC is frequently a slowly progressive malignancy, even when there is metastatic disease. As neither of the 2 approved agents is curative, there is broad consensus that “watchful waiting” is an appropriate course of action in many patients. It is not uncommon for MTC to progress slowly over years or decades. A helpful tool to understand the rate of progression is to plot serum CT and CEA, an indicator of tumor mass, over years/decades and to calculate a doubling time for these tumor markers (Laure Giraudet et al. 2008). Patients with a doubling time of greater than 2 years have a lower probability of dying from metastatic MTC, whereas those with a shorter doubling time and significant soft tissue metastasis are at risk for meaningful progression of metastatic disease and death and should be considered for therapy. However, even patients with a doubling time over 2 years may eventually develop bulky metastatic disease that causes hepatic dysfunction, compromise of pulmonary function, bone pain, or troublesome diarrhea. In patients in this category with a substantial “tumor burden,” intervention with tyrosine kinase inhibitor therapy may be appropriate. Indeed, in the phase II and phase III studies of vandetanib, there were patients in whom treatment of slowly progressive disease resulted in a reduction of tumor mass and a decrease of CT and CEA to a lower level with a concordant reduction of symptoms such as diarrhea and flushing. Thus, use of vandetanib or cabozantinib in a symptomatic patient with assessable metastasis is reasonable.
8.2 Rapid Escalation of Tumor Growth in a Patient with Prior Stable Disease
Rapid escalation of tumor markers and tumor growth following a prior period of stability is another situation in which kinase inhibitor therapy is appropriate. Figure 2 shows such an example. This patient had a 15-year period following thyroidectomy for MEN2B-associated MTC where there was no clinical evidence of disease other than minimal elevations of the serum CT and CEA where “watchful waiting” was the most appropriate approach. A rapid rise in serum CT and CEA was associated with development of hepatic and pulmonary metastasis. Although the patient had a substantial response to tyrosine kinase inhibitor therapy with marked reduction of pulmonary metastasis and had an additional year of quality life, tumor growth subsequently escalated on therapy and the patient succumbed a year and a half later. While the question can be asked of whether earlier low-dose therapy might have impacted the subsequent rate of tumor growth, there is, in fact, no current evidence that earlier therapy would be beneficial.
Watchful waiting. This patient with MEN2 underwent a total thyroidectomy and neck dissection for MTC. Serum CT and CEA (not shown) measurements remained stable over almost a 15-year period before increasing rapidly. This was associated with development of hepatic and pulmonary metastasis. Treatment with tyrosine kinase inhibitor therapy resulted in a return of serum CT and CEA to pretreatment levels with stability for slightly more than a year and resolution of pulmonary metastasis. The patient subsequently progressed rapidly while on therapy and died
8.3 Location of Metastatic Medullary Thyroid Carcinoma
Another feature that could drive a decision to initiate therapy is the location of the lesion. It is possible to have substantial metastases in the liver and lungs that grow slowly over time with little functional impairment. Figure 3 shows an example of patient with hepatic metastasis who had a prolonged and durable response to tyrosine kinase therapy over an extended period. In contrast, lesions that about the upper airway or sizeable lesions in the mediastinum can lead to significant impairment of respiratory or esophageal function. In a patient with such an inoperable lesion, tyrosine kinase therapy could be considered. One complication that has been observed is fistula development or bleeding associated with a lesion adjacent to the trachea or esophagus, particularly if there is a rapid response to therapy. As both vandetanib and cabozantinib target VEGFR2 receptors, their impact on tumor vascularity can cause hemorrhage in a patient with a lesion adjacent to and invading the trachea. Figure 4 shows an example of a patient with long-standing MTC who developed tracheal invasion that was not considered resectable without performance of a laryngectomy, which the patient declined. A decision was made, with full discussion with the patient of the potential risks of fistula development and/or hemorrhage, to initiate therapy with a tyrosine kinase inhibitor. The patient has tolerated the starting dose without toxicity for a period of almost 4 years with reduction in the size of the paratracheal mass.
