Abstract
In this chapter, we present our recently conceptualized model on Developmental Origins of Behavior, Health, and Disease (DOBHaD) in which we incorporate the results of four of our studies as examples to demonstrate how each topic influenced the model; in addition, we provide a brief overview of relevant literature. The study of DOBHaD encompasses both, short- and long-term consequences of conditions in the environment relevant to behavior, health, and disease risk and addresses research issues related to the interface between developmental, behavioral, and medical science. In the first section, one early and one later study from the Leuven prospective follow-up project are described. Study 1 examines the influence of maternal emotions on fetal and neonatal behavioral state-related activity and on infant activity. Study 2 examines the relationship between fetal behavioral states and self-regulation in childhood and adolescence. In the second section, two recent studies from the Tilburg prospective follow-up project are described. Study 3 explores how variation in both negative emotions (i.e., maternal anxiety) and positive emotions (i.e., maternal mindfulness) influence infant neurocognitive development. Study 4 explores the issue of how exposure to a past, resolved maternal anxiety disorder influences maternal heart rate variability during pregnancy as well as infant heart rate variability, which in turn influences infant temperament. In the final section we summarize our results, use them to explain applications of the DOBHaD model, and speculate on potential clinical implications.
“The idea that at birth the child is an individual is becoming more and more accepted. Prenatally, this individual is already unique because of his unique genotype and because for the past nine months it underwent the influences of his specific prenatal intra -uterine environment.” (Van den Bergh, 1981, p. XII)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Prenatal stress
- Maternal anxiety during pregnancy
- Maternal mindfulness during pregnancy
- Infant event related potentials (ERPs)
- Autonomic nervous system
- Infant temperament
- Fetal behavioral states
- Self-regulation
- Infant cognition
- Heart rate variability in pregnant women
- Infant heart rate variability
Introduction
To introduce his aggregated volume entitled Prenatal determinants of behavior, Joffe (1969, p. ix) wrote: “This book is an attempt to gather together from diverse sources the research which relates events prior to birth to effects on the postnatal behavior of organisms. Such studies are an extension of the widespread and intensive interests in the behavioral effects of events in the early environment to the organism’s earliest environment—the prenatal environment. It is hoped that bringing together studies which arose from a variety of experimental interests and appeared in a wide range of publications will stimulate further interest in a field which only now is being delineated as a research area in its own right.” When he wrote these words, one wonders whether Joffe could have foreseen how much interest the field of prenatal environmental influences was going to gain in the period that lay ahead. Joffe qualified his introductory sentences by stating that, over the centuries, there had been a fluctuation in the belief of prenatal influences and that apparently the status of the area did not depend so much on the available evidence as on the prevailing climate or opinion: “Medical opinion appears to have accepted the general proposition at times and ridiculed at others” (Joffe, 1969, p. 1). Likewise Stott (1958, p. 42) mentioned that effects of psychosomatic stress during pregnancy was “a topic which has fallen under the taboo of “old wives tales.” Although the general proposition of prenatal influences is now widely accepted, the underlying mechanism s of how the prenatal environment influences the developing organism and modulates brain structure–function relations, behavior, health, and disease risk are yet to be fully elucidated (e.g., Crews et al., 2012; Fox, Levitt, & Nelson, 2010; Hofer, 2014; Kolb et al., 2012; Lutz & Turecki, 2014; McEwen & Morrison, 2013; Meaney, 2010; O’Connor, Monk, & Fitelson, 2014; Reul et al., 2015; Schlotz, Jones, Godfrey, & Phillips, 2008; Zannas & West, 2014). Debates on which theoretical frameworks are best able to integrate most of the available research results in a coherent way are ongoing (e.g., Bock, Poeschel et al., 2014; Bock, Rether, Gröger, Xie, & Braun, 2014; Daskalakis, Bagot, Parker, Vinkers, & de Kloet, 2013; Daskalakis & Yehuda, 2014; Del Giudice, 2012; Del Giudice, Ellis, & Shirtcliff, 2011; Hanson & Gluckman, 2014; Lee & Goto, 2013; Lupien, McEwen, Gunnar, & Heim, 2009; Nederhof & Schmidt, 2012; Ortega-Martínez, 2015; Schlotz & Phillips, 2009).
An important reason for our scientific quest, which started some 35 years ago, was the question of when and how individual differences between people arise as well as when and how these processes can be studied. In common with Joffe (1969), we extended our search to the organism’s earliest environment and started to examine such issues as the variation in maternal emotional state during pregnancy and how this might lead to a variation in the behavior of the offspring before and after birth, including potential behavioral problems. To achieve this goal, concepts and methods were borrowed from the fields of developmental psychobiology /developmental behavioral neuroscience (e.g., Blumberg, Freeman, & Robinson, 2010; Gottlieb, 1997; Kolb et al., 2012; Lickliter, 2007; Michel & Moore, 1995), developmental cognitive neuroscience (Johnson, 2011; Johnson & de Haan, 2011), developmental affective neuroscience (e.g., Pollak, 2005; Schechter, 2012), developmental psychology (e.g., Kopp, 1982; Rothbart & Derryberry, 1981; Sameroff, 1975; Sameroff & Chandler, 1975), clinical psychology, and child psychiatry (e.g., Bayley, 2006; Rutter, 1987, 1995, 2002; Verhulst, van der Ende, & Koot, 1996). Our studies contributed to the efforts of interdisciplinary and multidisciplinary researchers examining the Developmental Origins of Health and Disease (DOHaD) hypothesis. This hypothesis originated from the “fetal programming of adult disease”—the hypothesis which states that an adverse fetal environment induces plastic responses that increase the risk of chronic diseases such as type 2 diabetes and coronary heart disease later in life (Barker, 1990, 1995, 2004; Barker & Osmond, 1986). Studies testing the DOHaD hypothesis include early prenatal and perinatal origins of a wide range of diseases and disorders, physical as well as mental, by adverse influences during sensitive periods of development (Gillman, 2005; Gluckman & Hanson, 2004; Gluckman, Hanson, & Beedle, 2007; Hanson & Gluckman, 2014; Nathanielsz, 1999; Meaney, 2010; Meaney, Szyf, & Seckl, 2007; Phillips & Jones, 2006; Seckl, 2007; Seckl & Holmes, 2007; Schlotz et al., 2008). The DOHaD research field was influenced by researchers studying the adaptive and/or maladaptive nature of neural, physiological, and behavioral responses to environmental stressors. Specifically, it was influenced by early life stress (ELS) research, targeting consequences of ELS and individual differences in resilience and vulnerability to stress and adversity later in life and the development of stress-related diseases (e.g., de Kloet, Claessens, & Kentrop, 2014; de Kloet, Joëls, & Holsboer, 2005; de Kloet, Karst, & Joëls, 2008; Gunnar & Quevedo, 2007; Heim & Nemeroff, 2001; Koolhaas et al., 2011; Lupien et al. 2009; McEwen & Morrison, 2013; Meaney, 2010; Meaney, Szyf, & Seckl, 2007; Reul et al., 2015; Swaab, Bao, & Lucassen, 2005). Most of this work demonstrated support for a “three-hit model” (Daskalakis et al., 2013). This is a model in which early life adversity does not directly or inevitably lead to disorder or disease but rather genetic factors (hit 1) in interaction with early life environmental inputs and experience -related factors (hit 2) lead to a certain phenotype with differential susceptibility to later-life challenges. In an individual with a certain phenotype when exposed to a later-life environment (hit-3), his or her mental functions may become compromised and a higher risk of psychiatric symptoms may arise (vulnerability); but when exposed to another type of environment the same individual is expected to be resistant to mental dysfunction (resilience) (Daskalakis et al., 2013, p. 1867).
Recently, we introduced a model to describe adaptation to environments in general rather than limited to early adversity (Van den Bergh, 2010, 2011a, b). Our model is compatible with the latter models (e.g., Daskalakis et al., 2013) in that it attempts to explain the association between early life events (typical as well as atypical; positive as well as negative) and physical and mental health as well as physical and mental health problems in a schematic way. Our model extended the concept of DOHaD by explicitly incorporating the study of behavior and brain-behavior relations in the DOHaD hypothesis (Van den Bergh, 2010, 2011a, b; see Fig. 14.1). The study of the Developmental Origins of Behavior, Health and Disease (DOBHaD) encompasses both short- and long-term consequences of conditions in the environment relevant to behavior, health, and disease risk and addresses research issues related to the interface between developmental, behavioral and medical science.
As demonstrated in this chapter, our DOBHaD model can be used to integrate both new and older data. It integrates the results of human literature on prenatal stress and relates them to results of preclinical, experimental animal studies which associated offspring outcome measures to changes in underlying neural circuits and causally related epigenetic processes (Bock, Poeschel et al., 2014; Bock, Rether et al., 2014; Weaver et al., 2004). The model illustrates that early life events will influence the development of organs , such as the heart, lungs, bones, kidneys and including development of the brain and neural circuitry, influence behavior and evolve into a certain programmed phenotype that finally leads to mental and physical health problems in some later-life environments and to mental and physical health in other environments.
During the prenatal and early postnatal life period the brain is subject to dramatic developmental processes; this period represents a phase of high susceptibility towards environmental influences. The specific physiological, neuroendocrine, and metabolic alterations that enable the individual to adapt to its early environment depend on the timing, duration, type, and magnitude of exposure of the organism to environmental factors and also are influenced by the individual’s genetic and epigenetic susceptibility. The latter implies that individuals differ in their susceptibility to early environmental factors (Daskalidas et al., 2013, Daskalakis & Yehuda, 2014; de Kloet et al., 2014; Nederhof & Schmidt, 2012). A causal pathway often studied in animal research is the alteration of the programming of the stress-regulating system [including epigenetic modifications in the hypothalamic–pituitary–adrenocortical (HPA)-axis and autonomic nervous system ] by early events. Adaptive developmental plasticity leads to changes in the limbic brain structures (hippocampus, amygdala) and the prefrontal cortex, which are involved in (stress) reactivity and regulation patterns, in emotional (e.g., anxiety, anger) and cognitive (e.g., sound perception, appraisal, learning , memory) processing and in temperament al variation in behavior (e.g., fearfulness or negative reactivity; surgency or positive reactivity; harm avoidance, novelty seeking) (Kolb et al., 2012). These changes in underlying circuits may influence how an individual “behaves” (i.e., senses, perceives, appraises, responds) in its environment in general. Moreover, situations of acute and chronic stress and adversity later in life will trigger its stress system in a particular way (Ladd et al., 1999; Lee & Goto, 2013; Seckl, 2007) and express the evolving “programmed phenotype .” Exposure of a person with a certain programmed phenotype to some types of environments may lead to behavioral problems, psychopathology, or more generally mental health problems (vulnerability) while the same phenotype exposed to another type of environment may lead to mental health or resistance to mental dysfunction (resilience).
Having developed a general model of adaptation to the early environment, the focus of this chapter is to demonstrate how research findings from our laboratory over 35 years were integrated into the model, describing four studies in particular which serve as exemplars (Braeken et al., 2014; Van den Bergh, 1989, 1990; Van den Bergh & Mulder, 2012; van den Heuvel, Donkers, Winkler, Otte, & Van den Bergh, 2014). Collectively, the research questions in the four studies focused on psychological and physiological processes and their interplay in examining individual differences in behavior in the offspring. Specifically, these longitudinal studies focused on describing neurobehavioral (Studies 1, 2, and 4), neurocognitive (Study 3), and neurophysiological (Studies 3 and 4) functioning from fetuses (Study 1) to infants (Studies 3 and 4) to adolescents (Study 2). It is important to note that, although all of the studies involved uncomplicated pregnancies in healthy pregnant women, there were a full range of both anxiety and mindfulness scores on the instruments used to measure these maternal variables (e.g., 25 % of women were highly anxious (Study 1); some women had a resolved anxiety disorder (Study 4). To show advances in research thinking and techniques over time, the chapter is divided into two major sections. In the first section, early and later studies from the Leuven prospective follow-up projectFootnote 1 are described (Van den Bergh, 1989, 1990; Van den Bergh & Mulder, 2012). Study 1 examines the influence of maternal emotions on fetal and neonatal behavioral state-related activity and on infant activity (Van den Bergh, 1989, 1990, 1992; Van den Bergh et al., 1989). Study 2 examines the relationship between fetal behavioral states and self-regulation in childhood and adolescence (Van den Bergh & Mulder, 2012). Because other results of the Leuven prospective follow-up project are reviewed elsewhere (see Van den Bergh, 2011b; Van den Bergh, Loomans, & Mennes, 2015), they were not included here. In the second section, recent studies from the Tilburg prospective follow-up project are described. Study 3 explores how variation in both negative emotions (i.e., maternal anxiety) and positive emotions (i.e., maternal mindfulness) influence infant neurocognitive development (van den Heuvel et al., 2014). Study 4, explores the issue of how exposure to a past, resolved maternal anxiety disorder (what is presumed to be an atypical maternal emotional state) influences maternal heart rate variability during pregnancy as well as infant heart rate variability, which in turn influences infant temperament (Braeken et al., 2013). In the final section, we summarize how exposure to maternal emotional state during pregnancy may have changed offspring behavior (i.e., his or her sensation, perception, appraisal, reactivity), use these results to explain the potential use of the DOBHaD model and speculate on potential clinical implications.
Section 1: Maternal Emotions , Fetal and Neonatal Behavioral States, and Child and Adolescent Self-Regulation
Historical Background
In the 1970s, the introduction of real-time ultrasound imaging enabled the direct, standardized, noninvasive study of the human fetus in utero. There was a resurgence of the work of early scientists who attempted to demonstrate classical conditioning (Ray, 1932; Spelt, 1948) and habituation (Peiper, 1925; Sontag & Wallace, 1934) in the fetus and those who examined whether maternal emotions influenced fetal behavior (Sontag, 1941, 1944). As knowledge that had been gained in various fields was brought together and reexamined with new techniques (Eskes, 1992), new paradigms emerged. Groundbreaking insights were reached, such as those on the developmental sequence of fetal movements, starting from 7.5 weeks gestational age (de Vries, Visser, & Prechtl, 1982, 1985) and on the development of fetal behavioral states (Nijhuis, Prechtl, Martin, & Bots, 1982).
