Abstract
The micromechanical properties of soft tissues and materials are of considerable interest for biomedical applications. Nanoindentation is a powerful technique for determining localized material properties of biological tissues and has been used widely for hard tissue and material characterization. However, the technique is much more challenging when utilized for soft tissues due to their compliance as well as due to the limitations of commercial instruments which were originally developed for stiff, engineering materials. This study explores the use of a dynamic indentation method with a cylindrical punch (100 μm diameter) to characterize gelatin gel and low molecular weight hydrogels. A Keysight Technologies DCM II actuator is used with the Continuous Stiffness Measurement (CSM) to determine the complex shear modulus of these gels. The method overcomes surface detection issues with standard quasi-static nanoindentation as a change in phase angle can be used to accurately detect the sample surface. The data collected in this study are found to be comparable with macroscopic rheology and demonstrates the utility of the method for characterization of hydrogels.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Nanoindentation is being used increasingly to characterise the mechanical properties of soft materials such as hydrogels [1] and compliant polymers [2] as well as soft tissues [3, 4]. For biomedical applications, accurate characterisation of the mechanical properties of hydrogels are required so that their properties can be tailored for optimal cellular behaviour [1] and so that they better mimic the properties of natural tissues that are being replaced for tissue engineering applications [5]. The advantages of nanoindentation are numerous including that small volumes of material can be tested with a high spatial resolution, ease of sample preparation, the ability to apply extremely low forces and the ability to map heterogeneous or spatially graded properties of materials [5]. Hence, the technique has significant potential over macroscopic mechanical tests such as rheology, which are typically used to characterise the shear properties of hydrogels. However, the technique, when applied to soft materials and tissues, has a number of limitations because commercial instruments were originally developed for characterising stiff, engineering materials. The main issues faced when characterising compliant materials is accurate surface detection and sample roughness [3].
Recently, dynamic nanoindentation testing has been used to characterise the storage and loss modulus of a range of hydrogel samples which had storage modulus values in the range of 3.5–8.9 MPa [5]. In this paper, we make use of an ultra-low nanoindentation head equipped with a dynamically compliant indenter head operated at its resonant frequency to test the properties of highly compliant gels including low molecular weight hydrogels, which have a shear modulus in the order of tens to hundreds of kPa. The application of this method for potential characterisation of spatially resolved properties of highly compliant gels which serve a range of biomedical applications is demonstrated.
17.2 Materials and Methods
17.2.1 Materials
17.2.1.1 Gelatin Gel
Gelatin (48723-500G-F, Sigma-Aldrich) gel was made with a concentration of 0.34 g/ml. The gel solution was poured into a backless tray and cured in the −20 °C refrigerator. The gel was tested 2 h after removal from the refrigerator.
17.2.1.2 Hydrogels
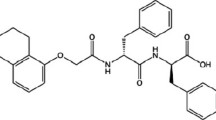
Two different gelators were utilised in this study (2-naphthalene-valine-glycine (2-NapVG) and 1-tetrahydronathyl-diphenylalanine (1-THNapFF). The gels were made by dissolving 5 mg/mL of the gelator using 1 molar equivalent of NaOH and then making up the solution to 2 mL with deionized water. The solutions were gently shaken until all the gelator was dissolved. Subsequently, 8 mg/mL of glucono-d-lactone (GdL) was added to the solutions to lower the pH and they were left to gel overnight. Nanoindentation tests were carried out on the gels the following day.
17.2.2 Experiment Setup
A Nanoindenter G200 system was used with a DCM-II head (Keysight Technologies, USA) for all experiments. A 100 μm flat-ended cylindrical punch tip (Synton-MDP Ltd, Nidau, Switzerland) was mounted on the DCM-II. A custom sample holder was designed into which the gel was transferred. The well in the sample holder was 1 cm2 and 4 mm deep.
