Abstract
Oil and water don’t mix, that is what everyone knows….but if you are able to convince them; it is very well possible to produce stable emulsions. For this you need the right technology, of which examples will be presented in this chapter, focusing both on established equipment (high pressure homogenization, rotor-stator systems, ultrasound) and technology that is currently developed (microfluidic technology, hybrid systems). Based on the droplet size that is generated and the energy that is required to do so, the technologies will be compared. Besides, attention is given to the emulsion ingredients that stabilize the oil-water interface, and prevent instability of the emulsion through sedimentation, flocculation, and/or coalescence. The chapter concludes with a short outlook on methods that are currently developed to determine emulsion stability, which we expect to become very useful, not only for emulsions but also for derived products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
8.1.1 What Are Emulsions?
Emulsions are dispersions of fluids that are considered immiscible, e.g., oil and water. The basic forms of emulsions are oil-in-water (abbreviated as O/W), and water-in-oil (W/O) emulsions. The droplet phase is called the dispersed phase (prior to emulsification, we will use the term to-be-dispersed phase), the surrounding phase is the continuous phase. Some important examples of emulsions in our daily life are paints, cosmetic crèmes, pharmaceutical ointments, and many food products (Fig. 8.1) of which we present a few in detail to give you a taste of what an emulsion is, how it is made, and in some cases how you could make them yourselves.
-
In raw milk, the fat is present in the form of milk fat globules, which are surrounded by a membrane consisting of a tri-layer of phospholipids, and many proteins derived from the lactating cell. When milk is homogenized in the factory, the globules are broken, and the fat is dispersed into smaller droplets. For stabilization of these newly formed droplets, the material that is initially present at the interface is not sufficient, and components from the plasma such as proteins adsorb and act as emulsifiers. The same processes also occur in cream, and various culinary products, which are concentrated milk fat emulsions; the fat concentration depends on the type of cream.
-
Salad dressings are made by whisking vegetable oil in an aqueous mixture that contains acid (e.g. vinegar or lemon) and other taste components such as mustard. When made at home, this emulsion is rather unstable because mustard is not a very efficient emulsifier: the droplets coalesce relatively quickly so one has to prepare just before use. Commercial variants are usually stabilized by other components that do keep the emulsion stable; in some cases also unstable mixtures may be found that need to be shaken before use.
-
Mayonnaise is a highly concentrated emulsion of oil droplets in an acidified water phase (lemon or vinegar are mostly used due to the low pH they create), which is stabilized by proteins from egg yolk (amongst others lecithin) that are maximally charged due to the acidic conditions of the water phase. Mayonnaise is very concentrated (70–80 % v/v), and in most recipes it is recommended to whisk in the oil drop by drop until completely dispersed. If the process does not go according to plan, at some stage the emulsion may even convert to a water-in-oil emulsion, and in that case the consistency is irreversibly lost. In a proper mayonnaise, the droplets are squeezed together but do not coalesce due to the charge of the stabilizers, which gives the mayonnaise its nice thick consistency. Note that the previously mentioned egg yolk is an emulsion of its own right; it consists of egg fat (and cholesterol) and protein in an aqueous solution, stabilized by a mixture of phospholipids.
-
Margarine is an emulsion of water droplets in fat, stabilized by a packing of needle-like crystals of fat inside the continuous fat phase. The same is also true for butter, although it should be mentioned that this product is obtained through phase inversion.
Various other examples of (food) emulsions have been described in literature, for further reference see e.g. the Encyclopedia of Emulsion Technology [5, 34, 92, 93].
8.1.2 Examples of Complex Emulsions and Other Related Colloidal Systems
Besides single emulsions (O/W, and W/O), it is possible to make water droplets in an oil phase that is subsequently emulsified into another water phase; this is called a water-in-oil-in-water emulsion (W/O/W), and it is also possible to make the reverse (O/W/O) emulsion. Such double or duplex emulsions are used in some medical applications (encapsulation of drugs), and in foods, to enhance the perception of for example fat, but also to mask the taste of for example bitter peptides that are encapsulated in the inner water phase, and to reduce the caloric load. These double or duplex emulsions tend to be very fragile and are very difficult to produce with the technology that is classically used for single emulsions. However, for microfluidic techniques many examples are reported in literature. Good and extensive reviews have been written by Vladisavljevic and Williams [88] and Vladisavljević and co-workers [89].
Foams are closely related to emulsions, and sometimes products contain both to give them a ‘lighter’ perception. In foams, the dispersed phase is a gas, and one can use similar techniques as applied for emulsion preparation. Well-known examples are e.g. whipped cream containing small air bubbles that are stabilized by small fat crystals which from a structure at the air interface, or ice cream, which consists of cream with a stabilizer (mostly gelatin) that captures the air bubbles incorporated in the liquid ice cream mix, and that is further stabilized by crystals [46, 62].
Finally, liposomes are also colloidal systems that may be used for encapsulation purposes in food or pharmaceutical applications. Liposomes are vesicles consisting of one or more bilayers of phospholipids that encapsulate an inner aqueous compartment. A few applications of liposomes are reported, for example, the encapsulation of water-soluble flavors [15] or the encapsulation of enzymes [24] or antimicrobials [7] in cheese technology.
8.1.3 Main Physical Characteristics of Emulsions
The most important physical characteristics of emulsions are the volume fraction of disperse phase and the droplet size distribution (see also section on stability). The dispersed phase volume fraction (or the concentration of droplets) determines to a large extent the macroscopic properties of emulsions, such as their appearance, texture and rheological behavior. The droplet size is also of great importance, as it affects the emulsion’s appearance, and also its flavor, stability, and shelf life. In food emulsions, the droplet size is often expressed as a droplet size distribution, since not all droplets have the same size (i.e., polydisperse emulsions).
Various techniques may be applied to determine particle size distributions; for a detailed reviewed please consult [45]. Briefly, microscopy techniques may be used, often to give a qualitative overview of the emulsion’s morphology and range of droplet sizes present. Depending on the emulsion sample and on the type of microscopy applied, none to elaborate sample preparation may be required. Light scattering is probably the most widely used technique to characterize particle size distributions in emulsions. Here we can distinguish between (i) static light scattering (also called laser diffraction), where the angular pattern and extent of light scattering by an emulsion sample can be related to the droplet size distribution, using a mathematical model; and (ii) dynamic light scattering, where the diffusion movement of small particles (typically, submicron emulsion droplets) causes fluctuations in the light scattering pattern in time, from which the droplet size distribution can be calculated using the Stokes-Einstein equation. Both these light scattering techniques generally require some emulsion dilution prior to the measurement, which should be carefully considered as any change in the emulsion droplet environment may alter the physical state and stability of emulsions.
Average droplet diameters can be calculated from the particle size distributions. Several calculations can be performed, that will give more or less weight to the largest or the smallest droplets. Probably most widely used in emulsion science is the area-volume mean diameter (or surface mean diameter, or Sauter diameter, noted d32), and for foams the volume-length diameter (or volume-weighted mean, noted d43) is found of greater importance. These values are calculated as follows:
Where d i is the diameter of droplets in each size-class, and n i is the number of particles in each size-class, for a given volume of emulsion. In principle, various average diameters can be calculated, and for emulsions mostly the d 32 is used, which is also convenient to calculate the required amount of surfactant.
Regarding instability, the largest droplets (or bubbles) will have the greatest effect; see also later sections in which instability in emulsions is discussed in greater detail. Here it is still relevant to mention that in foams unlike in emulsions, instability occurs mostly through Ostwald ripening, and in that process the largest droplets have even greater influence and that is why in that case the d 43 is preferred. From this it is clear that besides the average value, it is also important to characterize the width of the particle size distribution. For this, for example, the polydispersity value (P) of an emulsion [37] can be used:
Where d 50,V is the median diameter, i.e., the diameter for which the cumulative volume fraction is equal to 50 %. Although there is to the best of our knowledge, no fixed convention to set the limit between monodisperse and polydisperse emulsions, it has been considered that emulsions can reasonably be considered monodisperse for a polydispersity value below 25 % [37].
As mentioned above, many food emulsions are highly polydisperse. This can be a cause of emulsion instability, notably when a population of very large droplets is present (the detailed mechanisms of some instability phenomena are explained in the following paragraphs). A high polydispersity also makes it very difficult to understand the connection between the structural characteristics of emulsions (such as the droplet size) and their end-up properties in great detail [43, 44]. Some examples of average and ranges of droplet sizes in common foods are presented in Table 8.1.