Reviewing the literature a decade following this initial surge of research, Hepper (1992) suggested that the renewed interest in the fetal period may have arisen because of a change in the view of the capabilities of the newborn from being poorly developed and unable to adjust to his/her environment to having abilities exquisitely enabling adaptation. He attributed this reappraisal to the ability of scientists to ask the right questions. His ideas were reminiscent of Joffe’s (1969) interpretation of the importance of the prevailing climate or opinion regarding prenatal influences. In the same vein, Prechtl remarked that some writings of fetal behavior from around the turn of the nineteenth to twentieth century “were surprisingly modern, while writings from the 1920s and 1930 often seem extremely limited or obsolete”; the dominance of reflexology and behaviorism during the latter period may account for the prevailing view at that time (Prechtl, 1984, p. 5). One of the clear implications of psychobiological research being (re)-focused on the competences of the neonate and fetus was “the importance of early events in shaping subsequent development” (Smotherman & Robinson, 1995, p. 15), the study of which became a major topic of our research program.
We began our own empirical research program with an analysis and synthesis of the diverse literature on human maternal emotions during pregnancy (Van den Bergh, 1981, 1983, 1989). Many of the studies reviewed showed methodological shortcomings, such as: a failure , to specify the sample characteristics; insufficient control conditions; conclusions based on retrospectively obtained, inadequate assessment of predictor and outcome variables; lack of sound statistical methods; the problem of causation versus correlation; and the problem of rater bias, when the mother completed self-report questionnaires on her own emotional state or her offspring’s behavioral problems. Nevertheless, already at that time, results of the methodologically sound studies (e.g., Farber, Vaughn & Egeland, 1981 and see Carlson & Labarba, 1979 for a review) led to one general conclusion: negative maternal emotions during pregnancy may influence prenatal as well as postnatal behavior in the offspring and lead to behavioral problems and diseases later in life. Indeed, there was evidence that increased levels of negative emotionality and stress in the pregnant women may influence fetal brain development and behavior such as that shown by increased fetal heart rate (FHR) and motility (e.g., Copher & Huber, 1967; Sontag, 1941, 1966) and are associated with pregnancy and birth complications such as hyperemis gravidarum, toxemia, premature birth , and lowered birth weight (e.g., McDonald, 1968). Both negative maternal emotions and pregnancy and birth complications were shown to be associated with developmental irregularities and behavioral problems (e.g., Carlson & Labarba, 1979; Dörner, 1974; Erickson, 1971, 1976a, 1976b; Ferreira, 1960, 1965; Istvan, 1986; Knobloch & Pasamanick, 1966; Pasamanick & Knobloch, 1966; Pasamanick, Rogers, & Lilienfield, 1956; Sameroff & Zax, 1973; Sameroff & Chandler, 1975; Stott, 1958), altered mother–child interactions and adjustment (e.g., Davids & Holden, 1970; Davids, Holden, & Gray, 1963; Farber et al., 1981), childhood diseases (Stott, 1973; Stott & Latchford, 1976), and alterations in adult personality (Sontag, 1966). (Note: The link between maternal stress during pregnancy and childhood disease was recently confirmed in a very large scale (n = 66,203) prospective cohort study in Denmark (Tegethoff, Greene, Olsen, Schafner, & Meinlschmidt, 2012).
It was our aim to study processes that constitute offspring behavior in a prospective and standardized way as this would enable us to identify potential indices [or (bio)markers] of altered programmed phenotype and underlying mechanisms . Identifying indices and mechanisms is interesting from a basic scientific as well as from an applied, clinical point of view, namely to predict the risk of behavioral problems or disease in some environments later in life and, more generally, to set up innovative preventative and intervention strategies.
Study 1: Relationship Between Maternal Emotions and Fetal, Newborn , and Infant Behavior
In the early 1980s our research aims were to study two links which, according to our review, had not been systematically explored with a sound method, design or statistical technique: Can the influence of maternal emotions upon fetal behavior be established in the prenatal period? (first aim) and, is the prenatal influence established in the prenatal period reflected in the neonatal and infant behavior? (second aim)
While previous studies relied on maternal report of fetal movements or on fetal heart rate measures, the introduction of ultrasound in human research facilitated the study of these two links by enabling the direct measurement of fetal behavior for prolonged periods in pregnant women with varying levels of anxiety. Given the groundbreaking results from the work of colleagues in Groningen (de Vries et al., 1982, 1985; Prechtl, 1974, 1984; Visser, Poelman-Weesjes, Cohen, & Bekedam, 1987) and Leuven (e.g.,Casaer, 1979, 1993; Casaer & Devlieger, 1984; Casaer & Eggermont, 1985; Casaer, O’Brien, & Precht, 1973; Deprest et al., 1998; Van Assche, 1997; Van Assche, Holemans, & Aerts, 2001; Vandenberghe & Dewolf, 1990), we selected fetal and neonatal behavioral states and state-dependent movements as primary outcome variables. Fetal behavioral states emerge during the third trimester of pregnancy. They involve multiple interconnected neuronal networks. Functional (re)organization of sleep cycling likely occurs around 28–30 weeks gestational age (GA), 36 weeks GA, and 2 months of age (Nijhuis et al., 1999; Scher, 2008; Visser et al., 1987). From 36 weeks of gestation onward, the low-risk fetus exhibits two states of sleep and two states of wakefulness. (See Chap. 6 by Nijhuis this volume for a description of each state.) Fetuses typically pass through sleep cycles of non-REM (quiet) sleep and REM (active) sleep, lasting about 70–90 min (Visser, Mulder, & Prechtl, 1992). The time spent in wakefulness is usually less than 10 %. Typical fetal sleep states show a concordant (uninterrupted) association between the state parameters for a prolonged time and a simultaneous (synchronized) change of state parameters (≤3 min) at their beginning and end (transitions). The degree of sleep state stability and the duration of transitions into and out of a particular state are considered measures of neurophysiological development, integrity, and maturity (Mulder, Morssink, Van Der Schee, & Visser, 1998; Visser et al., 1992).
Our study was designed to examine both the issue of the influence of maternal emotions on fetal behavior (aim 1) and the effects of alterations in fetal behavior on neonatal and infant behavior (aim 2) in the same population. The sample included 86 Dutch women, 18–30 years of age, in their first pregnancy which was singleton, low-risk, with no medication or drug use. All pregnancies were dated using the last menstrual period and/or an ultrasonographic examination before 14 weeks. The course of pregnancy remained unremarkable with delivery from 36 to 41 weeks of gestation. All infants had a birth weight above the 10th percentile, a 5-min Apgar score of 9 or 10, and no postnatal medical complications while in hospital. Maternal pregnancy anxiety was measured at 12–22, 23–31, and 32–40 weeks GA and at 1 week, 10 weeks, and 7 months after delivery using the self-report state anxiety subscale of the Spielberger State Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970; Van der Ploeg & Defares, 1980). In a subsample of 37 women [Mean age (SD) = 26.6 (2.55)], simultaneous recordings of fetal heart rate (FHR), fetal generalized body movements (GM) and fetal rapid eye movements (REM) were made continuously for 2 h at 36–38 weeks GA. FHR recordings were collected using a cardiotocograph and scored visually into episodes of heart rate pattern (HRP) A, B, C, or D (Nijhuis et al., 1982). Fetal generalized body and eye movements were observed and video-recorded using real-time ultrasound scan. The presence of states (or coincidence) 1F–4F was identified according to predefined criteria (see Mulder, Visser, Bekedam, & Prechtl, 1987; Nijhuis et al., 1982). For some analyses, the data of state 2F and 4F, were combined (in order to have one measure of states in which fetuses are actively making movements); in other ones, state 2F, 3F, and 4F were combined to have one measure of state other than 1F/quiet sleep. On day 5–6 after birth, a comparable 2-h observation was carried out on the newborn. At 7 months of age, maternal report of the infant activity was dichotomized as problematic or not problematic. To examine our two research aims, we constructed and tested several sets of nested linear structural relation (LISREL)-models, including state and/or trait anxiety in each pregnancy trimester as predictor variable(s). To select the fetal behavioral measures to be included in the LISREL-models, component analyses were performed, with variamax rotation of components with eigenvalue >1 and the measures with the highest values on each of the components were selected; comparable neonatal measures were then introduced in the models. Fetal sex was introduced as a second predictor variable since our data showed that male fetuses were more active than female fetuses. Maternal anxiety postpartum had no significant association with neonatal behavioral measures and was not introduced in the neonatal LISREL model. However, as maternal anxiety 7 months after delivery was significantly associated with the infant activity measure, we introduced it as a second predictor to examine the link with infant activity at 7 months. The models were tested on n = 28 fetuses/neonates for whom all data were available.
As can be seen in Fig. 14.2—Model A, LISREL modelling showed that fetuses of high anxious mothers made more general movements and head movements and that male fetuses made more general movements than females. (Note: Only the final model of nested LISREL models are shown.) The prenatal influence of maternal State anxiety also was reflected in neonatal behavior; infants who made more head movements as fetuses, made more general movements and more head movements as neonates. The observation that the percentage of fetal head movements (rather than the percentage of fetal general movements) was significantly related to neonatal head and general movements may indicate that the newborns had difficulties in adjusting to gravidity, which may have more effect on making body movements than on making head movements (Michel & Moore, 1995; Prechtl, 1984). Model B illustrates that maternal Trait anxiety had a negative influence on the mean duration of epochs of coincidence of fetal State 1, and that infants who have shorter epochs of State 1F as fetuses also have shorter duration of epochs of State 1 as neonates . Model C reveals that at 7 months after birth, maternal anxiety during pregnancy had an indirect effect on activity level of the infant (i.e., via influencing fetal general movements) and maternal anxiety measured at 7 months after delivery had a direct influence on infant activity level. By this time, the mother and , infant would have had much more opportunity to interact than at four or five days after birth. Of course, these differences may have resulted because infant activity was reported by the mother and reflect her bias. In future research, direct observation of maternal–infant interactions at this age might be useful in untangling these effects.
LISREL model A,B,C concerning the relation between maternal anxiety during pregnancy, fetal and neonatal behavioral states and motor activity, and infant activity (adapted and translated from Van den Bergh, 1989). Legend: 7 months = 7 months after delivery; df = degrees of freedom; Epoch C1F-MD: Mean duration of epochs of coincidence State 1 in the fetus; Epoch 1N-MD: Mean duration of epochs of State 1 in the neonate ; % GM = percentage of general movement s; % HM = percentage of head movements; NoC1F = epochs of coincidence other than State 1F; C24F = epochs of coincidence 2F and 4F; Trim2 = Second trimester of pregnancy; Trim3 = Third trimester of pregnancy
In summary, the LISREL modelling, together with other results from this cohort (e.g., those showing that infants of high anxious pregnant mothers had a more difficult temperament at 10 weeks and cried more, were hungrier, had more stomach cramps at 7 months than infants of mothers who were less anxious, see Van den Bergh, 1989, 1990, 1992) answered our two research aims cited above in a positive way. Results of Study 1 can be integrated into our DOBHaD model (see Fig. 14.1) in the following way: prenatal exposure to maternal anxiety (early life events) in interaction with supposed (epi)genetic factors, may have an enduring influence on (or programs) fetal/neonatal brain development and behavioral functioning as reflected in higher fetal and neonatal reactivity. This higher reactivity may evolve into a programmed phenotype including seeking out arousal-inducing events. Moreover, the induced behavioral alterations observed in the offspring of mother’s with high anxiety levels may influence the quality of the interaction between mother and child (i.e., the caregiving environment) in a negative way, increasing the risk of subsequent offspring behavioral or mental health problems. [Further explanation of the (use of the) DOBHaD model is given in the final section which clarifies the i ntegration of the results of this study.]
A link between the level of stress and anxiety of the mother during pregnancy and ultrasonographically observed fetal behavior and fetal heart rate is now well established (for a review see DiPietro, Costigan, Pressman, & Doussard-Roosevelt, 2000; Kafalí, Derbent, Keskí, Símavlí, & Gözdemír, 2011; Monk et al., 2011; Van den Bergh, Mulder, Mennes, & Glover, 2005). Most studies have reported that increased maternal anxiety was associated with increased fetal arousal/wakefulness and increased FHR variability and % of body movements during states 2F and 4F . As an example, DiPietro and colleagues (2000) observed that fetuses of women with a positive versus negative attitude toward pregnancy exhibited different overall levels of motor activity (reduced versus increased, respectively). Although positive (pleasant, optimistic) emotions and negative stressors are believed to be regulated by the same physiological system [hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous system (ANS)], the negative emotions may have reflected chronic negative conditions, which were both unpredictable and uncontrollable and triggered a stress response, involving cortisol release (Koolhaas et al., 2011), while positive emotions (dispositional optimism) have been linked to lower levels of cortisol responses under stress (Jobin, Wrosch, & Scheier, 2014).
