Abstract
EDGE (Edge-based Droplet GEneration) emulsification systems with the ability to produce multiple droplets simultaneously from a single nozzle, were used for the preparation of monodispersed oil-in-water emulsions. The devices (with plateau height of 1 µm) were coated with metals (Cu, CuNi and CuNi/Cu) and had different surface roughness and wettability properties. This influenced the emulsification behavior significantly. The large surface roughness of the CuNi/Cu coated system resulted in stronger non-uniform filling of the plateau as compared to the smoother surfaces of Cu and less rough CuNi, and less droplet formation points in the CuNi/Cu coated system relative to the Cu and CuNi systems. The less hydrophilic CuNi surface, however, provided wider pressure stability than the more hydrophilic Cu and CuNi/Cu surface. A narrower pressure stability (Cu surface) and lower number of droplet formation points (CuNi/Cu surface) resulted in lower overall droplet formation frequency when compared with CuNi system. All metal coated EDGE systems reliably produced monodispersed droplets (with sizes being 6 times the plateau height), similar to the silicon-based EDGE systems having much smoother surfaces. The pressure stability for CuNi coated surfaces was wider, while the droplet formation frequency was comparable to that with the silicon system. This indicated that the use of metal is not a limitation in these systems as initially expected, but may be used for more robust and productive emulsification systems, which lend themselves well for scale-out to practical productivity rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Emulsions with uniformly dispersed droplets are important in the preparation of for example foods, cosmetics, pharmaceuticals and petrochemicals. Traditional techniques for emulsion preparation (colloid mills, high pressure homogenizers and rotor stator systems) are known for inefficient use of energy, and may negatively influence the product quality by heating and subjection of the product to shear stress, which limits the use of some ingredients (starch, proteins, etc.) in emulsion formulation (Charcosset et al. 2004). In addition, the produced emulsions are usually highly polydispersed; droplet sizes range from 1 to 5 µm for colloidal mills, 0.05–1 µm for high pressure homogenizers and 2–10 µm for blenders, with coefficients of variation (CV) approaching 40 % (Saito et al. 2006; Sugiura et al. 2002). This induces an increasing demand for systems that can provide a better control over droplet size and can produce emulsions using less energy.
Several microfluidic systems have been introduced in recent years which are able to produce uniformly sized droplets using much less energy as compared to the traditional emulsification systems (Lambrich and Schubert 2005). Most common examples are T-, Y- junctions, flow focussing devices and microchannels, all using different droplet formation mechanisms. In T- and Y-junctions, a cross flowing continuous phase is used to shear off a droplet growing from a narrow pore through which the disperse phase is pushed (van der Graaf et al. 2005; Steegmans et al. 2009a, b). In flow-focusing devices both the phases flow in the same direction with continuous phase flowing at much higher speed which causes longitudinal extension of the droplet resulting in droplet breakup (Utada et al. 2005, 2007). Droplet formation in microchannels takes place through a spontaneous mechanism driven by Laplace pressure differences (Sugiura et al. 2002; Kobayashi et al. 2002).
All mentioned systems are single droplet formation techniques (droplets are produced sequentially from one droplet formation unit) and produce highly monodispersed emulsions (CV < 10 %) (van Dijke et al. 2009). Their single-nozzle productivity is not sufficient to be appropriate for large scale applications, while scale-up of these systems through mass parallelization (especially for narrow structures meant to produce droplets of sizes <10 µm) is a challenge because of the difficulties related to the flow control of phases, fabrication inaccuracies and pressure gradients resulting in low working efficiency (van Dijke et al. 2010d; Kobayashi et al. 2008a).