Response of paratracheal mass to tyrosine kinase therapy. This patient with MTC presented with a paratracheal mass that was unresectable without a concurrent laryngectomy, which he declined. Treatment with tyrosine kinase inhibitor therapy resulted in a significant reduction in tumor size. The response has been maintained for a period of almost 4 year, but without further reduction in size after the first 20 months
8.4 Bone Metastasis
Bone metastasis occurs commonly in patients with metastatic MTC. As metastasis to bone is excluded from analysis using RECIST criteria for evaluation of tumor response, there is no formal analysis of response in the phase II or III trials of either vandetanib or cabozantinib (Wells et al. 2012; Elisei et al. 2013). Figure 5 shows a bone response to tyrosine kinase inhibitor therapy at 13 months with a reduction in size of multiple lesions and remineralization of lytic lesions, indicating potential efficacy.
Reduction in size of bone metastasis and partial healing of lytic lesions in a patient with metastatic MTC. This elderly patient had widespread metastatic MTC with extensive bone metastasis. This figure shows reduction in the size of pelvic bone metastasis and remineralization of lytic lesions over a 13-month period of treatment with tyrosine kinase inhibitor therapy. The patient subsequently developed recurrence while on therapy and died
9 Prediction of Response
The only criterion that predicts response is the presence of a somatic or germline RET codon M918T mutation as was demonstrated for both vandetanib and cabozantinib in the phase III trials (Wells et al. 2012; Elisei et al. 2013). In addition, there is evidence of an overall survival benefit for patients with RET codon M918T mutations treated with cabozantinib in recent results from the phase III trial (Schlumberger et al. 2015).
10 Duration of Response
There are no predictors of duration of response in the phase III trials of either vandetanib or cabozantinib. That durable responses occur has been documented. Figure 4 shows a durable response of almost 4 years for a paratracheal mass and Fig. 3 shows an example of an MEN2 patient with hepatic metastasis treated with a tyrosine kinase inhibitor who responded within a 12-month period and had no evidence of recurrence over a 7-year period. What is not clear is the frequency of such responses. Available evidence from the phase III trial of cabozantinib indicates that overall survival, a surrogate for response, is enhanced only in patients with a somatic or germline RET codon M918T mutation (Schlumberger et al. 2015). There are no similar data available for vandetanib at this time. Anecdotal experience suggests that responses lasting greater than 3–4 years will occur in a minority of patients.
11 Tyrosine Kinase Inhibitor Use in Pediatric Patients
A phase I/II trial of vandetanib in children and adolescents at a starting dose of 100 mg/m2 with a potential dose escalation to 150 mg/m2 after two 28-day cycles showed an objective partial response rate of 47 %, with a mean treatment duration of 27 cycles (Fox et al. 2013). Eleven of 16 patients entering the trial remained on therapy at the conclusion of the study. There have been no comparable trials of cabozantinib in the pediatric or adolescent populations.
12 Dose Reductions of Tyrosine Kinase Inhibitor
There is a long-standing tenet in the oncology community that starting at the highest effective and tolerable dose is preferred over starting at a lower dose and escalating the dose over time. This is based on the fact that some patients will have a substantial reduction in tumor size and vascularity during the first 3 months after initiation of therapy and there is concern that starting at a lower dose will impact negatively on this initial response. A significant percentage of patients will not tolerate the approved starting doses of vandetanib or cabozantinib because of toxicity and will require a dose reduction.
There have been no published studies directly comparing lower initial doses of either approved agent, although a comparative trial of vandetanib 150/day versus 300 mg/day is currently nearing completion. A prior phase II trial of vandetanib (100 mg/day) in hereditary MTC indicates that a lower starting dose has activity (Robinson et al. 2010). It therefore may be reasonable to consider a lower starting dosage with subsequent dose escalation in an individual with lower than normal body weight, poor performance status, or some other disease process (cardiac, pulmonary, dermatologic, or hepatic) that might predict toxicity.
The decision to lower the dose of either cabozantinib or vandetanib should be based on the development of significant toxicity. In the case of cabozantinib or vandetanib, this could include the development of hypertension not controlled by antihypertensives, deterioration in renal function or development of significant proteinuria, liver function abnormalities, unmanageable vomiting or diarrhea with profound weight loss, and severe dermatologic manifestations or other less common but significant side effects (Tables 2 and 3). Unique to cabozantinib is the development of palmar-plantar erythrodysesthesia (Cho and Chan 2013), whereas significant prolongation of the QT interval that is not remedied by the drug or supplement modifications mentioned earlier is an indication for vandetanib dose reduction (Shah et al. 2013). In general, when severe side effects occur, cessation of drug for a period of time to allow for normalization is mandatory followed by a dose reduction of 25–30 %.