A more physical chemical aspect is related to the properties of the interfacial layer that surrounds the emulsion droplets. This interfacial region, typically a few nm thick, is the preferred location for molecules that have dual affinity for oil and water (i.e., amphiphilic, or surface-active molecules). Therefore, the properties and structure of the interface (e.g., electrostatic charge, thickness, and fluidity) are largely governed by the type and concentration of adsorbed amphiphilic molecules. These properties can, in turn, tremendously affect the physical and chemical stability of emulsions as described in the following sections.
8.2 Emulsion Stability and Ingredients
Emulsions are, in essence, thermodynamically instable systems, because of the molecular incompatibility of both involved liquid phases. This incompatibility is responsible for the existence of an interfacial tension, denoted as σ (N/m). The total free energy of an emulsion (ΔG, J) thus depends on the total interfacial area (ΔA, m2), and on the interfacial tension:
ΔG is almost always positive, meaning that emulsions almost always tend to physically destabilize and go back to their demixed state. However, it is possible to retard destabilization and to obtain metastable emulsions (i.e., stable for a ‘reasonable’ period of time). This can be achieved, for example, by using emulsifiers. Emulsifiers are surface-active molecules (e.g., amphiphilic biopolymers or surfactants), which can adsorb at the oil-water interface and thereby reduce the interfacial tension.
Yet, emulsions can physically destabilize through a number of phenomena, such as creaming/sedimentation, agglomeration/flocculation and coalescence of droplets. In this section, these three aspects are discussed in some detail, and common methods to determine and quantify the level of destabilization are presented; we are very aware that we cannot be complete in our description of emulsion stability due to the variety of interactions that components may have. Still these selected aspects are the most prominent reasons why emulsions are or may become unstable.
8.2.1 Creaming/Sedimentation
The main reason why small droplets are required in emulsions is their creaming/sedimentation behavior, which is caused by the density difference between the two phases that normally is present in emulsion (only very few density matched emulsions have practical value). Obviously, this train of thought only holds for free flowing emulsions, and not for (partially) crystallized emulsions or gelled emulsions that exhibit a high enough yield stress to keep the droplets captured (see later sections). For simplicity reasons, we will use the term sedimentation from now on, but obviously, the same principle holds for creaming. For a droplet in a surrounding liquid the sedimentation velocity can be estimated using Stokes law:
where:
-
v s = sedimentation velocity (m/s)
-
g = gravitation constant (m/s2)
-
Δρ = density difference between the phases (kg/m3)
-
d d = droplet diameter (m)
-
η c = viscosity of the continuous phase (Pa s or kg/(m s)).
This equation directly shows that smaller droplets sediment less fast; that highly viscous continuous phases slow down sedimentation, and as mentioned before, that emulsions with a zero density difference between the two phases do not sediment. This also explains why the emulsions that are currently in the market have very small droplets, and mostly have a very viscous continuous phase. Moreover it should be mentioned that small droplets (<0.1 μm) also will move due to Brownian motion. This effect can substantially decrease the creaming/sedimentation rate in emulsions [43].
Besides, it is known that in emulsions with large numbers of droplets sedimentation slows down. This behavior has been described by multiple authors; good summaries can be found in the work of Walstra [91, 92]. Here, we illustrate the effect of multiple droplets on the sedimentation velocity through the so-called Krieger-Dougherty equation:
where:
-
v = velocity in a swarm of droplets (m/s)
-
v s = Stokes velocity for a single droplet (m/s)
-
φ = volume fraction of dispersed phase (−)
-
φ max = maximum volume fraction of dispersed phase, i.e. the volume fraction at which the droplets become closely packed (−) (for spherical, non-deformable droplets, φ max = 0.585)
-
k = proportionality constant. For a k-value of 6.5, this equation has been validated for volume fractions up to 40 %, and has shown to be good compared to other models [91].
A basic method to monitor creaming or sedimentation in emulsions consists of placing a certain volume of emulsion in a transparent test tube that is then left immobile for a certain period of time (or even brought under enhanced gravity for very stable emulsions). In the case of creaming, for instance, the droplets will move upwards over time, leading to the formation of a clear, droplet-depleted phase at the bottom of the tube (the serum layer), whereas oil droplets accumulate on the top of the sample, forming a creamed layer. The creaming index (CI, %) can be calculated as follows:
where:
-
h s = height of the serum layer
-
h t = total height of emulsion in the tube.
A more sophisticated way of quantifying the creaming (or sedimentation) of emulsions is to measure the amount of transmitted light throughout a transparent tube filled with emulsion, over time (possibly under accelerated gravity conditions), and all along the tube’s height. This is possible with automated instruments, such as the LUMiFuge® stability analyzer.
8.2.2 Bridging and Depletion Flocculation
To improve the physical stability of emulsions, it is of great importance to cover the available droplet surface that is formed during emulsification, and give it a protective coating. Yet, even if stabilizing components (in particular, emulsifiers) are present in the interface, this does not necessarily mean that the emulsion will be stable; this depends on the ‘quality’ of the coating. Various short-range interactions as described in the DLVO theory can allow droplets to approach and flocculate in a primary and secondary minimum [39], and these effects may become pronounced if multiple components (that, e.g., carry opposite charges) are used to stabilize an emulsion. Describing these systems is complex, and still part of an on-going debate in literature. Here we will describe the main effects that can be used to establish a good starting point for emulsion preparation; whether this will also work in practice will always have to be tested.
There are two situations that will lead to flocculation, namely one in which the surface is not sufficiently covered, and a surface-active molecule can attach to two or even more droplets (Fig. 8.2, left). The other occurs if a non-adsorbing molecule is overwhelmingly present; this is termed depletion flocculation (Fig. 8.2, middle and right) as described in, e.g., the work of Walstra [91]. The non-adsorbing component cannot come as close to the surface as an adsorbing species would. E.g., a polymer, will mostly remain the distance of its gyration radius away from the interface, and because of that, there will be a lower polymer concentration in this area (depicted by the dotted line in Fig. 8.2). The concentration difference implies that there is also an osmotic pressure difference in the system; the system will strive to minimize this effect and can do so through minimization of the area that is free of polymer, i.e. it does that through aggregation. For spherical droplets this energy gain may be not very big (but still sufficient), but for platelets this effect can be over-dominating.
Left: Schematic representation of bridging flocculation; one molecule attaches to two or more droplets due to insufficient surface coverage. Middle and right: illustration of depletion flocculation by non-adsorbing component. This component will have a non-accessible area near the droplets (indicated by the dotted line) that corresponds to the gyration radius of the component, and because of that, there is an osmotic pressure difference that may lead to aggregation (see text for further explanation)
In the group of Julian McClements (University of Massachusetts, USA) these effects have been very practically translated into so-called stability maps that are very useful in choosing appropriate compositions for emulsions prepared with multiple components, in particular in emulsions stabilized by multiple polyelectrolyte layers. In Fig. 8.3, examples are shown from the field of food, but that we recommend to be taken as a starting point for preparation of any emulsion. The first map (Fig. 8.3a) shows a plot of the critical polyelectrolyte concentration as a function of the dispersed phase fraction (ϕ). It was assumed that the droplets had a radius of 0.3 μm, and the non-adsorbed polyelectrolyte had a molecular weight of 100 kDa and effective radius of 30 nm. The second map (Fig. 8.3b) plots the critical polyelectrolyte concentration as a function of the droplet radius. It was assumed that the droplets had a volume fraction of 0.03 (3 vol.%), and the non-adsorbed polyelectrolyte had a molecular weight of 100 kDa and effective radius of 30 nm. The shaded area in both maps highlights the range of conditions where it should be possible to produce non-flocculated droplets. These plots nicely illustrate that a proper formulation strategy should be taken into account to identify not only the minimum concentration of surface-active molecules needed to cover the entire oil-water interface (C Ads ), but also the maximum concentration of such compounds beyond which any more excess induces depletion flocculation (C Dep ).
(a) Stability map showing the influence of droplet concentration on the critical polyelectrolyte concentrations for saturation, depletion, and adsorption. (b) Stability map showing the influence of droplet radius on the critical polyelectrolyte concentrations for saturation, depletion, and adsorption (Reprinted with permission by Elsevier, from Guzey and McClements [18])
8.2.3 Droplet Coalescence and Emulsion Ingredients
In order for droplets to coalesce, the interfacial film between them needs to drain, and subsequently the film needs to break. Various components that can be present in emulsions will prevent the droplets from approaching this closely that the film can break. This can occur through steric repulsion, or charge interactions of similarly charged components.