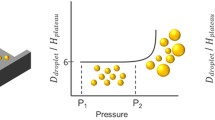
We have recently introduced a new droplet formation technique called EDGE (Edge-based Droplet Generation) which enables us to produce multiple monodispersed droplets concurrently from a single droplet formation unit (DFU) (van Dijke et al. 2010d). An EDGE DFU is a wide and flat cavity called a plateau situated in between an oil channel and a continuous phase channel. Droplet formation from a typical silicon-based EDGE device is shown in Fig. 1. The dispersed phase is pressurized through the dispersed phase channel, spreads over the plateau and on reaching the edge of the plateau it spontaneously forms monodispersed droplets at several locations along the entire length of the plateau. The size of the droplets is determined by the height of the plateau: a scaling factor of 6–8 has been observed. Single emulsions (O/W), double emulsions (W/O/W) and foams have been successfully prepared through EDGE emulsification (van Dijke et al. 2010c). The technique is simple in operation, stable within a reasonable pressure range and can easily and robustly be scaled-up (van Dijke et al. 2009).
Droplet formation through a typical EDGE device (reprinted from van Dijke et al. (2010d))
Several construction materials have been employed in the preparation of microfluidic devices; e.g. glass (Steegmans et al. 2009c; van der Graaf et al. 2006; Shah et al. 2008), silicon (Kobayashi et al. 2008b; van Dijke et al. 2010d) and polymer (Liu et al. 2004, 2005; Barbier et al. 2006) have been reported, but metals, being the preferred materials for industries, are still lacking in literature. Tong et al. (2001) have claimed the preparation of O/W microspheres using stainless steel microchannels. Microchannels with uniform dimensions could not be fabricated because of multicrystal property of stainless steel. Droplets produced from individual channels were monodispersed, however, they did not report on the effects of metal surface characteristics on emulsification behaviour.
In the current study, we move the EDGE technology a step forward using chips that have metallic surfaces (Cu and CuNi) and use them for the preparation of O/W emulsions with droplets of sizes <10 µm. The most important aspects which need to be considered are surface wettability and surface roughness, and to investigate these aspects before moving to a completely metal systems, we decided to use metal coated surfaces as a first step, and use that in combination with a glass cover plate to allow visual observation. We chose to use Cu and CuNi because these metals have been employed successfully in chips before, and we expect that changes in wettability and roughness will occur. We see this as first step toward stainless steel which is the preferred option for industry, but for which application in chips is still a challenge. We report here on the pressure stability of the semi-metal chips, the number of droplet formation points, and the droplet formation frequency in relation to surface properties (topography and wettability), and compare their behaviour to ‘standard’ silicon EDGE chips.
2 Materials and methods
2.1 Chemicals
n-Hexadecane (C16H34, 99 %) from Merck KGaA (Germany) was used as dispersed phase. MilliQ ultra-pure water with 0.5 % (wt./vol.) sodium dodecyl sulphate (SDS) from Sigma-Aldrich (Japan) was used as the continuous phase. The viscosities of the dispersed and continuous phases were 3.34 and 1.00 mPas, respectively, and were measured using a rheometer (MCR 301, Anton Paar, Gran, Austria) with Couette geometry. Rate sweeps were performed with shear rates from 2 to 100 s−1 at a controlled temperature of 20 °C. Each of the 29 shear rates were applied for a constant time of 5 s.
2.2 Chip design
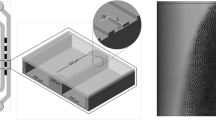
Construction of metal EDGE systems with precisely defined plateaus of micrometer size is currently not possible because of limitations in the precision of metal fabrication, even though the technology progresses fast. Therefore, to proceed with metal surfaces, we designed a system in which the plateau and the channels consisted of metals and were covered by a glass plate to allow the visual observation of droplet formation. The microstructures were fabricated on glass plates (1.5 × 1.5 cm) through a wet etching technique (Micronit Microfluidics, Enschede, The Netherlands) with a bottom plate having the supply channels (Fig. 2a) and the top plate carved with the plateau (Fig. 2b). The supply channels (with depth of 175 µm and width of 500 and 400 µm for dispersed and continuous phase channels, respectively) and the area specified for the plateau (in between the channels) were coated with Cu (300 nm), CuNi (300 nm) or CuNi/Cu (with 200 nm of Cu deposited on 100 nm seed layer of CuNi) using standard sputtering technique, as indicated by the orange color in Fig. 2. The systems were defined accordingly as EDGE-Cu, EDGE-CuNi and EDGE-CuNi/Cu. Both the plates were bonded together keeping the (engraved) plateau in between the dispersed and continuous phase channels such that the bottom of the plateau was metal and the top being the glass enabling visual inspection of the emulsification process. The plateaus used in current experiments were 500 µm wide and 1 µm high. The length of the plateaus ranged from 200 µm (in the center) to 300 µm (at the ends). All the chips were oxidized either through annealing (EDGE-CuNi and EDGE-CuNi/Cu) at high temperature (575 °C) or through plasma oxidation (EDGE-Cu) to render their surfaces hydrophilic.