Study 2: Relationship Between Fetal Behavioral State and Child and Adolescent Self-Regulation
Having observed in Study 1 that maternal anxiety did influence fetal behavioral state and was associated with alterations in state-related fetal activity level, we turned our attention to the influence of fetal states on self-regulation. Specifically, we addressed the question: Is fetal behavioral state organization a biological precursor of child and adolescent self-regulation? (Van den Bergh & Mulder, 2012)
Background
Sleep plays a critical role in early brain development, arousal regulation, attention and cognition (Graven & Browne, 2008; Mirmiran, Maas, & Ariagno, 2003; Mulder, Ververs, de Heus, & Visser, 2011; Peirano, Algarín, & Uauy, 2003) and the study of sleep ontogeny (i.e., behavioral state organization) can be used to identify patterns of brain maturation (Scher, 2008). For example, in one study (Scher, Steppe, & Banks, 1996), sleep measures of both the healthy preterm infant (assessed at term equivalent age) and the healthy full-term newborn were predictive of performance on the Bayley scales of mental development at 12 and 24 months. In another study (Holditch-Davis & Edwards, 1998), in high-risk premature infant s born at gestational ages from 27 to 29 weeks onwards, the degree of sleep state control after birth was associated with postnatal neurodevelopmental status at term equivalent age. The predictive value of these measures for behavioral developmental outcome in later life has remained unexplored due to a lack of long-term follow-up studies. Therefore, in a nonclinical sample (see Study 1 for a description of this sample), we examined whether differences in sleep state organization in the near term fetus could account for differences in child and adolescent self-regulation (Van den Bergh & Mulder, 2012).
Theories of self-regulation presume that human s, from prenatal life or birth onward, display individual differences in reactivity and regulation that have implications for subsequent development and adaptation (Calkins & Fox, 2002; Gunnar, Talge, & Herrera, 2009; Henrichs & Van den Bergh, 2015; Kochanska, Coy, & Murray, 2001; Kopp, 1982; Posner & Rothbart, 2000; Pruessner et al., 2010). Reactivity is understood as the arousability of physiological and behavioral systems, while self-regulation refers to neural and behavioral processes which function to modulate this reactivity. Interestingly, in some theories, temperament has been defined as constitutionally based individual differences in reactivity and regulation (Rothbart & Ahadi, 1994; Rothbart & Bates, 1998; Rothbart & Derryberry, 1981; Rothbart, Sheese, Rueda, & Posner, 2011). As the infant and child mature, later-developing neural structures become integrated into the existing neural organization, which involves reorganization of circuits (Michel & Moore, 1995). Due to this patterned reorganization, initial reactive forms of regulation are supplemented by an increasing capacity for volitional, effortful control or self-regulation (Derryberry & Rothbart, 1997). Much of the self-regulation development results from increasing volitional control over attention al processes and enhanced inhibitory control over motor behavior (Calkins & Fox, 2002). Starting in childhood and continuing throughout adolescence , executive function s such as attention al focusing, maintenance and shift of focusing, and inhibitory control become integrated into complex emotional and behavioral regulatory processes. These processes, in turn, are involved in planning and goal setting, responsible decision making, emotional and motivational changes, and interpersonal relationships (Nelson et al., 2002; Rothbart & Bates, 1998; Van den Bergh & Mulder, 2012, p. 585). Failure of self-regulation in one way or another is a characteristic feature of behavioral problems and mental disorders (Henrichs & Van den Bergh, 2015).
At the time of our study, we could find no empirical work on individual differences in typical fetal brain maturation processes, such as expressed in fetal behavioral state organization or in relation to the long-term consequences for self-regulation. Thus, the aim of this prospective longitudinal study was to examine which measures of fetal behavioral state organization in the normal, near-term fetus are predictors of measures of self-regulation obtained from the same individuals when 8–9 and 14–15 years of age.
A total of 73/86 offspring participated in this second study. Twenty-five mother–offspring pairs who had participated in the fetal observation part of the Leuven study detailed above and had complete data for both the fetal behavioral observation session at the end of pregnancy and a follow-up study on the offspring at ages 8–9 or 14–15 were included. The reference (i.e., comparison) group consisted of 48 mothers and their , children/adolescents who participated only in the follow-up study but not in the fetal observation study.
For the follow-up study reported here, the mothers completed Dutch versions of temperament questionnaires, measuring concepts of reactivity (i.e., positive reactivity (or surgency) and negative reactivity) and of self-regulation (i.e., effortful control). The Children’s Behavior Questionnaire (CBQ; Ahadi, Rothbart, & Ye, 1993; Rothbart, Ahadi, Hershey, & Fisher, 2001 translated and validated for a Dutch-speaking sample by Van den Bergh & Ackx, 2003) was used when their children were 8–9 years of age and the revised Early Adolescence Temperament Questionnaire (EATQ-R; Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001; translated and validated for a Dutch-speaking sample by Hartman, Oldehinkel, De Winter, & Ormel, 2002) when the children were 14–15 years old. Only the temperament data concerning self-regulation are used in this study. Statistical modelling of the fetal–child–adolescent data demonstrated that one behavioral state measure, namely the time a typically developing fetus takes to pass from quiet sleep (S1F) to active sleep (S2F) in the last month before birth, is associated with her/his degree of self-regulation in childhood and adolescence . In particular, fetuses exhibiting sharp, synchronous transitions from quiet sleep into active sleep , compared with fetuses showing non-synchronized transitions (lasting >3 min) reached a higher level of effortful control (i.e., higher than the reference group but within normal ranges) both at 8–9 years and 14–15 years. Although the mechanism s underlying fetal state transitions are yet unknown and in need of future study, our results demonstrate that studies of sleep ontogeny can provide insights into fetal brain maturational processes which have implications for later environmental adaptation as well as developmental consequences for behavior. The results of Study 2 can be integrated in our DOBHaD model (Fig. 14.1). The supposed interaction between fetal environmental and (epi)genetic factors is reflected in synchronous fetal state transitions from quiet into active sleep in some fetuses and in asynchronous ones in other fetuses; these types of transitions are one element of early brain-behavior processes. These early differences may evolve into a programmed phenotype implying optimal self-regulation in the former group and implying suboptimal self-regulation in the latter ones.
Section 2: Maternal Heart Rate Variability and Emotions Are Associated with Infant Cognition and Heart Rate Variability
Background
While the participants in the following studies were pregnant women and their offspring, the offspring were only measured after birth; however, the studies are included here because they have implications for fetal psychobiological development. The studies described below were part of the Prenatal Early Life Stress (PELS)-project, a multinational, European (i.e., Belgium, Netherlands, UK) project. The PELS-project was one of four projects of the “Stress and Mental Health” program (EuroSTRESS) in which the research questions aimed at increasing our knowledge of the basic mechanism s of stress-related mental disorders as well as advancing our understanding of how early life experience s, genetic makeup, and repeated traumatic events in adulthood might predispose a person to the development of mental health disorders. The ultimate objective of the EuroSTRESS-project was the use of this knowledge for the development of new treatment strategies and the prevention and/or amelioration of such disorders (for more information see: http://www.esf.org/coordinating-research/eurocores/completed-programmes/eurostress.html).
To identify specific maternal risk (and resiliency) factors during pregnancy having an influence on offspring neurodevelopment, a total of 151, 170, 190 pregnant women was recruited in the UK, Belgium and the Netherlands respectively. Each country gathered information on stress, anxiety and depression levels by having the pregnant women complete self-report questionnaires in addition to providing saliva samples for cortisol measures in each pregnancy trimester and at 2–4 and 9–12 months after delivery. For the infants, birth outcome data were collected; infant saliva cortisol was measured at 2–4 and 10–12 months-of-age. The Bayley Scales of Infant Development as well as a behavioral inhibition task were administered at 9–12 months after birth. In the Netherlands, infant event related potentials (ERP) and heart rate variability were measured at 2–4 and 9 months of age. Epigenetic analyses limited to one cohort (Belgium) showed that prenatal maternal emotional state, particularly pregnancy related anxiety, was associated with the methylation state of the NR3C1 gene in the child (Hompes et al., 2013; Hompes, 2014).
Study 3: Maternal Anxiety and Mindfulness During Pregnancy and Infant Neurocognitive Function
Electroencephalography (EEG), in general and event-related brain potentials (ERP ) specifically, are unique tools which can be employed to assess cognitive functions such as attention , habituation and memory in early infancy. Indeed, ERP recordings in infants and the analysis of the responses have become well developed in the past 25 years (e.g., Alho, Sainio, Sajaniemi, Reinikainen, & Näätänen, 1990; for a review, see Kushnerenko, Van den Bergh, & Winkler, 2013). Cognitive abilities may be shown through infant responses to auditory stimuli (e.g., such as those used in auditory oddball paradigms) that mimic important features of the postnatal environment (Smotherman & Robinson, 1995). We examined how exposure to variation in maternal emotional state during pregnancy might influence the early neurocognitive development of the offspring using auditory stimuli, basing our studies on the work of Winkler and collaborators (Winkler, 2007; Winkler, Háden, Ladinig, Sziller, & Honing, 2009; Winkler et al., 2003).
Auditory attention is a key aspect of early neurocognitive function as it is a prerequisite of important skills, such as learning to speak and communicate with others. Moreover, some non-speech sounds also require one’s attention because they may signal an opportunity or some danger and need further processing. Some sounds may be irrelevant for the current behavioral goals and their processing should be stopped or suppressed (Kushnerenko et al., 2013). Obviously, it is important to be able to differentiate between these types of sounds. While being vigilant is adaptive in a new and/or hostile environment, being constantly alert and vigilant even if the environment is more favorable may be maladaptive. The finding that individuals exposed to early life stress are more vigilant (which is a key characteristic of anxious individuals) is consistently found in animal studies (Gunnar & Quevedo, 2007; Lutz & Turecki, 2014; Weinstock, 2005, 2008), while the assocation between maternal anxiety during pregnancy and childhood anxiety or emotional problems is found in some human studies (O’Donnell, Glover, Holbrook, & O’Connor, 2014; O’Connor, Heron, Golding, & Glover, 2003; Van den Bergh & Marcoen, 2004). Thus, we looked for early markers of these traits or problems in infants exposed to varying levels of maternal anxiety during prenatal life. Moreover, as human DOBHaD studies have almost exclusively focused on the effects of negative maternal emotions during pregnancy on child neurocognitive function, the focus of this study was expanded to include exposure to positive maternal emotions to determine whether and how they might also influence fetal (brain) development. Such a focus was both theoretically interesting and clinically relevant. For instance, Lobel, DeVincent, Kaminer, and Meyer (2010) had shown that in women with high-risk pregnancies , optimism was a key protective factor against adverse pregnancy outcomes. Thus, a good candidate for our study was a positive trait, such as mindfulness (Keng, Smoski, & Robins, 2011). Being mindful refers to a state of mind consisting of two key elements: (1) An alert mode of perceiving all mental contents (i.e., perceptions, sensations, cognitions, and emotions) and (2) a friendly, accepting, and nonjudgmental attitude towards those mental contents (Kohls, Sauer, & Walach, 2009). During pregnancy, experiencing positive emotions due to these two factors may enhance a pregnant woman’s resilience against stress and adversity occuring during the pregnancy and hence constitute a prenatal environment that positively influences fetal brain development.
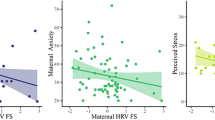
To study the relationship between maternal anxiety and mindfulness and offspring outcome, data from 79 Dutch mother–infant pairs were employed (van den Heuvel et al., 2014). At 20 weeks of gestation, women reported anxiety using the Symptom Checklist (SCL-90; Arrindell & Ettema, 1981, 2003) and mindfulness using the Freiburg Mindfulness Inventory (FMIs-14, Walach, Buchheld, Buttenmuller, Kleinknecht, & Schmidt, 2006). When their infants were 9 months-of age, EEG and auditory elicited ERPs were recorded using a passive auditory oddball paradigm. The stimulus sequences consisted of four different types of 200 ms sound events with an interstimulus interval of 300 ms, namely the standard sound and three deviant sounds (i.e., a white noise segment, a unique environmental sound such as slamming a door, and the same sound as the standard sound but with an interval of 100 ms). The frequent standard had a probability of 0.70 and the three types of deviants each had a probability of 0.10. A total of 1500 stimuli were delivered.
Mixed-mode ANOVAs were employed in two separate analyses, including the infants’ mean ERP amplitudes (elicited by each of the four types of sound events) and either maternal anxiety or maternal mindfulness as a predictor. Preliminary analyses showed no effects of gestational age, birth weight or maternal anxiety at 9 months after delivery and they were not included in subsequent modelling. The results showed that higher maternal mindfulness (during the second trimester) was associated with smaller infant N250 and higher infant P150 ERP amplitudes to the standard sound while higher maternal anxiety (during the second trimester) was associated with larger N250 amplitudes to the standard sound. No effects were found for the three deviant sound stimuli.
From these results, we concluded that infants prenatally exposed to higher levels of maternal mindfulness devote less in-depth processing to repeated sounds with low information content, suggesting fast habituation to these sounds. In contrast, infants prenatally exposed to higher levels of maternal anxiety processed such uninformative sounds more extensively and/or they habituated more slowly to these stimuli. We speculate that the 9 month-old infant ERP directional differences observed here to higher maternal mindfulness and anxiety during pregnancy might stem from infants prenatally exposed to higher maternal mindfulness pre-attentively forming more accurate perceptual representations, as reflected in higher P150 amplitudes to the standard sound. If so, a mindfulness intervention for pregnant women suffering from anxiety may be a desirable alternative or adjunct to pharmacological interventions. Clearly, firm conclusions await future research (van den Heuvel et al., 2014). The findings in relation to higher maternal anxiety during pregnancy are consistent with the results of other studies, namely with those showing that children prenatally exposed to high maternal anxiety have poorer language acquisition (King & Laplante, 2005; Laplante et al., 2004; Laplante, Brunet, Schmitz, Ciampi, & King, 2008) and are more anxious (O’Connor et al., 2003; Van den Bergh & Marcoen, 2004).
The results of Study 3 can be integrated in the DOBHaD model in the following way: prenatal exposure to maternal anxiety (early life events) in interaction with supposed (epi)genetic factors lead to altered emotion and (neuro)cognition and may evolve into increased vigilance (constituting the programmed phenotype ).