17.2.3 Nanoindentation Test Procedure and Analysis
The DCM-II head was used to perform dynamic (oscillatory) indentation and determine the complex shear modulus (G*), which has real and imaginary components, and gives the intrinsic elastic (G′) and viscous (G″) properties of the material:
The theoretical basis for this method has been covered in detailed elsewhere [6] and is summarised here. By indenting samples with a flat-ended cylindrical punch, the relationship between the shear modulus (G′), Poisson’s ratio (v), elastic contact stiffness (S), and punch diameter (D) can be determined using Sneddon’s analysis [7]:
And a similar relationship for G″ can be derived by considering the contact damping (Cw) [8–10]:
The contact stiffness (S) is obtained by subtracting the instrument stiffness (Ki) from the total measured stiffness (Ks):
The contact damping (Cw) is obtained by subtracting the instrument damping (Ciw) from the total measured damping (Csw):
Both the stiffness and damping are obtained by oscillating the indenter electromagnetically. Initially, the stiffness and damping of the head only are measured under the same conditions that are subsequently used for testing i.e., the same position, frequency and oscillation amplitude. The indenter head is subsequently moved down towards the sample until contact is sensed. At this point a pre-compression of is applied such that the indenter is in full contact with the sample surface. The indenter is then vibrated at fixed frequency and oscillation amplitude. Hence, with this two-step process the indenter head is calibrated in situ with the initial oscillation sequence. Stiffness and damping contributions from the head can then be subtracted from the measured signal to obtain the response of the contact only. Subsequently, the second oscillation sequence acquires the material properties.
For the experiments conducted in this study a pre-compression of 5 μm was selected. The indenter was fully in contact with the sample surface. The indenter was then vibrated at a frequency of 110 Hz (the resonant frequency of the indenter) and with an oscillation amplitude of 500 nm. The surface detection relies on a phase shift of the displacement measurement (Fig. 17.1).
As a criterion for detecting the surface, the phase shift was monitored over a number of data points in order to exclude random spikes which were found to occur in some instances over short time intervals (e.g., 2 data points) and thereby lead to an erroneous surface detection. Once the surface detection requirement had been fulfilled over a predefined number of data points the initial contact was determined from the first point in that sequence.
Ten indents were performed on each sample with 200 μm spacing between each sample. A Poisson’s ratio of 0.5 was assumed for each of the gel samples. G′, G″ and the loss factor [tan(δ)] i.e., ratio of G″/G′ were calculated for each indentation. After each indent, the tip was cleaned by indenting a piece of double-sided Scotch tape mounted on an adjacent sample puck before returning to the gel sample.
17.3 Results and Discussion
The gelatin gel which serves as a model for soft tissue [11] yielded values for G′ and G″ of 21.7 ± 2.7 kPa and 5.35 ± 0.5 kPa respectively. The loss factor was found to be 0.25 ± 0.01. These values are comparable with those reported in the literature with other techniques [12].
The data obtained with the two different hydrogels is shown in Fig. 17.2.
As shown in Fig. 17.2, the storage modulus (G′) dominates over the loss modulus (G″), which is the expected behaviour of a hydrogel [13]. 2NapVG was found to have a much higher elastic and viscous component than 1THNapFF as well as a slightly higher damping capacity (as determined from the loss factor, Fig. 17.1c). The rheology data for the 2NapVG hydrogel is presented as dipeptide 13 in Table 1 in [13]; G′ = 36 kPa and G″ = 8.1 kPa). The values obtained with nanoindentation are much higher for both G′ and G″ which is likely to be an effect of frequency dependency. The rheology experiments were conducted at 10 rad/s (1.6 Hz) whereas the nanoindentation measurements were performed at 110 Hz, which is the resonant frequency of the indenter. For the 1THNapFF gel, the elastic component of the shear modulus G′ was found to be very comparable with rheology measurements that were conducted previously (unpublished data; G′ = 73 kPa also at 10 rad/s using the method detailed in [13]). The value for G″ measured with nanoindentation was much higher than that determined with rheology (2.8 kPa) which is also likely to be related to the much higher frequency at which the nanoindentation tests were performed. Overall the data appear comparable with published data on similar hydrogel systems with conventional rheology [13]. Further, the modifications of the Keysight Technologies standard gel method that were implemented in this study allowed for a more robust way of accurately detecting the sample surface.
Future work will involve characterising the gels across a range of frequencies using both the nanoindentation method presented in this paper and rheology. The methodology may also be useful for soft tissue characterisation.