2.3 Experimental procedure
The emulsification process was observed with a high-speed camera (motionPro HS-4, Redlake MASD Inc., San Diego, CA) connected to a microscope (Axiovert 200 MAT, Carl Zeis B. V., Sliedrecht, The Netherlands). MotionPro Studio software (Redlake MASD Inc.) was used to control the camera. The maximum magnification was 2,500× and maximum frame rate was 10,000 frames s−1. The combination of magnification and frame rate was limited by the amount of light reaching the high-speed camera sensor. The microchip was fixed in a custom-made module (Micronit Microfluidics, Enschede, the Netherlands) and was placed on the microscope table. The continuous phase entered the system through 0.030 in. PEEK tubing (Grace Davison Discovery Sciences, Deerfield, IL) connected to a 10 mL Hamilton gastight Luer Lock syringe (Bonaduz, Switzerland) placed in a Harvard Apparatus (Holliston, MA) PHD 2000 syringe pump. Typical flow rates were between 300 and 1,000 µl/h. The dispersed phase was pumped through PEEK tubing (0.030 in.) connected to a pressurized vessel. A digital pressure controller controlled with Flowplot V3.25 and FlowView 2 V1.15 software (Bronkhorst, Ruurlo, The Netherlands) was used to set and regulate the applied pressure of the dispersed phase. Image analysis software (Image Pro plus 4.5) was used to measure the droplet size and size distribution.
2.4 Surface characteristics
2.4.1 Roughness
The roughness of the metal coated surfaces was measured using an AFM (Nanoscope Multimode IIIa, Bruker) provided with a standard V-shaped silicon nitride tip (NP, Bruker). Triplicate measurements were made for each surface; we report the root mean square roughness values, which are the average of these three measurements.
2.4.2 Contact angle
Because our plateaus consisted of metal at the bottom and glass at the top, contact angles were measured separately for both the metal and glass surfaces. Flat glass plates were coated separately with Cu, CuNi and CuNi/Cu using the standard sputtering technique applied in chip manufacture (Micronit Microfluidics, The Netherlands). For both (metal) coated and uncoated glass plates, the three phase contact angle was measured using contact angle measuring system (G10, KRUSS GmbH, Germany). The plates (annealed or plasma oxidized) were immersed in the continuous water phase containing 0.5 % (wt./vol.) SDS, and a drop of hexadecane was put on the surface. The contact angle was measured in triplicate after the shape of the drop became stable; the mentioned values are always the average of these three measurements.
3 Results and discussions
3.1 Surface characteristics
It is expected that topography and wettability of any microfluidic system and especially of the plateau in EDGE systems significantly influence the emulsification behavior. For successful emulsification through microfluidic devices, the continuous phase should preferentially wet the surface implying that the dispersed phase should have a three-phase contact angle of >90° (Tong et al. 2000; Kawakatsu et al. 2001; Kobayashi et al. 2003). Kawakatsu et al. (2001) investigated the effect of contact angle on w/o emulsification through microchannels and found that stable emulsification was only possible at contact angle of above 120°. We measured three-phase contact angles of oil (hexadecane) droplets on the surfaces dipped into water containing 0.5 % (wt./vol.) SDS (see Table 1). All the surfaces were reasonably hydrophilic (contact angles ≫90°, while still showing an appreciable difference in contact angle) and were therefore suitable for O/W emulsification.