Various components are used in practice to prepare emulsions, and they may induce both steric and charge effects. Surfactants are mostly low molecular weight components that have both a hydrophilic and hydrophobic part. The special structure of the surfactants causes that most of them cannot dissolve molecularly in one of the two phases of an emulsion. Often, a surfactant will form micelles; for example in water, the hydrophobic parts are clustered in the center and the polar parts are at the outside, in oil reversed micelles are formed. In an emulsion, only a limited amount of surfactant can reside in the interface (typically in the order of 0.1–10 mg/m2, depending on the surfactant and the available surface area). As soon as the surface is filled, the surplus of surfactant will remain in that phase for which it has most affinity.
In general, a suitable surfactant should be better soluble in the continuous phase of the emulsion. This is the basis of the so-called Bancroft rule. The solubility of a surfactant in oil or in water depends on its molecular structure, and can be quantified through the hydrophilic-lipophilic balance (HLB). This is an empirical value, which takes into account the type and amount of polar and non-polar groups present in the surfactant’s molecular structure. Low-HLB surfactants (e.g., polyglycerol polyricinoleate, HLB ~ 3) are more oil-soluble, and suitable for stabilizing W/O emulsions; whereas high-HLB surfactants (e.g., polysorbate 20, HLB ~ 16) are more water-soluble, and suitable for stabilizing O/W emulsions.
An excess of surfactant/stabilizer will be used to fill the interface rapidly when formed; and therewith prevent coalescence. When adsorbed onto an interface, ionic surfactants give mostly rise to electrostatic repulsion, and if they are bulky possibly also to steric repulsion between the droplets. Besides they lower the interfacial tension therewith reducing the energy of the system (Eq. 8.4).
A special class of surface active components is block copolymers, of which one part of the molecule has affinity to the interface, while the other part is extended in the continuous phase. Mostly these molecules form a steric barrier on the interface. Possibly also proteins could qualify as block-copolymers since they contain repetitive units but in this case many different amino acids would be present as units; however, these components are also known to be able to form viscoelastic films on the interface, therewith leading to very stable emulsions. Besides they are able to interact with other surface active components, and form networks that enhance stability. On the other hand, they may also be replaced by surfactants from the interface, and this may induce emulsion instability at longer time scales [93].
Polymers increase the viscosity of the continuous phase, which will reduce the droplet sedimentation velocity (Eq. 8.5), reduce film drainage, and increase through both effects the shelf life of the product. Alternatively, they may also give the product a yield stress, and if the strength of the weak gel is high enough, it can even prevent creaming.
Last but not least, also small particles that are partially wetted by both phases of the emulsion can accumulate in the interface, and give so-called Pickering stabilization, which is mostly a steric effect (recently reviewed by Berton-Carabin and Schroën [9]). Alternatively, in very few cases, it is possible to match the densities of both liquids, and therewith prevent creaming. Still, contact between droplets due to Brownian motion should be prevented in order to prevent coalescence.
More information on surfactants and their behavior can be found, e.g., in the work of Walstra [92], Walstra et al. [93], Lucassen-Reynders [38], and Guzey and McClements [18].
The coalescence of emulsion droplets can be monitored by measuring the particle size distribution (e.g., with a static light scattering instrument) at various time intervals. It should be pointed out that if the measured average particle size increases as compared to an initial value, it may be not only due to coalescence, but also to flocculation (in fact, such an instrument will consider a flock of emulsion droplets as one large particle). To distinguish between flocculation and coalescence, two simple strategies can be used. First, O/W emulsions can be diluted in a concentrated sodium dodecyl sulfate (SDS) solution prior to the measurement. SDS, an anionic surfactant, will rapidly cover the surface of emulsion droplets, providing them with a strong negative surface charge that will disrupt flocs of droplets, if any. Therefore, if a similar average particle size is measured without and with dilution of the emulsion in the SDS solution, most likely, coalescence occurred. Conversely, if the dilution of the emulsion in the SDS solution leads to a smaller particle size compared to the measurement on the non-diluted emulsion, most likely, flocculation occurred. Another strategy can simply be to observe the emulsion morphology under an optical microscope, which is often sufficient to assess whether the size of individual droplets did increase as compared to the initial sample, or if agglomerates (flocs) of small droplets are present. However, a limitation here is the resolution of optical microscopes and the number of droplets that can be viewed, which prevents obtaining accurate details on the structure and morphology of submicron particles.
8.3 Droplet Formation Mechanisms
As can be deduced from Eq. (8.4), making small droplets is hard, because it involves creating a large interfacial area, and a lot of energy is needed to do so. This is also caused by the fact that different time scales are relevant for the various droplet formation mechanisms that occur simultaneously and in relation to the stabilization of the interfaces, as will be discussed in the next section. Now, we focus first on the various mechanisms for droplet formation, both under laminar and turbulent conditions.
In general, when a continuous liquid flows around a discrete droplet, it induces a shear force onto the droplet, which may break the droplet if the exerted force is sufficiently large. That is also why most emulsification methods are designed to generate a very strong flow field that acts on a very small volume through which the emulsion passes.
As stated in Eq. (8.4), the amount of energy needed to create an interface between two phases costs an amount of energy that is proportional to the amount of interface generated, and that in turn is also related to the volume of oil that is present (V, m3) and with the droplet diameter (d d , m) this leads to A/V = 6/d d . It is also immediately makes clear that producing small droplets is more energy consuming.
In practice, much more energy than the minimal amount of energy from Eq. (8.4) is needed, because interfaces are not duly stabilized, and because energy will be lost due to heating up of the product. For more information on surface phenomena we recommend, e.g., the work of Hiemenz [19], Hiemenz and Rajagopalan [20] and Lyklema [39].
8.3.1 Interfacial Tension and Shear Forces
In order to break-up a large droplet into smaller droplets, the interfacial tension force that keeps the large droplet together needs to be exceeded by (any) shear force, be it laminar or turbulent. The interfacial tension force is related to the Laplace pressure (ΔP Laplace , Pa) which is defined as:
with R 1 and R 2 , the respective radii of curvature of an ellipsoid, and σ the interfacial tension (N/m). For a spherical droplet both curvature radii are equal to the droplet radius, R d , and the resulting Laplace pressure is equal to:
For any other oddly shaped droplet the Laplace pressure can be calculated using the actual curvatures.
As mentioned, the interfacial tension force needs to be overcome by shear forces exerted by the emulsification device. Often the Weber number , which is the ratio between the external disruptive stress and the internal coherent stress, is used to characterize droplet formation. Droplet size can be reduced under various conditions. Here we distinguish droplet break-up due to rotation, and elongation both under laminar conditions, or turbulent conditions.
8.3.2 Laminar Plain Shear Flow
In simple laminar flow, a droplet will be subjected to a flow field that leads to rotation of the droplet, and/or to its extension, as indicated in Fig. 8.4. In simple shear flow, the droplet is subjected to the flow field which initially rotates the droplet, which leads to distortion, and eventually the droplet will break up into smaller droplets.
The internal, coherent stress can be estimated with the help of the Laplace pressure in the droplets, equal to 2σ/ Rd as previously discussed (Eq. 8.9). The disruptive stress follows from:
where:
-
\( \dot{\gamma} \) = shear rate that is applied (1/s)
-
η c = viscosity of the continuous phase (Pa s)
-
v = velocity of the continuous phase (m/s)
-
z = position of the droplet (m) perpendicular to the solid surface, which is depicted in the bottom of Fig. 8.4a.
This leads to the following definition of the Weber number:
In order to achieve droplet break-up, this We-number should exceed a value, called the critical We-number. It was found that the critical Weber number is a function of the viscosity ratio of the water and oil phases. This is because the droplet will deform more when the dispersed phase viscosity is lower, which will give a higher Laplace pressure and a lower external stress, as is also shown in Fig. 8.5 [17, 92]. The figure will be discussed in detail in the section on extensional flow, where both flow patterns are compared.
8.3.3 Laminar Extensional Flow
This type of flow occurs when the liquid is squeezed through a small opening, during which the droplet is extended in the direction of flow, as could be the case in high pressure homogenization (under non-turbulent conditions). In general, during passage through the opening the droplet is extended to form a thread that breaks into many small droplets due to Rayleigh instabilities. In this case, the external force exerted on the droplet is equal to η c (dv/dy), where y is the coordinate in the direction of the extension, and the definition of the Weber number is the same as for simple shear flow.
In simple shear, energy is used to rotate the droplet, and besides the effective viscosity is a factor of two higher in extensional flow [92], leading to more efficient break-up in extensional flow. Therefore, the critical Weber number for extensional flow is lower than for simple shear flow. Figure 8.5 gives an impression of the values for both simple shear flow and for extensional flow; these curves are also known as the ‘Grace’ curves after the first author to publish them.