As well as investigating the effects of anxiety during pregnancy on later offspring development, we also examined the effects of resolved anxiety disorders during pregnancy on infant development.
Study 4: Heart Rate Variability in Pregnant Women and Their Infants
At the time that we began this research, from our own studies (see above) and that of others (e.g., Alder, Fink, Bitzer, Hösli, & Holzgreve, 2007; Ross & McLean, 2006), it was known that active anxiety disorders and experiencing a high anxiety level had long-term detrimental effects on pregnant mothers and their offspring. However, it was unknown if a resolved, nonactive, maternal anxiety disorder had similar effects. Anxiety-related conditions, such as reduced autonomic cardiac control, indicated by reduced heart rate variability (HRV) could persist despite disorder resolution, with long-term health implications for mothers and children (Braeken et al., 2013). The autonomic nervous system of the fetus seems to be susceptible to the influence of maternal cardiac characteristics (Young, 2002). Indeed, it has been shown that HRV of the developing fetus is altered in the offspring of mothers with a number of psychiatric conditions, including anxiety disorders, and these differences persist postnatally (Dierckx et al., 2009; DiPietro et al., 2000; Monk et al., 2004). Thus, we designed this study to test the hypothesis that pregnant mothers with a history of, but not current anxiety disorder, and their children have low HRV, predicting offspring anxiety-like temperament (Braeken, 2014; Braeken et al., 2013).
To test the hypothesis, a case-control study including 56, 1st trimester Dutch women (n = 22 with a history of anxiety disorder; n = 34 with no history of psychopathology determined using the Mini-International Neuropsychiatric Interview 6.0, Sheehan & Lecrubier, 2010) and their offsprings was carried out. Anxiety was measured with the State Trait Anxiety Inventory (Spielberger et al., 1970; Van der Ploeg & Defares, 1980) and maternal ECG (to obtain maternal HR and HRV) was measured continuously during rest and mental stress. Stress was induced during a mental task. Each mother participated in a 25-min task that consisted of five testing phases, lasting 5 min each (Vlemincx, Taelman, De Peuter, Van Diest, & Van Den Bergh, 2011). Stress was induced in the second and fourth phases, with the remainder being relaxation phases. The stress consisted of mentally solving a complex mathematical problem such as (361 + 11) ÷ (3 × 4) + 137 without verbalization and selecting the answer from three choices presented on a computer screen. The relaxation phases consisted of viewing pictures considered peaceful and listening to music considered restful. At 2–4 months of age, infant ECGs were recorded. At 9–10 months of age, infant fearfulness was assessed using the unpredictable mechanical toy paradigm of the fear subscale of the Laboratory Temperament Assessment Battery (Lab-TAB)-Locomotor Version (Goldsmith & Rothbart, 1999) (Braeken et al., 2013, p. 2–3).
Repeated measures ANOVA controlling for mother’s age and prepregnancy BMI, showed that HRV was lower in women in the past anxiety group compared to controls on both the root mean square of successive differences (RMSSD) and the high frequency (HF) measures of HRV. Regression analysis indicated that there was a significant relationship between maternal HRV measures and child HRV measures only in the anxiety group. Simple effects analysis showed that children of mothers with a past anxiety disorder had lower HRV (for both RMSSD and HF measures) than those born to mothers without a past anxiety disorder history. For all children, low HRV measures at 2–4 months were associated with a higher chance of fearful behavior at 9–10 months.
These results revealed that pregnant women with a past anxiety disorder had autonomic alterations (reduced parasympathetic function, indexed by HRV) early in pregnancy which may have influenced a subsequent physiological (reduced parasympathetic function, indexed by HRV) and/or psychological (fearful temperament ) attribute of their offspring. The findings were independent of variations in maternal state-anxiety, age, sex, or body mass index. Additionally, mother–child associations were not explained by the children’s birth weight or gestational age. The mechanism s by which a previous maternal anxiety disorder and/or HRV become associated with parasympathetic nervous system function in the offspring are unknown and a matter of speculation. It could be that altered autonomic function in pregnant women modulates their fetus’ development. There is some support for this postulate as reduced HRV has been shown to be associated with dysregulation of several allostatic systems, including glucose regulation , hypothalamic–pituitary–adrenal axis function and inflammatory processes (Thayer & Lane, 2007; Thayer & Sternberg, 2006; Thayer, Yamamoto, & Brosschot, 2010) all of which may modulate fetal development (Lupien et al., 2009; Matthews & Phillips, 2010; Meyer et al., 2006; Van den Bergh, 2011; Van den Bergh, Mennes, et al., 2005; Young, 2002). However, whether altered maternal ANS function is causative or simply the result of shared underlying processes is unknown. Alternatively, given that mothers and their children share genes and environmental exposures, maternal behavior also may be an important factor in the observed associations (Rutter, 2002; Stern, 2009; Weaver et al., 2004) as well as shared genes. For example, research has shown that the combination of a brain-derived neurotrophic factor (BDDNF) V/V genotype and early life stress predicts changes in brain structure that are associated with lower HRV and higher anxiety (Gatt et al., 2009). These findings may explain , in part, why the women with a history of an anxiety disorder in this study demonstrated lower HRV. Whether it may account for our observation of a relationship between maternal–infant HRV is unknown (Braeken et al., 2013, p. 6).
Our DOBHaD model (Fig. 14.1) shows the effects of prenatal exposure to resolved maternal anxiety and altered ANS function (early life events) leading to altered ANS function and fearful temperament , which may evolve into a pro-anxiety phenotype (constituting the programmed phenotype ).
Conclusion: The Use and Strength of the DOBHaD Model and Clinical Implications
In the past 30 years and especially in the last decade, an increasing number of studies have provided continuing evidence for an association between prenatal exposure to maternal stress, anxiety and depression , and altered behavior in the offspring. This body of evidence indicates that it may indeed be the case that events prior to birth, such as maternal emotions during pregnancy, influence the way offspring respond to their postnatal environment as demonstrated by correlations with infant outcome in the studies described above. The child of a highly anxious pregnant mother, by adapting to the early exposures when he/she was a fetus, reacts differently than the child of a low anxious pregnant mother, as reflected in newborn activity and EEG-responses, HRV, and temperament in infancy (see above studies), a delay in language development in toddlers and an enhanced risk for behavioral and emotional problems in childhood, specific cognitive problems, anxiety and depression in adolescence and young adulthood (for recent reviews see: Beydoun & Saftlas, 2008; Bock, Poeschel et al., 2014; Braeken, 2014; Charil, Laplante, Vaillancourt, & King, 2010; Glover, 2011, 2014, 2015; Glover, O’Connor, & O’Donnell, 2010; O’Donnell, O’Connor, & Glover, 2009; Graignic-Philippe, Dayan, Chokron, Jacquet, & Tordjman, 2014; Henrichs & Van den Bergh, 2015; Lewis, Galbally, Gannon, & Symeonides, 2014; Lewis & Olive, 2014; Loomans, 2013; Loomans et al., 2011, 2013; Mennes, 2008; O’Connor et al., 2014; Otte, 2013; Räikkönen, Seckl, Pesonen, Simons, Van den Bergh, 2011; Schlotz & Phillips, 2009; Van den Bergh & Henrichs, 2015; Van den Bergh et al., 2015; Van den Bergh, Mulder, et al., 2005; Weinstock, 2008).
To understand how exposure to prenatal (and early postnatal) environmental events may influence later behavior, health and disease, several models have been developed (see for example Bock, Rether et al., 2014; Nederhof & Schmidt, 2012). However, no firm conclusion can yet be drawn about the validity of the different models (Daskalakis et al., 2013). In recently generated models (Bock, Rether et al., 2014; Daskalakis et al., 2013; de Kloet et al., 2014; Hanson & Gluckman, 2014; Lewis et al., 2014), including our own (Van den Bergh, 2010, 2011a), early life events typically are seen as “conditional determinants” rather than as determinants which always/invariably lead to behavioral problems, disorder, or disease. Put simply, this means that an organism that was prenatally programmed (or organized) to be adapted to a particular environment, will gradually be behaving in an altered “biased” way. The “bias” constitutes his or her programmed phenotype and reflects the way the organism was adapted during its early development. Although early adversity will in some environments finally lead to disorder or disease, in other environments early adversity may constitute a possible source of adaptation (Daskalakis et al., 2013). For instance, according to the “mismatch hypothesis” only a mismatch between the early environment and later postnatal environment will lead to disorder and disease, while a match will not (Gluckman & Hanson, 2004; Hanson & Gluckman, 2014). Biological sensitivity (Boyce & Ellis, 2005) or differential susceptibility to the environment (Belsky & Pluess, 2009) models predict that some individuals are more susceptible than others to both the adverse and beneficial effects of, respectively, unsupportive and supportive environments. This genetic difference in sensitivity or susceptibility and the nature of the environment will influence how mental health or mental health problems are shaped; these processes covary with physical health and health problems.
The results of the four studies described above exemplified how “conditional determinants” (i.e., prenatal environmental events such as exposure to maternal anxiety, maternal mindfulness, a resolved maternal anxiety, and altered maternal ANS) might have influenced the course of fetal development, resulting in alterations in the function of the brain and motor systems as evidenced during fetal life and, gradually, in a “biased” response/altered phenotype to subsequent environmental inputs later in life. Using the results of Study 1 to illustrate, being a highly active fetus could be seen as an adaptation to the prenatal environment shaped by a highly anxious mother. The subsequent observation of an increase in neonatal activity might signify that the brain of the infants became shaped to facilitate a higher level of arousal during fetal life. It also could indicate an effect on self-regulation such that later in life, the infant, toddler and child would seek out arousal-inducing events. A consequence of arousal-seeking is that it could lead to hyperactivity and/or impulsivity , which places the child at risk for behavioral problems or attention deficit hyperactivity disorder (ADHD) in some, but not in other environments. For instance increased motor activity will in a (school) environment that requires restraint of impulsivity and motor activity be seen as inadequate behavior. However, in an environment that is stress-inducing and potentially harmful, or in a novel environment, increased motor activity (hyperactivity) may be adaptive (e.g., to explore the environment for threats and opportunities) (Jensen et al., 1997). In Study 3, infants of highly anxious pregnant women were more vigilant than those of low anxious pregnant women (i.e., ERPs indicated that they reacted stronger to a repeated, uninformative sound and seem to habituate less). In Study 4, infants of pregnant women with a resolved anxiety disorder who had lower HRV measures of RMSSD and HF during pregnancy, also showed similar lower HRV measures at 2 months of age, which predicted an anxious-like temperament at 9 months of age. When these infants, with an altered phenotype [i.e., ‘a bias in neurocognitive function (Study 3) and in sympathetic activity/fearfulness (Study 4)] encounter an anxiety or fear-inducing, environment such behavior may have an adaptive value. According to some authors (e.g., Hanson & Gluckman, 2014; Lee & Goto, 2013; Lewis et al., 2014; O’Connor et al., 2014) this bias, which also can be interpreted as a higher degree of stress reactivity, may in fact be promoted by maternal anxiety during pregnancy. The mother signals, with physiological changes accompanying anxiety, aspects of the environment to which the fetus adapts because it may have adaptive value if he or she encounters a similar (i.e., a matched) anxiety-inducing environment after birth/later in life (i.e., early life experience may program the brain for life to come). However if an individual is more vigilant in all types of environments (e.g., also in more favorable, safe ones), this biased (prenatally acquired) behavior may tax or compromise emotional and/or neurocognitive functioning and anxiety symptoms and poorer language acquisition may result. In order to develop adequate self-regulation skills, it will be vital that parents (and other educators) understand this fearfulness and/or heightened stress reactivity and try to induce changes in this behavior. Neural circuitry, molecular profiles, and neurochemistry can be (positively) changed by experiences ; these changes will in turn influence subsequent behavior (Bai & Repetti, 2015; McEwen & Morrison, 2013). If therapeutic interventions are needed, they might target the underlying mechanism s that produce heightened stress reactivity as well as strategies that might prevent subsequent related behavioral problems or psychopathology (Schechter, 2012). Importantly, the fact that phenotype s are programmed by adaptation to early life environments does not imply that they cannot be changed! To refer to the amazing plasticity of the developing brain, Seymour Levine once said “Nothing is written in stone” (Levine, 2005); this statement has frequently been cited in early life stress literature (e.g., Daskalakis et al., 2013).
A strength of the studies used to generate our DOBHaD model, including the ones detailed above, is that they focused on processes (constituting elements) of behavior that could be measured in an objective, standardized way (e.g., changes in fetal behavioral state; event related potentials during sensory stimulation ; heart rate variability measures). The importance of the findings and the model based on this line of research are their potential for identifying those maternal–offspring factors that could serve as markers of later mental health issues. A recent Danish population based study indicates that a person exposed to prenatal stress makes more use of primary health care than a person not exposed to prenatal stress (Li, Yang, Guldin, Vested, & Vestergaard, 2015). However, only when markers and underlying mechanism s are identified, may interventions be developed that are targeted to prevention and/or amelioration of specific health issues. Such interventions may focus on the care given to the mother during pregnancy, the mother herself, or the offspring. Recent randomized controlled trial studies of interventions to treat anxiety and depression during pregnancy show not only improvements in maternal mental health but in infant outcome (e.g., better self-regulation and stress reactivity) as well (e.g., Milgrom et al., 2015).
The human and economic toll of mental health issues in the population is substantive. A recent UK report (Bauer, Parsonage, Knapp, Iemmi, & Adelaja, 2014) calculated the costs of mental health problems (i.e., depression , anxiety, psychosis, post-traumatic stress disorder) during pregnancy and the first year after childbirth at about £8.1 billion for each 1-year cohort of births and the equivalent of just under £10,000 for a single birth. About 72 % of this cost relates to adverse effects on the child rather than the mother. It seems that, not only for fundamental scientific studies, but also for clinical studies and for society as a whole, the study of prenatal environmental influences on offspring outcome is critical. It has considerable potential for improving behavior and health outcomes because maternal anxiety, stress and lifestyle are modifiable.