Figure 3 shows the three-dimensional surface topography of the metal coated surfaces. The CuNi/Cu is clearly rougher than CuNi which is rougher than Cu; as is also evident from root mean square roughness shown in Table 1. All the surfaces were quite rough as compared to glass (and silicon).
3.2 Invasion of the plateau and location of droplet formation points
Water containing 0.5 % (wt./vol.) SDS was first introduced through the respective channel to wet the plateau with continuous phase. Subsequently, the pressure on the to-be-dispersed phase was increased to fill the dispersed phase channel with hexadecane. The pressure on the to-be-dispersed phase was then gradually increased throughout the emulsification process and the spread of the dispersed phase onto the plateau was observed as a function of the applied pressure. The dispersed phase entered the plateau at the invasion (Laplace) pressure and with higher pressures started filling up the plateau. On reaching the edge, the dispersed phase started generating monodispersed droplets in the continuous phase at the breakthrough pressure. Figure 4 shows the droplet formation in typical EDGE-Cu and EDGE-CuNi/Cu systems. The droplets retained their uniformity with increasing pressure until blow-up pressure was reached. At this pressure, the droplet size increased rapidly at some droplet formation locations, resulting in polydispersed emulsions.
Droplet formation through EDGE-Cu (a) and EDGE-CuNi/Cu micro devices (b) with magnification of a droplet formation point in the top left corner. Dispersed phase (oil) is pushed from the dispersed phase channel (right) to the continuous phase (water) channel (left) through the plateau (middle). Disperse phase behavior (fingering) can be seen on the plateau, while a clear image of droplets produced by EDGE-CuNi micro devices collected on a glass slide can be seen in (c)
In silicon-based EDGE systems (having smoother surfaces), the plateau was uniformly and regularly filled with the dispersed phase, and droplet formation was observed at seemingly regularly spaced droplet generation locations (van Dijke et al. 2010a). Similarly, in EDGE-Cu system, the plateau was also filled almost uniformly with dispersed phase. However, the dispersed phase, upon reaching the edge of the plateau, split into so-called (small) fingers (distributed throughout the length of the edge) with each finger giving rise to a droplet formation point. Droplet formation points were irregularly spaced with shortest and longest distances between them being 7 and 80 µm, respectively.
In the EDGE-CuNi system, the dispersed phase, after entering the plateau, moved towards its edge and almost in middle of the plateau it split into separate flow paths giving rise to fingers. Each finger, upon reaching the edge, started functioning as a droplet formation point. Initially, the fingers were unstable, i.e. the fingers detached from the rest of the dispersed phase on the plateau, while generating droplets until the entire length of the finger disappeared. Then, new fingers arose at approximately the same points. However, at somewhat increased pressures, the fingers became stable and stayed connected with the dispersed phase. At the breakthrough pressure only the middle part of the plateau was filled with dispersed phase and droplet formation was observed only there. With increasing pressure, the dispersed phase spreads towards the plateau ends; before blow-up pressure was reached the whole plateau was uniformly filled with dispersed phase and droplet formation points were present along the entire length of the edge. A total of 13 irregularly spaced droplet formation points were observed.
In Fig. 5a, the droplet formation points (DFP) are indicated in relation to the lower (−1) and upper (+1) end of the plateau; the lower and upper ends are defined relative to the stage of the microscope. The measurements were repeated with three different chips, and total number of droplet formation points was found to be almost same with a variation of ±1. In EDGE-CuNi most of the DFPs maintained their locations throughout the applied pressure range; however, in some cases, generation of new droplet formation points caused the surrounding DFPs to change their positions. This can be observed at 30 kPa where a DFP (triangles) was generated and surrounding DFPs adapted to it by slightly changing their positions. Some of the droplet formation points disappeared at some pressure and either completely vanished (filled circles) or re-appeared (multi) at higher pressure. The shortest and longest distances (at blow-up pressure) between droplet formation points (δ drop) were 11 and 48 µm, respectively.