For simple shear, the critical Weber number goes through a minimum. At high viscosity ratio it is difficult to make droplets due to the internal viscosity of the droplets that resists deformation, while at low viscosity ratio the droplets may be first deformed into very long threads before they break. Note that the lowest viscosity ratios in Fig. 8.5 correspond to the situation in foams.
In practice, one will always have a mixture of the two types of flow; and the critical We-number will have an intermediate value. Still the diagram is very useful to calculate best- and worst-case scenarios.
8.3.4 Turbulent Flow
As is clear from Figs. 8.7 and 8.8, under turbulent conditions that occur e.g. in high pressure homogenizers, the flow is much more erratic than described for laminar flow (Fig. 8.4). The liquid will start moving in a chaotic way and form swirls and eddies. As will be discussed later, the transition from laminar to turbulent conditions is related to the Reynolds number, and the critical values for transition may be different for different emulsification equipment, as discussed in the conventional emulsification devices section.
Given the chaotic nature of the process, it is more difficult to find a critical Weber-number, since the exact local flow-conditions cannot be determined, because experimentation and simulation on this scale is very difficult. Therefore an average value, the power density (symbol ε, with unit W/m3 or Pa/s), is taken as a measure for the intensity of the swirls and eddies.
At relatively low turbulence, the surrounding eddies impose shear on the droplets. The external disruptive force can be calculated with the Kolmogorov theory for turbulent flow. This force (τ, Pa) can be estimated as:
where:
-
ε = power density (Pa/s)
-
η c = viscosity of the continuous phase (Pa s).
The Weber number can be defined as before and the critical value now becomes [92]:
where:
-
τ = external stress
-
R d = droplet radius
-
σ = interfacial tension (N/m)
-
ε = power density (Pa/s)
-
η c = viscosity of the continuous phase (Pa s).
Assuming that the critical Weber number is one, the droplet size can be estimated.
When the turbulence becomes very intense, the inertia of the liquid surrounding the droplets becomes the dominant factor. In this case the external disruptive force can be calculated with the aid of the Bernouilli equation as [92]:
Again this external disruptive force can be substituted in the Weber equation, and by assuming that the critical Weber number will be around unity, one can estimate the droplet radius. The transition from viscosity-dominated break-up to inertia-dominated break-up takes place when the droplets are larger than [92]:
These flow types relate as follows to the emulsification techniques that are presented in the next section. Flow in a colloid mill can be laminar or turbulent, depending on the viscosity of the product, and the lay-out of the machine (toothed versus regular). Large-scale high-pressure homogenizers operate under turbulent flow and inertial forces dominate, while laboratory scale homogenizers operate in the laminar regime; therefore, translation of results obtained on laboratory scale is not straight forward [69, 94]. Emulsification with ultrasound is always based on inertial forces, created by cavitation of the vapor bubbles.
8.4 Conventional Emulsification Devices
There are many different machines available for emulsification, and they are reviewed in literature by e.g. Walstra (general review [92]), Behrend and Schubert (ultrasound [6]), Schubert and Armbruster (general review [66]), Arbuckle (general review [3]), Brennan (mechanical methods [10]), Karbstein and Schubert (continuous systems [22]). For more detailed information, we would like to refer to these papers. To make an emulsion, mostly all ingredients are mixed (very coarsely), after which a macro-emulsion is obtained that is not stable due to the large size of the droplets (Eq. 8.5). This emulsion needs to be further refined, by passage through an emulsification device. The resulting tiny droplets need rapid stabilization by surfactants in order to prevent coalescence. In industry, high-pressure homogenizers, rotor-stator systems, and ultrasound treatment are mostly used for this purpose (schematic representations are shown in Fig. 8.6), and depending on the formulation, multiple passages may be needed. In the high pressure homogenizer depicted on the left in Fig. 8.6, the premix emulsion is pushed through a constriction, which results amongst others in shear to break up the droplets. The rotor-stator system in the middle of Fig. 8.6 consists of two concave elements, of which one rotates and one is static. The velocity difference between elements creates the necessary shear for droplet break-up. In the ultrasound equipment on the right in Fig. 8.6, the ultrasound creates cavitation bubbles that collapse, and through which droplets are submitted to shear and break-up. More details are given in the respective sections.
Schematic representation of classic emulsification methods, from left to right, high pressure homogenizer, rotor-stator system, ultrasound ([80]; reprinted with permission from the author)
Cavitating fluid leaving a homogenization orifice at different homogenization pressure differences (Images recorded with a VKT Fastcam SAS; record rate, 50,000 fps; shutter speed, 1/216,000 s; total frame, 2001. Reprinted with permission from the author [57])
8.4.1 High Pressure Homogenizers
In the high-pressure homogenizer depicted on the left in Fig. 8.6, the pre-emulsion is pressurized and pushed through a tiny orifice. This creates local shear, while the liquid is subjected in addition to turbulence, and possibly cavitation (see Fig. 8.10), which all result in droplet break-up.
Since high pressures are used ranging from 10 to 50 MPa, very intense fields can be reached, that lead to submicron droplets [68]. Where small, lab-scale homogenizers still operate in the laminar flow regime, industrial-scale systems operate in the turbulent regime, which makes it difficult to translate results obtained in the lab to full-scale industrial emulsification. The transition between the two regimes can be derived from the Reynolds number (Re):
where:
-
ρ = liquid density (kg/m3)
-
v = average liquid velocity in the gap (m/s)
-
L = gap width (m)
-
Η = viscosity (Pa s).
From experiments it was deduced that transition from laminar to turbulent occurs at Re-number 1,500–3,000. The most used designs in industry are valve and nozzle systems. In a valve, a small gap is created by pressurizing the valve with a spring. In lab-scale systems, the gap width will be of the order of 1 μm; for industrial-scale systems, it will be 10–40 μm [69]. In the nozzle system, there is only a small hole through which the pre-mix emulsion is pushed, and in some cases there are a number of openings placed in series, or the liquid is split and recombined in the machine. Also in some cases the emulsion is passed repeatedly through the nozzle. This is all done to create as much shear as possible, and depending on the composition of the emulsion, different lay-outs of the high pressure homogenizers can be applied.
A lot of nice work in this field is carried out in the University of Karlsruhe, in Germany, within the group of prof. Schuchmann, formerly headed by prof. Schubert, and within the group of prof. Windhab at ETH Zurich, in Switzerland. Amongst others, the flow fields in various geometries are visualized, either through experimentation by, e.g., PIV, or through modeling. Both are very challenging, given the obviously extreme conditions in the machine. Besides there are limitations in simulations for which the Navier-Stokes equations need to be solved at a local level, using mathematical grids of nanometer scale [29, 67]. That, combined with the fact that droplets influence each other and local flow in their surroundings, makes these simulations very challenging.
Using local flow velocities, stresses acting on droplets may be calculated. This is illustrated in Fig. 8.8 for single phase flow through a spherical shaped orifice of 200 μm diameter, at 100-bar homogenization pressure [28]. These stresses can be compared to the critical values for the capillary number, which links the exerted shear to the interfacial tension force that resists deformation [17, 90]. The capillary and Weber number are discussed in more detail in the mechanisms section; it is important to remember that the critical capillary number is exceeded by far within microseconds in high pressure homogenizers, and oil-water interface will be readily made. Since interface formation is so rapid, surface active components will not (or hardly) have time to stabilize the formed interface (e.g., [8]), and this may lead to instability in the product unless droplet interactions can be delayed as long as is needed to cover the interface sufficiently. In high pressure homogenizers, this is not likely to be the case given the turbulent nature of the liquid flow (although the turbulent nature will favor rapid transport of surfactants as well). Calculating droplet size distributions that result from a widely distributed field of stresses is therefore one of the main challenges to food (and chemical) process engineers. An indication of the average size can be obtained using the estimates in the droplet formation mechanisms section.
Stresses acting on droplets (left bottom image) flowing through a spherical high pressure homogenization orifice (top left photograph) [25]. On the right, the local stresses on the middle axes as function of time: Stresses resulting from local turbulent energy dissipation ε, k as well as shear and elongational stresses in x- and y-direction are shown; the fluid leaves the orifice at time t = 0 μs [28] (Images are reprinted by courtesy of the authors [57])
8.4.2 Rotor-Stator Systems
By definition, rotor-systems consist of one part that is immobile and one that rotates; such as stirred vessels but also colloid mills qualify as such. The technology has been extensively reviewed by Urban and co-workers [74], and various examples can be found there by the interested reader.
In a stirred vessel, the liquid movement is rather chaotic and hard to predict, leading to very polydisperse emulsions with droplets typically larger than 10 μm, that are in general not very stable (Eq. 8.5). The energy density of stirrers is low; therefore, making very small droplets is outside the reach of this equipment. In a colloid mill, the central cone is static, while the outside cone rotates at high speed (see Fig. 8.9).