Generally, the development of primary hypothyroidism, hypocalcemia caused by calcium, vitamin D malabsorption, or hypomagnesemia can be managed without dose reduction of the kinase inhibitor by dose escalation of thyroid hormone in thyroidectomized patients or dose increases or supplementation with calcium, vitamin D, or magnesium preparations. Symptomatic hypocalcemia may require a temporary cessation of kinase inhibitor therapy. In a patient who had a bilateral adrenalectomy for pheochromocytoma in the context of MEN2 and is on corticosteroid and mineralocorticoid replacement, it may be challenging to differentiate between adrenal insufficiency and symptoms attributable to kinase inhibitor therapy. A dose increase of corticosteroid or mineralocorticoid may be needed. It is clear from published reports that vandetanib has profound effects to inhibit ectopic production of ACTH by MTC (Nella et al. 2014), thereby reversing Cushing’s syndrome. A similar effect was seen in a patient with MTC treated with sorafenib (Barroso-Sousa et al. 2014). It is less clear whether vandetanib has similar effects on the normal pituitary gland. One published report suggests that plasma ACTH concentrations are increased in vandetanib-treated patients (Brassard et al. 2011). The finding that vandetanib reverses ectopic ACTH production in MTC may have relevance to management of ectopic ACTH syndrome caused by lung cancer; earlier studies showed activity of this agent in the treatment of lung cancer (Tsao et al. 2013). One of the authors (RFG) has a patient with MEN2A and bilateral adrenalectomy who had two episodes of adrenal insufficiency while on cabozantinib, necessitating a dose reduction of the kinase inhibitor.
13 Combining Tyrosine Kinase Inhibitor Therapy with Other Treatment Modalities
The availability of agents that are capable of reducing tumor mass opens up the possibility of combining tyrosine kinase inhibitor therapy with other proven treatment modalities. In the example shown in Fig. 4, the question asked was whether treatment with a kinase inhibitor might make this patient’s tumor resectable at some future date. A similar logic could be applied to other neck or mediastinal lesions that threaten the airway. At present, this is uncharted territory, but is being actively considered. A potential concern is that treatment with these agents may make clean surgical margins more difficult to obtain. In addition, it is important to recognize the anti-VEGFR2 effects of these agents. Stoppage of these agents a suitable time prior to surgery is mandatory to facilitate wound healing. Similarly, treatment of a neck or mediastinal mass with kinase inhibitor therapy prior to radiation might reduce the size of the radiation field and hence reduce collateral radiation damage. Such strategies are being considered and will undoubtedly be pursued in the future.
14 The Future
One of the realities facing the MTC research community is the rarity of MTC and hence the lack of enthusiasm for pharmaceutical manufacturers to make a major commitment to new drug development for this tumor. However, that should not stop investigators in the field from pursuing the growing number of agents with known activity against RET, RAS, or BRAF, known to be mutated in MTC, or other potential targets such as mTOR, ATF4, or other factors that interact with RET-mediated signaling pathways. In addition, there is the possibility that other kinase inhibitors that target receptor tyrosine kinases may prove more effective than current agents. One such candidate is ponatinib, a multi-kinase inhibitor with substantial activity against RET and VEGFR2, which is currently under investigation. Finally, the potential for combining tyrosine kinase therapy with immunotherapy is largely unexplored. It is clear that the future is bright for progress in the treatment of this malignancy.