Notes
- 1.
The Leuven project was started in 1986 and examined the link between maternal anxiety during pregnancy and offspring development (i.e., fetal behavioral states and postnatal neurocognitive, behavioral and/or emotional development at the ages of 1 and 10 weeks, 7 months, and 8–9, 14–15, 17, and 20 years of age, e.g., Mennes, Stiers, Lagae, & Van den Bergh, 2006; Mennes, Van den Bergh, Lagae, & Stiers, 2009; Van den Bergh & Marcoen, 2004; Van den Bergh, Mennes, et al., 2005; Van den Bergh, Mulder, et al., 2005; Van den Bergh et al, 2008). Subsequently, the Tilburg prospective project was started in 2010 as part of the Prenatal Early Life Stress (PELS)-project (see section “Maternal Heart Rate Variability and Emotions Are Associated with Infant Cognition and Heart Rate Variability”) and aimed to examine the link between both negative and positive maternal emotions during pregnancy and offspring outcome.
References
Ahadi, S. A., Rothbart, M. K., & Ye, R. (1993). Children’s temperament in the US and China: Similarities and differences. European Journal of Personality, 7(5), 359–378. doi:10.1002/per.2410070506.
Alder, J., Fink, N., Bitzer, J., Hösli, I., & Holzgreve, W. (2007). Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. Journal of Maternal-Fetal and Neonatal Medicine, 20(3), 189–209. doi:10.1080/14767050701209560.
Alho, K., Sainio, K., Sajaniemi, N., Reinikainen, K., & Näätänen, R. (1990). Event-related brain potential of human newborns to pitch change of an acoustic stimulus. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 77(2), 151–155. doi:http://dx.doi.org/10.1016/0168-5597(90)90031-8.
Arrindell, W., & Ettema, H. (1981). Dimensionele structuur, betrouwbaarheid en validiteit van de Nederlandse bewerking van de Symptom Checklist (SCL-90): Gegevens gebaseerd op een fobische en een “normale’ populatie. [Dimensional Structure, Reliability and Validity of the Dutch Version of the Symptom Checklist (SCL-90): Data based on a phobic and an “normal’ population.]. Nederlands Tijdschrift voor de Psychologie en haar Grensgebieden, 36(2), 77–108.
Arrindell, W., & Ettema, J. (2003). Symptom checklist SCL-90: Handleiding bij een multidimensionele psychopathologie-indicator. [Symptom checklist. Manual of a multidimensional psychopathology-indicator]. Lisse: Swets Test.
Bai, S., & Repetti, R. L. (2015). Short-term resilience processes in the family. Family Relations, 64(1), 108–119. doi:10.1111/fare.12101.
Barker, D. J. (1990). The fetal and infant origins of adult disease. BMJ: British Medical Journal, 301(6761), 1111.
Barker, D. (1995). The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proceedings of the Royal Society Series B: Biological Sciences, 262, 37–43. doi:10.1098/rspb.1995.0173.
Barker, D. J. P. (2004). The developmental origins of well–being. Philosophical Transactions of the Royal Society, B: Biological Sciences, 359(1449), 1359–1366. doi:10.1098/rstb.2004.1518.
Barker, D. J. P., & Osmond, C. (1986). Infant mortality, childhood nutrition, and ischaemic heart disease and ischaemic heart disease in England and Wales. The Lancet, 327(8489), 1077–1081. doi:http://dx.doi.org/10.1016/S0140-6736(86)91340-1.
Bauer, A., Parsonage, M., Knapp, M., Iemmi, V., & Adelaja, B. (2014). Costs of perinatal mental health problems. London, UK: London School of Economics & Centre for Mental Health.
Bayley, N. (2006). Bayley scales of infant and toddler development. San Antonio, TX: Harcourt Assessment.
Belsky, J., & Pluess, M. (2009). Beyond diathesis-stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135, 885–908. doi:http://dx.doi.org/10.1037/a0017376.
Beydoun, H., & Saftlas, A. F. (2008). Physical and mental health outcomes of prenatal maternal stress in human and animal studies: A review of recent evidence. Paediatric and Perinatal Epidemiology, 22(5), 438–466. doi:10.1111/j.1365-3016.2008.00951.x.
Blumberg, M. S., Freeman, J. H., & Robinson, S. R. (2010). Oxford handbook of developmental behavioral neuroscience. New York: Oxford University Press.
Bock, J., Poeschel, J., Schindler, J., Börner, F., Shachar-Dadon, A., Ferdman, N., … Poeggel, G. (2014). Transgenerational sex-specific impact of preconception stress on the development of dendritic spines and dendritic length in the medial prefrontal cortex. Brain Structure and Function, 1–9. doi:10.1007/s00429-014-0940-4.
Bock, J., Rether, K., Gröger, N., Xie, L., & Braun, K. (2014). Perinatal programming of emotional brain circuits: An integrative view from systems to molecules. Frontiers in Neuroscience, 8, 11. doi:10.3389/fnins.2014.00011.
Boyce, W. T., & Ellis, B. J. (2005). Biological sensitivity to context: I. An evolutionary–developmental theory of origins and functions of stress reactivity. Developmental Psychopathology, 17, 271–301. doi:http://dx.doi.org/10.1017/S0954579405050145 DOI:10.1017/S0954579405050145#_blank.
Braeken, M. A. (2014). Psychological functioning and the autonomic nervous system during pregnancy. Impact on mother and child (PhD thesis). Tilburg University, Tilburg, the Netherlands.
Braeken, M. A., Kemp, A. H., Outhred, T., Otte, R. A., Monsieur, G. J., Jones, A., & Van den Bergh, B. R. (2013). Pregnant mothers with resolved anxiety disorders and their offspring have reduced heart rate variability: Implications for the health of children. PLoS One, 8(12), e83186. doi:10.1371/journal.pone.0083186.
Calkins, S. D., & Fox, N. A. (2002). Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and psychopathology, 14(03), 477–498. doi:http://dx.doi.org/10.1017/S095457940200305X.
Capaldi, D. M., & Rothbart, M. K. (1992). Development and validation of an early adolescent temperament measure. The Journal of Early Adolescence, 12(2), 153–173. doi:10.1177/0272431692012002002.
Carlson, D. B., & Labarba, R. C. (1979). Maternal emotionality during pregnancy and reproductive outcome: A review of the literature. International Journal of Behavioral Development, 2(4), 343–376. doi:10.1177/016502547900200402.
Casaer, P. (1979). Postural behaviour in newborn infants. London: William Heinemann Medical Books.
Casaer, P. (1993). Old and new facts about perinatal brain development. Journal of Child Psychology and Psychiatry, 34(1), 101–109. doi:10.1111/j.1469-7610.1993.tb00969.x.
Casaer, P., & Devlieger, H. (1984). The behavioural state in human perinatal life. Journal of Developmental Physiology, 6(3), 187–194.
Casaer, P., & Eggermont, E. (1985). Neonatal clinical neurological assessment. In S. Harel & N. J. Anastasiow (Eds.), The at-risk infant: Psycho/socio/medical aspects (pp. 197–220). Baltimore, MD: Brookes.
Casaer, P., O’Brien, M. J., & Prechtl, H. F. (1973). Postural behaviour in human newborns. Agressologie: Revue internationale de physio-biologie et de pharmacologie appliquées aux effets de l’agression, 14 (Spec B), 49–57.
Charil, A., Laplante, D. P., Vaillancourt, C., & King, S. (2010). Prenatal stress and brain development. Brain Research Reviews, 65(1), 56–79. doi:http://dx.doi.org/10.1016/j.brainresrev.2010.06.002.
Copher, D. E., & Huber, C. P. (1967). Heart rate response of the human fetus to induced maternal hypoxia. American Journal of Obstetrics and Gynecology, 98(3), 320–335.
Crews, D., Gillette, R., Scarpino, S. V., Manikkam, M., Savenkova, M. I., & Skinner, M. K. (2012). Epigenetic transgenerational inheritance of altered stress responses. Proceedings of the National Academy of Sciences, 109(23), 9143–9148. doi:10.1073/pnas.1118514109.
Daskalakis, N. P., Bagot, R. C., Parker, K. J., Vinkers, C. H., & de Kloet, E. R. (2013). The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38(9), 1858–1873. doi:http://dx.doi.org/10.1016/j.psyneuen.2013.06.008.
Daskalakis, N. P., & Yehuda, R. (2014). Site-specific methylation changes in the glucocorticoid receptor exon 1F promoter in relation to life adversity: Systematic review of contributing factors. Frontiers in Neuroscience, 8, 369. doi:10.3389/fnins.2014.00369.
Davids, A., & Holden, R. H. (1970). Consistency of maternal attitudes and personality from pregnancy to eight months following childbirth. Developmental Psychology, 2(3), 364–366. doi:http://dx.doi.org/10.1037/h0029192.
Davids, A., Holden, R. H., & Gray, G. B. (1963). Maternal anxiety during pregnancy and adequacy of mother and child adjustment eight months following childbirth. Child Development, 34(4), 993–1002. doi:10.2307/1126541.
de Kloet, E. R., Claessens, S. E. F., & Kentrop, J. (2014). Context modulates outcome of perinatal glucocorticoid action in the brain. Frontiers in Endocrinology, 5, 100. doi:10.3389/fendo.2014.00100.
de Kloet, E. R., Joels, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. doi:10.1038/nrn1683.
de Kloet, E. R., Karst, H., & Joëls, M. (2008). Corticosteroid hormones in the central stress response: Quick-and-slow. Frontiers in Neuroendocrinology, 29(2), 268–272. doi:10.1016/j.yfrne.2007.10.002.
de Vries, J. I. P., Visser, G. H. A., & Prechtl, H. F. R. (1982). The emergence of fetal behaviour. I. Qualitative aspects. Early Human Development, 7(4), 301–322. doi:http://dx.doi.org/10.1016/0378-3782(82)90033-0.
de Vries, J. I. P., Visser, G. H. A., & Prechtl, H. F. R. (1985). The emergence of fetal behaviour. II. Quantitative aspects. Early Human Development, 12(2), 99–120. doi:http://dx.doi.org/10.1016/0378-3782(85)90174-4.
Del Giudice, M. (2012). Fetal programming by maternal stress: Insights from a conflict perspective. Psychoneuroendocrinology, 37, 1641-1629. http://dx.doi.org/10.1016/j.psyneuen.2012.05.014
Del Giudice, M., Ellis, B.J., Shirtcliff, E.A., 2011. The adaptive calibration model of stress responsivity. Neuroscience Biobehavioral Reviews, 35, 1562-1592.doi:10.1016/j.neubiorev.2010.11.007.
Deprest, J. A., Van Ballaer, P. P., Evrard, V. A., Peers, K. H. E., Spitz, B., Steegers, E. A., & Vandenberghe, K. (1998). Experience with fetoscopic cord ligation. European Journal of Obstetrics & Gynecology and Reproductive Biology, 81(2), 157–164. doi:http://dx.doi.org/10.1016/S0301-2115(98)00181-X.
Derryberry, D., & Rothbart, M. K. (1997). Reactive and effortful processes in the organization of temperament. Development and Psychopathology, 9(04), 633–652. doi:http://dx.doi.org/10.1017/S0954579497001375.
Dierckx, B., Tulen, J. H., van den Berg, M. P., Tharner, A., Jaddoe, V. W., Moll, H. A., … Tiemeier, H. (2009). Maternal psychopathology influences infant heart rate variability: Generation R study. Psychosomatic Medicine, 71(3), 313–321. doi:10.1097/PSY.0b013e318198a82c.
DiPietro, J. A., Costigan, K. A., Pressman, E. K., & Doussard-Roosevelt, J. A. (2000). Antenatal origins of individual differences in heart rate. Developmental Psychobiology, 37(4), 221–228. doi:10.1002/1098-2302(2000)37:4<221::AID-DEV2>3.0.CO;2-A.
Dorner, G. (1974). Environment-dependent brain differentiation and fundamental processes of life. Acta Biologica et Medica Germanica, 33(2), 129–148.
Ellis, L. K., & Rothbart, M. K. (2001). Revision of the early adolescent temperament questionnaire. Paper presented at the 2001 Biennial Meeting of the Society for Research in Child Development, Minneapolis, MN.
Erickson, M. T. (1971). Risk factors associated with complications of pregnancy, labor, and delivery. American Journal of Obstetrics and Gynecology, 111, 658–662.
Erickson, M. T. (1976a). The influence of health factors on psychological variables predicting complications of pregnancy, labor and delivery. Journal of Psychosomatic Research, 20(1), 21–24. doi:http://dx.doi.org/10.1016/0022-3999(76)90096-9.
Erickson, M. T. (1976b). The relationship between psychological variables and specific complications of pregnancy, labor, and delivery. Journal of Psychosomatic Research, 20(3), 207–210. doi:http://dx.doi.org/10.1016/0022-3999(76)90022-2.
Eskes, T. K. A. B. (1992). Introduction. In J. G. Nijhuis (Ed.), Fetal behaviour: Developmental and perinatal aspects (pp. XV–XXI). New York, NY: Oxford University Press.
Farber, E. A., Vaughn, B., & Egeland, B. (1981). The relationship of prenatal maternal anxiety to infant behavior and mother-infant interaction during the first six months of life. Early Human Development, 5(3), 267–277. doi:http://dx.doi.org/10.1016/0378-3782(81)90034-7.
Ferreira, A. J. (1960). The pregnant woman’s emotional attitude and its reflection on the newborn. American Journal of Orthopsychiatry, 30(3), 553–561. doi:10.1111/j.1939-0025.1960.tb02070.x.