This system can be operated in continuous mode with the pre-emulsion entering from the bottom and the fine emulsion leaving from the top. The distance between both parts is small in order to generate high shear rates. A variation to the colloid mill is the so-called toothed mill; in this case, both the rotor and stator have openings, which generate turbulent conditions through which smaller droplets can be made, also at the high throughput that is required at industrial scale.
Since the distance between rotor and stator is very narrow (sometimes much less than a millimeter), the shear forces are very intense. Depending on the viscosity of the emulsion, the flow will be laminar (viscous liquids) or turbulent. The transition towards turbulent flow is again related to the Reynolds number , which characterizes the flow.
where:
-
L = gap width between rotor and stator (m)
-
v = tangential speed of the rotor (m/s)
-
ρ = density of the mixture (kg/m3)
-
η = viscosity of the mixture (Pa s).
At Re <370 the flow will be laminar, and at higher values turbulent. This is an experimental finding, as was the case for high pressure homogenizers.
8.4.3 Ultrasound
A third important emulsification method used in industry is ultrasound. At frequencies higher than 20 kHz and up till 100 kHz it can cause physical and chemical changes in matter. The sound is generated by an actuator resulting in pressure fluctuations (standing waves). When the sound is sufficiently intense, the pressure fluctuations become so large that in small regions, the pressure becomes lower than the vapor pressure of water, which induces the formation of small bubbles that implode almost immediately and cause intense, local turbulence (Fig. 8.10). The technology was reviewed by Canselier et al. [11]; the interested reader will find many relevant details there. Besides, various demonstrations can be found on the internet, with separated liquids being finely dispersed by ultrasound action.
Schematic representation of ultrasound effect. First the oil is dispersed into the water through surface waves, where it undergoes a second stage during which the droplets are further refined due to cavitation (Kendall [23]; image taken from the internet)
The effect of ultrasound is very local, so the premix emulsion needs to be brought into close proximity of the actuator where the field is strongest. As soon as the emulsion is led away from the actuator, the effect rapidly becomes less, and this also implies that the treatment chamber should be small in order to be efficient. Besides physical changes also chemical changes can be a result of ultrasound treatment; especially unsaturated fatty acids and oils are notoriously unstable in ultrasound. This technology is rarely used for large scale operation, but if the emulsion components allow, it can generate very small and stable droplets in specialty products.
The energy efficiency of all classic emulsification technologies in regard to the generated droplet size will be compared with those presented for emerging technologies in the following section, and the results can be found at the end of this chapter in the comparison paragraph.
8.5 Emerging Emulsification Technologies
Besides the established technologies that were presented earlier, there is a very lively field of research in which micro-structured devices are used to prepare emulsions, and also derived products, such as double emulsions, bubbles, particles, capsules etc. Mostly these emulsions are fairly monodisperse in droplet size; also the energy efficiency of these methods is rather high. However, these methods are currently not at such a level of development that they can be applied at large scale, although some are promising and closer to large scale application than others [65].
In this section, we will discuss various membrane emulsification techniques that use shear forces to make emulsions, together with microfluidic techniques that may use either shear-based or spontaneous droplet formation mechanisms. At the end of this chapter we will compare all presented methods based on their energy efficiency, but also on their ease of scale-up.
8.5.1 Membrane Emulsification
A membrane is a porous structure mostly used for separation but it is also used to make emulsions and related products. The two membranes that are most frequently applied for emulsification are the Shirazu Porous Glass membrane (SPG; [48]) and the ceramic membrane [63, 64, 86]; see Fig. 8.11, left and middle images. The SPG membrane consists of a matrix of interconnected pores that have similar size all through the membrane, while the ceramic membrane consists of a carrier with large pores onto which a layer with small pores is deposited. The right image in Fig. 8.11 is a microsieve, which has very uniform and also very thin pores. These microsieve membranes are made through photolytic techniques in a clean room environment. The typical pore sizes that can be made are from 0.1 to 100 μm (Aquamarijn microfiltration BV, http://www.aquamarijn.nl/).
(Left) Shirazu Porous Glass (SPG) membrane with interconnected tortuous pores of similar size all through the membrane (Image taken from internet). (Middle) Ceramic membrane with an open support structure and much finer top-layer. (Right) Microsieve made from silicon with a silicon nitride top layer, with uniform tailor-made pores ([85]; reprinted with permission from Aquamarijn Microfiltration BV)
The membranes can be used in cross-flow mode [48] or in pre-mix mode (reviewed by Nazir et al. [50]). During cross-flow emulsification (see Fig. 8.12; left image), the to-be-dispersed phase is pressed through the membrane where it forms small droplets on top of the membrane, that are consecutively sheared off by the cross-flowing continuous phase once they have reached a certain size. During pre-mix emulsification, the large droplets of a pre-mix emulsion are broken up into smaller ones as the liquid passes through the membrane, and are sheared off while doing so. The situation is rather similar to what happens with classic emulsification techniques, but now multiple ‘nozzles’ work in tandem (see Fig. 8.12, scheme on the right).
For both cross-flow and premix emulsification, the membrane needs to be wetted by the continuous phase of the emulsion. This implies using a hydrophilic membrane for O/W emulsions, and a hydrophobic membrane for W/O emulsions; and most importantly the wettability of the membrane should not change during operation, e.g., because of adsorption of surface-active components that are present in the emulsion.
In very limited cases, it is possible to induce phase inversion during pre-mix emulsification. To achieve this, the membrane should be compatible with the to-be-dispersed phase. Starting from an O/W pre-mix, the membrane needs to be hydrophobic; the oil droplets will wet the membrane, and the continuous water phase will be converted into the dispersed phase during passage through the membrane [73]. If this mode of operation is possible, emulsions with very high dispersed phase fraction may be obtained, but this strongly depends on the components in the emulsion mix, and on their interaction with the membrane.
To generate the required shear for droplet detachment, alternative designs have also been suggested, such as by Stillwell and co-workers [71], who investigated a stirred cell that generated rather polydisperse emulsions due to the differences in shear across the membrane. Eisner [14], Schadler [61], Aryantia and co-workers [4], and Yuan and co-workers [95] used a different approach and rotated (metal) membranes to shear off the droplets. As a result, better control over droplet size is achieved, although it should also be mentioned that compared to regular cross-flow emulsification, the droplets are much larger due to the larger pores in the metal sieves that are used; the mechanical stability required for rotating membranes requires this construction material.
For now, we focus on regular membrane emulsification that was invented in the group of prof. Nakashima in Japan [49]. Various good reviews have been published, and we recommend those by Joscelyne and Trägårdh ([21], general review), Charcosset and co-workers ([13] general review), Vladisavljevic and Williams ([88], general overview with many products), van der Graaf and co-workers ([77], double emulsions), and Charcosset ([12], specific for food). Most information on membrane emulsification is available for O/W emulsions, but also some authors have shown work on W/O emulsions, e.g., Vladisavljevic et al. [87]. On the other hand, pre-mix emulsification is not well documented; only very recently, a review became available by Nazir and co-workers [50], and not much is known about the droplet formation mechanisms.
The droplet formation mechanism during cross-flow membrane emulsification can also be described as a balance between the interfacial tension force that keeps the droplet connected to the pore, and the shear force that tries to remove the droplet, as was the case for the classic emulsification techniques. The first ones to describe this balance were Peng and Williams [55]. Later also more complex droplet formation mechanisms were reported in which two stages were distinguished. During stage one, a certain volume of liquid is pushed into the cross-flowing continuous phase and one reaching a certain value the droplet snap-off process starts. However, during this phase the droplet still grows due to its connection to the pore, and its size is not only determined by the cross-flowing continuous phase, but also by the applied pressure on the to-be-dispersed phase. In general, it can be said that the droplets that are generated are between two and ten times the diameters of the pore.
8.5.2 Microfluidic Techniques
Examples of microfluidic devices. Within the field of microfluidics, both shear based (T-shaped junctions, e.g., [78]; Y-shaped junctions, [70]; flow focusing devices, [1]) and spontaneous droplet formation (e.g., [72]) are used to generate droplets. A very extensive review, covering all these devices, has recently been published by Vladisavljević et al. [89]. In the present chapter we only touch briefly upon these devices since they seem to be still far away from large-scale application, although some may be used for the production of specialty products in the field of pharma.