References
Agrawal N, Jiao Y, Sausen M, Leary R, Bettegowda C, Roberts NJ, Bhan S, Ho AS, Khan Z, Bishop J, Westra WH, Wood LD, Hruban RH, Tufano RP, Robinson B, Dralle H, Toledo SP, Toledo RA, Morris LG, Ghossein RA, Fagin JA, Chan TA, Velculescu VE, Vogelstein B, Kinzler KW, Papadopoulos N, Nelkin BD, Ball DW (2013) Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab 98(2):E364–E369. doi:10.1210/jc.2012-2703
Bagheri-Yarmand R, Sinha KM, Gururaj AE, Ahmed Z, Rizvi YQ, Huang SC, Ladbury JE, Bogler O, Williams MD, Cote GJ, Gagel RF (2015) A novel dual kinase function of the RET proto-oncogene negatively regulates activating transcription factor 4-mediated apoptosis. J Biol Chem 290(18):11749–11761. doi:10.1074/jbc.M114.619833
Barroso-Sousa R, Lerario AM, Evangelista J, Papadia C, Lourenco DM Jr, Lin CS, Kulcsar MA, Fragoso MC, Hoff AO (2014) Complete resolution of hypercortisolism with sorafenib in a patient with advanced medullary thyroid carcinoma and ectopic ACTH (adrenocorticotropic hormone) syndrome. Thyroid Official J Am Thyroid Assoc 24(6):1062–1066. doi:10.1089/thy.2013.0571
Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Karlin N, Sideras K, Morris JC, 3rd, McIver B, Hay I, Fatourechi V, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Cher Goh B, Isham CR, Harris P, Erlichman C, Endocrine Malignancies Disease Oriented Group MCCC, the Mayo Phase C (2014) A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J Clin Endocrinol Metab 99(5):1687–1693. doi:10.1210/jc.2013-3713
Boichard A, Croux L, Al Ghuzlan A, Broutin S, Dupuy C, Leboulleux S, Schlumberger M, Bidart JM, Lacroix L (2012) Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab 97(10):E2031–E2035. doi:10.1210/jc.2012-2092
Brassard M, Neraud B, Trabado S, Salenave S, Brailly-Tabard S, Borget I, Baudin E, Leboulleux S, Chanson P, Schlumberger M, Young J (2011) Endocrine effects of the tyrosine kinase inhibitor vandetanib in patients treated for thyroid cancer. J Clin Endocrinol Metab 96(9):2741–2749. doi:10.1210/jc.2010-2771
Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M (2002) ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62(24):7284–7290
Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG (2010) Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res Official J Am Assoc Cancer Res 16(21):5260–5268. doi:10.1158/1078-0432.CCR-10-0994
Cho YT, Chan CC (2013) Cabozantinib-induced hand-foot skin reaction with subungual splinter hemorrhages and hypertension: a possible association with inhibition of the vascular endothelial growth factor signaling pathway. Eur J Dermatol EJD 23(2):274–275. doi:10.1684/ejd.2013.1930
Dadu R, Devine C, Hernandez M, Waguespack SG, Busaidy NL, Hu MI, Jimenez C, Habra MA, Sellin RV, Ying AK, Cote GJ, Sherman SI, Cabanillas ME (2014) Role of salvage targeted therapy in differentiated thyroid cancer patients who failed first-line sorafenib. J Clin Endocrinol Metab 99(6):2086–2094. doi:10.1210/jc.2013-3588
Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA Jr (1993) Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 2(7):851–856
Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol Official J Am Soc Clin Oncol 31(29):3639–3646. doi:10.1200/JCO.2012.48.4659
Faggiano A, Ramundo V, Dicitore A, Castiglioni S, Borghi MO, Severino R, Ferolla P, Crino L, Abbruzzese A, Sperlongano P, Caraglia M, Ferone D, Hofland L, Colao A, Vitale G (2012) Everolimus is an active agent in medullary thyroid cancer: a clinical and in vitro study. J Cell Mol Med 16(7):1563–1572. doi:10.1111/j.1582-4934.2011.01438.x
Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SM, Wells SA, Balis FM (2013) Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res Official J Am Assoc Cancer Res 19(15):4239–4248. doi:10.1158/1078-0432.CCR-13-0071
Gild ML, Landa I, Ryder M, Ghossein RA, Knauf JA, Fagin JA (2013) Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr Relat Cancer 20(5):659–667. doi:10.1530/ERC-13-0085
Goutas N, Vlachodimitropoulos D, Bouka M, Lazaris AC, Nasioulas G, Gazouli M (2008) BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res 28(1A):305–308
Hsu SC, Hung MC (2007) Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem 282(14):10432–10440. doi:10.1074/jbc.M610014200
Ibanez CF (2013) Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol 5(2). doi:10.1101/cshperspect.a009134
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Eng J Med 344(14):1052–1056. doi:10.1056/NEJM200104053441404
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, Pfister DG, Cohen EE, Janisch L, Nauling F, Hong DS, Ng CS, Ye L, Gagel RF, Frye J, Muller T, Ratain MJ, Salgia R (2011) Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol Official J Am Soc Clin Oncol 29(19):2660–2666. doi:10.1200/JCO.2010.32.4145