Ferreira, A. J. (1965). Emotional factors in prenatal environment: A review. The Journal of Nervous and Mental Disease, 141(1), 108–118.
Fox, S. E., Levitt, P., & Nelson, C. A., III. (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81(1), 28–40. doi:10.1111/j.1467-8624.2009.01380.x.
Gatt, J., Nemeroff, C., Dobson-Stone, C., Paul, R., Bryant, R., Schofield, P., … Williams, L. (2009). Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry, 14(7), 681–695. doi:10.1038/mp.2008.143.
Gillman, M. W. (2005). Developmental origins of health and disease. The New England Journal of Medicine, 353(17), 1848–1850. doi:10.1056/NEJMe058187.
Glover, V. (2011). Annual research review. Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry, 52(4), 356–367. doi:10.1111/j.1469-7610.2011.02371.x.
Glover, V. (2014). Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Practice & Research. Clinical Obstetrics & Gynaecology, 28(1), 25–35. doi:10.1016/j.bpobgyn.2013.08.017.
Glover, V. (2015). Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms. In M. C. Antonelli (Ed.), Perinatal programming of neurodevelopment (Chapter 10). Advances in neurobiology (Vol. 10, pp. 269–283). New York, NY: Springer. doi:10.1007/978-1-4939-1372_10.
Glover, V., O’Connor, T. G., & O’Donnell, K. (2010). Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews, 35(1), 17–22. doi:http://dx.doi.org/10.1016/j.neubiorev.2009.11.008.
Gluckman, P. D., & Hanson, M. A. (2004). Living with the past: Evolution, development, and patterns of disease. Science, 305(5691), 1733–1736. doi:10.1126/science.1095292.
Gluckman, P. D., Hanson, M. A., & Beedle, A. S. (2007). Early life events and their consequences for later disease: A life history and evolutionary perspective. American Journal of Human Biology, 19(1), 1–19. doi:10.1002/ajhb.20590.
Goldsmith, H., & Rothbart, M. (1999). The laboratory temperament assessment battery (Locomotor Version 3.1). Madison, WI: University of Wisconsin-Madison.
Gottlieb, G. (1997). Synthesizing nature–nurture: Prenatal roots of instinctive behavior. Mahwah, NJ: Lawrence Erlbaum Associates.
Graignic-Philippe, R., Dayan, J., Chokron, S., Jacquet, A. Y., & Tordjman, S. (2014). Effects of prenatal stress on fetal and child development: A critical literature review. Neuroscience & Biobehavioral Reviews, 43, 137–162. doi:http://dx.doi.org/10.1016/j.neubiorev.2014.03.022.
Graven, S. N., & Browne, J. V. (2008). Sleep and brain development: The critical role of sleep in fetal and early neonatal brain development. Newborn and Infant Nursing Reviews, 8(4), 173–179. doi:http://dx.doi.org/10.1053/j.nainr.2008.10.008.
Gunnar, M., & Quevedo, K. (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. doi:10.1146/annurev.psych.58.110405.085605.
Gunnar, M. R., Talge, N. M., & Herrera, A. (2009). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34(7), 953–967. doi:http://dx.doi.org/10.1016/j.psyneuen.2009.02.010.
Hanson, M. A., & Gluckman, P. D. (2014). Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiological Reviews, 94(4), 1027–1076. doi:10.1152/physrev.00029.2013.
Hartman, C. A., Oldehinkel, A. J., De Winter, A. F., & Ormel, J. (2002). Nederlandse vertaling van de Early Adolescent Temperament Questionnaire [Dutch translation of the Early Adolescent Temperament Questionnaire] (Internal Report] (TRAILS Research Group, Department of Psychiatry, University of Groningen, Trans.) Groningen, the Netherlands.
Heim, C., & Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry, 49(12), 1023–1039. doi:http://dx.doi.org/10.1016/S0006-3223(01)01157-X.
Henrichs, J., & Van den Bergh, B. R. H. (2015). Perinatal developmental origins of self-regulation. In G. H. E. Gendolla, M. Tops, & S. L. Koole (Eds.), Handbook of biobehavioral approaches to self-regulation (pp. 349–370). New York, NY: Springer.
Hepper, P. G. (1992). Fetal psychology: An embryonic science. In J. G. Nijhuis (Ed.), Fetal bebaviour: Developmental and perinatal aspects (pp. 129–146). Oxford: Oxford University Press.
Hofer, M. A. (2014). The emerging synthesis of development and evolution: A new biology for psychoanalysis. Neuropsychoanalysis, 16(1), 3–22. doi:10.1080/15294145.2014.901022.
Holditch-Davis, D., & Edwards, L. J. (1998). Temporal organization of sleep–wake states in preterm infants. Developmental Psychobiology, 33(3), 257–269. doi:10.1002/(SICI)1098-2302(199811)33:3<257::AID-DEV6>3.0.CO;2-Q.
Hompes, T. (2014). The effect of maternal prenatal emotional wellbeing and maternal cortisol on fetal and child development. An epigenetic study (PhD thesis), KU Leuven, Doctoral School of Biomedical Sciences, Leuven, Belgium.
Istvan, J. (1986). Stress, anxiety, and birth outcomes: A critical review of the evidence. Psychological Bulletin, 100(3), 331–348. doi:http://dx.doi.org/10.1037/0033-2909.100.3.331.
Jensen, P. S., Mrazek, D., Knapp, P. K., Steinberg, L., Pfeffer, C., Schowalter, J., & Shapiro, T. (1997). Evolution and Revolution in Child Psychiatry: ADHD as a Disorder of Adaptation. Journal of the American Academy of Child & Adolescent Psychiatry, 36(12), 1672–1681. doi:http://dx.doi.org/10.1097/00004583-199712000-00015.
Jobin, J., Wrosch, C., & Scheier, M. F. (2014). Associations between dispositional optimism and diurnal cortisol in a community sample: When stress is perceived as higher than normal. Health Psychology, 33(4), 382. doi:http://dx.doi.org/10.1037/a0032736.
Joffe, J. M. (1969). Prenatal determinants of behaviour. New York, NY: Pergamon.
Johnson, M. H. (2011). Interactive specialization: A domain-general framework for human functional brain development? Developmental Cognitive Neuroscience, 1(1), 7–21. doi:http://dx.doi.org/10.1016/j.dcn.2010.07.003.
Johnson, M. H., & de Haan, M. (2011). Developmental cognitive neuroscience (3rd ed.). Chichester, West Sussex: Wiley-Blackwell.
Kafalí, H., Derbent, A., Keskí, E., Símavlí, Z., & Gözdemír, E. (2011). Effects of maternal anxiety and music on fetal movements and fetal heart rate patterns. The Journal of Maternal-Fetal and Neonatal Medicine, 24(3), 461–464.
Keng, S.-L., Smoski, M. J., & Robins, C. J. (2011). Effects of mindfulness on psychological health: A review of empirical studies. Clinical Psychology Review, 31(6), 1041–1056. doi:http://dx.doi.org/10.1016/j.cpr.2011.04.006.
King, S., & Laplante, D. P. (2005). The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress: The International Journal on the Biology of Stress, 8(1), 35–45. doi:10.1080/10253890500108391.
Knobloch, H., & Pasamanick, B. (1966). Prospective studies on the epidemiology of reproductive casualty: Methods, findings, and some implications. Merrill-Palmer Quarterly of Behavior and Development, 12(1), 27–43.
Kochanska, G., Coy, K. C., & Murray, K. T. (2001). The development of self-regulation in the first four years of life. Child Development, 72(4), 1091–1111. doi:10.1111/1467-8624.00336.
Kohls, N., Sauer, S., & Walach, H. (2009). Facets of mindfulness—Results of an online study investigating the Freiburg mindfulness inventory. Personality and Individual Differences, 46(2), 224–230. doi:10.1016/j.paid.2008.10.009.
Kolb, B., Mychasiuk, R., Muhammad, A., Li, Y., Frost, D. O., & Gibb, R. (2012). Experience and the developing prefrontal cortex. Proceedings of the National Academy of Sciences, 109(Supplement 2), 17186–17193. doi:10.1073/pnas.1121251109.
Koolhaas, J. M., Bartolomucci, A., Buwalda, B., de Boer, S. F., Flügge, G., Korte, S. M., … Fuchs, E. (2011). Stress revisited: A critical evaluation of the stress concept. Neuroscience & Biobehavioral Reviews, 35(5), 1291–1301. doi:http://dx.doi.org/10.1016/j.neubiorev.2011.02.003.
Kopp, C. B. (1982). Antecedents of self-regulation: A developmental perspective. Developmental Psychology, 18(2), 199–214. doi:http://dx.doi.org/10.1037/0012-1649.18.2.199.
Kushnerenko, E. V., Van den Bergh, B. R. H., & Winkler, I. (2013). Separating acoustic deviance from novelty during the first year of life: A review of event-related potential evidence. Frontiers in Psychology, 4, 595. doi:10.3389/fpsyg.2013.00595.
Ladd, C.O., Huot, R.L., Thrivikraman, K.V., Nemeroff, C.B., Meaney, M.J., Plotsky, P.M. (1999). Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research, 122, 81–103. doi:10.1016/S0079-6123(08)62132-9.
Laplante, D. P., Barr, R. G., Brunet, A., Du Fort, G. G., Meaney, M. L., Saucier, J. F., … King, S. (2004). Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research, 56(3), 400–410. doi:10.1203/01.pdr.0000136281.34035.44.
Laplante, D. P., Brunet, A., Schmitz, N., Ciampi, A., & King, S. (2008). Project Ice storm: Prenatal maternal stress affects cognitive and linguistic functioning in 5½-year-old children. Journal of the American Academy of Child & Adolescent Psychiatry, 47(9), 1063–1072. doi:http://dx.doi.org/10.1097/CHI.0b013e31817eec80.
Lee, Y.-A., & Goto, Y. (2013). The effects of prenatal and postnatal environmental interaction: Prenatal environmental adaptation hypothesis. Journal of Physiology-Paris, 107(6), 483–492. doi:http://dx.doi.org/10.1016/j.jphysparis.2013.04.007.
Levine, S. (2005). Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology, 30, 939–946. doi:10.1016/j.psyneuen.2005.03.013.
Lewis, A., Galbally, M., Gannon, T., & Symeonides, C. (2014). Early life programming as a target for prevention of child and adolescent mental disorders. BMC Medicine, 12(1), 33. doi:10.1186/1741-7015-12-33.
Lewis, C. R., & Olive, M. F. (2014). Early-life stress interactions with the epigenome: Potential mechanisms driving vulnerability toward psychiatric illness. Behavioural Pharmacology, 25(5–6), 341–351. 310.1097/FBP.0000000000000057.
Li, J., Yang, H., Guldin, M.-B., Vedsted, P., & Vestergaard, M. (2015). Increased utilisation of primary healthcare in persons exposed to severe stress in prenatal life: A national population-based study in Denmark. BMJ Open, 5(1), e005657. doi:10.1136/bmjopen-2014-005657.
Lickliter, R. (2007). The dynamics of development and evolution: Insights from behavioral embryology. Developmental Psychobiology, 49(8), 749–757. doi:10.1002/dev.20270.
Lobel, M., DeVincent, C. J., Kaminer, A., & Meyer, B. A. (2000). The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychology, 19(6), 544. doi:http://dx.doi.org/10.1037/a0013242.
Loomans, E. M. (2013). From the Womb into the World. Early life influences on neurocognitive functioning and behaviour in five to six year olds. (PhD thesis), Tilburg University, Tilburg, the Netherlands.
Loomans, E., van der Stelt, O., van Eijsden, M., Gemke, R., Vrijkotte, T., & Van den Bergh, B. R. H. (2011). Antenatal maternal anxiety is associated with problem behaviour at age five. Early Human Development, 87, 565–570. doi:10.1016/j.earlhumdev.2011.04.014.
Loomans, E. M., van Dijk, A. E., Vrijkotte, T. G., van Eijsden, M., Stronks, K., Gemke, R. J., & Van den Bergh, B. R. (2013). Psychosocial stress during pregnancy is related to adverse birth outcomes: Results from a large multi-ethnic community-based birth cohort. The European Journal of Public Health, 23(3), 485–491.
Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. doi:10.1038/nrn2639.
Lutz, P. E., & Turecki, G. (2014). DNA methylation and childhood maltreatment: From animal models to human studies. Neuroscience, 264, 142–156. doi:http://dx.doi.org/10.1016/j.neuroscience.2013.07.069.
Matthews, S. G., & Phillips, D. I. (2010). Minireview: Transgenerational inheritance of the stress response: A new frontier in stress research. Endocrinology, 151(1), 7–13. doi:10.1210/en.2009-0916.
McDonald, R.L. (1968). The role of emotional factors in obstetric complications: a review. Psychosomatic Medicine, 30(2), 222-237.
McEwen, B. S., & Morrison, J. H. (2013). The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 79(1), 16–29. doi:http://dx.doi.org/10.1016/j.neuron.2013.06.028.
Meaney, M. J. (2010). Epigenetics and the biological definition of gene × environment interactions. Child Development, 81(1), 41–79. doi:10.1111/j.1467-8624.2009.01381.x.
Meaney, M. J., Szyf, M., & Seckl, J. R. (2007). Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in Molecular Medicine, 13(7), 269–277. doi:http://dx.doi.org/10.1016/j.molmed.2007.05.003.
Mennes, M. (2008). Longitudinal study on the effects of maternal anxiety during pregnancy: Neuropsychological and neurophysiological examination of cognitive control in the adolescent offspring. (PhD Thesis). Catholic University Leuven -KU Leuven, Leuven, Belgium.