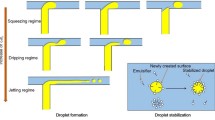
In the top part of Fig. 8.13, droplet formation in a T-junction is shown together with a simulation result obtained for the same system [76]. At the bottom, an artist’s impression is shown of droplet formation in a microchannel due to Laplace pressure differences in the system [79]. As was the case for cross-flow membrane emulsification, the droplet formation mechanisms in microfluidic devices mostly consist of two stages, one formation phase and a snap-off phase during which the droplet can still grow before actually being detached.
Top left row, images taken during cross-flow emulsification in a T-shaped junction. The to-be-dispersed phase is pushed through the top channel into the cross-flowing continuous phase that moves the to-be-dispersed phase into the direction of flow until the shear-force exceeds the interfacial tension force, and a droplet is formed (Reprinted with permission from Van der Graaf et al. [78]. Copyright 2005, American Chemical Society). Top right row, simulation results of the system shown on the left ([76]; reprinted with permission from Elsevier). Bottom image, the three stages of droplet formation in a spontaneous microchannel, stage A being intrusion onto the shallow terrace. At stage B, the to-be-dispersed liquid leaps from the terrace into a deeper channel, and at stage C the size of the droplet is such that the Laplace pressures in the system lead to droplet formation ([79]; reprinted with permission from the author)
When comparing shear-based and spontaneous droplet generation, it is important to notice that the size of the droplets is determined by flow of both phases in the shear-based systems. Both need to be monitored very carefully in order to have monodisperse droplets, which in general are produced at much higher throughput than in the spontaneous systems. In spontaneous droplet formation systems, the continuous phase does not need to flow, and only the dispersed phase should be controlled. Mostly there is a range of disperse phase pressures for which the droplet size is not affected. The droplet formation time in spontaneous systems is in general much longer as in shear-based systems, so the throughput is also accordingly lower.
Scalability of microfluidic emulsification devices. Although most of the investigations on emulsification in microfluidic devices are focused on single droplet formation units, also some examples are known in which multiple droplet formation units work in tandem as is the case is in straight through microchannels (e.g. [26, 27]), microsieves ([16, 84, 85]; see Fig. 8.11 right image), parallelised flow focusing devices [54] or even work simultaneously, as is the case in so-called EDGE chips [81–83]. These devices have also been prepared partly in metal to comply with industrial demands [37, 38], and it was demonstrated that these systems even have better pressure stability than the original EDGE design. Besides some derived systems have been proposed in which metal sieves as such or in combination with a glass bead bed are used for pre-mix emulsification, which can be operated at very high flux and reasonable monodispersity [51–53, 80].
Currently, these methods can only be applied on rather small scale, but considerable effort is put into surface modification methods that allow for stable wetting of Si-based surfaces that are used in microfluidics (e.g. [2, 58]). Besides, within our own research group we also work on bringing microfluidics toward metal devices, which are the material of choice in industry. Our first attempts were directed towards semi-metal chips, and they could successfully be applied [40–42]. The next step towards a completely metal chip still needs to be made; making micrometer structures at high precision in metal is a great challenge, but the results on semi-metal systems make us optimistic.
8.6 Comparison of Emulsification Techniques
All emulsification methods can be compared based on their energy usage in relation to the droplet size that is generated. Amongst others in the work of Nazir and co-workers [50], an illustrative diagram is shown that summarizes these effects; see Fig. 8.14. When comparing microfluidic emulsification with the more classic techniques, it is clear, that cross-flow emulsification is a much less energy consuming technique than the high pressure homogenizers reported by Lambrich and Schubert [35]. For dilute emulsions, the energy density may be even orders of magnitude smaller. The energy density of cross-flow membrane emulsification is determined by the number of droplets that need to be produced; each volume fraction having its own corresponding line, unlike other emulsification techniques, in which pressurization of the whole volume determines the amount of energy needed. Pre-mix emulsification seems to be more in line with the traditional techniques, but may be useful in the production of relatively large droplets, which could correspond to better defined pre-mixes that can be used in classic emulsification. Regarding microfluidic devices, they are expected to be similar in energy density as cross-flow emulsification. But given the large variety in designs and mode of operation, and also the relatively large droplets that are made compared to classic emulsification devices, it is hard to pinpoint them to the diagram.
Energy efficiencies of various emulsifying processes: cross-flow emulsification [35], (○) 1, (●) 5, (□) 10, (■) 20 and (◊) 50 vol.%; (×) pre-mix emulsification (5 vol.%) [80]; high pressure homogenization [35], (◆) orifice valve, (▲) flat valve homogenizer and (Δ) Microfluidizer (all 30 vol.%); (Reprinted with permission from Elsevier, from Nazir et al. [50])
8.7 Concluding Remarks
Various emulsification methods are available. Some are established, such as high pressure homogenization, others are still in their early stages of development, such as microfluidic devices. With all methods stable emulsions may be prepared, but only if the time scales of droplet formation are adequately matched with the time scale related to stabilization of the interface. This match can be reached by using stabilizing components that are able to lower the interfacial tension (surfactants), create a steric barrier (e.g. block co-polymers, particles), give rise to repulsive charge interactions (e.g. proteins, depending on the pH), or form a network (mostly through interactions of components).
It is very difficult to predict ab-initio which combination of emulsification device and emulsion composition needs to be chosen. In this chapter, some guidelines are given, such as the stability maps in Fig. 8.3. Still, this is far from perfect. In this light, the new developments in the field of microfluidics that allow emulsion stability testing, both under flow and under enhanced gravity, are very interesting methods that may lead to high-throughput testing of both ingredients and process conditions [31–33]. This is not only important for emulsions, but also for derived products such as double emulsions (a good review is by Muschiolik [47], capsules [59], particles [60], ultrasound contrast agents [30] and many more.
8.8 Definitions, Abbreviations and Symbols
Term | Definition |
|---|---|
DLVO theory | Theory describing the various interactions that play a role for colloidal particles |
Double emulsion: | Emulsion with three distinct phases, internal-, shell, and continuous phase. These emulsions are O/W/O or W/O/W |
Emulsion | Mixture of oil (O) and water (W) in which one phase is finely dispersed as droplets into the other. Emulsions can be oil in water (O/W) or water in oil (W/O) |
HLB balance | Indication of hydrophobicity/hydrophilicity of surfactant molecules. Is used in regard to their suitability to make O/W or W/O emulsion |
Interfacial tension | The energy related to a liquid/liquid interface |
Liposome | Water phase surrounded by a double layer of surfactant that can be used for encapsulation purposes |
Abbreviation | ||
|---|---|---|
Ca | Capillary number | Dimensionless ratio of viscous effects to interfacial tension effects |
Re | Reynolds number | Dimensionless ratio of inertial to viscous effects |
Recr is the critical value at which transition from laminar to turbulent flow occurs | ||
We | Weber number | Dimensionless ratio of fluid inertia effects to interfacial tension |
Wecr is the critical value that has to be overcome to get droplet break-up | ||
Symbol | Meaning | Unit | Remarks |
|---|---|---|---|
A | Interfacial area | m2 | |
ΔG | Gibbs free energy | J | |
L | Width | m | |
P | Polydispersity | ||
ΔP Laplace | Laplace pressure difference | Pa | |
R d | Droplet radius | m | |
d d | Droplet diameter | m | |
d i | Diameter of particles in each size-class | m | |
d 32 | Area-volume mean diameter | m | Also called: Sauter diameter, or surface-weighted mean diameter |
d 43 | Volume-length mean diameter | m | Also called volume-weighted mean size |
d 50,V | Median diameter of vol.-based distribution | m | |
g | Gravity acceleration | N/kg or m/s2 | |
n i | Number of particles in each size-class | m | |
v | Rate/velocity/speed | m/s | |
z | Height | m | |
ε | Power density | W/m3 | |
\( \dot{\gamma} \) | Shear rate/velocity gradient | 1/s | \( \dot{\gamma}=dv/dz \) |
η | Viscosity | Pa s | η c refers to the viscosity of the continuous phase; η d refers to the dispersed phase |
ρ | Density | kg/m3 | ρ c refers to the density of the continuous phase; ρ d refers to the dispersed phase |
σ | Interfacial tension | N/m | |
τ | Disruptive stress | Pa |
References
Anna, S.L., Bontoux, N., Stone, H.A.: Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 82(3), 364–366 (2003)
Arafat, A., Giesbers, M., Rosso, M., et al.: Covalent biofunctionalization of silicon nitride surfaces. Langmuir 23, 6233–6244 (2007)
Arbuckle, W.S.: Emulsification. In: Hall, C.W., Farral, A.W., Rippen, A.L. (eds.) Encyclopaedia of Food Engineering, pp. 286–288. Avi Publication Company, Westport (1986)
Aryantia, N., Williams, R.A., Houa, R., et al.: Performance of rotating membrane emulsification for o/w production. Desalination 200, 572–574 (2006)
Becher, P. (ed.): Encyclopedia of Emulsion Technology, vol. 1–4. Marcel Dekker, New York (1986)
Behrend, O., Schubert, H.: Influence of hydrostatic pressure and gas content on continuous ultrasound emulsification. Ultrason. Sonochem. 8, 271–276 (2001)
Benech, R.O., Kheadr, E.E., Laridi, R., Lacroix, C., Fliss, I.: Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl. Environ. Microbiol. 68, 3683–3690 (2002)
Bentley, B.J., Leal, L.G.: An experimental investigation of drop deformation and breakup in steady, two-dimensional linear flows. J. Fluid Mech. 176, 241–283 (1986)
Berton-Carabin, C.C., Schroën, K.: Pickering emulsions for food applications: background, trends and challenges. Ann. Rev. Food Sci. Technol. 6, 263–272 (2015). doi:10.1146/annurev-food-081114-110822
Brennan, J.G.: Emulsification, mechanical procedures. In: Hall, C.W., Farral, A.W., Rippen, A.L. (eds.) Encyclopaedia of Food Engineering, pp. 288–291. Avi Publication Company, Westport (1986)
Canselier, J.P., Delmas, H., Wilhelm, A.M., et al.: Ultrasound emulsification-an overview. J. Dispers. Sci. Technol. 23(1–3), 333–349 (2002)
Charcosset, C.: Preparation of emulsions and particles by membrane emulsification for the food processing industry. J. Food Eng. 92, 241–249 (2009)
Charcosset, C., Limayem, I., Fessi, H.: The membrane emulsification process—a review. J. Chem. Technol. Biotechnol. 79, 209–218 (2004)
Eisner, V.: Emulsion Processing with a Rotating Membrane (ROME). Dissertation ETH Zürich, number 17153 (2007).