As mentioned, in the EDGE-CuNi/Cu system, the dispersed phase split into fingers soon after entering the plateau, which was initially not smoothly filled with dispersed phase, widely spaced fingers occurred and droplet formation (at breakthrough pressure) was observed only in middle part of the plateau. At higher pressure, more droplet formation points were formed (as also indicated in Fig. 5b). The area covered by the dispersed phase increased, while the length of fingers decreased with increasing pressure. At blow-up pressure, most of the plateau was uniformly filled with dispersed phase and in total 10 fingers arising from the dispersed phase were making droplets along the edge as can be seen in Fig. 4b.
In the EDGE-CuNi/Cu system, the droplet formation points (DFPs), once they appeared, seemed to maintain their positions more than was observed in the EDGE-CuNi system throughout the applied pressure range. The positions did not change when a new DFP was generated in-between two already existing closely spaced DFPs, as can be seen at 35 kPa, where a DFP (−) appears between two narrowly spaced DFPs (open circles) and (diamonds) (Fig. 5b). As mentioned, the total number of DFPs was 10 at maximum pressure with shortest and longest distances between them (δ drop) being 21 and 70 µm, respectively.
van Dijk et al. (2010a) investigated the droplet formation using silicon-based EDGE systems and found that when a new DFP is generated by increasing pressure, all other points adopt to this resulting in a decrease in droplet formation distances and a shift in their positions. The distances between the droplet formation points were found to be scaling with 25× the plateau height. The DFPs were regularly spaced with distances between them being around 29 µm for a plateau height of 1.2 µm. This is different with the semi-metal EDGE chips investigated here: the droplet production seems here more irregular, but still has quite high pressure stability (except EDGE-Cu) and productivity (see Sect. 3.3).
The effect of the surface roughness on the flow of two immiscible fluids flowing through parallel plates having undulated surfaces was analyzed by Luo et al. (2006). It was found that roughness may cause fingering of the proceeding fluid interface because of local pinning of the contact line as a result of which the contact line locally moves more slowly when compared with the front tip of fluid–fluid interface. This results in strong deformation of the interface and fingering. This is also what we observed in semi-metal chips: the dispersed phase split into fingers when pushed onto the plateau. The higher surface roughness of the EDGE-CuNi/Cu system (see Table 1) resulted in fingers being formed soon after plateau invasion, as compared to the EDGE-CuNi system that formed fingers in middle of the plateau, and EDGE-Cu system that formed fingers very close to the edge. Early fingering caused irregular filling of the plateau, which resulted ultimately in a lower number of droplet formation points (10 vs. 13 for EDGE-CuNi). At low pressure (10 kPa) EDGE-Cu with minimum surface roughness had higher number of droplet formation points as compared to EDGE-CuNi and EDGE-CuNi/Cu (8 vs. 4 for both CuNi and CuNi/Cu systems); however, this value could not be greatly increased (maximum of 11) because of low pressure stability of the EDGE-Cu system (as will be discussed in next section). One should note that this fingering did not result in any coalescence or other instabilities during droplet formation. In that respect, the roughness (if not too high) may help in increasing the productivity of the system: the droplet formation points may be located at closer distances resulting in a higher number of droplet formation points per unit length of the edge.
3.3 Pressure stability and productivity of the system
In EDGE emulsification, the pressure applied on the to-be-dispersed phase gives important information on emulsification behavior. The minimum pressure required to initiate the plateau invasion and droplet generation can be calculated by Laplace’s law as (van Dijke et al. 2010a)
where σ is the interfacial tension, θ is the contact angle and R 1 and R 2 are the radii of curvature of the oil–water interface corresponding to plateau height (R 1) and its width (R 2) when completely filled. The effect of the applied pressure on the average droplet size and size distribution is shown in Fig. 6. For comparison, data points for a purely silicon-based system (EDGE-silicon) are also given in the same figure.