Mennes, M., Stiers, P., Lagae, L., & Van den Bergh, B. R. H. (2006). Long-term cognitive sequelae of antenatal maternal anxiety: Involvement of the orbitofrontal cortex. Neuroscience & Biobehavioral Reviews, 30(8), 1078–1086. doi:10.1016/j.neubiorev.2006.04.003.
Mennes, M., Van den Bergh, B. R. H., Lagae, L., & Stiers, P. (2009). Developmental brain alterations in 17 year old boys are related to antenatal maternal anxiety.Clinical. Neurophysiology, 120(6), 1116–1122. http://dx.doi.org/10.1016/j.neubiorev.2006.04.003.
Meyer, U., Nyffeler, M., Engler, A., Urwyler, A., Schedlowski, M., Knuesel, I., … Feldon, J. (2006). The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of Neuroscience, 26(18), 4752–4762. doi:10.1523/JNEUROSCI.0099-06.2006.
Michel, G. F., & Moore, C. L. (1995). Developmental psychobiology: An interdisciplinary science. Cambridge, MA: MIT Press.
Milgrom, J., Holt, C., Holt, C. J., Ross, J., Ericksen, J., & Gemmill, A. W. (2015). Feasibility study and pilot randomised trial of an antenatal depression treatment with infant follow-up. Archives of Womens Mental Health, 1–14. doi:10.1007/s00737-015-0512-5.
Mirmiran, M., Maas, Y. G. H., & Ariagno, R. L. (2003). Development of fetal and neonatal sleep and circadian rhythms. Sleep Medicine Reviews, 7(4), 321–334. doi:http://dx.doi.org/10.1053/smrv.2002.0243.
Monk, C., Fifer, W. P., Myers, M. M., Bagiella, E., Duong, J. K., Chen, I. S., … Altincatal, A. (2011). Effects of maternal breathing rate, psychiatric status, and cortisol on fetal heart rate. Developmental Psychobiology, 53(3), 221–233. doi: 10.1002/dev.20513.
Monk, C., Sloan, R. P., Myers, M. M., Ellman, L., Werner, E., Jeon, J., … Fifer, W. P. (2004). Fetal heart rate reactivity differs by women’s psychiatric status: An early marker for developmental risk? Journal of the American Academy of Child & Adolescent Psychiatry, 43(3), 283–290. doi: 10.1097/00004583-200403000-00009.
Mulder, E. J. H., Morssink, L. P., Van Der Schee, T., & Visser, G. H. A. (1998). Acute maternal alcohol consumption disrupts behavioral state organization in the near-term fetus. Pediatric Research, 44(5), 774–779. doi:10.1203/00006450-199811000-00022.
Mulder, E. J. H., Robles de Medina, P. G., Huizink, A. C., Van den Bergh, B. R. H., Buitelaar, J. K., & Visser, G. H. A. (2002). Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Human Development, 70(1–2), 3–14. doi:http://dx.doi.org/10.1016/S0378-3782(02)00075-0.
Mulder, E. J. H., Ververs, F. F. T., de Heus, R., & Visser, G. H. A. (2011). Selective serotonin reuptake inhibitors affect neurobehavioral development in the human fetus. Neuropsychopharmacology, 36(10), 1961–1971. doi:http://dx.doi.org/10.1038/npp.2011.67.
Mulder, E. J. H., Visser, G. H. A., Bekedam, D. J., & Prechtl, H. F. R. (1987). Emergence of behavioural states in fetuses of type-l diabetic women. Early Human Development, 15(4), 231–252. doi:10.1016/0378-3782(87)90082-X.
Nathanielsz, P. W. (1999). Life in the Womb.: The origing of health and disease. Ithaca, NY: Promethean Press.
Nederhof, E., & Schmidt, M. V. (2012). Mismatch or cumulative stress: Toward an integrated hypothesis of programming effects. Physiology & Behavior, 106(5), 691–700. doi:http://dx.doi.org/10.1016/j.physbeh.2011.12.008.
Nelson, C. A., Bloom, F. E., Cameron, J. L., Amaral, D., Dahl, R. E., & Pine, D. (2002). An integrative, multidisciplinary approach to the study of brain–behavior relations in the context of typical and atypical development. Development and Psychopathology, 14(03), 499–520. doi:10.1017/S0954579402003061.
Nijhuis, J. G., Prechtl, H. F. R., Martin, C. B., Jr., & Bots, R. S. G. M. (1982). Are there behavioural states in the human fetus? Early Human Development, 6(2), 177–195. doi:http://dx.doi.org/10.1016/0378-3782(82)90106-2.
Nijhuis, I. J. M., ten Hof, J., Nijhuis, J. G., Mulder, E. J. H., Narayan, H., Taylor, D. J., & Visser, G. H. A. (1999). Temporal organization of fetal behavior from 24-weeks gestation onwards in normal and complicated pregnancies. Developmental Psychobiology, 34(4), 257–268. doi:10.1002/(SICI)1098-2302(199905)34:2<257::AID-DEV2>3.0.CO;2-V.
O’Connor, T., Heron, J., Golding, J., Beveridge, M., & Glover, V. (2002). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry, 180, 502–508. doi:10.1192/bjp.180.6.502.
O’Connor, T. G., Heron, J., Golding, J., & Glover, V. (2003). Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. Journal of Child Psychology and Psychiatry, 44(7), 1025–1036. doi:10.1111/1469-7610.00187.
O’Connor, T. G., Monk, C., & Fitelson, E. M. (2014). Practitioner review: Maternal mood in pregnancy and child development—Implications for child psychology and psychiatry. Journal of Child Psychology and Psychiatry, 55(2), 99–111. doi:10.1111/jcpp.12153.
O’Donnell, K. J., Glover, V., Holbrook, J. D., & O’Connor, T. G. (2014). Maternal prenatal anxiety and child brain-derived neurotrophic factor (BDNF) genotype: Effects on internalizing symptoms from 4 to 15 years of age. Development and Psychopathology, 26(Special issue 4pt2), 1255–1266. doi:10.1017/S095457941400100X.
O’Donnell, K., O’Connor, T., & Glover, V. (2009). Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Developmental Neuroscience, 31, 285–292. doi: 10.1159/000216539.
Ortega-Martínez, S. (2015). Influences of prenatal and postnatal stress on adult hippocampal neurogenesis: The double neurogenic niche hypothesis. Behavioural Brain Research, 281(5), 309–317. doi:http://dx.doi.org/10.1016/j.bbr.2014.12.036.
Otte, R. A. (2013). Prenatal expsoure to maternal anxiety affects neurocognitiion in the first year of year (Phd thesis), Tilburg University, Tilburg, the Netherlands.
Otte, R. A., Winkler, I., Braeken, M. A. K. A., Stekelenburg, J. J., van der Stelt, O., & Van den Bergh, B. R. H. (2013). Detecting violations of temporal regularities in waking and sleeping two-month-old infants. Biological Psychology, 92(2), 315–322. doi:http://dx.doi.org/10.1016/j.biopsycho.2012.09.009.
Pasamanick, B., & Knobloch, H. (1966). Retrospective studies on the epidemiology of reproductive casualty: Old and new. Merrill-Palmer Quarterly of Behavior and Development, 12(1), 7–26.
Pasamanick, B., Rogers, M. E., & Lilienfield, A. M. (1956). Pregnancy experience and the development of behavior disorders in children? American Journal of Psychiatry, 112(8), 613–618. doi:10.1176/ajp.112.8.613.
Peiper, A. (1925). Sinnesempfindungen des Kindes vor seiner Geburt. Monatsschrift für Kinderheilkunde, 29, 236–241.
Peirano, P., Algarı́n, C., & Uauy, R. (2003). Sleep-wake states and their regulatory mechanisms throughout early human development. The Journal of Pediatrics, 143(4, Supplement), 70–79. doi:http://dx.doi.org/10.1067/S0022-3476(03)00404-9.
Phillips, D. I., & Jones, A. (2006). Fetal programming of autonomic and HPA function: Do people who were small babies have enhanced stress responses? The Journal of Physiology, 572(1), 45–50. doi:10.1113/jphysiol.2005.104695.
Pollak, S. D. (2005). Early adversity and mechanisms of plasticity: Integrating affective neuroscience with developmental approaches to psychopathology. Development and Psychopathology, 17(03), 735–752. doi:http://dx.doi.org/10.1017/S0954579405050352.
Posner, M. I., & Rothbart, M. K. (2000). Developing mechanisms of self-regulation. Development and Psychopathology, 12(03), 427–441. doi:http://dx.doi.org/10.1017/S0954579400003096.
Prechtl, H. F. R. (1974). The behavioural states of the newborn infant (a review). Brain Research, 76(2), 185–212. doi:http://dx.doi.org/10.1016/0006-8993(74)90454-5.
Prechtl, H. F. R. (1984). Continuity and change in early neural development. In H. F. R. Prechtl (Ed.), Continuity of neural functions from prenatal to postnatal life (1st ed., pp. 1–15). London: Spastics international Medical Publications: Oxford Blackwell Scientific.
Pruessner, J. C., Dedovic, K., Pruessner, M., Lord, C., Buss, C., Collins, L., … Lupien, S. J. (2010). Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology, 35(1), 179–191. doi:http://dx.doi.org/10.1016/j.psyneuen.2009.02.016.
Räikkönen, K., Seckl, J. R., Pesonen, A.-K., Simons, A., & Van den Bergh, B. R. H. (2011). Stress, glucocorticoids and liquorice in human pregnancy: Programmers of the offspring brain. Stress, 14(6), 590–603. doi:10.3109/10253890.2011.602147.
Ray, W. S. (1932). A preliminary report on a study of fetal conditioning. Child Development, 3(2), 175–177. doi:10.2307/1125392.
Reul, J. M. H. M., Collins, A., Saliba, R. S., Mifsud, K. R., Carter, S. D., Gutierrez-Mecinas, M., … Linthorst, A. C. E. (2015). Glucocorticoids, epigenetic control and stress resilience. Neurobiology of Stress, 1(0), 44–59. doi:http://dx.doi.org/10.1016/j.ynstr.2014.10.001.
Ross, L. E., & McLean, L. M. (2006). Anxiety disorders during pregnancy and the postpartum period: A systematic review. Journal of Clinical Psychiatry. doi:10.4088/JCP.v67n0818.
Rothbart, M. K., & Ahadi, S. A. (1994). Temperament and the development of personality. Journal of Abnormal Psychology, 103(1), 55–66. doi:http://dx.doi.org/10.1037/0021-843X.103.1.55.
Rothbart, M. K., Ahadi, S. A., Hershey, K. L., & Fisher, P. (2001). Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development, 72(5), 1394–1408. doi:10.1111/1467-8624.00355.
Rothbart, M. K., & Derryberry, D. (1981). Development of individual differences in temperament. In M. E. Lamb & A. L. Brown (Eds.), Advances in developmental psyvchology (Vol. 1, pp. 37–86). Hillsdale, NJ: Earlbaum.
Rothbart, M. K., Sheese, B. E., Rueda, M. R., & Posner, M. I. (2011). Developing mechanisms of self-regulation in early life. Emotion Review, 3(2), 207–213. doi:10.1177/1754073910387943.
Rothbart, Μ., & Bates, J. (1998). Temperament. In W. Damon (Series Ed.) & N. Eisenberg (Vol. Ed.), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (pp. 105–176). New York, NY: Wiley.
Rutter, M. (1987). Psychosocial resilience and protective mechanisms. American Journal of Orthopsychiatry, 75(3), 361–331.
Rutter, M. (1995). Clinical implications of attachment concepts: Retrospect and prospect. Journal of Child Psychology and Psychiatry, 36(4), 549–571. doi:10.1111/j.1469-7610.1995.tb02314.x.
Rutter, M. (2002). Nature, nurture, and development: From evangelism through science toward policy and practice. Child Development, 73(1), 1–21. doi:10.1111/1467-8624.00388.
Sameroff, A. J. (1975). Early influences on development: Fact or fancy? Merrill-Palmer Quarterly of Behavior and Development, 21, 267–294. doi: http://www.jstor.org/stable/23083878.
Sameroff, A. J., & Chandler, M. J. (1975). Reproductive risk and the continuum of caretaking casualty. In F. D. Horowitz (Ed.), Review of child development research (Vol. 4, pp. 187–244). Chicago, IL: The University of Chicago Press.
Sameroff, A. J., & Zax, M. (1973). Perinatal characteristics of the offspring of schizophrenic women. The Journal of Nervous and Mental Disease, 157(3), 191–199.
Schechter, D. S. (2012). The developmental neuroscience of emotional neglect, its consequences, and the psychosocial interventions that can reverse them. American Journal of Psychiatry, 169(5), 452–454. doi:10.1176/appi.ajp.2012.12020174.
Scher, M. S. (2008). Ontogeny of EEG-sleep from neonatal through infancy periods. Sleep Medicine, 9(6), 615–636. doi:http://dx.doi.org/10.1016/j.sleep.2007.08.014.
Scher, M. S., Steppe, D. A., & Banks, D. L. (1996). Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatric Neurology, 14(2), 137–144. doi:http://dx.doi.org/10.1016/0887-8994(96)00013-6.
Schlotz, W., Jones, A., Godfrey, K. M., & Phillips, D. I. W. (2008). Effortful control mediates associations of fetal growth with hyperactivity and behavioural problems in 7- to 9-year-old children. Journal of Child Psychology and Psychiatry, 49(11), 1228–1236. doi:10.1111/j.1469-7610.2008.01946.x.
Schlotz, W., & Phillips, D. I. W. (2009). Fetal origins of mental health: Evidence and mechanisms. Brain, Behavior, and Immunity, 23(7), 905–916. doi:http://dx.doi.org/10.1016/j.bbi.2009.02.001.
Seckl, J. R. (2007). Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Progress in Brain Research, 167, 17–34. http://dx.doi.org/10.1016/S0079-6123(07)67002-2.