Gibbs, B.F., Kermasha, S., Alli, I., Mulligan, C.N.: Encapsulation in the food industry. Int. J. Food Sci. Nutr. 50, 213–224 (1999)
Gijsbertsen-Abrahamse, A.J., Van der Padt, A., Boom, R.M.: Status of cross-flow membrane emulsification and outlook for industrial application. J. Membr. Sci. 230, 149–159 (2004)
Grace, H.P.: Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 14, 225–277 (1982)
Guzey, D., McClements, D.J.: Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid. Interface. Sci. 128–130, 227–248 (2006)
Hiemenz, P.C.: Principles of Colloid and Surface Chemistry. Marcel Dekker, New York (1986)
Hiemenz, P.C., Rajagopalan, R.: Principles of Colloid and Surface Chemistry. M. Dekker, New York (1997)
Joscelyne, S.M., Trägårdh, G.: Membrane emulsification—a literature review. J. Membr. Sci. 169, 107–117 (2000)
Karbstein, H., Schubert, H.: Developments in the continuous mechanical production of oil-in-water macro-emulsions. Chem. Eng. Proc. 34, 205–211 (1995)
Kendall, G.: http://blogs.nottingham.ac.uk/malaysiaknowledgetransfer/2013/06/25/what-is-pharmaceutical-nanoemulsion/. Visited 14 October 2014.
Kirby, C.F., Brooker, B.E., Law, B.A.: Accelerated ripening of cheese using liposome-encapsulated enzyme. Int. J. Food Sci. Technol. 22(4), 355–375 (1987)
Kissling, K., Schütz, S., Piesche, M.: Numerical investigation of the flow field and the mechanisms of droplet deformation and break-up in a high-pressure homogenizer. Proceedings of the 8th World Congress Chemical Engeneering, Montreal (2009).
Kobayashi, I., Neves, M.A., Uemura, K., et al.: Production characteristics of uniform large soybean oil droplets by microchannel emulsification using asymmetric through-holes. Procedia. Food. Sci. 2011(1), 123–130 (2011)
Kobayashi, I., Nakajima, M., Chun, K., et al.: Silicon array of elongated through-holes for monodisperse emulsion droplets. AICHE J. 48, 1639–1644 (2002)
Köhler, K.: Simultanes Emulgieren und Mischen. Logos Verlag, Berlin (2010). ISBN 978-3-8325-2716-7
Köhler, K.: In: Nagel, W.E., Kröner, D.B., Resch, M.M. (eds.) High Performance Computing in Science and Engineering’10. Springer, Heidelberg (2011)
Kooiman, K., Böhmer, M.R., Emmer, M.: Oil-filled polymer microcapsules for ultrasound-mediated delivery of lipophilic drugs. J. Control. Release 133, 109–118 (2009)
Krebs, T., Schroën, K., Boom, R.: Coalescence dynamics of surfactant-stabilized emulsions studied with microfluidics. Soft Matter 8(41), 10650–10657 (2012)
Krebs, T., Ershov, D., Schroen, C.G.P.H., et al.: Coalescence and compression in centrifuged emulsions studied with in situ optical microscopy. Soft Matter 9(15), 4026–4035 (2013)
Krebs, T., Schroen, K., Boom, R.: A microfluidic method to study demulsification kinetics. Lab Chip 12(6), 1060–1070 (2012)
Krog, N.J., Riisom, T.H., Larson, K.: Applications in food industry. In: Becher, P. (ed.) Encyclopedia of Emulsion Technology. Applications, vol. 2, pp. 58–127. Marcel Dekker, New York (1985)
Lambrich, U., Schubert, H.: Emulsification using microporous systems. J. Membr. Sci. 257, 76–84 (2005)
Langton, M., Jordansson, E., Altskar, A., et al.: Microstructure and image analysis of mayonnaises. Food Hydrocoll. 13, 113–125 (1999)
Leal-Calderon, F., Schmitt, V., Bibette, J.: Emulsion Science – Basic Principles, 2nd edn. Springer, New York (2007)
Lucassen-Reynders, E.H.: Dynamic interfacial properties in emulsification. In: Becher, P. (ed.) Encyclopedia of Emulsion Technology, vol. 4, pp. 63–90. Marcel Dekker, New York (1996)
Lyklema, J.: Fundamentals of Interface and Colloid Science. Academic, London (1991)
Maan, A.A., Schroën, K., Boom, R.: Spontaneous droplet formation techniques for monodisperse emulsions preparation – Perspectives for food applications (Review). J. Food Eng. 107(3–4), 334–346 (2011)
Maan, A.A., Boom, R., Schroën, K.: Preparation of monodispersed oil-in-water emulsions through semi-metal microfluidic EDGE systems. Microfluid. Nanofluid. 14(5), 775–784 (2013)
Maan, A.A., Schroën, K., Boom, R.: Monodispersed water-in-oil emulsions prepared with semi-metal microfluidic EDGE systems. Microfluid. Nanofluid. 14(1–2), 187–196 (2013)
McClements, D.J.: Food Emulsions: Principles, Practices and Techniques. CRC Press, Boca Raton (2005)
McClements, D.J., Chanamai, R.: Physicochemical properties of mono disperse oil-in-water emulsions. J. Dispers. Sci. Technol. 23(1–3), 125–134 (2002)
Merkus, H.G.: Particle Size Measurements – Fundamentals, Practice, Quality. Springer, New York (2009)
Merkus, H.G., Meesters, G.M.H. (eds.): Particulate Products – Tailoring Properties for Optimal Performance. Springer International Publishing, Switzerland (2014)
Muschiolik, G.: Multiple emulsions for food use. Curr. Opin. Colloid. Interface. Sci. 12, 213–220 (2007)
Nakashima, T., Shimizu, M.: Porous glass from calcium alumino boro-silicate glass. Ceram. Jpn. 21, 408 (1986)
Nakashima, T., Shimizu, M., Kukizaki, M.: Membrane emulsification by microporous glass. Key Eng. Mater. 61–62, 513 (1991)
Nazir, A., Schroën, K., Boom, R.: Pre-mix emulsification: a review. J. Membr. Sci. 362(1–2), 1–11 (2010)
Nazir, A., Schroën, K., Boom, R.: High-throughput premix membrane emulsification using nickel sieves having straight-through pores. J. Membr. Sci. 383(1-2), 116–123 (2011)
Nazir, A., Schroën, K., Boom, R.: The effect of pore geometry on premix membrane emulsifi-cation using nickel sieves having uniform pores. Chem. Eng. Sci. 93, 173–180 (2013)
Nazir, A., Boom, R., Schroën, K.: Droplet break-up mechanism in premix emulsification using packed beds. Chem. Eng. Sci. 92, 190–197 (2013)
Nisisako, T., Torii, T.: Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 8, 287–293 (2008)
Peng, S.J., Williams, R.A.: Controlled production of emulsions using a cross-flow membrane. Part I: droplet formation from a single pore. Trans. IChemE. 76, 894–901 (1998)
Perrechil, F., Santana, R., Fasolin, L.H., et al.: Rheological and structural evaluations of commercial Italian salad dressings. Cienc. Tecnol. Aliment. 30(2), 477–482 (2010)
Roos, Y.H., Fryer, P.J., Knorr, D., Schuchmann, H.P., Schroën, K., Schutyser, M.A.I., Trystram, G., Windhab, E.J.: Food engineering at multiple scales: case studies, challenges and the future—a European perspective. Food Eng Rev. doi:10.1007/s12393-015-9125-z (2015, in press)
Rosso, M., Giesbers, M., Arafat, A., et al.: Covalently attached organic monolayers on SiC and SixN4 surfaces: formation using UV light at room temperature. Langmuir 25, 2172–2180 (2009)
Sagis, L.M.C., De Ruiter, R., Rossier Miranda, F.J., et al.: Polymer microcapsules with a fiber-reinforced nanocomposite shell. Langmuir 24, 1608–1612 (2008)