Average droplet diameter (a) and coefficient of variation CV (b) as a function of pressure using the EDGE-Cu system (circles), EDGE-CuNi system (plus), EDGE-CuNi/Cu system (diamonds) and EDGE-silicon system (triangles). The results were repeated three times using different chips and were found to be the same in each repetition
With the EDGE-CuNi/Cu system, the breakthrough pressure was reached at 7 kPa; uniform droplets with an average size of 6.2 µm were produced at this pressure. At increasing pressures, the average droplet size remained constant albeit with a slight increase in CV, which stayed below 10 %. Above 30 kPa, the droplet size increased rapidly leading to polydispersity (CV > 10 %). Ultimately, blow-up occurred at 38 kPa, with droplet sizes of 25–30 µm produced at some points resulting in highly polydispersed emulsions.
With the EDGE-CuNi system, droplet formation started at 5.5 kPa with droplets that were highly uniform with average size of 6.3 µm. The droplet size remained constant and monodispersed (CV < 10 %) for the entire pressure range until the blow-up pressure (42.5 kPa) was reached. At this pressure, the behavior was similar to that of the EDGE-CuNi/Cu system. The EDGE-CuNi system was exceptionally stable as function of the pressure.
In contrast to EDGE-CuNi and EDGE-CuNi/Cu, pressure stability of EDGE-Cu was very low with breakthrough and blow-up pressures being 9 and 14 kPa, respectively. Within this pressure range, monodispersed droplets (CV < 8 %) with average size of 6.4 µm were produced. In fact, both EDGE-CuNi and EDGE-CuNi/Cu systems were much more stable when compared with the EDGE-Cu and EDGE-silicon as shown in Table 2. This stability is of great relevance for scale-up.
In addition to surface roughness which seems to facilitate the number of droplet formation points, the pressure stability of the systems may be linked to the contact angle or alternatively to the contact angle difference between top (glass) and bottom (silicon or metal coated) plate and it was found to increase with decreasing contact angle (or increasing contact angle difference). The EDGE-Cu system, having the largest contact angle (θ = 160°) and apparently no contact angle difference had the lowest pressure stability while the EDGE-CuNi system with its smallest contact angle and largest contact angle difference (Table 1) had the highest stability. These findings are contrary to the simulations of van Dijk et al. (2008) according to which the pressure stability decreases by decreasing dispersed phase contact angle. van Dijke et al. (2010a) also investigated the effect of the surface contact angle on the droplet size during EDGE emulsification. It was found that the droplet size decreases with increasing contact angle if the system is operated with a high viscosity ratio (i.e. above critical value reported by van Dijke et al. (2010b) and (Maan et al. 2012) as is the case here) and within the monodispersed droplet formation regime. The results of the present study are contrary to these findings: here, the droplet size was the same for all the systems (including silicon) even though the systems exhibited different contact angles. It might be that dynamic interfacial tension effects that were not covered in the geometric analysis, could be responsible for creating a higher Laplace pressure difference at higher expansion rates (frequency), therewith cancelling out the effect of the contact angle difference.
Table 2 shows a comparison of the pressure stability and productivity of the silicon- and metal-based systems. The maximum droplet formation frequency for the EDGE-CuNi system was comparable to that reported for silicon-based systems (when considering the frequency per droplet formation unit). As a result of the larger droplet size generated by the silicon EDGE system, which had a plateau height of 1.2 μm, the flux of the dispersed phase per hour per meter of the edge length is higher.
At low pressure (10 kPa) EDGE-Cu having minimum roughness had highest frequency. However, maximum droplet formation frequency found for the EDGE-CuNi/Cu and especially for EDGE-Cu system was low when compared with the EDGE-CuNi system. This can be attributed to the lower pressure stability (especially of EDGE-Cu system) and high roughness (of EDGE-CuNi/Cu). The surface roughness adds to the flow resistance on the plateau while the length of the fingers may effectively add to the total resistance in the system which is known to increase the pressure stability of microchannel emulsification systems (van Dijke et al. 2008). Low frequency of EDGE-CuNi (at 10 kPa) as compared to EDGE-CuNi/Cu can be attributed to unstable fingers in EDGE-CuNi at this pressure as already discussed in Sect. 3.2. In this respect, metal systems show similar productivity to the silicon systems, as long as their roughness is not too large. In addition, they exhibit wider pressure stability as compared to completely flat (silicon) systems, and this is an important step towards practical application of these systems.