Laure Giraudet A, Al Ghulzan A, Auperin A, Leboulleux S, Chehboun A, Troalen F, Dromain C, Lumbroso J, Baudin E, Schlumberger M (2008) Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol Eur Fed Endocr Soc 158(2):239–246. doi:10.1530/EJE-07-0667
Lemmon MA, Schlessinger J, Ferguson KM (2014) The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol 6(4):a020768. doi:10.1101/cshperspect.a020768
Lyra J, Vinagre J, Batista R, Pinto V, Prazeres H, Rodrigues F, Eloy C, Sobrinho-Simoes M, Soares P (2014) mTOR activation in medullary thyroid carcinoma with RAS mutation. Eur J Endocrinol Eur Fed Endocr Soc 171(5):633–640. doi:10.1530/EJE-14-0389
Moura MM, Cavaco BM, Pinto AE, Leite V (2011) High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 96(5):E863–E868. doi:10.1210/jc.2010-1921
Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L et al (1993) Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363(6428):458–460. doi:10.1038/363458a0
Nella AA, Lodish MB, Fox E, Balis FM, Quezado MM, Whitcomb PO, Derdak J, Kebebew E, Widemann BC, Stratakis CA (2014) Vandetanib successfully controls medullary thyroid cancer-related Cushing syndrome in an adolescent patient. J Clin Endocrinol Metab 99(9):3055–3059. doi:10.1210/jc.2013-4340
Pagliarini R, Shao W, Sellers WR (2015) Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep 16(3):280–296. doi:10.15252/embr.201439949
Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R (2010) Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab 95(6):2664–2671. doi:10.1210/jc.2009-2461
Santoro M, Carlomagno F (2013) Central role of RET in thyroid cancer. Cold Spring Harb Perspect Biol 5(12):a009233. doi:10.1101/cshperspect.a009233
Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JP, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Sherman SI (2009) Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol Official J Am Soc Clin Oncol 27(23):3794–3801. doi:10.1200/JCO.2008.18.7815
Schlumberger M, Jarzab B, Cabanillas ME (2012) A Phase II trial of the multitargeted kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer (MTC). J Clin Oncol Official J Am Soc Clin Oncol 30; Suppl; Abstr 5591
Schlumberger M, Elisei R, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI (2015) Final overall survival analysis of EXAM, an international, doubleblind,randomized, placebocontrolled phase III trial of cabozantinib (Cabo) in medullarythyroid carcinoma (MTC) patients with documented RECIST progression at baseline. J Clin Oncol 33 suppl; abstr 6012
Shah RR, Morganroth J, Shah DR (2013) Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf 36(5):295–316. doi:10.1007/s40264-013-0047-5
Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ, Motesanib Thyroid Cancer Study G (2008) Motesanib diphosphate in progressive differentiated thyroid cancer. N Eng J Med 359(1):31–42. doi:10.1056/NEJMoa075853
Sipple JH (1961) The association of pheochromocytoma with carccinoma of the thyroid gland. Am J Med 31:163–166
Stratton MR, Campbell PJ, Futreal PA (2009) The cancer genome. Nature 458(7239):719–724. doi:10.1038/nature07943
Tsao AS, Liu S, Lee JJ, Alden CM, Blumenschein GR Jr, Herbst R, Davis SE, Kim E, Lippman S, Heymach J, Tran H, Tang X, Wistuba I, Hong WK (2013) Clinical and biomarker outcomes of the phase II vandetanib study from the BATTLE trial. J Thorac Oncol Official Pub Int Assoc Study Lung Cancer 8(5):658–661. doi:10.1097/JTO.0b013e31828d08ae
Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, Skinner M, Krebs A, Vasselli J, Schlumberger M (2010) Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol Official J Am Soc Clin Oncol 28(5):767–772. doi:10.1200/JCO.2009.23.6604
Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol Official J Am Soc Clin Oncol 30(2):134–141. doi:10.1200/JCO.2011.35.5040
Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG (2015) Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid Official J Am Thyroid Assoc 25(6):567–610. doi:10.1089/thy.2014.0335
Wohllk N, Cote GJ, Bugalho MM, Ordonez N, Evans DB, Goepfert H, Khorana S, Schultz P, Richards CS, Gagel RF (1996) Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab 81(10):3740–3745. doi:10.1210/jcem.81.10.8855832
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dadu, R., Hu, M.N., Grubbs, E.G., Gagel, R.F. (2015). Use of Tyrosine Kinase Inhibitors for Treatment of Medullary Thyroid Carcinoma. In: Raue, F. (eds) Medullary Thyroid Carcinoma. Recent Results in Cancer Research, vol 204. Springer, Cham. https://doi.org/10.1007/978-3-319-22542-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-22542-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22541-8
Online ISBN: 978-3-319-22542-5
eBook Packages: MedicineMedicine (R0)