Sawalha, H., Purwanti, N., Rinzema, A.: Polylactide microspheres prepared by premix membrane emulsification – effects of solvent removal rate. J. Membr. Sci. 310, 484–493 (2008)
Schadler, V., Windhab, E.J.: Continuous membrane emulsification by using a membrane system with controlled pore distance. Desalination 189, 130–135 (2006)
Scholten, E.: Ice cream (Chapter 9). In: Merkus, H.G, Meesters, G.M.H. (eds.) Particulate Products – Tailoring Properties for Optimal Performance. Springer International Publishing, Switzerland (2014)
Schröder, V., Behrend, O., Schubert, H.: Effect of dynamic interfacial tension on the emulsification process using microporous, ceramic membranes. J. Colloid. Interface. Sci. 202, 334–340 (1998)
Schröder, V., Schubert, H.: Production of emulsions using microporous, ceramic membranes. Coll. Surf A Phys. Eng. Asp. 152, 103–109 (1999)
Schroën, K., Blyzniuk, O., Muijlwijk, K. et al.: Microfluidic emulsification devices: from micrometer insights to large-scale food emulsion production. Curr. Trends Food Sci. 3, 33–40 (2015)
Schubert, H., Armbruster, H.: Principles of formation and stability of emulsions. Int. Chem. Eng. 32, 14 (1992)
Schuchmann, H.P.: Food process engineering research and innovation in a fast changing world – paradigms/case studies. In: Advances in Food Process Engineering Research and Applications. Springer (2013).
Schuchmann, H.P., Hecht, L.L., Gedrat, M., et al.: High-pressure homogenization for the production of emulsions. In: Eggers, R. (ed.) Industrial High Pressure Applications. Processes, Equipment and Safety, pp. 97–118. Wiley-VCH Verlag, Weinheim (2012)
Smulders, P.E.A.: Formation and Stability of Emulsions Made with Proteins and Peptides. PhD thesis. Wageningen University, Wageningen, The Netherlands (2000).
Steegmans, M.L.J., Schroën, C.G.P.H., Boom, R.M.: Characterization of emulsification at flat microchannel Y junctions. Langmuir 25, 3396–3401 (2009)
Stillwell, M.T., Holdich, R.G., Kosvintsev, S.R., et al.: Stirred cell membrane emulsification and factors influencing dispersion drop size and uniformity. Ind. Eng. Chem. Res. 46, 965–972 (2007)
Sugiura, S., Nakajima, M., Iwamoto, S., et al.: Interfacial tension driven monodispersed droplet formation from microfabricated channel array. Langmuir 17, 5562–5566 (2001)
Suzuki, K., Hayakawa, K., Hagura, Y.: Preparation of high concentration o/w and w/o emulsions by the membrane phase inversion emulsification using PTFE membranes. Food Sci. Technol. Res. 5, 234–238 (1999)
Urban, K., Wagner, G., Schaffner, D., et al.: Rotor-stator and disc systems for emulsification processes. Chem. Eng. Technol. 29(1), 1–31 (2006)
Van Dalen, G.: Determination of the water droplet size distribution of fat spreads using confocal scanning laser microscopy. J. Microsc. 208, 116–133 (2002)
Van der Graaf, S., Nisisako, T., Schroën, C.G.P.H., et al.: Lattice Boltzmann simulations of droplet formation in a T-shaped micro-channel. Langmuir 22, 4144–4152 (2006)
Van der Graaf, S., Schroën, C.G.P.H., Boom, R.M.: Preparation of double emulsions by membrane emulsification—a review. J. Membr. Sci. 251, 7–15 (2005)
Van der Graaf, S., Steegmans, M.L.J., Van der Sman, R.G.J., et al.: Droplet formation in a T-shaped microchannel junction: a model system for membrane emulsification. Coll. Surf. A Phys. Eng. Asp. 266, 106–116 (2005)
Van der Zwan, E.A.: Emulsification with Microstructured Systems. Process Principles. PhD thesis. Wageningen University, Wageningen, The Netherlands (2008)
Van der Zwan, E.A., Schroën, C.G.P.H., Boom, R.M.: Pre-mix membrane emulsification by using a packed layer of glass beads. AICHE J. 54, 2190–2197 (2008)
Van Dijke, K.C., De Ruiter, R., Schroën, K., et al.: The mechanism of droplet formation in microfluidic EDGE systems. Soft Matter 6, 321–330 (2010)
Van Dijke, K.C., Schroën, C.G.P.H., Van der Padt, A., et al.: EDGE emulsification for food-grade dispersions. J. Food Eng. 97(3), 348–354 (2010)
Van Dijke, K.C., Veldhuis, G., Schroën, C.G.P.H., et al.: Parallelized edge-based droplet generation (EDGE) devices. Lab Chip 9, 2824–2830 (2009)
Van Rijn, C.J.M., Nano and Micro Engineered Membrane Technology. Membrane Science and Technology Series 10. Elsevier, Amsterdam. ISBN 0444514899, 9780444514899, 384 p (2004)
Van Rijn, C.J.M., Elwenspoek, M.C.: Micro filtration membrane sieve with silicon micro machining for industrial and biomedical applications. Proc. IEEE 29, 83–87 (1995)
Vladisavljevic, G.T., Schubert, H.: Preparation of emulsions with a narrow particle size distribution using microporous -alumina membranes. J. Disp. Sci. Technol. 24, 811–819 (2003)
Vladisavljevic, G.T., Tesch, S., Schubert, H.: Preparation of water-in-oil emulsions using microporous polypropylene hollow fibers: influence of some operating parameters on droplet dize distribution. Chem. Eng. Process. 41, 231–238 (2002)
Vladisavljevic, G.T., Williams, R.A.: Recent developments in manufacturing emulsions and particulate products using membranes. Adv. Colloid Interface. Sci. 113, 1–20 (2005)
Vladisavljević, G.T., Kobayashi, I., Nakajima, M.: Production of uniform droplets using membrane, microchannel, and microfluidic emulsification devices. Microfluid. Nanofluid. 13, 151–178 (2012)
Walstra, P.: Formation of emulsions. In: Becher, P. (ed.) Encyclopedia of Emulsion Technology. Basic aspects, vol. 1, pp. 58–127. Marcel Dekker, New York (1983)
Walstra, P.: Emulsion stability. In: Becher, P. (ed.) Encyclopedia of Emulsion Technology, vol. 4. Marcel Dekker, New York (1996)
Walstra, P.: Physical Chemistry of Foods. Marcel Dekker, New York (2003)
Walstra, P., Wouters, J.T.M., Geurts, T.J.: Dairy Science and Technology. Taylor & Francis, Boca Raton (2006)
Walstra, P., Smulders, P.E.A.: Emulsion formation. In: Binks, B.P. (ed.) Modern Aspects of Emulsion Science, pp. 56–99. The Royal Society of Chemistry, Cambridge (1998)
Yuan, Q., Houa, R., Aryantia, N., et al.: Manufacture of controlled emulsions and particulates using membrane emulsification. Desalination 224, 215–220 (2008)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Schroën, K., Berton-Carabin, C.C. (2016). Emulsification: Established and Future Technologies. In: Merkus, H., Meesters, G. (eds) Production, Handling and Characterization of Particulate Materials. Particle Technology Series, vol 25. Springer, Cham. https://doi.org/10.1007/978-3-319-20949-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-20949-4_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20948-7
Online ISBN: 978-3-319-20949-4
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)