4 Conclusion
Metal-coated microfluidic EDGE systems having different surface roughness and wettability were investigated for monodispersed oil-in-water emulsification. The plateau in the EDGE-CuNi/Cu system, having large surface roughness, was not filled uniformly, which resulted in a smaller number of droplet formation points per unit length. This ultimately leads to a lower droplet formation frequency. Droplet formation with the less hydrophilic EDGE-CuNi system was stable over a wider range of pressures than with the more hydrophilic EDGE-Cu and EDGE-CuNi/Cu surface. The productivity of the EDGE-CuNi system was found similar to the silicon-based systems.
The results indicate that a moderate surface roughness may help to decrease the distances between the locations at which droplet formation takes place and hence may increase the total number of droplet formation points per unit length of the edge. This, together with their high pressure stability, may increase the productivity of the system (compared to flat silicon based systems), which is significant for scale-out and the development of practical applications.
References
Barbier V, Tatoulian M, Li H, Arefi-Khonsari F, Ajdari A, Tabeling P (2006) Stable modification of PDMS surface properties by plasma polymerization: application to the formation of double emulsions in microfluidic systems (Langmuir). Langmuir 22(12):5230–5232. doi:10.1021/la053289c
Charcosset C, Limayem I, Fessi H (2004) The membrane emulsification process—a review. J Chem Technol Biotechnol 79(3):209–218
Kawakatsu T, Trägårdh G, Trägårdh C, Nakajima M, Oda N, Yonemoto T (2001) The effect of the hydrophobicity of microchannels and components in water and oil phases on droplet formation in microchannel water-in-oil emulsification. Colloid Surf A 179(1):29–37
Kobayashi I, Nakajima M, Chun K, Kikuchi Y, Fujita H (2002) Silicon array of elongated through-holes for monodisperse emulsion droplets. AIChE J 48(8):1639–1644
Kobayashi I, Nakajima M, Mukataka S (2003) Preparation characteristics of oil-in-water emulsions using differently charged surfactants in straight-through microchannel emulsification. Colloid Surf A 229(1–3):33–41
Kobayashi I, Takano T, Maeda R, Wada Y, Uemura K, Nakajima M (2008a) Straight-through microchannel devices for generating monodisperse emulsion droplets several microns in size. Microfluid Nanofluid 4(3):167–177
Kobayashi I, Uemura K, Nakajima M (2008b) Generation characteristics of highly uniform nonspherical droplets of soybean oil using microchannel array devices. Food Biophys 3(2):132–139
Lambrich I, Schubert H (2005) Emulsification using microporous systems. J Membr Sci 257:76–84
Liu H, Nakajima M, Kimura T (2004) Production of monodispersed water-in-oil emulsions using polymer microchannels. J Am Oil Chem Soc 81(7):705–711
Liu H, Nakajima M, Nishi T, Kimura T (2005) Effect of channel structure on preparation of a water-in-oil emulsion by polymer microchannels. Eur J Lipid Sci Technol 107(7–8):481–487
Luo X, Wang X-P, Qian T, Sheng P (2006) Moving contact line over undulating surfaces. Solid State Commun 139(11–12):623–629. doi:10.1016/j.ssc.2006.04.040
Maan A, Schroën K, Boom R (2012) Monodispersed water-in-oil emulsions prepared with semi-metal microfluidic EDGE systems. Microfluid Nanofluid 1–10. doi:10.1007/s10404-012-1037-0
Saito M, Yin LJ, Kobayashi I, Nakajima M (2006) Comparison of stability of bovine serum albumin-stabilized emulsions prepared by microchannel emulsification and homogenization. Food Hydrocolloid 20(7):1020–1028