17.4 Conclusions
In this study, a dynamic nanoindentation method which exploits the unique features of the DCM-II indenter head actuator has been found to be a suitable technique for characterising the elastic and viscous properties of low molecular weight hydrogels and similar materials.
References
Kaufman, J.D., Miller, G.J., Morgan, E.F., Klapperich, C.M.: Time-dependent mechanical characterization of poly (2-hydroxyethyl methacrylate) hydrogels using nanoindentation and unconfined compression. J. Mater. Res. 23, 1472–1481 (2008)
Selby, A., Maldonado-Codina, C., Derby, B.: Influence of specimen thickness on the nanoindentation of hydrogels: measuring the mechanical properties of soft contact lenses. J. Mech. Behav. Biomed. Mater. 35, 144–156 (2014)
Akhtar, R., Schwarzer, N., Sherratt, M.J., Watson, R.E., Graham, H.K., Trafford, A.W., et al.: Nanoindentation of histological specimens: mapping the elastic properties of soft tissues. J. Mater. Res. 24, 638–646 (2009)
Ebenstein, D.M., Pruitt, L.A.: Nanoindentation of soft hydrated materials for application to vascular tissues. J. Biomed. Mater. Res. A 69, 222–232 (2004)
Guglielmi, P.O., Herbert, E.G., Tartivel, L., Behl, M., Lendlein, A., Huber, N., Lilleodden, E.T.: Mechanical characterization of oligo (ethylene glycol)-based hydrogels by dynamic nanoindentation experiments. J. Mech. Behav. Biomed. Mater. 46, 1–10 (2015)
Hay J.: Complex Shear Modulus of Commercial Gelatin by Instrumented Indentation. Agilent Technologies (2011)
Sneddon, I.N.: The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 3, 47–57 (1965)
Loubet, J.L., Oliver, W.C., Lucas, B.N.: Measurement of the loss tangent of low-density polyethylene with a nanoindentation technique. J. Mater. Res. 15, 1195–1198 (2000)
Herbert, E.G., Oliver, W.C., Lumsdaine, A., Pharr, G.M.: Measuring the constitutive behavior of viscoelastic solids in the time and frequency domain using flat punch nanoindentation. J. Mater. Res. 24, 626–637 (2009)
Herbert, E.G., Oliver, W.C., Pharr, G.M.: Nanoindentation and the dynamic characterization of viscoelastic solids. J. Phys. D Appl. Phys. 41, 074021 (2008)
Quan, K.M., Christison, G.B., Mackenzie, H.A., Hodgson, P.: Glucose determination by a pulsed photoacoustic technique—an experimental-study using a gelatin-based tissue phantom. Phys. Med. Biol. 38, 1911–1922 (1993)
Markidou, A., Shih, W.Y., Shih, W.H.: Soft-materials elastic and shear moduli measurement using piezoelectric cantilevers. Rev. Sci. Instrum. 76, 064302 (2005)
Houton, K.A., Morris, K.L., Chen, L., Schmidtmann, M., Jones, J.T.A., Serpell, L.C., et al.: On crystal versus fiber formation in dipeptide hydrogelator systems. Langmuir 28, 9797–9806 (2012)
Acknowledgements
RA is grateful to the Royal Society for funding (Research Grant. RG130629), and also to the Academy of Medical Sciences for providing travel funding to present this paper at the SEM Annual Conference 2015. Kikelomo Moronkeji must be thanked for preparing the gelatin gel. ERD thanks the EPSRC for a DTA studentship. DJA thanks the EPSRC for a Fellowship (EP/L021978/1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 The Society for Experimental Mechanics, Inc.
About this paper
Cite this paper
Akhtar, R., Draper, E.R., Adams, D.J., Pfaff, H. (2016). Complex Shear Modulus of Hydrogels Using a Dynamic Nanoindentation Method. In: Tekalur, S., Zavattieri, P., Korach, C. (eds) Mechanics of Biological Systems and Materials, Volume 6. Conference Proceedings of the Society for Experimental Mechanics Series. Springer, Cham. https://doi.org/10.1007/978-3-319-21455-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-21455-9_17
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21454-2
Online ISBN: 978-3-319-21455-9
eBook Packages: EngineeringEngineering (R0)