Seckl, J. R., & Holmes, M. C. (2007). Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nature Clinical Practice Endocrinology & Metabolism, 3(6), 479–488. doi:10.1038/ncpendmet0515.
Sheehan, D., & Lecrubier, Y. (2010). The Mini International Neuropsychiatric Interview Version 6.0 (MINI 6.0. Jacksonville, FL: Medical Outcomes System.
Smotherman, W. P., & Robinson, S. R. (1995). Tracing developmental trajectories into the prenatal period. In J.-P. Lecanuet, W. P. Fifer, N. A. Krasnegor, & W. P. Smotherman (Eds.), Fetal development: A psychobiological perspective (pp. 15–32). Hillsdale, NJ: Lawrence Erlbaum Associates.
Sontag, L. W. (1941). The significance of fetal environmental differences. American Journal of Obstetrics & Gynecology, 42(6), 996–1003.
Sontag, L. W. (1944). Differences in modifiability of fetal behavior and physiology. Psychosomatic Medicine, 6, 151–154.
Sontag, L. W. (1966). Impliations of fetal behavior and environment for adult personalities. Annals of the New York Academy of Sciences, 134(2), 782–786. doi:10.1111/j.1749-6632.1966.tb43063.x.
Sontag, L. W., & Wallace, R. F. (1934). Preliminary report of the fels fund: Study of fetal activity. American Journal of Diseases of Children, 48(5), 1050–1057.
Spelt, D. K. (1948). The conditioning of the human fetus in utero. Journal of Experimental Psychology, 38(3), 338–346. doi:org/10.1037/h0059632.
Spielberger, C. D., Gorsuch, R. L., & Lushene, R. E. (1970). Manual for the state trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press.
Stern, D. N. (2009). The first relationship: Infant and mother. Cambridge, MA: Harvard University Press.
Stott, D. H. (1958). Some psychosomatic aspects of casualty in reproduction. Journal of Psychosomatic Research, 3(1), 42–55. doi:http://dx.doi.org/10.1016/0022-3999(58)90015-1.
Stott, D. H. (1973). Follow-up study from birth of the effects of prenatal stresses. Developmental Medicine & Child Neurology, 15(6), 770–787. doi:10.1111/j.1469-8749.1973.tb04912.x.
Stott, D. H., & Latchford, S. A. (1976). Prenatal antecedents of child health, development, and behavior: An epidemiological report of incidence and association. Journal of the American Academy of Child Psychiatry, 15(1), 161–191. doi:http://dx.doi.org/10.1016/S0002-7138(09)62267-6.
Swaab, D. F., Bao, A.-M., & Lucassen, P. J. (2005). The stress system in the human brain in depression and neurodegeneration. Ageing Research Reviews, 4(2), 141–194. doi:http://dx.doi.org/10.1016/j.arr.2005.03.003.
Tegethoff, M., Greene, N., Olsen, J., Schafner, E., & Meinlschmidt, G. (2011). Stress during pregnancy and offspring pediatric disease: A national cohort study. Environmental Health Perspectives, 11(9), 1647–1152.
Thayer, J. F., & Lane, R. D. (2007). The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology, 74(2), 224–242. doi:10.1016/j.biopsycho.2005.11.013.
Thayer, J. F., & Sternberg, E. (2006). Beyond heart rate variability. Annals of the New York Academy of Sciences, 1088(1), 361–372. doi:10.1196/annals.1366.014.
Thayer, J. F., Yamamoto, S. S., & Brosschot, J. F. (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131.
Van Assche, F. A. (1997). Birthweight as a risk factor for breast cancer. The Lancet, 349, 502.
Van Assche, F. A., Holemans, K., & Aerts, L. (2001). Long-term consequences for offspring of diabetes during pregnancy. British Medical Bulletin, 60(1), 173–182. doi:10.1093/bmb/60.1.173.
Van den Bergh, B. (1981). Factoren die de prenatale ontwikkeling beïnvloeden. Literatuurstudie aangaande factoren die het prenataal intra-uterien milieu bepalen en die te beschouwen zijn als prenatale determinanten van postnataal gedrag. [Factors influencing prenatal development. Review of the literature concerning factors constituting the prenatal intra-uterine environment that can be regarded as prenatal determinants of postnatal behavior] (Master thesis), Katholieke Universiteit Leuven, Leuven, Belgium.
Van den Bergh, B. (1983). De psychische toestand van de zwangere en de prenatale ontwikkeling. Literatuurstudie en schets van een heuristisch model. [The psychological state of the pregnant woman and prenatal development. Review of the literature and heuristic model]. Tijdschrift voor Orthopedagogie, Kinderpsychiatrie en Klinische Kinderpsychologie, 8(1), 18–37.
Van den Bergh, B. (1989). De emotionele toestand van de (zwangere) vrouw, obstetrische complicaties en het gedrag en de ontwikkeling van de foetus en van het kind tot de leeftijd van zeven maanden. [The emotional state of the (pregnant) woman, obstetrical complications and the behavior and development of fetus and child until seven months after birth] (PhD thesis), Katholieke Universiteit Leuven, Leuven, Belgium.
Van den Bergh, B. R. H. (1990). The influence of maternal emotions during pregnancy on fetal and neonatal behavior. Journal of Prenatal & Perinatal Psychology & Health, 5(2), 119–130.
Van den Bergh, B. R. H. (1992). Maternal emotions during pregnancy and fetal and neonatal behaviour. In J. G. Nijhuis (Ed.), Fetal behaviour. Developmental and perinatal aspects (pp. 157–178). New York, NY: Oxford University Press.
Van den Bergh, B.R.H. (2010). To become or to be? The duality of neurodevelopment has a perinatal and therefore also a societal dimension. Inaugural address at Tilburg University May 10, 2010. Prismaprint Tilburg University, Tilburg, the Netherlands.
Van den Bergh, B. R. H. (2011a). Developmental programming of early brain and behaviour development and mental health: A conceptual framework. Developmental Medicine & Child Neurology, 53, 19–23. doi:10.1111/j.1469-8749.2011.04057.x.
Van den Bergh, B. R. H. (2011b). Prenatal programming of cognition and emotion in humans: From birth to age 20. In A. Plagemann (Ed.), Perinatal programming: The state of the art (pp. 199–205). Berlin: Walter de Gruyter.
Van den Bergh, B. R. H., & Ackx, M. (2003). Een Nederlandse versie van Rothbarts’ Children’s Behavior Questionnaire’: Interne consistentie en driefactorenmodel van de subschalen. [Temperament measured using a Dutch version of Rothbart’s ‘Children’s Behavior Questionnaire’. Evidence for a three-factor structure of the subscales]. Kind en Adolescent, 24(2), 77–84.
Van den Bergh, B. R. H., Loomans, E. M., & Mennes, M. (2015). Early life influences on cognition, behavior, and emotion in humans: From birth to age 20. In M. C. Antonelli (Ed.), Perinatal programming of neurodevelopment (Chapter 15). Advances in neurobiology (Vol. 10, pp. 315–331). New York, NY: Springer. doi:10.1007/978-1-4939-1372-5_15.
Van den Bergh, B. R. H., & Marcoen, A. (2004). High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development, 75(4), 1085–1097. doi:10.1111/j.1467-8624.2004.00727.x.
Van den Bergh, B. R. H., Mennes, M., Oosterlaan, J., Stevens, V., Stiers, P., Marcoen, A., & Lagae, L. (2005). High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14-and 15-year-olds. Neuroscience & Biobehavioral Reviews, 29(2), 259–269. doi:10.1016/j.neubiorev.2004.10.010
Van den Bergh, B. R. H., & Mulder, E. J. H. (2012). Fetal sleep organization: A biological precursor of self-regulation in childhood and adolescence? Biological Psychology, 89(3), 584–590. doi:http://dx.doi.org/10.1016/j.biopsycho.2012.01.003.
Van den Bergh, B. R. H., Mulder, E. J. H., Mennes, M., & Glover, V. (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neuroscience & Biobehavioral Reviews, 29(2), 237–258. doi:http://dx.doi.org/10.1016/j.neubiorev.2004.10.007.
Van den Bergh, B. R. H., Mulder, E. J. H., Visser, G. H. A., Poelmann-Weesjes, G., Bekedam, D. J., & Prechtl, H. F. R. (1989). The effect of (induced) maternal emotions on fetal behaviour: A controlled study. Early Human Development, 19(1), 9–19. doi:org/10.1016/0378-3782(89)90100-X.
Van den Bergh, B. R. H., Van Calster, B., Smits, T., Van Huffel, S., & Lagae, L. (2008). Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology, 33(3), 536–545. doi:10.1038/sj.npp.1301450.
van den Heuvel, M. I., Donkers, F. C., Winkler, I., Otte, R. A., & Van den Bergh, B. R. (2014). Maternal mindfulness and anxiety during pregnancy affect infants’ neural responses to sounds. Social Cognitive and Affective Neuroscience, 91, 103–108. doi:10.1093/scan/nsu075.
Van der Ploeg, H., & Defares, P. (1980). ZBV: Handleiding bij de zelf-beoordelings vragenlijst: een Nederlandstalige bewerking van Spielberger state-trait anxiety inventory STAI-Y. Amsterdam: Harcourt.
Vandenberghe, K., & De Wolf, F. (1990). Ultrasonic assessment of fetal stomach function. In A. Kurjak (Ed.), Physiology and clinic, recent advances in ultrasound diagnosis, 2 (pp. 275–282). Amsterdam: Excerpta Medica.
Verhulst, F. C., van der Ende, J., & Koot, H. M. (1996). Handleiding voor the CBCL/14 – 18 [Manual for the CBCL/14 – 18] Rotterdam, the Netherlands: Afdeling Kinder-en Jeugdpsychiatrie, Sophia Kinderziekenhuis/Adacemisch Ziekenhuis/Erasmus Universiteit.
Visser, G. H. A., Mulder, E. J. H., & Prechtl, H. F. R. (1992). Studies on developmental neurology in the human fetus. Developmental Pharmacology and Therapeutics, 18(3-4), 175–183.
Visser, G. H. A., Poelman-Weesjes, G., Cohen, T. M. N., & Bekedam, D. J. (1987). Fetal behavior at 30 to 32 weeks of gestation. Pediatric Research, 22(6), 655–658. doi:10.1203/00006450-198712000-00009.
Vlemincx, E., Taelman, J., De Peuter, S., Van Diest, I., & Van Den Bergh, O. (2011). Sigh rate and respiratory variability during mental load and sustained attention. Psychophysiology, 48(1), 117–120. doi:10.1111/j.1469-8986.2010.01043.x.
Walach, H., Buchheld, N., Buttenmuller, V., Kleinknecht, N., & Schmidt, S. (2006). Measuring mindfulness—The Freiburg Mindfulness Inventory (FMI). Personality and Individual Differences, 40(8), 1543–1555. doi:10.1016/j.paid.2005.11.025.
Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., … Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854. doi:10.1038/nn1276.
Weinstock, M. (2005). The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity, 19, 296–308. doi:10.1016/j.bbi.2004.09.006.
Weinstock, M. (2008). The long-term behavioural consequences of prenatal stress. Neuroscience & Biobehavioral Reviews, 32(6), 1073–1086. doi:http://dx.doi.org/10.1016/j.neubiorev.2008.03.002.
Winkler, I. (2007). Interpreting the Mismatch Negativity. Journal of Psychophysiology, 21(3), 147–163. doi:10.1027/0269-8803.21.34.147.
Winkler, I., Háden, G. P., Ladinig, O., Sziller, I., & Honing, H. (2009). Newborn infants detect the beat in music. Proceedings of the National Academy of Sciences, 106(7), 2468–2471. doi:10.1073/pnas.0809035106.
Winkler, I., Kushnerenko, E., Horváth, J., Čeponienė, R., Fellman, V., Huotilainen, M., … Sussman, E. (2003). Newborn infants can organize the auditory world. Proceedings of the National Academy of Sciences, 100(20), 11812–11815. doi:10.1073/pnas.2031891100.
Young, J. B. (2002). Programming of sympathoadrenal function. Trends in Endocrinology & Metabolism, 13(9), 381–385. doi:10.1016/S1043-2760(02)00661-6.
Zannas, A. S., & West, A. E. (2014). Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience, 264, 157–170. doi:http://dx.doi.org/10.1016/j.neuroscience.2013.12.003.
Acknowledgements
I thank all parents and children of the Leuven and Tilburg cohorts for their participation in our studies and the many students who helped with the data collection. I am grateful to the (former) PhD students Drs. T. Billiet, Dr. H.R. Binderhagel, Dr.A. Bogaerts, Dr. M.A.K.A. Braeken, Dr. E.M. Loomans, Dr. K. Eggers, Dr. M.Mennes, Dr. R.A. Otte, Drs. M.I. van den Heuvel and to Dr. F. Donkers for their dedication to the longitudinal DOBHaD projects, their expertise and excellent collaboration. I thank all coauthors and collaborators for sharing their expertise and fruitful collaboration. These studies were realized with the financial support from of the Fund for Scientific Research (FWO, Flanders; grant agreement G.0211.3), the Katholieke Universiteit Leuven (KU Leuven IMPH/06/GHW and IDO 05/010 EEG-FMRI); Tilburg University–Babylab; the European Science Foundation—EruoSTRESS project, and EU Seventh Framework Programme (FP7, Health 20112.2.2-2, grant agreement No. 279281 BRAINAGE); we are grateful for this support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Van den Bergh, B.R.H. (2016). Maternal Anxiety, Mindfulness, and Heart Rate Variability During Pregnancy Influence Fetal and Infant Development. In: Reissland, N., Kisilevsky, B. (eds) Fetal Development. Springer, Cham. https://doi.org/10.1007/978-3-319-22023-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-22023-9_14
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22022-2
Online ISBN: 978-3-319-22023-9
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)