Shah RK, Shum HC, Rowat AC, Lee D, Agresti JJ, Utada AS, Chu L-Y, Kim J-W, Fernandez-Nieves A, Martinez CJ, Weitz DA (2008) Designer emulsions using microfluidics. Mater Today 11(4):18–27
Steegmans MLJ, Schroën CGPH, Boom RM (2009a) Generalised insights in droplet formation at T-junctions through statistical analysis. Chem Eng Sci 64(13):3042–3050
Steegmans MLJ, Schroën KCGPH, Boom RM (2009b) Characterization of emulsification at flat microchannel Y junctions. Langmuir 25(6):3396–3401. doi:10.1021/la8035852
Steegmans MLJ, Warmerdam A, Schroën KGPH, Boom RM (2009c) Dynamic interfacial tension measurements with microfluidic Y-junctions (Langmuir). Langmuir 25(17):9751–9758. doi:10.1021/la901103r
Sugiura S, Nakajima M, Seki M (2002) Effect of channel structure on microchannel emulsification. Langmuir 18(15):5708–5712. doi:10.1021/la025813a
Tong J, Nakajima M, Nabetani H, Kikuchi Y (2000) Surfactant effect on production of monodispersed microspheres by microchannel emulsification method. J Surfactants Deterg 3(3):285–293
Tong J, Nakajima M, Nabetani H, Kikuchi Y, Maruta Y (2001) Production of oil-in-water microspheres using a stainless steel microchannel. J Colloid Interface Sci 237(2):239–248
Utada AS, Lorenceau E, Link DR, Kaplan PD, Stone HA, Weitz DA (2005) Monodisperse double emulsions generated from a microcapillary device. Science 308(5721):537–541. doi:10.1126/science.1109164
Utada AS, Chu LY, Fernandez-Nieves A, Link DR, Weitz DA (2007) Dripping, jetting, drops, and wetting: the magic of microfluidics. MRS Bull 32:702–708
van der Graaf S, Steegmans MLJ, van der Sman RGM, Schroën CGPH, Boom RM (2005) Droplet formation in a T-shaped microchannel junction: a model system for membrane emulsification. Colloid Surf A 266(1–3):106–116
van der Graaf S, Nisisako T, Schroën CGPH, van der Sman RGM, Boom RM (2006) Lattice Boltzmann simulations of droplet formation in a T-shaped microchannel (Langmuir). Langmuir 22(9):4144–4152. doi:10.1021/la052682f
van Dijke KC, Schroën KCGPH, Boom RM (2008) Microchannel emulsification: from computational fluid dynamics to predictive analytical model. Langmuir 24(18):10107–10115. doi:10.1021/la801411x
van Dijke KC, Veldhuis G, Schroen K, Boom RM (2009) Parallelized edge-based droplet generation (EDGE) devices. Lab Chip 9(19):2824–2830. doi:10.1039/b906098g
van Dijke KC, de Ruiter R, Schroën K, Boom RM (2010a) The mechanism of droplet formation in microfluidic EDGE systems. Soft Matter 6(2):321–330
van Dijke KC, Kobayashi I, Schroën K, Uemura K, Nakajima M, Boom RM (2010b) Effect of viscosities of dispersed and continuous phases in microchannel oil-in-water emulsification. Microfluid Nanofluid 9:77–85
van Dijke KC, Schroën K, van der Padt A, Boom RM (2010c) EDGE emulsification for food-grade dispersions. J Food Eng 97(3):348–354
van Dijke KC, Veldhuis G, Schroën K, Boom RM (2010d) Simultaneous formation of many droplets in a single microfluidic droplet formation unit. AIChE J 56(3):833–836
Acknowledgments
This work was partially funded by Higher Education Commission (HEC) of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maan, A.A., Boom, R. & Schroën, K. Preparation of monodispersed oil-in-water emulsions through semi-metal microfluidic EDGE systems. Microfluid Nanofluid 14, 775–784 (2013). https://doi.org/10.1007/s10404-012-1097-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1097-1